Abstract
Quantitative estimates of the risk of lung cancer or mesothelioma in humans from asbestos exposure made by the U.S. Environmental Protection Agency (EPA) make use of estimates of potency factors based on phase-contrast microscopy (PCM) and obtained from cohorts exposed to asbestos in different occupational environments. These potency factors exhibit substantial variability. The most likely reasons for this variability appear to be differences among environments in fiber size and mineralogy not accounted for by PCM.
In this article, the U.S. Environmental Protection Agency (EPA) models for asbestos-related lung cancer and mesothelioma are expanded to allow the potency of fibers to depend upon their mineralogical types and sizes. This is accomplished by positing exposure metrics composed of nonoverlapping fiber categories and assigning each category its own unique potency. These category-specific potencies are estimated in a meta-analysis that fits the expanded models to potencies for lung cancer (KL's) or mesothelioma (KM's) based on PCM that were calculated for multiple epidemiological studies in our previous paper (Citation). Epidemiological study-specific estimates of exposures to fibers in the different fiber size categories of an exposure metric are estimated using distributions for fiber size based on transmission electron microscopy (TEM) obtained from the literature and matched to the individual epidemiological studies. The fraction of total asbestos exposure in a given environment respectively represented by chrysotile and amphibole asbestos is also estimated from information in the literature for that environment. Adequate information was found to allow KL's from 15 epidemiological studies and KM's from 11 studies to be included in the meta-analysis.
Since the range of exposure metrics that could be considered was severely restricted by limitations in the published TEM fiber size distributions, it was decided to focus attention on four exposure metrics distinguished by fiber width: “all widths,” widths > 0.2 μ m, widths < 0.4 μ m, and widths < 0.2 μ m, each of which has historical relevance. Each such metric defined by width was composed of four categories of fibers: chrysotile or amphibole asbestos with lengths between 5 μ m and 10 μ m or longer than 10 μ m. Using these metrics three parameters were estimated for lung cancer and, separately, for mesothelioma: KLA, the potency of longer (length > 10 μ m) amphibole fibers; rpc, the potency of pure chrysotile (uncontaminated by amphibole) relative to amphibole asbestos; and rps, the potency of shorter fibers (5 μ m < length < 10 μ m) relative to longer fibers.
For mesothelioma, the hypothesis that chrysotile and amphibole asbestos are equally potent (rpc = 1) was strongly rejected by every metric and the hypothesis that (pure) chrysotile is nonpotent for mesothelioma was not rejected by any metric. Best estimates for the relative potency of chrysotile ranged from zero to about 1/200th that of amphibole asbestos (depending on metric). For lung cancer, the hypothesis that chrysotile and amphibole asbestos are equally potent (rpc = 1) was rejected (p ≤ .05) by the two metrics based on thin fibers (length < 0.4 μ m and < 0.2 μ m) but not by the metrics based on thicker fibers.
The “all widths” and widths < 0.4 μ m metrics provide the best fits to both the lung cancer and mesothelioma data over the other metrics evaluated, although the improvements are only marginal for lung cancer. That these two metrics provide equivalent (for mesothelioma) and nearly equivalent (for lung cancer) fits to the data suggests that the available data sets may not be sufficiently rich (in variation of exposure characteristics) to fully evaluate the effects of fiber width on potency. Compared to the metric with widths > 0.2 μ m with both rps and rpc fixed at 1 (which is nominally equivalent to the traditional PCM metric), the “all widths” and widths < 0.4 μ m metrics provide substantially better fits for both lung cancer and, especially, mesothelioma.
Although the best estimates of the potency of shorter fibers (5 < length < 10 μ m) is zero for the “all widths” and widths < 0.4 μ m metrics (or a small fraction of that of longer fibers for the widths > 0.2 μ m metric for mesothelioma), the hypothesis that these shorter fibers were nonpotent could not be rejected for any of these metrics. Expansion of these metrics to include a category for fibers with lengths < 5 μ m did not find any consistent evidence for any potency of these shortest fibers for either lung cancer or mesothelioma.
Despite the substantial improvements in fit over that provided by the traditional use of PCM, neither the “all widths” nor the widths < 0.4 μ m metrics (or any of the other metrics evaluated) completely resolve the differences in potency factors estimated in different occupational studies. Unresolved in particular is the discrepancy in potency factors for lung cancer from Quebec chrysotile miners and workers at the Charleston, SC, textile mill, which mainly processed chrysotile from Quebec. A leading hypothesis for this discrepancy is limitations in the fiber size distributions available for this analysis. Citation recently analyzed by TEM archived air samples from the South Carolina plant to determine a detailed distribution of fiber lengths up to lengths of 40 μ m and greater. If similar data become available for Quebec, perhaps these two size distributions can be used to eliminate the discrepancy between these two studies.
INTRODUCTION
In the accompanying paper (Berman and Crump, Citation2008), estimates of the potency of asbestos for causing lung cancer or mesothelioma were developed from published studies (including raw data from three of the studies) of occupationally exposed cohorts. Results of this analysis were expressed as study-specific potency factors for lung cancer (denoted by KL) and mesothelioma (denoted by KM), along with “uncertainty bounds” for these factors that account for both statistical and nonstatistical uncertainty, including uncertainty in exposure to asbestos (Berman and Crump, Citation2008, and ). The KL's and KM's were derived using the same mathematical exposure-response models for lung cancer and mesothelioma that were used by the U.S. Environmental Protection Agency (EPA) in its health assessment document for asbestos (Nicholson, Citation1986, referred to herein as “the EPA 1986 update”).
Much relevant research has been published since the EPA 1986 update was completed, including several epidemiological studies of asbestos in heretofore unstudied populations, as well as additional years of follow-up for many of the epidemiological studies included in the EPA 1986 update. Berman and Crump (Citation2008) estimated KL's from 18 locations and KM's from 12 locations, compared to KL's from 12 locations and KM's from four locations in the EPA 1986 update. In addition, nine of the KL's and all four KM's in the EPA 1986 update were revised using results from more recent follow-up. The more recent data also encompass a much wider range of exposure conditions. For example, whereas the four KM's in the EPA 1986 update all came from environments where exposures were either to amphibole asbestos or to a substantial mixture of asbestos types, Berman and Crump (Citation2008) estimated KM's from six locations where exposures were principally to chrysotile.
As was also the case in the EPA 1986 update, Berman and Crump (Citation2008) found substantial variability among the KL's and KM's estimated from different locations. Among the 20 studies evaluated (from 18 unique environments), KL's varied by almost two orders of magnitude (ignoring one negative study that would otherwise make the range infinite) and KM's by more than three orders of magnitude. Various hypotheses have been advanced to account for this variation. In the EPA 1986 update, for example, it was concluded that if was primarily due to “statistical variability associated with small numbers, but also … uncertainties associated with methodology and exposure estimates.” However, potency factors computed from different environments remain incompatible, even after accounting for all of these uncertainties (Berman and Crump, Citation2008). This is particularly true among the potency factors for mesothelioma (KM's).
Patterns in the relative magnitudes of KL and KM estimates from specific types of environments suggest specific causes for the wide ranges in these values. KM's from environments where exposures were mainly to chrysotile are generally smaller than those from environments where exposures were primarily to amphibole asbestos. Other researchers have also noted that the risk of mesothelioma in workers exposed primarily to chrysotile is much lower than it is in populations exposed only to amphibole asbestos, even after adjusting for differences in exposures (see, e.g., Hodgson and Darnton, Citation2000).
The relative ability of chrysotile and amphibole asbestos fibers to cause mesothelioma has been studied and debated by multiple researchers in diverse research groups for more than 20 years (McDonald and Fry, Citation1982; Churg et al., Citation1984; Huncharek, Citation1987; McCoinnochie et al., 1987; Dunnigan, Citation1988; Becklake, Citation1988; Mancuso, Citation1988; McDonald, Citation1988; Churg, Citation1988a, Citation1988b; Sluis-Cremer, Citation1988; Langer and Nolan, Citation1989; Ohlson, Citation1989; McDonald et al., Citation1989; Sebastien et al., Citation1989; Case, Citation1991; Rogers et al., Citation1991; Tuomi, Citation1992; Roggli et al., Citation1993; Mossman, Citation1993; Elmes, Citation1994; Ross and McDonald, Citation1995; Berman et al., 1995; Case et al., Citation1997;, McDonald et al., Citation1997; Smith and Wright, Citation1996; Smith, 1998; Dumortier et al., Citation1998; Schneider et al., Citation1998; Miller et al., Citation1999). It is now generally agreed that amphibole asbestos is more potent than chrysotile toward the induction of mesothelioma (see, e.g., CitationERG, 2003). It has even been proposed that chrysotile does not cause mesothelioma and that the amphibole asbestos contamination in chrysotile explains the cases of mesothelioma observed among cohorts exposed predominantly to chrysotile (the “amphibole hypothesis”).
Limited evidence also suggests a difference between the potency of chrysotile and amphibole asbestos in causing lung cancer, although this evidence is much weaker than that for mesothelioma (see, e.g., Stayner et al., Citation1996; Hodgson and Darnton, Citation2000; Berman and Crump, Citation2003, Citation2008). In fact, of the KL's estimated by Berman and Crump (Citation2008), both one of the smallest (from Quebec miners and millers, Liddell et al., Citation1997) and one of the largest (from South Carolina textile workers, Hein et al., Citation2007) are from populations exposed to primarily chrysotile. Thus, differences in mineral type do not entirely explain the variation in lung cancer potency factors from different environments.
Size and shape also play a role in the potency of asbestos fibers.1 Considerable evidence that longer fibers are more carcinogenic in animals comes from work conducted initially by Stanton and coworkers (Stanton and Wrench, Citation1972; Stanton et al., Citation1977, Citation1981) and confirmed by many others (Bertrand and Pezerat, Citation1980; Bonneau et al., Citation1986; Bolton et al., Citation1982, Citation1984, Citation1986; Davis et al., Citation1985, Citation1986a, Citation1986b, Citation1987, Citation1988; Muhle et al., Citation1987; Pott, 1982; Pott et al., Citation1974, Citation1976, Citation1987; Wagner et al., Citation1976, Citation1982, Citation1985; Wylie et al., Citation1987, Citation1993, Berman et al., Citation1995a, Citation1995b).
Fiber size and shape are also likely to be important in determining disease outcomes in humans (see, for example, Case et al., Citation2000; McDonald, 1998; Sebastien et al., Citation1989; Berman and Crump, Citation2003; Stayner et al., Citation2007). For example, it has been proposed that the large differences in estimates of lung cancer potency between Quebec miners and millers and South Carolina textile workers (both cohorts exposed mainly to chrysotile) may be due to differences in the lengths of fibers between the two locations (see, for example, Berman and Crump, Citation2003, Appendix D). Stayner et al. (Citation2007) found that within the cohort of South Carolina textile workers lung cancer was most strongly associated with thin (≤ 0.2 μ m) and long (≥ 10 μ m) fibers.
In this article, the effects of fiber size and mineral type upon the exposure-response relationships for lung cancer and mesothelioma are examined formally by expanding the U.S. EPA lung cancer and mesothelioma models to permit KL's and KM's to depend upon fiber type and size. These expanded models are fit in a meta-analysis to the KL's and KM's obtained in individual studies, while taking into account the uncertainty intervals established for these values (Berman and Crump, Citation2008, and ). The goal of this work is to identify fiber size- and type-specific potencies for both lung cancer and mesothelioma that are biologically plausible and that reconcile, or help to reconcile, observed differences in the potency of asbestos for causing lung cancer and mesothelioma estimated in different environments. If such potency assignments can be found, they could be used to assess risk to humans in a wide variety of exposure environments with more confidence than with current methods available for assessing risk.
A major obstacle in this effort is the lack of data for characterizing the types of fibers and distribution of fiber sizes to which studied populations were exposed. In most epidemiological studies asbestos exposure is, at best, quantified in terms of air samples evaluated using phase-contrast microscopy (PCM). PCM only takes account of structures longer than 5 μ m, thicker than about 0.25 μ m, and with an aspect ratio ≥ 3:1, and does not distinguish between asbestos fibers and nonasbestos particles, among different mineralogical types of asbestos, or among the different size fractions of fibers that are included in the overall PCM counts (NIOSH, Citation1994a, Citation1994b). Note that it is not clear that the potency of fibers excluded from PCM counts can always be assumed to be negligible.
The lack of data on fiber sizes specific to individual epidemiological studies was addressed in the present analysis by matching fiber size distributions in the published literature obtained using transmission electron microscopy (TEM) to individual epidemiological studies. TEM can identify the thinnest asbestos fibers and can distinguish among the different mineralogical types of asbestos.2 Matching was based on such factors as location (in a limited number of cases), type of operation, and major fiber type. Even aside from the uncertainty introduced by this matching process, the fiber size distributions obtained from the literature have inherent limitations that preclude a full investigation of the relative potency of fiber size categories that are likely to be important determinants of risk. For example, the largest cut-point for length in the fiber size distributions obtained from the literature is 10 μ m, which precludes examining the hypothesis obtained from animal studies that fibers longer than 40 μ m are especially potent (Berman et al., Citation1995a, Citation1995b).
A number of studies currently are planned or in progress to obtain better data on fiber size for a number of key environments, such as the Quebec mines and mills (Liddell et al., Citation1997) and Libby, MT, vermiculite mines and mills (Sullivan, Citation2007). These studies will use TEM to analyze archived air samples and/or samples of reconstructed dusts. When data from these studies become available, the methods from the present analysis can be reapplied to the improved data base.
METHODS
The Expanded Lung Cancer and Mesothelioma Models
Although, for definiteness, the following discussion refers specifically to lung cancer, it also applies with only minor changes to mesothelioma. In the U.S. EPA lung cancer model the relative mortality risk can be written aswhere KL is the lung cancer potency, YE10 is the cumulative years of exposure lagged 10 years, CPCM is the average air concentration of PCM fibers (fibers counted by standard PCM counting methods) during those years of exposure, and α is the background relative risk. Note that YE10 × CPCM is simply the cumulative exposure lagged 10 years, which makes this expression equivalent to Eq. (2) of Berman and Crump (Citation2008).
We now generalize Eq. (1) to allow KL to depend upon the lengths, widths, and mineral types of asbestos fibers. Although potency is likely to be a continuous function of length and width, as well as a function of fiber type [i.e., KL = f (length, width, type)] the data available on fiber size and type only support a very limited investigation of the possible relationships. Consequently, fibers are divided into a few discrete categories defined by mineral type (chrysotile or amphibole asbestos) and two or three size categories. The set of categories of fibers that are assigned nonzero (positive) potencies will be called an “exposure metric.” For example, the exposure metric defined by PCM analysis consists of all fibers longer than 5 μ m, thicker than about 0.2 μ m, and with an aspect ratio > 3:1.
Although fibers not included in an exposure metric are assigned zero potency in this formalism, this is only an approximation that facilitates testing of exposure-response hypotheses with the limited data that are available. Similarly, assigning equal potency to all fibers within each category of a metric should also be interpreted as an approximation.
Given an exposure metric as described above, the lung cancer model [Eq. (1)] is generalized aswhere i indexes the categories in the exposure metric, Σi indicates a sum over all exposure categories, Ci is the average air concentration of fibers in category i, and KLi* is the lung cancer potency of fibers in category i. This equation retains both the time dependence and linear exposure-response assumptions in the original lung cancer model [Eq. (1)], but allows different mineral types and sizes of asbestos fibers to have different potencies.
Combining Eq. (1) and Eq. (2) results in the formal equalitywhich provides a relationship between the KL based on the PCM metric and the KLi* based on the expanded metric.
Since the epidemiological studies do not contain the information necessary for estimating the concentrations Ci of fibers in categories of interest, these concentrations are estimated using surrogate data, including fiber size distributions obtained from air samples collected in various occupational environments and analyzed using TEM. This work is described in the next subsection of this paper. For now it is assumed that estimates are available for each epidemiological study of the fraction fi of fibers in each fiber category, i, in the exposure metric in Eq. (3) and also of the fraction, fPCME, of PCM-equivalent (PCME) fibers. PCME fibers are those asbestos fibers identified using TEM that are in the same size range as those counted by PCM.
The use of these surrogate data requires two assumptions:
The distribution of sizes and types of fibers in the surrogate data matches the distribution in the epidemiological study.
The concentration, CPCME, of PCME fibers determined by TEM is the same as the concentration, CPCM, that would be determined using PCM.
The validity of these assumptions is considered in a later section.
From assumption (1) it follows thatand from assumption (2)
where fi and fPCME are the fractions of total fibers represented by fibers in category i and PCME fibers, respectively.
Dividing the first equation by the second results inUsing this relationship, Eq. (3) can be rewritten as
The subscript “L” indicative of lung cancer has been dropped in Eq. (4) because an identical equation holds for mesothelioma. This equation is the basis for a statistical model that is used to estimate general (epidemiological study-independent) potency factors for lung cancer and mesothelioma from the potency factors (KL and KM) obtained from individual epidemiological studies (Berman and Crump, Citation2008, and ), which are based on PCM. By applying this model using different exposure metrics, the relative ability of the different metrics to reconcile the disparate KL's and KM's obtained from different epidemiological studies is evaluated. Before explaining the details of this estimation process, the surrogate fiber size data and the specific exposure metrics that were evaluated (along with the rationale underlying the selection of these metrics) are described.
Characterization of Fiber Size and Type Using Surrogate Data
Data on Fiber Size
The objective was to find data in the published literature that could be used to characterize the distribution of asbestos fibers in various size categories [for use in estimating the fi and fPCME in Eq. (4)] for each of the epidemiological studies for which KL or KM was calculated (Berman and Crump, Citation2008, and ). Data considered to be pertinent to a studied environment consist of results from TEM analyses of samples containing comparable kinds of asbestos and collected in the same location or from an environment involving a similar operation (e.g., mining, textile manufacture, etc.). A literature search identified several potential sources of such data (Cherrie et al., Citation1979; Dement et al., Citation2007; Dement and Harris, Citation1979; Gibbs and Hwang, Citation1980; Hwang and Gibbs, Citation1981; Marconi et al., Citation1984; Roberts and Zumwalde, Citation1982; Rood and Scott, Citation1989; Snyder et al., Citation1987; Winer and Cossett, Citation1979).
To minimize variability resulting from differences in TEM analysis methodology used in different studies, it was decided to employ distributions from common studies conducted by common groups of researchers, to the extent that this could be accomplished without substantially reducing the number of size-distribution/epidemiological-study (SD/ES) pairs available for inclusion in the analysis. Size distributions from studies containing the best documented procedures were favored. In the earlier analysis (Berman and Crump, Citation2003) the size distributions selected for use came from only two studies, which were reported in three publications (Dement and Harris, Citation1979; Gibbs and Hwang, Citation1980; Hwang and Gibbs, Citation1981). Recently a more detailed size distribution has been developed for the South Carolina textile plant (Hein et al., Citation2007) based on archived air samples from that plant (Dement et al., Citation2007). In the present work analyses were conducted both using the size distribution assigned to that plant in our original work (Berman and Crump, Citation2003) and using the newer size distribution from Dement et al. (Citation2007).
shows the pairing of size distributions from these four studies with epidemiological studies from which KL's or KM's were estimated in Berman and Crump (Citation2008). Note that no matching size distributions were found for four of the epidemiological studies for which KL's or KM's were estimated (Laquet et al., 1980; Enterline et al., Citation1986; Liddell et al., Citation1997—factory only; Sullivan, Citation2007).
TABLE 1 Pairings of epidemiological studies with fiber size distributions and associated uncertainty factors
The fiber size distributions are not all of equal relevance to the respective epidemiological studies to which they were paired. As indicated in , some of the distributions are based on data collected at the same facility (e.g., Quebec mines and mills and South Carolina textile plant); others are based on data collected at a similar facility (e.g., Italian mine and mill), and still others are based on a combination of data from similar facilities (e.g., Connecticut plant). Uncertainty factors () were developed subjectively to quantify the relevance of each fiber size distribution to its paired epidemiological study, where larger factors indicate a less certain relevance.
It should also be kept in mind that, although these fiber size distributions are based on air samples collected over a fairly narrow time range, they are used to represent the fiber size distributions throughout the exposure period, which in most of the epidemiological studies covers many years.
presents the resulting bivariate (length by width) fiber size distributions derived from the published TEM data and the representative KL and KM values from the paired studies shown in . Also listed in are the upper and lower bounds of the “uncertainty intervals” derived in Berman and Crump (Citation2008) for the KL and KM values.
TABLE 2 Representative KL and KM Values Paired with Averaged TEM Fiber Size Distributions From Published Papers
For most of the environments relevant to the epidemiological studies listed in , size distributions were reported for multiple subareas/operations. Consequently, the distributions in were derived as the unweighted average of the distributions reported for each subarea of the corresponding environment. Thus, for example, the distributions paired with all mining and milling studies represent the averages of the distributions reported for mining, milling, and bagging the particular type of fiber handled (e.g., chrysotile or crocidolite) (Gibbs and Hwang, Citation1980; Hwang and Gibbs, Citation1981). Similarly, the distribution for mixed asbestos-cement product manufacturing is the average of the distributions reported for manufacturing and finishing (Hwang and Gibbs, Citation1981). Also, for the data derived from Dement and Harris (Citation1979):
The distribution for textile manufacturing is the average for preparation, twisting, and weaving.3
The distribution for friction product manufacturing is the average for mixing, forming, and finishing.
The distribution for chrysotile asbestos-cement product manufacturing is the average for forming, mixing, and finishing.
The distribution for amosite insulation manufacturing is the average for mixing, forming, and finishing.
In contrast, the distribution for amosite insulation application is the distribution for amosite insulation finishing alone.
Justification for the preceding approach of averaging over multiple subareas/operations within a plant derives from the observations that (1) workers at these types of plants typically spend some time working under conditions representative of all operations and (2) the cohorts studied at these facilities typically include workers from all operations within a facility.
In addition to the uncertain relevance of the fiber size distributions to the cohorts to which they were paired, the distributions themselves have some inherent limitations. Except for the distribution from Dement et al. (Citation2007), the maximum cut point for length is 10 μ m, which precludes the possibility of exploring the effects of fiber categories with cut points longer than 10 μ m. With regard to width, the largest cut point that can be supported by all of the available size distributions is 0.3 μ m, due to the restrictions on the size distributions for chrysotile mining and milling reported in Gibbs and Hwang (Citation1980). However, a width cut point of 0.4 μ m was added to these distributions by assuming that the fraction of fibers between 0.3 and 0.4 μ m in width (a small contribution in all cases) remains proportional to the fraction of fibers thinner than 0.3 μ m for each length category (with the proportionality constant interpolated from the diameter distribution graph for chrysotile in of Gibbs and Hwang (Citation1980). We were unable to add a width cut point of 0.4 μ m for the Dement et al. (Citation2007) data.
Cut points for widths greater than 0.4 μ m can only be supported for size distributions derived from the paper by Dement and Harris (Citation1979) and Dement et al. (Citation2007), which are available only for 9 of the 15 SD/ES pairs available for the lung cancer analysis and 7 of the 11 SD/ES pairs available for the mesothelioma analysis. Thus, because too many studies would need to be eliminated, exposure metrics with cut points greater than 0.4 μ m for fiber width were not evaluated.
Due to limitations in the set of cut points in the published size distributions reported in , fPCME is approximated by the fraction of all fibers longer than 5 μ m and thicker than 0.2 μ m. This cut point for thickness is close to the 0.25 μ m minimum width assumed identifiable by PCM in NIOSH Method 7402 (NIOSH, Citation1994b) and, consequently, the error in this approximation is expected to be small.
Another limitation of the size distributions used in this analysis is that they do not provide information on the morphological types of fibers (e.g., fibrils or single-crystal fibers, bundles, clusters, or matrices). Moreover, because the counting rules were not clearly documented in the original studies (Gibbs and Hwang, Citation1980; Hwang and Gibbs, Citation1981; Dement and Harris, Citation1979), it is neither known to what extent such complex fibers were included in the reported distributions nor whether such considerations were applied consistently across the studies. Thus, consideration of the effects of fiber morphology on potency cannot be addressed with the available data.
Potentially, one of the most important limitations to the use of the surrogate data is the need to assume that CPCM = CPCME. There are two reasons this assumption may not be a good one. First, because PCM fiber counts do not distinguish between asbestos and nonasbestos fibers, while PCME fiber counts do, to the extent that nonasbestos PCM fibers are present in an environment, PCM counts may not match the PCME counts. This concern is especially important for environments in which dusty materials in addition to asbestos are handled, such as during manufacture of asbestos-cement pipe where elongated particles not composed of an asbestos mineral may nevertheless be counted by PCM. The second reason CPCM may not match CPCME is that PCM and PCME counts are derived at different magnifications, which can result in different judgments concerning the morphology and dimensions of specific fibers and, thus, whether they should be included for counting.
Because of these potential differences between PCM and PCME, the NIOSH Method 7402 (NIOSH, Citation1994b), which is designed to determine PCM/PCME concentration ratios, recommends that fiber concentrations derived, respectively, by PCM and TEM (i.e., PCME) should not be directly compared. Instead, it is recommended that the ratio of PCME asbestos fibers to all fibers in the PCME size range (i.e., including nonasbestos fibers) be derived solely from the TEM data and that the resulting ratio then be used to adjust the PCM measurements.
Data on Fiber Type
None of the studies from which the fiber size distributions were obtained contain information on the relative amounts of chrysotile and amphibole asbestos in each environment. Consequently, it was necessary to estimate these values separately. presents estimates of the fraction of total asbestos exposure contributed by amphibole asbestos in each environment, based on information on each environment available in the literature. Three estimates are provided in each case: a best estimate, a lower bound and an upper bound. Note that, although some of the cohorts were assigned lower bounds of zero for the percent amphibole asbestos, these are clearly underestimates. For example, lung tissue samples from Quebec miners and millers show concentrations of tremolite and commercial amphibole asbestos as well as chrysotile, proving exposure to amphibole asbestos in this cohort (e.g., Case, Citation1991; Sebastien et al., Citation1989) that varies across different subgroups in the cohort (e.g., McDonald et al., Citation1997), and tremolite is a known contaminant in ore (Sebastien et al., Citation1986, William-Jones et al., Citation2001) in several of the mines covered by the Quebec cohort (Liddell et al., Citation1997). The source of the information used to develop each estimate, as well as a brief description of how the estimate was developed, is also provided in the table. Due to lack of data upon which to base an alternate assumption, the fraction of amphibole asbestos assigned to a particular exposure environment is assumed to be the same for all size categories.
TABLE 3 Estimated Fraction of Amphiboles in Asbestos Dusts
Selection of Exposure Metrics to Investigate
Ideally, a broad range of exposure metrics would be fit to the expanded lung cancer and mesothelioma equations to identify an optimal metric for each disease that can be used for predicting asbestos-related cancer risk. However, the limitations of the available data for characterizing asbestos exposures, which were described earlier, place severe restrictions on the metrics that can currently be evaluated.
There is strong evidence that not all fibers included in the PCM exposure metric have equal potency (Berman and Crump, Citation2003). Results from animal and human pathological studies (Berman and Crump, Citation2003, Chapter 6 and Appendix D) indicate that fibers longer than 20 μ m need to be distinguished from shorter fibers to adequately predict human cancer risk. Moreover, findings from a meta-analysis of rat inhalation data (Berman et al., Citation1995a, Citation1995b) suggest that an exposure metric may need to distinguish the effects of fibers longer than 40 μ m from shorter fibers to adequately predict cancer risk. In contrast, with a singular exception (as previously indicated), the size distributions paired here with the epidemiological studies () only discriminate among concentrations of fibers in categories up to 10 μ m in length, with all longer fibers grouped together.
Although the evidence on fiber width is not as strong as that for length, fiber widths that correlate best with biological activity are likely to be thinner than those included in the PCM metric. Moreover, the PCM metric does not exclude fibers that are too thick to be respirable or even deposited during mouth breathing (thicker than approximately 1.5 μ m; CitationERG, 2003). Stayner et al. (Citation2007) found that, among workers in the South Carolina textile factory, lung cancer was most strongly associated with long (> 10 μ m), thin (≤ 0.25 μ m) fibers. Similarly, Berman et al. (Citation1995a, Citation1995b) found that thin (≤ 0.4 μ m) fibers correlated best with lung cancer potency in animals. Unfortunately, the size distributions available for this analysis permit only a limited evaluation of the effect of width, as the only width cut points available are 0.2 μ m, 0.3 μ m, and 0.4 μ m.
An expert panel (CitationERG, 2003) reviewed a draft of a preliminary version of this work that proposed a metric composed of four categories of fibers thinner than 0.5 μ m (i.e., separate categories for amphibole asbestos and chrysotile in each of two length categories, one between 5 and 10 μ m and the other longer than 10 μ m). These size ranges were based on those derived from a meta-analysis of rat inhalation studies (Berman et al., Citation1995a, Citation1995b), except that the cutoff for the longest length category from the rat data (40 μ m) was reduced to 10 μ m to accommodate the available size data considered here and the width cut point was increased from 0.4 to 0.5 μ m because this was thought to facilitate reliable laboratory analysis. The panelists agreed that there is a considerably greater risk for lung cancer from fibers longer than 10 μ m than from shorter fibers. However, the panel was uncertain as to an exact cut point for length. They were also uncertain whether the optimal size categories for lung cancer and mesothelioma would precisely conform. A few panelists indicated that widths up to 1.5 μ m may be important because at least some of the fibers this thick can reach the deep lung during mouth breathing. There was general agreement that the diameter cutoff should be between 0.4 μ m and 1.5 μ m.
In light of the recommendations of the expert panel while given the severe constraints imposed by the limitations in the available fiber size data, it was decided to focus attention on exposure metrics incorporating four categories of fibers defined by two types of asbestos (chrysotile and amphibole asbestos) and two lengths (5 < length < 10 μ m, length > 10 μ m), within a specified width category. Four width categories were separately considered (< 0.2 μ m, < 0.4 μ m, > 0.2 μ m, and all widths).
Specific Approach for Estimating Category-Specific Potencies
The four categories of the exposure metrics defined, as described earlier, by two lengths categories of chrysotile and amphibole asbestos are linked with the notation of Eq. (4) by identifying the potencies (Ki*) of different fiber categories and fractions of fibers, fi, associated with these categories, as follows:
Since the relative proportion of chrysotile in each environment is assumed to be the same for short and long fibers, all four of the potencies listed in the preceding table cannot be estimated independently. Consequently the following parameters are introduced:
rps: The relative potency of short to long fibers.
rpc: The relative potency of chrysotile compared to amphibole asbestos. It needs to be emphasized that this refers to pure chrysotile uncontaminated by amphibole. Similarly, all estimates of the potency of chrysotile made herein refer to pure chrysotile. Thus, to estimate risks in real environments using our results, contributions from contaminating amphibole asbestos would have to be explicitly added.
This results in:where KA* is the lung cancer potency factor for long amphibole asbestos fibers.
The fraction of fibers in the short fiber category (fS) and the long fiber category (fL) in each environment are derived from the distributions in . Since these data do not provide separate information for chrysotile and amphibole asbestos, it was assumed that the relative fractions of fibers in the size intervals shown in are the same for chrysotile and amphibole asbestos. Separately, estimates of the relative amount of amphibole (fA) to which cohorts were exposed in each environment are provided in . The fraction of fibers in each of the four categories is then estimated as follows:
With this notation, Eq. (4) can be written aswhere the subscript j has been added to indicate quantities that are specific to a given epidemiological study and whose value is obtained from and .
Equation (5) forms the basis for a statistical model that is fitted to the potency values in for lung cancer (KL's) or mesothelioma (KM's) from the individual epidemiological studies to estimate the potency, KA*, of long amphibole fibers, the relative potency, rps, of short fibers compared to long fibers, and the relative potency, rpc, of chrysotile compared to amphibole asbestos.
To fit Eq. (5) to the data in and it is assumed that ln(Kj) has a normal distribution with mean given by the logarithm of the right side of Eq. (5). The standard deviation of ln(Kj) is assumed to be composed of two components. One component, σj, is study specific and reflects the uncertainty in the Ki values represented by the uncertainty bounds listed in and the uncertainty in the relevance of the size distributions applied to each environment, as reported in . Specifically, the upper bound of the uncertainty interval for each Kj in is multiplied by the uncertainty factor in and divided by the point estimate of Kj from . The log transform of the result, divided by 2, is defined as σj. A second component, σ, of the standard deviation, which is not study-specific, may be thought of as representing the uncertainty in the Kj estimates resulting from random variation across studies that is not represented in the σi. The overall standard deviation of ln(Kj) from study j is assumed to be (σj2+ σ 2)1/2.
The unknown parameters in the model (KLA*, rps, rpc, and σ) are estimated by the method of maximum likelihood and likelihood ratio tests are used to test hypotheses regarding these parameters (Cox and Hinkley, Citation1974). Separate analyses are conducted for lung cancer and mesothelioma.
RESULTS
Pairing of available size distributions with published epidemiological studies () allows for applying the statistical model defined by Eq. (5) to 15 studies for lung cancer and 11 studies for mesothelioma (). Since results using the original size distribution for South Carolina were very similar to those obtained using the Dement et al. (Citation2007) distribution, with one exception only, results based on the Dement et al. distribution are presented. The exception is the analysis for the metric with fiber widths < 0.4 μ m, which utilizes the original distribution (from Dement and Harris, Citation1979) because we do not have data from Dement et al. needed for that analysis. It should be kept in mind that potency estimates in are comparable only when based on the same category of fibers; otherwise, the relative abundance of fibers in each category affects the comparison. For example, a potency for a subcategory of widths (all else being equal) will necessarily be larger than a potency for all widths because there are fewer fibers in the subcategory.
TABLE 4 Results of fitting different exposure metrics to potency factors for mesothelioma and lung cancer
For mesothelioma, the hypothesis that chrysotile and amphibole asbestos are equally potent (rpc = 1) is strongly rejected for all metrics (). In the metrics examined, chrysotile is estimated as being nonpotent for three metrics and 900 or 2000 times less potent than amphibole asbestos in the remaining two analyses. The hypothesis that chrysotile has zero potency for mesothelioma is accepted for all metrics.
Although tests of hypotheses regarding width were not conducted (because the categories are not nested), some idea of the effect of width can be obtained from the values of the log-likelihoods.4 However, when comparing the values of the log-likelihoods in , it is important to remember that the metric with widths < 0.4 μ m was fitted to a data set in which the size distribution for the South Carolina cohort was from Dement and Harris (Citation1979) whereas all other metrics were fitted using the size distribution from Dement et al. (Citation2007) for this cohort. As previously indicated, this was due to lack of a cut point at a width of 0.4 μ m in the newer data set. Although not shown, results of tests of hypotheses and the relative values of the log-likelihoods for other metrics are comparable no matter which of the size distributions was used for the South Carolina cohort. However, the absolute values for the log-likelihoods vary somewhat depending on which distribution is used. Thus, effects due to differences in the size distribution employed for South Carolina must be considered when comparing the log-likelihood of the fit for widths < 0.4 μ m to fits for other widths.
The log-likelihood resulting from the fit of the “all widths” metric to the data set with only the older size distributions (−9.28) is virtually identical to that for the widths < 0.4 μ m (−9.30) fit to the same data set. Thus, the near unit difference in likelihoods for these two metrics apparent in is due entirely to differences in the data sets that were fitted so that both metrics in fact fit the data equally well and substantially better than any of the other metrics examined. That the “all widths” and widths < 0.4 μ m metrics fit the data equally well implies, among other things, that the available cohort studies may not be sufficiently rich (varied in exposure characteristics) to fully explore the effects of fiber width on mesothelioma potency.
As indicated by the log-likelihoods in , the fit of the traditional PCM metric is substantially worse than the fits using either the “all widths” or widths < 0.4 μ m metrics. This remains true even when rps and rpc are estimated (rather than fixed at 1). Thus, at least for mesothelioma, the “all widths” and widths < 0.4 μ m metrics provide a substantial improvement in their ability to predict risk over the PCM metric (even with rps and rpc estimated). The fit using the thinnest metric (widths < 0.2 μ m) is also substantially worse than the fit obtained using either the “all widths” or widths < 0.4 μ m metrics, which suggests that the thinnest metric potentially excludes fibers that are important contributors to mesothelioma risk.
For the “all widths” and widths < 0.4 μ m metrics, the hypothesis that rps = 1 is nearly rejected (p < .1 for both) and the best estimate for the relative potency of the shorter structures for both of these metrics is zero. This implies that, with fiber type and width addressed, fibers as long or longer than the maximum cut point available in the current database are the most potent.
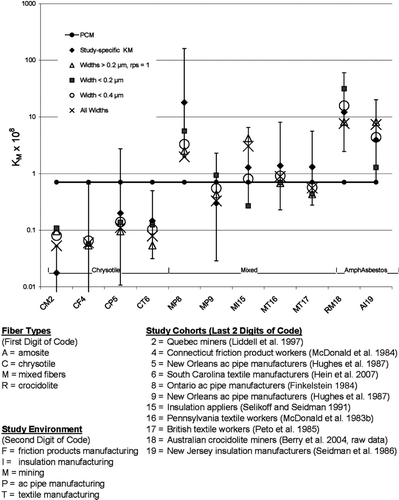
The findings reported thus far are further reinforced by the impressions gleaned from . compares the predicted study-specific potencies for mesothelioma computed using the right side of Eq. (5) for five of the metrics studied in to the KM's calculated from the epidemiological studies themselves (). The fiber size distributions and the percents of amphibole asbestos needed for this graph come from and . The vertical bars in the graph depict the uncertainty bounds for the study-specific Km's (derived from the individual epidemiological studies - ).
In , the traditional PCM metric, which assigns the same potency to all studies, clearly provides the worse fit. This metric overestimates the observed potencies (KM) for Quebec chrysotile mines and mills (Liddell et al., Citation1997) and the South Carolina textile plant (Hein et al., Citation2007) and underestimates the potencies for the Ontario factory (Finkelstein, Citation1984) and the Wittenoom, Australia, crocidolite mines (Berry, Citation2004). The predictions of the other four metrics all lie within the uncertainty bounds of all of the study-specific KM's except for Quebec mines and mills, although, even in this case, they are close. Thus, the “all widths” and widths < 0.4 μ m metrics proposed in this study offer a substantial improvement over the traditional PCM metric in the confidence with which mesothelioma risk can be predicted, although even further improvement appears possible.
presents the results of a limited sensitivity analysis of the mesothelioma model based on the “all widths” metric (which is the metric providing the best overall fit to the complete set of data incorporating Dement et al., Citation2007). It appears that the Quebec study (Liddell et al., Citation1997) is an outlier in , since the predictions of none of the metrics lie within the uncertainty bounds for that KM. Likewise, the Ontario study (Finkelstein, Citation1984) appears to be an outlier in the general trend of increasing KM's as one moves from environments involving primarily chrysotile exposures to environments involving exposures to mixed types of fibers, and then to environments involving exposures to predominantly amphibole asbestos. contains results from fitting the “all widths” metric to data sets with each of these studies, plus the South Carolina study (Hein et al., Citation2007), respectively, omitted. Also, to explore the effect of the values assumed for percent amphibole asbestos in each study, the “all widths” metric is applied after replacing the best estimate of percent amphibole asbestos in each study by the lower bounds or upper bounds of the estimated range of percent amphibole (), respectively.
TABLE 5 Results of sensitivity analysis
Replacing the estimates of percent amphibole asbestos by their lower bounds from causes the potency of long amphibole fibers (length > 10 μ m) towards mesothelioma to increase by about a factor of 2 and the potency of long chrysotile fibers to increase from zero to 1/350th of that of long amphibole asbestos (). The hypothesis that chrysotile is equally potent with amphibole asbestos (rpc = 1) remains strongly rejected. However, the hypothesis that chrysotile is non-potent (rpc = 0) is now rejected. This may be due to the lower bounds on percent amphibole asbestos having been assigned values of zero in several studies involving predominately exposure to chrysotile (), which is clearly an underestimate (as previously indicated, the bounds in are extreme). As a consequence, if even one mesothelioma was detected in only one such study, the hypothesis that rpc = 0 must be rejected because under the assumptions of the model [Eq. (5)] there is zero probability of a mesothelioma whenever rpc = 0 and no amphibole asbestos is present. Thus, that the hypothesis rpc = 0 was rejected under these conditions is to be expected. Nevertheless, even in this extreme case, chrysotile is still estimated as being far less potent than amphibole asbestos in causing mesothelioma.
Replacing percents of amphibole asbestos by their upper bounds () causes the potency of long amphibole fibers to decrease by about a factor of 2, worsens the overall fit somewhat, and causes rps to be estimated as nonzero (although not significantly different from zero), but otherwise has little effect. Individually omitting the Quebec, South Carolina, or Ontario cohort from the data set also has little effect other than causing the estimated potency of long chrysotile fibers to be positive in two cases (although still 200 or 1,000 times smaller than the potency of long amphibole fibers) and, when omitting either South Carolina or Quebec, causes rps to have an insignificant, positive value.
Turning now to lung cancer, long chrysotile was estimated as being less potent than long amphibole asbestos for lung cancer by factors ranging between 6 and 60 (). However, the confidence intervals for the two potencies overlap for all of the metrics except the one based on widths < 0.2 μ m. Hypotheses that the two fiber types are equally potent are rejected for the two metrics based on thin fibers (widths < 0.4 μ m or < 0.2 μ m) and nearly so for the metric including all widths (p < .07), but not for the metrics based on widths > 0.2 μ m (i.e., the PCM metric with or without rps estimated). The hypothesis that chrysotile has zero potency for lung cancer (rpc = 0) was rejected or nearly so (.01 ≤ p ≤ .06) with all metrics except the one based on fibers thinner than 0.2 μ m.
Based on a comparison of the log-likelihoods and remembering that the metric of fibers with widths < 0.4 μ m was fitted to a data set containing a size distribution from a different source (Dement and Harris, Citation1979, vs. Dement et al., Citation2007) for the South Carolina cohort, the effect of width on lung cancer potency can be evaluated. The log-likelihood resulting from the fit of the “all widths” metric to the data set with only the older size distributions (–15.65) is only half a unit smaller than that for the widths < 0.4 μ m (–16.16) fit to the same data set. Thus, the more than unit difference in likelihoods for these two metrics apparent in is largely due to differences in the data sets that were fitted so that the fit of the “all widths” metric to the lung cancer data is only marginally better than that for the metric with widths < 0.4 μ m and both provide better fits than the other metrics listed. Among other things, when coupled with the mesothelioma results, these results even more strongly reinforce the implication that the available set of cohorts are not sufficiently rich (varied in exposure characteristics) to fully explore the effects of fiber width on potency.
As indicated by the log-likelihoods in , the fit of the traditional PCM metric is somewhat worse than the fits using either the “all widths” or widths < 0.4 μ m metrics. However, when rpc and rps are allowed to vary, the fit with the PCM metric is improved so that the difference between this metric and either the “all widths” or the width > 0.4 μ m metrics becomes marginal. Thus, for lung cancer, the “all widths,” widths < 0.4 μ m, and widths > 0.2 μ m metrics all provide some improvement in their ability to predict risk over the traditional PCM metric, although the improvement is not as dramatic as for mesothelioma. The fit using the thinnest metric (widths < 0.2 μ m) is also marginally worse than the fits to the “all widths,” widths < 0.4 μ m, and widths > 0.2 μ m metrics, but again the differences in these fits are not as large as they are for mesothelioma. Among other things, this suggests that the affect of fiber width on potency may be somewhat different for lung cancer than for mesothelioma.
The hypothesis that rps = 1 is rejected for the widths < 0.4 μ m metric and nearly so for the “all widths” and widths < 0.2 μ m metrics (p < .1) and the best estimate of the relative potency of short fibers (5 μ m < L < 10 μ m) is zero for all of the metrics except the thinnest. For the thinnest metric, the relative potency of short fibers is estimated to be a little more than 1/20th of the longer fibers. Thus, with fiber type and width addressed, fibers as long as or longer than the maximum cut point available in the current database are the most potent for both lung cancer and mesothelioma (see earlier discussion).
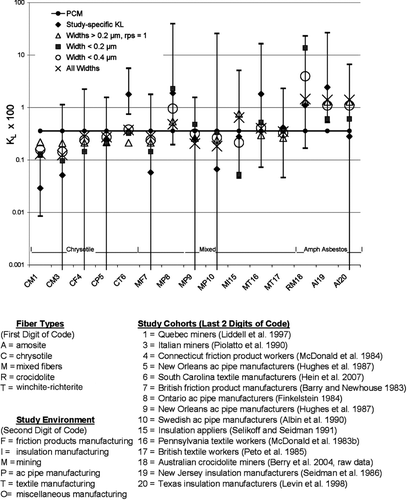
, constructed in a manner identical to , compares the predicted study-specific potencies for lung cancer computed using the right side of Eq. (5) for five of the metrics studied in to the KL's calculated from the epidemiological studies themselves (). In , the traditional PCM metric, which assigns the same potency to all studies, provides the worse fit. This metric overestimates the observed potencies (KL) for Quebec chrysotile mines and mills (Liddell et al., Citation1997) and underestimates the potencies for both the South Carolina textile plant (Hein et al., Citation2007) and the Paterson, NJ, insulation plant (Seidman et al., Citation1986). However, fits by the other metrics may be only marginally better, as none of the metrics reconciles the disparity between the Quebec and South Carolina studies; the predictions of all the metrics lie outside of the uncertainty bounds for both studies (although the overestimation for the Quebec study is marginal). Thus, it is apparent that the “all widths,” widths < 0.4 μ m, and widths > 0.2 μ m metrics evaluated in this study provide some improvement over the traditional PCM metric in the confidence with which lung cancer risk can be predicted, although further improvement is clearly possible. Specifically, a factor is needed to explain the difference between the lung cancer potencies observed among Quebec miners and millers and South Carolina textile workers.
also presents the results of a limited sensitivity analysis of the lung cancer model. Replacing the estimates of percent amphibole asbestos by either their lower or upper bounds results in little change from the fit with the best estimates of these percents. Potency estimates for both long amphibole and long chrysotile fibers vary by less than 25% from their original values in these runs, and none of the conclusions change concerning hypothesis testing for rpc = 1, rpc = 0, rps = 1, or rps = 0. Also, as previously noted, the best estimate for rps remains zero for these fits.
Individually omitting the Quebec cohort causes the estimate for the potency of long chrysotile fibers to approximately double, but otherwise has little effect on overall conclusions. Omitting the Ontario cohort has only marginal effects on any parameter estimate. Omitting the South Carolina cohort causes the estimate for the potency of long amphibole to approximately double, the potency of long chrysotile to decrease to about one-third of its original value, and causes the hypothesis that rpc = 1 to be strongly rejected.
Finally, to evaluate the potency of very short fibers, the four metrics based on “all widths,” and widths > 0.2 μ m, < 0.4 μ m, and < 0.2 μ m, were expanded by adding a third length category composed of fibers shorter than 5 μ m. The potency of fibers in this shortest category was estimated as zero for both lung cancer and mesothelioma for all metrics except that with widths > 0.2 μ m. For the metric with widths > 0.2 μ m, fibers shorter than 5 μ m were estimated as being 0.05 as potent as fibers longer than 10 μ m for mesothelioma and 0.001 as potent for lung cancer. In no case could the hypothesis that fibers shorter than 5 μ m are nonpotent be rejected. The hypothesis that all three categories of fiber length have equal potency was rejected (p ≤ .05) for all metrics for both lung cancer and mesothelioma except in the case of mesothelioma and lung cancer based on fibers with widths > 0.2 μ m, a metric that provides a poorer overall fit to the data in any case (discussed earlier).
DISCUSSION
The analyses presented herein provide consistently strong evidence that chrysotile is considerably less potent than amphibole asbestos in causing mesothelioma. The best estimates of the potency of chrysotile ranged from zero only up to 1/200th of the potency of amphibole asbestos. The hypothesis that chrysotile and amphibole asbestos are equally potency was firmly rejected in all cases. Furthermore, the hypothesis that chrysotile does not cause mesothelioma could not be rejected in any analysis that allowed at least some amphibole contamination in locations where exposures were principally to chrysotile. The situation was less clear for lung cancer. Although the best estimates of the potency of chrysotile were at least sixfold smaller than corresponding estimates for amphibole asbestos, the test of the hypothesis that the two fiber types are equally potent reached conventional levels of significance with some metrics but not with others. These analyses also provide evidence that the fibers longer than 10 μ m are more potent than shorter fibers for both mesothelioma and lung cancer, with the best estimate of the potency for short fibers being zero for the best fitting “all widths” and widths < 0.4 μ m metrics. Also for these metrics, the test of equal potency for the shorter and longer fibers (rps = 1) is rejected for the width < 0.4 μ m metric fit to the lung cancer data and nearly rejected for the “all widths” metric fit to lung cancer and both metrics fit to mesothelioma (p < .09).
The metric based on fibers with widths > 0.2 μ m and with rpc = rps = 1 () is equivalent to the PCM metric used in the EPA 1986 update. In the current analysis, this metric predicts a common potency across all studies ( and ), which is 0.71 × 10− 8 for mesothelioma and 0.36 × 10− 2 for lung cancer (). These values are both similar to, but somewhat smaller than, the values obtained in the EPA 1986 update (1.0 × 10− 8 for mesothelioma and 1 × 10− 2 for lung cancer), which may be due at least in part to the present analysis utilizing an expanded and more current database than was available for the 1986 update.
Note that in the metrics based on thin fibers (widths < 0.4 μ m or < 0.2 μ m) both overestimate the observed potency of mesothelioma in the Wittenoom cohort (Berry et al., Citation2004), whereas the metric based on fibers of all widths underestimates the observed potency. However, in other environments the metric based on fibers of all widths predicts a higher potency than the metrics based on thin fibers. This illustrates the important point that no metric can automatically be considered to be “more conservative” than others overall. In particular, metrics that incorporate expanded categories of fibers are not necessarily more health protective. Thus, for example, including fibers shorter than 5 μ m in a metric would not necessarily be health protective, even if these fibers actually make some contribution to risk. These points are explored more fully elsewhere (Berman, Citation2008).
None of the metrics evaluated in this study fully reconciles the observed disparities in study-specific KL's and KM's. In particular, these metrics provide only marginal improvement in reconciling the disparity in the KL's observed, respectively, among the Quebec mining cohort (Liddell et al., Citation1997) and the South Carolina textile factory cohort (Hein et al., Citation2007). Both of these studies are large and of high quality, as indicated by their relatively narrow uncertainty bounds. Likewise, although none of the metrics evaluated here fully reconcile the mesothelioma potencies (KM's) observed among Quebec miners and millers with the potencies observed in the other cohorts studied, the “all widths” and widths < 0.4 μ m metrics provide substantial improvements over the traditional PCM metric in the confidence that can be placed in predicting mesothelioma risk.
It is also interesting (although not entirely surprising) to note that the metric with the thinnest widths (< 0.2 μ m) gave a worse fit to the lung cancer data than either the metric with slightly thicker fibers (widths < 0.4 μ m) or the “all widths” metric. While recent evidence from a human study (Stayner et al., Citation2007) and earlier evidence from animal studies (e.g., Stanton and Wrench, Citation1972; Stanton et al., Citation1977, Citation1981) suggest that the thinnest fibers (those thinner than 0.25 μ m) best predict lung cancer risk, our earlier meta-analysis of the animal inhalation data (Berman et al., Citation1995a, Citation1995b; Berman and Crump, Citation2003, Appendix C) suggested that the dependence is a very strong function of the specific width cutoff and that the optimal range lies between 0.3 and 0.4 μ m (inclusive). These observations are probably a consequence of modeling what is likely a continuous function (of the effect of width on potency) by a severely restricted step function (defining a specific size range of fibers assigned equal potency with all excluded fibers assumed nonpotent). If the cutoff is set too narrow, too many fibers that contribute to potency may be excluded. If it is set to wide, too many fibers that contribute less substantially to potency may be included. At the same time, the good fit of the “all widths” metric found here for the human data may suggest that the current data set of cohorts is insufficiently rich (in the diversity of the exposure characteristics represented) to fully evaluate the effect of width.
It may be that the inability of these metrics to completely resolve the discrepancies among the study-specific KL's and KM's is due to limitations in the database available for this analysis (including the size distribution data in particular). It has been suggested by several authors (see Berman and Crump, Citation2003, Appendix D) that exposures among the Quebec miners and millers and the South Carolina textile factory workers differ primarily in the size of fibers, with those longer than the maximum cut point 10 μ m being particularly important. Thus, it may not be possible either to further improve understanding of the effects of fiber size and type on potency or to further reconcile the apparent disparities among study-specific potency estimates until better data describing fiber size and type become available.
Dement et al. (Citation2007) analyzed archived air samples from the South Carolina textile factory using TEM. By using analysis methods designed to enhance the counting of long fibers, they obtained a much more detailed characterization of the fiber size distribution for this plant than is available for other locations where epidemiological studies have been carried out. Supporting this distribution were > 3,000 fibers with lengths between 15 and 40 μ m and > 1,000 longer than 40 μ m. Thus, with these data it should be possible to characterize the fiber distribution rather accurately out to lengths > 40 μ m. Although these data were available for the present analysis, they could not be fully taken advantage of because the longest length category available in distributions for all other environments was only > 10 μ m. There is a particular need to obtain fiber size data for the Quebec mines and mills similar to that available for South Carolina in order to be able to determine whether the disparity in KL's from these two studies can be reconciled by considering metrics composed of categories of longer fibers.
Not only were the fiber size distributions utilized in this study limited in the size categories that could be examined, but some of the TEM procedures were not well documented and in some cases they were paired with epidemiological studies from locations other than from where the underlying air samples were collected. Given the limitations of the available data, perhaps the findings of this article should be considered a proof of concept more than a final result. The methods employed in this article can be used to incorporate better data, once they become available. Thus, the metrics proposed here should be considered as interim metrics with later refinements expected. At the same time, since these metrics show substantial improvement in performance relative to the traditional PCM metric (particularly toward the induction of mesothelioma), they should be considered for use in evaluating risk in exposed populations,4 subject to certain constraints indicated next, while further refinement continues.
Limitations to the Application of Metrics Evaluated in This Study
As indicated earlier, (1) arbitrarily applying metrics that incorporate counts of larger numbers of fibers does not automatically assure increased health protectiveness and (2) applying different metrics in different environments may result in shuffling of the relative risks predicted in such environments. To minimize the decision errors that may otherwise result from these effects, the range of conditions over which particular metrics may be applied to assess asbestos-related cancer risk needs to be carefully prescribed. For example, a metric should not be applied in environments where exposure characteristics vary radically from the range of characteristics included in the set of studies used to define the metric. These issues are particularly important when considering the assessment of risk in mixed-dust environments (environments in which dusts contain substantial numbers of both asbestiform fibers and nonasbestiform particles of similar mineralogy).
Except for the three mining studies (Liddell et al., Citation1997,; CitationPiolatto et al., 1990; Berry et al., Citation2004), all of the epidemiological studies evaluated here involve exposure to milled asbestos. As asbestos is milled explicitly to concentrate the highly fibrous components (Walton, Citation1982; La Ville de Thetford Mines, Citation1994; Smith, Citation1968), the three mining studies are likely to involve exposures containing the greatest fraction of nonasbestiform particles.
Surprisingly, the fractions of fibers thicker than 0.4 μ m (among fibers longer than 5 μ m) in the three mining environments () are among the smallest fractions of all the distributions presented. Given the characteristic distribution of widths anticipated among populations of asbestiform fibers and nonasbestiform particles (Veblen and Wylie, Citation1993; Wylie et al., Citation1982, Citation1993), a much larger fraction of thick fibers is expected in these environments. Whether this is due to an actual lack of nonasbestiform particles or to an artifact in the manner in which the size distributions were analyzed in these environments cannot currently be determined.
Among environments in which fibers are predominantly asbestiform, the majority of fibers thicker than 0.5 μ m are expected to be bundles (Veblen and Wylie, Citation1993; Virta et al., Citation1983; Wylie et al., Citation1982, Citation1993) and, as described in greater detail in the discussion of crystalline habit later, bundles may be substantially more potent toward the induction of cancer than single-crystal fibers. Therefore, it may not be appropriate to apply the metrics proposed here to environments in which the majority of thicker fibers may be (nonasbestiform) single crystals. Unfortunately, most the size distributions relied upon herein (Gibbs and Hwang, Citation1980; Hwang and Gibbs, Citation1981; Dement and Harris, Citation1979) do not distinguish between fibers and bundles, so that it is not currently possible to evaluate such considerations.
Inferences Regarding Future Refinements
Inferences gleaned from this study are supplemented by inferences derived from an earlier rat inhalation study (Berman et al., Citation1995a, Citation1995b), the recommendations of the peer consultation panel (CitationERG, 2003), and the broader literature, to suggest a range of modifications that may be needed to define metrics that adequately reconcile the human lung cancer and mesothelioma data. Such modifications may involve fiber length, fiber width, mineralogic type, crystallographic habit, and/or biodurability. These are each separately addressed next.
Fiber Length
The findings from this article, those from the earlier rat inhalation study (Berman et al., Citation1995a, Citation1995b), inferences from the general literature (see Berman and Crump, Citation2003, Chapter 6 and Appendix D), and recommendations from the peer-consultation panel (CitationERG, 2003) are all in general agreement with regard to fiber length. Based on these sources, as previously indicated, it is likely that length categories with minimum cut points substantially longer than 10 μ m contribute most heavily to both asbestos-induced lung cancer and mesothelioma. Much evidence indicates that fibers with minimum lengths at least as long 20 μ m will need to be separately delineated (see Berman and Crump, Citation2003, Chapter 6 and Appendix D), and some evidence also suggests fibers as long as 40 μ m need to be separately delineated (Berman et al., Citation1995a, Citation1995b).
Importantly, the preceding implications do not necessarily mean that shorter fibers are nonpotent. However, they do mean that short fibers are likely to be substantially less potent than longer fibers. Moreover, the relative weights to be assigned to the length categories of any future metrics should be explicitly determined by fitting the metric to the available human epidemiological data using methods similar to those illustrated herein.
Several researchers (for example, Lippmann, Citation1988, Citation1994, Citation1999; Timbrell, Citation1989; CitationERG, 2003) have suggested that the induction of lung cancer and the induction of mesothelioma depend differently on fiber length, so that separate metrics may ultimately need to be developed for each disease. Once suitable data become available, such hypotheses should be formally evaluated.
Fiber Width
The effect of fiber width on potency is substantially cloudier than that of fiber length. This is because the currently available evidence conflicts, because of limitations in the information on fiber width available from studies of both experimental animal and human populations exposed to asbestos, and because the consideration of width is unavoidably confounded with consideration of crystalline habit.
Clearly it would be appropriate for a metric to include only respirable fibers. Based on the best available evidence, the effective upper limit to respirability of asbestos fibers ranges somewhere between 0.7 and 1.0 μ m, with fibers as thick as 1.5 μ m potentially inhalable during mouth breathing. Inferences from both the general literature (Berman and Crump, Citation2003, Chapter 6 and Appendix D) and the recommendations of the peer-consultation panel (CitationERG, 2003) are in general agreement in this area. It should also be noted that defining an upper bound to width would facilitate reproducibility across laboratories because they would know more precisely which fibers to count and which not to count.
Beyond the points just dicussed, inferences from an analysis of rat inhalation data (Berman et al., Citation1995a, Citation1995b) suggest that factors in addition to respirability may further restrict the widths of asbestos fibers that contribute most substantially to cancer risk. In that study and the wider range of fits evaluated to support that study (see Berman and Crump, Citation2003, Appendix C), the ability to fit the animal tumor data tended to increase substantially with increasing widths up to a maximum of approximately 0.4 μ m and then decreased radically with further increases to width. Such effects may center on a somewhat different overall range of widths in humans than in rats, due to differences in physiology. However, the current inability to explore a broad range of width categories with the human data (due to the limitations of the published size distributions suitable for pairing with the human epidemiological data) suggests that any current exploration of the effects of width based on the human data should be interpreted with utmost caution.
Given all of the preceding description, it is likely that a range of width categories (with a maximum width of approximately 1.5 μ m) may need to be separately delineated to define metrics suitable for predicting human cancer risk. As some have suggested that fiber width differentially affects potency toward lung cancer and mesothelioma (for example, Lippmann, Citation1988, Citation1994, Citation1999; Timbrell Citation1989; CitationERG, 2003) and this is also suggested by the findings reported in this article, it is also likely that ultimately the metrics to predict each disease will reflect different dependence on width.
Unfortunately, as previously indicated, size-distribution data currently available for pairing with the human epidemiological data () do not allow for evaluation of width categories with the cut points needed to adequately evaluate the effects of width. Thus, additional fiber size distributions need to be developed to allow the effect of fiber width upon potency to be fully explored.
Fiber Mineralogy
The findings from this paper and the recommendations of the peer consultation group (CitationERG, 2003) suggest that chrysotile and amphibole asbestos exhibit substantially different potency toward the induction of mesothelioma and perhaps (much less dramatic) differences toward the induction of lung cancer. Inferences from the general literature are also in substantial agreement with these observations (Berman and Crump, Citation2003). As some have also suggested that amphibole asbestos should be further divided into its specific mineralogic subgroups (for example, Hodgson and Darnton, Citation2000), hypotheses concerning such differences should be considered to the extent that new data ultimately allow it.
Crystallographic Habit
The question of whether asbestiform fibers and nonasbestiform particles of similar size exhibit similar potency toward the induction of cancer is controversial (for example, OSHA, Citation1992; ATS, Citation1990; CitationERG, 2003). A major obstacle to resolving this issue is lack of a procedure for reliably distinguishing between individual particles of each type (Middendorf et al. Citation2007).6
At the same time, as previously discussed (Berman and Crump, Citation2003), identifying exposure metrics and their corresponding potency factors that adequately capture the relationships between fiber size, mineral type, and biological activity may potentially render this controversy moot. This is because, while it is difficult to distinguish among individual fibers that are asbestiform or not, populations of each of these types of particles exhibit distinct size distributions with only limited overlap (Virta et al., Citation1983). Consequently, the relative contributions of these two types of particles to cancer potency may be implicitly addressed by using exposure metrics that allow different size ranges of fibers to be assigned different potencies.
Bundles may pose a special problem in distinguishing between risk posed by asbestiform fibers and nonasbestiform particles, since for structures thicker than about 0.5 μ m, the majority of asbestiform fibers are comprised of bundles while virtually all nonasbestiform particles are single crystals (Veblen and Wylie, Citation1993; Virta et al., Citation1983; Wylie et al., Citation1982, Citation1993). This appears to be true even of amosite, as the mean thickness of single crystals in amosite may only be about 0.35 μ m and, given that such distributions are skewed, the median thicknesses are likely even narrower (Wylie et al., Citation1982). When single-crystal fibers get into the lung, they tend to behave as individual particles because they do not readily divide. In contrast, because they are held together primarily by Van der Waals forces, which are overcome by solvation, bundles tend to split longitudinally so that their effects are potentially magnified. Consequently, should the overall contributions to cancer risk from bundles prove important, it may be necessary to explicitly consider their contributions before exposure metrics can adequately predict lung cancer or mesothelioma risk in all environments. This might be accomplished, for example, by incorporating a coefficient for bundles that varies with bundle thickness and represents their magnified contribution to risk.
Some TEM methods explicitly require distinguishing between bundles and single-crystal fibers (fibrils) as part of routine analysis (for example, ISO, Citation1995). Moreover, protocols for distinguishing bundles from single crystals have been developed (for example, Van Orden, Citation2006; Van Orden et al., Citation2005), which can facilitate reproducibility across laboratories. However, the size distribution data available for the current analysis did not distinguish bundles from other fibers. Thus, this represents another important avenue for exploration, once better data become available for supporting development of exposure metrics for asbestos-related cancers.
Biodurability
A number of authors (e.g., Bernstein et al., Citation1996; Eastes and Hadley, Citation1995, Citation1996; Hesterberg et al., Citation1998a, Citation1998b) indicate that a correlation exists between a fiber's biodurability and its bioactivity (including carcinogenicity) and that the biodurability of a fiber is inversely related to its dissolution rate in biological fluids. However, many studies of this issue suffer from certain methodological limitations that limit their utility for quantitatively distinguishing among the effects of differing asbestos mineral types (Berman and Crump, Citation2003, Section 6.2.4). Nevertheless, taken as a whole, these studies suggest that biodurability can potentially explain the observed, radical differences in chrysotile and amphibole asbestos potency toward mesothelioma in humans and (to the extent they prove real) the more modest differences suggested in this study toward the induction of lung cancer.
Given the preceding, it may ultimately prove useful to consider an additional factor for future exposure metrics that reflects the relative biodurability of the fibers. Ideally, use of a biodurability factor holds the potential of making “mineralogical type” a dependent variable so that studies over differing mineral types might be explained by a single, common relationship that is a function of each mineral's measurable dissolution rate. This would eliminate the current need to develop separate potency factors for different minerals types.
Comparison With Other Studies
Several other reviews have also been published that address risk-related issues for asbestos, including questions concerning the identification of an appropriate exposure metric and the relative potency of varying fiber types. Such studies are reviewed briefly next.
Hodgson and Darnton (Citation2000)
Hodgson and Darnton conducted a comprehensive quantitative review of the potency of asbestos for causing lung cancer and mesothelioma in relation to fiber type. They concluded that amosite and crocidolite were, respectively, on the order of 100 and 500 times more potent for causing mesothelioma than chrysotile. They regarded the evidence for lung cancer to be less clear-cut, but concluded nevertheless that amphibole asbestos (amosite and crocidolite) was between 10 and 50 times more potent for causing lung cancer than chrysotile. In reaching this latter conclusion they discounted the high estimate of chrysotile potency obtained from the South Carolina cohort. Hodgson and Darnton concluded that interstudy comparisons for amphibole fibers suggested sublinear relationships (e.g., risk proportional to [cumulative exposure]K, with K > 1) for lung cancer and peritoneal mesothelioma, and a supralinear relationship (e.g., risk proportional to [cumulative exposure]K, with K < 1) for pleural mesothelioma. They considered that a linear relationship was possible for pleural mesothelioma and lung tumors, but not for peritoneal mesothelioma.
The Hodgson and Darnton study was based on 17 cohorts, 14 of which were among the 20 included in the present evaluation (Berman and Crump, Citation2008). This study had different goals from the present evaluation and used different methods of analysis. Hodgson and Darnton did not use the exposure-response information within a study. Instead, lung cancer potency was expressed as a cohort-wide excess mortality divided by the cohort mean exposure. Likewise, mesothelioma potency was expressed as the number of mesothelioma deaths divided by the expected total number of deaths, normalized to an age of first exposure of 30 years, and by the mean exposure for the cohort. These measures have the advantage of being generally calculatable from the summarized data available from a study. However, since they are not model based, it is not clear how they could be used to assess lifetime risk from a specified exposure pattern. In contrast, the goal of this current study is to define metrics suitable for incorporation into a protocol capable of assessing such lifetime risk.
Hodgson and Darnton also used average cohort exposure, which can cause biases in the estimates, if, for example, a large number of subjects were minimally exposed. Also, differences between studies may affect the reliability of conclusions concerning the shape of the exposure-response relationship based on comparisons of results across studies.
Hodgson and Darnton addressed uncertainty using statistical confidence bounds (based on a Poisson distribution for the number of observed lung cancer or mesothelioma deaths). These do not account for the additional, nonstatistical sources of uncertainty that are addressed by the uncertainty intervals constructed in our first paper (Berman and Crump, Citation2008) and incorporated in the analysis in this article.
Despite the different approaches and incorporation of a somewhat different suite of studies, by considering the effects of their use of average cohort exposures, their discounting of the South Carolina cohort, and especially their lack of consideration of nonstatistical sources of uncertainty, the overall findings of Hodgson and Darnton are not inconsistent with those of the current analysis.
Lippmann (Citation1988, Citation1994, Citation1999)
In these literature reviews, Lippmann concludes that it is longer fibers (those longer than approximately 5 μ m) that contribute to lung cancer and mesothelioma. He further indicates that, based primarily on the limits observed for fibers that can be phagocytized, fibers that contribute most to lung cancer are likely longer than 10 μ m. Based on a series of comparisons of mean and median dimensions reported for the relevant exposures across a broad range of studies, Lippmann draws several fairly specific conclusions on the ranges of fiber sizes that may contribute to various diseases (i.e., that the minimum length fibers that contribute to asbestosis, lung cancer, and mesothelioma are 2, 5, and 10 μ m, respectively). He also suggests that fibers that contribute to mesothelioma may need to be thinner than 0.1 μ m while those that contribute to lung cancer may need to be thicker than 0.15 μ m. While it is not clear that drawing such specific conclusions can be firmly supported by the kinds of qualitative comparisons across reported mean and median dimensions for exposures in various studies, Lippmann indicates that further, more formal study of the dose-response relationships that he posits is warranted. It is noted that many of the studies reviewed by Lippmann (Citation1999) are also incorporated in the present analysis.
In the earlier review, Lippmann (Citation1994) plotted lung tumor incidence as a function of inhaled animal dose for data from a series of broadly varying studies based, respectively, on fibers longer than 5, 10, and 20 μ m (no widths considered) and suggests that the quality of the fits are comparable. Lippmann further suggests, based on these plots, that PCM seems to provide a reasonable index of exposure. However, no formal goodness-of-fit tests were performed in this analysis and, based on visual inspection, none of the plots would likely show an adequate fit. Moreover, the plot of the tumor response vs. dose as a function of fibers longer than 5 μ m appears to be substantially worse than the other two plots; if one removes the single highest point in this plot, it appears that any correlation will largely disappear.
Lash et al. (Citation1997)
Lash et al. evaluated fits of a generalized exposure-response model for asbestos-induced lung cancer that also accounts for statistical error. The underlying model is equivalent to that described in Eq. (1) of this article. Lash and coworkers used their model to evaluate 21 studies, including many of the same studies evaluated in the present investigation, although they tended to consider as separate findings the results from a series of studies of the same cohort with differing periods of follow-up. Given the similarity of approach, it is not surprisingly that results reported herein are not inconsistent with those reported by Lash et al.
Stayner et al. (Citation1996)
In the context of evaluating the “amphibole hypothesis,” Stayner et al. (Citation1996) computed the excess relative risk of lung cancer per fiber per milliliter per year from 10 studies categorized by the fiber types to which the cohort was exposed. Each of these studies was also included in the present evaluation. Both the lowest and highest excess relative risks came from cohorts exposed exclusively to chrysotile. Based on their evaluation, they concluded that the epidemiological evidence did not support the hypothesis that chrysotile asbestos is less potent than amphibole asbestos for inducing lung cancer. However, based on a review of the percentage of deaths in various cohorts from mesothelioma, they concluded that amphibole asbestos was likely to be more potent than chrysotile in the induction of mesothelioma. They also noted that comparison of the potency of different forms of asbestos is severely limited by uncontrolled differences in fiber sizes. None of these conclusions is inconsistent with the findings from the analysis reported herein.
CONCLUDING REMARKS
Three of the metrics evaluated herein (those based on all widths, widths > 0.2 μ m, and widths < 0.4 μ m) show roughly similar performance in reconciling the disparate lung cancer potency factors reported in the published epidemiological studies, and two (the metrics based on all widths and widths > 0.4 μ m) show roughly similar performance in reconciling the mesothelioma potency factors. In all these cases, the best estimate for the effect of potency on length is that fibers shorter than 10 μ m should be excluded from the metric. Moreover, as these metrics show improved fits to the existing data relative to the traditional PCM metric (substantially so with regard to mesothelioma), their use as alternatives to the traditional PCM metric should be considered (within the cautions indicated earlier) as an interim measure while further refinements are developed.
At the same time, it must be remembered that none of these metrics fully resolve the discrepancies among the potency factors derived from different environments. In particular, the discrepancies between potency factors obtained from chrysotile mining environments and from the chrysotile textile operations were not resolved. Given that, it may be advisable to assess risk in chrysotile environments by giving weight to potency factors obtained from similar environments. For example, to assess risk in naturally occurring environments involving exposure to unmilled chrysotile fibers, it might be appropriate to emphasize potency factors obtained in mining environments, provided comparable methods of identifying and counting fibers are used in the two environments and that any potential contributions from any co-occurring amphibole asbestos are adequately addressed.
In addition, there may be environments in which these metrics give substantially different results. Use of these metrics in such an environment may be particularly problematic. Since the majority of environments included in this analysis involve exposures to milled asbestos and size distributions for the few environments involving unmilled asbestos that were included in this analysis contain a surprisingly high proportion of thin fibers, it is possible that the metrics discussed here could diverge when applied in environments involving substantial exposure to nonasbestiform particles in natural environments (which would be unmilled and would likely be predominantly thick). The metric based on fibers with widths < 0.4 μ m is likely to be less sensitive to contributions from nonasbestiform particles because it focuses on long, thin fibers that are relatively rare among nonasbestiform structures.
In general, the analyses presented herein further reinforce the recommendations of Berman and Crump (Citation2003) regarding the need to obtain better information on fiber size and type in environments in which epidemiological studies have been conducted in order to support development of exposure metrics that adequately predict asbestos-related cancer risk in all environments.
ACKNOWLEDGMENTS
We thank Corbett McDonald and acknowledge the help of Douglass Liddell (deceased) for making available the raw data on mesothelioma mortality for the cohort of Quebec chrysotile miners and millers. We thank Nicholas de Klerk for making available the raw data for the cohort of Wittenoom, Australia, crocidolite miners. We thank NIOSH, and Everett (Chip) Lehman in particular, for making available the raw data for the cohort of workers from the textile plant in Charleston, SC. We thank Misty Hein for her valuable and very timely assistance in providing data on background cancer rates and additional information on the South Carolina cohort. We gratefully acknowledge the guidance and support provided by Aparna Koppikar, Richard Troast, Chris Weis, and Paul Peronard. We are indebted to John Addison, John Dement, Agnes Kane, Bruce Case, Michel Camus, and Vanessa Vu for their input and assistance. We thank Phillip Benge for his technical assistance. Finally, we gratefully acknowledge the financial support for the earlier work provided by the U.S. EPA and the grant provided by the National Stone Sand and Gravel Association to prepare this manuscript. This project was supported in part by an appointment to the Research Participation Program at the National Center for Environmental Assessment, U.S. EPA, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. EPA. The financial support provided by any group should not be construed as endorsement of the results of this work.
Notes
1. As used here, the term “fiber” is intended to include not only single-crystal fibers (fibrils), but the bundles, clusters, and matrices that make up the full set of fibrous particles in an asbestos dust (ISO, 1995).
2. Distinguishing among the differing crystalline habits of true asbestos fibers (i.e., the asbestiform habit) and nonasbestos particles (i.e., cleavage fragments) potentially affects potency along with size and mineralogical type. However, as data for distinguishing among the crystalline habits of fibers in different environments are lacking, this consideration is not further addressed.
3. The distribution derived from Dement et al. (Citation2007) for the South Carolina plant also represents the unweighted average of distributions observed among the multiple plant processes analyzed in this study (Dement et al., Citation2007, ).
4. As a very approximate and informal marker, an improvement in a likelihood of 1.9 units is barely significant (p = .05) when adding a single estimated parameter to a model.
5. To illustrate how an exposure metric can be used in conjunction with exposure estimates from environments of interest to quantify human risk, see Chapter 8 and Appendix E of Berman and Crump (Citation2003).
6. Even whether various analyses are intended to include or exclude nonasbestiform particles is controversial. Documents in which PCM or PCME is proposed for estimating asbestos concentrations appear to vary in this regard. For example, NIOSH Method 7402, when first issued (NIOSH, Citation1986), explicitly listed “nonasbestiform amphiboles” as interferences. Later revisions of this method (NIOSH Citation1989, 1994) still list “massive amphiboles” as potential interferences, but provide varying CAS numbers to define what is supposed to be determined. More recently, the stated position of NIOSH is that (since 1990) the NIOSH definition of asbestos includes the nonasbestiform amphibole analogs (Middendorf et al., Citation2007). In contrast. nonasbestiform amphiboles were excluded from regulation under the OSHA final asbestos rule (OSHA, Citation1992). Yet compliance with the OSHA rule is typically based on use of NIOSH Methods.
REFERENCES
- Albin M., Jakobsson K., Attewell R., Johansson L., Welinder H. Mortality and cancer morbidity in cohorts of asbestos cement workers and referents. Br. J. Ind. Med. 1990; 79(9)602–610
- American Thoracic Society (ATS). Health effects of tremolite. Am. Rev. Respir. Dis. 1990; 142(6)1453–1458
- Becklake M. On Dr. Dunnigan's commentary linking chrysotile asbestos with mesothelioma. Am. J. Ind. Med. 1988; 14: 239–240
- Berman D. W. The implications of using alternate metrics to assess asbestos-related cancer risk. 2008, In preparation
- Berman D. W., Crump K. S. Update of potency factors for asbestos-related lung cancer and meoshtlioma. Crit. Rev. Toxicol. 2008; 38(S1)1–47
- Berman D. W., Crump K. S. Final draft: Technical support document for a protocol to assess asbestos-related risk. U. S. Environmental Protection Agency, Washington, DC 2003, Prepared for Mark Follensbee, Syracuse Research Corporation, Syracuse, NY, and the Office of Solid Waste and Emergency Response. EPA #9345.4-06. Limited revision draft
- Berman D. W., Crump K. S., Chatfield E. J., Davis J. M. G., Jones A. D. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 1995a; 15(2)181–195
- Berman D. W., Crump K. S., Chatfield E. J., Davis J. M. G., Jones A. D. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 1995b; 15(4)541
- Bernstein D. M., Morscheidt C., Grimm H. G., Thevenaz P., Teichert U. Evaluation of soluble fibers using the inhalation biopersistence, a nine-fiber comparison. Inhal. Toxicol. 1996; 8: 345–385
- Berry G., Newhouse M. L. Mortality of workers manufacturing friction materials using asbestos. Br. J. Ind. Med. 1983; 40: 1–7
- Berry G., de Klerk N. H., Reid A., Ambrosini G. L., Fritichi L., Olsen N. J., Merler E., Musk A. W. Malignant pleural and peritoneal mesotheliomas in former miners and millers of crocidolite at Wittenoom, Western Australia. Occup. Environ. Med. 2004; 61: 1–3
- Bertrand R., Pezerat H. Fibrous glass: Carcinogenicity and dimensional characteristics. Biological Effects of Mineral Fibres, J. C. Wagner. IARC Scientific Publications, Lyon 1980; 901–911
- Bolton R. E., Addison J., Davis J. M. G., Donaldson K., Jones A. D., Miller B. G., Wright A. Effects of the inhalation of dusts from calcium silicate insulation materials in laboratory rates. Environ. Res. 1986; 39: 26–43
- Bolton R. E., Davis J., Donaldson K., Wright A. Variation in the carcinogenicity of mineral fibres. Ann. Occup. Hyg. 1982; 26(1–4)569–582
- Bolton R. E., Davis J. M. G., Miller B., Donaldson K., Wright A. The effect of dose of asbestos on mesothelioma production in the laboratory rat. Proceedings of the 6th International Pneumonoconiosis Conference 1984; 2: 1028–1035
- Bonneau L., Malard C., Pezerat H. Studies on surface properties of asbestos. Environ. Res. 1986; 41: 268–275
- Case B. W. Health effects of tremolite: now and in the future. The Third Wave of Asbestos Disease: Exposure to Asbestos in Place, P. J. Kazemi, H. Kazemi. Ann. NY Acad. Sci, New York 1991; 643: 491–504
- Case B. W., Churg A., Dufresne A., Sebastien P., McDonald A., McDonald J. C. Lung fibre content for mesothelioma in the 1891–1920 birth cohort of Quebec chrysotile workers: A descriptive study. Ann. Occup. Hyg. 1997; 41: 231–236
- Case B. W., Dufresne A., McDonald A. D., McDonald J. C., Sebastien P. Asbestos fiber type and length in lungs of chrysotile textile and production workers: Fibers longer than 18 μ m. Inhal. Toxicol. 2000; 1(Suppl. 1)411–418
- Cherrie J. W., Dodgson J., Groat S., Carson M. Comparison of optical and electron microscopy for evaluating airborne asbestos. Institute of Occupational Medicine, Edinburgh 1979, (U. S. Department of Commerce)
- Churg A. Chrysotile, tremolite, and malignant mesothelioma in man. Chest 1988a; 93(3)621–628
- Churg A. Reply to Dr. Dunnigan. Am. J. Ind. Med. 1988b; 14: 235–238
- Churg A., Wiggs B., Depaoli L., Kampe B., Stevens B. Lung asbestos content in chrysotile workers with mesothelioma. Am. Rev. Respir. Dis. 1984; 130: 1042–1045
- Cox D. R., Hinkley D. V. Theoretical Statistics. Chapman and Hall, London 1974
- Davis J. M. G., Addison J., Bolton R. E., Donaldson K., Jones A. D., Miller B. G. Inhalation studies on the effects of tremolite and brucite dust in rats. Carcinogenesis 1985; 6(5)667–674
- Davis J. M. G., Addison J., Bolton R., Donaldson K., Jones A. D., Smith T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br. J. Exp. Pathol. 1986a; 67: 415–430
- Davis J. M. G., Addison J., Bolton R. E., Donaldson K., Jones A. D. Inhalation and injection studies in rats using dust samples from chrysotile asbestos prepared by a wet dispersion process. Br. J. Pathol. 1986b; 67: 113–129
- Davis J. M. G., Jones A. D., Smith T. Comparisons of the pathogenicity of long and short fibres of chrysotile asbestos in rats. Institute of Occupational Medicine. 1987, Institute for Research and Development of Asbestos, Montreal (ed.). Report No. TM-87/08
- Davis J. M. G., Bolton R. E., Douglas A. N., Jones A. D., Smith T. Effects of electrostatic charge on the pathogenicity of chrysotile asbestos. Br. J. Ind. Med. 1988; 45(5)292–309
- de Klerk N. H., Musk A. W., Armstrong B. K., Hobbs M. S. T. Diseases in miners and millers of crocidolite from wittenoom, Western Australia: A further follow-up to December 1986. Ann. Occup. Hyg. 1994; 38(Suppl. 1)647–655
- Dement J. M., Harris R. L. Estimates of pulmonary and gastrointestinal deposition for occupational fiber exposure. 1979, NTIS PB80-149644. U. S. HEW Contract 78-2438
- Dement J. M., Kuempel E., Zumwalde R., Smith R., Stayner L., Loomis D. Development of a fiber size-specific job-exposure matrix for airborne asbestos fibers. Occup. Environ. Med. 2007, November; doi:10.1136/oem.2007.033712
- Dunnigan J. Linking chrysotile asbestos with mesothelioma. Am. J. Ind. Med. 1988; 14: 205–209
- Dumortier P., De Vuyst P., Rey F., Boutin C. RE main asbestos type in pleural mesothelioma. Am. J. Ind. Med. 1998; 33(1)94–95
- Eastern Research Group, Inc. (ERG). Report on the peer consultation workshop to discuss a proposed protocol to assess asbestos-related risk. Final report. U. S. Environmental Protection Agency, Washington, DC May, 2003, Prepared for the Office of Solid Waste and Emergency Response
- Eastes W., Hadley J. G. A mathematical model of fiber carcinogenicity and fibrous in inhalation and intraperitoneal experiments in rats. Inhal. Toxicol. 1996; 8: 323–343
- Eastes W., Hadley J. G. Dissolution of fibers inhaled by rats. Inhal. Toxicol. 1995; 7: 179–196
- Elmes P. Mesotheliomas and chrysotile. Ann. Occup. Hyg. 1994; 38(4)547–553
- Enterline P. E., Harley J., Henderson V. Asbestos and Cancer—A cohort Followed to Death. Graduate School of Public Health, University of Pittsburgh. 1986
- Finkelstein M. M. Mortality among employees of an Ontario asbestos-cement factory. Am. Rev. Respir. Dis. 1984; 129: 754–761
- Gibbs G. W., Hwang C. Y. Dimensions of airborne asbestos fibers. Biological Effects of Mineral Fibers, J. C. Wagner. IARC Scientific Publication, Lyon 1980; 69–78
- Hein M. J., Stayner L. T., Leyman E., Dement J. M. Follow-up study of chrysotile textile workers: Cohort mortality and exposure-response. Occup. Environ. Med. 2007; 64: 616–625
- Hesterberg T. W., Chase G., Axten C., Miiller W. C., Musselman R. P., Kamstrup O., Hadley J., Morscheidt C., Bernstein D. M., Thevenaz P. Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicol. Appl. Pharmacol 1998a; 151: 262–275
- Hesterberg T. W., Hart G. A., Chevalier J., Miiller W. C., Hamilton R. D., Bauer J., Thevenaz P. The importance of fiber biopersistence and lung dose in determining the chronic inhalation effects of X607, RCF1, and chrysolite asbestos in rats. Toxicol. Appl. Pharmacol. 1998b; 153(1)68–82
- Hodgson J., Darnton A. The quantitative risk of mesothelioma and lung cancer in relation to asbestos exposure. Ann. Occup. Hyg. 2000; 44(8)565–601
- Hughes J. M., Weill H., Hammad Y. Y. Mortality of workers employed at two asbestos cement plants. Br. J. Ind. Med. 1987; 44: 161–174
- Huncharek M. Chrysotile asbestos exposure and mesothelioma. Br. J. Ind. Med. 1987; 44: 287–288
- Hwang C. Y., Gibbs G. W. The dimensions of airborne asbestos fibres—I. Crocidolite from Kuruman area, Cape Province, South Africa. Ann. Occup. Hyg 1981; 24(1)23–41
- International Organization for Standardization (ISO). Ambient air determination of asbestos fibres—Direct-transfer transmission electron microscopy method. 1995, Available at: http://www.complianceonline.com/ecommerce/control/product/~product_id=801483/~category_id=9960/~Ambient_air__Determination__asbestos_fibres;jsessionid=54646C5E80BE2712D005A0BFB0101493.jvm1 ISO 10312
- Lacquet L. M., Van der Linden L., Lepoutre J. Roentgenographic lung changes, asbestosis and mortality in a Belgian asbestos-cement factory. Biological Effects of Mineral Fibres, J. C. Wagner. IARC Scientific Publication, Lyon 1980; 783–793
- Langer A. M., Nolan R. P. Fiber type and mesothelioma risk. Symposium on Health Aspects of Exposure to Asbestos in Buildings. 1989; 91–140, December
- Lash T. L., Crouch E. A. C., Green L. C. A meta-analysis of the relation between cumulative exposure to asbestos and relative risk of lung cancer. Occup. Environ. Med. 1997; 54: 254–263
- La Ville de Thetford Mines. Thetford Mines a ciel ouvert: Histoire d'une ville miniere 1892–1992. La Ville de Thetford Mines, Thetford Mines, QuebecCanada 1994
- Levin J. L., McLarty J. W., Hurst G. A., Smith A. N., Frank A. L. Tyler asbestos workers: Mortality experience in a cohort exposed to amosite. Occup. Environ. Med. 1998; 55: 155–160
- Liddell F. D. K., McDonald A. D., McDonald J. C. The 1891–1920 birth cohort of Quebec chrysotile miners and millers: Development from 1904 and mortality to 1992. Ann. Occup. Hyg. 1997; 41: 13–36
- Lippmann M. Deposition and retention of inhaled fibres: effects on incidence of lung cancer and mesothelioma. Occup. Environ. Med. 1994; 51: 793–798
- Lippmann M. Review: asbestos exposure indices. Environ. Res. 1988; 46: 86–106
- Lippmann M. Asbestos and other mineral and vitreous fibers. Environmental Toxicants: Human Exposures and Their Health Effects, 2nd ed, M. Lippmanm. Wiley-Interscience, New York 1999; 65
- Mancuso T. F. Relative risk of mesothelioma among railroad machinists exposed to chrysotile. Am. J. Ind. Med. 1988; 13: 639–657
- Marconi A., Menichini E., Paoletti L. A comparison of light microscopy and transmission electron microscopy results in the evaluation of the occupational exposure to airborne chrysotile fibres. Ann. Occup. Hyg. 1984; 28(3)321–331
- McDonald J. C. Tremolite, other amphiboles, and mesothelioma. Am. J. Ind. Med. 1988; 14: 247–249
- McDonald J. C., Armstrong B., Case B., Doell D., McCaughey W. T. E., McDonald A. D., Sebastien P. Mesothelioma and asbestos fiber type—Evidence from lung tissue analyses. Cancer 1989; 63: 1544–1547
- McDonald A. D., Case B. W., Churg A., Dufresne A., Gibbs G. W., Sebastien P., McDonald J. C. Mesothelioma in Quebec chrysotile miners and millers: epidemiology and aetiology. Ann. Occup. Hyg. 1997; 41(6)707–719
- McDonald A. D., Fry J. S. Mesothelioma and fiber type in three american asbestos factories: preliminary report. Scand. J. Work Environ. Health 1982; 8(Suppl. 1)53–58
- McDonald A. D., Fry J. S., Woolley A. J., McDonald J. C. Dust exposure and mortality in an american chrysotile asbestos friction products plant. Br. J. Ind. Med. 1984; 41: 151–157
- McDonald A. D., Fry J. S., Wooley A. J., McDonald J. C. Dust exposure and mortality in an american chrysotile textile plant. Br. J. Ind. Med. 1983a; 39: 361–367
- McDonald A. D., Fry J. S., Woolley A. J., McDonald J. C. Dust exposure and mortality in an american factory using chrysotile, amosite, and crocidolite in mainly textile manufacture. Br. J. Ind. Med. 1983b; 40: 368–374
- McDonald J. C., McDonald A. D. Chrysotile, tremolite, and mesothelioma. Ann. Occup. Hyg. 1997; 41(6)699–705
- McDonald J. C., McDonald A. D. Chrysotile, tremolite, and mesothelioma. Science 1995; 267: 761–932
- Middendorf P., Zumwalde R., Castellan R. Asbestos and other mineral fibers: A roadmap for scientific research. NIOSH, Cincinnati, OH 2007, On behalf of: the National Institute of Occupational Safety and Health Fibers Work Group
- Miller B. G., Searl A., Davis J. M. G., Donaldson K., Cullen R. T., Bolton R. E., Buchanan D., Soutar C. A. Influence of fibre length, dissolution and biopersistence on the production of mesothelioma in the rat peritoneal cavity. Ann. Occup. Hyg. 1999; 43(3)155–166
- Mossman B. T. Mechanisms of asbestos carcinogenesis and toxicity: The amphibole hypothesis revisited. Br. J. Ind. Med. 1993; 50: 673–676
- Muhle H., Bellman B., Takenata S., Ziem Y. Inhalation and injection in rats to test the carcinogenicity of MMMF. Ann. Occup. Hyg. 1987; 31(4B)755–764
- National Institute for Occupational Safety and Health (NIOSH). Method for determination of asbestos in air using transmission electron microscopy. NIOSH, Cincinnati, OH 1986, NIOSH Method 7402. First publication (First issue): May 15
- National Institute for Occupational Safety and Health (NIOSH). Method for determination of asbestos in air using transmission electron microscopy. NIOSH, Cincinnati, OH 1989, NIOSH Method 7402. Revision 1: May 15
- National Institute for Occupational Safety and Health (NIOSH). Method for determination of asbestos in air using positive phase contrast microscopy. NIOSH, Cincinnati, OH 1994a, NIOSH Method 7400. First issued 1985. Current revision
- National Institute for Occupational Safety and Health (NIOSH). Method for determination of asbestos in air using transmission electron microscopy. NIOSH, Cincinnati, OH 1994b, NIOSH Method 7402. First issued 1986. Current revision (Issue 2)
- Nicholson W. J. Airborne asbestos health assessment update. U. S. Environmental Protection Agency, Washington, DC 1986, Report 600/8-84-003F
- Occupational Safety and Health Administration (OSHA). Final rule: occupational exposure to asbestos, tremolite, anthophyllite, and actinolite. Fed. Reg. 1992; 57(110)24310–24331
- Ohlson C. G. Is chrysotile a significant risk factor for mesothelioma?. Am. J. Ind. Med. 1989; 15: 351–352
- Peto J., Doll R., Hermon C., Binns W., Clayton R., Goffe T. Relationship of mortality to measures of environmental asbestos pollution in an asbestos textile factory. Ann. Occup. Hyg. 1985; 29(3)305–355
- Piolatto G., Negri E., LaVecchia C., Pira E., Decarli A., Peto J. An update of cancer mortality among chrysotile asbestos miners in Balangero, Northern Italy. Br. J. Ind. Med. 1990; 47: 810–814
- Pott F. Animal experiments with mineral fibers. Short and Thin Mineral Fibers: Identification, Exposure, and Health Effects, E. J. Chatfield. Mineralogical Association of Canada, Quebec 1983; 133–161
- Pott F., Huth F., Friedrichs K. H. Results of animal carcinogenesis studies after application of fibrous glass and their implications regarding human exposure. Occupational Exposure to Fibrous Glass. Washington, DC 1976; 183–191, U. S. HEW Publication No. 76–151
- Pott F., Huth F., Friedrichs K. H. Tumorigenic effect of fibrous dust in experimental animals. Environ. Health Perspect. 1974; 9: 313–315
- Pott F., Ziem U., Reiffer R. J., Huth F., Ernst H., Mohr U. Carcinogenicity studies on fibres, metal compounds, and some other dusts in rats. Exp. Pathol. 1987; 32: 129–152
- Roberts D. R., Zumwalde R. D. Industrial hygiene summary report of asbestos exposure assessment for brake mechanics. 1982, Available at: http://www.ntis.gov/# NIOSH Reports No. IWS-32-4A, Industrial Hygiene Section, NTIS# PB 87-105433
- Rogers A. J., Leigh J., Berry G., Ferguson D. A., Mulder H. B., Ackad M. Relationship between lung asbestos fiber type and concentration and relative risk of mesothelioma. For the National Institute of Occupational Health and Safety, Sydney, Australia. Cancer 1991; 67(7)1912–1920
- Roggli V. L., Pratt P. C., Brody A. R. Asbestos fiber type in malignant mesothelioma: An analytical scanning electron microscopic study of 94 cases. Am. J. Ind. Med. 1993; 23(4)605–614
- Rood A. P., Scott R. M. Size distributions of chrysotile asbestos in a friction products factory as determined by transmission electron microscopy. Ann. Occup. Hyg. 1989; 33(4)583–590
- Ross D., McDonald J. C. Occupational and geographical factors in the epidemiology of malignant mesothelioma. Monaldi Arch. Chest Dis. 1995; 50(6)458–463
- Schneider J., Roedelsperger K., Bruekel B., Kayser K., Woitowitz H. J. Environmental exposure to tremolite asbestos: Pleural mesothelioma in two Turkish workers in Germany. Rev. Environ. Health 1998; 13(4)213–220
- Sebastien P., McDonald J. C., McDonald A. D., Case B., Harley R. Respiratory cancer in chrysotile textile and mining industries: Exposure inferences from lung analysis. Br. J. Ind. Med. 1989; 46: 180–187
- Sebastien P., Plourde M., Robb R., Ross M., Nadon B., Wypruk T. Ambient air asbestos survey in Quebec mining towns. Part II: Main study. Canadian Environmental Protection Service, EnvironmentCanada 1986, Report No. EPS 5/AP/RQ/2E
- Seidman H., Selikoff I. J., Gelb S. K. Mortality experience of amosite asbestos factory workers: Dose-response relationships 5 to 40 years after onset of short-term work exposure. Am. J. Ind. Med. 1986; 10(5/6)479–514
- Selikoff I. J., Seidman H. Asbestos-associated deaths among insulation workers in the United States and Canada, 1967–1987. Ann. NY Acad. Sci. 1991; 643: 1–14
- Sluis-Cremer G. K. Linking chrysotile asbestos with mesothelioma. Am. J. Ind. Med. 1988; 14: 631–632
- Smith A. H. Amphibole fibers, chrysotile fibers, and pleural mesothelioma. Am. J. Ind. Med. 1998; 33(1)96
- Smith A. H., Wright C. C. Chrysotile asbestos is the main cause of pleural mesothelioma. Am. J. Ind. Med. 1996; 30: 252–266
- Smith G. W. The Thetford Mines. 1878–1967. George Washington Smith, Bell Asbestos Mines Ltd., Québec, Charrier & Dugal 1968; 103
- Snyder J., Virta R., Segreti J. Evaluation of the phase contrast microscopy method for the detection of fibrous and other elongated mineral particulates by comparison with a STEM technique. Am. Ind. Hyg. Assoc. J. 1987; 48(5)471–477
- Stanton M., Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J. Natl. Cancer Inst. 1972; 48: 797–821
- Stanton M., Layard M., Tegeris A., Miller E., May M., Kent E. Carcinogenicity of fibrous glass: Pleural response in the rat in relation to fiber dimension. J. Natl. Cancer Inst. 1977; 58(3)587–597
- Stanton M., Layard M., Tegeris A., Miller E., May M., Morgan E. Relation of particle dimension to carcinogenicity in amphibole asbestos and other fibrous minerals. J. Natl. Cancer Inst. 1981; 67(5)965–975
- Stayner L. T., Dankovic D. A., Lemen R. A. Occupational exposure to chrysotile asbestos and cancer risk: A review of the amphibole hypothesis. Am. J. Public Health 1996; 86(2)176–186
- Sullivan P. Vermiculite, respiratory disease, and asbestos exposure in Libby, Montana: Update of a cohort mortality study. Environ. Health Perspect. 2007; 115(4)579–585
- Timbrell V. Review of the significance of fibre size in fibre-related lung disease: A centrifuge cell for preparing accurate microscope-evaluation specimens from slurries used in inoculation studies. Ann. Occup. Hyg. 1989; 33(4)483–505
- Tuomi T. Fibrous minerals in the lungs of mesothelioma patients: Comparison between data on sem, tem, and personal interview information. Am. J. Ind. Med. 1992; 21(2)155–162
- Van Orden D. R. Asbestos. Environmental Forensics: Contaminant Specific Guide, R. D. Morrison, B. L. Murphy. Academic Press, New York 2006; 19–34
- Van Orden D. R., Lee R. J., Allison K. A. (2005) A review of the analysis of amphibole fibers. Presented at the SME Annual Meeting, Salt Lake City, UT, February 28–March 2, 2005, Pre-print 05-75
- Veblen D. R., Wylie A. G. Mineralogy of amphiboles and 1:1 layer silicates. Health Effects of Mineral Dusts, G. D. Guthrie, B. T. Mossman, 1993; 61–138, Reviews in Mineralogy and Geochemistry (1993); 28:61–137
- Virta R. L., Shedd K., Wylie A. G., Snyder J. G. Size and shape characteristics of amphibole asbestos (amosite) and amphibole cleavage fragments (actinolite, cummingtonite) collected on occupational air monitoring filters. Aerosols Mining Ind. Work Environ. 1983; 2: 633–643
- Wagner J. C., Berry G., Skidmore J. W. Studies of the carcinogenic effects of fiber glass of different diameters following intrapleural innoculation in experimental animals. 1976; 193–197, Proceedings of a symposium, HEW Publication Pub. No. 76-151 (National Institution of Occupational Safety and Health), Washington DC
- Wagner J. C., Berry G., Hill R., Munday D., Skidmore J. Animal experiments with MMM(V)F—Effects of inhalation and intrapleural inoculation in rats. Biological Effects of Man-Made Fibres—Proceedings of a WHO/IARC Conference, Copenhagen, 1982; 209–233
- Wagner J. C., Skidmore J. W., Hill R. J., Griffith D. M. Erionite exposure and mesotheliomas in rats. Br. J. Cancer 1985; 51: 727–730
- Walton W. H. The nature, hazards, and assessment of occupational exposure to airborne asbestos dust: A review. Ann. Occup. Hyg. 1982; 25: 117–247
- William-Jones A. E., Normand C., Clark J. R., Vali H., Martin R. F., Dufresne A., Nayebzadeh A. Controls of amphibole formation in chrysotile deposits: Evidence from the Jeffrey mine, Asbestos, Quebec. The Health Effects of Chrysotile Asbestos, R. P. Nolan, A. M. Langer, M. Ross, F. J. Wicks, R. F. Martin. The Mineralogical Association of Canada, Quebec 2001; 89–104, Spec Publ.5
- Winer A. A., Cossette M. The effect of aspect ration on fiber counts: A preliminary study. Ann. NY Acad. Sci 1979; 330: 661–672
- Wylie A. G., Bailey K. F., Kelse J. W., Lee R. J. The importance of width in asbestos fiber carcinogenicity and its implications for public policy. Am. Ind. Hyg. Assoc. J. 1993; 54: 239–252
- Wylie A. G., Shedd K. B., Taylor M. E. Measurement of the thickness of amphibole asbestos fibers with the scanning electron microscope and transmission electron microscope. Microbeam Analysis, K. F. J. Heinrich. San Francisco Press, San Francisco 1982; 181–187
- Wylie A. G., Virta R. L., Segretti J. M. Characterization of mineral population by index particle: Implications for the stanton hypothesis. Environ. Res. 1987; 43: 427–439