Abstract
The objective of this study was to evaluate the perceived stress index, quality of life, and hypothalamus-pituitary-adrenal axis activity in women with endometriosis and chronic pelvic pain. For the study, 93 women with endometriosis and 82 healthy women volunteered. The visual analogue scale (VAS) (0 = no pain; 10 = severe pain) was used to determine pain intensity; the perceived stress questionnaire (PSQ) defined stress index, and the health-related quality-of-life (HRQOL)-SF-36 questionnaire was used to evaluate quality of life. Salivary cortisol was measured at 0800, 1600, and 2000 h and the awakening cortisol response was assessed to evaluate the hypothalamus-pituitary-adrenal axis activity. The results show that women with endometriosis and chronic pelvic pain of moderate intensity (4.1 ± 0.58, mean ± SEM) have higher levels of perceived stress (0.55 ± 0.01 versus 0.42 ± 0.01, p < 0.05), a poorer quality of life expressed as lower scores for all items of the inventory and hypocortisolism. Lower levels of salivary cortisol were observed in all three samples collected, as well as in the awakening cortisol response, for women with endometriosis (0.19 ± 0.09 μg/dl) when compared with controls (0.78 ± 0.08 μg/dl, p < 0.05 l), and it was independent of pain intensity and Mental health (MH) scores in SF-36. We concluded that women with endometriosis and chronic pelvic pain show low concentrations of salivary cortisol and a high level of perceived stress, associated with a poor quality of life. Whether the hypocortisolism was an adaptive response to the aversive symptoms of the disorder or a feature related to the etiology of endometriosis remains to be elucidated.
Introduction
Endometriosis is a chronic inflammatory disease, which is found especially in young women with subfertility problems; it may affect as many as 30% of these women (Gazvani and Templeton Citation2002). This disease is estrogen-dependent and is characterized by endometrial proliferation outside the uterus, mainly in the abdominal cavity, causing continuous or intermittent pelvic pain, sometimes the one associated with intercourse and/or menstruation. The infertility and pelvic pain caused by endometriosis are a source of considerable physical and psychological stress because of their devastating effects on affective, sexual, and reproductive behavior, damaging the patient's self-esteem and impairing her quality of life (Marques et al. Citation2004).
Health-related quality-of-life (HRQOL) has been defined as the basis of attributes valued by patients. These include comfort or sense of well being, physical, emotional, and intellectual functioning, and the degree to which participation in valued activities is possible, within the family, in the workplace, or in the community (Naughton et al. Citation1996). The HRQOL-SF-36 questionnaire provides an established measure of success in health-related research and clinical trials (Ware Citation2000), and is used to estimate the burden of a disease, compare health profiles, calculate treatment effects, and monitor outcomes (Kelly et al. Citation2005).
Although stress has been classically associated with overactivation of the hypothalamus-pituitary-adrenal axis, more recent evidence has shown that insufficient glucocorticoid signaling or levels (hypocortisolism) may play a role in the development and expression of pathology in stress-related disorders, such as chronic fatigue syndrome, chronic pelvic pain, fibromyalgia, post-traumatic stress disorder (PTSD), irritable bowel syndrome, lower back pain, burnout, atypical depression, and chronic fatigue syndrome (Heim et al. Citation2000; Fries et al. Citation2005; Jerjes et al. Citation2006). When accompanied by hypocortisolemia, all of these disorders can share a syndrome characterized by a triad of enhanced stress sensitivity, pain, and fatigue (Fries et al. Citation2005).
To date, no treatment for endometriosis is considered successful, and there is no cure for the disease. The symptoms are not eliminated by treatment, and eventual hormone therapy has many side effects that also contribute to the poor quality of life and stress that are related to this disorder. Therefore, a better understanding of the mechanisms associated with endometriosis should help in the development of new therapeutic approaches that might improve patient well-being.
The objective of this study was to evaluate the perceived stress index, quality of life, and hypothalamus-pituitary-adrenal axis activity in a relatively large cohort of women with endometriosis and chronic pelvic pain.
Materials and methods
Subjects
In the study, 93 patients diagnosed as having endometriosis during laparoscopy or laparotomy (endometriosis group) and 82 healthy women (control group) participated. The patients with endometriosis had experienced a history of persistent pelvic pain not responding to surgical or pharmacological treatment, for at least 7 years prior to enrolling in the study. The severity of endometriosis among the patients enrolled in this study was distributed as follows: 33% had endometriosis level IV, 27% level III, 22% level II, and 18% level I. The healthy volunteers had no pain-related disease and were taking no hormonal anti-inflammatory medication at the time of the study.
This study was approved by the Internal Review Board of the School of Medical Sciences, State University of Campinas (UNICAMP), Campinas, São Paulo, Brazil, and was conducted according to the principles of the Helsinki Declaration. All volunteers signed an Informed Consent Form prior to admission.
Quantification of intensity of pain
Pain intensity was quantified daily for 1 week, using the Visual Analogue Scale (VAS) in which the patient records the intensity of pain on a scale from 0 (no pain) to 10 (severe pain). Pain was classified as: mild (1–3), moderate (4–7), or severe (8–10) (Huskisson Citation1974).
Health-related Quality-of-life (Hrqol-sf-36) Evaluation
The 36-Item Short Form Health Survey (SF-36) of the Medical Outcomes Study, which is a standardized assessment tool for HRQOL, was used in the study. This instrument provides a generic measure of HRQOL, which has proved to be reliable and valid. It is a short, multi-purpose, self-administered questionnaire composed of 36 questions examining various aspects of physical and Mental health (MH) in eight different domains. Each domain is scored from 0 to 100, with a higher score correlating with better physical functioning or MH. Norm-based methods were used to standardize the scores using means, standard deviations, and factor score coefficients for the scales (Ware et al. Citation1995, Citation2000).
Perceived stress questionnaire (PSQ)
The PSQ developed by Levenstein et al. (Citation1993) was specifically designed to measure stress in clinical psychosomatic research. It consists of 30 items developed by experienced clinicians and has been validated in Italian, English, and Spanish for populations of psychiatric inpatients, outpatients, students, and health workers. The PSQ index was obtained as follows: PSQ = (raw score − 30/90).
A free translation to Portuguese of the Spanish version of the PSQ, validated by Sanz-Carrillo et al. (Citation2002), was applied to the volunteers of the present study, considering the last 2 years. The translated version was examined by an expert in Portuguese for semantic validation, and then translated back to English by a native speaker. According to the Questionnaire authors, mean scores obtained from a sample of healthy individuals were 0.34 ± 0.13 (male, n = 43) and 0.36 ± 0.14 (female, n = 133). No significant differences were observed with respect to gender or age. PSQ scores < 0.25 fell into the lowest quartile for the validation sample as a whole, 0.25–0.34 in the second quartile, 0.35–0.44 in the third quartile, and ≥ 0.45 in the upper quartile (Levenstein et al. Citation1993). Data are presented as means ± SEM.
Salivary cortisol assay
Salivary cortisol concentrations and the salivary cortisol awakening response (CAR) were measured as an index of hypothalamic-pituitary-adrenal axis activity (Kirschbaum et al. Citation1999).
Saliva was collected in “salivettes” (Sarstedt, Numbrecht, Germany). Subjects were instructed to place a cotton pad in their mouth and leave it there until it became saturated with saliva (about 5 min). The cotton pad was subsequently returned to the plastic tube, which was then stored in the patient's home freezer until the next day, when the samples were taken to the laboratory, centrifuged and then kept frozen ( − 20°C) until salivary cortisol testing was carried out. Participants were told to collect three samples of saliva at 0800 h (before breakfast), 1600 h, and 2000 h (at least 2 h after dinner). One group of patients (eight women with endometriosis and eight healthy women) collected two additional samples, one immediately after waking up and rinsing their mouth only with water; and the second one, 30 min after awakening to determine CAR. This response was calculated as the difference between the concentrations of cortisol in the two samples (Pruessner et al. Citation1999). Subjects were instructed to avoid contamination of the saliva samples with blood, and not to have breakfast before collecting the two samples. Patients were also told not to smoke for at least 1 h before collecting any sample.
Hormone assay
Following thawing, the samples were centrifuged at 3500 rpm for 10 min to provide clear supernatant fractions. Cortisol assay was carried out using EIA kits supplied by Diagnostic Systems Laboratories, Inc. (Webster, TX, USA). The inter- and intra-assay CVs were 6.9 and 6.2%, respectively.
Experimental design
Patients were invited to participate in the study during one of their visits to the Physiotherapy and Psychology Departments of the Women's Health Center at the UNICAMP Teaching Hospital. Students and employees of the University were invited to participate in the control group, which was matched for gender and age to the endometriosis group.
Those who volunteered and who fulfilled inclusion criteria for the study signed the Informed Consent Form and received the salivettes for saliva collection, as well as detailed instructions, the PSQ and the HRQOL-SF-36 questionnaire. Women in the endometriosis group also received copies of the VAS and instructions to fill it out daily for 1 week. The salivettes containing saliva samples were kept in the patient's home freezer and were returned to the hospital during the following scheduled visit (endometriosis group) or on the next working day salivettes (control group).
The study staff immediately provided any additional information requested by the volunteers.
Statistical analysis
The salivary cortisol concentrations and PSQ index are presented as means, followed by the standard error of the mean. The area under the curve of salivary cortisol concentrations against time was calculated by the trapezoid method respective to the ground level (Pruessner et al. Citation2003b).
Data showing a normal distribution were compared using Student's t-test or Analysis of Variance for repeated measures (MANOVA), and other data were compared using the Mann–Whitney test. Scores on the SF-36 questionnaire are presented as median followed by the 25th and 75th percent quartiles and were compared using the Mann–Whitney test. The Spearman correlation between VAS score, perceived stress score, and salivary cortisol concentration was calculated (Conover Citation1998). Differences were considered significant when P < 0.05. Data were analyzed using the Graph Pad Instat software program, version 3.0.
Results
No significant differences were found with respect to the age, weight, height, and blood pressure between women in the control and the endometriosis groups ().
Table I. Anthropometric data, systolic (SBP) and diastolic (DBP) blood pressure of women in the control group and those with endometriosis.
In both the groups, ca. 80% of the volunteers were white, 10% were black and data regarding race were not given for the remaining 10%. In the control group, 50% were single, 64% nulliparous, and 86% had no history of spontaneous abortion; in the endometriosis group, 12.5% were single or divorced, 32% were nulliparous, and 76% had no history of spontaneous abortion.
More than 50% of the women in the endometriosis group had been experiencing constant pelvic pain while the rest suffered intermittent pain for at least 10 years. Some 40% reported low pain intensity (score 1–3 on the VAS), 37% reported moderate pain (score 4–7), and 20% reported severe pain (score 8–10). The mean ( ± SEM) pain intensity evaluated in the same week as the saliva sample collection was 4.10 ± 0.58, which is considered as moderate intensity (Huskisson Citation1974). Around 90% of these women reported having had dyspareunia for approximately 5 years.
In the control group, 32% of the women and 53% in the endometriosis group were using oral contraceptives. Medication for pain relief was regularly used by 32% of the women in the endometriosis group who reported pelvic pain occurring daily or associated with menstruation. Six women in the endometriosis group were under anti-depressant pharmacotherapy. None of them were under psychotherapy treatment.
HRQOL-SF-36 is scored from 0 to 100, with higher values indicating better quality of life (Ware Citation2000). Women in the control group scored higher than 60 for all of the questionnaire domains, which indicate a good quality of life. Endometriosis patients, however, scored higher than 60 only for the physical functioning domain; their scores were lower than 60 for all the other domains (). The total norm-based scoring (median followed by 25th and 75th percentiles) for the physical component were 52.4 (50.3–53.6) in the control group and 40.3 (37.9–41.5) in the endometriosis group. The total mental component norm-based scoring were 47.5 (43.3–47.6) in the control group and 33.3 (32.4–38.0) in the endometriosis group. Differences between groups were significant (P < 0.0001; Mann–Whitney test) for both the physical and the mental components of the SF-36 questionnaire.
Table II. Medians, 25th and 75th percentiles of the scores in the health-related quality of life 36-item short form (SF-36) questionnaire in healthy women (control) and those with endometriosis.
On the questionnaire evaluating perceived stress, scores were significantly higher (P < 0.05, Student's t-test) for women with endometriosis and chronic pelvic pain (0.55 ± 0.01; n = 82) than for control women (0.42 ± 0.01; n = 93, p < 0.05), indicating higher levels of perceived stress in women with the disease.
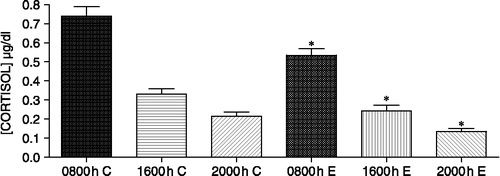
Salivary cortisol concentrations were assessed at 0800, 1600, and 2000 h on a single day in the luteal phase of the menstrual cycle, for both groups of women. Values for the control group were similar to those reported in other studies (Kirschbaum et al. Citation1999; Kudielka and Kirschbaum Citation2003; Young et al. Citation2004), whereas women with endometriosis had significantly lower salivary cortisol concentrations at all three times of measurement (). In both the groups, salivary cortisol levels were significantly higher at 0800 than at 1600 and 2000 h (F[1,174] 130.7, p < 0.001, MANOVA). The area under the curve was lower in the endometriosis group than in the control group (3.93 ± 0.24 μg/dl/h; n = 81 and 5.00 ± 0.25 μg/dl/h; n = 65, respectively, F[1,175] 21.5, p < 0.0001, MANOVA).
Figure 1 Salivary cortisol concentration (μg/dl) at 0800, 1600 and 2000 h in the control group (C, n = 82) and in the group of patients with endometriosis (E, n = 93). *p < 0.05 compared to the control group at the same time (MANOVA). Numbers inside the bars are the means ± SEM.
CAR was also determined for approximately 10% of the total number of participants since the test only came into common usage after the study was well underway. The salivary cortisol concentration of this subgroup was similar to that of the entire group (Student's t-test, p>0.05). CAR was lower in women with endometriosis than in the controls: 0.78 ± 0.08 μg/dl in the controls (n = 8) and 0.19 ± 0.09 μg/dl (n = 8) in the endometriosis group (p < 0.05; Student's t-test).
Since pain is a major symptom of endometriosis, the salivary cortisol concentration for the group of women with the disease was evaluated as a function of pain intensity. The results show that there was no effect of pain group (F[2,62] 1.21, p = 0.305, MANOVA), although the influence of time remained similar between groups (F[2,61] 50.2, p < 0.0001, MANOVA, ). The endometriosis group was also investigated according to the type of pelvic pain (constant versus intermittent), duration (1–3 years versus more than 3 years), frequency (daily, weekly, or associated with menstruation), and the presence or absence of dyspareunia. No significant differences in the salivary cortisol concentration were observed in relation to these parameters (data not shown). However, the PSQ score was higher (0.67 ± 0.02) for women with constant pain than for those with intermittent pain (0.54 ± 0.05; p < 0.05, Mann–Whitney test), even though the intensity of the pain was not significantly different for the two groups (VAS, 5.3 ± 0.73 versus 3.2 ± 0.78, respectively; p = 0.06, Mann–Whitney test).
Table III. Basal salivary cortisol concentration (μg/dl) in samples collected at 0800, 1600, and 2000 h in women with endometriosis suffering different levels of chronic pelvic pain, as determined by the visual analog scale of pain (VAS).
Moreover, other potential variables were also investigated: previous surgery (except for laparotomy or laparoscopy), use of oral contraceptives, use of other medication, regular physical activity, and quality of sleep. Infertility in the endometriosis group was also assessed. No statistically significant within-group differences were found for any of these variables (data not shown).
The literature links endometriosis with comorbid depression, and links such depression with a high plasma concentration of cortisol. We did not, however, examine the patients specifically for the presence or absence of depression, although we did consider the MH domain of the SF-36 as a probable indicator of mood disturbances, since it has been shown to be useful in screening for psychiatric disorders (Ware et al. Citation1995). We therefore divided the group of patients with endometriosis into those with a low ( ≤ 50) and a high (>50) MH score. No effects due to group were found for the salivary cortisol concentrations (F[1,91] 2.02, p = 0.158, MANOVA, ). Only six patients of the endometriosis group were under anti-depressant treatment, with fluoxetine being the drug used. The salivary cortisol concentrations of women using anti-depressants and that of women using no medication in the endometriosis group revealed no significant differences (p>0.05; Mann–Whitney test).
Table IV. Basal salivary cortisol concentration (μg/dl) in samples collected at 0800, 1600, and 2000 h in women with endometriosis scoring low or high in the mental health (MH) domain on the SF-36 questionnaire.
The Spearman test showed a negative correlation between MH and the PSQ score ( − 0.52), as well as between pain intensity and physical function ( − 0.51) and between pain intensity and general health ( − 0.46). There was no significant correlation either between PSQ scores and pain intensity (data not shown) or between PSQ and salivary cortisol concentration. However, there was a negative correlation ( − 0.42) between salivary cortisol concentrations and vitality in women with endometriosis.
Discussion
Young women at the peak of their reproductive life are the population most affected by endometriosis. In this study, women who had endometriosis and chronic pelvic pain, as well as dyspareunia, scored lower on the HRQOL-SF-36 questionnaire, indicating a poorer quality of life than that of control women; moreover, these individuals reported higher levels of perceived stress, as indicated by PSQ scores in the upper quartile of the values validated for a standard population (Levenstein et al. Citation1993; Sanz-Carrillo et al. Citation2002).
Stress is classically associated with high concentrations of glucocorticoids, the end products of the hypothalamus-pituitary-adrenal cortex cascade. However, neurobiological studies on the effects of stress have revealed a paradoxical phenomenon: hypocortisolism (Heim et al. Citation2000), which was first described in patients with post-traumatic stress disorder (Yehuda et al. Citation1997), and has been reported in patients suffering from burnout with physical complaints, fibromyalgia, asthma, chronic fatigue syndrome among others (Kirschbaum et al. Citation1990; Demitrack et al. Citation1991; Crofford et al. Citation1994; Kruger and Spiecker Citation1994; Jerjes et al. Citation2006; Tanriverdi et al. Citation2007), as well as in healthy individuals who live in conditions of constant, long-lasting stress (Heim et al. Citation2000; Fries et al. Citation2005). In contrast to the high concentrations of glucocorticoids traditionally associated with stress, this last condition involves low concentrations of cortisol.
In the present study, women with endometriosis and chronic pelvic pain were also found to have low concentrations of salivary cortisol as well as a blunted cortisol response to awakening, results similar to those reported by Heim et al. (Citation1998, Citation1999) for women with pelvic pain associated with pelvic adhesions.
Since hypocortisolism is characteristic of a family of physical disorders, Heim et al. (Citation2000) hypothesized that it might be a relevant factor in the pathogenesis of these disorders, especially since a lack of cortisol availability may promote increased vulnerability to, for example, autoimmune disorders, inflammation, chronic pain, asthma, and allergies. The secretion of cortisol under stressful conditions provides a protection for the organism, including mobilization of fuel supplies; moreover, it suppresses the immune and inflammatory responses.
Glucocorticoids can inhibit several steps of the inflammatory cascade (Fries et al. Citation2005). Therefore, the low salivary concentration of cortisol associated with endometriosis may contribute to the pathogenesis of this inflammatory disease. Although this low concentration may be deleterious, it can also be beneficial for health and survival for those individuals who are repeatedly or continuously exposed to intense immune stimuli, protecting the subject against the harmful effects of a high allostatic load at the expense of symptoms such as high stress sensitivity, pain, and fatigue (Fries et al. Citation2005; Tanriverdi et al. Citation2007).
We investigated only the basal salivary cortisol concentration, and not the etiology of the hypocortisolism detected in patients with endometriosis and chronic pelvic pain. However, the hypocortisolemic condition may reflect a deficiency in hypothalamus-pituitary-adrenal axis functioning, differences in cortisol metabolism, or even changes in the diurnal rhythm of cortisol, as was found in patients with chronic fatigue syndrome (Jerjes et al. Citation2006). Salivary as well as plasma cortisol concentrations vary greatly between individuals, but the number of patients analyzed in the present study (82 healthy female volunteers and 93 women with endometriosis and chronic pelvic pain) was large enough to compensate for this variation. The results presented here show a preserved but dampened diurnal rhythm of cortisol secretion in women with endometriosis, and the area under the curve of cortisol concentrations was smaller than that for control women. Therefore, the total secretion of cortisol during the day is decreased in women with endometriosis and chronic pelvic pain. This is the first report of low salivary cortisol concentrations in women suffering from endometriosis with chronic pelvic pain.
The results obtained for the CAR reinforce the presence of hypocortisolism in women suffering from endometriosis. Similar results were found in a group of emergency ward nurses experiencing high levels of stress (Yang et al. 2001), and in teachers experiencing burnout (Pruessner et al. Citation1999). The awakening cortisol response is a rather robust phenomenon and is easily replicated. However, the literature about this phenomenon is relatively new and there are certain inconsistencies, although some observations have clearly been replicated (Clow et al. Citation2004). Although this response was initially claimed not to suffer from the influence of age, the use of oral contraceptives, habitual smoking, time of awakening, total time slept, and use of an alarm clock (Wüst et al. Citation2000), it is now known that this response is sensitive to a range of confounding factors. The influence of gender, age, and smoking is still a matter of controversy in the literature (Clow et al. Citation2004), although certain other factors seem to be consistently related to increases in the awakening cortisol response, such as time of awakening and exposure to light before and after awakening, with early risers and people exposed to simulated dawn showing larger awakening cortisol responses. However, the most important confounding factor influencing the CAR seems to be strict adherence to the sample collection protocol (Kudielka et al. Citation2003; Broderick et al. Citation2004). Adherence to this kind of protocol tends to be low and data must be interpreted with caution. In this study, women with endometriosis were highly cooperative, and expressed a strong desire to participate, perhaps in the hope of being able to contribute to a solution to their health problems.
In the group of women with endometriosis evaluated here, the prevalence of dyspareunia was quite high (90%) in comparison with data reported by others. In the National Endometriosis Society survey, 66% of the sample reported pain during or after intercourse (Carlton Citation1996), whereas Flores et al. (Citation2007) reported dyspareunia in 52% of Puerto Rican women with endometriosis, and Matalliotakis et al. (Citation2007) indicated 49.5% in their survey. The higher prevalence of dyspareunia in the sample analyzed here might represent a bias of the study so that the present results might not extend to all patients with endometriosis. On the other hand, the lower prevalence registered in other studies could be due to the difficulty of identifying dyspareunia as a symptom during interview, since many women might not volunteer such personal information to a doctor, and few doctors ask women about it, reflecting a reticence to discuss issues relating to sexuality by both gynecologists and patients (Denny 2003; Denny and Mann Citation2007).
No evaluation of possible burnout or post-traumatic stress syndrome was made. Nevertheless, the women with endometriosis had a PSQ index located in the upper quartile for the sample as a whole (scores ≥ 0.45, Sanz-Carrillo, Citation2002), suggesting a chronically high level of perceived stress associated with their chronic pain and poor quality of life.
In women with endometriosis, there was a negative correlation between pain and physical function and general health although no significant correlation between pain and PSQ score or between pain and salivary cortisol levels was found. The low score in the physical functioning domain suggests a significantly limited performance in all physical activities, whereas the low score in relation to general health indicates a personal evaluation of an individual's health as poor and a belief that it is getting worse. Since the MH score was also low, the high PSQ index may suggest that these women are trying to cope with their symptoms but that they feel psychologically ill, experiencing symptoms such as nervousness, depression, and unhappiness. Moreover, they share two of the three symptoms of people with hypocortisolism associated with various other disorders (Fries et al. Citation2005); pain and fatigue, with the latter expressed as decreased vitality according to the SF-36 questionnaire. Sensitivity to stress was not evaluated in this study.
No specific instrument was used to evaluate depression in this group of women with endometriosis. Although depression is classically associated with the hypersecretion of cortisol, the results are still conflicting and certain recent results have shown no evidence of increased salivary cortisol levels in depressed subjects (Posener et al. Citation2000; Strickland et al. Citation2002). Therefore, some authors have suggested that hypersecretion of cortisol may be confined to severely depressed inpatients (Maes et al. Citation1994). On the other hand, a high awakening cortisol response was detected in a group of students self-reporting depressive symptomatology (Pruessner et al. Citation2003a) as well as in un-medicated depressed subjects recruited from primary care sources (Bhagwagar et al. Citation2005). When the patients with endometriosis were divided on the basis of scores in the MH item of the SF-36 questionnaire (high ≥ 50 and low < 50), no difference in the salivary cortisol concentrations was found. Although the use of anti-depressants can lower cortisol concentrations, only six of these patients were taking such medication, a percentage of the group that is too low to explain the low salivary cortisol concentrations observed in the endometriosis group.
In conclusion, we have shown that women suffering from endometriosis and chronic pelvic pain have higher levels of perceived stress and a lower quality of life than the healthy women; moreover, they reveal hypocortisolism. Whether this hypocortisolism plays a role in the development of the disorder is a part of the natural history of the disorder or is an adaptive response to the chronic stress associated with chronic pain and impaired sexuality remains to be determined.
Acknowledgements
This study was supported by the “Foundation for the Support of Research in the State of Sao Paulo” (FAPESP, Grant number 03/09173-3) and “The Brazilian Coordination for the Improvement of University Level Personnel” (CAPES), and reports some of the results of a master's thesis by Karina F. S. Petrelluzzi. The authors would like to acknowledge A. A. Marques, C. B. Lorençatto, and M. J. N. Vieira for permitting access to their patients. The authors also acknowledge the assistance of S. S. Moraes and L. Gentry El-Dash for the statistical analyses and linguistic revision of the text, respectively.
References
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology 2005; 182: 54–57
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: Comparison of patients and healthy volunteers. Psychoneuroendocrinology 2004; 29(5)636–650
- Carlton D. Awareness of endometriosis. Pract Nurse 1996; 12: 439–440
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress 2004; 7(1)29–37
- Conover WJ. Practical nonparametric statistics3rd ed. Wiley series in probability and statistics, IE Wiley, New York 1998, p. 584
- Crofford LJ, Pillemer SR, Kalogeras KT, Cash JM, Michelson D, Kling MA, Sternberg EM, Gold PW, Chrousos GP, Wilder RL. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum 1994; 37: 1583–1592
- Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, Chrousos GP, Gold PW. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab 1991; 73: 1224–1234
- Denny E, Mann CH. Endometriosis-associated dyspareunia: The impact of women's lives. J Farm Plann Reprod Health Care 2007; 33: 189–193
- Flores I, Abreu S, Abac S, Fourquet J, Laboy J, Rios-Nedoya C. Self-reported prevalence of endometriosis and its symptoms among Puerto Rican women. Int J Gynecol Obstet 2007, doi: 10.1016/j.ijgo.2007.08.010
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology 2005; 30: 1010–1016
- Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynecol Obstet 2002; 76: 117–126
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med 1998; 60: 309–318
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Psychological and endocrine correlates of chronic pelvic pain associated with adhesions. J Psychosom Obstet Gynecol 1999; 20: 11–20
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 2000; 25: 1–35
- Huskisson EC. Measurement of pain. Lancet 1974; 2: 1127–1131
- Jerjes WK, Peters TJ, Taylor NF, Wood PJ, Wessley S, Cleare AJ. Diurnal excretion of urinary cortisol, cortisone, and cortisol metabolites in chronic fatigue syndrome. J Psychos Res 2006; 60: 145–153
- Kelly MH, Brillante B, Kushner H, Collins MT. Physical function is impaired but quality of life preserved in patients with fibrous dysplasia of bone. Bone 2005; 37: 388–394
- Kirschbaum C, Steyer R, Eid M, Patalla U, Schwenkmezger P, Hellhammer DH. Cortisol and behavior: 2. Application of a latent state-trait model to salivary cortisol. Psychoneuroendocrinology 1990; 15: 297–307
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 1999; 61: 154–162
- Kruger U, Spiecker H. Die diagnostik der nebernnierenrindeninsuffizienz bei steroidpflichtigem Asthma bronchiale: Der CRH-test im Vergleich zu kortizol tagesprofil in serum und Kortizol im 24h-Urin. Pneumologie 1994; 48: 793–798
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology 2003; 28: 35–47
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med 2003; 65: 313–319
- Levenstein S, Prantera C, Varvo V, Scribano ML, Berto E, Luzi C, Andreoli A. Development of the perceived stress questionnaire: A new tool for psychosomatic research. J Psychosom Res 1993; 37: 19–32
- Maes M, Calabrese J, Meltzer HY. The relevance of the in- versus outpatients on HPA-axis in depression: Spontaneous hypercortisolism is a feature of major depressed inpatients and not of major depressed per se. Prog Neuropsychopharmacol Biol Psychiatry 1994; 18: 503–517
- Marques A, Bahamondes L, Aldrighi JM, Petta CA. Quality of life in Brazilian women with endometriosis assessed through a medical outcome questionnaire. J Reprod Med 2004; 49(2)115–120
- Matalliotakis JM, Cakmak H, Fragouli YG, Goumenou AG, Mahuttte NG, Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch Gynecol Obstet 2007, doi: 10.1007/s∅∅4∅4-∅∅7-∅479-1
- Naughton MJ, Shumaker SA, Anderson RT, Czajkowski SM. Quality of life and pharmacoeconomics in clinical trials2nd ed., B Spilker. Raven Press, New York 1996; 118–120
- Posener JA, DeBattista C, Williams GH, Chmura Kraemer H, Kalehzan BM, Schatzberg AF. 24-hour monitoring of cortisol and corticotrophin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry 2000; 57: 755–760
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress and cortisol responses to awakening. Psychosom Med 1999; 61: 197–204
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med 2003a; 65: 92–99
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formula for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003b; 28: 916–931
- Sanz-Carrillo C, Garcia-Campayo J, Rubio A, Santed MA, Montoro M. Validation of the Spanish version of the perceived stress questionnaire. J Psychosom Res 2002; 52: 167–172
- Strickland PL, Deakin JF, Percival C, Dixon J, Gater RA, Goldberg DP. Bio-social origins of depression in the community. Interactions between social adversity, cortisol and serotonin neurotransmission. Br J Psychiatry 2002; 180: 168–173
- Tanriverdi F, Karaca Z, Unluhizarci K, Kelestimur F. The hypothalamo-pituitary-adrenal axis in chronic fatigue symdrome and fibromyalgia syndrome. Stress 2007; 10(1)13–25
- Ware JE, Jr. SF-36 health survey update. Spine 2000; 25: 3130–3139
- Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med Care 1995; 33: AS264–279, Suppl
- Wüst S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response—Normal values and confounds. Noise Health 2000; 2(7)79–88
- Yehuda R, Schmeidler J, Siever LJ, Binder-Brynes K, Elkin A. Individual differences in posttraumatic stress disorder symptom profiles in Holocaust survivors in concentration camps or in hiding. J Trauma Stress 1997; 10: 453–463
- Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol 2004; 25: 69–76
