Abstract
Previous studies have demonstrated that 5-HT2A receptors may be involved in the central control of thermoregulation and of the cardiovascular system. Our aim was to test whether these receptors mediate thermogenic and tachycardiac responses induced by acute psychological stress. Three groups of adult male Hooded Wistar rats were instrumented with: (i) a thermistor in the interscapular area (for recording brown adipose tissue temperature) and an ultrasound Doppler probe (to record tail blood flow); (ii) temperature dataloggers to record core body temperature; (iii) ECG electrodes. On the day of the experiment, rats were subjected to a 30-min restraint stress preceded by s.c. injection of either vehicle or SR-46349B (a serotonin 2A receptor antagonist) at doses of 0.01, 0.1 and 1.0 mg/kg. The restraint stress caused a rise in brown adipose tissue temperature (from, mean ± s.e.m., 36.6 ± 0.2 to 38.0 ± 0.2°C), transient cutaneous vasoconstriction (tail blood flow decreased from 12 ± 2 to 5 ± 1 cm/s), increase in heart rate (from 303 ± 15 to 453 ± 15 bpm at the peak, then reduced to 393 ± 12 bpm at the steady state), and defaecation (6 ± 1 pellets per restraint session). The core body temperature was not affected by the restraint. Blockade of 5-HT2A receptors attenuated the increase in brown adipose tissue temperature and transient cutaneous vasoconstriction, but not tachycardia and defaecation elicited by restraint stress. These results indicate that psychological stress causes activation of 5-HT2A receptors in neural pathways that control thermogenesis in the brown adipose tissue and facilitate cutaneous vasoconstriction.
Introduction
Stress-induced hyperthermia, like all increases in body temperature, reflects increased heat production and/or decreased heat dissipation. In rats, sympathetically-mediated non-shivering thermogenesis in interscapular brown adipose tissue (iBAT) is a major means of increasing heat production and sympathetically-mediated tail artery vsoconstriction is a major means of decreasing heat dissipation. Stressful stimuli activate sympathetic outflow to both of these end organs (Shibata and Nagasaka Citation1984; Ootsuka and Blessing Citation2006). Our previous studies indicate that serotonin (5-HT) 2A receptors may be involved in controlling these two outputs as 5-HT2A receptor agonists cause iBAT thermogenesis and cutaneous vasoconstriction, in both conscious and anaesthetized animals (Blessing and Seaman Citation2003; Ootsuka et al. Citation2004; Ootsuka and Blessing Citation2006). Blockade of 5-HT2A receptors decreases cutaneous vasoconstriction elicited by sudden, short-lasting arousal stimuli (Blessing and Seaman Citation2003; Blessing Citation2005), but there have been no attempts to test the effects of 5-HT2A receptor blockade on stress-induced iBAT thermogenesis.
Psychological stress also affects heart rate, usually causing tachycardia. Involvement of serotonergic neurotransmission, and in particular 5-HT2A receptors, in cardiovascular control is well documented (McCall and Clement Citation1994; Ramage Citation2001). Inhibition of serotonergic function using the 5-HT1A receptor agonist 8-OH-DPAT prevents some forms of stress-induced tachycardia (Nalivaiko et al. Citation2005; van den Buuse and Wegener Citation2005), but so far no studies have determined whether 5-HT2A receptors are involved in cardiac responses to psychological stress.
Another autonomic manifestation of psychological stress is defaecation; this occurs due to activation of colonic motility. Here too, 5-HT neurotransmission may be involved, as activation of 5-HT1A receptors dramatically reduces stress-induced faecal output (Abe and Saito Citation1998). Whether 5-HT2A receptors play any role in the control of colonic motility during stress is not known.
Restraint is one common model of exposing a rat to a stressor, and it consistently elicits hyperthermia (Vidal et al. Citation1984; Long et al. Citation1991; Terlouw et al. Citation1996; Saiki et al. Citation1997; De Paula et al. Citation2000), tachycardia and an increase in arterial pressure (Barron and Van Loon Citation1989; Chen and Herbert Citation1995a; McDougall et al. Citation2005) as well as defaecation (Barone et al. Citation1990; Monnikes et al. Citation1992; Abe and Saito Citation1998). In the present experiments, we used restraint stress and tested the effects of the 5-HT2A receptor antagonist trans-4-((3Z)3-[(2-Dimethylaminoethyl)oxyimino]-3-(2-fluorophenyl)propen-1-yl)-phenol, hemifumarate (SR-46349B, Rinaldi-Carmona et al. Citation1992; also see PDSP Ki Database at http://pdsp.med.unc.edu/pdsp.php) on stress-induced changes in iBAT temperature, tail artery blood flow, heart rate and faecal pellet production.
Materials and methods
The study was performed on 17 adult (>14 weeks old) male Hooded Wistar rats weighing 250–300 g. All efforts were made to reduce animal pain or discomfort. Experiments were conducted in accordance with the European Community Council Directive of 24 November 1986 (86/609/EEC), and were approved by the Flinders University Animal Welfare Committee. Rats were kept on a reverse 12 h/12 h light–dark cycle (8 am off/8 pm on).
Preliminary surgery
Preliminary surgery was conducted under isoflurane (1.5 in 100% oxygen) (Veterinary Companies of Australia Pty. Ltd, NSW, Australia) anaesthesia. Three experimental groups were used. In the first group (TBAT and tail flow, n = 9), a Doppler ultrasonic flow probe (Iowa Doppler Products, IA, USA) was implanted around the tail artery about 3 cm from the base (Garcia et al. Citation2001). A thermistor (10 KΩ, beta = 3380 at 25°C, NTH5G10P, muRata) covered with silicone (3-1744, Dow Corning, Midland, ML, USA) was positioned in iBAT near Sulzer's vein after incision of the dorsal skin. The wires from the Doppler probe and from the thermistor were tunnelled under the skin and soldered to a headsocket; the latter was fixed to the skull using stainless steel screws and dental cement. One week after surgery, we validated the correct positioning of the iBAT probe in each rat, by placing the rats in a cold (5°C) environment for 30 min, and demonstrating that iBAT temperature increased by at least 0.5°C during this cold exposure. In the second experimental group (heart rate, n = 9), ECG electrodes were implanted according to the method described by Sgoifo et al. (Citation1996): one electrode was attached to the internal surface of the xyphoid processus, the other was positioned in the mediastinum along the trachea at the level of the left ventricle. Such electrode placement allows recovery of 95–99% of heartbeats, even in vigorously moving animals. The leads from data sensors or ECG electrodes were tunnelled subcutaneously to the back of the neck and soldered to a headsocket fixed to the skull as above. The latter was manufactured from a standard 8-pin electronic connector allowing easy connection to the cable that had a similar connector at its end. In the third experimental group (core body temperature, n = 6), Mini Temperature Dataloggers (SubCue, USA) were implanted intraperitoneally, with wires passed under the skin and exteriorised at the neck. After rats recovered from anaesthesia they were returned to the animal house for at least 1 week before experimental studies.
Experimental protocol
On the day of the experiments, rats were brought from the animal house, connected to the recording system via a flexible cable attached to a swivel, placed in a dark box (40 × 40 × 40 cm, maintained at 26°C) and remained undisturbed for at least 60 min. Rats from the third group were treated in the same way, except they were not connected to the swivel as their core body temperature was recorded by the dataloggers. Subsequently, all rats received subcutaneous injection of either vehicle or drug (see below), and 15 min later they were placed into the restrainer for 30 min. The restrainer consisted of transparent PVC tubing (i.d. 6 cm), and was illuminated by a table lamp. Rats from the first group (iBAT temperature/tail blood flow recording) were subjected to the restraint four times, at least 48 h apart, with the injection of either vehicle or SR46349B at concentrations of 0.01, 0.1 and 1.0 mg/kg (s.c.). To avoid serial effects, we used a rotational design. Rats from the two other groups (ECG recording and core body temperature recordings) were subjected to the restraint twice, with injection of either vehicle or the high dose of SR46349B (1.0 mg/kg s.c), in a counter-balanced manner. SR46349B, a generous gift from Sanofi–Aventis, was dissolved in 0.1 ml DMSO and then diluted by 0.4 ml Ringer solution. Injection volume was 0.5 ml. Injections were made in a skin fold on the back, at the level of the hindlimbs.
Data acquisition and analysis
Blood flow signal from the ultrasonic Doppler flow probe was recorded with a pulsed Doppler flowmeter (Model 202, Triton technology, Inc., CA, USA). The iBAT temperature signal was recorded with a custom made bridge amplifier. The ECG signal was recorded using a cardiomonitor (model 90603A, SpaceLab, USA). All analogue signals were acquired with a MacLab/4 and Chart (ADInstruments, Sydney, Australia), and digitised at a rate of 2 Hz (iBAT temperature), 40 Hz (tail flow) or 400 Hz (ECG). Heart rate was computed online from the ECG signal. Core body temperature was sampled every minute, and digital data were imported directly from Mini Dataloggers using SubCue Analyzer software (SubCue, USA). Data were analysed with Chart, Igor Pro (WaveMetrics, Lake Oswega, OR, USA) and Statview (SAS Institute, Cary, NC, USA) software.
Mean basal values were obtained during a 10-min period prior to drug or vehicle injections; pre-stress values were obtained during the 5-min period just prior to subjecting rats to the restraint stress. During the 30-min restraint, we computed mean values for each of six 5-min epochs. Treatment- and time-dependent effects on iBAT temperature, core body temperature, tail blood flow and heart rate were examined using two-way ANOVA for repeated measures. Effects on faecal output were assessed using one-way ANOVA for factorial measures. Fishers's post hoc protected t-test was used to determine significant differences. The significance threshold was set at the 0.05 level. All data are mean ± SEM.
Results
Effect of 5-HT2A receptor blockade on iBAT temperature changes induced by restraint
Just after the rats were placed in the experimental cage, iBAT temperature was 38.7 ± 0.1°C (n = 32 measurements in 8 rats), gradually decreasing by 2.0 ± 0.1°C (p < 0.01, n = 32) during the 90-min control period (, ). Baseline values for the iBAT temperature measured at this stage were 36.6 ± 0.2, 37.4 ± 0.3, 36.8 ± 0.3 and 36.8 ± 0.1°C for the vehicle and for the 3 incrementing doses of the drug, respectively; there were no significant differences between these values. Administration of either SR46349B (0.01, 0.1 or 1 mg/kg) or vehicle caused a transient increase in iBAT temperature from 36.7 ± 0.1 to 37.3 ± 0.1°C (p < 0.01, n = 31 measurements) (, ), with return to baseline pre-injection levels within 15 min. These iBAT temperature changes were not significantly different between treatments.
Figure 1 Restraint stress causes changes in the iBAT temperature (upper traces in each panel) and in the tail blood flow (bottom traces); both records are from the same rat on different days. In (A), vehicle was injected s.c. 15 min prior to the restraint; in (B), restraint was preceded by injection of SR-46349B at a high dose (1 mg/kg s.c.). SR-46349B attenuated the increase in iBAT temperature and the decrease in tail blood flow during restraint.
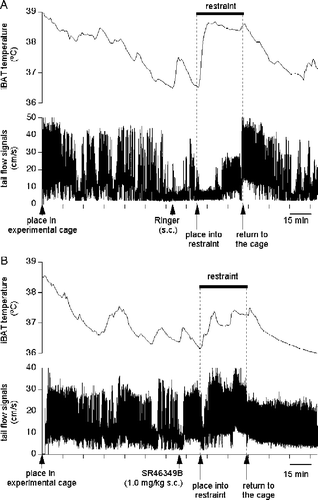
Figure 2 Blockade of 5-HT2A receptors with SR-46349B attenuates the stress-induced increase in iBAT temperature. Mean group data are expressed as delta values. (A) Averaged traces from 8 rats obtained after injection of either vehicle (○) or SR-46349B at a dose of 1 mg/kg (•) 15 min prior to the restraint. There was a significant difference (p < 0.01, n = 8) in the increase in iBAT temperature between vehicle and drug conditions. (B) mean values for stress-induced changes in the iBAT temperature for 4 different conditions (vehicle or SR-46349B at doses of 0.01, 0.1 and 1.0 mg/kg s.c.). **Significantly different, p < 0.01, respectively. Data are expressed as difference between basal (pre-injection) values and maximal temperature increases during restraint.
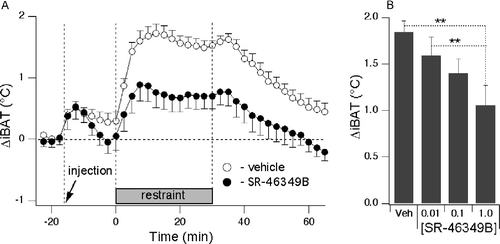
When the rats were placed in the restrainer, iBAT temperature increased in all treatment groups, peaking within 10–15 min, and then remained at the elevated level for the duration of the restraint (, ). Pre-treatment with the highest dose of SR46349B (1 mg/kg) significantly reduced the restraint-elicited increase in iBAT temperature compared to both vehicle and the lowest dose of the drug (p < 0.01, n = 8; ). SR46349B applied at lower doses (0.01 and 0.1 mg/kg) was without effect. The values for the iBAT temperature increase from the pre-drug background to the peak during restraint were +1.8 ± 0.1, +1.6 ± 0.3, +1.4 ± 0.2 and +1.0 ± 0.2°C for the vehicle and for the 3 incrementing doses of the drug, respectively. After the rats were removed from the restraint device and transferred back into the cage, iBAT temperature gradually returned towards the pre-restraint baseline level.
Effect of 5-HT2A receptor blockade on cutaneous vasoconstriction induced by restraint
Prior to the drug injection, mean Doppler flow signal from the tail artery was 12 ± 1 cm/s (n = 32 measurements in 8 rats). Administering either SR46349B (0.01, 0.1 or 1 mg/kg) or vehicle caused a transient decrease of the tail artery Doppler flow signal (, ). In rats treated with SR46349B at doses of 0.1 and 1.0 mg/kg, this transient fall in the tail blood flow quickly reversed, so that the pre-stress value was significantly higher compared to the baseline (for the dose of 0.1 mg/kg—baseline: 9.1 ± 1.3 cm/s; pre-restraint: 12.7 ± 1.1 cm/s; n = 8, p < 0.05; for the dose of 1.0 mg/kg—baseline: 13.4 ± 2.3 cm/s; pre-restraint: 18.5 ± 1.6 cm/s; n = 8, p < 0.05). There were no significant differences between basal and pre-stress values following vehicle or the low dose (0.01 mg/kg) of the drug. See for illustration and for data values.
Figure 3 Blockade of 5-HT2A receptors with SR-46349B attenuates stress-induced transient decreases in tail blood flow. (A) averaged traces from 8 rats obtained after injection of either vehicle (○) or SR-46349B at a dose of 1 mg/kg (•) 15 min prior to the restraint. There was a significant difference between vehicle and drug conditions during the first 5 min of restraint (p < 0.01, n = 8). (B) Mean data values obtained during the baseline period (Bas) are compared to the mean data values obtained during the first 5 min of the restraint, for 4 different conditions (vehicle or SR-46349B at doses of 0.01, 0.1 and 1.0 mg/kg s.c.). Restraint provoked significant decreases in the tail blood flow only after vehicle and the low dose of the drug (* and ** significantly different, p < 0.05 and < 0.01, respectively).

Table I. Effects of SR-46349B on the tail blood flow before and during restraint.
Placing rats into the restrainer caused a prompt decrease in the tail artery Doppler signal (, ). This started to recover within 4–5 min, and then reached a steady level within the next 10 min. During the first 5 min of restraint, tail blood flow was significantly lower compared to corresponding baseline values after vehicle and after the lowest dose of SR46349B. SR46349B applied at doses of 0.1 or 1.0 mg/kg attenuated and shortened the decreases in tail blood flow (Figures 1, 3A,B; ). There were also significant differences in absolute values of tail blood flow for the first 5 min of restraint between vehicle (3.8 ± 1.5 cm/s) and the middle and high doses of the drug (9.6 ± 1.1 cm/s, p < 0.05 and 12 ± 1.3 cm/s, p < 0.01, respectively; n = 8). It is important to emphasize here that while the larger fall from the pre-stress level after the high dose of the drug may suggest that the response was larger in this case, it is the absolute value of the flow that must be considered as an index of vasoconstriction. During the remainder of the restraint period (5–30 min), there were no significant differences between treatments. Data values for changes in the tail blood flow are presented in .
Effect of 5-HT2A receptor blockade on core body temperature
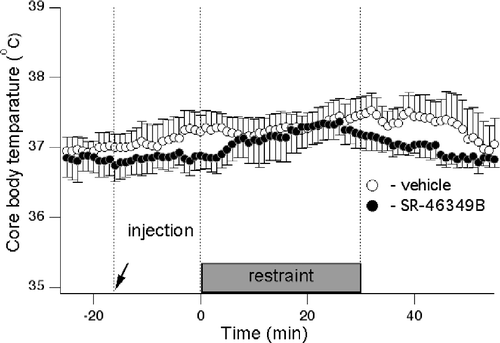
In this experiment, rats with temperature data loggers were subjected to the restraint twice—after either vehicle or the high dose of SR-46349B. Prior to drug injection, core body temperature was 36.9 ± 0.1°C (n = 12 measurements in 6 rats). While in some rats we observed small increases in core body temperature apparently associated with the restraint, there were no significant differences in mean group data values between control (pre-injection), pre-stress (post-injection) or stress periods. There were also no significant differences between drug and vehicle condition for each period. The data values were: baseline: 37.0 ± 0.1 and 36.8 ± 0.2°C for vehicle and drug, respectively; pre-stress: 37.1 ± 0.3 and 36.8 ± 0.2°C for vehicle and drug, respectively; stress period: 37.4 ± 0.3 and 37.1 ± 0.3°C for vehicle and drug, respectively. Averaged records of core body temperature are shown in .
Figure 4 Core body temperature is not affected by SR-46349B nor by the restraint stress. Averaged traces from 7 rats obtained after injection of either vehicle (○) or SR-46349B at a dose of 1 mg/kg (•) 15 min prior to restraint. Two-way ANOVA did not reveal any significant effects of either treatment or restraint on the core body temperature.
Effect of 5-HT2A receptor blockade on tachycardia induced by restraint
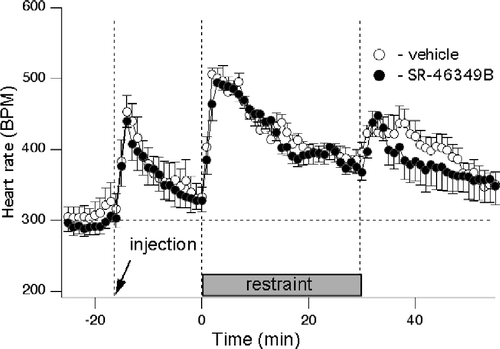
In this experiment, rats with ECG electrodes were subjected to the restraint twice—after either vehicle or the high dose of SR-46349B. Prior to vehicle or drug administration, heart rate was 294 ± 13 bpm (n = 14 measurements in 7 rats). Administration of SR46349B (1 mg/kg) or vehicle caused identical significant tachycardic responses (increasing to 452 ± 30 bpm after vehicle and to 440 ± 29 bpm after the drug, without significant difference between vehicle and drug; n = 7), with subsequent return to the basal level within 15 min as shown in . Placing rats into the restrainer elicited an initial transient increase in the heart rate (to 501 ± 15 and 495 ± 8 bpm for the vehicle and the drug, respectively) which reached a near steady state level within 15–20 min (393 ± 12 and 393 ± 12 bpm for the vehicle and the drug, respectively) There was no significant difference between vehicle and drug treatments. Tachycardia at the beginning and at the end of the restraint was equally significant for both conditions (p < 0.01, n = 7).
Figure 5 Blockade of 5-HT receptors with SR-46349B does not affect tachycardia elicited by the restraint stress. Averaged traces from 7 rats obtained after injection of either vehicle (○) or SR-46349B at a dose of 1 mg/kg (•) 15 min prior to the restraint. Heart rate was significantly elevated following vehicle or drug injection and during restraint compared to the baseline. There was no significant effect of SR46349B.
Effect of 5-HT2A receptor blockade on faecal pellet output induced by restraint
Placing rats into the restraint device elicited defaecation. The number of faecal pellets was not affected by SR46349B, being 6 ± 1, 6 ± 1, 5 ± 1 and 5 ± 1 pellets for vehicle and the three incremental doses of the drug, respectively (n = 32 measurements in 8 rats).
Discussion
5-HT2A receptor blockade differentially suppresses stress-induced autonomic changes
This is the first evidence that pharmacological blockade of 5-HT2A receptors differentially suppresses stress-elicited autonomic changes. SR-46349B, a mixed 5-HT2A/2C antagonist (Rinaldi-Carmona et al. Citation1992) attenuated, in a dose-dependent manner, the increase in iBAT temperature and the initial phase of cutaneous vasoconstriction, but not tachycardia and faecal pellet output, in rats subjected to restraint stress. This indicates that during restraint, 5-HT2A receptors are selectively engaged in some stress-induced autonomic changes.
The increases in heart rate, iBAT temperature, cutaneous vascular tone and in faecal output reported here presumably reflect autonomic arousal which accompanies the state of anxiety elicited by the restraint. There is now a substantial controversy with regard to potential involvement of 5-HT2A receptors in the genesis of anxiety (assessed in animal behavioural tests). Pharmacological studies in rats have not revealed anxiolytic properties of 5-HT2A antagonists (Griebel et al. Citation1997). In contrast, recent elegant experiments (Weisstaub et al. Citation2006) indicate that cortical 5-HT2A receptors are essential for the expression of anxiety in mice. Our results are consistent with Griebel's point of view, assuming that restraint stress-induced tachycardia and faecal output—both unaffected by the blockade of 5-HT2A receptors—are integral parts of the anxiety-generated visceral responses. We suppose that receptors responsible for attenuating thermogenic and tail vasoconstrictor effects must be located relatively low in the neuraxis, where pathways for different autonomic outputs run separately (see below).
iBAT temperature is a robust index of psychological arousal
Our study is the first direct measurement of iBAT temperature and cutaneous blood flow during prolonged psychological stress. We were the first to describe rapid and profound decreases in cutaneous vascular flow elicited by sudden short-lasting arousing and nociceptive stimuli (Yu and Blessing Citation1997). In the present experiments we anticipated that changes in the tail blood flow would last for the whole duration of the restraint, similar to tachycardia. However, we found that cutaneous vasoconstriction was relatively short-lasting. It may be that transient cutaneous vasoconstriction was elicited by the sudden event of placing the animal in the new environment—just like in several of our previous studies (Nalivaiko and Blessing Citation2003; Blessing Citation2005), whereas remaining in this new environment was not particularly stressful/anxiogenic, and thus did not activate the cutaneous vascular response.
Stress-induced increases in iBAT temperature observed in our study are in good accord with previous investigations in conscious rats (Shibata and Nagasaka Citation1984; Ootsuka and Blessing Citation2006). In both reports, the increase in iBAT temperature was greater and faster than that in core temperature, suggesting that the former reflects genuine iBAT thermogenesis rather than iBAT passive heating from some other sources. We did not observe any increase in the core body temperature, and thus we also consider the increase in iBAT thermogenesis to be the most likely explanation for the stress-related increase in iBAT temperature. In the present study, iBAT temperature was steadily and substantially elevated for the whole duration of the restraint stress, in contrast to the changes in cutaneous blood flow and in the heart rate which were most affected at the beginning of the restraint. Thus, iBAT temperature appears to be a robust autonomic index of extended psychological arousal—definitely more sensitive than core body temperature. The data regarding the latter are controversial: while some studies reported that restraint causes hyperthermia (Vidal et al. Citation1984; Long et al. Citation1991; Terlouw et al. Citation1996; Saiki et al. Citation1997; De Paula et al. Citation2000), others found hypothermic responses (Ushijima et al. Citation1985; Chen and Herbert Citation1995b). Our current finding of lack of significant core temperature changes further emphasizes this controversy. Its causes are unclear; one possibility is that in those studies where the core temperature decreased, the experimental condition allowed adequate heat dissipation: Ushijima et al. (Citation1985) wrapped their rats in metal mesh, and Chen and Herbert (Citation1995b) used restrainers with “large holes”. In rats, heat loss occurs principally via the skin of the tail, and we deliberately kept the tails of our rats outside the restrainer. During most of the restraint period the tail blood flow was high, and this likely prevented our animals from overheating.
It is of major interest to compare our results with those of Vianna and Carrive (Citation2005) who also examined changes in several autonomic parameters during prolonged (30 min) stress exposure. These authors reported that when fear-conditioned rats were placed in the same cage where they previously experienced electric footshocks, tail temperature (measured by infrared thermography) was reduced for the whole period of exposure. In contrast, when naïve rats were placed in the same cage, their tail temperature was reduced for only 10–15 min, and then returned to the basal level. As the tail temperature is defined by the amount of blood circulating through the tail vascular bed, it follows that cutaneous vasoconstriction in naïve animals was of significantly shorter duration compared to their fear-conditioned counterparts. These differences presumably reflected stress modality (novelty for naïve rats vs. fear for conditioned animals). Thus, the finding that tail vasculature was constricted for only a short period in our rats suggests that the “stressfulness” of the restraint procedure is closer to the exposure to the novel environment compared to conditioned fear.
In Vianna's and Carrive (Citation2005) paper, sustained tachycardia of a similar magnitude occurred in both naïve and fear-conditioned rats, and was also comparable to our results reported here (about 100 bpm) and in other rat restraint studies (McDougall et al. Citation2000). Hence, contrary to common assumption, heart rate alone appears to be one of the least sensitive indices of degree of psychological arousal. This is likely due to the complexity of neural mechanisms that control heart rate.
Possible location of 5-HT2A receptors that mediate the increase in iBAT temperature and cutaneous vasoconstriction
While localization of receptors responsible for anti-thermogenic and anti-vasocontrictor effects of SR-46349B is beyond the scope of our study, this is relevant to consider. Restraint-induced increase in iBAT temperature was abolished by sympathectomy (Shibata and Nagasaka Citation1984), and there are no reports demonstrating the presence of 5-HT2A receptors on iBAT tissue cells. In addition, the 5-HT2A antagonist SR46349B did not affect synaptic transmission in the cervical sympathetic ganglia which relay sympathetic influences to the rabbit ear vessels (Ootsuka and Blessing Citation2005) that serve the same thermoregulatory function as the tail vessels in the rat. While these data do not allow us to completely exclude peripheral localization of relevant 5-HT2A receptors, the data rather point to their central location. As already noted, differential sensitivity of several visceral responses to the selective blocker of 5-HT2A receptors suggests—though also indirectly—that they are not located at cortical or forebrain subcortical levels responsible for anxiogenesis.
Descending pathways that control iBAT thermogenesis and cutaneous vasoconstriction relay in the medullary raphe (Morrison Citation1999; Blessing and Nalivaiko Citation2000). Some of the labelled raphe-spinal neurons projecting to the tail are serotonergic (Toth et al. Citation2006). Most importantly, our own previous experiments demonstrated that cutaneous vasoconstriction elicited by activation of the medullary raphe could be inhibited by a 5-HT2A antagonist, and that spinal 5-HT2A receptors are involved in the raphe-elicited cutaneous vasoconstriction (Ootsuka et al. Citation2004; Ootsuka and Blessing Citation2005). Thus it is most likely that SR-46349B attenuated restraint stress-induced tail vasoconstriction by blocking 5-HT2A receptors located on the relevant sympathetic vasomotor neurons in the spinal cord. This is in accord with a recent report that intrathecal injection of serotonin increases activity in rat tail sympathetic nerves (Marina et al. Citation2006). It must be acknowledged that at the highest dose, SR-46349B nearly completely abolished stress-induced cutaneous vasoconstriction whereas the increase in the iBAT temperature was attenuated by only about 50%, suggesting involvement of some other receptors.
The lack of anti-tachycardiac action of SR-46349B is in accord with previously reported inconsistent effects of 5-HT2A agonists on the heart rate in rats (Dabiré et al. Citation1989; Alper Citation1990; Dedeolu and Fisher Citation1991; Chaouche-Teyara et al. Citation1993), and indicates that these receptors are not involved in mediation of stress-induced tachycardia. Our results also indicate that 5-HT2A receptors do not mediate a stress-induced increase in colonic motility.
In conclusion, selective blockade of 5-HT2A receptors attenuates the increase in iBAT temperature and the transient cutaneous vasoconstriction, but not tachycardia or the increased colonic motility elicited by restraint stress in rats. These results indicate that psychological stress causes activation of 5-HT2A receptors in the neural pathways that control thermogenesis and facilitate cutaneous vasoconstriction. The lack of effect of 5-HT2A receptor blockade on tachycardia and on colonic output favours the possibility that these receptors are not involved in anxiogenesis per se—otherwise their blockade would equally reduce all autonomic effects elicited by stress.
Acknowledgements
We are grateful for the excellent technical support provided by Ms Sarah Fitzpatrick and Ms Melissa Quinlan. The study was supported be the National Health and Medical Research Council and the National Heart Foundation of Australia, and by Flinders Medical Centre Foundation.
References
- Abe M, Saito K. Reduction of wrap restraint stress-induced defecation by MKC-242, a novel benzodioxan derivative, via 5-HT1A-receptor agonist action in rats. Jpn J Pharmacol 1998; 77: 211–217
- Alper RH. Hemodynamic and renin responses to (+/ − )-DOI, a selective 5-HT2 receptor agonist, in conscious rats. Eur J Pharmacol 1990; 175: 323–332
- Barone FC, Deegan JF, Price WJ, Fowler PJ, Fondacaro JD, Ormsbee HS. Cold-restraint stress increases rat fecal pellet output and colonic transit. Am J Physiol 1990; 258: G329–G337
- Barron B, Van Loon G. Role of sympathoadrenomedullary system in cardiovascular response to stress in rats. J Auton Nerv Syst 1989; 28: 179–187
- Blessing WW. Clozapine increases cutaneous blood flow and reduces sympathetic cutaneous vasomotor alerting responses (SCVARs) in rats: Comparison with effects of haloperidol. Psychopharmacology 2005; 181: 518–528
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: Patterning by medullary raphe, not ventrolateral medulla. J Physiol 2000; 524: 279–292
- Blessing WW, Seaman B. 5-hydroxytryptamine(2A) receptors regulate sympathetic nerves constricting the cutaneous vascular bed in rabbits and rats. Neuroscience 2003; 117: 939–948
- Chaouche-Teyara K, Fournier B, Safar M, Dabire H. Vascular and cardiac effects of alpha-methyl-5-HT and DOI are mediated by different 5-HT receptors in the pithed rat. Eur J Pharmacol 1993; 250: 67–75
- Chen X, Herbert J. Alterations in sensitivity to intracerebral vasopressin and the effects of a V1a receptor antagonist on cellular, autonomic and endocrine responses to repeated stress. Neuroscience 1995a; 64: 687–697
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience 1995b; 64: 675–685
- Dabiré H, Chaouche-Teyara K, Cherqui C, Fournier B, Laubie M, Schmitt H. Characterization of DOI, a putative 5-HT2 receptor agonist in the rat. Eur J Pharmacol 1989; 168: 369–374
- De Paula D, Steiner AA, Branco LG. The nitric oxide pathway is an important modulator of stress-induced fever in rats. Physiol Behav 2000; 70: 505–511
- Dedeolu A, Fisher LA. Central and peripheral injections of the 5-HT2 agonist, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane, modify cardiovascular function through different mechanisms. J Pharmacol Exp Ther 1991; 259: 1027–1034
- Garcia JN, Pedersen NP, Nalivaiko E, Blessing WW. Tail artery blood flow measured by chronically implanted Doppler ultrasonic probes in unrestrained conscious rats. J Neurosci Methods 2001; 104: 209–213
- Griebel G, Perrault G, Sanger D. A comparative study of the effects of selective and non-selective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacology 1997; 36: 793–802
- Long NC, Morimoto A, Nakamori T, Yamashiro O, Murakami N. Intraperitoneal injections of prostaglandin E2 attenuate hyperthermia induced by restraint or interleukin-1 in rats. J Physiol 1991; 444: 363–373
- Marina N, Taheri M, Gilbey MP. Generation of a physiological sympathetic motor rhythm in the rat following spinal application of 5-HT. J Physiol 2006; 571: 441–450
- McCall RB, Clement ME. Role of serotonin1A and serotonin2 receptors in the central regulation of the cardiovascular system. Pharmacol Rev 1994; 46: 231–243
- McDougall S, Paull J, Widdop R, Lawrence A. Restraint stress: differential cardiovascular responses in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 2000; 35: 126–129
- McDougall SJ, Lawrence AJ, Widdop RE. Differential cardiovascular responses to stressors in hypertensive and normotensive rats. Exp Physiol 2005; 90: 141–150
- Monnikes H, Schmidt BG, Raybould HE, Tache Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol 1992; 262: G137–G143
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol 1999; 276: R962–R973
- Nalivaiko E, Blessing WW. CRF1-receptor antagonist CP-154526 reduces alerting-related cutaneous vasoconstriction in conscious rabbits. Neuroscience 2003; 117: 129–138
- Nalivaiko E, Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in the medullary raphe reduces cardiovascular changes elicited by acute psychological and inflammatory stresses in rabbits. Am J Physiol 2005; 289: R596–R604
- Ootsuka Y, Blessing WW. Activation of slowly conducting medullary raphe-spinal neurons, including serotonergic neurons, increases cutaneous sympathetic vasomotor discharge in rabbit. Am J Physiol 2005; 288: R909–R918
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: Increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Lett 2006; 395: 170–174
- Ootsuka Y, Nalivaiko E, Blessing WW. Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxy-methamphetamine, “Ecstasy”) and its reversal by clozapine. Brain Res 2004; 1014: 34–44
- Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull 2001; 56: 425–439
- Rinaldi-Carmona M, Congy C, Santucci V, Simiand J, Gautret B, Neliat G, Labeeuw B, Le Fur G, Soubrie P, Breliere JC. Biochemical and pharmacological properties of SR 46349B, a new potent and selective 5-hydroxytryptamine2 receptor antagonist. J Pharmacol Exp Ther 1992; 262: 759–768
- Saiki Y, Watanabe T, Tan N, Matsuzaki M, Nakamura S. Role of central ANG II receptors in stress-induced cardiovascular and hyperthermic responses in rats. Am J Physiol 1997; 272: R26–R33
- Sgoifo A, Stilli D, Medici D, Gallo P, Aimi B, Musso E. Electrode positioning for reliable telemetry ECG recordings during social stress in unrestrained rats. Physiol Behav 1996; 60: 1397–1401
- Shibata H, Nagasaka T. Role of sympathetic nervous system in immobilization- and cold-induced brown adipose tissue thermogenesis in rats. Jpn J Physiol 1984; 34: 103–111
- Terlouw EM, Kent S, Cremona S, Dantzer R. Effect of intracerebroventricular administration of vasopressin on stress-induced hyperthermia in rats. Physiol Behav 1996; 60: 417–424
- Toth IE, Toth DE, Boldogkoi Z, Hornyak A, Palkovits M, Blessing WW. Serotonin-synthesizing neurons in the rostral medullary raphe/parapyramidal region transneuronally labelled after injection of pseudorabies virus into the rat tail. Neurochem Res 2006; 31: 277–286
- Ushijima I, Tanaka M, Tsuda A, Koga S, Nagasaki N. Differential effects of morphine on core temperature in stressed and non-stressed rats. Eur J Pharmacol 1985; 112: 331–337
- van den Buuse M, Wegener N. Involvement of serotonin1A receptors in cardiovascular responses to stress: A radio-telemetry study in four rat strains. Eur J Pharmacol 2005; 507: 187–198
- Vianna DM, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci 2005; 21: 2505–2512
- Vidal C, Suaudeau C, Jacob J. Regulation of body temperature and nociception induced by non-noxious stress in rat. Brain Res 1984; 297: 1–10
- Weisstaub N, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung J, Sibille E, Underwood M, Itohara S, Dauer W, Ansorge M, Morelli E, Mann J, Toth M, Aghajanian G, Sealfon S, Hen R, Gingrich J. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 2006; 313: 536–540
- Yu YH, Blessing WW. Cutaneous vasoconstriction in conscious rabbits during alerting responses detected by hippocampal theta-rhythm. Am J Physiol 1997; 272: R208–R216
