Abstract
Bidirectional associations between mood disorders and cardiovascular diseases are extensively documented. However, the precise physiological and biochemical mechanisms that underlie such relationships are not well understood. This review focuses on the neurobiological processes and mediators that are common to both mood and cardiovascular disorders. The discussion places an emphasis on the role of exogenous stressors in addition to: (a) neuroendocrine and neurohumoral changes involving dysfunction of the hypothalamic-pituitary-adrenal axis and the activation of the renin-angiotensin-aldosterone system, (b) immune alterations including activation of pro-inflammatory cytokines, (c) autonomic and cardiovascular dysregulation including increased sympathetic drive, withdrawal of parasympathetic tone, cardiac rate and rhythm disturbances, and altered baroreceptor reflex function, (d) central neurotransmitter system dysfunction involving the dopamine, norepinephrine and serotonin systems, and (e) behavioral changes including fatigue and physical inactivity. The review also discusses experimental investigations using preclinical disease models to elucidate the neurobiological mechanisms underlying the link between mood disorders and cardiovascular disease. These include: (a) the chronic mild stress model of depression, (b) a model of congestive heart failure, (c) a model of cardiovascular deconditioning, (d) pharmacological manipulations of body fluid and sodium balance, and (e) pharmacological manipulations of the central serotonergic system. In combination with an extensive human research literature, the investigation of mechanisms underlying mood and cardiovascular regulation using animal models will enhance understanding the association between depression and cardiovascular disease. This will ultimately promote the development of better treatments and interventions for individuals with co-morbid psychological and somatic pathologies.
Introduction
Converging lines of evidence from both experimental and epidemiological studies indicate that there is a bidirectional association between depression and cardiovascular disease. Cardiovascular disease, characterized by changes in homeostatic and neuroendocrine function, is a significant risk factor for disordered affective states, such as depression (Schleifer et al. Citation1989; Freedland et al. Citation2003). Also, depression, characterized by depressed mood, reduced responsiveness to pleasurable stimuli (anhedonia) and feelings of hopelessness, is a recognized risk factor for heart disease-related morbidity and mortality (Frasure-Smith et al. Citation1995; Barefoot et al. Citation1996; Barefoot and Schroll Citation1996; Everson et al. Citation1996; Penninx et al. Citation2001; Carney and Freedland Citation2003; Van der Kooy et al. Citation2007). The association between mood states and cardiovascular diseases has been shown to be independent of traditional cardiovascular risk factors such as hypertension, high cholesterol, increased body mass index, history of cardiac-related problems, and disease severity (Anda et al. Citation1993; Barefoot and Schroll Citation1996; Penninx et al. Citation2001; Frasure-Smith and Lespérance Citation2003), and exists in individuals with (Frasure-Smith et al. Citation1995; Barefoot et al. Citation1996; Carney and Freedland Citation2003; Frasure-Smith and Lespérance Citation2003) and without (Anda et al. Citation1993; Barefoot and Schroll Citation1996; Everson et al. Citation1996; Penninx et al. Citation2001) a history of cardiac pathophysiology.
During the past decade, increasing attention has been devoted to studying the link between mood states and cardiovascular diseases (Musselman et al. Citation1998; Nemeroff et al. Citation1998; Carney and Freedland Citation2003; Frasure-Smith and Lespérance Citation2003; Freedland et al. Citation2003; Johnson and Grippo Citation2006; Freedland et al. Citation2006; Glassman Citation2007), as well as attempts to disseminate information about this relationship to the general biomedical community and the lay public. In 2001, the National Institute of Mental Health presented a summary of recent data linking depression to cardiovascular disease and stated its commitment, along with the National Heart Lung and Blood Institute, to supporting research on the basic mechanisms involved in the co-morbidity of mental and physiological disorders (National Institute of Mental Health Citation2001). The American Psychological Association's Practice Directorate has a similar initiative to highlight the role of psychologists in contributing to research and treatment on the overlap of mental and physical well-being (see American Psychological Association Citation2004; Stambor Citation2006). A 2002 article appearing in My Generation, a periodical marketed toward individuals over 50 years of age, discussed the importance of cardiovascular diseases in producing depression (Doner Citation2002). Similar articles have appeared recently in Newsweek (Miller Citation2005), US News and World Report (Szegedy-Maszak Citation2003) and Chicago Tribune (Kotulak Citation2006).
In spite of the evidence that depression is associated with heart disease, the pathophysiological mechanisms underlying the association remain unclear. In combination with research that is focused on human populations, experimental approaches that focus on animal disease models provide novel methods for investigating causal and common mechanisms underlying the link between mood and cardiovascular regulation. In particular, the use of reliable and valid methodological procedures in animal models can produce results that have translational potential for understanding the association of depression and heart disease in humans.
The purpose of the present review is to provide a summary of common mechanisms in depression and altered cardiovascular regulation. For the past several years our laboratories have been investigating potential causal and common mechanisms that may be involved in this association using model systems in rodents. Our focus here is on clinical and experimental research, coupled with recent findings involving integrative research methods in rodent models. Additionally, we provide some recommendations for future mechanistic research involving animal models and multi-disciplinary studies.
Evidence for the association of depression and cardiovascular disease
The bidirectional association between mood disorders and heart disease is multifaceted, involving an integration of several central and peripheral processes. The presence of cardiovascular disease or dysfunction can directly and indirectly influence affective states, including signs of both anxiety and depression. Compared to a prevalence of approximately 2–3% (men) and 5–9% (women) of depression in the general population (American Psychiatric Association Citation2000), its prevalence in patients following a myocardial infarction may be approximately 45% (Schleifer et al. Citation1989), and might be even higher in patients with chronic cardiovascular conditions such as congestive heart failure (CHF) (Freedland et al. Citation2003). Cardiovascular disease-induced depression can result from psychological processes (e.g., contemplation of one's mortality or dealing with significant lifestyle changes) or physiological processes (e.g., changes in visceral afferent neural input or humoral factors released during states of cardiovascular pathophysiology), however, it is likely that both psychological and physiological mediators contribute to the association of cardiovascular disease and mood changes.
Aside from cardiovascular pathophysiology influencing depressive signs and symptoms, the presence of depression increases the likelihood of experiencing detrimental cardiac events in patients with established cardiovascular disease. Depending on the study, approximately 20–50% of patients who die from myocardial infarction may have experienced an episode of depression prior to the infarction (Lebovits et al. Citation1967; Greene et al. Citation1972; Glassman and Shapiro Citation1998). The presence of depression doubles the risk that patients with newly diagnosed cardiovascular disease will experience an adverse cardiovascular event within one year (Carney et al. Citation1988a). Depressed individuals are at a greater risk of death due to cardiac-related events for up to 10 years following the diagnosis of established cardiovascular disease, relative to non-depressed control subjects (Barefoot et al. Citation1996). Also, independent of risk factors such as arrhythmias and history of previous myocardial infarction, major depression is a significant predictor of mortality in patients at both 6 and 18 months following myocardial infarction (Frasure-Smith et al. Citation1993; Frasure-Smith et al. Citation1995). The predictive ability of depression on the occurence of subsequent cardiovascular events is similar to that of left ventricular dysfunction, history of previous myocardial infarction and smoking (Booth-Kewley and Friedman Citation1987; Frasure-Smith et al. Citation1993; Frasure-Smith et al. Citation1995), and the risk associated with this condition is independent of other cardiovascular risk factors including smoking, left ventricular ejection fraction, and severity of cardiovascular disease (Carney et al. Citation1988a).
Depression predicts the incidence of cardiovascular disease, as well as cardiac-related morbidity and mortality, in individuals with no prior history of cardiac pathophysiology. For instance, Carney and et al. (Citation1995a) have estimated that approximately one-half of patients who are depressed at the time cardiovascular disease is initially diagnosed have had one or more prior depressive episodes. Penninx and colleagues (Citation2001) showed that both major and minor depression increase the risk of cardiac-related mortality in patients without cardiac diseases at baseline, however, the excess mortality risk was twice as high for major depression versus minor depression. Anda et al. (Citation1993) showed that symptoms of depression, including depressed affect and moderate-to-severe hopelessness, predicted an increase in fatal and nonfatal heart disease in patients without a history of cardiac pathophysiology, independent of baseline medical variables, education, marital status, physical activity and smoking. Similarly, Barefoot et al. (Citation1996) showed that symptoms of depression predicted acute myocardial infarction and total mortality in a community sample of individuals. Furthermore, in this patient sample, the level of cardiovascular risk was directly related to the severity of depressive symptoms, suggesting that individual differences in vulnerability to psychopathology, pathophysiology, or both may be important in the link between depression and cardiovascular disease. Additional prospective studies, reviewed elsewhere (Rudisch and Nemeroff Citation2003), have found effects similar to those described here.
The role of exogenous stressors, neuroendocrine changes and immune dysfunction
Responsiveness to stressors interacts with neuroendocrine and immune function
The presence of exogenous stressors influences both mood and cardiovascular regulation. Specifically, chronic stressors, which do not favor behavioral or physiological adaptation, have been discussed in the context of depressive signs and symptoms (Anisman and Zacharko Citation1990; Grippo et al. Citation2003a) and cardiovascular dysregulation (Sgoifo et al. Citation2001). The predisposing influence of stressors on depression has been reviewed in detail elsewhere (Zacharko Citation1982, Citation1990, Citation1992; Swaab et al. Citation2005). Environmental stressors can lead to altered neurochemical function, such as disruptions in the synthesis and utilization of norepinephrine, changes in dopamine activity and altered synthesis of serotonin (5-HT) (Herman et al. Citation1982; Joseph and Kennett Citation1983; Irwin et al. Citation1986; Adell et al. Citation1988). Environmental stressors in humans, including marital conflicts, health problems and work overload, have been shown to be associated with depressive disorders (Bidzinska Citation1984). Additionally, exposure to acute stressors in rats leads to disrupted exploration, impaired escape performance and reduced appetitive responses to stimuli (Anisman and Zacharko Citation1992). These findings, considered together, provide a long history of evidence linking responsiveness to stressors with depressive syndromes. Hippocampal damage due to stress has been posited as a central nervous system mechanism leading to depressive symptoms via its influence on hypothalamic-pituitary-adrenal (HPA) axis activity (Vaidya Citation2000).
Stressors also contribute to cardiovascular diseases and their antecedent risk factors (see Knardahl et al. Citation1988; Johnson and Anderson Citation1990). There are several indices of interactions among environmental stressors and hypertension, arrhythmias, and sudden death. For example, behavioral stressors have been shown to lower the cardiac threshold for ventricular fibrillation (VF) in both normal and acutely ischemic hearts of dogs (Corbalan et al. Citation1974; Matta et al. Citation1976), which is a primary mechanism responsible for sudden cardiac death (Verrier and Lown Citation1984). Similarly, presenting food beyond the reach of a hungry animal has been shown to affect ventricular arrhythmias in the ischemic hearts of pigs (Carpeggiani and Skinner Citation1991). Environmental stressors also influence the pathogenesis of hypertension (see Sanders and Lawler Citation1992; Bedi et al. Citation2000).
While, stressors have been demonstrated to influence both mood and cardiovascular regulation, the specific central and peripheral mechanisms that contribute to these changes require further investigation. General and specific changes in neuroendocrine function may play a role in linking responsiveness to stressors, mood states, and cardiovascular function. Endocrine changes associated with depression – including activation of the HPA axis and abnormal feedback in this system – are similar to the body's physiological responses to stressors and have been linked directly or indirectly to cardiovascular regulation. The association of depressive syndromes with dysfunction of the hypothalamic-hypophysial axis was first described by Carroll (see Carroll Citation1976; Carroll et al. Citation1976a,Citation1976b). Alterations in corticotropin-releasing factor (CRF) level have been observed in the cerebrospinal fluid (Banki et al. Citation1992), hypothalamus (Raadsheer et al. Citation1995), and locus coeruleus (Bissette et al. Citation2003) of depressed patients. Similarly, central CRF administration induces depression- and anxiety-related behavorial changes both in rodents and non-human primates, including decreased food intake and sexual activity, disturbed sleep, altered motor behaviour, and impaired learning (Sirinathsinghji et al. Citation1983; Krahn et al. Citation1988; Glowa and Gold Citation1991; van Praag Citation2004). Several HPA axis-related changes, reviewed previously (Asnis et al. Citation1987; Maes et al. Citation1998; Weber et al. Citation2000), have been associated with depressive disorders, including: (a) dysregulated adrenocorticotropic hormone (ACTH) responses to CRF, (b) enhanced adrenal responses to ACTH, (c) elevated circulating cortisol or cortisone levels, and (d) lack of cortisol suppression in response to dexamethasone. Similar changes, including: (a) alterations in corticosterone and ACTH secretion, (b) impaired feedback control of HPA axis functioning, (c) impaired glucocorticoid receptor binding (increased mRNA expression and density of binding sites) in the hippocampus, cortex and dorsal raphe nucleus, and (d) altered CRF input to the dorsal raphe nucleus, have been observed in several validated animal models of depression (Froger et al. Citation2004; Maier and Watkins Citation2005; Grippo et al. Citation2005a,Citation2005b) [but also see (Azpíroz et al. Citation1999) for negative findings regarding circulating corticosterone levels]; some of these changes are further discussed in the following section.
The endocrine system interacts with the immune system; several of these interactions are common to mood disorders and heart disease and thus might play a role in the association of these conditions. Smith (Citation1991) initially proposed a macrophage theory of depression, suggesting that excessive secretion of monokines such as interleukin (IL)-1, tumor necrosis factor alpha (TNF-α) and interferon, contribute to the pathophysiology of depression. Several additional lines of evidence, reviewed in detail elsewhere (Maes Citation1995; Connor and Leonard Citation1998; Zorrilla et al. Citation2001; Pollak and Yirmiya Citation2002; Kronfol Citation2002; Dunn et al. Citation2005; Dantzer Citation2006; Hawkley et al. Citation2007), suggest that dysfunction of the immune system is associated with depression; however, the precise pathophysiological mechanisms that link immune dysregulation and depressive syndromes are not clear. TNF-α and interferon-α administered to humans induce depressive signs such as fatigue, malaise, lethargy, psychomotor retardation, irritability, and anorexia (Niiranen et al. Citation1988; Spriggs et al. Citation1988). Similar changes have been observed following administration of IL-1β (Cunningham and De Souza, Citation1993). Interferons and interleukins, such as IL-1β and IL-6, have been reported to be increased in the plasma of some depression patients, however, these changes have not been observed consistently across all studies (Dunn et al. Citation2005).
Experimental evidence, derived from non-human animal studies, may provide additional insight into the associations among immune dysregulation, the central nervous system, and depressive signs. Activation of the immune system, and particularly activation of pro-inflammatory cytokines, are associated with sickness behaviour, which includes signs of fatigue, anhedonia, anorexia, and reduced social interactions (Dantzer et al. Citation1998; Wichers and Maes Citation2002; De La Garza II, Citation2005; Dantzer Citation2006). As these signs in animals resemble to some extent depressive signs in humans, sickness behaviour may be a useful analog for investigating the precise role of cytokines in depression and cardiovascular dysregulation.
Activation of the immune system is linked directly to specific cardiovascular disorders. In the setting of acute myocardial infarction, the pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 are released into the systemic circulation (Das Citation2000). These have adverse affects on the heart and circulation (Kapadia et al. Citation1998; Ferrari Citation1999; Francis et al. Citation2004) and may act on the central nervous system in parallel to induce signs and symptoms of depression and endocrine and autonomic dysregulation. Peripheral cytokines influence the release and metabolism of several central nervous system neurotransmitters, including dopamine (Jarskog et al. Citation1997), norepinephrine (Kabiersch et al. Citation1988) and 5-HT (Clement et al. Citation1997). These neurotransmitters are involved in sympathetic nerve outflow to the cardiovascular system (Huangfu et al. Citation1994), and have also been implicated in the pathogenesis of depression (Nosjean et al. Citation1995) (see Role of central neurotransmitter systems section below for further discussion of central neurotransmitter dysregulation). Macrophages secrete ACTH and can influence HPA axis function (Nathan Citation1987). In addition, IL-1, which is produced by macrophages, directly acts on the hypothalamus and pituitary to regulate CRF and ACTH (Dunn et al. Citation1999). IL-6 and exogenous interferon-γ also activate the HPA axis (Cassidy and O'Keane Citation2000).
In addition to the immune system, the renin–angiotensin–aldosterone system (RAAS) is activated in some forms of heart disease such as CHF, resulting in high circulating levels of angiotensin II and aldosterone (Felder et al. Citation2001). Angiotensin and the pro-inflammatory cytokines both activate the HPA axis to increase circulating glucocorticoid and catecholamine levels (Wright et al. Citation1995; Pollak and Yirmiya Citation2002). These two stress-associated humoral systems interact in the brain, where aldosterone stimulates circulating TNF-α levels, and in the periphery, where cytokines prevent the feedback inhibition of renin release by circulating angiotensin II (Felder et al. Citation2001; Francis et al. Citation2001a).
Notably, the immune system and the RAAS also are activated in affective states and conditions of stress in the absence of cardiovascular disease (Connor and Leonard Citation1998; Pollak and Yirmiya Citation2002). For instance, circulating aldosterone levels were found to be elevated in a sample of patients who met formal criteria for major or minor depression (American Psychiatric Association Citation2000), compared to age- and gender-matched controls; plasma renin levels showed a slight (but nonsignificant) trend toward being elevated in this sample of depressed patients (Emanuele et al. Citation2005). Murck et al. (Citation2003) similarly suggest that increased aldosterone levels may be a sensitive marker of depression. The interactions of aldosterone with mineralocorticoid receptors in the brain and periphery may lead to adverse outcomes in depressed patients with a risk of cardiovascular dysfunction. Aldosterone can stimulate increased sympathetic drive and activation of pro-inflammatory cytokines, promoting vascular injury, endothelial dysfunction, myocardial necrosis, catecholamine release and cardiac arrhythmias (Stier et al. Citation2002; Francis et al. Citation2003a; Gomez-Sanchez Citation2004). Similarly, blockade of mineralocorticoid receptors with spironolactone reduces both sympathetic drive and circulating TNF-α levels in rats with experimental CHF (Francis et al. Citation2001a; Francis et al. Citation2003b), lowers mortality in humans with severe CHF (Pitt et al. Citation1999), and may improve cardiovascular status in patients with mild CHF symptoms (Baliga et al. Citation2006). These data suggest a potential pathophysiological role for central mineralocorticoid receptors in mediating autonomic and cardiovascular dysfunction in the context of cardiovascular diseases such as CHF (see Role of the autonomic nervous system section below for further discussion of autonomic dysfunction).
Stressor responsiveness interacts with neurohumoral, endocrine, and immune function in valid rodent models of disease
Studies from our laboratories have examined the role of stressor responsiveness, behaviour, neuroendocrine dysfunction, and immune alterations both in a rodent model of depression [chronic mild stress (CMS); (Willner Citation1997c; Willner Citation2005)] and in a rodent model of cardiovascular disease [experimental CHF; (Francis et al. Citation2001b; Felder et al. Citation2003)]. These investigations have provided insight into endocrine and immune changes that are common to both depressive disorders and cardiovascular diseases. In a recent study involving the CMS model of depression, we have experimentally validated the hypotheses of neuroendocrine and immune dysfunction in depression (Grippo et al. Citation2005a). Relative to an undisturbed control group, exposure of adult male rats to 4 weeks of CMS – mild and unpredictable stressors such as strobe light, white noise and damp bedding (e.g., ) – induced experimental anhedonia (reduced sucrose intake), and also led to HPA axis dysfunction, activation of the RAAS, and activation of pro-inflammatory cytokines. For instance, circulating corticosterone, aldosterone levels and plasma renin activity were elevated in the CMS group versus the unstressed control group. Furthermore, the pro-inflammatory cytokines TNF-α and IL-1β were elevated both in plasma and in the central nervous system of CMS rats (). Interestingly, in this study the cytokine levels also were systematically correlated with the degree of anhedonia in CMS rats; that is, a higher degree of anhedonia (lower level of sucrose intake) was correlated with higher levels of TNF-α and IL-1β. These relationships indicate that it might be useful to investigate individual differences in the behavioral and physiological vulnerability to environmental stressors using rodent models of depression.
Figure 1 Example of a chronic mild stress paradigm. Reprinted from (Grippo et al. Citation2006a); used with permission.
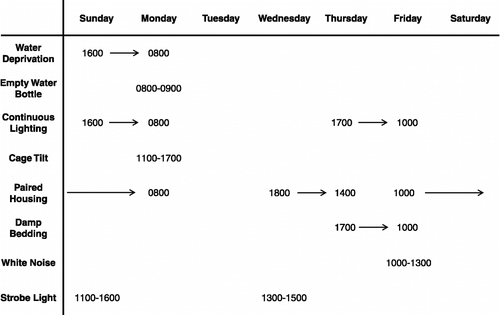
Figure 2 Mean ( + SEM) levels of tumor necrosis factor alpha (TNF-α) (Panels A and C) and interleukin-1beta (IL-1β (Panels B and D) in blood plasma (Panels A and B) and central nervous system (Panels C and D) in CMS and control groups following 4 weeks of CMS. Concentrations of both cytokines were increased in the plasma and specific central nervous system structures (*P < 0.05 vs. respective control value; #P < 0.1 vs. respective control value). Adapted from (Grippo et al. Citation2005a); used with permission.
The findings from this study suggest that interactions of cytokines and the RAAS with central processes, such as activation of the HPA axis, may be important mechanisms that influence both cardiovascular function and affective states. This intercommunication can influence downstream functions such as the release of corticosterone or catecholamines, regulation of circulating pro-inflammatory cytokines, and alterations in renin and aldosterone secretion (Bataillard et al. Citation1992; Wichers and Maes Citation2002; Francis et al. Citation2003b), in turn providing a potential physiological link between affective states and cardiovascular dysfunction.
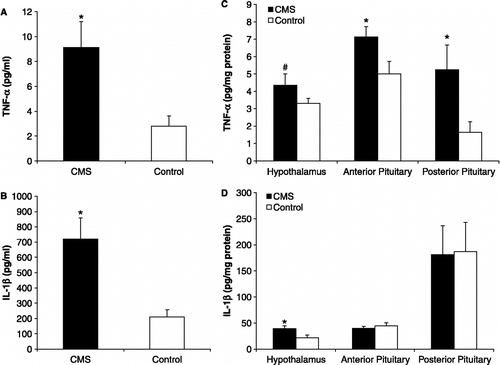
Although pro-inflammatory cytokine levels are elevated both in mood disorders and cardiovascular diseases, it is not clear whether these changes are a cause or consequence of the conditions. Therefore, we sought to examine the role of TNF-α in mediating a depression-like behaviour in adult male rats with CHF, which involved experimental occlusion of the left descending coronary artery (Grippo et al. Citation2003b). Serum levels of TNF-α are increased in rats with experimental CHF (Francis et al. Citation2004) and may be increased in humans with CHF associated with ischemic heart disease (Levine et al. Citation1990). This cytokine has been shown to contribute in part to left ventricular dysfunction, cardiomyopathy and pulmonary oedema (Kapadia et al. Citation1998). Similar to previous studies (Felder et al. Citation2003; Francis et al. Citation2003b), we found that experimental CHF, compared to sham occlusion procedures, produced a significant increase in circulating TNF-α levels. Furthermore, experimental CHF led to a significant reduction in responding for rewarding electrical brain stimulation in the lateral hypothalamus, indicative of anhedonia. When plasma TNF-α levels were lowered with the TNF-α antagonist etanercept (Adis International Limited Citation1999), the behavioral responding for rewarding electrical brain stimulation was restored to baseline values, indicating a reversal of the CHF-induced anhedonia (). These results suggest that CHF can induce anhedonia via a physiological mechanism involving activation of TNF-α.
Figure 3 Current-response functions illustrating the number of behavioral responses per minute at each level of standardized current (Panel A), and corresponding effective current 50 (ECu50) values showing the standardized current intensity that supports 50% of the maximum response rate (Panel B), in rats with CHF before and 24 h following etanercept (TNF-α antagonist) or vehicle treatment. CHF rats treated with vehicle displayed a parallel rightward shift in the current-response function and a corresponding increase in ECu50 value; CHF rats treated with etanercept displayed values similar to this group's respective baseline values and those of the control group (*P < 0.05 vs. Baseline Vehicle; #P < 0.05 vs. CHF + Etanercept). Modified from (Grippo et al. Citation2003b); used with permission.
Central TNF-α was not measured in this study, however it is possible that its behavioral effects were due in part to its actions in the brain. Receptors for TNF-α and IL-1, IL-2 and IL-6 have been found in the hippocampus and hypothalamus (Hopkins and Rothwell Citation1995; Rothwell and Hopkins Citation1995). The precise pathways of communication between the heart and brain involving the pro-inflammatory cytokines have not been elucidated and various routes have been proposed. For example, cytokines produced in the periphery may gain access to the central nervous system by way of the circumventricular organs, through the blood–brain barrier by selective saturable transport systems, actions on brain perivascular cells, or via neurally-mediated mechanisms involving visceral sensory nerves (Dantzer Citation1994; Banks et al. Citation1995; Maier and Watkins Citation1998; Schlitz and Sawchenko Citation2002; Felder et al. Citation2003). TNF-α may act directly or indirectly to affect neurotransmitters such as dopamine and 5-HT that are involved in reward mechanisms and the display of depressive signs, including dopamine and 5-HT signs (Connor et al. Citation1998; Dunn et al. Citation1999).
Role of the autonomic nervous system
Autonomic dysfunction influences cardiac changes associated with depression
Activation of neuroendocrine systems, manifests in both generalized and specific HPA axis dysfunction and RAAS activation, can induce autonomic nervous system changes that are common to both depression and cardiovascular disease. Depression may be characterized by changes in autonomic regulation of the heart, such as activation of the sympathetic nervous system, withdrawal of cardiac vagal tone, elevations in heart rate, reductions in heart rate variability, and altered baroreceptor reflex function (Esler et al. Citation1982; Rechlin et al. Citation1994a,Citation1994b; Carney et al. Citation1995b; Krittayaphong et al. Citation1997; Watkins and Grossman Citation1999; Pitzalis et al. Citation2001; Barton et al. Citation2007; Hausberg et al. Citation2007). Similar autonomic changes are associated with cardiovascular risk factors such as hypertension, increased body mass index and increased blood glucose level, and are observed in both acute and chronic cardiovascular conditions including atherosclerosis, myocardial ischemia, arrhythmias and heart failure (Ryan et al. Citation1976; Dyer et al. Citation1980; Gordon et al. Citation1981; Billman et al. Citation1982; Beere et al. Citation1984; Kannel et al. Citation1987; Schwartz et al. Citation1988; Carney et al. Citation1993; Kristal-Boneh et al. Citation1995; Palatini and Julius Citation1997; La Rovere et al. Citation1998; Esler and Kaye Citation2000; Tapanainen et al. Citation2002).
Autonomic imbalance is characterized by elevated sympathetic tone, decreased parasympathetic tone, or both. Previous research suggests that increased sympathetic and/or decreased parasympathetic tone are predisposing factors for ventricular fibrillation (VF) (Lown and Verrier Citation1976; Kjekshus et al. Citation1981; Kleiger et al. Citation1987). Disrupted autonomic balance can mediate cardiovascular states during behavioral challenges. Sgoifo et al. (Citation1998) showed that autonomic imbalance, characterized by exaggerated sympathetic tone and lower parasympathetic antagonism of sympathetic activation, may mediate ventricular arrhythmias during an acute social challenge (resident-intruder test).
Activation of the sympathetic nervous system and withdrawal of the parasympathetic limb lead to elevations in resting heart rate. An increase in basal heart rate is a prognostic indicator for morbidity and mortality related to heart disease (Ferrari et al. Citation2003). Elevated heart rate has been observed in depressed patients both with and without cardiovascular disease (Forbes and Chaney Citation1980; Carney et al. Citation1988b). Both hypertensive (Goldstein Citation1983) and normotensive (Lechin et al. Citation1995) depressed patients have been shown to exhibit higher heart rates than nondepressed individuals. Also, patients with both depression and cardiovascular disease display higher heart rates than nondepressed patients with cardiovascular disease (Carney et al. Citation1993). Heart rate may be altered in depression via norepinephrine and epinephrine acting on cardiac β-adrenergic receptors at the sinoatrial node, or from an increased sensitivity of β-adrenergic receptors in the heart (Kannel et al. Citation1987). While not all studies in depressed populations have shown significant end-organ changes, such as increases in heart rate (see Dawood et al. Citation2007; Barton et al. Citation2007), it is likely that the neural control of cardiac function is altered in depression. Indeed, increased catecholamine levels have been reported in the plasma and cerebrospinal fluid of patients with melancholic depression. Gold et al. (Citation2005) have observed significant increases in mean 24-h levels of cerebrospinal fluid norepinephrine, plasma norepinephrine, and plasma epinephrine in severely depressed patients versus controls. The authors also reported that plasma norepinephrine levels in severely depressed, unmedicated patients were high enough to be capable of increasing mortality in CHF. Also important, even mild-to-moderately depressed patients in this study displayed clinically relevant increases in arterial norepinephrine appearance rate. An elevation in spillover indicates increased secretion.
Neural control of autonomic and cardiac function associated with depression may also be present in the form of altered heart rate variability. A reduced variability in heart rate is a sign of impaired autonomic control of cardiovascular regulation. For example, a decreased variability in heart rate is found in patients with cardiovascular disease (Ryan et al. Citation1976; Kristal-Boneh et al. Citation1995), and has prognostic value for predicting outcomes in myocardial infarction and CHF (Kleiger et al. Citation1987; Wolk Citation1996; Tapanainen et al. Citation2002).
Heart rate variability has been shown to be reduced in depressed patients both with and without cardiovascular disease, compared to nondepressed individuals (Rechlin et al. Citation1994a,Citation1994b; Carney et al. Citation1995b; Pitzalis et al. Citation2001), however some studies have reported negative findings in this variable (Yeragani et al. Citation1991; Dawood et al. Citation2007). Differences in heart rate variability reported across studies may be related to the severity of depressive symptoms. For example, patients with higher depression scores on the Minnesota Multiphasic Personality Inventory-Depression (MMPI-D) showed lower heart rate variability than patients with lower scores on this instrument (Krittayaphong et al. Citation1997). Recent pharmacological data suggest that central serotonergic mechanisms may influence heart rate variability in depressed individuals (Khaykin et al. Citation1998). Furthermore, treatment of depressed patients with 5-HT reuptake inhibitors has been reported to lower sympathetic activation, evidenced by a reduction in whole body norepinephrine spillover (Barton et al. Citation2007). However, some studies have reported a lack of significant cardiovascular changes (neither positive nor negative) in depressed patients treated with 5-HT reuptake inhibitors (Nemeroff et al. Citation1998; Roose et al. Citation1998a,Citation1998b), whereas others have reported adverse effects (Dawood et al. Citation2007).
Cardiac reflex mechanisms, mediated in part by the autonomic nervous system, are important in regulating cardiovascular function, and may play a role in the link between cardiovascular disease and depression. A reduction in baroreceptor reflex (i.e., baroreflex) sensitivity has been shown to differentiate high- from low-risk patients recovering from myocardial infarction and CHF (Mortara et al. Citation1997). Also, the combination of both reduced baroreflex sensitivity and reduced heart rate variability was of additional prognostic value in a sample of post-myocardial infarction patients, compared to that of either marker alone (La Rovere et al. Citation1998). In animal models of heart disease, reduced baroreflex sensitivity is associated with an increased risk of VF during a brief ischemic episode (Billman et al. Citation1982; Schwartz et al. Citation1988). Also, coronary artery occlusion attenuates the baroreflex control of heart rate both in anesthetized dogs and in humans (Trimarco et al. Citation1987; Airaksinen Citation1999).
A few studies have demonstrated that depression in humans may be associated with reduced baroreflex sensitivity. Watkins and Grossman (Citation1999) reported that baroreflex sensitivity was altered in cardiac patients with depression. However, baroreflex dysfunction in depressed individuals has not been consistently demonstrated in all studies. A more recent study from Watkins et al. (Citation2002) found no association of baroreflex sensitivity with symptoms of depression in a sample of patients following acute myocardial infarction, however, symptoms of anxiety were associated with reduced baroreflex sensitivity in this patient sample. It is possible that symptoms of anxiety may mediate the apparent link between depression and altered baroreflex sensitivity that has been reported in some studies. However, Dawood et al. (Citation2007) showed no association between state or trait anxiety and altered baroreflex function in patients with major depression. Therefore, further studies are required to test this hypothesis. An increase in cardiac sympathetic tone may be an important mechanism underlying the reduced baroreflex sensitivity in depression, as reduced baroreflex sensitivity has been observed in unmedicated depressed patients, but not in depressed patients treated with β-adrenergic receptor antagonists, following myocardial infarction (Pitzalis et al. Citation2001).
Autonomic and baroreflex mechanisms are associated with depressive signs in animal models
The discrepant findings from human studies regarding the specific autonomic and cardiac disturbances associated with depression highlight the need for well-controlled, experimental investigations involving valid and reliable animal model systems. Because the CMS model of depression possesses both face and predictive validity (Willner Citation1997a; Citation1997c), it is a useful tool for investigating autonomic and cardiac dysfunction associated with depressive signs.
Recent studies with the CMS model indicate that specific autonomic mechanisms are likely to link depressive signs and cardiovascular dysregulation. Compared to unstressed control groups, exposure to 4 weeks of CMS in adult male rats induced several basal cardiac alterations, including elevated resting heart rate and reduced heart rate variability (Grippo et al. Citation2002, Citation2003a). Furthermore, when animals were perturbed with air jet stress (a novel stressor), we observed exaggerated pressor and heart rate reactivity in rats that were previously exposed to CMS versus the respective control groups. While, the behavioral changes associated with CMS are shown to recover within a few weeks following cessation of the CMS protocol, these cardiovascular disruptions persist for a longer period, suggesting that simple removal of the depressive signs is not associated with alleviation of the underlying cardiovascular pathophysiology. Consistent with these findings are those from Carney et al. (Citation2000), who suggest that while heart rate and heart rate variability may improve in treated depressed patients, they may never return to baseline levels.
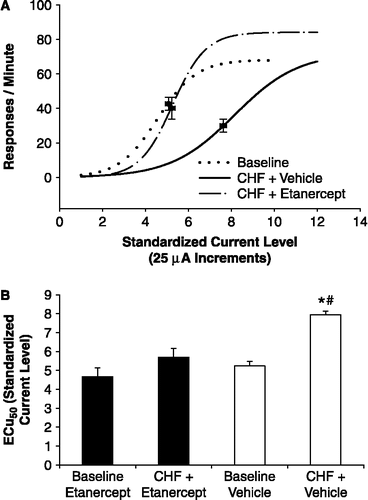
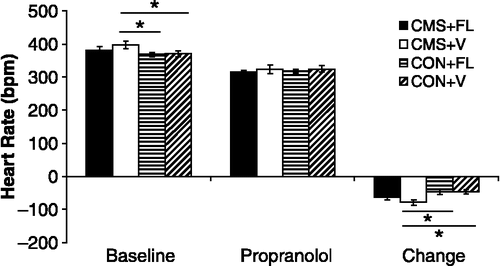
Based on our initial experiments, we have concluded that exposure to CMS induces several basal cardiac changes and exaggerated cardiovascular reactivity to environmental stressors. The elucidation of the underlying mechanisms for these changes will be extremely useful for developing novel and effective treatments for patients with depression and heart disease. Thus, we have investigated specifically the potential autonomic and cardiac mechanisms underlying these basal and stress-reactive changes in the CMS model, showing that the cardiac disturbances associated with CMS are mediated by elevated sympathetic drive (Grippo et al. Citation2002, Citation2003a). Protocols involving selective pharmacological autonomic blockade have demonstrated that CMS is associated with a greater attenuation of heart rate following β-adrenergic receptor blockade with propranolol (2 mg/kg, i.v.), relative to an unstressed control group (). β-Adrenergic receptor blockade with propranolol also abolished heart rate variability differences between CMS and control groups. These results suggest that cardiac sympathetic tone is elevated in CMS, mediating directly the elevated basal heart rate and heart rate variability changes observed in this animal model of depression.
Figure 4 Mean ( + SEM) absolute resting heart rate (HR; bpm: beats per minute; Panel A) and change in HR from baseline (Panel B) following selective and combined autonomic blockade (propranolol: β-adrenergic receptor antagonist; methylatropine: antagonist) cholinergic receptor in CMS and control groups. The CMS group displayed a greater bradycardia in response to propranolol administration, compared with the control group (*P < 0.05 vs. respective control value). Reprinted from (Grippo et al. Citation2002); used with permission.
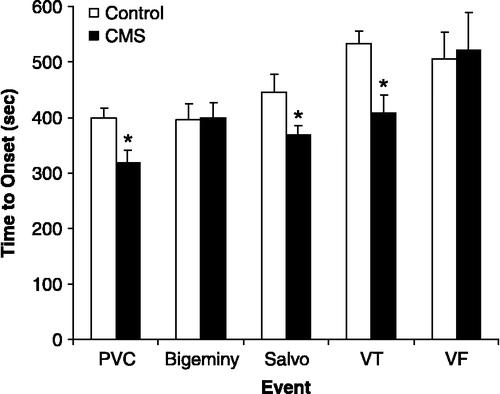
We have likewise investigated a potential cardiac mechanism, vulnerability to ventricular arrhythmias, in the link between depressive signs and cardiac dysregulation. Using the CMS model of depression, we examined the types and time course of ventricular arrhythmia development associated with anhedonia (Grippo et al. Citation2004). This study was conducted with an experimental protocol involving intravenous administration of aconitine, a pro-arrhythmic agent that induces ventricular arrhythmias by preventing the inactivation of sodium channels following generation of the action potential (Catterall and Ray Citation1976; Honerjager and Meissner Citation1983). Following exposure to 4 weeks of CMS or control conditions, aconitine was infused into anesthetized male rats over a period of several minutes, and the time to onset of different arrhythmias was recorded. Both simple premature ventricular contractions (PVC) and complex (salvos and ventricular tachycardia (VT)) arrhythmias developed earlier in the CMS group relative to the control group (). A re-examination of these data also indicates that a larger percentage of animals in the CMS group exhibited VT versus those in the control group in response to aconitine administration (80 versus 42%, respectively), suggesting that CMS is associated with an increased susceptibility to ventricular arrhythmias when the cardiovascular system is perturbed. These results are especially important when considered in the context of findings from human populations. Carney et al. (Citation1993) showed that patients with coronary artery disease and depression had a higher prevalence of VT versus those patients without corresponding depression. Similarly, post-myocardial infarct patients are at a greater risk of mortality if they have a combination of PVC (greater than 10 per hour) and a high score on the Beck Depression Inventory (BDI) relative to patients with fewer PVC or those with a low BDI score (Frasure-Smith et al. Citation1995). Taken together, the findings from humans and rodents suggest that signs and symptoms of depression may be directly associated with ventricular electrical instability, which can in turn influence cardiovascular function and disease outcomes.
Figure 5 Mean ( + SEM) time to the onset of PVC, bigeminy, salvos, VT, and VF following intravenous aconitine (a pro-arrhythmic agent) administration in control and CMS rats. CMS reduced the onset time to PVC, salvo and VT (*P < 0.05 vs. respective control value) following 4 weeks of CMS. Modified from (Grippo et al. Citation2004); used with permission.
Role of behavioral mechanisms
Behavioral mechanisms, including physical inactivity, may underlie depression and heart disease
Common behavioral mechanisms, only some of which can be modeled in animals, can underlie mood disorders, altered cardiovascular function, and potentially the links between these conditions. While not an exhaustive list, research from human studies and a limited number of non-human animal studies suggests that potential behavioral mechanisms can include: (a) poor adherence to medical regimens (Bollini et al. Citation2006; Rieckmann et al. Citation2006; Fenton and Stover Citation2006), (b) negative health behaviours such as smoking, alcohol use or poor diet (Everson-Rose et al. Citation2004; Pelloux et al. Citation2005; Fenton and Stover Citation2006), and (c) fatigue or physical inactivity (Appels and Mulder Citation1988; van Deist and Appels Citation1991; Bruce et al. Citation1994; Mendes de Leon et al. Citation1998; Kop Citation1999). For the purpose of the present discussion, we will focus on physical inactivity as a behavioral mechanism that is common to both mood disorders and cardiovascular disease.
Chronic fatigue – a syndrome which can be characterized by persistent fatigue that is not substantially relieved by rest, sleep that is not refreshing, poor memory or concentration, frequent or recurring sore throat, headache of a new type, swelling of lymph nodes, muscle or joint pain, and/or depression (Straus et al. Citation1985; Centers for Disease Control and Prevention and National Center for Infectious Diseases Citation2001) – is sometimes found in patients with depression (Puffer and McShane Citation1992). Some of the behavioral similarities of depression and chronic fatigue syndrome suggest that these two conditions may share aspects of the same physiologic abnormalities. The antidepressant fluoxetine, for instance, is a successful treatment for some chronic fatigue patients (Klimas et al. Citation1993). Chronic fatigue syndrome may occur in individuals with a premorbid vulnerability to depression; however, psychological disturbances may be a consequence, rather than a cause, of chronic fatigue (Hickie et al. Citation1990).
Excess fatigue, particularly in elderly individuals, may play an important underlying role in the association of altered mood and cardiovascular dysregulation. In a study comparing the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) classification of major depression with exhaustion in American patients with cardiovascular disease, over half of the exhausted patients did not meet criteria for depression, whereas nearly all patients meeting criteria for depression also met criteria for exhaustion (Kop Citation1999). Somatic symptoms of depression, including fatigue, may be responsible for affecting daily functioning in depressive disorders (Bruce et al. Citation1994). Also, the relationship between depressive symptoms and physical functioning may play an important role in the risk of cardiovascular disease in older women but not in older men (Mendes de Leon et al. Citation1998).
Excess fatigue also may be a precursor to myocardial infarction and sudden cardiac death (Rissanen et al. Citation1978; Appels and Mulder Citation1988). “Vital exhaustion” includes loss of vitality and libido, listlessness, tiredness and increased irritability (Appels and Mulder Citation1988; van Deist and Appels Citation1991). Exhaustion may be a short-term risk factor for recurrent myocardial infarction, and is predictive of future myocardial infarction independent of blood pressure, smoking, cholesterol, age and the use of antihypertensive medications (Appels and Mulder Citation1988). The possibility that fatigue and physical inactivity influence the relationship between depression and cardiovascular disease is supported by the finding that physical exercise is often included as a component of treatment programs for both depression (Perski et al. Citation1999; Paluska and Schwenk Citation2000) and heart disease (McCartney Citation1998; Braith Citation1998). Strength training has been shown to improve both mood and self-esteem in cardiac rehabilitation patients (Beniamini et al. Citation1997). Further, exercise training has been shown to significantly improve heart rate variability, baroreflex sensitivity, blood pressure and vascular function (Kristal-Boneh et al. Citation1995; Collins and DiCarlo Citation1997; Zoeller Citation2007), which has implications for both cardiovascular disease and depression.
Some (but not all) patients with chronic fatigue show similar immune deficiencies to depressed patients, including elevated levels of cytokines (IL-1 and TNF-α), reduced natural killer cytotoxicity and impaired cell-mediated immunity (Klimas et al. Citation1990; Partarca et al. Citation1993). Additionally, some immune abnormalities in chronic fatigue patients are normalized following fluoxetine treatment (Klimas et al. Citation1993). Further, IL-1 has been shown to cause prolonged slow-wave sleep in both rats (Tobler et al. Citation1984) and rabbits (Krueger et al. Citation1984). These findings indicate that the condition of fatigue is associated with immune dysfunction, which may mediate both depressive signs and cardiovascular dysfunction. However, it will be necessary to systematically investigate interactions of fatigue and activity level, mood states, and cardiovascular function to elucidate the precise immunological pathways involved in this link.
Physical inactivity and neurohumoral changes are observed in animal models
Previous studies from our laboratory have attempted to delineate the mechanisms underlying the associations among behavioral signs of depression, cardiovascular dysfunction, and fatigue or physical inactivity. For instance, in the CMS model, we have observed a reduction in spontaneous locomotor activity in adult male rats exposed to 4 weeks of CMS (Grippo et al. Citation2003b). This behavioral sign recovers approximately 3 weeks following cessation of the CMS protocol, which is similar to the time course in recovery of disrupted appetitive responses for rewarding stimuli (anhedonia) in the CMS model.
Physical inactivity may lead directly or indirectly to changes in autonomic and cardiovascular function. However, it is also possible that depression and heart disease share common features that are manifest in fatigue or associated with changes in physical activity. By using a model of disrupted sodium homeostasis, we have studied RAAS mechanisms associated with behavioral signs of depression and cardiovascular dysfunction (Grippo et al. Citation2006b). Disruption of body sodium and water balance has many behavioral, endocrine and autonomic effects (Johnson and Thunhorst Citation1997, Citation2007). We demonstrated that sodium depletion in rats, induced by two injections of the diuretic agent furosemide combined with a sodium-deficient diet, reduced responding for rewarding electrical brain stimulation in the lateral hypothalamus, indicative of anhedonia. In a parallel experiment, the same sodium depletion paradigm led to a significant elevation of resting heart rate and blood pressure, and a reduction in heart rate variability. Related research suggests that rats with experimental CHF display a sodium appetite (Francis et al. Citation2001b). In the clinical setting, hyponatraemia has been reported in patients with CHF, and has been shown to possess prognostic value for cardiovascular outcomes (LeJemtel and Serrano Citation2007; Gheorghiade et al. Citation2007a,Citation2007b). Further, improved outcomes in acute heart failure are related to an improvement of circulating sodium levels (Rossi et al. Citation2007). These data provide additional evidence for the interrelatedness of sodium intake and balance, activation of the RAAS and cardiovascular regulation.
Activation of the RAAS may play a role also in the hedonic deficit in animals with a sodium deficit. For instance, in a related series of experiments, administration of the mineralocorticoid agonist deoxycorticosterone acetate (DOCA), which induces a sodium appetite in the absence of any sodium deficiency, produced anhedonia in rats measured via two independent operational tests (responding for lateral hypothalamic rewarding electrical brain stimulation and ingestion of 2% sucrose) (Morris et al. Citation2006). Of particular importance in this study was the finding that DOCA-treated rats that were allowed ad libitum access to saline did not significantly reduce their responding for either rewarding electrical brain stimulation or sucrose, suggesting that a persistent sodium appetite (“craving” for sodium) may be capable of altering the behavioral sensitivity to other rewarding stimuli. Consistent with this hypothesis are findings from a study conducted by Willner et al. (Citation1998), showing that induction of depressive signs in both humans and rats led to an increased appetite (“craving”) for sweet rewards.
Role of central neurotransmitter systems
Central neurotransmitter system dysregulation is associated with depression and altered cardiovascular regulation
Central nervous system processes are ostensibly changed in both mood disorders and cardiovascular disease; evidence for some of these changes has been discussed in the preceding sections. Here we discuss the role of specific neurotransmitter system dysfunctions, with evidence derived primarily from pharmacological and anatomical studies.
Disrupted norepinephrine and dopamine function is implicated in the pathophysiology of depression (Lambert et al. Citation2000; Garlow and Nemeroff Citation2004), and increased norepinephrine levels may play a role in the elevated risk of CHF in some depressed patients (Gold et al. Citation2005). Treatment with the antihypertensive drug reserpine, which depletes monoamine stores, may induce depression (Duman Citation1999). Also, monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) increase synaptic levels of monoamines in the central nervous system. Individuals who are dexamethasone test non-suppressors, as compared to suppressors, exhibit higher basal plasma concentrations of norepinephrine and exaggerated levels of norepinephrine in response to a cold challenge (Roy et al. Citation1987). Pharmacological agents that affect central dopaminergic actions also are effective antidepressants (Muscat et al. Citation1992; Cheeta et al. Citation1994; Willner Citation1995; Willner Citation1997b; Foley et al. Citation2006). It is likely, therefore, that the pathophysiology of depression involves dysregulation of some monoamine systems in the brain. The precise central mechanisms involved in the pathogenesis of depressive syndromes, however, are not well understood.
For several years, research has focused on the role of the serotonergic system in depression. As reviewed elsewhere (Meltzer Citation1990; Maes and Meltzer Citation1995; Lucki Citation1998; Ressler and Nemeroff Citation2000), it has been reported that: (a) 5-HT plays a significant role in behaviours that are disrupted in depression, such as mood, sleep and appetite, (b) a decrease in brain 5-HT concentration can precipitate depression in recovering patients, and (c) depression is associated with multiple changes in central 5-HT metabolism and 5-HT receptors, including decreased tryptophan concentrations, impaired 5-HT synthesis or release, changes in 5-hydroxyindoleacetic acid, malfunctions at postsynaptic 5-HT receptors and alterations in 5-HT transporter density. Furthermore, several classes of pharmacological antidepressants, including MAOIs, TCAs and 5-HT reuptake inhibitors, as well as electroconvulsive shock therapy, increase serotonergic neurotransmission (Owens and Nemeroff Citation1994; Berman et al. Citation1999), and produce specific antidepressant-like effects in drug-screening models of depression such as the forced swim test (Cryan et al. Citation2005).
However, the precise central nervous system mechanisms of antidepressant treatments, and exactly how they act to alter depressive signs and symptoms, are not fully understood. Impaired function at the level of 5-HT type 1A (5-HT1A), 5-HT type 1B (5-HT1B) and 5-HT type 2 (5-HT2) receptors has been identified in several neurological and psychological disorders, including depression (Roth Citation1994; Lucki Citation1998). For instance, an enhancement of 5-HT2 receptor function has been described in depressed individuals (Mikuni et al. Citation1991), which may result from an increase in affinity or concentration of these receptors (Pandey et al. Citation1990; Kusumi et al. Citation1991). 5-HT2C receptor gene polymorphisms and altered editing of 5-HT2C receptors have been implicated in the pathophysiology of affective disorders (Lerer et al. Citation2001; Gutiérrez et al. Citation2001). Recent studies with a transgenic mouse model mis-expressing an RNA editing enzyme that affects the 5-HT2 receptor indicate changes on behavioral tests that are consistent with endogenous depression and anxiety (Singh and Johnson Citation2007). Also, it has been suggested that an impaired balance between 5-HT1A and 5-HT2A receptors may underlie depressive disorders (Berendsen Citation1995; Blier Citation2001).
5-HT reuptake inhibitors alter 5-HT neurotransmission and 5-HT receptor function in the brain. However, the effects of 5-HT reuptake inhibitors may differ depending on the brain region and the method of measurement. Daily injections of the 5-HT reuptake inhibitor fluoxetine for 3 weeks enhanced 5-HT synthesis and turnover in the hypothalamus, hippocampus and frontal cortex of the mouse brain; the mechanism for these changes may involve alterations at 5-HT1B autoreceptors (Stenfors and Ross Citation2002). In a more recent study, treatment of panic disorder patients with a 5-HT reuptake inhibitor led to a decrease in 5-HT turnover, using a measure of whole-brain turnover levels via 5-hydroxyindoleacetic acid spillover (Esler et al. Citation2007). Research from Van de Kar and colleagues (Van de Kar Citation1989; Zhang et al. Citation2000; Sullivan Hanley and Van de Kar Citation2003) suggests that functional changes at postsynaptic 5-HT1A receptors are important in the mechanisms underlying 5-HT reuptake inhibitors and in the development of depressive signs and symptoms. These functional changes may provide a partial explanation for the delay in clinical effectiveness in some depressed individuals, despite potentially rapid inhibition of the 5-HT transporter with 5-HT reuptake inhibitors.
The serotonergic system interacts with endocrine and autonomic function to influence cardiovascular regulation. In addition to their antidepressant properties, drugs that alter brain 5-HT function can alter circulating concentrations of ACTH and cortisol or corticosterone (Fuller Citation1996; Murphy et al. Citation1996). These effects are likely mediated by serotonergic innervation of CRF-containing neurons in the hypothalamic paraventricular nucleus (Bagdy and Makara Citation1994). 5-HT actions in the hypothalamus may play a role in regulating hormonal responses to stressors (Van de Kar and Blair Citation1999). For instance, antidepressant treatment restores the feedback inhibition of cortisol on the HPA axis that is dysregulated in some depressed patients (Barden et al. Citation1995). Similarly, destruction of hypothalamic 5-HT neurons with 5,7-dihydroxytryptamine has been shown to enhance the inhibitory effect of dexamethasone on the adrenocortical response to ether stress (Feldman and Weidenfeld Citation1991).
The hypothalamic paraventricular nucleus, which receives serotonergic innervation, projects to the intermediolateral cell column of the spinal cord, rostral ventrolateral medulla and dorsal vagal complex to influence both sympathetic and parasympathetic outflow (Swanson and Sawchenko Citation1980; Badoer Citation2001). Related evidence regarding 5-HT and cardiovascular function suggests that blood vessels which have been damaged by hypertension or atherosclerosis are hypersensitive to the vasoconstrictor effects of 5-HT (van Zwieten et al. Citation1990). Also, Sole et al. (Citation1983) observed altered 5-HT levels in the central nervous system of rodents with myocardial ischemia. More recently, Fumeron et al. (Citation2002) found that a specific 5-HT transporter gene polymorphism was associated with a higher risk of myocardial infarction in males who survived an initial heart attack. Finally, dysfunction of blood platelets, which are regulated in part by 5-HT and can influence the pathogenesis of cardiovascular disease, has been described in depression (Bruce and Musselman Citation2005; Mössner et al. Citation2007; Glassman Citation2007). Taken together, these findings point to a link between depressive disorders and neuroendocrine and autonomic regulation involving 5-HT, which can ultimately affect cardiovascular function.
Central neurotransmitter and receptor dysregulation is observed in animal models
Experimental research, involving whole-animal analyses of behaviour and physiology as a result of pharmacological manipulations of the serotonergic system, may increase our understanding of the involvement of 5-HT in mediating mood and cardiovascular function. Recent studies from our laboratory and our collaborators have focused on specific behavioral, neuroendocrine, and autonomic and cardiovascular effects of manipulation of 5-HT and 5-HT1A receptors in rodent models. Because 5-HT reuptake inhibitors have been an extremely effective class of antidepressants (Advanstar Communications Citation2007a,Citationb), we have focused on the effects of fluoxetine on both behavioral and cardiovascular consequences of CMS in rats (Grippo et al. Citation2006a). Compared to treatment with saline vehicle, daily administration of fluoxetine (10 mg/kg, sc; administered concurrently with the CMS paradigm) in animals exposed to 4 weeks of CMS prevented anhedonia (i.e., prevented the reduction in sucrose consumption), yet only partially prevented the cardiovascular consequences of the stressors. Specifically, while basal heart rate in the CMS group was significantly elevated (relative to an undisturbed control group), administration of fluoxetine only slightly attenuated this increase in CMS-exposed rats. Similarly, sympathetic drive was elevated in rats exposed to CMS, but fluoxetine administration only partially attenuated this increase (). These findings have implications for understanding the role of 5-HT in mediating depressive signs and cardiovascular dysfunction. Further, they provide additional evidence for the hypothesis that reduction of depressive signs does not automatically lead to a reduction in the underlying cardiovascular pathophysiology associated with this condition. However, reductions in heart rate have been described in patients with depression and cardiovascular disease treated with fluoxetine (Roose et al. Citation1998a), suggesting that this antidepressant may improve cardiac function in certain contexts.
Figure 6 Mean ( + SEM) absolute heart rate (HR; bpm: beats per minute) responses to β-adrenergic receptor blockade with propranolol (2 mg/kg, iv) and change in HR from baseline values, after 4 weeks of CMS, in CMS and control (CON) groups treated with either daily fluoxetine (FL; 10 mg/kg, sc; a 5-HT reuptake inhibitor or vehicle (V). Fluoxetine partially prevented the exaggerated reduction in HR following propranolol administration in the CMS group (horizontal lines denote paired t-tests; *P < 0.05 for the indicated comparisons). Reprinted from (Grippo et al. Citation2006a); used with permission.
In a related study, we investigated the effects of acute (4 days) fluoxetine administration (10 mg/kg, i.p.) on regulating sympathetic nervous system activity in a rodent model of cardiovascular deconditioning (hindlimb unloading) (Moffitt and Johnson Citation2004). Compared to saline vehicle, short-term administration of fluoxetine (10 mg/kg, i.p.) in animals exposed to 2 weeks of hindlimb unloading – consisting of elevating the hindlimbs of the animals with a harness – enhanced baroreceptor reflex control of lumbar sympathetic nerve activity. The results from this study suggest that, in animals with disrupted autonomic balance, fluoxetine administration may reduce sympathetic tone and restore autonomic balance (and consequently baroreflex function). When considered in the context of mood changes and cardiovascular dysfunction, these data indicate that alterations in serotonergic function, particularly via changes at the level of the 5-HT transporter, can influence directly autonomic control of cardiovascular function. Consistent with this hypothesis are data from Sauer et al. (Citation2003), demonstrating that the degree of 5-HT transporter affinity of a 5-HT reuptake inhibitor correlated positively with protection against myocardial infarction in users of 5-HT reuptake inhibitors following a first myocardial infarction.
We also have used the CMS model to investigate more directly the role of 5-HT receptor function in the control of HPA axis function (Grippo et al. Citation2005b). Activation of 5-HT1A receptors in the hypothalamus with the selective 5-HT1A receptor agonist (+)8-hydroxy-N,N-dipropyl-2-aminotetralin hydrobromide (8-OH-DPAT; 40 μg/kg, s.c.) attenuated the corresponding circulating ACTH response in both female and male rats following 4 weeks of CMS versus control conditions. This finding suggests that 5-HT1A receptor function may be altered in CMS, consistent with evidence from human depression and experimental studies involving the forced swimming model of depression in rodents (Lucki Citation1998). We have not yet extended our findings to investigate the potential autonomic and cardiovascular effects of 5-HT1A receptor activation in CMS; however, because the endocrine and autonomic nervous systems interact within the brain, it is possible that dysfunction of 5-HT1A receptors mediates the association of depressive signs and cardiovascular dysregulation. Future research should be focused more directly on the hypothalamic paraventricular nucleus, as this is a site of integration for behavioral, endocrine and autonomic regulation (Swanson and Sawchenko Citation1980; Van de Kar and Blair Citation1999; Sullivan Hanley and Van de Kar Citation2003).
Conclusions and recommendations for future research
Statistics from The Global Burden of Disease project (Murray and Lopez Citation1996; Mathers and Loncar Citation2005) list cardiovascular disease and psychological depression as two of the most detrimental diseases in developed countries. Furthermore, it has been estimated that approximately 75,000 deaths each year in the United States among patients discharged after an initial myocardial infarction may be attributable to co-morbid depression (Carney et al. Citation1999). The present discussion highlights the significance of the co-morbidity of depression and cardiovascular disease, and underscores the need for understanding the mechanisms responsible for this link to improve public health.
Advancing our understanding of the mechanisms involved in mood and cardiovascular function may be achieved by the systematic investigation of central and peripheral nervous system processes using integrative research techniques. Future research will benefit from a specific focus on factors that are common to both mood states and cardiovascular regulation, including CRF and the HPA axis, the RAAS, pro-inflammatory cytokines and central neurotransmitter systems, as well as the interactions of these systems.
The research described here, including studies from human populations and animal models relevant to depression and cardiovascular disease, provides a foundation for additional mechanistic investigations of brain-mind-heart interactions. It will prove profitable for future research to continue focusing on relevant animal model systems that have demonstrated validity and reliability. We recommend, similar to recent suggestions from Frazer and Morilak (Morilak and Frazer Citation2004; Frazer and Morilak Citation2005), that the development of an animal model focused on behavioral signs related to negative affect – i.e., the disposition to experience negative emotional states, including fear, hostility and sadness which underlies symptoms of mood and anxiety disorders (Watson and Clark Citation1984) – can lead to important investigations such as: (a) the neurobiological substrates of co-morbid affective disorders, such as depression and anxiety, (b) mechanisms underlying pharmacological actions in the central nervous system to modify the behavioral dimensions associated with affective disorders, and (c) mechanisms underlying the association of affective disorders with cardiovascular diseases and other general medical conditions. Suls and Bunde (Citation2005) have similarly suggested that generalized negative affect, rather than specific depressed or anxious states, may explain elevated disease risk (e.g., increased risk of cardiovascular disease) in individuals with symptoms of depression or anxiety. To promote further experimental investigations of this construct, recent studies have focused on behaviours relevant to negative affect using novel rodent models (see Overstreet et al. Citation2003; Grippo et al. Citation2007).
This discussion also highlights the need for increased collaboration among epidemiologists, health psychologists, physiologists and neuroscientists, similar to the recommendations of Suls and Bunde (Citation2005). Collaborations among clinical and experimental scientists can provide translational findings that have relevance for understanding mood and cardiovascular regulation in humans. This in turn will facilitate research that is focused on the integration of behaviour, physiology and brain function in the context of mood and cardiovascular dysregulation. To this end, cross-species comparative studies such as those described by Vaidya et al. (Citation2004) and Willner et al. (Citation1998), support the utility of parallel studies in human and preclinical animal models.
With continued experimental investigations and increased interdisciplinary and translational collaboration, a greater understanding of and appreciation for the processes that lead to altered mood states and cardiovascular dysregulation can be achieved. The continued focus on animal model systems, in combination with the suggestions recently put forth by Evans et al. (Citation2005) regarding a focus on both human research and clinical practice, will promote the development of more effective treatments, and ultimately improve the quality of life and physical well-being, in patients with co-morbid mood and cardiovascular disorders.
Acknowledgements
We thank our research collaborators: Terry Beltz, Gonzalo Carrasco, Zhuo Chen, James Crane, Katerina Damjanoska, Robert Felder, Joseph Francis, Fran Garcia, Ralph Johnson, James Martins, Julia Moffitt, Mike Morris, Nancy Muma, Elisa Na, Claudia Santos, Ju Shi, Nicole Sullivan, Robert Thunhorst, Louis Van de Kar, Shunguang Wei, Robert Weiss and Zhihua Zhang; and the following individuals for technical assistance: Tony Burnes, Lydia DonCarlos, Donna Farley, Lloyd Frei, Keith Miller, Brett Peterson, Doug Whyte, Brian Wulff and Kathy Zimmerman. Financial support was provided by: United States Public Health Service (DK-66086, GM-07069, HL-14388, HL-57472, HL-63915, MH-58448, MH-65839, MH-73233, MH-77581, and NS-34154); National Center for Research Resources (S10RR016652).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adell A, Garcia-Marquez C, Armario A, Gelpi E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem 1988; 50: 1678–1681
- Adis International Limited. Etanercept: Soluble tumour necrosis factor receptor, TNF receptor fusion protein, TNFR-Fc, TNR 001, Enbrel®. Drugs R&D 1999; 1: 258–261
- Advanstar Communications. Top 200 brand-name drugs by retail dollars in 2006. Drug Topics 2007a; 151, http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/072007-405100/article.pdf
- Advanstar Communications. Top 200 generic drugs by retail dollars in 2006. Drug Topics 2007b; 151, http://www.drugtopics.com/drugtopics/data/articlestandard/drugtopics/072007-405102/article.pdf
- Airaksinen KE. Autonomic mechanisms and sudden death after abrupt coronary occlusion. Ann Med 1999; 31: 240–245
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision. American Psychiatric Association, Washington, DC 2000
- American Psychological Association. For a healthy mind and body…talk to a psychologist. American Psychological Association, Washington, DC 2004
- Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology 1993; 4: 285–294
- Anisman H, Zacharko RM. Depression: The predisposing influence of stress. Behav Brain Sci 1982; 5: 89–137
- Anisman H, Zacharko RM. Multiple neurochemical and behavioral consequences of stressors: Implications for depression. Pharmacol Ther 1990; 46: 119–136
- Anisman H, Zacharko RM. Depression as a consequence of inadequate neurochemical adaptation in response to stressors. Br J Psychiatry 160 1992; 15(Suppl. 15)36–43
- Appels A, Mulder P. Excess fatigue as a precursor of myocardial infarction. Eur Heart J 1988; 9: 758–764
- Asnis GM, Halbreich U, Ryan ND, Rabinowicz H, Puig-Antich J, Nelson B, Novacenko H, Friedman JH. The relationship of the dexamethasone suppression test (1 mg and 2 mg) to basal plasma cortisol levels in endogenous depression. Psychoneuroendocrinology 1987; 12: 295–301
- Azpíroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferation response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology 1999; 24: 345–361
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 2001; 28: 95–99
- Bagdy G, Makara GB. Hypothalamic paraventricular nucleus lesions differentially affect serotonin-1A (5-HT1A) and 5-HT2 receptor agonist-induced oxytocin, prolactin, and corticosterone responses. Endocrinology 1994; 134: 1127–1131
- Baliga RR, Ranganna P, Pitt B, Koelling TM. Spironolactone treatment and clinical outcomes in patients with systolic dysfunction and mild heart failure symptoms: A retrospective analysis. J Card Fail 2006; 12: 250–256
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: Response to antidepressant treatment and relapse. Eur Neuropsychopharmacol 1992; 2: 107–113
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood–brain barrier. Neuroimmunomodulation 1995; 2: 241–248
- Barden N, Reul JM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic–pituitary–adrenocortical system?. Trends Neurosci 1995; 18: 6–11
- Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation 1996; 93: 1976–1980
- Barefoot JC, Helms MJ, Mark DB, Blumenthal JA, Califf RM, Haney TL, O'Connor CM, Siegler IC, Williams RB. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78: 613–617
- Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: Identifying those at increased cardiac risk?. J Hypertens 2007; 25: 2117–2124
- Bataillard A, del Rey A, Klusman I, Arditi GM, Besedovsky HO. Interleukin-1 stimulates aldosterone secretion: Involvement of renin, ACTH, and prostaglandins. Am J Physiol Regul Integr Comp Physiol 1992; 263: R840–R844
- Bedi M, Varshney VP, Babbar R. Role of cardiovascular reactivity to mental stress in predicting future hypertension. Clin Exp Hypertens 2000; 22: 1–22
- Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science 1984; 226: 180–182
- Beniamini Y, Rubenstein JJ, Zaichkowsky LD, Crim MC. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol 1997; 80: 841–846
- Berendsen HHG. Interactions between 5-hydroxytryptamine receptor subtypes: Is a disturbed receptor balance contributing to the symptomatology of depression in humans?. Pharmacol Ther 1995; 66: 17–37
- Berman RM, Belanoff JK, Charney DS, Schatzberg AF. Principles of the pharmacotherapy of depression. Neurobiology of mental illness, DS Charney, EJ Nestler, BS Bunney. Oxford University Press, New York 1999; 419–432
- Bidzinska EJ. Stress factors in affective diseases. Br J Psychiatry 1984; 144: 161–166
- Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: A predictor of sudden cardiac death. Circulation 1982; 66: 874–880
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology 2003; 28: 1328–1335
- Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry 2001; 62(Suppl. 15)12–17
- Bollini P, Pampallona S, Kupelnick B, Tibaldi G, Munizza C. Improving compliance in depression: A systematic review of narrative reviews. J Clin Pharm Ther 2006; 31: 253–260
- Booth-Kewley S, Friedman HS. Psychological predictors of heart disease: A quantitative review. Psychol Bull 1987; 101: 343–362
- Braith RW. Exercise training in patients with CHF and heart transplant recipients. Med Sci Sports Exerc 1998; 30(Suppl. 10)S367–S378
- Bruce EC, Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med 2005; 67(Suppl. 1)S34–S36
- Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur studies of successful aging. Am J Public Health 1994; 84: 1796–1799
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry 2003; 54: 241–247
- Carney RM, Rich MW, Freedland KE, Saini J, teVelde A, Simeone C, Clark K. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988a; 50: 627–633
- Carney RM, Rich MW, teVelde A, Saini J, Clark K, Freedland KE. The relationship between heart rate, heart rate variability and depression in patients with coronary artery disease. J Psychosom Res 1988b; 32: 159–164
- Carney RM, Freedland KE, Rich MW, Smith LJ, Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. Am J Med 1993; 95: 23–28
- Carney RM, Freedland KE, Rich MW, Jaffe AS. Depression as a risk factor for cardiac events in established coronary heart disease: A review of possible mechanisms. Ann Behav Med 1995a; 17: 142–149
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol 1995b; 76: 562–564
- Carney RM, Freedland KE, Veith RC, Jaffe AS. Can treating depression reduce mortality after an acute myocardial infarction?. Psychosom Med 1999; 61: 666–675
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med 2000; 62: 639–647
- Carpeggiani C, Skinner JE. Coronary flow and mental stress. Experimental findings. Circulation 1991; 83: II-90–II-93
- Carroll BJ. Limbic system-adrenal cortex regulation in depression and schizophrenia. Psychosom Med 1976; 38: 106–121
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression II. discrimination of depressed from nondepressed patients. Arch Gen Psychiatry 1976a; 33: 1051–1058
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression: I. limbic sytem-adrenocortical dysfunction. Arch Gen Psychiatry 1976b; 33: 1039–1044
- Cassidy EM, O'Keane V. Depression and interferon-alpha therapy. Br J Psychiatry 2000; 176: 494
- Catterall WA, Ray R. Interactions of neurotoxins with the action potential NA+ionophore. J Supramol Struct 1976; 5: 397–407
- Centers for Disease Control and Prevention and National Center for Infectious Diseases. Chronic fatigue syndrome. U.S. Department of Health and Human Services, Washington, D.C. 2001
- Cheeta S, Broekkamp C, Willner P. Stereospecific reversal of stress-induced anhedonia by mianserin and its (+)-enantiomer. Psychopharmacology 1994; 116: 523–528
- Clement HW, Buschmann J, Rex S, Grote C, Opper C, Gemsa D, Wesemann W. Effects of interferon-γ, interleukin-1β and tumor necrosis factor-α on the serotonin metabolism in the nucleus raphe dorsalis of the rat. J Neural Transm 1997; 104: 981–991
- Collins HL, DiCarlo SE. Daily exercise attenuates the sympathetic component of the arterial baroreflex control of heart rate. Am J Physiol Heart Circ Physiol 1997; 273: H2613–H2619
- Connor TJ, Leonard BE. Depression, stress, and immunological activation: The role of cytokines in depressive disorders. Life Sci 1998; 62: 583–606
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1β, ‐2, ‐6, and tumor necrosis factor-α administration on some behavioural, neurochemical, endocrine, and immune parameters in the rat. Neuroscience 1998; 84: 923–933
- Corbalan R, Verrier R, Lown B. Psychological stress and ventricular arrhythmias during myocardial infarction in the conscious dog. Am J Cardiol 1974; 34: 692–696
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 2005; 29: 547–569
- Cunningham ET, Jr, De Souza EB. Interleukin 1 receptors in the brain and endocrine tissues. Immunol Today 1993; 14: 171–176
- Dantzer R. How do cytokines say hello to the brain? Neural vs. humoral mediation. Eur Cytokine Netw 1994; 5: 271–273
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin 2006; 24: 441–460
- Dantzer R, Bluthé RM, Gheusi G, Cremona S, Layé S, Parnet P, Kelley KW. Molecular basis of sickness behavior. Ann NY Acad Sci 1998; 856: 132–138
- Das UN. Free radicals, cytokines and nitric oxide in cardiac failure and myocardial infarction. Mol Cell Biochem 2000; 215: 145–152
- Dawood T, Lambert EA, Barton DA, Laude D, Elghozi J-L, Esler MD, Haikerwal D, Kaye DM, Hotchkin EJ, Lambert GW. Specific serotonin reuptake inhibition in major depressive disorder adversely affects novel markers of cardiac risk. Hypertens Res 2007; 30: 285–293
- De La Garza R, II. Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: Focus on anhedonia. Neurosci Biobehav Rev 2005; 29: 761–770
- Doner K. Depression: Is it all in your heart?. My Generation 2002; 48, July-August
- Duman RS. The neurochemistry of mood disorders: Preclinical studies. Neurobiology of mental illness, DS Charney, EJ Nestler, BS Bunney. Oxford University Press, New York 1999; 333–347
- Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission: Comparison with the effects of stress. Cytokines, stress, and depression, R Dantzer, EE Wollmann, R Yirmiya. Kluwer Academic/Plenum, New York 1999; 117–127
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: What can we learn from animal studies?. Neurosci Biobehav Rev 2005; 29: 891–909
- Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA. Heart rate as a prognostic factor for coronary heart disease and mortality: Findings in three Chicago epidemiologic studies. Am J Epidemiol 1980; 112: 736–749
- Emanuele E, Geroldi D, Minoretti P, Coen E, Politi P. Increased plasma aldosterone in patients with clinical depression. Arch Med Res 2005; 36: 544–548
- Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J Cardiovasc Pharmacol 2000; 35(Suppl. 4)S1–S7
- Esler M, Turbott J, Schwarz R, Leonard B, Bobik A, Skews H, Jackman G. The peripheral kinetics of norepinephrine in depressive illness. Arch Gen Psychiatry 1982; 39: 295–300
- Esler M, Lambert E, Alvarenga M, Socratous F, Richards J, Barton D, Pier C, Brenchley C, Dawood T, Hastings J, Guo L, Haikerwal D, Kaye D, Jennings G, Kalff V, Kelly M, Wiesner G, Lambert G. Increased brain serotonin turnover in panic disorder patients in the absence of a panic attack: Reduction by a selective serotonin reuptake inhibitor. Stress 2007; 10: 295–304
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo W, Jr. Mood disorders in the medically ill: Scientific review and recommendations. Biol Psychiatry 2005; 58: 175–189
- Everson SA, Goldberg DE, Kaplan GA, Cohen RD, Pukkala E, Tuomilehto J, Salonen JT. Hopelessness and risk of mortality and incidence of myocardial infarction and cancer. Psychosom Med 1996; 58: 113–121
- Everson-Rose SA, House JS, Mero RP. Depressive symptoms and mortality risk in a national sample: Confounding effects of health status. Psychosom Med 2004; 66: 823–830
- Felder RB, Francis J, Weiss RM, Zhang ZH, Wei SG, Johnson AK. Neurohumoral regulation in ischemia-induced heart failure. Role of the forebrain. Ann NY Acad Sci 2001; 940: 444–453
- Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: New perspectives. Am J Physiol Regul Integr Comp Physiol 2003; 284: R259–R276
- Feldman S, Weidenfeld J. Depletion of hypothalamic norepinephrine and serotonin enhances the dexamethasone negative feedback effect on adrenocortical secretion. Psychoneuroendocrinology 1991; 16: 397–405
- Fenton WS, Stover ES. Mood disorders: Cardiovascular and diabetes comorbidity. Curr Opin Psychiatry 2006; 19: 421–427
- Ferrari R. The role of TNF in cardiovascular disease. Pharmacol Res 1999; 40: 97–105
- Ferrari R, Censi S, Mastrorilli F, Boraso A. Prognostic benefits of heart rate reduction in cardiovascular disease. Eur Heart J 2003; 5(Suppl. G)G10–G14
- Foley KF, DeSanty KP, Kast RE. Bupropion: Pharmacology and therapeutic applications. Expert Rev Neurother 2006; 6: 1249–1265
- Forbes LM, Chaney RH. Cardiovascular changes during acute depression. Psychosomatics 1980; 21: 472–477
- Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 2001a; 281: H2241–H2251
- Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 2001b; 281: R1734–R1745
- Francis J, Beltz TG, Johnson AK, Felder RB. Mineralocorticoids act centrally to regulate blood-borne tumor necrosis factor-alpha in nornal rats. Am J Physiol Regul Integr Comp Physiol 2003a; 285: R1402–R1409
- Francis J, Weiss RM, Johnson AK, Felder RB. Central mineralocorticoid receptor blockade decreases plasma TNF-α after coronary artery ligation in rats. Am J Physiol Regul Integr Comp Physiol 2003b; 284: R328–R335
- Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol 2004; 287: H791–H797
- Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry 2003; 60: 627–636
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: Impact on 6-month survival. JAMA 1993; 270: 1819–1825
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91: 999–1005
- Frazer A, Morilak DA. What should animal models of depression model?. Neurosci Biobehav Rev 2005; 29: 515–523
- Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med 2003; 65: 119–128
- Freedland KE, Miller GE, Sheps DS. The great debate, revisited. Psychosom Med 2006; 68: 179–184
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, Dutriez-Casteloot I, Enache M, Barden N, Cohen-Salmon C, Hamon M, Lanfumey L. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci 2004; 24: 2787–2796
- Fuller RW. Serotonin receptors involved in regulation of pituitary-adrenocortical function in rats. Behav Brain Res 1996; 73: 215–219
- Fumeron F, Betoulle D, Nicaud V, Evans A, Kee F, Ruidavets JB, Arveiler D, Luc G, Cambien F. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Temoins de l'Infarctus du Myocarde (ECTIM). Circulation 2002; 105: 2943–2945
- Garlow SJ, Nemeroff CB. The neurochemistry of mood disorders: Clinical studies. Neurobiology of mental illness2nd ed., DS Charney, EJ Nestler. Oxford University Press, New York 2004; 440–460
- Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC, OPTIMIZE-HF Investigators and Coordinators. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur Heart J 2007a; 28: 980–988
- Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Piña IL, Fonarow GC, DeMarco T, Pauly DF, Rogers J, DiSalvo TG, Butler J, Hare JM, Francis GS, Stough WG, O'Connor CM. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Int Med 2007b; 167: 1998–2005
- Glassman AH. Depression and cardiovascular comorbidity. Dialogues Clin Neurosci 2007; 9: 9–17
- Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry 1998; 155: 4–11
- Glowa JR, Gold PW. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides 1991; 18: 55–61
- Gold PW, Wong M-L, Goldstein DS, Gold HK, Ronsaville DS, Esler M, Alesci S, Masood A, Licinio J, Geracioti TD, Perini G, DeBellis MD, Holmes C, Vgontzas AN, Charney DS, Chrousos GP, McCann SM, Kling MA. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci USA 2005; 102: 8303–8308
- Goldstein DS. Plasma catecholamines and essential hypertension: An analytical review. Hypertension 1983; 5: 86–99
- Gomez-Sanchez EP. Brain mineralocorticoid receptors: Orchestrators of hypertension and end-organ disease. Curr Opin Nephrol Hypertens 2004; 13: 191–196
- Gordon D, Guyton JR, Karnovsky N. Intimal alterations in rat aorta induced by stressful stimuli. Lab Invest 1981; 45: 14–27
- Greene WA, Goldstein S, Moss AJ. Psychosocial aspects of sudden death. A preliminary report. Arch Int Med 1972; 129: 725–731
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol 2002; 282: R1333–R1341
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav 2003a; 78: 703–710
- Grippo AJ, Francis J, Weiss RM, Felder RB, Johnson AK. Cytokine mediation of experimental heart failure-induced anhedonia. Am J Physiol Regul Integr Comp Physiol 2003b; 284: R666–R673
- Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, Johnson AK. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol 2004; 286: H619–H626
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav 2005a; 84: 697–706
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, Chen Z, Garcia F, Muma NA, Van de Kar LD. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology 2005b; 179: 769–780
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry 2006a; 59: 309–316
- Grippo AJ, Moffitt JA, Beltz TG, Johnson AK. Reduced hedonic behavior and altered cardiovascular function induced by mild sodium depletion in rats. Behav Neurosci 2006b; 120: 1133–1143
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: Toward the development of a rodent model focused on co-occurring depression and anxiety. Depress Anxiety 2008, in press
- Gutiérrez B, Arias B, Papiol S, Rosa A, Fañanás L. Association study between novel promoter variants and the 5-HT2C receptor gene and human patients with bipolar affective disorder. Neurosci Lett 2001; 309: 135–137
- Hausberg M, Hillebrand U, Kisters K. Addressing sympathetic overactivity in major depressive disorder. J Hypertens 2007; 25: 2004–2005
- Hawkley LC, Bosch JA, Engeland CG, Marucha PT, Cacioppo JT. Loneliness, dysphoria, stress, and immunity: A role for cytokines. Cytokines: Stress and immunity2nd ed., NP Plotnikoff, RE Faith, AJ Murgo, RA Good, 2007; 67–85
- Herman JP, Guillonneau D, Dantzer R, Scatton B, Semerdjian-Rouquier L, LeMoal M. Differential effects of inescapable footshocks and of stimuli previously paired with inescapable footshocks on dopamine turnover in cortical and limbic areas of the rat. Life Sci 1982; 30: 2207–2214
- Hickie I, Lloyd A, Wakefield D, Parker G. The psychiatric status of patients with the chronic fatigue syndrome. Br J Psychiatry 1990; 156: 534–540
- Honerjager P, Meissner A. The positive inotropic effect of aconitine. Naunyn-Schmiedebergs Arch Pharmacol 1983; 322: 49–58
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system I: Expression and recognition. Trends Neurosci 1995; 18: 83–88
- Huangfu D, Hwang LJ, Riley TA, Guyenet PG. Role of serotonin and catecholamines in sympathetic responses evoked by stimulation of rostral medulla. Am J Physiol Regul Integr Comp Physiol 1994; 266: R338–R352
- Irwin J, Ahluwalia P, Anisman H. Sensitization of norepinephrine activity following acute and chronic footshock. Brain Res 1986; 379: 98–103
- Jarskog LF, Xiao H, Wilkie MB, Lauder JM, Gilmore JH. Cytokine regulation of embryonic rat dopamine and serotonin neuronal survival in vitro. Int J Dev Neurosci 1997; 15: 711–716
- Johnson AK, Anderson EA. Stress and arousal. Principles of psychophysiology: Physical, social, and inferential elements, JT Cacioppo, LG Tassinary. Cambridge University Press, Cambridge 1990; 216–252
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol 1997; 18: 292–353
- Johnson AK, Grippo AJ. Sadness and broken hearts: Neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol 2006; 57: 5–29, (Suppl. 11):
- Johnson AK, Thunhorst RL. The neuroendocrinology, neurochemistry and molecular biology of thirst and salt appetite. Handbook of neurochemistry and molecular neurobiology: Behavioral neurochemistry, neuroendocrinology and molecular neurobiology3rd ed, A Lajtha, JD Blaustein, 2007; 641–687
- Joseph MH, Kennett GA. Stress-induced release of 5-HT in the hippocampus and its dependence on increased tryptophan availability: An in vivo electrochemical study. Brain Res 1983; 270: 251–257
- Kabiersch A, del Rey A, Honegger CG, Besedovsky HO. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav Immun 1988; 2: 267–274
- Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: The Framingham study. Am Heart J 1987; 113: 1489–1494
- Kapadia S, Dibbs Z, Kurrelmeyer K, Karla D, Seta Y, Wang F, Bozkurt B, Oral H, Sivasubramanian N, Mann DL. The role of cytokines in the failing human heart. Cardiol Clin 1998; 16: 645–656
- Khaykin Y, Dorian P, Baker B, Shapiro C, Sandor P, Mironov D, Irvine J, Newman D. Autonomic correlates of antidepressant treatment using heart-rate variability analysis. Can J Psychiatry 1998; 43: 183–186
- Kjekshus JK, Blix AS, Grottum P, Aasen AO. Beneficial effects of vagal stimulation on the ischaemic myocardium during beta-receptor blockade. Scand J Clin Lab Invest 1981; 41: 383–389
- Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987; 59: 256–262
- Klimas NG, Salvato FR, Morgan R, Fletcher MA. Immunologic abnormalities in chronic fatigue syndrome. J Clin Microbiol 1990; 28: 1403–1410
- Klimas NG, Morgan R, Van Riel F, Fletcher MA. Observations regarding use of an antidepressant, fluoxetine, in chronic fatigue syndrome. Chronic fatigue and related immune deficiency syndromes, PJ Goodnick, NG Klimas. American Psychiatric Press, Inc, Washington, DC 1993; 95–108
- Knardahl S, Sanders BJ, Johnson AK. Effects of adrenal demedullation on stress-induced hypertension and cardiovascular responses to acute stress. Acta Physiol Scand 1988; 133: 477–483
- Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med 1999; 61: 476–487
- Kotulak R. The healing mind. Chicago Tribute Magazine 2006; 15–16: 29–31
- Krahn DD, Gosnell BA, Levine AS, Morley JE. Behavioral effects of corticotropin-releasing factor: Localization and characterization of central effects. Brain Res 1988; 443: 63–69
- Kristal-Boneh E, Raifel M, Froom P, Ribak J. Heart rate variability in health and disease. Scand J Work Environ Health 1995; 21: 85–95
- Krittayaphong R, Cascio WE, Light KC, Sheffield D, Golden RN, Finkel JB, Glekas G, Koch GG, Sheps DS. Heart rate variability in patients with coronary artery disease: Differences in patients with higher and lower depression scores. Psychosom Med 1997; 59: 231–235
- Kronfol Z. Immune dysregulation in major depression: A critical review of existing evidence. Int J Neuropsychopharmacol 2002; 5: 333–343
- Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol Regul Integr Comp Physiol 1984; 246: R994–R999
- Kusumi I, Koyama T, Yamashita I. Serotonin-stimulated Ca2+ response is increased in the blood platelets of depressed patients. Biol Psychiatry 1991; 30: 310–312
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998; 351: 478–484
- Lambert G, Johansson M, Ågren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: Evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry 2000; 57: 787–793
- Lebovits BZ, Shekelle RB, Ostfeld AM, Paul O. Prospective and retrospective psychological studies of coronary heart disease. Psychosom Med 1967; 29: 265–272
- Lechin F, van der Dijs B, Orozco B, Lechin ME, Báez S, Lechin AE, Rada I, Acosta E, Arocha L, Jiménez V, León G, García Z. Plasma neurotransmitters, blood pressure, and heart rate during supine-resting, orthostasis, and moderate exercise conditions in major depressed patients. Biol Psychiatry 1995; 38: 166–173
- LeJemtel TH, Serrano C. Vasopressin dysregulation: Hyponatremia, fluid retention and congestive heart failure. Int J Cardiol 2007; 120: 1–9
- Lerer B, Macciardi F, Segman RH, Adolfsson R, Blackwood D, Blairy S, Del Favero J, Dikeos DG, Kaneva R, Lilli R, Massat I, Milanova V, Muir W, Noethen M, Oruc L, Petrova T, Papadimitriou GN, Rietschel M, Serretti A, Souery D, Van Gestel S, Van Broeckhoven C, Mendlewicz J. Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorders. Mol Psychiatry 2001; 6: 579–585
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323: 236–241
- Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med 1976; 294: 1165–1170
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry 1998; 44: 151–162
- Maes M. Evidence for an immune response in major depression: A review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19: 11–38
- Maes M, Meltzer HY. The serotonin hypothesis of major depression. Psychopharmacology: The fourth generation of progress, FE Bloom, DJ Kupfer. Raven Press, New York 1995; 933–944
- Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, Biondi M, Bosmans E, Kenis G, Scharpe S. Increased 24-h uninary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatr Scand 1998; 98: 328–335
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev 1998; 105: 83–107
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 2005; 29: 829–841
- Mathers CD, Loncar D. Updated projections of global mortality and burden of disease, 2002–2030: Data sources, methods and results. World Health Organization, Geneva 2005
- Matta RJ, Lawler JE, Lown B. Ventricular electrical instability in the conscious dog: Effects of psychologic stress and beta adrenergic blockade. Am J Cardiol 1976; 38: 594–598
- McCartney N. Role of resistance training in heart disease. Med Sci Sports Exerc 1998; 30: S396–S402
- Meltzer HY. Role of serotonin in depression. Ann NY Acad Sci 1990; 600: 486–499
- Mendes de Leon CF, Krumholz HM, Seeman TS, Vaccarino V, Williams CS, Kasl SV, Berkman LF. Depression and risk of coronary heart disease in elderly men and women. Arch Int Med 1998; 158: 2341–2348
- Mikuni M, Kusumi I, Kagaya A, Kuroda Y, Mori H, Takahashi K. Increased 5-HT-2 receptor function as measured by serotonin-stimulated phosphoinositide hydrolysis in platelets of depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 1991; 15: 49–61
- Miller MC. The dangers of chronic distress. Newsweek 2005; 58–59, October 3
- Moffitt JA, Johnson AK. Short-term fluoxetine treatment enhances baroreflex control of sympathetic nervous system activity after hindlimb unloading. Am J Physiol Regul Integr Comp Physiol 2004; 286: R584–R590
- Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: A dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol 2004; 7: 193–218
- Morris MJ, Na ES, Grippo AJ, Johnson AK. The effects of deoxycorticosterone-induced sodium appetite on hedonic behaviors in the rat. Behav Neurosci 2006; 120: 571–579
- Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: Clinical and hemodynamic correlates and prognostic implications. Circulation 1997; 96: 3450–3458
- Mössner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Müller N, Fallgatter AJ, Riederer P. Consensus paper of the WFSBP Task Force on Biological Markers: Biological markers in depression. World J Biol Psychiatry 2007; 8: 141–174
- Murck H, Held K, Ziegenbein M, Künzel H, Koch K, Steiger A. The renin-angiotensin-aldosterone system in patients with depression compared to controls - a sleep endocrine study. BMC Psychiatry 2003; 3: 15–23
- Murphy DL, Aulakh C, Mazzola-Pomietto P, Briggs NC. Neuroendocrine responses to serotonergic agonists as indices of the functional status of central serotonin neurotransmission in humans: A preliminary comparative analysis of neuroendocrine endpoints versus other endpoint measures. Behav Brain Res 1996; 73: 209–214
- Murray CJL, Lopez AD. The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard University Press, Cambridge 1996
- Muscat R, Papp M, Willner P. Antidepressant-like effects of dopamine agonist in an animal model of depression. Biol Psychiatry 1992; 31: 937–946
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease. Arch Gen Psychiatry 1998; 55: 580–592
- Nathan CF. Secretory products of macrophages. J Clin Invest 1987; 79: 319–327
- National Institute of Mental Health. Depression can break your heart. National Institutes of Health, Bethesda 2001
- Nemeroff CB, Musselman DL, Evans DL. Depression and cardiac disease. Depress Anxiety 1998; 8(Suppl. 1)71–79
- Niiranen A, Laaksonen R, Iivanainen M, Mattson K, Fäkkilä M, Cantell K. Behavioral assessment of patients treated with alpha-interferon. Acta Psychiatr Scand 1988; 78: 622–626
- Nosjean A, Franc B, Laguzzi R. Increased sympathetic nerve discharge without alteration in the sympathetic baroreflex response by serotonin3 receptor stimulation in the nucleus tractus solitarius of the rat. Neurosci Lett 1995; 186: 41–44
- Overstreet DH, Commissaris RC, De La Garza R, II, File SE, Knapp DJ, Seiden LS. Involvement of 5-HT1A receptors in animal tests of anxiety and depression: Evidence from genetic models. Stress 2003; 6: 101–110
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clin Chem 1994; 40: 288–295
- Palatini P, Julius S. Association of tachycardia with morbidity and mortality: Pathophysiological considerations. J Hum Hypertens 1997; 11(Suppl. 1)S19–S27
- Paluska SA, Schwenk TL. Physical activity and mental health: Current concepts. Sports Med 2000; 29: 167–180
- Pandey GN, Pandey SC, Janicak PG, Marks RC, Davis JM. Platelet serotonin-2 receptor binding sites in depression and suicide. Biol Psychiatry 1990; 28: 215–222
- Partarca R, Fletcher MA, Klimas NG. Immunological correlates of chronic fatigue syndrome. Chronic fatigue and related immune deficiency syndromes, PJ Goodnick, NG Klimas. American Psychiatric Press, Inc, Washington, DC 1993; 1–21
- Pelloux Y, Hagues G, Costentin J, Duterte-Boucher D. Helplessness in the tail suspension test is associated with an increase in ethanol intake and its rewarding effect in female mice. Alcoholism: Clin Exp Res 2005; 29: 378–388
- Penninx BWJH, Beekman ATF, Honig A, Deeg DJH, Schoevers RA, van Eijk JTM, van Tilburg W. Depression and cardiac mortality: Results from a community-based longitudinal study. Arch Gen Psychiatry 2001; 58: 221–227
- Perski A, Osuchowski K, Andersson L, Sanden A, Feleke E, Anderson G. Intensive rehabilitation of emotionally distressed patients after coronary by-pass grafting. J Int Med 1999; 246: 253–263
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999; 341: 709–717
- Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, Massari F, Mastropasqua F, Russo GD, Rizzon P. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am Heart J 2001; 141: 765–771
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: Implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol 2002; 5: 389–399
- Puffer JC, McShane JM. Depression and chronic fatigue in athletes. Clin Sports Med 1992; 11: 327–338
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJG, Tilders FJH, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer's disease and depression. Am J Psychiatry 1995; 152: 1372–1376
- Rechlin T, Weis M, Claus D. Heart rate variability in depressed patients and differential effects of paroxetine and amitriptyline on cardiovascular autonomic functions. Pharmacopsychiatry 1994a; 27: 124–128
- Rechlin T, Weis M, Spitzer A, Kaschka WP. Are affective disorders associated with alterations of heart rate variability?. J Affect Disord 1994b; 32: 271–275
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety 2000; 12(Suppl. 1)2–19
- Rieckmann N, Kronish IM, Haas D, Gerin W, Chaplin WF, Burg MM, Vorchheimer D, Davidson KW. Persistent depressive symptoms lower aspirin adherence after acute coronary syndromes. Am Heart J 2006; 152: 922–927
- Rissanen V, Romo M, Siltanen P. Premonitory symptoms and stress factors preceding sudden death from ischaemic heart disease. Acta Med Scand 1978; 204: 389–396
- Roose SP, Glassman AH, Attia E, Woodring S, Giardina EGV, Bigger JT, Jr. Cardiovascular effects of fluoxetine in depressed patients with heart disease. Am J Psychiatry 1998a; 155: 660–665
- Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT, Jr, Pollock BG, Gaffney A, Narayan M, Finkel MS, McCafferty J, Gergel I. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998b; 279: 287–291
- Rossi J, Bayram M, Udelson JE, Lloyd-Jones D, Adams KF, O'Connor CM, Stough WG, Ouyang J, Shin DD, Orlandi C, Gheorghiade M. Improvement in hyponatremia during hospitalization for worsening heart failure is associated with improved outcomes: Insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) trial. Acute Card Care 2007; 9: 82–86
- Roth BL. Multiple serotonin receptors: Clinical and experimental aspects. Ann Clin Psychiatry 1994; 6: 67–78
- Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: Actions and mechanisms of action. Trends Neurosci 1995; 18: 130–136
- Roy A, Guthrie S, Pickar D, Linnoila M. Plasma norepinephrine responses to cold challenge in depressed patients and normal controls. Psychiatry Res 1987; 21: 161–168
- Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry 2003; 54: 227–240
- Ryan C, Hollenberg M, Harvey DB, Gwynn R. Impaired parasympathetic responses in patients after myocardial infarction. Am J Cardiol 1976; 37: 1013–1018
- Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: A review and update. Neurosci Biobehav Rev 1992; 16: 207–217
- Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation 2003; 108: 32–36
- Schleifer SJ, Macari-Hinson MM, Coyle DA, Slater WR, Kahn M, Gorlin R, Zucker HD. The nature and course of depression following myocardial infarction. Arch Int Med 1989; 149: 1785–1789
- Schlitz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci 2002; 22: 5606–5618
- Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation 1988; 78: 969–979
- Sgoifo A, De Boer SF, Buwalda B, Korte-Bouws G, Tuma J, Bohus B, Zaagsma J, Koolhaas JM. Vulnerability to arrhythmias during social stress in rats with different sympathovagal balance. Am J Physiol Heart Circ Physiol 1998; 275: H460–H466
- Sgoifo A, Pozzato C, Costoli T, Manghi M, Stilli D, Ferrari PF, Ceresini G, Musso E. Cardiac autonomic responses to intermittent social conflict in rats. Physiol Behav 2001; 73: 343–349
- Singh M, Johnson AK. Depression- and anxiety-related behaviors in transgenic mice overexpressing the RNA editing enzyme ADAR2. 2007, Program No. 907.16. 2007 Neuroscience Meeting Planner. SanDiego, CA: Society for Neuroscience Online
- Sirinathsinghji DJ, Rees LH, Rivier J, Vale W. Corticotropin-releasing factor is a potent inhibitor of sexual receptivity in the female rat. Nature 1983; 305: 232–235
- Smith RS. The macrophage theory of depression. Med Hypotheses 1991; 35: 298–306
- Sole MJ, Versteeg DH, de Kloet ER, Hussain N, Lixfeld W. The identification of specific serotonergic nuclei inhibited by cardiac vagal afferents during acute myocardial ischemia in the rat. Brain Res 1983; 265: 55–61
- Spriggs DR, Sherman ML, Michie H, Arthor KA, Imamura K, Wilmore D, Frie E, Kufe DW. Recombinant human tumor necrosis factor administered as a 24-h intravenous infusion. A phase 1 and pharmacology study. J Natl Cancer Inst 1988; 80: 1039–1044
- Stambor Z. Stressed out nation. Monitor on Psychology 2006; 37: 28–29
- Stenfors C, Ross SB. Evidence for involvement of 5-hydroxytryptamine(1B) autoreceptors in the enhancement of serotonin turnover in the mouse brain following repeated treatment with fluoxetine. Life Sci 2002; 71: 2867–2880
- Stier CT, Jr, Chander PN, Rocha R. Aldosterone as a mediator in cardiovascular injury. Cardiol Rev 2002; 10: 97–107
- Straus SE, Tosato G, Armstrong G, Lawley T, Preble OT, Henle W, Davey R, Pearson G, Epstein J, Brus I, Blaese RM. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Int Med 1985; 102: 7–16
- Sullivan Hanley NR, Van de Kar LD. Serotonin and the neuroendocrine regulation of the hypothalamic–pituitary–adrenal axis in health and disease. Vitam Horm 2003; 66: 189–255
- Suls J, Bunde J. Anger, anxiety and depression as risk factors for cardiovascular disease: The problems and implications of overlapping affective dispositions. Psychol Bull 2005; 131: 260–300
- Swaab DF, Bao A-M, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev 2005; 4: 141–194
- Swanson LW, Sawchenko PE. Paraventricular nucleus: A site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 1980; 31: 410–417
- Szegedy-Maszak M. Hearts and minds. US News and World Report 2003; 68–70, December 1
- Tapanainen JM, Thomsem PEB, Køber L, Torp-Pedersen C, Mäkikallio TH, Still A-M, Lindgren KS, Huikuri HV. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol 2002; 90: 347–352
- Tobler I, Borbely AA, Schwyzer M, Fontana A. Interleukin-1 derived from astrocytes enhances slow wave activity in sleep EEG of the rat. Eur J Pharmacol 1984; 104: 191–192
- Trimarco B, Ricciardelli B, Cuocolo A, Volpe M, De Luca N, Mele AF, Condorelli M. Effects of coronary occlusion on arterial baroreflex control of heart rate and vascular resistance. Am J Physiol Heart Circ Physiol 1987; 252: H749–H759
- Vaidya VA. Stress, depression, and hippocampal damage. J Biosci 2000; 25: 123–124
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: The role of reward sensitivity. Ann NY Acad Sci 2004; 1021: 395–398
- Van de Kar LD. Neuroendocrine aspects of the serotonergic hypothesis of depression. Neurosci Biobehav Rev 1989; 13: 237–246
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol 1999; 20: 1–48
- van Deist R, Appels A. Vital exhaustion and depression: A conceptual study. J Psychosom Res 1991; 35: 535–544
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: Systematic review and meta analysis. Int J Geriatr Psychiatry 2007; 22: 613–626
- van Praag HM. Can stress cause depression?. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 891–907
- van Zwieten PA, Blauw GJ, van Brummelen P. Pathophysiological and pharmacotherapeutic aspects of serotonin and serotonergic drugs. Clin Physiol Biochem 1990; 8(Supp. 3)1–18
- Verrier RL, Lown B. Behavioral stress and cardiac arrhythmias. Annu Rev Physiol 1984; 46: 155–176
- Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. Am Heart J 1999; 137: 453–457
- Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J 2002; 143: 460–466
- Watson D, Clark LA. Negative affectivity: The disposition to experience aversive emotional states. Psychol Bull 1984; 96: 465–490
- Weber B, Lewicka S, Deuschle M, Colla M, Vecsei P, Heuser I. Increased diurnal plasma concentrations of cortisone in depressed patients. J Clin Endocrinol Metab 2000; 85: 1133–1136
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol 2002; 5: 375–388
- Willner P. Dopaminergic mechanisms in depression and mania. Psychopharmacology: The fourth generation of progress, FE Bloom, DJ Kupfer. Raven Press, New York 1995; 921–931
- Willner P. The chronic mild stress procedure as an animal model of depression: Valid, reasonably reliable, and useful. Psychopharmacology 1997a; 134: 371–377
- Willner P. The mesolimbic dopamine system as a target for rapid antidepressant action. Int Clin Psychopharmacol 1997b; 12(Suppl. 3)S7–S14
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology 1997c; 134: 319–329
- Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005; 52: 90–110
- Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, Morgan M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology 1998; 136: 272–283
- Wolk R. Central origin of decreased heart rate variability in patients with cardiovascular diseases. Med Hypotheses 1996; 46: 479–481
- Wright JW, Krebs LT, Stobb JW, Harding JW. The angiotensin IV system: Functional implications. Front Neuroendocrinol 1995; 16: 23–52
- Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Jung I, Sherwood P. Heart rate variability in patients with major depression. Psychiatry Res 1991; 37: 35–46
- Zhang Y, Raap DK, Garcia F, Serres F, Ma Q, Battaglia G, Van de Kar LD. Long-term fluoxetine produces behavior anxiolytic effects without inhibiting neuroendocrine responses to conditioned stress in rats. Brain Res 2000; 855: 58–66
- Zoeller RF, Jr. Physical activity: Depression, anxiety, physical activity, and cardiovascular disease: What's the connection?. Am J Lifestyle Med 2007; 1: 175–180
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav Immun 2001; 15: 199–226
