Abstract
Although SARS-CoV-2, responsible for COVID-19, is primarily a respiratory infection, a broad spectrum of cardiac, pulmonary, neurologic, and metabolic complications can occur. More than 50 long-term symptoms of COVID-19 have been described, and as many as 80% of patients may develop ≥1 long-term symptom. To summarize current perspectives of long-term sequelae of COVID-19, we conducted a PubMed search describing the long-term cardiovascular, pulmonary, gastrointestinal, and neurologic effects post-SARS-CoV-2 infection and mechanistic insights and risk factors for the above-mentioned sequelae. Emerging risk factors of long-term sequelae include older age (≥65 years), female sex, Black or Asian race, Hispanic ethnicity, and presence of comorbidities. There is an urgent need to better understand ongoing effects of COVID-19. Prospective studies evaluating long-term effects of COVID-19 in all body systems and patient groups will facilitate appropriate management and assess burden of care. Clinicians should ensure patients are followed up and managed appropriately, especially those in at-risk groups. Healthcare systems worldwide need to develop approaches to follow-up and support patients recovering from COVID-19. Surveillance programs can enhance prevention and treatment efforts for those most vulnerable.
Introduction
The COVID-19 pandemic has caused devastating loss of life and profoundly impacted the global economy; as of February 6, 2023, >754 million confirmed cases of COVID-19 and >6.8 million deaths worldwide have occurred (WHO Coronavirus \(COVID-19\) Dashboard 2021). The causative virus, SARS-CoV-2, belongs to the betacoronavirus clade that includes SARS-CoV and MERS-CoV, which can cause severe and sometimes fatal respiratory syndrome (Chen et al. Citation2020; Lu et al. Citation2020; Verdecchia et al. Citation2020).
A large proportion of patients recovering from acute COVID-19 continue to have cardiac, pulmonary, neurologic, and other non-specific symptoms as a post-acute condition, even after mild/uncomplicated COVID-19 (Lopez-Leon et al. Citation2021; Havervall et al. Citation2021; Jacobson et al. Citation2021). More than 50 long-term COVID-19 symptoms are described; up to 80% of patients may develop ≥1 long-term symptom, and ∼30% have persistent symptoms up to 6 months post-symptom onset, although heterogeneity in symptom prevalence is noted (Lopez-Leon et al. Citation2021; Logue et al. Citation2021; Hayes et al. Citation2021; Chen et al. Citation2022). While the lung is the primary organ likely to be impaired or damaged in acute COVID-19, evidence suggests broad tropism of SARS-CoV-2 to the cardiac, renal, digestive, and nervous systems (Bradley et al. Citation2020; De Felice et al. Citation2020).
The purpose of this narrative review is to summarise current perspectives and mechanistic insights of long-term COVID-19 sequelae based on organ system, treatment, and patient population. We also explore how the pandemic indirectly affects healthcare provision and long-term patient management.
Methods
This is a focussed narrative review with no formal literature search protocol. Publications were identified from a PubMed search of long-term pulmonary, cardiovascular, neurologic, and gastrointestinal effects post-SARS-CoV-2 infection and treatment. Publications describing risk factors and underlying mechanisms for the above-mentioned sequelae were also identified. Additional relevant references were added from authors’ personal collections. The initial search was performed in October 2020, with subsequent searches in March/October 2021 and November 2022; however, given the rapidly evolving body of knowledge around COVID-19, additional articles of interest were included as they were identified.
COVID-19 onset, clinical course, and prolonged symptoms
Although most people with COVID-19 arising from the ancestral SARS-CoV-2 strain had mild-to-moderate illness, the disease became severe in 10%−15% of cases, with 5% becoming critically ill (Coronavirus Update 36: What We Know About the Long-Term Effects of COVID-19 Citation2020). Notably, the Omicron variant, which was first identified in late 2021 and quickly becoming the predominant strain, is more transmissible yet less virulent than earlier strains, but has strained healthcare systems because of rapid rises in case numbers; reasons for the decrease in disease severity are multifactorial, but include increased vaccination uptake (Iuliano et al., Citation2022).
Acute COVID-19 lasts up to 4 weeks, symptomatic COVID-19 can last up to 12 weeks, and post-COVID-19 condition is defined as signs/symptoms developing typically about 3 months from COVID-19 onset, continuing for >3 months, and which cannot be explained by alternative diagnoses (Condition WHOCCDWGoP-C 2022; National Institute for Health and Care Excellence \(NICE, Citation2022) “Long COVID” is a commonly used term to describe symptoms beyond the acute stage (National Institute for Health and Care Excellence: clinical Guidelines Citation2020); however, many different ‘long COVID’ definitions exist (Yong Citation2021; Condition WHOCCDWGoP-C 2022). This review considers long COVID to be signs/symptoms continuing or developing >4 weeks post-acute COVID-19 (NICE Citation2022).
Estimates of the percentage of recovered individuals developing long-term COVID-19 symptoms vary widely from ∼10%−80%, depending on the patient population analysed, inclusion of controls, and follow-up duration (Lopez-Leon et al., Citation2021; Parums Citation2021; Chen et al. Citation2022). Estimates at the upper end of the scale (i.e. 80%) tend to be derived from selected patient samples lacking a control group and/or systematic reviews that include such studies, compounding potential selection biases (Lopez-Leon et al. Citation2021; Buonsenso et al. Citation2021; Amin-Chowdhury and Ladhani Citation2021). More conservative estimates of long COVID ranging from 13%−42% are reported in studies utilizing case–control matching to individuals with no SARS-CoV-2 infection (Sudre et al. Citation2021; Taquet et al. Citation2021). An estimated 6.2% of individuals may experience long COVID symptom clusters, including 3.7%, 3.2%, and 2.2% with ongoing respiratory problems, persistent fatigue with body pain or mood swings, and cognitive issues, respectively (Global Burden of Disease Long CC 2022). Additionally, symptom prevalence can vary depending on the time period (e.g. 4 − 12 vs >12 weeks post-acute COVID-19) (Jennings et al. Citation2021).
Pulmonary effects
SARS-CoV-2 enters lungs via ACE2-expressing type 2 pneumocytes, initiating acute systemic inflammatory responses and cytokine storm, leading to lung-resident dendritic cell activation and antiviral cytokine release by activated T lymphocytes, ultimately resulting in lung injury (Zhao et al. Citation2020). While long-term pulmonary COVID-19 complications are not fully understood, studies of other respiratory viral infections indicate that lasting effects can occur (e.g. permanent pulmonary damage post-acute pneumonia with SARS and avian flu H7N9 infections) (Ngai et al. Citation2010; Salehi et al. Citation2020; Shaw et al. Citation2021).
Post-mortem studies of COVID-19 patients from Switzerland, Italy, and Germany report diffuse alveolar damage in the majority of autopsies (Carsana et al. Citation2020; Menter et al. Citation2020; Schaller et al. Citation2020). A Chinese study of 165 patients with chest CT results reported diffuse alveolar damage in 14% of patients, although long-term consequences are unknown (Jin et al. Citation2020). A Chinese study of 55 COVID-19 survivors reported radiologic abnormalities in 71% of patients and interstitial thickening in 27% of patients 3 months post-discharge (Zhao et al. Citation2020). A prospective longitudinal cohort study of 83 patients hospitalized for severe COVID-19 but not requiring mechanical ventilation found that 33% had pulmonary diffusion abnormality and 24% had persistent radiologic abnormalities 12 months post-discharge (Wu et al., Citation2021).
CT images in patients post-SARS-CoV-2 infection identified long-term pulmonary sequelae, including interstitial pulmonary fibrosis, reduced pulmonary function, and diffuse alveolar damage (Zhao et al. Citation2020; Salehi et al. Citation2020; Jin et al. Citation2020). A meta-analysis of 15 studies of 102 − 44,799 patients, and median study follow-up times ranging from 14 − 110 days, found that lung disease symptoms were common in recovered patients with prevalence (95% CI) of 19% (7%−34%) for cough, 16% (10%−22%) for chest pain/discomfort, 10% (6%−16%) for reduced pulmonary diffusing capacity, and 5% (3%−8%) for pulmonary fibrosis (Lopez-Leon et al. Citation2021). Another meta-analysis of 6770 patients from 18 studies with a 6-month and 12 studies with a 12-month follow-up found that impaired diffusion capacity (35% prevalence) was the most common abnormality, which was most likely observed in those with severe COVID-19 (Lee et al. Citation2022). Prospective studies are further evaluating these complications and will help identify those most at risk (Fraser Citation2020; Shaw et al. Citation2021).
Cardiovascular effects
Multiple direct and indirect cardiovascular complications may continue to have long-term consequences post-resolution of SARS-CoV-2 infection, including acute myocardial injury, acute coronary syndrome, cardiogenic shock, myocarditis, heart failure, dysrhythmias, and venous thromboembolism (Driggin et al. Citation2020; Yancy and Fonarow Citation2020; Cormican et al. Citation2021). SARS-CoV-2 entry into cardiovascular cells is facilitated by ACE2 on surfaces of myocytes, coronary endothelial cells, pericytes, and arterial smooth muscle, increasing risk of organ damage and myocardial infarction (Chen et al. Citation2020; Cormican et al. Citation2021). SARS-CoV-2 may also infect myocardial cells directly, causing cytopathic effects and tissue destruction, or by immune dysregulation and hyperactivity (Shah et al. Citation2021). Long-term effects of this damage are unclear, and further study warranted.
A German autopsy study of 39 individuals with COVID-19 found frequent infection (62% of individuals) of the myocardium with SARS-CoV-2 using RT-PCR; however, none were diagnosed antemortem with myocarditis (Lindner et al. Citation2020). Comorbidities in these patients included hypertension (44%), coronary artery disease (82%), and diabetes mellitus (18%). Further investigation is warranted on long-term consequences of cardiac infection.
Myocarditis is an important cause of sudden death during exercise in athletes, and is a concern following SARS-CoV-2 infection. Multicentre registry data documenting CV outcomes in athletes following SARS-CoV-2 infection are being collected to assess cardiac pathology, including those with asymptomatic or mild infection (Kim et al. Citation2021). In a German study, 100 patients who recently recovered from SARS-CoV-2 infection, confirmed by real-time RT-PCR, were evaluated for myocardial injury (Puntmann et al. Citation2020). Cardiovascular involvement was detected by cardiac magnetic resonance in 78/100 patients and myocardial inflammation (abnormal native T1 and T2 measures) in 60/100 patients (Puntmann et al. Citation2020).
In a large case–control cohort utilising the US Department of Veterans Affairs (DVA) electronic healthcare database, long-term cardiovascular sequelae identified in individuals with COVID-19 included hypertension, cardiac dysrhythmias (particularly tachycardia), circulatory defects, chest pain, coronary atherosclerosis, and heart failure (Al-Aly et al. Citation2021; Ståhlberg et al. Citation2021). Notably, these cardiovascular conditions were more likely to occur in individuals hospitalized for COVID-19 versus those who had not (Al-Aly et al. Citation2021). In another case–control cohort study using the same database, those surviving the first 30 days post-COVID-19 had increased risk at 12 months for cardiovascular disease including cardiac disorders (stroke, transient ischaemic attacks), dysrhythmias (atrial fibrillation, sinus tachycardia and bradycardia, ventricular arrhythmia, atrial flutter), pericarditis/myocarditis, ischaemic heart disease (acute coronary disease, ischaemic cardiomyopathy, angina), other cardiac disorders (heart failure, non-ischaemic cardiomyopathy, cardiac arrest, cardiogenic shock), and thromboembolic disorders (pulmonary embolism, deep vein and superficial vein thrombosis) (Xie et al. Citation2022). In a cross-sectional study of individuals who recovered from COVID-19, patients reporting palpitations and/or fatigue with exercise in the post-acute COVID-19 phase had significantly higher NT-proBNP levels, significantly lower nitric oxide levels, and regional increases in 18F-FDG uptake on cardiac PET, compatible with myocardial fatigue (Sarıçam et al. Citation2021). Decreased oxygen consumption in COVID-19 cases, compared with controls, particularly during exercise, has been tied to chronotropic incompetence in combination with stroke volume limitations largely attributed to diminished increases in left ventricular end-diastolic volume and insufficient increases in ejection fraction (Szekely et al. Citation2021).
Neurologic effects
Many studies have investigated sensory deficits, mainly loss of smell and taste; however, pathogenesis is still unclear (Mastrangelo et al. Citation2021). A systematic review found anosmia and hyposmia prevalence to be 12% and 30%, respectively, >12 weeks post-COVID-19; corresponding percentages for ageusia and hypogeusia were 12% and 31% (Trott et al. Citation2022). Sensory deficit symptoms can persist for several weeks, but little is known about long-term sequelae (Patel et al. Citation2020; Kosugi et al. Citation2020; Paderno et al. Citation2020; Lee et al. Citation2020; Otte et al. Citation2020; Meini et al. Citation2020; Sayin and Yazici, 2020). There are high rates of COVID-19 − related olfactory and gustatory dysfunction resolution in the first month post-symptom onset, with 90% of individuals recovering smell and taste senses at 90 days (Tan et al. Citation2022). However, complete resolution may not be achieved in a notable percentage of individuals (10%−15% after 45 days follow-up) (Paderno et al. Citation2020). Patients often had ongoing symptoms at study end, suggesting further follow-up and longer-term prospective studies are needed (Patel et al. Citation2020; Kosugi et al. Citation2020; Paderno et al. Citation2020).
Currently available evidence suggests SARS-CoV-2 can cause nervous system damage and alterations (Liotta et al. Citation2020; Troyer et al. Citation2020). Long-term consequences are unknown, but may potentially include cognitive deficits, and delayed neurologic complications, such as Parkinson’s disease, Alzheimer’s disease, and neuropsychiatric disorders (Troyer et al. Citation2020; Abboud et al. Citation2020; Heneka et al. Citation2020; Lennon Citation2020; Serrano-Castro et al. Citation2020; Ali Awan et al. Citation2021). Some evidence exists of cognitive decline 2 − 3 weeks post-infection in recovered patients, which could be a result of inflammatory responses to severe infection, leading to cytokine storm syndrome, and vascular injury causing stroke, hypoxia, or delirium (Zhou et al. Citation2020; Cothran et al. Citation2020;Ali Awan et al. Citation2021; Ceban et al. Citation2022). Other suggested mechanisms leading to cerebral sequelae associated with SARS-CoV-2 infection include viral neurotropism (e.g. leading to infiltration of virus-laden immune cells, blood − brain barrier breaks, or olfactory system infiltration), and pandemic-associated psychological burden caused by stressors affecting cognitive ability (Ali Awan et al. Citation2021; Douaud et al. Citation2022).
Longitudinal studies are therefore needed to determine long-term effects of COVID-19 on neurological and cognitive function (Abboud et al. Citation2020; Serrano-Castro et al. Citation2020; Zhou et al. Citation2020). A Swedish study of 323 patients with mild COVID-19 reported the most common long-term symptoms were loss of smell (15%), fatigue, and loss of taste (both 8%) (Havervall et al. Citation2021). Loss of smell persisted for ≥8 months in 9% of patients (Havervall et al. Citation2021). In an Italian study of 312 COVID-19 survivors, 86% of whom were hospitalised but not requiring ICU admission, 75% of evaluable patients showed impairment in ≥1 of 6 cognitive functions at 6 months (COVID-19 BioB Outpatient Clinic Study group, 2022). Moreover, COVID-19 survivors scored lower than healthy controls in psychomotor coordination, attention and speed-of-information processing, verbal fluency, and executive functioning (COVID-19 BioB Outpatient Clinic Study group 2022). Several studies and systematic reviews have investigated prevalence of neurologic and cognitive function sequelae in long COVID. A Dutch online survey study of individuals experiencing long COVID-19 symptoms found 37%, 36%, and 47% were at risk of PTSD, anxiety, and depression, respectively, 3 months post-infection (Houben-Wilke et al. Citation2022). A systematic review and meta-analysis of 21 studies assessing health-related quality of life and psychiatric problems in COVID-19 survivors identified a pooled prevalence of PTSD, depression, and anxiety of 18%, 12%, and 17%, respectively (Dorri et al. Citation2021). Another systematic review and meta-analysis, which included 18 studies of >10,000 COVID-19 survivors, reported fatigue (37%), brain fog (32%), sleep disturbances (31%), and memory issues (28%) to be the most frequent long-term neurologic sequelae post-infection (Premraj et al. Citation2022). Notably, these post-acute neurologic and psychiatric sequelae are similar to post-acute ICU syndrome, which includes new or worsening mental (predominantly PTSD, anxiety, depression) and cognitive (predominantly memory and executive function dysfunction) health impairments (Schwab et al. Citation2022).
Gastrointestinal effects
While gastrointestinal symptoms of acute COVID-19, such as diarrhoea, constipation, vomiting, abdominal pain, and acid reflux, are recognised, comparatively less data are available on these symptoms in long COVID (Choudhury et al. Citation2022; Gang et al. Citation2022). In a systematic review and meta-analysis of 50 studies, the frequency of overall gastrointestinal symptoms was 12% and 22% in acute COVID-19 and long COVID, respectively, and gastrointestinal manifestations in long COVID did not appear related to acute COVID-19 severity (Choudhury et al. Citation2022). Proposed mechanisms associated with gastrointestinal symptoms from SARS-CoV-2 infection include activation of signal transduction pathways in epithelial cells, triggering vascular endothelial growth factor production and then leading to vascular permeability and inflammation (Gang et al. Citation2022). For patients with long COVID, gastrointestinal symptoms have been associated with diminished richness of the gut microbiota (Gang et al. Citation2022).
Long-term consequences in children
Prevalence, associated mechanisms, and outcomes of long COVID in children are not well elucidated, although a milder phenotype compared with older populations has been described (Stephenson et al. Citation2022). Long COVID prevalence in children varies greatly (1.6%−70%), with fatigue, headache, arthro-myalgia, dyspnoea, and smell and taste alterations most commonly experienced (Pellegrino et al. Citation2022). Female sex, having underlying comorbidities, and increasing age appear associated with persistent COVID-19 symptoms among children (Behnood et al. Citation2022). Frequencies of many reported persistent COVID-19 symptoms in children are similar in SARS-CoV-2 − positive cases and controls, (Behnood et al. Citation2022) which may be related to difficulties in distinguishing long COVID effects from those of extended lockdown measures in this population.
Notably, multisystem inflammatory syndrome in children (MIS-C) is a newly reported illness that appears associated with SARS-CoV-2 (Belot et al. Citation2020). MIS-C, which has similar characteristics to toxic shock syndrome and Kawasaki disease, (Rowley et al. Citation2020) can appear several weeks post-SARS-CoV-2 infection. A French study of 21 children and adolescents with MIS-C reported a median (range) of 36 (18 − 45) days between reported COVID-19 contact and onset of MIS-C symptoms (Toubiana et al. Citation2020). MIS-C has been associated with cardiovascular complications. In this French study, 76% of patients had myocarditis and 10% showed significant electrocardiographic changes (Toubiana et al. Citation2020). A US study conducted in 186 children (median age 8.3 years) with MIS-C associated with SARS-CoV-2, in whom 70% were SARS-CoV-2 RT-PCR or antibody positive, reported common cardiovascular involvement (in 80% of children), and 8% of children had coronary artery aneurysms (Feldstein et al. Citation2020). A New York State study of 99 patients ≤20-years-old with MIS-C reported coronary artery aneurysms in 9% of patients (Dufort et al. Citation2020). In children with COVID-19-associated MIS-C, 14% had coronary lesions and 12%−25% giant aneurysms (Singh-Grewal et al. Citation2020). A meta-analysis of 11 observational studies assessing cardiovascular outcomes in 547 patients with MIS-C, found generally favourable mid-term outcomes in the majority of patients, including low mortality rate and normalization of left ventricular systolic dysfunction, but some patients showed persistent abnormalities and mitral regurgitation at 6 months (Yasuhara et al. Citation2023). The long-term effects of MIS-C are unclear. A preliminary report suggests that cardiac abnormalities tend to resolve in most children by 2 months post-infection (Capone et al. Citation2021). However, large ongoing observational cohort studies will provide better insight into long-term consequences of MIS-C (e.g. the MUSIC study) (Truong et al. Citation2022).
Other body systems
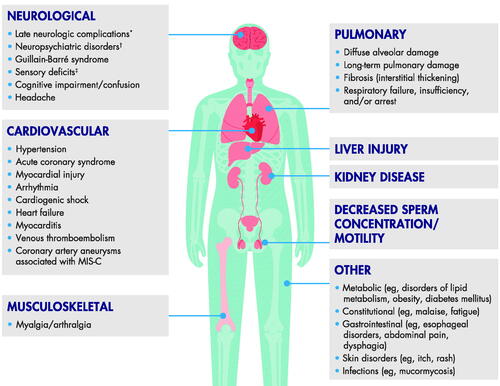
While the nature and extent of long-term COVID-19 consequences are unclear, many other affected body systems and clinical manifestations may emerge, including diabetes mellitus and mucormycosis () (Coronavirus Update 36: What We Know About the Long-Term Effects of COVID-19, Citation2020; Cothran et al. Citation2020; Adapa et al. Citation2020; Long-term sequelae and COVID-19 – what we know so far Citation2020; Samidoust et al. Citation2020; Segars et al. Citation2020; Al-Aly et al. Citation2021; Mansoor et al. Citation2021; Yasmin et al. Citation2021; Lai et al. Citation2022; Wrona and Skrypnik Citation2022. However, the increasingly diverse number of reports across multiple organ systems and subspecialties goes beyond the scope of this review.
Possible mechanisms underlying long COVID
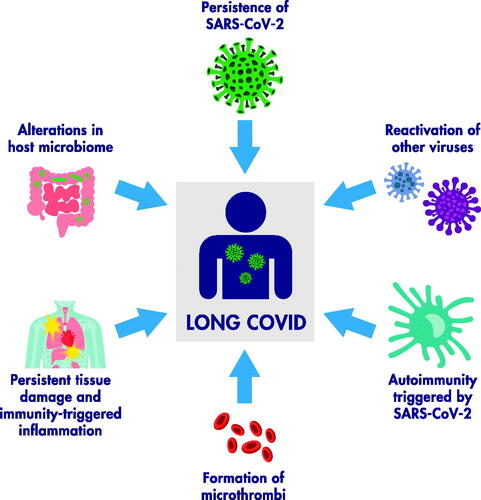
Several published reviews have in-depth discussions of potential pathophysiological mechanisms of long-term sequelae; here we provide a succinct overview (). Candidate mechanisms potentially contributing to pathogenesis include SARS-CoV-2 persistence; reactivation of other viruses (in particular Epstein-Barr virus); virus-triggered autoimmunity; persistent tissue damage and immunity-triggered inflammation; and formation of microthrombi in the vascular bed of different tissues (e.g. endothelial cell activation) (Covid-19 Commission of the Accademia Nazionale dei Lincei Citation2022; Monje and Iwasaki Citation2022; Newell and Waickman Citation2022). Host microbiome alterations may also play a role (Batiha et al. Citation2022; Liu et al. Citation2022). Endothelial damage and subsequent endothelial dysfunction may be a mechanism as long-term viral infection/chronic hypoxia/inflammatory response can lead to persistent vascular endothelial injury followed by coagulation and microthrombosis, and finally systemic functional impairments and clinical sequelae (Wang et al. Citation2022). Impaired endothelial function has been observed in convalescent COVID-19 patients, (Ambrosino et al. Citation2022) while elevation of ANG-1 and P-SEL may represent a long-term angiogenesis response for wound-repair of endothelial injuries (Patel et al. Citation2022).
Risk factors
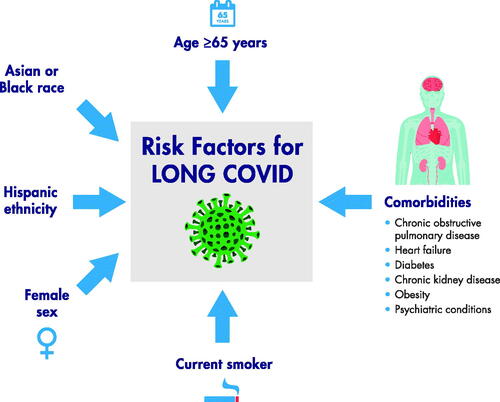
Risk factors for development of severe COVID-19 include age >60 years, and chronic health conditions, including hypertension, diabetes, underlying cardiovascular, respiratory, and kidney diseases, and malignancy (Driggin et al. Citation2020; Chen et al. Citation2020; Grasselli et al. Citation2020; Suleyman et al. Citation2020; World Health Organization Citation2020; Cormican et al. Citation2021). Risk factors for prolonged illness are emerging; these include age ≥65 years, female sex, Black or Asian race, Hispanic ethnicity, and active smoking () (Coronavirus Update 36: What We Know About the Long-Term Effects of COVID-19, Citation2020; Lavery et al. Citation2020; Sigfrid et al. Citation2021; Bai et al. Citation2022). An app-based 2020 study of >4000 users reported the likelihood of long COVID increased with older age and female sex (Sudre et al. Citation2020). In a US DVA electronic healthcare database study of >180,000 patients, burden of long COVID manifestations was higher in veterans with poor health status and increased by acute COVID-19 severity (Xie et al. Citation2021). In a longitudinal, prospective cohort study of 1038 patients with laboratory-confirmed SARS-CoV-2 infection, COVID-19-associated hospitalization, having diabetes, and higher BMI were associated with development of long COVID (Yoo et al. Citation2022).
Long-term effects are not restricted to these vulnerable groups; younger individuals (<50 years) with no or few comorbidities can have prolonged illness, even after mild or uncomplicated COVID-19 (Tenforde et al. Citation2020; Jacobson et al. Citation2021). For instance, a meta-analysis that included 20 studies and 7840 patients found that risk of persistent cough, chest pain, anosmia, and palpitation was not associated with the severity of acute COVID-19 (Dirican and Bal Citation2022).
Implications
Globally, almost 650 million people have recovered from COVID-19; among them, many will have long-term health consequences, perhaps for life. These long-term health consequences will not only affect these people and their families but also, collectively, add a sizable burden to healthcare systems. The pandemic has resulted in widespread job losses and school closures (Nicola et al. Citation2020). Children have suffered from being away from a learning environment, especially those from lower income households (Townsend Citation2020; Bayham and Fenichel Citation2020; Masonbrink and Hurley Citation2020; Van Lancker and Parolin Citation2020). Stay-at-home orders may have other negative impacts, such as decline in mental health, increased alcohol consumption, and increased weight in some populations, (Flanagan et al. Citation2021) while delay or avoidance of routine medical care because of COVID-19 may lead to poor management of underlying conditions, missed routine vaccinations, missed early detection, and delayed diagnoses of new conditions (Czeisler et al. Citation2020). Because of the nature of their jobs, healthcare workers (HCWs) have particularly experienced detrimental pandemic-related effects, including stress, anxiety, depression, and insomnia, constrained resources, working to exhaustion, and being at personal risk of infection (Awan et al. Citation2021). In a meta-analysis of 47 studies, which compared anxiety and depression prevalence among frontline versus non-frontline HCWs, prevalence was higher among the former and nurses had higher rates than doctors (Sun et al. Citation2021).
Barriers to recovery from long-term COVID-19 effects include pre-existing health status, health disparities, and access to care. The latter may vary by geographic area, age, and socioeconomic group (Nunez et al. Citation2020). Quality of care may vary, and some regions may have difficulties accessing healthcare, during a hospital surge, or obtaining vaccines. COVID-19-associated health disparities can be due to population size, Black race, poverty, disability, and education level; these may be at the individual level or on a systemic level (Parcha et al. Citation2020; Johnson et al. Citation2020; Abedi et al. Citation2021). Economic consequences of long COVID are also far reaching. For instance, the annual cost of treatment has been estimated at ∼$9000/person; additionally, affected individuals often need to leave employment or work reduced hours, representing direct earning losses (Cutler Citation2022).
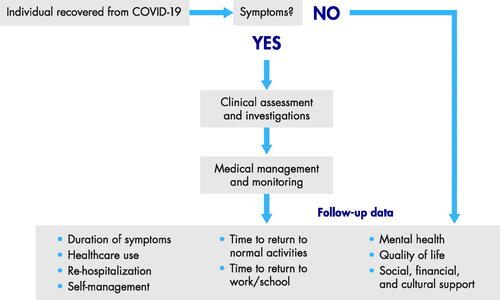
To improve our understanding of long-term COVID-19 health effects, large-scale standardised data collection is vital, and study of COVID-19 risk and long-term effects is underway at 37 US academic centres (Study of COVID-19 Risk and Long-Term Effects Underway at 37 U.S. Academic Medical Centers Citation2021). Follow-up studies of people previously infected with SARS-CoV-2 are also underway, including an international, prospective, observational study of patients across 42 countries hospitalised for COVID-19 who agree to regularly complete standardised survey instruments for ≤3 years to measure long-term physical and psychosocial sequelae (Cardiovascular Consequences After COVID-19 Citation2021; Evaluate Long Term Cardiovascular and Pulmonary Complications After COVID-19 With Point of Care Ultrasound Citation2021; Sigfrid et al. Citation2021). Ideally a well-defined, long-term, follow-up and surveillance program should include clinical assessment, investigations, and ongoing medical management and monitoring (). Such a program could provide more information on persistent medical conditions post-COVID-19 and whether COVID-19 exacerbated underlying conditions. Measurement metrics could include duration of long-term adverse effects, need for follow-up medical care (e.g. medical appointments, long-term medications), re-hospitalizations, and time to return to normal daily living activities and to work or school. Surveillance and follow-up programs can inform actionable steps, including enhancing prevention and treatment efforts for the most vulnerable. Surveillance and follow-up will also allow researchers to determine whether any new SARS-CoV-2 variants of concern have different long-term effects than the ancestral virus. For instance, a UK case-controlled, observational study found that the long COVID risk was lower after Omicron versus Delta infection (reduced by ∼24%−50% depending on age and time since vaccination) (Antonelli et al. Citation2022).
Prevention of COVID-19 altogether with vaccination is the best way to reduce long COVID risk. This goal is now attainable with availability of approved vaccines, the administration of >13 billion vaccination doses worldwide, and the COVAX initiative, which aims to guarantee fair and equitable access of vaccines globally (WHO Coronavirus \(COVID-19\) Dashboard 2021; COVAX Citation2021). Although rare side effects have been reported, (Bozkurt et al. Citation2021; Palaiodimou et al. Citation2021) the benefit-risk balance is favourable for vaccination across ages and sexes (Bozkurt et al. Citation2021). It is unknown whether individuals who contract COVID-19 post-vaccination are less likely to suffer from long-term consequences, although data from systematic literature reviews and database studies are emerging (Notarte et al. Citation2022; Gao et al. Citation2022; Taquet et al. Citation2022). Interestingly, early reports of vaccination in individuals with long COVID suggest that vaccination is safe in this group, but also that persistent symptoms improve in a subset of individuals (Arnold et al. Citation2021).,Additionally, vaccination among adults was reported to result in decreased likelihood of experiencing long COVID (Ayoubkhani et al. Citation2022). Further research is ongoing in this area.
Conclusions
With almost 650 million recovered COVID-19 patients, a need exists for further research into the different patterns and underlying mechanisms of long COVID. Prospective studies will examine these effects; meanwhile, clinicians should ensure that patients are followed-up and managed appropriately, especially those at risk. Healthcare systems worldwide should follow-up and support patients recovering from COVID-19. Surveillance programs could enhance vaccination and treatment efforts for the most vulnerable, measure and understand treatment protocols, and determine ways to improve treatment and management. Such steps may help improve patients’ health and quality of life and avoid further healthcare resource strains.
Disclosure statement
No potential conflict of interest was reported by the authors.SS
Additional information
Funding
References
- Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A. 2020. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 140:49–53.
- Abedi V, Olulana O, Avula V, Chaudhary D, Khan A, Shahjouei S, Li J, Zand R. 2021. Racial, economic, and health inequality and COVID-19 infection in the United States. J Racial Ethn Health Disparities. 8(3):732–742.
- Adapa S, Chenna A, Balla M, Merugu GP, Koduri NM, Daggubati SR, Gayam V, Naramala S, Konala VM. 2020. COVID-19 Pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. 12(6):352–361.
- Al-Aly Z, Xie Y, Bowe B. 2021. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 594(7862):259–264.
- Ali Awan H, Najmuddin Diwan M, Aamir A, et al. 2021. SARS-CoV-2 and the brain: what do we know about the causality of 'cognitive COVID? J Clin Med. 10(15):3441.
- Ambrosino P, Sanduzzi Zamparelli S, Mosella M, Formisano R, Molino A, Spedicato GA, Papa A, Motta A, Di Minno MND, Maniscalco M, et al. 2022. Clinical assessment of endothelial function in convalescent COVID-19 patients: a meta-analysis with meta-regressions. Ann Med. 54(1):3234–3249.
- Amin-Chowdhury Z, Ladhani SN. 2021. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med. 27(7):1129–1130.
- Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. 2022. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 399(10343):2263–2264.
- Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. 2021. Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: a case series. Ann Intern Med. 174(9):1334–1336.
- Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. Are vaccines safe in patients with Long COVID? A prospective observational study. medRxiv 2021. 2021.03.11.21253225.
- Awan S, Diwan MN, Aamir A, Allahuddin Z, Irfan M, Carano A, Vellante F, Ventriglio A, Fornaro M, Valchera A, et al. 2021. Suicide in healthcare workers: determinants, challenges, and the impact of COVID-19. Front Psychiatry. 12:792925.
- Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, Khunti K, Alwan NA, Walker AS. 2022. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 377:e069676.
- Bai F, Tomasoni D, Falcinella C, et al. 2022. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 28:611 e9–e16.
- Batiha GE, Al-Kuraishy HM, Al-Gareeb AI, Welson NN. 2022. Pathophysiology of post-COVID syndromes: a new perspective. Virol J. 19(1):158.
- Bayham J, Fenichel EP. 2020. Impact of school closures for COVID-19 on the US health-care workforce and net mortality: a modelling study. Lancet Public Health. 5(5):e271–e8.
- Behnood SA, Shafran R, Bennett SD, Zhang AXD, O'Mahoney LL, Stephenson TJ, Ladhani SN, De Stavola BL, Viner RM, Swann OV. 2022. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J Infect. 84(2):158–170.
- Belot A, Antona D, Renolleau S, et al. 2020. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 25:2001010.
- Bozkurt B, Kamat I, Hotez PJ. 2021. Myocarditis with COVID-19 mRNA vaccines. Circulation. 144(6):471–484.
- Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, et al. 2020. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 396(10247):320–332.
- Buonsenso D, Espuny Pujol F, Munblit D, McFarland S, Simpson F. 2021. Clinical characteristics, activity levels and mental health problems in children with long COVID: a survey of 510 children. Preprints. 2021:030271.
- Capone CA, Misra N, Ganigara M, Epstein S, Rajan S, Acharya SS, Hayes DA, Kearney MB, Romano A, Friedman RA, et al. 2021. Six month follow-up of patients with multi-system inflammatory syndrome in children. Pediatrics. 148(4):e2021050973.
- Cardiovascular Consequences After COVID-19. 2021. (Accessed 15 March 2021, at https://clinicaltrials.gov/ct2/show/NCT04452630?term=observational%2C+long-term%2C+recovered&cond=Covid19&draw=2&rank=5.)
- Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, et al. 2020. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 20(10):1135–1140.
- Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, Rodrigues NB, Subramaniapillai M, Di Vincenzo JD, Cao B, et al. 2022. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 101:93–135.
- Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. 2022. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 226(9):1593–1607.
- Chen L, Li X, Chen M, Feng Y, Xiong C. 2020. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 116(6):1097–1100.
- Chen Y, Liu Q, Guo D. 2020. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 92(4):418–423.
- Choudhury A, Tariq R, Jena A, Vesely EK, Singh S, Khanna S, Sharma V. 2022. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Therap Adv Gastroenterol. 15:17562848221118403.
- Cormican DS, Winter D, McHugh S, Sonny A, Crowley J, Yu R, Barrack F, Núñez-Gil IJ, Ramakrishna H. 2021. Severe acute respiratory syndrome coronavirus-2 cardiovascular complications: implications for cardiothoracic anesthesiology. J Cardiothorac Vasc Anesth. 35(3):932–943.
- Coronavirus Update 36: What We Know About the Long-Term Effects of COVID-19 2020. Accessed March 31, 2021at https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-36-long-term-symptoms.pdf?sfvrsn=5d3789a6_2.)
- Cothran TP, Kellman S, Singh S, Beck JS, Powell KJ, Bolton CJ, Tam JW. 2020. A brewing storm: the neuropsychological sequelae of hyperinflammation due to COVID-19. Brain Behav Immun. 88:957–958.
- COVAX 2021. (Accessed July 1, 2021, at. Working for global equitable access to COVID-19 vaccines. https://www.who.int/initiatives/act-accelerator/covax.).
- Cutler DM. 2022. The costs of long COVID. JAMA Health Forum. 3(5):e221809.
- Czeisler MÉ, Marynak K, Clarke KEN, Salah Z, Shakya I, Thierry JM, Ali N, McMillan H, Wiley JF, Weaver MD, et al. 2020. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 69(36):1250–1257.
- De Felice FG, Tovar-Moll F, Moll J, Munoz DP, Ferreira ST. 2020. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 43(6):355–357.
- Dirican E, Bal T. 2022. COVID-19 disease severity to predict persistent symptoms: a systematic review and meta-analysis. Prim Health Care Res Dev. 23:e69.
- Dorri M, Mozafari Bazargany MH, Khodaparast Z, Bahrami S, Seifi Alan M, Rahimi F, Kamipoor Z, Niksima MM, Dehghan H, Rastad H. 2021. Psychological problems and reduced health-related quality of life in the COVID-19 survivors. J Affect Disord Rep. 6:100248.
- Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JLR, Griffanti L, Duff E, et al. 2022. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 604(7907):697–707.
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, Der Nigoghossian C, Zidar DA, Haythe J, et al. 2020. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 75(18):2352–2371.
- Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, et al. 2020. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 383(4):347–358.
- Evaluate Long Term Cardiovascular and Pulmonary Complications After COVID-19 With Point of Care Ultrasound 2021. (Accessed 15 March 2021, at https://clinicaltrials.gov/ct2/show/NCT04756193?term=observational%2C+long-term%2C+recovered&cond=Covid19&draw=2&rank=7.)
- Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, et al. 2020. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 383(4):334–346.
- Flanagan EW, Beyl RA, Fearnbach SN, Altazan AD, Martin CK, Redman LM. 2021. The impact of COVID-19 stay-at-home orders on health behaviors in adults. Obesity (Silver Spring). 29(2):438–445.
- Fraser E. 2020. Long term respiratory complications of covid-19. BMJ. 370:m3001.
- Gang J, Wang H, Xue X, Zhang S. 2022. Microbiota and COVID-19: long-term and complex influencing factors. Front Microbiol. 13:963488.
- Gao P, Liu J, Liu M. 2022. Effect of COVID-19 vaccines on reducing the risk of long COVID in the real world: a systematic review and meta-analysis. Int J Environ Res Public Health. 19(19):12422.
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, et al. 2020. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 323(16):1574–1581.
- Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, Thålin C. 2021. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 325(19):2015–2016.
- Hayes LD, Ingram J, Sculthorpe NF. 2021. More than 100 persistent symptoms of SARS-CoV-2 (long COVID): a scoping review. Front Med (Lausanne). 8:750378.
- Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. 2020. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 12:69.
- Houben-Wilke S, Goërtz YM, Delbressine JM, Vaes AW, Meys R, Machado FV, van Herck M, Burtin C, Posthuma R, Franssen FM, et al. 2022. The impact of long COVID-19 on mental health: observational 6-month follow-up study. JMIR Ment Health. 9(2):e33704.
- Iuliano AD, Brunkard JM, Boehmer TK, Peterson E, Adjei S, Binder AM, Cobb S, Graff P, Hidalgo P, Panaggio MJ, et al. 2022. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 71(4):146–152.
- Jacobson KB, Rao M, Bonilla H, Subramanian A, Hack I, Madrigal M, Singh U, Jagannathan P, Grant P. 2021. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 73(3):e826–e9.
- Jennings G, Monaghan A, Xue F, Mockler D, Romero-Ortuno R. 2021. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 10(24):5913.
- Jin C, Tian C, Wang Y, Wu CC, Zhao H, Liang T, Liu Z, Jian Z, Li R, Wang Z, et al. 2020. A pattern categorization of CT findings to predict outcome of COVID-19 pneumonia. Front Public Health. 8:567672.
- Johnson SF, Tiako MJN, Flash MJE, Lamas DJ, Alba GA. 2020. Disparities in the recovery from critical illness due to COVID-19. Lancet Psychiatry. 7(8):e54–e5.
- Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, Thompson PD, Baggish AL. 2021. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 6(2):219–227.
- Kosugi EM, Lavinsky J, Romano FR, Fornazieri MA, Luz-Matsumoto GR, Lessa MM, Piltcher OB, Sant’Anna GD. 2020. Incomplete and late recovery of sudden olfactory dysfunction in COVID-19. Braz J Otorhinolaryngol. 86(4):490–496.
- Lai H, Yang M, Sun M, Pan B, Wang Q, Wang J, Tian J, Ding G, Yang K, Song X, et al. 2022. Risk of incident diabetes after COVID-19 infection: a systematic review and meta-analysis. Metabolism. 137:155330.
- Lavery AM, Preston LE, Ko JY, Chevinsky JR, DeSisto CL, Pennington AF, Kompaniyets L, Datta SD, Click ES, Golden T, et al. 2020. Characteristics of hospitalized COVID-19 patients discharged and experiencing same-hospital readmission - United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 69(45):1695–1699.
- Lee JH, Yim JJ, Park J. 2022. Pulmonary function and chest computed tomography abnormalities 6-12 months after recovery from COVID-19: a systematic review and meta-analysis. Respir Res. 23(1):233.
- Lee Y, Min P, Lee S, Kim SW. 2020. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 35(18):e174.
- Lennon JC. 2020. Neurologic and immunologic complications of COVID-19: potential long-term risk factors for Alzheimer’s disease. J Alzheimers Dis Rep. 4(1):217–221.
- Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss H-P, et al. 2020. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 5(11):1281–1285.
- Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ. 2020. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 7(11):2221–2230.
- Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC-Y, Ng SSS, Zhang F, Li AYL, Lu W, Hui DS-C, et al. 2022. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 71(3):544–552.
- Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY. 2021. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 4(2):e210830.
- Long-term sequelae and COVID-19 – what we know so far 2020. (Accessed March 31, 2021at https://www.publichealthontario.ca/-/media/documents/ncov/covid-wwksf/2020/07/what-we-know-covid-19-long-term-sequelae.pdf?la=en.)
- Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo P, Cuapio A, Villapol S. 2021. [Epub ahead of print]. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Res Sq. 11(1):16144.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224):565–574.
- Mansoor E, Perez A, Abou-Saleh M, Sclair SN, Cohen S, Cooper GS, Mills A, Schlick K, Khan A. 2021. Clinical characteristics, hospitalization, and mortality rates of coronavirus disease 2019 among liver transplant patients in the United States: a multicenter research network study. Gastroenterology. 160(1):459–462 e1.
- Mantovani A, Morrone MC, Patrono C, Santoro MG, Schiaffino S, Remuzzi G, Bussolati G, Covid-19 Commission of the Accademia Nazionale dei Lincei 2022. Long Covid: where we stand and challenges ahead. Cell Death Differ. 29(10):1891–1900.
- Masonbrink AR, Hurley E. 2020. Advocating for children during the COVID-19 school closures. Pediatrics. 146(3):e20201440.
- Mastrangelo A, Bonato M, Cinque P. 2021. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett. 748:135694.
- Meini S, Suardi LR, Busoni M, Roberts AT, Fortini A. 2020. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 277(12):3519–3523.
- Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, et al. 2020. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 77(2):198–209.
- Monje M, Iwasaki A. 2022. The neurobiology of long COVID. Neuron. 110(21):3484–3496.
- National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), Royal College of General Practitioners (RCGP). COVID-19 rapid guideline: managing the long-term effects of COVID-19 2022.
- National Institute for Health and Care Excellence: clinical Guidelines 2020. COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: national Institute for Health and Care Excellence (UK) Copyright © NICE 2020.;
- Newell KL, Waickman AT. 2022. Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infectionImmunity and inflammaion in post-acute sequelae of SARS-CoV-2 infection. Curr Opin Immunol. 77:102228.
- Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. 2010. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 15(3):543–550.
- Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. 2020. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 78:185–193.
- Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, Peligro PJ, Casimiro M, Guerrero JJ, Gellaco MML, et al. 2022. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine. 53:101624.
- Nunez A, Madison M, Schiavo R, Elk R, Prigerson HG. 2020. Responding to healthcare disparities and challenges with access to care during COVID-19. Health Equity. 4(1):117–128.
- Otte MS, Klussmann JP, Luers JC. 2020. Persisting olfactory dysfunction in patients after recovering from COVID-19. J Infect. 81(3):e58.
- Paderno A, Mattavelli D, Rampinelli V, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T, Taboni S, Zorzi S, Del Bon F, et al. 2020. Olfactory and gustatory outcomes in COVID-19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg. 163(6):1144–1149.
- Palaiodimou L, Stefanou M-I, Katsanos AH, Aguiar de Sousa D, Coutinho JM, Lagiou P, Michopoulos I, Naska A, Giannopoulos S, Vadikolias K, et al. 2021. Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: a systematic review and meta-analysis. Neurology. 97(21):e2136–e47.
- Parcha V, Malla G, Suri SS, Kalra R, Heindl B, Berra L, Fouad MN, Arora G, Arora P. 2020. Geographic variation in racial disparities in health and coronavirus disease-2019 (COVID-19) mortality. Mayo Clin Proc Innov Qual Outcomes. 4(6):703–716.
- Parums DV. 2021. Editorial: long COVID, or post-COVID syndrome, and the global impact on health care. Med Sci Monit. 27:e933446.
- Patel A, Charani E, Ariyanayagam D, Abdulaal A, Denny SJ, Mughal N, Moore LSP. 2020. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. 26(9):1236–1241.
- Patel MA, Knauer MJ, Nicholson M, Daley M, Van Nynatten LR, Martin C, Patterson EK, Cepinskas G, Seney SL, Dobretzberger V, et al. 2022. Elevated vascular transformation blood biomarkers in Long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol Med. 28(1):122.
- Pellegrino R, Chiappini E, Licari A, Galli L, Marseglia GL. 2022. Prevalence and clinical presentation of long COVID in children: a systematic review. Eur J Pediatr. 181(12):3995–4009.
- Poletti S, Palladini M, Mazza MG, De Lorenzo R, Furlan R, Ciceri F, Rovere-Querini P, Benedetti F, COVID-19 BioB Outpatient Clinic Study group 2022. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur Arch Psychiatry Clin Neurosci. 272(5):773–782.
- Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, Suen J, Robba C, Fraser J, Cho S-M. 2022. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J Neurol Sci. 434:120162.
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, et al. 2020. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5(11):1265–1273.
- Rowley AH, Shulman ST, Arditi M. 2020. Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J Clin Invest. 130(11):5619–5621.
- Salehi S, Reddy S, Gholamrezanezhad A. 2020. Long-term pulmonary consequences of coronavirus disease 2019 (COVID-19): what we know and what to expect. J Thorac Imaging. 35(4):W87–W89.
- Samidoust P, Samidoust A, Samadani AA, Khoshdoz S. 2020. Risk of hepatic failure in COVID-19 patients. A Systematic Review and Meta-Analysis. Infez Med. 28:96–103.
- Sarıçam E, Dursun AD, Türkmen Sarıyıldız G, Can N, Bozkurt E, Gönüllü U, Basay N, Türkmen M, Denli A, Ünlü M. 2021. Laboratory and imaging evaluation of cardiac involvement in patients with post-acute COVID-19. Int J Gen Med. 14:4977–4985.
- Sayin I, Yazici ZM. 2020. Taste and smell impairment in SARS-CoV-2 recovers early and spontaneously: experimental data strongly linked to clinical data. ACS Chem Neurosci. 11(14):2031–2033.
- Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B, Claus R. 2020. Postmortem examination of patients with COVID-19. JAMA. 323(24):2518–2520.
- Schwab K, Schwitzer E, Qadir N. 2022. Postacute sequelae of COVID-19 critical illness. Crit Care Clin. 38(3):455–472.
- Segars J, Katler Q, McQueen DB, Kotlyar A, Glenn T, Knight Z, Feinberg EC, Taylor HS, Toner JP, Kawwass JF, et al. 2020. Prior and novel coronaviruses, Coronavirus Disease 2019 (COVID-19), and human reproduction: what is known? Fertil Steril. 113(6):1140–1149.
- Serrano-Castro PJ, Estivill-Torrús G, Cabezudo-García P, Reyes-Bueno JA, Ciano Petersen N, Aguilar-Castillo MJ, Suárez-Pérez J, Jiménez-Hernández MD, Moya-Molina MÁ, Oliver-Martos B, et al. 2020. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia (Engl Ed). 35(4):245–251.
- Shah KS, Hale Hammond ME, Drakos SG, Anderson JL, Fang JC, Knowlton KU, Shaw RM. 2021. SARS-CoV-2 as an inflammatory cardiovascular disease: current knowledge and future challenges. Future Cardiol. 17(7):1277–1291.
- Shaw B, Daskareh M, Gholamrezanezhad A. 2021. The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol Med. 126(1):40–46.
- Sigfrid L, Cevik M, Jesudason E, Lim WS, Rello J, Amuasi J, Bozza F, Palmieri C, Munblit D, Holter JC, et al. 2021. What is the recovery rate and risk of long-term consequences following a diagnosis of COVID-19? A harmonised, global longitudinal observational study protocol. BMJ Open. 11(3):e043887.
- Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Lim WS, Gillesen A, Berry C, Lowe DJ, McPeake J, et al. 2021. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health Eur. 8:100186.
- Singh-Grewal D, Lucas R, McCarthy K, Cheng AC, Wood N, Ostring G, Britton P, Crawford N, Burgner D. 2020. Update on the COVID-19-associated inflammatory syndrome in children and adolescents; paediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2. J Paediatr Child Health. 56(8):1173–1177.
- Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, Condition WHOCCDWGoP-C 2022. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 22(4):e102–e7.
- Ståhlberg M, Reistam U, Fedorowski A, Villacorta H, Horiuchi Y, Bax J, Pitt B, Matskeplishvili S, Lüscher TF, Weichert I, et al. 2021. Post-COVID-19 tachycardia syndrome: a distinct phenotype of post-acute COVID-19 syndrome. Am J Med. 134(12):1451–1456.
- Stephenson T, Shafran R, Ladhani SN. 2022. Long COVID in children and adolescents. Curr Opin Infect Dis. 35(5):461–467.
- Study of COVID-19 Risk and Long-Term Effects Underway at 37 U.S. Academic Medical Centers 2021. (Accessed 15 March 2021, at. https://www.cuimc.columbia.edu/news/nationwide-study-covid-19-risk-and-long-term-effects-underway-37-academic-medical-centers.)
- Sudre CH, Murray B, Varsavsky T, et al. 2020. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv 202010.19.20214494.
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, et al. 2021. Attributes and predictors of long COVID. Nat Med. 27(4):626–631.
- Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Demertzis Z, Hanna Z, Failla A, Dagher C, et al. 2020. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open. 3(6):e2012270.
- Sun P, Wang M, Song T, Wu Y, Luo J, Chen L, Yan L. 2021. The psychological impact of COVID-19 pandemic on health care workers: a systematic review and meta-analysis. Front Psychol. 12:626547.
- Szekely Y, Lichter Y, Sadon S, Lupu L, Taieb P, Banai A, Sapir O, Granot Y, Hochstadt A, Friedman S, et al. 2021. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr. 34(12):1273–1284 e9.
- Tan BKJ, Han R, Zhao JJ, Tan NKW, Quah ESH, Tan CJ-W, Chan YH, Teo NWY, Charn TC, See A, et al. 2022. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ. 378:e069503.
- Taquet M, Dercon Q, Harrison PJ. 2022. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun. 103:154–162.
- Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. 2021. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 18(9):e1003773.
- Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS, Smithline HA, et al. 2020. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 69(30):993–998.
- Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, et al. 2020. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 369:m2094.
- Townsend E. 2020. Debate: the impact of school closures and lockdown on mental health in young people. Child Adolesc Ment Health. 25(4):265–266.
- Trott M, Driscoll R, Pardhan S. 2022. The prevalence of sensory changes in post-COVID syndrome: A systematic review and meta-analysis. Front Med (Lausanne). 9:980253.
- Troyer EA, Kohn JN, Hong S. 2020. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 87:34–39.
- Truong DT, Trachtenberg FL, Pearson GD, Dionne A, Elias MD, Friedman K, Hayes KH, Mahony L, McCrindle BW, Oster ME, et al. 2022. The NHLBI study on long-term outcomes after the multisystem inflammatory syndrome in children (MUSIC): design and objectives. Am Heart J. 243:43–53.
- Van Lancker W, Parolin Z. 2020. COVID-19, school closures, and child poverty: a social crisis in the making. Lancet Public Health. 5(5):e243–e4.
- Verdecchia P, Cavallini C, Spanevello A, Angeli F. 2020. COVID-19: ACE2centric infective disease? Hypertension. 76(2):294–299.
- Wang C, Yu C, Jing H, Wu X, Novakovic VA, Xie R, Shi J. 2022. Long COVID: the nature of thrombotic sequelae determines the necessity of early anticoagulation. Front Cell Infect Microbiol. 12:861703.
- WHO Coronavirus (COVID-19) Dashboard 2021. (Accessed February 06, 2023, at. https://covid19.who.int/.)
- World Health Organization 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)
- Wrona M, Skrypnik D. 2022. New-onset diabetes mellitus, hypertension, dyslipidaemia as sequelae of COVID-19 infection-systematic review. Int J Environ Res Public Health. 19(20):13280.
- Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, Ni F, Fang S, Lu Y, Ding X, et al. 2021. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 9(7):747–754.
- Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, Ballouz T, Blyuss O, Bobkova P, Bonsel G, Borzakova S, Global Burden of Disease Long CC, et al. 2022. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 328(16):1604–1615.
- Xie Y, Bowe B, Al-Aly Z. 2021. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 12(1):6571.
- Xie Y, Xu E, Bowe B, Al-Aly Z. 2022. Long-term cardiovascular outcomes of COVID-19. Nat Med. 28(3):583–590.
- Yancy CW, Fonarow GC. 2020. Coronavirus disease 2019 (COVID-19) and the heart-is heart failure the next chapter? JAMA Cardiol. 5(11):1216–1217.
- Yasmin F, Najeeb H, Naeem A, Dapke K, Phadke R, Asghar MS, Shah SMI, De Berardis D, Ullah I. 2021. COVID-19 associated mucormycosis: a systematic review from diagnostic challenges to management. Diseases. 9(4):65.
- Yasuhara J, Masuda K, Watanabe K, Shirasu T, Takagi H, Sumitomo N, Lee S, Kuno T. 2023. Longitudinal cardiac outcomes of multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Cardiol. 44(4):892–907.
- Yong SJ. 2021. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 53(10):737–754.
- Yoo SM, Liu TC, Motwani Y, Sim MS, Viswanathan N, Samras N, Hsu F, Wenger NS. 2022. Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic COVID-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med. 37(8):1988–1995.
- Zhao Y-M, Shang Y-M, Song W-B, Li Q-Q, Xie H, Xu Q-F, Jia J-L, Li L-M, Mao H-L, Zhou X-M, et al. 2020. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 25:100463.
- Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, Shi C, Hu S. 2020. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 129:98–102.