Abstract
Since the brain neurotransmitter changes characterising panic disorder remain uncertain, we quantified brain noradrenaline and serotonin turnover in patients with panic disorder, in the absence of a panic attack. Thirty-four untreated patients with panic disorder and 24 matched healthy volunteers were studied. A novel method utilising internal jugular venous sampling, with thermodilution measurement of jugular blood flow, was used to directly quantify brain monoamine turnover, by measuring the overflow of noradrenaline and serotonin metabolites from the brain. Radiographic depiction of brain venous sinuses allowed differential venous sampling from cortical and subcortical regions. The relation of brain serotonin turnover to serotonin transporter genotype and panic disorder severity were evaluated, and the influence of an SSRI drug, citalopram, on serotonin turnover investigated.
Brain noradrenaline turnover in panic disorder patients was similar to that in healthy subjects. In contrast, brain serotonin turnover, estimated from jugular venous overflow of the metabolite, 5-hydroxyindole acetic acid, was increased approximately 4-fold in subcortical brain regions and in the cerebral cortex (P < 0.01). Serotonin turnover was highest in patients with the most severe disease, was unrelated to serotonin transporter genotype, and was reduced by citalopram (P < 0.01). Normal brain noradrenaline turnover in panic disorder patients argues against primary importance of the locus coeruleus in this condition. The marked increase in serotonin turnover, in the absence of a panic attack, possibly represents an important underlying neurotransmitter substrate for the disorder, although this point remains uncertain. Support for this interpretation comes from the direct relationship which existed between serotonin turnover and illness severity, and the finding that SSRI administration reduced serotonin turnover. Serotonin transporter genotyping suggested that increased whole brain serotonin turnover most likely derived not from impaired serotonin reuptake, but from increased firing in serotonergic midbrain raphe neurons projecting to both subcortical brain regions and the cerebral cortex.
Introduction
Although the brain neurotransmitter changes characterising panic disorder are uncertain, available evidence does point to a possible importance of noradrenergic and serotonergic neuronal systems. The ascending noradrenergic neurons of the locus coeruleus have been implicated in anxiety responses, arousal and autonomic activation (Redmond and Huang Citation1979; Ziegler et al. Citation1999), as have rostral serotonergic projections from the brainstem raphe nuclei (Shimizu et al. Citation1992; Kirby et al. Citation1995; Graeff et al. Citation1996; Rueter and Jacobs Citation1996). The 5-HT2C agonist, m-chlorophenylpiperazine (m-CPP), has anxiogenic effects and can induce panic attacks (Bagdy Citation1998).
Given the strength of this experimental evidence indicating brain noradrenergic and serotonergic neurons participate in anxiety responses, there is a need to evaluate these neuronal systems in panic disorder, but it has not proven easy to quantify noradrenaline, and in particular serotonin turnover in the human brain. Serotonin sampled in plasma mainly originates from platelets activated in the blood collection process (unpublished personal observations). The brain is the source of less than 10% of the major serotonin metabolite, 5-hydroxyindole acetic acid (5-HIAA) found in plasma and urine (Lambert et al. Citation1995a,Citationb, Citation2002), rendering systemic plasma measurements and urine collections unsuitable for studying brain serotonin turnover. Serotonin in lumbar CSF is derived primarily from the terminals of descending serotonergic neuronal projections to the spinal cord, not from the brain (Tork Citation1990). Testing for brain serotonin receptor binding, to infer receptor occupancy and serotonin release, with PET scanning methodology using serotonin agonist PET ligands is one direct and valid approach (Nash et al. Citation2004; Neumeister et al. Citation2004), but serotonin binding can also be altered by changes in the serotonin receptors of the brain.
In the present study we teamed with psychiatrists and psychologists and have drawn on our technical skills as cardiologists to use an alternative, direct methodology based on sampling from the internal jugular veins (Maas et al. Citation1979a,Citationb) to quantify brain noradrenaline and serotonin turnover. In untreated patients with panic disorder we have measured the overflow into the internal jugular veins of noradrenaline and its major lipophilic metabolites, 3-methoxy-4-hydroxyphenylglycol (MHPG) and 3,4-dihydroxyphenylglycol (DHPG), and that of the principal brain serotonin metabolite, 5-HIAA (Ferrier et al. Citation1993; Lambert et al. Citation1995a,Citationb, Citation1999). Noradrenaline and serotonin metabolites in internal jugular venous plasma originate almost exclusively from brain neurons, not from the cerebrovascular sympathetic nerves (Ferrier et al. Citation1993; Lambert et al. Citation1995a,Citationb, Citation1998).
Testing was done, in the absence of a panic attack, to test whether there might be a neurochemical substrate to panic disorder which involved these neurotransmitters. To separately quantify cortical and subcortical monoamine turnover using jugular venous sampling, delineation of the venous drainage pattern of the brain is needed. This was achieved with a technetium-99 venous sinus scan (Lambert et al. Citation1991), which indicates whether the sagittal sinus carrying the bulk of cortical blood drains into the right or left internal jugular vein thereby allowing differential venous sampling from cortical and subcortical brain regions (Ferrier et al. Citation1993; Aggarwal et al. Citation2002).
The serotonin transporter gene is subject to a 44 base pair deletion–insertion promoter region polymorphism which influences gene activity (Lesch et al. Citation1996). Presence of the “short form” of the gene might increase serotonin synthesis rates through impairment of neuronal serotonin reuptake. To facilitate interpretation of brain serotonin turnover measurements in the panic disorder patients studied, serotonin transporter genotyping was performed. Psychometric testing was also done on all patients, searching for links between brain monoamine turnover and clinical characteristics. In an additional 10 patients with panic disorder, jugular venous sampling was performed twice, first while untreated, then during treatment with the selective serotonin reuptake inhibitor (SSRI), citalopram, given as sole therapy in 4 patients, and combined with cognitive behaviour therapy (CBT) in 6. This was done to ascertain whether treatment response under SSRI dosing was associated with “normalization” of any abnormality in brain monoamine turnover which might be present.
Methods
Research subjects
Thirty-four patients meeting DSM IV (American Psychological Association Citation1994) diagnostic criteria for panic disorder, who were either never treated or had received no medication in the preceding 3 months, and 24 healthy volunteers participated. The recruited panic disorder patients had responded to requests for patient volunteers that had accompanied medical articles on panic disorder published in Melbourne major daily newspapers. Clinical evaluation was independently performed by a clinical psychologist and psychiatrist participating in the study. After initial screening, a structured clinical interview based on DSM-IV was used to make a clinical diagnosis of panic disorder (Brown et al. Citation1994). Patient selection attempted to minimize psychiatric comorbidity. Possible comorbidity was evaluated with the anxiety disorders interview schedule for DSM-IV (ADIS-IV) (American Psychological Association Citation1994), which allows for discrimination between anxiety disorders as well as for the determination of primary and secondary diagnoses based on the participant's responses and severity scores on measures of symptomatology. Ratings were considered to be clinically significant with a score of 4 or above on the Likert-type scale. Fourteen panic disorder volunteers had a secondary diagnosis of depression, three had a secondary diagnosis of social avoidant disorder, two had a secondary diagnosis of post-traumatic stress disorder, two a secondary diagnosis of generalized anxiety disorder and one, obsessive compulsive disorder. These diagnoses were mild, scoring between 0 and 2 points on a 9-point severity scale (Brown et al. Citation1994).
Avoidance of potential confounding by season, sunlight and adiposity
Brain serotonin releasing neurons subserve diverse, although incompletely understood functions related to feeding and adiposity (Tecott et al. Citation1995) and to light stimulation (Kennaway and Moyer Citation1998). We have previously demonstrated an influence of these on whole brain serotonin turnover in humans (Lambert et al. Citation1999, Citation2002). By appropriate selection and matching of a healthy reference population, with matching of healthy subjects and panic disorder patients in terms of body mass index and sunlight hours on the study day, we were able to avoid a confounding effect of serotonergic neuronal systems activated by sunlight and in obesity, as we tested for differences in the level of activity of anxiogenic serotonergic systems. In all participants body mass index was < 28 to exclude any confounding influence of obesity on brain serotonin turnover (Lambert et al. Citation1999). Body mass index was 23.6 ± 0.7 kg/m2 (mean, SE) in panic disorder patients and 23.4 ± 0.6 kg/m2 in healthy subjects. With the assistance of the Melbourne Bureau of Meteorology (Lambert et al. Citation2002) healthy volunteers were chosen from a large pool of subjects such as to achieve matching for sunlight hours on the day of the catheter sampling study, 5.7 ± 0.8 h in panic disorder patients and 5.0 ± 0.9 h in the reference population ().
Table I. Subject matching and brain serotonin turnover.
Central venous catheter placement
The research procedure was performed with the participants resting in the supine position. They had earlier eaten a standardized light breakfast. Tea, coffee and alcohol were withheld for a minimum of 12 h prior to the study. High internal jugular venous sampling, with the catheter tip placed beyond the angle of the jaw to exclude sampling from the venous drainage of the face, and measurement of internal jugular blood flow by thermodilution was performed using our previously described techniques (Ferrier et al. Citation1993). The central venous catheter, a 7F coronary sinus thermodilution catheter (Webster laboratories, type CCS-7U-90B) introduced via an antecubital venous sheath, was percutaneously inserted under local anaesthesia, and placed with fluoroscopic control high in one internal jugular vein (bilateral sampling in six panic disorder patients).
Measurement of brain monoamine turnover
Simultaneous arterial and jugular venous blood samples were obtained in the 34 panic disorder patients and in the 24 healthy subjects. Jugular venous overflow of noradrenaline, MHPG, DHPG and 5-HIAA was calculated using the Fick principle, from venous–arterial plasma concentration differences (arterial sampling from an indwelling radial or brachial artery cannula), and from internal jugular blood flows (Ferrier et al. Citation1993; Lambert et al. Citation1995a,Citationb). Measurements were made at quiet rest, in the absence of a panic attack. Assay of noradrenaline, DHPG, MHPG and 5-HIAA was by high performance liquid chromatography, using previously described methods (Lambert et al. Citation1995a,Citationb).
Cerebral venous scan
Human cerebral venous drainage is asymmetrical, in that the two internal jugular veins typically drain anatomically distinct brain regions, one (typically the right) representing the continuation of the superior sagittal sinus and predominantly draining the cortex, and the other draining suprabulbar subcortical regions (Lambert et al. Citation1991; Ferrier et al. Citation1993; Aggarwal et al. Citation2002). With assay of monoamines and their metabolites in plasma separately sampled from the two internal jugular veins this provides a basis for the differential analysis of brain norepinephrine and serotonin turnover in cortical and subcortical brain areas. We used a technetium-99 cerebral venous sinus scan to study venous sinus flow patterns and establish which internal jugular vein was a continuation of the sagittal sinus and which largely drained subcortical regions (). The brainstem drains to the spinal veins, so that monoamine turnover in the brainstem is not represented in internal jugular plasma metabolite concentrations.
Figure 1 Patterns of cerebral venous sinus drainage demonstrated by cerebral sinus scans with technetium-99, with images (posterior view) at 10–16 s after peripheral venous injection of tracer. Top, drainage of the superior sagittal sinus (carrying the bulk of cortical venous return) is primarily into the left internal jugular vein (“left dominant” pattern of drainage). Subcortical venous flow is primarily to the right internal jugular vein. Bulbar venous return (not demonstrated in the scan) is to the spinal veins. Middle, drainage of the superior sagittal sinus is largely into the right internal jugular vein “right dominant”). Subcortical flow is to the left jugular vein. Bottom, non-lateralizing venous sinus flow, with symmetrical drainage of the superior sagittal sinus into the right and left internals jugular veins, due to admixture of blood at the confluence of the sinuses.
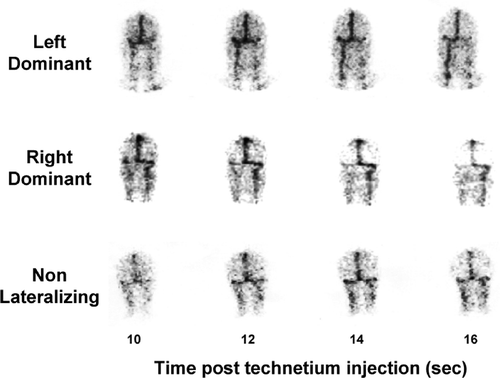
The cerebral venous sinus scans were performed as we have reported earlier (Lambert et al.Citation1991; Ferrier et al. Citation1993; Aggarwal et al. Citation2002), using the patients' own harvested red cells, which were labelled with technetium-99, then reinjected for the single photon emission computed tomographic (SPECT) scan. In, approximately one third of cases the cerebral venous drainage was non-lateralizing (), due to vascular interconnection at the confluence of the transverse sinuses, so that in this circumstance both internal jugular veins contained blood mixed from cortical and subcortical regions. In the presence of this vascular anatomy, regionalized analysis of brain monoamine turnover was not possible (Ferrier et al. Citation1993).
Serotonin transporter genotyping
The human serotonin transporter (5-HTT), encoded by a single gene on chromosome 17q11.2, is expressed in both the brain and in peripheral blood cells. A 5-HTT-linked gene promoter region (5-HTTLPR) insertion/deletion polymorphism with long (l) and short (s) forms has been demonstrated to influence expression and function of the 5-HTT (Heils et al. Citation1996; Lesch et al. Citation1996; Greenberg et al. Citation1999). The observation that 5-HTT expression and serotonin uptake are impaired but no different in cells with homozygous and heterozygous short genotypes suggests that the s variant exerts a dominant influence on 5-HTT function.
Genomic DNA was isolated from whole blood using the Flexigene DNA Kit (Qiagen, Hilden, Germany). Genotyping for the 5-HTTLPR was performed after amplification by polymerase chain reaction (PCR) using sense and antisense primers as previously described (Lesch et al. Citation1996; Greenberg et al. Citation1999). The PCR products were resolved in a 2% agarose gel containing 0.5 μg/ml ethidium bromide in TAE buffer (40 mmol/l Tris–acetate, 1 mmol/l EDTA, pH 8.0) following electrophoresis at 120 V for approximately 40 min. Each gel contained one lane of a 50 base pair ladder in order to identify the 484/528- bp fragment of the 5-HTTLPR, corresponding to the s and l variants respectively. Bands were visualized by ultraviolet illumination. The identity of each band was confirmed by automated DNA sequencing.
Assessment of panic disorder clinical severity
Assessment of overall panic disorder severity was obtained using the patient self-rating panic disorder severity scale (PDSS) (Shear et al. Citation1997) and with a clinician-applied scale, ADIS-IV (Brown et al. Citation1994). The PDSS self-rating scale, modelled on the Yale-Brown Obsessive Compulsive Scale (Goodman et al. Citation1989), contains seven items that assess the severity of seven dimensions of panic disorder. The scale provides a quantitative measure of panic severity. Each scale item is rated 0 (“not at all”)–4 (“very severely”). A composite score is derived by averaging the scores on the seven items (Shear et al. Citation1997). Inter-rater reliability with this instruments has been shown to be 0.87, with an intraclass correlation coefficient of 0.88 and internal consistency of 0.65 (Shear et al. Citation2001).
Investigation of the effects of serotonin uptake-blockade on brain serotonin turnover
In a parallel study, ten additional panic disorder patients were treated with serotonin uptake-blocking medication alone (n = 4), or in combination with CBT, which was performed weekly by a clinical psychologist skilled in this form of therapy. The chosen SSRI drug was citalopram, because of the selectivity of its blockade of serotonin reuptake. Patients were reviewed weekly for the purposes of the study, or more frequently if required on clinical grounds. Dosage was determined according to clinical response; the dose range at the time of repeat research testing, 20–60 mg citalopram, was well tolerated in all.
Brain monoamine turnover was estimated, on the principles described above, on clinical presentation, then after patients had been maintained on medication for a minimum of 3 months, when their panic disorder was in partial or complete clinical remission. A sequential rather than crossover design was chosen on ethical grounds, to avoid prolonged withholding of treatment. As the coronary sinus thermodilution catheter (enabling measurement of internal jugular vein blood flow) was now no longer in commercial production, a sampling catheter without thermodilution capability was used in this component of the study. Brain serotonin turnover was estimated from the 5-HIAA venous–arterial plasma concentration difference across the brain (Lambert et al. Citation1991, Citation1999). Unlike in the primary study, this could not be converted to serotonin turnover, in the absence of jugular flow measurements.
Research ethics
Given that this study involved the placement of a central venous catheter and an arterial cannula in participants for who this was not indicated on clinical grounds, comment on research ethics is in order. The central issues in clinical research ethics are the quality of the research, the potential for harm to an individual from the experiment, and the degree of safeguarding of the participant's autonomy. In the present study we investigated aspects of brain neurotransmitter function in panic disorder which are potentially of clinical importance, using state-of-the-art research methodology. There is no less invasive method for validly doing this than we applied. In our very extensive research experience with central venous catheters (Esler et al. Citation1984; Meredith et al. Citation1991; Wilkinson et al. Citation1998; Lambert et al. Citation2002), involving in excess of 2100 studies performed over 24 years, we have found the procedure to be invariably associated with negligible risk. The procedures are always performed by cardiologists who are expert in the technique. The process of written consent, to which an honest, open and explicit participant information sheet was central, conformed to the standards expected to preserve the autonomy of the participants. The research was approved by the Alfred Hospital Medical Research Ethics Committee.
Statistics
All results, unless otherwise specified, are expressed as mean (standard error of mean). Tests of significance were carried out using ANOVA, paired t-tests or distribution-free nonparametric tests where appropriate. The possible relation between variables was evaluated using least squares linear regression analysis. All statistical tests were two-tailed and statistical significance was set at a probability level of 0.05.
Results
Brain monoamine turnover
Brain noradrenaline turnover, calculated with bilateral jugular venous sampling, or from doubling the combined unilateral jugular venous overflow of norepinephrine, MHPG and DHPG (findings in volunteers having bilateral sampling justified this adjustment), was similar in panic disorder patients, 246 ng/min, SEM 24 (1454 pmol/min, SEM 142) and healthy volunteers, 226 ng/min, SEM 13 (1336 pmol/min, SEM 77). In contrast, for brain serotonin turnover, estimated from bilateral jugular sampling or doubled unilateral values, the jugular venous overflow of 5-HIAA, was increased approximately 4-fold in panic disorder, 347 ng/min, SEM 81 (1815 pmol/min, SEM 424) compared with 69 ng/min, SEM 14 (361 pmol/min, SEM 73) in healthy subjects (P < 0.01).
Arterial plasma 5-HIAA concentrations were not elevated in panic disorder. Indicative of greater release of serotonin in the brain, however, the veno–arterial plasma concentration step-up across the brain was higher (). It seemed unlikely that this difference was simply due to greater test anxiety in the patient group, induced by the invasive nature of the testing, in that an applied laboratory mental stress, in the form of difficult mental arithmetic (Wilkinson et al. Citation1998) did not increase brain serotonin turnover in either experimental group (results not presented here). To this point jugular venous sampling has not been performed during a panic attack.
Regionalized serotonin turnover
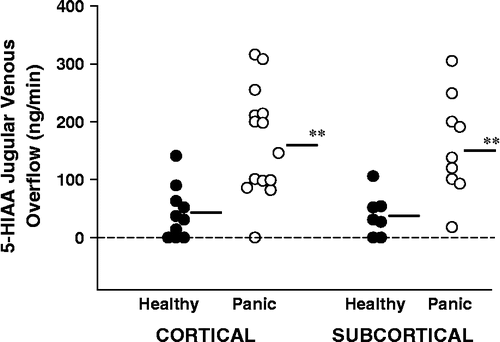
In those participants in whom the cerebral sinus venous scan demonstrated lateralized drainage into the internal jugular veins it was possible to compare serotonin turnover separately in cortical and subcortical regions. Serotonin turnover in panic disorder patients was similarly increased (approximately 4-fold) in cortical and subcortical brain regions. Based on the cerebral venous sinus scan depiction of brain venous drainage, cortical serotonin turnover (10 values in healthy subjects, 14 in panic disorder) was 165 ng/min, SEM 25 (863 pmol/min, SEM 131) in panic disorder patients and 43 ng/min, SEM 14 (225 pmol/min, SEM 73) in healthy volunteers (P < 0.001; Student's t-test)(). Subcortical serotonin turnover (seven values in healthy subjects, nine in panic disorder) was 157 ng/min, SEM 29 (821 pmol/min, SEM 152) in panic disorder patients and 38 ng/min, SEM 14 (199 pmol/min, SEM 73) in healthy volunteers (P = 0.005; Student's t-test).
Figure 2 5-HIAA overflow from the brain into the internal jugular veins characterised on the venous sinus scan in terms of whether this was from a predominantly cortical or subcortical field of venous drainage. In those cases where the cerebral venous drainage was non-lateralizing due to vascular interconnection at the confluence of the transverse sinuses, regionalized analysis of brain serotonin turnover was not possible. Brain serotonin turnover was increased approximately 4-fold in both cortical and subcortical brain regions in panic disorder. SI conversion; ng/min = 5.23 pmol/min. **P < 0.01.
Influence of 5-ht transporter genotype
The elevated brain serotonin turnover in panic disorder was not a consequence of impairment of neuronal serotonin reuptake. Serotonin turnover was no higher in patients with ss and sl serotonin transporter genotypes (), in which serotonin reuptake is reduced (Lesch et al. Citation1996).
Table II. Influence of serotonin transporter gene promoter region insertion/deletion. Polymorphism on brain serotonin turnover in panic disorder.
Panic disorder clinical severity
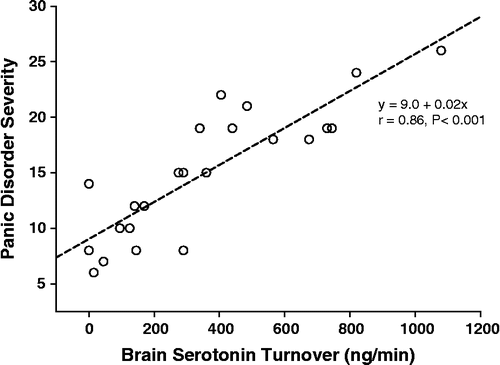
Severity of panic disorder, scored with the patient self-rating panic disorder severity scale was strongly and directly correlated with brain serotonin turnover; r = 0.86, P < 0.001 (). The clinician-based ADIS-IV severity scale also showed a direct correlation of panic disorder severity with serotonin turnover, but at a somewhat lower order; r = 0.66, P < 0.001.
Influence of SSRI treatment on brain monoamine turnover
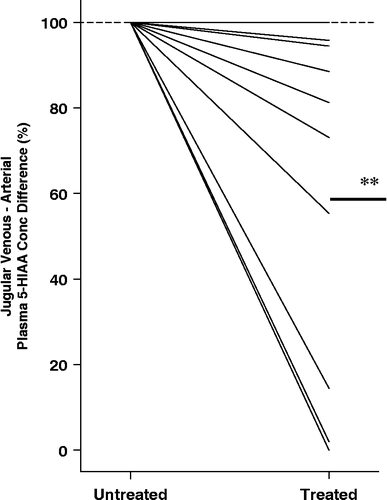
In the ten patients with panic disorder studied before and during SSRI dosing with citalopram, the transcerebral 5-HIAA plasma concentration difference, which while untreated was markedly higher than in normal volunteers, was reduced in the treatment phase, falling in 9 of 10 patients, and into the normal range in 5 (P < 0.01)().
Figure 4 The effect of SSRI dosing in panic disorder, with citalopram alone or in combination with CBT, on brain serotonin turnover. The baseline veno–arterial 5-HIAA plasma concentration difference is listed as 100%, with treated serotonin turnover shown as a percentage of the initial value. A fall was seen in 9 of 10 patients (**P < 0.01).
Discussion
The brain neurotransmitter changes characterising panic disorder remain uncertain. Ascending noradrenergic neurons of the locus coeruleus (Redmond and Huang Citation1979; Ziegler et al. Citation1999), and rostral serotonergic projections from the brainstem raphe nuclei (Shimizu et al. Citation1992; Kirby et al. Citation1995; Graeff et al. Citation1996; Rueter and Jacobs Citation1996) are possibly involved. Measurement of interstitial brain serotonin concentrations with microdialysis in studies in animals has demonstrated the release of serotonin in several sites, including the locus coeruleus, forebrain and cortex during the application of a range of stressors (Shimizu et al. Citation1992; Kirby et al. Citation1995; Rueter and Jacobs Citation1996; Singewald et al. Citation1997). The serotonin release in the locus coeruleus (Singewald et al. Citation1997) provides evidence of linkage of raphe nuclei and the locus coeruleus during evoked anxiety.
We used direct methodology to quantify brain noradrenaline and serotonin turnover in untreated patients with panic disorder, based on the overflow into the internal jugular veins of noradrenaline and its major lipophilic metabolites MHPG and DHPG, and of the principal brain serotonin metabolite, 5-HIAA (Ferrier et al. Citation1993; Lambert et al. Citation1995a,Citationb; Aggarwal et al. Citation2002). The primary analysis was done in patients who were either never treated or had received no medication in the preceding 3 months. Brain noradrenaline turnover, estimated with bilateral jugular venous sampling, or from doubling the unilateral jugular venous overflow of noradrenaline, MHPG and DHPG, was similar in panic disorder patients and healthy volunteers, perhaps arguing against participation of the locus coeruleus, although measurements were made only in the absence of a panic attack. Study of the locus coeruleus during a panic attack clearly would be more pertinent. Brain serotonin turnover, estimated from the jugular venous overflow of 5-HIAA, was increased approximately 4-fold in panic disorder. Although brain transmitter changes during a panic attack remain unknown, it is possible that the pronounced increase in serotonin turnover noted at rest in panic sufferers represents an important underlying neurotransmitter substrate for the disorder, but it was important to exclude a confounding influence of serotonergic neurons with functions unrelated to anxiety.
Brain serotonin releasing neurons subserve diverse functions related to nutrition (Tecott et al. Citation1995) and season (Kennaway and Moyer Citation1998). We have previously demonstrated an influence of feeding and adiposity (Lambert et al. Citation1999) and of sunlight (Lambert et al. Citation2002), on whole brain serotonin turnover in humans. By appropriate selection and matching of the healthy reference population for mean body mass index and for sunlight hours on the day of the study, we were able to avoid potential confounding effects of serotonergic neuronal activation by sunlight and in obesity, facilitating detection of activation of anxiogenic serotonergic neurons in patients with panic disorder.
Arterial plasma 5-HIAA concentrations were not elevated in panic disorder, but only 6–8% of the serotonin metabolite in plasma derives from the brain (Lambert et al. Citation1995a). Similar to panic disorder, the effect of sunlight and season on serotonin turnover is also confined to the brain, and is not reflected in changed arterial concentration of 5-HIAA (Lambert et al. Citation2002). Indicative of greater release of serotonin in the brain, however, in both contexts the veno–arterial plasma concentration step-up across the brain is higher. The bulk of whole-body serotonin turnover occurs in the intestinal tract and its innervation, so that approximately 65% of the 5-HIAA in systemic plasma enters via the hepatic vein (Lambert et al. Citation1995a).
Our use of cerebral venous scans to delineate the pattern of venous drainage of the brain allowed the separate quantification of cortical and subcortical monoamine turnover based on jugular venous sampling. We used a technetium-99 venous sinus scan, which indicated whether the sagittal sinus carrying the bulk of cortical blood drains into the right or left internal jugular vein (Ferrier et al. Citation1993; Aggarwal et al. Citation2002). In those participants in whom the cerebral sinus venous scan demonstrated lateralized drainage into the internal jugular veins, without mixing at the confluence of the sinuses, it was possible to compare serotonin turnover separately in cortical and subcortical regions. We found serotonin turnover in panic disorder patients to be proportionally increased (3–5-fold) in cortical and subcortical brain regions, suggesting that there was increased firing in midbrain raphe serotonergic neurons projecting to both the cerebral cortex and to subcortical brain regions (Graeff et al. Citation1997). It will be crucial in the future to derive more definitive topographic information on the sites of increased brain serotonin turnover in panic disorder than this methodology allows. Serotonin in the brain can be very “site-specific” in its actions. Contradictory influences of serotonin on fear have been demonstrated in the rat, with serotonin enhancing conditioned fear in the amygdala, dorsal raphe nucleus serotonergic projections to the amygdala and frontal cortex augmenting conditioned fear, while inhibiting innate fear in the periaqueductal grey (PAG) (Graeff et al. Citation1997), with the raphe projections to the PAG inhibiting fight and flight responses to asphyxia and pain (Graeff et al. Citation1996). Further, the effects of serotonin depend also on the type of 5-HT receptor bound. Studies in knockout mice (Zhuang et al. Citation1999; Olivier et al. Citation2001) suggest the 5-HT1A receptor mediates anxiolytic responses while the 5-HT1B receptor can subserve anxiogenic ones.
Applying PET scanning methodology in parallel with the jugular venous technique would be instructive. PET studies, ideally utilising both 5-HT1A and 5-HT1B serotonergic ligands, should provide regional measures of receptor occupancy which would be complementary to the measures of serotonin turnover based on jugular venous sampling. We anticipate that high receptor occupancy and high serotonin turnover will be linked. Scanning methodology should provide brain topography information, not available at this point, which might further help in the differentiation of brain serotonin neurons related to anxiety from those serotonergic neuronal pools related to seasonality and to feeding/satiety.
The serotonin transporter gene is subject to a 44 base pair deletion–insertion promoter region polymorphism which influences gene activity (Heils et al. Citation1996; Lesch et al. Citation1996; Greenberg et al. Citation1999). We tested whether presence of the “short form” of the gene might have increased brain serotonin turnover rates through impairment of neuronal serotonin reuptake, but the elevated brain serotonin turnover in panic disorder was not a consequence of impairment of neuronal serotonin reuptake. Serotonin turnover was no higher in patients with ss and sl serotonin transporter genotypes, in which serotonin reuptake is reduced (Heils et al. Citation1996; Lesch et al. Citation1996; Greenberg et al. Citation1999), indicating that the increased whole brain serotonin turnover most likely resulted from increased firing in serotonergic neurons rather than impaired serotonin reuptake.
Psychometric testing was also done on all patients, searching for links between brain monoamine turnover and patient clinical characteristics. Severity of panic disorder, scored with both patient self-rating panic disorder severity scale (Shear et al. Citation2001) and the clinician-based scale ADIS IV scale (Brown et al. Citation1994) was strongly and directly correlated with brain serotonin turnover. This makes more plausible the notion that brain serotonergic activation might be the prime mover in panic disorder, but it could still be consequential on other suggested primary causes of panic disorder, such as cardiac sympathetic nerve sensitization by faulty neuronal noradrenaline reuptake, which might lead to cardiac symptoms and the enhanced vigilance which accompanies them (Alvarenga et al. Citation2006).
The finding of increased brain serotonin turnover in panic disorder is contrary to the commonly held view that brain serotonin release is diminished (Maron et al. Citation2005), but does fit well with some clinical and experimental observations. Common clinical experience is that SSRIs can cause agitation and increased frequency of panic attacks as treatment is started (Burghardt et al. Citation2004). Clinical improvement occurs typically only after a few weeks of dosing. As panic disorder appears from our results to be characterised by increased brain neuronal serotonin release, this early clinical deterioration is as would be expected from a drug class increasing the synaptic concentration of serotonin. The phase of clinical improvement seen later possibly coincides with inhibition of firing of serotonergic neurons, by an extrasynaptic inhibitory action of serotonin on the neuronal cell body (Norman Citation1999), although this interpretation does remain speculative.
We have tested whether SSRI drugs do cause serotonergic inhibition. In ten patients with panic disorder, additional to the primary study group, jugular venous sampling was performed twice, first untreated, then during treatment with the SSRI, citalopram. During SSRI dosing the transcerebral 5-HIAA plasma concentration difference, which while untreated was markedly higher than in normal volunteers, was reduced in the treatment phase, falling into the normal range in 5 of 10 patients. Perhaps surprisingly, in no patient was there an increase in brain serotonin turnover, which might have been expected with serotonin reuptake block. Whether there was a contribution of CBT, administered concurrently with SSRI dosing in 6 of these patients, to reduce serotonin release does remain uncertain.
In summary, the pronounced increase in serotonin turnover we find in panic disorder, in the absence of a panic attack possibly represents an important underlying neurotransmitter substrate for the disorder, especially given the direct relationship which existed between serotonin turnover and illness severity, and the observation that SSRI administration reduces serotonin turnover. Measurement of serotonin concentrations previously in CSF has given often contradictory results, but a very recent report does describe increased CSF 5-HIAA levels when depressive illness is accompanied by comorbid panic disorder (Sullivan et al. Citation2006). Serotonin transporter genotyping demonstrates that the increased brain serotonin turnover we document is not due to impaired serotonin reuptake, but most likely results from increased firing in midbrain raphe serotonergic neurons projecting to both the cerebral cortex and to subcortical brain regions.
Limitations
Brain serotonin neurons subserve diverse functions, but appropriate subject matching allowed us to avoid confounding by neuronal pools linked to season, sunlight and feeding, with our focus on serotonin turnover linked to anxiety/panic. Beyond differentiating between serotonin turnover in cortical and subcortical brain regions we were, however, unable to provide specific topographical information. The linear trial design used in the SSRI dosing part of the study, done to avoid the withholding of necessary treatment, does leave open the possibility of artefact arising from sequential measurements, given that these were made without dosing crossover. The veno–arterial plasma concentration step-up across the brain for 5-HIAA, on which the measurement of brain serotonin turnover is based was rather small (mean 11% in panic disorder patients), but well within the precision of the assay used.
Acknowledgements
This research was financially supported by a Project Grant from the National Health and Medical Research Council of Australia, by a Senior Principal Research Fellowship from the NHMRC to ME, a Career Development Award from the NHMRC to GL and EL and by a Grant-in-Aid from the National Heart Foundation of Australia. Prof. Murray Esler takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Aggarwal A, Esler MD, Lambert GW, Hastings J, Johnston L, Kaye DM. Norepinephrine turnover is increased in suprabulbar subcortical brain regions and is related to whole-body sympathetic activity in human heart failure. Circulation 2002; 105: 1031–1033
- Alvarenga ME, Richards JC, Lambert G, Esler MD. Psychophysiological mechanisms in panic disorder: A correlative analysis of noradrenaline spillover, neuronal noradrenaline reuptake, power spectral analysis of heart rate variability, and psychological variables. Psychosom Med 2006; 68: 8–16
- American Psychological Association. Diagnostic and statistical manual of mental disorders, fourth edition. American Psychiatric Association, Washington DC 1994
- Bagdy G. Serotonin, anxiety, and stress hormones. Focus on 5-HT receptor subtypes, species and gender differences. Ann NY Acad Sci 1998; 851: 357–363
- Brown TA, DiNardo PA, Barlow DH. Anxiety disorders interview schedule for DSM-IV (ADIS-IV). Graywind Publications, San Antonio 1994
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JN, Le Doux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: A comparison with tianeptine. Biol Psychiatry 2004; 55: 1171–1178
- Esler M, Jennings G, Korner P, Blombery P, Sacharias N, Leonard P. Measurement of total and organ-specific norepinephrine kinetics in humans. Am J Physiol 1984; 247: E21–E28
- Ferrier C, Jennings GL, Eisenhofer G, Lambert G, Cox HS, Kalff V, Kelly M, Esler MD. Evidence for increased noradrenaline release from subcortical brain regions in essential hypertension. J Hypertens 1993; 11: 1217–1227
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown obsessive compulsive scale I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46: 1006–1011
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 1996; 54: 129–141
- Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev 1997; 21: 791–799
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet 1999; 88: 83–87
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66: 2621–2624
- Kennaway DJ, Moyer RW. Serotonin 5-HT2c agonists mimic the effect of light pulses on circadian rhythms. Brain Res 1998; 806: 257–270
- Kirby LG, Allen AR, Lucki I. Regional differences in the effects of forced swimming on extracellular levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res 1995; 682: 189–196
- Lambert GW, Eisenhofer G, Cox HS, Horne M, Kalff V, Kelly M, Jennings GL, Esler MD. Direct determination of homovanillic acid release from the human brain, an indicator of central dopaminergic activity. Life Sci 1991; 49: 1061–1072
- Lambert GW, Kaye DM, Cox HS, Vaz M, Turner AG, Jennings GL, Esler MD. Regional 5-hydroxyindoleacetic acid production in humans. Life Sci 1995a; 57: 255–267
- Lambert GW, Kaye DM, Vaz M, Cox HS, Turner AG, Jennings GL, Esler MD. Regional origins of 3-methoxy-4-hydroxyphenylglycol in plasma: Effects of chronic sympathetic nervous activation and denervation, and acute reflex sympathetic stimulation. J Auton Nerv Syst 1995b; 55: 169–178
- Lambert GW, Kaye DM, Thompson JM, Turner AG, Cox HS, Vaz M, Jennings GL, Wallin BG, Esler MD. Internal jugular venous spillover of noradrenaline and metabolites and their association with sympathetic nervous activity. Acta Physiol Scand 1998; 163: 155–163
- Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet 2002; 360: 1840–1842
- Lambert GW, Vaz M, Cox HS, Turner AG, Kaye DM, Jennings GL, Esler MD. Human obesity is associated with a chronic elevation in brain 5-hydroxytryptamine turnover. Clin Sci (Lond) 1999; 96: 191–197
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274: 1527–1531
- Maas JW, Hattox SE, Greene NM, Landis DH. 3-Methoxy-4-hydroxyphenethyleneglycol production by human brain in vivo. Science 1979a; 205: 1025–1027
- Maas JW, Hattox SE, Martin DM, Landis DH. A direct method for determining dopamine synthesis and output of dopamine metabolites from brain in awake animals. J Neurochem 1979b; 32: 839–843
- Maron E, Lang A, Tasa G, Liivlaid L, Toru I, Must A, Vasar V, Shlik J. Associations between serotonin-related gene polymorphisms and panic disorder. Int J Neuropsychopharmacol 2005; 8: 261–266
- Meredith IT, Broughton A, Jennings GL, Esler MD. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med 1991; 325: 618–624
- Nash JR, Sargen PA, Rabiner EA, Hood SD, Argyropoulos SV, Grasby RM, Nutt DI. Altered 5HT1A binding in panic disorder demonstrated by positron emission tomography. Eur Neuropsychopharm 2004; 14: S322–S323
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci 2004; 24: 589–591
- Norman TR. The new antidepressants—mechanisms of action. Aust Prescrib 1999; 22: 106–108
- Olivier B, Pattij T, Wood SJ, Oosting R, Sarnyai Z, Toth M. The 5-HT(1A) receptor knockout mouse and anxiety. Behav Pharmacol 2001; 12: 439–450
- Redmond DE, Jr, Huang YH. Current concepts. II. New evidence for a locus coeruleus–norepinephrine connection with anxiety. Life Sci 1979; 25: 2149–2162
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res 1996; 739: 57–69
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Gorman JM, Papp LA. Multicenter collaborative panic disorder severity scale. Am J Psychiatry 1997; 154: 1571–1575
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V, Vander Bilt J, Houck P, Wang T. Reliability and validity of the panic disorder severity scale: Replication and extension. J Psychiatr Res 2001; 35: 293–296
- Shimizu N, Take S, Hori T, Oomura Y. In vivo measurement of hypothalamic serotonin release by intracerebral microdialysis: Significant enhancement by immobilization stress in rats. Brain Res Bull 1992; 28: 727–734
- Singewald N, Kaehler S, Hemeida R, Philippu A. Release of serotonin in the rat locus coeruleus: Effects of cardiovascular, stressful and noxious stimuli. Eur J Neurosci 1997; 9: 556–562
- Sullivan GM, Oquendo MA, Huang YY, Mann JJ. Elevated cerebrospinal fluid 5-hydroxyindoleacetic acid levels in women with comorbid depression and panic disorder. Int J Neuropsychopharmacol 2006; 9: 547–556
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 1995; 374: 542–546
- Tork I. Anatomy of the serotonergic system. Ann NY Acad Sci 1990; 600: 9–34
- Wilkinson DJ, Thompson JM, Lambert GW, Jennings GL, Schwarz RG, Jefferys D, Turner AG, Esler MD. Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch Gen Psychiatry 1998; 55: 511–520
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 1999; 21(Suppl 2)52S–60S
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic–pituitary–adrenocortical axis responses to stress. J Neuroendocrinol 1999; 11: 361–369