Abstract
Brain oxytocin (OT) regulates aspects of emotionality and stress coping including maternal behavior and maternal aggression. Maternal aggression correlates with the amount of OT released within the paraventricular nucleus (PVN) and the central amygdala (CeA). OT, a key neurotransmitter or neuromodulator, is likely to modulate other neurotransmitter systems. Here, we investigated the dynamic changes in extracellular concentrations of the amino acids aspartate, glutamate, γ-aminobutyric acid (GABA), serine, histidine, arginine and taurine in the PVN and CeA in lactating rats bred for high (HAB) and low (LAB) anxiety-related behavior under basal conditions and during maternal aggression. Further, to determine whether local OT is involved in the regulation of amino acid release we infused a selective OT receptor antagonist (OTA) via local retrodialysis. Within the CeA, HAB and LAB dams differed in the basal release of glutamate and arginine. Infusion of a selective OTA increased the concentrations of glutamate and aspartate in LAB dams and GABA in HAB dams. In OTA-treated HAB and LAB dams taurine levels increased during maternal aggression. Within the PVN, the highly-aggressive HAB dams showed a more pronounced increase in aspartate and serine levels; the latter being attenuated by local OTA administration. However, OTA did not affect the level of any amino acid in the LAB dams. Thus, the extracellular concentrations of selected amino acids differed between lactating HAB and LAB dams under both basal conditions and following maternal aggression. The effects of OT within the CeA and PVN on maternal aggressive behavior might be related to its regulation of local amino acid release.
Introduction
The neuropeptide oxytocin (OT) is known to play an important role in physiological and behavioral functions related to female reproduction and affiliation at the brain level (Richard et al. Citation1991; Insel Citation1992; Landgraf and Neumann Citation2004). OT is released within certain brain regions, including the hypothalamic paraventricular nucleus (PVN) both during parturition and in lactation (Moos et al. Citation1989; Neumann and Landgraf Citation1989; Neumann et al. Citation1993). Specifically, after parturition OT is thought to induce (Pedersen and Prange Citation1979; van Leengoed et al. Citation1987) and fine-tune the maintenance (Pedersen and Boccia Citation2002; Numan and Insel Citation2003) of maternal behavior of the rat dam.
Enhanced aggressive behavior towards an intruder is a part of the complex patterns of maternal behavior in most mammals (Erskine et al. Citation1978; Numan Citation1994; Rosenblatt et al. Citation1994; Lonstein and Gammie Citation2002; Numan and Insel Citation2003). Although the neuropeptidergic regulation of maternal aggression is not well understood, and in part controversial (Consiglio and Lucion Citation1996; Giovenardi et al. Citation1998; Johns et al. Citation1998; Elliott et al. Citation2001; Lubin et al. Citation2003), we recently demonstrated that OT is released within the PVN of lactating rats when exposed to a virgin female intruder for 10 min (maternal defense test; Bosch et al. Citation2004a, Citation2005). Interestingly, the level of maternal aggression was found to depend on the dam's innate level of anxiety with high anxiety (HAB) dams displaying more aggressive behaviors towards the intruder than dams of the low anxiety breeding line (LAB; Bosch et al. Citation2005). Thereby, the level of maternal aggression during the maternal defense test directly correlates with the amount of locally released OT, which is higher in HAB dams both in the PVN and the central amygdala (CeA; Bosch et al. Citation2005). Furthermore, application of a selective OT receptor antagonist (OTA) in these areas reduced maternal aggression in HAB rats, while local OT elevated the level of maternal aggression in LAB dams (Bosch et al. Citation2005). Thus, these data demonstrate that brain OT is a key regulatory factor in maternal aggression.
Importantly, exposure to the maternal defense test has been recently shown to present a relevant stimulus for lactating rats resulting in elevated plasma ACTH and corticosterone concentrations (Neumann et al. Citation2001), with even more pronounced hormonal and neuronal responses in HAB compared with LAB rats (unpublished).
As an established neurotransmitter/neuromodulator in the brain (Landgraf and Neumann Citation2004), OT is likely to modulate other neurotransmitter systems such as amino acids. Of these, glutamate and γ-aminobutyric acid (GABA) are particularly interesting candidates for modulation by OT because their levels were found to be altered, for example, in the CeA (Singewald et al. Citation2000) and locus coeruleus (Singewald et al. Citation1995) during emotional stress. Moreover, it has been shown that the stress-induced release of some amino acids including GABA within the hypothalamic supraoptic nucleus (SON; Engelmann et al. Citation2004), and glutamate and GABA in the CeA (Ebner et al. Citation2005) is regulated by local OT in male rats.
Therefore, in the present study we aimed to extend these findings and investigated: (i) whether extracellular concentrations of the amino acids in the PVN and/or CeA differ between lactating HAB and LAB dams indicating differences in local release, (ii) whether the release of selected amino acids is altered in lactating HAB and LAB residents in response to maternal defense, and (iii) whether local OT is involved in the regulation of amino acid release under both basal and stressful conditions. For this purpose, we monitored the dynamics of extracellular concentrations of various amino acids with and without established local neurotransmitter function including aspartate, glutamate, GABA, serine, histidine, arginine and taurine in the PVN and CeA of lactating HAB and LAB rats in the presence or absence of a selective OTA applied via retrodialysis.
Material and methods
Animals
Experiments were performed on adult female HAB and LAB Wistar rats (see review by Landgraf and Wigger (Citation2002)). Both rat lines were treated identically in terms of care, mating and behavioral testing over the last 10 years. At the age of 10 weeks, virgin female rats of both breeding lines were tested on the elevated plus-maze to confirm their high and low anxiety-related behavior, respectively, with the criterion of percentage time spent in the open arms below 10% (about 85% of HAB offspring) or above 40% (about 90% of LAB offspring) for the respective lines. The animals used in the present study are identical to the rats of an earlier study investigating the influence of OT on maternal aggression in HAB and LAB dams (Bosch et al. Citation2005). All surgical, sampling and behavioral protocols were approved by the Committee on Animal Health and Care of the local government, and are in accordance with the guide for the care and use of laboratory animals by the NIH.
Virgin female HAB and LAB rats (250–280 g body weight) were mated with an experienced male breeder (Wistar rat unselected for anxiety, 300–350 g; Charles River, Sulzfeld, Germany). Pregnant rats were housed in groups of four in standard rat cages (40 × 60 × 20 cm); from day-18 of pregnancy rats were housed individually. On day 22 or 23 of pregnancy the females delivered 8–16 pups and litters were culled to 8 pups. Rats were kept under standard laboratory conditions (12:12 h light–dark cycle, lights on at 06:00 h, 22°C, 50% humidity and free access to water and standard rat chow).
Maternal defense test
The maternal defense test was performed with lactating HAB and LAB residents and virgin intruders (Wistar rats unselected for anxiety, 230–250 g body weight; Charles River, Sulzfeld, Germany) between 10:00 and 12:00 h on lactation day 3. The virgin intruder was placed into the resident's cage for 10 min in the presence of the pups. After the 10-min testing period the virgin intruder was returned to its home cage. The behavior was recorded by a digital camera/video setup for subsequent analysis (Neumann et al. Citation2001; Bosch et al. Citation2004a, Citation2005).
Implantation of microdialysis probes
Lactating HAB and LAB rats were bilaterally fitted with microdialysis probes targeting the PVN or CeA. Specifically, on day 1 post partum, U-shaped microdialysis probes (U-length ca. 2 mm; dialysis membrane: molecular cutoff of 18 kDa; Hemophan, Gambro Dialysatoren, Hechingen, Germany; for details see Neumann et al. Citation1993) were stereotaxically implanted under isoflurane (Isofluran-Baxter, Baxter Deutschland GmbH, Unterschleissheim, Germany) anesthesia. The implantation coordinates were set relative to bregma with incisor bar at − 3.5 mm: 1.6 mm caudal, 1.8 mm lateral to midline, 9.1 mm beneath the surface of the skull, angle of 10° to avoid sagittal sinus damage for the PVN () and 2.5 mm caudal, 4.0 mm lateral, 8.5 mm deep for the CeA (), respectively, according to the stereotaxic atlas of Paxinos and Watson (Citation1998). Probes were fixed to the skull with two stainless steel screws and dental cement.
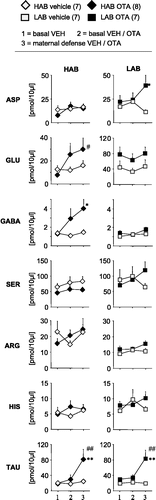
Figure 1 Concentrations of the amino acids aspartate, glutamate, GABA, serine, arginine, histidine and taurine in 30-min microdialysates obtained from the hypothalamic PVN of day 3 lactating HAB and LAB rats before (mean basal from sample 1 and 2) and during maternal defense (sample 3). The microdialysis probes were perfused with Ringer's solution at 1 μl/min. At the beginning of dialysis period 3, a virgin female intruder unselected for anxiety was introduced into the home cage of the lactating resident for 10 min with ongoing microdialysis. Data are expressed as mean + SEM. Number of rats was n = 6 and n = 7 in HABs and LABs, respectively. ##p < 0.01, #p < 0.05 vs. respective mean basal. Right: Representative microphotograph of a cresyl-stained coronal section of the rat brain showing bilateral localisation of the tips of the membranes of the microdialysis probes (arrows) within the PVN. Scale bar, 500 μm.
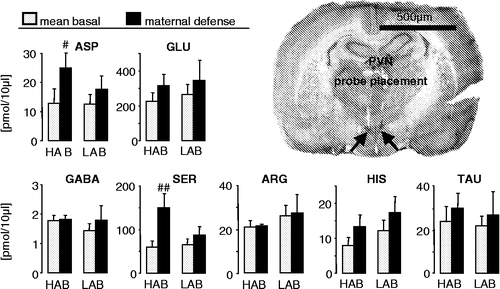
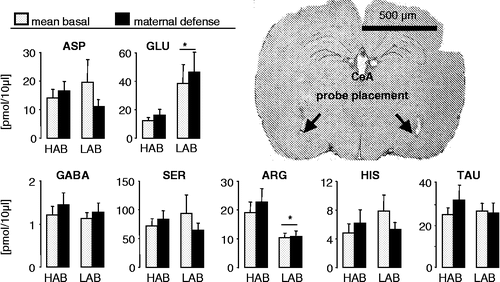
Figure 2 Concentrations of the amino acids aspartate, glutamate, GABA, serine, arginine, histidine and taurine in 30-min microdialysates obtained from the CeA of day 3 lactating HAB and LAB rats before (mean basal from sample 1 and 2) and during maternal defence (sample 3). For details see . Data are expressed as mean + SEM. Number of rats was n = 7 per group. *p < 0.05 vs. respective HAB. Right: Representative microphotograph of a cresyl-stained coronal section of the rat brain showing bilateral localisation of the tips of the membranes of the microdialysis probes (arrows) within the CeA. Scale bar, 500 μm.
Prior to implantation, probes were flushed and filled with sterile Ringer's solution (pH adjusted to 7.4; Fresenius, Bad Homburg, Germany). Subsequently, the two ends of each probe were attached to PE-20 polyethylene tubing (5 cm long) filled with Ringer's solution. After surgery, rats received a subcutaneous depot injection of antibiotic (Tardomyocel, Bayer, Leverkusen, Germany) in order to prevent infections. Rats were kept together with their litters in polycarbonate observation cages (38 × 22 × 35 cm). The following day, they were handled carefully to habituate them to the microdialysis procedure and to minimize nonspecific stress responses during the experiment.
Monitoring of amino acid concentrations within the PVN or CeA during maternal defense: Effect of local oxytocin receptor antagonist (OTA)
Two days after surgery at 08:00 h, the inflow tubing of the microdialysis probe located within the PVN or the CeA was connected to a syringe mounted onto a microinfusion pump via PE-20 tubing. The outflow was equipped with a tube holder that allowed direct sample collection into a 1.5 ml Eppendorf tube.
The microdialysis probes were perfused at a rate of 1.0 μl/min with sterile Ringer's solution for 2 h prior to the first sample collection. Three consecutive 30-min dialysates were then collected from each lactating rat. Sample 1 was taken under basal (undisturbed) conditions. During perfusion period 2, rats of both lines were dialyzed either with Ringer's solution (control groups) or with Ringer's solution supplemented with the OTA (des–Gly–NH2, d(CH2)5[Tyr(Me)2, Thr4]OVT; 10 μg/ml dissolved in Ringer's solution; Manning et al. Citation1989). At the beginning of sample 3, rats were exposed to the maternal defense test for 10 min with ongoing microdialysis of the respective perfusion medium, while their behavior was recorded by a video system as described above. Microdialysates were directly collected in Eppendorf tubes and immediately frozen on dry ice and stored at − 80°C until quantification of amino acids was performed.
Histology
After completion of the microdialysis experiments, rats were killed with an overdose of isoflurane. Brains were removed, quickly frozen in pre-chilled n-methylbutane on dry ice and stored at − 20°C. For histological verification of probe placement, 40-μm coronal brain sections were cryostat-cut and stained with cresyl violet. The rat brain atlas of Paxinos and Watson (Citation1998) was used to assist identification of the PVN and CeA. Only data for rats where microdialysis probes were confirmed to be localized in the PVN () and CeA (), respectively, were included in the study.
Quantification of amino acid content in microdialysates
The content of amino acids in microdialysates collected from the left brain hemisphere was determined by high performance liquid chromatography (HPLC) and fluorimetric detection as previously described (Singewald et al. Citation1995). Briefly, samples maintained at 7°C were derivatized with o-phthaldialdehyde/2-mercaptoethanol by an autoinjector (L-7250, Merck Hitachi). Reproducibility of the derivatization was controlled by adding S-carboxymethyl-1-cysteine as an internal standard to each sample. The amino acids aspartate, glutamate, GABA, serine, histidine, arginine and taurine were separated by gradient HPLC (L-6200 gradient pump, Merck-Hitachi) using a sodium acetate buffer (0.05 M, pH 6.9) and methanol. Excitation and emission wavelengths for the fluorescence detector (L-7485, Merck-Hitachi) were set at 330 and 450 nm, respectively. Quantification of amino acids in microdialysates was performed by an integrator (D 2500, Merck-Hitachi) against external standards at two different concentrations. Blank samples were treated identically and showed negligible amounts of the respective amino acids.
Statistics
For comparison of behavioral parameters (maternal defense test) a one-way analysis of variance (ANOVA) was performed followed by Newman–Keuls post hoc analysis where appropriate. For the comparison of amino acid release a one-way (factor time) or two-way ANOVA for repeated measurements (factors breeding line × time; factors treatment × time) was performed followed by Bonferroni post hoc analysis where appropriate. Significance was accepted at p ≤ 0.05. All statistics were performed using a computer software package (GB-Stat 6.0, Dynamic Microsystems, Silver Springs, USA; SPSS 13.1, Apache Software Foundation, Chicago, USA).
Results
Histology
Histological analysis revealed that in 24 out of 31 rats and in 29 out of 36 rats the implanted microdialysis probes were bilaterally localized within the PVN () and CeA (), respectively.
Extracellular microdialysate concentrations of amino acids from the PVN and the CeA
PVN
The content of the amino acids quantified in microdialysates including aspartate, glutamate, GABA, serine, arginine, histidine and taurine did not differ between lactating HAB and LAB rats under basal conditions (). However, when dams were exposed to maternal defense, the concentrations of aspartate (factor time: F1,78.93, p = 0.02) and serine (F1,711.6, p = 0.01) were significantly increased in HAB, but not LAB dams.
CeA
The microdialysate concentrations of the amino acids glutamate (one-way ANOVA, factor line: F1,125.32, p = 0.04) and arginine (F1,129.94, p = 0.01) differed between HAB and LAB dams under basal conditions (). Specifically, compared to LAB rats basal concentration of glutamate was lower (p < 0.05) while the concentration of arginine was higher (p < 0.05) in lactating HABs. Maternal defense did not affect the concentration of any of the amino acids studied, including glutamate and arginine in the CeA of HAB and LAB dams.
Effects of an oxytocin receptor antagonist within the PVN or the CeA on maternal aggression and the microdialysate concentrations of amino acids
PVN
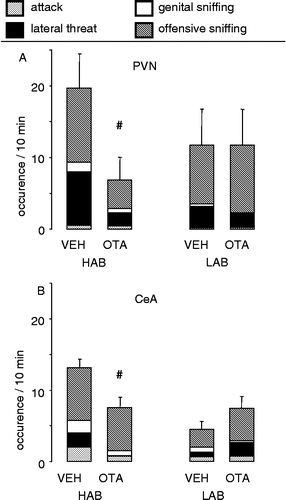
Retrodialysis of the OTA bilaterally into the PVN of lactating HAB rats significantly reduced the level of the overall offensive behavior compared with vehicle-treated control HAB dams (one-way ANOVA: F1,115.22; p = 0.04; ). In contrast, in LAB dams, the infusion of the OTA into the PVN did not change the amount of offensive behavior (; data adapted from Bosch et al. (Citation2005)).
Figure 3 Consequences on maternal aggression of infusion of the OTA bilaterally into (A) the PVN or (B) the CeA of lactating HAB and LAB residents via retrodialysis. The occurrence of attacks, lateral threats, genital sniffing after attack and offensive sniffing during the 10-min maternal defense test were monitored. Data are expressed as the sum of the aggressive behaviors + SEM. Number of rats was 6–8 per group. #p < 0.05 vs. respective VEH. Data partly reprinted from Bosch et al. (Citation2005) with permission. Copyright 2005 by the Society for Neuroscience.
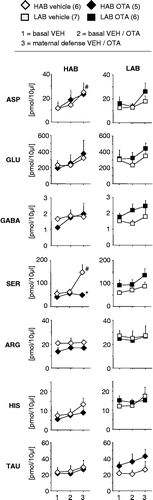
Local administration of OTA into the PVN did not affect the local concentration of any of the amino acids under basal conditions in both lactating groups (). However, in HAB dams the microdialysate concentration of serine changed over time depending on the treatment (two-way ANOVA, factors treatment x time: F2,1810.4; p = 0.001); the increase in intra-PVN serine concentration induced by maternal aggression (see above) was abolished by OTA (p < 0.05).
Figure 4 Effects of local OTA on the microdialysate concentrations of the amino acids aspartate, glutamate, GABA, serine, arginine, histidine and taurine from the PVN of lactating HAB and LAB dams, under basal conditions and in response to maternal defense. During dialysis periods 2 (basal 2) and 3 (maternal defense), either Ringer's solution (VEH) or Ringer's solution containing the OTA was used as perfusion fluid. At the beginning of dialysis period 3, a virgin female intruder was introduced into the lactating resident's home cage for 10 min with ongoing microdialysis. Vehicle-treated control animals are the same as in Figure 1. Data are presented as mean + SEM. Numbers of rats are given in parenthesis. #p < 0.05 vs. basal sample 2 of the same group; *p < 0.05 vs. respective VEH.
CeA
Retrodialysis of the OTA bilaterally into the CeA of lactating HAB dams resulted in a significant decrease in the overall offensive behavior in HAB (one-way ANOVA: F1,14 8.40; p = 0.01; ), but not in LAB dams compared with their respective controls (; data adapted from Bosch et al. (Citation2005)).
The microdialysate content of GABA in HAB dams (factors treatment × time: F2,144.78; p = 0.03; ), and of aspartate in LAB dams (F2,243.85; p = 0.04), as well as of taurine both in HAB (F2,263.41; p = 0.048) and LAB (F2,244.76; p = 0.02) dams changed over time depending on the treatment. Local infusion of OTA into the CeA increased the content of GABA in HAB dams during maternal aggression (p < 0.05) and of aspartate in LAB dams during aggression(p < 0.05). Furthermore, local retrodialysis of OTA increased the concentration of taurine during maternal defense in both lactating HAB (p < 0.01) and LAB (p < 0.01) rats. Separate statistics on OTA-treated dams only revealed that compared with basal vehicle levels the combination of blocked OT receptors and maternal aggression increased glutamate concentration in HAB dams (F2,144.32; p = 0.04).
Figure 5 Effects of local OTA on the microdialysate concentrations of the amino acids aspartate, glutamate, GABA, serine, arginine, histidine and taurine within the CeA of lactating HAB and LAB dams, under basal conditions and in response to maternal defense. For details see . Vehicle-treated control animals are the same as in . Data are presented as mean + SEM. Numbers of rats are given in parenthesis. ##p < 0.01, # < 0.05 vs. basal sample 1 of the same group; **p < 0.01, *p < 0.05 vs. respective VEH.
Discussion
The present study extends a previous one demonstrating that the protective maternal behavior of HAB dams (Neumann et al. Citation2005) is paralleled by a high level of maternal aggression during the resident-intruder test and is directly correlated with the local release of OT (Bosch et al. Citation2005). Here, we report that a possible mechanism of action of OT in the PVN and the CeA involves the regulation of local amino acid release. Specifically, we show that HAB and LAB dams differ in the basal extracellular concentrations of the amino acids glutamate and arginine in the CeA. In response to maternal defense, amino acid release within the CeA was not found to be elevated in HAB or LAB rats. However, when OT receptors in the CeA were blocked during maternal aggression, the concentrations of glutamate and GABA in HABs, of aspartate in LABs, and of taurine in both lines was increased. In contrast to the CeA, the basal release of the amino acids studied did not differ between HAB and LAB dams in the PVN. The display of a high level of maternal aggression seen in HAB dams was accompanied by a more pronounced release of aspartate and serine in the PVN irrespective of whether the OTA was applied or not. In LAB dams, neither intra-PVN OT receptor blockade nor maternal defense altered the release of any of the amino acids studied.
Thus, the finding of differences in the dynamics of changes in concentration of selected amino acids within the PVN and CeA of HAB and LAB dams especially during exposure to the maternal defense may at least in part explain their differences in maternal aggression. Moreover, differences in local OXT release patterns are likely to contribute to the regulation of local extracellular concentrations of amino acids.
In the CeA, the basal concentrations of glutamate and arginine, two amino acids known to regulate emotional responses (Masood et al. Citation2003, Citation2004; for review see Cortese and Phan (Citation2005)), was found to differ between HAB and LAB dams: glutamate was lower and arginine, a natural substrate for nitric oxide synthase, was higher in HABs. The latter finding is interesting since increased release of arginine in HAB dams may result in increased levels of nitric oxide, which is suggested to contribute to those mechanisms that mediate maternal behavior and aggression (Gammie and Nelson Citation1999; Popeski and Woodside Citation2004; Gammie et al. Citation2006), both found to be greater in HAB dams. Alternatively, it may well be that lower extracellular arginine in LABs could indicate an increased arginine–citrulline conversion and, thus, higher nitric oxide levels in LAB rats. As CSF citrulline and/or nitric oxide concentrations were not determined in HAB and LAB animals, future measurements may be helpful to exclude one or other possibility. In contrast, the hyperanxious, but highly-aggressive HAB dams showed lower amygdaloid glutamate levels than LABs. Yet, those glutamate levels found in HAB dams are comparable to the basal glutamate release in the CeA of male rats unselected for anxiety (Ebner et al. Citation2005). Glutamate provides one of the principal sources of synaptic transmission in the amygdala (Davis et al. Citation1994; Maren Citation1996; Sah et al. Citation2003) and, together with GABA, has been implicated in the modulation of emotional behavior (Davis et al. Citation1994; Singewald et al. Citation2000). Interestingly, the low level of glutamate seen in the CeA of HAB dams seems to be under inhibitory control by local OT, since local application of the OTA elevated glutamate release during exposure to the maternal defense test. In the same way, the local application of a selective OTA has been shown to disinhibit the stress-induced release of glutamate in male rats unselected for anxiety (Ebner et al. Citation2005). In addition, a reduced depolarisation-induced glutamate release from the hypothalamic SON in vitro is found after OT application (Currás-Collazo et al. Citation2003) further supporting an inhibitory effect of OT on glutamate neurotransmission. Since excitatory amino acid neurotransmission within the amygdala is stress sensitive (Singewald et al. Citation2000) and might be critically involved in stress- and anxiety-related behaviors (Adamec et al. Citation1999; Walker et al. Citation2002), the interaction between OT and glutamate within the amygdala could modulate behavioral responses to acute stress.
Importantly, in the present study local application of OTA into the CeA elevated the release of GABA in HAB dams during the maternal defense test, while, at the same time, the level of maternal aggression was reduced. In contrast, in LAB dams, an effect of OTA on local GABA release and on maternal aggression (see Bosch et al. Citation2005) was absent (). GABAergic neurotransmission within the CeA has previously been shown to be involved in OT-induced anxiolytic effects in virgin female rats (Bale et al. Citation2001). However, the contribution of locally released GABA to the regulation of maternal aggressive behavior, specifically in lactating HAB dams, remains to be shown. GABA has been more extensively studied in males, where it has been reported to inhibit isolation-induced aggressive behavior within several parts of the limbic system (DaVanzo and Sydow Citation1979; Bolin and DaVanzo Citation1982).
Either with or without OTA treatment, the amount of the inhibitory amino acid taurine sampled within the CeA was found to be similar between HAB and LAB dams under basal conditions (, ). Maternal defense did not affect taurine levels in control dams. In contrast, when OT receptors were antagonized during maternal defense, taurine release was elevated in both lines. In untreated male rats taurine levels increase in the SON during social defeat (Engelmann et al. Citation2004). In this hypothalamic brain region, however, taurine has been shown not to interact with the spontaneous activity of OT neurons or the release of OT, but to decrease stressor-induced release of vasopressin (Engelmann et al. Citation2001), a regulator of aggressive behavior in male rats (Elkabir et al. Citation1990; Everts et al. Citation1997; Ferris Citation2005; Veenema et al. Citation2005). Since the highly-aggressive behavior of HAB dams during maternal defense is associated with increased vasopressin release within the CeA as previously shown (Bosch et al. Citation2004b), it may be speculated that the increased release of OT within the CeA during maternal aggression (Bosch et al. Citation2005) inhibits the release of taurine (this study), which might disinhibit vasopressin release (Bosch et al. Citation2004b). Such a circuit would enable both neuropeptides—OT and vasopressin—to promote maternal aggression in the CeA. It remains to be shown to what extent the increased taurine release in the CeA contributes to the low-level of maternal aggression seen in LAB dams. With respect to the origin of extracellular taurine, it could be either released as a neurotransmitter from neurons, but also from glial cells as shown within the SON (Bres et al. Citation2000; Engelmann et al. Citation2001)
To our knowledge, nothing is known so far about the regulation of amino acid release within the hypothalamus of the female brain during stressful situations such as exposure to the maternal defense test and the display of maternal aggression. In the present study, the basal release of the amino acids investigated did not differ between HAB and LAB dams while aspartate and serine levels increased only in the PVN of HAB dams in response to maternal defense. Similarly, though not directly comparable, increased hypothalamic aspartate and serine tissue levels have been reported in the aggressive Spanish fighting-bull relative to the non-aggressive Friesian strains (Munoz-Blanco et al. Citation1986) supporting our idea. Moreover, since blockade of the OT receptors in the PVN of HAB dams, which has previously been shown to attenuate the level of maternal aggression against the virgin intruder (Bosch et al. Citation2005), reduced the release of serine, this is the first evidence for a possible involvement of hypothalamic OT interacting with amino acids in the control of maternal aggression. Although interactions with other relevant neuroactive substances including vasopressin (Neumann et al. Citation2006), noradrenaline (Kendrick et al. Citation1992; Lipschitz et al. Citation2004; for review see Onaka (Citation2004)), and prolactin (Egli et al. Citation2006; Kokay et al. Citation2006) can not be excluded, an interplay between OT and particularly serine at the level of the PVN is likely to mediate, at least in part, maternal aggressive behavior. Indeed, d-serine has been shown to be an endogenous co-agonist at N-methyl-d-aspartate (NMDA) receptors (Mothet et al. Citation2000) enhancing, for example, long-term potentiation (Krasteniakov et al. Citation2005) and Ca2 + -responses (Chaban et al. Citation2004), and NMDA stimulates the release of OT in the PVN of the lactating rat (Parker and Crowley Citation1995).
Finally, in lactation the expression and the release of OT itself as well as the expression (Young et al. Citation1997) and binding (Insel Citation1992; Freund-Mercier et al. Citation1994) of OT receptors are generally enhanced in various brain regions. These alterations are believed to contribute to the profound adaptations in emotionality, in general stress responsiveness, and in the onset and maintenance of maternal behavior including maternal aggressive behavior (for review see Neumann (Citation2002)). However, the expression of OT receptors within relevant brain areas like the PVN and CeA did not differ between lactating HAB and LAB rats (Bosch et al. Citation2005). Therefore, the differences between HAB and LAB dams with respect to: (i) local OT release, and (ii) OT interactions with local amino acid release may contribute to the display of varying patterns of maternal aggression.
In summary, our results demonstrate for the first time that the release of selected amino acids like glutamate and arginine (CeA) and aspartate and serine (PVN) differs between lactating HAB and LAB dams. The differential release of OT within the PVN and the amygdala demonstrated before is likely to contribute to the regulation of maternal aggression via selective inhibition of glutamate and GABA release within the CeA, and excitation of serine release within the PVN. In future studies, retrodialysis of specific antagonists for the receptors of, e.g. GABA or glutamate into the PVN or CeA during maternal defense could provide us more information on the role of these amino acids in the OT-mediated aggression in rat dams.
Acknowledgements
We are grateful to Dr M. Manning for his generous gift of the OT receptor antagonist, and to Dr D. Slattery for critical comments on the manuscript. We thank M. Fuchs and G. Schindler (Regensburg) for excellent technical help.
References
- Adamec RE, Burton P, Shallow T, Budgell J. Unilateral block of NMDA receptors in the amygdala prevents predator stress-induced lasting increases in anxiety-like behavior and unconditioned startle-effective hemisphere depends on the behavior. Physiol Behav 1999; 65: 739–751
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: Importance in regulation of anxiety and sex behaviour. J Neurosci 2001; 21: 2546–2552
- Bolin P, DaVanzo JP. The influence of isolation and aminooxyacetic acid (AOAA) on GABA in muricidal rats. Psychopharmacology (Berlin) 1982; 76: 367–370
- Bosch OJ, Krömer SA, Brunton P, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience 2004a; 124: 439–448
- Bosch OJ, Meddle SL, Neumann ID. Local vasopressin release in the maternal brain during pup defence and its behavioural relevance for maternal aggression. Forum Eur Neuro Sci Abstr 2004b; A163.2
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. J Neurosci 2005; 25: 6807–6815
- Bres V, Hurbin A, Duvoid A, Orcel H, Moos FC, Rabie A, Hussy N. Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells. Br J Pharmacol 2000; 130: 1976–1982
- Chaban VV, Li J, Ennes HS, Nie J, Mayer EA, McRoberts JA. N-methyl-d-aspartate receptors enhance mechanical responses and voltage-dependent Ca2+ channels in rat dorsal root ganglia neurons through protein kinase C. Neuroscience 2004; 128: 347–357
- Consiglio AR, Lucion AB. Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol Behav 1996; 59: 591–596
- Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr 2005; 10: 14–15
- Currás-Collazo MC, Gillard ER, Jin J, Pandika J. Vasopressin and oxytocin decrease excitatory amino acid release in adult rat supraoptic nucleus. J Neuroendocrinol 2003; 15: 182–190
- DaVanzo JP, Sydow M. Inhibition of isolation-induced aggressive behavior with GABA transaminase inhibitors. Psychopharmacology (Berlin) 1979; 62: 23–27
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci 1994; 17: 208–214
- Ebner K, Bosch OJ, Krömer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behaviour and the release of excitatory amino acids. Neuropsychopharmacology 2005; 30: 223–230
- Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME. Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab 2006; 290: E566–E572
- Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept 1990; 28: 199–214
- Elliott JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: Implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides 2001; 35: 127–134
- Engelmann M, Ludwig M, Singewald N, Ebner K, Sabatier N, Lubec G, Landgraf R, Wotjak CT. Taurine selectively modulates the secretory activity of vasopressin neurons in conscious rats. Eur J Neurosci 2001; 14: 1047–1055
- Engelmann M, Bull PM, Brown CH, Landgraf R, Horn TF, Singewald N, Ludwig M, Wotjak CT. GABA selectively controls the secretory activity of oxytocin neurons in the rat supraoptic nucleus. Eur J Neurosci 2004; 19: 601–608
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav Biol 1978; 23: 206–218
- Everts HG, De Ruiter AJ, Koolhaas JM. Differential lateral septal vasopressin in wild-type rats: Correlation with aggression. Horm Behav 1997; 31: 136–144
- Ferris CF. Vasopressin/oxytocin and aggression. Novartis Found Symp 2005; 268: 190–198
- Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: Histoautoradiographic detection in the lactating rat. J Physiol 1994; 480: 155–161
- Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci 1999; 19: 8027–8035
- Gammie SC, Auger AP, Jessen HM, Vanzo RJ, Awad TA, Stevenson SA. Altered gene expression in mice selected for high maternal aggression. Genes Brain Behav 2006, [Epub ahead of print]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: Effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav 1998; 63: 351–359
- Insel TR. Oxytocin—a neuropeptide for affiliation: Evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 1992; 17: 3–35
- Johns JM, Noonan LR, Zimmerman LI, McMillen BA, Means LW, Walker CH, Lubin DA, Meter KE, Nelson CJ, Pedersen CA, Mason GA, Lauder JM. Chronic cocaine treatment alters social/aggressive behavior in Sprague–Dawley rat dams and in their prenatally exposed offspring. Ann NY Acad Sci 1998; 846: 399–404
- Kendrick KM, Keverne EB, Hinton MR, Goode JA. Oxytocin, amino acid and monoamine release in the region of the medial preoptic area and bed nucleus of the stria terminalis of the sheep during parturition and suckling. Brain Res 1992; 569: 199–209
- Kokay IC, Bull PM, Davis RL, Ludwig M, Grattan DR. Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol Regul Integr Comp Physiol 2006; 290: 1216–1225
- Krasteniakov NV, Martina M, Bergeron R. Role of the glycine site of the N-methyl-d-aspartate receptor in synaptic plasticity induced by pairing. Eur J Neurosci 2005; 21: 2782–2792
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 2004; 25: 150–176
- Landgraf R, Wigger A. High vs. low anxiety-related behavior rats: An animal model of extremes in trait anxiety. Behav Genet 2002; 32: 301–314
- Lipschitz DL, Crowley WR, Bealer SL. Differential sensitivity of intranuclear and systemic oxytocin release to central noradrenergic receptor stimulation during mid- and late gestation in rats. Am J Physiol Endocrinol Metab 2004; 287: E523–E528
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev 2002; 26: 869–888
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci 2003; 117: 195–201
- Manning M, Kruszynski M, Bankowski K, Olma A, Lammek B, Cheng LL, Klis WA, Seto J, Haldar J, Sawyer WH. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J Med Chem 1989; 32: 382–391
- Maren S. Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits. Mol Neurobiol 1996; 13: 1–22
- Masood A, Banerjee B, Vijayan VK, Ray A. Modulation of stress-induced neurobehavioral changes by nitric oxide in rats. Eur J Pharmacol 2003; 458: 135–139
- Masood A, Banerjee B, Vijayan VK, Ray A. Pharmacological and biochemical studies on the possible role of nitric oxide in stress adaptation in rats. Eur J Pharmacol 2004; 493: 111–115
- Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res 1989; 76: 593–602
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 2000; 97: 4926–4931
- Munoz-Blanco J, Yusta B, Cordoba F. Differential distribution of neurotransmitter amino acids from the limbic system of aggressive and non-aggressive bull strains. Pharmacol Biochem Behav 1986; 25: 71–75
- Neumann ID. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamo–pituitary–adrenal axis. Prog Brain Res 2002; 139: 147–162
- Neumann I, Landgraf R. Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious lactating rat during suckling. J Neuroendocrinol 1989; 1: 305–308
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: A microdialysis study. Neuroscience 1993; 53: 65–75
- Neumann ID, Toschi N, Ohl F, Torner L, Krömer SA. Maternal defence as an emotional stressor in female rats: Correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci 2001; 13: 1016–1024
- Neumann ID, Krömer S, Bosch OJ. Differential effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters of lactating rats bred for high versus low stress vulnerability. Psychoneuroendocrinology 2005; 30: 791–806
- Neumann ID, Torner L, Toschi N, Veenema AH. Oxytocin actions within the supraoptic and paraventricular nuclei: Differential effects on peripheral and intranuclear vasopressin release. Am J Physiol Regul Integr Comp Physiol 2006; 291: R29–R36
- Numan M. Maternal behaviour. The physiology of reproduction2nd ed., E Knobil, JD Neill. Raven Press, New York 1994; 221–301
- Numan M, Insel TR. Hormones, brain and behavior. The neurobiology of parental behavior. Springer, Secaucus, NJ 2003
- Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. J Neuroendocrinol 2004; 16: 308–312
- Parker SL, Crowley WR. Central stimulation of oxytocin release in the lactating rat by N-methyl-d-aspartate: Requirement for coactivation through non-NMDA glutamate receptors or the glycine coagonist site. Neuroendocrinology 1995; 62: 467–478
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates4th ed. Academic Press, Sydney 1998
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress 2002; 5: 259–267
- Pedersen CA, Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA 1979; 76: 6661–6665
- Popeski N, Woodside B. Central nitric oxide synthase inhibition disrupts maternal behavior in the rat. Behav Neurosci 2004; 118: 1305–1316
- Richard P, Moos F, Freund-Mercier MJ. Central effects of oxytocin. Physiol Rev 1991; 71: 331–370
- Rosenblatt JS, Factor E, Mayer AD. Relationship between maternal aggression and maternal care in the rat. Aggress Behav 1994; 20: 243–255
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiol Rev 2003; 83: 803–834
- Singewald N, Zhou GY, Schneider C. Release of excitatory and inhibitory amino acids from the locus coeruleus of conscious rats by cardiovascular stimuli and various forms of acute stress. Brain Res 1995; 704: 42–50
- Singewald N, Kouvelas D, Mostafa A, Sinner C, Philippu A. Release of glutamate and GABA in the amygdala of conscious rats by acute stress and baroreceptor activation: Differences between SHR and WKY rats. Brain Res 2000; 864: 138–141
- Veenema AH, Beiderbeck DI, Neumann ID. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for high and low anxiety. Soc Neurosci Abstr 2005; 6868
- Walker DL, Rattiner LM, Davis M. Group II metabotropic glutamate receptors within the amygdala regulate fear as assessed with potentiated startle in rats. Behav Neurosci 2002; 116: 1075–1083
- Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol 1997; 9: 859–865
- van Leengoed E, Kerker E, Swanson HH. Inhibition of postpartum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol 1987; 112: 275–282
