Abstract
Diabetes mellitus type 2 (DM type 2) is associated with depressive symptomatology and intermittent hyperfunction of the hypothalamic–pituitary–adrenal (HPA) axis. DM type 2 is also accompanied by increased tissue levels of angiotensin II (Ang II), which stimulates the HPA axis through the Ang II type 1 receptors (AT1). We investigated the effect of candesartan, an angiotensin receptor blocker (ARB) that crosses the blood brain barrier, on the activity of the HPA axis and on the affect of 17 patients with DM type 2, aged 40–65 years, who were treated with 4 mg/day candesartan per os for at least 3 months. Before and after candesartan administration, a corticotropin-releasing hormone (CRH) stimulation test and psychological tests were performed. In response to hCRH, time-integrated secretion of ACTH was not altered by candesartan administration, however, the cortisol response was decreased significantly compared to baseline (mean ± SEM, 2327 ± 148.3 vs. 1943 ± 131.9 μg/dl, P = 0.005) suggesting reduced sensitivity of the adrenals to ACTH. In parallel, there was a significant improvement in interpersonal sensitivity (0.91 ± 0.16 vs. 0.70 ± 0.15, P = 0.027) and depression scores (0.96 ± 0.15 vs. 0.71 ± 0.10, P = 0.026). We suggest that candesartan resets the HPA axis of patients with DM type 2 and improves their affect.
Introduction
Angiotensin II (Ang II), the final effector of the renin–angiotensin system (RAS) (Lavoie and Sigmund Citation2003) plays major roles in fluid and electrolyte homeostasis and the regulation of the hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous system (Phillips Citation1987; Saavedra Citation1992; Phillips and Sumners Citation1998). Ang II exerts its effects through angitotensin receptors 1 and 2 (AT1 and AT2 receptors). These G protein-coupled receptor subtypes are present in various proportions in Ang II target tissues. In the brain, AT1 and AT2 are widely distributed in structures that are both outside and inside the blood brain barrier (Landas et al. Citation1980; Tsutsumi and Saavedra Citation1991A,CitationB,CitationC). Ang II modulates CRH neurons directly in the rat hypothalamus, through AT1 receptors, which are located in the perikarya of the periventricular and parvicellular divisions of the paraventricular nucleus (Aguilera et al. Citation1995; Latchford and Ferguson Citation2005). In rodents and primates, AT1 receptors have also been described on the pituitary corticotrophs (Hauger et al. Citation1982; Lenkei et al. Citation1999; Latchford and Ferguson Citation2005; Pawlikowski Citation2006), with Ang II directly stimulating ACTH secretion in vitro (Gaillard et al. Citation1981; Ganong Citation1993). The in vivo effect of Ang II on ACTH secretion is probably mediated primarily by CRH, since an increase in plasma ACTH requires relatively high doses of peripherally administered Ang II, while intracerebroventricular injection of low doses (1 ng) of this hormone suffice to elicit large increases in plasma ACTH concentration (Spinedi and Negro-Vilar Citation1983; Dessi-Fulgheri et al.Citation1985; Spinedi and Rodriguez Citation1986; Murakami and Ganong Citation1987; Ganong Citation1993; Volpi et al. Citation1996; Coiro et al. Citation1998). Furthermore, Ang II stimulates the formation and release of vasopressin in the paraventricular nucleus (Phillips Citation1987; Veltmar et al. Citation1992; Lenkei et al. Citation1995). Vasopressin secreted into the hypophysial portal circulation synergizes with CRH, enhancing the secretion of ACTH (Gillies et al. Citation1982; Liu et al. Citation1983). Ang II also has direct effects on the adrenal gland, via the splanchnic nerves, stimulating the secretion of cortisol from the zona fasciculate (Rabano et al. Citation2004), of aldosterone from the zona glomerulosa (Aguilera Citation1993) and of catecholamines from the adrenal medulla (Livett et al. Citation1990). Furthermore, there are intrinsic RAS systems in the adrenal zona glomerulosa and medulla through which locally produced Ang II mediates the release of aldosterone and the catecholamines (Plunkett et al. Citation1985; Deschepper et al. Citation1986; Livett and Marley Citation1993; Phillips et al. Citation1993). In the rat adrenal medulla, AT1 and AT2 receptors regulate norepinephrine and epinephrine formation and release (Jezova et al. Citation2003). In addition, centrally administered Ang II increases the transcription rate of tyrosine hydroxylase, the rate-limiting enzyme of catecholamine formation in the locus coeruleus (Seltzer et al. Citation2004).
In diabetes mellitus type 2, (DM type 2) the circulating RAS is usually normal or suppressed, while tissue Ang II level is increased (Henriksen et al. Citation2001; Kersaw and Flier Citation2004; Shinozaki et al. Citation2004; Giacchetti et al. Citation2005). This increased tissue Ang II induces oxidative stress, endothelial damage and disease pathology including vasoconstriction, thrombosis, inflammation and vascular remodeling. Furthermore, insulin resistance is associated with upregulation of the AT1 receptor and an increase in oxygen free radicals in endothelial tissue caused by activation of NAD(P)H oxidase. Treatment with an AT1 receptor blocker (ARB) normalizes oxidase activity and improves endothelial function (McFarlane et al. Citation2003; Giacchetti et al. Citation2005; Zanchetti and Elmfeldt Citation2006). DM type 2 is a chronic disease associated with protracted metabolic dyshomeostasis of different degrees. Patients with this disorder have intermittent hyperfunction of their HPA axis expressed as non-suppression of plasma cortisol by dexamethasone, an increased 24 h urinary free cortisol (UFC) excretion rate and/or an abnormal CRH stimulation test (Roy et al. Citation1993; Tsigos et al. Citation1993). Subtle abnormalities in cortisol action may be one of the missing links among factors contributing to development of the metabolic syndrome and its manifestations in patients with obesity, hypertension, coronary heart disease, hyperlipidemia and DM type 2 (Andrews et al. Citation2002). Also it is known that cortisol administration impairs cholinergic vasodilation (Mangos et al. Citation2000) and suppresses nitric oxide (NO) release by down-regulating endothelial NO synthase proteins (eNOs) (Rogers et al. Citation2002). Interestingly, chronic CRH hypersecretion and/or mild hypercortisolism also characterize chronic anxiety (Tsigos and Chrousos Citation2002; Contoreggi et al. Citation2004) and major depression (Tsigos and Chrousos Citation1994; Habib et al. Citation2001; Makino et al. Citation2002; Tsigos and Chrousos Citation2002), while in DM type 2 there is an increased prevalence of both of these disorders (Wells et al. Citation1989; Thomas et al. Citation2003; Charmandari et al. Citation2005).
We hypothesized that an ARB that crosses the blood brain barrier could reset the HPA axis by inhibiting both peripheral and central AT1 receptors in disorders such as DM type 2 characterized by mild intermittent hyperactivity of this axis and mild affective symptomatology (Nishimura et al. Citation2000; Gohlke et al. Citation2002). To test the ability of candesartan to normalize the activity of the HPA axis in patients with DM type 2 to decrease their affective symptomatology, and improve arterial wall properties, we administered candesartan at a low dose and examined the patients and their HPA axis function before and after at least 3 months of candesartan administration.
Materials and methods
Patients
We studied 17 patients, 6 women and 11 men, aged 40–65 years (mean ± SEM, 53.9 ± 2.44 years) with DM type 2 of less than 5 years duration, who first were diagnosed and regularly followed at Diabetes Clinics of the Athens University Medical School. The diagnosis of DM type 2 was based on the criteria established by the World Health Organization in 1996 and modified by the American Diabetes Association in 1997 and 2003 (American Diabetes Association Standards of medical care in diabetes Citation2006).
Patients were included in the study provided that: (a) their DM type 2 was controlled by diet and/or oral antidiabetic medication, and (b) they had no clinical or biochemical evidence of retinopathy, nephropathy, or neuropathy. Patients that were receiving antidiabetic medication(s) continued their treatment. Patients were excluded if they had concurrent hypertension, an allergic, inflammatory or autoimmune disorder, or were pregnant or lactating. In addition, patients were excluded if they were receiving synthetic glucocorticoids, non-steroidal anti-inflammatory drugs, or psychotropic medication. Five of the 6 women included were menopausal and received no hormone therapy, while one was pre-menopausal not on oral contraceptives.
The patients' hemoglobin (Hb) A1c and albumin to creatinine ratio in a spot urine sample was < 7% (mean ± SEM, 6.5 ± 0.26%), with < 30 μg/mg creatinine (mean ± SEM, 18.0 ± 0.84 μg/mg), respectively (American Diabetes Association Standards of medical care in diabetes Citation2006).
Protocol
On the first visit, a complete medical history was obtained, while a physical examination, screening laboratory examinations (a complete blood count and standard chemistry) and an electrocardiogram were performed. To exclude retinopathy, patients underwent eye fundoscopy. Nephropathy was excluded by plasma measurements of creatinine and urea and spot urine measurements of the albumin-to-creatinine ratio (as above; American Diabetes Association Standards of medical care in diabetes Citation2006). Neuropathy was excluded both by a neurological examination and the use of a vibrameter for the assessment of sensory perception thresholds.
Patients received 4 mg/day candesartan per os for at least 3 months. To ensure compliance with candesartan administration, patients were asked to note down the daily taking of the medicine. During this time, and throughout the study, patients receiving medication(s) for diabetes type 2 continued their standard treatment. Before and at the end of candesartan administration, patients underwent standard anthropometric measurements (body mass index, BMI; height, waist to hip ratio), laboratory examinations (glucose, HbA1c, urea, creatinine, lipids, C-reactive protein (CRP), serum amyloid A protein (SAA), serum insulin, cortisol-binding globulin (CBG), albumin and creatinine in the urine free cortisol (UFC); estimation of indices of insulin resistance and sensitivity by homeostasis model assessment (HOMA) and quantitative insulin sensitive check index (QUICKI), respectively, endocrine (CRH test), psychological tests [symptom checklist-90-Revised (SCL-90-R), state-trait-anxiety-inventory (STAI), Zung Depression scale, Whiteley index, Leyton obsessional inventory and visual analogue scales] and peripheral hemodynamic measurements [flow-mediated dilation of the brachial artery (FMD), Carotid–femoral pulse wave velocity (PWV), heart rate, systolic and diastolic blood pressure).
Methods
Anthropometry
Height in centimeters was measured on a portable stadiometer calibrated with a machine meter rod, and weight in kilograms was measured with an electronic scale. Body mass index (BMI) was calculated by dividing body weight in kilogram by height in meters squared. Waist to hip circumference ratio was also obtained.
Biochemical tests
Blood samples were drawn in the morning (0800 h) from an antecubital vein after at least 8 h of fasting and before taking any medication(s). Peripheral venous blood samples (20 ml) were drawn into plastic syringes under sterile conditions and transferred immediately to appropriate tubes for measurements of glucose, HbA1c, urea, creatinine, SAA, CRP, lipids, insulin and CBG. UFC was determined in 24 h urine collections, while albumin and creatinine were also determined in urine spot collections for ratio calculation. All measurements were performed before and after chronic candesartan administration.
HOMA index
Insulin resistance was estimated by the HOMA index by employing the following formula: [Glucose (mmol/l) × Insulin (mIU/ml)/22.5] (Hanley et al. Citation2002).
Quicki Index
Insulin sensitivity was defined using the quantitative insulin sensitivity check index by employing the following formula: 1/(log10 [fasting Glucose] + log10 [fasting Insulin] (Katz et al. Citation2000).
CRH stimulation test
This was performed for the evaluation of HPA axis function. At 1600 h, patients were placed at bed rest for the duration of the procedure, and an i.v. catheter was inserted 40 min before CRH administration. At 1700 h, human (h)CRH was administered at a dose 1 μg/kg as an i.v. bolus over 2 min. Blood for ACTH determinations was drawn and placed in pre-chilled EDTA containing tubes 15 and 1 min before and 5, 15, 30, 60, 90 and 120 min after administration of hCRH and placed on ice. Blood for cortisol determinations was also drawn and placed in appropriate tubes, at the same time-points. Plasma and serum were separated in a refrigerated centrifuge within 3 h of withdrawal, stored in polystyrene tubes and frozen at − 20°C until assayed for ACTH and cortisol.
Assessment of endothelial function
This was measured as FMD of the brachial artery. All subjects were studied in the morning, having abstained from alcohol, caffeine, food and tobacco use for 8 h before the study as well as, before they had taken their medication(s). The patient rested supine in a quiet room for at least 30 min before endothelial function was assessed. The procedure was performed according to recently published guidelines. Optimal imaging of the right brachial artery was obtained and a resting scan was recorded using an Echo–Doppler ultrasound machine (Vivid 7, GE, Horton, Norway) and a 7–10 MHz transducer. Reactive hyperemia was then induced by inflation of a blood pressure cuff on the forearm for 5 min and subsequent deflation; the brachial artery was scanned continuously 30 s before and 90 s after cuff deflation. All images were recorded on super VHS videotape for analysis. Artery diameter measurements were made using electronic calipers from the anterior to the posterior mid-line. Flow-mediated dilation was calculated as the percent increase in arterial diameter during hyperemia as compared to the resting scan. At the end images were obtained 4 min after sublingual nitroglycerine (0.4 mg) for measurement of nitrate-induced, endothelium-independent vasodilation (NID). The inter- and intra-observer variability for brachial diameter measurements in our laboratory is 0.1 ± 0.12 and 0.08 ± 0.19 mm respectively, while FMD variability measured on two different days was 1.1 ± 1% (Corretti et al. Citation2002).
PWV for measurement of arterial stiffness
All patients were examined in the morning hours and after a 10 min rest period. Patients did not receive their medications on the day of examination. PWV was measured automatically using Complior apparatus (Artech Medical France). PWV determination is based on the simultaneous recording of pulse waves in the common carotid and femoral arteries by two transducers, and calculated as the distance separating the two transducers divided by the time delay between the onset (foot) of the two recorded waves (Protogerou et al. Citation2006).
Peripheral hemodynamics measurements—brachial blood pressure and heart rate
Brachial systolic blood pressure, diastolic blood pressure and heart rate were recorded from the right arm using an automated sphygmomanometer (after 15 min of supine rest in a quiet room).
Psychometring testing
A battery of self-administered questionnaires was given to all patients. The following tests were employed:
Symptom checklist-90 revised (SCL-90-R). This provides 9 sub-scores and a total score. It is a general psychopathology test, as describes somatization, obsessive-compulsive symptoms, interpersonal sensitivity, depression, anxiety, aggression, phobic anxiety, paranoid ideation and psychoticism (Derogatis Citation1983).
State-trait-anxiety-inventory (STAI). This questionnaire assesses anxiety and provides separate scores for “state” (X1) and “trait” (X2) Anxiety (Spielberger et al. Citation1970).
Zung. Depression scale. This provides a total score of depressive symptomatology, focused on the bodily manifestations of depression (Zung Citation1965).
Whiteley index. This provides a total estimation of hypochondriacal manifestations (Pilowski Citation1967).
Leyton obsessional inventory (trait portion). This provides a total score of obsessional personality traits (no-symptoms) (Cooper Citation1970).
Visual analogue scales. This provides a subjective evaluation of the individual across a line connecting two opposite aspects of a notion (Mottola Citation1993).
The study was approved by the Bioethics Committee of the Laikon and Aretaieion University Hospitals, as well as by the Greek National Drug Administration (EOF). All patients gave informed consent of participation.
Assays
Glucose, total cholesterol (Chol), triglycerides (TG), high density lipoprotein-cholesterol (HDL-C), urea and creatinine as well as urine albumin and creatinine were measured using the Siemens Advia 1650 Clinical Chemistry System (Siemens Medical Solutions, Erlangen, Germany). Cholesterol bound to low density lipoprotein (LDL) was estimated by the Friedewald equation. Internal quality control for lipids was carried out according to the laboratory manual of the Lipid Research Clinics Programme. Whole blood HbA1c levels were measured with cation exchange HPLC (HA8121 HPLC system, Arkray Inc., Kyoto, Japan).
The concentrations of SAA and CRP in serum were measured by particle-enhanced immunonephelometric assays (BN ProSpec nephelometer, Dade Behring, Liederbach, Germany).
The serum concentration of insulin was measured by the automated chemiluminescence system ACS: 180 (Siemens Medical Solutions, Erlangen, Germany). Assays for serum cortisol concentrations were performed on previously frozen serum with the same system as for insulin. UFC was also measured with this system. Conversion of the results for the direct urine sample provided by the system in μg/dl, to μg/24 h, was made using the following equation: direct urinary cortisol (μg/24 h) = assay result (μg/dl) × 10 × V, where V = volume of urine in liters excreted per 24 h. Normal values for UFC measured by the ACS 180, are 28.5–213.7 μg/24 h.
Serum levels of cortisol-binding globulin (CBG) were measured by radioimmunoassay “RIA” (BioSourse Europe SA, Belgium). The analytical sensitivity (lower detection limit) was 0.25 μg/ml.
Plasma levels of ACTH were measured by the electrochemiluminescence immunoassay “ECLIA” using the Roche Elescys 2010 immunoassay analyzer (Roche Diagnostics Mannheim, Germany).
Intra- and inter-assay imprecision were less than 5% for all assays.
Statistical analyses
Values are expressed as mean ± SEM, as indicated. ACTH and cortisol measured during the CRH test performed before and after candesartan administration were compared using ANOVA repeated-measures. Dunnett's post-hoc test was applied. The total area under the curve (AUC) of plasma ACTH and serum cortisol concentrations vs. time during CRH tests was estimated using the trapezoid method, a numerical integration method. The AUC of the ACTH/cortisol ratios during CRH tests was also estimated. The AUCs of ACTH, cortisol and ACTH/cortisol during CRH tests performed before and after candesartan administration, were compared using paired Student t-test analysis. Biochemical (cholesterol, TG, HDL, LDL, HbA1c, glucose, CRP, SAA, insulin, CBG, UFC and the ratio albumin to creatinine of a spot urine sample) and clinical (body weight, BMI, waist/hip ratio) parameters, indices of insulin sensitivity (QUICKI) and resistance (HOMA) measurements of the hemodynamic tests and scores of psychometric tests before and after candesartan administration were compared with paired Student's t-test. Relationships between the psychometric test scores were investigated by calculation of Pearson's correlation coefficient. Statistical significance was set at P < 0.05.
To quantify the difference between two groups and taking into account the variability of the measurements of the study, we calculated effect size for the number of patients participating in this study.
Results
Anthropometric and biochemical characteristics
Body weight, BMI and the waist to hip ratio of the patients remained unchanged with candesartan administration ().
Table I. Anthropometric, clinical and biochemical characteristics before and after candesartan administration (BMI, body mass index; UFC, urine free cortisol; CBG, cortisol-binding globulin; Glu, glucose; HOMA, the homeostasis model assessment; HbA1c, glycosylated Hb A1c; QUICKI, quantitative insulin sensitivity check index; SEM, standard error of the mean).
Twenty four-hour UFC, circulating CBG, cholesterol, HDL, LDL, TG, fasting serum insulin and fasting glucose concentrations, albumin to creatinine ratio of a spot urine sample, whole blood HbA1c levels and Quicki and HOMA indices did not change significantly between baseline and after candesartan administration (). The effect sizes of the parameters weight, BMI, CRP, insulin, HOMA and QUICKI ranged between 0.30 and 0.60, whereas the remainder were below 0.2.
Plasma ACTH and cortisol responses to hCRH test
The time-course of ACTH and cortisol responses to an i.v. bolus of hCRH are illustrated in (A),(B). A statistically significant difference was found only at time-point +120 min for the ACTH response to hCRH (P = 0.017). At other time-points, the plasma ACTH response to hCRH was unchanged after at least 3 months of candesartan administration ((A)). The time-integrated secretion AUC for ACTH did not differ at the two testing times ((a)). Statistically significant differences were found in the cortisol response to hCRH before and after candesartan administration at time-points t = − 1 (P = 0.031), t = +5 (P = 0.021), t = +15 (P = 0.022), t = +30 (P = 0.034) and t = +90 min (P = 0.041) ((B)). The time-integrated secretion AUC of cortisol after candesartan administration was significantly suppressed compared to baseline (P = 0.005; (b)). The effect size for the time-course of ACTH responses to hCRH was greater for the time-points t = +5, +15, +30, +60 min (0.32–0.45). The effect size for the time-course of cortisol responses to hCRH was greater for the time-points t = − 1, +5, +15, +30, +60 and +90 min (0.42–0.75). The estimated effect size of the time-integrated secretion AUC of ACTH and cortisol were 0.24 and 0.91, respectively. The effect size for cortisol was particularly significant.
Figure 1 (A) Mean ± SEM plasama ACTH (pg/mL) response to hCRH in DM type 2 patients before (closed squares) and after (open squares) candesartan administration. Asterisks indicate statistically significant difference at the indicated time-point. P = 0.0017, n = 17. (a) Mean ± SEM AUC values of plasma ACTH in DM type 2 patients before (black bars) does not differ from that after (white bars) candesartan administration. (n = 17). (B) Mean ± SEM serum cortisol (μg/dl) response to hCRH in DM type 2 patients before (closed squares) and after (open squares) candesartan administration. Asterisks indicate statistically significant difference at the indicated time-point. − 1 min: P = 0.031; 5 min: P = 0.021; 15 min: P = 0.022; 30 min: P = 0.034; 90 min: P = 0.041; n = 17. (b) Mean ± SEM AUC values of serum cortisol in DM type 2 patients before (black bars) and after (white bars) candesartan administration. Asterisks indicate statistically significant difference at the indicated time-point. P = 0.005, n = 17. (C) Mean ± SEM plasma ACTH (expressed in pg/ml)/serum cortisol (expressed in μg/dl) ratios at the different time-points of the hCRH test in DM type 2 patients before (closed squares) and after (open squares) candesartan administration. Asterisks indicate statistically significant difference at the indicated time-point. − 1 min: P = 0.045; 5 min: P = 0.01; 90 min: P = 0.034; 120 min: P = 0.021; n = 17. (c) Mean ± SEM AUC values of plasma ACTH (expressed in pg/ml)/serum cortisol (expressed in μg/dl) ratios in DM type 2 patients before (black bars) and after (white bars) candesartan administration. Asterisks indicate statistically significant difference at the indicated time-point. P = 0.017, n = 17.
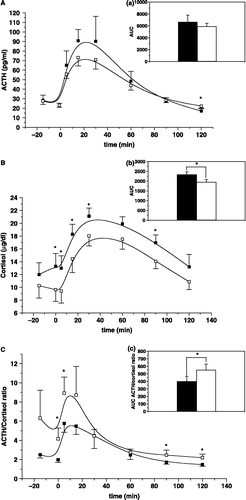
As an index of a change in adrenocortical sensitivity to ACTH the ratios of the circulating ACTH to cortisol concentrations were calculated at the different time-points after hCRH both before and after candesartan administration ((C)). Statistically significant differences were found at the time-points t = − 1 (P = 0.045), t = +5 (P = 0.010), t = +90 (P = 0.034) and t = +120 min (P = 0.021). The time-integrated AUC of the ratios of the circulating ACTH to cortisol concentrations after hCRH was significantly increased compared to baseline after candesartan administration (P = 0.017; (c)). The estimated effect size for the ratios of the circulating ACTH to cortisol concentrations, as well as of the time-integrated AUC of these ratios, calculated at the different time-points after hCRH, before and after candesartan administration, was greater for points t = − 15, − 1, +90, +120 min and above 0.4 for these time-points and 0.28 for the ratio of the AUCs, respectively.
Vascular endothelial function before and after candesartan administration
Systolic (124.20 ± 3.10 vs. 119.20 ± 4.60 mmHg) and diastolic (79.10 ± 2.60 vs. 74.60 ± 3.50 mmHg) blood pressure did not decrease significantly with candesartan. Candesartan significantly decreased heart rate (P = 0.01), but did not alter FMD (5.42 ± 1.12 vs. 4.95 ± 1.15) and arterial stiffness (10.27 ± 1.38 vs. 11.37 ± 1.86). The estimated effect sizes for diastolic blood pressure, heart rate at baseline and after hyperemia and nitrate-induced-hyperemia, were above 0.5.
Psychological profile of the patients before and after candesartan administration
After candesartan administration, there was an improvement in the interpersonal sensitivity (0.91 ± 0.16 vs. 0.70 ± 0.15, P = 0.027) and depression (0.96 ± 0.15 vs. 0.71 ± 0.10, P = 0.026) scores obtained by the SCL-90-R. There were positive correlations between several of the psychometric scores reflecting psychological co-morbidities (data not shown). The effect sizes of the psychometric results from the psychological tests performed gave values above 0.4 for depression, hypochondriacal manifestations and obsessional traits.
No significant differences were found between male and female subjects, when the means of the main measures were compared.
Discussion
The patients with DM type 2 we studied were not clinically depressed, had no recent history of hospitalization for any reason and had no condition that might have affected their HPA axis activity in a major fashion. Furthermore, all DM type 2 patients had well-controlled, mild DM type 2, with a mean blood Hb A1c less than 7%. Hence, it was unsurprising that they had UFC values within the normal range. They showed a decreased cortisol response to a bolus infusion of hCRH after at least 3 months of candesartan administration, compared to the baseline response. This is consistent with previous findings indicating that dysregulation of the HPA axis may be present among patients with DM type 2 (Roy et al. Citation1993; Tsigos et al. Citation1993; Andrews et al. Citation2002), characterized by chronic intermittent mild hypercortisolism and a concomitantly altered HPA axis response to CRH and suggests that candesartan administration reset the HPA axis closer to normal. The unchanged baseline UFC excretion levels after candesartan administration indicates a properly functioning glucocorticoid negative feedback system before and after candesartan administration and a complete, time-integrated quantitative reset of the HPA axis. This is consistent with the unchanged plasma ACTH response to hCRH with candesartan administration, and the increased ACTH to cortisol response ratio, suggesting that candesartan decreased sensitivity of the adrenal cortex to ACTH. This is compatible with a chronically decreased central stimulation of the HPA axis and, presumably, a consequently smaller adrenal cortex (Bornstein et al. Citation1998; Bornstein and Chrousos Citation1999).
Endothelial function assessed by FMD did not change significantly after candesartan treatment. Hypercortisolemia leads to endothelial dysfunction, which is ameliorated by inhibition of cortisol production by metyrapone (Broadley et al. Citation2005, Citation2006), and candesartan at a dose of 8–16 mg daily improves endothelial function in postmenopausal women (Wassmann et al. Citation2006), and in patients with hypertension and non-insulin dependent DM (Rizzoni et al. Citation2005). The lack of FMD improvement in our study could be explained by the lower dose of candesartan employed (4 mg daily), which in turn had a mild but statistically non-significant hypotensive effect. PWV did not change with candesartan administration either, indicating no effect of low dose candesartan on arterial stiffness. The short period of treatment with candesartan could explain the lack of improvement in arterial stiffness. Sasamura et al. (Citation2006) observed that PWV values decreased after 1 year of treatment with candesartan in hypertensive patients, which is in accordance with observations by Spoelstra-de Man et al. (Citation2006) in hypertensive type 2 diabetic patients. In contrast, in the present study there was a significant decrease in heart rate, probably due to a decrease in sympathetic system activity that is in accordance with the hCRH test results showing a decrease in the responsiveness of the HPA axis. An increase in vagal tone was previously described after administration of losartan only in rats with myocardial infarction and heart failure, but not in control rats (Du et al. Citation1998). Ang II does not induce a decrease in vagal tone in normal subjects either (Sander-Jensen et al. Citation1988). Therefore, the decrease in heart rate observed in our study in patients with normal left ventricular function after administration of candesartan is unlikely to be due to increased vagal tone. Finally, in this study after chronic low dose candesartan administration there was a tendency for improvement of the affective psychopathology of patients with DM. Recently, Saavedra et al. (Citation2005) showed that in rats long-term pretreatment with candesartan prevented the cortical CRH and benzodiazepine responses to isolation stress and was anxiolytic in the elevated plus-maze, whereas AT2 knockout mice exhibited anxiety behaviour (Okuyama et al. Citation1999). Furthermore, there is some preliminary clinical evidence for a possible role of Ang II in depression. Indeed, the angiotensin-converting enzyme (ACE) inhibitor captopril improved mood in hypertensive patients with depression, an effect that was not observed with other anti-hypertensive drugs (Zubenko and Nixon Citation1984; Deicken Citation1986; Germain and Chouinard Citation1988).
There is a large amount of evidence from preclinical studies that clearly shows the anti-stress effects of ARB in acute stress disorders (Saavedra and Pavel Citation2005). Armando et al. (Citation2001) demonstrated that candesartan prevented HPA axis and sympathetic stimulation during isolation stress, while administration of an ARB protected against stress-induced gastric ulcers in rats (Bregonzio et al. Citation2003).
Chronic activation of the stress system includes changes in both the HPA axis and the sympathetic nervous system and a resetting of the entire system to greater activity (Tsigos and Chrousos Citation1994, Citation2002; Habib et al. Citation2001;). Ang II stimulates HPA axis via AT1 receptors regulating CRH release. Through the sympathetic system, brain Ang II increases heart rate and arterial blood pressure and (Phillips Citation1987; Reid Citation1992; Saavedra Citation1992; Phillips and Sumners Citation1998), via the splanchnic nerves and the intrinsic RAS in the adrenal zona glomerulosa and medulla (Plunkett et al. Citation1985; Deschepper et al. Citation1986; Livett and Marley Citation1993; Phillips et al. Citation1993) stimulates the adrenal medulla and, indirectly, the adrenal cortex (Livett et al. Citation1990; Ehrhart-Bornstein et al. Citation1995; Bornstein et al. Citation1998; Bornstein and Chrousos Citation1999; Jain et al. Citation2004). The adrenal zona fasciculata is thus stimulated both by circulating ACTH and the stress-activated medulla (Tsigos and Chrousos Citation1994; Bornstein et al. Citation1998; Bornstein and Chrousos Citation1999). A chronically activated stress system is associated with structural changes of the adrenals characterized by hypervascularization and cellular hypertrophy and hyperplasia of the cortices, a larger size and a greater responsiveness to ACTH (Bornstein et al. Citation1998; Bornstein and Chrousos Citation1999). Naturally the glucocorticoid negative feedback system is also activated, which exerts a restraining influence upon the stress system by inhibiting ACTH and central sympathetic activity (Tsigos and Chrousos Citation1994, Citation2002; Habib et al. Citation2001; Jain et al. Citation2004). In our subjects, candesartan exerted effects on both limbs of the stress system, resetting both the HPA axis and the sympathetic system at a lower level of activity.
Our results are in accordance with previous studies, examining decrease of HPA axis activity in association with other centrally acting drugs. Thus, chronic imipramine administration to healthy, non-depressed volunteers resulted in decreased ACTH and cortisol responses to ovine CRH and AVP without changes in indices of time-integrated HPA axis function, including UFC excretion (Michelson et al. Citation1997). This action of imipramine is potentially related to its therapeutic effects in melancholic depression, a disorder that is associated with hypothalamic hypersecretion of CRH. Similarly, alprazolam, a triazolobenzodiazepine, administered to jacketed, intravenously cannulated nonhuman primates and to normal humans suppressed HPA axis activity (Kalogeras et al. Citation1990). This most likely reflects suppression of the CRH neuron rather than the pituitary corticotroph, explaining its efficacy in the treatment of chronic anxiety.
Our results are also in accordance with previous studies suggesting that Ang II is an important stress hormone, implicated in the response to different stressors. Ang II contributes to the regulation of the sympathoadrenal and hormonal responses to stress, through stimulation of the brain and peripheral AT1 receptor. In rats, the effects of centrally administered Ang II on the sympathetic nervous system are mediated via an increase in oxidative stress in brain regions involved in the noradrenergic control of blood pressure (Campese et al. Citation2005). A two-week edaravone treatment significantly restored systolic blood pressure and lipid peroxidation to normal and enhanced the catalase and superoxide dismutase activity in diabetic rats suggests the involvement of hydroxyl radical stress in augmented responses of Ang II in diabetic animals (Saini et al. Citation2006). On the other hand, Ang II participates in the response to isolation stress (Armando et al. Citation2007). Furthermore, Ang II seems to regulate not only the acute (Armando et al. Citation2001) but also the chronic stress reaction (Uresin et al. Citation2004). In addition, blockade of the RAS by losartan significantly prevented the elevation of plasma glucose levels induced by chronic immobilization stress (Uresin et al. Citation2004). Furthermore, a large body of evidence suggests a possible link between Ang II-dependent end-organ damage and the advanced glycation end products in vivo (Bohlender et al. Citation2005).
In conclusion, candesartan administration for at least 3 months decreased central HPA axis activity and improved the affect of patients with DM type 2. We suggest that ARB at higher doses might be useful as antidepressants. We suggest that candesartan resets the HPA axis of patients with DM type 2 and improves their affect, possibly protecting these patients from long-term CRH hypersecretion.
Acknowledgements
We would like to acknowledge Anastasios Boutsiadis for the calculation of the effect sizes.
References
- Aguilera G. Factors controlling steroid biosynthesis in the zona glomerulosa of the adrenal. J Ster Biochem Mol Biol 1993; 45: 147–151
- Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology 1995; 61: 437–444
- American Diabetes Association Standards of medical care in diabetes-2006. Diab Care 2006; 29: S4–S42
- Andrews RC, Herlihy O, Livingstone DE, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. JCEM 2002; 87: 5587–5593
- Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terron JA, Falcon-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic–pituitary–adrenal response to isolation stress. Endocrinology 2001; 142: 3880–3889
- Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotrophin-releasing factor response to isolation stress. Brain Res 2007; 20(1142)92–99, Epub 2007 January 19
- Bohlender J, Franke S, Sommer M, Stein G. Advanced glycation end products: A possible link to angiotensin in an animal model. Ann NY Acad Sci 2005; 1043: 681–684
- Bornstein SR, Chrousos GP. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: Neural and immune inputs. JCEM 1999; 84: 1729–1736
- Bornstein SR, Webster EL, Torpy DJ, Richman SJ, Mitsiades N, Igel M, Lewis DB, Rice KC, Joost HG, Tsokos M, Chrousos GP. Chronic effects of a nonpeptide corticotropin-releasing hormone type I receptor antagonist on pituitary–adrenal function, body weight, and metabolic regulation. Endocrinology 1998; 139: 1546–1555
- Bregonzio C, Armando I, Ando H, Jecova M, Baiardi G, Saavedra JM. Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Psysiol Gastrointest Liver Physiol 2003; 285: 414–423
- Broadley AJ, Korszum A, Abdelaal E, Moskvina V, Jones CJ, Nash GB, Ray C, Deanfield J, Frenneaux MP. Inhibition of cortisol production with metyrapone prevents mental stress-induced endothelial dysfunction and baroreflex impairment. JACC 2005; 19: 344–350
- Broadley AJ, Korszum A, Abdelaal E, Moskvina V, Deanfield J, Jones CJ, Frenneaux MP. Metyrapone improves endothelial dysfunction in patients with treated depression. JACC 2006; 48: 170–175
- Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 2005; 46: 533–539, Epub 2005 August 22
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Ann Rev Physiol 2005; 67: 259–284
- Coiro V, Volpi R, Capretti L, Caffarri G, Colla R, Giuliani N, Chiodera P. Stimulation of ACTH and GH release by angiotensin II in normal men is mediated by the AT1 receptor subtype. Regul Pept 1998; 74: 27–30
- Contoreggi C, Rice KC, Chrousos G. Nonpeptide corticotropin-releasing hormone receptor type 1 antagonists and their applications in psychosomatic disorders. Neuroendocrinology 2004; 80: 111–123
- Cooper J. The leyton obsessional inventory. Psychol Med 1970; 1: 48–64
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. JACC 2002; 39: 257–265
- Deicken RF. Captopril treatment of depression. Biol Psychiat 1986; 21: 1425–1428
- Derogatis LR. SCL-90-R administration, scoring and procedures manualIInd ed. Procedures psychometric research. 1983, Baltimore, MD
- Deschepper CF, Mellon SH, Cumin F, Baxter JD, Ganong WF. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal and pituitary of the rat. Proc Natl Acad Sci USA 1986; 83: 7552–7556
- Dessi-Fulgheri P, Alagna S, Madeddu P, Glorioso N, Masala A, Rovasio PP, Leoni C, Rappelli A. Blunted adrenocorticotrophic hormone release during captopril treatment. J Hypert (Supplement) 1985; 3: 125–127
- Du XJ, Cox HS, Dart AM, Esler MD. Depression of efferent parasympathetic control of heart rate in rats with myocardial infraction: Effect of losartan. J Cardiovasc Pharmacol 1998; 31: 937–944
- Ehrhart-Bornstein M, Bornstein SR, Gonzalez-Hernandez J, Holst JJ, Waterman MR, Scherbaum WA. Sympathoadrenal regulation of adrenocortical steroidogenesis. Endocr Res 1995; 21: 13–24
- Gaillard RC, Grossman A, Gillies G, Rees LH, Besser GM. Angiotensin II stimulates the release of ACTH from dispersed rat anterior pituitary cells. Clin Endocrinol (Oxf) 1981; 15: 573–578
- Ganong WF. Blood, pituitary and brain renin–angiotensin systems and regulation of secretion of anterior pituitary gland. Front Neuroendocrinol 1993; 14: 233–249
- Germain L, Chouinard G. Captopril treatment of major depression with serial measurements of blood cortisol concentrations. Biol Psychiat 1988; 25: 489–493
- Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin–angiotensin–aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metabol 2005; 16: 120–126
- Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 1982; 299: 355–357
- Gohlke P, Kox T, Jurgensen T, von Kugelgen S, Rascher W, Unger T, Culman J. Peripherally applied candesartan inhibits central responses to angiotensin II in conscious rats. Naunyn Schmiedebergs Arch Pharmacol 2002; 365: 477–483
- Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metabol Clin North Amer 2001; 30: 695–728
- Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio heart study. Diab Care 2002; 25: 1177–1184
- Hauger RL, Aguilera G, Baukal AJ, Catt KJ. Characterization of angiotensin II receptors in the anterior pituitary gland. Mol Cell Endocrinol 1982; 25: 203–212
- Henriksen EJ, Jacob S, Kinnick RT, Teachey MK, Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese zucker rats. Hypertension 2001; 38: 884–890
- Jain P, Armando I, Juorio AV, Barden N, Benicky J, Saavedra JM. Decreased hypothalamic and adrenal angiotensin II receptor expression and adrenomedullary catecholamines in transgenic mice with impaired glucocorticoid receptor function. Neuroendocrinology 2004; 80: 171–180
- Jezova M, Armando I, Bregonzio C, Yu ZX, Qian S, Ferrans VJ, Imboden H, Saavedra JM. Angiotensin II AT (1) and AT (2) receptors contribute to maintain basal adrenomedullary norepinephrine synthesis and tyrosine hydroxylase transcription. Endocrinology 2003; 144: 2092–2101
- Kalogeras KT, Calogero AE, Kuribayiashi T, Khan I, Gallucci WT, Kling MA, Chrousos GP, Gold PW. In vitro and in vivo effects of the triazolobenzodiazepine alprazolam on hypothalamic–pituitary–adrenal function: Pharmacological and clinical implications. JCEM 1990; 70: 1462–1471
- Katz A, Nambi S, Mather K, Baron A, Follman D, Sullivan G, Quon M. Quantitive insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. JCEM 2000; 85: 2402–2410
- Kersaw EE, Flier JS. Adipose tissue as an endocrine organ. JCEM 2004; 89: 2548–2556
- Landas S, Phillips MI, Stamler JF, Raizada MK. Visualization of specific angiotensin II binding sites in the brain by fluorescent microscopy. Science 1980; 14: 791–793
- Latchford KJ, Ferguson AV. Angiotensin depolarizes parvocellular neurons in paraventricular nucleus through modulation of putative nonselective cationic and potassium conductances. Am J Physiol Reg Integr Comp Physiol 2005; 289: R52–R58
- Lavoie JL, Sigmund CD. Minireview: Overview of the renin–angiotensin system—an endocrine and paracrine system. Endocrinology 2003; 144: 2179–2183
- Lenkei Z, Corvol P, Llorens-Cortes C. Comparative expression of vasopressin and angiotensin type-1 receptor mRNA in rat hypothalamic nuclei: A double in situ hybridization study. Brain Res Mol Brain Res 1995; 34: 135–142
- Lenkei Z, Nuyt AM, Grouselle D, Corvol P, Llorens-Cortes C. Identification of endocrine cell populations expressing the AT1B subtype of angiotensin II receptors in the anterior pituitary. Endocrinology 1999; 140: 472–477
- Liu JH, Muse K, Contreras P, Gibbs D, Vale W, Rivier J, Yen SS. Augmentation of ACTH-releasing activity of synthetic corticotropin releasing factor (CRF) by vasopressin in women. JCEM 1983; 57: 1087–1089
- Livett BG, Marley PD. Noncholinergic control of adrenal catecholamine secretion. J Anat 1993; 183: 277–289
- Livett BG, Marley PD, Wan DC, Zhou XF. Peptide regulation of adrenal medullary function. J Neural Transm (Supplementum) 1990; 29: 77–89
- Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav 2002; 73: 147–158
- Mangos GJ, Walker BR, Kelly JJ, Lawson JA, Webb DJ, Whitworth JA. Cortisol inhibits cholinergic vasodilation in the human forearm. Am J Hypertens 2000; 13: 1155–1160
- McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-coverting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol 2003; 91: 30H–37H
- Michelson D, Galliven E, Hill L, Demitrack M, Chrousos G, Gold P. Chronic imipramine is associated with diminished hypothalamic–pituitary–adrenal axis responsivity in healthy humans. JCEM 1997; 82: 2601–2606
- Mottola CA. Measurement strategies: The visual analogue scale. Decubitus 1993; 6: 56–58
- Murakami K, Ganong WF. Site at which angiotensin II acts to stimulate ACTH secretion in vivo. Neuroendocrinol 1987; 46: 231–235
- Nishimura Y, Ito T, Hoe K, Saavedra JM. Chronic peripheral administration of the angiotensin II AT (1) receptor antagonist candesartan blocks brain AT (1) receptors. Brain Res 2000; 871: 29–38
- Okuyama S, Sakagawa T, Chaki S, Imagawa Y, Ichiki T, Inagami T. Anxiety-like behaviour in mice lacking the angiotensin II type-2 receptor. Brain Res 1999; 821: 150–159
- Pawlikowski M. Immunohistochemical detection of angiotensin receptors AT1 and AT2 in normal rat pituitary gland, estrogen-induced rat pituitary tumor and human pituitary adenomas. Folia Histochem Cytobiol 2006; 44: 173–177
- Phillips MI. Functions of angiotensin in the central nervous system. Ann Rev Physiol 1987; 49: 413–435
- Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept 1998; 78: 1–11
- Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin–angiotensin systems. Regul Pept 1993; 22: 1–20
- Pilowski I. Dimensions of hypochondriasis. Br J Psych 1967; 113: 89–93
- Plunkett LM, Correa FM, Saavedra JM. Quantitative autoradiographic determination of angiotensin-converting enzyme binding in rat pituitary and adrenal glands with 125I-351A, a specific inhibitor. Regul Pept 1985; 28: 263–272
- Protogerou AD, Blacher J, Aslangul E, Le Jeunne C, Lekakis J, Mavrikakis M, Safar ME. Gender influence on metabolic syndrome's effects on arterial stiffness and pressure wave reflections in treated hypertensive subjects. Atherosclerosis 2006; 23, Epub ahead of print
- Rabano M, Pena A, Brizuela L, Macarulla JM, Gomez-Munoz A, Trueba M. Angiotensin II-stimulated cortisol secretion is mediated by phospholipase D. Mol Cell Endocrinol 2004; 222: 9–20
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol 1992; 262: E763–E778
- Rizzoni D, Porteri E, De Ciuceis C, Sleiman I, Rodella L, Rezzani R, Paiardi S, Bianchi R, Rugeri G, Boari GE, Muiesan ML, Salvetti M, Zani F, Miclini M, Rosei EA. Effect of treatment with candesartan or enapril on subcutaneous small artery structure in hypertensive patients with noninsulin-dependent diabetes mellitus. Hypertension 2005; 45: 659–665
- Rogers KM, Bonar CA, Estrella JL, Yang S. Inhibitory effect of glucocorticoid on coronary artery endothelial function. Am J Physiol Heart Circul Physiol 2002; 283: H1922–H1928
- Roy MS, Roy A, Gallucci WT, Collier B, Young K, Kamilaris TC, Chrousos GP. The ovine corticotropin-releasing hormone-stimulation test in type I diabetic patients and controls: Suggestion of mild chronic hypercortisolism. Metabolism 1993; 42: 696–700
- Saavedra JM. Brain and pituitary angiotensin. Endocr Rev 1992; 13: 329–380
- Saavedra JM, Pavel J. Angiotensin II AT1 receptor antagonists inhibit the angiotensin–CRF–AVP axis and are potentially useful for the treatment of stress-related and mood disorders. Drug Devel Res 2005; 65: 237–269
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sanchez-Lemus E. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology 2005; 31: 1123–1134
- Saini AK, Patel RJ, Sharma SS, H SAK. Edaravone attenuates hydroxyl radical stress and augmented angiotensin II response in diabetic rats. Pharmacol Res 2006; 54: 6–10, Epub 2006 march 20
- Sander-Jensen K, Secher NH, Astrup A, Christensen NJ, Damkjaer-Nielsen M, Giese J, Warberg J, Bie P. Angiotensin II attenuates reflex decrease in heart rate and sympathetic activity in man. Clin Physiol 1988; 8: 31–40
- Sasamura H, Ktamura Y, Nakamura M, Ryuzaki M, Saruta T. Effects of the angiotensin receptor blocker candesartan on arterial stiffness and markers of extracellurar matrix metabolism in patient with essential hypertension. Clin Exp Hypert 2006; 28: 511–520
- Seltzer A, Bregonzio C, Armando I, Baiardi G, Saavedra JM. Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res 2004; 28: 9–18
- Shinozaki K, Kazuhide A, Yoshihiko N, Takeshi S, Atsunori K, Tomio O. Evidence for causal role of the renin–angiotensin system in vascular dysfuction associated with insulin resistance. Hypertension 2004; 43: 255–262
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Palo Alto, CA 1970, 53
- Spinedi E, Negro-Vilar A. Angiotensin II and ACTH release: Site of action and potency relative to corticotropin releasing factor and vasopressin. Neuroendocrinol 1983; 37: 446–453
- Spinedi E, Rodriguez G. Angiotensin II and adrenocorticotropin release: Mediation by endogenous corticotropin-releasing factor. Endocrinology 1986; 119: 1397–1402
- Spoelstra-de Man AM, van Ittersum FJ, Schram MT, Kamp O, van Dijk RA, Ijzerman RG, Twisk JW, Brouwer CB, Stehouwer CD. Aggressive antihypertensive strategies based on hydrochlorothiazide, candesartan or lisinopril decrease left ventricular mass and improve arterial compliance in patients with type II diabetes mellitus and hypertension. J Hum Hypert 2006; 20: 599–611
- Thomas J, Jones G, Scarinci I, Brantley P. A descriptive and comparative study of the prevalence of depressive and anxiety disorders in low-income adults with type 2 diabetes and other chronic illnesses. Diab Care 2003; 26: 2311–2317
- Tsigos C, Chrousos GP. Physiology of the hypothalamic–pituitary–adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metabol Clin North Am 1994; 23: 451–466
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002; 53: 865–871
- Tsigos C, Young RJ, White A. Diabetic neuropathy is associated with increased activity of the hypothalamic–pituitary–adrenal axis. JCEM 1993; 76: 554–558
- Tsutsumi K, Saavedra JM. Angiotensin-II receptor subtypes in median eminence and basal forebrain areas involved in regulation of pituitary function. Endocrinology 1991A; 129: 3001–3008
- Tsutsumi K, Saavedra JM. Characterization of AT2 angiotensin II receptors in rat anterior cerebral arteries. Am J Physiol 1991B; 261: 667–670
- Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol 1991C; 261: 209–216
- Uresin Y, Erbas B, Ozek M, Ozkok E, Gurol AO. Losartan may prevent the elevation of plasma glucose, corticosterone and catecholamine levels induced by chronic stress. J Renin Angiotensin Aldosterone Syst 2004; 5: 93–96
- Veltmar A, Culman J, Qadri F, Rascher W, Unger T. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II-induced vasopressin release. J Pharmacol Exp Therap 1992; 263: 1253–1260
- Volpi R, Chiodera P, Capretti L, Caiazza A, Caffarri G, Magoti MG, Boni S, Coiro V. Inhibition by somatostatin of the growth hormone, but not corticotrophin response to angiotensin II in normal men. Horm Res 1996; 45: 269–272
- Wassmann K, Ghiassi A, Wassmann S, Bohm M, Nickening G. AT1 receptor antagonism improves endothelial dysfunction in postmenopausal women. Maturitas 2006; 20: 176–183
- Wells KB, Golding JM, Burnam MA. Affective, substance use, and anxiety disorders in persons with arthritis, diabetes, heart disease, high blood pressure, or chronic lung conditions. Gen Hosp Psych 1989; 11: 320–327
- Zanchetti A, Elmfeldt D. Findings and implications of the study of cognition and prognosis in the elderly (SCOPE)—a review. Blood Press 2006; 15: 71–79
- Zubenko GS, Nixon RA. Mood-elevating effect of captopril in depressed patients. Am J Psychiat 1984; 141: 110–111
- Zung WWK. A self-rating depression scale. Arch Gen Psychiat 1965; 12: 63–70
