Abstract
The impact of a lifelong absence of the neuronal nitric oxide synthase (nNOS) in the neuroendocrine stress response was investigated in nNOS knockout (KO) and wild type (WT) mice under basal conditions and in response to forced swimming. In the hypothalamic paraventricular nucleus oxytocin and corticotropin-releasing-hormone mRNA levels did not differ between these genotypes under resting conditions, whereas vasopressin mRNA levels were significantly lower in nNOS KO than in WT animals. Also, in the adrenal glands basal levels of tyrosine hydroxylase protein, the rate-limiting enzyme for catecholamine biosynthesis, and of phenylethanolamine N-methyltransferase, which converts norepinephrine to epinephrine, were significantly reduced in nNOS KO mice. Plasma adrenocorticotropin, corticosterone, norepinephrine and epinephrine levels were similar in the KO and WT genotypes under resting conditions. In response to forced swimming, a similar increase in plasma adrenocorticotropin and corticosterone was observed in KO and WT animals. Stressor exposure triggered also an increased epinephrine release in WT animals, but did not significantly alter plasma epinephrine levels in KO mice. These data suggest that the chronic absence of nNOS reduces the capacity of epinephrine synthesising enzymes in the adrenal gland to respond to acute stressor exposure with an adequate epinephrine release.
Introduction
The hypothalamic paraventricular nucleus (PVN) is the pivotal structure that coordinates the neuroendocrine stress response. Parvocellular PVN neurones constituting the central nervous part of the hypothalamic–pituitary–adrenal (HPA)-axis secrete corticotropin-releasing hormone (CRH; Vale et al. Citation1981) into the portal vasculature supplying the anterior pituitary. CRH triggers the secretion of adrenocorticotropin (ACTH) from the pituitary to stimulate the release of glucocorticoids, like corticosterone, from the adrenal cortex (Korte Citation2001). In addition, parvocellular PVN neurones synthesise and release also arginine-vasopressin (AVP), which increases the ACTH releasing action of CRH not only in case of chronic and prolonged stress (for review, see: Antoni Citation1993), but also in response to acute stressor exposure (Paulmyer-Lacroix et al. Citation1994; Aubry et al. Citation1999).
The PVN participates also in regulating the activity of the sympatho-adrenal system (Yamashita et al. Citation1984; Jansen et al. Citation1995; Ranson et al. Citation1998; Pyner and Coote Citation2000), which contributes to the endocrine stress response via the secretion of epinephrine and norepinephrine (Chrousos and Gold Citation1992). The catecholamines are synthesised by a sequence of four enzymes, including the rate-limiting tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT). It is of note that chromaffin cells are of ectodermal origin like neurones, and express high levels of neuronal nitric oxide synthase (nNOS), the enzyme that catalyses the production of nitric oxide (NO) in neurones (Palacios et al. Citation1989; Oset-Gasque et al. Citation1994).
The PVN harbours also magnocellular neurones belonging to the hypothalamic–neurohypophyseal system (HNS), which secrete AVP and oxytocin from the neurohypophysis into the blood. Growing evidence indicates that in particular at the level of the hypothalamus neurones of the HNS are activated by defined stressor exposure (Lang et al. Citation1983; Wotjak et al. Citation1996; Wotjak et al. Citation1998), and interact with the HPA-axis to orchestrate a finely tuned stress response (Wotjak et al. Citation2001; Engelmann et al. Citation2004). The activity of both the HNS and the HPA-axis is known to be shaped by a variety of neuromodulators, amongst which NO has been suggested to be a key regulatory molecule (Givalois et al. Citation2002; Kadekaro Citation2004). Previously, evidence has been accumulated that NO of nNOS origin modulates the endocrine stress response (Sanchez et al. Citation1994; Miyagawa et al. Citation1994; Barnes et al. Citation2001; Vicente et al. Citation2002). However, several studies addressing this question yielded contradictory results, depending upon whether in vitro or in vivo models and which pharmacological tools were used (Hashimoto et al. Citation1995; Giordano et al. Citation1996; Lee et al. Citation1999).
In the present study, we used genetically modified mice to examine the effect of a life-long absence of NO of nNOS origin on the regulation of the endocrine stress response. Since no residual NOS enzyme activity is present in the hypothalamus of nNOS knockout (KO) mice (Eliasson et al. Citation1997), the use of these animals may provide new insight by circumventing some of the limitations associated with the administration of pharmacological agents (Horn et al. Citation1994; Alderton et al. Citation2001). Indeed, previous reports showed that nNOS KO mice displayed altered behavioural parameters (Nelson et al. Citation1995; Chiavegatto et al. Citation2001; Chiavegatto and Nelson Citation2003), as well as impaired cognitive performance under stressful conditions (Weitzdoerfer et al. Citation2004). Moreover, it has been reported that nNOS KO mice show an altered pattern of c-Fos expression in the PVN after forced swimming (Salchner et al. Citation2004). These findings indicate that nNOS gene inactivation may affect the functionality of stress-related hypothalamic neurones.
Firstly, we examined by in situ hybridisation the effect under resting conditions of nNOS gene inactivation on neurones of the PVN, as well as on protein levels of catecholamine biosynthetic enzymes in the adrenal gland. Secondly, we investigated whether the in vivo release of ACTH, corticosterone and catecholamines is altered in nNOS KO mice under both basal conditions and in response to the forced swimming stressor to monitor the secretory activity of the HPA axis and the sympatho-adrenomedullary system in the absence of NO of nNOS origin.
Materials and methods
Animals
Adult nNOS KO and wild type (WT) male mice from our breeding colony were used in this study. The colony was originally established with breeders derived from the Cardiovascular Research Center, General Hospital, Massachusetts, USA. nNOS KO mice bear a targeted disruption of the nNOS gene that was achieved by homologous recombination and show >95% loss of nNOS production in the brain (Huang et al. Citation1993). Their genetic background is derived from multiple backcrossings with C57BL/6J mice. Animals were housed in groups of six under standard laboratory conditions (22 ± 1°C, 60 ± 5% humidity, 12-h light: 12-h dark cycle with lights on at 06:00 h, food and water ad libitum) and were single-housed a week before the experiments to avoid stress responses of cage cohort members by removing individuals for experimental manipulations. Experimental protocols were approved by the local governmental body and were in accordance with the European Communities Council Directives (86/609/EEC). The status of each nNOS KO and WT mouse was verified by genotyping. Briefly, genomic DNA was isolated from mouse tails (Invisorb Spin Tissue Mini Kit, Invitek). PCRs were carried out with approximately 200 ng genomic DNA in a total volume of 25 μl containing 100 mM Tris–HCl, pH 8.8, 500 mM KCl, 15 mM MgCl2, 200 μM of each of the four dNTPs, 2 U Taq polymerase (PeqLab GmbH, Erlangen, Germany), and 0.3 μM each primer, respectively. Primer sequences for nNOS were used as detailed by Huang, Harvard Medical School, Charlestown, Massachusetts (personal communication): B1 primer 5′-CCTTAGAGAGTAAGGAAGGGGGCGGG-3′ and B2 primer 5′-GGGCCGATCATTGACGGCGAGAATGATG-3′, giving rise to a 404 bp PCR product. The sequence of the standard Neo primers was 5′-TGCCGAGAAAGTATCCATCATGGCTGATGC-3′ and 5′-CAGAAGAACTCGTCA AGAAGGCGATAGAAGG-3′ producing a 460 bp product.
Stressor exposure
Forced swimming was performed in a glass cylinder (27 cm high and 15 cm wide) which was filled with tap water to a height of 15 cm. Fresh water was used in every trial. We decided to use a low water temperature (20 ± 1°C), as it is perceived more aversive by the animals. Mice were separated in three different groups and forced to swim for either 5 min (T5) or 10 min (T15 and T60). The animals of group T5 were immediately killed at the end of a 5 minute-swimming session, whereas those of groups T15 and T60 were gently dried with a towel after a 10-minute swimming session and returned to their home cages for 5 and 50 min, respectively, before being killed. The different time points were selected with the aim of investigating the hormonal responses of the HPA-axis and the sympatho-adrenomedullary system during 1 h following the defined stressor exposure.
In situ hybridisation
Mice (WT = 6, nNOS KO = 5) were deeply anaesthetised by isoflurane inhalation (Abbott GmbH, Wiesbaden, Germany) and quickly decapitated. Brains were immediately frozen at − 40°C in dry ice-chilled methylbutane and stored at − 80°C until sectioning. Serial 25 μm coronal cryosections throughout the rostrocaudal extension of the PVN were cut, with every fourth and seventh slice being thaw-mounted on a single RNA-free glass slide (i.e. three series, four sections/slide). In situ hybridisation using 35S uracil triphosphate-labelled ribonucleotide probes was performed as described elsewhere (Schafer and Day Citation1995) with some modifications. If not otherwise indicated, substances were obtained from Sigma-Aldrich, Munich, Germany. Briefly, sections were fixed in 4% paraformaldehyde for 1 h at room temperature. After two washing steps in PBS, sections were treated with proteinase K (0.1 μg/ml) in Tris–HCl pH 8.0, 50 mM EDTA for 10 min at 37°C. Subsequent washing steps were performed in DEPC-treated water for 5 min, 0.1 M triethanolamine pH 8.0 for 5 min, 0.1 M triethanolamine pH 8.0 with freshly added acetic acid anhydride for 10 min and finally 2 × standard saline citrate for 5 min. Brain sections were dehydrated in increasing concentrations of ethanol and air dried. The two vectors containing the sequence of DNA specific either for oxytocin or AVP were kindly provided by Dr Evita Mohr, Institute of Cellular Biochemistry and Clinical Neurobiology, Hamburg-Eppendorf University, whereas the CRH plasmid was available from previous experiments in our laboratory. The linearised DNA templates were used in a transcription reaction to produce 35S-uracil triphosphate-cRNA radioactive probes and 106 cpm/30 μl of AVP, oxytocin or CRH cRNA probe were applied onto each slide. Hybridisation was carried out at 55°C overnight in humid chambers with 75% formamide. The following day, the sections were rinsed in 2 × standard saline citrate, treated with RNAase A (40 μg/ml) and washed in increasingly stringent standard saline citrate solutions at room temperature.
The slides were dipped in Kodak NTB-2 nuclear emulsion (Eastman Kodak Co., Rochester, NY) diluted 1:1 in 0.5% glycerol and exposed for 4 days (AVP), 7 days (oxytocin) or 31 days (CRH) at 4°C. Sections were subsequently developed in Kodak Developer D19 (Eastman Kodak Co., Rochester, NY), fixed with Kodak fixer (Eastman Kodak Co., Rochester, NY) at 14°C, counterstained with Mayer's hematoxylin solution and eosin and finally coverslipped with DePeX (Serva, Heidelberg, Germany).
Grey levels were measured bilaterally as arbitrary units (AU) in dark-field images (AxioVision 4.2, Carl Zeiss Vision, Jena, Germany) at three different representative PVN levels (from Bregma − 0.70 to − 0.94; Paxinos and Franklin Citation1997), and cell numbers were counted when possible. Each image was adjusted for equal background. The hybridisation signal of nNOS in the PVN of KO mice was in the range of background levels in all cases (data not shown).
Blood sampling and hormone assays
Blood samples were collected between 8:00 and 11:00 a.m. Mice (n = 6–8 per group) were rapidly anaesthetised by isoflurane and blood samples were obtained by heart puncture within 45 s of first touching the cage at the following time points: after 5 min (T5), after 15 min (T15) or after 60 min (T60) from stressor onset. We decided to use heart puncture for blood collection because the volume required cannot be provided by retro-orbital or tail vein bleeding. Furthermore, unilateral occlusions caused by catheterization of either the carotid artery or jugular vein cause severe motor impairment in mice (Csete et al. Citation1986; Rainer Landgraf, MPI Munich, Germany, personal communication). This would have made it impossible to apply a 10 min forced swimming session and, moreover, to measure appropriate behavioural parameters (Orlando et al. Citation2007). The latter holds true also for the catheterisation of the femoral vessels, which impairs the motility of the respective limb and was, therefore, also not suitable with respect to our stressor paradigm. Other investigators reported the use of heart puncture as a reliable blood sampling technique for measuring plasma catecholamine levels in mice (Thomas and Palmiter Citation1997; Bornstein et al. Citation2000; Dash et al. Citation2001).
Blood was collected in ice chilled-EDTA-coated vials (Kabe Labortechnik, Nümbrecht-Elsenroth, Germany) containing 10 μl of Trasylol (Bayer, Leverkusen, Germany) and centrifuged (5000g, 5 min at 4°C) to separate plasma from cellular components. Control mice remained undisturbed in their home cages until blood sampling was performed. Aliquots of the supernatants were stored frozen at − 80°C until peptide content analysis. Plasma corticosterone and ACTH values were measured using radioimmunoassay kits (ICN Biomedicals, Inc., Costa Mesa, CA). Plasma norepinephrine and epinephrine levels were determined by ELISA (2 CAT EIA kit; LDN GmbH, Nordhorn, Germany).
Western blot analysis
A group of naïve mice was used for Western blot analysis. Mice (WT, n = 7, nNOS KO, n = 8) were deeply anaesthetised by isoflurane and quickly decapitated. Adrenal glands were excised, carefully freed from fat, homogenised in a lysis buffer containing 50 mM K+-/Na+-phosphate buffer (pH 6.7), 0.2% Triton X-100 and a cocktail of protein inhibitors (Roche Diagnostics GmbH, Mannheim, Germany) and finally centrifuged at 4°C 10,000g for 20 min. Only the supernatant (soluble proteins) was used for Western blot. Protein concentration was determined using a bicinchoninic acid protein assay kit (BCA kit, Pierce, Rockford, IL). Samples (5 μg for TH and 10 μg for PNMT) were electrophoresed using SDS-PAGE (gradient gel from 5 to 20%) and then transferred to nitrocellulose membranes. Samples from WT and KO mice were loaded on the same gel for comparison.
The membranes were blocked with 5% non-fat dry milk in Tris buffered saline with 0.1% Tween-20 and then incubated with polyclonal antibodies raised in rabbits against either TH (1:500, Chemicon, Chandlers Ford, UK) or PNMT (1:200, Acris Antibodies GmbH, Hiddenhausen, Germany) at 4°C overnight, followed by goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:10,000, Jackson ImmunoResearch Lab., West Grove, PA) at room temperature for 2 h. To assure equal sample loading, the membrane blots were co-incubated with mouse anti-beta-actin monoclonal antibody (1:2500, Sigma-Aldrich, Munich, Germany). After three washing steps, TH and PNMT were visualised by enzymatic chemiluminescence (ECL assay kit, Amersham Biosciences, Little Chalfont, UK). Blots were exposed to hyperfilm ECL autoradiographic film for 5 s (TH) or 1 min (PNMT) and bands were quantitated using Kodak 1D Image Analysis Software (Kodak, Rochester, NY). Graphs indicate densitometric analysis normalised to beta-actin values.
Statistical analysis
Plasma hormone values were submitted to a completely randomised two-way analysis of variance (ANOVA, GraphPad Software, San Diego, California; genotype × time points) followed by Fisher's LSD post hoc test (GB-Stat 6.0, Dynamic Microsystems, Silver Spring, MD), whereas in situ hybridisation and Western blot data were analysed using the Mann–Whitney test (GraphPad Software, San Diego, California). If not stated otherwise, all values are expressed as mean+SEM. p < 0.05 was considered statistically significant.
Results
Basal expression of AVP, oxytocin and CRH mRNAs in the PVN
Avp Mrna
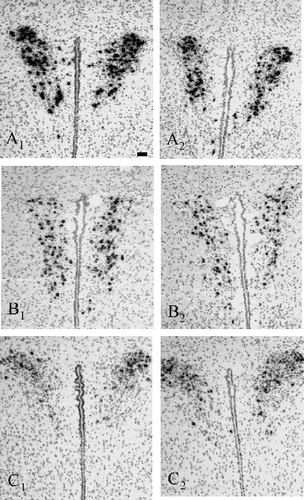
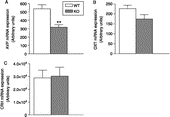
The relatively high intensity of the hybridisation signal did not allow an accurate cell count. Quantification of the emulsion-dipped slices revealed that in the PVN AVP mRNA grey value intensity was significantly lower in nNOS KO than in WT mice (318 ± 30 AU vs. 537 ± 49 AU, respectively; Mann–Whitney U-test, U = 0, p < 0.01; 1, A and 2AFigures 1, A2 and ).
Oxytocin mRNA
Neither the number of oxytocin mRNA-containing cells (data not shown) nor the overall oxytocin mRNA expression in the PVN differed significantly between WT and nNOS KO mice (174 ± 23 AU vs. 226 ± 17 AU; Mann–Whitney U-test, U = 6, p = 0.12; 1, 2 and 2BFigure 1, 1B2 and ).
Crh Mrna
Representative photomicrographs of CRH mRNA in situ hybridisation in WT and KO mice are shown in 1,1C2. shows the CRH mRNA grey values in the two groups. The intensity of the hybridisation signal in nNOS KO mice was similar to that in WT mice (3 × 104 AU ± 7 × 103 AU vs. 2.8 × 104 AU ± 0.6 × 103 AU, respectively; Mann–Whitney U-test, U = 15, p = 1.00).
Plasma neuroendocrine hormone measurements
ACTH
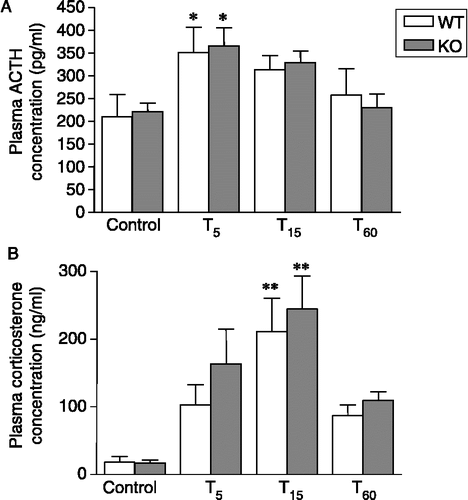
Plasma ACTH concentrations did not differ between the genotypes, either under resting conditions or in response to forced swimming: ACTH levels were found to be increased at T5 and returned close to resting levels at T15 and T60 (Factor sample time points: F3,51 = 5.10, p < 0.01; p < 0.05, Fisher's LSD test compared to control; Factor sample genotype: F1,51 = 0.01, p = 0.91; Factor sample interactions: F3,51 = 0.11, p = 0.94; ).
Figure 3 Plasma concentrations of (A) ACTH and (B) corticosterone in nNOS KO and WT mice under resting conditions (control) and 5, 15 and 50 min after a 10-min forced swimming session (T5, T15 and T60, respectively). Data are expressed as means ± SEM (n = 6–8 mice per group). In (A): *p < 0.05 vs. the respective control. In (B): **p < 0.01 vs. the respective controls. Two-way ANOVA followed by Fisher's LSD post hoc analysis.
Corticosterone
Both resting plasma concentrations and the release profile triggered by stressor exposure were similar in nNOS KO and WT mice. In both genotypes, a slight increase was observed at T5, whereas peak levels were reached at T15 (Factor sample time points: F3,57 = 13.46, p < 0.01; p < 0.01, Fisher's LSD test compared to control; Factor sample genotype: F1,57 = 1.47, p = 0.23; Factor sample interaction: F3,57 = 0.26, p = 0.84; ).
Epinephrine
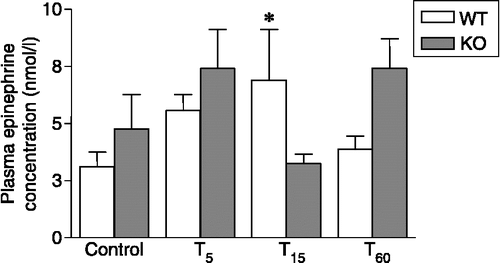
Plasma epinephrine levels in KO control mice (4.76+1.50 nmol/l) were slightly, but not significantly, higher than in WT (3.11+0.65 nmol/l). In WT animals, forced swimming caused an increase in epinephrine plasma values, which peaked 15 min after stressor onset (Factor sample interaction: F3,50 = 2.86, p = 0.04; p < 0.05, Fisher's LSD test compared to control; Factor sample time points: F3,50 = 1.39, p = 0.25; Factor sample genotype: F1,50 = 1.19, p = 0.28; ). In contrast, we failed to observe an increase of epinephrine in KO animals after forced swimming. Rather, the hormone concentration measured at all time points failed to differ from resting levels (F3,50 = 2.86, p = 0.04; p < 0.05, Fisher's LSD test compared to T5 and T60; ).
Figure 4 Plasma concentrations of epinephrine in nNOS KO and WT mice under resting conditions (control) and 5, 15 and 50 min after a 10-min forced swimming session (T5, T15 and T60, respectively). Data are expressed as means ± SEM (n = 6–8 mice per group). *p < 0.05 vs. WT control and T60 and vs. KO T15. Two-way ANOVA followed by Fisher's LSD post hoc analysis.
Norepinephrine
Plasma norepinephrine concentrations from WT (71.80+33.86 nmol/l) did not differ significantly from KO mice (87.10+24.66 nmol/l) under resting conditions and were not significantly altered by stressor exposure (Factor sample genotype: F1,53 = 3.57, p = 0.06; Factor sample time points: F3,53 = 0.34, p = 0.79; Factor sample interaction: F3,53 = 0.54, p = 0.65; data not shown).
Western blot analysis of adrenal gland enzymes
Tyrosine hydroxylase
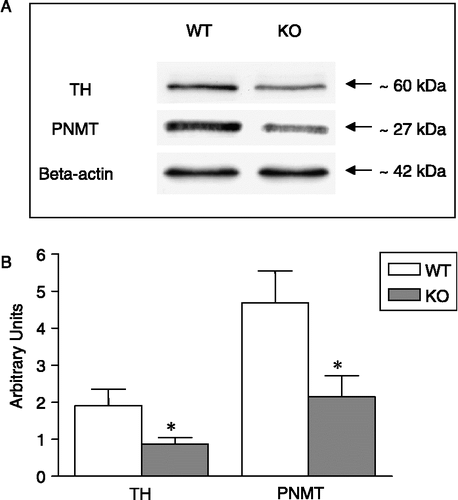
The TH signal was identified as a single band at ∼60 kDa (). Densitometric analysis revealed a reduced expression of TH in adrenal gland homogenates of nNOS KO mice compared to WT (Mann–Whitney U-test, U = 9, p < 0.05; ).
Figure 5 Western blot analysis of TH and PNMT in adrenal gland homogenates pooled from WT (n = 7) and KO mice (n = 8). (A) Gel lanes for TH, PNMT and beta-actin. The positions of the respective molecular weight markers are indicated. (B) Semi-quantitative histogram depicting TH and PNMT expression. Data were normalised to the respective beta-actin values. *p < 0.05 vs. WT, Mann–Whitney U-test.
Phenylethanolamine N-methyltransferase
The PNMT signal was seen at ∼27 kDa (). Similar to TH, quantitative analysis of PNMT-stained immunoblots revealed a significantly lower expression of this enzyme in nNOS KO mice compared to WT (Mann–Whitney U-test, U = 7, p < 0.05; ).
Discussion
This study was designed to gain further insight into the role that NO of nNOS origin plays in the regulation of endocrine systems involved in the mammalian hormonal stress response. We used conventional KO mice to evaluate the impact of a lifelong absence of nNOS on the basal expression of relevant neuropeptide mRNAs in the PVN and on catecholamine producing enzyme proteins in the adrenal gland. Moreover, we measured the concentration of selected stress hormones under resting conditions and in response to forced swimming.
nNOS KO mice displayed a significantly reduced AVP mRNA content in the PVN under resting conditions (). Previous studies investigating this parameter failed to detect such a difference (Bernstein et al. Citation1998; Nomura et al. Citation2005). However, these studies were conducted using oligonucleotides, whereas we employed cRNA probes, which are known to result in an enhanced sensitivity and reduced background signal. Together, this is likely to have contributed to the detection of differences that have not been observed previously. Our result is supported by the finding that AVP transcriptional activity was enhanced after intracerebroventricular injection of a NO donor predominantly in the PVN in rats (Lee et al. Citation1999) and suggests that, in the mouse, endogenous NO of nNOS origin contributes to the control of AVP mRNA expression in the PVN in a stimulatory manner. Evidently, magnocellular neurones of the PVN rather than the SON release oxytocin into the general circulation under stress conditions (Jezova et al. Citation1993), and AVP neurones in the SON rather than the PVN respond to osmotic stimulation (Burbach et al. Citation1984; Sherman et al. Citation1986; Gulya et al. Citation1991). The observation of reduced mRNA levels for AVP in the PVN of nNOS KO mice () is consistent with the reported reduced plasma AVP levels in response to forced swimming in this genotype (Orlando et al. Citation2007). This further suggests that not only oxytocin, but also AVP release into plasma during stress originates predominantly from PVN magnocellular neurones. This suggestion is supported by results showing higher AVP mRNA levels in the SON of nNOS KO mice (Orlando et al. Citation2007). At the same time, these findings provide evidence for a differential contribution of NO of nNOS origin on the expression of the mRNA coding for the same neuropeptide in the PVN versus the SON. Based on the information available, it is difficult to identify the mechanism that might have contributed to this difference. Further studies should investigate for instance the impact of nNOS absence during early development of AVP versus oxytocin neuronal populations within the two hypothalamic nuclei.
In contrast to AVP, oxytocin mRNA levels in KO mice did not differ significantly from WT. Obviously, the impact of congenital absence of nNOS on magnocellular oxytocin neurones is different from that on AVP cells, a functional heterogeneity that has been observed previously (Roberts et al. Citation1993; Orlando et al. Citation2007).
The involvement of NO in the modulation of ACTH secretion might be due to the subcellular localisation of nNOS in the PVN (Torres et al. Citation1993; Siaud et al. Citation1994; Hatakeyama et al. Citation1996), which suggests that NO may participate in an autocrine and/or paracrine manner to the regulation of CRH release into the portal blood. Indeed, it has been suggested that NO is involved in the control of the CRH neurosecretory system (Riedel Citation2000). However, some investigators reported no effect of NO precursor or NOS inhibitor treatment on basal CRH release (Costa et al. Citation1993). We have extended these findings by showing that in nNOS KO mice baseline mRNA level for CRH in the PVN is comparable to that of WT mice. Consistent with this observation, plasma ACTH and corticosterone levels both under resting conditions and in response to stressor exposure were equivalent in the two genotypes (). These results suggest that the responsiveness of the HPA-axis has not been impaired by nNOS gene disruption. Although this holds for the net output of the HPA axis, the literature suggests that normal ACTH plasma values might result from complementary effects of NO at the levels of the median eminence, where NO might have an inhibitory action (Rivier and Shen Citation1994), and at the anterior pituitary, where NO might play a stimulatory role (Brunetti et al. Citation1993; Keilhoff et al. Citation2001; Akasaka et al. Citation2006). It may be that the absence of nNOS in the KO mice might have caused opposite effects in the brain and at the anterior pituitary that compensate each other, and thus has no net effect on ACTH secretion.
Gross anatomical and histological investigation of the adrenal gland provided no evidence for possible differences between the genotypes (unpublished observations). This is in line with the data of Huang et al. (Citation1993), who first described nNOS KO mice and reported that the anatomy of the adrenal gland of these animals appears normal. The physiological role NO may play in controlling the secretory activity of adrenal chromaffin cells under resting conditions is still a matter of discussion (Uchiyama et al. Citation1994; Oset-Gasque et al. Citation1994; Marley et al. Citation1995; Rodriguez-Pascual et al. Citation1996; Ward et al. Citation1996). An earlier study showed that KO mice displayed a reduced number of beta-endorphin positive cells and fibers in the hypothalamus (Bernstein et al. Citation1998). Beta-endorphin has been reported to stimulate central sympathetic outflow, thereby promoting catecholamine secretion (Van Loon et al. Citation1981a,Citationb; Appel et al. Citation1984). However, the activity of the sympatho-adrenal system, measured in terms of plasma norepinephrine and epinephrine values, appeared normal under resting conditions. It has to be mentioned that we failed to observe a significant effect of stressor exposure on plasma norepinephrine levels in either genotype. This failure is most likely due to technical limitations of our experimental approach as (1) our basal norepinephrine values (approximately 72 and 87 nmol/l for WT and KO, respectively) are high compared to the levels reported in the literature (4–124 nmol/l; Grouzmann et al. Citation2003) and (2) we cannot rule out that we missed a fast and transient increase of norepinephrine in response to acute stressor exposure (Sgoifo et al. Citation1996) due to our sampling protocol (i.e. first “stress” sample taken 5 min after stressor onset).
In contrast to norepinephrine, we were able to measure significantly increased epinephrine plasma levels in WT animals triggered by forced swimming, whereas nNOS KO mice showed no significant response (). The basal epinephrine levels (approximately 3–5 nmol/l; ) are in the range of samples obtained via arterial catheters in mice (approximately 1 nmol/l: Grouzmann et al. Citation2003). This implies that blood sampling performed by heart puncture in the present study provides reliable epinephrine plasma concentrations. Therefore, the observed difference between WT and KO in the ability to mount an epinephrine response to forced swimming could be attributed to a limited capacity of the epinephrine synthesising machinery that is unable to respond adequately to acute stressor exposure in nNOS KO mice. Indeed, we measured significantly reduced TH and PNMT protein levels in the adrenal glands in KO mice. Thus, the missing response of plasma epinephrine observed in KO mice 15 min after stressor onset may be ascribed to reduced amounts of both TH and PNMT, which seem be sufficient to ensure normal resting levels of epinephrine, but become inadequate under acute stress conditions. Although nNOS is preferentially expressed by noradrenergic cells in the adrenal medulla of different mammalian species (Dun et al. Citation1993; Heym et al. Citation1994), adrenergic cells constitute the main target of NO action (Oset-Gasque et al. Citation1998). Thus, the life-long lasting absence of NO of nNOS origin is prone to affect the function of these adrenergic chromaffin cells. Moreover, the activity of the catecholamine biosynthetic enzymes is regulated by different protein kinases through phosphorylation (Zigmond et al. Citation1989) and gene expression (Sabban and Kvetnansky Citation2001). Previous reports have shown that NO up-regulates activity and transcript levels of these enzymes through a cyclic GMP (cGMP)/G protein kinase (PKG)-activated pathway (Kim et al. Citation2003). Based on the results obtained here, there is good evidence that the lifelong absence of NO originating from nNOS in the adrenal glands of KO mice reduces the capacity of the epinephrine synthesising pathway to ensure an adequate response to acute stressors.
In conclusion, we have shown here that, in the whole animal, nNOS gene inactivation reduced the expression of AVP mRNA in the PVN, whereas HPA axis activity remained indistinguishable from that of WT mice in response to forced swimming. More importantly, the constitutive absence of NO of nNOS origin was associated with lower basal TH and PNMT protein levels in the adrenal gland, and affected the secretion of epinephrine from the adrenal medulla following acute stressor exposure. Further studies should focus on the possible contribution of the altered epinephrine response to the behavioural alterations seen in KO mice during forced swimming under less challenging conditions (Salchner et al. Citation2004) with respect to the balance between active and passive coping.
Acknowledgements
The authors gratefully thank Regina Dobrowolny, Rita Murau and Andrea Rudloff for their excellent technical assistance. This work was sponsored by the Graduiertenkolleg Project of the Otto von Guericke University, Magdeburg, and by the Bundesministerium für Bildung und Forschung (BMBF; grant number 01ZZ0407).
References
- Akasaka S, Nomura M, Nishii H, Fujimoto N, Ueta Y, Tsutsui M, Shimokawa H, Yanagihara N, Matsumoto T. The hypothalamo-pituitary axis responses to lipopolysaccharide-induced endotoxemia in mice lacking inducible nitric oxide synthase. Brain Res 2006; 1089: 1–9
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. Biochem J 2001; 357: 593–615
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol 1993; 14: 76–122
- Appel NM, Kiritsy-Roy JA, Van Loon GR. Hypothalamic opioid peptide regulation of catecholamine secretion. Neuropeptides 1984; 5: 287–290
- Aubry JM, Bartanusz V, Jezova D, Belin D, Kiss JZ. Single stress induces long-lasting elevations in vasopressin mRNA levels in CRF hypophysiotrophic neurones, but repeated stress is required to modify AVP immunoreactivity. J Neuroendocrinol 1999; 11: 377–384
- Barnes RD, Ward LE, Frank KP, Tyce GM, Hunter LW, Rorie DK. Nitric oxide modulates evoked catecholamine release from canine adrenal medulla. Neuroscience 2001; 104: 1165–1173
- Bernstein HG, Keilhoff G, Seidel B, Stanarius A, Huang PL, Fishman MC, Reiser M, Bogerts B, Wolf G. Expression of hypothalamic peptides in mice lacking neuronal nitric oxide synthase: Reduced beta-END immunoreactivity in the arcuate nucleus. Neuroendocrinology 1998; 68: 403–411
- Bornstein SR, Tian H, Haidan A, Bottner A, Hiroi N, Eisenhofer G, McCann SM, Chrousos GP, Roffler-Tarlov S. Deletion of tyrosine hydroxylase gene reveals functional interdependence of adrenocortical and chromaffin cell system in vivo. Proc Natl Acad Sci USA 2000; 97: 14742–14747
- Brunetti L, Preziosi P, Ragazzoni E, Vacca M. Involvement of nitric oxide in basal and interleukin-1 beta-induced CRH and ACTH release in vitro. Life Sci 1993; 53: PL219–PL222
- Burbach JP, De Hoop MJ, Schmale H, Richter D, De Kloet ER, Ten Haaf JA, De Wied D. Differential responses to osmotic stress of vasopressin-neurophysin mRNA in hypothalamic nuclei. Neuroendocrinology 1984; 39: 582–584
- Chiavegatto S, Nelson RJ. Interaction of nitric oxide and serotonin in aggressive behavior. Horm Behav 2003; 44: 233–241
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci USA 2001; 98: 1277–1281
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992; 267: 1244–1252
- Costa A, Trainer P, Besser M, Grossman A. Nitric oxide modulates the release of corticotropin-releasing hormone from the rat hypothalamus in vitro. Brain Res 1993; 605: 187–192
- Csete K, Kovacs GL, Szekeres L. Disturbance of motoric function as behavioral measure of impaired cerebral circulation in mice. Physiol Behav 1986; 36: 409–412
- Dash R, Kadambi V, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, Lorenz JN, Dorn GWn, Kranias EG. Interactions between phospholamban and beta-adrenergic drive may lead to cardiomyopathy and early mortality. Circulation 2001; 103: 889–896
- Dun NJ, Dun SL, Wu SY, Forstermann U. Nitric oxide synthase immunoreactivity in rat superior cervical ganglia and adrenal glands. Neurosci Lett 1993; 158: 51–54
- Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: Prominent functional localizations in the brain. Proc Natl Acad Sci USA 1997; 94: 3396–3401
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: An old concept revisited. Front Neuroendocrinol 2004; 25: 132–149
- Giordano M, Vermeulen M, Trevani AS, Dran G, Andonegui G, Geffner JR. Nitric oxide synthase inhibitors enhance plasma levels of corticosterone and ACTH. Acta Physiol Scand 1996; 157: 259–264
- Givalois L, Li S, Pelletier G. Central nitric oxide regulation of the hypothalamic-pituitary-adrenocortical axis in adult male rats. Brain Res Mol Brain Res 2002; 102: 1–8
- Grouzmann E, Cavadas C, Grand D, Moratel M, Aubert JF, Brunner HR, Mazzolai L. Blood sampling methodology is crucial for precise measurement of plasma catecholamines concentrations in mice. Pflugers Arch 2003; 447: 254–258
- Gulya K, Dave JR, Hoffman PL. Chronic ethanol ingestion decreases vasopressin mRNA in hypothalamic and extrahypothalamic nuclei of mouse brain. Brain Res 1991; 557: 129–135
- Hashimoto K, Nishioka T, Tojo C, Takao T. Nitric oxide plays no role in ACTH release induced by interleukin-1 beta, corticotropin-releasing hormone, arginine vasopressin and phorbol myristate acetate in rat pituitary cell cultures. Endocr J 1995; 42: 435–439
- Hatakeyama S, Kawai Y, Ueyama T, Senba E. Nitric oxide synthase-containing magnocellular neurons of the rat hypothalamus synthesize oxytocin and vasopressin and express Fos following stress stimuli. J Chem Neuroanat 1996; 11: 243–256
- Heym C, Colombo-Benckmann M, Mayer B. Immunohistochemical demonstration of the synthesis enzyme for nitric oxide and of comediators in neurons and chromaffin cells of the human adrenal medulla. Ann Anat 1994; 176: 11–16
- Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: Cardiovascular and neurochemical implications. Am J Physiol 1994; 266: R306–R313
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 1993; 75: 1273–1286
- Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: Basis of the fight-or-flight response. Science 1995; 270: 644–646
- Kadekaro M. Nitric oxide modulation of the hypothalamo-neurohypophyseal system. Braz J Med Biol Res 2004; 37: 441–450
- Keilhoff G, Seidel B, Reiser M, Stanarius A, Huang PL, Bogerts B, Wolf G, Bernstein HG. Lack of neuronal NOS has consequences for the expression of POMC and POMC-derived peptides in the mouse pituitary. Acta Histochem 2001; 103: 397–412
- Kim D, Choi HJ, Kim SW, Cho SW, Hwang O. Upregulation of catecholamine biosynthetic enzymes by nitric oxide. J Neurosci Res 2003; 72: 98–104
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev 2001; 25: 117–142
- Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 1983; 37: 314–316
- Lee S, Kim CK, Rivier C. Nitric oxide stimulates ACTH secretion and the transcription of the genes encoding for NGFI-B, corticotropin-releasing factor, corticotropin-releasing factor receptor type 1, and vasopressin in the hypothalamus of the intact rat. J Neurosci 1999; 19: 7640–7647
- Marley PD, McLeod J, Anderson C, Thomson KA. Nerves containing nitric oxide synthase and their possible function in the control of catecholamine secretion in the bovine adrenal medulla. J Auton Nerv Syst 1995; 54: 184–194
- Miyagawa A, Okamura H, Ibata Y. Coexistence of oxytocin and NADPH-diaphorase in magnocellular neurons of the paraventricular and the supraoptic nuclei of the rat hypothalamus. Neurosci Lett 1994; 171: 13–16
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 1995; 378: 383–386
- Nomura M, Tsutsui M, Shimokawa H, Fujimoto N, Ueta Y, Morishita T, Yanagihara N, Matsumoto T. Effects of nitric oxide synthase isoform deletion on oxytocin and vasopressin messenger RNA in mouse hypothalamus. Neuroreport 2005; 16: 413–417
- Orlando GF, Langnaese K, Landgraf R, Spina MG, Wolf G, Engelmann M. Neural nitric oxide gene inactivation affects the release profile of oxytocin into the blood in response to forced swimming. Nitric Oxide 2007; 16: 64–70
- Oset-Gasque MJ, Parramon M, Hortelano S, Bosca L, Gonzalez MP. Nitric oxide implication in the control of neurosecretion by chromaffin cells. J Neurochem 1994; 63: 1693–1700
- Oset-Gasque MJ, Vicente S, Gonzalez MP, Rosario LM, Castro E. Segregation of nitric oxide synthase expression and calcium response to nitric oxide in adrenergic and noradrenergic bovine chromaffin cells. Neuroscience 1998; 83: 271–280
- Palacios M, Knowles RG, Palmer RM, Moncada S. Nitric oxide from l-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun 1989; 165: 802–809
- Paulmyer-Lacroix O, Anglade G, Grino M. Insulin-induced hypoglycaemia increases colocalization of corticotrophin-releasing factor and arginine vasopressin mRNAs in the rat hypothalamic paraventricular nucleus. J Mol Endocrinol 1994; 13: 313–320
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego, USA 1997
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 2000; 100: 549–556
- Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res 1998; 120: 164–172
- Riedel W. Role of nitric oxide in the control of the hypothalamic–pituitary–adrenocortical axis. Z Rheumatol 2000; 59(Suppl 2)II/36–II/42
- Rivier C, Shen GH. In the rat, endogenous nitric oxide modulates the response of the hypothalamic–pituitary–adrenal axis to interleukin-1 beta, vasopressin, and oxytocin. J Neurosci 1994; 14: 1985–1993
- Roberts MM, Robinson AG, Fitzsimmons MD, Grant F, Lee WS, Hoffman GE. c-Fos expression in vasopressin and oxytocin neurons reveals functional heterogeneity within magnocellular neurons. Neuroendocrinology 1993; 57: 388–400
- Rodriguez-Pascual F, Miras-Portugal MT, Torres M. Effect of cyclic GMP-increasing agents nitric oxide and C-type natriuretic peptide on bovine chromaffin cell function: Inhibitory role mediated by cyclic GMP-dependent protein kinase. Mol Pharmacol 1996; 49: 1058–1070
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: Dynamics of transcriptional events. Trends Neurosci 2001; 24: 91–98
- Salchner P, Lubec G, Engelmann M, Orlando GF, Wolf G, Sartori SB, Hoeger H, Singewald N. Genetic functional inactivation of neuronal nitric oxide synthase affects stress-related Fos expression in specific brain regions. Cell Mol Life Sci 2004; 61: 1498–1506
- Sanchez F, Alonso JR, Arevalo R, Blanco E, Aijon J, Vazquez R. Coexistence of NADPH-diaphorase with vasopressin and oxytocin in the hypothalamic magnocellular neurosecretory nuclei of the rat. Cell Tissue Res 1994; 276: 31–34
- Schafer MK-H, Day R. In situ hybridization techniques to map processing enzymes. Methods Neurosci 1995; 23: 16–44
- Sgoifo A, de Boer SF, Haller J, Koolhaas JM. Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: Relationship with aggression. Physiol Behav 1996; 60: 1403–1407
- Sherman TG, McKelvy JF, Watson SJ. Vasopressin mRNA regulation in individual hypothalamic nuclei: A northern and in situ hybridization analysis. J Neurosci 1986; 6: 1685–1694
- Siaud P, Mekaouche M, Ixart G, Balmefrezol M, Givalois L, Barbanel G, Assenmacher I. A subpopulation of corticotropin-releasing hormone neurosecretory cells in the paraventricular nucleus of the hypothalamus also contain NADPH-diaphorase. Neurosci Lett 1994; 170: 51–54
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell 1997; 91: 583–592
- Torres G, Lee S, Rivier C. Ontogeny of the rat hypothalamic nitric oxide synthase and colocalizatio with neuropeptides. Mol Cell Neurosci 1993; 4: 155–163
- Uchiyama Y, Morita K, Kitayama S, Suemitsu T, Minami N, Miyasako T, Dohi T. Possible involvement of nitric oxide in acetylcholine-induced increase of intracellular Ca2+ concentration and catecholamine release in bovine adrenal chromaffin cells. Jpn J Pharmacol 1994; 65: 73–77
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981; 213: 1394–1397
- Van Loon GR, Appel NM, Ho D. Beta-endorphin-induced increases in plasma epinephrine, norepinephrine and dopamine in rats: Inhibition of adrenomedullary response by intracerebral somatostatin. Brain Res 1981a; 212: 207–214
- Van Loon GR, Appel NM, Ho D. Beta-endorphin-induced stimulation of central sympathetic outflow: Beta-endorphin increases plasma concentrations of epinephrine, norepinephrine, and dopamine in rats. Endocrinology 1981b; 109: 46–53
- Vicente S, Gonzalez MP, Oset-Gasque MJ. Neuronal nitric oxide synthase modulates basal catecholamine secretion in bovine chromaffin cells. J Neurosci Res 2002; 69: 327–340
- Ward LE, Hunter LW, Grabau CE, Tyce GM, Rorie DK. Nitric oxide reduces basal efflux of catecholamines from perfused dog adrenal glands. J Auton Nerv Syst 1996; 61: 235–242
- Weitzdoerfer R, Hoeger H, Engidawork E, Engelmann M, Singewald N, Lubec G, Lubec B. Neuronal nitric oxide synthase knock-out mice show impaired cognitive performance. Nitric Oxide 2004; 10: 130–140
- Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: A novel mechanism of regulating adrenocorticotropic hormone secretion?. J Neurosci 1996; 16: 7725–7732
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: New insights into the secretory capacities of peptidergic neurons. Neuroscience 1998; 85: 1209–1222
- Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci 2001; 13: 2273–2281
- Yamashita H, Inenaga K, Koizumi K. Possible projections from regions of paraventricular and supraoptic nuclei to the spinal cord: Electrophysiological studies. Brain Res 1984; 296: 373–378
- Zigmond RE, Schwarzschild MA, Rittenhouse AR. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci 1989; 12: 415–461