Abstract
Being the largest archipelago country in the world, with a tropical climate and a unique flora and fauna, Indonesia habitats one of the most diverse biome in the world. These characteristics make Indonesia a popular travel destination, with tourism numbers increasing yearly. These characteristics also facilitate the transmission of zoonosis and provide ideal living and breading circumstances for arthropods, known vectors for viral diseases. A review of the past 10 years of literature, reports of the Ministry of Health, Republic of Indonesia and ProMED-mail shows a significant increase in dengue infection incidence. Furthermore, chikungunya, Japanese encephalitis and rabies are proven to be endemic in Indonesia. The combination of cohort studies, governmental data and ProMED-mail reveals an integrated overview for those working in travel medicine and public health, focusing on both endemic and emerging acute virus infections. This review summarizes the epidemiology of acute virus infections in Indonesia, including outbreak reports, as well as public health response measurements and their potential or efficacy. Knowledge about human behaviour, animal reservoirs, climate factors, environment and their role in emerging virus infection are discussed. We aim to support public health authorities and health care policy makers in a One Health approach.
Introduction
Comprising thousands of islands spread across the equator, Indonesia is the largest archipelago country in the world with 257.5 million inhabitants (Worldbank Indonesia Data Citation2016). With a broad offer of both culture and nature, Indonesia is a popular travel destination for travellers all over the world, with the number of travellers going to Indonesia increasing yearly (World Economic Forum Citation2015). Traditionally, tourists visit both Java and Bali Island, but other islands, such as Flores, Lombok, Sumatra, and Sulawesi gain popularity, most likely due to effective advertisement of the islands. However, its tropical climate and subsequent relative high humidity are both favourable conditions for vector-borne disease transmission. In addition, Indonesia yields a large and growing population of notorious reservoirs of zoonotic infections like poultry, rodents, wild birds, dogs, pigs, and monkeys (Konishi et al. Citation2009; Yamanaka et al. Citation2010; Dash et al. Citation2013; Townsend et al. Citation2013; Lane-DeGraaf et al. Citation2014).
This constant tread of infection and potential local outbreaks or even pandemics compromise both the people living in Indonesia as well as travellers visiting the archipelago. Infectious diseases remain one of the leading causes of morbidity and mortality worldwide, especially in countries with a (sub)tropical climate (WHO Citation2014b). Its burden is a result of a constant ground of established infections in the past period, combined with epidemics of emerging infectious diseases (EID) (Morens et al. Citation2004). An EID is characterized as an infectious disease that appeared and affected a population for the first time or existed before but increased rapidly in case numbers, or geographical spread. They often have the characteristic to easily spread over large areas, infecting people and/or animals with potential significant morbidity, and mortality (WHO-SEARO Citation2005).
In the past decade, outbreaks of multiple emerging infectious virus diseases (EIVD) have been reported in Indonesia. Especially of note were those of avian influenza, dengue, chikungunya, and rabies (Soepandi et al. Citation2010; Kosasih et al. Citation2013; Townsend et al. Citation2013; Karyanti et al. Citation2014). As a result, the Indonesian government has put much effort in trying to control many of these diseases, for instance by implementing surveillance and vector control programmes, or in the case of rabies culling campaigns. This resulted in data of specific interest for those working with infectious disease patients in Indonesia, public health or those working in travel medicine outside of Indonesia. Data from local outbreak reports and from published epidemiological studies give a unique insight in how to optimize EIVD prevention in Indonesia. This data potentially can be used to support public health authorities and health care policy makers.
EI(V)D outbreaks are hard to predict since they are the result of a complex interaction between host, vector, pathogen, and environment. Furthermore, infections in humans often happen unnoticed, such as via contaminated foods, mosquito bites, or inhalation of virus-containing aerosols. From the One Health concept, we know that six out of every 10 infectious diseases in humans are spread from animals (CDC Citation2016). An optimal insight in this interaction is crucial for executing adequate preventive methods, moreover since no treatment is available for the majority of acute EIVDs. A current overview of EIVDS in Indonesia for (international) clinicians and healthcare authorities does not seem to be available. Furthermore, we observed that authorities from other countries advising their inhabitants for pre-travel vaccinations and precautions could not adequately assess their advices since insight in local literature and governmental data for this region is lacking ().
Table 1. Vaccine-preventable diseases as recommendation from the Ministry of Health Republic of Indonesia, Centers for Disease Control and Prevention, Dutch, Swiss, Singapore’s and Australian perspective.
We, therefore, have reviewed acute (E)IVD outbreaks in Indonesia in the past decade, using published cohort data, ministry of health reports and reports from the online Programme for Monitoring Emerging Diseases (ProMED-mail). ProMED-mail is open to all sources; it includes a variety of reports ranging from local media to science. Data should be interpreted with care, as reports are susceptible for information bias. Viral diseases able to unnoticeably transmit from vector to human or inter human are discussed as well. summarizes the discussed viruses, incidence, transmission route, incubation period, symptomatology, protective actions, and diagnostic methods.
Table 2. Overview of the major discussed viruses including epidemiology, symptomatology, protective actions, and diagnostics.
Methods
Based on manuscripts studying the epidemiology of virus infections in South East Asia and ProMED-mail reports, hepatitis A, West Nile virus, Japanese encephalitis, dengue, chikungunya, measles, rabies, hantavirus, avian influenza, and seasonal influenza were considered to be predominantly associated with acute human viral disease in SEA region (Cleton et al. Citation2012; WHO Citation2014a). Since more recently diseases, such as Zika, hepatitis E, and MERS-CoV (re-)emerged in the South-East Asia region; these were taken into consideration as well. We conducted a search of the literature for research performed in Indonesia and to human cases with a history of recent travel to Indonesia using the PubMed (including MesH) and Embase databases from May 12 to 19 2016. Search strategy is available as supplemental data (Supplemental data 1). Two authors independently selected articles written in English and Bahasa Indonesia by first reading title/abstract from human and animal/vector studies published from May 2006 to 2016. We checked reference lists for references to relevant first published data (i.e. first virus detection or clusters of cases) and we extracted data when available. Scientific literature about selected human research is available as supplementary data (Supplemental data 2). represents a map of Indonesia with a visual overview of the locations where the discussed cohort studies were conducted. We accessed Ministry of Health, Republic of Indonesia (MoH-RI) and World Health Organization (WHO) information at May 12 2016. MoH-RI reports for 2006–2015 were available for retrieval. Where applicable, WHO reports are referred to in text. Furthermore, ProMED-mail was searched for reports from Indonesia at Oct 21 2016. ProMED-mail together with MoH-RI data is visually presented in to give insight in the trends in reporting for some EIVDs of interest and is also available as supplemental data (Supplemental data 2).
Figure 1. Map of Indonesia with location overview of the discussed cohort studies conducted or reported from 2006 until May 2016 - not including case reports and/or export cases.
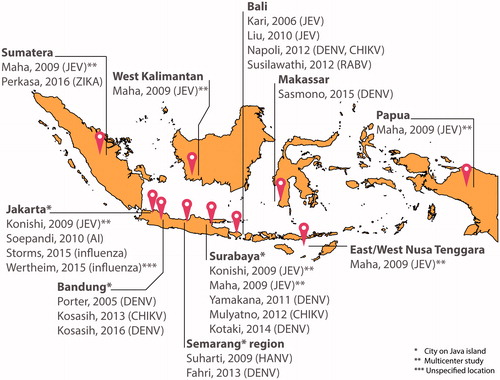
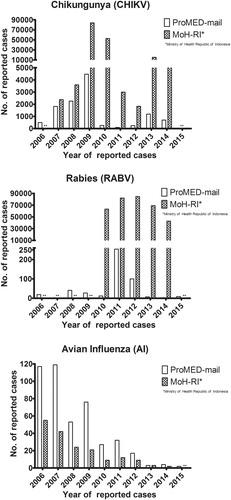
Figure 2. Trends in ProMED-mail and Ministry of Health Republic of Indonesia for the reporting of chikungunya, rabies and avian influenzain Indonesia Reporting up till 21st October 2016. Source data ProMED-mail available as supplementary data 2. Restricted to human cases (both laboratory confirmed and unconfirmed), not including summarizing outbreak reports. **indicates no data available.
Dengue virus (DENV)
Dengue virus (DENV) is a Flavivirus (genus including yellow fever and Zika virus) belonging to the family of Flaviviridae. DENV infection is a mosquito-borne infection caused by one of the four DENV serotypes (DENV 1–4) and is primarily transmitted to humans by the Aedes mosquito. DENV has a worldwide distribution, is geographically concentrated around the equator and is endemic in Southeast Asia. The estimated number of infections worldwide is 50–100 million, with estimated 500 000 people requiring hospitalization each year. The incubation period is 5–8 d. Severe DENV could potentially lead to haemorrhagic shock and organ failure. Most of the symptoms become manifest around the time of defervescence. Treatment is supportive and mainly directed to maintenance of the body fluid volume. Safe and effective DENV vaccines are under development in several stages of research (Martina et al. Citation2009; Meltzer and Schwartz Citation2009; Ooi and Gubler Citation2009; Chen and Wilson Citation2010; Mangold and Reynolds Citation2013; Vannice et al. Citation2016). Currently, Dengvaxia® (a tetravalent, live attenuated, chimeric dengue vaccine in a yellow fever 17D backbone developed by Sanofi Pasteur, Swiftwater, PA) is registered for use in individuals 9–45 years of age living in endemic areas (WHO Citation2016b). However, current data does not support implementation in the Indonesian vaccination programme.
The nationwide DENV surveillance programme started in 1968. Besides increasing DENV incidence numbers, surveillance showed an annual increase of dengue haemorrhagic fever (DHF), classified as DHF according to former classifications with at least two of four clinical manifestations (fever, haemorrhagic manifestations/positive tourniquet test, hepatomegaly, and circulatory failure combined with thrombocytopenia) from 0.05/100 000 to 35–40/100 000 in 2013. DHF incidence increased significantly in people aged 15 or above (Karyanti et al. Citation2014).
DENV import cases from Indonesia were reported in literature (Napoli et al. Citation2012). For instance, in Norway 2 DENV infections were reported from 2008 to 2010 (Vainio et al. Citation2010). Furthermore, in Italy (2010) an imported case was reported with viremia lasting for several days. The authors stress out the importance of performing diagnostics and the potential for a local outbreak since Aedes mosquitos are present in Italy (and other parts of Europe) (Rovida et al. Citation2011).
There is data available suggesting a succeeding episode of DENV infection may lead to detrimental clinical disease, the so-called antibody depended enhancement theory. However, virulence seems mainly determined by difference in genotypes and increased virulence (Martina et al. Citation2009). Multiple studies report the continuous change of DENV serotypes and their lineages. Both clustering of lineages and subtypes during outbreaks (e.g. lineage 4 of DENV-2 between December 2011 and March 2012 (Yamanaka et al. Citation2011; Kotaki et al. Citation2014b; Ernst et al. Citation2015)) as well as the concurrent presence of all four DENV genotypes is reported in Indonesia (Kotaki Citation2014a; Kotaki et al. Citation2014b).
Clinical studies on the epidemiology of febrile illness in Indonesian cohorts report various rates of molecular, antigenic or serological evidence for DENV. Overall, for both urban and rural areas, DENV is detected in 12–55% of all tested febrile cases; this includes all four DENV genotypes. With a follow-up period up to seven years in specific reports (Porter et al. Citation2005; Fahri et al. Citation2013; Sasmono et al. Citation2015; Kosasih et al. Citation2016).
The MoH-RI national control programme in the DENV epidemic includes surveillance of mosquito larvae in households and eradication of mosquito breeding spots, as a community-based programme (MoH-RI Citation2009). The importance of such interventions is emphasized by several studies: Stahl et al. showed that indirect costs take up to 44% of the total costs (est. 2 979 902 US$) per DENV outbreak (Stahl et al. Citation2013). Tourism and labour flow contribute to DENV incidence, as is shown in field research studies in Bali – one of the most popular islands as tourism destination. It is suggested to reduce vector populations and spread knowledge of vector transmittable diseases, though in this study no effect measurements are done (Yoshikawa and Kusriastuti Citation2013).
To add up, multiple manuscripts suggest adjusting for underreporting DENV cases during an epidemic and to analyse currently available data from cohort studies or MoH-RI with expansion factors (Shepard et al. Citation2013; Undurraga et al. Citation2013). Possible resistance to larvicides, such as temephos (Mulyanto et al. Citation2012), understanding the mosquito population (Rasic et al. Citation2015), and the ability to predict the number of DENV cases in advance (Halide and Ridd Citation2008; Wijayanti et al. Citation2016) all could play an important role in further understanding of DENV outbreaks.
West Nile virus (WNV)
West Nile virus (WNV), an arthropod-borne Flavivirus, is a zoonotic infection mainly of birds and horses. Birds serve as reservoir and transmission occur via mosquitoes. WNV is found in West Asia, as well as Africa, Europe, Middle East, and North America (Chancey et al. Citation2015). Mammals are considered dead-end hosts that do not contribute to the epidemic spread of the pathogen (van der Meulen et al. Citation2005). The average incubation period lasts 2–14 d. Of humans infected, 20% will experience a mild, non-specific disease presentation including high fever, headache, myalgia, sometimes with rash, and lymphadenopathy; <1% will develop severe neurological symptoms (Watson et al. Citation2004).
In Indonesia, the data regarding WNV epidemiology is scarce. WNV was isolated from a 15-year-old patient with fever in 2004. Phylogenetic analysis showed a relation with WNV strains isolated in Africa (Myint et al. Citation2014). Only limited reports of WNV in humans in Indonesia are available. For instance, based on the work of Nasronudin et al. (Citation2014) and Wicaksono et al. (Citation2014) there is a suggestion of WNV infection in human in Indonesia. However, these studies offer a low level of evidence since they used a sub-optimal methodology and confirmation by a third party lab was not performed. No surveillance programme exists for WNV and no entries exist on ProMED-mail, thus only current case studies suggest potential circulation of WNV in Indonesia.
Japanese encephalitis (JEV)
Japanese encephalitis virus (JEV) is an arthropod-borne Flavivirus. It is the leading cause of encephalitis in most of South-East Asia and Western Pacific regions, where 24 countries are known to have endemic JEV transmission (WHO Citation2015b). The incubation period of JEV is 6–16 d. The majority of cases are non-specific or asymptomatic; only less than 1% of the individuals develop clinical symptoms, such as fever, headache, altered mental state, and convulsions. When encephalitis occurs, mortality rises from 30 to 40–50% in comatose patients. JEV is known for long-term neuropsychological sequelae in 30–50% of those who recover from intensive care support. Vaccination is considered the single and most important control measure for JEV (Unni et al. Citation2011; Tiwari et al. Citation2012).
The first manuscript describing JEV in humans in Indonesia dates from 1974 (Van Peenen et al. Citation1974). From then, a number of cases were reported in the 80–90s in both returning travellers (Macdonald et al. Citation1989; Wittesjo et al. Citation1995; Buhl et al. Citation1996) as well in Indonesian inhabitants (Yoshida et al. Citation1999). Early research to JEV was done on the reservoir (pigs) (Van Peenen et al. Citation1975), vector (detected in both Culex and Anopheles species) (Van Peenen et al. Citation1975; Olson et al. Citation1985a; Olson et al. Citation1985b), and humans (Van Peenen et al. Citation1974). Studies of Yamanaka et al. investigated JEV viremia and antibodies in 219 pigs from Bali and East Java in 2008. Of these pigs 49% in Bali and 6% in Java had viremia and 24% of 123 pig samples tested in Bali showed positive JEV IgM antibodies (none in the Java population) (Yamanaka et al. Citation2010). In contrast to the above-mentioned, only two human cases exist in ProMED-mail in 2011 and 2015, whereas sero-surveillance studies from 1999 to 2001 on Java Island detected 2.2% JEV antibodies (titres ≥1:160) involving over 2000 samples (Konishi et al. Citation2009).
A number of clinical studies report a noteworthy incidence of JEV cases in children, of which a significant number report subsequent neurological sequelae. A study performed between 2001 and 2003, involving all healthcare facilities on Bali island, reported an average incidence rate of 7.1 per 100 000 children aged 10 or below (Kari et al. Citation2006). Another study followed up 65 out of 72 cases of laboratory-confirmed JEV in children between 2001 and 2004. In this study, a total of 16 children were deceased at the follow-up assessment (average 13 months after discharge, range 4–24 months). No less than 25% of the children had sequelae in such way, that they were dependent on their daily functioning and only 25% of the children were considered to be fully recovered (Maha et al. Citation2009). A case-control study of Liu et al. confirmed JEV in about one-third of the clinically identified viral encephalitis or aseptic meningitis in children aged 0–11 years. Multivariate analysis revealed that pig ownership by their family, close proximity to the rice fields and older age was significantly associated with the incidence risk of JEV in children (Liu et al. Citation2010). No such studies seem to have been carried out in adults in the past decade.
Despite being discussed at World Health Organization’s meetings (Tsai Citation2000) and its relevance as pointed out above, currently, no surveillance programme nor a vaccination programme exist in Indonesia, even while the latter was reported as cost-effective for a two-dose regime on Bali island (Liu et al. Citation2008). To our knowledge, the MoH-RI is advancing towards implementation of a childhood vaccine for JEV; it is, however, unclear what a surveillance programme will look like.
Chikungunya (CHIKV)
Chikungunya virus (CHIKV) is an Alphavirus belonging to the Togaviridae family, transmitted in Asia by mosquitos of the Aedes family (Caglioti et al. Citation2013). After an incubation period of 2–4 d (range 1–12 d) (Caglioti et al. Citation2013; Thiberville et al. Citation2013), the majority of the infected patients become symptomatic. CHIKV is characterized by an onset of acute febrile illness and maculopapular rash. Severe joint pain is described as main symptom and is present in almost all cases; arthralgia in specific cases can last up to 24 months (Kucharz and Cebula-Byrska Citation2012; Thiberville et al. Citation2013). Treatment is symptomatic. The re-emergence of CHIKV was reported in Indonesia in serological studies in 1999 (Porter et al. Citation2004) and in outbreak reports from 2001 to 2003 (Laras et al. Citation2005). Furthermore, CHIKV was isolated from patient sera in febrile illness cases from prospective cohorts from 2000 to 2008 – with incidence rates of 10.1/1000 CHIKV infections per person-years (Mulyatno et al. Citation2012; Kosasih et al. Citation2013). When comparing MoH-RI reported incidence numbers of CHIKV in 2014 (MoH-RI Citation2015) to confirmed cases in a cohort study of 2010–2011, there is reason to believe that there is underreporting of CHIKV in Indonesia. This is likely the result due to the similarities in clinical presentation for both DENV and CHIKV (Mulyatno et al. Citation2012), the sparse availability of routine diagnostics and not the trends in the CHIKV outbreak highlights these trends for CHIKV, avian influenza, and rabies. Both MoH-RI and ProMED-mail data are prone to reporting bias; the figure shows clear trends and discrepancies in reporting between MoH-RI and ProMED-mail. For CHIKV, there seems a fixed ratio of ProMED-mail reports versus MoH-RI data in 2007 and 2008, whereas in later years, it is definitely questionable how to adequately assess the real incidence numbers, since ProMED-mail numbers decline and MoH-RI numbers both drop and raise.
Measles (MV)
Measles virus (MV), a Morbillivirus belonging to the Paramyxoviridae, is a highly contagious virus, with the number of new infections that can result from one infected person ranging from 12 to 16 (also referred to in literature as the R0). It spreads directly from human to human by virus-containing aerosols. Especially at risk are people naïve to the virus, being those not vaccinated and children losing passive antibodies after birth. The infection is characterized by illness with fever, cough, coryza, conjunctivitis, and Koplik’s spots. A characteristic erythematous and maculopapular rash appears which lasts for 3–5 d (Moss and Griffin Citation2012). In 40% of the cases, measles infection is complicated, for instance by pneumonia, due to a relative immunosuppression after measles virus infection (de Vries et al. Citation2012; Mina et al. Citation2015). The incidence number of MV is successfully being reduced by vaccination, although outbreaks do occur, both in countries with and without vaccination programme due to vaccine effectiveness and vaccine coverage (WHO Citation2012; Durrheim et al. Citation2014). The implementation of a regular immunization programme for MV led to vaccination coverages above 90% in most of Indonesia, a target as set by WHO, with a declining number of provinces not meeting this target over the past years (Dalimunthe et al. Citation1990; Serquina-Ramiro et al. Citation2001; MoH-RI Citation2014). When comparing these data to WHO reports, it can be depicted that the overall 1st dose coverage among one-year-olds is still below the 90% target (WHO Citation2016a). Also, ProMED-mail reports show no evidence of MV outbreaks, while MoH-RI still reports a number of cases totalling to an incidence rate of about 5–6 per 100 000 inhabitants (MoH-RI Citation2014, Citation2015).
Rabies (RABV)
Rabies (RABV) is caused by a number of Lyssaviruses including the rabies virus and bat Lyssaviruses. Saliva from infected animals can transmit the virus to humans and eventually cause a lethal infection of the brains. In the majority of cases, dogs transmit the virus; however, transmission of rabies via other wildlife should be taken into consideration (Wang et al. Citation2014). RABV causes an estimated number of deaths between fifty and a hundred thousand per year. The Asian and African continent account for 95% of the worldwide deaths of the total reported infections (Leung AK et al. Citation2007).
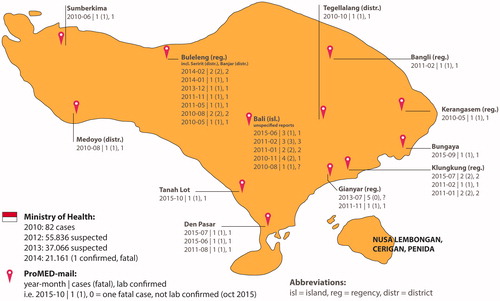
RABV is endemic in Indonesia, especially in Bali and East Nusa Tenggara and is likely present since the 1880s, based on multiple manuscripts describing clinical cases following bites from “mad dogs” (Ward Citation2014). After being last described in late 1980s (Waltner-Toews et al. Citation1990) an outbreak in Bali and surrounding islands commenced in 2008 (MoH-RI Citation2009). Up to hundred human cases and a case fatality rate of 100% in laboratory confirmed human cases were reported during the outbreak until 2010 (Susilawathi et al. Citation2012). Of interest is the data from GeoSentinel and EuroTravNet sites; 12.1% of all post-exposure prophylactic vaccination for RABV was requested by travellers returning from Bali. However, their data shows that the majority of animal-related injuries in travellers returning from Bali are associated with exposure to monkeys, and not dog bites/scratches (Gautret et al. Citation2011). shows trends in RABV reporting to MoH-RI and ProMED-mail. The trends in reporting to both entities are less well correlated as compared to those in CHIKV. shows outbreak locations in Bali as reported in ProMED-mail and compared to MoH-RI incidence numbers. It could be beneficial to combine Ministry of Health, ProMED-mail and possibly WHO data to get a thorough overview of areas at risk in case of outbreaks since locational data could be of relevance for governmental interventions or travel-related risks.
Figure 3. Rabies outbreak heat map to demonstrate the benefit of combining Ministry of Health and ProMED-mail data.
To further understand the endemic, a number of animal studies were carried out. During the 2008–2010 outbreak, Dibia and colleagues showed that Indonesian RABV originated from Java Island and were (re-)introduced in several other islands including Bali (Dibia et al. Citation2015). Also, a study has been conducted to estimate the owned and unowned dog populations and dog bite cases in (RABV free) Lombok island (Mustiana et al. Citation2015) in order to get insight in the (potential) spread. From 2008, early preventive measurements to stop the outbreak were implemented. Despite control – including mass vaccinations and culling for dogs – the outbreak continued for four years. This largely had to do with the fact that the dog population in Bali is rather large and the human-dog relationship in Bali is believed to be multifaceted (i.e. for cultural reasons, as guard, but also different point-of-views in dog care and best practices after a bite wound) (Widyastuti et al. Citation2015). Also culling of dogs seemed to be ineffective. The island-wide vaccination, aiming for a vaccine coverage rate of 70% in dogs, did, however, reduce the incidence and spread of rabies (Putra et al. Citation2013). Still, a number of RABV cases are reported over the last years, as is summarized in and .
Hantavirus (HV)
Hantaviruses (HV) belong to the family of the Bunyaviridae. In humans, the rodent-borne HVs cause two different diseases. In North and South America inhalation of virus-containing aerosols with new world hantaviruses could lead to the hantavirus cardio pulmonary syndrome, a syndrome characterized by fever and acute respiratory distress associated with high case fatality rates (Bi et al. Citation2008). Of importance for Indonesia, seem to be the so-called Old-World HVs which can cause haemorrhagic fever with renal syndrome (HFRS) (Watson et al. Citation2014). A triad of fever, haemorrhage and acute kidney injury classically characterizes this syndrome. It is crucial to initiate prompt and proper symptomatic and supportive treatment for HFRS. This includes monitoring of fluid balance, diuresis, kidney function, and the use of fresh frozen plasma/transfusions in case of haemorrhagic complications (Goeijenbier et al. Citation2013). Small trials and case reports have shown that ribavirin treatment may be useful in the very early phase of HFRS by reducing the risk of haemorrhagic events and the severity of renal insufficiency. Larger trials are needed to corroborate these findings (Moreli et al. Citation2014). In routine diagnostics, Old World HV infections are diagnosed by both serological detection of IgM and/or IgG antibodies and molecular detection of the virus. However, the extent of viremia varies per HV species and in some cases might be limited to a short period after onset of illness (Bi et al. Citation2008). Currently, (comparative) virus neutralization tests remain the gold standard in HV serology to confirm an infection with a specific HV species. Neutralization tests remain an absolute necessity since current available serological HV diagnostics suffer from a high percentage of false-positive results, and, therefore, reports solely relying on ELISA or IFA results should be interpreted with care (Goeijenbier et al. Citation2014). Earlier studies suggested HV infection in humans and rodents in Indonesia (Ibrahim et al. Citation1996; Plyusnina et al. Citation2004; Plyusnina et al. Citation2009; Kosasih et al. Citation2011; Ibrahim et al. Citation2013) and a number of cases were found in hospital-based cohort studies conducted between 1995 and 2005 (Groen et al. Citation2002; Suharti et al. Citation2009; Kosasih et al. Citation2011). However, this evidence of human HV infection in Indonesia is based on only IgM ELISA proven cases. For instance, Suharti et al. report ELISA response of a recent infection in 4.2% of DENV suspected patients (Suharti et al. Citation2009). Although not conclusive, this data does suggest symptomatic human HV infection in Indonesia, which is also supported by the results of Groen et al. who reported serological evidence, based on EIA, ELISA, and IFA results in 11% in a DENV suspected cohort (Groen et al. Citation2002). Furthermore, serological evidence of HV infection was reported in a clustered case of 2005 in Western Java (Kosasih et al. Citation2011). In this case, Rattus sp. trapped around the living area of the patient showed the molecular presence of Seoul HV, known to cause HFRS in Europe. Seoul virus has been detected by PCR in Rattus norvegicus, the known reservoir of the pathogenic Seoul virus (Plyusnina et al. Citation2004).
Furthermore, a newly discovered HV in the Thousand Island region in Indonesia might be pathogenic to humans (Ibrahim et al. Citation2013). Urgent studies are needed to estimate the burden of HV disease in humans in Indonesia and for adequate prevention methods (i.e. rodent trapping and human behaviour changes).
Avian influenza (AI)
Avian influenza (AI) infection in human, caused by non-seasonal influenza A viruses of the subtypes H5N1 and H7N9 belonging to the Orthomyxoviridae family, is clinically characterized by respiratory disease. The first AI H5N1 reported cases date from 1997 influenza in Hong Kong. Since then, the virus has spread throughout the South East of Asia with Indonesia being hit the hardest (WHO Citation2015a). In Indonesia, the epidemic in poultry started in December 2003 on Java Island, with now 31 out the 33 provinces being considered endemic. AI in humans was first detected in July 2005. Despite control measures to prevent further transmission among poultry, the infection rate among humans declined from 5 to 3 cases per month in the reports of 2008.
Up to 2015, a total of 195 cases have been WHO confirmed in Indonesia, of which 165 were fatal – mainly in the period of 2005–2009 (Sedyaningsih et al. Citation2008; WHO Citation2015a). Reports over 2014 show a continuation of the decline and only 2 cases are reported in the archipelago (MoH-RI Citation2015). Refer to for an overview of MoH-RI reports and trends in ProMED-mail reporting of AI. The importance of early ProMED-mail reports is clearly shown here, as the number of reports constantly seems to exceed MoH-RI data, which could indicate impaired implementation of a governmental programme (underreporting to the government). Unfortunately, one cannot rule out (unverifiable) over reporting in ProMED-mail.
Transmission of the disease is considered to be caused by direct exposure to infected poultry. Several family case clusters (Kandun et al. Citation2008) have been reported suggesting the possibility of human-to-human transmission in a study of Yang et al. (Citation2007). However, transmission estimates were only based on one cluster. In addition, blood relatives were shown to be at risk of infection in household outbreaks, supporting the hypothesis for genetic host susceptibility (Aditama et al. Citation2011; Aditama et al. Citation2012). Recent data showed that only a couple mutations in current circulating highly pathogenic AI H5N1 viruses, would be enough to enhance human-to-human airborne transmission (Herfst et al. Citation2012). This illustrates the pandemic potential and possible concerns for international public health.
Several measures have been taken to control disease, comprising vaccination of poultry, and implementation of a participatory disease surveillance (PDS) programme, a community-based reporting system (Azhar et al. Citation2010; Mariner et al. Citation2014). At the beginning of the outbreaks, control of disease has been problematic due to poor gathering of epidemiological information. Since the start of PDS, registration of new cases has been greatly enhanced. Taking social and cultural context of dynamics and distribution of disease into account have been valuable in this process (Mariner et al. Citation2014).
Hepatitis A (HAV)
Indonesia was generally considered to be part of the Hepatitis A (HAV) endemic regions of the world. This food-borne infection with HAV, related to decreased sanitation conditions, infects over 90% of children under the age of 10 years in endemic regions (Barzaga Citation2000). Since the infection before the age of five is rather asymptomatic, the burden of disease for inhabitants in endemic regions is rather low, resulting in a high level of community-based immunity for acute HAV infections. Therefore, hardly any outbreaks occur within the community. In upcoming economies like Indonesia, however, the number of infected children drops with a subsequent increase in symptomatic infections on an older age as a results of a clear increase in level of sanitation (Vranckx et al. Citation1997). This decline in seroprevalence amongst children and adolescents has been reported in Indonesia and other countries on the continent (Kunasol et al. Citation1998). This change in epidemiologic pattern has clinical and public health implications, where at some point the introduction of nation-wide HAV vaccination might be cost-effective compared to treatment of acute HAV infection (Suwantika et al. Citation2014), which is symptom driven. For persons traveling to Indonesia, HAV vaccination is generally recommended by travel medicine guidelines (refer to ) – especially for adults, and imported cases of HAV from travellers to and from Indonesia have been broadly reported in literature (Boggild et al. Citation2010; Utsumi et al. Citation2014).
Seasonal influenza and influenza-like illnesses
Influenza viruses, the most well-known members of Orthomyxoviridae family, cause respiratory disease in humans predominantly. Those mimicking the symptoms of Influenza are known as non-influenza viruses, causing influenza-like illnesses. Of the three influenza virus types (A, B, and C), influenza A is the best known for its ability to drift, re-assort, and the cause of seasonal human flu on a yearly base. Influenza A affects 5–15% of the human population every year. In the tropical Indonesia climate, no true seasonality of influenza is present (Nicholson et al. Citation2003). The virus seems to be constantly present and cause respiratory disease throughout the whole year. However, Storms et al. studied the prevalence of influenza virus in patients seeking care for respiratory disease in Jakarta, Java over a one-year period starting in 2011 and reported the highest incidence during December–May with the proportion of positive PCR being 76% for patients presenting with classic influenza-like illness. Of those, 36% had more severe disease during the weeks with highest influenza activity (Storms et al. Citation2015). Most likely, seasonality exists in line with rainy season. Currently, no country -based policies regarding influenza vaccination (such as for risk groups, healthcare professionals, etc.) are implemented in Indonesia.
MoH-RI discusses the recommendation of antivirals like oseltamivir or vaccination policies in Indonesia, since several studies report this high percentage of complicated influenza cases in Indonesia (up to 15%) (Kosasih et al. Citation2014; Ampofo et al. Citation2015; Storms et al. Citation2015). Of interest for clinicians dealing with travellers with travel plans to Indonesia, comes from data of the Geosentinel network. It shows that travellers travelling to South East Asia have a sevenfold higher change of being infected with seasonal influenza compared to those staying at home (Boggild et al. Citation2012). It, therefore, seems reasonable to vaccinate travellers and inhabitants; however, a yearly seasonal influenza vaccine is currently not implemented in any Indonesian immunization schedule () (Goeijenbier et al. Citation2017).
During a one-year study from 2008, just above 200 respiratory specimens from patients hospitalized in Indonesia (total 1222 patients including hospitals in Thailand and Vietnam) with suspected influenza or influenza-like illness were collected and analysed. In the cohort, 18.7% of the samples tested positive for Rhinovirus, in particular in March. Furthermore, respiratory syncytial virus (RSV, 11.8%) was found from July to November. Other detected viruses included bocavirus (16.4%), parainfluenza (11.5%), adenovirus (8.4%), influenza A and B (7.6%), and coronaviruses (1.8%) (Wertheim et al. Citation2015).
Zika virus infection, hepatitis E, MERS corona
Three cases of Zika virus infection, caused by a Flavivirus transmitted by Aedes aegypti mosquitoes, in Indonesia are reported in literature. These infections, clinically characterized by fever, headache, malaise, arthralgia, myalgia, and rash, were all documented in returning travellers from Jakarta (2012, after 9-d holiday) (Kwong et al. Citation2013), Bali (2013, after monkey bite in Ubud Monkey Forest) (Leung GH et al. Citation2015), and Malaysian Borneo (2014, after a 3-week holiday) (Tappe et al. Citation2015).
Also, seven cases of probable Zika virus infection in inpatient inhabitants of Central Java were reported based on antibody diagnostics (Olson et al. Citation1981). In samples collected in 2014–2015, during a DENV outbreak in Jambi (n = 103), one DENV negative sample was tested positive for Zika (Perkasa et al. Citation2016). Once, a monkey bite was proposed as a route of transmission, while Zika is generally considered to be a mosquito-borne disease (Leung GH et al. Citation2015). Since both DENV and Zika manifest clinically similar, it remains unclear whether Zika virus infection is rare in Indonesia or is often mistaken for DENV. Only limited, if none, diagnostics are carried out to Zika and no regular surveillance programmes exist (MoH-RI Citation2015).
Three outbreaks of hepatitis E infections, transmitted faecal-orally and associated with low standards of sanitation, have been described in West-Kalimantan and Java (Corwin et al. Citation1995; Corwin et al. Citation1997; Sedyaningsih-Mamahit et al. Citation2002). All three outbreaks were related to dependence to polluted river water. Anti-hepatitis E virus seropositivity is shown to be more prevalent in regions where pigs and swines are more incorporated in daily life, as is the case in Bali Island, compared to Java. Samples were taken from farms in Yogyakarta (Central Java), Tulungagung (East Java) and Mengwi (Bali) (Utsumi et al. Citation2011; Widasari et al. Citation2013). Also, hepatitis E virus has been detected in wild rats, however, whether their role in human infection is significant or not remains unclear (Mulyanto et al. Citation2013).
Middle East respiratory syndrome corona virus (MERS-CoV) causes upper respiratory infections in humans with case fatality rates up to 35%. MERS-CoV is prevalent in Arabian countries, including Saudi-Arabia, which is visited by Indonesian inhabitants for hajj (pilgrimage) (Widagdo et al. Citation2017). So far, no cases of MERS corona virus infections in Indonesia are reported in literature.
Implications and future actions
Summarized data from scientific literature, WHO reports, reports from the Ministry of Health of the Republic of Indonesia and ProMED-mail, presented in this review, provide a unique insight in the past, current and potential of relevant viral pathogens for the Indonesian archipelago. Indonesia seems to be prone to vector-borne and zoonotic diseases, due to environmental conditions suited for arthropod vectors and the presence of reservoirs for zoonotic viruses. Viruses like DENV have been endemic for many years while re-emergence of AI and RABV have drawn attention from governmental institutes and scientists. Relevant for this region, but underreported in MoH reports and literature are HV, HAV, and JEV. Furthermore, only limited data is available about the incidence of influenza and influenza-like illnesses – while these viruses are known to have a significant burden on society in due to economical losses. Furthermore, these viruses most likely account for a substantial number of hospital admissions for the very young or elderly, as they do in Europe and USA.
The description of a (re-)emerging virus is expected to happen in the order of ProMED-mail reports, scientific studies and governmental data (MoH-RI and CDC or WHO). Only parts of the ProMED-mail data regarding outbreak reports were confirmed or became of scientific interest in cohort studies (i.e. this holds especially true for avian influenza). Ministry of Health (MoH-RI) data seems to be partially in line with these findings. ProMED-mail has proven to be a valuable tool in the (early) detection of outbreaks all over the world. For Indonesia, this is very well illustrated with AI reports, for which, in many cases, the ProMED-mail reports are ahead of official statements and literature. Interestingly, ProMED-mail reporting for the rabies outbreak in Bali started in 2010 (), while most likely the outbreak started in 2008. Given the goal of ProMED-mail to report emerging diseases on a low-threshold base, it relies heavily on the awareness and possibilities to report (unknown) cases by local healthcare workers. This shows the importance for adequate on-site diagnostics and surveillance programmes. To our experience, the rabies under reporting seems to be an exception in ProMED-mail.
We noticed some discrepancies in MoH-RI data as compared to WHO and CDC data. In MoH-RI data it is not always clear whether data was not available (i.e. not reported to the government) or no cases occurred at all. Furthermore, limited insight is given in number of suspected cases versus laboratory-confirmed cases. In case of RABV, the number of suspected cases (as measured by the number of animal bites) versus administration of RABV vaccine and number of confirmed and fatal cases is reported. Information about the used diagnostic methods lacks, as well as the number of tested samples versus laboratory confirmed samples. Since data is not available for local persons exposed to RABV, it is impossible to draw conclusions based on these observations. However, we do know, with special focus on Bali Island, that RABV post exposure is the number two most prevalent post-travel health care problem in travellers as per Geosentinel data – just above influenza and below travellers’ diarrhoea. The number of actual RABV cases, luckily, is actually one of the more rare problems. We also observed over reporting in ProMED-mail, as only few reports of DENV in ProMED-mail exist but dozens of reports for CHIKV and AI were added; we were unable to settle this discrepancy, moreover, both are reported to the government and taken to MoH-RI reports over the past years. ProMED-mail entries should be interpreted with care, as its form is prone to reporting bias and no peer-review system exists.
There is a clear unmet need for cohort studies in a number of primary, secondary, and tertiary care centres evenly spread over the archipelago. In particular, some well-studied acute viral diseases, such as influenza and influenza-like illnesses are only limitedly researched in Indonesia. Furthermore, up-to-date scientific information regarding for instance HAV and MV antibody prevalence – and hence – the risk for those unvaccinated seems to lack for this region.
Incidence numbers as reported in scientific studies serve a different purpose than those reported in MoH-RI (or CDC, WHO) data, due to the selection of a specific population (i.e. a poultry market, hospital-based, or clinical symptoms). These data should guide healthcare workers in their decisions when assessing patients in the general practice or emergency department. Healthcare workers should, therefore, be able to rely on solid surveillance data from the MoH-RI as well as scientific literature.
In recent years, CHIKV (2013, Caribbean islands), Ebola (2014, West Africa), and Zika (2015, South America and 2016, Asia), have proved to be of relevance for both developed and developing countries. No exception exists for Indonesia, especially since Indonesia is an upcoming economy and a popular travel destination. Vector control is relevant in order to control outbreaks. The MoH-RI sets targets to lower the number of larvae around houses as well as targets for lowering the incidence rate for new cases (e.g. DENV) on a yearly base. Next, to this challenging task, there is need for a functional and validated surveillance programme. Government reference laboratories are only available in limited numbers and the geographic layout in terms of the archipelago, including distant areas, and a variety in both economic strength and availability of health care facilities, poses another challenge to get an accurate oversight EIVD epidemiology.
Changes to the Indonesian vaccination programme will be needed to be made in the future, as new vaccines and epidemiological data become available. This especially holds true for new DENV vaccines and existing JEV (refer to , comparing the MoH-RI immunization scheme to travel immunization advice). Preparedness for EI(V)Ds can only be achieved with an adequate insight and control of current pathogens, a solid surveillance system including reference laboratories and the ability to scale up the resources needed to adequately diagnose, treat, and follow-up patients.
To our findings, resources should be directed to the One Health concept as it is of utmost importance to achieve the correct balance for people, animals, and the environment. Given recent outbreaks in the world, the existence of vectors in Indonesia and the ability to spread diseases in humans, poultry, or goods, it might just be a matter of time before other well-known viral diseases, such as yellow fever or other emerging diseases will be engrafted in Indonesia.
Wesley_de_Jong_et_al._Supplementary_files.zip
Download Zip (180.5 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Aditama TY, Samaan G, Kusriastuti R, Purba WH, Misriyah, Santoso H, Bratasena A, Maruf A, Sariwati E, Setiawaty V, et al. 2011. Risk factors for cluster outbreaks of avian influenza A H5N1 infection, Indonesia. Clin Infect Dis. 53:1237–1244.
- Aditama TY, Samaan G, Kusriastuti R, Sampurno OD, Purba W, Misriyah, Santoso H, Bratasena A, Maruf A, Sariwati E, et al. 2012. Avian influenza H5N1 transmission in households, Indonesia. PLoS One. 7:e29971.
- Ampofo WK, Azziz-Baumgartner E, Bashir U, Cox NJ, Fasce R, Giovanni M, Grohmann G, Huang S, Katz J, Mironenko A, et al. 2015. Strengthening the influenza vaccine virus selection and development process: report of the 3rd WHO informal consultation for improving influenza vaccine virus selection held at WHO headquarters, Geneva, Switzerland, 1–3 April 2014. Vaccine. 33:4368–4382.
- Azhar M, Lubis AS, Siregar ES, Alders RG, Brum E, McGrane J, Morgan I, Roeder P. 2010. Participatory disease surveillance and response in Indonesia: strengthening veterinary services and empowering communities to prevent and control highly pathogenic avian influenza. Avian Dis. 54:749–753.
- Barzaga BN. 2000. Hepatitis A shifting epidemiology in South-East Asia and China. Vaccine. 18:S61–S64.
- Bi Z, Formenty PB, Roth CE. 2008. Hantavirus infection: a review and global update. J Infect Dev Ctries. 2:3–23.
- Boggild AK, Castelli F, Gautret P, Torresi J, von Sonnenburg F, Barnett ED, Greenaway CA, Lim PL, Schwartz E, Wilder-Smith A, et al. 2010. Vaccine preventable diseases in returned international travelers: results from the GeoSentinel Surveillance Network. Vaccine. 28:7389–7395.
- Boggild AK, Castelli F, Gautret P, Torresi J, von Sonnenburg F, Barnett ED, Greenaway CA, Lim PL, Schwartz E, Wilder-Smith A, et al. 2012. Latitudinal patterns of travel among returned travelers with influenza: results from the GeoSentinel Surveillance Network, 1997–2007. J Travel Med. 19:4–8.
- Buhl MR, Black FT, Andersen PL, Laursen A. 1996. Fatal Japanese encephalitis in a Danish tourist visiting Bali for 12 days. Scand J Infect Dis. 28:189.
- Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, Bordi L. 2013. Chikungunya virus infection: an overview. New Microbiol. 36:211–227.
- CDC. 2016. One Health. Centers for Disease Control and Prevention; [accessed 2016]. https://www.cdc.gov/onehealth/
- Chancey C, Grinev A, Volkova E, Rios M. 2015. The global ecology and epidemiology of West Nile virus. Biomed Res Int. 2015:376230.
- Chen LH, Wilson ME. 2010. Dengue and chikungunya infections in travelers. Curr Opin Infect Dis. 23:438–444.
- Cleton N, Koopmans M, Reimerink J, Godeke GJ, Reusken C. 2012. Come fly with me: review of clinically important arboviruses for global travelers. J Clin Virol. 55:191–203.
- Corwin A, Jarot K, Lubis I, Nasution K, Suparmawo S, Sumardiati A, Widodo S, Nazir S, Orndorff G, Choi Y, et al. 1995. Two years’ investigation of epidemic hepatitis E virus transmission in West Kalimantan (Borneo), Indonesia. Trans R Soc Trop Med Hyg. 89:262–265.
- Corwin A, Putri MP, Winarno J, Lubis I, Suparmanto S, Sumardiati A, Laras K, Tan R, Master J, Warner G, et al. 1997. Epidemic and sporadic hepatitis E virus transmission in West Kalimantan (Borneo), Indonesia. Am J Trop Med Hyg. 57:62–65.
- Dalimunthe S, Tjipta GD, Lubis IZ, Manoeroeng SM, Lubis CP. 1990. Immunization in the Well Baby Clinic of Dr. Pirngadi Hospital Medan. Paediatr Indones. 30:42–53.
- Dash AP, Bhatia R, Sunyoto T, Mourya DT. 2013. Emerging and re-emerging arboviral diseases in Southeast Asia. J Vector Borne Dis. 50:77–84.
- de Vries RD, McQuaid S, van Amerongen G, Yuksel S, Verburgh RJ, Osterhaus AD, Duprex WP, de Swart RL. 2012. Measles immune suppression: lessons from the macaque model. PLoS Pathog. 8:e1002885.
- Dibia IN, Sumiarto B, Susetya H, Putra AA, Scott-Orr H, Mahardika GN. 2015. Phylogeography of the current rabies viruses in Indonesia. J Vet Sci. 16:459–466.
- Durrheim DN, Crowcroft NS, Strebel PM. 2014. Measles - the epidemiology of elimination. Vaccine. 32:6880–6883.
- Ernst T, McCarthy S, Chidlow G, Luang-Suarkia D, Holmes EC, Smith DW, Imrie A. 2015. Emergence of a new lineage of dengue virus type 2 identified in travelers entering Western Australia from Indonesia, 2010–2012. PLoS Negl Trop Dis. 9:e0003442.
- Fahri S, Yohan B, Trimarsanto H, Sayono S, Hadisaputro S, Dharmana E, Syafruddin D, Sasmono RT. 2013. Molecular surveillance of dengue in Semarang, Indonesia revealed the circulation of an old genotype of dengue virus serotype-1. PLoS Negl Trop Dis. 7:e2354.
- Gautret P, Lim PL, Shaw M, Leder K. 2011. Rabies post-exposure prophylaxis in travellers returning from Bali, Indonesia, November 2008 to March 2010. Clin Microbiol Infect. 17:445–447.
- Goeijenbier M, Hartskeerl RA, Reimerink J, Verner-Carlsson J, Wagenaar JF, Goris MG, Martina BE, Lundkvist Å, Koopmans M, Osterhaus AD, et al. 2014. The hanta hunting study: underdiagnosis of Puumala hantavirus infections in symptomatic non-travelling leptospirosis-suspected patients in the Netherlands, in 2010 and April to November 2011. Euro Surveill. 19:20878.
- Goeijenbier M, van Genderen P, Ward BJ, Wilder-Smith A, Steffen R, Osterhaus AD. 2017. Travellers and influenza: risks and prevention. J Travel Med. 24:taw078.
- Goeijenbier M, Wagenaar J, Goris M, Martina B, Henttonen H, Vaheri A, Reusken C, Hartskeerl R, Osterhaus A, Van Gorp E. 2013. Rodent-borne hemorrhagic fevers: under-recognized, widely spread and preventable - epidemiology, diagnostics and treatment. Crit Rev Microbiol. 39:26–42.
- Groen J, Suharti C, Koraka P, van Gorp EC, Sutaryo J, Lundkvist A, Osterhaus AD. 2002. Serological evidence of human hantavirus infections in Indonesia. Infection. 30:326–327.
- Halide H, Ridd P. 2008. A predictive model for dengue hemorrhagic fever epidemics. Int J Environ Health Res.18:253–265.
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 336:1534–1541.
- Ibrahim IN, Shimizu K, Yoshimatsu K, Yunianto A, Salwati E, Yasuda SP, Koma T, Endo R, Arikawa J. 2013. Epidemiology of hantavirus infection in thousand islands regency of Jakarta, Indonesia. J Vet Med Sci. 75:1003–1008.
- Ibrahim IN, Sudomo M, Morita C, Uemura S, Muramatsu Y, Ueno H, Kitamura T. 1996. Seroepidemiological survey of wild rats for Seoul virus in Indonesia. Jpn J Med Sci Biol. 49:69–74.
- Kandun IN, Tresnaningsih E, Purba WH, Lee V, Samaan G, Harun S, Soni E, Septiawati C, Setiawati T, Sariwati E, et al. 2008. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 372:744–749.
- Kari K, Liu W, Gautama K, Mammen MP, Jr., Clemens JD, Nisalak A, Subrata K, Kim HK, Xu ZY. 2006. A hospital-based surveillance for Japanese encephalitis in Bali, Indonesia. BMC Med. 4:8.
- Karyanti MR, Uiterwaal CS, Kusriastuti R, Hadinegoro SR, Rovers MM, Heesterbeek H, Hoes AW, Bruijning-Verhagen P. 2014. The changing incidence of dengue haemorrhagic fever in Indonesia: a 45-year registry-based analysis. BMC Infect Dis. 14:412.
- Konishi E, Sakai Y, Kitai Y, Yamanaka A. 2009. Prevalence of antibodies to Japanese encephalitis virus among inhabitants in Java Island, Indonesia, with a small pig population. Am J Trop Med Hyg. 80:856–861.
- Kosasih H, Alisjahbana B, Nurhayati, de Mast Q, Rudiman IF, Widjaja S, Antonjaya U, Novriani H, Susanto NH, Jusuf H, et al. 2016. The epidemiology, virology and clinical findings of dengue virus infections in a cohort of Indonesian adults in Western Java. PLoS Negl Trop Dis. 10:e0004390.
- Kosasih H, Bratasena A, Pangesti K, Laras K, Samaan G. 2014. Managing seasonal influenza: oseltamivir treatment policy in indonesia? Acta Med Indones. 46:58–65.
- Kosasih H, de Mast Q, Widjaja S, Sudjana P, Antonjaya U, Ma'roef C, Riswari SF, Porter KR, Burgess TH, Alisjahbana B, et al. 2013. Evidence for endemic chikungunya virus infections in Bandung, Indonesia. PLoS Negl Trop Dis. 7:e2483.
- Kosasih H, Ibrahim IN, Wicaksana R, Alisjahbana B, Hoo Y, Yo IH, Antonjaya U, Widjaja S, Winoto I, Williams M, et al. 2011. Evidence of human hantavirus infection and zoonotic investigation of hantavirus prevalence in rodents in western Java, Indonesia. Vector Borne Zoonotic Dis. 11:709–713.
- Kotaki T, Yamanaka A, Mulyatno KC, Churrotin S, Labiqah A, Sucipto TH, Soegijanto S, Kameoka M, Konishi E. 2014a. Continuous dengue type 1 virus genotype shifts followed by co-circulation, clade shifts and subsequent disappearance in Surabaya, Indonesia, 2008–2013. Infect Genet Evol. 28:48–54.
- Kotaki T, Yamanaka A, Mulyatno KC, Labiqah A, Sucipto TH, Churrotin S, Soegijanto S, Konishi E, Kameoka M. 2014b. Phylogenetic analysis of dengue virus type 3 strains primarily isolated in 2013 from Surabaya, Indonesia. Jpn J Infect Dis. 67:227–229.
- Kucharz EJ, Cebula-Byrska I. 2012. Chikungunya fever. Eur J Intern Med. 23:325–329.
- Kunasol P, Cooksley G, Chan VF, Isahak I, John J, Loleka S, Villar EP, Poovorawan Y, Seong NH, Sulaiman HA, et al. 1998. Hepatitis A virus: declining seroprevalence in children and adolescents in Southeast Asia. Southeast Asian J Trop Med Public Health. 29:255–262.
- Kwong JC, Druce JD, Leder K. 2013. Zika virus infection acquired during brief travel to Indonesia. Am J Trop Med Hyg. 89:516–517.
- Lane-DeGraaf KE, Putra IG, Wandia IN, Rompis A, Hollocher H, Fuentes A. 2014. Human behavior and opportunities for parasite transmission in communities surrounding long-tailed macaque populations in Bali, Indonesia. Am J Primatol. 76:159–167.
- Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R, Djauzi, Wandra T, Master J, Kosasih H, Hartati S, et al. 2005. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 99:128–141.
- Leung AK, Davies HD, Hon KL. 2007. Rabies: epidemiology, pathogenesis, and prophylaxis. Adv Ther. 24:1340–1347.
- Leung GH, Baird RW, Druce J, Anstey NM. 2015. Zika virus infection in Australia following a monkey bite in Indonesia. Southeast Asian J Trop Med Public Health. 46:460–464.
- Liu W, Clemens JD, Kari K, Xu ZY. 2008. Cost-effectiveness of Japanese encephalitis (JE) immunization in Bali, Indonesia. Vaccine. 26:4456–4460.
- Liu W, Gibbons RV, Kari K, Clemens JD, Nisalak A, Marks F, Xu ZY. 2010. Risk factors for Japanese encephalitis: a case-control study. Epidemiol Infect. 138:1292–1297.
- Macdonald WB, Tink AR, Ouvrier RA, Menser MA, de Silva LM, Naim H, Hawkes RA. 1989. Japanese encephalitis after a two-week holiday in Bali. Med J Aust. 150:334–336.
- Maha MS, Moniaga VA, Hills SL, Widjaya A, Sasmito A, Hariati R, Kupertino Y, Artastra IK, Arifin MZ, Supraptono B, et al. 2009. Outcome and extent of disability following Japanese encephalitis in Indonesian children. Int J Infect Dis.13:e389–e393.
- Mangold KA, Reynolds SL. 2013. A review of dengue fever: a resurging tropical disease. Pediatr Emerg Care. 29:665–669.
- Mariner JC, Jones BA, Hendrickx S, El Masry I, Jobre Y, Jost CC. 2014. Experiences in participatory surveillance and community-based reporting systems for H5N1 highly pathogenic avian influenza: a case study approach. Ecohealth. 11:22–35.
- Martina BE, Koraka P, Osterhaus AD. 2009. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 22:564–581.
- Meltzer E, Schwartz E. 2009. A travel medicine view of dengue and dengue hemorrhagic fever. Travel Med Infect Dis. 7:278–283.
- Mina MJ, Metcalf CJ, de Swart RL, Osterhaus AD, Grenfell BT. 2015. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 348:694–699.
- MoH-RI. 2009. [Indonesia Health Profile 2008]. Jakarta (Indonesia): Ministry of Health – Republic of Indonesia (MoH-RI); [accessed 2016 May 12]. http://www.depkes.go.id/folder/view/01/structure-publikasi-pusdatin-profil-kesehatan.html
- MoH-RI. 2014. [Indonesia Health Profile 2013]. Jakarta (Indonesia): Ministry of Health – Republic of Indonesia (MoH-RI); [accessed 2016 May 12]. http://www.depkes.go.id/folder/view/01/structure-publikasi-pusdatin-profil-kesehatan.html
- MoH-RI. 2015. [Indonesia Health Profile 2014]. Jakarta (Indonesia): Ministry of Health – Republic of Indonesia (MoH-RI); [accessed 2016 May 12]. http://www.depkes.go.id/folder/view/01/structure-publikasi-pusdatin-profil-kesehatan.html
- Moreli ML, Marques-Silva AC, Pimentel VA, da Costa VG. 2014. Effectiveness of the ribavirin in treatment of hantavirus infections in the Americas and Eurasia: a meta-analysis. Virusdisease. 25:385–389.
- Morens DM, Folkers GK, Fauci AS. 2004. The challenge of emerging and re-emerging infectious diseases. Nature. 430:242–249.
- Moss WJ, Griffin DE. 2012. Measles. Lancet. 379:153–164.
- Mulyanto KC, Ngadino AY, Konishi E. 2012. Resistance of Aedes aegypti (L.) larvae to temephos in Surabaya, Indonesia. Southeast Asian J Trop Med Public Health. 43:29–33.
- Mulyanto KC, Depamede SN, Sriasih M, Takahashi M, Nagashima S, Jirintai S, Nishizawa T, Okamoto H. 2013. Frequent detection and characterization of hepatitis E virus variants in wild rats (Rattus rattus) in Indonesia. Arch Virol. 158:87–96.
- Mulyatno KC, Susilowati H, Yamanaka A, Soegijanto S, Konishi E. 2012. Primary isolation and phylogenetic studies of Chikungunya virus from Surabaya, Indonesia. Jpn J Infect Dis. 65:92–94.
- Mustiana A, Toribio JA, Abdurrahman M, Suadnya IW, Hernandez-Jover M, Putra AA, Ward MP. 2015. Owned and unowned dog population estimation, dog management and dog bites to inform rabies prevention and response on Lombok Island, Indonesia. PLoS One. 10:e0124092.
- Myint KS, Kosasih H, Artika IM, Perkasa A, Puspita M, Ma’roef CN, Antonjaya U, Ledermann JP, Powers AM, Alisjahbana B. 2014. West Nile virus documented in Indonesia from acute febrile illness specimens. Am J Trop Med Hyg. 90:260–262.
- Napoli C, Salcuni P, Pompa MG, Declich S, Rizzo C. 2012. Estimated imported infections of chikungunya and dengue in Italy, 2008 to 2011. J Travel Med. 19:294–297.
- Nasronudin, Aksono B, Lukito BD, Rachman BE, Noordiansyah, Indrawati R, Rahayu RP, Lusida MI. 2014. West Nile Virus (WNV) as a potential new threat to HIV/AIDS patients in Indonesia. 32nd World Congress of Internal Medicine (WCIM). Seoul: Korean Association of Internal Medicine.
- Nicholson KG, Wood JM, Zambon M. 2003. Influenza. Lancet. 362:1733–1745.
- Olson JG, Ksiazek TG, Lee VH, Tan R, Shope RE. 1985a. Isolation of Japanese encephalitis virus from Anopheles annularis and Anopheles vagus in Lombok, Indonesia. Trans R Soc Trop Med Hyg. 79:845–847.
- Olson JG, Ksiazek TG. Suhandiman, Triwibowo. 1981. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 75:389–393.
- Olson JG, Ksiazek TG, Tan R, Atmosoedjono S, Lee VH, Converse JD. 1985b. Correlation of population indices of female Culex tritaeniorhynchus with Japanese encephalitis viral activity in Kapuk, Indonesia. Southeast Asian J Trop Med Public Health. 16:337–342.
- Ooi EE, Gubler DJ. 2009. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention. Cad Saúde Pública. 25(1):S115–S124.
- Perkasa A, Yudhaputri F, Haryanto S, Hayati RF, Ma'roef CN, Antonjaya U, Yohan B, Myint KS, Ledermann JP, Rosenberg R, et al. 2016. Isolation of Zika virus from febrile patient, Indonesia. Emerg Infect Dis. 22:924–925.
- Plyusnina A, Ibrahim IN, Plyusnin A. 2009. A newly recognized hantavirus in the Asian house rat (Rattus tanezumi) in Indonesia. J Gen Virol. 90:205–209.
- Plyusnina A, Ibrahim IN, Winoto I, Porter KR, Gotama IB, Lundkvist A, Vaheri A, Plyusnin A. 2004. Identification of Seoul hantavirus in Rattus norvegicus in Indonesia. Scand J Infect Dis. 36:356–359.
- Porter KR, Beckett CG, Kosasih H, Tan RI, Alisjahbana B, Rudiman PI, Widjaja S, Listiyaningsih E, Ma'Roef CN, McArdle JL, et al. 2005. Epidemiology of dengue and dengue hemorrhagic fever in a cohort of adults living in Bandung, West Java, Indonesia. Am J Trop Med Hyg. 72:60–66.
- Porter KR, Tan R, Istary Y, Suharyono W, Sutaryo, Widjaja S, Ma’Roef C, Listiyaningsih E, Kosasih H, Hueston L, et al. 2004. A serological study of chikungunya virus transmission in Yogyakarta, Indonesia: evidence for the first outbreak since 1982. Southeast Asian J Trop Med Public Health. 32:408–415.
- Putra AA, Hampson K, Girardi J, Hiby E, Knobel D, Mardiana IW, Townsend S, Scott-Orr H. 2013. Response to a rabies epidemic, Bali, Indonesia, 2008–2011. Emerging Infect Dis. 19:648–651.
- Rasic G, Endersby-Harshman N, Tantowijoyo W, Goundar A, White V, Yang Q, Filipovic I, Johnson P, Hoffmann AA, Arguni E. 2015. Aedes aegypti has spatially structured and seasonally stable populations in Yogyakarta, Indonesia. Parasit Vectors. 8:610.
- Rovida F, Percivalle E, Campanini G, Piralla A, Novati S, Muscatello A, Baldanti F. 2011. Viremic dengue virus infections in travellers: potential for local outbreak in Northern Italy. J Clin Virol. 50:76–79.
- Sasmono RT, Wahid I, Trimarsanto H, Yohan B, Wahyuni S, Hertanto M, Yusuf I, Mubin H, Ganda IJ, Latief R, et al. 2015. Genomic analysis and growth characteristic of dengue viruses from Makassar, Indonesia. Infect Genet Evol. 32:165–177.
- Sedyaningsih-Mamahit ER, Larasati RP, Laras K, Sidemen A, Sukri N, Sabaruddin N, Didi S, Saragih JM, Myint KS, Endy TP, et al. 2002. First documented outbreak of hepatitis E virus transmission in Java, Indonesia. Trans R Soc Trop Med Hyg. 96:398–404.
- Sedyaningsih ER, Isfandari S, Soendoro T, Supari SF. 2008. Towards mutual trust, transparency and equity in virus sharing mechanism: the avian influenza case of Indonesia. Ann Acad Med Singapore. 37:482–488.
- Serquina-Ramiro L, Kasniyah N, Inthusoma T, Higginbotham N, Streiner D, Nichter M, Freeman S. 2001. Measles immunization acceptance in Southeast Asia: past patterns and future challenges. Southeast Asian J Trop Med Public Health. 32:791–804.
- Shepard DS, Undurraga EA, Halasa YA. 2013. Economic and disease burden of dengue in Southeast Asia. PLoS Negl Trop Dis. 7:e2055.
- Soepandi PZ, Burhan E, Mangunnegoro H, Nawas A, Aditama TY, Partakusuma L, Isbaniah F, Ikhsan M, Swidarmoko B, Sutiyoso A, et al. 2010. Clinical course of avian influenza A(H5N1) in patients at the Persahabatan Hospital, Jakarta, Indonesia, 2005–2008. Chest. 138:665–673.
- Stahl HC, Butenschoen VM, Tran HT, Gozzer E, Skewes R, Mahendradhata Y, Runge-Ranzinger S, Kroeger A, Farlow A. 2013. Cost of dengue outbreaks: literature review and country case studies. BMC Public Health.13:1048.
- Storms AD, Kusriastuti R, Misriyah S, Praptiningsih CY, Amalya M, Lafond KE, Samaan G, Triada R, Iuliano AD, Ester M, et al. 2015. The East Jakarta Project: surveillance for highly pathogenic avian influenza A(H5N1) and seasonal influenza viruses in patients seeking care for respiratory disease, Jakarta, Indonesia, October 2011–September 2012. Epidemiol Infect. 143:3394–3404.
- Suharti C, van Gorp EC, Dolmans WM, Groen J, Hadisaputro S, Djokomoeljanto RJ, D MEO, van der Meer JW. 2009. Hanta virus infection during dengue virus infection outbreak in Indonesia. Acta Med Indones. 41:75–80.
- Susilawathi NM, Darwinata AE, Dwija IB, Budayanti NS, Wirasandhi GA, Subrata K, Susilarini NK, Sudewi RA, Wignall FS, Mahardika GN. 2012. Epidemiological and clinical features of human rabies cases in Bali 2008–2010. BMC Infect Dis. 12:81.
- Suwantika AA, Beutels P, Postma MJ. 2014. Cost-effectiveness of hepatitis A vaccination in Indonesia. Hum Vaccin Immunother. 10:2342–2349.
- Tappe D, Nachtigall S, Kapaun A, Schnitzler P, Gunther S, Schmidt-Chanasit J. 2015. Acute Zika virus infection after travel to Malaysian Borneo, September 2014. Emerg Infect Dis. 21:911–913.
- Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, de Lamballerie X. 2013. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 99:345–370.
- Tiwari S, Singh RK, Tiwari R, Dhole TN. 2012. Japanese encephalitis: a review of the Indian perspective. Braz J Infect Dis.16:564–573.
- Townsend SE, Sumantra IP, Pudjiatmoko Bagus GN, Brum E, Cleaveland S, Crafter S, Dewi AP, Dharma DM, Dushoff J, et al. 2013. Designing programs for eliminating canine rabies from islands: Bali, Indonesia as a case study. PLoS Negl Trop Dis. 7:e2372.
- Tsai TF. 2000. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine. 18(2):1–25.
- Undurraga EA, Halasa YA, Shepard DS. 2013. Use of expansion factors to estimate the burden of dengue in Southeast Asia: a systematic analysis. PLoS Negl Trop Dis. 7:e2056.
- Unni SK, Ruzek D, Chhatbar C, Mishra R, Johri MK, Singh SK. 2011. Japanese encephalitis virus: from genome to infectome. Microbes Infect. 13:312–321.
- Utsumi T, Hayashi Y, Lusida MI, Amin M, Soetjipto Hendra A, Soetjiningsih Yano Y, Hotta H. 2011. Prevalence of hepatitis E virus among swine and humans in two different ethnic communities in Indonesia. Arch Virol. 156:689–693.
- Utsumi T, Yano Y, Amin M, Lusida MI, Soetjipto Hotta H, Hayashi Y. 2014. Acute hepatitis due to hepatitis A virus subgenotype IA as an imported infectious disease from Indonesia. Kobe J Med Sci. 60:E43–E47.
- Vainio K, Noraas S, Holmberg M, Fremstad H, Wahlstrøm M, Ånestad G, Dudman S. 2010. Fatal and mild primary dengue virus infections imported to Norway from Africa and south-east Asia, 2008–2010. Euro Surveill. 15:19666.
- van der Meulen KM, Pensaert MB, Nauwynck HJ. 2005. West Nile virus in the vertebrate world. Arch Virol. 150:637–657.
- Van Peenen PF, Anderson KE, See R, Nasution R. 1974. Serological evidence of arbovirus infection in febrile hospitalized patients in Jakarta, Indonesia during 1970–71. J Trop Med Hyg. 77:244–246.
- Van Peenen PF, Joseph PL, Atmosoedjono S, Irsiana R, Saroso JS. 1975. Japanese encephalitis virus from pigs and mosquitoes in Jakarta, Indonesia. Trans R Soc Trop Med Hyg. 69:477–479.
- Vannice KS, Durbin A, Hombach J. 2016. Status of vaccine research and development of vaccines for dengue. Vaccine. 34:2934–2938.
- Vranckx R, Alisjahbana A, Devillé W, Meheus A. 1997. Hepatitis A antibodies in Indonesian neonates and children. Int J Infect Dis. 2:31–33.
- Waltner-Toews D, Maryono A, Akoso BT, Wisynu S, Unruh DHA. 1990. An epidemic of canine rabies in central Java, Indonesia. Prev Vet Med. 8:295–303.
- Wang L, Tang Q, Liang G. 2014. Rabies and rabies virus in wildlife in mainland China, 1990–2013. Int J Infect Dis. 25:122–129.
- Ward MP. 2014. Rabies in the Dutch East Indies a century ago - a spatio-temporal case study in disease emergence. Prev Vet Med. 114:11–20.
- Watson Pertel PE, Jones RC, Siston AM, Paul WS, Austin CC, Gerber SI. 2004. Clinical characteristics and functional outcomes of West Nile Fever. Ann Intern Med. 141:360–365.
- Watson DC, Sargianou M, Papa A, Chra P, Starakis I, Panos G. 2014. Epidemiology of Hantavirus infections in humans: a comprehensive, global overview. Crit Rev Microbiol. 40:261–272.
- Wertheim HF, Nadjm B, Thomas S, Agustiningsih A, Malik S, Diep NN, Vu TV, Kinh NV, Chau NV, Liem NT, et al. 2015. Viral and atypical bacterial aetiologies of infection in hospitalised patients admitted with clinical suspicion of influenza in Thailand, Vietnam and Indonesia. Influenza Other Respir Viruses. 9:315–322.
- WHO-SEARO. 2005. Combating emerging infectious diseases in the South-East Asia Region. New Delhi (India): World Health Organization Regional Office for South-East Asia (WHO-SEARO).
- WHO. 2012. Global measles and rubella strategic plan: 2012–2020. Geneva (Switzerland): World Health Organization.
- WHO. 2014a. A brief guide to emerging infectious diseases and zoonoses. Delhi (India): World Health Organization Regional Office for South-East Asia; [accessed 2016 May]. http://www.searo.who.int/entity/emerging_diseases/ebola/a_brief_guide_emerging_infectious_diseases.pdf
- WHO. 2014b. The top 10 causes of death. Geneva (Switzerland): WHO; [accessed 2014]. http://www.who.int/mediacentre/factsheets/fs310/en/
- WHO. 2015a. Human cases of influenza at the human-animal interface, January 2014–April 2015. Wkly Epidemiol Rec. 90:349–362.
- WHO. 2015b. Japanese encephalitis. Geneva (Switzerland): WHO; [accessed 2016]. http://www.who.int/mediacentre/factsheets/fs386/en/
- WHO. 2016a. Immunization surveillance, assessment and monitoring (Measles 1st dose (MCV1) immunization coverage among 1-year olds, 1980–2015 (%): 2015. Geneva (Switzerland): World Health Organization; [accessed 2016]. http://gamapserver.who.int/gho/interactive_charts/immunization/mcv/atlas.html
- WHO. 2016b. Immunization, Vaccines and Biologicals Dengue vaccine research. Geneva (Switzerland): WHO; [accessed 2016]. http://www.who.int/immunization/research/development/dengue_vaccines/en/
- Wicaksono EO, Soekarman B, Hadi U. 2014. A patient with acute disseminated encephalopathy caused by West Nile infection. 32nd World Congress of Internal Medicine (WCIM). Seoul: Korean Association of Internal Medicine.
- Widagdo W, Okba NMA, Stalin Raj V, Haagmans BL. 2017. MERS-coronavirus: from discovery to intervention. One Health. 3:11–16.
- Widasari DI, Yano Y, Utsumi T, Heriyanto DS, Anggorowati N, Rinonce HT, Utoro T, Lusida MI, Soetjipto, Asmara W, et al. 2013. Hepatitis E virus infection in two different community settings with identification of swine HEV genotype 3 in Indonesia. Microbiol Immunol. 57:692–703.
- Widyastuti MD, Bardosh KL, Sunandar Basri C, Basuno E, Jatikusumah A, Arief RA, Putra AA, Rukmantara A, Estoepangestie AT, et al. 2015. On dogs, people, and a rabies epidemic: results from a sociocultural study in Bali, Indonesia. Infect Dis Poverty. 4:30.
- Wijayanti SP, Sunaryo S, Suprihatin S, McFarlane M, Rainey SM, Dietrich I, Schnettler E, Biek R, Kohl A. 2016. Dengue in Java, Indonesia: relevance of mosquito indices as risk predictors. PLoS Negl Trop Dis. 10:e0004500.
- Wittesjo B, Eitrem R, Niklasson B, Vene S, Mangiafico JA. 1995. Japanese encephalitis after a 10-day holiday in Bali. Lancet. 345:856–857.
- Worldbank Indonesia Data. 2016. [accessed 2016]. http://data.worldbank.org/country/indonesia
- World Economic Forum. 2015. Travel and Tourism Competitiveness Report. Cologny (Switzerland): World Economic Forum; [accessed 2016]. http://reports.weforum.org/travel-and-tourism-competitiveness-report-2015/economies/#economy=IDN
- Yamanaka A, Mulyatno KC, Susilowati H, Hendrianto E, Ginting AP, Sary DD, Rantam FA, Soegijanto S, Konishi E. 2011. Displacement of the predominant dengue virus from type 2 to type 1 with a subsequent genotype shift from IV to I in Surabaya, Indonesia 2008–2010. PLoS One. 6:e27322.
- Yamanaka A, Mulyatno KC, Susilowati H, Hendrianto E, Utsumi T, Amin M, Lusida MI, Soegijanto S, Konishi E. 2010. Prevalence of antibodies to Japanese encephalitis virus among pigs in Bali and East Java, Indonesia, 2008. Jpn J Infect Dis. 63:58–60.
- Yang Y, Halloran ME, Sugimoto JD, Longini IM. Jr. 2007. Detecting human-to-human transmission of avian influenza A (H5N1). Emerg Infect Dis. 13:1348–1353.
- Yoshida M, Igarashi A, Suwendra P, Inada K, Maha MS, Kari K, Suda H, Antonio MT, Arhana BN, Takikawa Y, et al. 1999. The first report on human cases serologically diagnosed as Japanese encephalitis in Indonesia. Southeast Asian J Trop Med Public Health. 30:698–706.
- Yoshikawa MJ, Kusriastuti R. 2013. Surge of dengue virus infection and chikungunya Fever in bali in 2010: the burden of mosquito-borne infectious diseases in a tourist destination. Trop Med Health. 41:67–78.

