Abstract
This review examines the large body of toxicological and epidemiological information on human exposures to chlorpyrifos, with an emphasis on the controversial potential for chlorpyrifos to induce neurodevelopmental effects at low doses. The results of this review demonstrate that the use of urinary 3,5,6-trichlorpyridinol (TCPy), a metabolite of chlorpyrifos as a biomarker of nonoccupational exposure is problematic and may overestimate nonoccupational exposures to chlorpyrifos by 10-to 20-fold because of the widespread presence of both TCPy and chlorpyrifos-methyl in the food supply. Current “background” (nonoccupational) levels of exposure to chlorpyrifos are several orders of magnitude lower than those required to inhibit plasma cholinesterase activity, which is a more sensitive target than nervous system cholinesterase. However, several in vitro studies have identified putative neurodevelopmental mechanisms that are altered at concentrations of chlorpyrifos below those that inhibit cholinesterases. Although one human cohort study reported an association between maternal and cord blood chlorpyrifos levels and several measures of neurodevelopment, two other cohort studies that utilized urinary TCPy as a surrogate for chlorpyrifos exposure did not demonstrate an association. Although the weight of the scientific evidence demonstrates that current levels of chlorpyrifos exposure will not have any adverse effects on neurodevelopment that might result from inhibition of nervous system cholinesterases, several recent studies propose alternative mechanisms. Thus, further in vivo investigation on neurodevelopment in an appropriate animal model is needed; additional epidemiological studies may be warranted if a suitable, chlorpyrifos-exposed cohort can be identified and more rigorous measures of exposure are utilized.
EXECUTIVE SUMMARY
The authors were asked to review the literature on chlorpyrifos toxicology and address several specific questions regarding: (1) the strength of the scientific evidence supporting the hypothesis put forward by others that chlorpyrifos is capable of causing adverse neurodevelopmental outcomes in humans at current, background exposure levels; (2) whether there is scientific evidence to support a mechanism for neurodevelopmental effects other than AChE inhibition; and (3) whether limiting chlorpyrifos exposures to levels that protect against AChE inhibition would be adequate to protect against any potential neurodevelopmental outcomes. As with most toxicological assessments, there are not clear-cut answers to these questions, as limitations and uncertainties in the existing data preclude firm answers. This article provides an extensive review of the published literature, as well as key internal documents requested of and provided by Dow AgroSciences. The paragraphs that follow summarize the key observations and conclusions derived from the detailed review that follows.
Past and Current Uses
Chlorpyrifos, first introduced into the marketplace in 1965, has been widely used globally as an insecticide to control crop pests in agriculture, reduce household pests such as termites, reduce insect damage to turf on lawns and golf courses, and for mosquito control. Residential use of chlorpyrifos was eliminated in the United States in 2001, and is being phased out in the European Union (EU). In 2006, Dow AgroSciences, the major manufacturer of chlorpyrifos in the United States and the EU, began a global phase-out of nonagricultural uses of chlorpyrifos. It continues to be used to control crop damage from insects in agriculture worldwide. Although chlorpyrifos is registered for use on dozens of different crops; over 60% of chlorpyrifos use in the United States is on 3 crops, corn (39%), tree nuts (15%), and soybeans (9%). Use on tree fruits accounts for an additional 10% of total use. Today, nonagricultural uses account for less than 3% of total chlorpyrifos applications, and are limited to mosquito control for public health purposes and insect control on golf courses. However, other registrants and manufacturers may continue to support residential uses outside of the United States and the EU.
Past and Current Exposures
Over the past 40 years, numerous studies have assessed potential human exposures to chlorpyrifos. Exposure pathways include ingestion, inhalation and dermal exposure. Dietary exposures to trace levels of chlorpyrifos on food products appear to be the main source of nonoccupational exposures to chlorpyrifos, both past and present. In the past, inhalation exposure to chlorpyrifos in indoor air likely contributed significantly to total exposures following indoor application of chlorpyrifos for residential pest control. However, this pathway of exposure is no longer relevant in the United States and the EU, as residential use of chlorpyrifos has been discontinued. Chlorpyrifos can be detected in outdoor air following agricultural applications, but the concentrations are such that community exposures via this pathway are likely to be very low, relative to diet. Inhalation exposure in the occupational environment may contribute substantially to total exposure for applicators, and potentially for farm workers and those family members with whom they live. Dermal exposure, and secondary ingestion of house dust in children, may have contributed a small amount to total exposures prior to 2001, but is not likely to be a significant pathway for exposure today.
For the past two decades, exposure assessment for chlorpyrifos has relied upon the use of the urinary biomarker 3,5,6-trichloro-2-pyridinol (TCPy) a metabolic breakdown product of chlorpyrifos. Until recently, nearly all studies that measured TCPy in urine assumed that chlorpyrifos was the sole source of TCPy, and thus equated molar equivalents of TCPy in the urine to daily exposures to chlorpyrifos. Based on this assumption, it appeared that some level of exposure to chlorpyrifos was commonplace, since most population-based studies with limits of detection greater than 1 μ g/L (ppb) found TCPy in > 95% of urine samples. The vast majority of studies that have analyzed urine for TCPy collected the samples in the period 1995–2001. Typical urinary concentrations of TCPy averaged 3–5 ppb, and it was not unusual to find levels 10 times higher. Several studies demonstrated large sample-to-sample variability in the same individual over the course of several days, suggesting highly variable intake. However, it is now recognized that TCPy is an inadequate biomarker for chlorpyrifos exposure in nonoccupational settings because: (1) residues of TCPy itself frequently occur on fruits and vegetables, often at concentrations 10–20 times greater than chlorpyrifos, and TCPy appears to be efficiently absorbed and eliminated in the urine; (2) chlorpyrifos-methyl (an insecticide structurally related to chlorpyrifos) is widely used in the protection of grains, and relatively frequent contamination of dietary staples (e.g., bread and other grain products) with low levels of chlorpyrifos-methyl may occur. Chlorpyrifos-methyl also is converted to TCPy. Estimates of dietary exposures to chlorpyrifos-methyl derived from residue analyses of foods suggest that chlorpyrifos-methyl in the diet could easily account for 50% or more of the urinary TCPy. It is also possible that a significant portion of chlorpyrifos-methyl reported on grain products was actually TCPy. Thus, for studies in which urinary samples were collected and analyzed for TCPy prior to 2002, it is likely that chlorpyrifos exposures were overestimated by at least 10-to 20-fold. This conclusion is supported by “mass balance” calculations for the few studies that measured both urinary TCPy and multiple pathways of exposure to chlorpyrifos (e.g., directly measured intake of chlorpyrifos in air and food, and then analyzed urine in the same period of time following the measured exposure). The amount of TCPy recovered in urine was approximately 10–20 times greater than the measured amount of chlorpyrifos intake. Unfortunately, these studies did not measure the intake of TCPy itself, or the intake of chlorpyrifos-methyl, but dietary analyses suggest that these two sources of exposure could readily account for the difference between measured urinary excretion of TCPy and the measured intake of chlorpyrifos.
Surprisingly, few published studies have assessed chlorpyrifos exposures or urinary TCPy since the elimination of residential use of chlorpyrifos in 2001. Even recently published studies have reported on samples collected prior to 2002. Thus, there are few data from which to assess current exposures to chlorpyrifos and TCPy. However, it seems likely that urinary TCPy levels will be somewhat reduced from those measured prior to 2002 because of the elimination of both the residential use of chlorpyrifos and a reduction in agricultural use of chlorpyrifos-methyl. This is an important gap in our ability to assess current risks of chlorpyrifos.
Some previous estimates for daily intake of chlorpyrifos, based solely on urinary TCPy, have suggested that typical chlorpyrifos intake was in the range of 1–5 μ g/kg-day. Based on this presumed intake rate, it has been suggested that typical exposures to chlorpyrifos exceed an “acceptable daily intake” rate established by the U.S. Environmental Protection Agency (EPA). In 1988 the EPA established a “reference dose” (RfD)Footnote1 of 3 μ g/kg-day, although other agencies have determined acceptable daily intakes (ADIs) substantially greater than this (see summary discussion below on “dose-response analysis”). In 2000, the EPA recommended lowering the reference dose by a factor of 10, to 0.3 μ g/kg-day. However, if reliance on urinary TCPy overestimates actual “background” exposures (e.g., urinary TCPy 1–5 ppb) to chlorpyrifos by 10-to 20-fold, as suggested in this review, then typical exposures should be well below the EPA's current RfD. In this review we estimate that typical exposures to chlorpyrifos in the past were on the order of 0.001–0.01 μ g/kg-day, and were derived largely from diet. Current exposures are likely to be lower. However, it is possible that specific exposures to chlorpyrifos (e.g., beyond dietary background levels, such as might occur with farm worker families living in agricultural areas subject to intensive applications of chlorpyrifos) could add substantially to total chlorpyrifos exposure, even though it might be difficult to identify this by urinary monitoring of TCPy because of the potentially high background from direct exposure to dietary TCPy. For example, an increase in urinary TCPy from a “background” of 2 ppb (of which perhaps only 0.1 –0.2 ppb would be from chlorpyrifos) to 4 ppb would represent a 10-to 20-fold increase in chlorpyrifos exposure if all of the additional 2 ppb of urinary TCPy were derived from direct exposure to chlorpyrifos. Thus, future exposure studies should attempt to distinguish between direct exposures to TCPy from exposures to chlorpyrifos.
Nonneurological Effects of Chlorpyrifos
The primary target organ for chlorpyrifos toxicity is the central and peripheral nervous system, due to the ability of the chlorpyrifos-oxon metabolite to inhibit the enzyme activity of acetylcholinesterase, which terminates neurotransmission at cholinergic synapses (see later discussion). There is very little evidence to indicate that chlorpyrifos has toxicological effects in tissues other than the nervous system. Standard toxicological bioassays have not found significant toxicity in organ systems other than the nervous system, at least at doses less than those causing frank neurological effects. Chlorpyrifos is not considered to be teratogenic at doses that do not cause frank maternal toxicity.
Although mutagenicity and chronic animal bioassays for carcinogenicity of chlorpyrifos were largely negative, a recent epidemiological study of pesticide applicators reported a significant exposure response trend between chlorpyrifos use and lung and rectal cancer. However, the positive association was based on small numbers of cases, i.e., for rectal cancer an excess of less than 10 cases in the 2 highest exposure groups. The lack of precision due to the small number of observations and uncertainty about actual levels of exposure warrants caution in concluding that the observed statistical association is consistent with a causal association. This association would need to be observed in more than one study before concluding that the association between lung or rectal cancer and chlorpyrifos was consistent with a causal relationship.
There is no evidence that chlorpyrifos is hepatotoxic, nephrotoxic, or immunotoxic at doses less than those that cause frank cholinesterase poisoning.
One series of clinical studies in human males attending a fertility clinic found an inverse correlation between urinary TCPy levels and several measures of male reproductive health. Urinary TCPy levels in the population were within the range of the normal U.S. population, so it is unlikely that exposures were unusual. Although these studies raise potential concerns, the collection of exposure-related data and outcome data at the same point in time precludes an appropriate temporal sequence, and “within-individual” variability and other sources of error in single test results for both the urinary and sperm measures represent serious limitations to the study. Thus, it can only be concluded that this report describes correlations between urinary TCPy and various male reproductive outcomes that should be pursued in subsequent more rigorous studies.
Mechanism of Action and the Determination of the Reference Dose for Chlorpyrifos
The primary, but not necessarily the only, mechanism of action for the toxic effects of chlorpyrifos is related to the ability of the oxon metabolite of chlorpyrifos to bind to and irreversibly inhibit acetylcholinesterase (AChE) in target tissues. The nervous system is the primary target because AChE and/or butlycholinesterase (BuChE) catabolyze the neurotransmitter acetylcholine, thereby terminating its synaptic function. There is substantial evidence to demonstrate that, in humans, the enzyme activity of plasma BuChE is substantially more sensitive to inhibition by chlorpyrifos (oxon) than erythrocyte AChE, which is widely recognized as more reflective of AChE in brain, spinal cord, and the peripheral somatic and autonomic nervous system. Although a specific physiological role for BuChE has not been firmly established, there is a growing body of evidence that BuChE participates in several neurophysiological processes such as modulating acetylcholine levels in the brain and periphery, binding to certain brain proteins, and providing protection against certain exogenous substances (e.g., the drug succinylcholine and certain plant toxins). Thus it is reasonable that the more sensitive plasma BuChE, rather than erythrocyte AChE, be used as the principal biomarker to assess inhibition of cholinesterases by chlorpyrifos-oxon.
Coulston and associates Citation(1972) carried out a 28-day controlled clinical study of the effects of several different daily oral doses (0.10 mg/kg-day for 9 days; 0.03 mg/kg-day for 20 days; 0.014 mg/kg-day for 28 days) of 99.5% chlorpyrifos on the enzyme activities of both plasma BuChE and erythrocyte AChE of 16 healthy adult male volunteers. The lowest dose with a marginal, nonsignificant effect on BuChE—the cholinesterase with enzyme activity most sensitive to chlorpyrifos—was 0.014 mg/kg-day for 28 days. After review of the original Coulston data and other published studies on chlorpyrifos inhibition of cholinesterase, as well as a review of the literature examining the putative physiological functions of BuChE, we support the use of plasma BuChE activity to establish a no-effect level for target tissue cholinesterase inhibition by chlorpyrifos. From a detailed evaluation of the literature, we conclude that the weight of the evidence suggests that repeated exposures to chlorpyrifos at a daily dose of less than 14 μ g/kg-day would have little or no effect on either acetyl or butyryl cholinesterase activity in target tissue in adults. Therefore, repeated daily exposures to chlorpyrifos of less than ∼ 10 μ g/kg-day would be expected to have no discernable effects on the enzyme activity of target tissue AChE or BuChE.
It has been suggested that alternative mechanisms of action for chlorpyrifos could potentially contribute to toxic effects, so it is important to consider whether such putative mechanisms of toxicity are likely to occur in vivo at doses less than those that would inhibit target tissue AChE.
Alternative Mechanisms of Action for Chlorpyrifos Toxicity, Proposed From In Vitro Studies
Numerous potential molecular targets for chlorpyrifos, besides AChE, have been identified in in vitro studies, including cytotoxicity, effects on macromolecule synthesis (DNA, RNA, proteins), interactions with neurotransmitter receptors, interactions with signal transduction pathways, effects on neuronal differentiation, interactions with various enzymes, other neurochemical effects (e.g., neurotransmitter release or uptake), and other effects (e.g. oxidative stress, effects on microtubules). For the vast majority of these endpoints, effects are not seen at concentrations below those necessary to cause significant inhibition of AChE, and thus protection against cholinesterase inhibition would also protect against these effects. However, two targets have been identified for which chlorpyrifos may cause effects at concentrations below those necessary to inhibit AchE activity.
The serine hydrolase enzyme KIAA1363 appears to be important in an ether lipid signaling network involving platelet activating factor and is highly expressed in cancer cells. KIAA1363 activity was inhibited by chlorpyrifos-oxon at nanomolar concentrations—similar to concentrations causing AChE inhibition. In vivo administration of chlorpyrifos-oxon to mice demonstrated that brain AChE was inhibited to a greater extent than KIAA1363, suggesting that, in vivo, brain AChE inhibition is relatively more sensitive to inhibition than KIAA1363. It is unclear how inhibition of KIAA1363 activity might contribute to delays in neurodevelopment, and thus it is not possible to conclude from these in vitro observations alone that inhibition of KIAA1363 by chlorpyrifos would contribute to neurodevelopmental effects at concentrations below those that inhibit plasma BuChE or AChE, especially since in vivo studies found brain AChE to be somewhat more sensitive to inhibition by chlorpyrifos than KIAA1363.
A second example is the phosphorylation of CREB in rat cortical neurons, which was reported to occur at extremely low concentrations: In vitro treatment of rat cortical neurons with chlorpyrifos or chlorpyrifos-oxon induced a threefold increase in CREB phosphorylation at concentrations more than 1,000-fold lower than those necessary to cause cholinesterase inhibition. CREB plays a major role in regulating gene expression, especially genes whose products mediate synaptic plasticity, which is correlated with the ability to learn. TCPy also caused increased CREB phosphorylation in vitro, although it was much less potent than either chlorpyrifos or chlorpyrifos-oxon. Chlorpyrifos-oxon was more than 1000 times more potent than chlorpyrifos, suggesting a similar mechanism of action to cholinesterase inhibition. The functional significance of such increase in pCREB is unknown, and the authors suggest that it may represent a neuroprotective response to subtle metabolic stress, operational only in neurons in culture. To date, no in vivo studies have demonstrated alterations in pCREB phosphorylation in nervous tissue at doses less than those that cause inhibition of plasma BuChE, and thus it is difficult to draw firm conclusions about the relevance of this putative mechanism of neurodevelopmental toxicity at chlorpyrifos doses that do not inhibit plasma BuChE.
Several other in vitro studies have observed effects of chlorpyrifos on neuronal growth in tissue culture at concentrations that do not cause significant inhibition of AChE. Both chlorpyrifos (0.1–1 nM) and chlorpyrifos-oxon (0.001 nM) decreased axonal length, but not the number of axons, while TCPy was devoid of effects, in a study using sympathetic neurons dissociated from the superior cervical ganglia of GD 20–PND 1 rats. These effects were seen at concentrations a thousand fold less than concentrations necessary to inhibit AChE activity. Another study with sensory neurons derived from embryonic dorsal root ganglia found that both chlorpyrifos and chlorpyrifos-oxon decreased axonal length without affecting other cell growth parameters or AChE enzymatic activity. The authors concluded that low concentrations of chlorpyrifos (1 nM) and of chlorpyrifos-oxon (0.01 nM) inhibit axonal growth by interfering with the morphogenic rather than the enzymatic activity of AChE. Collectively, these studies are of interest as they suggest a novel mechanism by which chlorpyrifos and chlorpyrifos-oxon may interfere with axonal growth at concentrations substantially below those necessary to inhibit AChE enzymatic activity. Whether such effects on axonal outgrowth occur in vivo with low-dose exposure has yet to be examined but bears experimental study as it could impact synaptogenesis in the developing brain.
Several intriguing new mechanisms for potential neurodevelopmental effects of “lower dose” chlorpyrifos have been proposed based on in vitro studies. The potential relevance of such effects on the developing nervous system should not be dismissed, but await confirmation in in vivo studies using sensitive parameters of axonal growth and neurodevelopment in animal models, and/or sensitive measures of cognitive function in humans exposed in utero (see later description).
Thus, the weight of evidence from mechanistic studies still supports the utilization of plasma cholinesterase inhibition as a point of departure for establishing the RfD for chlorpyrifos. Because there is no evidence demonstrating that inhibition of plasma BuChE itself is an “adverse effect,” the use of inhibition of plasma BuChE as an effects-related biomarker of organophosphorus pesticide exposure should be considered as a highly sensitive indicator of potential adverse effects in target tissues (relative to use of erythrocyte AChE), and thus fewer uncertainty factors may be necessary to ensure that allowable exposures are fully protective of inhibition of target tissue cholinesterases that are responsible for the adverse effects of chlorpyrifos.
Differential Sensitivity of Young Versus Adult Animals to Chlorpyrifos Toxicity
Studies in laboratory animals demonstrate that young animals are more sensitive to acute cholinergic toxicity of chlorpyrifos than older animals. This appears to be related to differences in absorption, biotransformation, and tissue distribution, rather than age-related differences in intrinsic sensitivity of AChE to inhibition by chlorpyrifos-oxon. Expression levels of paraoxonase and carboxylesterase are lower in newborns, and this could contribute to the enhanced susceptibility to chlorpyrifos-mediated inhibition of AChE. However, such differences in sensitivity are not evident at lower, repeated-dose (subchronic) exposures (< 1 mg/kg/day), probably because at lower doses detoxication pathways in young animals are adequate to protect against cholinesterase inhibition. In addition, the rate of AChE synthesis is higher in young animals than in adults, leading to a faster recovery of AChE activity. Thus, the in vivo animal data suggest that an additional safety factor of 10 may not be necessary for the protection of infants and children. However, it remains possible, with some in vitro mechanistic support, that the early developing nervous system could be relatively more susceptible to chlorpyrifos than the more developed nervous system in infants and children. Thus, it is reasonable to consider pregnant women as a potentially susceptible subpopulation.
Neurodevelopmental Effects of Chlorpyrifos in Animal Studies
Numerous in vivo developmental toxicity studies in rats, and several in mice, confirm that administration of chlorpyrifos to pregnant dams at doses sufficient to inhibit brain cholinesterases cause some adverse neurodevelopmental outcomes in offspring. Common effects observed have been: decreased muscarinic and nicotinic receptors, decreased choline acetyltransferase activity, and changes in 5-HT function, including increased or decreased 5-HT receptors. Functional behavior changes include hyperactivity in females and an increase in memory errors. Thus, the animal data provide strong evidence that inhibition of AChE during prenatal development is associated with adverse developmental outcomes. As noted earlier, in vitro studies have demonstrated some effects of chlorpyrifos on brain tissue at concentrations well below those required to inhibit AChE. In this context, the limitations of the animal studies in identifying subtle cognitive dysfunction that might occur during development should be noted, as well as the observation that relatively few in vivo neurodevelopmental studies have utilized doses below those that cause substantial inhibition of brain AChE.
From the available in vivo animals studies, the lowest no-observable-adverse-effect level (NOAEL) was established for developmental toxicity at 1 mg/kg-day, based upon a study in Sprague-Dawley rats. Adverse effects were only observed at levels where inhibition of both plasma and erythrocyte AChE activity occur at doses lower than that causing growth impairment.
Epidemiological Studies Examining Neurodevelopmental Effects of Chlorpyrifos in Humans
Three cohort studies, at Columbia University (Perrera, Whyatt, and coworkers), University of California (UC)–Berkeley (Eskenazi and coworkers), and Mount Sinai Medical Center (Berkowitz, Wolff, and coworkers), have examined pregnant women for possible associations between in utero exposure to chlorpyrifos and adverse neurodevelopmental outcomes of their children. Although significant effects were reported in two of these studies, no consistent associations were observed when outcomes of the three studies were compared.
Estimates of exposure to chlorpyrifos in these cohorts demonstrate that exposure levels were well below those known to cause any measurable inhibition of AChE. The Columbia, New York inner-city cohort, from which several statistically significant associations between estimates of chlorpyrifos exposure and adverse neurodevelopmental outcomes were noted, had presumed exposure to chlorpyrifos from residential use prior to 2001. However, the estimates of exposure in the Columbia cohort study, even in the “worst case,” were not likely to exceed the average dietary exposure by more than 10-fold (which would also be within the range of variation in background dietary exposure).
The UC-Berkeley cohort consisted of families living in an agricultural area with presumed intensive exposures to chlorpyrifos and other pesticides. No significant abnormal measures of neurodevelopment were correlated with measures of chlorpyrifos exposure (assessed by urinary TCPy concentrations) in this cohort.
The Children's Environmental Cohort Study (CECS) is a prospective multiethnic birth cohort study of mothers and infants delivered at Mount Sinai Hospital in New York City. Samples of maternal blood and urine were obtained during the third trimester and cord blood samples were obtained at birth and both were analyzed for PON1 activity and PON1 polymorphisms. The Mount Sinai cohort, like the Columbia cohort, was presumed to have exposures from residential use of chlorpyrifos. Urine samples were analyzed for pesticide metabolites, including dialkylphosphates and TCPy. The median urinary TCPy level, 7.6 μ g/L, was about 5-fold greater than the U.S. (NHANES III) mean, but within the 95% confidence limits (mean = 1.5; UCL 1–11 μ g/L). There was no significant association between the level of TCPy with birth weight, birth length, or head circumference. However, there was a small but significant reduction in head circumference when comparing infants of women with low PON1 activity and the highest tertile of urinary TCPy with infants of women with high PON1 activity and the lowest tertile of urinary TCPy. There was no significant association between TCPy and PON1 activity for birth weight or birth length. In addition, there was no association for infant PON1 genotype with birth weight or for maternal or infant PON1 genotypes with head circumference or birth length.
In the Columbia cohort, small but statistically significant decreases in birth weight and birth length were associated with increased chlorpyrifos exposure, assessed by blood concentrations of chlorpyrifos in maternal and/or cord blood, from a single blood sample taken at the time of delivery. Follow-up studies of this cohort reported an association between chlorpyrifos blood levels and measures of impairment of cognition, motor function, and behavior. However, the magnitude of the observed effects was modest and not out of the normal range. The authors attempted to control for confounding factors, including other known neurodevelopmental risk factors in this inner-city cohort, such as maternal perinatal smoking and alcohol; nevertheless, it is difficult to dismiss the contribution of these and perhaps other confounding factors. It is noteworthy that only a single measurement of blood chlorpyrifos was obtained at the time of delivery. Given that the initial elimination rate of chlorpyrifos from plasma is very rapid (plasma half-life of chlorpyrifos is very short on the order of 1 h or less), it is likely that the levels of chlorpyrifos detected in maternal and cord blood at the time of delivery are reflective of a “steady-state” value that is greatly influenced by an equilibrium between adipose tissue and blood lipids. However, little is known about the terminal half-life of chlorpyrifos after steady state is achieved following repeated, relatively low-level exposure. If the terminal half-life was measured in months, then the values collected at the time of delivery could be reasonable indicators of the levels of exposure throughout pregnancy. Conversely, if the terminal half-life was measured in days, then the values obtained at the time of delivery might have little relationship to exposure levels that were present during most the pregnancy. Thus, it is unclear whether this single measurement of chlorpyrifos in plasma, collected at the time of delivery, provides a relevant measure of chlorpyrifos exposure during the period of prenatal central nervous system (CNS) development (last 3–4 months of pregnancy).
The correspondence in findings between the UC-Berkeley and Mount Sinai groups for an increase in total urinary diethylphosphate (DEP) metabolites with increased numbers of abnormal reflexes could be important. Although chlorpyrifos exposure will contribute to urinary DEP, the actual contribution of chlorpyrifos to total urinary DEP metabolites is unknown. Thus, while the potential clinical significance of these findings must be acknowledged, the contribution of chlorpyrifos exposure to them is unknown. Additional studies of women exposed to chlorpyrifos during pregnancy would be needed to fully resolve this controversy. However, it will be difficult, if not impossible, to identify a suitably sized cohort of pregnant agricultural workers exposed to chlorpyrifos to complete such a study, as efforts clearly should be in place to minimize occupational exposures to chlorpyrifos (or any pesticide) during pregnancy.
Continued use of TCPy as a biomarker of chlorpyrifos exposure should be approached with an understanding of the uncertainties of this measurement. For occupational exposures or other scenarios when exposure is likely to exceed typical dietary background levels (e.g., urinary TCPy > ∼5–10 ppb) TCPy is likely to be a reasonably good surrogate for chlorpyrifos exposure. However, either negative or positive associations between urinary TCPy and specific health metrics must be viewed with suspicion if urinary TCPy levels are less than ∼ 10 ppb, unless efforts to assess actual intake of chlorpyrifos, chlorpyrifos-methyl, and TCPy are included in the study design.
Finally, it should be noted that the conclusions of this review are based on: (1) controlled studies of well-nourished laboratory animals, (2) healthy humans treated with chlorpyrifos or metabolites, (3) epidemiological studies, and (4) case series/reports of humans with incidental or deliberate exposures to chlorpyrifos. Findings relating to human neurodevelopment rely almost solely on populations residing in high-income nations—albeit comprising people of diverse ethnic and geographic origins—that may lack the magnitude of environmental stressors (malnutrition, infectious agents) experienced in low-income nations where chlorpyrifos is also widely deployed. Data on the impact of chlorpyrifos in individuals or offspring with preexisting illness and/or low nutrition are not available, a significant absence given that several clinical states can markedly reduce the levels of plasma BuChE, which may play a role in protecting the nervous system from agents with anticholinesterase activity and potential neurotoxicity. Additionally, certain genetic variants of BuChE that exist in human populations also impart substantially greater sensitivity to the acute clinical effects of chemicals with anticholinesterase properties.
In summary, based on review of a remarkably large volume of scientific studies on chlorpyrifos in both animals and humans, we offer the following answers to questions posed at the beginning of this review:
What is the strength of the scientific evidence supporting the hypothesis put forward by others that chlorpyrifos is capable of causing adverse neurodevelopmental outcomes in humans at current, “background” exposure levels?
Current background levels of exposure to chlorpyrifos are derived largely from the diet, and are several orders of magnitude lower than doses that could have measurable effects on plasma BuChE and thus any significant effect on nervous tissue AChE.
Is there sufficient scientific evidence to support a mechanism for neurodevelopmental effects other than AChE inhibition?
Several in vitro studies have identified putative neurodevelopmental mechanisms or effects on neuronal growth in vitro that occur at concentrations of chlorpyrifos (oxon) below those necessary to inhibit AChE. One human cohort study of infants exposed in utero to chlorpyrifos, in part from residential use, reported an association between maternal and cord blood chlorpyrifos levels and several measures of neurodevelopment. Two other cohort studies using urinary TCPy as a surrogate for chlorpyrifos exposure did not find a significant association between chlorpyrifos exposure and measures of neurodevelopment. Limitations common to such epidemiological studies make it very difficult to draw a causal connection between chlorpyrifos and adverse neurodevelopment. The mechanistic data suggesting plausible alternative (noncholinergic) mechanisms and the one epidemiological study suggesting an association between blood chlorpyrifos and certain measures of neurodevelopment at exposures well below those that could inhibit cholinesterase warrant further in vivo studies on neurodevelopment in a suitable animal model; further epidemiological investigation would also be warranted if a suitable, chlorpyrifos-exposed cohort can be identified and rigorous measures of exposure are utilized.
Will limiting chlorpyrifos exposures to levels that protect against target tissue AChE inhibition be adequate to protect against any potential neurodevelopmental outcomes?
Based on the weight of the scientific evidence, it is highly unlikely that current levels of chlorpyrifos exposure in the United States would have any adverse neurodevelopmental effects in infants exposed in utero to chlorpyrifos through the diet. This assumes that the sole mechanism of neurodevelopmental effects is via inhibition of the enzymatic activity of target tissue AChE; since other mechanisms of neurodevelopmental chlorpyrifos toxicity plausibly may exist, reevaluation of this conclusion is recommended as relevant data become available.
I. BACKGROUND AND GENERAL CHARACTERISTICS OF CHLORPYRIFOS
I.A. Purpose
The Science Partners Evaluation Group (Evaluation Group)Footnote2 conducted an independent analysis of the insecticide chlorpyrifos, relative to its potential to cause adverse effects in humans at current levels of use, with an emphasis on human exposure assessment and potential neurodevelopmental toxicity in humans. Review of published and unpublished toxicology, clinical reports, and field studies comprised the core of the analysis. These studies were evaluated by the Evaluation Group for their quality and consistency of findings, mechanism of action, and relevance to humans. The exposure models used in past studies to estimate human exposure to chlorpyrifos were reviewed in detail.
I.B. Introduction
Chlorpyrifos is one of the most widely used organophosphorus insecticides in the world. Numerous papers published in the past decade have reported on a possible association between chlorpyrifos exposure and neurodevelopmental effects. This article provides an overview of the basic toxicology of chlorpyrifos, followed by an in-depth analysis of human exposures to chlorpyrifos and a critical evaluation of the toxicological and epidemiological data that address the potential for chlorpyrifos to induce neurodevelopmental effects in humans. For a more detailed review of the basic toxicology of chlorpyrifos, the reader is referred to several exhaustive reviews of the literature, including: CitationATSDR (1997), IPCS (undated; [http://www.inchem.org/DOCUMENTS/JMPr/jmpmono/v072 pr10.htm]), and Australian Pesticides and Veterinary Medicine Authority (The NRA Review of Chlorpyrifos—Toxicology Assessment; available at http:www.apvma.gov.au/chemrev/downloads/chlorpyrifos_;tox.pdf).
I.C. Chemical and Physical Properties
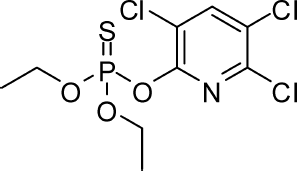
Chlorpyrifos (chlorpyrifos, O,O-diethyl O-3,5,6-trichloropyridin-2-yl phosphorothioate, O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate, chlorpyrifos-ethyl; ) belongs to the organophosphorus (OP) class of pesticides, serving as an insecticide and acaricide. Toxicity of OP pesticides, primarily via activated metabolites, is principally due to persistent inhibition of the enzyme activity of acetylcholinesterase (AChE) in the brain and peripheral nervous system. AChE is primarily located in the postsynaptic membrane of central and peripheral cholinergic synapses. Such enzyme inhibition results in decreased neurotransmitter degradation (acetylcholine) and, consequently, overstimulation of the associated synaptic systems. In humans, acetylcholine transmits electrical information from preganglionic and postganglionic neurons in both the sympathetic and parasympathetic divisions of the peripheral nervous system, from peripheral somatic motor nerves to skeletal muscle fibers, and, of special relevance here, to, from, and within the brain and spinal cord. Although mammals relative to insects more efficiently detoxify chlorpyrifos, and consequently are less sensitive to acute intoxication, some OP pesticides have a high potential for acute toxicity in humans. For example, sarin, soman, and tabun are highly potent organophosphorus cholinesterase inhibitors that have found use as chemical warfare agents.
Chlorpyrifos is the active component in a wide array of pesticide formulations (). According to the U.S. Environmental Protection Agency (EPA), at one time there were over 400 different commercial products that contained chlorpyrifos as an active ingredient.Footnote3 The compound is a colorless to white crystalline solid, has a mild mercaptan-like odor, and is only slightly soluble in water but soluble in most organic solvents. Chlorpyrifos is present as the active ingredient in a variety of formulations and delivery systems, including basic formulations such as emulsifiable concentrates, granular and wettable powders, water-dispersible granulars, micro-encapsulated suspensions, and gel-based products.
TABLE 1 Formulations containing chlorpyrifos and alternate chemical names
Although chlorpyrifos is combustible, the National Fire Protection Agency has not assigned chlorpyrifos a flammability rating. Heat, sparks, and open flame, however, do contribute to its instability (CitationNIOSH, 1995). Further physicochemical and other substance-related data are given in .
TABLE 2 Chlopyrifos—Physical/chemical properties
I.D. Uses of Chlorpyrifos
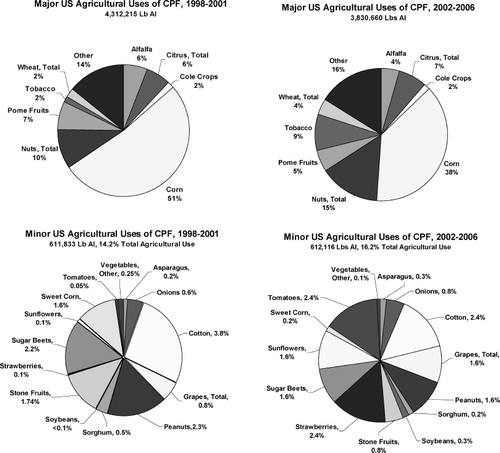
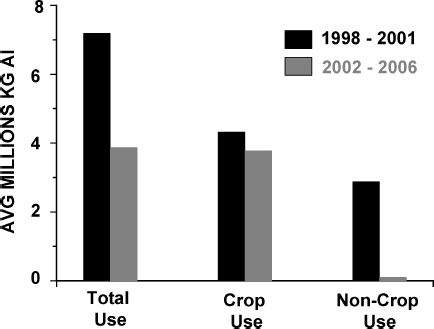
Chlorpyrifos was introduced into the market in 1965, and was formerly used in both agricultural and nonagricultural environments (CitationATSDR, 1997). In the home, it was applied to control insect pests (e.g., cockroaches, fleas, and termites) and sometimes as a component in tick and flea collars for pets. On the farm, chlorpyrifos has occasional use for the control of ticks on cattle but its major use is in crop protection. Once among the most widely applied pesticides in U.S. homes, such uses were restricted in 2001 (fully implemented in 2002). As shown in , after the restriction of noncrop usage in 2001, the average annual use of chlorpyrifos (referred to as active ingredient, AI) in the United States was about one half that of the 1998–2001 period. This reduction was largely due to a sharp decline in chlorpyrifos use for nonagricultural purposes, which declined to only 3% of prerestriction quantities. Within the same time frame, chlorpyrifos use in crop protection was only slightly reduced to just below 90%, so that the percentage of agricultural use relative to total use changed from about 60% of total AI in 1998–2001 to 97% in 2002–2006. Thus, potential exposures to chlorpyrifos are now largely limited to agricultural sources, such as residues on food crops, or residence on or near agricultural lands, or through occupational exposures to agricultural applicators and farm workers.
FIG. 2 Distribution of chlorpyrifos uses before and after the restriction of indoor application. (Data provided by Dow AgroSciences.)
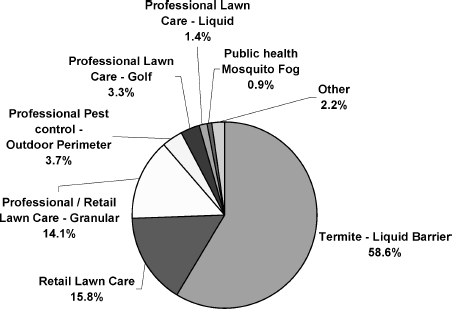
In 2002–2006, the United States accounted for 16% of total worldwide agricultural uses of chlorpyrifos ().Footnote4 The corresponding U.S. share was higher at 26% with regard to nonagricultural use, although the absolute amounts used for these purposes (97,273 kg AI, annual average) were still low in comparison with those associated with crop protection. Prior to the restrictions on chlorpyrifos use in the United States in 2001, residential application for termite control accounted for nearly 60% of total nonagricultural uses, with lawn care treatment accounting for an additional 30% (). Use of chlorpyrifos for public health (mosquito control) is still allowed and the amounts used for this purpose (∼ 27,000 kg AI per year) in 2002–2006 are similar to those used in 1998–2001. Use of chlorpyrifos for professional care of golf courses also continues (70,000 kg AI annual average), with most of this use occurring in the United States.
FIG. 3 U.S. Share in global agricultural and non-agricultural uses of chlorpyrifos from 2002–2006. Values are given as annual averages, in kg active ingredient (Data provided by Dow AgroSciences.)
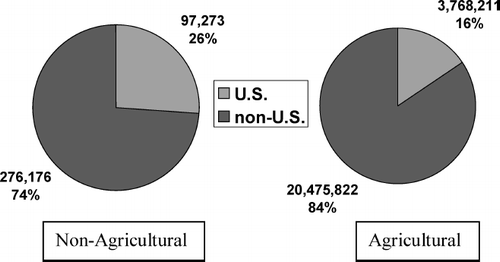
FIG. 4 Former use of chlorpyrifos in nonagricultural settings in the U.S. during the period of 1998–2001, i.e., prior to restriction of home use. (Data provided by Dow AgroSciences.)
In the period 2002–2006, 85% of total chlorpyrifos use was on seven “major” crops (crops associated with average chlorpyrifos amounts of more than 100,000 kg active ingredient (AI) per year; , upper panels). The crop associated with the highest chlorpyrifos use was corn, accounting for over a third of total agriculturally applied chlorpyrifos. Tree nuts, soybeans, and citrus fruit each account for 5% to 15% of total chlorpyrifos use. A comparison with the prerestriction period of 1998–2001 () reveals a substantial decrease in the relative contribution of corn, with larger relative amounts of chlorpyrifos use on nuts, wheat, and particularly soybeans. In addition to these “major crop” uses, chlorpyrifos is registered for use on over a dozen other agricultural products, including fruits and vegetables, in relatively small amounts (, lower panels).
I.E. Overview of Metabolic and Environmental Degradation of Chlorpyrifos
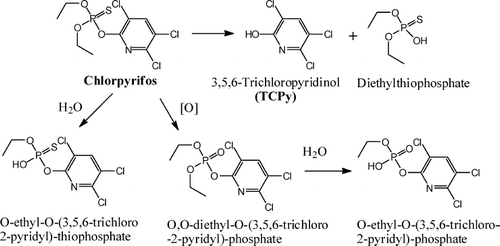
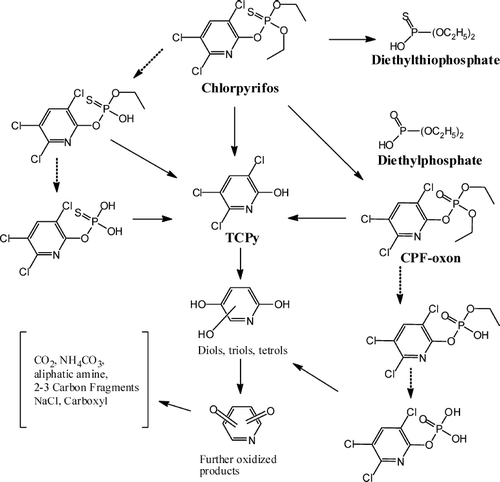
Like most OP pesticides, chlorpyrifos is oxidized to its oxon form, chlorpyrifos-oxon (; reviewed in Section II), which is generally regarded as the principal toxic metabolite, and is responsible for inhibition of cholinesterases. Chlorpyrifos-oxon is either enzymatically or spontaneously hydrolyzed to form the diethylphosphate and 3,5,6-trichloro-2-pyridinol (TCPy). In addition to the formation of chlorpyrifos-oxon, chlorpyrifos is oxidized via cytochrome(s) P-450 to an unstable intermediate that spontaneously hydrolyses to form diethylthiophosphate and TCPy. These metabolites are excreted in the urine, or form glucuronide and sulfate conjugates, which are also excreted in the urine ().
FIG. 6 Degradation pathways for chlorpyrifos. Dashed arrows show theoretical metabolites that have not been rigorously identified as environmental degradation products.
I.E.1. Environmental Distribution
Following application to crops, chlorpyrifos quickly binds to soil and plants. Though it typically degrades rapidly in the environment, residual levels of chlorpyrifos can last for long periods of time. Human exposure pathways from agricultural applications include dermal, oral, or inhalation (discussed in Section V).
I.E.2. Stability and Environmental Fate
The vapor pressure of chlorpyrifos (1.9 × 10− 5 mm Hg at 25°C) suggests that it will quickly volatilize into the atmosphere. Chlorpyrifos is poorly soluble in water and rapidly binds to particles in the soil or on plants, so very little enters any surrounding water sources. If chlorpyrifos enters a water system it will typically volatilize from the surface of the water.
Chlorpyrifos undergoes a number of degradation pathways () while in the environment (reviewed in CitationATSDR, 1997). Chlorpyrifos and its metabolites are susceptible to photodegradation, with a half-life of approximately 3 days; in the presence of hydroxyl radicals in the atmosphere the half-life is lowered to about 6 h. Upon entering surface water, chlorpyrifos degradation is associated with abiotic hydrolysis or photosensitized oxidation. In soil, photodegradation plays a role in hydrolysis, dechlorination, and oxidation of chlorpyrifos. However, in indoor environments, chlorpyrifos can persist for several months because of the relative lack of sunlight, water, and/or soil microorganisms that contribute to its rapid degradation in the outdoor environment.
II. ABSORPTION, METABOLISM, DISTRIBUTION, EXCRETION, AND PHARMACOKINETICS OF CHLORPYRIFOS
In considering the relevance of individual pharmacokinetic and metabolism studies, key considerations are: the route of exposure, the exposure dose, the form of exposure, and the methodology employed (including the choice of species, the sampling and analytical procedures employed, and processing of data).
Doses of chlorpyrifos used in toxicological investigations in laboratory animals have been typically at least two orders of magnitude higher than those experienced during occupational exposures, which are generally several orders of magnitude greater than exposure to the general population that may occur from dietary residues and agricultural uses. The majority of long-term toxicological studies have been conducted using the oral route of administration with doses in the range 0.05–25 mg/kg body weight/day. Shorter term studies using inhalation exposures have used concentrations ranging from 3 to 5300 mg/m3. In view of the very large differences between the doses used in most toxicological studies and the levels to which the general public is exposed, it is important to consider the extent to which the toxicokinetic and metabolism findings at high doses can be extrapolated to low doses and vice versa.
Experimental animals (mainly rats and mice) have been extensively used to identify the toxicological properties of chlorpyrifos and to elucidate the mechanisms associated with these properties. A number of toxicokinetic and metabolism studies have also been carried out in farm animals with the primary purpose of identifying the conditions required to achieve acceptable residues in meat and milk.
II.A. Absorption
II.A.1. Oral Absorption
Oral Absorption in Experimental Animals. Most uptake studies have been conducted by treating rats at relatively high dose levels. Some caution must be adopted in extrapolating findings at high doses to much lower dose levels because frank toxic effects may affect gastrointestinal absorption and pharmacokinetics. At the relatively low doses reflective of typical human exposures, in vivo absorption has most commonly been determined by measurement of blood or urine levels of TCPy, diethyl-thiophosphate (DETP), or diethylphosphate (DEP) or by determining the disappearance of chlorpyrifos at the exposure site. At high doses the most widely used approach to characterize absorption has been to correlate the anticholinesterase effects with the exposure dose.
Chlorpyrifos administered in corn oil appears to be well absorbed (around 80%) over a range of dose levels (CitationTimchalk et al., 2002b). At an oral dose level of 50 mg/kg body weight (a dose that produced some inhibition of cholinesterase with consequent adverse effects) peak blood levels of the metabolites DEP, DETP and TCPy were found 3h after dosing. In this study no attempt was made to measure either chlorpyrifos or chlorpyrifos-oxon in the blood. In a study with pregnant rats (CitationMattsson et al., 2000), following oral administration of 5 mg/kg body weight, the peak blood level of chlorpyrifos itself was 109 ng/g. The oxon was detected (1 ng/g) in a single animal.
CitationTimchalk et al. (2007) have shown for rats treated orally with TCPy, DEP, or DETP (140 umol/kg body weight) that all three compounds are well absorbed with peak blood levels occurring between 1 and 3 h after administration. This is of relevance to biomonitoring since these compounds are routinely measured in urine as biomarkers of exposure to chlorpyrifos. CitationCook and Shenoy (2003) used a single-pass intestinal perfusion method to assess the intestinal uptake of chlorpyrifos. Using a 100-fold concentration range they found that chlorpyrifos was well absorbed over the entire length of the small intestine. They consider absorption to be at least 99% based on their findings in rats and a comparative study in rats and humans (CitationFagerholm et al., 1996). However, a decrease in permeability at the highest concentration in the duodenum and ileum implies that, although passive diffusion is the predominant mode of uptake, a saturable transport mechanism may also be involved in the ileal and possibly the duodenal absorption of chlorpyrifos. It is noted that chlorpyrifos and its oxon interact with the efflux protein P-glycoprotein (CitationLanning et al., 1996) that is found in the intestine. However, whether this saturable interaction is the transport mechanism proposed by Cook and Shenoy is uncertain (CitationCook and Shenoy, 2003).
CitationCometa et al. (2007) examined the metabolism and anticholinesterase effects in mice of acute and repeated dosing of chlorpyrifos at doses between 12.5 and 100 mg/kg body weight. With single doses of 12.5 mg/kg or 25 mg/kg, observable effects arising from the inhibition of blood and brain AChE started 2 h after treatment, with the peak inhibition observed at 6 h after dosing. Repeated dosing for 5 days at 1.56–25 mg/kg-day resulted in a slight initial depression of hepatic carboxylesterases and a significant reduction in glutathione levels (about 80% of the control value). However, after 30 days there were no significant differences from the control animals, suggesting that induction of glutathione and carboxylesterase biosynthesis may have occurred as part of an adaptive response.
Although no specific studies on absorption have been identified, one oral feeding study in Fischer 344 rats found that chlorpyrifos-oxon was substantially less effective as a cholinesterase inhibitor than chlorpyrifos (CitationCieslak, 1999); yet in vitro the oxon is at least 1000 times more potent, and the oxon is also a much more potent cholinesterase inhibitor following dermal application than chlorpyrifos (CitationCole et al., 2005). Thus, chlorpyrifos-oxon may degrade in the acid environment of the stomach and/or undergoes extensive first-pass metabolism in the gut and liver.
Oral Absorption in Rodent Weanlings. CitationTimchalk et al. (2006) investigated the absorption of chlorpyrifos in rats of 5, 12, and 17 days of age using oral doses of 1 and 10 mg/kg body weight. At both dose levels rapid absorption and metabolism was evident, with peak blood levels of chlorpyrifos and its metabolites occurring between 3 and 6 h at each age investigated. Blood levels of chlorpyrifos were independent of the age of the weanlings and there was no indication of nonlinear kinetics in this study over the dose range investigated.
Absorption in the Rodent Fetus. Pregnant rats treated daily with chlorpyrifos (7 mg/kg/day, p.o.) on gestational days (GD) 14–18 showed similar peaks in terms of time of inhibition of brain AChE for the dams and the fetuses (CitationLassiter et al., 1998b), demonstrating that chlorpyrifos can pass into the fetus and inhibit fetal brain AChE. The extent of enzyme inhibition was substantially lower in the fetus than the dam (4.7-fold). They concluded that the main reason for the lower inhibition seen in the dams was the higher ability of the fetus to synthesize new cholinesterase protein, although the difference may also partially reflect the ability of the mother to detoxify chlorpyrifos. Several other authors have also investigated the transplacental disposition of chlorpyrifos in pregnant rats. CitationAkhtar et al. (2006) fed pregnant rats 14C-labeled chlorpyrifos at doses ranging from 9.6 to 15 mg/kg-day. No major malformations were found although changes were noted in fetal weight. The levels of residues in the fetus, based on distribution of radioactivity, were: liver, 0.0531 μ g/g; brain 0.0364 μ g/g; placenta 0.04 μ g/g; and amniotic fluid 0.001 μ g/g. Overall the total residue in the fetus was higher than in the mother by approximately fourfold. Unfortunately, while it is evident that the placenta does not serve as a major barrier to passage of material into the fetus, this study did not define whether chlorpyrifos or one or more of its metabolites was responsible. CitationHunter et al. (1999) examined the metabolite profile in the fetus during late (14–18 days) gestation. They found that the highest level of TCPy and the greatest cholinesterase inhibition occurred at the same time, 5 h. The concentration in the maternal liver was approximately five times that in fetal liver, whereas the concentration of TCPy in the brain was two-to fourfold higher in the fetus than in the mother. The half-life for TCPy was similar in all the tissues investigated, indicating that conjugation of TCPy was effective in the late fetus.
Oral Absorption in Humans. Chlorpyrifos appears to be relatively well absorbed from the intestine. CitationNolan et al. (1984) estimated bioavailability of orally administered chlorpyrifos to be at least 70% as that amount was recovered in the urine following a single oral dose. The actual bioavailability may have been greater, as some fraction of the dose may have been eliminated by other pathways (e.g., bile/feces; breath) or retained in the body through lipid partitioning and or protein binding. However, in a more recent study CitationTimchalk et al. (2002b) administered a single dose of 0.5, 1, or 2 mg/kg chlorpyrifos to 12 human volunteers. Blood and urine was collected over 168 h. They recovered only about 20 to 35% of the administered dose as TCPy in the urine, suggesting that a significant fraction of the administered dose was not absorbed. No chlorpyrifos or chlorpyrifos-oxon was detected in the urine. Based on the differences in formulation between their study and that of CitationNolan et al. (1984), they concluded that the extent of oral absorption of chlorpyrifos is dependent upon its physical form and other formulation characteristics. In a human volunteer study involving a single oral dose of 0.5 mg/kg body weight, peak blood chlorpyrifos levels were less than 30 ng/ml, and represented only a fraction of the levels of TCPy. Peak TCPy levels (939 ng/ml) were achieved after 6 h (CitationNolan et al., 1984). In incidents where accidental high level exposure has occurred the time to effect (confusion, headaches, dizziness, nausea, vomiting, etc.) is less than 1 h, indicating rapid absorption and distribution to the brain following high-dose exposures (CitationCochran, 2002).
II.A.2. Dermal Absorption
Dermal Absorption in Experimental Animals. Dermal uptake of chlorpyrifos has been demonstrated in several animal species. The application of various combinations of chlorpyrifos and cypermethrin to the tails of rats reportedly caused inhibition of cholinesterase activity as well as pyknosis of brain neurons (CitationLatuszynska et al., 2001).
Dermal Absorption in Humans. In a study of dermal absorption in 6 human volunteers, 1.28 ± 0.75% of the dermally applied dose of chlorpyrifos was recovered in the urine as metabolites after 24 h (CitationNolan et al., 1984). Another study found that approximately 1% of the metabolites of chlorpyrifos was recovered in the urine (CitationGriffin et al., 1999). Not all of the absorbed dose, however, will be eliminated in the urine. Consequently, the percent of the dose absorbed following dermal application in these studies may have been higher than that estimated by urinary metabolites alone. Additional limitations to interpretation of these dermal absorption studies were discussed by CitationMage (2006). The absorption of chlorpyrifos was determined after application of either 5 or 15 mg of chlorpyrifos to the forearms of human volunteers (CitationMeuling et al., 2005). The chlorpyrifos was applied in ethanol to 100 cm2 and left for 4 h, after which the skin was washed. The nonabsorbed fraction amounted to 42–67% of the applied dose. In individuals exposed to 5 mg of chlorpyrifos approximately 4.3% of the dose was excreted as TCPy. The amount of TCPy excreted after a dose of 15 mg was similar to that at 5 mg, indicating that the percutaneous absorption rate over this exposure range was independent of dose. Clearance was not complete until 5 days postexposure. This implies that chlorpyrifos was either partly retained in the skin or in the body after dosing. This may reflect its lipophilicity and/or ability to bind to various proteins (see Section II.B).
The percutaneous penetration of chlorpyrifos has also been studied in vitro using cadaveric human skin samples (CitationGriffin et al., 2000). A commercial formulation of chlorpyrifos (dissolved in water to a final concentration of 52.15 mM) was compared to the same concentration of chlorpyrifos dissolved in ethanol. A 20-μ l aliquot was placed on 0.5 cm2 of cadaveric skin in a flow-through apparatus. The rates of penetration of chlorpyrifos through the skin were 9.0 and 4.9 nmol/cm2-h in the commercial formulation and the ethanolic preparation, respectively. These data suggest that formulation has a significant influence on dermal penetration rates, at least in vitro. Between 11 and 20% of chlorpyrifos was found in the perfusate and a substantial amount remained bound to the skin even after 24 h. No investigation was conducted of the ability of the skin to metabolize chlorpyrifos, although some metabolism would be expected.
II.A.3. Absorption via Inhalation Exposure
Chlorpyrifos is generally assumed to be well absorbed through the lung following airborne exposure (CitationGeer et al., 2004), although no direct measurements verified this assumption.
CitationBrenner et al. (1989) evaluated the prevalence of certain illnesses and symptoms in 175 employees involved in the production of chlorpyrifos. The occupational cohort was subdivided into three exposure intensity groups based on air-monitoring data and job title. Time-weighted average airborne concentrations of chlorpyrifos ranged from 10 to 1100 μ g/m3 in the workplace. Exposed workers were categorized into one of three groups: low, medium, and high exposure, although a specific definition of these categories of exposure relative to airborne measurements was not provided. When these groups were compared with 335 matched controls with no history of exposure to OP pesticide chemicals, no statistically significant differences in the prevalence of central and peripheral nervous system symptoms were found. Exposure was assumed to be via inhalation and dermal routes, but no individual markers of exposure, such as TCPy or personal air monitors, were obtained, so it was not possible to assess the extent of airborne exposure and bioavailability, if any. However, baseline plasma cholinesterase was obtained for each worker assigned to jobs with potential exposure, and monthly plasma BuChE activity was monitored. Workers in the “low” exposure category had an average reduction from baseline BuChE of 19.1 ± 2.1%, whereas workers in the medium and high categories had reductions of 32.1 ± 2.8 and 32.0 ± 5.3%, respectively.
Animal studies demonstrate that high-dose acute inhalation exposure to chlorpyrifos can result in significant cholinesterase inhibition, although the absorption rate and/or bioavailability via this route of exposure have not been determined. CitationCorley et al. (1989) exposed rats (nose-only) to 0, 0.075, 0.148, or 0.295 mg/m3 chlorpyrifos for 6 h/day, 5 days/week for 13 weeks. These exposure levels did not inhibit erythrocyte or plasma cholinesterase activity. Thus, although inhalation exposure to chlorpyrifos undoubtedly can result in systemic absorption, the actual extent of absorption (bioavailability) is not known with certainty, and it seems unlikely that airborne concentrations of chlorpyrifos of less than 10 μ g/m3 (10,000 ng/m3) could significantly inhibit plasma BuChE or erythrocyte AChE. For comparative purposes, airborne concentrations measured in indoor residential air after crack-and-crevice application of chlorpyrifos ranged from 0.1 to 0.8 μ g/m3 (CitationByrne et al., 1998), or approximately 10–750 times lower than the lowest airborne concentration used in the CitationCorely et al. (1989) study. Other studies have found indoor air concentrations of chlorpyrifos typically ranging from 0.001 to 0.1 μ g/m3 (see Section V, Exposure Assessment).
II.B. Distribution
II.B.1. Distribution Studies in Experimental Animals
The highest concentrations of chlorpyrifos are found in the fat and fatty tissues. Chlorpyrifos also binds to various proteins: e.g., plasma albumin. As a consequence, the free levels of chlorpyrifos in the blood that are available for distribution into other body compartments are rather low. In an oral dosing study in rats, chlorpyrifos was found to accumulate in the adipose tissue but not in other tissue compartments (CitationBakke et al., 1976). This would be expected because of its high lipophilicity.
In a detailed study of the tissue distribution of chlorpyrifos (CitationTimchalk, 2002b), partition coefficients for chlorpyrifos between various tissues and blood were calculated (not measured), based on octanol:water partition coefficients and lipid content of tissues (CitationPoulin and Krishnan, 1995), as follows:
Brain 33:1
Liver 22:1
Kidney 10:1
Fat 435:1
Chlorpyrifos-oxon, which is less lipophilic than the parent compound, has slightly lower calculated tissue:blood partition coefficients (brain 26:1, liver 11.8:1, kidney 8:1, and fat 324:1). However, it should be noted that these represent calculated—not measured—tissue distributions based solely on the octanol:water partition coefficients for chlorpyrifos and chlorpyrifos-oxon. Because of extensive protein binding in the plasma, specific blood:brain partitioning could be substantially different from that estimated based only on lipid partitioning.
Radiolabeled [14C]-chlorpyrifos (5 mg/kg), administered intravenously to pregnant rats as a single injection at various gestational ages, was used to investigate the distribution of chlorpyrifos and its metabolite in the mother and fetus (CitationAbdel-Rahman et al., 2002). Radioactivity and TCPy were identified in all tissues five minutes after dosing; however, the parent compound was only identified in maternal plasma and liver, indicating rapid metabolism. In the maternal plasma the peak level of radioactivity occurred 5 min after injection, whereas in the fetus the peak level of radioactivity occurred after 15 min and the level was about 1% of that in the mother's plasma. In tissues (maternal blood, liver, brain, and placenta, and fetus), the maximum concentration was found in the maternal liver. However, the levels in the fetus and the placenta were only marginally lower. Analysis of the toxicokinetics indicated the best fit to be bi-exponential with a rather slow second phase. It was speculated that lipid storage may be the primary determinant of the overall elimination rate.
II.B.2. Distribution Studies in Humans
There are no definitive studies of the tissue distribution of chlorpyrifos and its metabolites in humans. However, in an epidemiology study of pregnant women and their newborn infants exposed to chlorpyrifos from indoor use (discussed in detail later; Section V.C), maternal and umbilical cord blood levels of chlorpyrifos were comparable and highly correlated among mother–child pairs, indicating that chlorpyrifos readily passes through the placenta (CitationWhyatt et al., 2005).
II.C. Biotransformation of Chlorpyrifos
II.C.1. General Aspects of Biotransformation of Chlorpyrifos (Experimental Animals)
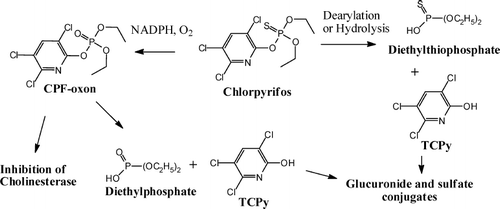
The biotransformation pathways of chlorpyrifos in the body are illustrated in . Of particular importance is the desulfuration to chlorpyrifos-oxon since this metabolite must be formed to express potent anticholinesterase activity; chlorpyrifos by itself is unable to inhibit AChE to any significant extent (CitationChambers, 1992). The main route of inactivation of the oxon is by hydrolysis to form 3,5,6-trichloro-2-pyridinol (TCPy). A key role in this pathway is played by the enzyme paraoxonase. The parent compound is also converted in vitro to TCPy through CYP-mediated dearylation (CitationSultatos et al., 1984). Thus, both oxidative dearylation of chlorpyrifos by CYP enzymes and hydrolysis of chlorpyrifos-oxon by paraoxonase (and other esterases) contribute to the formation of TCPy, which is the major chlorpyrifos metabolite identified in urine.
The metabolism of chlorpyrifos has been the subject of many in vivo and in vitro studies. A number of authors have used the inhibition of cholinesterase activity to estimate both the rate of chlorpyrifos-oxon formation from the parent compound and its subsequent detoxication. Differences have been observed in the inhibition of cholinesterase activity with species, gender, and age (CitationPope et al., 1991b; CitationMoser and Padilla, 1998).
An overview of mechanisms involved in the metabolism of chlorpyrifos is given next.
Desulfuration. As with other phosphorothioates, oxidative desulfuration of chlorpyrifos appears to be primarily a cytochrome P-450-dependent reaction. Activated sulfur atoms formed during the process of desulfuration have the potential to bind irreversibly to the cytochrome P-450 molecule, catalyzing the reaction. Thus, chlorpyrifos acts as a “suicide substrate” (CitationDeMatteis, 1974) and as a consequence, a time-related reduction in cytochrome P-450 activity may occur (CitationHalpert et al., 1980; CitationSultatos, 1994). The rate of desulfuration of chlorpyrifos by rat liver microsomes was found to be 100 times greater than that by rat brain microsomes (CitationChambers and Chambers, 1989). No sex differences in the rate of metabolism were noted for the brain microsomes. However, for rat liver microsomes, those from males were approximately three times more active than those from female rats (CitationChambers and Chambers, 1989). Comparable sex differences have been found using perfused rat livers (CitationSultatos, 1991).
Dearylation and Hydrolysis. Detoxication of the oxon can be brought about by several enzymes, notably oxonases (A-esterases such as paraoxonase), which catalyze the hydrolysis, and cytochromes P-450, which catalyze dearylation of chlorpyrifos to DETP and TCPy (CitationJokanovic, 2001). Specific isoforms of cytochrome P-450 have different capacities to form the oxon and to detoxify chlorpyrifos and its oxon. This issue is discussed in detail later, in regard to human metabolism.
Oxonases (EC 3.1.1.2) are calcium-dependent enzymes that hydrolyze oxons in a nonstoichiometric manner (CitationAldridge, 1953). There are substantial species differences in serum A-esterase activity, with species with high activity tending to be less vulnerable to OP pesticide toxicity (CitationCosta et al., 1990).
Carboxylesterase levels appear to correlate with age-related differences in the inhibition of AChE activity due to chlorpyrifos, which indicates an important role for this detoxication enzyme in the weanling rat (CitationMoser et al., 1998). Because of the potential relevance of known human polymorphisms in the human gene for “paraoxonase” to chlorpyrifos detoxification, a detailed discussion of the animal and human studies on the paraoxonase enzyme is provided below.
Glutathione Conjugation. Glutathione conjugation is mediated by glutathione S-transferases. This reaction appears to be comparable to the well-researched glutathione conjugation reaction involving a chlorine atom next to a nitro group in an aromatic ring. It is likely that in rats the glutathione conjugate is formed principally in the liver and excreted in the bile. It is likely, however, that the glutathione conjugate will also be produced in other tissues too. Subsequently, the glutathione conjugate excreted in the bile may be anticipated to undergo cleavage of the glutamic acid and glycine residues in the gut resulting in the formation of a cysteine conjugate. This reaction may be mediated by enzymatic and gut microfloral action. The cysteine conjugate may be reabsorbed and further metabolized. In the kidney the cysteine moiety may be acetylated to form the mercapturic acid or may lose alanine resulting in a metabolite with a free sulfhydryl group (CitationDekant, 2001). Glutathione conjugates of chlorpyrifos were detected in the livers of mice treated intraperitoneally with chlorpyrifos, (CitationFujioka and Casida, 2007), suggesting that this could be a significant pathway for metabolism of chlorpyrifos (see later discussion on human metabolism of chlorpyrifos).
Conjugation With Glucuronic Acid and Sulfate. The free hydroxyl group, arising from the hydrolysis of TCPy or thio-TCPy, may be conjugated in various tissues with glucuronic acid (mediated by glucuronyl transferases) or sulfate (by means of sulfur transferases). These reactions result in a much more water-soluble product that may be excreted in either the bile or the urine. There is some limited evidence that methylation of the hydroxyl group may occur.
II.C.2. Influence of Animal Age and Characteristics of Dosing
Comparison of the metabolic capability of young rats (5, 12, and 17 days old) and adult rats has been made by CitationTimchalk et al. (2006). For each weanling age group and for adults the rate of detoxication via formation of TCPy significantly exceeded the rate of formation of the toxic metabolite, chlorpyrifos-oxon. It can be concluded that even in animals as young as 5 days old the overall detoxication capacity exceeds that for intoxication at levels between 1 and 10 mg/kg body weight. Nonetheless, it has been observed that the blood levels of chlorpyrifos are somewhat lower in adult rats than younger animals treated, with a dose ranging from 1 to 10 mg/kg body weight, indicating that the latter have a lower overall capacity to metabolize chlorpyrifos (CitationTimchalk et al., 2002b).
The chlorpyrifos-oxonase activities have been shown to be considerably lower in rat fetal brain, as compared with their mothers, although the toxicological significance of this difference is not clear (CitationLassiter et al., 1998a).
Metabolite Profiles at Different Dose Levels. It would be important to identify the dose dependency of each of the several metabolic pathways, whether or not saturation of one or more metabolic pathways occurs, and, if so, which metabolite(s) of interest are formed at high dose levels. However, no studies have been identified that enable conclusions to be made on this question.
II.C.3. Overview of Active/Toxic Metabolites of Chlorpyrifos (Experimental Animals)
The main metabolites, DEP, DETP, and TCPy and its glucuronic acid and sulfate conjugates, are considered to make no significant contribution to the adverse effects of chlorpyrifos. It is noted, however, that this conclusion is based on rather limited evidence and that relatively few studies have examined the toxicity of TCPy or other chlorpyrifos metabolites (see Section III.D). Phosphorothioates are very weak inhibitors of AChE due to the relatively poor electron-withdrawing ability of the sulfur atom compared with an oxygen atom. In adult rats and in weanlings the principal active metabolite of chlorpyrifos is chlorpyrifos-oxon. This metabolite is considered to cause the irreversible inhibition of AchE activity through binding to the enzymes active site. Not surprisingly, the vast majority of the research on active metabolites of chlorpyrifos has focused on the formation and further metabolism of this oxon. It should be recognized, however, that the reaction of the oxon is with serine at the active site. There are many other serine hydrolases that are potential targets for the oxon. CitationCasida and Quistead (2005) have reviewed the evidence for the effects of chlorpyrifos-oxon on some of these other serine hydrolases. It may be concluded from the evidence that the serine hydrolases studied are unlikely to be significantly inhibited at typical environmental concentrations. However, knowledge of the role of the majority of serine hydrolases and the consequences of their inhibition is very limited.
Several other putative active metabolites can arise potentially as a result of chlorpyrifos biotransformation, namely:
As a consequence of the desulfuration of chlorpyrifos an active sulfur moiety is released. It is presumably this metabolite that is responsible for the noncompetitive inhibition of cytochromes P-450. It is uncertain however whether the extent of inhibition of an individual isoform of cytochrome P-450 is directly correlated with its ability to form the oxon. Nor is it clear the extent to which the active sulfur moiety can interact with nearby tissue components. Taken at face value it appears that the production of the oxon by a particular isoform of cytochrome P-450 may be self-limiting. If this is the case, it may enable chlorpyrifos to be metabolized by noncytochrome P-450-dependent enzymes, resulting in an altered profile of metabolites. Evidence that this is the case is very limited.
The identification of glutathione and cysteine conjugates indicates that further reactive metabolite(s) may arise as a consequence of the dechlorination at the 5-position. Whether this is a suicide substrate for glutathione transferase(s), resulting in loss of transferase activity, is unknown.
The range of sulfur conjugates found (see later discussion) raises the possibility of metabolites being formed that have free –SH groups as a result of β-lyase activity on the cysteine conjugates. By virtue of the free –SH group they should be considered as possible reactive metabolites since there is evidence that some products ofβ-lyase can cause renal toxicity. β-Lyase is present in brain.
To elucidate whether such metabolites have any role in the adverse effects of chlorpyrifos in humans it is important to identify the dose-response relationship for the formation of each of these putative active metabolites and their rates of formation compared to their rates of detoxication in the target organs for toxicity.
Several papers have indicated that chlorpyrifos can promote glutathione depletion and/or promote active oxygen formation and lipid peroxidation in various in vitro preparations, including regions of rat brain (CitationVerma and Srivastava, 2001). Initiation of such oxidative stress is commonly associated with either the formation of an active metabolite or further metabolism that generates reactive oxygen species. Chlorpyrifos-induced oxidative stress has been proposed to cause or contribute to developmental neurotoxicity, based on the in vitro observation that chlorpyrifos itself was considerably more potent than chlorpyrifos-oxon (CitationCrumpton et al., 2000b). Since chlorpyrifos itself is a chemically stable molecule it is reasonable to propose that it is the desulfuration of chlorpyrifos or a subsequent metabolite with the phosphorus–sulfur bond still intact that is responsible for the oxidative stress. The relevance of these findings to adverse effects of chlorpyrifos in vivo has yet to be studied. Chlorpyrifos (CitationGultekin et al., 2000) causes lipid peroxidation and significant changes in antioxidant enzyme activity in erythrocytes. Studies are needed to identify the relationship between lipid peroxidation and organophosphorus structure in order to identify the metabolic step(s) that account(s) for lipid peroxidation produced by chlorpyrifos and to elucidate the dose-response relationship. The in vivo relevance of such findings also awaits investigation.
II.C.4. Biotransformation Studies in Humans
Studies have examined various facets of the metabolism of chlorpyrifos in humans. CitationBicker et al. (2005a) examined the metabolite profile in the urine of a 59-year-old woman who accidentally drank from a bottle of Dursban E (containing 20–25% of chlorpyrifos in a hydrocarbon mixture). Fifteen urinary metabolites were identified:
TCPy (3,5,6 trichloro-2-pyridinol)
Mono-O-deethyl-chlorpyrifos
Mono-O-deethyl-chlorpyrifos-oxon
3,5-Dichloro-6-methylthio-2-pyridinol
Diethyl phosphate
Diethylthiophosphate
Cysteine S-conjugate of 6-dechloro-chlorpyrifos
Cysteine S-conjugate of 6-dechloro-mono-O-deethyl-chlor-pyrifos
Cysteine S-conjugate of 6-dechloro-chlorpyrifos-oxon
Cysteine S-conjugate of 6-dechloro-mono-O-deethyl-chlor-pyrifos-oxon
Cysteine S-conjugate of 6-dechloro-3,5-dichloro-2-pyridinol
Mercapturic acid conjugate of 6-dechloro-3,5-dichloro-2-pyri-dinol
S-3,5-Dichloro-2-hydroxyl-6-pyridinyl-N-diethoxythiopho-sporyl cysteine
O-Glucuronide of 3,5,6-trichloro-2-pyridinol
O-Glucuronide of 3,5-dichloro-6-methylthio-2-pyridinol
The authors consider that these metabolites were produced via three metabolic routes: cleavage at the aromatic phosphoester bond, cleavage of the alkyl diester bonds, and nucleophilic substitution of the 6-chlorine atom on the aromatic ring with glutathione. Only the first route had previously been described in humans. Neither the parent compound nor chlorpyrifos-oxon was detected in the urine in this subject. Some species differences in the urinary profile of metabolites between humans and rats may be expected because the molecular weight threshold in much lower in rats (around 300–350) than in humans (approximately 550) (CitationWilliams et al., 1965).
It is important to identify the roles of individual metabolism enzymes to understand the potential rate limiting steps in activation and detoxication of chlorpyrifos, because differences in these rates contribute substantially to both species and interindividual differences in sensitivity to chlorpyrifos. Data on the kinetics of each of these enzymes are also needed for physiologically based pharmacokinetic (PBPK) modeling.
Human Cytochrome P-450-Mediated Dearylation. Over the past decade there has been intense activity to elucidate which isoforms of cytochrome P-450 (CYP) are expressed in human tissues, particularly the liver. Very large variations in the activity of individual CYP isoforms have been reported. For example, expression of CYP3A4/5 and CYP2C8 varies by 150-fold (CitationMutch and Williams, 2004). It is also well established that the levels of CYP isoforms are susceptible to both differential inhibition and induction. The CYP3A family is comprised principally of CYP3A4, CYP3A5, and CYP3A7. CYP3A4 is the main isoform found in human liver and intestine. CYP3A7 is the main form found in fetal liver. Expression of the CYP3A7 gene is usually switched off after birth and CYP3A4 expression becomes dominant (CitationStevens et al., 2003). However, small amounts of CYP3A7 protein persist in the liver and intestine of adults (CitationBurk et al., 2002). The levels of CYP3A5show the greatest interindividual differences, due to the presence of a common genetic polymorphism that results in the expression of a truncated, nonfunctional protein. While the xenobiotic metabolism profile for the various isoforms is relatively well defined, knowledge of the physiological substrates is still limited. Relatively little is known about the CYP metabolizing capacity in human brain. The major hepatic, adrenal, and gonadal CYP isozymes contribute very little to the overall content of CYP in brain, levels of which are 0.5–2% of that in liver (CitationHedlund et al., 2001). CYPs are primarily neuronal and participate in the regulation of neurotransmitters and steroids, and in brain maintenance of cholesterol homeostasis (CitationDutheil et al., 2008).
Several studies have examined the roles of the various peripheral CYP isoforms in the metabolism of OP pesticides (CitationSams et al., 2000; CitationBuratti and Testai, 2003; CitationBuratti et al., 2006; CitationMutch and Williams, 2006). CitationMutch and Williams (2006) found a wide variation in the in vitro activity of liver samples from different individuals to form chlorpyrifos-oxon from chlorpyrifos (100 uM). Similar variation was found in the deactivation of chlorpyrifos to TCPy.
The human isoforms that metabolize chlorpyrifos are CYPs 1A2, 2B6, 2C9, 2C19, and 3A4. A role for 2D6 has also been suggested (CitationSams et al., 2000). Each isoform is involved in both the formation of the oxon and in its detoxification, although the balance of these activities varies between isoforms (CitationRose and Hodgson, 2005; CitationSams et al., 2004). CYP 2B6 and 3A4, both of which are polymorphic, are the most important in respect to formation of the oxon. The enzyme most important for formation of TCPy is CYP2C19, which is also polymorphic (CitationSams et al., 2004; CitationMutch and Williams, 2006). In experiments with recombinant human CYPs, CitationMutch and Williams (2006) reported that 2D6, 3A5, 2B6, and 3A4were most effective in producing chlorpyrifos-oxon, whereas CYPs 2C19, 2D6, 3A5, and 3A4 were most effective at producing TCPy. The apparent KM for activation of chlorpyrifos was 30.2 μ M, and for the detoxication to TCPy was 14.4 μ M. Another study using human liver microsomes found that while azinphos-methyl, diazinon, and parathion had two distinct phases in the desulfuration reaction characterized by different affinity constants, only one high-affinity component could be identified for chlorpyrifos, with an apparent KM of 0.27–0.94 μ M (CitationBuratti and Testai, 2003). The high affinity indicates that the enzyme will be active in metabolizing chlorpyrifos even at low substrate concentrations. Neither the activation nor the detoxication of chlorpyrifos correlated with protein expression, indicating that multiple forms of CYP are involved in chlorpyrifos metabolism (CitationMutch and Williams, 2006). The patterns of activation and detoxication reactions of chlorpyrifos were consistent with the relative amounts of the main CYP isoforms (CitationRose et al., 2005).
CitationSams et al. (2004) found that the catalytic efficiency (kcat; Vmax/Km) of human liver microsomes for the CYP-mediated dearylation was four times greater than the oxidative desulfuration of chlorpyrifos, suggesting that hepatic CYPs are relatively more effective at detoxifying than activating chlorpyrifos. Individual cDNA-expressed human CYPs 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, and 3A4 were also evaluated in this study. The results demonstrated that CYP2B6 had the highest catalytic efficiency for desulfuration of chlorpyrifos and formed only chlorpyrifos-oxon, whereas human CYP2C19 was the most effective at dearylation, and formed about 10 times more TCPy than chlorpyrifos-oxon. CYP 1A2 was relatively effective at forming both metabolites, whereas 2A6, 2C9, 2D6, and 3A4formed small amounts of chlorpyrifos-oxon and little TCPy.
Thus, the profile of CYP isoforms may be an important contributor to interindividual differences in human susceptibility to the adverse effects of chlorpyrifos. Although the literature is not completely consistent on the relatively importance of specific human CYP enzymes in activation and/or detoxication of chlorpyrifos, there is general agreement that CYP2B6 has the highest activity toward activation of chlorpyrifos to chlorpyrifos oxon, whereas CYP2C19 has the ability to dearylate chlorpyrifos to TCPy. However, the levels of expression of CYP genes in a given tissue, as well as the catalytic efficiency of each CYP protein, are important determinants of the overall contribution of a specific CYP protein to biotransformation of chlorpyrifos. Thus, the potential range of differences in the ratio of overall activation to detoxication that could be expected across the human population is unfortunately hard to gauge.
The catalytic activity of recombinant human fetal liver CYP3A family has been examined (CitationBuratti et al., 2006). It was shown that CYP3A7 is able to produce significant levels of the oxon metabolite of chlorpyrifos. The metabolism of chlorpyrifos to TCPy by CYP3A7 at low substrate concentrations was considerably greater than that for the formation of the oxon. At rather high substrate levels (50 μ M) the ratio of TCPy to oxon was substantially reduced.
Effects of Induction and Inhibition of CYPs on the Metabolism of Chlorpyrifos. A further consideration is the potential for inhibition and induction of the key CYP isoforms. Human CYPs 1A1, 1A2, 2B6, and 3A4 are inducible by chlorpyrifos (CitationRose and Hodgson, 2005). What is less clear is the level of chlorpyrifos intake required for the induction of these enzymes. It appears likely that the levels involved are rather higher than those of normal human exposure. Chlorpyrifos appears to act both as an inducer and as a suicide substrate for CYPs; the balance of these conflicting activities might be expected to differ between an acute exposure and a more prolonged one. The practical significance of this, in terms of toxicity, is difficult to judge based on the available literature.
Studies using human liver microsomes have also shown that chlorpyrifos can significantly inhibit the metabolism of some other chemicals such as fibronil, nonane (CitationJoo et al., 2007) and carbaryl (CitationTang, 2000) as a direct consequence of its interaction with CYPs. This is to be anticipated since each requires CYP3A4 and /or CYP2B6 for its oxidative metabolism. Inhibition of activity was significantly greater following inhibition of enzyme with chlorpyrifos than when co-incubation was employed. This may be expected, as chlorpyrifos has been shown in vitro to be an irreversible noncompetitive inhibitor of human CYP isoforms.
Formation of Glutathione–Chlorpyrifos Conjugates in Humans. As noted previously, CitationBicker et al. (2005b) identified glutathione-derived metabolites in urine from an individual acutely poisoned with chlorpyrifos (CitationBicker et al., 2005b). Several other in vitro studies with human liver have further evaluated the role of glutathione conjugation in the overall disposition of chlorpyrifos. In a detailed study of chlorpyrifos biotransformation in human hepatocytes and pooled S9 liver fractions, CitationChoi et al. (2006) identified 15 chlorpyrifos-derived and 6 chlorpyrifos-oxon-derived metabolites by liquid chromatography (LC)–mass spectroscopy (MS)/MS analysis. The pyridinyl-2-glutathione conjugate of chlorpyrifos and chlorpyrifos-oxon were identified when pooled samples of human liver S9 were incubated with chlorpyrifos or chlorpyrifos-oxon. Incubations of human hepatocytes with chlorpyrifos yielded multiple different metabolites derived from glutathioneS-transferase (GST) conjugation, including γ-glutamyl-cysteine, cysteinyl-glycine, and cysteine conjugates of chlorpyrifos, and the glutathione and cysteine conjugates of TCPy (CitationChoi et al., 2006).
Recently, CitationFujioka and Casidy (2007) conducted a detailed analysis of the GST-mediated conjugation of chlorpyrifos, chlorpyrifos-oxon and TCPy using various sources of GSTs and LC-ESI-MS analysis of metabolites. They demonstrated that GSTs can catalyze the O-deethylation of both chlorpyrifos and chlorpyrifos-oxon, as well as dearylation to form S-3,5,6-trichloropyridin-2-yl)glutathione or S-(3,5-dichloro-6-hydroxypyridin-2-yl)glutathione. In the absence of an oxidation system (e.g., microsomal CYPs and NADPH), they found that equine cytosolic GSTs were able to directly conjugate chlorpyrifos and chlorpyrifos-oxon, yielding a GSH conjugate in which the 6-chloro substituent is displaced by GSH. They also found a dichlorpyrdinyl-GSH metabolite, presumably derived by GST-mediated dechlorination of TCPy (or the parent compound, followed by dearylation) by attack at carbon 6. In all, the authors identified eight different glutathione-derived conjugates of the parent chlorpyrifos molecule from attack at the 2or 6 position of the pyridinyl moiety, or via O-deethylation to give rise to desethylchlorpyrifos and ethyl-S-glutathione. In the presence of equine GST, glutathione (GSH), human microsomes, and NADPH, the predominant metabolite was the chlorpyrifos-6-S-glutathione conjugate, with only 5% present as theO-deethylated metabolite. No GSH conjugates of chlorpyrifos-oxon were identified. The various GSH conjugates were formed by both cytosolic and microsomal fractions of human liver, indicating that both soluble and membrane-bound human GSTs may play a role in GSH-mediated biotransformation of chlorpyrifos. In contrast, GST-mediated biotransformation of chlorpyrifos-oxon (either O-deethylation or dearylation) occurred only with human cytosolic GSTs. The authors did not evaluate which specific GSTs participated in the reactions.
To date, no studies have examined in which specific human GSTs participate in the biotransformation of chlorpyrifos, although clues from studies of another aryl-OP, methyl-parathion, might be informative. Eaton (2005) examined the potential role of specific human GSTs in the GST-mediated O-dealkylation of methyl-parathion, with an emphasis on hGSTs M1-1 and T1-1, since deletion polymorphisms occur commonly in these genes. No correlation between hGSTM1/T1genotypes and methyl-parathion O-dealkylation activities of the 10 human liver cytosolic samples was seen. That report also measured O-dealkylation activities of several purified recombinant GSTs belonging to the alpha (human GSTs A1-1 and A2-2, mouse GSTA3-3, rat GSTA5-5), mu (human GSTs M1a-1a, M2-2, M3-3, M4-4), pi (human GSTP1-1, mouse GSTs P1-1, P2-2), and theta (human GSTT1-1) classes toward methyl-parathion. At 1 mM glutathione and 300 μ Mmethyl-parathion concentrations, hGSTT1-1 and hGSTA1-1 exhibited the highest O-dealkylation activities, with hGSTT1-1 possessing nearly 10 times higher catalytic activity that hGSTA1-1. Human GSTT1-1 is poorly expressed in human liver, and thus when the expression level and enzymatic activity are considered, it was estimated that hGSTA1-1 is responsible for the majority of methyl parathion O-dealkylation in human hepatic cytosol. However, it was noted that in target organs such as brain and skeletal muscle, where hGSTT1-1 is expressed, hGSTT1-1-mediated dealkylation of OPs may contribute to protection against OP-mediated inhibition of cholinesterases (Eaton, 2005). Whether human GSTs exhibit similar isoform-specific metabolism of chlorpyrifos is not known.
Formation of Glucuronide and Sulfate Conjugates of Chlorpyrifos and Its Metabolites. Several in vivo studies in animals have identified the glucuronide conjugate of TCPy in urine and or bile (CitationBakke et al., 1976; CitationBakke and Price, 1976; CitationBarron et al., 1991; Abdel Rahman et al., 1993), but few studies have evaluated the role of glucuronidation and/or sulfation in the disposition of chlorpyrifos in humans. Bicker et al. (200b) identified the O-glucuronide of both TCPy and 3,5-dichloro-6-methylthio-2-pyridinol (DCPy) in the urine of a patient who had ingested a large quantity of chlorpyrifos. During the first 72 h following exposure, approximately 30% of the urinary TCPy was present as free TCPy, whereas after 96 h, less than 10% was free, suggesting that either the rate of glucuronide conjugation of TCPy is slow, and/or that glucuronidation capacity was saturated at relatively high substrate concentration.
CitationChoi et al. (2006) also identified the O-glucuronides of both TCPy and DCPy following incubation of human hepatocytes with chlorpyrifos. Interestingly, they also identified an S-glucuronide conjugate of chlorpyrifos, which they proposed was the result of secondary displacement of 2-aminopropanoic acid from the chlorpyrifos–cysteine conjugate that was derived from the chlorpyrifos-glutathione conjugate.
Human Esterases. A key feature of the biological properties of OP pesticides is their ability to interact with a variety of esterases. In addition to AChE these include neuropathy target esterase (NTE), BuChE, A-esterases, and other B-esterases. Two classes of esterase are involved in the metabolism of chlorpyrifos, carboxylesterases, and PON-1.
Carboxylesterases. Carboxylesterases (CEs), like AChE, are members of the α,β-serine hydrolase multigene family (CitationCygler et al., 1993). These enzymes have in common serine, histidine, and glutamic acid at their active site and are capable of hydrolyzing a wide range of substrates, including esters, amides, and thioesters.
Carboxylesterases are glycoproteins, and may be classified into four groups based on phylogenetic analysis: CES1, CES2, CES3, and CES4 (CitationSatoh and Hosokawa, 1998). The two major forms of carboxylesterase in humans are hCE1 and hCE2; these belong to classes CES1 and CES2, respectively (CitationSatoh et al., 2002). Using Northern blots hCE-1 gives a single band (2.1), whereas hCE-2 is comprised of three bands (2, 3, 4.1). Examining different tissues, the levels of hCE-1 expression, as indicated by the intensities of the hCE-1 bands, were in the order liver > > heart > stomach > testes = kidney = spleen > colon > other tissues. In the case of hCE-2 the bands showed a different distribution in different tissues. The intensity of the band at 2 kb was in the order: liver > colon > small intestine > heart. For the band at 3 kb the order was: liver > small intestine > colon > heart; the 4.2-kb band was found only in the brain, testis, and kidney. hCE-2 was not found in other tissues (CitationSatoh et al., 2002). In human liver, hCE1 and hCE2 are primarily located in the endoplasmic reticulum, although lower amounts may be found in the cytosol.
In humans, carboxylesterases are not found in the serum (CitationLi et al., 2005). The two main human carboxylesterases have two principal binding sites that may be described as the “alcohol” and “acyl” or acid sites. These largely dictate the substrate specificity of the two enzymes.
Much less is known about the properties of the minor carboxylesterases (CitationRoss and Crow, 2007). A cytosolic esterase is responsible for the metabolism of S-formylglutathione (CitationRoss and Crow, 2007).
The activities of hCE-1 and hCE-2 appear to be rather constant in human liver from the age of two months to maturity (CitationPope et al., 2005). It is less clear whether these enzymes preserve their activity in old age. Interindividual differences appear to be small (CitationRoss and Crow, 2007).
Comparison of Rodent and Human Carboxylesterases. The distribution of carboxylesterases in animals differs considerably from humans. In rats and mice the plasma contains high levels of carboxylesterases. Female rats have more plasma carboxylesterase activity than males (CitationChanda et al., 1997). There are multiple forms of carboxylesterases in rodent liver (CitationCrow et al., 2007) that vary in their substrate specificity (CitationChanda et al., 1997). In animals carboxylesterase activity is considerably lower in weanling animals than in adults. Indeed, the considerable difference in response to chlorpyrifos with age in rodents appears to be correlated with carboxylesterase activity (CitationKaranth and Pope, 2000).
Carboxylesterases and Chlorpyrifos Toxicity. Chlorpyrifos-oxon binds to and irreversibly inhibits major carboxylesterases. Because these carboxylesterases occur in a number of tissues at much higher concentrations than AChE, it has been proposed that they act as a “metabolite sink” (“metabolic scavengers”) for chlorpyrifos-oxon, thereby reducing its toxic impact (CitationChanda et al., 1997). However, plasma BuChE, a primary target for chlorpyrifos-oxon binding, has been shown to have important physiological functions, at least in the absence of AChE, since AChE knockout mice with normal BuChE activity were highly sensitive to chlorpyrifos-oxon, and BuChE knockout mice were highly sensitive to the cholinergic effects of cholinesterase inhibitors used in the treatment of Alzheimer's disease (CitationDuysen et al., 2007). Other proteins such as albumin will also act in this way; however, the affinity of the active site of carboxylesterases is of a similar order to AChE, whereas for other “sinks” the affinity is lower.
In mice, repeated dosing with chlorpyrifos resulted in a small reduction in total carboxylesterase activity. The reduction was only statistically significant at 13 days (CitationCometa et al., 2007). Whether this finding should be interpreted as indicating enhanced synthesis of the carboxylesterases that were primarily inhibited (e.g., turnover rate of specific inhibited carboxylesterases was enhanced) or the synthesis of other carboxylesterases is uncertain.
The physiological role(s) of carboxylesterases remains to be fully established. Recent research indicates that they may play a part in lipid metabolism (CitationRoss and Crow, 2007). Thus, the biological consequences of the irreversible inhibition of one or more of these enzymes by OP pesticides has yet to be determined.
II.C.5. Paraoxonase (PON1)
PON1 (EC 3.1.8.1) is a member of a family of proteins that also includes PON2 and PON3. PON1 hydrolyzes paraoxon, the substrate that provides its name, as well as the active metabolites of several other OP insecticides, including chlorpyrifos oxon. Two polymorphisms were observed in the PON1 coding sequence: a Gln(Q)/Arg(R) substitution at position 192, and a Leu(L)/Met(M) substitution at position 55 (CitationHumbert et al., 1993; CitationAdkins et al., 1993). The polymorphism at position 192 has been the most studied, with gene frequencies of PON1Q192 ranging from 0.75 for Caucasians of Northern European origin, to 0.31 for some Asian populations (CitationBrophy et al., 2002). The Q/R polymorphism at position 192 significantly affects the catalytic efficiency of PON1. Initial studies indicated that the PON1R192 allozyme hydrolyzed paraoxon more readily than PON1Q192 (CitationHumbert et al., 1993; CitationAdkins et al., 1993). Further studies indicated that this polymorphism was substrate dependent, as the PON1Q192alloform was found to hydrolyze the nerve agents sarin and soman more rapidly than PON1R192 in vitro (CitationDavies et al., 1996). Under physiological conditions both PON1 alloforms hydrolyze diazoxon with the same efficiency (CitationLi et al., 2000). As with paraoxon, the PON1R192 allozyme hydrolyzes chlorpyrifos oxon more readily than PON1Q192 (CitationLi et al., 2000). The PON1 status of an individual encompasses the two factors that affect PON1levels or activity, the Q192R polymorphism and plasma alloform levels (CitationRichter and Furlong, 1999). Among several other polymorphisms in the noncoding region a significant one is that at position –108, with the –108C allele providing levels of PON1 about twice as high as those seen with the –108T allele (CitationLeviev and James, 2000; CitationSuehiro et al., 2000; CitationBrophy et al., 2001).
Although the ability of PON1 to hydrolyze a number of OP substrates in vitro has been established for some time, evidence that the enzyme plays a role in modulating the toxicity of the same OPs in vivo has emerged more slowly. Administration (via the tail vein) of the enzyme (purified from rabbit serum) to rats increased serum PON1 activity toward chlorpyrifos oxon by 50-fold (CitationCosta et al., 1990). Thirty minutes after PON1 injection, rats were challenged with an acute dose of chlorpyrifos oxon. Four hours later, at sacrifice, AChE activity measurements indicated a much lower degree of inhibition in animals that had been pretreated with PON1. Additional experiments in mice showed that iv administration of pure rabbit PON1 increased serum chlorpyrifos oxonase activity by 30-to 40-fold, and protected animals toward AChE inhibition by dermally applied chlorpyrifos oxon (CitationLi et al., 1993). Rabbit PON1 also provided some degree of protection against the toxicity of the parent compound chlorpyrifos (CitationLi et al., 1993, Citation1995). Further experiments showed that PON1, when given 30 min after dermal administration of chlorpyrifos, prevented the reduction of AChE activity in all tissues (CitationLi et al., 1995). PON1 knockout and transgenic animals have provided important new tools to investigate the role of PON1 in modulating OP toxicity. Plasma and liver from PON1 knockout mice have no detectable hydrolytic activity toward paraoxon and diazoxon, and very limited chlorpyrifos-oxonase activity (CitationLi et al., 2000). As predicted, PON1 knockout mice showed a dramatically increased sensitivity to chlorpyrifos oxon, and a slightly increased sensitivity to the toxicity of chlorpyrifos (Shih et al., 1988). Administration of human PON1R192 provided significantly better protection than PON1Q192 toward chlorpyrifos oxon, a finding confirmed by a subsequent study by CitationCowan et al. (2001), who administered recombinant adenoviruses containing PON1-LQ or PON1-LR genes to BALB/c mice before challenge with chlorpyrifos oxon. Results from kinetic analysis of substrate hydrolysis by purified human alloforms indicated that in the case of chlorpyrifos oxon, the catalytic efficiency of both PON1 alloforms was very high, and was higher for the PON1R192alloform (CitationLi et al., 2000). Additional experiments were carried out in PON1 transgenic mice (mice expressing either human PONQ192 or human PON1R192 on a knockout background). As predicted, hPON1R192-TG mice (expressing human PON1R192 on a knockout background) were significantly less sensitive to the toxicity of chlorpyrifos oxon than hPON1Q192-TG mice, despite having the same level of PON1 protein in liver and plasma (CitationCole et al., 2005).
Direct confirmation in humans of the relevance of PON1 status in determining relative sensitivity to OP toxicity is still elusive. In recent years, some studies have started to investigate the potential role of PON1 in modulating the toxicity of OPs in humans. Such studies have dealt primarily with the nerve agent sarin, in relationship to exposure in the terrorist attacks in Japan (CitationYamada et al., 2001) or the Gulf War Syndrome (Haley et al., 2000; CitationMackness et al., 2000), and diazinon, in relationship to the use of this OP in sheep dipping (CitationCherry et al., 2002; CitationMackness et al., 2003). These studies are discussed in detail elsewhere (CitationCosta et al., 2003). None of these studies examined PON1 in relationship to exposure to chlorpyrifos (but see studies on PON1 and TCPy, Mount Sinai cohort; CitationBerkowitz et al., 2003, Citation2004).
Age is a major determinant of PON1 activity (CitationCosta et al., 2005). Studies in rodents have shown that serum and liver PON1 activity is very low at birth, and increases up to postnatal day 21, with a parallel increase in liver mRNA (CitationMortensen et al., 1996a; CitationLi et al., 1997; CitationMoser et al. 1998; CitationKaranth and Pope, 2000), and similar increases were also seen in transgenic mice expressing either the human PON1R192 or the PON1Q192 (CitationCole et al., 2003c). Studies in humans have also shown that serum PON1 activity is very low at birth and increases over time, reaching a plateau between 6 and 15 months of age (CitationAugustinsson and Barr, 1963; CitationEcobichon and Stephens, 1973; CitationMueller et al., 1983; CitationCole et al., 2003a: Chen et al., 2003). Low PON1 activity during development could represent a relevant risk factor for increased susceptibility to the acute toxicity of certain OP insecticides. OP toxicity is influenced by age, with young animals being more sensitive than adults to the effects of acute exposure (CitationHarbison, 1975; CitationPope and Liu, 1997; CitationMoser et al., 1998). Studies with chlorpyrifos have indicated that a lower hydrolytic detoxication by PON1 accounts for the differential age-related sensitivity in acute toxicity (CitationMortensen et al., 1996a; CitationMoser et al., 1998; CitationPadilla et al., 2000). The finding of low PON1 activity in neonates (CitationCole et al., 2003a) suggested that PON1 levels may be even lower before birth, as indeed indicated by data showing a 24% lower activity in premature babies using phenylacetate as a substrate (33–36 weeks of gestation) compared to term babies (CitationEcobichon and Stephens, 1973). In addition, an expectant mother with low PON1 status would be predicted not to be able to provide protection for her fetus against exposure to some OPs (Cole et al., 2003). In a recent study, PON1 status was established for 130 Latina women and their newborns (CitationFurlong et al. 2006). Among newborns, levels of PON1 (measured as arylesterase activity) varied by 26-fold (4.3–110.7 U/ml), and among mothers by 14-fold (19.8–281.4 U/ml). On average, newborns' PON1 levels were fourfold lower than the mothers' PON1 levels. Average PON1 levels in newborns were comparable with hPON1 levels in transgenic mice expressing PON1Q192 or PON1R192, allowing for prediction of relative sensitivity to chlorpyrifos oxon; the predicted variability in sensitivity between newborns and mothers was 131-to 164-fold (CitationFurlong et al., 2006). These findings suggest that most newborns and many of the mothers in this cohort may be more susceptible to the acute adverse acute effects of certain OPs due to their PON1 status.
The studies summarized in the previous sections indicate that PON1 status is an important determinant in modulating the acute toxicity of chlorpyrifos. All experiments (and extrapolations from such experiments; see CitationFurlong et al., 2006) were carried out utilizing relatively high doses of chlorpyrifos oxon or chlorpyrifos. An issue that needs to be addressed is whether PON1 status may play a role in chlorpyrifos metabolism and toxicity at lower, environmentally relevant, dose levels. CitationTimchalk et al. (2002a) carried out a Monte Carlo analysis of the human PON1192 polymorphism, using a physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model. The single doses of chlorpyrifos utilized in this model were 0.005, 0.05, 0.5, and 5.0 mg/kg. These doses range from those above an effect on plasma cholinesterase inhibition to below the reference dose for acute exposure. The theoretical brain chlorpyrifos oxon concentrations were calculated for the QQ, QR, and RR PON1 polymorphisms and are shown in .
TABLE 3 Theoretical human brain chlorpyrifos-oxon levels utilizing Monte Carlo simulation for the QQ, QR, and RR PON1 polymorphism following a single exposure to different doses of chlorpyrifos
As expected, brain concentrations of chlorpyrifos oxon increase with increasing doses of chlorpyrifos. The results also indicate that differences in PON1 activity have a significant impact on chlorpyrifos oxon brain dosimetry at higher doses. In contrast, no effect is seen at the low dose of 0.005 mg/kg (5 μ g/kg). To further evaluate the potential implications of the PON1 Q192R polymorphism, the impact of repeated dietary exposures was also simulated for the QQ genotype (low chlorpyrifos metabolizers) (CitationTimchalk et al., 2002a). The ratios of theoretical peak brain chlorpyrifos oxon concentrations between single exposure and repeated (30 days) exposure were: 1.16 (0.005 mg/kg), 2.27 (0.05 mg/kg), 1.56 (0.5 mg/kg), and 8.37 (5.0 mg/kg). These data indicate that at the low dose of chlorpyrifos (5 μ g/kg), in the “worst case” scenario (QQ), concentrations of chlorpyrifos oxon are predicted to increase only minimally upon repeated exposure as compared to a single exposure.
Altogether, these findings suggest that at low doses of chlorpyrifos, PON1 192 polymorphism does not appear to play a significant role in modulating chlorpyrifos metabolism, and that other detoxication pathways would be capable of compensating for the interindividual differences in PON1 activity due to the PON1 Q192R polymorphism. These conclusions were also echoed by CitationCole et al. (2005). It should be noted that the low dose of CPF used in this Monte Carlo simulation (0.005 mg/kg) is slightly above the RfD for CPF (0.003 mg/kg/day).
II.C.6. Balance of Activation and Detoxication Pathways
Inhibition of cholinesterases, and hence toxicity of chlorpyrifos, ultimately results from the balance of activation and detoxication pathways. Bioactivation is entirely dependent on oxidative desulfuration of chlorpyrifos to chlorpyrifos oxon by various CYPs; in contrast, detoxication of the oxon occurs through multiple pathways, including various CYPs, PON1, and carboxylesterases. CitationSultatos et al. (1984) showed that the ability of rat hepatic microsomes to detoxify chlorpyrifos oxon (dearylation) exceeded their capacity to generate this metabolite from chlorpyrifos (desulfuration) by a factor of 7.6. Similarly, in a study by CitationTang et al. (2001), rat, mouse, and human microsomes were found to produce TCPy more readily from chlorpyrifos oxon than from chlorpyrifos. Several CYPs are involved in both desulfuration and dearylation of chlorpyrifos and its oxon, respectively. Most CYPs have higher dearylation activity, with the exception of CYP1A2 and CYP2B6 (CitationTang et al., 2001). Investigations of human liver microsomes of an individual with high CYP2B6 levels confirmed a slightly higher desulfuration activity (ratio desulfuration/dearylation = 1.10). However, in four other individuals (one with high CYP2B6 levels) the same ratios ranged from 0.26 to 0.74, indicating a preponderance of dearylation over desulfuration (CitationTang et al., 2001). Polymorphisms of CYPs influence their catalytic activity toward chlorpyrifos and chlorpyrifos oxon. For example, CYP2C19 has a high dearylation activity (ratio desulfuration/dearylation = 0.11). Variants of CYP2C19 have significantly decreased (–70 to –90%) dearylation activity, but no detectable desulfuration activity (CitationTang et al., 2001). Variants of CYP3A4 have either higher or lower metabolic activities than the wild-type enzyme, but in all cases detoxication (dearylation) is higher than activation (desulfuration) (CitationDai et al., 2001). Thus, an analysis of CYP-mediated metabolism would suggest that the balance of activation to detoxication favors the latter. As noted previously, both PON1 and carboxylesterases represent major pathways of chlorpyrifos oxon detoxication (CitationFurlong, 2007). These would shift the balance toward detoxication even further. Studies in rats (CitationSultatos et al., 1984) and humans (CitationChoi et al., 2006) have indeed indicated that chlorpyrifos oxon does not escape the liver, confirming that the active metabolite, once formed, is readily detoxified. Extrahepatic sites of activation and detoxication (with the exception of plasma PON1 and carboxylesterases) have not been studied to a great extent, and should be further investigated.
II.C.7. Role of Cholinesterases in Chlorpyrifos Toxicity
Acetylcholinesterase (AChE), the key enzyme in terminating the neurotransmitter function of acetylcholine at central and peripheral synapses and at neuromuscular junctions, is generally recognized to be the primary target associated with acute neurotoxicity of organophosphates, including chlorpyrifos. It has been thought for many years that cholinesterases on the surface of erythrocytes and other cholinesterases in plasma (notably BuChE) serve as a sink for organophosphorus compounds and thereby reduce the amount of the neurotoxic agent that can reach the nervous system (CitationMisulis et al., 1993). The total activities of AChE in human plasma and cerebrospinal fluid (CSF) are similar, but activity of BuChE in plasma is 120-to 500-fold higher than that in CSF (CitationAtack et al., 1987). Recent experimental studies provide empirical support for the concept that blood cholinesterase activity, particularly plasma BuChE, is a critically important line of defense that serves to protect the nervous system from organophosphorus agents. Rats injected intraperitoneally with equine serum BuChE show dose-dependent increases in plasma BuChE activity that reduce the degree of erythrocyte and brain AChE inhibition induced by inhalation of sublethal doses of sarin, an organophosphorus compound of high acute neurotoxic potency (CitationBajgar et al., 2007). Factors that influence cholinesterase activity in blood and other peripheral tissues—outlined in the following sections—are therefore important variables in determining how well the nervous system is protected against chlorpyrifos-oxon.
Plasma Cholinesterase (E.C. 3.1.1.8, Pseudocholinesterase; Butyrylcholinesterase, BuChE). This glycoprotein is present in all human and mouse tissues, and is more abundant than AChE in all tissues except brain. Blood BuChE, which comprises most of the blood cholinesterase activity, is synthesized in the liver and has a replacement time in humans of ∼ 50 days (CitationSidell, 1992). Levels at birth are one-fourth that in adults, but these levels increase rapidly reaching adult levels by the second month of life. In adults, enzyme activity is reported to show large temporal variations over the course a year, is higher in men than in women, and is lower in women taking oral contraceptives and in individuals with liver disease (30–50% in acute and longstanding chronic hepatitis) (CitationWhittaker et al., 1971; CitationSidell and Kaminskis, 1975; CitationHayes, 1982; CitationSidell, 1992; CitationKisicki, 1999). Decreases of 50–70% occur in advanced cirrhosis and carcinoma with metastasis to the liver (CitationKisicki, 1999). Essentially normal levels are seen in chronic hepatitis, mild cirrhosis, and obstructive jaundice (CitationKisicki, 1999). Decreased levels of the serum enzyme are also found in patients with acute infections, pulmonary embolism, and muscular dystrophy, and after surgical procedures. After myocardial infarction, the enzyme level decreases until the fifth day and then begins a slow rise to normal. Decreased levels are also seen in chronic renal disease and in pregnancy (CitationKisicki, 1999). A marginal increase in enzyme levels may be observed in patients with nephritic syndrome. Marginal increases are also seen in thyrotoxicosis and hemochromatosis, in the obese diabetic state, and in patients with anxiety and other psychiatric states (CitationKisicki et al., 1999).
Some 65 variants of plasma BuChE are described (CitationChatonnet and Lockridge, 1989; CitationMikami et al., 2008), one of which is a point mutation at the anionic active site that changes aspartate to glycine (CitationMcGuire et al., 1989). While homozygotes for this genetic polymorphism are rare (1:2500), heterozygotes make up 4–5% of the general population (CitationWhittaker, 1986). Subjects with BuChE polymorphism may have reduced binding capacity for, and increased susceptibility to, anticholinesterase agents (such as succinylcholine). Pyridostigmine bromide, a carbamate anticholinesterase drug, triggered a cholinergic crisis in an Israeli soldier found to be homozygous for the aforementioned BuChE genotype (CitationLoewenstein-Lichtenstein et al., 1995).
Recent studies with BuChE knock-out mice suggest the enzyme may have a physiological function in hydrolyzing excess neurotransmitter acetylcholine in tissues such as diaphragm, cardiac muscle, and brain, which leads to the conclusion that BuChE plays a role in neurotransmission (CitationDuysen et al., 2007). In this study, the presence of a functional gene for BuChE reduced the extent of inhibition of AChE in plasma and lessened the extent of cholinergic signs in mice treated with cholinesterase inhibitors, including chlorpyrifos-oxon. Whether the apparent protection against chlorpyrifos-oxon inhibition afforded by a functional BuChE enzyme is due to scavenging of chlorpyrifos-oxon in the plasma or because of functional catalytic activity of BuChE in plasma and/or target tissues where AChE activity is low, is uncertain. However, since BuChE protected against Huperzine A-induced cholinergic toxicity, it is clear that BuChE can functionally substitute for brain AChE enzyme activity, since Huperzine A does not bind to and inhibit BuChE. In contrast, chlorpyrifos-oxon is a relatively more effective inhibitor of BuChE than AChE and thus plasma binding and/or catalytic activity toward chlorpyrifos-oxon could contribute to the protection of AChE afforded by BuChE. Additionally, AChE(−/−) mice treated with chlorpyrifos-oxon lost all BuChE activity, showed severe cholinergic signs and died of convulsions. This indicates that BuChE is essential for survival in the absence of AChE (CitationDuysen et al., 2007). Patients with Alzheimer's disease improve cognitively with central inhibition of cholinesterase function, and studies of this phenomenon support a role for central BuChE in addition to AChE inhibition in modulating cholinergic function in this disease (CitationGiacobini et al., 2002 (see also VI.c, pp. 96–99)).
Acetylcholinesterase (E.C. 3.1.1.7; AChE). There may be some temporal variation in erythrocyte AChE; enzyme activity over the course of a year varied by 11% in male and 16% in female subjects (CitationSidell and Kaminskis, 1975). AChE activity is depressed in certain hematological disorders, such as pernicious anemia (CitationHayes, 1982). This may be of particular relevance to low-income populations exposed to OPs. The smallest measurable decrease in erythrocyte AChE with one pre-exposure measure is estimated to be ∼ 15% (CitationCallaway et al., 1951). Recovery of enzyme activity depends on replacement of blood cells (approximately 1% per day in humans (CitationSidell, 1992). While blood AChE estimation provides a valuable diagnostic tool, there may be a poor correlation between enzyme activity and clinical manifestations of acute cholinergic neurotoxicity (CitationHolmstedt, 1959; CitationYokoyama et al., 1996). There is some evidence that prolonged low-level exposure to organophosphorus pesticides may result in tolerance to their potential adverse effects, and individuals with markedly depressed erythrocyte AChE activity may lack signs or symptoms of toxicity (CitationSumerford et al., 1953; CitationBarnes, 1954; CitationDulaney et al., 1985; CitationHoskins and Ho, 1992). There is no evidence of tolerance to CNS AChE in rodents receiving repeated systemic treatment with the potent organophosphorus compound soman (CitationHartgraves and Murphy, 1992).
II.D. Excretion of Chlorpyrifos
II.D.1. Urinary Excretion
Studies in Experimental Animals. Following oral exposure, measurable levels of chlorpyrifos metabolites can be found in the urine of rats at 24 h. At 48 h DEP and TCPy are still detected. In the urine the maximum excretion was around 12 h but significant excretion occurred even at 72 h (CitationTimchalk et al., 2007). The major urinary metabolites were TCPy (62%) and diethylthiophosphate (40%); diethylphosphate was a rather minor metabolite (4%). Chlorpyrifos could not be detected in the urine. In view of its lipophilicity and rapid metabolism this is not surprising.
Studies in Humans. As described in Section II.C, numerous chlorpyrifos metabolites have been identified in urine in a subject who ingested a near lethal dose of chlorpyrifos, including numerous glutathione-derived metabolites (CitationBicker et al., 2005a). CitationVasilic et al. (1992) investigated the urinary excretion of diethylphosphate and diethylthiophosphate in three individuals who ingested toxic doses of chlorpyrifos and were treated with oximes. No parent compound was detected in the urine. It was not determined whether the treatment of these patients with oximes affected the metabolite profile for chlorpyrifos. Other authors have also reported that unchanged chlorpyrifos is not excreted in the urine in humans (CitationNolan et al., 1984), while urinary elimination of TCPy is the major pathway for excretion of chlorpyrifos metabolites. Section V provides an in-depth discussion of human studies that have utilized urinary elimination of the chlorpyrifos metabolite TCPy as a biomarker for chlorpyrifos exposure.
II.D.2. Biliary and/or Fecal Elimination
Studies in Experimental Animals. Based on molecular weight considerations and findings for comparable compounds, the glutathione and glucuronic acid conjugates identified earlier would be expected to be excreted to a significant extent in the bile in rats (CitationHirom et al., 1972). In the rat intestine microbial action is likely to cleave the conjugates (CitationWilliams et al., 1965). In the case of the glutathione conjugate this would result in a cysteine conjugate. Hydrolysis of the glucuronides causes regeneration of the aglycones. It is uncertain whether enterohepatic recirculation of one or more of these cleavage products occurs. This is relevant because in the case of the aglycones it could result in a delay in their final clearance from the body. For the cysteine conjugate further metabolism is likely that, as discussed earlier, could result in an active metabolite.
Studies in Humans. There are no studies addressing biliary and/or fecal elimination of chlorpyrifos in humans.
II.D.3. Excretion in Milk
Studies in Experimental Animals. Chlorpyrifos levels in milk of lactating rats were measured following the oral administration of 0.3, 1.0, or 5.0 mg of chlorpyrifos (CitationMattsson et al., 2000). Levels in milk were up to 200 times those found in blood during the perinatal period. At the highest oral dose the chlorpyrifos concentration in the milk was ∼ 3 μ g/g. In terms of dose to the neonate (assuming a 25-g neonate consumes 1 ml milk per day) this concentration would result in a daily intake of chlorpyrifos of around 0.12 mg/kg body weight. The excreted concentration was not linear with dose; at the lowest dose a concentration of approximately 20 ng/g of milk was detected. Following the perinatal period, chlorpyrifos levels were not detectable in most milk samples.
Studies in Humans. CitationAnderson and Wolff (2000) assumed that chlorpyrifos residues would be found because it is a relatively lipophilic substance and the metabolite TCPy is commonly found in urine, although they did not identify any studies that assessed the levels of chlorpyrifos or its metabolites in breast milk. It might be expected that the levels would relate to the fat content of the breast milk. CitationSanghi et al. (2003) reportedly identified measurable levels of chlorpyrifos in breast milk from nursing mothers in India. Among other pesticides, chlorpyrifos was detected in milk samples of all 12 women studied: values (mean ± SE) were: 230 ± 24 μ g/L milk (range: 8.5–355 μg/L). For these women it was calculated that a newborn (2.8 kg, consuming 500 ml milk/day) could be exposed to an average of 40 μ g/kg chlorpyrifos (range 2–60 μ g chlorpyrifos/kg). Relative to other sources of exposure, this would be a remarkably high projected dose of chlorpyrifos for a nursing infant, and requires further study to verify this initial observation and determine whether chlorpyrifos appears commonly in breast milk.
A number of toxic metals and pesticides, including chlorpyrifos, were reportedly identified in the meconium fluid of newborn infants (collected for the first 2 days following birth) from nursing mothers in the Philippines (CitationOstrea et al., 2002). Chlorpyrifos was reportedly identified in 11% of 426 samples, with a median value of 8.26 μ g/ml. However, this is an extraordinarily high concentration and seems unlikely without some known source of direct exposure. A recent study by this same group (CitationOstrea et al., 2008) reanalyzed these same samples and an additional 212 samples, and did not find chlorpyrifos above the detection limit in any of 638 samples of meconium. Thus, the first report appears to have been in error.
II.E. Comparison of Kinetics of Chlorpyrifos in Animals and Humans
No clear qualitative species differences in the blood or urinary profiles of chlorpyrifos and its metabolites have been identified. However there are significant quantitative differences across species in the relative activities of a number of the enzymes involved, which is reflected in the ratio of metabolites between species and with age. It is evident that all species are able to form the oxon responsible for AChE inhibition.
Measurement of Blood Concentrations of Chlorpyrifos in Humans. Only two studies have attempted to measure chlorpyrifos directly in blood following controlled exposures to humans (CitationNolan et al., 1984; CitationTimchalk et al., 2002b). In the CitationNolan et al. (1984) study, 6 human volunteers were given 0.5 mg/kg of chlorpyrifos orally, and blood and urine samples were collected over a period of 4 days to obtain pharmacokinetic data. The authors noted that chlorpyrifos concentrations in blood were “very low” (< 30 ng/g, or 30,000 pg/g), although the limit of detection (∼ 30 ppb) was quite high, relative to today's methods. However, plasma TCPy was readily measured, and peaked at 6 h following the oral dose, indicating relatively rapid metabolism of chlorpyrifos to TCPy. Peak plasma concentrations of TCPy at 6 h were approximately 1 μ g/g (range, 0.51–1.35 μ g/g). Converting to molar equivalents of chlorpyrifos, this would be equal to a blood chlorpyrifos concentration of approximately 1,800 ng/g. Based on these values, the molar ratio of peak TCPy to peak chlorpyrifos concentrations in blood in this study must have been greater than 60 (1800/30), indicating the relatively rapid conversion of chlorpyrifos to TCPy. In the second controlled human exposure that measured chlorpyrifos in blood, CitationTimchalk et al. (2002b) administered single oral doses of chlorpyrifos of 0, 0.5, 1, or 2 mg/kg to 6 male and 6 female volunteers. Blood was collected at 2, 4, 8, 12, 24, 36, 48, 72, 96, 122, and 168 h and analyzed for chlorpyrifos and TCPy. Only 5 of the 12 individuals had measurable chlorpyrifos concentrations in blood, but again, the limit of detection, although much better than that of CitationNolan et al. (1984) was quite high (3 mmol/L, which is equivalent to ∼ 1 ng/ml, or 1 ppb) and not adequate to measure CPF in the blood for more than 2 h following the highest dose. The results of the five subjects with measurable levels were presented graphically in the paper using log dose versus time plots. Although exact concentrations are difficult to discern from the plots, in 4 of the 5 subjects, values determined in the initial samples (2, 4, 8 h) were approximately 0.01–0.02 μ mol/L, which is equal to 3500–7000 pg/g of blood. TCPy was also measured in blood, and appeared rapidly at concentrations in excess of 100-fold greater than chlorpyrifos even at the earliest time points, indicating very rapid metabolism of chlorpyrifos to TCPy in these subjects, consistent with that reported by CitationNolan et al. (1984). In contrast to the rapid elimination of chlorpyrifos from blood, the half-life of TCPy appears to be on the order of 27 h. Thus, based on the CitationTimchalk et al. (2002b) and CitationNolan et al. (1984) studies, it appears that the initial half-life of chlorpyrifos is quite short—probably only an hour or less—but the half-life of TCPy is much longer, on the order of 24–27 h.
More recently, a cohort study conducted at Columbia University evaluated the concentrations of chlorpyrifos in maternal and cord blood of women living in an “inner-city” area of New York City (the Columbia cohort, discussed in detail in Sections IV and VI). In the development of the assay used in the Columbia cohort studies, CitationBarr et al. (2002) developed a new and very sensitive assay, with a detection limit ∼ 1000 times more sensitive than that used by CitationTimchalk et al. (2002b), and used this to analyze a pooled sample of 9 different blood (serum) specimens obtained from the Cincinnati Red Cross. They reported a “background” concentration of chlorpyrifos of 9 pg/g of serum. Although the “steady-state” dose of chlorpyrifos received by the women and newborn infants in the Columbia cohort is uncertain, the levels measured in maternal and cord blood averaged approximately 4 pg/ml, or 0.02 nM (CitationWhyatt et al., 2003b), a concentration approximately 1000-fold lower than the blood concentrations (∼ 3500 pg/g) achieved from a single oral dose of 1 mg/kg in humans (CitationTimchalk et al., 2002b). To put this blood concentration in perspective with that of other neurotoxic substances, the molar concentration of lead in blood at the current CDC action level of 10 μ g/dl is 480 nM, or approximately 22,000 times greater than the average molar concentration of chlorpyrifos measured in maternal and cord blood in the Columbia cohort. This calculation illustrates the remarkable sensitivity of the assay used to measure chlorpyrifos in blood. It is likely that the concentrations of chlorpyrifos measured in the blood of the Columbia cohort women and infants was at a pseudo steady state, following daily inhalation and dietary exposures to trace levels of chlorpyrifos in air and food. Exactly what intake rate would be necessary to achieve a steady-state concentration of ∼ 4 pg/g is uncertain, although some estimation can be made with several assumptions regarding the toxicokinetics of chlorpyrifos. As noted earlier, the initial rate of clearance of chlorpyrifos from the blood appears quite rapid, with an initial elimination half-life of perhaps an hour or less. However, the distribution and elimination of chlorpyrifos in the body follow a two-compartment model, such that there is a much slower elimination of a portion of chlorpyrifos from the body because it is both partitioned into body fat, and is tightly bound to plasma proteins. If this were not the case, then the exposure of women and their unborn child to chlorpyrifos in the home would have had to have been quite high, approaching acutely toxic levels, since the blood samples from which chlorpyrifos was measured were collected for at least 10–12 h after leaving the homes (with a half-life of 1 h or less, 99.9% would have been eliminated after 10 h). Alternatively, the chlorpyrifos measured in the blood samples in the Columbia cohort could have reflected exposures that occurred in the hospital hours prior to delivery. Given the rather universal presence of trace levels of chlorpyrifos in the blood of both the Columbia cohort women and infants, and the presence of chlorpyrifos in blood bank samples (CitationBarr et al., 2002) that were approximately twice the average levels found in the Columbia cohort, it seems more likely that the low (parts per trillion) concentrations of chlorpyrifos in blood represent a “background,” steady-state level due to partitioning of chlorpyrifos in body fat that is then more slowly eliminated from the body. What the “terminal” half-life, β, is for chlorpyrifos in humans has not been determined. However, Abdel-CitationRahman et al. (2002) characterized the beta elimination rate of chlorpyrifos from blood in pregnant rats that received a single intravenous dose of 5 mg/kg chlorpyrifos. The terminal plasma disappearance half-life for chlorpyrifos and TCPy was 18 and 16 h, respectively. Thus, if one were to assume that the ratio of terminal elimination half-lives of chlorpyrifos to TCPy was similar between rats and humans, a terminal plasma elimination half-life in humans would be approximately 30 h, since the terminal TCPy elimination half-life in humans was estimated to be 27 h (CitationTimchalk et al., 2002b).
No clear qualitative differences between species in the blood or urinary profiles of chlorpyrifos have been identified. However, there are significant quantitative differences in the relative activities of a number of the enzymes involved, which is reflected in the ratio of metabolites between species and with age. It is evident that all species are able to form chlorpyrifos-oxon which is responsible for AChE inhibition. There is no reason to anticipate qualitative differences in the formation of other putative active metabolites discussed earlier.
II.F. Summary of Conclusions Regarding ADME and PK of Chlorpyrifos in Animals and Humans
Chlorpyrifos is well absorbed from the intestine and from the lung in both humans and experimental animals. Very limited uptake (less than 5%) occurs after dermal exposure.
Chlorpyrifos is rapidly metabolized. The principal metabolites are: TCPy and its conjugates, DEP, DETP and chlorpyrifos-oxon. In addition, glutathione conjugates and subsequent metabolites have been identified. There are interindividual differences in the metabolism and toxicokinetics of chlorpyrifos.
Some of these metabolites have either been shown to interact with biological components or may be anticipated to do so. Chlorpyrifos-oxon is considered to be the sole active metabolite responsible for the inhibition of AChE. Other possible reactive metabolites, potentially with a different mode of action, include reactive sulfur, a product of 5-dechlorination, and one or more additional metabolites of the glutathione conjugate. In this regard it is noted that chlorpyrifos promotes lipid peroxidation in some in vitro systems. Several of these putative active metabolites are likely to be formed in the liver, the brain, and the developing fetus. Unfortunately, the published literature does not enable a conclusion to be reached on the dose of chlorpyrifos required to generate significant levels of each of these metabolites, the potential they have to translocate between tissues, and their relevance to adverse effects in humans.
The overall balance of the enzyme activities in the liver, brain, and developing fetus of laboratory animals favors detoxication, although there are substantial differences in the activities of individual enzymes between species and with age and gender.
Chlorpyrifos and chlorpyrifos-oxon are only detectable in blood and the liver following oral dosing of animals with chlorpyrifos. The concentration of chlorpyrifos in lipid-rich tissues, including the brain, is higher at steady state than the concentration in blood. Initial clearance of chlorpyrifos from the blood occurs very rapidly, whereas total body clearance appears to occur more slowly. It appears that there is a much slower elimination from a “second compartment,” which is likely due to extensive binding to plasma proteins and to distribution of chlorpyrifos in poorly perfused adipose tissue.
Because of the apparent partitioning of a small fraction of a dose of chlorpyrifos to lipids, chlorpyrifos would be expected to occur in breast milk at concentrations higher than plasma. One study in India indicated a relatively high level of chlorpyrifos in breast milk of nursing mothers, warranting further study of this potentially important route of exposure.
Chlorpyrifos is able to irreversibly inhibit certain isoforms of both cytochromes P-450 and carboxylesterases. Since these enzymes also have physiological substrates the possibility exists that their metabolism could be modified by exposure to chlorpyrifos. However, there is no good evidence of adverse consequences from this interaction.
Paraoxonase (PON1) status has been shown in animal studies to play a relevant role in the acute toxicity of chlorpyrifos oxon and, to a minor extent, of chlorpyrifos. However, its role in modulating chlorpyrifos toxicity at low levels of exposure appears to be negligible.
Blood cholinesterases, notably BuChE, serve as an important line of defense against the neurotoxic property of organophosphorus compounds by reducing the amount of circulating agent that is available to access the nervous system. This defensive role is likely to be shared with cholinesterases in other tissues, including cerebrospinal fluid.
III. TOXICITY ASSESSMENT OF CHLORPYRIFOS
III.A. Acute and Subchronic Toxicity Studies
The basic acute and subchronic toxicology of chlorpyrifos has been extensively reviewed previously (e.g., CitationATSDR, 1997; IPCS; Australian Pesticides and Veterinary Medicine Authority; URLs cited previously), and are not reviewed in detail here. As the primary focus of this review is on the potential neurodevelopmental effects of chlorpyrifos, the following section covering basic observational studies of acute and subchronic chlorpyrifos toxicity is included to provide an general overview of other toxicological aspects of chlorpyrifos, but is not intended to be a critical and exhaustive review of “nonneurological” or “nonneurodevelopmental” effects of chlorpyrifos seen in animal studies. However, in some instances, human studies for endpoints other than neurodevelopment have been evaluated, and a critical review of those studies is included in this section.
III.A.1. Acute Lethality Studies—Experimental Animals
Oral Administration. The acute oral LD50 for chlorpyrifos has been assessed in many species. A summary of the data is listed in . Death was observed in 4 out of 47 pregnant CF-1 mice treated with 25 mg/kg-day Dursban F during gestational days (GD) 6–15, while one animal also died in the 1-mg and 10-mg dose groups (CitationDeacon et al., 1980).
TABLE 4 Acute oral LD50 values in different species
Toxicity in pregnant rodents was found to be higher than in nonpregnant animals. Oral exposure to doses as high as 25 mg/kg-day to pregnant CF-1 mice on GD 6–10 significantly decreased the mean body weight gain for GD 10–15 (33.3%) and overall (14%).
Long-Evans rats treated with a single dose of 100 mg/kg experienced a 13% decrease in body weights in male rats by 24 h postdosing. Decreased body weight was not seen at doses of 50 mg/kg or less.
Intermediate-duration oral treatment with chlorpyrifos has been shown to cause death in rodents. Six out of 10Long-Evans rats treated orally with 100 mg/kg-day chlorpyrifos in corn oil for 3 days followed by 75 mg/kg-day for 1–4 weeks died (CitationChiappa et al., 1995). No treatment-related deaths were observed in a multigeneration study where both male and female rats received up to 5 mg/kg-day chlorpyrifos in feed (CitationBreslin et al., 1996), and in SD rats receiving up to 1.2 mg/kg-day for 120–135 days (CitationDow, 1983b). Similarly, exposure up to 15 mg/kg-day Dursban for 13weeks caused no death in Fisher 344 rats (CitationDow, 1983b). In a chronic-duration oral exposure study, no deaths were observed in Sherman rats or beagle dogs exposed to up to 3 mg/kg-day chlorpyrifos in feed for up to 2 years (CitationMcCollister et al., 1974).
Inhalation Exposures in Experimental Animals. The LD50 for acute inhalation exposure to chlorpyrifos has been determined for mice and rats (CitationBerteau and Deen, 1978). In mice, an LD50 of 94 mg/kg has been determined for whole-body inhalation exposure by treatment with 6700–7900 mg/m3 chlorpyrifos for varying length of time (single treatment). However, because the vapor pressure of chlorpyrifos is relatively low, it is not possible that this high of concentration of chlorpyrifos could exist in the vapor phase, and thus the relevance of this study to airborne exposure in the workplace and general environment is limited. Using a similar type of treatment, an LD50 of 78 mg/kg was determined for virgin female Sprague-Dawley rats. Mortality was observed in both male and female SD rats exposed to a lower concentration of chlorpyrifos but for longer duration, with the mortality rate being higher in male than female rats.
Short-duration inhalation exposure to commercial chlorpyrifos (Pyrenone-Dursban) was investigated in male and female SD rats. Death occurred in 80% of the males following exposure to 5300 mg/m3 for 4 h, whereas no effect was observed in animals treated with 2500 mg/m3 (CitationDow, 1983a). Both male and female rats lost weight, approximately 10%, but surviving rats consequently gained weight within normal ranges (CitationDow, 1983a). No body weight loss was observed in the 2500 mg/m3 group (CitationDow, 1984). The body weight was not affected by intermediate-duration, nose-only exposure of up to 0.295 mg/m3 chlorpyrifos 6 h/day, 5 days/week for 13 weeks. Exposure levels in this study were insufficient to inhibit erythrocyte or plasma cholinesterase activities (CitationCorley et al., 1989). No data were located for body weight effects following chronic-duration inhalation exposure to chlorpyrifos. It should be noted that the reported concentrations of aerosolized chlorpyrifos greatly exceed the concentration of volatilized chlorpyrifos that could occur, based on its vapor pressure and saturation of air. Thus, the exact dose the animals received is somewhat uncertain and the exposure must have included both liquid aerosol as well as volatilized chlorpyrifos (Dow AgroSciences, personal communication).
Acute Toxicity Following Dermal Exposure to Chlorpyrifos in Experimental Animals. Short-duration dermal exposure to chlorpyrifos was determined in Sherman rats giving an LD50 of 202 mg/kg (CitationGaines, 1969).
A study of newborn piglets showed that the toxicity following dermal exposure was dependent on the time after birth of treatment, with high mortality when given 1–3 h after birth, but did not show any effect when the piglets were treated 30 h after birth. However, the dose was not reported in this study (CitationLong et al., 1986). No data are available for the effects of prolonged dermal exposure to chlorpyrifos.
Subcutaneous administration of chlorpyrifos to pregnant rats (GD 17–20) evoked fetal body weight changes at ≥ 10 mg/kg-day, whereas maternal growth impairment was observed at ≥ 5 mg/kg-day. In the same studies, chlorpyrifos inhibited brain AChE, a marker normally less sensitive than plasma and erythrocyte AChE, at ≥ 2 mg/kg-day in the F0 dams (CitationGarcia et al., 2002)
III.A.2. Acute and Subchronic Toxicity—Organ System Toxicity (Other Than Reproductive and Nervous Systems)
Effects on Body Weight. Male and female Fisher 344 rats exposed to up to 15 mg/kg-day of a commercial formulation of chlorpyrifos (Dursban) for 13 weeks (CitationDow, 1993) did not exhibit any significant changes in body weight. Similarly, in a multigeneration study in male and female Sprague-Dawley rats, no effects were seen in the parental animals or first generation offspring exposed to up 1.2 mg/kg-day Dursban for 135 and 120 days, respectively. In nonrodent studies, a 25% decrease in body weight was observed in chickens exposed to 10 mg/kg-day for 20 days, whereas no effect was observed in beagle dogs fed up to 3 mg/kg-day for 1–2 years.
Dams treated with 1 or 10 mg/kg-day chlorpyrifos gained body weight comparable to the control group. Pregnant Fischer 344 rats exposed via gavage to 15 mg/kg-day Dursban on GD 6–15 experienced a statistically significant decrease in mean body weight gain for GD 9–12 (44%). The body weight gain of dams exposed to 0.1 and 0.3mg/kg-day chlorpyrifos was comparable to controls.
Thus, the highest dose with no evidence of adverse effects (NOAEL) on body weight was 1.2 mg/kg-day following subchronic exposure in female SD rats.
Effects on the Respiratory System. Respiratory effects of short-duration inhalation exposure to commercial chlorpyrifos (Pyrenone-Dursban) were investigated in male and female SD rats. Death occurred in 80% of the males following exposure to 5300 mg/m3for 4 h. Morphological changes were observed in the lungs of the dead animals. Fibrinous pleurisy was observed in one female rat that died 14 days posttreatment (CitationDow, 1983a). No respiratory effects were observed in animals exposed to 2500 mg/m3 (CitationDow, 1984).
The effects of intermediate-duration exposure (nose-only) were assessed in male and female Fisher 344 rats (0–0.295mg/m3) treated for 6 h/day, 5 days/week for 13 weeks. These exposure levels did not inhibit RBC AChE or plasma BuChE activity, and histopathological evaluation of the lungs revealed normal lung histology (CitationCorley et al., 1989). No histopathological lesions of the lung were noted following acute-duration exposure to 40 mg/kg in cats.
A NOAEL of 2500 mg/m3 chlorpyrifos for respiratory effects was based upon acute inhalation exposure in rats. No effects on the lung were noted following chronic-duration exposure of Sherman rats and beagle dogs to as much as 3mg/kg-day chlorpyrifos in feed (CitationMcCollister et al., 1974).
Respiratory effects were observed in piglets acutely exposed (by spraying) to an undetermined amount of chlorpyrifos at 0, 1–3, 24–30, or 36 h after birth. High mortality was observed in the early treatment groups, and dyspnea, resulting from cholinergic overstimulation, was observed in the animals that eventually died. No morphological lesions in the lungs were observed (CitationLong et al., 1986).
Cardiovascular Effects. The cardiovascular effects of short-duration inhalation exposure to commercial chlorpyrifos (Pyrenone-Dursban) were investigated in both male and female SD rats. No cardiotoxicity was noted in male rats, whereas 1 female rat died of pericarditis 14 days after exposure to 5300 mg/m3.
No histopathological lesions were noted in the heart of cats following short-duration treatment with 40 mg/kg chlorpyrifos (CitationHooser et al., 1988). Similarly, no heart weight changes or histopathological lesions were observed following chronic-duration exposure of Sherman rats and beagle dogs fed up to 3 mg/kg-day.
Gastrointestinal Effects. Limited gastrointestinal effects were noted in female Fisher 344 rats treated with 5 mg/kg-day chlorpyrifos in feed for 13 weeks, while no effect was observed in male rats fed up to 15 mg/kg-day. It was anticipated that this effect in female rats may be related to cholinesterase inhibition (CitationDow, 1993). No histopathological lesions of the stomach were noted following chronic-duration exposure in either male cats, up to 40 mg/kg chlorpyrifos (CitationHooser et al., 1988) or Sherman rats and beagle dogs feed up to as much as 3 mg/kg-day (CitationMcCollister et al., 1974).
Gastrointestinal effects were observed in piglets acutely exposed (by spraying) to an undetermined amount of chlorpyrifos at 0, 1–3, 24–30, or 36 h after birth. High mortality was observed in the early treatment groups, and diarrhea resulting from cholinergic over-stimulation was observed in the animals that actually died. Necropsy revealed increased fluid in the intestine of some but not all piglets treated at 1–3 h after birth. Severe diarrhea was also seen in 2 of 4 bulls treated with testosterone for 84 days prior to dermal exposure to 0.33 ml/kg of a chlorpyrifos solution (CitationHaas et al., 1983).
A NOAEL of 1 mg/kg-day for gastrointestinal effects following oral exposure to chlorpyrifos was established based upon intermediate exposure to female Fisher-344 rats and perineal soiling.
Hematological Effects. No effects on hematological parameters were seen in Fisher 344 rats exposed by inhalation up to 0.295 mg/m3chlorpyrifos 6 h/day, 5 days/week for 13 weeks. Similarly no effects on hematological parameters were observed following short-or intermediate-duration oral exposure to chlorpyrifos, including Sherman rats and beagle dogs fed up to 3 mg/kg-day for 1–2 years (CitationMcCollister et al., 1974).
There is no information concerning hematological effects in animals given intermediate or chronic-duration dermal exposure to chlorpyrifos. No effects were observed in bulls treated with 0.33 ml/kg of a chlorpyrifos solution corresponding to 0.04 mg/kg (CitationHaas et al., 1983).
No data were located for hematological effects for animals following intermediate-, or chronic-duration dermal exposure to chlorpyrifos.
Musculoskeletal Effects. No data are available for musculoskeletal effects for animals following short-, intermediate-, or prolonged-duration inhalation or oral exposure to chlorpyrifos.
Hepatic Effects. The effect of intermediate-duration inhalation exposure to chlorpyrifos on liver histology was assessed in male and female Fisher 344 rats exposed up to 0.295 mg/m3 chlorpyrifos 6 h/day, 5 days/week for 13 weeks. Histology in the controls and treated animals was similar. These exposure levels were not sufficient to inhibit erythrocyte AChE or plasma BuChE activities. No data were located for hepatic effects in animals following acute-or chronic inhalation exposure to chlorpyrifos.
No effects on liver weight and relative liver weight were noted in pregnant CF-1 mice following acute-duration oral treatment with doses as high as 25 mg/kg-day chlorpyrifos from GD 6–15 compared to untreated animals. Hepatic effects were noted in pregnant female Fisher 344 rats dosed by gavage with up to 15 mg/kg-day of technical grade chlorpyrifos (Dursban F) on GD 6–15. Porphyrin deposits around the eyes (chromodachtyorhea) was observed in rats treated with 15 mg/kg-day, but not in the lower dose groups (CitationBreslin et al., 1996). No hepatic histological lesions or liver weight changes were observed in Sherman rats and beagle dogs chronically exposed to 3 mg/kg-day.
A NOAEL of 3 mg/kg-day for hepatotoxicity following oral exposure was established based upon chromodachtyorhea in female rats.
Renal Effects. The effect of intermediate-duration inhalation exposure to chlorpyrifos on urinary chemistry was assessed in male and female Fisher 344 rats treated with up to 0.295 mg/m3 chlorpyrifos 6 h/day, 5 days/week for 13 weeks. These levels of chlorpyrifos were not sufficient to inhibit either erythrocyte AChE or plasma BuChE activities. The urinary chemistry of the treated animals and that of the controls were similar. Furthermore, the treatment had no effect on kidney weight and kidney histopathology.
Renal toxicity was rarely observed following oral administration of chlorpyrifos in the feed or via gavage in vegetable oil. Urine staining was observed in the perineal region of pregnant Fisher 344 rats exposed to 15mg/kg-day of Dursban F, while no renal effects were observed in animals feed up to 3 mg/kg-day (CitationBreslin et al., 1996). In a chronic-duration oral exposure study, no renal effects were seen in Sprague-Dawley rats exposed up to 1.2 mg/kg-day chlorpyrifos for 135 days (CitationDow, 1983b). Similarly no kidney toxicity was observed following chronic-duration oral exposure to chlorpyrifos in male and female Sherman rats and beagle dogs feed up to 3mg/kg-day for 1–2 years (CitationMcCollister et al., 1974).
A NOAEL of 1.2 mg/kg-day was established for renal toxicity based upon a study in SD rats.
Dermal Effects. No information was found concerning dermal toxicity in animals following acute-or chronic-duration inhalation, oral, or dermal exposure to chlorpyrifos.
Immunological and Lymphoreticular Effects. No immunological and lymphoreticular effects were observed following oral administration of chlorpyrifos. In a multigeneration study in Sprague-Dawley rats treated with doses up to 5 mg/kg-day, no treatment-related histopathological changes in thymus, spleen, mesenteric lymph node was observed in either the F0 or F1 adults. Similarly, no effects on the histopathology of spleen was seen in Sherman Rats or beagle dogs chronically exposed up to 3 mg/kg-day chlorpyrifos for 1–2 years (CitationMcCollister, 1974).
Blood and lymphocytes from 29 individuals diagnosed by their physicians with “multiple chemical sensitivity” were examined for various immune markers (lymphocyte CD markers, autoantibodies, and lymphocyte mitogenesis) (CitationThrasher et al., 2002). Blood samples were collected “1–4.5 years following exposure.” The nature of the exposures to chlorpyrifos was only generally described as related to accidents, spills or other uses of chlorpyrifos, and no specific exposure assessment for the individuals was performed. The authors stated, “No attempt was made to correlate symptoms with the data because this was beyond the scope of our study.” Two unmatched “control” groups consisted of “volunteer asymptomatic chiropractic students (aged 29 ± 9 yr; 15 males, 13 females)” and “healthy volunteer home dwellers (aged 54 ± 19 yr; 13 males, 16 females).” A third “positive control” group was represented by a group of 12 individuals stated to have had exposure to chlorpyrifos in a previous report from the same author 9 years earlier (CitationThrasher et al., 1993). However, there were no individual exposure details in the previous report, so it is impossible to make any assessment of “dose-response” for either the “positive controls” or the subjects evaluated in the 2002 report. The authors reported a significant increase in the expression of the CD26 receptor on lymphocytes in the “chlorpyrifos exposed” group compared to the two control groups. Given the lack of any individual exposure assessment, and numerous other limitations of both studies, it is difficult to draw any conclusions from these studies regarding “cause–effect” association between chlorpyrifos and immune toxicity in humans.
III.B. Mutagenicity and Carcinogenicity
III.B.1. Mutagenicity
DNA Damage, DNA Mutations. Numerous publications report on studies on the genotoxicity and mutagenicity of chlorpyrifos (CitationSimmon et al., 1977; CitationWaters et al., 1981; CitationMoriya et al., 1983; CitationSandhu et al., 1985; CitationGarrett et al., 1992). Chlorpyrifos and other pesticides have been tested utilizing standard batteries on a variety of relevant endpoints. Chlorpyrifos was found not to induce reverse mutation, with and without metabolic activation, in Salmonella TA 1535, 1537, 1538, 100, 98, and in Escherichia coli WP2. The Drosophila melanogaster sex-linked recessive lethal test was negative. Evidence suggesting DNA damage by chlorpyrifos was obtained in differential toxicity assays that compared repair-proficient and-deficient (uvrB neg) E. coli and Bacillus subtilis strains, but not by testing for unscheduled DNA synthesis (UDS) in human fetal lung fibroblasts WI-38. Also, no evidence of mitotic recombination in Saccharomyces cerevisiae was obtained. Based on these studies, the authors classified chlorpyrifos as an organophosphorus compound with mainly negative results.
Chlorpyrifos was investigated in standard test batteries using common major endpoints, including reverse gene mutation in bacteria (Ames test) and forward mutation in mammalian cells (CHO, HGPRT locus), chromosomal aberrations in rat lymphocytes, DNA damage by testing for UDS in rat hepatocytes, and micronucleus formation in vivo (mouse bone marrow) (CitationGollapudi et al., 1995). All tests included positive and negative controls. Tests in vitro were done with and without metabolic activation (except the hepatocyte assay). Chlorpyrifos did not produce evidence of genotoxic or mutagenic activity in any of the tests.
Several other research groups studied chlorpyrifos for potential genotoxic activity in less comprehensive investigations using endpoints for which no or no “official” guidelines exist.
Chlorpyrifos was negative in a modified Ames test in which reverse mutation of a plasmid carrying a mutated lactamase gene introduced into Salmonella strains was used as an endpoint (CitationHour et al., 1998). In contrast to the results just described, CitationPatnaik and Tripathy (1992) reported on induction of sex-linked recessive lethal mutations and mosaic wing spots in Drosophila by a farm-grade formulation of chlorpyrifos; the composition of the formulation is not indicated, and pure chlorpyrifos was not tested for comparison.
Induction of DNA damage by chlorpyrifos was assessed by unscheduled DNA synthesis (UDS) performed in primary hepatocytes from male Fischer-344 rats following protocols of CitationWilliams (1977) and CitationWilliams et al. (1977). Several concentrations of chlorpyrifos from 0.35 to 35.0 μ g/ml were tested, with signs of toxicity seen at the two highest concentrations. The compound was not completely soluble that the highest concentration. Based on the evaluation of 30 cells per treatment none of the concentrations resulted in an increase of UDS (CitationGollapudi et al., 1995). In human WI38 cells no UDS was induced by chlorpyrifos (CitationWaters et al., 1981). In contrast to the study of CitationWaters et al. (1981) in repair-proficient and-deficient Bacillus subtilis (already described), no evidence of DNA damage by chlorpyrifos was obtained in this assay by CitationShirasu et al. (1976), while positive results were reported in Jones et al. (1984) as described in CitationGollapudi et al. (1995). CitationXu and Schurr (1990) tested 22 pesticides for their ability to induce DNA damage by using the SOS response in E. coli as endpoint. Chlorpyrifos at different dose levels did not elicit a response; in contrast, some of the other pesticides tested positive in this mutagenesis assay.
Chlorpyrifos and similar organophosphorus esters have been hypothesized to alkylate DNA. CitationWellman and Kramer (2004) investigated the ability of chlorpyrifos and its primary metabolite chlorpyrifos-oxon to bind to calf thymus DNA in an in vitro system. Thermal denaturation of DNA was monitored after incubation with the agent, and no shifts in denaturation curves were observed. It was concluded that the compounds do not bind to DNA or bind only with weak affinity. CitationCui et al. (2006) incubated chlorpyrifos with calf thymus DNA or with isolated mouse hepatocytes. They found no evidence of formation of DNA adducts and DNA–protein or DNA interstrand cross-links. Formaldehyde used as a positive control produced these alterations. The detection limits of the assays used were not described in either study (CitationWellman and Kramer, 2004; CitationCui et al., 2006).
The comet or single-cell gel electrophoresis assay was also used to study chlorpyrifos for formation of DNA damage. This type of mutagenesis assay detects the ability of compounds to interact with alkali-labile DNA sites and induce DNA strand breaks. CitationRahman et al. (2002) treated mice orally with single doses of chlorpyrifos ranging from 0.28 to 8.96 mg/kg. In blood leukocytes dose-dependent increases of comet tail length were seen after 24 h. These changes decreased after 48 and 72 h and disappeared after 96 h. The authors discuss formation of reactive oxygen species (ROS) as a possible mechanism responsible for DNA damage, as numerous studies have shown that chlorpyrifos increases indicators of oxidative stress or leads to oxidative damage to various tissues or cells (CitationOncu et al., 2002; CitationVerma and Srivastava, 2003; CitationWielgomas and Krechniak, 2007). CitationRahman et al. (2002) did not test for the presence of oxidized bases in DNA using specific enzymes (FPG, Endo III). Also, no other tests such as the micronucleus assay were performed to confirm the results. Increases in lipid peroxidation and DNA single-strand breaks (measured after alkaline elution of DNA from isolated liver and brain nuclei of rats treated with 2 doses of 41 mg/kg chlorpyrifos each) were reported by CitationBagchi et al. (1995), supporting the hypothesis put forward by Rahman. Thus, the DNA damage observed in these studies may be due to indirect effects by reactive oxygen.
In an epidemiological study, sperm from 260 men attending an infertility clinic was subjected to the comet assay (CitationMeeker et al., 2004b). Spermatozoa DNA was de-condensed and liberated from proteins by an extensive lysis step. The neutral comet assay was used to avoid strand breaks by alkaline pH. To estimate exposure to chlorpyrifos, urinary levels of the metabolite TCPy were measured as described earlier and in Section V. Urine and semen samples were collected on the same day. Exposures to another pesticide (carbaryl) and smoking, but not to other agents, were assessed. Urinary TCPy levels (in interquartile ranges) were tested for correlation with three different parameters of DNA integrity in the comet assay. The percentage of DNA in the comet tail showed a significant increase with TCPy levels, while the tail distributed moment decreased significantly and the comet extent decreased insignificantly with TCPy levels. The authors suggest that the different parameters of the comet assay may measure different types of DNA damage. Whether or not the study population is representative of the general male population is uncertain. It is also uncertain whether methodological difficulties inherent to analyses of sperm DNA using the comet assay contributed to the correlations observed. This is an interesting clinical survey, but the collection of exposure-related data and outcome data at the same point in time precludes an appropriate temporal sequence. Within-individual variability and other sources of error in a single test result for both the urinary and sperm measures were not addressed. Additional studies with more definitive chlorpyrifos exposure assessment techniques are needed to replicate these observations.
In a case-control study, blood samples from 30 women occupationally exposed to chlorpyrifos and possibly other pesticides in the process of banana packaging were examined for DNA damage in lymphocytes (CitationRamirez and Cuenca, 2002). Exposure was not confirmed analytically. Comet assays on blood lymphocytes were performed following exposure of cases for up to 5, 5–10, 10–15, and more than 15 years. Large interindividual differences were noted, with a wide range of results. Overall no difference was seen between cases and controls. The authors state that subgroups exposed for between 5 and 15 years showed an increase of DNA strand breaks but these subgroups consisted of only 8 and 6 subjects, including controls. The data are therefore insufficient to draw firm conclusions on the genotoxicity of chlorpyrifos.
The primary metabolite of chlorpyrifos, TCPy, was also tested for genotoxicity according to appropriate guidelines. Mutation assays in bacteria and CHO cells as well as the UDS assay in rat hepatocytes were negative (; Dow, 2002).
TABLE 5 Summary of genotoxicity studies with TCPy (from CitationDow, 2002)
Chromosomal Alterations. CitationMuscarella et al. (1984) studied the frequency of sister chromatid exchanges (SCE) in 3-day chick embryos treated with chlorpyrifos and its two major metabolites, TCPy and chlorpyrifos-oxon. Three doses including the toxic/lethal range were used. No increases of SCE were seen. Likewise, in Chinese hamster ovary cells none of the three agents, at any concentration, enhanced SCE frequencies. In the same study bovine blastocysts obtained from superovulated cows crossed with bulls treated with Dursban 44 were tested for chromosomal aberrations. In total, 4 bulls were treated topically on the skin at the withers with 16 ml Dursban 44, a commercial product containing 43.2% chlorpyrifos and inert ingredients with petroleum distillate. This treatment apparently was performed once and induced severe clinical signs of poisoning. Four months after treatment semen was collected and inseminated into 13untreated cows. Blastocysts obtained 8 days after insemination showed no evidence of chromosome aberrations.
The in vitro chromosomal aberration test was performed with primary rat lymphocytes according to protocols described by CitationSinha et al. (1989). Cells were cultured with and without metabolic activation by S9 preparations from Aroclor 1254-treated rats. Concentrations of chlorpyrifos evaluated were 16.7, 50.0, and 167 μ M/ml; higher concentrations blocked mitosis. No evidence of clastogenic activity was seen with chlorpyrifos, while positive controls (mitomycin c and cyclophosphamide) induced numerous chromosomal aberrations (CitationGollapudi et al., 1995). Effects of chlorpyrifos on chromosome alterations and SCE of human blood cells from three donors were studied by CitationNelson et al. (1990). Four different concentrations of chlorpyrifos were tested, but no positive controls were included. No relevant increases of the parameters studied were seen. CitationAmer and Aly (1992) utilized primary mouse spleen cells to assess chromosomal effects of chlorpyrifos in the absence of metabolic activation. The four concentrations tested (range 0.5–4 μ g/ml) induced dose-dependent increases of both chromosome aberrations and SCE.
Chromosome alterations and SCE were measured in blood cells of 8 persons exposed to chlorpyrifos after household exposure to chlorpyrifos for periods between 1 week and 7 months (CitationLieberman et al., 1998). The control group consisted of 141 individuals without known exposure, but no further data were provided. To characterize this group, the authors reported that chromosome alterations were outside the normal range in 7 out of 8 individuals. Because no quantitative analysis of exposure was performed, the significance of the findings in this study cannot be assessed.
Micronucleus formation in mouse bone marrow in vivo after chlorpyrifos dosing was tested and evaluated according to CitationSchmid (1976). Single doses of chlorpyrifos (7, 22, 70, and 90 mg/kg) were administered to male and female mice. The 70-and 90-mg/kg doses correspond to 60 and 80% of the LD50 reported in an initial experiment. No evidence of micronucleus formation was found at 24, 48, or 72 h after treatment with chlorpyrifos, whereas cyclophosphamide induced a strong increase (CitationGollapudi et al., 1995) In the micronucleus study of CitationNi et al. (1993) a series of 22organophosphorus pesticides including chlorpyrifos was investigated in bone marrow of strain “615” mice and in vitro using Chinese hamster lung cells. Treatment in vivo consisted of 4 doses given on consecutive days, each representing 10, 20, 40, or 80% of the LD50. The authors reported that chlorpyrifos was positive both in vitro and in vivo although the concentrations used for the studies in vitro are not indicated. The significance of this study cannot be evaluated because details on methods, resulting data, and statistical analyses were not reported for any of the agents studied.
Micronucleus formation was studied in 3-day mouse blastocysts following maternal exposure to chlorpyrifos (CitationTian and Yamauchi, 2003). The agent was administered ip at doses of 40 and 80 mg/kg on day 0 of pregnancy. It is reported that the number of blastocysts containing at least 1 micronucleus increased from approx. 10% in controls to 25% after maternal treatment. In a subsequent study CitationTian et al. (2007) used the same samples to demonstrate that Giemsa staining provided similar results as DAPI staining applied in the 2003 study. The results obtained in this unusual test system are difficult to evaluate, as the doses administered were sufficient to produce maternal toxicity.
In an agriculturally oriented investigation, chlorpyrifos was tested in the Tradescantia micronucleus (Trad-MCN) assay that indicates chromosome breakage in meiotic pollen mother cells. Exposure of Tradescantiacuttings to nutrient solutions containing 10 and 50 ppm, but not 1 ppm, chlorpyrifos enhanced the formation of micronuclei (CitationRodrigues et al., 1998). In the absence of positive results in tests that conform to standard testing guidelines, the relevance of these data is questionable.
Chlorpyrifos was tested for induction of mitotic recombination in Saccharomyces cerevisae D3; the results were negative (CitationWaters et al., 1981; CitationSandhu et al., 1985).
A metabolite of chlorpyrifos, TCPy, was tested in two studies for micronucleus formation. As shown in , no evidence of clastogenicity was found (taken from submission dossier of EU active substance review of trichlopyr; CitationDow, 2002).
III.B.2. Carcinogenicity
Several publications address the potential carcinogenicity of chlorpyrifos. Chlorpyrifos was administered daily via the feed to male and female Fischer 344 rats for 2 years according to U.S. EPA guidelines (CitationYano et al., 2000). Doses were 0, 0.05, 0.1, 1.0, and 10 mg/kg-day. The highest dose produced considerable and significant inhibition of AChE in several organs and some mild signs of toxicity; no increase in mortality was noted. No significant increases or decreases in the incidence of tumours were found. Likewise, no increases in incidence of tumours in any organ were observed in earlier oncogenicity studies in rats given 0.01, 0.03, 0.1, 1.0, and 3.0 mg/kg-day for up to 2 years (CitationMcCollister et al., 1974), or in CD-1 mice receiving doses of 0.05, 5.0, and 15 mg/kg-day for 2 years (CitationWarner, 1980). In addition, the chlorpyrifos-containing products Pyrinex technical and Dursban were tested in rats, and Pyrinex technical in mice in 2-year bioassays. No tumorigenic effects were reported (CitationDow, 2002).
In another study, mixtures of 20 pesticides including chlorpyrifos were evaluated for modifying effects on carcinogenesis in medium-term protocols in rats (CitationIto et al., 1996). This mixture of pesticides was prepared by adding to the diet concentrations of each pesticide to achieve “acceptable daily intake” (ADI) levels, When carcinogenesis was initiated by a single dose of diethylnitrosamine, treatment with the mixture of pesticides at their respective “ADI” concentrations had no effect on the development of preneoplastic lesions in the liver. However, when 100 times the ADI level was added to the diet, significant increases in number and size of these lesions were observed, suggesting a tumour promoting effect. In a second approach, a multi-organ carcinogenicity protocol with 5 potent carcinogens exhibiting different organ specificities was used for initiation. A mixture of 40different pesticides (including chlorpyrifos) in the diet at their respective ADI levels was fed for 28 weeks. No evidence of carcinogenesis in any organ was observed. These studies suggest the absence of a tumour promoting effect of the mixture in any of the target organs investigated. In this context it is of interest that chlorpyrifos did not activate the estrogen receptor in MCF 7 cells (CitationVinggaard et al., 1999), suggesting that chlorpyrifos should not cause promotion of estrogen-dependent tumours.
Several human epidemiology studies have evaluated a possible association between cancer risk and chlorpyrifos exposure. The prospective Agricultural Health Study is an investigation of approximately 55,000 certified pesticide applicators to examine a possible relationship between agricultural pesticides and incidence of cancer. Detailed information on exposure to 50 different pesticides was obtained by self-administered questionnaires before enrollment (1993–1997). Exposure assessment was based on calculations made from the number of exposure days, number of exposure years, application method, equipment repair status, use of personal protective equipment and other variables, as recalled by respondents. Cancer incidence was recorded from enrollment through 2001 (6.4 years). The number of all cancers combined was not increased (rate ratio = .97). A significant exposure-incidence trend was observed for lung cancer, with a 2.18-fold increase of risk in the highest lifetime exposure category. This association apparently was restricted to current smokers (CitationAlavanja et al., 2004; CitationLee et al., 2004).
A small telephone-based case-control study, largely limited to proxy respondents, reported elevated odds ratios for glioma among farmers exposed to chlorpyrifos (CitationLee et al., 2005). This was a nested case control study within the prospective Agricultural Health Study.
In a recent publication on the Agricultural Health study (study period from enrollment through 2002, i.e., 7.3years) chlorpyrifos use showed a significant exposure response trend for rectal cancer, rising to a 2.5-and 2.7-fold increase in risk in the 2 highest exposure categories, while no increase was seen with colon cancer (CitationLee et al., 2007). These associations between chlorpyrifos and lung and rectal cancer were unexpected in view of the absence of an increased incidence of intestinal or other tumors in animal studies, and the authors conclude that their findings should therefore be interpreted cautiously. Indeed, the positive association is based on small numbers of cases, i.e., an excess of less than 10 cases in the 2 highest exposure groups in the study of CitationLee et al. (2007). Additional uncertainty arises from the limitations in exposure assessment, which is estimated from information recalled by surrogate respondents. Therefore, more research is needed before a causal association between rectal cancer and chlorpyrifos can be assessed.
III.B.3. Summary and Conclusions—Genotoxicity and Carcinogenesis
Chlorpyrifos was tested for genotoxicity according to Good Laboratory Practice (GLP) and appropriate guidelines covering the endpoints considered relevant today. Results obtained in these studies consistently revealed no evidence of genotoxicity. Further investigations were conducted using test systems and endpoints not described in accepted guidelines and mostly not systematically validated with compounds known for presence or absence of genotoxic activity. While a few were positive, the majority of these studies yielded negative results with chlorpyrifos. Possibly, under certain conditions chlorpyrifos may have some potential to cause DNA damage via an indirect mechanism, namely, formation of reactive oxygen, although the studies supporting this hypothesis are far from convincing. Overall, the weight of evidence indicates that chlorpyrifos (and its metabolite TCPy) do not possess direct genotoxic activity.
Chlorpyrifos was also tested for carcinogenicity in 2-year bioassays in conformity with appropriate guidelines in several studies on rats and mice. No compound-dependent increase of tumor formation in any organ was detected. Also, when chlorpyrifos was fed at ADI levels in mixtures of 20 or 40 different pesticides there was no evidence for tumour-enhancing/promoting activity in any organ.
In conclusion, the data available do not provide convincing evidence for genotoxic or carcinogenic activity of chlorpyrifos. However, the observed relationship between self-reported chlorpyrifos exposure and glial tumors of the CNS as well as lung and rectal cancer warrants further investigation.
III.C. Teratogenic and Reproductive Effects
III.C.1. General Teratogenic and Reproductive Toxicity Assays in Experimental Animals
This section examines evidence for the potential of chlorpyrifos to cause prenatal toxicity, including major congenital malformations. Effects on fertility and reproduction are also included.
Experimental Studies in Rats. The most comprehensive study was conducted in rats (CitationQuellette et al., 1983). Experimental groups containing 24–29pregnant Fischer 344 rats per group were treated orally with 0, 0.1, 3, or 15 mg/kg-day (in corn oil [vehicle], by gavage) on days 6–15 of pregnancy. Litter data were evaluated on day 21 of pregnancy. The highest dose used in this study (15 mg/kg) is about half of the dose (30 mg/kg) reported to induce lethality in pregnant rats. All pregnant rats survived, but some showed typical signs of OP pesticide intoxication (excessive salivation, urine staining in the perineal region, porphyrin deposits around the eyes [chromodactyorrhea], and tremors) throughout the dosing period. Body weights and feed consumption were not different from controls within the groups treated with 0.1 or 3mg/kg. There was, however, a slight reduction in body weight (∼ 4%) in the 15-mg/kg group. No effects on the general appearance or demeanor of the animals were noted at or below 3 mg/kg-day. There was no evidence for increased embryo-/fetotoxicity (e.g., prenatal mortality or decreased fetal weight) up to the highest dose level tested, and the incidence of malformations was not increased at any dose.
Another study using the CD rat strain was performed by Rubin et al. (1987a). Sufficiently large groups of pregnant rats were treated orally (via gavage) with 0, 0.5, 2.5, or 15 mg/kg Pyrinex Technical (purity 96.1%) on days 6–15 of pregnancy. Litter data were evaluated on day 20 of pregnancy. Satellite groups (treated identically) were used for measurement of plasma cholinesterase on day 15. The study was conducted under GLP conditions. The highest dose produced tremors in 3/21 rats, and small changes in feed consumption and maternal body weight gain. Therefore, a maternal functional NOAEL was estimated to be 2.5 mg/kg. No increased rate of malformations was observed, but a slight increase in early resorptions was found at the highest dose level. From these data the authors estimated a developmental NOAEL for Pyridex to be 2.5 mg/kg.
The potential for technical grade chlorpyrifos (97%) to cause teratogenic effects was also examined in Wistar rats (CitationAkhtar et al., 2006). Pregnant animals received chlorpyrifos (0, 9.6, 12, 15 mg/kg-day) in corn oil via gavage from day 0 to 20 of pregnancy. Signs of maternal toxicity including tremor and reduced body weight gain were observed at the highest dose level during the dosing period. Reduced body weight gain was significant in animals that received chlorpyrifos at 12 mg/kg-day. At 15 mg/kg-day increased prenatal toxicity and signs of fetal growth retardation were reported. Both effects were associated with signs of maternal toxicity. The incidence of morphological, visceral and skeletal fetal malformations was not increased at any dose.
The negative findings of the previous reports are supported by an additional study in which Fischer 344 rats were given 0, 5, 15, 25 mg/kg by gavage on days 6–15 of pregnancy (CitationFarag et al., 2003). Fetuses were evaluated on prenatal day 21. Maternal weight reduction of 9–13% was found during late pregnancy at doses of 15 and 25mg/kg-day. Maternal brain-AChE activity was reduced by 30% and 49%, respectively, in the two high-dose groups. Increased prenatal losses were found only in the group that received the highest dose. Increased rates of “anophthalmia” and “ectodactyly” were observed in underweight fetuses. Both of these defects were found at somewhat lower incidence in controls. No increased incidence of malformations was reported at doses less than 15 mg/kg-day.
Newborn Sprague-Dawley rats directly treated subcutaneously on postnatal days 1–4 with 1 mg/kg chlorpyrifos (in 1ml/kg bw in dimethyl sulfoxide, DMSO), a dose at which no general toxicity was observed, were reported to exhibit altered cell signaling within the brain, when the animals where tested in adulthood (110 days of age). Also an elevation in plasma cholesterol and triglycerides, without the alterations in nonesterified free fatty acids and glycerol and hyperinsulinemia, was observed in males (CitationSlotkin et al., 2005). It should be noted that this study was not performed for regulatory purposes and thus did not follow standard “guidelines” for toxicological evaluation. This and other similar studies are discussed in more detail in the following section on neurodevelopmental toxicology.
Multigeneration Study in Rats (Development and Fertility). Multigenerational studies in rats have been conducted to assess the potential for chlorpyrifos to adversely affect reproductive capacity, including fertility (Breslin et al., 1991). Groups of 30 male and female Sprague-Dawley rats were treated with chlorpyrifos (97.8% purity) via the feed 10 weeks before and during mating (F0). Females were further exposed during pregnancy and lactation (until weaning of the F1 generation on postnatal day 21); F1 rats raised the F2 generation (until weaning). Thus, exposure was over the entire reproductive life, providing 7 days/week doses of 0, 0.1, 1, and 5 mg /kg. Analyzed in the F1 and F2 generation were development, cholinesterase activities in blood and brain, and treatment-related alterations in nonspecific tests of behavior or demeanor. The concentration of the test substance in the feed was reduced during the lactation period (2nd week to 1/2, 3rd week to 1/3). With the exposure extending over the entire pregnancy, no indication of increased prenatal mortality was reported up to 5 mg chlorpyrifos/kg body weight (bw) (the same number of viable offspring). There was no evidence for impairment of the reproductive functions (fertility) within the F0/F1 or the F1/F2 generation, up to a daily dose of 5 mg chlorpyrifos/kg, which, according to previous studies, represents the threshold for inducing effects on both neonatal development (body weight and survival) and maternal toxicity. Animals mated with normal frequency and exhibited normal pregnancies, offspring and lactation. A few deviations from controls were reported for offspring of mothers exposed to 5 mg/kg but were not consistent in F1 pups and F2 pups, The changes overlapped with values of historical controls, or were little different from them.
Data from the multigeneration study also allowed assessment of possible effects on fertility in rats: Female and male mating indices and female and male conception indices were within the range of historical controls.
Experimental Studies in Mice. The mouse is not a preferred species in developmental toxicology because many strains are abnormally sensitive to test agents and have high and variable incidence of spontaneous malformations (e.g., exencephaly and cleft palate). These compromise attempts to evaluate borderline effects. Such limitations were evident in several studies with chlorpyrifos.
Two studies were performed using groups of 23 to 36 pregnant mice treated orally via gavage on days 6–15 of pregnancy and evaluated on day 18 (Dow K-44 793-32). In the first study animals were administered doses of 0, 1, 10, or 25 mg/kg-day. The highest dose represented about 70% of the oral LD50 [gavage] in pregnant mice of the strain used. This dose was maternally toxic (9% of mice died during the study), and typical signs of inhibition of cholinesterase, including increased salivation and urination, tremors, ataxia, lethargy in about 70% of mice. The study was repeated using the same dosing protocol with a maximum dose of 10 mg/kg-day; maternal toxicity was decreased at this dose. Nevertheless, these studies did not give evidence that chlorpyrifos adversely effects prenatal development. The defects reported (especially cleft palate and exencephaly) are known to occur “spontaneously” and at varying frequency in various mouse strains. In addition, the incidence of abnormalities showed no dose dependency—the highest incidence of major malformations was observed at the lowest dose (i.e., 1mg/kg-day dose) in the initial study. Lack of dose dependency further suggests that no cause-and-effect relationship was evident from these studies. Reproducibility was lacking between the two studies, with the second study failing to confirm the results of the initial investigation.
In another study, Crj:SD-1 mice were administered a single high dose of 40 or 80 mg/kg chlorpyrifos on day 10 of pregnancy via i.p., injection (CitationTian et al., 2005). (It should be noted that intraperitoneal administration is not generally considered to be an appropriate route of administration for toxicological studies on reproduction and development.) An increased incidence of major malformations including cleft palate and open eyelids was observed at the dose of 80 mg/kg. No effects were seen at the lower dose (40 mg/kg). As with the previous study, both types of observed major malformations (cleft palate and open eyelids) occur spontaneously in mice, and can be increased by nonspecific stress. Because the incidence of these malformations in this laboratory was not specified, the significance of the findings in the high-dose group is unknown. Nevertheless, it is possible that extraordinarily high does of chlorpyrifos may produce congenital malformations.
Experimental Studies in Rabbits. CitationRubin et al. (1987b) conducted a study to examine the effects of chlorpyrifos on prenatal development in rabbits. Chlorpyrifos (Pyrinex Technical, purity 96.1%) was administered to groups (n: 13–17) of New Zealand White rabbits (HY/CR) via gavage in corn oil at 1, 9, 81, or 140 mg/kg on days 7–19 of pregnancy. Control animals received vehicle only. Additional satellite groups were used for measurement of plasma BuChE, which was reduced at all dose levels. The slight increase in postimplantation loss reported in this study is difficult to judge because no dose-response relationship was demonstrated, and a normal number of viable fetuses was seen at the highest dose. Signs of retardation of fetal growth were coincident with reduced maternal weight gain. Therefore, maternal NOAEL and developmental NOAEL were 81 mg/kg in this study. There was no evidence to indicate that chlorpyrifos had a teratogenic effect in the rabbit.
Effects on Reproductive Organs of Animals. No effects on testicular weight and histology were reported in Fischer 344 rats treated by inhalation with a dose of chlorpyrifos (0.3 mg/m3, 6 h/day, 5 days/week, for 13 weeks) insufficient to inhibit erythrocyte or plasma cholinesterase activity (CitationCorley et al., 1989).
Some maternal toxicity was observed in pregnant CF-1 mice following oral exposure to chlorpyrifos (up to 25 mg/kg) on GD 6, GD 6–10, or GD 6–15. Thirty-two of 47 mice treated with 25 mg/kg-day exhibited signs of cholinergic over stimulation (CitationDeacon et al., 1980). No reproductive effects, with the exception of vaginal bleeding in high-dose animals, were observed in pregnant Fisher 344 rats treated with up to 15 mg/kg-day Dursban on GD 6–15.
No effect on testes weight or reproductive organ histology was seen in Sherman rats or beagle dogs chronically treated with up to 3 mg/kg-day chlorpyrifos for 1–2 years (CitationMcCollister et al., 1974).
The reproductive effect of dermal exposure to chlorpyrifos was investigated in young bulls. The animals were treated with an undetermined amount of Dursban-44 and sperm parameters were analyzed. An unspecified increase in nonmotile sperm, and decreased sperm mobility and ejaculate volume, were observed at 6 months postexposure, whereas no effect was observed 7–12 months postexposure (CitationEverett, 1982).
III.C.2. Reproductive/Teratogenic Effects in Humans
Observations on Malformations in Potentially Exposed Females. Although it is likely that pregnant women have been exposed to substantial doses of OP pesticides (including attempted suicides), reliable data from systematic surveys on malformation rates have not been reported.
Based on multiple retrospective case reports (CitationSherman, 1995, Citation1996, Citation1999) a malformation syndrome linked to in utero exposure to chlorpyrifos (Dursban-LO or-TC) was postulated. The eight children suffered from brain defects, as well as abnormalities of the eyes and other organ systems. Most exhibited growth retardation. If true, the variety of malformations reported would have originated during various stages of development, from embryonic to late fetal stages. Unfortunately, spontaneous genetic causes are a more likely cause of some of the malformation. The validity of these studies has been challenged by others (CitationGibson, 1999; CitationClegg et al., 1999; CitationAlbers et al., 1999).
CitationRull et al. (2006) completed a retrospective evaluation of the potential association between incidence of neural-tube defects (NTD; spina bifida, anencephaly, craniorrhachischisis) found in deliveries from 1987–1991, and maternal residential proximity to agricultural fields (within a circle of 1000 m) with potential applications of one or more of 59 specific pesticides (chlorpyrifos being one of them [n = 49]), as compared with mother/child pairs with no NTD (n = 43). No individual or overall measurements of exposure were documented. There were differences between cases and controls in ethnicity, and fewer mothers of cases consumed vitamins, e.g., folic acid. The odds ratio calculated depended on the regression model used ([a] OR 1.5; 95% CI 1.0–2.3, [b] OR 1.35; 95% CI 0.7–2.3, [c] OR 1.2; 95% CI 0.7–1.9): Only for [a] the lower limit of the CI is > 1.0, and thereby statistically significant.
Male Reproductive Effects in Humans. Meeker and colleagues published several reports from cross-sectional analyses conducted on a clinical series of 322men seen in a fertility clinic between 2000 and 2003 (CitationMeeker et al., 2004a, Citation2004b, Citation2006a, Citation2006b). Among eligible males, 65% consented to participate and provided a spot urine sample for analysis of TCPy, and semen blood samples for analyses of reproductive and thyroid hormones.
In the first report (CitationMeeker et al., 2004a), the relationship of exposures in 272 participants to either carbaryl or chlorpyrifos with semen quality was examined through a single analysis of the urinary metabolites 1-naphthol (for carbaryl and naphthalene) and TCPy for chlorpyrifos and chlorpyrifos-methyl. Semen quality was assessed as sperm concentration, percent motile sperm, and percent sperm with normal morphology, as well as several other measures of sperm motility. Statistical analyses for both continuous and dichotomous measures for the three sperm parameters used reference norms (sperm concentration < 20 million/ml, sperm motility < 50% motile, and sperm morphology < 4% normal morphology) and were conducted using men (n = 157) with values above these reference values for all three sperm parameters as the comparison subjects. For TCPy metabolite values below the limit of detection (LOD), corresponding to 0.25 μ g/L, an imputed value equal to one-half of the LOD was used. Compared to men in the three “abnormal” semen parameter groups, the comparison subjects were younger, more likely to be Caucasian and nonsmokers, and a lower proportion of males in this group had been previously examined for infertility. These variables, plus body mass index (BMI) and abstinence times (days), were treated as potential confounders. In the statistical analysis, there was evidence of confounding for only age and abstinence time in some of the models. In contrast to the results for the carbaryl urinary biomarker 1N, the results for TCPy were not statistically significant for the three semen parameters, and the odds ratios (OR) were fairly consistent around the null value of 1.0. The authors concluded that there were suggestive associations between TCPy concentration and sperm motility, “but they are difficult to interpret because there are currently limited human and animal data.” Additional considerations in the interpretation of these data are the relatively small number of observations for some comparisons and only one sample of urine and semen, collected at the same time, thus preventing consideration of temporality.
It is noted that biomarkers of exposure for chlorpyrifos, i.e., the urinary metabolite TCPy, may reflect the exposures of the previous 24–48 h and therefore may not represent exposures relevant for the time of initiating overt disease or alterations in physiologic function.
In the second report from Meeker and his colleagues (CitationMeeker et al., 2004b) on this clinical case series, sperm samples from 260 men examined at the infertility clinic were subjected to the comet assay to assess DNA damage. This study was discussed in detail in the previous section on mutagenesis studies of chlorpyrifos.
The third report (CitationMeeker et al., 2006b) examined the association of male reproductive hormones with TCPy exposures using the results of urinary metabolites as described earlier in a sample of 268 males seen at the fertility clinic between 2000 and 2003. Reproductive hormones, specifically follicle-stimulating hormone (FSH), luteinizing hormone (LH), inhibin B, testosterone, and sex hormone-binding globulin (SHBG), were measured in serum. The statistical analysis using regression models indicated an inverse relationship between TCPy and testosterone concentration; an interquartile range increase in TCPy was associated with a difference of 25 ng/dl in testosterone concentration (95% CI = –40 to –10). The association appeared to be dose related for TCPy. The authors also state that they observed an inverse relationship between TCPy and free androgen index and a suggestive inverse association between TCPy and LH, but the data for free androgen index and TCPy are of modest significance (coefficients ranging from .93 to .96 and 95% CIs ranging from 0.89–0.98 to 0.91–0.99) for the adjusted and unadjusted coefficients. The data for the association of TCPy concentrations and LH by quintiles of exposure were not consistent with an association of TCPy exposure with level of LH.
The final report by Meeker and associates looked at the association of chlorpyrifos exposure with thyroid hormones in 268 male patients (CitationMeeker et al., 2006a). Urine and nonfasting blood samples were collected between the hours of 9:00 a.m. and 4:00 p.m. Serum samples were analyzed for free T4, total T3, and thyroid-stimulating hormone (TSH). The authors report an association between TCPy and TSH; an interquartile range difference of TCPy was associated with a 9% (CI = 0–18%) difference in TSH. The adjusted coefficient for TSH was 1.09 (95% CI = 1.00–1.18). They also describe a suggestive inverse relationship between TCPy and free T4; the adjusted coefficient for free T4 was –0.031 (95% CI = –0.063 to –0.0003). The authors note the limitations on their findings and suggest that more research is necessary to substantiate the observed associations.
It should be noted that the concentrations of chlorpyrifos observed in this case series fall within the range of the general U.S. population, according to data from the NHANES III survey (see Section V). As discussed earlier, further caution is advised with respect to calculations using TCPy as a quantitative measure of chlorpyrifos exposure.
It has also been hypothesized that chlorpyrifos could inhibit testosterone metabolism (CitationRose and Hodgson, 2005). The concentration of chlorpyrifos necessary to produce such inhibition in vivo must be established in order to ascertain its possible physiological relevance.
III.C.3. Summary of Teratogenic and Reproductive Studies on Chlorpyrifos (Excluding Possible Neurodevelopmental Effects—Discussed in Section IV)
Animal studies to assess possible teratogenic effects of chlorpyrifos have been conducted with rats, mice and rabbits. In most studies the test substance was administered orally (via gavage). Pregnant rodents seem to be more susceptible to chlorpyrifos than nonpregnant animals. The highest doses used in these studies (i.e., 15 mg/kg-day in rats and 25 mg/kg-day in mice) were greater than half of the maternal LD50 and were associated with signs of OP pesticide poisoning in these species. Observations at the end of pregnancy have been extended to the analysis of adverse effects in pups after delivery (F1 and F2) in a two-generation study in rats. No effect on fertility was found.
Taken together, the studies found no consistent evidence for teratogenicity or abnormal reproduction with daily oral dose of chlorpyrifos up to 5 mg/kg-day. Indications of prenatal growth retardation and increased pre-/perinatal death were seen in some studies at 5 mg/kg, associated with signs of maternal toxicity. At higher dose levels, signs of maternal toxicity occur in the majority of experimental animals, and this may be associated with increased pre-/perinatal mortality and growth retardation.
At oral doses of ≥ 5 mg chlorpyrifos/kg, substantial inhibition of maternal (erythrocytes, plasma, brain) and fetal cholinesterases occurs, and histological changes are regularly reported within maternal adrenal glands. Inhibition of plasma BuChE is one of the early biomarkers of exposure to chlorpyrifos, and this indication may be observable at 0.1–0.3 mg/kg in rats.
Results of human surveys on malformation rates after chlorpyrifos exposure are few, and existing data are too limited to allow firm conclusions.
III.D. Toxicity Assessment of TCPy
Very few toxicity studies on TCPy have been reported. Two internal studies from Dow Chemical Company (CitationHanley et al., 1998, Citation2000) indicated that TCPy has no cholinesterase inhibiting activity and relatively low acute toxicity. The oral LD50 in rats is approximately 800 mg/kg, and the dermal LD50 in rabbits is greater than 2000 mg/kg (CitationHanley et al., 1998, Citation2000). The primary target organs for subacute (4-week dietary exposure) toxicity were the liver and kidneys, with hepatic and renal effects seen at dose levels of 120 mg/kg-day and greater (unreported data, Dow Chemical Company; cited in CitationHanley et al., 2000). Because of the widespread exposure of the general population to low levels of TCPy, a reproductive toxicity study was done on TCPy. Groups of 32–34 bred female rats were treated by oral gavage with 0, 50, 100, or 150 mg TCPy/kg-day on GD 6–15. The TCPy was > 99.7% pure. On GD 21, pups were delivered by cesarean section and evaluated for standard developmental measures, including complete gross postmortem examination and the weight of the maternal liver, kidneys, and gravid uterus. The number of corpora lutea and number and position of implantations, resorptions, and live or dead fetuses were also recorded. Uteri with no visible implantations were stained and examined for evidence of early resorptions. All fetuses were euthanized by CO2 asphyxiation and examined for external malformations/variations. Individual fetuses were weighed, sexed, and approximately one-half of the fetuses in each litter were evaluated for visceral malformations/variations. Dose-dependent decrease in maternal weight gain was noted in the 100-and 150-mg/kg-day dose groups. A decrease in food consumption was also noted in these animals, and may have explained the decrease in maternal weight gain. No treatment-related changes in organ weight were noted. No adverse effects on fetal development were seen at any dose level, and reproductive parameters (pregnancy rates, resorption rates, litter size, etc.) among pregnant rats were unaffected by treatment. Although the number of pups born in the high-dose group was less than that in the other groups, the number of implantations was proportionately lower as well, since exposures did not start until postimplantation; this could not have been treatment related. Thus, there was no evidence of reproductive toxicity in this study.
CitationHanley et al. (2000) also conducted a reproductive toxicity assessment in New Zealand White rabbits. Groups of 16 pregnant rabbits were exposed from GD 7 to 19 to 0, 25, 100, and 250 mg/kg-day of TCPy by oral gavage, and the same types of analyses as described earlier were completed. Consistent with the results in rats, no adverse effects on reproductive parameters were observed. Examination of the fetuses from this study revealed a number of malformations in the various treatment groups; there were 7 malformations in 3 fetuses from the same liter in the 100-mg/kg-day dose group. There were a total of seven fetuses from six different litters with malformations, including five fetuses with CNS anomalies in the 250-mg/kg-day dose group. According to the authors, “there were, however, no statistically significant increases in the incidence of any developmental effects in any of the treated groups when compared to the controls. Examination of those end points that are typically associated with delayed fetal development (i.e., ossification of sternebrae, vertebral centra, etc. which represent the last centers to ossify) did not indicate any evidence of a treatment-related effect. Low incidences (one or two in a single treatment group) of minor variations such as pale or bilobed spleen, irregular patterns of ossification, or extra sites of ossification normally seen in rabbits at a low incidence were not included in the table. There was no pattern in the incidence of malformations (either singly or combined) to suggest any association with TCP administration” (CitationHanley et al., 2000, p. 105). The authors noted that the apparent increase in CNS anomalies in the two high-dose groups was not out of the range of what was seen in historical controls, and that there was no indication of similar malformations in studies with chlorpyrifos that would have generated dose equivalents of TCPy of 80 mg/kg-day.
Because there was no indication of any increase in malformations at the 25-mg/kg-day dose rate, this could be viewed as the NOAEL for developmental effects of TCPy in rabbits. As discussed in Section V, human urinary TCPy levels are typically in the range of 1–10 μ g/L (ppb), with levels up to 100 ppb possible in highly exposed populations. A 24-h urine concentration of 1 ppb TCPy would be equivalent to a daily intake of approximately 2.4 μ g TCPy (assuming 1.7 L of urine volume per day, and 70% absorption), or for a 70-kg human, a dose of 0.04 μ g/kg-day for every 1 ppb urinary TCPy. Therefore, human exposures to TCPy, either directly or via conversion of chlorpyrifos/chlorpyrifos-methyl to TCPy, would range from 0.04 μ g/kg-day to 4 μ g/kg-day of TCPy. Thus, the rabbit NOAEL for developmental effects of TCPy of 25 mg/kg-d is approximately 62,500 to 625,000 times greater than the doses of TCPy that would result in the typical urinary TCPy concentrations seen in the U.S. population (based on typical urinary TCPy values of 1–10 ppb; see Section V). Even exposures that would result in urinary concentrations TCPy as high as 100 ppb would be 6250 times less than the rabbit NOAEL for developmental effects.
TCPy was not mutagenic when tested in several in vitro mutagenicity assays (), as discussed in Section III.B.1. TCPy has also been examined in several in vitro cytotoxicity and biochemical assays, and has generally been found to be substantially less toxic than chlorpyrifos (see Section IV.C.2 for descriptions of studies that have assessed toxicity of TCPy in various in vitro systems).
Thus, the toxicological assessment of TCPy thus far does not indicate that TCPy has toxicological properties that would be of concern.
III.E. Toxicity Assessment of Direct Exposure to Chlorpyrifos-Oxon
Chlorpyrifos-oxon is a metabolite of chlorpyrifos, formed by oxidative desulfuration of chlorpyrifos through cytochromes P-450 (see Section II.C). Like all oxygen analogs of OPs, chlorpyrifos-oxon is considered the“active/toxic” metabolite of chlorpyrifos, as it inhibits esterases (AChE, BuChE, carboxylesterases) with high potency (1–10 nM). Direct administration of chlorpyrifos-oxon would thus be expected to cause rapid and severe cholinesterase inhibition, and ensuing cholinergic toxicity. For example, upon dermal exposure of PON1-R192 mice (the least sensitive to chlorpyrifos-oxon toxicity) to 1.5 or 3.0 mg/kg chlorpyrifos-oxon, dissolved in acetone, brain AChE activity was decreased by 20 and 60%, respectively. Inhibition was already maximal at 30 min after administration (CitationCole et al., 2005). In the same study, dermal doses of chlorpyrifos of 100 and 150 mg/kg were necessary to cause 30 and 60% inhibition of brain AChE, respectively, and morbidity paralleled the results of AChE inhibition.
In contrast to the findings following dermal exposure to chlorpyrifos-oxon and chlorpyrifos, oral administration of chlorpyrifos-oxon revealed a much lower toxicity of this metabolite. In an unpublished study conducted according to Good Laboratory Practices (CitationCieszlak, 1999), chlorpyrifos-oxon was given orally to Fischer 344 rats. The acute LD50was calculated as > 100 mg/kg in male rats, and 300 mg/kg in female rats. These values are comparable to those reported for the acute oral LD50 of chlorpyrifos in rats (118–163 mg/kg; see ). These results suggest that upon oral exposure, chlorpyrifos-oxon undergoes extensive hepatic metabolism (first-pass effect), which would limit its entry into circulation and ensuing inhibition of target organ AChE.
IV. NEUROTOXICITY OF CHLORPYRIFOS
IV.A. General Neurotoxicity of Organophosphorus (OP) Insecticides
IV.A.1. Acute Cholinergic Syndrome
OP insecticides have high acute toxicity, with oral LD50 values in rat often below 50 mg/kg, although for some compounds (e.g., malathion) toxicity is much lower, due to effective detoxication. The primary targets for OPs are cholinesterases (BuChE and AChE), whose physiological role is that of hydrolyzing acetylcholine, a major neurotransmitter in the central and peripheral nervous systems. Acetylcholine transmits information via nicotinic and muscarinic receptors from neurons in both the sympathetic and parasympathetic divisions of the peripheral nervous system, from somatic motor nerves to skeletal muscle fibers, and, of special relevance here, to, from, and within the brain and spinal cord. Inhibition of cholinesterases by OPs causes accumulation of acetylcholine at cholinergic synapses, resulting in over-stimulation of cholinergic receptors of the muscarinic and nicotinic type. As these receptors are localized in most organs of the body, a “cholinergic syndrome” ensues, which includes increased sweating and salivation, profound bronchial secretion, bronchoconstriction, miosis, increased gastrointestinal motility, diarrhea, tremors, muscular twitching, and various central nervous system effects. When death occurs, this is believed to be due to respiratory failure caused by inhibition of respiratory centers in the brainstem, bronchoconstriction and increased bronchial secretion, and flaccid paralysis of respiratory muscles. At the molecular level, OPs with a P = O moiety phosphorylate a hydroxyl group on serine in the active (esteratic) site of the enzyme, thus impeding its action on the physiological substrate. Phosphorylated AChE is hydrolyzed by water at a very slow rate, but hydrolysis can be facilitated by certain chemicals (oximes) that are utilized in the treatment of OP poisoning. However, oximes are ineffective at reactivating phosphorylated AChE once the enzyme–inhibitor complex has “aged.” Aging consists of the loss (by nonenzymatic hydrolysis) of one of the two alkyl groups. When phosphorylated AChE has aged, the enzyme can be considered to be irreversibly inhibited, and the only means of replacing its activity is through synthesis of new enzyme, a process that may take days. Atropine, a cholinergic muscarinic antagonist, is the main antidote for OP poisoning; by blocking muscarinic receptors, it prevents the action of accumulating acetylcholine on these receptors. As said, oximes, such as pralidoxime, are also used in the therapy of OP poisoning, and in some cases diazepam is also used to relieve anxiety or antagonize convulsions.
IV.A.2. Intermediate Syndrome
In addition to the acute cholinergic syndrome, OPs may also cause an intermediate syndrome, which is seen in 20–50% of acute OP poisoning cases. The syndrome develops a few days after the poisoning, during recovery from usually severe cholinergic manifestations, or in some cases, when patients are completely recovered from the initial cholinergic crisis. Prominent features of the intermediate syndrome are a marked weakness of respiratory, neck, and proximal limb muscles. The intermediate syndrome is not a direct effect of AChE inhibition, and its precise underlying mechanisms are unknown. A plausible mechanism is that muscle weakness results from repairable damage secondary to excessive stimulation at neuromuscular junctions during the phase of cholinergic toxicity, such that the intermediate syndrome represents a reversible subjunctional myopathy.
IV.A.3. Delayed Peripheral Neuropathy
A third neurotoxic syndrome associated with exposure to certain OPs is peripheral neuropathy, known in the experimental literature as OP pesticide-induced delayed polyneuropathy (OPIDP). Peripheral neuropathy most commonly occurs following a prior episode of cholinergic toxicity and the intermediate syndrome; however, it remains possible that neuropathies could develop in the absence of frank cholinergic toxicity since the underlying mechanisms of these phenomena are unrelated to the genesis of the nerve fiber degeneration that is responsible for clinical signs of neuropathy. OPs with high acute potency may induce severe cholinergic toxicity such that death may intervene before sufficient time has elapsed for peripheral neuropathy to surface. However, if cholinergic toxicity is effectively treated, thereby allowing the animal or human to survive, peripheral neuropathy may thereby be allowed to surface weeks later.
Of the many laboratory species that develop axonal neuropathy after single or multiple exposures to OP pesticides, the young adult hen has been the subject of much experimental study. This work has identified a neuronal protein, neuropathy target esterase (NTE), as an important molecular target of OP pesticides that is also present in other tissues, including lymphocytes. Lymphocyte NTE inhibition induced by chlorpyrifos correlates with peripheral nerve NTE inhibition (CitationOsterloh et al., 1983). Studies with hens and other species demonstrate that clinical neuropathy develops in hens if > 50–90% (depending on the compound) of NTE enzyme activity is inhibited in nervous tissue within hours of dosing (CitationJohnson, 1990; CitationLotti, 1991). Similar degrees of neural NTE inhibition are required to induce neuropathy after single or smaller, repeated OP exposures, and prolonged NTE inhibition to a degree insufficient to trigger nerve damage fails to induce tolerance to the subsequent induction of OP neuropathy. While the relationship between neural NTE inhibition and the subsequent appearance of neuropathy is strong, a clear cause–effect relationship has not been unequivocally established. For any given OP pesticide, the degree of NTE enzyme inhibition correlates with the severity of the resulting neuropathy. OP neuropathy can be blocked by pretreatment with agents (carbamates, sulfonates, and phosphinates) that inhibit NTE but have little or no ability to induce neuropathy in their own right, but the same agents can potentiate (promote) the neuropathy-inducing activity of OPs (phosphates, phosphoroamidates, phosphonates). Other experimental animal studies show that carbamate and phosphinate promoters of OP-induced axonal neuropathy also magnify other toxic and traumatic axonopathies, suggesting that these NTE inhibitors impair a mechanism involved in axonal homeostasis (CitationLotti, 2000).
Systemic exposure to neuropathic OPs results in a symmetrical distribution of nerve damage, with the largest diameter and longest nerve fibers the first to undergo distal degeneration. Neuropathological study of peripheral neuropathy in humans and laboratory animals reveals the presence of distal, retrograde axonal degeneration of elongate nerve fibers in spinal pathways as well as limb nerves, a pattern known as CNS–PNS distal axonopathy (CitationCavanagh, 1963; CitationSpencer and Schaumburg, 1976). The motor pathway from frontal cortex to spinal cord, and from spinal cord to extremity muscles, is heavily affected. Primary sensory neurons that contribute axons in dorsal spinal columns and peripheral nerves are also involved. The resulting clinical picture of peripheral neuropathy is dominated by a symmetrical, distal distribution of motor weakness, with some involvement of peripheral sensory and autonomic function. The clinical appearance of neuropathy in adults characteristically appears a few weeks after a single large exposure and advances for a period of time before stabilizing and recovering to a greater or lesser extent. Peripheral neuropathy may also appear in the setting of chronic exposure to OP pesticides. After exposure ceases, recovery of strength and sensory function is associated with the growth and end-organ connection of regenerating peripheral axons; central axons in pyramidal and dorsal spinal tracts fail to regenerate, thereby resulting in the paradoxical appearance during recovery from severe neuropathy of lower-extremity spasticity. Residual sensory and autonomic dysfunction may persist for years after cessation of exposure. Children are reported to be resistant to and recover rapidly from peripheral neuropathy induced by the OP tricresyl phosphate (CitationSenanayake, 1981).
IV.B. Neurotoxicity of Chlorpyrifos
IV.B.1. Acute Cholinergic Toxicity: Controlled Studies in Human and Nonhuman Primates
A controlled clinical study of the short-term effects on overnight-fasted healthy male and female adult (18–55 years old) of a single oral dose of chlorpyrifos (99.8% purity, the 0.2% component of “active” and “nonactive” ingredients, not stated) was performed by Dow AgroSciences to determine the NOEL for erythrocyte AChE inhibition (CitationKisicki et al., 1999). The study was a double-blind, randomized, placebo-controlled, rising-dose design conducted in two phases separated by 14 days. Phase 1 included 6 males and 6 females randomly assigned to each of three groups receiving chlorpyrifos in dosages of 1.00 mg/kg, 0.5 mg/kg, or 0.00 mg/kg (lactose powder). Phase 2 employed 6 male and 6 female volunteers randomly assigned to each of two groups receiving chlorpyrifos dosages of 0.00 mg/kg (placebo) or 2.0 mg/kg. Clinical data included signs and symptoms termed adverse events, vital signs, electrocardiogram, clinical laboratory measurements, and determination of the enzyme activity of erythrocyte AChE. Blood samples were collected at 10 h and 0 h pretreatment and at 2, 4, 8, 12, 24, 36, 48, 72, 96, 120, 144, and 168 h post chlorpyrifos treatment. Total urine was voided at 4 time periods prior to (–48 h to 0 h) chlorpyrifos dosing and at 15 time periods after (0–168 h) chlorpyrifos treatment.
There was no convincing evidence of chlorpyrifos-related signs or symptoms in either the Phase 1 or Phase 2 study. Group comparisons revealed no statistical differences in erythrocyte AChE activity (on the basis that a minimum decrease of 17.3% is needed to identify a statistical depression when there are two preexposure measurements; CitationGallo and Lawryk, 1991). One female subject given 2.0 mg chlorpyrifos/kg body weight showed a 28% decrease at 12 h posttreatment, some recovery at 36 h and 48 h posttreatment, but had withdrawn from the study at later time points. The study authors concluded that a single dose of 1 mg chlorpyrifos/kg body weight is the NOAEL for erythrocyte AChE inhibition (reversible), with 2.0 mg/kg representing a threshold dose.
A pharmacokinetic study of chlorpyrifos (99.8% pure) was performed with 6 healthy male Caucasians 27–50 years old (CitationNolan et al., 1984). One pilot subject was given a single 0.5 mg/kg oral dose and, 1 month later, a single dermal dose of 0.5 mg/kg chlorpyrifos in methylene chloride, followed 2 weeks later by a second 0.5-mg/kg dermal dose in dipropylene glycol methyl ether (DPGME). Less than 5% of either dermal dose was absorbed. Other subjects received an oral dose of 0.5 mg/kg oral dose followed 4 weeks later by a dermal dose of 5.0 mg/kg chlorpyrifos in DPGME. No signs or symptoms of toxicity were observed in any subject. Plasma cholinesterase in the pilot subject was depressed to 29% of predose levels and to a mean of 15% of predose levels in the other subjects. No change was reported in erythrocyte cholinesterase levels, although a possible modest depression of enzyme activity is evident from the data provided.
A controlled clinical study of the short-term effects of repeated oral dose of chlorpyrifos (DOWCO 179, 99.5% pure) on 16 healthy adult male volunteers was performed at a New York correctional facility (CitationCoulston et al., 1972).Footnote5 Individuals received oral daily (number unstated) doses in tablet form of 0.10 mg/kg (9 days), 0.03 mg/kg (20 days), 0.014 mg/kg (28 days), or 0.00 mg/kg (48 days of placebo) chlorpyrifos administered with a breakfast meal. Twice-weekly heparinized blood samples were obtained from each volunteer for determination of plasma and erythrocyte cholinesterase activity. Additional blood samples were obtained weekly for hematology and routine serum chemistry determinations, the results of which were unremarkable. For plasma cholinesterase, the study authors state there was no significant reduction of enzyme activity (micromoles acetate/min/ml) in men treated with 0.014 mg/kg-day for 28 days. However, on days 13, 16, 23 and 27, all tested subjects had enzyme activities that were 10–20% lower than their individual mean baseline values. Men receiving daily doses of 0.03 mg/kg-day for 21 days were reported to have plasma BuChE values that averaged 87% of concurrent controls and 70% of baseline levels, with 2 of the 4 subjects showing 30–50% reduction on day 20. Individuals receiving 0.10 mg/kg-day chlorpyrifos averaged 34% of baseline values at 9 days. Taken in concert, these data suggest a dose-time-response relationship, with some variations across individuals, in which large reductions in plasma BuChE activity occur over relatively short periods (< 10 days) of 0.1 mg/kg-day, with smaller reductions over longer periods (> 10 days) with ∼ 10% smaller doses. For plasma BuChE inhibition, therefore, the effect threshold for adult men with prolonged daily brief oral exposure of chlorpyrifos is likely to lie at or below 0.01 mg/kg-day. This is three times lower than the level (0.03 mg/kg-day) proposed by the authors. Data for recovery of plasma cholinesterase enzyme activity in individual test subjects showed an inverse relationship between recovery rate and chlorpyrifos dosage. For erythrocyte AChE activity (micromoles acetate/min/ml), no changes of toxicological significance were observed during the treatment or recovery phases. Similarly, no clinical manifestations were reported except for subject number 3 (0.10 mg/kg-day chlorpyrifos), who complained on day 10 of “runny nose, blurred vision, and a feeling of faintness” and who, after treatment for a cold, was asymptomatic at the end of the day. While the symptoms are consistent with erythrocyte AChE inhibition, the value on day 9 was within normal limits when plasma BuChE activity was depressed from baseline by ∼ 70%. Baseline urine samples and urine samples obtained during the administration of DOWCO 179 were examined for the presence of DOWCO 179, its oxygen analog, and TCPy, but none of the compounds was detected in urine in any of the samples tested.
summarizes available studies in humans and a long-term study in rhesus monkey (CitationCoulston, 1971). Conclusions are: (a) The enzyme activity of plasma BuChE is more sensitive than the enzyme activity of erythrocyte AChE and (b) the threshold dosage for repeated oral exposure is 1–2 orders of magnitude (20-to 50-fold) lower than the threshold dosage for a single oral exposure.
TABLE 6 Controlled primate studies: Single dose and repeated dose
IV.B.2. Peripheral Neuropathy With or Without Intermediate Syndrome
OP pesticides are far better inhibitors of AChE than of NTE. CitationLotti (2000) has proposed a method of comparing the enzyme-inhibiting properties of a range of OP compounds by the ratio for the hen of the LD50/neuropathy dose, where the latter represents the dose required to induce hind limb weakness. When the ratio for the LD50/the neuropathy dose is > 1, the OP induces neuropathy at doses that do not cause fatal cholinergic toxicity in 50% of animals treated with single doses. Conversely, a ratio of < 1 describes compounds that induce neuropathy but only if the animal is treated with drugs (atropine, oximes) to curtail otherwise fatal cholinergic toxicity. Chlorpyrifos like other commercial pesticides has an LD50/neuropathy dose ratio of < 0.1. Cholinergic toxicity is therefore the limiting factor for development of chlorpyrifos neuropathy in hens. OP pesticides (including chlorpyrifos) therefore only cause peripheral neuropathy with doses that inevitably cause cholinergic toxicity (CitationLotti and Moretto, 2005).
The “rules” established in hens appear to hold in humans, although one report of a man with high oral exposure to commercial chlorpyrifos suggested that induction of clinical neuropathy might be associated with a lower degree of inhibition of NTE enzyme activity in the human relative to the hen (CitationLotti et al., 1986). As predicted from hen studies, acute exposures of adult humans to commercial chlorpyrifos resulting in cholinergic toxicity that required treatment with atropine and oxime have eventuated in the appearance of pure or predominantly motor neuropathy. Reports of sensory and sensory-motor neuropathies associated with subchronic chlorpyrifos exposures apparently insufficient to induce manifestations of acute cholinergic toxicity have been challenged on the unlikely grounds that chlorpyrifos has the potential only to induce a purely motor neuropathy (CitationMoretto and Lotti, 1998; CitationAlbers et al., 2004a). CitationDick et al. (2001) reported possible proprioceptive or vestibular differences in exposed pesticide applicators versus referents.
Several studies have sought to establish a relationship between exposure to chlorpyrifos, changes in the activity of targeted enzymes in blood, notably erythrocyte AChE and plasma BuChE, and the appearance of the chlorpyrifos metabolite TCPy in urine as an indicator of exposure (study details summarized in ). CitationLotti (2000) states that urinary metabolites have little value other than proving OP exposure, and that the inhibition of plasma BuChE has no neurotoxic significance and should not be used to establish NOAELs. Controlled studies of adult humans treated with small single or repeated oral or dermal doses of high-purity chlorpyrifos show that plasma BuChE enzyme activity declines in the absence of changes in erythrocyte AChE enzyme activity (CitationCoulston et al., 1972; CitationNolan et al., 1984); comparable studies examining changes in NTE enzyme activity have not been found. In a controlled study of Australian pest control operators, Dyer and colleagues (CitationDyer et al., 2001) found that Australian and British controls had similar values for erythrocyte AChE activity but dissimilar values for lymphocyte NTE (∼ 50% lower) and plasma AChE (23% lower) enzyme activities. The mean level of lymphocyte NTE was higher in exposed than referents.
TABLE 7 Summary of reports of the effects of uncontrolled exposures to chlorpyrifos
Case reports of acute exposure to commercial chlorpyrifos provide no further information on the differential susceptibility of human plasma BuChE, AChE, and NTE. A 1-year prospective study of adults engaged in chlorpyrifos manufacture demonstrated a modest mean depression of plasma BuChE enzyme activity and an absence of peripheral neuropathy or other clinical deterioration. Chronic exposures of workers to chlorpyrifos sufficient to induce mild depression of BuChE activity have failed to induce overt or subclinical evidence of sensory or motor neuropathy (CitationAlbers et al., 2004b). Axonal damage therefore seems most unlikely to occur in adults at chlorpyrifos exposures insufficient to inhibit the enzyme activity of erythrocyte AChE or plasma BuChE, the more sensitive enzyme. Nolan and colleagues (1984) have proposed that plasma BuChE is a sensitive indicator of chlorpyrifos exposure.
Taken together, systemic exposure to commercial chlorpyrifos may induce a self-limiting and largely reversible motor or sensorimotor neuropathy in adult humans exposed acutely to levels of the pesticidal agent that induce cholinergic toxicity. While peripheral neuropathy generally does not occur without prior depression of erythrocyte AChE activity, it is not possible with confidence to rule out minor, subclinical nerve damage in the absence of florid cholinergic toxicity.
In addition to the well-known OP-induced peripheral neuropathy, there are two reports of reversible bilateral vocal cord paralysis delayed in onset after single oral exposure to commercial chlorpyrifos and treatment for acute cholinergic toxicity (CitationAiuto et al., 1993; Citationde Silva, 1994) (). One case, a 3-year-old child with reportedly normal plasma BuChE within 3 days of chlorpyrifos exposure and treatment for cholinergic toxicity, developed areflexia and vocal cord paralysis during hospitalization days 11–18. The second case, a 21-year-old man, developed recurrent laryngeal paralysis 28 days after chlorpyrifos exposure and severe cholinergic toxicity that was sufficient to trigger a constellation of additional signs known as the intermediate syndrome. Known best from nonchlorpyrifos cases of OP toxicity, the intermediate syndrome is characterized by paralysis of proximal limb muscles, neck flexors, motor cranial nerves, and the muscles of respiration. Believed to arise from excessive activity at synaptic junctions during the phase of cholinergic toxicity, the clinical effects of this apparently subjunctional myopathy usually surface 1–4 days after severe OP-induced cholinergic toxicity, may be delayed for up to 15 days, and usually recover within 2 weeks (CitationLotti, 2000). The intermediate syndrome is mechanistically distinct from both acute OP-induced cholinergic toxicity and delayed-onset OP-induced peripheral neuropathy. Indeed, in the instance of the chlorpyrifos-poisoned man who developed intermediate syndrome requiring 10 days of intubation and intermediate positive-pressure ventilation, cranial nerve function was reported to be normal prior to the development of recurrent laryngeal paralysis. Notably, in both the man and the boy with this condition, electrophysiological and clinical studies revealed vocal cord paralysis with no evidence of distal symmetrical neuropathy. Absent F latencies in the young child raised the possibility of a proximal (demyelinating) neuropathy. The explanation for this phenomenon is unknown, but it is noteworthy that both subjects required prolonged intubation and ventilatory support.
Experimental studies suggest that OP pesticides can act directly on peripheral nerves to induce axonal degeneration. This is consistent with a report of asymmetric peripheral neuropathy following localized dermal exposure to chlorpyrifos (CitationMeggs, 2003) ().
IV.B.3. Effects of Chronic Chlorpyrifos Exposure on Cognitive Function in Adult Animals
CitationSamsam et al. (2005) evaluated the effects of chronic exposure to chlorpyrifos on learning and attention in 3-month-old Long-Evans rats, as well as the consequence of intermittent acutely toxic doses. No effects on learning or memory were observed in rats that received chlorpyrifos in the diet at a daily dose of 0, 1, or 5 mg/kg for 1 year. However, animals that received additional, “high-dose” chlorpyrifos (initial dose of 60 mg/kg, followed every 2 months with single doses of 45 mg/kg) did exhibit deficits in learning. The authors concluded that “learning the contingency between an action and reward may be accelerated by chronic exposure to chlorpyrifos and inhibited by previous symptomatic exposure to [chlorpyrifos], and that persistent cognitive impairment may follow if chlorpyrifos exposure inhibits brain ChE activity and is accompanied by acute doses sufficient to induce signs of toxicity.” Because of the large doses used and the necessity for frank cholinergic toxicity to be present on repeated occasions, these results have little relevance to potential concerns for low-dose exposures to chlorpyrifos in the diet, but could be relevant to high-dose exposures that give rise to serious cholinergic toxicity, such as intentional exposures (suicide attempts) or occupational accidents. Numerous other studies have also failed to find persistent cognitive dysfunction (e.g., effects that persist after recovery from cholinesterase inhibition) following single or repeated exposures to chlorpyrifos at doses that inhibit brain AChE, although neurological and behavioral effects were clearly evident during periods of significant inhibition of brain AChE (CitationBushnell et al., 1993; Citation1994; CitationCohn and MacPhail, 1997; CitationMattsson et al., 1996; CitationMaurissen et al., 2000; CitationMoser and Padilla, 1998; CitationTerry et al., 2003).
IV.C. Age-Dependent Sensitivity to the Acute Cholinergic Toxicity of Chlorpyrifos
Several investigators have shown that young animals are more susceptible than adults to the acute toxicity of chlorpyrifos (CitationPope et al., 1991b; CitationWhitney et al., 1995; CitationMoser et al., 1996; CitationZheng et al., 2000). For example, the LD50 of chlorpyrifos in neonatal [postnatal day (PND) 7] rats was reported to be 10-fold lower than in adult animals (CitationZheng et al., 2000). The maximal tolerated dose (MTD) of chlorpyrifos was estimated to be 15 mg/kg in 10-day-old rats versus 100 mg/kg in adult rats (ratio = 6.7) (CitationMoser and Padilla, 1998). Similarly, CitationPope and Chakraborti (1992) reported MTD values for chlorpyrifos of 45 mg/kg in 7-day-old rats and of 279 mg/kg in adult rats (ratio = 6.2). CitationVidair (2004) reported that in almost all comparisons between young and adult rats, young animals were more sensitive than adults. As indicated later in this review (see section on studies by Pope et al.), age-related differences are much less pronounced (or even nonexistent) in case of repeated exposures to chlorpyrifos.
Sensitivity of brain AChE to chlorpyrifos-oxon inhibition is similar in neonate (PND 4) and in adult rats, with IC50 values of 5.2 and 4.6 nM, respectively (CitationMortensen et al., 1996b, Citation1998b). Similarly, CitationAtterberry et al. (1997) reported that chlorpyrifos-oxon inhibits cerebral cortex AChE with IC50s of 3.7 and 3.8 nM in PND1 and PND 80 rats, respectively. This indicates that intrinsic differences in brain AChE do not account for the age-related differences in susceptibility to acute chlorpyrifos toxicity. A recent study (CitationKousba et al., 2007) reported a Ki for AChE inhibition by chlorpyrifos-oxon in brain homogenates that was fivefold higher in PND 5 rats than in PND 17 animals, implying an age-related difference in AChE sensitivity. However, this result may be due to a differential contribution of extrinsic factors (that may bind to and/or inactivate chlorpyrifos-oxon), present in the crude homogenate, and displaying age-related differences (CitationPadilla et al., 2000; CitationKousba et al., 2007). For example, in brain membranes from wild type mice, the IC50 for AChE inhibition was reported to be 4 nM; however, it was 0.7 nM in brain tissue from mice lacking KIAA1363, a serine hydrolase that binds chlorpyrifos-oxon with relatively high affinity (CitationNomura et al., 2006).
As discussed also in Section II.C.2., differential detoxication abilities, due in large part to differences in expression of paraoxonase in young animals compared to adults, are believed to be the major determinant of the age-dependent susceptibility to acute cholinergic toxicity of chlorpyrifos.
IV.C.1. Developmental Neurotoxicity of Chlorpyrifos: In Vivo Animal Studies
The possible developmental neurotoxicity of chlorpyrifos has been investigated in a substantial number of studies in rodents, mostly in rats. Only one or two studies were carried out according to regulatory guidelines for developmental neurotoxicity testing. Doses and routes of administration of chlorpyrifos vary, as do the periods of exposure (e.g., pre-and postnatal) and measured endpoints (biochemical, molecular, behavioral).
However, plasma BuChE activity at birth was found to be one-fourth that of adult levels in humans (CitationKisicki et al., 1999). If true, this would substantially increase the amount of unbound chlorpyrifos in the newborn and, presumably, prior to birth—which could modify susceptibility to acute cholinergic toxicity of chlorpyrifos.
Studies of Chambers et al. Chambers and her colleagues carried out a series of studies involving exposure to chlorpyrifos during development in rats. The main findings of these studies are summarized in . Changes in muscarinic receptors, choline transporters, and locomotor activity were found. In all studies, the doses of chlorpyrifos utilized caused significant inhibition of brain AChE in pups.
TABLE 8 In vivo developmental neurotoxicity studies with chlorpyrifos (Chambers et al.)
Studies of Pope et al. Pope and collaborators () focused primarily on AChE inhibition by chlorpyrifos in young and adult rats, and in several cases assessed other neurochemical features of the cholinergic system and behavioral endpoints. Some studies aimed at determining maximum tolerated doses (MTDs) of chlorpyrifos in neonatal and adult rats are not included in , which summarizes only results of developmental neurotoxicity studies.
TABLE 9 In vivo developmental neurotoxicity studies with chlorpyrifos (Pope et al.)
Changes were found in muscarinic receptors, acetylcholine synthesis, and adenylate cyclase. Of particular interest is a study in which the effects of acute and repeated chlorpyrifos administrations were compared in neonatal (PND 7) and adult rats (CitationZheng et al., 2000). Upon acute exposure, the ED50s for brain and plasma AChE inhibition were 1.5–2.9 mg/kg in neonates and 3.9–4.4 mg/kg in adults, with a ratio of NOEL values between neonates and adults of ∼ 10. This finding confirms the higher susceptibility of young rats to the acute toxicity of chlorpyrifos. With repeated exposures (14 daily doses from PND 7 or in adults), however, the ED50s were 1.2–2.2 mg/kg-day in neonates and 0.5–3.3 mg/kg-day in adults, and the ratios of NOEL values, based on AChE inhibition, between neonates and adults were 0.2 (erythrocyte) and 2 (brain). This indicates that differences in sensitivity appear to be lesser in magnitude with lower level repeated exposures. This has been ascribed to the more rapid recovery of AChE activity following exposure in neonates (CitationPope et al., 1991a; CitationMoser and Padilla, 1998; CitationLiu et al., 1999; CitationAshry et al., 2002). This in turn may be due to a higher basal rate of protein synthesis in the developing animal (CitationMichalek et al., 1982).
Studies of Abou-Donia et al. Abou-Donia and his colleagues carried out a series of studies investigating developmental effects associated with prenatal chlorpyrifos exposure in rats. The principal findings are summarized in . In general, the doses of chlorpyrifos utilized caused significant inhibition of AChE activity. In one study (CitationAbou-Donia et al., 2006), behavioral and histological changes were found at PND 90 in rats exposed in utero to chlorpyrifos [1 mg/kg-day on gestational day (GD) 4–20]. At PND 90, AChE activity in the brainstem was increased. However, no information was provided on AChE activity at earlier time points.
TABLE 10 In vivo developmental neurotoxicity studies with chlorpyrifos (Abou-Donia et al.)
Studies of Slotkin et al. , , and summarize the main findings obtained by Slotkin and colleagues in a large number of studies utilizing different pre-and postnatal exposure protocols. In all these studies chlorpyrifos was dissolved in dimethyl sulfoxide (DMSO) and administered by subcutaneous (sc) injection to rat pups. Four main protocols of exposure can be identified: (1) exposure on PND 1–4 to 1 mg/kg-day chlorpyrifos; (2) exposure on PND 11–14 to 5 mg/kg-day of chlorpyrifos; (3) prenatal exposure on GD 9–12 to 1 or 5 mg/kg-day chlorpyrifos; and (4) prenatal exposure on GD 17–20 to 1 or 5 mg/kg-day chlorpyrifos. In addition, a few studies utilized slightly different protocols, mainly involving additional dose levels. Biochemical, molecular, and behavioral endpoints were assessed at different intervals upon termination of exposure to determine short-and long-term effects of chlorpyrifos exposure.
presents a summary of the findings obtained upon postnatal exposure to 1 mg/kg-day chlorpyrifos on PND 1–4. This exposure to chlorpyrifos was found to cause a number of biochemical, molecular, and behavioral effects. These include changes in RNA content, in DNA synthesis, in neurochemical parameters related to the cholinergic, serotoninergic, and noradrenergic systems, in the expression of glial markers proteins and of growth factors, in signal transduction systems (adenylate cyclase), and in various behavioral endpoints. In two studies, brain AChE activity following the treatment was determined. CitationSong et al. (1997a) measured AChE activity in the brainstem on PND 5 (24 h after the last chlorpyrifos administration) and on PND 10 (6 days after the last administration of chlorpyrifos); AChE activity was decreased by 25% and 10%, respectively (see in reference, CitationSong et al., 1997b). Given the rapid recovery of AChE activity in neonatal rats upon inhibition by an OP pesticide (CitationPope et al., 1991a; CitationMoser and Padilla, 1998; CitationLiu et al., 1999; CitationAshry et al., 2002), inhibition of AChE would be predicted to be even greater shortly after exposure to chlorpyrifos. Indeed, in another study (CitationDam et al., 2000), AChE activity was measured at 2 and 4 h following the first sc administration of 1 mg/kg chlorpyrifos on PND 1. At 2 h AChE activity was decreased by ∼ 75% in the brainstem, ∼ 60% in the forebrain, and ∼ 75% in the cerebellum ( in CitationDam et al., 2000). Inhibition of AChE was greater in male pups than in females, and was less at 4 h than at 2 h, indicating a rapid recovery. Thus, the dosage protocol of 1 mg/kg-day chlorpyrifos on PND 1–4, which has been reported to not cause any overt sign of toxicity or changes in body weight (CitationSlotkin et al., 2006), does cause significant inhibition of AChE activity in the brain of rat pups, which appears to recover somewhat between daily doses and/or over repeated dosing, from the maximal inhibition that occurs hours after dosing.
TABLE 11 In vivo studies with chlorpyrifos (1 mg/kg-day on PND 1–4) (Slotkin et al.)
presents a summary of the effects observed upon chlorpyrifos exposure on PND 11–14 at the dose of 5 mg/kg-day, administered by the subcutaneous route. Numerous biochemical, molecular, and behavioral changes, mostly in the same domains as those investigated in the earlier exposure, were found. Measurements of AChE activity were reported in two studies. CitationSong et al. (1997b) indicated that AChE activity in the brainstem was decreased by 65% 24 h after the last dose of chlorpyrifos on PND 14, and was still 25% inhibited on PND 20, 6 days after termination of treatment. CitationDam et al. (2000) showed that chlorpyrifos caused a 10% (brainstem), 20% (forebrain), and 25% (cerebellum) inhibition of brain AChE at 2 and 4 h after the first injection (on PND 11). These findings differ from those obtained in the earlier study, in which inhibition of AChE was higher at earlier time-points after chlorpyrifos administration. This suggests that repeated administrations of chlorpyrifos have a “cumulative inhibitory” effect on AChE activity. Nevertheless, as for the earlier exposure, administration of 5 mg/kg-day chlorpyrifos on PND 11–14 caused significant inhibition of brain AChE.
TABLE 12 In vivo studies with chlorpyrifos (5 mg/kg-day on PND 11–14) (Slotkin et al.)
summarizes the effects seen after prenatal exposure to chlorpyrifos. In most studies, two dose levels of chlorpyrifos were used (1 or 5 mg/kg-day) during early (GD 9–12) or late (GD 17–20) gestation. In some studies additional higher dose levels were also used. Early prenatal exposure to chlorpyrifos (1 or 5 mg/kg-day, GD 9–12) was shown to cause a number of effects such as changes in cholinergic, serotoninergic, and noradrenergic neurochemistry, and behavioral changes (CitationAldridge et al., 2003a; CitationMeyer et al., 2003; CitationAldridge et al., 2004; CitationIcenogle et al., 2004; CitationQiao et al., 2004; CitationSlotkin and Seidler, 2007). However, no information on AChE activity in brain or blood of dams or fetuses upon such exposure was provided. The investigators, however, stated that “the effects seen required doses closer to the threshold for fetal weight loss; this implies a lower vulnerability in the fetal compared with neonatal brain” (CitationQiao et al., 2002a).
TABLE 13 Summary of studies on effects of prenatal exposures to chlorpyrifos PND 17–20 (Slotkin et al.)
Prenatal exposure to chlorpyrifos (1 or 5 mg/kg-day) on GD 17–20 was also shown to cause a number of biochemical and behavioral alterations (). Information on AChE activity was provided in one study (CitationQiao et al., 2002a). In brain of fetuses on GD 21 (24 h after the last chlorpyrifos administration) chlorpyrifos caused a dose-dependent inhibition of brain AChE: no significant inhibition at 1 mg/kg-day; 15–20% at 2 mg/kg-day; 40–50% at 5 mg/kg-day; 60–70% at 10 mg/kg-day; 80% at 20 mg/kg-day; and > 80% at 40 mg/kg-day ( in reference, CitationQiao et al., 2002b). Thus, the 1-mg/kg-day dose appears to be a NOEL for sustained fetal brain AChE inhibition, notwithstanding the considerations that because of the rapid recovery of AChE during brain development, actual transient inhibition at earlier time points (e.g., during exposure or at 2–4 h following the last exposure) is likely to occur (CitationDam et al., 2000). A few effects were seen upon exposure to the low dose of chlorpyrifos (1 mg/kg-day on GD 17–20). These include an increase in serotonin receptors and an increased response of adenylate cyclase to serotonin at GD 21 in forebrain (CitationAldridge et al., 2003b; CitationMeyer et al., 2004); an increase in serotonin and dopamine turnover (CitationMeyer et al., 2004; CitationAldridge et al., 2005); and behavioral changes such as a decreased latency in the spontaneous alternation task, and in working memory errors in the radial arm maze (CitationLevin et al., 2002).
Other Studies. In a study by CitationMaurissen et al. (2000) carried out according to the U.S. EPA developmental neurotoxicity study guidelines, chlorpyrifos was given in corn oil by gavage to pregnant rats from GD 6 through lactational day 10, at doses of 0.3, 1.0 or 5.0 mg/kg-day. Exposure of pups thus occurred only in utero and through maternal milk. Toxicity was evident in the dams and pups at the high dose. No observable effects were found at any dose level in a number of behavioral tests (motor activity, auditory startle, delayed spatial alternation). Neuropathological examination was also unremarkable. Blood levels of chlorpyrifos and TCPy and brain AChE activity were also measured (CitationMattsson et al., 2000). In pups, AChE activity was significantly decreased in brain (∼ 60%) and blood (> 80%) on GD 20 at the high dose (5 mg/kg-day). Significant inhibition was still present in brain on PND 1 and in blood on PND 11. No AChE inhibition was found in pups in either brain or blood, at the two lower doses of chlorpyrifos. AChE activity in dams was decreased by 1 and 5 mg/kg-day chlorpyrifos in brain and blood, and only in blood by the lowest dose. The highest level of chlorpyrifos in blood of fetuses (46.1 ng/g) was found on GD 20 in the 5-mg/kg-day group. By PND 1, chlorpyrifos level had decreased to 12.4 ng/g, and chlorpyrifos was undetectable at later time points. Concentrations of TCPy in the blood of fetuses or pups on GD 20 and PND1 ranged from 97 to 1782 ng/g, and from 50 to 638 ng/g, respectively, depending on the dose of chlorpyrifos. These values are in agreement with those reported by other investigators (e.g. (CitationHunter et al., 1999; CitationTimchalk et al., 2006)). This study thus did not identify any neurotoxic effect of chlorpyrifos upon developmental exposure utilizing EPA-implemented protocols, even at dose levels that caused significant inhibition of AChE. In a follow-up study by the same group (CitationMarty et al., 2007), levels of chlorpyrifos and TCPy were quantified in pup's blood after administration of a single dose of chlorpyrifos (1 mg/kg on PND 5). Depending on the mode of administration (by gavage in corn oil or in milk; sc in DMSO), blood chlorpyrifos levels ranged between 9 and 49 ng/g (0.02–0.14 μ M). TCPy levels ranged from 171 to 320 ng/g (0.8–1.5 μ M).
CitationMoser and Padilla (1998) reported a decrease in muscarinic receptors in different brain areas following an acute exposure to chlorpyrifos (15 mg/kg in corn oil by gavage) on PND 17. This dose caused significant (50–90%) inhibition of brain and blood AChE and behavioral signs of cholinergic toxicity.
CitationMoser (2000) compared behavioral effects of chlorpyrifos given to PND 17 rats (4, 10, 20 mg/kg in corn oil by gavage), PND 27 rats (10, 25, 50 mg/kg), and adult rats (10, 50, 100 mg/kg). The doses were selected to provide a range giving a no-or low-effect level and the MTD for each age group. The high doses in each group caused 85–90% inhibition of brain AChE (CitationMoser et al., 1998). A similar degree of AChE inhibition was associated with a similar magnitude of dysfunction for some endpoints (e.g., gait, tremor), but not for others. For example, salivation, pupil response, and click response were not altered in the young rats.
Carr and his colleagues (CitationBetancourt and Carr, 2004; CitationBetancourt et al., 2006) treated rat pups on PND 1–6 with 1.5 or 3.0 mg/kg-day chlorpyrifos (by gavage in corn oil). Decreased levels of mRNA for NGF, reelin and muscarinic M1 receptors, and an increase in GFAP mRNA were found in forebrain on PND 7. These doses of chlorpyrifos caused no overt signs of toxicity, but significantly (28, 43%) inhibited brain AChE activity.
In a study by CitationGultekin et al. (2007), chlorpyrifos at the dose of 40 mg/kg was administered sc to juvenile (PND 30) rats. This dose, which caused > 50% decrease of plasma AChE activity on PND 32, caused a small increase in hippocampal glutamate NMDA receptor subunits NR2A and NR2B on PND 44.
In contrast to the studies just described, in which developmental neurotoxic effects, if seen, were associated with significant decreases in AChE activity, a few studies were identified in which developmental exposure to chlorpyrifos was associated with effects at dose levels that did not cause any apparent or persistent AChE inhibition. In a study by CitationJett et al. (2001), rats were injected sc with chlorpyrifos (dissolved in corn oil) on PND 7, 11, and 15 or on PND 22 and 26. In both cases, doses were 0.3 and 7.0 mg/kg. Behavioral testing (the Morris swim task) was conducted between PND 24 and PND 28 (i.e., 9 days after the last injection in the first group, and during chlorpyrifos treatment in the second group). The investigators state that these exposure protocols were chosen to investigate effects of exposure before and after weaning (which occurs at PND 21). Upon preweaning exposure, statistically significant effects (in time latency and time spent in training quadrant) were found upon exposure to the high dose (7.0 mg/kg) but not to the low dose (0.3 mg/kg) of chlorpyrifos. When exposure occurred after weaning, significant effects in the same parameters were found at both dose levels of chlorpyrifos. However, despite a 23-fold difference in dose levels, no dose response was observed, as both doses caused identical changes in the measured parameters. No changes in muscarinic receptors in different brain regions were found on PND 16 in the preweaning-dosed rats. None of the treatments caused any apparent inhibition of AChE activity in different brain areas at 3 h or 24 h after chlorpyrifos administration. The lack of AChE inhibition upon exposure to the high dose of chlorpyrifos (7.0 mg/kg) is surprising, as others have shown significant inhibition of brain AChE upon oral exposure to similar and even lower doses (CitationZheng et al., 2000). In this study, chlorpyrifos was dissolved in corn oil and given by sc injection, and the absorption and bioavailability of chlorpyrifos upon such exposure are not known.
CitationRicceri et al. (2003) examined the effects of postnatal exposure to chlorpyrifos on PND 1–4 or PND 11–14 in mice. Chlorpyrifos was dissolved in DMSO and injected sc at the doses of 1 or 3 mg/kg-day. Solvent, route of administration, and the low dose are also identical to those utilized by Slotkin and his colleagues. These exposures had no effect on body weight gain and did not cause any overt sign of toxicity. The early treatment (PND 1–4, at either dose) had no effect on a number of behavioral endpoints (locomotor activity on PND 25, novelty seeking on PND 35–38, passive avoidance on PND 60). However, treatment with chlorpyrifos (PND 11–14) caused an increase in locomotor activity on PND 25, and an increased agonistic response in male mice at both dose levels. AChE activity was measured in whole brain at different intervals after chlorpyrifos exposure. On PND 4, both 1 and 3 mg/kg-day chlorpyrifos caused 20–23% inhibition of AChE activity 1 h after the last treatment, with a complete recovery by 4 h. This degree of AChE inhibition is less than that previously observed in rats upon the same exposure protocol (CitationSong et al., 1997a; CitationDam et al., 2000). This is somewhat surprising since mice are generally more susceptible than rats to the acute toxicity of chlorpyrifos (e.g., LD50 = 60 mg/kg in mice; 90–240 mg/kg in rats). No brain AChE inhibition was seen on PND 14, 1 h following exposure to 1 or 3 mg/kg chlorpyrifos on PND 1–14. As indicated earlier, a slightly higher dose (5 mg/kg-day) caused significant AChE inhibition in rats (CitationSong et al., 1997a; CitationDam et al., 2000). Furthermore, using the same doses of chlorpyrifos (1 and 3 mg/kg-day on PND 11–14), a subsequent study (CitationRicceri et al., 2006; see later description) reported a 25–45% inhibition of serum AChE (in this latter study chlorpyrifos was dissolved in peanut oil instead of DMSO and measurements were done 1 day later, on PND 15). Nevertheless, results of this study indicate behavioral effects of chlorpyrifos at doses causing no apparent inhibition of brain AChE activity.
In a subsequent study, CitationRicceri et al. (2006) treated pregnant mice with chlorpyrifos (3 or 6 mg/kg-day on GD 15–18), and offspring were tested on PND 70 for spontaneous locomotor activity in an open field, in a plus maze test for anxiety, and for socioagonistic (aggressive) behavior. Chlorpyrifos was dissolved in peanut oil and administered by gavage. Groups of control mice, as well as mice exposed prenatally to chlorpyrifos, were also exposed to chlorpyrifos postnatally on PND 11–14 (1 or 3 mg/kg-day in peanut oil by sc injection). Prenatal exposure to 6 mg/kg-day chlorpyrifos caused hyperactivity; this effect was also present in mice that, in addition to prenatal exposure, were also given 1 mg/kg-day chlorpyrifos on PND 11–14. Surprisingly, however, hyperactivity was not seen if prenatal exposure was followed by PND 11–14 exposure to a higher dose (3 mg/kg-day). In animals treated only postnatally, the 3 mg/kg-day dose caused hyperactivity, as reported also in the previous study (CitationRicceri et al., 2003). Male mice exposed prenatally or postnatally to the high dose of chlorpyrifos (6 and 3 mg/kg-day chlorpyrifos, respectively) displayed increased agonistic behavior. In female mice, postnatal exposure only (both doses) increased maternal behavior in virgin animals. In the plus maze, percent time spent in the open arms increased in female mice exposed postnatally to 3 mg/kg-day chlorpyrifos, suggesting a decrease in anxiety. AChE activity was measured in brain and serum in all treatment groups. Upon prenatal exposure, AChE activity in brain of pups on the day of birth was not affected by the treatment, but serum AChE activity was significantly inhibited (percentage not specified, p < .001 for both doses). On PND 15, both brain and serum AChE activities were unchanged by prenatal exposure to chlorpyrifos. Still, on PND 15, mice exposed prenatally to solvent and on PND 11–14 to chlorpyrifos (1 or 3 mg/kg-day) showed no changes in brain AChE activity (as in CitationRicceri et al., 2003), but a significant decrease (25 and 45%, respectively) of serum AChE. In animals that were exposed both prenatally and postnatally to chlorpyrifos, AChE activity was not affected in brain, but was decreased by 40 to 50% in serum.
Utilizing the same exposure protocol as CitationRicceri et al. (2003), CitationVenerosi et al. (2006) investigated effects of developmental exposure to chlorpyrifos on a social recognition test in female mice on PND 120. Small but statistically significant effects were found in mice that were exposed to 6 mg/kg-day in utero (GD 15–18), followed by a 3-mg/kg-day exposure on PND 11–14, but not in any other group. As indicated earlier, this treatment protocol was reported to inhibit plasma AChE activity by 40–50%, without affecting brain AChE (CitationRicceri et al., 2003).
In summary, most in vivo studies with chlorpyrifos reported neurochemical and/or behavioral effects that were seen upon exposure to doses of chlorpyrifos that caused inhibition of brain and/or plasma AChE activity. However, a handful of studies exist where effects were seen at dose levels that did not cause any apparent AChE inhibition (notwithstanding the caveats of the kinetics of AChE inhibition and fast recovery at early ages) or where information on AChE inhibition was not reported. These studies would imply that mechanism(s) other than AChE inhibition may be responsible for the observed effects of chlorpyrifos, due to chlorpyrifos itself, and/or its metabolites. The potential roles of BuChE or KIAA1363 were not examined in these developmental studies.
IV.C.2. Developmental Neurotoxicity of Chlorpyrifos: Mechanistic Studies
Several studies have examined in vitro effects of chlorpyrifos, and/or chlorpyrifos-oxon, and in some cases also of TCPy. Various models were utilized, and several endpoints were measured; these are summarized in , which also indicates effective concentrations for each compound (or IC50 values).
TABLE 14 Summary of in vitro effects of chlorpyrifos and chlorpyrifos metabolites
A wide variety of effects was seen with all three compounds. Data summarized in are divided into eight different “domains”: These include cytotoxicity, effects on macromolecule synthesis (DNA, RNA, proteins), interactions with neurotransmitter receptors, interactions with signal transduction pathways, effects on neuronal differentiation, interactions with various enzymes, other neurochemical effects (e.g., neurotransmitter release or uptake), and other effects (e.g. oxidative stress, effects on microtubules). Studies related to interactions with NTE are discussed elsewhere. In general, for all compounds, the effects were seen at concentrations that exceeded those found in vivo upon exposure to chlorpyrifos, or, in the case of chlorpyrifos-oxon, sufficient to inhibit AChE. However, a few exceptions exist, and these are discussed more in detail later.
In the case of chlorpyrifos, the effects were usually seen at low micromolar (1–50 μ M) concentrations. TCPy was usually less potent than chlorpyrifos, with effects seen at higher (15–150 μ M) micromolar concentrations. Chlorpyrifos-oxon was equally or more potent than chlorpyrifos, with effective concentrations ranging mostly from 0.1 to 30 μ M.
In humans, upon exposure to 1–2 mg/kg chlorpyrifos, blood chlorpyrifos concentrations are 1–10 nM, while at exposure equivalent to the RfD for chlorpyrifos (0.003 mg/kg-day), blood chlorpyrifos levels are estimated at 0.01nM (CitationDybowski et al., 2001). Upon exposure of PND 5 rats to 1 mg/kg chlorpyrifos, a dose causing AChE inhibition, blood chlorpyrifos concentrations range between 20 and 140 nM, depending on the mode of administration (CitationTimchalk et al., 2006; CitationMarty et al., 2007). Thus, the effects of chlorpyrifos reported in vitro occur mostly at higher concentrations than those found following in vivo exposure.
Upon exposure to 7 mg/kg chlorpyrifos (oral) in pregnant rat on GD 14–18 (a dose level that causes AChE inhibition), the highest concentration of TCPy found in brains of fetuses was 250 ng/g (CitationHunter et al., 1999). Other studies reported concentrations of TCPy of 50–1782 ng/g (CitationMattsson et al., 2000), of 171–320 ng/g (0.8–1.5 μ M) (CitationMarty et al., 2007), and of 3.6 μ M (CitationTimchalk et al., 2006), depending on the dose, route of administration, and time of measurement. These concentrations are lower than those found to be effective in in vitro experiments.
With regard to chlorpyrifos-oxon, very limited information is available on its levels following in vivo chlorpyrifos exposure, as it would be expected to phosphorylate AChE and other esterases shortly after its formation. In one study, levels of chlorpyrifos-oxon of 1 ng/g were detected in GD 20 fetal blood following exposure to 3 mg/kg chlorpyrifos from GD 6 (CitationMattsson et al., 2000). AChE inhibition in vitro is seen at concentrations of chlorpyrifos-oxon of 1–10 nM (CitationMortensen et al., 1996a; CitationAtterberry et al., 1997; CitationMortensen et al., 1998a), while chlorpyrifos and TCPy are practically devoid of AChE inhibitory activity (inhibition of AChE observed with chlorpyrifos is most likely due to contamination with chlorpyrifos-oxon, while upon prolonged incubation of cells with chlorpyrifos some degree of metabolic activation of chlorpyrifos to chlorpyrifos-oxon may occur). In vitro effects of chlorpyrifos-oxon are usually (with a few exceptions) seen at concentrations higher than those sufficient to inhibit AChE.
Cytotoxicity, measured by different approaches, was usually observed at micromolar concentrations in case of chlorpyrifos. For this endpoint, chlorpyrifos-oxon appears to be only slightly more potent than chlorpyrifos, while TCPy displays the least cytotoxicity. All three compounds were found to inhibit macromolecule synthesis, in particular DNA synthesis, at micromolar concentrations. Again, chlorpyrifos and chlorpyrifos-oxon appear to be essentially equipotent, while TCPy displayed a much lower potency. Decreased DNA synthesis has also been reported following in vivo developmental exposure to chlorpyrifos (CitationDam et al., 1998).
A few studies investigated the interaction of chlorpyrifos and its metabolites with cholinergic muscarinic receptors. Chlorpyrifos displayed a low ability to displace agonists or antagonists from muscarinic receptors in binding studies. In contrast, chlorpyrifos-oxon displaces agonist binding to muscarinic M2 receptors with relatively high potency (IC50s = 7–22 nM), while being ineffective toward binding of muscarinic antagonists. It should be noted that these studies utilized the heart as a source of muscarinic M2 receptors. Nevertheless, these concentrations are only slightly above those necessary to inhibit the enzyme activity of AChE. Alterations (decreased density) of muscarinic M2 receptors have been observed in various studies following developmental exposure to chlorpyrifos (CitationLiu et al., 1999; CitationTang et al., 1999; CitationQiao et al., 2002b; CitationRhodes et al., 2004; CitationRichardson and Chambers, 2004, Citation2005). However, as these changes were associated with AChE inhibition, the role played by direct interaction of chlorpyrifos-oxon with the receptors (vs. the effect of accumulated acetylcholine) cannot be readily discerned. Chlorpyrifos-oxon was also reported to interact with cannabinoid CB1 receptors (IC50 = 14 nM), while chlorpyrifos was active only at micromolar concentrations. No information on TCPy is available. Administration of chlorpyrifos in vivo, in adult mice, caused inhibition of cannabinoid receptors, but only at a dose causing signs of cholinergic intoxication (CitationQuistad et al., 2002).
Different signal transduction pathways were found to be affected by chlorpyrifos-oxon. Of note is inhibition of adenylate cyclase activity, possibly mediated by activation of muscarinic M2 receptors, where IC50 values for chlorpyrifos-oxon ranged from 15 to 155 nM. Effects of chlorpyrifos on the adenylate cyclase system following exposure during prenatal or postnatal development have been reported in several studies (CitationSong et al., 1997a; CitationAldridge et al., 2003a; CitationMeyer et al., 2004, Citation2005). Alterations of nuclear transcription factors AP-1 and Sp1 have also been reported both in vitro and after in vivo administration of chlorpyrifos (CitationCrumpton et al., 2000a; CitationGarcia et al., 2001).
The effects of chlorpyrifos and chlorpyrifos-oxon on neuronal differentiation were investigated in various cellular systems. Both compounds inhibited neuronal differentiation at micromolar concentrations. However, studies by CitationHoward et al. (2005) and by CitationYang et al. (2008) reported effects of chlorpyrifos and chlorpyrifos-oxon in superior cervical ganglia and dorsal root ganglia at nanomolar, or less than nanomolar concentrations (see later discussion).
Various enzymes, a few related to aspects of signal transduction, were found to be inhibited by chlorpyrifos-oxon, some at medium to high nanomolar concentrations. One in particular, the serine hydrolase KIAA1363, was inhibited by chlorpyrifos-oxon with an IC50 of 8 nM, well within the range of AChE inhibition. However, upon in vivo administration of chlorpyrifos-oxon to mice, a 39% inhibition of brain KIAA1363 was associated with a 98% inhibition of AChE, and lethality (CitationNomura et al., 2006). On the other hand, KIAA1363 may serve as an important detoxication mechanism for chlorpyrifos-oxon (much like serum and liver paraoxonase-1 and carboxylesterase) in brain. Indeed, as mentioned earlier, the IC50 for chlorpyrifos-oxon for mouse AChE inhibition was sixfold higher in brain from wild-type mice than from KIAA1363 knockout mice, indicating a protective role for this enzyme (CitationNomura et al., 2006).
In other neurochemical parameters that were examined, related to the cholinergic system or other neurotransmitter systems, effects of chlorpyrifos or chlorpyrifos-oxon were seen at micromolar concentrations. Some of the changes (e.g., ChAT) have also been observed following developmental exposure to chlorpyrifos (CitationDam et al., 1999; CitationSlotkin et al., 2001; CitationRichardson and Chambers, 2003, Citation2005). Finally, various other in vitro effects have also been reported for these three compounds (). All were observed at micromolar concentrations, with the one exception being the phosphorylation of CREB in rat cortical neurons, which was reported to occur at extremely low concentrations (CitationSchuh et al., 2002). This study, which also reports effect of TCPy at picomolar concentrations (i.e., six orders of magnitude less the any other study), is discussed later.
As stated previously, two studies reported effects of chlorpyrifos, chlorpyrifos-oxon and TCPy at very low concentrations. In a study by CitationSchuh et al. (2002), rat cortical and hippocampal neurons were utilized to test the effect of chlorpyrifos, chlorpyrifos-oxon, and TCPy on the phosphorylation of CREB (Ca2 +/cAMP response element binding protein), a transcription factor suggested to play a role in synaptic plasticity and in cell survival and differentiation (see references in CitationSchuh et al., 2002). In cortical neurons, a 1-h exposure to chlorpyrifos (dissolved in ethanol) was found to induce a threefold increase in pCREB, with an IC50 of ∼ 0.06nM. Both chlorpyrifos-oxon and TCPy produced the same effect, with IC50 values of ∼ 0.00003 nM and ∼ 0.03 nM, respectively. Similar effects were seen in hippocampal neurons, but at higher concentrations (chlorpyrifos, 1–10 nM; chlorpyrifos-oxon, 0.01–0.1 nM). No effects were seen in cortical astrocytes. None of the three compounds affected cell viability, while the lowest concentrations causing AChE inhibition were 1 μ M (chlorpyrifos) and 1 nM (chlorpyrifos-oxon). TCPy (up to 10 μ M) did not inhibit AChE. The functional significance of such increase in pCREB is unknown, and the authors suggest that it may represent a neuroprotective response to subtle metabolic stress, operational only in neurons in culture.
CitationHoward et al. (2005) examined the effect of chlorpyrifos, chlorpyrifos-oxon, and TCPy on differentiation of sympathetic neurons dissociated from the superior cervical ganglia of GD 20–PND 1 rats. Differentiation of neurons was induced by bone morphogenetic protein-7. All three compounds were dissolved in DMSO and utilized at concentrations of 0.1 nM–10 μ M (chlorpyrifos and TCPy) or 0.0001–10 nM (chlorpyrifos-oxon). Two experimental protocols were utilized: (1) a 24-h incubation, 1 h after plating, to investigate effects on axonal growth; and (2) a 72-h incubation at 5 days in vitro, to investigate effects on dendrites. Both chlorpyrifos (0.1–1nM) and chlorpyrifos-oxon (0.001 nM) decreased axonal length, but not the number of axons, while TCPy was devoid of effects. At higher concentrations (chlorpyrifos and TCPy, 1 μ M; chlorpyrifos-oxon, 1 nM) these compounds increased dendritic length per neuron, without affecting the number of dendrites. AChE activity was inhibited by 1μ M chlorpyrifos and by 1 nM chlorpyrifos-oxon, but not by TCPy.
In a subsequent study, CitationYang et al. (2008) examined the effects of chlorpyrifos and chlorpyrifos-oxon on axonal growth in sensory neurons derived from embryonic dorsal root ganglia (DRG). They found that both compounds decreased axonal length without affecting the number of axons per cell, protein synthesis, cell viability, or AChE enzymatic activity. In DRG neurons from AChE knockout mice, axonal growth was impaired, but neither chlorpyrifos nor chlorpyrifos-oxon had any further effect on axonal length. The authors concluded that low concentrations of chlorpyrifos (1 nM) and of chlorpyrifos-oxon (0.01 nM) inhibit axonal growth in DRG neurons by interfering with the morphogenic rather than the enzymatic activity of AChE. These latter two studies are of interest as they suggest a novel mechanism by which chlorpyrifos and chlorpyrifos-oxon may interfere with axonal growth at concentrations below those necessary to inhibit AChE enzymatic activity. It should be noted, however, that upon exposure to chlorpyrifos at doses equivalent to the initial EPA RfD (0.003 mg/kg-day), tissue concentrations of chlorpyrifos are estimated at 0.01 nM (CitationDybowski et al. 2001). Furthermore, human exposure to chlorpyrifos is estimated to be at least an order of magnitude lower than the initial EPA RfD (see section on exposure), suggesting that brain concentrations of chlorpyrifos, and of chlorpyrifos-oxon, may be even lower.
In summary, most in vitro studies report effects of chlorpyrifos, chlorpyrifos-oxon and TCPy at concentrations that exceed those found after exposure to chlorpyrifos in vivo. Three studies, just summarized here, describe effects on neuronal differentiation at concentrations of chlorpyrifos-oxon below those required to cause AChE inhibition. These studies are of interest and would deserve further scrutiny and investigations. However, as mentioned earlier, even these effects are seen at concentrations of chlorpyrifos/chlorpyrifos-oxon that exceed by several orders of magnitude the concentrations that could occur in human tissues following typical “background” (diet) exposure of human populations to chlorpyrifos.
IV.C.3. Developmental Neurotoxicity of Chlorpyrifos: Human Studies
A number of epidemiology studies have looked for associations between OP insecticide exposures and the development of the human nervous system (summarized in ). Several studies report findings of direct relevance to chlorpyrifos exposure. In some, chlorpyrifos metabolites were measured specifically while in others the common metabolites of chlorpyrifos and other OPs were examined. We focus specifically on the associations tested for chlorpyrifos and its specific metabolite TCPy and for the diethyl metabolites of chlorpyrifos: DEP and DETP. Diethyldithiophosphate (DEDTP) is not a metabolite of chlorpyrifos (CitationTimchalk et al., 2007). The studies were carried out by three groups of investigators: Whyatt, Perera, and colleagues at Columbia University; Eskenazi and colleagues at the University of California at Berkeley; and Berkowitz, Wolff, and colleagues at Mount Sinai School of Medicine. These studies are reviewed next with an emphasis on: (1) the hypothesis tested; (2) study design and methods; (3) the findings; and (4) the author's discussion. In Section V, a detailed description of the chlorpyrifos exposure assessment for each of these cohorts is provided. In Section VI, the findings and conclusions of the combined studies are interpreted in terms of their demonstrated or possible clinical significance. Finally, important gaps in knowledge that could motivate additional studies of the biological and clinical neurological significance of chlorpyrifos and OPs for fetuses, neonates and children, are identified.
TABLE 15 Major cohort studies on chlorpyrifos, with summary of authors' reported finding
The Columbia Center for Children's Environmental Health Study. There are several reports from the investigators of the Columbia Center for Children's Environmental Health. Of these publications, the four most relevant for chlorpyrifos exposures and birth and developmental outcomes are the following reports.
Perera et al., 2003
Hypothesis: Exposure to environmental pollutants, including insecticides, is adversely associated with birth weight, length, and head circumference.
Study design and methods: In an ongoing prospective cohort study of inner-city minority mothers and infants born between February 1998 and May 2002, Perera, Whyatt, and colleagues evaluated the impact of exposure to several environmental toxicants on infant and children's health. These individuals were believed to be at high risk for adverse birth outcomes and more likely to be exposed to a number of environmental contaminants, including pesticides. Indeed, an earlier survey of pesticide use in this same cohort indicated that pesticide exposure was frequent (CitationWhyatt et al., 2002). In that study, a large proportion of the mothers [266 of 314 (85%)] reported that pest control measures were used in the home during pregnancy and most (90%) pesticide applications were for cockroach control. Personal air monitoring for 48 h was conducted for 72 women during the third trimester of pregnancy. All women monitored had detectable levels of exposure to chlorpyrifos (range 0.7 to 193 ng/m3), as well as several other pesticides, notably diazinon, propoxur, and o-phenylphenol. Exposures were generally associated with the level of housing disrepair and were higher among African-Americans than among Dominicans. (See Section V for further discussion of exposure assessment in this cohort.)
CitationPerera et al. (2003) evaluated the association of chlorpyrifos exposure with adverse birth outcomes among African-American and Dominican mothers and newborns residing in Washington Heights, Central Harlem, and the South Bronx, New York. All women were enrolled in the study between 1998 and 2002. All infants were delivered at the New York City Presbyterian Medical Center, Harlem Hospital, or associated satellite clinics. Eligible mothers were nonsmokers (classified by self-report and validated by blood cotinine levels of < 15 ng/ml), aged 18–35 years, self-identified as Black or Dominican registered at prenatal clinics by the 20th week of pregnancy, free of diabetes, hypertension, or known HIV infection, no documented or reported history of drug abuse, and residing in the study area for 1 year or longer, and were asked to participate in the study. Participation rates are not provided but 648 women consented to participate. A personal interview was used to administer a questionnaire in which various relevant data were collected, including demographic information, residential history, travel history, history of smoking and alcohol use, and exposure to polycyclic aromatic hydrocarbons. Women were excluded if there was evidence of active smoking as revealed by levels of plasma cotinine. Subjects included in the study were those in which samples of cord or maternal blood were available and for which there were complete questionnaire data and birth outcome data. The number of mother-infant pairs was 263. Pesticide concentrations were evaluated in blood collected from the mothers within 1 day postpartum and in umbilical cord blood collected at delivery. Pesticide levels in these biospecimens were determined by the Centers for Disease Control and Prevention (CDC). Chlorpyrifos itself was analyzed using isotope dilution gas chromatography–high resolution mass spectrometry. The analytical method for identification of chlorpyrifos in blood used in these studies was reported by CitationBarr et al. (2002), with the limit of detection for chlorpyrifos of 0.5 to 1 pg/g. Maternal and cord plasma concentrations of chlorpyrifos were significantly correlated. In cases where umbilical cord levels were not available, the mother's values were used, with adjustments, as detailed in the study. The relationships between exposure variables and birth outcomes were examined by multiple regression analyses, adjusting for known or potential confounders.
Findings: Chlorpyrifos was detected in blood samples from 98% of mothers and in 94% of cord samples with means of 7.1 pg/g in maternal blood and 7.6 pg/g in cord blood. In the development of the method for analysis of chlorpyrifos in blood, CitationBarr et al. (2002) reported a concentration of 9 pg/g chlorpyrifos in a pooled sample of blood obtained from a Red Cross Blood Bank in Cincinnati, OH (CitationBarr et al., 2002). In the overall sample, chlorpyrifos levels were significantly associated with decreased birth weight and reduced birth length, but not with head circumference. Estimated chlorpyrifos exposure was associated with lower birth weight (p = .01) and shorter birth length (p = .003) in the combined group of African-Americans and Dominicans, and with lower birth weight among African-Americans (p = .04) and shorter birth length in Dominicans (p = .001). Exposure to polyaromatic hydrocarbons (PAH) was also examined in these analyses and was associated with lower birth weight (p = .003) and smaller head circumference (p = .01) among African-Americans. In these analyses exposures to PAH and chlorpyrifos appeared to be independently and significantly associated with the birth outcomes assessed.
Authors' discussion: The principal conclusions of this study were that chlorpyrifos exposure is associated with reduced birth weight and birth length. Although it is difficult in the type of cross-sectional assessment conducted (i.e., with exposure status and birth outcomes determined simultaneously) to establish that the exposure to chlorpyrifos preceded the birth outcomes assessed, chlorpyrifos was viewed by the authors as a significant adverse determinant of birth outcomes.
CitationWhyatt et al., 2004a. CitationWhyatt et al. (2004a) expanded on the earlier analyses reported by CitationPerera et al. (2003) to include additional insecticides (diazinon and propoxur), a larger sample size (n = 314 mother–newborn pairs), and chlorpyrifos measurements in maternal air during pregnancy as well as in umbilical cord plasma at delivery.
Hypothesis: Environmental exposure of pregnant women to insecticides is inversely associated with birth weight, length, and head circumference.
Study design and methods: This study was carried out with the same cohort as for CitationPerera et al. (2003) with the number of mother–infant pairs now increased to 314. The eligibility criteria were the same but women were excluded if they had a history of illicit drug use, and the questionnaire also now gathered information about occupational history. Self-reported residential pesticide use during pregnancy was obtained and included whether or not any pest control measures were used by an exterminator or by others during pregnancy and, if so, what types of pesticides were used. Personal prenatal ambient air samples were taken using a small backpack containing an air monitor. Monitoring was for two 24-h periods during the third trimester. A sample of maternal blood was obtained within two days postpartum. Samples of umbilical cord blood were collected at delivery. Samples were analyzed at the CDC as in CitationPerera et al. (2003) using the sensitive analytical method described in CitationBarr et al. (2002). As before, subjects were excluded if there was evidence of active smoking. A summary of the results of these analytical determinations is provided in .
Statistical examination involved the use of multiple regression analyses to estimate the contribution of antenatal insecticide exposure to birth outcomes. As noted in CitationPerera et al. (2003), pesticide levels in maternal and umbilical cord plasma samples were significantly correlated. For chlorpyrifos, levels in umbilical cord blood were available for 256 newborns and were imputed from maternal blood levels for 31. Insecticide levels in both maternal personal air and blood samples were available for 82% of the mother–newborn pairs. Values below the limit of detection for chlorpyrifos were imputed by assigning a value of one-half of the limit of detection. Thirty-one percent of all chlorpyrifos levels were below the limit of detection. These samples were assigned to a “lowest exposure” group and the remaining subjects were placed into tertiles of increasing exposure.
Findings: Controlling for potential confounding exposures, the investigators reported that there were no significant correlation between chlorpyrifos levels in maternal prenatal personal air samples and birth outcomes, notably birth weight, birth length and head circumference. This was the case whether or not the data were stratified on the basis of year of delivery. There were, however significant inverse correlations between chlorpyrifos levels in maternal sera and cord blood with birth weight and birth length, but not head circumference. Thus, birth weight was 42.6 g lower and birth length 0.24 cm shorter for each log unit increase in cord plasma chlorpyrifos. When stratified into four exposure groups based on increasing levels of chlorpyrifos in cord plasma, the effects were principally seen among newborns with highest exposures. Indeed, in these analyses, a significant difference in birth weight was detected only when comparing the group in which chlorpyrifos was not detected with that in which exposure was greatest; the birth weight of the latter was 150.1 g less than those in which chlorpyrifos levels were not detected. For birth length, again only in comparing the two groups with the highest and the lowest levels of exposure was a marked difference evident; the magnitude of this change was of borderline significance (p = .07). The absolute difference in birth length between these groups was 0.75 cm.
The EPA began “risk mitigation” actions limiting residential applications of chlorpyrifos in June 2000 (CitationEPA, 2002), and acted to eliminate the sale of chlorpyrifos for residential use effective December 31, 2000. The apparent result of this restriction was a marked decrease in chlorpyrifos exposure as measured in both air and plasma blood levels in this study cohort (CitationWhyatt et al., 2005). Indeed, CitationWhyatt et al. (2003a) detected a decrease in the levels of chlorpyrifos in maternal air samples and in umbilical cord samples by 2001. For this reason, data for chlorpyrifos were stratified by whether the delivery of the newborn took place before or after January 1, 2001. Chlorpyrifos in personal air samples decreased from geometric mean values of 8 to 4.9 mg/m3 between the two periods for evaluation. In umbilical cord blood samples the geometric levels were 2.5 pg/g before January 1, 2001, and 0.6 pg/g after this date (see data; ).
When data were stratified for year of birth, those born before January 1, 2001 showed significant correlations between chlorpyrifos levels with both birth weight and birth length. However, for newborns delivered after January 1, 2001, there was no evident relationship between chlorpyrifos levels and either birth weight or birth length.
Authors' discussion: Three principal findings emerged from this study. The first is that the results confirmed earlier findings of an inverse association between chlorpyrifos levels in umbilical cord plasma with birth weight and length. The second is that there is a exposure-response relationship between chlorpyrifos levels and birth weight and birth length, but not head circumference. Finally, the authors note that after 2001, when exposures to chlorpyrifos were lower, decreases in exposure to chlorpyrifos were associated with the loss of significant associations with birth weight and length. The authors conclude that higher exposure to chlorpyrifos during the perinatal period is significantly linked to adverse birth outcomes.
The authors do not comment on how the lack of an association between birth weight, birth length, head circumference, and insecticide exposures (including chlorpyrifos) estimated from the temporally more relevant prenatal maternal personal air monitoring might limit the interpretation of the associations noted between cord plasma levels and birth weight and birth length in the cross-sectional type analysis.
TABLE 16 Summary of mean chlorpyrifos concentration in air and blood of Columbia cohort maternal–child pairs
Hypothesis: Prenatal exposure to chlorpyrifos is significantly and inversely correlated with performance on the Bayley Scales Infant Development II (BSID-II), and this exposure is positively correlated with behavioral problems as measured though maternal reports collected using the Child Behavior Checklist (CBCL).
In the study by CitationPerera et al. (2006), prenatal exposure to airborne polycyclic aromatic hydrocarbons is significantly and inversely correlated with performance on the Bayley Scales Infant Development II (BSID-II), and this exposure is positively correlated with behavioral problems as measured though maternal reports collected using the Child Behavior Checklist (CBCL).
Study design and methods: The study population for both of these studies is described earlier, as are the details regarding how women were recruited, interviewed, and blood samples obtained. Of the 648 consenting women, 536 were active participants in the ongoing study at the time of this study, and 254 of their children had reached the age of 3 years with data collected for: (1) prenatal maternal interview data, (2) biomarkers of chlorpyrifos exposure levels from maternal and/or cord blood samples obtained at time of delivery, (3) postnatal observational data on the quality of the home caretaking environment, and (4) a neurobehavioral outcome assessment at one or more yearly evaluations (12, 24, and 36 months). All requisite data were available for 228 of the 254 at 12 months, for 227 at 24 months, and for 228 children at 36 months. For 189 children, all the required data were available for all three time points.
The BSID-II was used to assess cognitive and psychomotor development. Use of this tool generates a Mental Development Index (MDI) and a corresponding Psychomotor Development Index (PDI). Scores on these tests are classified as either normal or delayed (scores of ≤ 85). The Child Behavior Checklist (CBCL) is informed by a mother's responses to 99 items relating to children's behavior during the 2 months preceding the interview. Measures of maternal intelligence and quality of the home environment were also made. For purposes of statistical analysis, chlorpyrifos levels were categorized into four groups—undetectable, lowest tertile, middle tertile, and highest tertile. Prenatal exposures to airborne PAH were monitored during pregnancy by personal air sampling and were analyzed by quartiles. On the basis of preliminary analysis, chlorpyrifos exposure was dichotomized such that subjects were classified as belonging to a high exposure group whose level was > 6.17 pg/g, versus lower exposure (≤ 6.17 pg/g). Multivariate linear regression was used to evaluate continuous measures and logistic regression was used for categorical outcomes.
Findings: Of the entire sample, 20.6% was classified as having a “high” chlorpyrifos exposure. Chlorpyrifos levels in maternal and umbilical cord blood samples were strongly correlated (r = .76; p < .001), although the correlation coefficient of .76 still indicates that 42% of the variation between the two measures remains unexplained (r-squared minus 1 = .42). As in prior studies, birth weight was significantly reduced (by 111 g) in the high versus low exposure group. There were no statistically significant differences in birth length or in head circumference, though both measures were lower in the high exposure group.
There was no significant association with chlorpyrifos exposure level and mean Mental Development Index (MDI) scores at any age of testing. However, the 36-month mean Psychomotor Development Index (PDI) score was significantly lower in children in the high exposure group as compared to those in the low exposure group. Also at 36 months there was a significantly greater proportion of children in the high exposure group with developmental delays in cognition (45.5% versus 29.9%) and psychomotor function (24.4 versus 7.1%). When multivariate linear regression models were examined, an adverse effect of prenatal chlorpyrifos exposure on mean MDI scores was suggested (p = .06) at 36 months of age. The same analysis demonstrated a significant negative effect of chlorpyrifos on mean PDI score at 36 months in children with high exposure. At 36 months, in comparison with children with low exposure, the odds of highly exposed children having mental delays were 2.4 times greater and the odds of motor delays were 4.9 times greater. Age-related analysis indicated that changes in MDI scores showed improvement between age 24 months and 36 months in those that had low levels of exposure versus a smaller degree of improvement in those with high exposure. This difference underlies the difference in scores that was evident at 36 months. The age-related changes in PDI scores showed a decrease in children with high exposure between 24 and 36 months, but an increase for those with low exposure. This change was associated with the significant difference between the groups at 36 months.
The Child Behavior Checklist (CBCL) at 36 months of age detected significant differences in the proportion of high versus low exposure for attention problems and for attention deficit/hyperactivity disorder (ADHD). Of all five domains tested, the proportion of children in the clinical problem range was less for those exposed to lower levels of chlorpyrifos than for those with high exposure. It is only for attention problems and ADHD that the differences reached statistical significance. Using logistic regression analyses, there was a significant association of chlorpyrifos exposure and attention problems, ADHD, and pervasive developmental disorder (PDD).
Examining the effect of decreasing exposure to chlorpyrifos before and after January 2001, the authors conducted a one-way analysis of variance to examine decreases in mean chlorpyrifos blood levels at delivery in the period prior to removal of residential use of chlorpyrifos, the period that spanned the phase-out of residential use, and the period following removal of residential use of chlorpyrifos. A statistically significant difference in levels was apparent. Significant increases in the 36-month BSID-II MDI scores were evident in comparing the pre-chlorpyrifos restriction period to the mid-chlorpyrifos restriction period and in PDI scores from the pre-chlorpyrifos restriction period to the mid-chlorpyrifos period. No significant differences were seen in comparing the mid-chlorpyrifos period to the post-chlorpyrifos restriction period for either MDI or for PDI.
The authors' conclusions: At 3 years of age children exposed to higher, compared with lower, chlorpyrifos levels in umbilical cord plasma scored on average 6.5 points lower on the Bayley PDI and 3.3 points lower on the Bayley MDI. The authors point to the following findings: (1) By 3 years of age there were significantly greater proportions of children in the “high exposure” group (> 6.17 pg chlorpyrifos/g blood) that score in the range of mental and motor delays compared with those with lower chlorpyrifos exposure (< 6.17 pg chlorpyrifos/g blood); (2) the developmental trajectory for PDI and MDI scores differentiated between children with high versus low chlorpyrifos exposure between ages 24 and 36 months; (3) children with high chlorpyrifos exposure were more likely to demonstrate problems with attention, ADHD, and PDD; and (4) multivariate tests showed that the effects of chlorpyrifos exposure could not be attributed to known confounders.
The authors assessed the magnitude of the effect on MDI scores as modest but comparable to studies of prenatal intoxication with cocaine. In addition, while the actual differences in PDI and MDI scores for both high versus low exposures differed by only a few points, the proportion of affected children with scores in the abnormal range was 5 times greater for the PDI and 2.4 times greater for the MDI. They conclude that the higher chlorpyrifos exposure is correlated with significant adverse neurobehavioral outcomes, but note the limitations of this analysis for attention problems and ADHD. Notably, the CBCL criteria for ADHD are derived from the DSM–IV, which has a low sensitivity for assessing the inattentiveness of preschool-aged children. A very small number of children (n = 9) included in the final analyses had scores above the clinical cutoff value; this resulted in very imprecise estimates of effect, i.e., wide confidence intervals.
Center for the Health Assessment of Mothers and Children of Salinas Study. CitationEskenazi et al., 2004.
Hypothesis: In utero exposure to pesticides, including chlorpyrifos, adversely effects birth outcomes and length of gestation.
Study design and methods: This report is the first of several from the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a longitudinal birth cohort study of the effects of pesticides and other environmental exposures on the health of pregnant women and their children living in the Salinas Valley, a largely agricultural area of California. Pregnant women receiving care at Natividad Medical Center or in one of the five clinical centers within this geographic area were screened for eligibility between October 1999 and October 2000. Eligible women were above 18 years of age, greater than 20 weeks gestation at enrollment, and planning to deliver at Natividad Medical Center. Of 1130 eligible women, 601 (53.2%) agreed to participate. Excluded from the study were women with gestational or preexisting diabetes, hypertension, twin births, stillbirths, and 11 cases in which infants were diagnosed with congenital anomalies at birth. The sample size was 488. Interviews were conducted during pregnancy and repeated after delivery to obtain demographic information, to obtain estimated use of alcohol, tobacco, drugs and caffeine during pregnancy, and to assess the extent to which women were exposed to agricultural work or agricultural workers during pregnancy. Other medical pregnancy complications were also assessed. Maternal blood samples were taken twice during pregnancy—at the time of the second interview and prior to delivery. The umbilical cord blood sample was taken at the time of delivery. Maternal urine samples were obtained twice during pregnancy at the time of the pregnancy interviews.
Maternal OP pesticide exposure was assessed by: (1) measuring OP dialkyl phosphate metabolites (DAPs) in maternal urine during pregnancy; (2) measuring maternal urinary pesticide-specific metabolites, including TCPy; and (3) assessing the activity of cholinesterase (ChE) in whole blood and BuChE in plasma collected during pregnancy and at delivery from umbilical cord blood. DAPs were measured in spot urine samples, with analysis conducted at the CDC using standard methods. Total DAPs were calculated, as were the levels of the three dimethyl metabolites (DMP metabolites = DMP, DMTP, DMDTP, and the three diethyl metabolites [DEP metabolites = DEP, DETP, and DEDTP]). Metabolite levels below the limit of detection were assigned a value that was the limit of detection divided by the square root of 2. Total DAP and DMP metabolites were available for 485 women, while for 486 women the levels of DEP metabolites could be examined. The median levels of total DAPs, total DMP metabolites, and total DEP metabolites were 136, 101, and 22 nmol/L, respectively. Also measured in urine was TCPy, the metabolite of chlorpyrifos and chlorpyrifos-methyl. Measurements of cholinesterase activity were conducted using standard methods in 292 samples available for these measurements during pregnancy, 357 at delivery, and 340 from umbilical cord blood.
Logistic regression was used to test for associations between exposure measurements and low birth weight, preterm delivery, and small-for-gestational-age births. Cholinesterase activity was analyzed as a continuous variable. DAPs were analyzed as continuous variables. For TCPy, subjects were assigned as having either no detectable levels (DL ∼ 0.2 μ g/L), detectable levels below the median, or detectable levels above the median. For TCPy the median level was 3.3 μ g/L. A number of potential confounders was included in multivariate models.
Findings: Only one woman had no evidence of DAP metabolites in urine during pregnancy. Surprisingly, increases in infant body length and in head circumference were associated with a 10-fold increase in average DAP metabolite concentration. The same increases were detected when DEP metabolites were examined separately, although this increase was not statistically significant. While a 10-fold increase in average DMP metabolites was associated with a significant decrease of 3 days in duration of gestation, the same association was not detected for total DAP or DEP metabolites. For neither total DAPs, nor DMP metabolites, nor DEP metabolites, was there a significant inverse relationship with birth weight, birth length, head circumference, or ponderal index. In fact, all three measures of exposure were associated with an increase in head circumference of about 0.25 cm with p values ranging from .07 (for DMP and DEP metabolites) to .03 (for total DAPs).
TCPy was detected in ∼ 77% of urine samples. No adverse associations of fetal growth or gestational age were detected for TCPy exposure. A lower ChE level in umbilical cord blood was associated with a shorter length of gestation, a change that was significant, as well as with increased risk of preterm delivery. No parameters of fetal growth were associated significantly with either maternal or umbilical cord blood ChE or BuChE levels.
Authors' discussion: The authors detected a significant decrease in gestational duration with the DMP metabolites of OP pesticides as well as with decreased levels of umbilical blood ChE. There was no evidence of an adverse relationship between fetal growth and in utero OP exposure. In particular, there was no link between DEP metabolites and any adverse outcome. Nor was there evidence for an adverse association of the metabolite of chlorpyrifos and chlorpyrifos-methyl with birth outcomes. While a concern arises over the possible effect of pesticide exposure on gestational duration, the authors suggest that the risks must be viewed in the context of the evidence showing no adverse effect, and indeed enhanced values for some measures of fetal growth. The latter includes head circumference.
Hypothesis: Exposure to pesticides results in adverse effects on neonatal development as measured by the Brazelton Neonatal Behavioral Assessment Scale (BNBAS).
Study design and methods: This study assesses subjects followed in the CHAMACOS longitudinal birth cohort study. The inclusion and exclusion criteria are as described in CitationEskenazi et al. (2004). The sample size for this study was 381 full-term, singleton infants who were less than or equal to 62 days old at the time of testing. The BNBAS consists of a number of different behavioral items that are scored on a 9-point scale, as well as an 18-item reflex battery. The BNBAS measures were reduced to seven clusters, as follows: habituation, orientation, motor performance, range of state, regulation of state, autonomic stability, and reflexes. On the first six clusters, better scores represent improvement, while a higher score on the reflex cluster suggests less optimal neurological function. The nonspecific metabolites of OPs were evaluated in maternal urine specimens collected at the time of the pre-and postdelivery interviews. Metabolite levels were examined as per CitationEskenazi et al. (2004).
Findings: The median values for total DAPs, DMP metabolites, and DEP metabolites during pregnancy were 132, 27, and 21 nmol/L, respectively. The median values for these same metabolites post delivery were 222, 160, and 27 nmol/L, respectively. The only significant association between metabolite levels and neurobehavioral outcomes as measured by BNBAS was for the reflex battery. Increasing total DAP, or DMP, or DEP metabolite levels during pregnancy were all significantly associated with an increased number of abnormal reflexes in infants. When the analysis was extended to examine time of reflex testing post delivery, it was only for infants examined after the first 3 days of life that adverse effects were seen between the levels of exposure and reflex activity. For all three categories of metabolites (i.e., total DAPs, DMP metabolites, and DEP metabolites), increasing levels of exposure were correlated significantly with the probability of three or more abnormal reflexes. A 10-fold increase in metabolite level increased the odds of more than three abnormal reflexes by 4.9-fold for total DAP, 3.2-fold for DMP metabolites, and 3.4-fold for DEP metabolites. In most cases, abnormalities reflected a failure to elicit a reflex response or induce a hypoactive response. Post-delivery urinary metabolite measurements were not significantly associated with any aspect of performance on the BNBAS, whether the analysis involved the entire sample or only infants examined after 3 days.
Authors' discussion: The authors conclude that the findings point to a detrimental impact of in utero OP exposure on neurological function, as indicated by the presence of abnormal reflex activity in infants greater than 3 days of age. Increases in both the number of abnormal reflexes and the proportion of infants affected were significant. Conversely, none of the other elements tested in BNBAS showed a significant correlation with in utero exposure to OPs. The authors' conclusion is that exposure to OPs may adversely impact some aspects of neural development.
CitationEskenazi et al., 2007.
Hypothesis: Exposure to OP pesticides, including chlorpyrifos, adversely affects neurodevelopmental outcomes beyond the early postnatal period.
Study design and methods: This study was a continuation of the CHAMACOS longitudinal cohort study. Inclusion and exclusion criteria, interview parameters, and other methodological issues are essentially identical to those described earlier. The Bayley Scales of Infant Development, Second Edition (BSID-II), was used to assess aspects of infant development. The MDI portion of the BSID-II was used to characterize cognitive abilities and the PDI to characterize fine and gross motor coordination. In addition, CBCL was used for detecting emotional and behavioral problems and competencies. Pesticide exposure measurements were assessed in urine, as described in earlier studies of this group, with maternal samples taken at the times of prenatal and postnatal interviews and from children at interviews at 6, 12, and 24 months of age.
Findings: Children's exposure to OPs increased with age with total DAPs at 6, 12, and 24 months averaging 45.5, 59.5, and 70.9 nmol/L, respectively. DEP metabolites in children were 10.6, 15.2, and 10.5 nmol/L, at these same ages. For DMP metabolites the values were 23.8, 32.9, and 48.6 nmol/L. Approximately 91% of mothers had detectable serum TCPy during pregnancy with a median level of approximately 4 μ g/L.
There was no significant association between metabolite levels and PDI at any age. Regarding the MDI, significant negative associations were detected at 24 months with the prenatal levels of total DAPs and with DMP metabolites. Remarkably, the levels of these metabolites in the urine of children were positively associated with the MDI. DEP metabolites were positively associated with MDI using postnatal measurements at the 12-month time point but not at other times of assessment using either prenatal samples or samples from children.
Using the CBCL, the authors observed a significant negative association between children with higher prenatal and postnatal total DAPs and the risk of PDD. At 24 months, there was about a twofold increase in risk for each 10-fold increase in prenatal or childhood levels of total DAPs. Similarly, at this same age, prenatal DMP metabolites and children's levels of DEP metabolites conferred a significant increased risk for PDD. No significant association was detected between any of the BSID-II or CBCL measures and the maternal level of TCPy.
Authors' discussion: The authors suggest that exposure to OPs is associated with changes in cognitive performance and behavioral outcomes as measured using validated tests of childhood performance. Because there was no significant association of TCPy levels with any neurodevelopmental measure, there does not appear to be a contribution of chlorpyrifos to the adverse findings.
Children's Environmental Cohort Study, Mount Sinai Hospital, New York City. CitationBerkowitz et al., 2004.
Hypothesis: Prenatal pesticide exposure, paraoxonase (PON1) polymorphisms, and enzyme activity are associated with infant growth and neurodevelopment.
Study design and methods: The Children's Environmental Cohort Study (CECS) is a prospective multiethnic birth cohort study of mothers and infants delivered at Mount Sinai Hospital in New York City. An ethnically diverse cohort of mothers was recruited consecutively during early pregnancy through clinics associated with Mount Sinai Hospital from March 1998 to March 2002. Only first pregnancies with singleton births were included. Mothers were included if they had their first prenatal visit before 26 weeks gestation, were without serious chronic illnesses, and did not develop a serious pregnancy complication that could affect fetal growth. Mothers were excluded if they consumed more than a modest amount of alcohol or used illegal drugs. Mother–infant pairs were also excluded if the child was born with a congenital malformation or was severely premature. Of a total of 479 prenatal patients, 404 births were available for analysis. Pesticide and other environmental exposures, sociodemographic characteristics, and aspects of maternal health and lifestyle were assessed using a prenatal questionnaire that was administered during the third trimester. Information from this questionnaire was linked to information on delivery characteristics and birth outcomes from medical records. Samples of maternal blood were obtained during the third trimester and cord blood samples were obtained at birth. They were analyzed for PON1 activity and PON1 polymorphisms. Maternal urine samples were obtained at the same time as blood samples and metabolites of TCPy and other pesticides were measured. PON1 activity was assessed using a published method. PON1 genotypes were determined using RFLPs or allele-specific PCR. For multivariate analyses, covariates were selected from those known to be associated with abnormalities of fetal growth or with pesticide exposure.
Findings: Approximately 72% of mothers were classified as having potential exposure to chlorpyrifos on the basis of the results of the questionnaire. Neither the self-reported exposure data nor the pesticide metabolite levels were associated with any of the fetal growth indices or gestational age. The median level of TCPy uncorrected for creatinine was 7.6 μ g/L; corrected for creatinine the value was 11.5 μ g/L. There was no significant association of the level of TCPy with birth weight, birth length, or head circumference. When the level of maternal PON1 activity is considered, maternal levels of TCPy above the limit of detection coupled with low maternal PON1 activity were associated with a significant but small reduction in head circumference. Comparing low versus high PON1 activity in these mothers, the difference in infant head circumference was –0.8 cm. There was also a statistically nonsignificant decrease in head circumference of approximately the same magnitude (–0.6 cm), which was correlated with low versus high PON1 activity in mothers whose TCPy levels were below the level of detection. Interestingly, maternal PON1 activity alone was positively associated with head circumference. Mean head circumference for those with the lowest levels of PON1 activity was 33.5 cm, while for those with the highest levels of PON1 activity the head circumference was 34.1 cm (p = .004), a difference of 0.6 cm. In testing for interaction between PON1 activity, TCPy levels, and head circumference no significant association was evident. Also, there was no significant association between TCPy and PON1 activity for birth weight or birth length. There was a weak association between birth weight and maternal PON1 genotype; lower mean birth weight was associated with the low activity allele. There was no association for infant genotype with birth weight or for maternal or infant genotypes with head circumference or birth length.
Authors' discussion: A small but significant decrease in head circumference was evident when the levels of PON1 activity and TCPy were considered concurrently. Nevertheless, the tested interactions were not statistically significant. It is noteworthy that the interaction between PON1 activity and head circumference was positive independent of any TCPy levels. Moreover, a trend of absolute magnitude of approximately the same size was seen for TCPy levels greater than the level of detection and TCPy levels below the level of detection when examined with respect to PON1 activity. The authors conclude that these data suggest that chlorpyrifos may have a detrimental effect on fetal neurodevelopment among mothers who exhibit low PON1 activity. In pointing to findings indicating that the level of PON1 activity in infants is only about one-third that of mothers (CitationChen et al., 2003a), the authors suggest that infants may be more susceptible to OP toxicity.
Hypothesis: Prenatal exposure to OPs and other pesticides is associated with adverse outcomes with respect to birth weight, birth length, ponderal index, head circumference, and gestational age.
Study design and methods: This study continues the CECS investigation of an ethnically diverse cohort of mother–infant pairs enrolled at Mount Sinai Hospital. The criteria for inclusion of pairs are identical to that for Berkowitz et al., as reviewed earlier. Sample size was 404. Maternal blood and urine samples were obtained during the third trimester and cord blood samples at the time of delivery. Maternal urine samples in this study were analyzed for six DAP metabolites at the CDC using established methods. Levels of DMP or for DEP metabolites were added to calculate summed DMP and summed DEP levels. Blood samples were used to measure PON1 activity and PON1 genotypes.
Findings: The median of the sum of DEP metabolites was 18.8 nmol/L without adjustment for creatinine, and 22.1 nmol/L after adjustment. Eighty-eight percent of women had levels of DEP metabolites greater than the level of detection. The sum of DEP metabolites was weakly and nonsignificantly adversely associated with birth weight and ponderal index. The sum of all total OP metabolites (i.e., summed DMP plus summed DEP = total DAPs) was weakly associated with a small decrease in head circumference (0.26 cm; p = .045). The same weak associations were seen after correcting for creatinine but now the association between total DAPs and head circumference was not significant. As in CitationBerkowitz et al. (2004), there was a strong association between maternal PON1 activity and head circumference (p = .004). Examining PON1 activity in tertiles, head circumference in the lowest tertile was 0.62 cm smaller than in the highest tertile (p = .009). In addition, head circumference was smaller for infants born of mothers with the slow/slow allelotype than with the fast/fast allelotype. Birth length was also shorter in the slow/slow allelotype.
To examine interactions between OP metabolites and PON1 activity and genotype, further statistical analyses were carried out. Examining the sum of DEP metabolites and birth weight, there was no significant interaction between maternal PON1 activities, as examined in tertiles, versus either low or high maternal exposure. However, when considering the extremes of interaction, i.e., low maternal exposure (below the median level) with high PON1 activity versus high maternal exposure (equal to or above the median level) with low PON1 activity, a statistically significant decrease in birth weight was detected. A similar analysis for PON1 allelotype also showed a significant difference. The absolute decrease in birth weight was 163 g in the first analysis and 199 g in the second. In addition, examining only the fast/fast alleles for PON1, increased exposure, as measured by DEP metabolites equal to or greater than the median, showed a significant decrease in birth weight of approximately 220g. There was a significant interaction between DMP metabolites and PON1 activity for birth length. Among mothers with low PON1 activity, those exposed to higher levels of DMP metabolites had infants that were on average 0.9 cm shorter than those exposed to lower levels. Neither head circumference nor ponderal index showed a significant interaction between OP metabolites and PON1 activity.
Authors' discussion: The principal findings were a marginal, nonsignificant effect on birth weight and ponderal index when examining DEP metabolites and a small although significant association between total DAPs and decreased head circumference. This paper supports the earlier evidence that PON1 activity and PON1 genotype are significantly related to head circumference. In addition, there were statistically significant interactions of PON1 activity and PON1 genotypes with the level of DEP metabolites for birth weight and with DMP metabolites for birth length. TCPy was not reported in this study, so it is uncertain what contribution chlorpyrifos may have provided to total DEP metabolite concentration.
Hypothesis: Prenatal exposure to OPs adversely effects brain maturation as measured in tests administered at or near the time of birth.
Study design and methods: This study continues CECS, an infant–mother pair cohort study carried out at Mount Sinai Hospital. Sample size was 404 with the same inclusion criteria and methods of sampling as outlined in CitationWolf et al. (2007). The Brazelton Neonatal Behavioral Assessment Scale (BNBAS) was administered to infants before hospital discharge. This scale includes 28 behavioral items and 18 primitive reflexes and usually is examined through the scoring of seven clusters, as detailed earlier. As in an earlier study, OP metabolites were classified as the sum of DEP metabolites, the sum of DMP metabolites, and the sum of all metabolites (DAPs).
Findings: During pregnancy, approximately 89% of women were exposed to OPs whose breakdown produces DEP metabolites. Examining total DAPs, fully 96.5% of the women had detectable levels in urine. The median level for DEP metabolites was 24.7 nmol/L. Increased exposure to OP pesticides, as examined with respect to quartiles of total DAP, DMP, and DEP metabolites, was associated with an increased number of abnormal reflexes. This was significant for mothers with total DEP metabolites above the median; these mothers delivered infants that were 2.3 times more likely to have at least two abnormal reflexes. The relative risk for abnormal reflexes, adjusting for PON1 activity and creatinine was 1.49. There was also a statistically nonsignificant increase in relative risk for total DAPs and summed DMPs. Higher total levels of DEP metabolites were significantly associated with an abnormal “crawling” reflex. While there was no evident interaction between DEP metabolites with PON1 activity with respect to abnormal reflexes, such interactions were detected for DMP and total DAPs. None of the other six test clusters showed an adverse correlation with OP metabolites. The same caveat as earlier is noted here: Neither urinary TCPy nor blood chlorpyrifos was measured, so the contribution of chlorpyrifos exposure to total DEP metabolites is uncertain.
Authors' discussion: There was an increased risk of abnormal reflexes in infants born to mothers with higher levels of exposure to OPs, including those with DEP metabolites. Among the battery of tests included in the BNBAS, only changes in reflexes were detected as statistically significant. In view of the fact that the literature suggests that the observation of two or more abnormal reflexes is clinically significant, the authors raise concern for a more lasting effect of pesticide exposure on neural development.
V. ASSESSMENT OF HUMAN EXPOSURES TO CHLORPYRIFOS
V.A. Overview of Exposure Assessment in Human Populations
Exposure to chlorpyrifos may occur via any of the common pathways of exposure: (1) through ingestion of residues in the diet or through secondary ingestion of contaminated house dust/soil, or hand-to-mouth contact, (2) via inhalation of vapors or aerosols, or (3) via dermal absorption following contact with the skin. Although dermal and inhalation exposure pathways are likely to dominate occupational exposures to pesticides, at the present time ingestion is likely to be the predominant pathway for exposure to the general public, especially including children. As noted previously, indoor use of chlorpyrifos for residential insect control was restricted in 2001 (Federal Register, January 25, 2001). The restrictions were fully implemented in 2002, and resulted in nearly complete elimination of chlorpyrifos sales to the public (CitationCarlton et al., 2004). Thus, it is likely that the primary sources of current exposure to chlorpyrifos are via the diet and agricultural exposures (occupational, or rural residential from “take home” or drift).
As discussed earlier in Section IV, several large birth cohort studies were initiated in the late 1990s to examine putative pathways of exposure of women and children to chlorpyrifos. Several of these studies carefully evaluated inhalation exposure via measurement of chlorpyrifos in indoor and/or “personal” air samples. Some have also calculated chlorpyrifos “loading” on surfaces (determined as ng chlorpyrifos/cm2 surface area) such as floors, toys, and body surfaces (e.g., hands and arms), and several have included dietary assessments of chlorpyrifos exposure as well. Many of these and other studies have utilized urinary concentrations of TCPy as a biomarker for exposure to chlorpyrifos. A summary of urinary TCPy concentrations in these and other studies is provided in .
TABLE 17 Urinary TCPy Concentrations (μ ug/L, or ppb)
Most of the published exposure studies focused on populations that are likely to have exposures that exceed “typical” exposures of the general public in the United States. For example, several of the studies evaluated chlorpyrifos residues and urinary TCPy following use of chlorpyrifos for insect control in and around homes and thus do not represent exposure circumstances today. However, several of the studies have focused on agricultural families living on or near farms that utilize chlorpyrifos for insect control, and thus represent potential exposures that are relevant today.
V.B. Designs of the Major Children's Cohort Studies That Have Evaluated Chlorpyrifos Exposures
V.B.1. NHEXAS
Following the passage of the Food Quality Protection Act of 1996, the U.S. EPA began a multimedia, multi-pathway exposure assessment of chlorpyrifos and other pesticides as part of the National Human Exposure Assessment Survey (NHEXAS). For chlorpyrifos exposure, NHEXAS involved studies in Minnesota [referred to as the Minnesota Children's Pesticide Exposure Study, or MNCPES (CitationAdgate et al., 2000; CitationLioy et al., 2000; CitationQuackenboss et al., 2000; CitationBuck et al., 2001; CitationFreeman et al., 2001; CitationRigas et al., 2001; CitationClayton et al., 2003; CitationPellizzari et al., 2003; CitationSexton et al., 2003)], Arizona [NHEXAS-AZ (CitationGordon et al., 1999; CitationRobertson et al., 1999; CitationBuck et al., 2001; CitationMoschandreas et al., 2001a; CitationMoschandreas et al., 2001b; CitationMoschandreas et al., 2002)], and Maryland [NHEXAS-Maryland (CitationMacIntosh et al., 2001a; CitationPang et al., 2002; CitationEgeghy et al., 2005)].
NHEXAS-MN (MNCPES). The MNCPES “was designed to evaluate the feasibility and practicality of conducting a population-based exposure monitoring study in children, and to examine the utility of associated measurements for assessing and comparing children's multi-pathway pesticide exposures (air, water, food, soil, residential surfaces)” (CitationQuackenboss et al., 2000). The study involved telephone screening of 1388 households, from which 348 families were interviewed, and 294 completed detailed questionnaires. From this, 102 households with children participated in intensive exposure monitoring. Of the 102 households, 72 were from urban environments and 30 were “nonurban” (CitationQuackenboss et al., 2000). The MNCPES studies evaluated exposures to chlorpyrifos, diazinon, malathion, and atrazine. Many of the sampling and analytical protocols used in these studies were derived from the NHEXAS Phase I field studies (CitationPellizzari et al., 2003). For the MNCPES study, indoor air, outdoor air, and personal air monitoring was conducted over 7 days. Kitchen tap water and other beverages (e.g., fruit juice) were also analyzed for pesticide residues. Diets (duplicate composite samples of solid foods consumed over the first 4 days), surface wipes, carpet dust, soil (outside), and dermal rinses (hand washed with isopropyl alcohol on day 4) were collected. First morning void urine samples were also collected on days 3, 5, and 7.
The results of the MNCPES study reported on numerous exposure parameters, including surface loading sampling and analysis (CitationLioy et al., 2000); assessment of children's behaviors (CitationFreeman et al., 2001); urinary TCPy analyses (CitationAdgate et al., 2000); modeling estimates of exposure for all routes (CitationBuck et al., 2001); analytical approaches and quality assurance/quality control (QA/QC) (CitationPellizzari et al., 2003); pharmacokinetic modeling of exposure, based on urinary TCPy (CitationRigas et al., 2001); characterization of multiple exposure pathways (CitationClayton et al., 2003); and the ability of questionnaires to predict children's' exposure to pesticides (CitationSexton et al., 2003). Key summary findings of the exposure parameters measured for chlorpyrifos in this study are presented in –, and are discussed next, by each pathway of exposure.
NHEXAS-MD. As part of the NHEXAS-MD study, MacIntosh et al. (Citation2001a, Citation2001b) conducted a stratified probability sample of the diets of 80 individuals older than 10 years in the greater Baltimore metropolitan area. Subjects participated in an extensive diet collection study in which duplicate portions of each subject's diet were collected for analysis of chlorpyrifos residues over a 7-day period. Sampling was repeated up to six times for each subject over the period of a year to provide a longitudinal assessment, and to provide insights into seasonal variation in dietary pesticide exposure. All food items were combined and homogenized for analysis. The results of this study are discussed below under “dietary pathways.” In addition to the relatively extensive sampling of diet, the NHEXAS-MD study also collected indoor air via periodic 10-min sampling intervals over 7 days, with a total air collection period of 24 h. House dust samples were also collected by high-volume small-surface sampler, with careful attention to the area sampled. When possible, outdoor soil samples from the subjects' residence were also obtained. This information was used to construct an aggregate exposure assessment for chlorpyrifos (CitationPang et al., 2002). The exposure pathway results are summarized in and , and are discussed by pathway, and by aggregate analyses, next.
NHEXAS-AZ. CitationGordon et al. (1999) reported on extensive sampling of indoor air (n = 122), outdoor air (n = 42), house dust (n = 218), dermal wipes (n = 149) and yard soil (n = 281) for residues of chlorpyrifos. Results are shown in and . Extensive dietary analysis of chlorpyrifos in 19 subpopulations in Arizona was also completed in this cohort (CitationMoschandreas et al., 2002), and is discussed in the section on dietary exposures. An aggregate exposure analysis was also completed (CitationMoschandreas et al., 2001a, Citation2001b), and is discussed later.
V.B.2. Urban Minority Women Cohort (Columbia University)
A cohort of inner city women and newborn infants exposed to chlorpyrifos via indoor application for pest control has been followed by Perrera, Whyatt, and colleagues (CitationWhyatt et al., 2002; CitationPerera et al., 2003; CitationWhyatt et al., 2003a; CitationWhyatt et al., 2003b; CitationPerera et al., 2004; CitationRauh et al., 2004a; CitationWhyatt et al., 2004a; CitationWhyatt et al., 2004b; CitationPerera et al., 2005; CitationWhyatt et al., 2005; CitationPerera et al., 2006; CitationRauh et al., 2006; CitationWhyatt et al., 2007). Chlorpyrifos was among 21 pesticides that were evaluated. Since the study began in 1997, the study has recruited over 600 African-American and Dominican women and their newborns (CitationWhyatt et al., 2007). Numerous reports from this cohort have been published, and are discussed in Sections IV and VI.
V.B.3. Children's Total Exposure to Persistent Pesticides and Other Persistent Organic Pollutants (CTEPP) Cohort (North Carolina)
An extensive assessment of exposure to persistent organic pollutants and certain pesticides in urban homes and day-care centers in North Carolina was initiated in 1997 (CitationMorgan et al., 2005). The initial study examined several persistent organic pollutants in 10 child day-care centers in the Raleigh–Durham–Chapel Hill area of North Carolina (CitationWilson et al., 2001). From this original cohort of 10 day-care centers, two centers were chosen that demonstrated the highest and lowest overall levels of target pollutants. From these two day-care centers nine children, each from a different household (but in two different day-care centers), completed the study. Samples of indoor and outdoor air, floor dust, play area soil, and hand wipes were collected. In addition, duplicate diet samples and six spot urine samples were collected from each child (CitationWilson et al., 2003). A substantially larger study of 129 children was published in 2005 (CitationMorgan et al., 2005). Thus, the CTEPP study as reviewed here will focus on the single larger study of CitationMorgan et al. (2005) with study design and methods described in the earlier report (CitationWilson et al., 2004). The design for the larger cohort study was similar to that described earlier for the nine day-care children (indoor and outdoor air, house dust, soil, hand wipes, liquid and solid food, and several spot urine samples from each child/household/day care), except that half of the participants were children who attended day care, while the other half did not. Based on questionnaire results, it was determined that 18 of the 129 participants had applied pesticides in their homes within 7 days preceding field sample collections. Results of the CTEPP exposure analyses are shown in –, and are discussed below by pathway of exposure.
V.B.4. Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) Cohort (UC-Berkeley)
In another series of studies of Mexican-American children living in an intensive agricultural area of California, Eskenazi and coworkers evaluated several metrics of exposure to numerous insecticides, including chlorpyrifos, and a variety of neurodevelopmental health outcomes in children, as discussed in Section IV (CitationBradman et al., 1997; CitationEskenazi et al., 1999; CitationCastorina et al., 2003; CitationEskenazi et al., 2004; CitationHarnly et al., 2005; CitationDuramad et al., 2006; CitationFurlong et al., 2006; CitationHolland et al., 2006; CitationEskenazi et al., 2007). CHAMACOS is “a prospective cohort study of the association of pesticides and other environmental exposures on the health of pregnant women and their children living in the Salinas Valley, CA.” In this series of studies, 531 pregnant women were followed to delivery of a surviving newborn. An exposure assessment was conducted in the households of 20 children in 2002, and reported recently (CitationBradman et al., 2007). In this study the authors collected house dust, indoor and outdoor air, dislodgeable residues from surfaces and toys, residues on clothing (sock and union suits), food, and spot and overnight diaper urine samples. They measured chlorpyrifos and 28 other common agricultural and home use pesticides in multiple exposure media samples and food (), and analyzed urine samples for OP pesticide metabolites, including TCPy (). In addition they assessed the effectiveness of a questionnaire, food diaries, home inspection reports, and a self-administered child activity timeline as field-based exposure assessment instruments (CitationBradman et al., 2007). The results of their findings for chlorpyrifos in source media are included in and and discussed later by pathways of exposure.
TABLE 19 Chlorpyrifos in source media—Soil, Housedust and food [mean, SD; (maximum or range)]
V.B.5. Children's Post-Pesticide Application Exposure Study (CPPAES; New Jersey)
The CPPAES study is a “detailed multimedia / multipathway 10-home residential study … conducted to provide information on the release and movement of chlorpyrifos … within a residential environment and within children living in this environment over time after an application” (CitationHore et al., 2005). Commercial formulations of chlorpyrifos (Dursban 2.E. or Dursban L.O.) were applied to homes in a 0.25–0.5% water-based solution by a commercial pesticide applicator by the “crack and crevice” mode. Approximately 60–700 ml was applied in each home, although analysis of the material applied indicated that 2 homes had very low applications (0.4 and 4.3 μ g) compared to the other 8 homes (70–600 mg of chlorpyrifos). In each CPPAES home, measurements were taken in two rooms that had been treated, and time-weighted average measurements of chlorpyrifos vapor/aerosol were collected from the indoor air. Samples were collected prior to application, and again at 0–1, 1–2, 2–3, 3–5, 5–7, 7–9, and 9–11 days after application. Surface wipe samples were also collected over this same time period from surfaces that were not directly targeted for application. Several “plush” toys were also placed in the home prior to application and were analyzed for chlorpyrifos residues after treatment. Results of the indoor air sampling are summarized in , and discussed later. A modeling exercise using the MENTOR-SHEDS “physically based probabilistic population model” simulation was used to predict exposure levels, based on input data from the CPPAES study (CitationHore et al., 2006). This is discussed later, in the “aggregate exposures” section.
TABLE 18 Chlorpyrifos in source media–air, ng/m3 [mean (SD/range; maximum)]
V.B.6. Iowa Farm Study
This is a relatively small cohort of Iowa farm and nonfarm families to investigate “take-home” pesticide exposure. The study consisted of 47 fathers, 48 mothers and 117 children from 25 farm households and 25 nonfarm households (CitationCurwin et al., 2002, Citation2005a, Citation2005b, Citation2007b, Citation2007a). For the urinary assessment of pesticide metabolites, including TCPy, each household was visited on two occasions, one within 1–5 days after a pesticide application, and the second 3–5 weeks (average 4 weeks) after the application. Two spot urine samples were collected from participants at each visit, one in the morning and one in the evening (CitationCurwin et al., 2007b). The results of the urinary analyses are shown in , and are discussed later in the context of other studies that have measured TCPy as a biomarker for chlorpyrifos exposure.
V.B.7. Farm Family Exposure Study (FFES): Iowa and South Carolina
This study was initiated in 1999 as a pilot study of 5 families living on farms that regularly utilized pesticides (CitationBaker et al., 2005). It was extended to include 95 families during 2001–2002 (CitationAcquavella et al., 2004; CitationBaker et al., 2004, Citation2005; CitationMandel et al., 2005). Eligibility for the FFES required that: “1) the family lived on a farm and consisted of the farmer, a spouse and at least one child between the ages of 4 and 17 years; 2) the applicator … planned to apply one or more of the study pesticides (2,4-D, glyphosate or chlorpyrifos) during the study period as part of their normal operation; 3) the chemical would be applied to at least 10 acres of land where some of the field was within one mile of the family home, and 4) the family members were willing to collect 24 h urine samples for 5 days (1 day before through 3 days after the application).” This is the only exposure assessment study of chlorpyrifos that utilized a 24-h urine collection—a potentially important design consideration to be discussed later. The study selected farmers who were planning to apply chlorpyrifos in the second year. The study successfully enrolled farmers who applied chlorpyrifos 34 different times over the 2-year study period, 28 of which were in South Carolina and 6 in Minnesota. Study participants were asked to complete a detailed questionnaire at the beginning and end of the study that included numerous questions pertaining to farm characteristics, pesticide practices, relevant activities before and during application, use of personal protective equipment, and activities of children that might bring them into direct contact with the pesticide application (CitationBaker et al., 2005). During the applications, a member of the study team made observations and recorded pertinent information on all aspects of the application (type of equipment used, meteorological conditions, location of mixing and application sites, personal protective equipment used, etc). All participants were provided with 500-ml containers and detailed instructions on how to collect 24-h urine samples. A subset of urine samples was utilized to “field fortify” with known amounts of analytical-grade pesticide metabolites, including TCPy, for analytical validation. Urinary TCPy was determined by GC/MS methods using a stable isotope-label of TCPy as an internal standard. The limit of quantitation for TCPy was 1 ppb (1 μ g/L). Because this study collected 24-h urine samples rather than spot urine samples, from farmers who applied chlorpyrifos, as well as from their spouses and children, and samples were collected prior to, the day of, and 3 days following application, it represents the most thorough and relevant assessment of chlorpyrifos exposures to date. The results of this exposure assessment study for chlorpyrifos were presented recently (CitationAlexander et al., 2006) and are discussed later.
V.B.8. The Children's Environmental Cohort Study (CECS, Mount Sinai, New York City)
The CECS was a prospective study that followed an ethnically diverse cohort of mother–infant pairs at Mount Sinai Hospital in New York City, as described in Section IV (CitationBerkowitz et al., 2003, Citation2004). A unique aspect of this study is that the authors considered the potential role of genetic variability in chlorpyrifos metabolism by paraoxonase by genotyping and phenotyping mothers and infants (CitationBerkowitz et al., 2003; CitationChen et al., 2003a; CitationBerkowitz et al., 2004). The principal focus of this study was whether residential application of chlorpyrifos was associated with effects on in utero development. Exposure was assessed quantitatively only via urinary TCPy measurements, and via questionnaires that addressed pesticide use around the home.
V.B.9. Other Chlorpyrifos Exposure Assessment Studies
Fenske, Lu, and coworkers (CitationSimcox et al., 1995; CitationYuknavage et al., 1997; CitationLu and Fenske, 1998; CitationLu and Fenske, 1999; CitationFenske et al., 2002a; CitationFenske et al., 2002b; CitationLu et al., 2004; CitationKissel et al., 2005; CitationLu et al., 2006; CitationRodriguez et al., 2006) have completed several studies of chlorpyrifos exposure in the State of Washington, focusing primarily on agricultural exposures to farm workers and farm families. The results of these studies are shown in –, and discussed laer.
V.C. Specific Pathways of Exposure
V.C.1. Urinary TCPy as a Biomarker of Chlorpyrifos Exposure
As discussed in Section I, chlorpyrifos, as well as chlorpyrifos-methyl and triclopyr, are metabolized in the body to 3,5,6-trichlorpyridinol (TCPy; ), which is then excreted in the urine. Numerous studies, including most, but not all, of the large cohort studies discussed earlier, have utilized TCPy as a biomarker of exposure to chlorpyrifos, on the assumption that chlorpyrifos is the only source of TCPy in urine and that most (70% is commonly used) of the absorbed dose is converted to TCPy and eliminated in the urine (). Perhaps the most striking observation from the data in is the surprisingly high frequency with which TCPy is identified in the urine. In studies with detection limits in the 1–2 ppb (μ g/L urine) range, TCPy is frequently identified, suggesting widespread exposure to chlorpyrifos. For example, in the National Health and Nutrition Examination Survey (NHANES II) of urine samples collected in 1999–2001, TCPy was identified in more than 98% of urine samples collected from over 4,500 residents in the United States. Since the NHANES subjects represent a statistically determined cross section of the U.S. population, these values are likely to represent “typical” background values for urinary TCPy in the United States during that period. Several conclusions about urinary TCPy concentrations are evident from the NHANES data: (1) Urinary concentrations of TCPy in the U.S. population at that time were typically in the range of 1–10 ppb, with median and geometric mean values from 2 to 4 ppb; (2) however, levels substantially above 10 ppb were present, since the upper 95% confidence limit ranged from 11 to 26 ppb (depending on year and age group); (3) children (ages 6–18) had, on average, nearly twice the concentration of urinary TCPy as adults. Another observation from the summary data in is that populations with known exposures to chlorpyrifos through residential application (CitationAdgate et al., 2001; CitationBerkowitz et al., 2003; CitationClayton et al. 2003, CitationHore et al., 2005; CitationMorgan et al. 2005) have, on average, urinary TCPy values in the range of 6–8 ppb, or approximately 2–3 times greater than the “background” levels seen in the NHANES II study. Similarly, farmers who applied chlorpyrifos tended also to have urinary TCPy values about twofold greater than the NHANES II “background” levels, suggesting that some exposure to chlorpyrifos does occur (Curwin et al., 2005; CitationAlexander et al., 2006). However, exposures to farm worker families in those same studies did not appear to add substantially to the background levels of TCPy in urine.Footnote6
A critical evaluation of TCPy as a biomarker for chlorpyrifos exposure is provided at the end of this section, after consideration of specific routes of exposure to chlorpyrifos that give rise to urinary TCPy.
V.C.2. Inhalation (Indoor and Outdoor Air)
A list of studies with summary data in which concentrations of chlorpyrifos were measured in indoor and outdoor air is provided in . The findings in these studies and their significance are described next.
Indoor Air. In the NHEXAS-MNCPES cohort, chlorpyrifos was identified in indoor air (both area and personal air samplers) in over 90% of 82 households, with a median concentration of 1.7 ng/m3, with 90% of the samples collected below 16 ng/m3.
Indoor air concentrations of chlorpyrifos were also measured in the Urban Minority Women Cohort (Columbia University) studies. For the Whyatt et al. (2004) study, indoor air samples were collected between February 1998 and May 2002, a time when chlorpyrifos was widely used for residential insect control. Indeed, 85% of these women reported using insecticides in the home, with 56% reporting use of spray cans, pest bombs, or sprays by an exterminator. Insecticide levels in both maternal personal air and blood samples were reported for 82% of the 314 mother–newborn pairs, and one or the other of the two measures (either personal air or blood) of chlorpyrifos exposure were available for the remaining 18% (55 pairs). Analysis of personal air samples for chlorpyrifos demonstrated substantial intersubject variability, with a mean and standard deviation of 15.3 ± 31.8 ng/m3 among 271 subjects. A geometric mean of 8 and 4.3 ng/m3 was reported in women evaluated prior to and after January 1, 2001, respectively (). The decrease in personal air concentrations of chlorpyrifos presumably was a result of the declining use of chlorpyrifos after January 1, 2001. The CitationWhyatt et al. (2005) study reported air concentrations of an additional 123 subjects, for a total of 394. However, the paper indicated that the time frame of collection of these samples was the same as reported in the 2004 paper (between 1998 and 2002). The chlorpyrifos levels in these 394 personal air samples were 14.3 ± 30.7, with a range of 0.1 to 345 ng/m3 ().
These indoor air concentrations are approximately an order of magnitude higher than what was observed in the MNCPES cohort. Subsequent studies of this cohort showed somewhat decreasing indoor air concentrations over the period 2000–2004 (). For example, 32 homes sampled in 2001 had mean chlorpyrifos indoor air concentrations of 12.4 ± 29 ng/m3, whereas in 2004 the concentrations were 2.4 ± 1.9 ng/m3 (CitationWhyatt et al., 2007), almost an order of magnitude lower than those measured in the first study (CitationWhyatt et al., 2002) and similar to indoor air concentrations reported in the MNCPES cohort. As chlorpyrifos was widely used in residential application for pest control until 2001, and the subjects in this cohort were selected based in part on the presumed use of pesticides in these residences, the higher levels of chlorpyrifos in indoor air during the late 1990s, compared to 2004, is not surprising. Numerous other studies have also examined indoor air concentrations of chlorpyrifos after residential use (e.g., crack and crevice treatment or in some cases broadcast application). Concentrations in indoor air after such uses were frequently in excess of 100 ng/m3 (CitationByrne et al., 1998; CitationHore et al., 2005), with one report (CitationGurunathan et al., 1998) finding levels in the 4000–6000 ng/m3 range after extensive indoor treatment (direct application of chlorpyrifos formulation to floor surfaces, with approximately 12,000 mg chlorpyrifos applied in each of two apartments).
In studies where indoor use of chlorpyrifos was not recent, indoor air levels were of course much lower than studies where it had been applied recently. For example, the CTEPP (North Carolina) cohort found typical indoor air concentrations of less than 10 ng/m3 (5 ± 13.3 in 129 urban homes; 2.6 ± 3.3 in 13 day cares).
In the CHAMACOS Cohort (CitationBradman et al., 1997), indoor air concentrations of chlorpyrifos measured in the homes of 20 children had a median concentration of 11 ng/m3 (range 4.0–36).
Fenske, Lu, and coworkers have evaluated both indoor air concentrations of chlorpyrifos in homes adjacent to agricultural areas. Indoor air concentrations in 6 agricultural households were 3 ± 4 ng/m3 (maximum 10), essentially no different than the indoor air concentration of 7 reference urban households (2 ± 4 ng/m3, maximum 5) (CitationLu et al., 2004).
As discussed earlier, most of the studies that have measured indoor air levels for chlorpyrifos have been done on cohorts where indoor application of chlorpyrifos had occurred recently. It appears from the limited studies that have been done in households that have not recently used chlorpyrifos for insect control that indoor air concentrations of chlorpyrifos are typically less than 10 ng/m3. In agricultural areas, indoor air is not likely to be significantly greater than outdoor air, unless there is extensive “take home” of the pesticide by occupational users (farmers, pest applicators).
Outdoor Air. Relative to indoor air concentrations, fewer studies have reported measuring concentrations of chlorpyrifos in outdoor air (). As would be expected, concentrations of chlorpyrifos in outdoor urban air are substantially lower than indoor air following indoor residential use. In the MNCPS cohort (CitationClayton et al., 2003) only 10% of outdoor air samples exceed the detection limit, and the air concentration of the 90th percentile of samples was 0.07 ng/m3. The CTEPP cohort study (CitationMorgan et al., 2005) also measured outdoor air concentrations of chlorpyrifos at urban homes and day-care centers of 0.5 ± 1 and 0.2 ± 0.2 ng/m3, respectively.
Harnly et al. (2005, 2006) measured chlorpyrifos and chlorpyrifos-oxon in outdoor air samples collected in the southeastern portion of California's Central Valley, an agriculturally intensive area. Although few specific airborne concentration data are provided, they reported a median air concentration of 33 and 22 ng/m3 for chlorpyrifos and chlorpyrifos oxon, respectively. They also reported correlations between airborne concentrations of chlorpyrifos and chlorpyrifos-oxon and agricultural use. CitationLee et al. (2002) reported on chlorpyrifos levels in ambient air in urban and rural locations in California as part of the California Air Resources Board ambient air monitoring program. Chlorpyrifos levels (which apparently combine chlorpyrifos and chlorpyrifos-oxon measurements) were substantially higher in this report than in any other ambient air studies. Mean concentrations of chlorpyrifos/chlorpyrifos-oxon in ambient air were 15 and 100 ng/m3 in urban and rural areas, respectively.
Several studies have also measured ambient airborne levels of chlorpyrifos and chlorpyrifos oxon in air sheds impacted by agricultural use of pesticides—including chlorpyrifos. Chlorpyrifos was identified in outdoor air in the Sierra Nevada foothills during summer months, with concentrations ranging from 0.1 to 100 ng/m3, depending on elevation and distance from agricultural sources (CitationAston and Seiber, 1997). Chlorpyrifos-oxon was also detected in most samples, with concentrations often equal to or somewhat greater than chlorpyrifos itself. Chlorpyrifos was also detected in outdoor air in the vicinity of agricultural areas in southern Manitoba, Canada, after periods of agricultural application, with peak concentrations measured between 10 and 110 ng/m3 (CitationRawn and Muir, 1999). Concentrations generally declined to low levels (< 1 ng/m3) within a week after peak concentrations were seen.
From these various studies it is possible to make a rough estimate of “typical” exposures of children and adults to chlorpyrifos via the inhalation pathway under current use conditions (e.g., no current use of chlorpyrifos in residential pest control, but possible residual indoor air exposures from past uses or other sources, and continued use in agriculture). Assuming a body weight of 20 kg for a child, and an inhalation rate over 24 h of 8 m3, for every 1 ng/m3 of chlorpyrifos a dose rate of 0.0004 μ g/kg-day would be achieved. Assuming first order elimination in the urine, a urinary elimination half-life of 27 h (as TCPy), and a dosing interval of 24 h, the steady-state body burden of (per ng/m3) would be approximately 0.00065 μ g equivalents of chlorpyrifos/kg body weight.Footnote7 [As the half-life for chlorpyrifos is much shorter than TCPy, probably on the order of 1 h or less (CitationTimchalk et al., 2002b), most of the body burden will actually be as TCPy, rather than chlorpyrifos, although chlorpyrifos may partition to lipid compartments in the body.] Further assuming that urinary excretion accounts for 70% of the elimination, and a urine volume for a 20-kg toddler of 0.5 L, and correcting for the differences in formula weight between chlorpyrifos and TCPy (ratio of 1.77), 24-h urine TCPy concentrations would be approximately 0.010 ppb for every 1 ng/m3 airborne chlorpyrifos. Thus, a constant airborne concentration of chlorpyrifos of 10 ng/m3 would be expected to yield a urinary TCPy concentration of approximately 0.1 ppb in a 20-kg child, whereas a continuous air concentration of 100 ng/m3 would produce a urinary concentration of about 1 ppb. Similar calculations for adults (assuming 20 m3 air per day and 70 kg body weight, and urine volume of 1.7 L) yields approximately the same daily dose rate as children (0.0003 μ g/kg-day per 1 ng/m3 chlorpyrifos air concentration) and slightly lower 24-h urinary TCPy concentrations (∼ 0.008 ppb increase for each ng/m3 air concentration of chlorpyrifos).
Similar calculations can be used to assess the relative contributions of inhalation exposure to total daily intake in the women studied in the Columbia Cohort, since air concentrations were measured. As an approximate estimation of daily intake from the airborne exposures in this cohort, an adult woman who breathes 15 m3/day containing 14 ng/m3 would have an intake of approximately 200 ng over the course of the day (assuming that most of the inhaled dose was absorbed), or a dose rate of approximately 0.003 μ g/kg-day for a 70-kg woman. It is important to recognize that there was undoubtedly a large subject-to-subject and day-to-day variability in exposures for both diet and inhalation exposure in the Columbia population, as evidenced by the large standard deviation and range of values reported (SD > mean; over 100-fold range in blood concentrations, and > 1000-fold range in airborne concentrations). The maximum value for airborne exposure measured from personal air monitors in the Columbia cohort (345 ng/m3) yields an estimated maximum intake (assuming 15 m3 per day, 70 kg body weight) of about 0.07 μ g/kg-day.
These estimates are in close agreement with the estimates of exposure from crack and crevice application of chlorpyrifos provided by CitationByrne et al. (1998). In that study, three homes were treated with chlorpyrifos according to standard practice, and indoor air concentrations of chlorpyrifos were monitored over time. Typical indoor air concentrations over a 2-week period ranged between 50 and 400 ng/m3, consistent with, but somewhat higher than, the range of levels reported in the Columbia University cohort (maximum of 345 ng/m3). CitationByrne et al. (1998) also estimated daily doses from inhaled chlorpyrifos in residents of these homes in the days following application, after correcting for background exposures from diet, using pre-and post-application assessment of urinary TCPy (the authors assumed that all urinary TCPy was derived from chlorpyrifos). These authors estimated that the inhalation exposure in adults contributed only slightly to total urinary TCPy, and calculated daily intake rates from inhalation of chlorpyrifos was 0.002–0.09 μ g/kg-day (CitationByrne et al., 1998). Thus, the calculated daily exposures from inhalation exposure using a simple analysis of the Columbia Cohort exposure data (0.003–0.07 μ g/kg-day) is quite consistent with measured values in a small study of humans exposed through residential use of chlorpyrifos for insect control (similar to that in the Columbia Cohort, where 85% of the subjects reported indoor use of insecticides). However, these values are substantially lower than estimates made by CitationKrieger et al. (2001) after crack and crevice application in a family, using urinary TCPy as the biomarker of exposure. In that study, they estimated daily intake of chlorpyrifos in the 0.8–5.3 μ g/kg-day range, although their “pre-spray” values were also substantially higher (0.3–2.1 μ g/kg-day).
V.C.3. House Dust and Soil (Dermal and Oral)
Household dust as a potential pathway for exposure to chlorpyrifos, especially for children, has been considered in several papers (CitationBradman et al., 1997; CitationLioy et al., 2000; CitationButte and Heinzow, 2002; CitationFenske et al., 2002b; CitationSexton et al., 2003). (See CitationButte and Heinzow [2002] for a review of house dust as a potential source of pollutant exposures.)
CitationBradman et al. (1997) found residues of chlorpyrifos in house dust and hand wipe samples from children living in homes of farm workers. Although one sample from a linoleum floor was very high (33,000 ng/g), house dust levels were much lower (230–710 ng/g) and similar to those seen in other studies (19). For example, in the MNCPES study, chlorpyrifos was identified in 62% of house dust samples collected from surfaces (e.g., floors), with a median surface loading of 1100 ng/cm2 (CitationLioy et al., 2000). Subsequent studies from the MNCPES cohort found that house dust exposure was not a significant contributor to aggregate total exposure to chlorpyrifos (CitationClayton et al., 2003). CitationFenske et al. (2002b) found that median house dust concentrations for chlorpyrifos were highest in applicator homes (400 ng/g), followed by farm-worker homes (300 ng/g) and then nonagricultural “reference” homes (100 ng/g). However, there was no correlation between house dust chlorpyrifos concentrations and children's urinary TCPy levels, although the detection limit for urinary TCPy was relatively high (8 μ g/L) and thus many of the samples were below the limit of detection. In another study of agricultural families, CitationLu et al. (2004) identified trace levels of chlorpyrifos in house dust (detectable, but below the limit of quantitation) in six agricultural homes and one nonagricultural home.
With the elimination of indoor residential use of chlorpyrifos, it seems likely that dermal exposure and secondary (nondietary) oral exposure of children to chlorpyrifos is not likely to contribute significantly to their overall exposures to chlorpyrifos. For example, using the same “steady-state” approach as described earlier for airborne exposures, one can estimate both steady-state “body burden” and urinary concentrations from ingestion of house dust or soil. For example, if a 20-kg toddler ingested 50 mg of dust/soil on a daily basis that contained 100 ng chlorpyrifos/gram of house dust, and further assuming that 70% of the ingested dose was absorbed, the daily intake rate of chlorpyrifos would be about 0.0002 μ g/kg-day, and the “steady state” body burden would be about 0.0003 μ g chlorpyrifos equivalents/kg. From this, an estimated 24-h urine concentration at steady state would be about 0.006 ppb. Even if one were to assume a much higher chlorpyrifos concentration (5000 ppb) and much higher soil ingestion rate (200 mg/day), the daily intake rate, steady-state body burden of chlorpyrifos equivalents, and 24-h urine concentration of TCPy would be 0.035 μ g chlorpyrifos equivalents/kg-day, 0.06 μ g chlorpyrifos equivalents/kg-day, and 1.28 ppb TCPy, respectively. These values are useful in evaluating the importance of different routes of exposure to aggregate exposure, as discussed later.
It is also possible that dermal contact to chlorpyrifos could contribute to total exposures, especially in children who come in contact with chlorpyrifos residues in house dust, or even direct contact with sprayed solutions of chlorpyrifos.
CitationZartarian et al. (2000) utilized a stochastic model to simulate children's exposure to chlorpyrifos via dermal contact and secondary (nondietary) ingestion of dust from surfaces following simulated pesticide application via broadcast or “crack and crevice” treatments. The model incorporated, in the author's words, an “extremely simplified model of the absorption, metabolism and elimination” of chlorpyrifos, as well as numerous other model input simulations of age-specific activity data derived from “object contact events” and children's diaries, and videotaped activities of children. Surface loading values for chlorpyrifos (ng/cm2) were estimated from fitted probability distributions to literature values for residential chlorpyrifos surface loading using Crystal Ball software. The model gave estimates of chlorpyrifos exposure following 1 day of crack and crevice treatment with chlorpyrifos of 5–50 μ g of chlorpyrifos, with a predicted urinary TCPy concentration of 1–10 μ g/L. It should be noted that these values are based on a very large number of assumptions, and the estimates were provided as an initial test of the model, rather than for the purposes of determining actual exposure levels of children to chlorpyrifos.
CitationKrieger et al. (2001) also evaluated the extent of absorbed dose of chlorpyrifos following indoor discharge of fogger canisters in two residences. Surface loading in this study resulted in surface deposition of chlorpyrifos from 1 to 17 μ g/cm2. Two additional homes were evaluated in which chlorpyrifos was used in broadcast spray or via “crack-and-crevice” application by a pesticide applicator. Urinary TCPy values were determined in family members before application, and then 1, 2, 3, 4, and 7 days after application. Results were converted to daily exposures to chlorpyrifos (correcting for formula weight differences between TCPy and chlorpyrifos), using creatinine-adjusted values and assuming 1 and 1.7 g creatinine excretion per day for adult females and males, respectively, and 0.08 g creatinine/day/year of age for children. The results, averaged for all family members for each of the three types of application, are shown in . The standard assumption that all urinary TCPy was from ingestion of chlorpyrifos was utilized. Thus, the pre-spray values are likely to have been a substantial overestimate of actual chlorpyrifos exposures, but the difference between pre-and postexposure values is likely to represent increased exposures to chlorpyrifos from the application. These data clearly demonstrate that all three residential exposure methods can result in a substantial increase in daily exposure from chlorpyrifos, beyond what occurs via the diet.
FIG. 9 Pre-versus postapplication exposure to chlorpyrifos from residential use (from Kreiger et al., 2001).
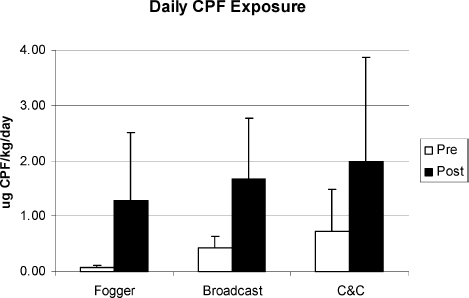
This same study demonstrated that urinary TCPy levels remained elevated above the background for about a month following thorough fogging of a home with chlorpyrifos.
Dermal absorption of chlorpyrifos from floor surfaces was examined in individuals using an exercise facility in which the carpet had been treated with chlorpyrifos via fogging (CitationKrieger et al., 2000). Individuals wearing swimsuits during exercise reportedly received an average absorbed dose of chlorpyrifos of 176 μ g/person. The authors concluded that the absorbed dose depended on a number of factors, including the extent of dermal contact and the rate of absorption. On average, urinary excretion of TCPy increased from two-to fourfold each day, for each of the 3 days following application, relative to the amount excreted the day before application. However, there were large interindividual differences in both the level of TCPy in the urine prior to exposure, and the magnitude of change following exposure. Nevertheless, the data provided convincing evidence that some dermal absorption of chlorpyrifos can occur following routine contact with surfaces after aerosol dispersion of chlorpyrifos (fogger application).
Given the current restrictions on chlorpyrifos use for residential housing, it seems unlikely that such exposures could occur from dermal contact in the absence of an accident. “Surface loading” measurements (), most of which were obtained from homes that had recently used chlorpyrifos for pest control, or in agricultural homes, generally have reported values of less than 10 ng/cm2, although in one farm-worker home a value of 210 ng/cm2 was reported (CitationBradman et al., 1997). Using these data and several assumptions, it is possible to provide a “ballpark” estimate of the potential contribution of dermal absorption following surface contact with chlorpyrifos to total urinary TCPy. For example, if one assumed that “surface loading” occurred directly to a child's skin at a rate of 10 ng/cm2, and 100 cm2 of skin were in contact at that rate, and further assumed that 5% of the exposed dose was absorbed over the course of a day, the daily dose for a 20-kg child would be 0.0025 μ g/kg-day.Footnote8 If repeated dermal exposure occurred at that rate, the steady-state body burden would be approximately 0.004 μ g chlorpyrifos equivalents/kg, and the average 24-h urine TCPy concentration would be approximately 0.06 ppb (assuming a 24-h urine volume of 0.5 L for a 20-kg child, and that 70% of the absorbed dose is eliminated as TCPy in urine). These represent conservative assumptions that are likely to substantially overestimate dermal exposure to a child, but are useful in assessing the contribution of dermal exposure to aggregate exposure to children, and suggest that dermal exposure is unlikely to contribute significantly to urinary TCPy values, relative to other sources, as discussed later.
Not surprisingly, the more recent cohort studies that have assessed the correlation between surface loading and/or house dust concentrations of chlorpyrifos in homes and urinary TCPy concentrations have not found any significant correlations between either surface loading or house dust concentrations of chlorpyrifos and urinary concentrations of TCPy, suggesting that dermal exposures to chlorpyrifos do not contribute significantly to total urinary TCPy values.
V.C.4. Diet
Chlorpyrifos Residues on Foods. Chlorpyrifos is frequently used to control pests in many areas of agriculture, especially in the growing of fruits and vegetables. Thus, residues of chlorpyrifos are present in food. The U.S. Food and Drug Administration (FDA) has conducted a “Market Basket Survey” of a wide variety of food items for the past 20 years (referred to as the Total Diet Study; CitationGunderson, 1995a, Citation1995b). Samples of market products, including fresh and frozen fruits and vegetables, processed foods, and a wide variety of packaged food, are collected periodically and analyzed for a number of potential contaminants, including pesticides such as chlorpyrifos and chlorpyrifos-methyl. Since 1991, a total of 285 different types of foodstuffs purchased from supermarkets or grocery stores four times a year have been analyzed for chlorpyrifos residues. The samples were collected in four geographic regions of the country, and each “market basket” is a composite of foods purchased in three cities in a given region. provides a summary of the pertinent findings of the Total Diet Study for chlorpyrifos and chlorpyrifos-methyl.Footnote9
TABLE 20 Summary statistics for chlorpyrifos and chlorpyrifos-methyl residues in foods, FDA Total Diet Study, 1991–2003
Of the nearly 6000 food samples analyzed (representing 285 different food types), chlorpyrifos was detected at trace or quantifiable levels in approximately 18% of samples, and was above a quantifiable level in slightly less than 5% of the samples. None of these samples exceeded the EPA tolerance level for chlorpyrifos. Not surprisingly, the primary source of dietary intake for chlorpyrifos occurs from residues on fruits and vegetables.
However, the data just described represent a compilation of samples collected over the period 1991–2003, and agricultural use patterns for chlorpyrifos have changed significantly in that time period. For example, “post-bloom” use of chlorpyrifos on apples and all uses on tomatoes were prohibited after January 1, 2001 (CitationEPA, 2002). Thus, the residue levels shown earlier for products containing apples and tomatoes are primarily of historical relevance in determining TCPy values in urinary biomarker studies in which samples were collected prior to 2001.
Chlorpyrifos-Methyl Residues on Foods. In a summary of the 2003 Total Diet Study (CitationFDA, 2005) the FDA noted that chlorpyrifos-methyl and chlorpyrifos ranked 4th and 5th, respectively, among the 18 pesticides found to occur in 1049 food samples collected in 2003 as part of the market basket survey. The range of concentrations was from 0.5 to 92 and from 0.2 to 110 ppb for chlorpyrifos-methyl and chlorpyrifos, respectively. Because the urinary metabolite of chlorpyrifos-methyl is the same as that for chlorpyrifos (TCPy), chlorpyrifos-methyl residues in the diet need to be considered in studies that rely upon TCPy as a biomarker for chlorpyrifos exposure. Chlorpyrifos-methyl was registered by the EPA in 1985 for use on stored grain, seed treatment, and grain bin and warehouse use. Based on 1989 through 1998 usage information, the EPA estimated that total domestic usage of chlorpyrifos-methyl averaged approximately 80,000 lb active ingredient, of which about 80% is applied to wheat. Approximately 8% of all stored wheat, 5% of sorghum, and 5% of barley were treated with chlorpyrifos-methyl (EPA, 2001). As such, it was a common trace contaminant of flour-based consumer products. Registration of chlorpyrifos-methyl for use in storage of grains and most other applications was proposed for voluntary cancellation by the registrants in 2001. However, because of the lack of a suitable alternative to protect grains from pests during storage, the EPA postponed the cancellation of the registration of chlorpyrifos-methyl until a suitable alternative could be developed. In December 2007, the registration of several chlorpyrifos-methyl products was enacted, although not all products and uses were canceled.Footnote10
Several products containing chlorpyrifos-methyl as one of the active ingredients continue to be used for grain storage protection, generally in combination with pyrethroid insecticides. Existing stocks of the canceled products may be used until December 2009.
Although the number of types of food products in the Total Diet Study that contained chlorpyrifos-methyl residues above the detection limit was less than that seen for chlorpyrifos (31% for chlorpyrifos-methyl, vs. 52% for chlorpyrifos), for those products that did contain chlorpyrifos-methyl, both the frequency of detection and the level of contamination was substantially greater (). As would be expected based on its approved uses, most of the dietary chlorpyrifos-methyl residues are derived from flour-based products. Many common food products had a very high frequency of detection for chlorpyrifos-methyl. This becomes an important consideration when evaluating the many cohorts that have estimated chlorpyrifos exposures based largely or solely on urinary TCPy concentrations, because chlorpyrifos-methyl will yield the identical metabolite in urine (as does direct exposure to TCPy that is present as a residue on fruits and vegetables).
Contributions of Diet to Chlorpyrifos and Chlorpyrifos-Methyl Total Exposures, and Urinary TCPy Levels. The U.S. Department of Agriculture and the FDA have developed “typical” dietary food consumption values for various ages in the US population for most of the foodstuffs collected in the Total Diet Study (data available at http://www.cfsan.fda.gov/∼comm/tds-hist.html). Although diets obviously vary greatly from one individual to another based on personal food preferences, it is instructive to determine the estimated “typical” dietary intake for specific age groups in the United States by combining the food intake data with the chlorpyrifos and chlorpyrifos-methyl residue data. By multiplying the quantity of food (in grams) by the average concentration of chlorpyrifos or chlorpyrifos-methyl in each food item, it is possible to estimate “typical” average daily exposures to chlorpyrifos via the diet. These can then be added together to obtain an estimate of the total average daily intake for each age group that is reflective of the dietary contaminant levels present at the time period when the samples were collected. Further assuming typical body weights (70 kg for adults/lifetime average body weight; 20 kg for 2-year-old toddler, 10 kg for infants 6–11 mo old), one can further project approximate daily intake rates of chlorpyrifos and chlorpyrifos-methyl for the various age groups, in μ g/kg-day. Finally, with additional assumptions regarding the pharmacokineticsFootnote11 of chlorpyrifos and chlorpyrifos-methyl it is possible to make “ballpark” estimates of urinary TCPy values from the estimated dietary intake, using the standard equation for steady-state body burden and urinary concentration, used previously.
illustrates these calculations for adults, toddlers (age ∼ 2 years), and infants (age 6–11 months). For chlorpyrifos, an average daily intake of approximately 0.35 μ g/day is estimated for adults, yielding a body-weight-adjusted daily intake of 0.005 μ g/kg-day. This estimate is very similar to the estimate provided by Gunderson (CitationGunderson, 1995a) in an analysis of the TDS study data from 1986–1991. For infants, the intake is estimated to be slightly less than 0.1 μ g/day, yielding a weight-adjusted intake rate of ∼ 0.009 μ g/kg-day for a 10-kg infant. For chlorpyrifos-methyl, average daily intakes are approximately four-to fivefold higher in adults and toddlers, but 10 times higher for infants. There is of course substantial variability in dietary samples, as well as varying eating habits. To provide a reasonable “upper bound” on these estimated intake rates, one could use the maximum residue detected for each food group (rather than the mean), times the average intake rate of that food item. This provides an estimate approximately 4–15 times greater than if the mean values are used (). Thus, based on the Total Diet Study data from 1993–2003, average daily intake rates from food for adults, toddlers, and infants would be predicted to be about 0.005, 0.014, and 0.009 μ g/kg-day for chlorpyrifos. For chlorpyrifos-methyl, the corresponding rates would be 0.021, 0.086, and 0.010 μ g/kg-day. The projected urinary TCPy values will be discussed in detail in the following section.
TABLE 21 Estimates of daily chlorpyrifos/chlorpyrifos-methyl intake based on food intake rates and residue concentrations
To estimate dietary exposure to chlorpyrifos, the EPA used the Dietary Exposure Evaluation Model (DEEM). DEEM incorporates consumption data generated in USDA's Continuing Surveys of Food Intakes by Individuals (CSFII), 1989–91. For chlorpyrifos, inputs to the DEEM analysis also include DAS's National Food Survey (NFS, 1993–1994), the U.S. Department of Agriculture Pesticide Data Program (PDP) monitoring data (1994–1999), the Food and Drug Administration (FDA) Surveillance Monitoring Program data (1992–1998), and field trial residue data. “Percent crop treated” data were supplied by the EPA Biological and Economic Analysis Division. Where percent crop treated estimates indicated no chlorpyrifos use, a default assumption of 1% crop treated was applied. In general, when residues on commodities were nondetectable, one-half the limit of detection (LOD) was assumed. All available processing and cooking factors were incorporated into the dietary exposure analysis.Footnote12 Although the EPA document does not provide specific intake values, it does provide an estimate of the percentage of the chronic “population adjusted dose” (defined as the chronic reference dose divided by an appropriate uncertainty factor), for the 99.9% distribution of dietary exposure. Thus, for children 1–6 years, the estimated exposure at the 99.9% distribution was 81% of the cPAD. Since the cPAD was stated to be 0.03 μ g/kg-day for 1–6 year olds, then 81% of this value is 0.024 μ g/kg-day. This value is about twice what was calculated earlier in this review as an average daily intake for toddlers, but the EPA value represents the upper 99.9% distribution, so is very consistent with the estimate of 0.014 μ g/kg-day dietary intake for toddlers. Similarly, the EPA estimated that the 99.9% population distribution for adults was 4% of the adult cPAD of 0.3 μ g/kg-day, or 0.012 μ g/kg-day, again about twice the “average daily exposure” estimated earlier for the general population (0.005 μ g/kg-day). Thus, the average daily dietary exposures estimated from the TDS diet illustrated earlier are consistent with the EPA estimates using the DEEM model (EPA, 2000). It should be noted that the EPA further restricted the use of chlorpyrifos on tomatoes, grapes, and apples in June 2000, and predicted that the additional restrictions would result in a 30–40% reduction in dietary exposures to chlorpyrifos. Thus, current levels of exposure to chlorpyrifos from the diet are expected to be somewhat less than those measured in 2000 and before.
The dietary exposure data discussed earlier are derived from the FDA's Total Diet Study, which is the most comprehensive and “representative” analysis of pesticide residues in the U.S. diet available. However, as noted earlier, several other cohort studies on chlorpyrifos have measured chlorpyrifos residues in diets of adults and children during the study period, generally as homogenates of all foods consumed. As part of the NHEXAS-MD study, MacIntosh et al. (2001a, 2001b) conducted a stratified probability sample of the diets of 80 individuals older than 10 years in the greater Baltimore metropolitan area. Subjects participated in an extensive diet collection study in which duplicate portions of each subject's diet were collected for analysis over a 7-day period. Sampling was repeated up to six times for each subject over the period of a year to provide a longitudinal assessment and provide insights into seasonal variation in dietary pesticide exposure. All food items were combined and homogenized for analysis, in contrast to the FDA Total Diet Study, which analyzed each food item separately. Chlorpyrifos was detected in 38% of the 379 samples collected, with 10% of samples having chlorpyrifos concentrations greater than 1.8 ppb, and 1% greater than 7.7 ppb, with a maximum concentration of 24.3 ppb (CitationMacIntosh et al., 2001b). Based on the self-reported body weights of the subjects, the authors calculated mean body-weight adjusted daily intake rates of 0.007 μ g/kg-day. This value is very close to the intake rate of 0.005 μ g/kg-day estimated for adults from the FDA Total Diet Study. A somewhat later report of this same cohort reported the mean concentration of chlorpyrifos in food of 0.75 ± 2.2 ppb, with a maximum of 24 ppb (CitationPang et al., 2002). CitationPang et al. (2002) included food analysis in his NHEXAS-MD report on aggregate exposures, and these probably represent the same or a subset of the NHEXAS-MD food data reported by MacIntosh et al. (2001b).
In the NHEXAS-MN (MNCPES) study, CitationClayton et al. (2003) measured chlorpyrifos residues in 96 solid food samples, and reported detectable chlorpyrifos in 57% of the samples, with a median concentration of 0.2 ppb, and 90% of samples less than 0.6 ppb (). They did not identify measurable chlorpyrifos in any of 101 beverage samples.
In the CTEPP cohort, CitationMorgan et al. (2005) evaluated chlorpyrifos concentrations in solid and liquid food in both the home environment (n = 129) and day-care centers (n = 24). The mean concentration was 0.6 ppb (SD 1.8), with a geometric mean of 0.2 ppb for home samples, with significantly lower concentrations found in the day care food (0.2 ± 0.3 ppb). Chlorpyrifos was below the limit of detection in nearly all of the liquid food samples collected.
CitationLu et al. (2006) recently compared the urinary TCPy levels in children fed a diet containing fruits and vegetables from standard agricultural practices (e.g., marketplace produce not identified as “organic”) with a diet where fruits and vegetables were obtained from a certified organic source. The study involved 23 children ages 3–11 years recruited from schools in the greater Seattle, WA, area. The study was conducted in 3 phases over 16 days. For phases I and III of the study, the children were given the conventional diet for 3 days (days 1–4 and days 10–16). For phase II of the study (days 5–9) fruits and vegetables obtained from “certified organic” sources replaced fruits and vegetables from conventional sources. The types of fruits and vegetables consumed in phase 2 were matched as closely as possible with that of their conventional diet (phase I and III). Samples of all the organic food items were analyzed for OP pesticides and verified to be free of measurable levels of OP pesticides, including chlorpyrifos. Unfortunately, neither the conventional diet nor the “certified organic” diet was analyzed for the presence of TCPy and only the “certified organic” diet (not the conventional diet) was analyzed for chlorpyrifos in the food itself. Urine samples were analyzed for TCPy at the National Center for Environmental Health at the CDC. The results are shown in .
TABLE 22 Urinary TCPy concentrations in children consuming conventional and “certified organic” diet (from CitationLu et al., 2006)
It is interesting to note that the urinary concentrations of TCPy during the organic diet phase, while significantly less than that observed during both conventional diet phases, still showed measurable levels of TCPy in 50% of the samples, and that the mean value (1.7 ± 2.7 ppb) was only modestly lower than the mean urinary TCPy values measured in NHANES III in 2001–02 for children 6–12 years old (3.1 ± 4.2 ppb). The authors also reported the data by each day of urinary collection. Although the mean urinary TCPy value during the first day of the “organic” diet was substantially higher than the subsequent 4 days (consistent with a half-life of TCPy somewhat greater than 1 day), the concentration of TCPy in urine in most of the samples was greater than 1 ppb. The source of this urinary TCPy is not clear, since the analysis of the organic diet indicated no detectable residues of chlorpyrifos. However, based on the TDS data on chlorpyrifos-methyl discussed earlier, chlorpyrifos-methyl and/or TCPy derived from chlorpyrifos-methyl may have been present in grain-derived produce consumed by these children, thereby contributing to background urinary TCPy. Nevertheless, the results of this study clearly indicate that chlorpyrifos and/or TCPy residues on fruits and vegetables obtained from conventional sources are a major contributor to total urinary TCPy seen commonly in the U.S. population (as indicated in the NHANES and other studies).
A follow-up study of this same urban cohort of children was recently reported (CitationLu et al., 2008), in which seasonal influences of dietary chlorpyrifos on urinary TCPy levels were reported. The results of this study basically confirmed their earlier report demonstrating that urinary TCPy levels were significantly lower in children who consumed diets containing “certified organic” fruits and vegetables, relative to “conventional” diets. Mean levels of urinary TCPy over 2 years were 5.1 ± 5.0 μ g/L. Seasonal variation in urinary TCPy was evident, with samples collected in the fall (2.6 ± 3.1) averaging about half of the mean values seen in other seasons (mean values of 5.1, 5.6 and 6.4 μ g/L for winter, spring, and summer, respectively). Replacing conventional produce with certified organic produce lowered the urinary TCPy concentrations by approximately 80% during the summer months, when consumption of fresh fruits and vegetables was highest. Although samples of the diet were collected for analyses, the actual levels of chlorpyrifos residues were not reported (although this may be the subject of a future publication). It would be of particular interest to analyze the food samples for both TCPy and chlorpyrifos (and for chlorpyrifos-methyl) to determine the relative contribution of dietary TCPy to total urinary TCPy.
To determine the relative contribution of different food types to urinary TCPy, CitationKieszak et al. (2002) analyzed TCPy data in the NHANES III cohort and dietary “Food Frequency Questionnaires” that were also part of the NHANES III evaluation. When “highest consumers” were compared with “lowest consumers” of specific food items, TCPy concentrations were significantly higher for the highest consumers of fruits and vegetables (using creatinine-corrected values; uncorrected values were not different). The authors concluded that they were unable to establish a relationship between food consumption of specific food groups and urinary pesticide/metabolite (including TCPy) levels using the NHANES III data.
V.C.5. Limitations of Use of TCPy as a Biomarker for Chlorpyrifos Exposure
As noted earlier, urinary TCPy levels have been widely used as a “biomarker” for chlorpyrifos exposure (17), and several studies attempted to assess the relative contribution of various pathways of exposure to chlorpyrifos from urinary TCPy. For example, in the NHEXAS-MD cohort, the authors estimated “partial aggregate median intake” (from inhalation and diet) for chlorpyrifos of 304 ng/day (90% less than 648). When adjusted for age and body weight, partial aggregate daily intake rates were 0.012 μ g/kg-day, of which approximately 94% was derived from diet and 6% was from inhalation exposures. Earlier modeling exercises that incorporated surface loading and hand wash data along with numerous assumptions about exposures via the dermal and inhalation pathways (CitationBuck et al., 1996) yielded an estimate of a median exposure of 0.09 μ g/kg-day (∼ 8 times greater than the measured exposures of Clayton et al., 2003), from which 81% was estimated to be due to inhalation exposure, and 19% from diet (CitationBuck et al., 1996). The modeling exercise of Buck et al. suggested that soil ingestion and the dermal pathways were relatively insignificant pathways for exposure (each contributing less than 0.1% of total estimated exposure). The Buck et al. report relied upon airborne concentrations measured in previous studies where chlorpyrifos had been applied indoors for insect treatment (CitationFenske et al., 1990; CitationLewis et al., 1994), which likely accounts for the much higher levels of inhalation exposure modeled by Buck et al. in 2001. Thus, the Clayton et al. data are likely to be much more relevant to exposure that might be encountered today from agricultural use of chlorpyrifos. CitationClayton et al. (2003) also included the urinary TCPy data in this cohort, reported previously by Adgate et al. (2001; ).
It is instructive to compare a “mass balance” for chlorpyrifos/TCPy in the MNCPES cohort, since this is perhaps the only chlorpyrifos exposure study that measured both urinary TCPy and had reasonably good estimates of chlorpyrifos exposure from all sources (including diet) at the time that the urine samples were collected. The median estimated daily intake of chlorpyrifos from both diet and inhalation exposure in the MNCPES cohort of children was 304 ng/day, and the median urinary concentration of TCPy for all three days (day 3, 5 and 7) was approximately 7 μ g/L. Assuming that the concentration of TCPy in urine was at somewhat of a steady state, and further assuming that a 3-to 5-year-old child produces approximately a half a liter of urine per day, and adjusting for the differences in molecular weight between chlorpyrifos and TCPy (it takes 1.7 μ g chlorpyrifos to yield 1μ g TCPy), the estimated amount of TCPy excreted in urine per day (∼ 3500 ng) is almost 20 times more than the estimated aggregate exposure (median of 304 ng chlorpyrifos/day, or 178 ng TCPy equivalents/day). Thus, the estimates of exposure obtained from direct measurements of chlorpyrifos in air and diet account for only about 5% of the TCPy that was actually measured in urine. Indeed, the Spearman correlation between partial aggregate exposure and urinary TCPy was low (0.15) and not statistically significant. There are several possible explanations for this apparent discrepancy between measured pathways of exposure (air and diet) and the biomarker (TCPy) of exposure. One possibility is that dermal and/or soil ingestion plays a far greater role in exposures than was predicted by the modeling exercise of CitationBuck et al. (1996). However, this seems highly unlikely, since the exposure assumptions were somewhat conservative, and it is unlikely that the estimates would be off by several orders of magnitude (which would be required for these pathways to account for > 90% of the “excess” TCPy found in urine). As discussed earlier, even highly conservative assumptions about dermal absorption and soil ingestion yield estimates of urinary TCPy of less than 1 ppb at steady state.
A second explanation may be that first morning voids substantially underestimate the previous day's exposure. Although 24-h urine sampling is certainly a preferred method for such analyses, it is seldom done for logistical reasons. To evaluate the potential magnitude of errors in using spot urine samples to estimate daily excretion rates, CitationScher et al. (2007) compared the TCPy concentration in 24-h urine collections to “spot” urine samples (first morning void) in farmers and farm children potentially exposed to chlorpyrifos. They concluded that “A consistent bias towards overprediction of pesticide concentration was found among the MVs [morning voids], likely in large part due to the pharmacokinetic time course of the analytes in urine,” and noted that this was true for both farmers and their children. However, their results did demonstrate a reasonably good correlation between MV and 24-h urine samples, suggesting that the magnitude of the error is not large. The geometric mean of the ratios of [MV]/[24h] was 1.5 for the first 24-h collection in both children and farmers, with the ratio slightly higher (1.6) in farmers who had applied chlorpyrifos, relative to their children (1.3). Thus, it is unlikely that the seemingly large differences in estimates of chlorpyrifos intake versus the amount of TCPy excreted in urine in the MNCPS study can be explained by underestimates of chlorpyrifos intake due to spot urine collection. The data from Scher et al. in fact suggest that use of first morning urine voids will overestimate the previous day's exposure by perhaps as much as 50%.
A third possible explanation is that much of the TCPy found in the urine is actually from exposure to TCPy, rather than from chlorpyrifos, and/or from other dietary pesticides that are also metabolized to TCPy, such as chlorpyrifos-methyl (see earlier discussion on dietary sources of exposure). Indeed, CitationMorgan et al. (2005) identified concentrations of TCPy in fruits and vegetables that were 12–29 times higher than chlorpyrifos residues in the same produce. CitationWilson et al. (2003) also measured both chlorpyrifos and TCPy in food samples from nine children in a day-care setting. Whole duplicate meals were collected over several days and homogenized (solid foods and liquid foods kept separately). Morgan et al. (1995) found that the mean concentration of chlorpyrifos in solid food was 0.65 ng/g (ppb), with a range of 0.21–1.65 (n = 4), TCPy concentrations measured in the same food samples averaged 16 ppb, with a range of 14–18. These findings are remarkably similar to those of CitationMorgan et al. (2005) as the average TCPy levels were ∼ 20 times greater than the average chlorpyrifos concentrations. These are extremely important findings, as nearly all studies on chlorpyrifos exposure have relied upon the assumption that the ONLY source of TCPy in the urine is from chlorpyrifos exposure.
In the NHEXAS-MD study, detailed dietary assessment was made, and urinary TCPy was also determined. Children in that study (aged 6–11 years) had significantly higher concentrations of TCPy than adults and adolescents, similar to what was reported in the NHANES III study (). When the authors compared chlorpyrifos intake measured directly in food (estimated mean value of 0.46 μ g/day) to the amount of TCPy excreted in urine (6.3 μ g/day), they noted that “dietary intake of chlorpyrifos accounted for approximately 7% of TCPy in this population, consistent with what was measured by CitationMorgan et al. (2005) and CitationWilson et al. (2003), and concluded that “intake of chlorpyrifos from food is a minor contributor to TCPy in urine.” However, they did not measure TCPy in the food. Further, they did not consider the widespread presence of chlorpyrifos-methyl in the diet during the time this study was done, which would likely add substantially to dietary sources of urinary TCPy, as discussed earlier. If the ratio of chlorpyrifos-methyl to chlorpyrifos in the diet of the children in the NHEXA-MD study was similar to that estimated earlier for the FDA Total Diet Study (ratio of 4 for adults and 6 for toddlers), then the amount of chlorpyrifos-methyl ingested would have been approximately 2.5 μ g/day. If the ratio of TCPy to chlorpyrifos in food was similar to that measured by CitationMorgan et al. (2005) and CitationWilson et al. (2003), most of the TCPy in the urine may have come from exposure to chlorpyrifos-methyl and TCPy, with chlorpyrifos itself representing only a minor component, perhaps less than 5%. Interestingly, although TCPy was also identified in indoor and outdoor air as well as in house dust samples, it was found in concentrations similar to or somewhat less than chlorpyrifos, in contrast to the diet, where concentrations of TCPY were 11–28 times greater than chlorpyrifos (CitationWilson et al., 2003; CitationMorgan et al., 2005).
CitationTimchalk et al. (2007) completed a detailed pharmacokinetic study of both chlorpyrifos and TCPy in rats, and concluded that “Assuming similar pharmacokinetics in humans, it is plausible that total urinary chlorpyrifos metabolite levels may be reflective of not only an individual's contact with the parent OP pesticide, but also exposure with intact metabolites present in the environment.” Thus, a mass balance analysis of TCPy and chlorpyrifos exposure determined the MNCPES cohort (CitationClayton et al., 2003), coupled with the direct measurement of TCPy in fruits and vegetables described by CitationMorgan et al. (2005) and CitationWilson et al. (2003), provides consistent and convincing evidence that 90–95% of urinary TCPy derived from dietary exposure is actually from exposure to TCPy, and not chlorpyrifos. Dietary exposures to chlorpyrifos-methyl also likely contributed substantially to urinary TCPy (perhaps as much or more than chlorpyrifos itself). These data clearly indicate that urinary TCPy is not a useful biomarker of chlorpyrifos exposure in populations in which exposure is primarily via the diet, since sources of TCPy other than chlorpyrifos may contribute to > 95% of urinary TCPy.
CitationBarr and Angerer (2006) provide an excellent review of the many nuances associated with using specific urinary metabolites as biomarkers of exposure for specific “nonpersistent” pollutants such as chlorpyrifos. The authors note, “In particular, the timing of biological sample collection is very critical and highly dependent on both the exposure scenario and the uptake and elimination kinetics of the individual pesticides that may depend largely on the pesticide's chemical and physical characteristics.” One should add a note of caution that all sources of the biomarker should be considered. As stated earlier, it appears that the vast majority of urinary TCPy is derived from direct dietary exposure to TCPy as a breakdown product of chlorpyrifos (and potentially chlorpyrifos-methyl, although no studies to date have reported on the ratio of TCP-methyl to TCPy in commodities), and from dietary exposure to TCP-methyl in grain-based foods.
Ambient air concentrations in the absence of local agricultural applications are generally less than 1 ng/m3. Assuming a “steady-state” exposure to1 ng/m3, urinary TCPy concentration would not likely exceed 0.01 μ g/L (∼ 0.02 μ g/day), which would be not be detectably different from “background” due to the substantially higher levels of urinary TCPy that results from dietary exposure to a combination of chlorpyrifos, chlorpyrifos-methyl, and TCPy. Thus, in spite of the problems in using TCPy as a biomarker of chlorpyrifos exposure, chlorpyrifos residues in the diet appear to be the major source of exposure to chlorpyrifos (and chlorpyrifos-methyl) for the general population, based on a large number of studies that have relied upon urinary TCPy as biomarkers of exposure to chlorpyrifos. NHANES data suggest that typical urinary TCPy concentrations are in the range of 1–25 μ g/L, with typical (mean, median, and geometric mean) concentrations of 2–3 ppb. For a typical adult, a 24-h urinary concentration of 2 ppb would be consistent with an exposure to approximately 10 μ g chlorpyrifos (assuming 1.6 L of urine volume, 70% absorption, and a ratio of formula weights of 1.77). However, the FDA Total Diet Study estimates of dietary chlorpyrifos exposure suggest that typical daily intake of chlorpyrifos is approximately 0.3–0.4 μ g per day, which is thus only 3–4% of that predicted based on urinary excretion of TCPy (again, these data are consistent with others suggesting that > 95% of urinary TCPy is derived from sources other than chlorpyrifos). The restrictions on use of chlorpyrifos for residential pest control have also reduced nondietary exposures to the general population today. Thus, a conservative estimate of current daily exposures of chlorpyrifos among the general adult population with no specific sources of exposure would be 0.004–0.006 μ g/kg-day. The Total Diet Study data for chlorpyrifos suggests that young children might have somewhat higher exposures on a body weight basis, perhaps on the order of 0.01–0.02 μ g/kg-day. Although toddler exposure to chlorpyrifos-methyl in the past appears to be substantially greater than for chlorpyrifos (), this source of exposure is likely to be significantly reduced with the expected replacement of chlorpyrifos-methyl as a grain protectant, once a suitable alternative has been identified.
The assessment earlier indicates that background exposures to chlorpyrifos and TCPy are common in the diet, but do not address the potential contributions of inhalation exposure to chlorpyrifos that might occur in people living on or near agricultural areas. Thus, assessment of public (nonoccupational) exposures to chlorpyrifos should consider not only the general public, from which dietary exposures via chlorpyrifos residues in food constitute the primary source of exposure, but also farm-worker families and others residing on or near agricultural areas that utilize chlorpyrifos, where additional exposure from “take home” pathways or drift could add to the general population exposure.
Curwin et al. (Citation2007a, Citation2007b) recently provided a detailed dose estimate of farm and nonfarm children, based solely on spot urine measurements of TCPy. As shown in , there was no difference between urinary TCPy levels in farm and “nonfarm” children. Similar findings were reported by CitationAlexander et al. (2006) and Fenske et al. (Citation2002a, Citation2002b). However, the geometric mean concentrations of TCPy in the Curwin et al. study were three to five times greater than seen in other studies, probably because they utilized an enzyme-linked immunosorbent assay (ELISA), rather than direct chemical detection (HPLC-MS). Immunoassay of TCPy appears to yield different quantitative results for reasons that are not clear. A direct comparison of the same samples using the two different methods also found that the immunoassay gave results that were ∼ 4 times higher than the HPLC method (CitationCurwin et al., 2007b). In the CPPAES study (CitationHore et al., 2005), there were no significant differences in urinary TCPy levels in children when measured the day before, and then several days after, crack and crevice application of chlorpyrifos (), even though there was a substantial increase in indoor air () and surface contamination () with chlorpyrifos in most of the C&C treated homes. Thus, most studies of indirectly exposed populations (e.g., farm-worker families, families living in homes after crack and crevice application, etc.) have found that total exposures, as assessed by urinary TCPy, are not significantly higher among the population with “extra” exposures, relative to urinary TCPy levels that appear to be due to diet alone (see ). In light of the likely contribution of chlorpyrifos-methyl and direct exposure to TCPy residues in the diet, as discussed earlier, to urinary TCPy, this is perhaps not surprising.
Whether children will have lower or higher urinary concentrations of TCPy following the same “exposed” doses, measured on a body weight basis, is not clear. Although CitationScher et al. (2007) assumed that children produce a somewhat larger volume of urine per kilogram body weight than adults, other studies have utilized an estimated volume of urine production for small children (e.g., 18–20 kg) of 0.5 L per day, or 25–33 ml/kg-day (CitationLentner, 1981; CitationHore et al., 2005). Daily urine volume in healthy adults is typically about 1.7 L per day, or about 25 ml/kg-day. Urine volume is highly variable, especially in children, and the concentration of TCPy in urine will depend not only on the amount of chlorpyrifos + chlorpyrifos-methyl + TCPy exposure, but also the volume of fluid intake and the time of sample collection. This was illustrated by CitationKissel et al. (2005), who evaluated both within-child and between-child variability in urinary TCPy in 13 children. Spot urine samples were obtained at four different times throughout the day, including the night before bed and first morning void, in a randomly selected subset of four children (). It is evident that very large differences in urinary TCPy concentrations occur over the period of 24 h. In general, “between-sample” variability in the same child was as great as interindividual variability across the four subjects. In two of the subjects, urinary TCPy concentrations ranged from approximately 1 to 24 μ g/L over the period of a day, even though the mean values (approximately 12) of these two children (11.5 and 11.8) were not very different from the mean of the group (9.0). Fortunately, first morning voids, which have been used in the majority of studies examining chlorpyrifos exposure using TCPy as biomarker, appear to be relatively more reliable than spot urine samples collected at other times of the day, consistent with the conclusions of CitationScher et al. (2007). The Kissel et al. study also found that “corrections” of urine volume using creatinine were of relatively little value.
TABLE 23 Variability in urinary TCPy at different times of sample collection (from CitationKissel et al., 2005)
A relatively detailed urinary TCPy time course analysis was conducted by Hore et al. as part of the CPPAES study (CitationHore et al., 2005, Citation2006). Urine samples were collected the day before application of chlorpyrifos by “crack and crevice” treatment in 10 homes, and urinary TCPy concentrations were then measured daily for the following 12 days. The results of the individual samples are shown in .
FIG. 10 Urinary TCPy concentrations in children following Crack and Crevice treatment of homes with chlorpyrifos. Data from CitationHore et al. (2005).
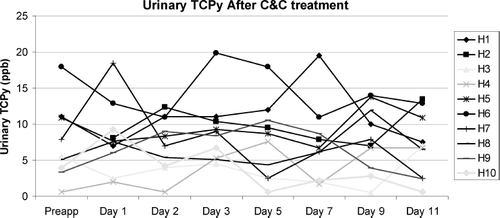
The authors reported daily excretion rates as μ g/kg-day for each individual child. Body weights were provided; thus estimated urine concentrations were obtained by taking μ g/kg-day × kg body weight, divided by an assumed daily urine volume of 0.5 L/day (CitationLentner, 1981). It is evident on inspection of these data that individual urinary TCPy values varied substantially from day to day in the same individual, and there is no obvious correlation between urinary TCPy and chlorpyrifos application (preapplication vs. postapplication). The authors concluded that “Although an increase was observed in the amount of chlorpyrifos measured from the CPPAES homes after the pesticide application, CPPAES findings indicated that the children living within the crack-and-crevice–treated homes were actually not coming into contact with most of the chlorpyrifos that was present in the indoor environment,” and noted that the TCPy in urine must be coming from other sources.
As discussed in sections III.B and III.C, CitationMeeker et al. (2005) examined the temporal variability of TCPy in 10 males with “background” exposures to chlorpyrifos (presumably from the diet) over a period of 3 months. They noted that within subject variability was large, with intraclass correlation coefficients of only 0.15 to 0.21. The authors speculated that highly variable dietary intake of chlorpyrifos and TCPy are likely to contribute to substantial day-to-day variation in urinary TCPy. They attempted to correlate urinary TCPy values with reported intake of food items with historically relatively high chlorpyrifos residues (grapes, tomatoes strawberries), as well as self-reported pesticide use. Some seasonal effect (higher TCPy values in the fall) relative to other seasons was noted. Eating grapes and cheese was also significantly correlated with TCPy. The authors concluded that, although within subject variability was large, single spot urine samples “performed adequately” in classifying a subject in to highest or lowest exposure tertiles. However, the sources of TCPy in the urine (chlorpyrifos, TCPy, and/or chlorpyrifos-methyl exposure) were not addressed. Nor was the importance of sample size when the classification of exposure is subject to a large amount of misclassification considered.
Finally, although urinary TCPy values are problematic in terms of specific biomarkers of chlorpyrifos exposure due to potentially high background exposure to TCPy itself, even an apparently small increase in total urinary TCPy could be indicative of a substantial increase in exposure to chlorpyrifos. Take, for example, the CHAMACOS cohort, in which the average urinary TCPy values (3.5 μ g/L) were in the range of typical United States “background” levels of TCPy in children as measured in numerous other studies (), including the NHANES III cohort, most of which is likely the result of exposures directly to TCPy and chlorpyrifos-methyl, rather than chlorpyrifos. Although the average urinary concentration of TCPy in this cohort was not significantly higher than that seen in the NHANES III cohort, it is possible that some individuals within the cohort had substantially higher exposures to chlorpyrifos on specific occasions, such as the day of application of the pesticide. To illustrate this, assume that urinary TCPy concentration in a child from all “background” exposure (primarily dietary TCPy, chlorpyrifos, and chlorpyrifos-methyl) was 3 ppb, and 5%, or 0.15 ppb, of that was due to dietary chlorpyrifos exposure. If, following an application of chlorpyrifos, the child's urinary concentration increased from 3 ppb to 6 ppb, and all of the increase was from additional exposure to chlorpyrifos from the agricultural application, the actual increase in chlorpyrifos exposure is 40-fold (from 0.15 to 6 ppb), not 2-fold. Yet a change in the urinary concentration of TCPy from 3 ppb to 6 ppb is relatively modest and well within “day-to-day” variability, as shown in the CitationKissel et al. (2005) and CitationHore et al. (2005) studies.
V.D. Use of Blood Levels of Chlorpyrifos as Biomarkers of Exposure
As discussed in Section III, at least at relatively high doses, chlorpyrifos is quickly metabolized to chlorpyrifos-oxon and TCPy, with an apparent half-life of 1 h or less, although some fraction of chlorpyrifos may have a relatively longer elimination rate due to extensive plasma protein binding and/or partitioning into lipids. Very few studies have attempted to measure chlorpyrifos in blood, and only one of the birth cohort studies, the Columbia cohort, has utilized blood chlorpyrifos as a biomarker of exposure. However, because of the limitations noted earlier in using TCPy as a specific biomarker of chlorpyrifos exposure, blood chlorpyrifos could be of potentially greater value, although the resulting exposure estimate would be more limited in terms of relevant time because of the much shorter half-life. It is important to note that there is very little information on the toxicokinetics of chlorpyrifos in blood following repeated, low level exposures. The studies of CitationTimchalk et al. (2002b) and CitationNolan et al. (1984) provide limited information on chlorpyrifos toxicokinetics in humans following single exposures, suggesting that the plasma elimination of chlorpyrifos at relatively high doses is quite rapid, with a half-life on the order of a few hours, although both of these studies suffered from relatively poor detection limits, and thus were not able to measure chlorpyrifos in blood for more than 1–2 h following exposure. In contrast, the method developed by CitationBarr et al. (2002) is highly sensitive, with a detection limit more than 1000 times lower than that used by CitationTimchalk et al. (2002b). Thus, it is now possible to carefully evaluate the kinetics of chlorpyrifos in blood over time following repeated, relatively low-dose, exposures (e.g., μ g/day seen from diet, vs. mg/day used in Nolan et al. and Timchalk et al. studies). This is important because chlorpyrifos exhibits substantial lipid solubility, so it is likely that there is some partitioning of chlorpyrifos into adipose tissue following repeated exposures. A steady-state concentration of chlorpyrifos in adipose tissue will be achieved following repeated exposures, which would be in equilibrium with blood lipids (CitationNeedham, 2005). If the terminal half-life (rate of elimination of chlorpyrifos from the lipid compartment) were measured in months, then single values collected at the time of delivery could be reasonable indicators of blood levels present over a period of months. However, if the terminal half-life is measured in days, then the values obtained at a given point in time might have little relationship to blood levels that were present weeks or months previous to the collection.
In the development of the method used in the Columbia cohort studies for measuring chlorpyrifos in blood, CitationBarr et al. (2002) identified chlorpyrifos at a concentration of 9 pg/g in a sample of 9 pooled blood samples. As noted earlier, the GC-MS method developed for this cohort is extremely sensitive, and the authors provided excellent quality control data that ensures confidence in the analytical data. [Although the extraction efficiency of chlorpyrifos from the blood was relatively low, ∼ 18%, the sensitivity of the analysis plus the incorporation of deuterated internal standards allowed for reliable estimates of blood concentrations of chlorpyrifos. The method has detection limits in the low picogram/gram range and coefficients of variation of typically less than 20% (CitationBarr et al., 2002).] Cord blood samples were collected at the time of delivery, and the maternal blood samples were collected 1 day following delivery. Chlorpyrifos was determined by GC-MS at the Centers for Disease Control and Prevention as described by CitationBarr et al. (2002). Since births were in the hospital, maternal blood samples were collected at least 24 h, and more likely > 30 h from the time of last exposure in the residence. This is an important consideration, given the relatively short plasma half-life of chlorpyrifos itself. CitationWhyatt et al. (2005) reported a mean plasma chlorpyrifos concentration of 3.9 and 3.7 pg/g, in maternal and cord blood, respectively, with a correlation between maternal and cord blood samples of 0.79 (). Intersubject variability was quite large, with standard deviations greater than the mean, and a range that covered more than 2 orders of magnitude. There was a very weak, but statistically significant, correlation between indoor air measurements of chlorpyrifos and maternal (r = 0.21) and cord (r = 0.19) cord blood. (Note: With correlation coefficients of this size, a considerable amount of the variation in the relationship remains unexplained; i.e., 1 minus r2 = variation unexplained by relationship.) A previous report from this group (CitationPerera et al., 2003) reported mean blood levels of chlorpyrifos (measured in plasma) of 7.1 and 7.6 pg/g in maternal and cord blood, respectively. (A comparison of these blood concentrations with those determined in a controlled human exposure to chlorpyrifos by Timchalk et al. [2002b] is discussed in Section II.E.1.)
VI. SUMMARY EVALUATION OF THE TOXICOLOGICAL EFFECTS OF CHLORPYRIFOS
The authors of this report were to review the literature on chlorpyrifos toxicology and then address several specific questions regarding: (1) the strength of the scientific evidence supporting the hypothesis put forward by others that chlorpyrifos is capable of causing adverse neurodevelopmental outcomes in humans at current, “background” exposure levels; (2) whether there is scientific evidence to support a mechanism for neurodevelopmental effects other than AChE inhibition; and (3) whether limiting chlorpyrifos exposures to levels that protect against AChE inhibition would be adequate to protect against any potential neurodevelopmental outcomes. As noted previously, answers to these questions are not clear-cut, as limitations and uncertainties in the existing data preclude firm answers, but general conclusions can be derived from the weight of available evidence. Here we provide summary evaluations of the key issues addressed in this review.
VI.A. Summary of Exposure Characterization
For most of the general population, exposure to chlorpyrifos occurs principally via the diet. Prior to 2002, residential application of chlorpyrifos for insect control was widespread, and numerous studies evaluated whether such residential use contributed substantially to total exposure. Although indoor air and house dust exposures may have contributed significantly to total chlorpyrifos exposures in homes where chlorpyrifos had been used for insect control, these pathways are unlikely to contribute significantly to chlorpyrifos exposures of children in urban settings today. Studies of “take home” pathways for agricultural workers, combined with possible airborne and house dust exposures in homes in heavily agricultural areas, may add somewhat to total exposures of infants and children to chlorpyrifos. Today, it is likely that dietary exposure accounts for the vast majority of current nonoccupational exposures to chlorpyrifos, especially among children.
However, the extent of dietary exposures to chlorpyrifos reported in numerous studies in the past may have been substantially overestimated, since nearly all studies have relied upon urinary TCPy as a biomarker of chlorpyrifos exposure. Both “mass balance” analysis of dietary chlorpyrifos/urinary TCPy from several studies and the studies of CitationMorgan et al. (2005) and CitationWilson et al. (2003), which directly analyzed both TCPy and chlorpyrifos in the diet and TCPy in the urine of children, suggest that estimated daily intakes of chlorpyrifos based on urinary TCPy may overestimate actual chlorpyrifos exposures by 10-to 20-fold. Based on this review of the extensive data on chlorpyrifos exposures (most of which is based on urinary TCPy exposures measured in samples collected prior to 2001), a conservative estimate of current daily exposures of chlorpyrifos among the general adult population with no specific sources of exposure would be 0.004–0.006 μ g/kg-day. The Total Diet Study data for chlorpyrifos suggest that young children might have somewhat higher background exposures on a body weight basis, perhaps on the order of 0.01 μ g/kg-day. Toddler exposure to chlorpyrifos-methyl in the past appears to be substantially greater than for chlorpyrifos. Studies of farm-worker families (excluding direct occupational exposures of applicators) suggest that the levels of exposure associated with living on or near intensive agricultural areas do not appreciably add to the total daily exposures to chlorpyrifos as measured by urinary TCPy. However, in the absence of dietary exposures to chlorpyrifos-methyl, the relative contribution to total exposures of living on or near agricultural areas may contribute substantially to total urinary TCPy levels in the future (although total urinary TCPy levels are expected to decline when chlorpyrifos-methyl use on grain products is replaced by alternative controls).
Thus, for purposes of assessing current risks to chlorpyrifos, daily exposures rates in the general population, excluding farm workers and their children, are expected to be less than 0.01 μ g/kg-day. Exposures for adults, when adjusted on a body weight basis, would be expected to be somewhat less than this, perhaps on the order of 0.004–0.006 μ g/kg-day.
For adults and children with additional sources of exposure to chlorpyrifos, such as farm-worker families and/or populations living near agriculturally intensively areas, it is possible that chlorpyrifos exposures could be substantially greater than the “background” exposures from trace residues in the diet. For example, an increase in urinary TCPy from a “background” of 2 ppb (of which perhaps only 0.1–0.2 ppb would be from chlorpyrifos) to 4 ppb would represent a 10-to 20-fold increase in chlorpyrifos exposure if all of the additional 2 ppb of urinary TCPy were derived from direct exposure to chlorpyrifos. Thus, future studies should attempt to distinguish between exposures to TCPy from exposures to chlorpyrifos.
VI.B. Dose-Response Analysis From Controlled Human Exposures to Chlorpyrifos
Although substantial inhibition (up to 80%) of plasma BuChE occurs following a single oral dose of 0.5 mg/kg of chlorpyrifos (CitationNolan et al., 1984), inhibition of erythrocyte AChE (generally regarded as a more relevant measure of potential toxicity of cholinesterase inhibitors) appears to require chlorpyrifos doses greater than 1 mg/kg, since none of the 12 subjects receiving either 0.5 or 1 mg/kg chlorpyrifos had any evidence of erythrocyte AChE inhibition, and only one of the 12 subjects receiving 2 mg/kg chlorpyrifos had a significant decrease in erythrocyte AChE in the CitationTimchalk et al. (2002b) study. The one subject with significant erythrocyte AChE inhibition appeared to have a substantially higher absorption of the oral dose, relative to other subjects, which would account for the observed inhibition. Overall, the estimated oral bioavailability of chlorpyrifos in the 12 subjects in the Timchalk et al. study, based on recovery of TCPy in the urine, was only 20–35%, compared to the 70% of the administered dose recovered in the urine in the study by CitationNolan et al. (1984). Although these studies only provide data following a single dose, it is clear from these studies that a single dose of 0.5 mg/kg has measurable effects on plasma BuChE, but not erythrocyte (and thus presumably not brain) AChE. CitationCoulston et al. (1972), as reported in CitationZhao et al. (2006), measured plasma (BuChE) and erythrocyte (AChE) cholinesterase in human subjects give repeated daily doses of chlorpyrifos at 0, 0.014, 0.03, or 0.1 mg/kg-day for 49, 28, 21, and 9 days, respectively. Erythrocyte AChE was not inhibited at any dose level. However, at the highest dose of 0.1 mg/kg-day, plasma BuChE activity was inhibited by up to 64% after 9 days of treatment. At the next lowest dose, 0.03 mg/kg day, there was some inhibition toward the latter part of the 21-day study (values for some subjects on days 16, 18, and 20 were ∼ 10% below the baseline) but none reached statistical significance. There was no evidence of plasma BuChE inhibition at the 0.014 mg/kg-day dose, even after 28 days of dosing.
Chlorpyrifos Exposure Estimates Based on Urinary TCPy. Studies that relied solely on urinary TCPy as an index of chlorpyrifos exposure have typically yielded estimates of daily chlorpyrifos exposure of approximately 0.1–2 μ g/kg-day for urinary concentration of TCPy between 2 and 20 μ g/L (CitationHill et al., 1995; CitationGibson et al., 1998; CitationShurdut et al., 1998; CitationQuackenboss et al., 2000; CitationTimchalk et al., 2002b). For example, in the Farm Family Study, CitationCurwin et al. (2007b) estimated the “absorbed daily dose” of chlorpyrifos of 0.27–1.96 and 0.24–1.36 μ g/kg-day in 25 farm and 25 nonfarm children, respectively. The calculations were based on urinary TCPy levels from spot urine samples collected on two occasions (evening and first morning void). However, as discussed in detail earlier, it is very likely that > 95% of urinary TCPy is due to direct exposure to TCPy and/or to exposure to chlorpyrifos-methyl from consumption of grain-based foodstuffs in samples collected prior to 2002. The authors noted this in their discussion: “It is probable that the chlorpyrifos doses were over-estimated as a result of direct exposure to TCPy, a metabolite of chlorpyrifos; however, the calculated chlorpyrifos doses assume that the TCPy excreted in urine came entirely from chlorpyrifos exposure.” Thus, actual exposures to chlorpyrifos would then be 10-to 20-fold lower, or in the range of 0.005–0.1 μ g/kg-day, and possibly considerably lower than this if chlorpyrifos-methyl contributed substantially to urinary TCPy. As discussed earlier, the estimated daily exposures to chlorpyrifos in all of the cohorts for which chlorpyrifos exposure was directly measured, is in this same range. Thus, in all of the community-based studies performed to date, the existing data indicate that exposures to chlorpyrifos were generally below 0.01 μ g/kg-day or at least 3000 times lower than the lowest dose shown in the CitationCoulston et al. (1972) study to have a slight effect on plasma BuChE, and at least 30-fold lower than the EPA's proposed chronic reference dose of 0.3 μ g/kg-day (0.0003 mg/kg-day), which incorporates a 100-fold safety factor. Since substantial decreases (perhaps > 75%) in plasma BuChE are required before significant inhibition of erythrocyte (and presumably brain) AChE occurs, and erythrocyte AChE shows substantial inhibition prior to detectable changes in target tissue AChE, it is inconceivable that absorbed doses of chlorpyrifos of 0.01 μ g/kg-day could produce any biologically relevant inhibition of AChE in maternal or fetal brain during development.
VI.C. Is Inhibition of BuChE Appropriately Considered an “Adverse Effect”?
A common approach taken in regulatory toxicology is that risk assessments are based on the “most sensitive” adverse effect. Generally, the various observed responses are identified, and the one that occurs at the lowest dose is used for standard setting. Most regulatory guidelines for toxicity testing require that “no-observable-adverse-effect levels” (NOAELS) be established for various toxicity endpoints, and generally the lowest NOAEL is then used as the “point of departure” for subsequent risk assessments, with the presumption that an “allowable” exposure level that protects against the most sensitive response will thus protect against all possible adverse responses. Although this sounds straightforward, there are many nuances to establishing NOAELs for regulatory purposes. In the early days of regulatory toxicology, “no-observable-effect levels” (NOELs) were utilized. However, the scientific community and regulators recognized that there were frequently “observable” effects that had little toxicological significance, and thus guidelines were revised to specifically address those effects that were deemed to be “adverse.” This is of particular relevance to risk assessment of chlorpyrifos, because there has been substantial debate about whether inhibition of BuChE is indeed an adverse effect.
There has been considerable controversy over endpoints and data sets to be used for determination of acceptable intake rates, or “reference doses” (RfDs). In 1986, EPA initially established a reference dose for chlorpyrifos of 3 μ g/kg-day based on a NOAELFootnote13 of 30 μ g/kg-day and an uncertainty factor (UF) of 10. The NOAEL was based on the lack of statistically significant inhibition of plasma BuChE in male humans given 0.03 mg/kg-day for 20 days (the CitationCoulston et al. [1972] study), although a slight inhibition was apparent in some subjects, leading others to consider the next lower dose, 14 μ g/kg-day, to be a NOAEL. However, in 2000 EPA proposed a reference dose of 0.3 μ g/kg-day, 10 times lower than the initial RfD established in 1986 (see note 1), and this value is now used for regulatory purposes.
CitationZhao et al. (2006) recently reviewed the current global regulatory values utilized for chlorpyrifos. The estimate of “safe” exposures to chlorpyrifos depends on the particular endpoint used, the specific study and species used, and the magnitude of the perceived uncertainty in the endpoint/study selected, which in turn dictates the uncertainty (or safety) factor used. The most “conservative” acceptable daily dose was 0.3 μ g/kg-day, based on plasma cholinesterase inhibition in dogs and an uncertainty factor of 100.
Van Gemert et al. (2001) reported the results of an expert panel convened by the manufacturer of chlorpyrifos to address four key questions pertaining to establishing a RfD for chlorpyrifos. The panel concluded that: “1) inhibition of BuChE is not an adverse effect, and the RfD for chlorpyrifos should be based on AChE inhibition; (2) the RfD for chlorpyrifos should be based on the three available human studies, which are also supported by animal data; (3) the extra FQPA safety factor should be reduced to 1, because chlorpyrifos shows no pre-or postnatal toxicity of concern at relevant human exposure conditions; and (4) the extra 10-fold safety factor for interspecies variation appears overly conservative because no differences in species sensitivity to chlorpyrifos is evident” (van Gemert et al., 2001).
However, as noted in Section II, recent studies suggest that BuChE may have some physiological role above and beyond the critical defense against acute OP neurotoxicity provided by BuChE in blood and probably other peripheral tissues. It is thus important to reevaluate the scientific evidence supporting or refuting the position that inhibition of the enzyme activity of BuChE should be considered an adverse effect.
BuChE is a serine hydrolase glycoprotein that catalyzes the hydrolysis of several choline esters (CitationDarvesh et al., 2003; CitationLane et al., 2006). It is the product of a single gene that resides on chromosome 3q26, and consists of 4 exons and 3 large introns. It is relevant to note that the BuChE gene is quite structurally diverse from the AChE gene, which is found on a different chromosome (e.g., BuChE is not simply present because of an ancient gene duplication event of AChE, and thus would be expected to have functions quite different from AChE). BuChE undergoes posttranslational modification, and exists in tissues in monomeric or multimeric forms. Although it has some activity toward acetylcholine, it is much less effective than AChE and is generally not thought to contribute substantially to normal acetylcholine metabolism in nervous tissue, at least in the presence of normal levels of AChE. BuChE also catalyzes the hydrolysis of acyl amides of aromatic amines, suggesting that it has additional enzymatic activity that is distinct from its esterase activity (CitationDarvesh et al., 2003).
Because there are common polymorphisms in BuChE that are not associated with any obvious phenotype, it has been assumed for years that it does not play any important physiological role. Commenting on this in a comprehensive review of BuChE, CitationDarvesh et al. (2003) state: “However, recent observations suggest that BuChE could have more specific functions than previously recognized. For example, BuChE is expressed in distinct populations of neurons, is a co-regulator of cholinergic neurotransmission, and seems to be involved in some aspects of the development of the nervous system. Structural similarities between BuChE, AChE and cell-adhesion molecules have raised the possibility of a noncatalytic role for cholinesterases, a property that might explain their involvement in the development of the nervous system. Moreover, the biochemical properties of BuChE are altered in neurodegenerative diseases such as Alzheimer's disease (AD). Consequently, the possible role of BuChE in normal physiological functions and its involvement in neurodegenerative diseases is now gaining recognition.”
In another recent review, CitationLane et al. (2006) also suggest that BuChE may have a physiological function, due in part to the relatively high levels of expression of the gene in specific regions of the brain (embedded references can be found in CitationLane et al., 2006):
The majority of ChE in the human brain is AChE. However, it is now known that BuChE has more widespread distribution than previously thought (Mesulam et al., 2002a). In the normal brain, BuChE activity has been located in all regions that receive cholinergic innervation. It is mainly found in glial cells and in endothelial cells, whereas AChE is in neurons and axons (Mesulam et al., 2002a). Although AChE containing neurons are widely distributed and more numerous than BuChE neurons, there is a particularly high expression of BuChE immunopositive neurons in the hippocampus, thalamus and amygdala (Darvesh et al., 1998, 2003a; Mesulam, 2000). In these structures, both AChE and BuChE have widespread but different distributions (CitationDarvesh and Hopkins, 2003). For example, thalamic nuclei (anteroventral, mediodorsal, ventral anterior, lateral, and pulvinar) show particularly high BuChE expression; 90% or more neurons show intense immunostaining for BuChE (CitationDarvesh and Hopkins, 2003). … [Lane concluded by stating:] The physiological roles of the different splice variants or molecular forms of BuChE and AChE also require further clarification. Screening for protein partners that interact with various molecular forms of AChE and BuChE may identify possible biochemical pathways in which they participate. (pp. 105–106)
Nevertheless, a specific physiological role for BuChE in these regions of the brain has not been identified (Silver, 1974). In their 2003 review, Darvish et al. specifically address this point (numbered references can be found in CitationDarvesh et al., 2003):
The fact that people with the silent variants of BuChE are apparently normal (1) has prompted the suggestion that this enzyme might not have a vital role in normal brain function. However, the term “silent” simply describes the inability of this variant to catalyse the hydrolysis of esters of choline. No studies have examined whether these variants lack other properties such as aryl acylamidase activity. The widespread and differential distribution of neuronal BuChE, in comparison to AChE, suggests a role for this enzyme in the human nervous system, particularly within the amygdala, hippocampal formation and thalamus in which large populations of BuChE-positive neurons are present. Consistent with this suggestion, accumulating evidence points to several possible roles for BuChE in diverse neural and nonneural functions (1,84,94). Several lines of evidence indicate that BuChE might be a co-regulator of the activity of the neurotransmitter acetylcholine (16,17,37,38,50,95). Although BuChE can catalyse the hydrolysis of acetylcholine, it does so with less efficiency than AChE (1). However, a regulatory role for BuChE in the hydrolysis of acetylcholine is consistent with the demonstration that inhibition of BuChE leads to a dose-dependent increase in the levels of acetylcholine in the brain (16). In the absence of AChE, it seems that BuChE can compensate for some of the functions of AChE, particularly with respect to the cholinergic system (17,37). For example, AChE-null mice are viable with special postnatal care, and retain their cholinergic neurons. These mice also display normal neuronal BuChE (17). Given the fact that complete inhibition of both AChE and BuChE is lethal, primarily due to cholinergic over-activity, it has been suggested that BuChE has a prominent role in hydrolyzing acetylcholine in AChE-null animals (17). BuChE has been shown to be closely associated with other proteins such as albumin, transferrin and peptidases (94,96,97). The association of peptidases in cholinesterase preparations has led to the suggestion of possible protein–protein interactions between cholinesterases and other enzymes (98), and that some or all of the peptidase activity is probably related to enzymes distinct from BuChE (98). Recent evidence indicates that an association between BuChE and proteases such as trypsin could have functional significance in terms of proteolytic activity (99). For example, BuChE significantly enhances the proteolytic activity of trypsin, and the addition of increasing amounts of BuChE to a fixed amount of trypsin leads to a concentration-dependent enhancement of trypsin activity. This stimulation of trypsin by BuChE shows a saturation profile that is consistent with a 1:1 protein–protein interaction between the two enzymes. This observation is of significance to nervous system function because trypsin is present in the normal human brain and might be involved in the pathology of AD (100). So, BuChE could have functions that depend on its interaction with other proteins in addition to cholinergic co-regulation. (p. 136)
There is also evidence that BuChE collocalizes with beta-amyloid fibrils and neurofibrillary tangles, a neuropathological hallmark of AD (CitationMateo et al., 2008). Additionally, recombinant BuChE prolongs the persistence of soluble forms of amyloid precursors, thereby reducing their aggregation to form the multimers that are required for amyloid plaque formation, another neuropathological hallmark of AD (CitationPodoly et al., 2008).
Controlled studies of selective BuChE inhibition of cognitively normal adults would be of significant interest (although difficult to justify) in clarifying the neurophysiological role of brain BuChE. While these observations lead one to suspect a priori that minimal inhibition of brain BuChE would be supportive of or perhaps even enhance cognitive function in normal subjects, such a conclusion is justified only in the presence of concrete experimental evidence from human or animal studies. Extrapolation to the developing brain, where the function of BuChE is poorly understood, would be even more uncertain. In the developing rat brain, histochemical evidence of low neuronal BuChE activity is present in neurons at birth, but the distribution and intensity of regional neuronal enzyme activity change markedly during postnatal development. The transient presence of BuChE activity at very low levels in all neurons supports a role for BuChE in cellular development rather than neurotransmission (CitationGeula and Nagykery, 2007). The expression of genes coding for cholinesterases during embryonic development, their roles in morphogenesis and apoptosis, and the effects of their regulation in developing and proliferating cells, support a developmental, rather than a neurotransmitter, role for cholinesterases in cell cycle control and in neuronal maturation and growth (CitationVidal, 2005; CitationYang et al., 2008).
Numerous functional single-nucleotide polymorphisms (SNPs) have been identified in the BuChE gene, and these gene variations code for proteins with or without diminished enzymatic activity—up to 80% loss of activity in the case of the recently reported G333C mutation (CitationMikami et al., 2008). The most common is an SNP in codon 70 that results in a substitution of aspartic acid with glycine (D70G), commonly referred to as the “atypical” variant. This amino acid substitution results in a loss of ∼ 30% of the activity of the enzyme for some substrates, including succinylcholine chloride, a membrane depolarizing drug used commonly in surgical procedures to paralyze skeletal muscles. Another mutation in codon 539 results in the replacement of alanine with threonine (A529T), and is referred to as the “K variant.” This amino acid substitution results in the loss of about 33% of the cholinesterase activity. The “J variant” of BuChE has a change in codon 497 that substitutes a glutamic acid with valine (E497V). This variant has 66% lower enzymatic activity relative to the “wild-type” enzyme. Finally, the “H variant,” which replaces the valine residue at codon 142 with a methionine (V142M), has only 10% of the activity of the wild-type enzyme. There are at least 35 other known mutations, including at least a dozen mutations that result in truncated proteins with no activity. These mutations have invariably been identified not because of an overt phenotype, but because of either follow-up to an adverse response to paralytic drugs such as succinylcholine, or through pharmacogenetic studies of normal populations screened in vitro for low BuChE activity, with subsequent sequencing of the gene in those samples identified with low activity. The allele frequency of the “atypical” (D70G) variant is approximately 10%, with homozygote frequencies ranging from 2 to 5%. The K (A529T) variant is somewhat more common, with allele frequencies from 15 to 35%, with 3–6% homozygotes. All of the other variants occur at allele frequencies of less than 1% and thus are considered rare. Most of these have not been extensively studied for phenotype associations, although any obvious phenotype (beyond susceptibility to succinylcholine) would likely have been identified and reported in the literature. Although there is no overt phenotype, homozygotes clearly have significantly lower plasma BuChE activity, and are susceptible to prolonged apnea and paralysis following normal therapeutic doses of succinylcholine.
Numerous studies have evaluated whether the variant forms of BuChE are associated with disease susceptibility. Of particular interest has been whether BuChE variant forms are associated with Alzheimer's disease risk or progression of disease, since loss of cholinergic activity in parts of the brain appears to be an important contributor to the pathology of the disease. Indeed, the most recent therapeutic approaches to AD have been the use of centrally acting cholinergic drugs or cholinesterase inhibitors to increase cholinergic function (CitationLane et al., 2006). Numerous studies have examined whether BuChE variants (usually the “atypical” and/or K variants) are associated with Alzheimer's disease. The results have not been consistent (Lehmann et al., 2001), although several have found that the low activity alleles seem to be associated with lower susceptibility to AD and/or later age of onset (CitationAlvarez-Arcaya et al., 2000; CitationLane et al., 2006, Citation2008; CitationMateo et al., 2008), or a significantly reduced rate of progression of cognitive decline in individuals with severe AD (CitationHolmes et al., 2005). The presence of the K variant has also been associated with lower benefits from rivastigmine treatment (CitationBullock et al., 2005). Thus, some studies do suggest a role for BuChE in a disease process (Alzheimer's disease), especially in association with other genetic risk factors such as ApoE4 (CitationLane et al., 2008), although, as noted earlier, the putative direction of the effect is that the presence of BuChE activity is detrimental rather than protective, and some authors have called for studies to identify cholinesterase inhibitors that are selective for BuChE as potentially advantageous in treatment of AD (CitationLane et al., 2006). A recent report found that while the combination of the BuChE-K variant and ApoE4 allele conferred extra risk of cognitive decline, hippocampal volume loss, and AD progression, the presence of BuChE-K had no independent effect on disease progression (CitationLane et al., 2008).
Few studies have examined the possible association between BuChE deficiency and risk for other diseases. One report did identify a significant positive association between the presence of the K allele of BuChE and type II diabetes (CitationHashim et al., 2001), although a subsequent, much larger study failed to find any association (CitationJohansen et al., 2004). Serum BuChE activity was associated with parameters of adiposity, serum lipid profile, and degree of insulin resistance in Japanese subjects (Iwaskai et al., 2007); however, BuChE(−/−) mice were at risk for obesity when placed on a high fat diet (CitationLi et al., 2008). A large case-control study of Iranian subjects with and without type II diabetes concluded that the BuChE-K allele, even in the heterozygous form, exacerbates the risk of coronary artery disease (CitationVaisi-Raygani et al., 2008)
Thus, although there is no established phenotype associated with decreased (or even complete lack of) BuChE activity in plasma of humans, there is certainly evidence that reduced activity of BuChE in plasma can greatly enhance susceptibility to certain drugs (e.g., succinylcholine and mivacarium) and plant-derived toxins (Solanaceae alkaloids) that are substrates for BuChE. Furthermore, the tissue-specific expression and other putative functional roles for BuChE, especially a potential role in neurodevelopment, argue that inhibition of BuChE should be considered as a potential adverse effect. However, it is recognized that there is, to date, no scientific proof that inhibition of the enzymatic activity of BuChE per se is associated with increased risk for disease or other type of adverse effect (except for enhanced susceptibility to certain other toxic agents). On the other hand, there is experimental animal evidence that elevating blood BuChE reduces the acute neurotoxic effects of the potent organophosphorus nerve agent sarin (CitationBajgar et al., 2007; see also Section II). The value of inhibition of BuChE as an effects-related biomarker of exposure is not disputed, and it is logical to use this easy and specific effects-related biomarker for regulatory purposes to both establish protective exposure levels and monitor work-place populations. Because inhibition of serum BuChE by chlorpyrifos occurs at doses lower than those that inhibit brain AChE (which is clearly an adverse effect), the “conservative” nature of the selection of BuChE inhibition as a point of departure for risk assessment should be recognized. The choice of that endpoint may thus already incorporate some of the elements of uncertainty that are typically adjusted by the use of uncertainty factors to arrive at a reference dose.
VI.D. Discussion of Potential Nonneurodevelopmental Effects of Chlorpyrifos From Cohort Studies
In general, clinical and epidemiologic studies using various clinical tests as the outcomes of interest often make two broad types of comparisons: (1) comparison of the proportion of study participants among those exposed to various levels (percentiles) who have an abnormal clinical test result (as determined using some reference value of normal values or medically recognized upper bound of normal values usually determined as the 95th percentile of results of the test in a healthy population), and (2) comparison of the mean or median values of clinical test results among groups of people with different levels of exposure. Correlations resulting from both types of comparison are difficult to interpret because of the reasons described earlier.
Regardless, of the approach used, results obtained from studies using clinical tests as the medical or health outcome are difficult to interpret with regard to a cause–effect association because of their cross-sectional design. Cross-sectional surveys typically obtain a single measurement of exposure and outcome (via a clinical test) at a single point in time among patients who agree to participate. If positive associations are observed, it is not possible to ensure that the exposure of interest preceded the clinical test by an appropriate time interval. Thus, the necessary cause-to-effect temporal sequence cannot be demonstrated, and the causal nature of such an association cannot be determined. On the other hand, when no association (correlation) is observed, it may not be possible to determine if the reason is due to: (1) lack of a causal relationship between the exposure and the clinical parameters of interest, (2) selection bias due to higher refusal rates among medically affected patients, or (3) other errors such as misclassification of exposure or disease (test results).
In epidemiologic research, it is extremely difficult to collect and analyze data on all factors that could affect clinical tests of physiologic function on a given day in a particular individual and this is rarely done. Even when potential confounding factors are adjusted in the statistical analysis, there may still remain residual confounding due to inadequate adjustment. Thus, a single clinical test result in an individual, even if the result is “abnormal,” cannot be construed as an indicator of an adverse effect (i.e.,. functional impairment or illness). Moreover, other factors may affect clinical tests/chemistries, e.g. age, physical activity, energy balance, longer term alcohol, dietary and medications exposures and obesity. When a study identifies a correlation or statistical association between an exposure and a clinical test, such an association may be noncausal and due instead to differences in the other confounding factors rather than the exposure of interest.
There is very little evidence to indicate that chlorpyrifos has relevant toxicological effects in tissues other than the nervous system. Standard toxicological bioassays have not found significant toxicity in organ systems other than the nervous system, at least at doses less than those causing neurotoxicity. Chlorpyrifos is not considered to be teratogenic at doses that do not cause frank maternal toxicity.
Although mutagenicity and chronic animal bioassays for carcinogenicity of chlorpyrifos were largely negative, a recent epidemiological study of pesticide applicators reported a significant exposure response trend between chlorpyrifos use and rectal cancer. However, the positive association was based on small numbers of cases, i.e., an excess of less than 10 cases in the 2 highest exposure groups, and other uncertainties about actual levels of exposure warrant caution in making any inference about a causal association. Thus, more research is needed before an association between rectal cancer and chlorpyrifos can be assumed, especially in the absence of any other positive evidence of carcinogenicity or genotoxicity of chlorpyrifos.
There is also no evidence that chlorpyrifos is hepatotoxic, nephrotoxic, or immunotoxic at doses less than those that cause cholinesterase poisoning.
One series of studies in human males attending a fertility clinic found an association between urinary TCPy levels and several measures of male reproductive health (CitationMeeker et al., 2004a, Citation2004b, Citation2005, Citation2006a, Citation2006b). Urinary TCPy levels in the population were within the range of the normal U.S. population, so exposures were not unusual. In their conclusions the authors recognize that studies in support of their findings are limited and more research is necessary to substantiate the observed associations. Such future work might involve investigating the association between hormone levels and semen quality and the relationship between serum thyroid hormones and reproductive hormones in this same case series, as well as replication studies in other case series. The authors attempted to synthesize the results of their various studies, and although the correlations between T4 and testosterone were slightly stronger in “subnormal men,” than in “normal” men, these differences were not statistically significant. Thus, they conclude that “although TCPy was associated with a decrement in both T4 and testosterone, the hypothesis that these decrements in the hormones contribute to a decline in semen quality remains unclear.” It is recognized that biomarkers of physiologic function are more difficult to interpret than data on overt disease. This is true for several reasons. Most importantly, biomarkers of physiologic function may normally vary within the same individual on a day-to-day basis, and often are affected on a short-term basis by a number of factors such as diet, smoking, use of prescription and nonprescription drugs, alcohol consumption, and common acute illnesses, especially those associated with fevers. Laboratory processing or analytical errors, as well as subject characteristics such as abstinence or fasting status, and time of the day of testing can also produce spurious results. Thus, in clinical practice, a single isolated clinical test is not accepted as definitive. A preferred approach to obtaining stable measures requires conducting several tests and computing an average value, or, at minimum, repeated testing in situations where the result of a single test is abnormal.
VI.E. Discussion of Potential Neurodevelopmental Effects of Chlorpyrifos at Current Levels of Exposure
VI.E.1. Considerations Based on In Vivo and In Vitro Animal Studies on Chlorpyrifos Developmental Neurotoxicity
It is well established that young animals are more sensitive than adults to the acute cholinergic toxicity of chlorpyrifos, as well as to other OPs. This is due primarily to the lower detoxication ability of the young, ascribed in particular to low PON1 and carboxylesterase activities, and potentially because of low plasma BuChE which may afford some protection to brain AChE. However, in vivo comparisons of the level of chlorpyrifos needed to inhibit brain, red blood cell, or plasma cholinesterase by 20% following repeated exposures found approximately equal sensitivity of adults and neonatal rats (CitationZhao et al., 2005). Thus, developmental sensitivity may depend on the particular measure of effect (e.g., brain vs. plasma, vs. erythrocyte cholinesterase), and the most sensitive indicator may be the most relevant.
Most experimental studies reporting biochemical, molecular, and behavioral effects following repeated prenatal and/or postnatal exposure to chlorpyrifos employed doses of chlorpyrifos that caused significant AChE inhibition. Regulatory standards are intended to prevent AChE inhibition; the current U.S. EPA chronic RfD of 0.0003 mg/kg/day (0.3 μ g/kg/day) is based on a NOEL for AChE inhibition of 0.03 mg/kg/day (30 μ g/kg/day) and a 100-fold uncertainty factor (CitationZhao et al., 2006). Other regulatory agencies have established higher acceptable exposure levels, in the range of 1–10 μ g/kg/day. Because of the likely overestimation of chlorpyrifos exposures based on urinary TCPy, the exposure in the United States today is expected to be less than 0.01 μ g/kg/day. In Japan, exposure of children to chlorpyrifos has been estimated to be 0.007 μ g/kg/day (CitationKawahara et al., 2007).
Thus, developmental neurotoxic effects that arise as a consequence of AChE inhibition in target tissue (the developing nervous system) would not be expected to occur at doses below those that inhibit AChE. Because plasma BuChE is inhibited at doses considerably lower than those that inhibit target tissue AChE, protection against BuChE would be expected to protect against neurodevelopmental effects that are a consequence of AChE inhibition. This is the current basis for the established RfD. However, a few animal studies were identified in which chlorpyrifos was reported to cause developmental neurotoxic effects at dose levels that did not cause any apparent inhibition of AChE.
Three different hypotheses may be formulated to address possible mechanisms that contribute to the effects observed at “non-AChE inhibitory doses” of chlorpyrifos, and that imply that chlorpyrifos developmental neurotoxicity could be unrelated to AChE inhibition.
The developmental neurotoxicity of chlorpyrifos is due to chlorpyrifos-oxon, which, in addition to inhibiting AChE, has the ability to interact with other targets and exert other effects at concentrations below those required to cause AChE inhibition. This hypothesis is not supported by most in vitro studies (see ), which indicate that effects of chlorpyrifos-oxon occur at higher concentrations than those required to inhibit AChE (1–10 nM). Exceptions are, however, three in vitro studies (CitationSchuh et al., 2002; CitationHoward et al., 2005); (CitationYang et al., 2008) where effects of chlorpyrifos-oxon were seen at concentrations in vitro of < 1 nM. However, levels of chlorpyrifos-oxon found in blood after in vivo exposure to 5 mg/kg chlorpyrifos were 1 ng/g, or approximately 3 nM (CitationMattsson et al., 2000).
The developmental neurotoxicity of chlorpyrifos is due to the metabolite TCPy, which may have biological effects on its own, unrelated to, and independent of, AChE inhibition. Formation of TCPy results from phosphorylation of AChE and subsequent hydrolysis, or from hydrolysis of chlorpyrifos-oxon by paraoxonase. Additionally, direct exposure to TCPy can also occur and contributes to urinary levels of TCPy found in humans (see Section V, Exposure Assessment). Although few studies have examined TCPy, one in vivo developmental study with TCPy did not identify any indication of developmental toxicity (CitationHanley et al., 2000). In vitro effects of TCPy have been observed at high micromolar concentrations (which greatly exceed TCPy levels found with in vivo exposure to chlorpyrifos) at doses that also cause AChE inhibition. However, a single in vitro study reported a biochemical effect of TCPy (increase in pCREB) in neurons at concentrations as low as 30 pM (CitationSchuh et al., 2002). Whether this observed in vitro effect would occur in vivo and would have any biological relevance to neurodevelopment remains unknown. Thus, the current “weight of evidence” suggests that the levels of TCPy found in humans (which, as said, may also result from exposure to TCPy) are below those shown to have any in vivo biological effects on neurodevelopment.
The developmental neurotoxicity of chlorpyrifos is due to chlorpyrifos itself, not to its metabolites, and is independent of AChE inhibition. In rat pups, exposure to 1 mg/kg chlorpyrifos on PND 5 produced blood chlorpyrifos levels of 120–140 nM (CitationTimchalk et al., 2006; CitationMarty et al., 2007). In humans, levels of chlorpyrifos in blood upon exposure to 1–2 mg/kg (doses that are 300–600 higher than the RfD) are 1–10 nM, and are estimated to be 0.01 nM upon exposure to 3 μ g/kg/day (CitationDybowski et al., 2001). The blood concentration of chlorpyrifos measured in the Columbia cohort averaged approximately 4 pg/g, which is equivalent to a concentration of 0.01 nM, but was as high as 35 pg/g, or 0.1 nM. However, CitationTimchalk et al. (2002b) calculated a brain/blood partition coefficient of 33 in rats, suggesting that brain concentrations of chlorpyrifos could be approximately 0.3 nM at a blood concentration of 0.01 nM, and 3.3 nM at 35 pg/g blood. It should be noted that almost all in vitro studies report effects of chlorpyrifos at micromolar concentration, i.e., higher than the measured or estimated levels following in vivo exposure. However, three studies were identified that reported effects [increase of pCREB in neurons (CitationSchuh et al., 2002) and inhibition of neurite outgrowth (CitationHoward et al., 2005; CitationYang et al., 2008)] at chlorpyrifos concentration < 1 nM.
The great majority of in vivo studies report developmental neurotoxic effects of chlorpyrifos that are observed in the presence of AChE inhibition. As noted earlier, current regulatory guidelines are intended to limit exposure to levels well below those causing AChE inhibition. As such, these in vivo studies have no immediate relevance to the human situation.
A small number of experimental studies exist where chlorpyrifos caused developmental neurotoxic effects at dose levels that did not cause apparent AChE inhibition (and others in which no AChE measurements were reported). Mechanisms unrelated to AChE inhibition, and involving chlorpyrifos itself, chlorpyrifos-oxon, or TCPy, have been suggested as possible explanations for such findings. As the parameters affected (neuronal CREB and axonal outgrowth) are involved in important neural developmental processes, it is important to determine whether these findings extend to in vivo developmental studies. The identification and subsequent in vivo validation of a mechanism for neurodevelopmental toxicity of chlorpyrifos at doses that do not inhibit cholinesterases in blood or target tissue are of some importance, since epidemiological studies that have examined an association between chlorpyrifos exposure in pregnant women and adverse neurodevelopmental outcomes have been completed in populations in which the exposures were well below levels that have been associated with inhibition of cholinesterases, as is discussed later.
In summary, the weight of evidence from animal studies and in vitro mechanistic studies suggests that many of the neurodevelopmental effects of chlorpyrifos are secondary to inhibition of AChE in target tissues such as the developing brain, although plausible alternative mechanisms have been proposed, based on in vitro studies.
VI.E.2. Discussion of Human Studies Related to Neurodevelopmental Effects of Chlorpyrifos
In addition to the substantial animal bioassay and mechanistic data, numerous human studies have been completed, and several have evaluated the possible association between chlorpyrifos exposure and neurodevelopmental effects. The following discussion provides a critical evaluation of the weight of evidence from the human studies that examined the hypothesis that chlorpyrifos exposure can induce neurodevelopmental effects in humans. This analysis focuses on evidence for associations between exposure to chlorpyrifos, as revealed through levels of chlorpyrifos in blood, or its metabolite TCPy (specific to chlorpyrifos and chlorpyrifos-methyl) and its nonspecific metabolites DEP and DETP.
Reviewing the data for in utero development and chlorpyrifos, the Columbia investigators reported inverse associations between blood chlorpyrifos level (single sample obtained at birth) and birth length and birth weight. In contrast, no significant negative associations for chlorpyrifos exposure were demonstrated in the birth cohort studies by the UC-Berkeley or Mount Sinai investigators. Similarly, when comparing the studies from the CitationRauh et al. (2006) study from the Columbia cohort and the CitationEskenazi et al. (2007) study from the Berkeley cohort, the findings were inconsistent for the association of chlorpyrifos with neurobehavioral functions of young children.
Because of the importance of determining whether or not chlorpyrifos exposure predisposes to abnormal brain development, it is essential to attempt to decipher the reasons for the apparent discrepancies.
The first question pertains to what was measured in the three studies. The Berkeley and Mount Sinai groups measured the chlorpyrifos-derived metabolite TCP, but did not measure chlorpyrifos. The most important question is whether or not the levels of exposure varied significantly between these studies. Unfortunately, there is no method for interpolating between the studies to define confidently the extent of exposures. As shown by CitationZhao et al. (2005), the efficacy of the biomarkers used to estimate chlorpyrifos exposure differed between the studies. The Columbia cohort examined chlorpyrifos in blood, whereas in the UC-Berkeley and Mount Sinai cohorts the urinary levels of TCPy were used as biomarkers of chlorpyrifos exposure. Each group of investigators chose rationally, but the measures used were not ideal. Ignoring for the moment the constraints that attend observational studies in human populations, an ideal exposure measure for humans would have the following properties: (1) It is specifically and quantitatively correlated with the levels of chlorpyrifos and chlorpyrifos-methyl—i.e., the only compounds for which there is evidence of potential injury to the nervous system; (2) it has pharmacodynamic properties that have been elucidated in studies on humans and in carefully correlated studies in animals so that one can validly predict the levels of chlorpyrifos that result from all sources of exposure (i.e., inhalation, ingestion, dermal contact) to the maternal blood, and thence to placenta, to fetal blood, to fetal brain; (3) its pharmacodynamics also allow for estimations of the half-life for chlorpyrifos in the brain; (4) it can be measured at regular intervals during pregnancy, with sampling during all trimesters; (5) the tissue/fluid source(s) for the marker can be readily accessed—e.g., urine or blood; and (6) the methods for detection are sensitive enough to detect levels in the nanomolar and even subnanomolar range. As yet, such a biomarker for chlorpyrifos exposure does not exist. No marker of chlorpyrifos exposure, including chlorpyrifos itself, satisfies these ideal properties. However, the data available allow us to make reasonable, though necessarily imprecise, estimates of exposure in the studies reviewed herein.
It could be argued that cord blood levels of chlorpyrifos are the most relevant and best current measure of exposure (CitationNeedham, 2005). This was measured in the Columbia studies and the sensitivity of the assay methods was excellent. Lacking in these studies, however, are repeated measures of chlorpyrifos during the course of the pregnancy. Of course, it would have been impossible to repeatedly measure cord blood levels, but the reported correlation between maternal and cord blood levels suggests that maternal blood levels could be considered a surrogate and these could be obtained at regular intervals. How can one relate the level in maternal blood to the levels in fetal brain? Chlorpyrifos is lipophilic. Therefore, once distributed throughout the body via the blood, much chlorpyrifos would be found associated lipids in blood, brain, and other tissues (CitationNeedham, 2005). The half-life of chlorpyrifos in the adipose tissue of rats is 62 h (Smith et al., 1967); since the brain is also lipid rich, a similar value may apply. Needham has argued that the concentrations of chlorpyrifos in maternal blood and adipose tissue are likely to be in steady state, unless the extent of chlorpyrifos exposure has changed near the time of sampling (CitationNeedham, 2005). With this in mind, and absent a marked change in recent exposure, the level of chlorpyrifos measured in the Columbia study at or very near the time of delivery would reflect exposure only during the past 6 half-lives, or about 15 days prior to admission to hospital—i.e., during very late pregnancy. While it is possible that maternal blood levels may not have differed significantly at early times in pregnancy, the data do not allow this conclusion. Nevertheless, assuming for the moment that the blood levels at birth were representative, one can estimate the fetal brain level of chlorpyrifos from the blood level. For the overall study, the arithmetic mean for cord blood was 3.7 pg/g and the geometric mean was 1.5 pg/g. Taking the higher value, and rounding to 4.0 pg/g, the molar concentration was 0.01 nM. Interestingly, this level of blood chlorpyrifos cannot be viewed as high relative to other U.S. populations. Indeed, using the same assay methods, a mean of 9.0 pg/g was measured in a pooled blood sample obtained from the Cincinnati Red Cross (Barr et al., 2002b). Accepting a ratio of brain to blood levels of chlorpyrifos of 33 (CitationTimchalk et al., 2002b), the mean level of chlorpyrifos in brain would be about 0.33 nM. Taking the worst-case scenario, if all chlorpyrifos in the brain were metabolized to the oxon, and the oxon was quite stable, using an IC50 for brain AChE for the oxon of 4.9 nM predicts that this level of chlorpyrifos exposure would cause less that 10% inhibition of AChE. Somewhat more than 10% of activity would be lost if only the value for mothers delivered prior to January 1, 2001 (equating to a brain level of 0.61 nM), is considered. Using the geometric means would result in overall exposures producing a brain level of 0.13 nM and for early exposures a level of 0.33nM. Whether this concentration of chlorpyrifos in the brain would be sufficient to induce inhibition of AchE at levels that result in neurodevelopmental toxicity is unknown. Nor is it clear whether this concentration of chlorpyrifos would have any effect on brain BuChE activity, which may be somewhat more sensitive to inhibition than AChE. However, almost all in vitro studies report effects of chlorpyrifos at a micromolar concentration, i.e., several orders of magnitude higher than the measured or estimated levels of brain chlorpyrifos following in vivo community exposure levels, based on chlorpyrifos blood levels reported in the blood bank samples in CitationBarr et al. (2002) and maternal and cord blood samples from the Columbia cohort. However, other “noncholinesterase” targets whose inhibition might be induced by nanomolar or subnanomolar concentrations of chlorpyrifos have been suggested (see Section IV) and theoretically could contribute to neurodevelopmental toxicity.
In contrast to the Columbia studies, the Mount Sinai and Berkeley investigators collected urine specimens to assay urinary metabolites of chlorpyrifos, including DAP and TCPy. The utility of urine as a source for defining exposures has many advantages, including the ease of sample collection, which facilitates repeated measures (CitationNeedham, 2005). Unfortunately, neither the Berkeley nor the Mount Sinai groups took advantage of this to conduct measurements throughout the pregnancy. A more significant limitation in these studies, as discussed in Section V, is that urinary TCPy, has potentially serious limitations as a specific biomarker of exposure to chlorpyrifos, because much of the exposure may be due to TCPy itself, and not to chlorpyrifos or chlorpyrifos-methyl. Thus, TCPy levels may not accurately reflect exposure to chlorpyrifos and, because of this, even substantial changes in exposure to chlorpyrifos or chlorpyrifos-methyl could be masked.
Though no accurate analysis of exposure across studies is possible, one measure of exposure that does allow a comparison is the personal air samples collected by the Columbia and Berkeley groups (see and ), assuming that dietary exposures to chlorpyrifos in both cohorts were similar. Although, as pointed out, the exact types of exposure differed in the Columbia and Berkeley studies (CitationNeedham, 2005), this measure of exposure should capture the increased exposure from residential use of chlorpyrifos in the Columbia studies and was made during the third trimester—i.e., at a more relevant developmental window. Moreover, it may serve as a guide for the increased exposure in the Mount Sinai studies because self-reported pesticide exposure in the home during pregnancy was similar—71% in the Mount Sinai study, and approximately 85% in the Columbia study. In the Columbia studies (), the geometric mean was 6.3 ng/m3. The arithmetic mean was 14.3 ± 30.7 ng/m3. If there was complete absorption of inhaled chlorpyrifos, the amount ingested by an adult woman (70 kg; breathing 15 m3 per day) can be estimated to be 210 ng, for a dose of 0.003 μ g/kg-day (). The values for air samples taken during the early years of the study are modestly higher than those for the study overall, but would change these estimates by only about 20% (). No air samples were collected by the Mount Sinai group but, as suggested earlier from reports of pesticide exposure, it seems likely that they would be similar to those recorded by the Columbia group. The Columbia values can be compared to those reported by CitationBradman et al. (2007) for the Berkeley group, among which about 42% of mothers reported working either in agricultural fields (28%) or in other agricultural settings (∼ 14%). In the Berkeley study the median indoor air concentration for chlorpyrifos was 11 ng/m3 (range 4.0 to 36) and for outdoor air was 6.0 (ND to 36) (CitationBradman et al., 2007). If we use the median indoor air value, the amount of chlorpyrifos intake by inhalation in the home can be estimated to be 165 ng, for a dose of 0.002 μ g/kg-day (0.165 ng/70 kg; ). Using the maximum value measured the dose would be 0.007 μ g/kg-day. It is quite possible that even greater exposures were experienced by those working in fields in which chlorpyrifos was applied. In any case, the difference in exposure from chlorpyrifos in the air in homes was, at most, small. It is noteworthy, furthermore, that for all three study populations, the levels of exposure were modest and not dissimilar from exposures among the U.S. population in general, based on urinary TCPy levels as examined in NHANES III (CitationNHANES III, 2003), or the blood levels measured in a blood bank sample reported in CitationBarr et al. (2002). Finally, it should be noted that the Columbia study did not find an association between birth weight, birth length, and head circumference and chlorpyrifos exposure estimated from maternal personal air monitoring.
TABLE 24 Comparison of exposure parameters for three epidemiology cohort studies evaluating potential neurodevelopmental effects of chlorpyrifos
There is no reason to believe that dietary exposure to chlorpyrifos in the Columbia cohort was dissimilar to that in other populations. This is likely to have been the case for the Berkeley studies as well, although in a study of children of farm workers in the Salinas Valley in 2002, CitationBradman et al. (2007) found only trace levels of chlorpyrifos in a small percentage of food samples analyzed (detection limit of ∼ 1 ppb).
Although specific dietary intake of chlorpyrifos in these cohorts is uncertain, it is possible to make approximations of “typical” intake rates based on food frequency intake data, and the frequency and levels of chlorpyrifos residues in foods, based on FDA market basket surveys (see Section V). Using such an approach for chlorpyrifos, an average daily intake of approximately 0.35 μ g/day is estimated for adults consuming a typical U.S. diet (), yielding a body-weight-adjusted daily intake of 0.005 μ g/kg-day for an average adult (). If the estimated dietary exposure is added to the estimate for inhalation exposure noted earlier, an average daily exposure to chlorpyrifos is estimated to be ∼ 0.56 μ g/day or 0.008 μ g/kg-day for the mothers in the Columbia study and ∼ 0.52 μ g/day or 0.007 μ g/kg-day for the mothers in the Berkeley CHAMACOS study, with estimated inhalation exposures contributing approximately one-third of the total exposures in both cases (). When chlorpyrifos-methyl in the diet is considered as an additional source of exposure to chlorpyrifos, the total exposure still amounts to about 0.03 μ g/kg/day in both the Columbia and Berkeley cohorts.
As discussed earlier, inhibition of plasma BuChE can be taken as a “no-effect level” for tissue cholinesterase inhibition. The estimated exposures in these cohort studies to chlorpyrifos are approximately 4000 times lower than the daily dose (30 μ g/kg-day for 28 days) shown to have a modest (nonsignificant) inhibition on BuChE, and approximately 12,000 times lower than the dose (100 μ g/kg-day for 9 days) shown to have significant (up to 64%) inhibition of BuChE in human volunteers (CitationCoulston et al., 1972). The combined exposures to chlorpyrifos in these cohorts thus fall far below those associated with inhibition of plasma BuChE in humans. The margin of safety with respect to AChE would be even greater, as no inhibition of erythrocyte AChE was seen in the CitationCoulston et al. (1972) study at the highest dose (100 μ g/kg-day for 9 days), even though BuChE was substantially inhibited. Considering the exposures to both chlorpyrifos and chlorpyrifos-methyl combined, the level would be ∼ 1000-fold lower than the dose shown to have a modest effect on BuChE activity. As indicated earlier, however, it is not possible to rule out the existence of novel targets whose inhibition is induced by the low levels of chlorpyrifos exposure experienced in these settings.
Recognizing the limitations inherent in using TCPy in the urine as a biomarker of chlorpyrifos exposure, it is useful to compare the studies with respect to the levels of TCPy that would be expected to result from inhalation exposure to chlorpyrifos. Using the indoor air concentrations to estimate inhaled chlorpyrifos for the Columbia study, the amount of TCPy present in the urine would be about 0.03 μ g/L using the geometric mean (6.3ng/m3) and 0.07 μ g/L using the arithmetic mean (14.3 ng/m3). Inhaled chlorpyrifos in the Berkeley CHAMACOS study (median 11 ng/m3) would have resulted in a TCPy urinary level of 0.055 μ g/L. Given the earlier discussion of urinary TCPy as a marker of chlorpyrifos exposure, it is not surprising that inhaled chlorpyrifos is a small percentage of the TCPy measured in the Berkeley study (1.7%). While the level of TCPy in urine samples from the Mount Sinai group was somewhat greater (7.6 μ g/L) than that for the Berkeley study (3.3μ g/L), these differences are small considering the variability in the measurements in each study, and the large “within subject” variability that can occur from typical dietary exposures (CitationKissel et al., 2005; ). If we assume that exposure to inhaled chlorpyrifos was equal to that in the Columbia group, less than 1% of urinary TCPy in the Mount Sinai study population would have been contributed by air exposures.
Notwithstanding this analysis, two additional aspects of the Columbia studies were striking. CitationWhyatt et al. (2004a) noted that the effects of chlorpyrifos on birth length and birth weight were correlated with the extent of exposure. Second, these studies showed changes over time in the inverse correlations between chlorpyrifos exposure and developmental outcomes. With the use of chlorpyrifos restricted for residential use after January 1, 2001, there were decreases in chlorpyrifos in blood samples and previously significant correlations were no longer demonstrable. Comparing samples obtained before and after January 2001, CitationWhyatt and colleagues (2004a) reported significant decreases in chlorpyrifos in air samples and in blood; earlier inverse correlations of exposure with birth weight and length were absent. Similarly, Rauh and co-workers (2006) found significant improvements in MDI and PDI scores in infants born following January 1, 2001. Taken together, these data provide evidence that changes in exposure, at least as reflected in chlorpyrifos levels, appear to be correlated with outcomes. What is not clear is whether it is the decreasing levels of chlorpyrifos or some other covariate that was responsible for these changes in the birth and neurodevelopmental outcomes. If it was chlorpyrifos, the effect would have to be observed at concentrations more than 4,000 fold lower than the estimated “No Effect Level” of chlorpyrifos for inhibition of plasma BuChE, which is generally considered to be the most sensitive in vivo biological effect of chlorpyrifos. If not chlorpyrifos, then other environmental factors must be considered. As the authors comment, further studies will be needed to assess the impact of restricting chlorpyrifos on developmental outcomes.
In principle, many other environmental exposures could affect birth weight and neurobehavioral measures, and exposure levels to such risk factors could differ between the two cohorts, and over time. Among the factors that could play a role are exposure to tobacco smoke and maternal alcohol intake. The evidence is compelling that smoking or exposure to tobacco fumes impacts birth weight. In a study involving more than 3000 women, CitationEngland et al. (2001) provided evidence to suggest that the relationship between smoking and decreased birth weight is not linear (CitationEngland et al., 2001). Self reports of less than 5 cigarettes smoked per day as well as relatively low levels of cotinine, a specific metabolite of nicotine, in urine were associated with ∼ 200 g decreases in birth weight. This suggests that rather low-level exposure to tobacco smoke has a significant impact on birth weight. In another large study, a smaller (45 g) and nonsignificant effect on birth weight was seen when the infants of nonsmoking and nonexposed women were compared to mothers who reported exposure to tobacco smoke but were not themselves smokers (CitationEskenazi et al., 1995). Similar findings were reported in an earlier study (CitationHaddow et al., 1988). The infants of mothers who were not exposed to tobacco delivered infants that were significantly larger (143 g) than those of smoking women whose plasma cotinine levels were in the lowest tertile of exposure (2 to 10 ng/ml). In this study, the potentially confounding effect of consumption of alcohol was considered. In a more recent study, CitationWang et al. (2002) reported that infants of mothers who smoked during the entire pregnancy were significantly smaller at birth (377 g) than infants of nonsmoking mothers. Similarly, when compared to nonsmoking pregnancies, smoking throughout pregnancy resulted in significant shortening of gestation (1 week) and intrauterine growth retardation. Thus, even modest differences in exposure to tobacco smoke between cohorts, or between subjects within a cohort, are a potent risk factor and a potentially important confounder in studies of birth weight and other birth outcomes.
Increasing maternal alcohol consumption is also associated with decreased birth weight. For example, CitationWindham et al. (1995) reported that three or more drinks per week was associated with a birth weight decrement of 143 g. Interestingly, the infants of drinking mothers that were nonsmokers showed little effect on weight, while mothers who also smoked more than 10 cigarettes per day delivered smaller infants; the evidence suggests that the effects of smoking and alcohol consumption were more than additive (CitationWindham et al., 1995). Pregnancies in which more than one drink per day was consumed resulted in nearly a tripling of intrauterine growth retardation (IUGR), an effect that persisted after controlling for smoking.
Increased alcohol consumption has also been associated with a reduction in gestational age after adjusting for smoking (CitationSood et al., 2001). Women attending a university-based maternity clinic were screened for alcohol and drug use. The sample included 506 mother–child pairs who later completed a questionnaire detailing day-by-day alcohol intake in the periconceptual period and for the 2 weeks preceding the visit. Mean gestational age and birth weight were significantly negatively associated with increasing maternal alcohol exposure. Tobacco smoke exposure increased with increasing alcohol exposure, as did blood lead levels. The Child Behavior Checklist (CBCL) was used to assess the extent of behavioral problems. Adjusting for potential confounding variables, including exposure to tobacco smoke and lead, alcohol exposure was a significant predictor of adverse behaviors considered as a group and for attention problems in particular (CitationSood et al., 2001). The authors provide evidence to suggest that even one drink per week has an effect. Thus, providing adequate control for exposures to both tobacco smoke and alcohol in studies of neurobehavioral outcomes is an extremely important consideration.
Recognizing that exposures to tobacco smoke and alcohol could have independent adverse impacts on the birth measures being examined in studies of chlorpyrifos exposure, it is important to attempt to compare the extent of reported or objectively measured evidence of exposure to smoking and alcohol in the chlorpyrifos study populations examined in the three cohorts. The Columbia investigators attempted to eliminate active smokers by excluding from the analysis women or infants with plasma cotinine levels of > 25 ng/ml (CitationPerera et al., 2003). In this sample, ∼ 43% of mothers reported a smoker in the home, and about the same percent had cotinine values that indicated exposure to tobacco. It is noteworthy, however, that the half-life of cotinine metabolism during pregnancy is 8.8 h and that plasma samples were obtained within 1 day (CitationPerera et al., 2003) or 2 days (CitationWhyatt et al., 2004a) of delivery. Because plasma samples were not obtained at the time of admission to hospital, it is possible that cotinine values underestimated the actual exposures, perhaps considerably. In addition, the entire sample tested positive for PAHs combustion by-products of organic matter, including tobacco, air contaminants, and PAH-containing meat. Fully 24% of mothers reported drinking alcohol during pregnancy, but the extent of use was not specified. In a subsequent report, 25% reported drinking but only 2% reported “heavy drinking”; the latter term was not defined (CitationWhyatt et al., 2004b). In the UC-Berkeley group, 6.1% of women reported active smoking and only 1% reported alcohol use in the 2004 paper. In the 2007 paper reported alcohol use was the same but 5.2% reported active smoking and 8.3% lived with a smoker. It is noteworthy, however, that no tests for cotinine were done, possibly due to the low rate of smoking among this predominantly Latina population (84% Mexican born). Among women in the Mount Sinai cohort, ∼ 5% reported active smoking. Women who drank more than two alcoholic beverages per day were excluded from the study; this would eliminate only those with heavy consumption, leaving uncertainty with respect to the extent of exposure within the rest of the cohort.
When the three cohorts are compared, it appears that the groups were substantially different with respect to exposure to tobacco smoke and alcohol, with higher levels of exposure to both tobacco smoke and alcohol in the Columbia cohort. Focusing specifically on tobacco, and relying simply on maternal reports, active smoking and exposure to tobacco smoke was present for ∼ 43% of the subjects in the Columbia cohort, for 13.5% in the UC-Berkeley cohort, and in perhaps about the same (∼ 13–15%) in the Mount Sinai cohort. It is difficult to assess the differences in exposure to alcohol across all reports, but alcohol exposure also appears to have been greater for mothers in the Columbia cohort than in the UC-Berkeley CHAMACOS cohort.
The Columbia investigators did attempt to control for tobacco exposure in their studies by examining cotinine levels in specimens obtained at time or shortly after delivery. Cotinine measurements, however, were taken 24–36 h after nicotine exposures were likely to have occurred. There was no reported association of cotinine with birth outcomes. CitationPerera et al. (2003) report that while PAH exposure shared with chlorpyrifos exposure a significantly negative association with birth weight, the effects of the two appeared to be independent. In this same study population, CitationWhyatt et al. (2004a) included a history of active smoking as a covariate, but not alcohol exposure. The authors stated that few women drank and that alcohol consumption did not predict birth outcomes. In subsequent analyses with this cohort, CitationRauh et al. (2004b) also controlled for tobacco smoke exposure in their analysis of chlorpyrifos on behavioral outcomes but noted that exposure to smoking was positively associated with exposure to chlorpyrifos. In this analysis there also was no attempt to control for alcohol exposure. In summary, while the investigators made attempts to control for the potential confounding by two well-known potent neurodevelopmental toxicants—tobacco smoke and ethanol—there were clear limitations in what was possible with the data for these cohorts.
What is not adequately explained by unrecognized or underestimated exposures to smoking and alcohol is the absence of evidence of adverse birth outcomes and improvement of neurobehavioral results in subjects examined after January 2001 in the Columbia cohort, the time at which residential use of chlorpyrifos was restricted. That other factors might also have distinguished the two periods is suggested by CitationWhyatt et al. (2004a) by their observation that other pesticide exposures also decreased. Indeed, in spite of the fact that mothers reported no significant change in pesticide use, maternal air samples showed significant decreases in the amounts of diazinon and propoxur and umbilical blood samples showed a significant decrease in the metabolites of propoxur. Moreover, while the sum of chlorpyrifos and diazinon exposures was significantly negatively correlated with birth weight and length before January 1, 2001, the sums of these exposures were not significantly associated with these measures for infants born after this date. Finally, total sample size was smaller in the portion of the cohort born after January 1, 2001; while total sample size was 314, only 77 samples constituted the post-January 1, 2001, subgroup. It is also possible that changes in exposures to other known and suspected neurodevelopmental risks, such as lead, perfluorooctanoic acid (PFOA; Apelberg et al., 2007), methylmercury (CitationJohansson et al., 2007), or any number of environmental factors, might also have contributed to differences in birth outcomes and neurobehavioral measures in these later pregnancies. Thus, although the findings in the Columbia cohort provide support for the hypothesis that chlorpyrifos exposures are associated with adverse neurodevelopmental outcomes, further studies with more rigorous exposure assessment and careful control of other strong confounding factors are needed to clarify this association.
Finally, it should be noted that the magnitude of reported effects in the three birth cohort studies is modest, which could contribute to either “false negative” or “false positive” results. For example, in the Columbia cohort, a difference in birth weight of 43 g and in birth length of 0.24 cm was observed for each 10-fold increment in chlorpyrifos exposure. In comparing the extremes of chlorpyrifos exposure, the differences were 150 g for birth weight and 0.75 cm for birth length. These changes are of modest size, representing changes in average birth weight of ∼ 4% and length of ∼ 1%. With regard to the differences in behavior reported for the Columbia cohort (CitationRauh et al., 2006), the difference in mean PDI score at 36 months is relatively modest; comparing scores for the high versus the low exposure groups a difference of ∼ 6.5 points is reported. While significant differences associated with chlorpyrifos exposure were detected by bivariate analysis for attention problems and ADHD, relatively few children in the low exposure group scored in the clinical range compared to national norms, i.e., ADHD, 3 to 5% national versus 3.9% in this study (as referenced therein). As indicated by the authors, while the findings are of potential concern, in view of the small sample size it will be important to study further the possible relationship of chlorpyrifos exposure to relevant behavioral outcomes.
The findings reviewed earlier for the three birth cohort studies suggest the possibility that prior chlorpyrifos exposures may be associated with adverse birth and neurodevelopmental outcomes; however, the evidence for this association is inconsistent between studies and the weight of available evidence argues against the proposed association as being of causal significance.
While it is difficult at present to judge the significance, or lack thereof, of current levels of chlorpyrifos exposures in the United States on the development of the nervous system in humans, we cannot rule out chlorpyrifos-induced human neurodevelopmental perturbation. Indeed, important unanswered questions exist with respect to: (1) the extent of current childhood exposures to chlorpyrifos; (2) the spectrum of active metabolites of various pesticides potentially adversely linked to neurodevelopmental outcomes; (3) the levels of these metabolites, relative to the activities of the enzymes that produce and degrade them; (4) the best methods for objectively assessing the neurodevelopmental consequence of environmental (including pesticide) exposures; and (5) elucidating the interactions of chlorpyrifos and its metabolites with other environmental factors that have known adverse actions on fetal growth and child development. While it might be informative to conduct studies of these associations in children born to mothers in low-resource countries where exposures to pesticides and other environmental factors might be higher, these studies would be more challenging for a number of reasons, including ethical considerations for what is acceptable for human subjects. In addition, pregnancies in these settings may differ in terms of the levels of nutrition, health, and medical supports available to women in high-resource countries. Obviously, the contribution of nutrition, medical care, and other exposures to the birth and developmental outcomes assessed would need to be considered in the interpretation of study findings.
Ideally, building upon the available body of knowledge, future studies could incorporate (1) more refined and specific measures of exposure and methods for assessing individual time-relevant exposures; and (2) sufficient sample sizes and objective measures for assessing health outcomes. Such studies could be carried out in exposed agricultural worker populations to resolve whether or not prenatal exposure to current levels of chlorpyrifos is causally associated with neurobehavioral deficits. It is recognized, however, that it may be difficult, if not impossible, to identify a sufficiently large cohort of potentially exposed pregnant women for such a follow-up study.
Beyond concerns for potential effects attributable to chlorpyrifos, the studies reviewed suggest that prenatal exposure to OP pesticides, as determined by measures of urinary metabolites, may be associated with adverse birth outcomes. Indeed, the correspondence in findings from studies by the UC-Berkeley and Mount Sinai investigators of an association between levels of total DEP metabolites with increased numbers of abnormal reflexes is persuasive. In addition, Grandjean and colleagues (2006) found neurobehavioral abnormalities in children in northern Ecuador that were associated with prenatal or postnatal exposure to pesticides. While the clinical significance of these findings is uncertain, the importance of the observations must be acknowledged and pursued. We note, however, that it is unknown whether or not chlorpyrifos exposure contributed to the outcomes reported in these studies.
In summary, based on a review of a remarkably large volume of scientific studies on chlorpyrifos in both animals and humans, we offer the following answers to the questions posed at the beginning of this review: (1) What is the strength of the scientific evidence supporting the hypothesis put forward by others that chlorpyrifos is capable of causing adverse neurodevelopmental outcomes in humans at current, “background” exposure levels? (2) Does the scientific evidence to support a mechanism for neurodevelopmental effects other than AChE inhibition? (3) Would limiting chlorpyrifos exposures to levels that protect against AChE inhibition be adequate to protect against any potential neurodevelopmental outcomes?
Current “background” levels of exposure to chlorpyrifos are derived largely from the diet, and are several orders of magnitude lower than doses that could have measurable effects of plasma BuChE (and thus any significant effect on nervous tissue AChE). Thus, based on the weight of the scientific evidence, it is highly unlikely that background levels would have any adverse neurodevelopmental effects in infants exposure in utero to chlorpyrifos through the diet, if the sole mechanism of neurodevelopmental effects is via inhibition of target tissue AChE. However, several in vitro studies have identified putative neurodevelopmental mechanisms or effects on neuronal growth in vitro that occur at concentrations of chlorpyrifos (oxon) below those necessary to inhibit AChE. One epidemiological cohort study of infants exposed in utero to chlorpyrifos, in part from residential use, reported an association between maternal and cord blood chlorpyrifos levels and several measures of neurodevelopment. However, the association was based on a single measure obtained months after the critical period of neurodevelopment. Other limitations common to such epidemiological studies make it very difficult to infer a causal relationship between chlorpyrifos and adverse neurodevelopmental effects based on this cohort study. Nevertheless, neither the mechanistic data suggesting plausible alternative mechanisms (i.e., not through inhibition of the enzyme activity of AChE and BuChE) nor the epidemiological studies suggesting an association between blood chlorpyrifos and certain measures of neurodevelopment can be ignored. Rather, they warrant further attempts to (1) define an ideal marker(s) for exposure whose pharmacodynamics allow for valid predictions for chlorpyrifos concentrations in fetal blood and brain, whose levels can be measured sensitively and with great precision, and for which samples can be collected during the entire course of the pregnancy; and (2) conduct in vivo studies to examine a comprehensive list of relevant neurodevelopmental measures and outcomes in a suitable animal model(s). Further epidemiological investigation would be warranted if a suitable, chlorpyrifos-exposed cohort can be identified and more specific and objective measures of exposure are utilized.
ACKNOWLEDGMENTS
Science Partners LLC is an independent company dedicated to establishing scientific and medical consensus in public health. Science Partners is composed of more than 160 of the United States' and Europe's leading clinicians, toxicologists, epidemiologists, medical scientists, biomedical engineers, and other manufacturing and risk experts. This study was funded by Dow AgroSciences (DAS). Science Partners LLC independently selected the experts for the analysis, including determining the areas of disciplinary expertise required for its conduct. At the end of our review and drafting process and immediately prior to submission of the document to the publisher, a copy of the final draft was sent to Dow and Science Partners asked Dow to review the draft solely for any factual errors. They made (minor) comments but they did not influence, modify or contribute to the opinions and conclusions in any way. There was no communication or interaction of any kind between members of the Evaluation Group and any employee of DAS during the analysis, with the exception of requests for data. The conclusions and opinions of Science Partners LLC expressed herein are premised upon the enumerated, publicly available literature and data as of the date of this analysis, and the specified data, studies, and literature provided by Dow AgroSciences. To the extent there is additional information relevant to the topics reviewed, Science Partners LLC reserves the right to reconsider and alter its opinions and conclusions.
The authors thank Dr. Robert Croy (MIT), Dr. Wolfgang Huber (Medical University of Vienna), and Dr. Eric Elmquist (MIT) for their excellent technical assistance in compiling background information and editing of the manuscript. We thank Dr. Robin Whyatt for her generous contribution of time and data included in . We also thank Nancy Elder, head Life Science Librarian at the University of Texas, for her assistance in bibliographic database searches; Dr. Dongren Yang, Oregon Health Sciences University, for his assistance in bibliographic database searches of the relevant Chinese databases; Zhong Wang, Case Western Reserve University, for translating Chinese documents; and Mihran Aroian and Jeremy Griffiths with Science Partners LLC for their exceptional guidance in project management.
Notes
1The U.S. EPA defines the oral reference dose (RfD) as “an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime”. See http://www.epa.gov/ncea/iris/subst/0026.htm
2At the request of Dow AgroSciences (DAS), Science Partners, LLC, assembled a team of scientists with extensive expertise in pediatric neurology, child psychiatry, teratology, epidemiology, toxicology, and neurotoxicology to conduct this review. Science Partners LLC independently selected the experts for the analysis, including determining the areas of disciplinary expertise required for its conduct. Each member of the team was paid a fee by Science Partners, LLC, for their time and effort. There was no direction given by DAS on the content of our analysis nor did they review the opinions and conclusions reached by our team prior to its completion. There was no communication or interaction of any kind between members of the Evaluation Group and any employee of DAS during the analysis. Request for data from DAS was channeled through Science Partners, LLC. Science Partners, LLC, is an independent company dedicated to establishing the scientific and medical consensus. Science Partners, LLC, operations and structure are designed to provide maximum objectivity and independence, and the firm maintains the right to publish findings regardless of the outcome or approval of clients.
3U.S. EPA, “Interim Reregistration Eligibility Decision for Chlorpyrifos,” September 2001; http:www.epa.gov/pesticides/op.
4Use statistics for chlorpyrifos provided by Dow AgroSciences.
5Although this study followed research guidelines in place at the time, it is recognized that intentional exposures to humans at the dose levels used in this study would not be approved by Institutional Review Boards under current Office of Human Research Protections guidelines.
6It should be noted that the Curwin et al. (2007) studies of farm worker families used an immunoassay for TCPy, which for unexplained reasons appears to yield urinary TCPy concentrations approximately 3–4 times greater than those determined by direct chemical analysis (HPLC or GC-MS). Thus, no direct comparison of the Curwin et al. (2007) values with other values in the table should be made.
7Body burden [ss] = [T1/2* 1.44* D]/[tau]. If D = 0.0004 μ g/kg-day, then BB[ss] = 0.00065 μ g/kg with a half-life of 27 h and a dosing interval [tau] of 24 h.
8100 cm2 × 10 ng/cm2 × 5%/(20 kg) = 2.5 ng/kg-day, or 0.0025 μ g/kg-day.
9It is recognized that these data are now more than 10 years old. Agricultural use of chlorpyrifos has declined only slightly between 1998–2001 and 2002–2006 (), although the distribution of use among some food crops has changed substantially. Thus, it is not clear how representative food exposure data obtained in 1995 are for today. However, the evaluation is pertinent to understanding the extensive number of exposure assessment studies that were completed in this time frame (1995–2002).
10See Federal Register Vol. 72, No. 233, Wednesday, December 5, 2007, pp. 68580–81.
11Assumptions include: steady-state body burden, determined by the equation: [BB]ss = T1/2 × 1.44 × D/Tau, where half-life is based on the elimination half-life for TCPy of 27 h, D is the dose rate, expressed in μ g chlorpyrifos/kg-day, and Tau is the dosing interval, set at 24 h. Converting to urinary TCPy concentration assumes 70% of the daily dose of chlorpyrifos is eliminated in the urine, and adjusts for the difference in formula weight between chlorpyrifos and TCPy, a value of 1.77. Twenty-four-hour urine volumes are assumed to be 1.7 L for adults, 0.5 L for toddlers, 0.25 L for infants.
12Procedures described in EPA 738-R-01-007, Interim Reregistration Eligibilibility Decision for Chlorpyrifos.
13See EPA Integrated Risk Information System, docket CASRN 2921-88-2, available at http://www.epa.gov/iris/subst/0026.htm.
REFERENCES
- A.A. Abdel-Rahman, G.M. Blumenthal, S.A. Abou-Donia, F.A. Ali, A.E. Abdel-Monem, and M.B. Abou-Donia. (2002). Pharmacokinetic profile and placental transfer of a single intravenous injection of [(14)C]chlorpyrifos in pregnant rats.. Arch. Toxicol. 76:452–459.
- A. Abdel-Rahman, A.M. Dechkovskaia, H. Mehta-Simmons, J.M. Sutton, X. Guan, W.A. Khan, and M.B. Abou-Donia. (2004). Maternal exposure to nicotine and chlorpyrifos, alone and in combination, leads to persistently elevated expression of glial fibrillary acidic protein in the cerebellum of the offspring in late puberty.. Arch. Toxicol. 78:467–476.
- A. Abdel-Rahman, A. Dechkovskaia, H. Mehta-Simmons, X. Guan, W. Khan, and M. Abou-Donia. (2003). Increased expression of glial fibrillary acidic protein in cerebellum and hippocampus: Differential effects on neonatal brain regional acetylcholinesterase following maternal exposure to combined chlorpyrifos and nicotine.. J. Toxicol. Environ. Health A 66:2047–2066.
- M.B. Abou-Donia, W.A. Khan, A.M. Dechkovskaia, L.B. Goldstein, S.L. Bullman, and A. Abdel-Rahman. (2006). In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult offspring rats.. Arch. Toxicol. 80:620–631.
- A.W. Abu-Qare, A. Abdel-Rahman, C. Brownie, A.M. Kishk, and M.B. Abou-Donia. (2001). Inhibition of cholinesterase enzymes following a single dermal dose of chlorpyrifos and methyl parathion, alone and in combination, in pregnant rats.. J. Toxicol. Environ. Health A 63:173–189.
- A.W. Abu-Qare, and M.B. Abou-Donia. (2001). Inhibition and recovery of maternal and fetal cholinesterase enzyme activity following a single cutaneous dose of methyl parathion and diazinon, alone and in combination, in pregnant rats.. J. Appl. Toxicol. 21:307–316.
- J.F. Acquavella, B.H. Alexander, J.S. Mandel, C. Gustin, B. Baker, and P. Chapman. (2004). Glyphosate biomonitoring for farmers and their families: Results from the Farm Family Exposure Study.. Environ. Health Perspect. 12:321–326.
- J.L. Adgate, D.B. Barr, C.A. Clayton, L.E. Eberly, N.C. Freeman, P.J. Lioy, L.L. Needham, E.D. Pellizzari, J.J. Quackenboss, A. Roy, and K. Sexton. (2001). Measurement of children's exposure to pesticides: Analysis of urinary metabolite levels in a probability-based sample.. Environ. Health Perspect. 109:583–590.
- J.L. Adgate, A. Kukowski, C. Stroebel, P.J. Shubat, S. Morrell, J.J. Quackenboss, R.W. Whitmore, and K. Sexton. (2000). Pesticide storage and use patterns in Minnesota households with children.. J. Expos. Anal. Environ. Epidemiol. 10:159–167.
- S. Adkins, K.N. Gan, M. Mody, and B.N. La Du. (1993). Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: Glutamine or arginine at position 191, for the respective A or B allozymes.. Am.J. Hum. Genet. 52:598–608.
- L.A. Aiuto, S.G. Pavlakis, and R.A. Boxer. (1993). Life-threatening organophosphate-induced delayed polyneuropathy in a child after accidental chlorpyrifos ingestion.. J. Pediatr. 122:658–660.
- N. Akhtar, M.K. Srivastava, and R.B. Raizada. (2006). Transplacental disposition and teratogenic effects of chlorpyrifos in rats.. J. Toxicol. Sci. 31:521–527.
- M.C. Alavanja, M. Dosemeci, C. Samanic, J. Lubin, C.F. Lynch, C. Knott, J. Barker, J.A. Hoppin, D.P. Sandler, J. Coble, K. Thomas, and A. Blair. (2004). Pesticides and lung cancer risk in the agricultural health study cohort.. Am. J. Epidemiol. 160:876–885.
- J.W. Albers, S. Berent, D.H. Garabrant, B. Giordani, S.J. Schweitzer, R.P. Garrison, and R.J. Richardson. (2004a). The effects of occupational exposure to chlorpyrifos on the neurologic examination of central nervous system function: A prospective cohort study.. J. Occup. Environ. Med. 46:367–378.
- J.W. Albers, P. Cole, R.S. Greenberg, J.S. Mandel, R.R. Monson, J.H. Ross, W.R. Snodgrass, A. Spurgeon, and M. van Gemert. (1999). Analysis of chlorpyrifos exposure and human health: Expert panel report.. J. Toxicol. Environ. Health B 2:301–324.
- J.W. Albers, D.H. Garabrant, J.L. Mattsson, C.J. Burns, S.S. Cohen, C. Sima, R.P. Garrison, R.J. Richardson, and S. Berent. (2007). Dose-effect analyses of occupational chlorpyrifos exposure and peripheral nerve electrophysiology.. Toxicol. Sci. 97:196–204.
- J.W. Albers, D.H. Garabrant, S. Schweitzer, R.P. Garrison, R.J. Richardson, and S. Berent. (2004b). Absence of sensory neuropathy among workers with occupational exposure to chlorpyrifos.. Muscle Nerve 29:677–686.
- J.E. Aldridge, E.D. Levin, F.J. Seidler, and T.A. Slotkin. (2005a). Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression.. Environ. Health Perspect. 113:527–531.
- J.E. Aldridge, E.D. Levin, F.J. Seidler, and T.A. Slotkin. (2005b). Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression.. Environ. Health Perspect. 13:527–531.
- J.E. Aldridge, A. Meyer, F.J. Seidler, and T.A. Slotkin. (2005c). Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure.. Environ. Health Perspect. 113:1027–1031.
- J.E. Aldridge, F.J. Seidler, and T.A. Slotkin. (2003b). Changes in serotonin receptors in brain regions of rats after neonatal exposure to chlorpyrifos.. Toxicol. Sci. 72:124–125.
- J.E. Aldridge, F.J. Seidler, and T.A. Slotkin. (2004). Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood.. Toxicologist 78:273.
- J.E. Aldridge, F.J. Seidler, A. Meyer, I. Thillai, and T.A. Slotkin. (2003a). Serotonergic systems targeted by developmental exposure to chlorpyrifos: Effects during different critical periods.. Environ. Health Perspect. 111:1736–1743.
- W.N. Aldridge. (1953). Serum esterases.I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination.. Biochem. J. 53:110–117.
- B.H. Alexander, C.J. Burns, M.J. Bartels, J.F. Acquavella, J.S. Mandel, C. Gustin, and B.A. Baker. (2006). Chlorpyrifos exposure in farm families: Results from the Farm Family Exposure Study.. J. Expos. Sci. Environ. Epidemiol. 16:447–456.
- A. Alvarez-Arcaya, O. Combarros, J. Llorca, M. Sánchez-Guerra, J. Berciano, C. Fernández-Viadero, and N. Peña. (2000). The butyrylcholinesterase K variant is a protective factor for sporadic Alzheimer's disease in women.. Acta Neurol. Scand 102:350–353.
- S.M. Amer, and F.A. Aly. (1992). Cytogenetic effects of pesticides. IV. Cytogenetic effects of the insecticides Gardona and Dursban.. Mutat. Res. 279:165–170.
- R.G. Ames, S.K. Brown, J. Rosenberg, R.J. Jackson, J.W. Stratton, and S.G. Quenon. (1989). Health symptoms and occupational exposure to flea control products among California pet handlers.. Am. Ind. Hyg. Assoc.J. 50:466–472.
- H.A. Anderson, and M.S. Wolff. (2000). Environmental contaminants in human milk.. J. Expos. Anal. Environ. Epidemiol. 10:755–760.
- B.J. Apelberg, F.R. Witter, J.B. Herbstman, A.M. Calafat, R.U. Halden, L.L. Needham, and L.R. Goldman. (2007). Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth.. Environ. Health Perspect. 115:1670–1676.
- K.M. Ashry, A.W. Abu-Qare, F.R. Saleem, Y.A. Hussein, S.M. Hamza, A.M. Kishk, and M.B. Abou-Donia. (2002). Inhibition and recovery of maternal and fetal cholinesterase enzymes following a single oral dose of chlorpyrifos in rats.. Arch. Toxicol. 76:30–39.
- L.S. Aston, and J.N. Seiber. (1997). Fate of summertime airborne organophosphate pesticide residues in the Sierra Nevada mountains.. J. Environ. Qual. 26:1483–1492.
- J.R. Atack, E.K. Perry, J.R. Bonhan, and R.H. Perry. (1987). Molecular forms of acetylcholinesterase and butyrylcholinesterase in human plasma and cerebrospinal fluid. J. Neurochem 48:1845–1850.
- ATSDR. Toxicological profile for chlorpyrifos. Agency for Toxic Substances and Disease Registry, AtlantaGA, (1997).
- T.T. Atterberry, W.T. Burnett, and J.E. Chambers. (1997). Age-related differences in parathion and chlorpyrifos toxicity in male rats: Target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism.. Toxicol. Appl. Pharmacol. 147:411–418.
- K.B. Augustinsson, and M. Barr. (1963). Age variation in plasma arylesterase activity in children.. Clin. Chim. Acta 8:568–573.
- J.C. Axelrad, C.V. Howard, and W.G. McLean. (2002). Interactions between pesticides and components of pesticide formulations in an in vitro neurotoxicity test.. Toxicology 173:259–268.
- D. Bagchi, M. Bagchi, E.A. Hassoun, and S.J. Stohs. (1995). In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides.. Toxicology 104:129–140.
- J. Bajgar, L. Bartosova, K. Kuca, D. Jun, and J. Fusek. (2007). Changes of cholinesterase activities in the rat blood and brain after sarin intoxication pretreated with butyrylcholinesterase.. Drug Chem. Toxicol 30:351–359.
- B.A. Baker, B.H. Alexander, J.S. Mandel, J.F. Acquavella, and P. Chapman. (2004). Pesticide exposure in spouses from the Farm Family Exposure Study.. J. Toxicol. Clin. Toxicol. 42:802.
- B.A. Baker, B.H. Alexander, J.S. Mandel, J.F. Acquavella, R. Honeycutt, and P. Chapman. (2005). Farm Family Exposure Study: Methods and recruitment practices for a biomonitoring study of pesticide exposure.. J. Expos. Anal. Environ. Epidemiol. 15:491–499.
- J.E. Bakke, V.J. Feil, and C.E. Price. (1976). Rat urinary metabolites from O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate.. J. Environ. Sci. Health Bull. 3:225–230.
- J.E. Bakke, and C.E. Price. (1976). Metabolism of O,O-dimethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in sheep and rats and of 3,5,6-trichloro-2-pyridinol in sheep.. J. Environ. Sci. Health B. 11:9–22.
- J.M. Barnes. (1954). Organo-phosphorous insecticides. The toxic action of organophosphorous insecticides in mammals.. Chem. Ind. Jan 2.
- D.B. Barr, and J. Angerer. (2006). Potential uses of biomonitoring data: A case study using the organophosphorus pesticides chlorpyrifos and malathion.. Environ. Health Perspect. 114:1763–1769.
- D.B. Barr, J.R. Barr, V.L. Maggio, R.D. Whitehead, M.A. Sadowski, R.M. Whyatt, and L.L. Needham. (2002). A multi-analyte method for the quantification of contemporary pesticides in human serum and plasma using high-resolution mass spectrometry.. J. Chromatogr. B 778:99–111.
- M.G. Barron, S.M. Plakas, and P.C. Wilga. (1991). Chlorpyrifos pharmacokinetics and metabolism following intravascular and dietary administration in channel catfish.. Toxicol. Appl. Pharmacol. 108:474–482.
- G.M. Benke, and S.D. Murphy. (1975). The influence of age on the toxicity and metabolism of methyl parathion and parathion in male and female rats.. Toxicol. Appl. Pharmacol. 31:254–269.
- G.S. Berkowitz, J.G. Wetmur, E. Birman-Deych, J. Obel, R.H. Lapinski, J.H. Godbold, I.R. Holzman, and M.S. Wolff. (2004). In utero pesticide exposure, maternal paraoxonase activity, and head circumference.. Environ. Health Perspect. 112:388–391.
- G. Berkowitz, J. Wetmur, E. Birman-Deych, I. Holzman, and M. Wolff. (2003). Maternal exposure to indoor pesticides, paraoxonase activity, and fetal growth.. Am.J. Epidemiol. 157:S67.
- P.E. Berteau, and W.A. Deen. (1978). A comparison of oral and inhalation toxicities of four insecticides to mice and rats.. Bull. Environ. Contam. Toxicol. 19:113–120.
- A.M. Betancourt, and R. Carr. (2004). The effect of repeated oral ingestion of chlorpyrifos on cholinesterase and carboxylesterase activity in adult rats.. Toxicologist 78:276.
- A.M. Betancourt, S.C. Burgess, and R.L. Carr. (2006). Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain.. Toxicol. Sci. 92:500–506.
- W. Bicker, M. Lammerhofer, and W. Lindner. (2005b). Determination of chlorpyrifos metabolites in human urine by reversed-phase/weak anion exchange liquid chromatography-electrospray ionisation-tandem mass spectrometry.. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 822:160–169.
- W. Bicker, M. Lammerhofer, D. Genser, H. Kiss, and W. Lindner. (2005a). A case study of acute human chlorpyrifos poisoning: Novel aspects on metabolism and toxicokinetics derived from liquid chromatography-tandem mass spectrometry analysis of urine samples.. Toxicol. Lett. 159:235–251.
- J.A. Bomser, and J.E. Casida. (2001). Diethylphosphorylation of rat cardiac M2 muscarinic receptor by chlorpyrifos oxon in vitro.. Toxicol. Lett. 119:21–26.
- J.A. Bomser, G.B. Quistad, and J.E. Casida. (2002). Chlorpyrifos oxon potentiates diacylglycerol-induced extracellular signal-regulated kinase (ERK 44/42) activation, possibly by diacylglycerol lipase inhibition.. Toxicol. Appl. Pharmacol. 178:29–36.
- J. Bomser, and J.E. Casida. (2000). Activation of extracellular signal-regulated kinases (ERK 44/42) by chlorpyrifos oxon in Chinese hamster ovary cells.. J. Biochem. Mol. Toxicol. 14:346–353.
- A. Bradman, D. Whitaker, L. Quiro'S, R. Castorina, B.C. Hebb, M. NIishoka, J. Morgan, D.B. Barr, M. Harnly, J.A. Brisben, L.S. Sheldon, T.E. Mckone, and B. Eskenazi. (2007). Pesticides and their metabolites in the homes and urine of farmworker children living in the Salinas Valley, CA.. J. Expos. Sci. Environ. Epidemiol. 17:331–349.
- M.A. Bradman, M.E. Harnly, W. Draper, S. Seidel, S. Teran, D. Wakeham, and R. Neutra. (1997). Pesticide exposures to children from California's Central Valley: Results of a pilot study.. J. Expos. Anal. Environ. Epidemiol. 7:217–234.
- F.E. Brenner, G.G. Bond, E.A. McLaren, S. Green, and R.R. Cook. (1989). Morbidity among employees engaged in the manufacture or formulation of chlorpyrifos.. Br. J. Ind. Med 46:133–137.
- W.J. Breslin, A.B. Liberacki, D.A. Dittenber, and J.F. Quast. (1996). Evaluation of the developmental and reproductive toxicity of chlorpyrifos in the rat.. Fundam. Appl. Toxicol. 29:119–130.
- V.H. Brophy, R.L. Jampsa, J.B. Clendenning, L.A. McKinstry, G.P. Jarvik, and C.E. Furlong. (2001). Effects of 5' regulatory-region polymorphisms on paraoxonase-gene (PON1) expression.. Am.J. Hum. Genet. 68:1428–1436.
- V.H. Brophy, G.P. Jarvik, and C.E. Furlong. PON 1 polymorphisms.Paraoxonase (PON1) in Health and Disease: Basic and Clinical Aspects L.G. Costa, and C.E. Furlong. Kluwer Academic Publishers, NorwellMA, (2002)53–77.
- J.A. Buck, L.W. Brewer, M.J. Hooper, G.P. Cobb, and R.J. Kendall. (1996). Monitoring great horned owls for pesticide exposure in southcentral Iowa.. J. Wildl. Manage. 60:321–331.
- R.J. Buck, H. Ozkaynak, J. Xue, V.G. Zartarian, and K. Hammerstrom. (2001). Modeled estimates of chlorpyrifos exposure and dose for the Minnesota and Arizona NHEXAS populations.. J. Expos. Anal. Environ. Epidemiol. 11:253–268.
- R. Bullock, J. Touchon, H. Bergman, G. Gambina, Y. He, G. Rapatz, J. Nagel, and R. Lane. (2005). Rivastigmine and donepezil treatment in moderate to moderately-severe Alzheimer's disease over a 2-year period.. Curr. Med. Res. Opin 21:1317–1327.
- F.M. Buratti, and E. Testai. (2003). The inhibition of malathion detoxication by isomalathion and other OPTs in human liver microsomes.. Toxicol. Lett. 144:s152.
- F.M. Buratti, C. Leoni, and E. Testai. (2006). Foetal and adult human CYP3A isoforms in the bioactivation of organophosphorothionate insecticides.. Toxicol. Lett. 167:245–255.
- O. Burk, H. Tegude, I. Koch, E. Hustert, R. Wolbold, H. Glaeser, K. Klein, M.F. Fromm, A.K. Nuessler, P. Neuhaus, U.M. Zanger, M. Eichelbaum, and L. Wojnowski. (2002). Molecular mechanisms of polymorphic CYP3A7 expression in adult human liver and intestine.. J. Biol. Chem. 277:24280–24288.
- C.J. Burns, J.B. Cartmill, B.S. Powers, and M.K. Lee. (1998). Update of the morbidity experience of employees potentially exposed to chlorpyrifos.. Occup. Environ. Med. 55:65–70.
- P.J. Bushnell, C.N. Pope, and S. Padilla. (1993). Behavioral and neurochemical effects of acute chlorpyrifos in rats: Tolerance to prolonged inhibition of cholinesterase. J. Pharmacol. Exp. Ther 266:1007–1017.
- P.J. Bushnell, K.L. Kelly, and TR. Ward. (1994). Repeated inhibition of cholinesterase by chlorpyrifos in rats: Neurochemical, pharmacological, and behavioral indices of tolerance. J. Pharmacol. Exp. Therap 270:15–25.
- W. Butte, and B. Heinzow. (2002). Pollutants in house dust as indicators of indoor contamination.. Rev. Environ. Contam. Toxicol. 175:1–46.
- S.L. Byrne, B.A. Shurdut, and D.G. Saunders. (1998). Potential chlorpyrifos exposure to residents following standard crack and crevice treatment.. Environ. Health Perspect. 106:725–731.
- S. Callaway, D.R. Davies, and J.P. Rutland. (1951). Blood cholinesterase levels and range of personal variation in a healthy adult population.. Br. Med. J. 2:812–816.
- C.G. Campbell, F.J. Seidler, and T.A. Slotkin. (1997). Chlorpyrifos interferes with cell development in rat brain regions.. Brain Res. Bull. 43:179–189.
- E.J. Carlton, H.L. Moats, M. Feinberg, P. Shepard, R. Garfinkel, R. Whyatt, and D. Evans. (2004). Pesticide sales in low-income, minority neighborhoods.. J. Commun. Health 29:231–244.
- R.L. Carr, H.W. Chambers, J.A. Guarisco, J.R. Richardson, J. Tang, and J.E. Chambers. (2001). Effects of repeated oral postnatal exposure to chlorpyrifos on open-field behavior in juvenile rats.. Toxicol. Sci. 59:260–267.
- J.E. Casida, and G.B. Quistad. (2004). Organophosphate toxicology: Safety aspects of nonacetylcholinesterase secondary targets.. Chem. Res. Toxicol. 17:983–998.
- J.E. Casida, and G.B. Quistad. (2005). Serine hydrolase targets of organophosphorus toxicants.. Chem. Biol. Interact. 157–158:277–283.
- R. Castorina, A. Bradman, T.E. McKone, D.B. Barr, M.E. Harnly, and B. Eskenazi. (2003). Cumulative organophosphate pesticide exposure and risk assessment among pregnant women living in an agricultural community: A case study from the CHAMACOS cohort.. Environ. Health Perspect. 111:1640–1648.
- A. Caughlan, K. Newhouse, U. Namgung, and Z. Xia. (2004). Chlorpyrifos induces apoptosis in rat cortical neurons that is regulated by a balance between p38 and ERK/JNK MAP kinases.. Toxicol. Sci. 78:125–134.
- J.B. Cavanagh. (1963). Organo-phosphorus neurotoxicity: A model “dying back” process comparable to certain human neurological disorders.. Guys Hosp. Rep. 112:303–319.
- T.K. Chakraborti, J.D. Farrar, and C.N. Pope. (1993). Comparative neurochemical and neurobehavioral effects of repeated chlorpyrifos exposures in young and adult rats.. Pharmacol. Biochem. Behav. 46:219–224.
- H.W. Chambers. Organophosphorus compounds; An overview, Organophosphates: Chemistry, Fate and Effects J. Chambers, and P. Levi. Academic Press, New York, (1992)11–12.
- J.E. Chambers, and H.W. Chambers. (1989). Oxidative desulfuration of chlorpyrifos, chlorpyrifos-methyl, and leptophos by rat brain and liver.. J. Biochem. Toxicol. 4:201–203.
- S.M. Chanda, P. Harp, J. Liu, and C.N. Pope. (1995). Comparative developmental and maternal neurotoxicity following acute gestational exposure to chlorpyrifos in rats.. J. Toxicol. Environ. Health 44:189–202.
- S.M. Chanda, S.R. Mortensen, V.C. Moser, and S. Padilla. (1997). Tissue-specific effects of chlorpyrifos on carboxylesterase and cholinesterase activity in adult rats: An in vitro and in vivo comparison.. Fundam. Appl. Toxicol. 38:148–157.
- A. Chatonnet, and O. Lockridge. (1989). Comparison of butyrylcholinesterase and acetylcholinesterase.. Biochem. J. 260:625–634.
- J. Chaudhuri, T.K. Chakraborti, S. Chanda, and C.N. Pope. (1993). Differential modulation of organophosphate-sensitive muscarinic receptors in rat brain by parathion and chlorpyrifos.. J. Biochem. Toxicol. 8:207–216.
- J. Chen, M. Kumar, W. Chan, G. Berkowitz, and J.G. Wetmur. (2003a). Increased influence of genetic variation on PON1 activity in neonates.. Environ. Health Perspect. 111:1403–1409.
- Q. Chen, S.E. Reis, C.M. Kammerer, D.M. McNamara, R. Holubkov, B.L. Sharaf, G. Sopko, D.F. Pauly, C.N. Merz, and M.I. Kamboh. (2003b). Association between the severity of angiographic coronary artery disease and paraoxonase gene polymorphisms in the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) study.. Am. J. Hum. Genet. 72:13–22.
- W.L. Chen, J.J. Sheets, R.J. Nolan, and J.L. Mattsson. (1999). Human red blood cell acetylcholinesterase inhibition as the appropriate and conservative surrogate endpoint for establishing chlorpyrifos reference dose.. Regul. Toxicol. Pharmacol. 29:15–22.
- N. Cherry, M. Mackness, P. Durrington, A. Povey, M. Dippnall, T. Smith, and B. Mackness. (2002). Paraoxonase (PON1) polymorphisms in farmers attributing ill health to sheep dip.. Lancet 359:763–764.
- K.P. Chiang, S. Niessen, A. Saghatelian, and B.F. Cravatt. (2006). An enzyme that regulates ether lipid signaling pathways in cancer annotated by multidimensional profiling. Chem. Biol 13:1041–1050.
- S. Chiappa, S. Padilla, C. Koenigsberger, V. Moser, and S. Brimijoin. (1995). Slow accumulation of acetylcholinesterase in rat brain during enzyme inhibition by repeated dosing with chlorpyrifos.. Biochem. Pharmacol. 49:955–963.
- K. Choi, H. Joo, R.L. Rose, and E. Hodgson. (2006). Metabolism of chlorpyrifos and chlorpyrifos oxon by human hepatocytes.. J. Biochem. Mol. Toxicol. 20:279–291.
- F.S. Cieszlak. Chlorpyrifos oxon: Acute oral toxicity in Fischer 344 rats, DECO HET K-045925-004 (C006) GLP. , , (1999) Unpublished..
- C.A. Clayton, E.D. Pellizzari, R.W. Whitmore, J.J. Quackenboss, J. Adgate, and K. Sefton. (2003). Distributions, associations, and partial aggregate exposure of pesticides and polynuclear aromatic hydrocarbons in the Minnesota Children's Pesticide Exposure Study (MNCPES).. J. Expos. Anal. Environ. Epidemiol. 13:100–111.
- D.J. Clegg, and M. Van Gemert. (1999). Determination of the reference dose for chlorpyrifos: Proceedings of an expert panel.. J. Toxicol. Environ. Health B 2:211–255.
- R.C. Cochran. (2002). Appraisal of risks from nonoccupational exposure to chlorpyrifos.. Regul. Toxicol. Pharmacol. 35:105–121.
- J. Cohn, and R.C. MacPhail. (1997). Chlorpyrifos produces selective learning deficits in rats working under a schedule of repeated acquisition and performance.. J. Pharmacol. Exp. Ther 283:312–320.
- T.B. Cole, R.L. Jampsa, B.J. Walter, T.L. Arndt, R.J. Richter, D.M. Shih, A. Tward, A.J. Lusis, R.M. Jack, L.G. Costa, and C.E. Furlong. (2003a). Expression of human paraoxonase (PON1) during development.. Pharmacogenetics 13:357–364.
- T.B. Cole, C. Pettan-Brewer, T.M. BurbAChEr, L.G. Costa, and C.E. Furlong. (2003b). Paraoxonase (PON1) polymorphisms and their significance for the developmental toxicity of organophosphates.. Neurotoxicol. Teratol. 25:390.
- T.B. Cole, B.J. Walter, L.G. Costa, R.J. Richter, C. Pettan-Brewer, D.M. Shih, A. Tward, A.J. Lusis, and C.E. Furlong. (2003c). Contribution of paraoxonase (PON1) levels and Q192R genotype to organophosphate detoxication: Evidence from humans and “humanized” transgenic mice.. Toxicol. Sci. 72:100.
- T.B. Cole, B.J. Walter, D.M. Shih, A.D. Tward, A.J. Lusis, C. Timchalk, R.J. Richter, L.G. Costa, and C.E. Furlong. (2005). Toxicity of chlorpyrifos and chlorpyrifos oxon in a transgenic mouse model of the human paraoxonase (PON1) Q192R polymorphism.. Pharmacogenet. Genom. 15:589–598.
- M.F. Cometa, F.M. Buratti, S. Fortuna, P. Lorenzini, M.T. Volpe, L. Parisi, E. Testai, and A. Meneguz. (2007). Cholinesterase inhibition and alterations of hepatic metabolism by oral acute and repeated chlorpyrifos administration to mice.. Toxicology 234:90–102.
- T.J. Cook, and S.S. Shenoy. (2003). Intestinal permeability of chlorpyrifos using the single-pass intestinal perfusion method in the rat.. Toxicology 184:125–133.
- R.A. Corley, L.L. Calhoun, D.A. Dittenber, L.G. Lomax, and T.D. Landry. (1989). Chlorpyrifos: A 13-week nose-only vapor inhalation study in Fischer 344 rats.. Fundam. Appl. Toxicol. 13:616–618.
- L.G. Costa, B.E. McDonald, S.D. Murphy, G.S. Omenn, R.J. Richter, A.G. Motulsky, and C.E. Furlong. (1990). Serum paraoxonase and its influence on paraoxon and chlorpyrifos-oxon toxicity in rats.. Toxicol. Appl. Pharmacol. 103:66–76.
- L.G. Costa, R.J. Richter, W.F. Li, T. Cole, M. Guizzetti, and C.E. Furlong. (2003). Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity.. Biomarkers 8:1–12.
- L.G. Costa, A. Vitalone, T.B. Cole, and C.E. Furlong. (2005). Modulation of paraoxonase (PON1) activity.. Biochem. Pharmacol. 69:541–550.
- F. Coulston, L. Golberg, and T. Griffinn. Safety evaluation of Dowco 179 in human volunteers. , , (1972) Unpublished report from the Institute of Experimental Pathology and Toxicology, Albany Medical College..
- F. Coulston, L. Goldberg, R. Abraham, K.F. Benitz, T.B. Griffin, and M. Norvell. Final report on safety evalulation and metabolic studies on Dowco 179.. Albany Medical College, AlbanyN.Y., (1971).
- J. Cowan, C.M. Sinton, A.W. Varley, F.H. Wians, R.W. Haley, and R.S. Munford. (2001). Gene therapy to prevent organophosphate intoxication.. Toxicol. Appl. Pharmacol. 173:1–6.
- J.A. Crow, A. Borazjani, P.M. Potter, and M.K. Ross. (2007). Hydrolysis of pyrethroids by human and rat tissues: Examination of intestinal, liver and serum carboxylesterases.. Toxicol. Appl. Pharmacol. 221:1–12.
- T.L. Crumpton, F.J. Seidler, and T.A. Slotkin. (2000a). Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: Effects on nuclear transcription factors involved in cell replication and differentiation.. Brain Res. 857:87–98.
- T.L. Crumpton, F.J. Seidler, and T.A. Slotkin. (2000b). Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos?. Brain Res. Dev. Brain Res. 121:189–195.
- Y. Cui, J. Guo, B. Xu, and Z. Chen. (2006). Potential of chlorpyrifos and cypermethrin forming DNA adducts.. Mutat. Res. 604:36–41.
- B.D. Curwin, M.J. Hein, W.T. Sanderson, D.B. Barr, D. Heederik, S.J. Reynolds, E.M. Ward, and M.C. Alavanja. (2005a). Urinary and hand wipe pesticide levels among farmers and nonfarmers in Iowa.. J. Expos. Anal. Environ. Epidemiol. 15:500–508.
- B.D. Curwin, M.J. Hein, W.T. Sanderson, M.G. Nishioka, S.J. Reynolds, E.M. Ward, and M.C. Alavanja. (2005b). Pesticide contamination inside farm and nonfarm homes.. J. Occup. Environ. Hyg. 2:357–367.
- B.D. Curwin, M.J. Hein, W.T. Sanderson, C. Striley, D. Heederik, H. Kromhout, S.J. Reynolds, and M.C. Alavanja. (2007a). Pesticide dose estimates for children of Iowa farmers and non-farmers.. Environ. Res. 105:307–315.
- B.D. Curwin, M.J. Hein, W.T. Sanderson, C. Striley, D. Heederik, H. Kromhout, S.J. Reynolds, and M.C. Alavanja. (2007b). Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa.. Ann. Occup. Hyg. 51:53–65.
- B. Curwin, W. Sanderson, S. Reynolds, M. Hein, and M. Alavanja. (2002). Pesticide use and practices in an Iowa farm family pesticide exposure study.. J. Agric. Safety Health 8:423–433.
- M. Cygler, J.D. Schrag, J.L. Sussman, M. Harel, I. Silman, M.K. Gentry, and B.P. Doctor. (1993). Relationship between sequence conservation and three-dimensional structure in a large family of esterases, lipases, and related proteins.. Protein Sci. 2:366–382.
- D. Dai, J. Tang, R.L. Rose, E. Hodgson, R.J. Bienstock, H.W. Mohrenwasser, and J.A. Goldstein. (2001). Identification of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos.. J. Pharmacol. Exp. Ther. 299:825–831.
- K. Dam, S.J. Garcia, F.J. Seidler, and T.A. Slotkin. (1999). Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways.. Brain Res. Dev. Brain Res. 116:9–20.
- K. Dam, F.J. Seidler, and T.A. Slotkin. (1998). Developmental neurotoxicity of chlorpyrifos: Delayed targeting of DNA synthesis after repeated administration.. Brain Res. Dev. Brain Res. 108:39–45.
- K. Dam, F.J. Seidler, and T.A. Slotkin. (2000). Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity.. Brain Res. Dev. Brain Res. 121:179–187.
- K. Dam, F.J. Seidler, and T.A. Slotkin. (2003). Transcriptional biomarkers distinguish between vulnerable periods for developmental neurotoxicity of chlorpyrifos: Implications for toxicogenomics.. Brain Res. Bull. 59:261–265.
- S. Darvesh, D.A. Hopkins, and C. Geula. (2003). Neurobiology of butyrylcholinesterase.. Nat. Rev. Neurosci 4:131–138.
- K.P. Das, and S. BaroneJr.. (1999). Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: Is acetylcholinesterase inhibition the site of action?. Toxicol. Appl. Pharmacol. 160:217–230.
- H.G. Davies, R.J. Richter, M. Keifer, C.A. Broomfield, J. Sowalla, and C.E. Furlong. (1996). The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin.. Nature Genet. 14:334–336.
- M.M. Deacon, J.S. Murray, M.K. Pilny, K.S. Rao, D.A. Dittenber, J.R. Hanley, and J.A. John. (1980). Embryotoxicity and fetotoxicity of orally administered chlorpyrifos in mice.. Toxicol. Appl. Pharmacol. 54:31–40.
- W. Dekant. (2001). Chemical-induced nephrotoxicity mediated by glutathione S-conjugate formation.. Toxicol. Lett. 124:21–36.
- F. DeMatteis. (1974). Covalent binding of sulphur to microsomes and loss of cytochrome P450 during oxidative desulphuration of several chemicals.. Mol. Pharmacol 10:849–854.
- H.J. de Silva, P.S. Sanmuganathan, and N. Senanayake. (1994). Isolated bilateral recurrent laryngeal nerve paralysis: A delayed complication of organophosphorous poisoning.. Hum. Exp. Toxicol 13:171–173.
- R.B. Dick, K. Steenland, E.F. Krieg, and C.J. Hines. (2001). Evaluation of acute sensory-motor effects and test sensitivity using termiticide workers exposed to chlorpyrifos.. Neurotoxicol. Teratol. 23:381–393.
- Dow. Acute inhalation toxicity study in rats exposed to pyrenone Dursban aqueous base. Cosmopolitan Safety Evaluation, Inc., LafayetteNJ, (1983a).
- Dow. Dursban insecticide: Assessment of neonatal survival in a two generation reproduction study in rats. Dow Chemical USA, Lake Jackson Research Center, FreeportTX, (1983b).
- Dow. Acute inhalation study in rats exposed to non-volatile portion of pyrenone-Dursban W.B. pressurized spray. Cosmopolitan Safety Evaluation, Inc., LafayetteNJ, (1984).
- Dow. Cholrpyrifos: 13-week neurotoxicity study in Fisher 344 rats. Dow Chemical Co., Health and Environmental Sciences, MidlandMI, (1993).
- Dow. Toxicology studies on metabolites; Chapter 5.8.1, Toxicological and metabolism studies on the active substance. Dow Agrosciences, MidlandMI, (2002).
- M.D. DulaneyJr., B. Hoskins, and I.K. Ho. (1985). Studies on low dose subacute administration of soman, sarin and tabun in the rat.. Acta Pharmacol. Toxicol. (Copenh.) 57:234–241.
- P. Duramad, I.B. Tager, J. Leikauf, B. Eskenazi, and N.T. Holland. (2006). Expression of Th1/Th2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and allergen Der p1.. J. Appl. Toxicol. 26:458–465.
- F. Dutheil, P. Beaune, and M.A. Loriot. (2008). Xenobiotic metabolizing enzymes in the central nervous system: Contribution of cytochrome P450 enzymes in normal and pathological human brain. Biochimie 90: 42636. Epub 2007 Oct 22.
- E.G. Duysen, B. Li, S. Darvesh, and O. Lockridge. (2007). Sensitivity of butyrylcholinesterase knockout mice to (–)-huperzine A and donepezil suggests humans with butyrylcholinesterase deficiency may not tolerate these Alzheimer's disease drugs and indicates butyrylcholinesterase function in neurotransmission.. Toxicology 233:60–69.
- J.A. Dybowski, S.R. Mortensen, and J.L. Mattsson. (2001). Comparison of chlorpyrifos (CPF) concentrations for in vitro and in vivo effects.. Toxicologist 60:325. (abstr. 1546)
- S.M. Dyer, M. Cattani, D.L. Pisaniello, F.M. Williams, and J.W. Edwards. (2001). Peripheral cholinesterase inhibition by occupational chlorpyrifos exposure in Australian termiticide applicators.. Toxicology 169:177–185.
- D.L. Eaton. (2000). Biotransformation enzyme polymorphism and pesticide susceptibility.. Neurotoxicology 21:101–111.
- D.J. Ecobichon, and D.S. Stephens. (1973). Perinatal development of human blood esterases.. Clin. Pharmacol. Ther. 14:41–47.
- P.P. Egeghy, J.J. Quackenboss, S. Catlin, and P.B. Ryan. (2005). Determinants of temporal variability in NHEXAS-Maryland environmental concentrations, exposures, and biomarkers.. J. Expos. Anal. Environ. Epidemiol. 15:388–397.
- A.H. El-Sebae, N.S. Ahmed, and S.A. Soliman. (1978). Effect of pre-exposure on acute toxicity of organophosphorus insecticides to white mice.. J. Environ. Sci. Health B 13:11–24.
- S.M. Engel, G.S. Berkowitz, D.B. Barr, S.L. Teitelbaum, J. Siskind, S.J. Meisel, J.G. Wetmur, and M.S. Wolff. (2007). Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort.. Am.J. Epidemiol. 165:1397–1404.
- L.J. England, J.S. Kendrick, P.M. Gargiullo, S.C. Zahniser, and W.H. Hannon. (2001). Measures of maternal tobacco exposure and infant birth weight at term.. Am.J. Epidemiol. 153:954–960.
- EPA. Report on FQPA Tolerance Reassessment Progress and Risk Management Decision for Chlorpyrifos Methyl. U.S. EPA, WashingtonDC, (2001).
- EPA. Interim Reregistration Eligibility Decision for Chlorpyrifos. U.S. EPA, WashingtonDC, (2002).
- B. Eskenazi, A. Bradman, and R. Castorina. (1999). Exposures of children to organophosphate pesticides and their potential adverse health effects.. Environ. Health Perspect. 107 (Suppl 3):409–419.
- B. Eskenazi, K. Harley, A. Bradman, E. Weltzien, N.P. Jewell, D.B. Barr, C.E. Furlong, and N.T. Holland. (2004). Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population.. Environ. Health Perspect. 112:1116–1124.
- B. Eskenazi, A.R. Marks, A. Bradman, K. Harley, D.B. Barr, C. Johnson, N. Morga, and N.P. Jewell. (2007). Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children.. Environ. Health Perspect. 115:792–798.
- B. Eskenazi, A.W. Prehn, and R.E. Christianson. (1995). Passive and active maternal smoking as measured by serum cotinine: The effect on birthweight.. Am. J. Public Health 85:395–398.
- R.W. Everett. (1982). Effect of Dursban 44 on semen output of Holstein bulls.. J. Dairy Sci. 65:1781–1794.
- U. Fagerholm, M. Johansson, and H. Lennernas. (1996). Comparison between permeability coefficients in rat and human jejunum.. Pharm. Res. 13:1336–1342.
- A.T. Farag, A.M. El Okazy, and A.F. El-Aswed. (2003). Developmental toxicity study of chlorpyrifos in rats.. Reprod. Toxicol. 17:203–208.
- FDA. Food and Drug Administration Pesticide Program Residue Monitoring 2003. U.S. Food and Drug Administration, WashingtonDC, (2005).
- R.A. Fenske, K.G. Black, K.P. Elkner, C.L. Lee, M.M. Methner, and R. Soto. (1990). Potential exposure and health risks of infants following indoor residential pesticide applications.. Am J. Public Health 80:689–693.
- R.A. Fenske, G. Kedan, C. Lu, J.A. Fisker-Andersen, and C.L. Curl. (2002a). Assessment of organophosphorous pesticide exposures in the diets of preschool children in Washington State.. J. Expos. Anal. Environ. Epidemiol. 12:21–28.
- R.A. Fenske, C. Lu, D. Barr, and L. Needham. (2002b). Children's exposure to chlorpyrifos and parathion in an agricultural community in central Washington State.. Environ. Health Perspect. 110:549–553.
- N.C.G. Freeman, M. Jimenez, K.J. Reed, S. Gurunathan, R.D. Edwards, A. Roy, J.L. Adgate, E.D. Pellizzari, J. Quackenboss, K. Sexton, and P.J. Lioy. (2001). Quantitative analysis of children's microactivity patterns: The Minnesota Children's Pesticide Exposure Study. J. Expos. Anal. Environ. Epidemiol. 11:501–509.
- K. Fujioka, and J.E. Casida. (2007). Glutathione S-transferase conjugation of organophosphorus pesticides yields S-phospho-, S-aryl-, and S-alkylglutathione derivatives.. Chem. Res. Toxicol. 20:1211–1217.
- C.E. Furlong. (2007). Genetic variability in the cytochrome P450-paraoxonase (PON1) pathways for the detoxication of organophosphorus compounds.. J. Biochem. Mol. Toxicol. 21:197–205.
- C.E. Furlong, N. Holland, R.J. Richter, A. Bradman, A. Ho, and B. Eskenazi. (2006). PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity.. Pharmacogenet. Genom. 16:183–190.
- C.E. Furlong, R.J. Richter, S.L. Seidel, and A.G. Motulsky. (1988). Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon.. Am. J. Hum. Genet. 43:230–238.
- T.B. Gaines. (1969). Acute toxicity of pesticides.. Toxicol. Appl. Pharmacol 14 (3):515–534.
- M.A. Gallo, and N.J. Lawryk. Organic phosphorous pesticides, Handook of Pesticide Toxicology, Vol 2, Classes of PesticidesW.J. Hayes, and E.R. LawsJr.. Academic Press, New York, (1991)917–1123.
- K.N. Gan, A. Smolen, H.W. Eckerson, and B.N. La Du. (1991). Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities.. Drug Metab. Dispos. 19:100–106.
- S.J. Garcia, F.J. Seidler, and T.A. Slotkin. (2003). Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: Effects on neurospecific proteins indicate changing vulnerabilities.. Environ. Health Perspect. 111:297–303.
- S.J. Garcia, F.J. Seidler, T.L. Crumpton, and T.A. Slotkin. (2001). Does chlorpyrifos target glial cell development?. Neurotoxicology (Little Rock) 22:526.
- S.J. Garcia, F.J. Seidler, D. Qiao, and T.A. Slotkin. (2002). Chlorpyrifos targets developing glia: Effects on glial fibrillary acidic protein.. Brain Res. Dev. Brain Res. 133:151–161.
- N.E. Garrett, H.F. Stack, M.A. Jackson, and M.D. Waters. Genotoxic and Carcinogenic Potential of Anticholinesterases, Clinical and Experimental Toxicology of Organophosphates and CarbamatesB. Ballantyne, and T.C. Marrs. , , (1992).
- D.A. Gearhart, D.W. Sickles, J.J. Buccafusco, M.A. Prendergast, and A.V. TerryJr.. (2007). Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility.. Toxicol. Appl. Pharmacol. 218:20–29.
- L.A. Geer, N. Cardello, M.J. Dellarco, T.J. Leighton, R.P. Zendzian, J.D. Roberts, and T.J. Buckley. (2004). Comparative analysis of passive dosimetry and biomonitoring for assessing chlorpyrifos exposure in pesticide workers.. Ann. Occup. Hyg. 48:683–695.
- C. Geula, and N. Nagykery. (2007). Butyrylcholinesterase activity in the rat forebrain and upper brainstem: Postnatal development and adult distribution.. Exp. Neurol. 204:640–657.
- E. Giacobini, R. Spiegel, A. Enz, A.E. Veroff, and N.R. Cutler. (2002). Inhibition of acety-and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer's disease by rivastigmine: Correlation with cognitive benefit.. J. Neural Trans. 109:1053–65.
- E. Giacobini. (2001). Selective inhibitors of butyrylcholinesterase: A valid alternative for therapy of Alzheimer's disease?. Drugs Aging 18:891–898.
- J.E. Gibson, R.K. Peterson, and B.A. Shurdut. (1998). Human exposure and risk from indoor use of chlorpyrifos.. Environ. Health Perspect. 106:303–306.
- J.E. Gibson. (1999). Chlorpyrifos (Dursban) and Dow employees: Response.. Environ. Health Perspect. 107:A134.
- G. Giordano, Z. Afsharinejad, M. Guizzetti, A. Vitalone, T.J. Kavanagh, and L.G. Costa. (2007). Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency.. Toxicol. Appl. Pharmacol. 219:181–189.
- B.B. Gollapudi, A.L. Mendrala, and V.A. Linscombe. (1995). Evaluation of the genetic toxicity of the organophosphate insecticide chlorpyrifos.. Mutat. Res. 342:25–36.
- S.M. Gordon, P.J. Callahan, M.G. Nishioka, M.C. Brinkman, M.K. O'Rourke, M.D. Lebowitz, and D.J. Moschandreas. (1999). Residential environmental measurements in the national human exposure assessment survey (NHEXAS) pilot study in Arizona: Preliminary results for pesticides and VOCs.. J. Expos. Anal. Environ. Epidemiol. 9:456–470.
- P. Grandjean, R. Harari, D.B. Barr, and F. Debes. (2006). Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children.. Pediatrics 117:e546–556.
- P. Griffin, H. Mason, K. Heywood, and J. Cocker. (1999). Oral and dermal absorption of chlorpyrifos: A human volunteer study.. J. Occup. Environ. Med. 56:10–13.
- P. Griffin, M. Payne, H. Mason, E. Freedlander, A.D. Curran, and J. Cocker. (2000). The in vitro percutaneous penetration of chlorpyrifos.. Hum. Exp. Toxicol. 19:104–107.
- M. Guizzetti, S. Pathak, G. Giordano, and L.G. Costa. (2005). Effect of organophosphorus insecticides and their metabolites on astroglial cell proliferation.. Toxicology 215:182–190.
- F. Gultekin, I. Karakoyun, R. Sutcu, E. Savik, G. Cesur, H. Orhan, and N. Delibas. (2007). Chlorpyrifos increases the levels of hippocampal NMDA receptor subunits NR2A and NR2B in juvenile and adult rats.. Int. J. Neurosci. 117:47–62.
- F. Gultekin, M. Ozturk, and M. Akdogan. (2000). The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro).. Arch. Toxicol. 74:533–538.
- E.L. Gunderson. (1995a). Dietary intake of pesticides, selected elements, and other chemicals: FDA total diet study, June 1984–April 1986.. J. AOAC International 78:910–921.
- E.L. Gunderson. (1995b). FDA total diet study, July 1986-April 1991, dietary intakes of pesticides, selected elements, and other chemicals.. J. AOAC Int. 78:1353–1363.
- S.X. Guo-Ross, J.E. Chambers, E.C. Meek, and R.L. Carr. (2007). Altered muscarinic acetylcholine receptor subtype binding in neonatal rat brain following exposure to chlorpyrifos or methyl parathion.. Toxicol. Sci. 100:118–127.
- S. Gurunathan, M. Robson, N. Freeman, B. Buckley, A. Roy, R. Meyer, J. Bukowski, and P.J. Lioy. (1998). Accumulation of chlorpyrifos on residential surfaces and toys accessible to children.. Environ. Health Perspect. 106:9–16.
- P.J. Haas, W.B. Buck, J.E. Hixon, R.D. Shanks, W.C. Wagner, P.G. Weston, and H.L. Whitmore. (1983). Effect of chlorpyrifos on Holstein steers and testosterone-treated Holstein bulls.. Am. J. Vet. Res. 44:879–881.
- J.E. Haddow, G.J. Knight, G.E. Palomaki, and P.K. Haddow. (1988). Estimating fetal morbidity and mortality resulting from cigarette smoke exposure by measuring cotinine levels in maternal serum.. Prog. Clin. Biol. Res. 281:289–300.
- R.W. Haley, S. Billecke, and B.N. La Du. (1999). Association of low PON1 type Q (type A) arylesterase activity with neurologic symptom complexes in Gulf War veterans.. Toxicol. Appl. Pharmacol. 157:227–233.
- J. Halpert, D. Hammond, and R.A. Neal. (1980). Inactivation of purified rat liver cytochrome P-450 during the metabolism of parathion (diethyl p-nitrophenyl phosphorothionate).. J. Biol. Chem. 255:1080–1089.
- T.R. HanleyJr., E.W. Carney, and E.M. Johnson. (1998). 3,5,6-Trichloro-2-pyridinol: Developmental toxicity studies in rats and rabbits.. Toxicologist 42:255.
- T.R. HanleyJr., E.W. Carney, and E.M. Johnson. (2000). Developmental toxicity studies in rats and rabbits with 3,5,6-trichloro-2-pyridinol, the major metabolite of chlorpyrifos.. Toxicol. Sci. 53:100–108.
- R.D. Harbison. (1975). Prenatal development of human blood esterases.. Clin. Pharmacol. Ther. 14:41–47.
- M. Harnly, R. McLaughlin, A. Bradman, M. Anderson, and R. Gunier. (2005). Correlating agricultural use of organophosphates with outdoor air concentrations: A particular concern for children.. Environ. Health Perspect. 113:1184–1189.
- M. Harnly, R. McLaughlin, R. Gunier, A. Bradman, and M. Anderson. (2006). Organophosphates and outdoor air: Harnly et al. respond [4].. Environ. Health Perspect. 114:A339–A340.
- S.L. Hartgraves, and M.R. Murphy. Behavioral effects of low-dose nerve agents, Chemical Warfare AgentsS.M. Somani. Academic Press, San Diego, (1992)125.
- Y. Hashim, D. Shepherd, S. Wiltshire, R.R. Holman, J.C. Levy, A. Clark, and C.A. Cull. (2001). Butyrylcholinesterase K variant on chromosome 3 q is associated with Type II diabetes in white Caucasian subjects.. Diabetologia 44:2227–2230. Erratum in: Diabetologia 2002;45(3):459
- C. Hassett, R.J. Richter, R. Humbert, C. Chapline, J.W. Crabb, C.J. Omiecinski, and C.E. Furlong. (1991). Characterization of cDNA clones encoding rabbit and human serum paraoxonase: The mature protein retains its signal sequence.. Biochemistry 30:10141–10149.
- M. Hayes. Pesticides Studies in Man. Williams & Wilkins, BaltimoreMD, (1982).
- E Hedlund, J.A. Gustafsson, and M. Warner. (2001). Cytochrome P450 in the brain; A review.. Curr. Drug Metab 2:245–263.
- R.H.J. Hill, S.L. Head, S. Baker, M. Gregg, D.B. Shealy, S.L. Bailey, C.C. Williams, E.J. Sampson, and L.L. Needham. (1995). Pesticide residues in urine of adults living in the United States: Reference range concentrations.. Environ. Res. 71:99–108.
- P.C. Hirom, P. Millburn, R.L. Smith, and R.T. Williams. (1972). Species variations in the threshold molecular-weight factor for the biliary excretion of organic anions.. Biochem. J. 129:1071–1077.
- M.J. Hodgson, G.D. Block, and D.K. Parkinson. (1986). Organophosphate poisoning in office workers.. J. Occup. Med. 28:434–437.
- N. Holland, C. Furlong, M. Bastaki, R. Richter, A. Bradman, K. Huen, K. Beckman, and B. Eskenazi. (2006). Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns.. Environ. Health Perspect. 114:985–991.
- C. Holmes, C. Ballard, D. Lehmann, A. David Smith, H. Beaumont, I.N. Day, M. Nadeem Khan, S. Lovestone, M. McCulley, C.M. Morris, D.G. Munoz, K. O'Brien, C. Russ, T. Del Ser, and D. Warden. (2005). Rate of progression of cognitive decline in Alzheimer's disease: effect of butyrylcholinesterase K gene variation.. J. Neurol. Neurosurg Psychiatry 76:640–643.
- B. Holmstedt. (1959). Pharmacology of organophosphorus cholinesterase inhibitors.. Pharmacol. Rev. 11:567–688.
- S.B. Hooser, V.R. Beasley, J.P. Sundberg, and K. Harlin. (1988). Toxicologic evaluation of chlorpyrifos in cats.. Am. J. Vet. Res. 49:1371–1375.
- P. Hore, M. Robson, N. Freeman, J. Zhang, D. Wartenberg, H. Ozkaynak, N. Tulve, L. Sheldon, L. Needham, D. Barr, and P.J. Lioy. (2005). Chlorpyrifos accumulation patterns for child-accessible surfaces and objects and urinary metabolite excretion by children for 2 weeks after crack-and-crevice application.. Environ. Health Perspect. 113:211–219.
- P. Hore, V. Zartarian, J. Xue, H. Ozkaynak, S.-W. Wang, Y.-C. Yang, P.-L. Chu, L. Sheldon, M. Robson, L. Needham, D. Barr, N. Freeman, P. Georgopoulos, and P.J. Lioy. (2006). Children's residential exposure to chlorpyrifos: Application of CPPAES field measurements of chlorpyrifos and TCPy within MENTOR/SHEDS-pesticides model.. Sci. Total Environ. 366:525–537.
- B. Hoskins, and I.K. Ho. Tolerance of organophosphate inhibitors, Organophosphates: Chemistry, Fate and EffectsJ.E. Chambers, and P.E. Lei. Academic Press, New York, (1992)285.
- T.-C. Hour, L. Chen, and J.-K. Lin. (1998). Comparative investigation on the mutagenicities of organophosphate, phthalimide, pyrethroid and carbamate insecticides by the Ames and lactam tests.. Mutagenesis 13:157166.
- A.S. Howard, R. Bucelli, D.A. Jett, D. Bruun, D. Yang, and P.J. Lein. (2005). Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures.. Toxicol. Appl. Pharmacol. 207:112–124.
- M.D. Howard, and C.N. Pope. (2002). In vitro effects of chlorpyrifos, parathion, methyl parathion and their oxons on cardiac muscarinic receptor binding in neonatal and adult rats.. Toxicology 170:1–10.
- R.A. Huff, J.J. Corcoran, J.K. Anderson, and M.B. Abou-Donia. (1994). Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum.. J. Pharmacol. Exp. Ther. 269:329335.
- R. Humbert, D.A. Adler, C.M. Disteche, C. Hassett, C.J. Omiecinski, and C.E. Furlong. (1993). The molecular basis of the human serum paraoxonase activity polymorphism.. Nat. Genet. 3:73–76.
- D.L. Hunter, T.L. Lassiter, and S. Padilla. (1999). Gestational exposure to chlorpyrifos: Comparative distribution of trichloropyridinol in the fetus and dam.. Toxicol. Appl. Pharmacol. 158:16–23.
- L.M. Icenogle, N.C. Christopher, W.P. Blackwelder, D.P. Caldwell, D. Qiao, F.J. Seidler, T.A. Slotkin, and E.D. Levin. (2004). Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation.. Neurotoxicol. Teratol. 26:95–101.
- N. Ito, A. Hagiwara, S. Tamano, M. Futacuchi, K. Imaida, and T. Shirai. (1996). Effects of pesticide mixtures at the acceptable daily intake levels on rat carcinogenesis.. Food Chem. Toxicol. 34:1091–1096.
- T. Iwasaki, M. Yoneda, A. Nakajima, and Y. Terauchi. (2007). Serum butyrylcholinesterase is strongly associated with adiposity, the serum lipid profile and insulin resistance.. Intern. Med 46:1633–1639.
- R.R. Jameson, F.J. Seidler, and T.A. Slotkin. (2007). Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: Chlorpyrifos, chlorpyrifos oxon, and diazinon.. Environ. Health Perspect. 115:65–70.
- R.R. Jameson, F.J. Seidler, D. Qiao, and T.A. Slotkin. (2006). Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: Critical stages targeting differentiation in PC12 cells.. Environ. Health Perspect. 114:667–672.
- D.A. Jett, R.V. Navoa, R.A. Beckles, and G.L. McLemore. (2001). Cognitive function and cholinergic neurochemistry in weanling rats exposed to chlorpyrifos.. Toxicol. Appl. Pharmacol. 174:89–98.
- A. Johansen, E.M. Nielsen, G. Andersen, Y.H. Hamid, D.P. Jensen, C. Glümer, T. Drivsholm, K. Borch-Johnsen, T. Jørgensen, T. Hansen, and O. Pedersen. (2004). Large-scale studies of the functional K variant of the butyrylcholinesterase gene in relation to Type 2 diabetes and insulin secretion.. Diabetologia 47:1437–1441.
- C. Johansson, A.F. Castoldi, N. Onishchenko, L. Manzo, M. Vahter, and S. Ceccatelli. (2007). Neurobehavioural and molecular changes induced by methylmercury exposure during development.. Neurotoxicol. Res. 11:241–260.
- D.E. Johnson, F.J. Seidler, and T.A. Slotkin. (1998). Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chloropyrifos.. Brain Res. Bull. 45:143–147.
- M.K. Johnson. (1990). Organophosphates and delayed neuropathy—Is NTE alive and well?. Toxicol. Appl. Pharmacol. 102:385–399.
- M. Jokanovic. (2001). Biotransformation of organophosphorus compounds.. Toxicology 166:139–160.
- H. Joo, K. Choi, R.L. Rose, and E. Hodgson. (2007). Inhibition of fipronil and nonane metabolism in human liver microsomes and human cytochrome P450 isoforms by chlorpyrifos.. J. Biochem. Mol. Toxicol. 21:76–80.
- J.G. Kaplan, J. Kessler, N. Rosenberg, D. Pack, and H.H. Schaumburg. (1993). Sensory neuropathy associated with Dursban (chlorpyrifos) exposure.. Neurology 43:2193–2196.
- S. Karanth, and C. Pope. (2000). Carboxylesterase and A-esterase activities during maturation and aging: Relationship to the toxicity of chlorpyrifos and parathion in rats.. Toxicol. Sci. 58:282–289.
- S. Karanth, and C. Pope. (2003). Age-related effects of chlorpyrifos and parathion on acetylcholine synthesis in rat striatum.. Neurotoxicol. Teratol. 25:599–606.
- J. Kawahara, J. Yoshinaga, and Y. Yanagisawa. (2007). Dietary exposure to organophosphorus pesticides for young children in Tokyo and neighboring area.. Sci. Total Environ. 378:263–268.
- S.M. Kieszak, L.P. Naeher, C.S. Rubin, L.L. Needham, L. Backer, D. Barr, and M. McGeehin. (2002). Investigation of the relation between self-reported food consumption and household chemical exposures with urinary levels of selected nonpersistent pesticides.. J. Expos. Anal. Environ. Epidemiol. 12:404–408.
- J.C. Kisicki, C.W. Seip, and M.L. Combs. A rising dose toxicology study to determine the no-observable effect levels (NOEL) for erythrocyte acetylcholinesterase (AChE) inhibition and cholinergic signs and symptoms of chlorpyrifos at three dose levels. Dow AgroSciences LLC, , (1999).
- J.C. Kissel, C.L. Curl, G. Kedan, C. Lu, W. Griffith, D.B. Barr, L.L. Needham, and R.A. Fenske. (2005). Comparison of organophosphorus pesticide metabolite levels in single and multiple daily urine samples collected from preschool children in Washington State.. J. Expos. Anal. Environ. Epidemiol. 15:164–171.
- A.A. Kousba, T.S. Poet, and C. Timchalk. (2007). Age-related brain cholinesterase inhibition kinetics following in vitro incubation with chlorpyrifos-oxon and diazinon-oxon.. Toxicol. Sci. 95:147–155.
- R.I. Krieger, C.E. Bernard, T.M. Dinoff, L. Fell, T.G. Osimitz, J.H. Ross, and T. Ongsinthusak. (2000). Biomonitoring and whole body cotton dosimetry to estimate potential human dermal exposure to semivolatile chemicals.. J. Expos. Anal. Environ. Epidemiol. 10:50–57.
- R.I. Krieger, C.E. Bernard, T.M. Dinoff, J.H. Ross, and R.L. Williams. (2001). Biomonitoring of persons exposed to insecticides used in residences.. Ann Occup Hyg 45 (Suppl 1):S143–153.
- R. Lane, H.H. Feldman, J. Meyer, Y. He, S.H. Ferris, A. Nordberg, T. Darreh-Shori, H. Soininen, T. Pirttilä, M.R. Farlow, N. Sfikas, C. Ballard, and N.H. Greig. (2008). Synergistic effect of apolipoprotein E epsilon4 and butyrylcholinesterase K-variant on progression from mild cognitive impairment to Alzheimer's disease.. Pharmacogenet Genomics 18:289–298.
- R.M. Lane, S.G. Potkin, and A. Enz. (2006). Targeting acetylcholinesterase and butyrylcholinesterase in dementia.. Int. J. Neuropsychopharmacol 9:101–124.
- C.L. Lanning, R.L. Fine, C.W. Sachs, U.S. Rao, J.J. Corcoran, and M.B. Abou-Donia. (1996). Chlorpyrifos oxon interacts with the mammalian multidrug resistance protein, P-glycoprotein. J. Toxicol. Environ. Health 47:395–407.
- T.L. Lassiter, S. Padilla, S.M. Chanda, K. Das, N. Haykal-Coates, D. Hunter, R. Marshall, and S. BaroneJr.. (1998a). Dose response study of the effects of chlorpyrifos on various biochemical measures in the fetus and dam during late gestation.. Toxicologist 42:159.
- T.L. Lassiter, S. Padilla, S.R. Mortensen, S.M. Chanda, V.C. Moser, and S. BaroneJr.. (1998b). Gestational exposure to chlorpyrifos: Apparent protection of the fetus?. Toxicol. Appl. Pharmacol. 152:56–65.
- J. Latuszynska, S. Luty, G. Raszewski, M. Tokarska-Rodak, D. Przebirowska, E. Przylepa, and A. Haratym-Maj. (2001). Neurotoxic effect of dermally-applied chlorpyrifos and cypermethrin in Wistar rats.. Ann. Agric. Environ. Med. 8:163–170.
- S. Lee, R. McLaughlin, M. Harnly, R. Gunier, and R. Kreutzer. (2002). Community exposures to airborne agricultural pesticides in California: Ranking of inhalation risks.. Environ. Health Perspect. 110:1175–1184.
- W.J. Lee, A. Blair, J.A. Hoppin, J.H. Lubin, J.A. Rusiecki, D.P. Sandler, M. Dosemeci, and M.C. Alavanja. (2004). Cancer incidence among pesticide applicators exposed to chlorpyrifos in the Agricultural Health Study.. JNCI 96:1781–1789.
- W.J. Lee, J.S. Colt, E.F. Heineman, R. McComb, D.D. Weisenburger, W. Lijinsky, and M.H. Ward. (2005). Agricultural pesticide use and risk of glioma in Nebraska, United States.. Occup. Environ. Med. 62:786–792.
- W.J. Lee, D.P. Sandler, A. Blair, C. Samanic, A.J. Cross, and M.C. Alavanja. (2007). Pesticide use and colorectal cancer risk in the Agricultural Health Study.. Int.J. Cancer 121:339–346.
- C. Lentner. Geigy Scientific Tables. Ciba Geigy Corporation, West CaldwellNJ, (1981).
- I. Leviev, and R.W. James. (2000). Promoter polymorphisms of human paraoxonase PON1 gene and serum paraoxonase activities and concentrations.. Arterioscler. Thromb. Vasc. Biol. 20:516–521.
- E.D. Levin, N. Addy, A. Baruah, A. Elias, N.C. Christopher, F.J. Seidler, and T.A. Slotkin. (2002). Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations.. Neurotoxicol. Teratol. 24:733–741.
- R.G. Lewis, R.C. Fortmann, and D.E. Camann. (1994). Evaluation of methods for monitoring the potential exposure of small children to pesticides in the residential environment.. Arch. Environ. Contam. Toxicol. 26:37–46.
- B. Li, M. Sedlacek, I. Manoharan, R. Boopathy, E.G. Duysen, P. Masson, and O. Lockridge. (2005). Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma.. Biochem. Pharmacol. 70:1673–1684.
- B. Li, E.G. Duysen, and O. Lockridge. (2008). The butyrylcholinesterase knockout mouse is obese on a high-fat diet. Chem Biol Interact. in press
- W.F. Li, L.G. Costa, and C.E. Furlong. (1993). Serum paraoxonase status: A major factor in determining resistance to organophosphates.. J. Toxicol. Environ. Health 40:337–346.
- W.F. Li, L.G. Costa, R.J. Richter, T. Hagen, D.M. Shih, A. Tward, A.J. Lusis, and C.E. Furlong. (2000). Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds.. Pharmacogenetics 10:767–779.
- W.F. Li, C.E. Furlong, and L.G. Costa. (1995). Paraoxonase protects against chlorpyrifos toxicity in mice.. Toxicol. Lett. 76:219–226.
- W.F. Li, C. Matthews, C.M. Disteche, L.G. Costa, and C.E. Furlong. (1997). Paraoxonase (PON1) gene in mice: Sequencing, chromosomal localization and developmental expression.. Pharmacogenetics 7:137–144.
- W. Li, and J.E. Casida. (1998). Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells.. Toxicol. Lett. 98:139–146.
- Z. Li, R. Yang, and Z. Chen. (1996). [Effects of iodine and thyroid hormone deficiency during brain development on activities of cholinergic neurone-related enzymes in central nervous system of rats].. Zhonghua Yu Fang Yi Xue Za Zhi 30:337–339.
- A.D. Lieberman, M.R. Craven, H.A. Lewis, and J.H. Nemenzo. (1998). Genotoxicity from domestic use of organophosphate pesticides.. J. Occup. Environ. Med. 40:954–957.
- P.J. Lioy, R.D. Edwards, N. Freeman, S. Gurunathan, E. Pellizzari, J.L. Adgate, J. Quackenboss, and K. Sexton. (2000). House dust levels of selected insecticides and a herbicide measured by the EL and LWW samplers and comparisons to hand rinses and urine metabolites.. J. Expos. Anal. Environ. Epidemiol. 10:327–340.
- J. Liu, and C.N. Pope. (1996). Effects of chlorpyrifos on high-affinity choline uptake and [3H]hemicholinium-3 binding in rat brain.. Fundam. Appl. Toxicol. 34:84–90.
- J. Liu, T. Chakraborti, and C. Pope. (2002). In vitro effects of organophosphorus anticholinesterases on muscarinic receptor-mediated inhibition of acetylcholine release in rat striatum.. Toxicol. Appl. Pharmacol. 178:102–108.
- J. Liu, K. Olivier, and C.N. Pope. (1999). Comparative neurochemical effects of repeated methyl parathion or chlorpyrifos exposures in neonatal and adult rats.. Toxicol. Appl. Pharmacol. 158:186–196.
- Y. Loewenstein-Lichtenstein, M. Schwarz, D. Glick, B. Norgaard-Pedersen, H. Zakut, and H. Soreq. (1995). Genetic predisposition to adverse consequences of anti-cholinesterases in 'atypical' BCHE carriers.. Nat. Med. 1:1082–1085.
- G.G. Long, A.B. Scheidt, R.J. Everson, and F.W. Oehme. (1986). Age related susceptibility of newborn pigs to the cutaneous application of chlorpyrifos.. Vet. Hum. Toxicol. 28:297–299.
- M. Lotti. (1991). The pathogenesis of organophosphate delayed polyneuropathy. Crit. Rev. Toxicol 21:465–487.
- M. Lotti. Organophosphate compounds, Experimental and Clinical NeurotoxicologyP.S. Spencer, and H.S. Schaumburg. Oxford University Press, New York, (2000)897.
- M. Lotti, and A. Moretto. (2005). Organophosphate-induced delayed polyneuropathy.. Toxicol. Rev. 24:37–49.
- M. Lotti, A. Moretto, R. Zoppellari, R. Dainese, N. Rizzuto, and G. Barusco. (1986). Inhibition of lymphocytic neuropathy target esterase predicts the development of organophosphate-induced delayed polyneuropathy.. Arch. Toxicol. 59:176–179.
- C. Lu, and R.A. Fenske. (1998). Air and surface chlorpyrifos residues following residential broadcast and aerosol pesticide applications.. Environ. Sci. Technol. 32:1386–1390.
- C. Lu, and R.A. Fenske. (1999). Dermal transfer of chlorpyrifos residues from residential surfaces: Comparison of hand press, hand drag, wipe, and polyurethane foam roller measurements after broadcast and aerosol pesticide applications.. Environ. Health Perspect. 107:463–467.
- C. Lu, G. Kedan, J. Fisker-Andersen, J.C. Kissel, and R.A. Fenske. (2004). Multipathway organophosphorus pesticide exposures of preschool children living in agricultural and nonagricultural communities.. Environ. Res. 96:283–289.
- C. Lu, K. Toepel, R. Irish, R.A. Fenske, D.B. Barr, and R. Bravo. (2006). Organic diets significantly lower children's dietary exposure to organophosphorus pesticides.. Environ. Health Perspect. 114:260–263.
- C. Lu, D.B. Barr, M.A. Pearson, and L.A. Waller. (2008). Dietary intake and its contribution to longitudinal organophosphorour pesticide exposure in urban/suburban children. Environ. Health Perspect. 116:537–542.
- T. Ma, and J.E. Chambers. (1994). Kinetic parameters of desulfuration and dearylation of parathion and chlorpyrifos by rat liver microsomes.. Food Chem. Toxicol. 32:763–767.
- D.L. MacIntosh, C.W. Kabiru, and P.B. Ryan. (2001b). Longitudinal investigation of dietary exposure to selected pesticides.. Environ. Health Perspect. 109:145–150.
- D.L. MacIntosh, C. Kabiru, S.L. Echols, and P.B. Ryan. (2001a). Dietary exposure to chlorpyrifos and levels of 3,5,6-trichloro-2-pyridinol in urine.. J. Expos. Anal. Environ. Epidemiol. 11:279–285.
- B. Mackness, P.N. Durrington, and M.I. Mackness. (2000). Low paraoxonase in Persian Gulf War Veterans self-reporting Gulf War Syndrome.. Biochem. Biophys. Res. Commun. 276:729–733.
- B. Mackness, P. Durrington, A. Povey, S. Thomson, M. Dippnall, M. Mackness, T. Smith, and N. Cherry. (2003). Paraoxonase and susceptibility to organophosphorus poisoning in farmers dipping sheep.. Pharmacogenetics 13:81–88.
- D.T. Mage. (2006). Dermal absorption of chlorpyrifos.. Ann. Occup. Hyg 50:638–639.
- J.S. Mandel, B.H. Alexander, B.A. Baker, J.F. Acquavella, P. Chapman, and R. Honeycutt. (2005). Biomonitoring for farm families in the farm family exposure study.. Scand. J. Work Environ. Health 31 (Suppl 1):98–104. discussion 163–105.
- M.S. Marty, J.Y. Domoradzki, S.C. Hansen, C. Timchalk, M.J. Bartels, and J.L. Mattsson. (2007). The effect of route, vehicle, and divided doses on the pharmacokinetics of chlorpyrifos and its metabolite trichloropyridinol in neonatal Sprague-Dawley rats.. Toxicol. Sci. 100:360–373.
- I. Mateo, J. Llorca, J. Infante, E. Rodríguez-Rodríguez, J. Berciano, and O. Combarros. (2008). Gene-gene interaction between 14-3-3 zeta and butyrylcholinesterase modulates Alzheimer's disease risk.. Eur. J. Neurol 15:219–222.
- J.L. Mattsson, J.W. Wilmer, M.R. Shankar, N.M. Berdasco, J.W. Crissman, J.P. Maurissen, and D.M. Bond. (1996). Single-dose and 13-week repeated-dose neurotoxicity screening studies of chlorpyrifos insecticide.. Food Chem. Toxicol 34:393–405.
- J.L. Mattsson, J.P. Maurissen, R.J. Nolan, and K.A. Brzak. (2000). Lack of differential sensitivity to cholinesterase inhibition in fetuses and neonates compared to dams treated perinatally with chlorpyrifos.. Toxicol. Sci. 53:438–446.
- J.P. Maurissen, A.M. Hoberman, R.H. Garman, and T.R. HanleyJr.. (2000). Lack of selective developmental neurotoxicity in rat pups from dams treated by gavage with chlorpyrifos.. Toxicol. Sci. 57:250–263.
- S.B. McCollister, R.J. Kociba, C.G. Humiston, D.D. McCollister, and P.J. Gehring. (1974). Studies of the acute and long-term oral toxicity of chlorpyrifos (O,O-diethyl-O(3,5,6-trichloro-2-pyridyl) phosphorothioate).. Food Cosmet. Toxicol 12:45–61.
- M.C. McGuire, C.P. Nogueira, C.F. Bartels, H. Lightstone, A. Hajra, A.F. Van der Spek, O. Lockridge, and B.N. La Du. (1989). Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase.. Proc. Natl. Acad. Sci. USA 86:953–957.
- J.D. Meeker, D.B. Barr, and R. Hauser. (2006a). Thyroid hormones in relation to urinary metabolites of non-persistent insecticides in men of reproductive age.. Reprod. Toxicol. 22:437–442.
- J.D. Meeker, D.B. Barr, L. Ryan, R.F. Herrick, D.H. Bennett, R. Bravo, and R. Hauser. (2005). Temporal variability of urinary levels of nonpersistent insecticides in adult men.. J. Expos. Anal. Environ. Epidemiol. 15:271–281.
- J.D. Meeker, L. Ryan, D.B. Barr, and R. Hauser. (2006b). Exposure to nonpersistent insecticides and male reproductive hormones.. Epidemiology 17:61–68.
- J.D. Meeker, L. Ryan, D.B. Barr, R.F. Herrick, D.H. Bennett, R. Bravo, and R. Hauser. (2004a). The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality.. Environ. Health Perspect. 112:1665–1670.
- J.D. Meeker, N.P. Singh, L. Ryan, S.M. Duty, D.B. Barr, R.F. Herrick, D.H. Bennett, and R. Hauser. (2004b). Urinary levels of insecticide metabolites and DNA damage in human sperm.. Hum. Reprod. 19:2573–2580.
- W.J. Meggs. (2003). Permanent paralysis at sites of dermal exposure to chlorpyrifos.. J. Toxicol. Clin. Toxicol. 41:883–886.
- S.M. Mense, A. Sengupta, C. Lan, M. Zhou, G. Bentsman, D.J. Volsky, R.M. Whyatt, F.P. Perera, and L. Zhang. (2006). The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes.. Toxicol. Sci. 93:125–135.
- W.J.A. Meuling, L.C. Ravensberg, L. Roza, and J.J. van Hemmen. (2005). Dermal absorption of chlorpyrifos in human volunteers.. Int. Arch. Occup. Environ. Health 78:44–50.
- A. Meyer, F.J. Seidler, J.E. Aldridge, and T.A. Slotkin. (2005). Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide chlorpyrifos.. Toxicol. Appl. Pharmacol. 203:154–166.
- A. Meyer, F.J. Seidler, J.E. Aldridge, C.A. Tate, M.M. Cousins, and T.A. Slotkin. (2004). Critical periods for chlorpyrifos-induced developmental neurotoxicity: Alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure.. Environ. Health Perspect. 112:295–301.
- A. Meyer, F.J. Seidler, M.M. Cousins, and T.A. Slotkin. (2003). Developmental neurotoxicity elicited by gestational exposure to chlorpyrifos: When is adenylyl cyclase a target?. Environ. Health Perspect. 111:1871–1876.
- H. Michalek, A. Meneguz, and G.M. Bisso. (1982). Mechanisms of recovery of brain acetylcholinesterase in rats during chronic intoxication by isoflurophate.. Arch. Toxicol. Suppl. 5:116–119.
- L.R. Mikami, S. Wieseler, R.L. Souza, L.M. Schopfer, F. Nachon, O. Lockridge, and E.A. Chautard-Freire-Maia. (2008). Five new naturally occurring mutations of the BCHE gene and frequencies of 12 butyrylcholinesterase alleles in a Brazilian population.. Pharmacogenet. Genom 18:213–218.
- J. Miranda, I. Lundberg, R. McConnell, E. Delgado, R. Cuadra, E. Torres, C. Wesseling, and M. Keifer. (2002a). Onset of grip-and pinch-strength impairment after acute poisonings with organophosphate insecticides.. Int. J. Occup. Environ. Health 8:19–26.
- J. Miranda, R. McConnell, E. Delgado, R. Cuadra, M. Keifer, C. Wesseling, E. Torres, and I. Lundberg. (2002b). Tactile vibration thresholds after acute poisonings with organophosphate insecticides.. Int. J. Occup. Environ. Health 8:212–219.
- K.E. Misulis, J. Sussman, S. Bon, and I. Silman. (1993). Structure and functions of acetylcholinesterase and butyrylcholinesterase.. Prog. Brain Res 98:139.
- F. Monnet-Tschudi, M.G. Zurich, B. Schilter, L.G. Costa, and P. Honegger. (2000). Maturation-dependent effects of chlorpyrifos and parathion and their oxygen analogs on acetylcholinesterase and neuronal and glial markers in aggregating brain cell cultures.. Toxicol. Appl. Pharmacol. 165:175–183.
- A. Moretto, and M. Lotti. (1998). Poisoning by organophosphorus insecticides and sensory neuropathy.. J. Neurol. Neurosurg. Psychiatry 64:463–468.
- M.K. Morgan, L.S. Sheldon, C.W. Croghan, P.A. Jones, G.L. Robertson, J.C. Chuang, N.K. Wilson, and C.W. Lyu. (2005). Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments.. J. Expos. Anal. Environ. Epidemiol. 15:297–309.
- M. Moriya, T. Ohta, K. Watanabe, T. Miyazawa, K. Kato, and Y. Shirasu. (1983). Further mutagenicity studies on pesticides in bacterial reversion assay systems.. Mutat. Res. 116:185–216.
- S.R. Mortensen, S. Brimijoin, M.J. Hooper, and S. Padilla. (1998a). Comparison of the in vitro sensitivity of rat acetylcholinesterase to chlorpyrifos-oxon: What do tissue IC50 values represent?. Toxicol. Appl. Pharmacol. 148:46–49.
- S.R. Mortensen, S.M. Chanda, and S. Padilla. (1996b). Age-related sensitivity to the anticholinesterase pesticide chlorpyrifos studies on the developmental changes in acetylcholinesterase AChE and a-esterase arylesterase activities.. J. Neurochemistry 66:S17.
- S.R. Mortensen, S.M. Chanda, M.J. Hooper, and S. Padilla. (1996a). Maturational differences in chlorpyrifos-oxonase activity may contribute to age-related sensitivity to chlorpyrifos.. J. Biochem. Toxicol. 11:279–287.
- S.R. Mortensen, M.J. Hooper, and S. Padilla. (1998b). Rat brain acetylcholinesterase activity: Developmental profile and maturational sensitivity to carbamate and organophosphorus inhibitors.. Toxicology 125:13–19.
- D.J. Moschandreas, S. Karuchit, M.R. Berry, M.K. O'Rourke, D. Lo, M.D. Lebowitz, and G. Robertson. (2002). Exposure apportionment: Ranking food items by their contribution to dietary exposure.. J. Expos. Anal. Environ. Epidemiol. 12:233–243.
- D.J. Moschandreas, S. Karuchit, Y. Kim, H. Ari, M.D. Lebowitz, O.R. MK, S. Gordon, and G. Robertson. (2001a). On predicting multi-route and multimedia residential exposure to chlorpyrifos and diazinon.. J. Expos. Anal. Environ. Epidemiol. 11:56–65.
- D.J. Moschandreas, Y. Kim, S. Karuchit, H. Ari, M.D. Lebowitz, M.K. O'Rourke, S. Gordon, and G. Robertson. (2001b). In-residence, multiple route exposures to chlorpyrifos and diazinon estimated by indirect method models.. Atmos. Environ. 35:2201–2213.
- N. Moser, A. Wevers, D.E. Lorke, S. Reinhardt, A. Maelicke, and H. Schroder. (1996). Alpha4-1 subunit mRNA of the nicotinic acetylcholine receptor in the rat olfactory bulb: Cellular expression in adult, pre-and postnatal stages.. Cell Tissue Res. 285:17–25.
- V.C. Moser. (1998). Age-related differences in aldicarb neurotoxicity in the rat.. Neurotoxicol. Teratol. 20:364.
- V.C. Moser. (2000). Dose-response and time-course of neurobehavioral changes following oral chlorpyrifos in rats of different ages.. Neurotoxicol. Teratol. 22:713–723.
- V.C. Moser, and S. Padilla. (1998). Age-and gender-related differences in the time course of behavioral and biochemical effects produced by oral chlorpyrifos in rats.. Toxicol. Appl. Pharmacol. 149:107–119.
- V.C. Moser, S.M. Chanda, S.R. Mortensen, and S. Padilla. (1998). Age-and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities.. Toxicol. Sci. 46:211–222.
- R.F. Mueller, S. Hornung, C.E. Furlong, J. Anderson, E.R. Giblett, and A.G. Motulsky. (1983). Plasma paraoxonase polymorphism: A new enzyme assay, population, family, biochemical, and linkage studies.. Am.J. Hum. Genet. 35:393–408.
- S.D. Murphy. (1982). Toxicity and hepatic metabolism of organophosphate insecticides in developing rats.. Banbury Rep. 11:125–136.
- D.E. Muscarella, J.F. Keown, and S.E. Bloom. (1984). Evaluation of the genotoxic and embryotoxic potential of chlorpyrifos and its metabolites in vivo and in vitro.. Environ. Mutagen. 6:13–23.
- E. Mutch, and F.M. Williams. (2004). Do multiple P450 isoforms contribute to parathion, diazinon and chlorpyrifos metabolism in man?. Drug Metab. Rev. 36:265.
- E. Mutch, and F.M. Williams. (2006). Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver.. Toxicology 224:22–32.
- M.C. Nelson, S.M. Jalal, and O.R. Larson. (1990). Genotoxicity of the organophosphorous insecticide chlorpyrifos based on human lymphocyte culture. Cytologia (Tokyo) 55:588–592.
- NHANES III. Third National Health and Nutrition Examination Survey. National Center for Health Statistics Centers for Disease Control and Prevention, AtlantaGA, (2003).
- L.L. Needham. (2005). Assessing exposure to organophosphorous pesticides by biomonitoring in epidemiological studies of birth outcomes.. Environ. Health Perspect. 113:494–498.
- Z. Ni, S. Li, Y. Liu, Y. Tang, and D. Pang. (1993). [Induction of micronucleus by organophosphorus pesticides both in vivo and in vitro].. Hua Xi Yi Ke Da Xue Xue Bao 24:82–86.
- NIOSH. Occupational Safety and Health Guidelines for Chlorpyrifos. National Institute for Occupational Safety and Health, CincinnatiOH, (1995).
- R.J. Nolan, D.L. Rick, N.L. Freshour, and J.H. Saunders. (1984). Chlorpyrifos: Pharmacokinetics in human volunteers.. Toxicol. Appl. Pharmacol. 73:8–15.
- D.K. Nomura, K.A. Durkin, K.P. Chiang, G.B. Quistad, B.F. Cravatt, and J.E. Casida. (2006). Serine hydrolase KIAA1363: Toxicological and structural features with emphasis on organophosphate interactions.. Chem. Res. Toxicol. 19:1142–1150.
- K. OlivierJr., J. Liu, and C. Pope. (2001). Inhibition of forskolin-stimulated cAMP formation in vitro by paraoxon and chlorpyrifos oxon in cortical slices from neonatal, juvenile, and adult rats.. J. Biochem. Mol. Toxicol. 15:263–269.
- M. Oncu, F. Gultekin, E. Karaoz, I. Altuntas, and N. Delibas. (2002). Nephrotoxicity in rats induced by chlorpryfos-ethyl and ameliorating effects of antioxidants.. Hum. Exp. Toxicol. 21:223–230.
- J. Osterloh, M. Lotti, and S.M. Pond. (1983). Toxicologic studies in a fatal overdose of 2,4-D, MCPP, and chlorpyrifos.. J. Anal. Toxicol. 7:125–129.
- E.M. Ostrea, V. Morales, E. Ngoumgna, R. Prescilla, E. Tan, E. Hernandez, G.B. Ramirez, H.L. Cifra, and M.L. Manlapaz. (2002). Prevalence of fetal exposure to environmental toxins as determined by meconium analysis.. Neurotoxicology 23:329–339.
- E.M. OstreaJr., D.M. Bielawski, N.C. PosecionJr., M. Corrion, E. Villanueva-Uy, Y. Jin, J.J. Janisse, and J.W. Ager. (2008). A comparison of infant hair, cord blood and meconium analysis to detect fetal exposure to environmental pesticides.. Environ. Res 106:277–283.
- S. Padilla, J. Buzzard, and V.C. Moser. (2000). Comparison of the role of esterases in the differential age-related sensitivity to chlorpyrifos and methamidophos.. Neurotoxicology 21:49–56.
- Y. Pang, D.L. MacIntosh, D.E. Camann, and P.B. Ryan. (2002). Analysis of aggregate exposure to chlorpyrifos in the NHEXAS-Maryland investigation.. Environ. Health Perspect. 110:235–240.
- K.K. Patnaik, and N.K. Tripathy. (1992). Farm-grade chlorpyrifos (Durmet) is genotoxic in somatic and germ-line cells of Drosophila.. Mutat. Res. 279:15–20.
- E.D. Pellizzari, D.J. Smith, A.C. Clayton, and J.J. Quackenboss. (2003). Assessment of data quality for the NHEXAS—Part II: Minnesota chlidren's pesticide exposure study (MNCPES).. J. Expos. Anal. Environ. Epidemiol. 13:465–479.
- F.P. Perera, V. Rauh, W.Y. Tsai, P. Kinney, D. Camann, D. Barr, T. Bernert, R. Garfinkel, Y.H. Tu, D. Diaz, J. Dietrich, and R.M. Whyatt. (2003). Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population.. Environ. Health Perspect. 111:201–205.
- F.P. Perera, V. Rauh, R.M. Whyatt, D. Tang, W.Y. Tsai, J.T. Bernert, Y.H. Tu, H. Andrews, D.B. Barr, D.E. Camann, D. Diaz, J. Dietrich, A. Reyes, and P.L. Kinney. (2005). A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures.. Neurotoxicology 26:573–587.
- F.P. Perera, V. Rauh, R.M. Whyatt, W.Y. Tsai, D. Tang, D. Diaz, L. Hoepner, D. Barr, Y.H. Tu, D. Camann, and P. Kinney. (2006). Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children.. Environ. Health Perspect. 114:1287–1292.
- F. Perera, V.A. Rauh, D. Tang, D.B. Barr, D. Camann, P.L. Kinney, H. Andrew, and R.M. Whyatt. (2004). Relationship between prenatal environ-mental exposures, birth outcomes and cognitive development in an urban minority cohort.. Neurotoxicology (Amsterdam) 25:667–668.
- J.R. Playfer, C. Powell, and D.A. Evans. (1977). Plasma paroxonase activity in old age.. Age Ageing 6:89–95.
- E. Podoly, T. Bruck, S. Diamant, N. Melamed-Book, A. Weiss, Y. Huang, O. Livnah, S. Langermann, H. Wilgus, and H. Soreq. (2008). Human recombinant butyrylcholinesterase purified from the milk of transgenic goats interacts with beta-amyloid fibrils and suppresses their formation in vitro.. Neurodegener Dis 5:232–236.
- C.N. Pope. (2005). Organophosphorus and carbamate cholinesterase inhibitors: Uses and misuses of a common mechanism.. Toxicol. Sci. 84:204.
- C.N. Pope, and T.K. Chakraborti. (1992). Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures.. Toxicology 73:35–43.
- C.N. Pope, and J. Liu. (1997). Age-related differences in sensitivity to organophosphorus pesticides.. Environ. Toxicol. Pharmacol. 4:309–314.
- C.N. Pope, T.K. Chakraborti, M.L. Chapman, J.D. Farrar, and D. Arthun. (1991a). Comparison of In vivo Cholinesterase Inhibition in Neonatal and Adult Rats by Three Organophosphorothioate Insecticides. NTIS/PB92-110550, 110513. Govt Reports Announcements & Index Issue 02, 1992
- C.N. Pope, T.K. Chakraborti, M.L. Chapman, J.D. Farrar, and D. Arthun. (1991b). Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides.. Toxicology 68:51–61.
- C.N. Pope, S. Karanth, J. Liu, and B. Yan. (2005). Comparative carboxylesterase activities in infant and adult liver and their in vitro sensitivity to chlorpyrifos oxon.. Regul. Toxicol. Pharmacol. 42:64–69.
- P. Poulin, and K. Krishnan. (1995). An algorithm for predicting tissue: Blood partition coefficients of organic chemicals from n-octanol: water partition coefficient data.. J. Toxicol. Environ. Health 46:117–129.
- M.A. Prendergast, R.L. Self, K.J. Smith, L. Ghayoumi, M.M. Mullins, T.R. Butler, J.J. Buccafusco, D.A. Gearhart, and A.V. TerryJr.. (2007). Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity.. Neuroscience 146:330–339.
- D. Qiao, F.J. Seidler, Y. Abreu-Villaca, C.A. Tate, M.M. Cousins, and T.A. Slotkin. (2004). Chlorpyrifos exposure during neurulation: Cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood.. Brain Res. Dev. Brain Res. 148:43–52.
- D. Qiao, F.J. Seidler, and T.A. Slotkin. (2001). Developmental neurotoxicity of chlorpyrifos modeled in vitro: Comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells.. Environ. Health Perspect. 109:909–913.
- D. Qiao, F.J. Seidler, and T.A. Slotkin. (2002b). Developmental neurotoxicity of chlorpyrifos: Defining the vulnerable period.. Toxicologist 66:130–131.
- D. Qiao, F.J. Seidler, E.D. Levin, S. Padilla, and T.A. Slotkin. (2002a). Developmental neurotoxicity of chlorpyrifos: Defining the vulnerable period.. FASEB J. 16:A949.
- D. Qiao, F.J. Seidler, C.A. Tate, M.M. Cousins, and T.A. Slotkin. (2003). Fetal chlorpyrifos exposure: Adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood.. Environ. Health Perspect. 111:536–544.
- J.J. Quackenboss, E.D. Pellizzari, P. Shubat, R.W. Whitmore, J.L. Adgate, K.W. Thomas, N.C. Freeman, C. Stroebel, P.J. Lioy, A.C. Clayton, and K. Sexton. (2000). Design strategy for assessing multi-pathway exposure for children: The Minnesota Children's Pesticide Exposure Study (MNCPES).. J. Expos. Anal. Environ. Epidemiol. 10:145–158.
- J.H. Quellette, D.A. Dittenber, P.M. Kloes, and J.A. John. Chlorpyrifos: Oral teratology study in Fisher 344 rats. Study K-44793-47, Tox. Res. Lab. Dow. , MidlandMI, (1983).
- G.B. Quistad, R. Klintenberg, P. Caboni, S.N. Liang, and J.E. Casida. (2006a). Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice.. Toxicol. Appl. Pharmacol. 211:78–83.
- G.B. Quistad, S.N. Liang, K.J. Fisher, D.K. Nomura, and J.E. Casida. (2006b). Each lipase has a unique sensitivity profile for organophosphorus inhibitors.. Toxicol. Sci. 91:166–172.
- G.B. Quistad, D.K. Nomura, S.E. Sparks, Y. Segall, and J.E. Casida. (2002). Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides.. Toxicol. Lett. 135:89–93.
- G.B. Quistad, S.E. Sparks, and J.E. Casida. (2001). Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides.. Toxicol. Appl. Pharmacol. 173:48–55.
- M.F. Rahman, M. Mahboob, K. Danadevi, B. Saleha Banu, and P. Grover. (2002). Assessment of genotoxic effects of chloropyriphos and acephate by the comet assay in mice leucocytes.. Mutat. Res. 516:139–147.
- K.W. Raines, F.J. Seidler, and T.A. Slotkin. (2001). Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos.. Brain Res. Dev. Brain Res. 130:65–72.
- V. Ramirez, and P. Cuenca. (2002). [DNA damage in female workers exposed to pesticides in banana plantations at Limon, Costa Rica].. Rev. Biol. Trop. 50:507–518.
- V.A. Rauh, R. Garfinkel, F.P. Perera, H.F. Andrews, L. Hoepner, D.B. Barr, R. Whitehead, D. Tang, and R.W. Whyatt. (2006). Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children.. Pediatrics 118:e1845–1859.
- V.A. Rauh, D. Tang, D.B. Barr, D. Camann, P.L. Kinney, H. Andrews, and R.M. Whyatt. (2004b). Relationship between prenatal environmental exposures, birth outcomes and cognitive development in an urban minority cohort.. Neurotoxicology 25:667–668.
- V. Rauh, F. Perera, D. Tang, D.B. Barr, D. Camann, P.L. Kinney, H. Andrews, and R.M. Wyatt. (2004a). Relationship between prenatal environmental exposures, birth outcomes and cognitive development in an urban minority cohort.. Epidemiology 15:S87.
- D.F.K. Rawn, and D.C.G. Muir. (1999). Sources of chlorpyrifos and dacthal to a small Canadian prairie watershed.. Environ. Sci. Technol 33:3317–3323.
- M.C. Rhodes, F.J. Seidler, D. Qiao, C.A. Tate, M.M. Cousins, and T.A. Slotkin. (2004). Does pharmacotherapy for preterm labor sensitize the developing brain to environmental neurotoxicants? Cellular and synaptic effects of sequential exposure to terbutaline and chlorpyrifos in neonatal rats.. Toxicol. Appl. Pharmacol. 195:203–217.
- L. Ricceri, N. Markina, A. Valanzano, S. Fortuna, M.F. Cometa, A. Meneguz, and G. Calamandrei. (2003). Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice.. Toxicol. Appl. Pharmacol. 191:189–201.
- L. Ricceri, A. Venerosi, F. Capone, M.F. Cometa, P. Lorenzini, S. Fortuna, and G. Calamandrei. (2006). Developmental neurotoxicity of organophosphorous pesticides: Fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice.. Toxicol. Sci. 93:105–113.
- J.R. Richardson, and J.E. Chambers. (2003). Effects of repeated developmental exposure to chlorpyrifos on presynaptic neurochemical components of the cholinergic system.. Neurotoxicology (Little Rock) 24:305.
- J.R. Richardson, and J.E. Chambers. (2004). Neurochemical effects of repeated gestational exposure to chlorpyrifos in developing rats.. Toxicol. Sci. 77:83–90.
- J.R. Richardson, and J.E. Chambers. (2005). Effects of repeated oral postnatal exposure to chlorpyrifos on cholinergic neurochemistry in developing rats.. Toxicol. Sci. 84:352–359.
- R.J. Richter, and C.E. Furlong. (1999). Determination of paraoxonase (PON1) status requires more than genotyping.. Pharmacogenetics 9:745–753.
- M.L. Rigas, M.S. Okino, and J.J. Quackenboss. (2001). Use of a pharmacokinetic model to assess chlorpyrifos exposure and dose in children, based on urinary biomarker measurements.. Toxicol. Sci. 61:374–381.
- G.L. Robertson, M.D. Lebowitz, M.K. O'Rourke, S. Gordon, and D. Moschandreas. (1999). The National Human Exposure Assessment Survey (NHEXAS) study in Arizona—Introduction and preliminary results.. J. Expos. Anal. Environ. Epidemiol. 9:427–434.
- G.S. Rodrigues, D. Pimentel, and L.H. Weinstein. (1998). In situ assessment of pesticide genotoxicity in an integrated pest management program: II. Maize waxy mutation assay.. Mutat. Res. 412:245–250.
- T. Rodriguez, L. Younglove, C. Lu, A. Funez, S. Weppner, D.B. Barr, and R.A. Fenske. (2006). Biological monitoring of pesticide exposures among applicators and their children in Nicaragua.. Int.J. Occup. Environ. Health 12:312–320.
- R.L. Rose, and E. Hodgson. (2005). Pesticide metabolism and potential for metabolic interactions.. J. Biochem. Mol. Toxicol. 19:276–277.
- R.L. Rose, J. Tang, J. Choi, Y. Cao, A. Usmani, N. Cherrington, and E. Hodgson. (2005). Pesticide metabolism in humans, including polymorphisms.. Scand.J. Work Environ. Health 31 (Suppl 1):156–163. discussion 119–122
- M.K. Ross, and J.A. Crow. (2007). Human carboxylesterases and their role in xenobiotic and endobiotic metabolism.. J. Biochem. Mol. Toxicol. 21:187–196.
- T.S. Roy, J.E. Andrews, F.J. Seidler, and T.A. Slotkin. (1998). Chlorpyrifos elicits mitotic abnormalities and apoptosis in neuroepithelium of cultured rat embryos.. Teratology 58:62–68.
- T.S. Roy, F.J. Seidler, and T.A. Slotkin. (2004). Morphologic effects of subtoxic neonatal chlorpyrifos exposure in developing rat brain: Regionally selective alterations in neurons and glia.. Brain Res. Dev. Brain Res. 148:197–206.
- T.S. Roy, V. Sharma, F.J. Seidler, and T.A. Slotkin. (2005). Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats.. Brain Res. Dev. Brain Res. 155:71–80.
- Y. Rubin, N. Gal, T. Waner, and A. Nyska. Pyrinex, teratogenicity study in the rat. Life Science Res. Israel Ltd., Ness ZionaIsrael, (1987a).
- Y. Rubin, A. Nyska, and T. Waner. Pyrnex, teratogenicity study in the rabbit. Life Science Res. Ltd., Ness ZionaIsrael, (1987b).
- R.P. Rull, B. Ritz, and G.M. Shaw. (2006). Neural tube defects and maternal residential proximity to agricultural pesticide applications.. Am.J. Epidemiol. 163:743–753.
- M. Sachana, J. Flaskos, E. Alexaki, P. Glynn, and A.J. Hargreaves. (2001a). The toxicity of chlorpyrifos towards differentiating mouse N2a neuroblastoma cells.. Toxicol. In Vitro 15:369–372.
- M. Sachana, J. Flaskos, and A.J. Hargreaves. (2005). Effects of Chlorpyrifos and Chlorpyrifos-Methyl on the Outgrowth of Axon-Like Processes, Tubulin, and GAP-43 in N2a Cells. Toxicol. Mechanisms Methods 15:405–410.
- M. Sachana, J. Flaskos, E. Nikolaidis, A. Hargreaves, and E. Alexaki-Tzivanidou. (2001b). Inhibition of rat platelet 5-hydroxytryptamine uptake by chlorpyrifos and carbaryl.. Pharmacol. Toxicol. 89:195–200.
- C. Sams, J. Cocker, and M.S. Lennard. (2004). Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP).. Xenobiotica 34:861–873.
- C. Sams, H.J. Mason, and R. Rawbone. (2000). Evidence for the activation of organophosphate pesticides by cytochromes P450 3A4 and 2D6 in human liver microsomes.. Toxicol. Lett. 116:217–221.
- T.E. Samsam, D.L. Hunter, and P.J. Bushnell. (2005). Effects of chronic dietary and repeated acute exposure to chlorpyrifos on learning and sustained attention in rats. Toxicol.. Sci 87:460–468.
- S.S. Sandhu, M.D. Waters, V.F. Simmon, K.E. Mortelmans, A.D. Mitchell, T. Jorgenson, D.L. Jones, R. Valencia, and F. Stack. (1985). Evaluation of the genotoxic potential of certain pesticides used in Pakistan. Basic and applied mutagenesis.. Basic Life Sci. 34:185–219.
- R. Sanghi, M.K. Pillai, T.R. Jayalekshmi, and A. Nair. (2003). Organochlorine and organophosphorus pesticide residues in breast milk from Bhopal, Madhya Pradesh, India.. Hum. Exp. Toxicol. 22:73–76.
- T. Satoh, and M. Hosokawa. (1998). The mammalian carboxylesterases: From molecules to functions.. Annu. Rev. Pharmacol. Toxicol. 38:257–288.
- T. Satoh, P. Taylor, W.F. Bosron, S.P. Sanghani, M. Hosokawa, and B.N. La Du. (2002). Current progress on esterases: From molecular structure to function.. Drug Metab. Dispos. 30:488–493.
- D.P. Scher, B.H. Alexander, J.L. Adgate, L.E. Eberly, J.S. Mandel, J.F. Acquavella, M.J. Bartels, and K.A. Brzak. (2007). Agreement of pesticide biomarkers between morning void and 24-h urine samples from farmers and their children.. J. Expos. Sci. Environ. Epidemiol. 17:350–357.
- W. Schmid. The micronucleus test for cytogenetic analysis, Chemical Mutagens: Principles and Methods for their DetectionA. Hollaender. Plenum, New York, (1976)Vol. 431–53.
- R.A. Schuh, P.J. Lein, R.A. Beckles, and D.A. Jett. (2002). Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: Altered phosphorylation of Ca2 +/cAMP response element binding protein in cultured neurons. Toxicol. Appl. Pharmacol. 182:176–185.
- N. Senanayake. (1981). Tri-cresyl phosphate neuropathy in Sri Lanka: A clinical and neurophysiological study with a three year follow up.. J. Neurol. Neurosurg. Psychiatry 44:775–780.
- K. Sexton, J.L. Adgate, L.E. Eberly, C.A. Clayton, R.W. Whitmore, E.D. Pellizzari, P.J. Lioy, and J.J. Quackenboss. (2003). Predicting children's short-term exposure to pesticides: Results of a questionnaire screening approach.. Environ. Health Perspect. 111:123–128.
- , J.D. Sherman. (1995). Organophosphate pesticides—Neurological and respiratory toxicity.. Toxicol. Ind. Health 11:33–39.
- J.D. Sherman. (1996). Chlorpyrifos (Dursban)-associated birth defects: Report of four cases.. Arch. Environ. Health 51:5–8.
- J.D. Sherman. (1999). Chlorpyrifos (Dursban) and Dow employees.. Environ. Health Perspect. 107:A132–134.
- D.M. Shih, L. Gu, Y.R. Xia, M. Navab, W.F. Li, S. Hama, L.W. Castellani, C.E. Furlong, L.G. Costa, A.M. Fogelman, and A.J. Lusis. (1998). Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis.. Nature 394:284–287.
- Y. Shirasu, M. Moriya, K. Kato, A. Furuhashi, and T. Kada. (1976). Mutagenicity screening of pesticides in the microbial system.. Mutat. Res. 40:19–30.
- B.A. Shurdut, L. Barraj, and M. Francis. (1998). Aggregate exposures under the Food Quality Protection Act: An approach using chlorpyrifos.. Regul. Toxicol. Pharmacol. 28:165–177.
- F.R. Sidell. Clinical considerations in nerve agent intoxication, Chemical Warfare AgentsS.M. Somani. Academic Press, San Diego, (1992)155.
- F.R. Sidell, and A. Kaminskis. (1975). Influence of age, sex, and oral contraceptives on human blood cholinesterase activity.. Clin. Chem. 21:1393–1395.
- N.J. Simcox, R.A. Fenske, S.A. Wolz, I.C. Lee, and D.A. Kalman. (1995). Pesticides in household dust and soil: Exposure pathways for children of agricultural families.. Environ. Health Perspect. 103:1126–1134.
- V.F. Simmon, A.D. Mitchell, and T.A. Jorgenson. (1977). Evaluation of selected pesticides and chemical mutagens. In vitro and in vivo studies.. Natl. Tech. Inform. Serv. PB 268:
- A.K. Sinha, B.B. Gollapudi, V.A. Linscombe, and M.L. McClintock. (1989). Utilization of rat hepatocytes f or the in vitro chromosomal aberration assay.. Mutagenesis 4:147–153.
- T.A. Slotkin, K.K. Brown, and F.J. Seidler. (2005). Developmental exposure of rats to chlorpyrifos elicits sex-selective hyperlipidemia and hyperinsulinemia in adulthood.. Environ. Health Perspect. 113:1291–1294.
- T.A. Slotkin, M.M. Cousins, C.A. Tate, and F.J. Seidler. (2001). Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure.. Brain Res. 902:229–243.
- T.A. Slotkin, E.D. Levin, and F.J. Seidler. (2006). Comparative developmental neurotoxicity of organophosphate insecticides: Effects on brain development are separable from systemic toxicity.. Environ. Health Perspect. 114:746–751.
- T.A. Slotkin, and F.J. Seidler. (2007). Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: Critical periods for regional and sex-selective effects.. Reprod. Toxicol. 23:421–427.
- T.A. Slotkin, F.J. Seidler, and F. Fumagalli. (2007). Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: Similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily.. Environ. Health Perspect. 115:909–916.
- T.A. Slotkin, M.C. Southard, S.J. Adam, M.M. Cousins, and F.J. Seidler. (2004). Alpha7 nicotinic acetylcholine receptors targeted by cholinergic developmental neurotoxicants: nicotine and chlorpyrifos.. Brain Res. Bull. 64:227–235.
- T.A. Slotkin, C.A. Tate, M.M. Cousins, and F.J. Seidler. (2002). Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure.. Brain Res. Dev. Brain Res. 133:163–173.
- X. Song, F.J. Seidler, and T.A. Slotkin. (1997b). Cellular mechanisms for developmental toxicity of chlorpyrifos: Targeting the adenylyl cyclase signaling cascade.. Toxicologist 36:100.
- X. Song, F.J. Seidler, J.L. Saleh, J. Zhang, S. Padilla, and T.A. Slotkin. (1997a). Cellular mechanisms for developmental toxicity of chlorpyrifos: Targeting the adenylyl cyclase signaling cascade.. Toxicol. Appl. Pharmacol. 145:158–174.
- X. Song, J.D. Violin, F.J. Seidler, and T.A. Slotkin. (1998). Modeling the developmental neurotoxicity of chlorpyrifos in vitro: Macromolecule synthesis in PC12 cells.. Toxicol. Appl. Pharmacol. 151:182–191.
- B. Sood, V. Delaney-Black, C. Covington, B. Nordstrom-Klee, J. Ager, T. Templin, J. Janisse, S. Martier, and R.J. Sokol. (2001). Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. Dose-response effect.. Pediatrics 108:E34.
- P.S. Spencer, and H.H. Schaumburg. Central-peripheral distal axonopathy—the pathology of dying-back polyneuropathies, Progress in NeuropathologyH.M. Zimmerman. Grune and Stratton, New York, (1976)253.
- K. Steenland, B. Jenkins, R.G. Ames, M. O'Malley, D. Chrislip, and J. Russo. (1994). Chronic neurological sequelae to organophosphate pesticide poisoning.. Am.J. Public Health 84:731–736.
- J.C. Stevens, R.N. Hines, C. Gu, S.B. Koukouritaki, J.R. Manro, P.J. Tandler, and M.J. Zaya. (2003). Developmental expression of the major human hepatic CYP3A enzymes.. J. Pharmacol. Exp. Ther. 307:573–582.
- T. Suehiro, T. Nakamura, M. Inoue, T. Shiinoki, Y. Ikeda, Y. Kumon, M. Shindo, H. Tanaka, and K. Hashimoto. (2000). A polymorphism upstream from the human paraoxonase (PON1) gene and its association with PON1 expression.. Atherosclerosis 150:295–298.
- L.G. Sultatos. (1991). Metabolic activation of the organophosphorus insecticides chlorpyrifos and fenitrothion by perfused rat liver.. Toxicology 68:1–9.
- L.G. Sultatos. (1994). Mammalian toxicology of organophosphorus pesticides.. J. Toxicol. Environ. Health 43:271–289.
- L.G. Sultatos, M. Shao, and S.D. Murphy. (1984). The role of hepatic biotransformation in mediating the acute toxicity of the phosphorothionate insecticide chlorpyrifos.. Toxicol. Appl. Pharmacol. 73:60–68.
- W.T. Sumerford, W.J. HayesJr., J.M. Johnston, K. Walker, and J. Spillane. (1953). Cholinesterase response and symptomatology from exposure to organic phosphorus insecticides.. AMA Arch. Ind. Hyg. Occup. Med. 7:383–398.
- J. Tang. (2000). The neurochemical effects of repeated oral exposures to chlorpyrifos or methyl parathion on rat brain cholinergic and dopaminergic systems during early postnatal development. ix. 110 leaves
- J. Tang, Y. Cao, R.L. Rose, A.A. Brimfield, D. Dai, J.A. Goldstein, and E. Hodgson. (2001). Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes.. Drug Metab. Dispos. 29:1201–1204.
- J. Tang, R.L. Carr, and J.E. Chambers. (1999). Changes in rat brain cholinesterase activity and muscarinic receptor density during and after repeated oral exposure to chlorpyrifos in early postnatal development.. Toxicol. Sci. 51:265–272.
- A.V. TerryJr., J.D. Stone, J.J. Buccafusco, D.W. Sicles, A. Sood, and M.A. Prendergast. (2003). Repeated exposures to subthreshold doses of chlorpyrifos in rats: Hippocampal damage, impaired axonal transport, and deficits in spatial learning.. J. Pharmacol. Exp. Ther. 305:375–384.
- J.D. Thrasher, G. Heuser, and A. Broughton. (2002). Immunological abnormalities in humans chronically exposed to chlorpyrifos.. Arch. Environ. Health 57:181–187.
- J.D. Thrasher, R. Madison, and A. Broughton. (1993). Immunologic abnormalities in humans exposed to chlorpyrifos: Preliminary observations.. Arch. Environ. Health 48:89–93.
- Y. Tian, and T. Yamauchi. (2003). Micronucleus formation in 3-day mouse embryos associated with maternal exposure to chlorpyrifos during the early preimplantation period.. Reprod. Toxicol. 17:401–405.
- Y. Tian, H. Ishikawa, T. Yamaguchi, T. Yamauchi, and K. Yokoyama. (2005). Teratogenicity and developmental toxicity of chlorpyrifos. Maternal exposure during organogenesis in mice.. Reprod. Toxicol. 20:267–270.
- Y. Tian, L. Shen, Y. Gao, T. Yamauchi, X.-m. Shen, and N. Ma. (2007). Comparison of 4',6'-diamidino-2-phenylindole and Giemsa stainings in preimplantation mouse embryos micronucleus assay including a triple dose study.. Ind. Health 45:343–347.
- C. Timchalk, A. Busby, J.A. Campbell, L.L. Needham, and D.B. Barr. (2007). Comparative pharmacokinetics of the organophosphorus insecticide chlorpyrifos and its major metabolites diethylphosphate, diethylthiophosphate and 3,5,6-trichloro-2-pyridinol in the rat.. Toxicology 237:145–157.
- C. Timchalk, A. Kousba, and T.S. Poet. (2002a). Monte Carlo analysis of the human chlorpyrifos-oxonase (PON1) polymorphism using a physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model.. Toxicol. Lett. 135:51–59.
- C. Timchalk, R.J. Nolan, A.L. Mendrala, D.A. Dittenber, K.A. Brzak, and J.L. Mattsson. (2002b). A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans.. Toxicol. Sci. 66:34–53.
- C. Timchalk, T.S. Poet, and A.A. Kousba. (2006). Age-dependent pharmacokinetic and pharmacodynamic response in preweanling rats following oral exposure to the organophosphorus insecticide chlorpyrifos.. Toxicology 220:13–25.
- A. Vaisi-Raygani, Z. Rahimi, H. Entezami, H. Kharrazi, F. Bahrhemand, H. Tavilani, M. Rezaei, A. Kiani, B. Nomanpour, and T. Pourmotabbed. (2008). Butyrylcholinesterase K variants increase the risk of coronary artery disease in the population of western Iran. Scand.J. Clin. Lab. Invest 68:123–129.
- M. van Gemert, M. Dourson, A. Moretto, and M. Watson. (2001). Use of human data for the derivation of a reference dose for chlorpyrifos.. Regul. Toxicol. Pharmacol. 33:110–116.
- Z. Vasilic, V. Drevenkar, V. Rumenjak, B. Stengl, and Z. Frobe. (1992). Urinary excretion of diethylphosphorus metabolites in persons poisoned by quinalphos or chlorpyrifos.. Arch. Environ. Contam. Toxicol. 22:351–357.
- A. Venerosi, G. Calamandrei, and L. Ricceri. (2006). A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure.. Neurotoxicol. Teratol. 28:466–471.
- R.S. Verma, and N. Srivastava. (2001). Chlorpyrifos induced alterations in levels of thiobarbituric acid reactive substances and glutathione in rat brain.. Indian J. Expos. Biol. 39:174–177.
- R.S. Verma, and N. Srivastava. (2003). Effect of chlorpyrifos on thiobarbituric acid reactive substances, scavenging enzymes and glutathione in rat tissues.. Indian J. Biochem. Biophys. 40:423–428.
- C.A. Vidair. (2004). Age dependence of organophosphate and carbamate neurotoxicity in the postnatal rat: Extrapolation to the human.. Toxicol. Appl. Pharmacol. 196:287–302.
- C.J. Vidal. (2005). Expression of cholinesterases in brain and non-brain tumours.. Chem. Biol. Interact. 157–158:227–232.
- A.M. Vinggaard, V. Breinholt, and J.C. Larsen. (1999). Screening of selected pesticides for oestrogen receptor activation in vitro.. Food Addit. Contam. 16:533–542.
- X. Wang, B. Zuckerman, C. Pearson, G. Kaufman, C. Chen, G. Wang, T. Niu, P.H. Wise, H. Bauchner, and X. Xu. (2002). Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight.. JAMA 287:195–202.
- S.D. Warner, C.G. Gerbig, R.J. Strebing, and J.A. Molello. Results of a two-year toxicity and oncogenicity study of chlorpyrifos administered to CD-1 mice in the diet. Dow Chemical Co., MidlandMI, (1980).
- M.D. Waters, S. Nesnow, V.F. Simmon, A.D. Mitchell, T.A. Jorgenson, and R. Valencia. (1981). Pesticides: Mutagenic and carcinogenic potential.. Pesticide Chemist and Modern Toxicology, ACS Symposium Series No 160:89–113.
- S.E. Wellman, and R.E. Kramer. (2004). Absence of DNA binding activity of methyl parathion and chlorpyrifos.. Toxicol. Mech. Methods 14:247–251.
- K.D. Whitney, F.J. Seidler, and T.A. Slotkin. (1995). Developmental neurotoxicity of chlorpyrifos: Cellular mechanisms.. Toxicol. Appl. Pharmacol. 134:53–62.
- M. Whittaker. (1986). Cholinesterase.. Monogr. Hum. Genet. 2:1.
- M. Whittaker, A.R. Charlier, and S. Ramaswamy. (1971). Changes in plasma cholinesterase isoenzymes due to oral contraceptives.. J. Reprod. Fertil. 26:373–375.
- R.M. Whyatt, D.B. Barr, D.E. Camann, P.L. Kinney, J.R. Barr, H.F. Andrews, L.A. Hoepner, R. Garfinkel, Y. Hazi, A. Reyes, J. Ramirez, Y. Cosme, and F.P. Perera. (2003b). Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns.. Environ. Health Perspect. 111:749–756.
- R.M. Whyatt, D.E. Camann, P.L. Kinney, A. Reyes, J. Ramirez, J. Dietrich, D. Diaz, D. Holmes, and F.P. Perera. (2002). Residential pesticide use during pregnancy among a cohort of urban minority women.. Environ. Health Perspect. 110:507–514.
- R.M. Whyatt, D. Camann, F.P. Perera, V.A. Rauh, D. Tang, P.L. Kinney, R. Garfinkel, H. Andrews, L. Hoepner, and D.B. Barr. (2005). Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth.. Toxicol. Appl. Pharmacol. 206:246–254.
- R.M. Whyatt, R. Garfinkel, L.A. Hoepner, D. Holmes, M. Borjas, M.K. Williams, A. Reyes, V. Rauh, F.P. Perera, and D.E. Camann. (2007). Within-and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City.. Environ. Health Perspect. 115:383–389.
- R.M. Whyatt, V.A. Rauh, D. Camann, D. Tang, P.L. Kinney, R. Garfinkel, H. Andrews, L. Hoepner, and F.P. Perera. (2004b). Residential pesticide exposure, fetal growth and neurocognitive development among urban minorities.. Neurotoxicology (Amsterdam) 25:683.
- R.M. Whyatt, V. Rauh, D.B. Barr, D.E. Camann, H.F. Andrews, R. Garfinkel, L.A. Hoepner, D. Diaz, J. Dietrich, A. Reyes, D. Tang, P.L. Kinney, and F.P. Perera. (2004a). Prenatal insecticide exposures and birth weight and length among an urban minority cohort.. Environ. Health Perspect. 112:1125–1132.
- R. Whyatt, V. Rauh, D. Barr, D. Camann, H. Andrews, and F. Perera. (2003a). Prenatal insecticide exposure adversely affects fetal growth in an urban minority cohort.. Am.J. Epidemiol. 157:S68.
- B. Wielgomas, and J. Krechniak. (2007). Effect of alpha-cypermethrin and chlorpyrifos in a 28-day study on free radical parameters and cholinesterase activity in Wistar rats.. Polish J. Environ. Stud. 16:91–95.
- G.M. Williams. (1977). Detection of chemical carcinogens by unscheduled DNA synthesis in rat liver primary cell cultures.. Cancer Res 37:1845–1851.
- G.M. Williams, E. Bermudez, and D. Scaramuzzino. (1977a). Rat hepatocyte primary cultures III. Improved dissociation and attachment techniques and the enhancement of survival by culture medium.. In Vitro 13:809–817.
- R.T. Williams, P. Millburn, and R.L. Smith. (1965). The influence of enterohepatic circulation on toxicity of drugs.. Ann. NY Acad. Sci. 123:110–124.
- N.K. Wilson, J.C. Chuang, and C. Lyu. (2001). Levels of persistent organic pollutants in several child day care centers.. J. Expos. Anal. Environ. Epidemiol. 11:449–458.
- N.K. Wilson, J.C. Chuang, R. Iachan, C. Lyu, S.M. Gordon, M.K. Morgan, H. Ozkaynak, and L.S. Sheldon. (2004). Design and sampling methodology for a large study of preschool children's aggregate exposures to persistent organic pollutants in their everyday environments.. J. Expos. Anal. Environ. Epidemiol. 14:260–274.
- N.K. Wilson, J.C. Chuang, C. Lyu, R. Menton, and M.K. Morgan. (2003). Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home.. J. Expos. Anal. Environ. Epidemiol. 13:187–202.
- G.C. Windham, L. Fenster, B. Hopkins, and S.H. Swan. (1995). The association of moderate maternal and paternal alcohol consumption with birthweight and gestational age.. Epidemiology 6:591–597.
- M.S. Wolff, S. Engel, G. Berkowitz, S. Teitelbaum, J. Siskind, D.B. Barr, and J. Wetmur. (2007). Prenatal pesticide and PCB exposures and birth outcomes.. Pediatr. Res. 61:243–250.
- Y.K. Won, J. Liu, K. OlivierJr., Q. Zheng, and C.N. Pope. (2001). Age-related effects of chlorpyrifos on acetylcholine release in rat brain.. Neurotoxicology 22:39–48.
- Y.J. Wu, P. Harp, X.R. Yan, and C.N. Pope. (2003). Nicotinic autoreceptor function in rat brain during maturation and aging: Possible differential sensitivity to organophosphorus anticholinesterases.. Chem. Biol. Interact. 142:255–268.
- H.H. Xu, and K.M. Schurr. (1990). Genotoxicity of 22 pesticides in mictrotitration SOS chromotest.. Toxicity Assess. 5:1–14.
- Y. Yamada, T. Takatori, M. Nagao, H. Iwase, N. Kuroda, J. Yanagida, and T. Shinozuka. (2001). Expression of paraoxonase isoform did not confer protection from acute sarin poisoning in the Tokyo subway terrorist attack.. Int.J. Legal Med. 115:82–84.
- D. Yang, A. Howard, D. Bruun, M. Ajua-Alemanj, C. Pickart, and P.J. Lein. (2008). Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase.. Toxicol. Appl. Pharmacol. 228:32–41.
- B.L. Yano, J.T. Young, and J.L. Mattsson. (2000). Lack of carcinogenicity of chlorpyrifos insecticide in a high-dose, 2-year dietary toxicity study in Fischer 344 rats.. Toxicol. Sci. 53:135–144.
- R.A. Yeary, J. Eaton, E. Gilmore, B. North, and J. Singell. (1993). A multiyear study of blood cholinesterase activity in urban pesticide applicators.. J. Toxicol. Environ. Health 39:11–25.
- K. Yokoyama, A. Yamada, and N. Mimura. (1996). Clinical profiles of patients with sarin poisoning after the Tokyo subway attack.. Am.J. Med. 100:586.
- J.G. Young, B. Eskenazi, E.A. Gladstone, A. Bradman, L. Pedersen, C. Johnson, D.B. Barr, C.E. Furlong, and N.T. Holland. (2005). Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates.. Neurotoxicology 26:199–209.
- K.L. Yuknavage, R.A. Fenske, D.A. Kalman, M.C. Keifer, and C.E. Furlong. (1997). Simulated dermal contamination with capillary samples and field cholinesterase biomonitoring.. J. Toxicol. Environ. Health 51:35–55.
- V.G. Zartarian, H. Ozkaynak, J.M. Burke, M.J. Zufall, M.L. Rigas, and E.J. FurtawJr.. (2000). A modeling framework for estimating children's residential exposure and dose to chlorpyrifos via dermal residue contact and nondietary ingestion.. Environ. Health Perspect. 108:505–514.
- H. Zhang, J. Liu, and C.N. Pope. (2002). Age-related effects of chlorpyrifos on muscarinic receptor-mediated signaling in rat cortex.. Arch. Toxicol. 75:676–684.
- Q. Zhao, M. Dourson, and B. Gadagbui. (2006). A review of the reference dose for chlorpyrifos.. Regul. Toxicol. Pharmacol. 44:111–124.
- Q. Zhao, B. Gadagbui, and M. Dourson. (2005). Lower birth weight as a critical effect of chlorpyrifos: A comparison of human and animal data.. Regul. Toxicol. Pharmacol. 42:55–63.
- Q. Zheng, K. Olivier, Y.K. Won, and C.N. Pope. (2000). Comparative cholinergic neurotoxicity of oral chlorpyrifos exposures in preweanling and adult rats.. Toxicol. Sci. 55:124–132.