Abstract
The ILSI Research Foundation convened a cross-disciplinary working group to examine current approaches for assessing dose-response and identifying safe levels of intake or exposure for four categories of bioactive agents—food allergens, nutrients, pathogenic microorganisms, and environmental chemicals. This effort generated a common analytical framework—the Key Events Dose-Response Framework (KEDRF)—for systematically examining key events that occur between the initial dose of a bioactive agent and the effect of concern. Individual key events are considered with regard to factors that influence the dose-response relationship and factors that underlie variability in that relationship. This approach illuminates the connection between the processes occurring at the level of fundamental biology and the outcomes observed at the individual and population levels. Thus, it promotes an evidence-based approach for using mechanistic data to reduce reliance on default assumptions, to quantify variability, and to better characterize biological thresholds. This paper provides an overview of the KEDRF and introduces a series of four companion papers that illustrate initial application of the approach to a range of bioactive agents.
INTRODUCTION
Protection of the general population and susceptible subpopulations from bioactive agents that may pose health risks is a primary public health challenge. The agents of potential concern include food allergens, pathogens, environmental chemicals (i.e., chemicals introduced into the environment via industrial processes, consumer products, etc.), and high intakes of certain nutrients. For each of the many potential exposure scenarios—chemical contaminants in consumer products, pathogens in water, allergens in food, nutrients in dietary supplements, etc.—public health authorities must make judgments regarding “safe levels” (i.e., levels of exposure or intake where risk is considered negligible).
Ideally, determinations regarding safe levels are informed by a thorough knowledge of the dose-response relationship for the agent and population of concern. In practice, however, decisions must often be made without complete information, requiring the use of extrapolation and assumptions. Also, each type of bioactive agent has its own particular challenges in assessing dose-response. In the case of pathogenic microorganisms, for example, the bioactive agent may multiply after intake, substantially increasing the dose. With essential nutrients, adverse effects may potentially result from deficient intake as well as excess intake. With food allergens, both the nature and the severity of the response to a given dose vary considerably even within the susceptible subpopulation. In general, discipline-specific approaches for identifying safe levels have evolved. However, the disciplines share some fundamental challenges regarding dose-response assessment, and there is a common need to advance methodology and to harmonize approaches to the extent possible.
To address this need, the International Life Sciences Institute Research Foundation (ILSI RF) convened a working group with experts from a range of disciplines, including cancer risk assessment, microbial risk assessment, food allergies, nutrition, and dose-response modeling. Working group participants are listed in .
Table 1 ILSI RF Threshold Working Group
The members of the group were asked to consider two main questions:
-
How can we make better use of current data and methods to advance understanding of dose-response relationships, especially with regard to dose levels relevant to public health?
-
How can we make practical use of an improved understanding of dose-response for assessing risk, developing regulatory standards, designing nutrition programs, etc.?
Initial discussions by the group considered a range of agent-endpoint combinations (e.g., chloroform and liver carcinogenicity, Listeria monocytogenes and fetal death), focusing on what is known about the fundamental biology. The cross-disciplinary approach promoted a fresh look at long-held assumptions and traditional approaches to assessing dose-response. In these initial discussions it became clear, however, that the term “threshold” is commonly used to refer to several diverse concepts. Thus, definitions were adopted for terms used in this effort, in particular, the biological threshold and the population threshold. These are discussed in the Appendix.
Despite the wide range of agents considered, the group came to develop and adopt a common analytical framework that is based on mode-of-action concepts. This paper describes this analytical approach, referred to as the “Key Events Dose-Response Framework,” and provides a summary of the general findings and conclusions from this effort.
THE KEY EVENTS DOSE-RESPONSE FRAMEWORK
Relationship to Mode-of-Action
The Key Events Dose-Response Framework (KEDRF) is largely based on “mode-of-action” (MOA) concepts, where MOA refers to the fundamental biological events and processes that underlie the effect of a bioactive agent. MOA is relevant to the range of life science disciplines, and investigations into MOA have a long history in pharmacology, toxicology, and medicine (CitationDuBois et al., 1949; CitationBueding and Mansour, 1957; CitationDaniel et al., 1966; CitationEldefrawi et al., 1970, CitationErlanger and Goode, 1967). In the field of risk assessment, MOA information has been particularly useful for evaluating potential chemical carcinogens (CitationCohen, 1995; CitationSonich-Mullin, 2001; CitationEPA, 2005). In recent years, the use of MOA information for risk assessment purposes has expanded substantially with the development of frameworks for evaluating the human relevance of MOA in experimental animals for carcinogens (CitationCohen et al. 2003; CitationKlaunig et al., 2003, CitationMeek et al., 2003; CitationBoobis et al., 2006) and for non-cancer effects (CitationSeed et al., 2005; CitationBoobis et al., 2008).
In the field of chemical risk assessment, MOA analysis starts with the identification of the specific effect of concern, and then identifies the series of key events that lead to this effect. The term “key event” has been defined as an empirically observable precursor step that is itself a necessary element of the MOA, or a biologically based marker for such an element (CitationSonich-Mullin et al., 2001). Hence, a key event is a necessary, though not a sufficient, step in a process that results in a specific adverse effect. In some MOA assessments for evaluating potential carcinogens, the focus has been on dynamic events. For example, in the EPA Guidelines for Carcinogen Risk Assessment (CitationEPA, 2005), the toxicokinetic processes that lead to formation of the active agent or its distribution to the target tissue are considered in estimating dose, but they are not considered as part of the mode of action.
Similar to a MOA analysis, the first step in a Key Events Dose-Response Analysis (KEDRA) involves specifying the effect of concern for the agent of interest.Footnote 1 Also, similarly, the Key Events in a KEDRA are necessary precursor steps. But, as illustrated by some of the papers in this series, a KEDRA may also include substantial consideration of kinetic events (i.e., absorption, distribution, metabolism, etc.) in addition to dynamic events in the target tissue. The novel contribution of the KEDRF is that it outlines a systematic analytical approach to examining in detail the fundamental determinants of dose-response relationships, including determinants of variability in such relationships. The KEDRF provides a foundation for more rigorous and quantitative descriptions of dose-response at dose levels relevant to public health. Moreover, this analytical approach, which focuses at the level of key events, is applicable across a range of bioactive agents.
Framework Elements and Process
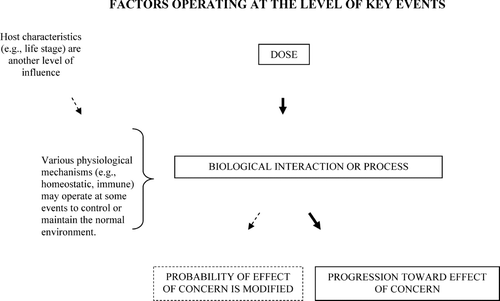
As noted above, one of the first tasks in conducting a KEDRA is to describe the pathway of key events occurring between the initial exposure and the effect of concern. The analysis then proceeds to examine what is known about the dose-response relationship for each individual event, including whether there is scientific evidence for the existence of a threshold at any of the events. Generally, the key events pathway is composed of several kinetic and dynamic steps, which can be described with respect to relevant receptors, enzymes, etc. As shown in , at each event in the pathway several elements can be examined with regard to their influence on the dose-response relationship. These elements include i) the level and frequency of the dose (for the agent itself, its metabolite, or ultimate effector); ii) the specific biological interaction that occurs; and iii) the range of factors that may affect outcome of the interaction. These factors include various physiological mechanisms that serve to control or maintain the normal physiological environment, for example, homeostatic, repair, adaptive or immune mechanisms. In addition, there are a variety of host characteristics (for example, life stage, disease state, genetic makeup) that correspond to, and underlie, the inter- and intra-individual variability often observed in the dose-response. Note that one way in which these host characteristics may contribute to variability is by altering the effectiveness of physiological control mechanisms at individual events in the pathway.
Figure 1 Factors operating at the level of Key Events. In addition to dose, other factors may influence the outcome of an individual event. In combination, they may affect the likelihood of progression to the next event, or they may affect the magnitude of the ultimate response of concern.
Understanding the interplay of the various factors (dose, control mechanisms, host characteristics) at the level of individual events in the pathway is a prerequisite to refining the understanding of dose-response for both individuals and populations. In theory, it should be possible to describe a dose-response relationship for each individual event in the pathway, and to study i) how these individual dose-response relationships combine to generate the overall dose-response curve for the effect of concern, and ii) how interspecies, interindividual, and intraindividual differences affect specific events in the pathway, and consequently alter dose-response relationships.
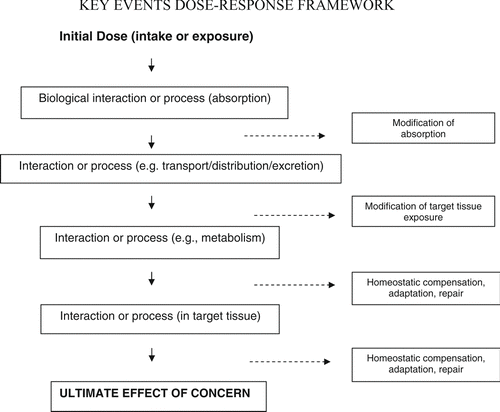
A schematic of the overall Key Events Dose-Response Framework is shown in . While the overall pathway consists of a chain of conditional events, certain events may be considered “control points” in that they engage one or more mechanisms (homeostatic, repair, etc.) to control or maintain the physiological environment. In order for the cascade of events to proceed toward the ultimate effect of concern, it appears these control mechanisms must be overwhelmed by dose, or otherwise fail. Understanding the conditions (i.e., the specific combination of dose level, homeostatic capacity, etc.) under which control can be lost is likely central to characterizing thresholds, including variability in their values. Theoretically, certain control points may play an especially critical role in a given pathway, i.e., they may be “determining events.” The outcome of a determining event greatly influences the likelihood of the ultimate effect of concern; it tends to “determine” whether the effect will occur given the initial dose.Footnote 2 Starting with this framework, the KEDRF proceeds to ask questions about individual events, and also about the overall series of events.
Figure 2 The Key Events Dose-Response Framework organizes available information on the multiple kinetic and dynamic events that occur between an initial dose and the effect of concern. Events are indicated generically here; but, for a given pathway, many specific kinetic and dynamic events may occur.
Questions about individual events may include:
-
What physiological mechanisms (homeostatic, immune response, adaptive, or repair) may come into play at this event, and how do they affect the outcome of the event? Can a “high enough” dose potentially overwhelm such mechanisms? How could this be further studied?
-
What host characteristics, for example, life stage, disease state, genetic makeup, exposure patterns, may modify (diminish or enhance) effectiveness of homeostatic or other such mechanisms at this event? To what extent might these factors explain the inter- and intra-individual variability? How could this be further studied?
-
What data would be needed to better characterize the dose-response relationship at this individual event? What evidence would be needed to support or refute the existence of a biological threshold at this event? What data would be needed to characterize the location/value of a threshold?
Questions about the overall pathway of events may then be asked, including:
-
Which events appear to be “control points,” i.e., they engage specific mechanisms that may influence the ultimate outcome (either the magnitude of the outcome, or the probability of the outcome)?
-
Is any particular event a potential “determining event,” i.e., its outcome has a disproportionate influence (compared to other events) on whether the ultimate effect of concern occurs?
-
Does the dose-response relationship at any particular event appear to drive the shape, slope, or position of the overall dose-response curve?
-
What metabolites or other biomarkers would indicate that control points have failed? Are these, potentially, early indicators of toxicity? How could they be studied?
The questions raised in the KEDRF may not generate ready answers; however, this type of analysis helps identify the data and knowledge most critically needed to better understand the determinants of the overall dose-response relationship.
Value of the Key Events Dose-Response Framework
The KEDRF is an analytical approach for evaluating currently available data and for focusing future research. It promotes an iterative scientific process that can be expected to continually strengthen the scientific basis for public health decision-making and regulatory standards. More specifically, this conceptual framework is expected to advance dose-response assessment in the following ways.
Refining Understanding of Dose-Response and Biological Thresholds
The KEDRF approach “deconstructs” the overall dose-response relationship into its essential elements—its Key Events, and the multiple factors influencing those events (i.e., dose level and frequency, homeostatic mechanisms, etc.). This systematic analytical approach sheds light on the fundamental determinants of the dose-response curve. Thus, the KEDRF provides a strategy for substantially refining dose-response assessment. This approach also promotes new insights and hypotheses regarding drivers of the dose-response relationship, early indicators of increased risk of an adverse effect (e.g., biomarkers indicating loss of homeostatic control) and first indicators of the effect.
Reducing Uncertainty in Population Thresholds
Uncertainty in population dose-response curves comes from many sources—inter- and intra-individual variability, interspecies differences, model uncertainty (e.g., extrapolation to low dose levels), etc. Fundamentally, this uncertainty is due to a lack of knowledge regarding exactly how the individual biological processes that underlie dose-response are affected by species, interindividual differences, etc. By promoting examination of these issues at their fundamental level, the KEDRF provides a bridge between available mechanistic data and practical use of such data to refine dose-response assessment.
Leveraging Data and Knowledge
The KEDRF approach promotes the best use of all available science. It complements and builds on the standard empirical, mechanistic, and modeling approaches currently used to study dose-response. Such approaches inform and refine the understanding of key events. A refined understanding in turn generates new hypotheses. Thus, the KEDRF promotes an iterative process that integrates available information, reveals critical knowledge gaps, and makes clear how newly generated data will be useful.
The KEDRF also leverages data across disciplines, and across bioactive agents. It provides a platform for sharing knowledge about cellular and molecular pathways that are relevant to diverse agents. In addition, the KEDRF approach helps draw connections between underlying biological processes and the effects observed at the population level. This leverages knowledge across distinct biological levels (e.g., the use of epidemiological methods to corroborate mechanistic studies of biomarkers).
General Conclusions
As noted initially, two broad questions were posed at the outset of this ILSI RF project: How can we utilize current science to improve understanding of dose-response, and how can we translate improved understanding into practical advances in risk assessment and public health decision-making? The working group answered with the development of an analytical framework, a way of thinking about dose-response that suggests a path forward. The KEDRF illuminates the connections between the processes occurring at a fundamental biological level and the outcomes observed at the individual and population levels. It is a mode-of-action, evidence-based approach that promotes use of data on key events to characterize dose-response relationships, including thresholds, and to reduce reliance on default assumptions.
It is important to note that the KEDRF's analytical approach complements the vision and strategy for toxicity testing outlined in a recent NRC report (CitationNational Research Council, 2007). While the NRC report focuses on chemicals, the developments it discusses in bioinformatics, systems biology, epigenetics, and computational toxicology are relevant to the range of bioactive agents considered in the present study. These developments are expected to transform the paradigm for toxicity testing into a system that integrates data generated at multiple levels (molecular and cellular assays, in vivo studies, in silico models, epidemiological observations, etc.). The KEDRF provides a platform for utilizing such data specifically to advance dose-response assessment for the range of agents of concern in public health and for informing the assessment of one agent by knowledge obtained from the assessment of others.
Initial Application of the Key Events Dose-Response Framework
The next four papers in this series describe how the KEDRF may be applied to a range of bioactive agents including DNA-reactive and non DNA-reactive carcinogens, endocrine disruptors, microorganisms, food allergens, and nutrients (CitationBoobis et al., 2009; CitationBuchanan et al., 2009; CitationTaylor et al., 2009; CitationRoss et al., 2009). Some of these studies consider specific agents (chloroform, an environmental toxicant; Listeria monocytogenes, a pathogenic microorganism; and Vitamin A, a nutrient). These agents were selected to illustrate the approach, and were not intended to be representative of all agents in their class. Other studies in this series discuss the application of the KEDRF approach to broad categories of agents (DNA-reactive carcinogens, food allergens, and endocrine disruptors). While most of the case studies consider agents found in food products, the KEDRF should be readily applicable to other scenarios and routes of exposure as well. Also, while the case studies consider only effects from excess intake or exposure, the KEDRF should also be applicable to examining the effects from inadequate intake of nutrients.
The general finding from this initial investigation is that the KEDRF is a promising analytical approach applicable to a wide range of bioactive agents. As will be seen in the documents that follow, the principal value of the approach at this time differs depending on the type of agent. For example, for food allergens, clinical data clearly demonstrate the presence of biological thresholds. The interindividual variability in threshold levels is remarkable, however. Thus for allergens, the KEDRF is likely to be especially valuable in pinpointing specific biological processes responsible for variability, or perhaps biomarkers that can differentiate levels of sensitivity. In contrast to food allergens, for invasive or toxicoinfectious microorganisms, the widely accepted assumption is that ingestion of even a single pathogenic cell has the potential to cause illness. Even within susceptible subpopulations, however, ingestion does not lead invariably to adverse effects. A rigorous examination of the dynamic interplay between the pathogen and the host at specific key events is needed to tease out the pathogen and host variables that determine whether illness will occur. For nutrients, kinetic processes designed to accommodate daily variations in dose (nutrient intake) may play an especially critical role in determining whether high intake levels lead to adverse effects. Further investigation of how these processes operate at control points in the key events cascade should contribute important insights for characterizing tolerable upper levels. For carcinogens, the KEDRF provides further insight into the types of information that would obviate the need for the default assumption of linear low-dose extrapolation in the risk assessment of a chemical. It would also enable better integration of chemical-specific information into such assessments.
APPENDIX
Threshold Concepts and Definitions
The term “threshold” is used frequently in the fields of health risk assessment, food safety, and nutrition. Multiple regulatory agencies and public health organizations use the term, and in some cases define the term. Some examples of definitions are provided in .
Table A-1 Some authoritative definitions of “threshold”
As is apparent from , the term “threshold” is commonly interpreted to mean a dose below which no adverse effect is observed or expected. It is important to recognize both aspects—observation and expectation—as observation alone usually cannot establish a threshold, due to limitations of the sensitivity of measurement methods. “Expectation” of a threshold is based on knowledge of the underlying biology of the relevant processes.
Also, none of the definitions listed in differentiate between two distinct but related concepts, biological thresholds and population thresholds. Thus, for purposes of the present project, the ILSI RF working group developed two definitions.
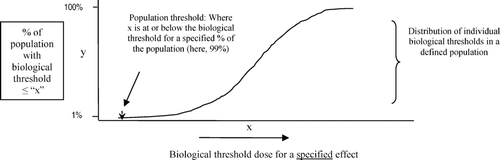
As used in this project, a biological threshold is the transition point (observed or expected) between the highest dose that will not elicit a given biological effect, and the lowest dose that will.Footnote 3 Biological thresholds are not equivalent to No-Observed-Effect-Levels (NOELs). The location or value of a biological threshold, the actual point of transition between two qualitatively different physiological states, is determined by fundamental properties of an organism, whereas the values of NOELs are affected by study design, for example, dose selection or sample size. NOELs may, however, help to locate the dose range in which a biological threshold likely exists, that is, a biological threshold for a given effect is generally assumed to be greater than its NOEL but lower than its Lowest-Observed-Effect-Level (LOEL). However, due to statistical variation and lack of sensitivity or precision in measuring techniques, it is seldom possible to identify the exact location of a biological threshold. Biological thresholds can be further described by the following characteristics:
-
Biological thresholds can occur at various levels of biological organization, for example, at subcellular, cellular, tissue, and organism levels.
-
There is a fundamental interconnection between biological thresholds and the maintenance of homeostasis. Homeostatic feedback mechanisms engage in response to a perturbation when physiological conditions exceed a set point, and disengage when the physiological environment is restored to within tolerable parameters. The points at which such mechanisms engage, and then disengage, are examples of biological thresholds.
As used in this project, a population threshold is an estimate of a biological threshold for a specified effect that holds for a specified proportion of a defined population. Thus, at doses above the population threshold, y% of the population will show the response of interest, and (100-y)% will not. In theory, the population threshold is derived from the distribution of biological thresholds for individuals in the population; thus it takes into account interindividual variability (in genetic makeup, exposure patterns, disease states, etc), as illustrated in .
ACKNOWLEDGMENTS
This paper is one of the work products of an international group of experts convened by the International Life Sciences Institute (ILSI) Research Foundation. ILSI is a nonprofit, worldwide organization established in 1978 to advance the understanding of scientific issues relating to nutrition, food safety, toxicology, risk assessment, and the environment. The ILSI Research Foundation was established in 1984 to create a vehicle for ILSI to support research. Its risk assessment program sponsors, organizes, and participates in a wide range of activities to develop and disseminate new scientific knowledge, encourage exchange of ideas, and build consensus among scientists from academia, industry, government, and public interest groups in its efforts to improve the scientific basis of risk assessment.
Financial support for this project from the following sources is gratefully acknowledged: ILSI Research Foundation, Health Canada, Ajinomoto, Coca-Cola Company, Groupe Danone, Kellogg Company, Kraft Foods, Inc., Mars, Inc., Mead Johnson Nutritionals, Monsanto Company, Nestlé, PepsiCo, Inc., The Procter & Gamble Company, Syngenta Ltd., Flavor Extract Manufacturers Association, and Grocery Manufacturers Association. Assistance from Ms. Julie Fitzpatrick with coordination of the final preparation of these papers is also gratefully acknowledged.
Declaration of Interests : All authors declare that they have no relevant interests to disclose.
Notes
*Threshold Project Steering Committee Member.
† Currently, Scientific Consultant, Rockville, MD, USA.
1 Note that the KEDRA approach may also be applied to categories of agents or endpoints, as is shown in the allergen case study (CitationTaylor et al., 2009).
2 The concept of a determining event is discussed further in a separate paper in this series (CitationRoss et al., 2009).
3 “Dose” may refer to the initial dose, exposure or intake level – or to an internal dose of the parent compound, its metabolite, or a resulting effector.
* Currently, Scientific Consultant, Rockville, MD
REFERENCES
- Boobis , A. R. , Cohen , S. M. , Dellarco , V. , McGregor , D. , Meek , M. E. , Vickers , C. , Willcocks , D. and Farland , W. 2006 . IPCS framework for analyzing the relevance of a cancer mode of action for humans . Crit. Rev. Toxicol. , 36 ( 10 ) : 781 – 792 .
- Boobis , A. R. , Doe , J. E. , Heinrich-Hirsch , B. , Meek , M. E. , Munn , S. , Ruchirawat , M. , Schlatter , J. , Seed , J. and Vickers , C. 2008 . IPCS framework for analyzing the relevance of a noncancer mode of action for humans . Crit. Rev. Toxicol. , 38 ( 2 ) : 87 – 96 .
- Boobis , A. R. , Daston , G. , Preston , J. and Olin , S. 2009 . Application of Key Events Analysis to Chemical Carcinogens and Noncarcinogens . Crit. Rev. Food Sci. Nutr. , 49 : 690 – 707 .
- Buchanan , R. L. , Havelaar , A. H. , Smith , M. A. , Whiting , R. C. and Julien , E. 2009 . The Key Events Dose-Response Framework: Its potential for application to foodborne pathogenic microorganisms . Crit. Rev. Food Sci. Nutr. , 49 : 718 – 728 .
- Bueding , E. and Mansour , J. M. 1957 . The relationship between inhibition of phosphofructokinase activity and the mode of action of trivalent organic antimonials on Schistosoma mansoni . Br. J. Pharmacol. Chemother. Jun , 12 ( 2 ) : 159 – 165 .
- Cohen , S. M. 1995 . Role of urinary physiology and chemistry in bladder carcinogenesis . Food Chem. Toxicol. , 33 ( 9 ) : 715 – 730 .
- Cohen , S. M. , Meek , M. E. , Klaunig , J. E. , Patton , D. E. and Fenner-Crisp , P. A. 2003 . The human relevance of information on carcinogenic modes of action: Overview . Crit. Rev. Toxicol. , 33 ( 6 ) : 581 – 589 .
- Daniel , M. R. , Dingle , J. T. , Glauert , A. M. and Lucy , J. A. 1966 . The action of excess of vitamin A alchohol on the fine structure of rat dermal fibroblasts . J. Cell Biol. , 30 ( 3 ) : 465 – 475 .
- DuBois , K. P. , Doull , J. , Salerno , P. R. and Coon , J. M. 1949 . Studies on the toxicity and mechanism of action of p-nitro phenyl diethyl thione phosphate (parathion) . J. Pharmacol. Exp. Ther , 95 : 79 – 91 .
- Eldefrawi , M. E. , Eldefrawi , A. T. and O'Brien , R. D. 1970 . Mode of action of nicotine in the housefly . J. Agric. Food Chem. , 18 ( 6 ) : 1113 – 1116 .
- EPA (U.S. Environmental Protection Agency) . 2005 . Guidelines for Carcinogen Risk Assessment Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=116283 EPA/630/P-03/001F. Risk Assessment Forum. Washington, DC [Accessed June 3, 2009]
- Erlanger , B. F. and Goode , L. 1967 . Mode of action of penicillin . Nature , 213 : 183 – 184 .
- Klaunig , J. E. , Babich , M. A. , Baetcke , K. P. , Cook , J. C. , Corton , J. C. , David , R. M. , DeLuca , J. G. , Lai , D. Y. , McKee , R. H. , Peters , J. M. , Roberts , R. A. and Fenner-Crisp , P. A. 2003 . PPAR alpha agonist-induced rodent tumors: Modes of action and human relevance . Crit. Rev. Toxicol. , 33 ( 6 ) : 655 – 780 .
- Meek , M. E. , Bucher , J. R. , Cohen , S. M. , Dellarco , V. , Hill , R. N. , Lehman-McKeeman , L. D. , Longfellow , D. G. , Pastoor , T. , Seed , J. and Patton , D. E. 2003 . A framework for human relevance analysis of information on carcinogenic modes of action . Crit. Rev. Toxicol. , 33 ( 6 ) : 591 – 653 .
- National Research Council . 2007 . Toxicity Testing in the 21st Century: A Vision and A Strategy , Washington, DC : National Academies Press .
- Ross , A. C. , Russell , R. M. , Miller , S. A. , Munro , I. C. , Rodricks , J. V. , Yetley , E. A. and Julien , E. 2009 . Application of a Key Events Dose-Response Analysis to nutrients: A case study with vitamin A (retinol) . Crit. Rev. Food Sci. Nutr. , 49 : 708 – 717 .
- Seed , J. , Carney , E. W. , Corley , R. A. , Crofton , K. M. , DeSesso , J. M. , Foster , P. M. , Kavlock , R. , Kimmel , G. , Klaunig , J. , Meek , M. E. , Preston , R. J. , Slikker , W. Jr. , Tabacova , S. , Williams , G. M. , Wiltse , J. , Zoeller , R. T. , Fenner-Crisp , P. and Patton , D. E. 2005 . Overview: Using mode of action and life stage information to evaluate the human relevance of animal toxicity data . Crit. Rev. Toxicol. , 35 ( 8–9 ) : 664 – 672 .
- Sonich-Mullin , C. , Fielder , R. , Wiltse , J. , Baetcke , K. , Dempsey , J. , Fenner-Crisp , P. , Grant , D. , Hartley , M. , Knaap , A. , Kroese , D. , Mangelsdorf , I. , Meek , M. E. , Rice , J. M. and Younes , M. 2001 . International Programme on Chemical Safety. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis . Regul. Toxicol. Pharmacol. , 34 ( 2 ) : 146 – 152 .
- Taylor , S. L. , Gendel , S. M. , Houben , G. F. and Julien , E. 2009 . The Key Events Dose-Response Framework: A foundation for examining variability in elicitation thresholds for food allergens . Crit. Rev. Food Sci. Nutr. , 49 : 729 – 739 .