Abstract
The ability to discriminate between spatial contexts is crucial for survival. This ability can be succinctly tested in the paradigm of fear renewal. In this paradigm, a change of spatial context results in robust renewal of conditioned fear, even if the conditioned fear has been previously extinguished. Chronic stress and environmental enrichment are known to affect learning and memory in opposite directions, with the former generally being deleterious. In this study, we examined the effects of chronic stress and enrichment on fear renewal in rats. Fear was evaluated as freezing responses to an auditory conditioning stimulus initially associated with footshocks in context A; fear extinction was evaluated in a novel spatial context (B) without the conditioned stimulus, and renewal in a third context (C) with the auditory cue. Specifically, we aimed to test if environmental enrichment can oppose the effects of chronic stress on fear renewal. We exposed different groups of adult male Wistar rats (6–12 per group) to 10 days of chronic stress (immobilization for 2 h daily), 14 days of enrichment, or a combination of both. We report that chronic stress compromised fear extinction and renewal. In contrast, enrichment re-established fear renewal in chronically stressed rats. Enhanced contextual modulation of fear memories in animals experiencing environmental enrichment while stressed could reflect an adaptive response. This could allow greater flexibility to optimize vigilance in differing spatial contexts.
Introduction
The ability to discriminate between two spatial contexts is crucial for survival, because it allows flexibility to manifest different behavioral responses in different environments. This becomes particularly important when contextual discrimination enables animals to inhibit or exhibit previously learned defensive behaviors. Because of the importance of contextual information, animals encode information about spatial contexts during learning. This contextual information is used to gate future retrieval of memory. Such contextual gating has been demonstrated in a variety of species, ages and behavioral tasks, suggesting a privileged role for contextual processing (Rovee-Collier and Dufault Citation1991; Bouton Citation2004; Bouton et al. Citation2006; Parker et al. Citation2006; Effting and Kindt Citation2007).
Fear renewal is a succinct model to study the interplay of context and memory retrieval (Bouton Citation2004; Bouton et al. Citation2006). In this paradigm, a change of context after extinction results in robust return of the conditioned response. In the first stage, animals are conditioned to associate a stimulus with an aversive event such as electrical foot shock. In the second stage, the contingency between conditioned stimulus and unconditioned stimulus is weakened by repeated presentation of the conditioned stimulus alone. This results in formation of the new memory of fear extinction that overlays previous fear conditioning. In the third stage, the conditioned stimulus is presented in a context different from where extinction occurred. This results in renewed expression of fear, demonstrating that extinction memory is gated by contextual information. The dorsal hippocampus and its influence on the amygdala and prefrontal cortex are important for contextual gating in this model (Bouton et al. Citation2006; Ji and Maren Citation2007).
A variety of environmental changes leave their mark on behavior. Exposure to chronic stress, for example, interferes with hippocampus dependent memory (Luine et al. Citation1994; Diamond et al. Citation1996; Kim et al. Citation2007). In contrast, exposure to an enriched environment enhances hippocampus dependent behavioral performance (Bruel-Jungerman et al. Citation2005; Gaulke et al. Citation2005; Leggio et al. Citation2005; Segovia et al. Citation2006; Wright and Conrad Citation2007; Yang et al. Citation2007).
These studies indicate that chronic stress and enrichment can have opposing effects upon spatial tasks dependent on contextual cues and upon structure of the hippocampus, a brain region important for contextual processing. Despite these indications, it is not known if these environmental manipulations can affect contextual gating of memories. In the present report, we investigated the effects of chronic stress and enrichment on acuity of contextual processing in rats. Specifically, we investigated if stress and enrichment affect fear renewal, i.e., contextual modulation of memory after extinction. We hypothesized that chronic stress will compromise fear renewal and enrichment of stressed animals will re-establish fear renewal. Thus, rats were exposed to chronic stress, enrichment or a combination of both. Subsequently, fear renewal in a novel context was tested by measuring fear response to a conditioned auditory cue that had been previously extinguished.
Materials and methods
Animals
Adult male Wistar rats (10 weeks old at start of experiment) were obtained from a commercial supplier (Charles River, Wilmington, MA, USA). Upon arrival, rats were housed in standard laboratory cages (3 rats per cage) with food and water ad libitum and a day-night cycle of 14:10 h (lights on at 7 am). After 2–3 days of habituation in standard cages, rats were divided into four treatment groups: (1) controls (no treatment); (2) enriched (14 days of environmental enrichment); (3) stress (10 days of chronic repeated immobilization stress); and (4) stress and enrichment running concurrently. All procedures related to animal maintenance and experimentation were approved by the Stanford University's Administrative Panel on Laboratory Animal Care (APLAC) and were in accordance with animal care standards outlined in National Institute of Health (USA) guidelines.
Treatment
Chronic stress consisted of 2 h of immobilization stress (10 am–12 noon) daily, repeated for 10 days. Rats were completely immobilized in Harvard immobilization bags (Restraint Cones; Harvard Apparatus, South Natick, MA, USA). Immobilization involved placing the rat in a cone-shaped plastic restraint bag, with an opening in the narrow end to permit breathing. The wide end of the bag was closed by tape at the base of the tail. This stress paradigm is known to induce behavioral alterations and structural changes in the brain (Mitra et al. Citation2005; Vyas et al. Citation2003). Stress reduced relative body weight gain of the rats (average % gain in body weight over 14 days: 19% in controls and 11% in the stress group, p < 0.01, Student's t-test).
Enrichment consisted of 14 days of housing in an enriched environment. The enriched environment composed of placing rats (3/cage) in a bigger cage (dimension; 60 × 60 × 60 cm) compared to the standard laboratory cage (45 × 24 × 20 cm) with wire-net walls for climbing in the enrichment-cages. The enrichment cages were provided with cylindrical burrowing tubes, toys, nesting material, climbing planks, steel chains, jingle-bells, fruit-flavored chews, water and regular food (rat chow) mixed with flavored cereals, and sunflower seeds. Every 4 days, the arrangement of the environmental stimuli was changed. Enrichment did not affect relative body weight gain of the rats (average % gain in body weight: 19% in Controls and 18% with enrichment).
Rats undergoing both stress and enrichment were housed in the enriched environment and removed daily for the immobilization procedure. After termination of the repeated stress (10 days), rats were housed in enriched cages for four additional days (total of 14 days in enriched cages) before behavioral testing began. This duration of enrichment (14 days) was chosen to match closely the duration of stress (10 days). We did not know, a priori the length of enrichment necessary to reverse the stress effect. Thus, we started by testing effects of enrichment on stressed rats at two weeks after the start of experiment. Enriched rats were housed in enriched cages throughout the period of behavioral testing; non-enriched rats (groups: stress alone and no stress, no enrichment) were housed in the standard laboratory cages. Control rats were not exposed to either stress or enrichment.
Behavioral apparatus
The experiments were conducted in two identical modified observation chambers (30 × 24 × 21 cm; Med Associates, St. Albans, VT, USA), placed in sound-attenuating cabinets. Construction of the observation chamber was similar to that described previously (Ji and Maren Citation2005). The floor of the chambers consisted of steel rods placed 1.5 cm apart. These rods were attached to a shock-generator and a solid-state shock scrambler to deliver electrical foot- shocks. A loudspeaker mounted on the wall of the chamber delivered the auditory tones used for conditioning.
Fear was quantified in terms of percentage freezing, i.e., cessation of all movements except breathing (Ji and Maren Citation2005; LeDoux et al. Citation1984). A load-cell platform recorded locomotor activity of the rats, as measured by the chamber displacement. Activity samples were collected at 5 Hz (one sample every 200 ms). Load-cell amplifiers of both chambers were calibrated to a fixed displacement. Before the start of the experiment, rats of similar weight and age were used for the calibration. Gain of amplifiers was calibrated to provide for the highest resolution possible at the lower range of locomotor activity. A pre-defined freezing threshold was applied to the amplifier output in order to separate freezing from movement. An experimental rat had to show activity below this threshold for at least one second (five successive sample points at 5 Hz) before it was deemed to be frozen. Values for freezing obtained by this method were comparable to those obtained by an experienced observer by visual examination of the video records.
Three different contexts were used. For the first context (context A), chambers were placed opposite a wall with red plastic strips, a house light mounted near the chambers was switched on the chambers were cleaned with 70% alcohol, the room lights were kept lit and the floor of the chamber consisted of exposed steel grids. Rats were transported to context A in standard laboratory rat cages (45 × 24 × 20 cm) without bedding.
The second context (context B) had white walls facing the chambers, the chamber light and room light were switched off, an exhaust fan mounted on the chamber provided constant background noise the chambers were cleaned with 1% acetic acid and the steel grids on the chamber floor were covered by a perforated plastic sheet. Rats were transported to Context B in standard laboratory mice cages (28 × 17.5 × 12 cm) with bedding.
Finally, the third context (context C) consisted of a distinct set of visual cues including cabinets and tables on the room floor, the door of the sound-attenuating cabinets were kept open, the chamber light was switched off, the room light was switched on, the chambers were cleaned with 5% ammonium hydroxide and the chamber floor was covered with wire mesh. Rats were transported in this context in thermocol boxes lined with animal bedding.
These contexts differed from each other in terms of spatial arrangement, prevailing odor, presence or absence of background noise generated by a fan on the wall of the chamber, the type of boxes used to carry rats to the observation room, ambient light conditions and the texture of the chamber floor.
Behavioral testing
On the day of training, rats were placed in the observation chamber in a specific context (context A). Beginning 3 min after being placed in the chamber, the rats were presented with three successive auditory tones (5 kHz, 80 dB, 10 s and inter-trial duration = 90 s) co-terminating with footshock (1 mA, 1 s). This resulted in Pavlovian conditioning to the tone. Rats were returned to their home-cage 200 s after the termination of the final footshock.
One day after training, the strength of conditioning to the training context (context A) was measured by placing rats in the same context for 10 min. The next day, the strength of conditioning to the auditory cue was measured as percentage freezing in response to a continuous tone (duration = 3 min, 5 kHz, 80 dB; starting three minutes after being placed in the chamber) in a novel context (context B). Thus, testing for the contextual conditioning preceded the testing for cued conditioning. Two days after initial training, rats were placed in context B and presented with 30 successive auditory tones (5 kHz, 80 dB, duration = 10 s, inter-trial duration = 50 s) to measure the extinction of cued fear conditioning. The first presentation of the tone started three minutes after rats were placed in the chamber.
After extinction of cued fear in context B, rats were placed in another novel context (context C). Renewal of fear in this novel context was measured as freezing in response to five successive conditioned tones (5 kHz, 80 dB, 10 s, inter-trial duration = 50 s).
Statistical analysis
Values are reported as means ± SEM. Statistical analysis was performed using two-way analysis of variance (ANOVA) with stress and enrichment as inter-subject sources of the variance. In circumstances where an intra-subject source of variance was present, analysis was performed using two-way ANOVA with repeated measures. Omnibus F-values and significance levels achieved with ANOVA are listed in . In addition to calculating omnibus F-values, we also conducted two planned comparisons: (1) between control and stress rats; and (2) between stress rats with or without enrichment. Planned comparisons were conducted using Student's t-test.
Table I. F-values and degrees of freedom (df) derived from ANOVA.
Results
Neither stress nor enrichment affected freezing during conditioning
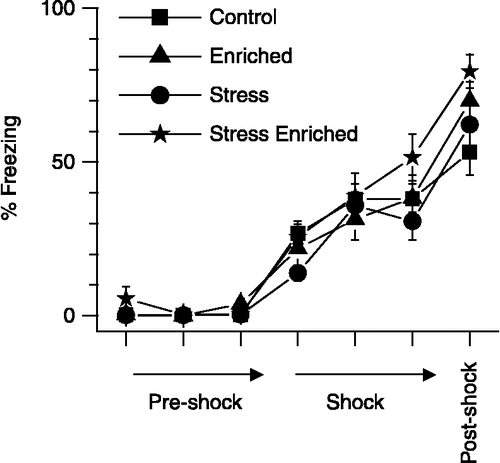
On the day of training, rats were presented with three pairs of tone and footshock in context A. This allowed conditioning of both tone and spatial context with aversive foot shocks. During the course of this training, footshocks elicited significant freezing that was similar across all experimental groups (). Freezing exhibited by all experimental groups was similar in magnitude both at the presentation of the last tone-footshock pair and during the post-shock period.
Figure 1 Freezing exhibited by rats exposed to stress and enrichment, during conditioning (tone and footshock pairing). The ordinate (y-axis) depicts percentage freezing before (first three points; inter-block interval = 60 s; pre-shock), during (next three points; inter-block intervel = 90 s; shock) and after (last point; 180 s post-shock) the presentation of tone and footshock pairs. Neither stress nor enrichment affected freezing during conditioning. Two-way ANOVA with repeated measures; n = 11 − 12 rats per group. Values are group means ± SEM.
Neither stress nor enrichment affected fear conditioning to auditory cue or to training context
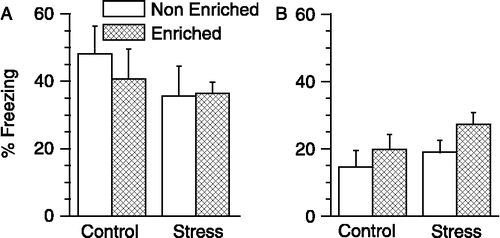
Two days after the conditioning, rats were placed in a novel unconditioned context (context B) and exposed to the conditioned tone. This allowed us to measure cued conditioning (conditioning to the tone) without interference from previously conditioned context (i.e., context A). The conditioned tone elicited similar freezing in all experimental groups (see ). Preceding this, one day after conditioning, rats were exposed to context A, without the presentation of tone. This allowed us to measure contextual fear conditioning without interference from conditioned cue (tone). All experimental groups exhibited significant and similar freezing in the conditioned context (see ).
Figure 2 Effects of stress and enrichment on cued and contextual fear conditioning. The ordinate depicts percentage freezing during the presentation of the conditioned tone. The freezing observed is specific to the conditioning, in view of a low level of freezing obtained in a novel context before presentation of tone-shock pairs (Figure 1, pre-shock). Neither chronic stress nor environmental enrichment affected fear conditioning to an auditory cue (A) or to the training context (B). Two-way ANOVA. n = 11 − 12 rats per group. Values are means ± SEM.
Stress reduced freezing during extinction of cued fear
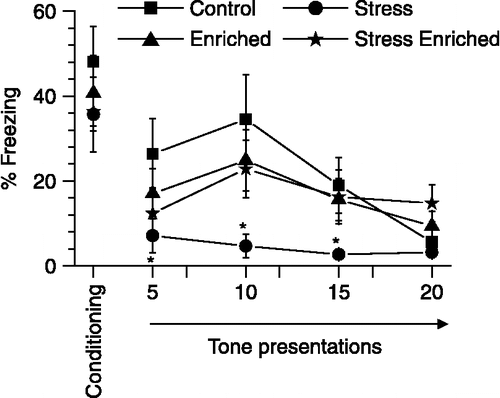
One day later, the rats were again exposed to the conditioned tone while in context B. The cued fear response in this spatial context extinguished (i.e., the time spent in freezing declined with repeated tone presentation, as compared with the level of freezing at the end of conditioning; ), such that by the 20th presentation, all experimental groups spent less than 15% of time freezing. No significant differences between experimental groups were evident at this time point (One-way ANOVA, percentage freezing obtained between 16 and 20th presentation of tone. F(3,43) = 2.5, p > 0.05). Additionally, a two-way ANOVA with repeated measures revealed that the amount of freezing exhibited across presentations decreased (main effect of blocks, p < 0.05). Significant interaction between blocks, stress and enrichment was not evident (p > 0.35). Interestingly, exposure to stress appeared to reduce freezing across all presentations, although this effect failed to reach statistical significance (p = 0.075 for main effect of stress) in two-way ANOVA. Nevertheless, planned comparison revealed that stressed rats exhibited significantly less freezing than control rats during the first 15 trials (p < 0.05; ) during extinction, with differences between them becoming insignificant during the 16–20th tone presentation. Enrichment of stressed rats showed significantly more freezing than non-enriched stressed rats during the last 15 trials of extinction (p < 0.05).
Figure 3 Effects of stress and enrichment on extinction of cued fear. The ordinate depicts percentage freezing obtained during first tone presentation (also depicted in Figure 2A) and four consecutive bins (1 bin = 5 tone presentations) while rats were in a context different from that for training. Stress alone reduced freezing to the tone during extinction trials. Two-way ANOVA with bins as repeated measures. n = 11 − 12 rats/group. *p < 0.05, planned comparison between stress and control rats without enrichment. Values are group means ± SEM.
Stress and enrichment had opposing effects on renewal of fear in a novel spatial context
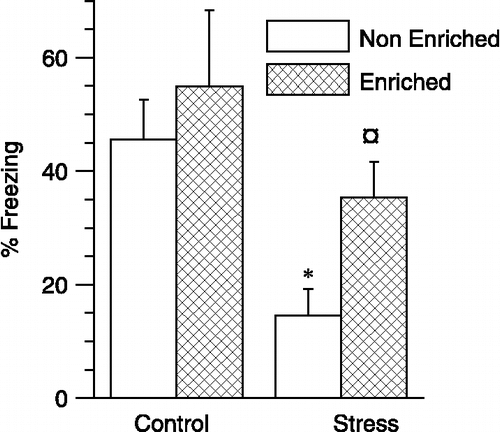
One day later, rats were exposed to five successive conditioned tones in a novel spatial context (context C). Control rats (i.e., without exposure to stress or enrichment) exhibited robust freezing to the cued stimulus in this context (). Indeed, the level of freezing obtained during fear renewal for control rats (46.6 ± 6.9%) was similar to that obtained during the initial testing for cued fear conditioning prior to extinction in Context B (48.1 ± 8.3%; p > 0.4, paired t-test). A two-way ANOVA revealed that stress significantly reduced fear renewal, as manifested by reduced freezing to the conditioned auditory cue in Context C (; F(1,36) = 11.9, p < 0.05). In contrast to the effects of stress, exposure to enrichment enhanced fear renewal (main effect of enrichment, two-way ANOVA; F(1,36) = 4.2, p < 0.05). Interaction between stress and enrichment was not statistically significant. The planned comparison revealed that exposure to stress reduced fear renewal in non-enriched rats, as demonstrated by a 68% reduction in freezing response to the tone (p < 0.01). Enrichment of stressed rats reinstated fear renewal (a 143% increase in freezing compared to stress alone group; p < 0.05), bringing the level of fear renewal comparable to that exhibited by control rats ().
Figure 4 Effects of stress and enrichment on renewal of cued fear. The ordinate depicts percentage freezing obtained during five tone presentations in a novel spatial context different from that for training and extinction. Stress reduced and enrichment enhanced renewal of cued fear. Two-way ANOVA. n = 10 rats for control, six rats for enriched, 12 rats for stress and 12 rats for stress+ enriched. *p < 0.05, planned comparison between stress and control rats without enrichment. ¤p < 0.05, planned comparison between stress rats with and without enrichment. Values are group means ± SEM.
Discussion
We report that stress and enrichment have opposing effects on fear renewal in rats. While stress, in itself, reduced renewal of fear in a novel context, environmental enrichment for stressed rats blocked this effect. Thus, when chronic stress and enrichment were combined, effects of enrichment predominated over those of stress.
There is now significant evidence that Pavlovian extinction does not involve destruction of original associative learning, and that much of the original conditioning survives extinction (Bouton Citation2004; Bouton et al. Citation2006). It has been suggested that extinction results in formation of a new memory that is overlaid on the original conditioning. A potent argument in favor of this view is context-specificity of the extinction memory. If animals are tested in an environment distinct from the extinction context, the conditioned response can be reinstated. Hence, in a way, extinction leaves the conditioned stimulus with two available associations, and spatial context is crucial in determining which one of these associations is retrieved during behavioral performance (Bouton Citation2004; Bouton et al. Citation2006). Thus, this paradigm offers a useful way to test if an experimental manipulation affects contextual processing and acuity. Our results show that stress reduced fear renewal and enrichment of stressed rats blocked this stress effect. Indeed, the conditioned response observed in enriched rats during renewal was comparable to that observed before the extinction. This indicates that enrichment during stress could enhance the ability of rats to discriminate between contextual cues relative to rats undergoing stress alone. It is advantageous for an animal to learn the association between spatial context and presence or absence of danger, since this allows animals to optimize vigilance and arousal. Thus, we suggest that the re-establishment of fear renewal in stressed animals by environmental enrichment represents an adaptive response in naturalistic circumstances.
The effects of environmental manipulations, like stress and enrichment, on fear renewal have not been previously studied. However, several recent reports have investigated neural mechanisms of renewal, mainly suggesting a crucial role for the dorsal hippocampus (Corcoran and Maren Citation2004; Corcoran et al. Citation2005; Ji and Maren Citation2005; Ji and Maren Citation2007). For example, both the renewal of conditioned response and the increase in lateral amygdala neuronal firing caused by context shift are impaired if the dorsal hippocampus is lesioned before testing (Bouton et al. Citation2006). It is relevant that both stress and enrichment affect the dorsal hippocampus. Chronic stress causes dendritic retraction in CA3 neurons and suppresses neurogenesis in the dentate gyrus (Magarinos and McEwen Citation1995; McEwen Citation1999; McEwen Citation2001; Lee et al. Citation2006; Kim et al. Citation2007; Thomas et al. Citation2007), while environmental enrichment has the opposite effects (Berman et al. Citation1996; Rampon et al. Citation2000; Bruel-Jungerman et al. Citation2005; Gaulke et al. Citation2005; Leggio et al. Citation2005; Segovia et al. Citation2006; Bindu et al. Citation2007). Moreover, while stress compromises hippocampal-dependent spatial memory (Luine et al. Citation1994; Diamond et al. Citation1996; Kim et al. Citation2007;), enrichment has a positive outcome on spatial memory and other cognitive tasks (Rampon et al. Citation2000; Leggio et al. Citation2005; Wright and Conrad Citation2007; Yang et al. Citation2007). It is possible that the compromised fear renewal brought about by stress and the increased fear renewals brought about by enrichment in these animals are related to the effects of each of these manipulations on the dorsal hippocampus. An alternative explanation will be that in the presence of enrichment, a stressful stimulus is less effective. Thus, it appears likely that the effects of enrichment are related to counteracting damaging effects of stress on these same brain regions as well as countering the effects of stress per se.
While stress and enrichment affected renewal of conditioned fear in the present study, contextual fear memory was not affected. Previous studies have shown that extinction learning is more context-specific than original conditioning itself (Harris et al. Citation2000; Bouton Citation2004). Indeed, it has been suggested that extinction enables contextual gating of a conditioned response (Bouton Citation2004; Bouton et al. Citation2006; Ji and Maren Citation2007). Thus, it is likely that demands on contextual processing are greater in the case of fear renewal than in initial contextual fear conditioning; this would make it likely that the effects of treatment will be more readily observed in fear renewal.
The amygdala and its interactions with the prefrontal cortex are involved in extinction of conditioned fear (Corcoran and Quirk Citation2007). Both of these structures also exhibit plastic changes in response to stress, including reduced spine density in the medial prefrontal cortex and increased density in the amygdala (Mitra et al. Citation2005; Murmu et al. Citation2006; Radley et al. Citation2006). Since the prefrontal cortex exerts a facilitating influence on fear extinction through its interaction with the amygdala (Akirav and Maroun Citation2007), we expected that stress would induce a slower extinction. On the contrary, we observed that stress did not affect fear conditioning per se and, in fact, caused a faster extinction, relative to levels of freezing at the end of conditioning among the groups. It should also be noted here, that chronic stress has previously been reported to enhance fear conditioning (Servatius and Shors Citation1994; Conrad et al. Citation1999; Rodriguez Manzanares et al. Citation2005). This discrepancy in effects of stress on fear conditioning and extinction may be due to differences between the previous and the present studies in the type of stressor (immobilization in our study in comparison to inescapable and acute restraint stress; Conrad et al. Citation1999; Corcoran et al. Citation2005), length of stress paradigms (2 h/day immobilization for 10 days in this report in comparison with 21 days of restraint stress or prenatal stress Murmu et al. Citation2006; Radley et al. Citation2006), and time elapsed between termination of stress and behavioral testing (4–6 days after the termination of stress in comparison with two days or several week after termination of stress; Servatius and Shors Citation1994; Murmu et al. Citation2006).
Stressed rats exhibited a low level of freezing compared to the other three groups during the extinction trials. Since no differences between control and stressed rats were discerned during the training and conditioning trials, reduced freezing during extinction appears specific to extinction learning. Therefore, stressed rats exhibited faster extinction. Alternatively, it can be argued that a low level of freezing during extinction could influence the freezing of the stressed rats during renewal. Therefore, it could be possible that in the case of the stressed rats, the association between tone and fear was too weak by the end of the extinction procedure, hence the magnitude of renewal was compromised. Due to a paucity of relevant literature, we cannot discount any of these possibilities. Even in the face of these alternative interpretations, it is important to emphasize that the effects of stress in the present study were blocked by enrichment during fear extinction and renewal.
Different forms of stress have also been shown to influence fear extinction (Izquierdo et al. Citation2006; Miracle et al. Citation2006). Some of them specifically affect extinction without affecting conditioning (Izquierdo et al. Citation2006), similar to the present findings. However, the effects of stress on extinction and also the stress paradigms used previously are distinct from our study. It is interesting to note that some stress paradigms influence conditioning, some influence extinction and some influence both. Hence, the nature of the stress directs the outcome on specific forms of fear memory.
In this study, for the first time, we examined the effects of two different environmental manipulations, stress and enrichment presented concurrently, on conditioned fear and its retrieval. While stress or enrichment had no effect on fear conditioning, stress compromised both extinction and renewal. Enrichment on the other hand did not affect extinction and re-established context-dependent fear renewal in stressed rats.
In conclusion, we have demonstrated that environmental enrichment can affect contextual modulation of fear memories. Moreover, such enrichment can enhance contextual processing in fear renewal and predominate over the opposing effects of stress.
Acknowledgements
We thank Dr I. Hairston for helpful suggestions. This work was supported by the National Institute of Health, USA (RO1 AGO20633).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akirav I, Maroun M. The role of the medial prefrontal cortex–amygdala circuit in stress effects on the extinction of fear. Neural Plast 2007; 30873
- Berman RF, Hannigan JH, Sperry MA, Zajac CS. Prenatal alcohol exposure and the effects of environmental enrichment on hippocampal dendritic spine density. Alcohol 1996; 13: 209–216
- Bindu B, Alladi PA, Mansooralikhan BM, Srikumar BN, Raju TR, Kutty BM. Short-term exposure to an enriched environment enhances dendritic branching but not brain-derived neurotrophic factor expression in the hippocampus of rats with ventral subicular lesions. Neuroscience 2007; 144: 412–423
- Bouton ME. Context and behavioral processes in extinction. Learn Mem 2004; 11: 485–494
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry 2006; 60: 352–360
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci 2005; 21: 513–521
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 1999; 113: 902–913
- Corcoran KA, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem 2004; 11: 598–603
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr 2007; 12: 200–206
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci 2005; 25: 8978–8987
- Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci 1996; 110: 661–672
- Effting M, Kindt M. Contextual control of human fear associations in a renewal paradigm. Behav Res Ther 2007; 45: 2002–2018
- Gaulke LJ, Horner PJ, Fink AJ, McNamara CL, Hicks RR. Environmental enrichment increases progenitor cell survival in the dentate gyrus following lateral fluid percussion injury. Brain Res Mol Brain Res 2005; 141: 138–150
- Harris JA, Jones ML, Bailey GK, Westbrook RF. Contextual control over conditioned responding in an extinction paradigm. J Exp Psychol Anim Behav Process 2000; 26: 174–185
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 2006; 26: 5733–5738
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem 2005; 12: 270–276
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus 2007; 17: 749–758
- Kim JJ, Lee HJ, Welday AC, Song E, Cho J, Sharp PE, Jung MW, Blair HT. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proc Natl Acad Sci USA 2007; 104: 18297–18302
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci 1984; 4: 683–698
- Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, Moon BH, Cho E, Lee MS, Chun BG, Shin KH. Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Exp Mol Med 2006; 38: 44–54
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res 2005; 163: 78–90
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639: 167–170
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience 1995; 69: 83–88
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci 1999; 22: 105–122
- McEwen BS. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann NY Acad Sci 2001; 933: 265–277
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 2006; 85: 213–218
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA 2005; 102: 9371–9376
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci 2006; 24: 1477–1487
- Parker LA, Limebeer CL, Slomke J. Renewal effect: context-dependent extinction of a cocaine- and a morphine-induced conditioned floor preference. Psychopharmacology (Berl) 2006; 187: 133–137
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 2006; 16: 313–320
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1–knockout mice. Nat Neurosci 2000; 3: 238–244
- Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci 2005; 25: 8725–8734
- Rovee-Collier C, Dufault D. Multiple contexts and memory retrieval at three months. Dev Psychobiol 1991; 24: 39–49
- Segovia G, Yague AG, Garcia-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res Bull 2006; 70: 8–14
- Servatius RJ, Shors TJ. Exposure to inescapable stress persistently facilitates associative and nonassociative learning in rats. Behav Neurosci 1994; 108: 1101–1106
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci 2007; 27: 2734–2743
- Vyas A, Mitra R, Chattarji S. Enhanced anxiety and hypertrophy in basolateral amygdala neurons following chronic stress in rats. Annals of New York Academy of Sciences 2003; 985: 554–555
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav Brain Res 2007; 187: 41–47
- Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, Xu L, Li L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem 2007; 87: 257–263
