Abstract
Chronic stress is associated with hippocampal atrophy and cognitive dysfunction. This study investigates how long-lasting administration of corticosterone as a mimic of experimentally induced stress affects psychometric performance and the expression of the phosphatidylethanolamine binding protein (PEBP1) in the adult hippocampus of one-year-old male rats. Psychometric investigations were conducted in rats before and after corticosterone treatment using a holeboard test system. Rats were randomly attributed to 2 groups (n = 7) for daily subcutaneous injection of either 26.8 mg/kg body weight corticosterone or sesame oil (vehicle control). Treatment was continued for 60 days, followed by cognitive retesting in the holeboard system. For protein analysis, the hippocampal proteome was separated by 2D electrophoresis (2DE) followed by image processing, statistical analysis, protein identification via peptide mass fingerprinting and gel matching and subsequent functional network mapping and molecular pathway analysis. Differential expression of PEBP1 was additionally quantified by Western blot analysis. Results show that chronic corticosterone significantly decreased rat hippocampal PEBP1 expression and induced a working and reference memory dysfunction. From this, we derive the preliminary hypothesis that PEBP1 may be a novel molecular mediator influencing cognitive integrity during chronic corticosterone exposure in rat hippocampus.
Introduction
Glucocorticoids are steroid hormones that can influence the nervous system (Melcangi and Panzica Citation2003). Specifically, high circulating glucocorticoid levels can adversely affect cognition and reduce memory capacity (Newcomer et al. Citation1994; Wolf Citation2003). Sustained high glucocorticoid levels may also be associated with the progression of CNS diseases such as stroke, Alzheimer's disease, or depression (O' Brien et al. Citation1996; Murialdo et al. Citation2000; Marklund et al. Citation2004) and have been the target of advanced therapeutic approaches (Kaufer et al. Citation2004).
Hippocampal circuits are particularly susceptible to glucocorticoids, and hippocampal structural and cellular changes (McEwen Citation2005) vary with different stress qualities such as chronic, repeated and severe forms vs. milder forms, as quantified by lower blood glucocorticoids levels (Kempermann Citation2002; Swaab et al. Citation2005). Specifically, effects of glucocorticoids on the brain depend on glucocorticoid and mineralocorticoid receptors (GR and MR, respectively) target action. Under resting conditions (low glucocorticoid concentrations) the primarily MR mediated effect is rather modest, under elevated stress amplitudes (high glucocorticoid concentrations) and depending on the brain region, however, GR effects can prevail which may impair hippocampal long-term potentiation (LTP). On the other hand, stress may also improve hippocampal function (Joels et al. Citation2006), thus, an imbalance in activation of the two receptor types may therefore have important modulatory consequences for higher brain network qualities such as behavior and cognitive performance (De Kloet et al. Citation1999). The U-shaped dose dependency of glucocorticoid action (Joels Citation2006) indicates that behavioral, cellular and molecular effects in the hippocampus may depend on the concentration of glucocorticoid (low, moderate, high), in addition to quality of exposure (acute or chronic).
Our present knowledge of the molecular determinants that regulate proteomic expression and functional changes in hippocampal cells underlying chronically elevated corticosterone (the principal glucocorticoid in rodents) exposure is still limited. In a recent study, the hippocampal proteome was analyzed after 14 days of corticosterone treatment (Skynner et al. Citation2006). The goal of the present study was to provide a preliminary map of candidate proteins that are involved in chronic corticosterone-mediated effects for a longer period of time (60 days) by using differential proteome analysis and molecular pathway analysis. Such longer-dated data could provide the basis for future specific and targeted investigations of adaptive and compensatory processes in brain cells in response to chronic stress, should be applicable in models of chronic neurodegenerative diseases and yield more insights into their mechanisms in the hippocampus. Some of these diseases exhibiting cognitive impairment such as Alzheimer's disease have also been associated with a decline in the expression of the hippocampal cholinergic neurostimulating peptide precursor protein (HCNPpp)-1 (phosphatidylethanolamine binding protein; PEBP1) (Maki et al. Citation2002; George et al. Citation2006) which may link chronic neurodegeneration with learning and memory dysfunction in the hippocampus. Its exact mechanisms and cellular roles including those under prolonged stress, however, are still unknown. Alzheimer's disease and chronic stress share some common ground regarding their impact on long-term cellular survival affecting degeneration. Thus, the present study sought to investigate in a rat model whether extended corticosterone administration, as a mimic of experimentally induced chronic stress, entails a decrease in hippocampal PEBP1 expression and how this might be accompanied by cognitive changes. For this purpose, hippocampal PEBP1 protein expression was analyzed with two independent methods along with psychometric testing.
Materials and methods
Animals
After approval by the local administrative authority (Regierungspraesidium Karlsruhe, Germany), all animal experiments were carried out in accordance with the European Communities Council Directives of 24 November 1986 (86/609/EEC) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
For the experiments, 1 year old male Wistar rats were used (n = 14, breeder: Thomae, Biberach, Germany). The age of 1 year was chosen to follow a previous study on the effects of chronic corticosterone-induced stress on NF-kB (Plaschke et al. Citation2006). Rats were weighed daily; initial body weight was 576 ± 64 g (mean ± SD; n = 14). Rats were housed in individual cages in a temperature-controlled animal room (22.0 ± 0.5°C) with a reversed 12 h:12 h light/dark cycle (lights on at 7 p.m.). Psychometric experiments were conducted during the dark phase of the cycle after 2 weeks of adaptation. Free access to food and water was provided throughout the experimental period. All animals underwent psychometric testing (Lannert and Hoyer Citation1998; Plaschke et al. Citation1999), after they were handled 3 times within 5 days. During habituation (5 days), training (3 days), and testing periods (1 day), food was restricted to 5 g/day ( = 20–30% of normal food intake; Lannert and Hoyer Citation1998) to enhance motivation to perform the holeboard tests. After the first handling and training period, rats were divided into two subgroups: corticosterone administration (n = 7, treatment group) and vehicle (n = 7, sham-treated group). Psychometric testing in the second period was performed identically before the end of the experiments at day 60 after the first injection.
At the end of the experiment, blood samples were drawn from the femoral vein into heparinized vials under 1.5 vol% halothane anaesthesia and nitrous oxide/oxygen (70:30) shortly before the rats were killed between 1 and 2 p.m. To determine corticosterone levels, the blood was rapidly centrifuged at 4000 rpm for 10 min at 4°C, the supernatant separated and stored at − 80°C until further analysis. Rats were decapitated under anaesthesia with 2.5 vol% halothane and nitrous oxide/oxygen (70:30) and hippocampi were dissected, frozen in isopentane, and stored at − 80°C.
Chronic corticosterone administration
Rats received daily s.c. injections of corticosterone (Sigma, Germany) at 26.8 mg/kg body weight (equivalent to ca. 15 mg/day per rat, in 1 ml sesame oil) alternately on the right or left side of the neck (corticosterone group) for 60 days. For sham-treated rats, 1 ml of the vehicle sesame oil was administered daily between 8–9 a.m. for 60 days. For the daily injections, rats were briefly anaesthetized (1–2 min) with 1.5 vol% halothane (O2:N2O = 30:70), which does not significantly affect the hypothalamo–pituitary–adrenal axis, including corticosterone secretion (Karuri et al. Citation1998; De Haan et al. Citation2002).
Measurement of corticosterone
Plasma corticosterone concentrations were measured after 60 days of corticosterone treatment using a specific radioimmunoassay (RIA) as previously published (Vecsei Citation1979; Vollmayr et al. Citation2001). Briefly, 100 μl of 5% aqueous ethanol were added to 10 μl plasma and tritium-labeled corticosterone (to determine individual loss), and the mixture was extracted with 1 ml of cyclohexane/dichloromethane (2:1, v/v). The organic extract was separated, evaporated to dryness, dissolved in 1 ml of 5% aqueous ethanol and quantified with a specific RIA. Intra-assay variation was 12.4%, inter-assay variation 14.3%. Each result was corrected for the individually determined procedural loss. The antisera used for the RIA were raised in this laboratory and extensively characterized, especially with respect to cross-reactivity with potentially interfering endo- and exogenous steroids.
Psychometric testing
Memory ability was measured with the psychometric holeboard memory test (Lannert and Hoyer Citation1998; Plaschke et al. Citation1999). Briefly, habituation, training, and retesting of memory measurements were performed in a holeboard box (70 × 70 × 40 cm). The square flat closed-field area contained 16 holes in a 4 × 4 array. Each hole contained a metal cup (3.5 cm inner diameter, 3 cm deep) which had a perforated bottom; holes were of the same diameter as the outer diameter of the cups (4 cm). A starting box was attached on one side of the holeboard box and separated from the testing area by means of a remotely operated guillotine door. For habituation, rats were placed in the starting box and allowed to enter the testing area to explore the holeboard in which all 16 holes were baited with 50 mg of food pellet (Altromin, standard no. 1320, Lage, Germany). Tests were carried out between 1 and 5 p.m. during the active phase of the rats in the dark. A trial started when the door was opened and ended when the rat had dipped into all 16 holes. Even if the rat did not find all food pellets, the trial ended after 10 min.
After repeating the habituation process five times, rats were trained to search in 4 out of 16 baited holes in a fixed order. Each of the 16 holes supplied with food pellets was covered by a false bottom (a metal cup, 3 cm deep) to mask potential odor from the “reward” in the baited holes. Thus, rats were unable to distinguish between baited and unbaited holes by olfactory stimuli. The trial was terminated when the rat had found all food pellets or when 5 min had elapsed, whichever occurred first. Two training trials were performed each day. For (re)testing, rats were tested by the same procedure as during training, however with different combinations of food holes to avoid the possibility of habituation as experienced for the baited set of food holes during the training period. Working memory ratio in % (number of food-rewarded visits/number of visits and revisits to the baited set of holes) and reference memory (number of visits and revisits to the baited set of holes/number of visits and revisits to all holes) were determined at the beginning and after the end of the corticosterone treatment and were calculated as published (Van Der Staay et al. Citation1990). For example, the task for working memory was fulfilled by 100% if all 4 out of 16 pellets were found without revisiting food- and non-food-baited holes. All behavioral tests in the second psychometric testing period were carried out at least 4 h after the daily injections.
Statistically significant differences in performance in the holeboard test were calculated by a repeated-measures analysis of variance (two-way ANOVA) using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was assumed at the P < 0.05 level.
Proteomic analysis
Sample preparation
In order to obtain high-yield protein extracts, a modified protocol was employed as published previously (Maurer et al. Citation2003). Dissected hippocampi were dissolved in a detergent lysis buffer containing 7M urea, 2M thiourea, 4% (w/v) CHAPS, 0.5% (v/v) Triton X-100, 0.5% (v/v) IPG buffer pH 3–10 (GE Healthcare, formerly Amersham, Uppsala, Sweden), 100 mM DTT and 1.5 mg/ml Complete protease inhibitor (Roche, Mannheim, Germany) for 60 min at 18°C in an orbital shaker. The lysate was centrifuged at 21,000g for 30 min and the protein content in the supernatant determined by the Bradford assay (Ramagli Citation1999).
2-DE, image analysis and protein identification
For proteomic analysis of the hippocampal protein lysates, samples of the sham-treated group (n = 7) and the corticosterone group (n = 7), respectively, were pooled and equal amounts of protein were combined from each group. Separation of proteins in the corticosterone and sham pools by 2DE, with each gel loaded with 250 μg (5–15 μl) of protein extract, protein spot visualization and image analysis were then performed essentially using standard protocols (Maurer et al. Citation2003). Statistical analysis of the spot volumes defined as the integral of the spot area multiplied by optical density was performed comparing normalized means ± SD from 3 gels of each group (sham and corticosterone) by Student's t-test for unpaired data (Maurer Citation2006). Level of significance was set to P < 0.05. To account for the fact that some protein expression changes may lie within the natural range of technical variability, only spots that were up- or down-regulated by at least 20% were included in the continuing analysis (variability cutoff). Differentially expressed spots of interest were identified by peptide mass fingerprinting (PMF) using matrix-assisted time-of-flight mass spectrometry (MALDI-TOF) and gel-matching via polynomial image warping. For PMF, protein spots were automatically located using the HT-Analyzer software (Genomic Solutions, Ann Arbor, MI, USA) and excised with an automated spot-picker Flexys (Genomic Solutions, Ann Arbor, MI, USA). Destaining, in-gel digestion with trypsin (Promega, Madison, WI, USA) and mass spectrometry was then performed as described in (Feldmann et al. Citation2005). The Mascot-delivered probability based score was regarded as a quality parameter for the correct identification. Additional spots were identified by gel matching through polynomial image warping (Maurer et al. Citation2004) using proteomic reference material from adult rat hippocampal stem cells (Maurer et al. Citation2003).
Functional network mapping and molecular pathway analysis
For the biological context of the hippocampal stress proteome data, a systematic functional network mapping and molecular pathway analysis was carried out through the use of ingenuity pathways analysis (IPA) (Ingenuity Systems, Redwood City, CA, USA; www.ingenuity.com). This web-based application enables the exploration of pathway networks relevant to experimental protein expression array data. The proteins differentially regulated in the hippocampus under chronic stress were uploaded (focus proteins) and used as starting points for building biological networks. For this, the application queries the ingenuity pathways knowledge base for interactions between the regulated and all other proteins stored in the database to generate networks using computational algorithms. The basis for the determination of the most significant interaction networks were known protein–protein interactions retrieved from published data. After mapping, IPA compounded a score for the network according to the fit of the set of proteins. Taking into account the number of focus proteins and the size of the computed network, this score measured the probability of the focus proteins being found together in a network at random; values above 2 reflect a confidence of at least 99% for the network map not to have been produced by chance. Also, biological functions assigned to each network are ranked according to the significance of that function to the network. Key proteins of the generated network and their function then became the focus of more detailed functional inspection by western blot analysis.
Western blot analysis
Western blot analysis was performed as previously published (Maurer et al. Citation2006). Briefly, protein extracts of the hippocampal lysates were suspended in Laemmli sample buffer containing 0.5 M Tris, 10% SDS, 10% glycerol, 0.05% bromophenol blue, and 5% 2-mercaptoethanol and denaturated at 95°C for 5 min. Fifty microgram of protein were separated in 12.5% polyacrylamide gels and transferred to 0.2 μm nitrocellulose membranes by wet blotting (150 mA for 50 min). Membranes were blocked with blocking buffer (1x TBS, 0.1% Tween-20, 5% (w/v) non-fat dry milk) for 1 h at room temperature and stained with the specific antibody for PEBP1 (0.5 μg/ml; Abcam, Cambridge, UK). The primary antibody was detected by a secondary antibody, HRP-IgG donkey-anti-goat (1:5000; Dianova, Hamburg, Germany). Chemiluminescence was visualized by mixing 0.45 mM p-coumaric acid, 12.5 mM luminol [5-amino-2,3-dihydro-1,4-phthalazinedione] in 100 mM Tris, pH 8.5, with 0.018% H2O2 in 100 mM Tris, pH 8.5, and exposure on X-ray films (MRDM; Eastman Kodak, Rochester, NY, USA) for 30–60 s. Molecular size gauging was achieved using Kaleidoscope Prestained Standards (Bio-Rad, Hercules, CA, USA). Expression data were quantified densitometrically using the PG200 software (Nonlinear Dynamics, Newcastle-upon-Tyne, UK) after background subtraction and normalization.
Results
Effects of corticosterone on physiological and psychometric parameters
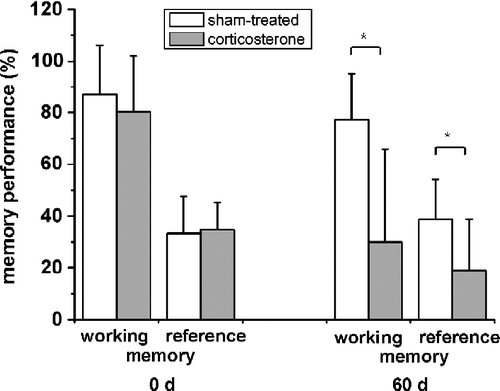
Specific RIA analysis yielded a 1.7-fold increase in plasma corticosterone concentration after 60 days of daily corticosterone injection (n = 7; P < 0.05) (). Body weight of the corticosterone-injected rats decreased about 20% (n = 7; P < 0.05). Also, daily corticosterone injection led to significant changes in cognition. Both hippocampus-sensitive memory capacities, working and reference memory, were markedly reduced under treatment (n = 7; P < 0.05) (). The measure of working memory decreased to about 50% in the corticosterone group (n = 7; P < 0.05), while reference memory was reduced to about 60% as compared to sham-treated animals.
Table I. Changes in plasma corticosterone, body weight and protein expression.
Figure 1 Decreasing memory performance after chronic corticosterone treatment for 60 days. Whereas no difference between rats is seen at the start of the experiment, both working memory (number of food-rewarded visits/number of visits and revisits to the baited set of holes in % with SD) and reference memory (number of visits and revisits to the baited set of holes/number of visits and revisits to all holes in % with SD) performances decrease after 60 days of corticosterone treatment (n = 7; *, P < 0.05). Values are mean ± SD.
Differential changes, functional network mapping and PEBP1 expression in the hippocampal proteome after chronic corticosterone
Total protein content in the hippocampal lysate showed no differences between the groups. The soluble whole-cell proteome incorporated an average of 1989 ± 397 (n = 3) protein spots (). Comparing the corticosterone group to the sham-treated group yielded 102 differentially expressed spots. Fifty-seven spots were upregulated and 45 spots were downregulated under chronic corticosterone. Relative expression strength of a protein between the corticosterone and sham-treated group ranged from 1.23- to 9.17-fold for the upregulated proteins, and from 0.80- to 0.03-fold for the downregulated proteins. Forty of the 102 spots were identified by peptide mass fingerprinting (30 proteins) and polynomial image warping (10 proteins) (). Polynomial image warping is a mathematical method comparing unknown protein spots arrays with previously identified ones by similarity, is indirect and therefore less reliable than peptide mass fingerprinting.
Figure 2 Differential expression of hippocampal proteins after chronic corticosterone. (A) The 2D gel electropherogram shows both spots of the corticosterone group (green) and the sham-treated group (purple) in false-color coding. Overlaid spots appear white. The position of phosphatidylethanolamine binding protein (PEBP1, MW approx. 21 kDa) is indicated by an arrow. (B) Three-dimensional reconstruction of the PEBP1 expression data from the 2D gels show decreased PEBP1 expression after 60 days of corticosterone treatment to roughly half (46%) as compared to the sham-treated group.
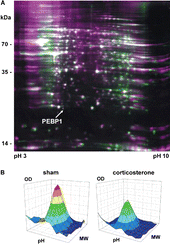
Table II. Differentially expressed proteins in the rat hippocampus after chronic corticosterone treatment.
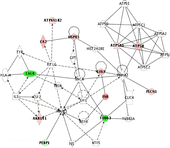
Proteins were mapped with regard to their biological networking and function using IPA which yielded a score 25 network with 12 identified focus proteins out of 35 network candidates () and functional activities in cell-to-cell signaling and interaction, cellular development, cellular growth and proliferation, which are ranked in the order of significance in the network. One protein in the lower left section was identified to be PEBP1. Differential proteome analysis highlighted a decrease in its expression to roughly half of the sham-treated group value after corticosterone treatment (IF = − 2.17, ).
Figure 3 Functional network mapping of differentially expressed hippocampal proteins after chronic corticosterone. Proteins with expression altered after 60 days of corticosterone treatment were analysed regarding their biological functional network using the IPA (Ingenuity Systems, www.ingenuity.com). The network is displayed as nodes (proteins) and lines (biological relationships between the nodes). Focus proteins which are downregulated under corticosterone treatment are shown in green, proteins upregulated under chronic corticosterone in red. Protein abbreviations can be inferred from . The network contains the phosphatidylethanolamine binding protein-1, PEBP1, and other putative chronic corticosterone target proteins such as calreticulin, CALR, various components associated with synaptic plasticity (internexin alpha, INA; fascin 1, FSCN1; tubulin alpha, TUBA3; glial acidic fibrillary protein, GFAP), as well as metabolic factors (carbonic anhydrase 2, CA2; aldo-keto reductase family 1 member A1, AKR1A1; mitochondrial H+-transporting ATPase F1 alpha isoform 1, ATP5A1, and beta isoform, ATP5B, as well as H+-transporting ATPase V1 subunit B isoform 2, ATP6V1B2) and chaperones (heat shock protein 1, HSPD1).
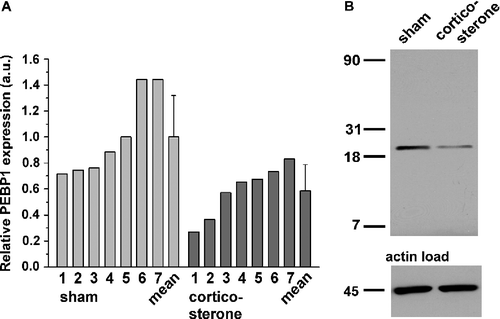
Thus, to acquire additional data to ascertain how chronic administration of corticosterone influences PEBP1, Western blotting was conducted for PEBP1 expression changes. PEBP1 expression was significantly decreased in the hippocampus of corticosterone-treated rats (). shows the distribution of PEBP1 expression in both groups. The IPA network also comprised the calcium binding calreticulin (CALR) as a putative chronic corticosterone target protein which showed downregulation (IF = − 8.33) (). Other proteins subjected to expression changes included various cytomorphological components associated with synaptic plasticity. Here, constituents such as internexin (INA), twinfilin-2, fascin-1 (FSCN1), actins, sirtuin, tubulin (TUBA3), collapsing response mediator protein-2 (CRMP-2) or glial acidic fibrillary protein (GFAP) underwent up- or down-regulation, besides diverse factors of canonical metabolism and chaperones (). This array of differential protein expression corresponds with the most significant global protein functions associated with the focus proteins, i.e. cell-to-cell signaling and interaction, cellular development, cellular growth and proliferation, yielded by the IPA knowledge base query.
Figure 4 Western blot analysis of PEBP1 expression in the rat hippocampus after 60 days of corticosterone treatment. (A) The panel shows the relative quantification of PEBP1 expression in sham- and corticosterone-treated rats in arbitrary units (a.u.; n = 7). Bars above the mean show the standard deviation. Of note, the largest PEBP1 expression value in the corticosterone group is only slightly greater than the smallest expression value in the sham-treated group. (B) Original gel image obtained by western blotting of the lysates and respective levels of a housekeeping protein. A single PEBP1 band is located at about 22 kDa.
Discussion
The present study identifies preliminary candidate proteins that are involved in chronic corticosterone-mediated effects in adult rat hippocampus including the expression of PEBP1, that are accompanied by impaired hippocampus-dependent psychometric performance. The chronic administration of corticosterone achieved a significant increase in plasma corticosterone level and decreased body weight, as expected from other studies (Czeh et al. Citation2002; Plaschke et al. Citation2006; Skynner et al. Citation2006).
Corticosterone-sensitive hippocampal proteins
The global hippocampal proteome screening yielded 102 differentially expressed proteins after chronic corticosterone treatment. Several proteins identified in the present study such as internexin alpha, phosphoglycerate kinase 1, glial fibrillary acidic protein, glucose-related protein grp75, fructose-bisphosphate aldolase C, enolase 1, or β-actin have also been shown to be regulated in rat hippocampal tissue after repeated exposure to psychosocial stress (Carboni et al. Citation2006). In addition, others have been recognized as corticosterone-responsive genes in the hippocampus in transcriptional profiling (Datson et al. Citation2001; Morsink et al. Citation2006). From these, the genes F1-ATPase alpha isoform 1, NADH dehydrogenase 1, heat shock protein 1, heat shock 90 kDa protein β and brain creatine kinase induce products that were also corticosterone target proteins in the present study. Along with aspartate transaminase and carbonic anhydrase, the latter two proteins were also identified as regulated in the, to our knowledge, only other chronic corticosterone-sensitive proteome study available (Skynner et al. Citation2006), although these authors utilized solid pellets to apply corticosterone and reported a shorter observation time of 14 days compared to 60 days in the present study. Another global expression study on hippocampal gene expression under chronic stress (Alfonso et al. Citation2004) yielded no direct congruence with our data. Reasons may be that gene and protein expression dynamics change rapidly in time (Morsink et al. Citation2006) and the shorter time window of 28 days. Hippocampal expression also exhibits strong inter-individual differences (Alfonso et al. Citation2002) and gene and protein expression do not always correlate linearly. In addition, directions of gene and protein regulation, i.e. up- or downregulation, do not always coincide in the above studies. It must be noted, however, that regulation of protein expression in the hippocampus and elsewhere in the brain is a delicate interplay of multiple influential factors of which only marginal alterations may suffice to induce expression changes.
Although a few proteins exhibited strong regulation, expression of other candidates in this study showed moderate degrees of change. Reasons for that may in part lie in the cellular and structural heterogeneity of the investigated tissue. The hippocampal formation contains different cell types all of which have their tailored metabolism which was frozen at the time point of lysis. Also, by technical nature of the applied screen, investigated lysates contain pools of many different types of hippocampal neurons, only some of which contain MR and GR. These limitations may mask some of the corticosterone effects on the expression dynamics, in particular when proteins are in low abundance. Furthermore, replicate gels in the screen can be considered only as technical replicates that provide information about the method; biological, inter-animal variability, however, must be considered as a factor that was not assessed. Thus, proteomic data in this study are reported and discussed as preliminary findings that need to be validated in future experiments.
We hypothesize that regulation of target proteins by corticosterone in this study includes extensive long-range adaptive cellular after-effects rather than immediate receptor actions of corticosterone. This is supported by the observation that proteomic profile alterations after long-term corticosterone treatment have also been detected in brain regions outside the hippocampus (Skynner et al. Citation2006) which exhibit different distribution patterns of glucocorticoid receptors (Morimoto et al. Citation1996).
Pathway mapping resulted in the association of regulated proteins with cellular development and growth. Our data show corticosterone regulation of various components associated with structural formation such as synaptic plasticity, axonogenesis, and neurogenesis (internexin, twinfilin-2, fascin 1, actins, sirtuin, tubulin, CRMP, glial fibrillary acidic protein), and are supported by other studies reporting similar results (Stein-Behrens et al. Citation1994; Skynner et al. Citation2006). Inasmuch as the regulation of cytoarchitectural changes can be represented by a set of regulated proteins, alterations in morphology attending the long-term adaptation processes can be assumed. Skynner et al. (Citation2006) for example, showed a marked reduction of hippocampal weight under chronic corticosterone treatment which corresponds with data on hippocampal volume under stress and depression shown by others (Feldmann et al. Citation2007). Specific information about how hippocampal corticosterone-responsive cells undergo morphological changes due to functional adaptation including stimulation of specific cellular pathways (e.g. apoptosis, neurogenesis), however, cannot be deduced from the present screening results. More specific experiments are necessary to elucidate this in the future. Of note, several of the cytoskeletal proteins, folding agents and (energy) metabolic components found to be altered in this study were also found to be differentially expressed in adult hippocampal stem cells undergoing in vitro differentiation (Maurer et al. Citation2004), which suggests involvement of these proteins in hippocampal neuron plastic responses to chronic corticosterone in vivo. This highlights findings on the role of glucocorticoids in the modulation of neurogenesis (Mirescu and Gould Citation2006).
Besides expression changes in structural proteins, a strong downregulation of calreticulin (CALR) was registered after chronic corticosterone treatment (IF = − 8.33). Calreticulin plays a prominent role as a transcriptional regulator for nuclear glucocorticoid receptor actions (Holaska et al. Citation2001). Its overexpression can inhibit glucocorticoid response-mediated transcriptional activation of sensitive genes (Burns et al. Citation1994). The strong decrease in expression in the present study may point to an involvement in adjuvant action on the slow gene-mediated glucocorticoid receptor signaling pathway in the hippocampus under corticosterone activation. Additional experiments will be necessary to substantiate this in the future.
PEBP1 as a molecular translator in hippocampal response to chronic corticosterone exposure?—A first hypothetical model
The present study found that rats subjected to chronic corticosterone treatment showed a decrease in hippocampal PEBP1 expression and impaired cognition. This is in agreement with other studies finding similar results in chronic neurodegenerative diseases exhibiting cognitive decline such as Alzheimer's disease (Maki et al. Citation2002; Reddy et al. Citation2004; George et al. Citation2006). Since Alzheimer's disease and chronic stress share some principal similarities with respect to long-term cellular survival and degeneration, this analogy may be indicative of a more prominent role of PEBP1 in hippocampal cell management under certain conditions, ultimately influencing cognitive outcome. Here, we propose a first hypothetical model and concept for a role of PEBP1 after chronically increased corticosterone exposure, and perhaps chronic stress.
PEBP1, formerly known as HCNPpp, is the substrate of a local endopeptidase which occurs exclusively in the hippocampus and generates the hippocampal cholinergic neurostimulating peptide (HCNP) (Otsuka and Ojika Citation1996). HCNP stimulates the differentiation of cholinergic neurons in the septo-hippocampal system of the basal forebrain (Ojika et al. Citation2000). Being importantly integrated into the hippocampal network, this major cholinergic system converges with several neural (e.g. serotonin, dopamine, noradrenaline, enkephalins) and hormonal inputs that are involved in stress response homeostasis, thereby modulating hippocampal circuitry for normal function (Cobb and Davies Citation2005). One final common output from these afferents is represented by cholinergic terminals which mediate part of the hippocampal (limbic) adaptation to stressful stimuli, a response which is primarily adaptive in nature (Gilad Citation1987; Dutar et al. Citation1995). This highly conserved cholinergic transmission (Pick et al. Citation2004) is believed to be modulated and altered by stress (Gilad Citation1987; Kaufer et al. Citation1998) as a long-lasting component of changes in the central nervous system upon stress, yet, appears to be especially prone to damage induced by corticosterone (Imperato et al. Citation1989; Hortnagl et al. Citation1993). This may link hippocampal activity with declining basal forebrain function (Hortnagl et al. Citation1993). Based on feedback mechanisms, changes in PEBP1 and subsequently HCNP expression could be part of regulatory mechanisms of cellular homeostasis in the hippocampus: an increase in expression can stimulate the differentiation of cholinergic neurons in the septo-hippocampal system (Ojika et al. Citation2000) whereas a decrease restrains their formation but suppresses inhibition, i. e. enhances mitogen activated protein kinase (MAPK) in addition. Assuming that excess corticosterone entails functional (ir)reversible cell damage or loss (atrophy), attenuated HCNP during corticosterone exposure (enhancing MAPK) could enable the proliferation of new hippocampal precursors to prepare for accepting neuronal commitment at a later time, whereas enhanced HCNP with low corticosterone level (suppressing MAPK) could stimulate mature yet still not terminally differentiated neurons to develop into cholinergic phenotypes. Periods of anastasis from stress could thereby leave room for fine tuning septo-hippocampal system circuit repair and maintenance, whereas periods of chronic stress could entail alternative regeneration of the basal progenitor reservoir needed for that repair to counteract ongoing glucocorticoid-induced damage and atrophy of the hippocampus. PEBP1 expression may thus act as a physiological mediator responding to changes in supply and demand (damage) of septo-hippocampal components, such as during stress with high corticosterone levels.
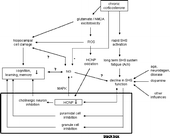
As part of an adaptive hippocampal response to (chronic) stress, the association of PEBP1 decline and cognitive decline might be explained in the following simplified manner using a black box (): corticosterone induces rapid (and chronic) activation of the septo-hippocampal system (Gilad Citation1987), resulting in long-term (acetylcholine) system fatigue over time and progressive decline of septo-hippocampal system function (system negative override), similar to states of aging or neurodegenerative diseases (Hortnagl and Hellweg Citation1997). The effect of a weakened septo-hippocampal system (black box input) then feeds forward to an increase in inhibition of granule cells and pyramidal cells (black box transformation function) as the main septo-hippocampal system projection targets (Hortnagl and Hellweg Citation1997), contributing to the cognitive impairment in learning and memory (black box output). On the other hand, declining septo-hippocampal system function is reflective of the PEBP1 decrease (Iwase et al. Citation2001) (alternative black box transformation function) thus potentially depressing cognition in addition (Kesner Citation1988; Fibiger Citation1991). Together with hippocampal atrophy and regulation of adult stem cell proliferation/neurogenesis, these mechanisms may act cooperatively or influence each other. Since suppression of cognitive functions by corticosterone is supported by other studies (Lupien and Mcewen Citation1997; Belanoff et al. Citation2001), this concept proposes PEBP1 to be a novel molecular mediator influencing cognitive integrity under chronic corticosterone management in the hippocampus. Its underlying cellular activities are in congruence with the functional changes in protein expression due to corticosterone as predicted by the network analysis, namely cell-to-cell signaling and interaction, cellular development, cellular growth and proliferation. Testing such a direct link between cognitive impairment and PEBP1 levels needs additional experiments.
Figure 5 Model of PEBP1 as a mediator in adaptive chronic glucocorticoid-induced response of the brain. Via long-term (acetylcholine) system fatigue upon chronic corticosterone impact, a septo-hippocampal system (SHS) negative override can occur. This results in impaired function over time (right branch). Its feed-forward contributes to the inhibition of several transmitter systems in the hippocampus (black box) leading to circuit defects and reduction of hippocampus-sensitive memory capacities. An alternative transformation function may include regulation of PEBP1 (HCNP) expression, possibly acting together with stem cell proliferation and neurogenesis (NG) to influence circuit integrity. In parallel, PEBP1 inhibition may result from glutamate excitotoxicity and subsequent effects of reactive oxygen species (ROS) (middle branch). Likely, PEBP1 expression is regulated on the levels of the genome, proteome and physiome, i.e. integrated biological function, and makes up only one component of a dynamic response variability that includes manifold interactions with different stages of response to chronic corticosterone (or stress). Question marks indicate unclear relations,+/ − signs stimulatory/inhibitory effects.
Summary
In summary, this study shows that chronic administration of corticosterone for 60 days suppressed the expression of PEBP1 in rat hippocampus and induced cognitive impairment. These changes are paralleled by regulation of cytoskelatal proteins, folding agents and metabolic components reflecting active proliferative and differentiative protein turnover in the hippocampus during chronic stress. We hypothesize PEBP1 expression to be part of a regulative mechanism in adaptive management of chronic stress in the brain, acting as physiological mediator of response to changes in supply and demand of cholinergic components during stress. As part of a putative PEBP1 feed-back/feed-forward cycle in neuroprotective response, it may thus be a novel hippocampal mediator influencing cognitive integrity under chronic corticosterone, possibly acting via the septo-hippocampal system, neurogenesis, and/or reactive oxygen species downstream of the glutamate/NMDA receptor excitotoxicity cascade.
Acknowledgements
The authors are indebted to Roland Galmbacher for animal handling and preparation and Maria Harlacher and Mathilde Lorenz for help with 2DE and WB.
References
- Alfonso J, Aguero F, Sanchez DO, Flugge G, Fuchs E, Frasch AC, Pollevick GD. Gene expression analysis in the hippocampal formation of tree shrews chronically treated with cortisol. J Neurosci Res 2004; 78: 702–710
- Alfonso J, Pollevick GD, Castensson A, Jazin E, Frasch AC. Analysis of gene expression in the rat hippocampus using real time PCR reveals high inter-individual variation in mRNA expression levels. J Neurosci Res 2002; 67: 225–234
- Belanoff JK, Gross K, Yager A, Schatzberg AF. Corticosteroids and cognition. J Psychiatr Res 2001; 35: 127–145
- Burns K, Duggan B, Atkinson EA, Famulski KS, Nemer M, Bleackley RC, Michalak M. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature 1994; 367: 476–480
- Carboni L, Piubelli C, Pozzato C, Astner H, Arban R, Righetti PG, Hamdan M, Domenici E. Proteomic analysis of rat hippocampus after repeated psychosocial stress. Neuroscience 2006; 137: 1237–1246
- Cobb SR, Davies CH. Cholinergic modulation of hippocampal cells and circuits. J Physiol 2005; 562: 81–88
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 2002; 52: 1057–1065
- Datson NA, Van Der Perk J, De Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci 2001; 14: 675–689
- De Haan M, Van Herck H, Tolboom JB, Beynen AC, Remie R. Endocrine stress response in jugular-vein cannulated rats upon multiple exposure to either diethyl-ether, halothane/O2/N2O or sham anaesthesia. Lab Anim 2002; 36: 105–114
- De Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys?. Trends Neurosci 1999; 22: 422–426
- Dutar P, Bassant MH, Sesnut MC, Lamour Y. The septohippocampal pathway: Structure and function of a central cholinergic system. Physiol Rev 1995; 75: 393–427
- Feldmann RE, Jr., Bieback K, Maurer MH, Kalenka A, Burgers HF, Gross B, Hunzinger C, Kluter H, Kuschinsky W, Eichler H. Stem cell proteomes: A profile of human mesenchymal stem cells derived from umbilical cord blood. Electrophoresis 2005; 26: 2749–2758
- Feldmann RE, Jr., Sawa A, Seidler GH. Causality of stem cell based neurogenesis and depression—to be or not to be, is that the question?. J Psychiatr Res 2007; 41: 713–723
- Fibiger HC. Cholinergic mechanisms in learning, memory and dementia: A review of recent evidence. Trends Neurosci 1991; 14: 220–223
- George AJ, Holsinger RM, Mclean CA, Tan SS, Scott HS, Cardamone T, Cappai R, Masters CL, Li QX. Decreased phosphatidylethanolamine binding protein expression correlates with Abeta accumulation in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Aging 2006; 27: 614–623
- Gilad GM. The stress-induced response of the septo-hippocampal cholinergic system. A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinology 1987; 12: 167–184
- Holaska JM, Black BE, Love DC, Hanover JA, Leszyk J, Paschal BM. Calreticulin Is a receptor for nuclear export. J Cell Biol 2001; 152: 127–140
- Hortnagl H, Berger ML, Havelec L, Hornykiewicz O. Role of glucocorticoids in the cholinergic degeneration in rat hippocampus induced by ethylcholine aziridinium (AF64A). J Neurosci 1993; 13: 2939–2945
- Hortnagl H, Hellweg R. Insights into the role of the cholinergic component of the septohippocampal pathway: What have we learned from experimental lesion studies?. Brain Res Bull 1997; 43: 245–255
- Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: Role of corticosterone. Eur J Pharmacol 1989; 165: 337–348
- Iwase T, Ojika K, Matsukawa N, Nishino H, Yamamoto T, Okada H, Fujimori O, Ueda R. Muscarinic cholinergic and glutamatergic reciprocal regulation of expression of hippocampal cholinergic neurostimulating peptide precursor protein gene in rat hippocampus. Neuroscience 2001; 102: 341–352
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 2006; 27: 244–250
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work?. Trends Cogn Sci 2006; 10: 152–158
- Karuri AR, Engelking LR, Kumar MS. Effects of halothane and methoxyflurane on the hypothalamic–pituitary–adrenal axis in rat. Brain Res Bull 1998; 47: 205–209
- Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 1998; 393: 373–377
- Kaufer D, Ogle WO, Pincus ZS, Clark KL, Nicholas AC, Dinkel KM, Dumas TC, Ferguson D, Lee AL, Winters MA, Sapolsky RM. Restructuring the neuronal stress response with anti-glucocorticoid gene delivery. Nat Neurosci 2004; 7: 947–953
- Kempermann G. Regulation of adult hippocampal neurogenesis—implications for novel theories of major depression. Bipolar Disord 2002; 4: 17–33
- Kesner RP. Reevaluation of the contribution of the basal forebrain cholinergic system to memory. Neurobiol Aging 1988; 9: 609–616
- Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci 1998; 112: 1199–1208
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Brain Res Rev 1997; 24: 1–27
- Maki M, Matsukawa N, Yuasa H, Otsuka Y, Yamamoto T, Akatsu H, Okamoto T, Ueda R, Ojika K. Decreased expression of hippocampal cholinergic neurostimulating peptide precursor protein mRNA in the hippocampus in Alzheimer disease. J Neuropathol Exp Neurol 2002; 61: 176–185
- Marklund N, Peltonen M, Nilsson TK, Olsson T. Low and high circulating cortisol levels predict mortality and cognitive dysfunction early after stroke. J Intern Med 2004; 256: 15–21
- Maurer MH. Software analysis of two-dimensional electrophoretic gels in proteomic experiments. Curr Bioinform 2006; 1: 255–262
- Maurer MH, Feldmann RE, Jr., Futterer CD, Butlin J, Kuschinsky W. Comprehensive proteome expression profiling of undifferentiated versus differentiated neural stem cells from adult rat hippocampus. Neurochem Res 2004; 29: 1129–1144
- Maurer MH, Feldmann RE, Jr., Fütterer CD, Kuschinsky W. The proteome of neural stem cells from rat hippocampus. Proteome Sci 2003; 1: 4–10
- Maurer MH, Geomor HK, Burgers HF, Schelshorn DW, Kuschinsky W. Adult neural stem cells express glucose transporters GLUT1 and GLUT3 and regulate GLUT3 expression. FEBS Lett 2006; 580: 4430–4434
- McEwen BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism 2005; 54: 20–23
- Melcangi RC, Panzica G. Steroids and the nervous system. Ann NY Acad Sci 2003; 1007: 1–5
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus 2006; 16: 233–238
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistochemical and in situ hybridization study. Neurosci Res 1996; 26: 235–269
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER, Datson NA. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol 2006; 18: 239–252
- Murialdo G, Nobili F, Rollero A, Gianelli MV, Copello F, Rodriguez G, Polleri A. Hippocampal perfusion and pituitary–adrenal axis in Alzheimer's disease. Neuropsychobiology 2000; 42: 51–57
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci 1994; 14: 2047–2053
- O'Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, Tress B. Clinical and magnetic resonance imaging correlates of hypothalamic–pituitary–adrenal axis function in depression and Alzheimer's disease. Br J Psychiatry 1996; 168: 679–687
- Ojika K, Mitake S, Tohdoh N, Appel SH, Otsuka Y, Katada E, Matsukawa N. Hippocampal cholinergic neurostimulating peptides (HCNP). Prog Neurobiol 2000; 60: 603–783
- Otsuka Y, Ojika K. Demonstration and characterization of hippocampal cholinergic neurostimulating peptide (HCNP) processing enzyme activity in rat hippocampus. Neurochem Res 1996; 21: 369–376
- Pick M, Flores-Flores C, Soreq H. From brain to blood: Alternative splicing evidence for the cholinergic basis of Mammalian stress responses. Ann NY Acad Sci 2004; 1018: 85–98
- Plaschke K, Feindt J, Djuric Z, Heiland S, Autschbach F, Lewicka S, Martin E, Bardenheuer HJ, Nawroth PP, Bierhaus A. Chronic corticosterone-induced deterioration in rat behaviour is not paralleled by changes in hippocampal NF-kappaB-activation. Stress 2006; 9: 97–106
- Plaschke K, Yun SW, Martin E, Hoyer S, Bardenheuer HJ. Interrelation between cerebral energy metabolism and behaviour in a rat model of permanent brain vessel occlusion. Brain Res 1999; 830: 320–329
- Ramagli LS. Quantifying protein in 2-D PAGE solubilization buffers. Methods Mol Biol 1999; 112: 99–103
- Reddy PH, Mcweeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: Up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum Mol Genet 2004; 13: 1225–1240
- Skynner HA, Amos DP, Murray F, Salim K, Knowles MR, Munoz-Sanjuan I, Camargo LM, Bonnert TP, Guest PC. Proteomic analysis identifies alterations in cellular morphology and cell death pathways in mouse brain after chronic corticosterone treatment. Brain Res 2006; 1102: 12–26
- Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R, et al. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci 1994; 14: 5373–5380
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 2005; 4: 141–194
- Van Der Staay FJ, Van Nies J, Raaijmakers W. The effects of aging in rats on working and reference memory performance in a spatial holeboard discrimination task. Behav Neural Biol 1990; 53: 356–370
- Vecsei P. Glucocorticoids: Cortisol, cortisone, corticosterone, compound S, and their metabolites. Methods of hormone radioimmunoassays, BM Jaffe, HR Behrmann. Academic Press, New York, NY 1979; 767–792
- Vollmayr B, Faust H, Lewicka S, Henn FA. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry 2001; 6: 471–474
- Wolf OT. HPA axis and memory. Best Pract Res Clin Endocrinol Metab 2003; 17: 287–299
