Abstract
Given recent findings on the potential for detrimental effects of chronic stress on the prefrontal cortex, additional research on the relationship between chronic stress and performance on executive functioning tasks (dependent on prefrontal functioning) is needed. Eighty-one undergraduate students completed a self-report measure of stress over the previous month (perceived stress scale—PSS) and the comprehensive trail-making test (CTMT, Trials 3 and 5). Results revealed a statistically significant positive correlation between PSS score and time needed to complete Trial 5 of the CTMT, which places demands on the set-shifting component of executive functioning. This finding adds to a growing body of work that suggests a relationship between chronic stress and executive functioning, and extends these findings to include set-shifting performance.
Introduction
The experience of stress has been linked to numerous biological functions, such as deleterious cardiovascular, metabolic, and immunosuppressive consequences (McEwen Citation1998). Stress has also been associated with altered cognitive performance, particularly in the areas of memory and attention. While cardiovascular, metabolic, and immunosuppressive changes due to stress may impair cognition, the bulk of the research literature has focused on the increased release of glucocorticoids, which have been shown to have a negative impact on both memory and attention (Reus et al. Citation1985; Lupien and Lepage Citation2001).
It has been well documented that stress, whether induced experimentally or experienced in response to naturalistic stressors, leads to changes in levels of corticosteroids in the brain (Bohnen et al. Citation1990; Newcomer et al. Citation1994; Kirschbaum et al. Citation1996; Vedhara et al. Citation2000). To summarize this process, stress sets off a chain reaction in the brain that begins with the release of corticotropin-releasing hormone (CRH), which signals the pituitary gland to release adrenocorticotropic hormone (ACTH). As circulating ACTH levels increase, corticosteroids are secreted from the adrenal cortex. The interaction of corticosteroid hormones at the receptor sites located in the hippocampus and prefrontal cortex during the hypothalamic–pituitary–adrenal (HPA) axis stress response is one factor that is thought to contribute to cognitive performance differences associated with stress (Fuchs et al. Citation2006).
The hippocampus is an area of the brain that is a particular focus in the stress-cognition literature because it plays an important role in both the negative feedback control of the HPA stress response, as well as memory function. In the animal literature, it has been shown that, in both rats and other primates, elevated levels of corticosteroids cause a reduction in hippocampal volume over time (Hoschl and Hajek Citation2001), as well as “remodeling” (dendritic retraction) of hippocampal neurons (McKittrick et al. Citation2000; Conrad Citation2006). In rats, it has been reported that “social defeat” stress induces major structural changes in the hippocampus (Buwalda et al. Citation2005). In humans, hippocampal volume reduction has been reported in patients with a history of psychiatric disorders typically associated with elevated cortisol levels (Sheline et al. Citation1996; Herbert et al. Citation2006). Hippocampal atrophy has also been reported in a subset of older adults with elevated cortisol levels (Lupien et al. Citation1998). Although this research suggests that elevated cortisol levels are associated with damage to the hippocampus over time, one acknowledged limitation is that it is still not possible to estimate the critical duration of corticosteroid hyper-exposure necessary for structural changes in the hippocampus to occur (Hoschl and Hajek Citation2001). Furthermore, although these studies suggest a link between cortisol and hippocampal functioning, none of these studies specifically linked stress and memory performance per se.
Although the HPA stress response is a well established process, the nature of the relationship between stress and cognition is not as well understood, and research in this area has yielded somewhat inconsistent and sometimes contradictory findings (Mendl Citation1999). For example, increased cortisol levels have been found to enhance some aspects of cognition (e.g. memory consolidation) while appearing to have a detrimental effect on others (e.g. memory retrieval) both in rats and humans (de Kloet et al. Citation1999; Roozendaal Citation2002). There are several possible reasons for this, including whether stress was induced, imitated experimentally, or occurred in the natural environment. It appears that the majority of studies examining the relationship of stress and cognition have involved either administering hormones that are known to immediate the stress response (i.e. cortisol), or have induced stress by subjecting participants to a stressful situation (e.g. a videotaped speaking performance). A number of studies that have imitated or induced stress (e.g. pharmacologically or stressor-induced) have reported that induced stress is related to reduced performance, or no change, on a number of measures of cognitive performance (Bohnen et al. Citation1990; Newcomer et al. Citation1994; Kirschbaum et al. Citation1996; Lupien and McEwen Citation1997; Lupien et al. Citation1997; Newcomer et al. Citation1999; Skosnik et al. Citation2000; Wolf et al. Citation2001Braunstein-Bercovitz Citation2003; Domes et al. Citation2005; Elzinga et al. Citation2005), although at least one study reported improved cognitive performance (on a variation of the Stroop task) in relation to increased acute stress (Chajut and Algom Citation2003). Another study that administered cortisol during memory encoding also reported that individuals who received an oral form of cortisol had improved recall for emotional pictures compared to those who received a placebo (Buchanan and Lovallo Citation2001).
However, relatively little work has been reported that focuses on chronic self-perceived stress and cognitive performance. In the animal literature, it appears as though the negative consequences of chronic stress on cognition are much more pronounced than the effects of acute stress, which tend to have mixed impairing and enhancing properties (Wolf Citation2003). A study that measured self-reported stress levels in humans using the perceived stress scale (PSS; Cohen et al. Citation1983) found that increased self-perceived stress over the past month was correlated with reduced performance on tasks of selective and divided attention, which is dependent on prefrontal cortex functioning (Vedhara et al. Citation2000). In a study of older adults, it has been reported that increased self-perceived psychological distress was associated with general cognitive decline (Wilson et al. Citation2005).
Thus, a growing body of research suggests a neurobiological mechanism for deleterious chronic effects of stress on the prefrontal cortex. Consistent with this theory, several studies have shown that both acute and chronic administration of cortisol impairs working memory, as well complex attention (Lupien et al. Citation1999; Young et al. Citation1999), both of which rely on prefrontal functioning. However, relatively little research has examined the relationship between self-perceived chronic stress and other executive functioning abilities—which would help determine if such a relationship is found across most, if not all, types of tasks that place significant demands on prefrontal functioning.
One well established neuropsychological task that reflects executive functioning ability (among other cognitive constructs) is the CTMT. The CTMT has been used to assess a wide range of cognitive skills, including different subtypes of attention, psychomotor speed, and mental flexibility. The task contains five different subtests, each of which aims to emphasize different dimensions of those cognitive constructs. Trial 5 of the CTMT is modeled after Part B of the trail-making test (TMT) of the Halstead-Reitan Neuropsychological Test Battery (Reitan and Wolfson Citation1993). While the TMT Part B (TMT-B) involves attention and visuomotor processing speed that is also found in Part A, TMT-B included additional set-shifting demands—a component of more general executive functioning. Set-shifting has been defined as the ability to move back and forth between multiple tasks, operations, or mental sets (Miyake et al. Citation2000). One study found that patients with dorsolateral prefrontal lesions show slower performance on the TMT-B (Stuss et al. Citation2001). Similarly, a recent fMRI study of the TMT found that distinct left-hemisphere dorsolateral and medial frontal activity distinguished Trail B from the more generalized network found with Trail A (Zakzanis et al. Citation2005).
Another aim of the study was to examine a wide spectrum of stress levels, as opposed to dichotomized groups, in order to investigate the continuous relationship with executive functioning ability. To examine this continuum of chronic stress, we screened over 1000 adults using the 10-item PSS (Cohen and Williamson Citation1988), an established self-report measure of perceived stress experienced over the past month. We then recruited participants representing a wide range of PSS scores to complete CTMT Trial 3 (similar to TMT-A) and Trial 5 (similar to TMT-B). While both of these trials include demands on focal attention and visuomotor processing speed, Trial 5 includes additional set-shifting demands. Since, stress has been reported to interfere with the attentional component shared by both Trials 3 and 5, we hypothesized that there would be a positive correlation between time needed to complete both Trials 3 and 5 with the continuous PSS score. As a more recent study suggests a specific effect of stress on the prefrontal cortex (Lupien et al. Citation2005), we hypothesized that there would be a stronger correlation between PSS and Trial 5 completion time, as Trial 5 requires additional set-shifting (executive functioning) demands (Reynolds Citation2002). We hypothesized that this additional effect would be most evident in a correlation between a Trial 5 − Trial 3 difference score with the PSS score.
Methods
Participants
Participants in this study were recruited from a screening phase during which over 1000 undergraduate students completed online versions of the PSS, as well as an 8-item Infrequency Scale—a validity measure modelled after the Infrequency Scale of the Personality Research Form (Jackson Citation1984; Calkins et al. Citation2004). The infrequency scale was used to exclude participants who may have not adequately attended to item content (endorsing more than one item in the wrong direction). These items were mixed in with the PSS questions in a fixed random order. A smaller number of participants were recruited for the in-person phase of the study from this larger participant pool with the goal of including a wide range of PSS scores, while attempting to evenly distribute age, gender, and race across the range of PSS scores. All participants were undergraduate students enrolled in psychology courses who received either academic credit or $10 for their participation.
Overall, 81 participants completed the CTMT—58.0% female; 72.8% Caucasian; 7.4% African American; 16.0% Hispanic; 2.5% Asian; and 1.2% “Other/Mixed”. The age range of participants was 18–38 years (mean = 20.9 ± 4.0, SD years). The mean PSS score at the time of cognitive testing was 17.25 ± 6.9, SD; scores ranged from 5, indicating very little perceived stress, to 31, indicating substantial perceived stress (the highest score possible being 40). As a result of the recruiting procedures, there was no statistically significant difference in PSS scores between genders and no statistically significant correlation between age and PSS scores.
Procedures
All participants were screened for visual acuity, using a standard Snellen wall chart, and only those with acuity of 20/30 or better were included in the study, to ensure adequate visual acuity for the visual stimuli. After completing informed consent procedures, participants completed the PSS (paper version) again to use in the analysis as a more current reflection of the perceived stress over the previous month. Participants then completed a battery of cognitive tests, which included the CTMT (Trials 3 and 5), described below. The entire session lasted about 30 min. Testing occurred at random times throughout the day, ranging from 9:00 am to 5:00 pm. The sample consisted of two smaller subsets of participants who received different cognitive batteries (from two consecutive studies). The only task common to both cognitive batteries, which comprised this larger sample, was the CTMT task. Therefore, additional cognitive performance data on this larger sample is not available.
Perceived stress scale
The global measure of perceived stress is one of the most widely used psychological instruments for measuring the perception of stress (Cohen et al. Citation1983). The present study used an abbreviated form of the original scale called the PSS (Cohen and Williamson Citation1988). The abbreviated PSS is a 10-item measure of the degree to which situations in one's life are appraised as stressful within the past month. Specifically, the items were designed to measure the degree to which respondents found their lives unpredictable, uncontrollable and overloaded. The measure utilizes a Likert scale with five ratings on how often respondents have experienced certain feelings such as “control of irritations” and “confidence in ability to handle personal problems”, with answer choices ranging from “never” to “very often”.
The CTMT, Trials 3 and 5
The CTMT is an alternate version of the original TMT (Reynolds Citation2002). This version was developed in order to remedy multiple perceived shortcomings of the original form, the TMT (Partington and Leiter Citation1949). According to Reynolds (Citation2002), the CTMT was designed to highlight and isolate specific components of performance because the original TMT may be “too brief and too general.” Only Trials 3 and 5 of the CTMT were administered in this study. Trial 3 requires participants to draw a line to connect the numbers 1–25 (contained in black circles on the page) in order, as quickly as possible, while avoiding distracter circles on the page. Trial 5 is more cognitively demanding, as participants have to draw a line to connect the numbers 1–13 (chronologically) and letters A–L (alphabetically) in alternating order, also while avoiding distracter circles. The inclusion of blank distracter circles is the primary difference between the original TMT Parts A and B and the CTMT Trials 3 and 5. In addition to requiring focal attention and psychomotor speed, Trial 5 adds additional executive functioning (set-shifting) demands. Participants were administered abbreviated practice trials (with feedback) prior to completing each scored trial. The performance index for each of the trials is the number of seconds required for successful completion. If errors are made, the examiner instructs the examinee to start back from the last correct location, contributing to a longer completion time. In our sample of college students, error rates were relatively low—for CTMT Trial 3, participants made an average of 0.1 (SD = 0.3; range 0–4) errors; for CTMT Trial 5, participants averaged 0.6 (SD = 1.3; range 0–6) errors.
Statistical analysis
Correlations were performed using SPSS v. 12.0 in order to investigate the relationship between PSS score and performance on the CTMT-3, CTMT-5, and difference score between CTMT Trials 3 and 5. As PSS scores and CTMT performance scores approximated a Gaussian distribution, Pearson correlations were used in the analyses.
Results
Results of the main analyses revealed a statistically significant positive correlation between PSS score and time (in seconds) needed to complete Trial 5 of the CTMT (r = 0.25, p = 0.02; R2 = 0.06; ). An additional partial correlation was run, controlling for age and gender of the participants. The partial correlation revealed a similar statistically significant relationship (r = 0.24, p = 0.03; R2 = 0.06). In contrast, there was no suggestion of a statistically significant correlation between the PSS and time to complete Trial 3 (r = 0.10, p = 0.36). The CTMT difference score (Trial 5 minus Trial 3), approached statistical significance (r = 0.20, p = 0.08).
Figure 1 Relationship between PSS score and time needed for completion of the CTMT—Trial 5. r = 0.25, p = 0.02; R2 = 0.06. Data from 81 participants.
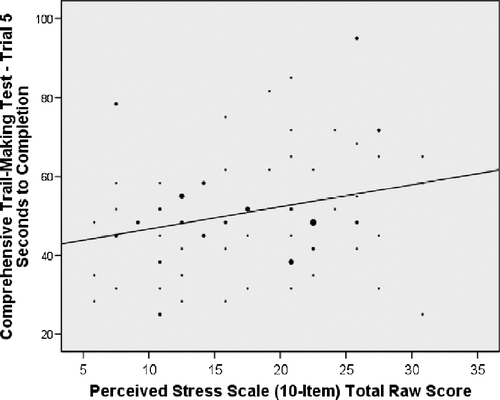
The relationship between PSS score and Trial 5 was then examined for non-linear trends. Results of this analysis revealed that a linear function provided the best fit for the data.
Discussion
The present study was designed to examine whether self-reported recent chronic stress was related to performance on the set-shifting component of executive functioning. Contrary to our hypothesis, the difference score between CTMT Trial 5 and 3 did not show the strongest relationship to self-reported stress, although it did appear as a statistical trend in this sample size (N = 81). However, consistent with our hypothesis, we found a statistically significant positive relationship between Trial 5 and self-reported stress, which did not substantially change when covarying for gender and age. There was no suggestion of a statistically significant relationship between Trial 3 and self-reported stress. Since, the correlation between Trial 3 and PSS was also positive (although non-significant, r = 0.10), the difference score may have removed variance shared between both Trials 3 and 5, that related to the PSS score. The variance that these trials shared may relate to common cognitive requirements such as focal attention and psychomotor processing speed, suggesting that stress may have some relationship with these basic cognitive processes, while having an even stronger relationship with executive functioning (unique to Trial 5).
Our findings are consistent with a number of previous studies that explored the relationship between stress (imitated by cortisol injections) and cognitive performance related to the prefrontal cortex in humans. Young et al. (Citation1999) examined 20 human male adults on a brief neuropsychological battery following either twice-daily administration of hydrocortisone (cortisol) over 10 days or placebo. Results indicated a reversible deficit on tasks that rely on prefrontal functioning (i.e. spatial working memory strategy) that was linked to the elevated cortisol. Another study examined the effects of acute hydrocortisone administration on working and declarative memory in 40 healthy young male adults (Lupien et al. Citation1999). The participants received infusions of saline (placebo group) or hydrocortisone. Three tasks were included in the cognitive testing, an item-recognition working memory task, a word-pair declarative memory task, and a continuous performance vigilance task. The results revealed that, at the highest cortisol dose, cortisol selectively impaired performance on the working memory (prefrontal) task, without impairing performance on either the declarative memory or vigilance tasks.
Previous work investigating stress and attentional set-shifting in rats has reported that chronic stress induced a selective impairment in set-shifting ability, which was evidenced by behavioral performance and dendritic remodeling in the prefrontal cortex following a 21 day period of induced stress (Liston et al. Citation2006). In humans, previous studies have investigated the relationship between stress and measures of executive functioning, namely working memory and divided attention; however, relatively few studies have examined set-shifting. In one recent study, it was reported that increased examination stress (as measured by the Spielberger State Anxiety Questionnaire) actually facilitated performance on two tasks requiring set-shifting (a spatial task-switching paradigm and the Stroop task) in a healthy college population (Kofman et al. Citation2006). In contrast, the current study reported reduced set-shifting capacity as self-reported chronic stress increased. Differences between the two studies include the use of an anxiety measure in the previous study to measure stress (versus a measure more specific to stress), and differences in tasks used to measure set-shifting. The Stroop task, and to a lesser degree, the task switching task utilized in the Kofman et al. (Citation2006) study, included other components of executive function (i.e. response inhibition), as compared to the CTMT used in the current study—which primarily measured set-shifting, as well as focal attention and psychomotor speed. Thus, the current study appears to be the first to report a negative relationship between chronic self-reported stress and set-shifting ability in healthy humans. While this may relate to the experimental findings of increases in cortisol levels that result from stress affecting brain functioning, it is also plausible that initial inefficient brain functioning leads to increased stress. Unfortunately, the correlational design of the current study prohibits inferences about causality.
A number of limitations of the current study warrant consideration. The study participants were all college students, and these findings may be exclusive to this population, whose age range was restricted (18–38 years of age) to that of young adults. Additionally, their intelligence was likely to be above that of the average population, as evidenced by their acceptance into an institute of higher education. In addition, participants were not screened for psychiatric illness, substance use, or chronic diseases, which may have introduced noise to the data and reduced the potential effect size. Another limitation was reliance on the self-report measure of chronic stress. The use of repeated cortisol measurements over an extended period of time (e.g. 1–2 months) may provide a more objective measure of biological chronic stress that is, theoretically, more directly related to changes in cognitive function. Finally, while our main finding reported here was statistically significant, the effect size obtained was small (r = 0.25). Considering the limitations associated with sampling from a college population (e.g. restricted age, IQ), it is possible that in a less restricted community sample, our findings might have been stronger given the greater range of CTMT performance and stress levels one might expect in such a sample.
Further research that includes a broader battery of executive functioning tasks may clarify whether this relationship is limited to select measures of prefrontal functioning. In addition, future research may clarify the casual mechanisms of the relationship between stress and prefrontal functioning, as well as potential differences between the effects of acute and chronic stress. Similarly, research that examines the longitudinal course of brain changes related to stress, including possible recovery of function following the removal of stress, would clarify long-term cognitive consequences of both acute and chronic stress. For example, reversibility of some of the effects of chronic stress, namely dendritic remodeling in the prefrontal cortex, has been reported in rats (Radley et al. Citation2005). Future research should also address whether these changes are limited to subtle performance differences on formal laboratory measures, or extend to functional impairment in ecologically valid settings such as the workplace.
References
- Bohnen N, Houx P, Nicolson N, Jolles J. Cortisol reactivity and cognitive performance in a continuous mental task paradigm. Biol Psychol 1990; 31: 107–116
- Braunstein-Bercovitz H. Does stress enhance or impair selective attention? The effects of stress and perceptual load on negative priming. Anxiety Stress Coping 2003; 16: 345–357
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 2001; 26: 307–317
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, et al. Long-term effects of social stress on brain and behavior: A focus on hippocampal functioning. Neurosci Biobehav Rev 2005; 29: 83–97
- Calkins ME, Curtis CE, Grove WM, Iacono WG. Multiple dimensions of schizotypy in first degree biological relatives of schizophrenia patients. Schizophr Bull 2004; 30: 317–325
- Chajut E, Algom D. Selective attention improves under stress: Implications for theories of social cognition. J Pers Soc Psychol 2003; 85: 231–248
- Cohen S, Williamson G. Perceived stress in a probability sample of the united states. The social psychology of health, S Spacapan, S Oskamp. Sage, Newbury Park, CA 1988; 31–67
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus?. Behav Cogn Neurosci Rev 2006; 5: 41–60
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys?. Trends Neurosci 1999; 22: 422–426
- Domes G, Rothfischer J, Reichwald U, Hautzinger M. Inverted-U function between salivary cortisol and retrieval of verbal memory after hydrocortisone treatment. Behav Neurosci 2005; 119: 512–517
- Elzinga BM, Bakker A, Bremner JD. Stress-induced cortisol elevations are associated with impaired delayed, but not immediate recall. Psychiatry Res 2005; 134: 211–223
- Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci 2006; 11: 2746–2758
- Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, et al. Do corticosteroids damage the brain?. J Neuroendocrinol 2006; 18: 393–411
- Hoschl C, Hajek T. Hippocampal damage mediated by corticosteroids—a neuropsychiatric research challenge. Eur Arch Psychiatry Clin Neurosci 2001; 251(Suppl 2)II81–II88
- Jackson DN. Personality research form manual3rd ed. Research Psychologists Press, Port Huron, MI 1984
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 1996; 58: 1475–1483
- Kofman O, Meiran N, Greenberg E, Balas M, Cohen H. Enhanced performance on executive functions associated with examination stress: Evidence from task-switching and Stroop paradigms. Cogn Emot 2006; 20: 577–595
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Brain Res Rev 1997; 24: 1–27
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can't live with it, can't live without it. Behav Brain Res 2001; 127: 137–158
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NP, et al. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. J Clin Endocrinol Metab 1997; 82: 2070–2075
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998; 1: 69–73
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behav Neurosci 1999; 113: 420–430
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology 2005; 30: 225–242
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998; 338: 171–179
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse 2000; 36: 85–94
- Mendl M. Performing under pressure: Stress and cognitive function. Appl Anim Behav Sci 1999; 65: 221
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognit Psychol 2000; 41: 49–100
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci 1994; 14: 2047–2053
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry 1999; 56: 527–533
- Partington JE, Leiter RG. Partington's pathway test. Psychol Ser Center Bull 1949; 1: 9–20
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol 2005; 196: 199–203
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation2nd ed. Neuropsychology Press, Tucson, AZ 1993
- Reus VI, Peeke HV, Miner C. Habituation and cortisol dysregulation in depression. Biol Psychiatry 1985; 20: 980–989
- Reynolds CR. Comprehensive trail making test: Examiner's manual. PRO-ED, Austin, TX 2002
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 2002; 78: 578–595
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996; 93: 3908–3913
- Skosnik PD, Chatterton RT, Jr., Swisher T, Park S. Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. Int J Psychophysiol 2000; 36: 59–68
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The trail making test: A study in focal lesion patients. Psychol Assess 2001; 13: 230–239
- Vedhara K, Hyde J, Gilchrist ID, Tytherleigh M, Plummer S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology 2000; 25: 535–549
- Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology 2005; 30: 11–17
- Wolf OT. HPA axis and memory. Best Pract Res Clin Endocrinol Metab 2003; 17: 287–299
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 2001; 26: 711–720
- Young AH, Sahakian BJ, Robbins TW, Cowen PJ. The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology 1999; 145: 260–266
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the trail making test. Neuropsychologia 2005; 43: 1878–1886
