Abstract
The International Agency for Research on Cancer (IARC) published a monograph in 2015 concluding that glyphosate is “probably carcinogenic to humans” (Group 2A) based on limited evidence in humans and sufficient evidence in experimental animals. It was also concluded that there was strong evidence of genotoxicity and oxidative stress. Four Expert Panels have been convened for the purpose of conducting a detailed critique of the evidence in light of IARC’s assessment and to review all relevant information pertaining to glyphosate exposure, animal carcinogenicity, genotoxicity, and epidemiologic studies. Two of the Panels (animal bioassay and genetic toxicology) also provided a critique of the IARC position with respect to conclusions made in these areas. The incidences of neoplasms in the animal bioassays were found not to be associated with glyphosate exposure on the basis that they lacked statistical strength, were inconsistent across studies, lacked dose-response relationships, were not associated with preneoplasia, and/or were not plausible from a mechanistic perspective. The overall weight of evidence from the genetic toxicology data supports a conclusion that glyphosate (including GBFs and AMPA) does not pose a genotoxic hazard and therefore, should not be considered support for the classification of glyphosate as a genotoxic carcinogen. The assessment of the epidemiological data found that the data do not support a causal relationship between glyphosate exposure and non-Hodgkin’s lymphoma while the data were judged to be too sparse to assess a potential relationship between glyphosate exposure and multiple myeloma. As a result, following the review of the totality of the evidence, the Panels concluded that the data do not support IARC’s conclusion that glyphosate is a “probable human carcinogen” and, consistent with previous regulatory assessments, further concluded that glyphosate is unlikely to pose a carcinogenic risk to humans.
Introduction
Background on glyphosate
Glyphosate, or N-(phosphonomethyl)glycine (CAS# 1071-83-6), is a widely used broad-spectrum, nonselective post-emergent herbicide that has been in use since 1974. Glyphosate effectively suppresses the growth of many species of trees, grasses, and weeds. Glyphosate works by interfering with the synthesis of the aromatic amino acids phenylalanine, tyrosine, and tryptophan, through the inhibition of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS). Inhibition of the synthesis of these amino acids stops growth of plants such as weeds. Importantly, EPSPS is not present in mammals, which obtain their essential aromatic amino acids from the diet.
A wide variety of new uses have been developed for glyphosate in agricultural, industrial, and home & garden applications. Glyphosate accounts for approximately 25% of the global herbicide market (http://www.glyphosate.eu). Glyphosate is currently marketed under numerous trade names by more than 50 companies in several hundreds of crop protection products around the world. More than 160 countries have approved uses of glyphosate-based herbicide products (http://www.monsanto.com). To further enhance the effectiveness of glyphosate in agriculture, a number of genetically modified crop varieties have been developed which are tolerant to glyphosate (i.e. allows for application after emergence of the crops). In addition, given its effectiveness and broad-spectrum activity, glyphosate is also used worldwide for forestry, rights of way, landscape, and household control of weeds.
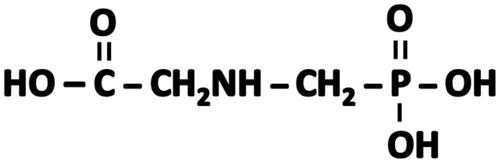
Glyphosate is a relatively simple molecule which consists of the amino acid glycine and a phosphonomethyl moiety (). As such, glyphosate has no structural alerts for chromosomal damage, genotoxicity, mutagenicity, or carcinogenicity when analyzed by DEREK (Deductive Estimation of Risk from Existing Knowledge) (Kier & Kirkland Citation2013). It is a polar molecule that is incompletely (15–36%) absorbed orally, undergoes very little biotransformation, and is rapidly excreted unmetabolized (Williams et al. Citation2000). A molecule with these characteristics would be expected to exhibit, if any, only a low order of toxicity. The results from toxicity studies and regulatory risk assessments have been consistent with that expectation (JMPR Citation1987, Citation2006; US EPA Citation1993; WHO Citation1994; Williams et al. Citation2000; European Commission Citation2002; EFSA Citation2015).
Previous assessments of the carcinogenicity of glyphosate
The safety, including the potential carcinogenicity, of glyphosate has been reviewed by scientists and regulatory authorities worldwide, including the US Environmental Protection Agency (US EPA), the European Commission, and the Canadian Pest Management Regulatory Agency (Health and Welfare Canada Citation1991; US EPA Citation1993, Citation2013; WHO Citation1994; Williams et al. Citation2000; European Commission Citation2002; Kier & Kirkland Citation2013; EFSA Citation2015; Health Canada Citation2015; JMPR Citation2016). The conclusion of all these reviews is that proper use of glyphosate and glyphosate-based formulations (GBFs) does not pose a genotoxic or carcinogenic hazard/risk to humans.
The first assessment of glyphosate’s carcinogenic potential was undertaken by the US EPA in 1985. This review was done by a US EPA panel that then was called the Toxicology Branch Ad Hoc Committee, which comprised members of the Toxicology Branch of the Hazard Evaluation Division. At that time, two chronic animal bioassays were available: a combined chronic toxicity/carcinogenicity study in Sprague-Dawley rats and a carcinogenicity study in CD-1 mice. The Agency concluded that the data did not demonstrate a carcinogenic response in rats. However, the US EPA also concluded that the dose levels used in that study were inadequate for assessing glyphosate’s carcinogenic potential in this species. The US EPA concluded that there was limited evidence of an increased incidence of renal tubule adenomas in male mice at the high-dose level (4841 mg/kg/day), a dose that greatly exceeds the limit dose level (1000 mg/kg/day) for carcinogenicity testing with pesticides (OECD Citation2009). Based on this information, the Agency initially classified glyphosate as a Group C (Possibly Carcinogenic to Humans: Agents with limited animal evidence and little or no human data) carcinogen (see US EPA Citation1991a).
The kidney slides from the mouse study were subsequently reexamined by a consulting pathologist (Dr. Marvin Kuschner M.D., Dean, School of Medicine, State University of New York at Stony Brook), and three other scientists (Dr. Robert A. Squire, Robert A. Squire Associates Inc., Ruxton Maryland; Dr. Klaus L. Stemmer M.D., Kettering Laboratory, University of Cincinnati Medical Center; Dr. Robert E. Olson, M.D., Ph.D., Professor of Medicine and Pharmacological Sciences, State University of New York at Stony Brook) also reviewed the slides and/or the chronic toxicity data. All these scientists concluded that there was no relationship to treatment (US EPA, Citation1986a). In addition, a Pathology Working Group (PWG), consisting of 5 pathologists (Dr. RM Sauer, Dr. MR Anver, Dr. JD Strandberg, Dr. JM Ward, and Dr. DG Goodman), was also assembled and they issued the following conclusion: “This PWG firmly believes and unanimously concurs with the original pathologist and reviewing pathologist that the incidences of renal tubular cell neoplasms in this study are not compound related” (US EPA Citation1986a).
All available information was presented to an US EPA FIFRA Science Advisory Panel (SAP) in February 1986. The SAP determined that the carcinogenic potential of glyphosate could not be determined from the existing data and proposed that a chronic rat and/or mouse study be conducted in order to clarify these unresolved questions; the panel also proposed that glyphosate be categorized as Group D or having “inadequate animal evidence of oncogenicity” (US EPA Citation1986b).
After considering the SAP’s conclusions and recommendations, the US EPA requested that a new 2-year rat oncogenicity study be conducted. In 1991, after the new rat study was completed, the US EPA re-convened its Carcinogenicity Peer Review Committee to review the results of this study as well as all of the relevant scientific data on glyphosate (US EPA Citation1991a). The Committee concluded that glyphosate should be classified in Group E (evidence of non-carcinogenicity) based upon the lack of a carcinogenic response in two animal species. Subsequent reevaluations by US EPA (Citation1993, Citation2012, Citation2013) have re-affirmed the Agency’s earlier conclusion.
After Monsanto had marketed glyphosate-based herbicide products for a number of years, other companies entered the glyphosate market; as a result, some of them generated substantial, or even complete, additional toxicology databases. The first additional databases that became available were generated by Cheminova and Syngenta in the mid- to late 1990s timeframe. Additional data packages were subsequently generated by other companies (e.g. Arysta, Excel, Feinchemie, Nufarm) and became available in the mid- and late 2000s timeframe.
In addition to new studies conducted to meet regulatory guidelines and support various re-registration processes globally, new epidemiology and genotoxicity studies (testing glyphosate and glyphosate-based herbicide formulations) began to appear in the scientific literature in the late 1990s and early 2000s. One of the first epidemiological investigations of interest involving glyphosate published in the scientific literature was that of Hardell and Eriksson (Citation1999), and other epidemiology studies were periodically published after 2000 up until the present. Genetic toxicology studies of glyphosate and GBFs began to appear in the literature in increasing numbers throughout the 1990s and were reviewed by Williams et al. (Citation2000). The occurrence of such studies has increased during the 2001–2015 timeframe: approximately 125 such genotoxicity studies were reviewed by Kier and Kirkland (Citation2013), and an additional 40 genotoxicity biomonitoring studies of GBFs were reviewed by Kier (Citation2015).
As glyphosate underwent reregistration processes by major national regulatory authorities and additional reviews by other health agencies after 2000, these evaluations included more and more of the new toxicology, genotoxicity, and epidemiology information generated after the initial Monsanto animal bioassay studies. For example, a 2004 Joint Meeting of the FAO Panel of Experts on Pesticide Residues (JMPR) in Food and the Environment and the WHO Core Assessment Group concluded that there was an absence of carcinogenic potential in animals and a lack of genotoxicity in standard tests; thus, “the Meeting concluded that glyphosate is unlikely to pose a carcinogenic risk to humans” (JMPR Citation2006). The Australian Pesticides and Veterinary Medicines Authority (APVMA) evaluated the active ingredient and concluded that the evidence shows that glyphosate is not genotoxic or carcinogenic (APVMA Citation2013). The US EPA conducted a comprehensive Human Health Risk Assessment in 2012 (US EPA Citation2012). The Agency noted that “no evidence of carcinogenicity was found in mice or rats,” and US EPA concluded that “glyphosate does not pose a cancer risk to humans” (US EPA Citation2013). Health Canada’s Pesticide Management Regulatory Agency (PMRA) completed a comprehensive review of glyphosate as part of the reregistration process in that country. PMRA concluded that “the overall weight of evidence indicates that glyphosate is unlikely to pose a human cancer risk” (Health Canada Citation2015). The complete genotoxicity, carcinogenicity, and human epidemiology databases were evaluated by the German Federal Institute for Risk Assessment (BfR) for the European Commission on the Annex 1 renewal of glyphosate. The BfR concluded that glyphosate is unlikely to pose a carcinogenic risk to humans (Markard Citation2014). This conclusion was supported by the peer review evaluation conducted by the European Food Safety Authority (EFSA) both before and after a mandate from the European Commission to consider the findings from IARC regarding glyphosate’s carcinogenic potential (EFSA Citation2015). Most recently, JMPR (Citation2016) reviewed the data and concluded that: “glyphosate is unlikely to pose a carcinogenic risk to humans from exposure through the diet.”
IARC assessment of the carcinogenicity of glyphosate
The International Agency for Research on Cancer (IARC) in 2015 undertook an evaluation of the oncogenic potential of glyphosate as part of its Monograph Programme. Glyphosate, along with four other pesticides (the insecticides diazinon, malathion, parathion, and tetrachlorvinphos), was considered by an IARC Working Group, which met in March 2015 at IARC in Lyon, France. A brief summary of IARC’s conclusions was initially published in The Lancet Oncology on 20 March 2015 (Guyton et al. Citation2015), and the full IARC Monograph (Volume 112) was published online on 29 July 2015 (IARC Citation2015). IARC concluded that glyphosate is “probably carcinogenic to humans (Group 2A)” based on limited evidence in humans and sufficient evidence in experimental animals; it was also concluded that there was strong evidence of genotoxicity and oxidative stress (IARC Citation2015).
Expert Panel critique of the IARC assessment and review of relevant data
Since the IARC conclusions were found to be in such stark contrast to those from all other assessments of carcinogenic potential, it was decided that a thorough review should be conducted by scientists in the area of cancer risk assessment, critiquing IARC’s processes where appropriate. Toward that end, Intertek Scientific & Regulatory Consultancy (Intertek, Mississauga, Ontario, Canada) was commissioned by the Monsanto Company to assemble panels of scientific experts in the four areas considered by IARC: exposure; epidemiology; cancer in experimental animals; mechanistic and other relevant data (focused on genotoxicity and oxidative stress).
Fifteen scientific experts were selected on the basis of their expertise and standing within the international scientific community (i.e. publication history, participation in scientific and regulatory committees, and familiarity with regulatory authorities) and recruited by Intertek to participate on these Expert Panels. Panelists were recruited and assigned to one of the four areas considered by IARC (noted above) based on their areas of expertise; two panelists participated in two areas. A sixteenth scientific expert from Intertek participated on the Expert Panels and served as the overall organizer and facilitator for the panel meetings. A listing of the experts, their affiliations, and the specific “Panel” on which they served is presented in .
Table 1. Composition of the four Expert Panels.
Prior to the meeting, all key studies/publications cited by IARC were made available to the panelists for their review; panelists were told to request any additional information they felt was necessary for them to conduct a thorough evaluation. The epidemiology panel conducted its own independent literature search. The scientists were asked to closely examine the studies/data that IARC used to come to their conclusions; panelists were also advised to examine any additional information needed to come to an overall conclusion in their respective areas.
Based on the scope of the information to be evaluated, it was decided that the panels would meet over a 2-day period to discuss all relevant information and make appropriate conclusions regarding the carcinogenic potential of glyphosate. As needed, the expert scientists held pre-meeting phone conferences and communicated via email to establish and plan how they would prepare for and conduct their review at the Expert Panels review meeting. Since the amount, nature, and quality of the data used by IARC varied considerably across the four areas, the evaluation approaches used by the expert panelists in their specialist areas varied somewhat as well. The Expert Panels Meeting was held on 27–28 August 2015 at Intertek in Mississauga, Canada. On the first day of the meeting, the discussions focused on the exposure and human epidemiology data. The second day of the meeting began with a summation of epidemiology and exposure discussions/conclusions and then focused on the animal bioassay and genotoxicity/oxidative stress data. After the Expert Panels met, the reports for the four individual areas were developed by designated scientists; the content of these reports was finalized through additional phone conferences and email communications as necessary with the other panel members. As indicated previously, due to the large amount of data and information evaluated by the individual panels and the subsequent length of the individual reports, it was decided to prepare four separate specialist manuscripts covering the methodologies applied and their respective outcomes and conclusions. This report presents a summary of the deliberations, and conclusions reached, by the Expert Panels in the four areas of research. Prior to publishing the Expert Panels findings, they were presented at the Society for Risk Analysis Annual Meeting at Arlington, Virginia on 7 December 2015.
As a preface to the remainder of the document, the process by which IARC identifies and reviews data must be compared with that employed by the Expert Panel(s). IARC only reviews data included in: “reports that have been published or accepted for publication in the openly available scientific literature” or “data from governmental reports that are publicly available” (IARC Citation2006). In addition, IARC reviews and assesses these data in the context of hazard (i.e. inherent carcinogenic potential) not risk (i.e. the likelihood of carcinogenic effects at exposure levels humans may encounter). As a result, the conclusion of IARC is often solely associated with hazard. In contrast to IARC, toxicology, mechanism, and exposure Expert Panels evaluated all of the available scientific data, including the results of a number of unpublished reports, some of which have been submitted to and reviewed by regulatory authorities. These reports document GLP- and OECD/FDA Redbook guideline compliant studies, conducted to assess the genotoxic and carcinogenic potential of glyphosate. In essence, these studies provide the highest quality of documentation and verification; hence, a balanced assessment requires the inclusion of such studies in the review process. The third panel (epidemiology) took an approach consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews (Moher et al. Citation2009), standard approaches to critically evaluating epidemiologic studies (Aschengrau & Seage Citation2003a,Citationb; Sanderson et al. Citation2007) and well-recognized interpretative methods – e.g. the criteria-based methods of causal inference (Hill Citation1965, Citation1971) – sometimes referred to as “weight of evidence” (WoE) methods (Weed Citation2005). In addition to the identification of hazard potential, the Expert Panels assessed exposure data to provide a perspective from which to comment on potential risk. In the absence of carcinogenic hazard, however, no risk is present regardless of exposure. The conclusions reached by the Expert Panels and IARC clearly differ. However, in the opinion of the Expert Panel(s) this is not due to differences in process (hazard versus risk assessment), but rather the result of the exclusion from the IARC review process of key data (animal bioassay and genotoxicity) or differences in the interpretation of the data that was assessed particularly in regard to the animal bioassay results. Given these differences, even without the data IARC did not include, there is no support for IARC’s conclusion that glyphosate is “probably carcinogenic to humans.” This critique is presented and discussed in the context of the Expert Panels’ assessment of the totality of the data.
Exposures to glyphosate
Unpublished reports of studies on exposure to glyphosate in applicators were provided by Monsanto Company which covered uses in agriculture and forestry (see Solomon Citation2016 for additional details and bibliography). Other data on exposures were obtained from the open literature as a result of searches in PubMed®, references in reviews, and Google Scholar®. These papers and reports were grouped into sources of exposures and the data analyzed as described below.
Only one paper reported concentrations of glyphosate in air. In a study conducted in Iowa, Mississippi, and Indiana in 2007 and 2008, concentrations of glyphosate and its major environmental degradate, aminomethylphosphonic acid (AMPA), were measured in air and precipitation (Chang et al. Citation2011). For estimation of human exposure, it was assumed that there was 100% absorption of glyphosate from the air into the body of a 70 kg human breathing 8 m3 air (half a day for an adult) (US EPA Citation2009). Also, surface water measurements of glyphosate as part of the National Water-Quality Assessment (NAWQA) program (USGS Citation2015) since 2002 were downloaded from the NAWQA data warehouse and then sorted by concentration. All values measured across the US between 2002 and 2014 were pooled for the analysis. Where concentrations were less than the level of detection (0.02 μg glyphosate acid equivalents (a.e.)/L), these values were substituted with a dummy value of “zero.” Although chlorine and ozone are highly effective in removing glyphosate and AMPA during purification of drinking water (Jönsson et al. Citation2013), it was assumed that treatment did not remove any glyphosate. The estimated concentrations are thus a worst-case.
Studies documenting exposures through food and to “bystanders” (persons who are located within or directly adjacent to areas where pesticides are applied but who are not actively involved in the process) were reviewed and data extracted (Acquavella et al. Citation2004; Curwin et al. Citation2007; Mesnage et al. Citation2012; Hoppe Citation2013; Honeycutt & Rowlands Citation2014; Niemann et al. Citation2015). For those measurements, publications that provided actual systemic dose calculations were used rather than estimates calculated from default exposure factors (e.g. body weight (bw), water consumption, breathing rate, etc.). Where dietary exposures were calculated the urinary concentration was used to calculate the systemic dose on the assumption of 2 L of urine per day and a 60 kg person (Niemann et al. Citation2015). In 2013, the JMPR reviewed dietary exposures to glyphosate (glyphosate, N-acetyl glyphosate, AMPA, and N-acetyl AMPA) and calculated the international estimated daily intakes (IEDI) of glyphosate for 13 regional food diets (JMPR Citation2014). These IEDIs were based on estimated mean residues from supervised trials under normal or good agricultural practice. The US EPA has calculated exposures to glyphosate using the Dietary Exposure Evaluation Model (DEEM, ver 7.81), based on tolerance levels for all commodities and modeled estimates of exposures from food and drinking water for the overall US population (US EPA Citation2012). For studies using dosimetry, the normalization to systemic dose was conducted using the following assumptions: 70 kg adult, 2.1 m2 surface area for a 70 kg male (US EPA Citation2009), 10% penetration through clothing if not actually measured, 1% dermal penetration. The estimated systemic doses were ranked from smallest to largest and a cumulative frequency distribution derived. These values were plotted on a log-probability scale. The median (50th centile) and 90th centile values were calculated from the raw data using the Excel function < =percentile>.
Where an applicator makes a single application, the systemic dose of glyphosate can be estimated from the total amount of glyphosate excreted in the urine over the 4 or 5 days following and including the day of application (Acquavella et al. Citation2004). If applications are conducted every day, the amount excreted each day provides a time-weighted average for daily exposures. Because glyphosate is applied infrequently in normal agricultural practice, the assumption of a single initial exposure is considered appropriate for risk assessment purposes.
Exposures via air
Based on the above assumptions, inhaling glyphosate in air at the maximum measured concentration would result in an exposure of 1.04 × 10−6 mg/kg body mass (bm)/day. This is about five orders of magnitude less than the systemic ADI proposed by EFSA (Citation2015).
Exposures via water
The concentrations of glyphosate measured in US surface waters ranged from 0.02 to 73 μg/L. The 90th centile value was 0.79 μg/L (see Solomon (Citation2016) for details of the calculations), more than four orders of magnitude less than the EFSA ADI.
Exposures from food and in bystanders
Estimates of glyphosate exposures to bystanders and the general public have been reported by various investigators (Curwin et al. Citation2007; Mesnage et al. Citation2012; Hoppe Citation2013; Honeycutt & Rowlands Citation2014; Krüger et al. Citation2014; Markard Citation2014). In these studies, the range for estimates of systemic doses was 0.000022–0.00063 mg/kg/day. These values are all less than the ADI suggested by EFSA.
Exposure of applicators
The 90th centile in the dosimetry studies was 0.021 mg/kg/day; about five-times less than the systemic EFSA ADI. The range of values for the systemic doses determined by biomonitoring was smaller than for the passive dosimeters. The 90th centile was 0.0014 mg/kg b.m./day; about 70-times less than the systemic EFSA ADI.
In summary, there is a robust dataset on glyphosate exposures to humans. Even when using worst-case assumptions, systemic exposures to applicators, bystanders, and the general public are very small. Based on current RfDs and ADIs and measured exposures, there is an extremely large margin of safety from exposure to glyphosate via normal uses.
Epidemiological data
The epidemiology Expert Panel conducted a systematic review of the published glyphosate literature for the two cancers that were the focus of IARC’s epidemiology review: non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM) (see Acquavella et al. (Citation2016) for additional details). Initially, an exhaustive search of the medical literature was performed to identify all epidemiological studies that examined the relationships between reported use of glyphosate and NHL or MM. This resulted in seven unique studies for NHL and four studies for MM after removal of duplicates and focusing on the most recent findings for study populations that were the subject of more than one publication. The relevant studies are listed in . Each study was then reviewed individually according to key validity considerations specified a priori and the results for NHL and MM were separately and systematically evaluated according to widely used criteria for judging causal associations from epidemiologic studies (Hill Citation1965).
Table 2. Relevant studies for glyphosate review: non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM).
Data abstracted from each study included: first author, year of publication, outcome (NHL, MM), study design, study size, statistical methods, results (measure of relative risk [RR] with accompanying 95% confidence interval [95% CI]), exposure-response findings, and variables controlled in the analyses. Each study was evaluated for key features that relate to study validity, most importantly: recall bias, proxy respondents, selection bias, adequate statistical control for confounding factors, and evaluation of dose response ().
Table 3. Key validity considerations in glyphosate epidemiological studies.
Of the seven NHL studies, only one study – the Agricultural Health Study (AHS) cohort study (de Roos et al. Citation2005) – was devoid of major concerns about recall bias and selection bias by virtue of the design (prospective versus retrospective), was controlled comprehensively for confounding factors, and extensively considered RR by frequency and duration of glyphosate use. This study of more than 50 000 licensed pesticide farmers and applicators collected information about pesticide use before follow-up for health outcomes, had only first-hand respondents reporting about pesticide use (viz. no proxy respondents), had minimal potential for selection bias, and included statistical analyses that controlled confounding factors by myriad personal characteristics and non-glyphosate occupational exposures. In addition, de Roos et al. (Citation2005) were the only investigators who conducted exposure-response analyses while controlling extensively for confounding exposures. In contrast, the NHL case–control studies had major validity concerns including the strong potential for recall bias, selection bias (either appreciably lesser participation for controls than cases or selecting controls that clearly did not reflect the population that gave rise to the cases [e.g. hospitals controls from rheumatology and orthopedic departments]), proxy respondents, and uncontrolled confounding factors in the statistical analyses. Indeed, in many of the case–control studies virtually every pesticide exposure studied was associated with increased risk for NHL (or MM) – a clear indication of widespread systematic bias.
With these considerations in mind, for NHL, the results of the de Roos et al. (Citation2005) cohort study were considered the only reliable epidemiologic findings. As de Roos et al. (Citation2005) concluded “… the available data provided evidence of no association between glyphosate exposure and NHL incidence.” Results from this study were the basis for the Panel’s conclusion of no epidemiologic support for a causal relationship between reported glyphosate use and NHL.
The glyphosate literature for MM is appreciably sparser than the literature for NHL, both in terms of the number of available studies (one cohort and three case–control studies) and the number of cases in those studies with reported glyphosate use. The three case–control studies had important validity concerns, as noted for the NHL case–control studies, and were unable to adjust analyses comprehensively for confounding factors due to the very small number of exposed cases. The AHS cohort study (de Roos et al. Citation2005 and re-analyzed by Sorahan Citation2015) found that glyphosate users had about the same rate of MM as non-users adjusting for confounding factors, but had too few exposed cases to conduct informative exposure response analyses.
In summary, the epidemiology Expert Panel concluded that the glyphosate epidemiologic literature does not indicate a causal relationship between glyphosate exposure and NHL. For MM, the evidence was considered too sparse to judge a relationship between MM and reported glyphosate use. The panel’s conclusion for NHL differed from that of the IARC working group primarily because the null findings from the AHS (cohort) study were the only epidemiologic findings considered likely to be valid.
Cancer bioassays
The carcinogenicity Expert Panel reviewed all listed cancer bioassays reviewed by Greim et al. (Citation2015) and IARC (2015). The recommended method for evaluating the results of an extensive database of toxicology and carcinogenicity bioassays, as exist for glyphosate, involves the application of a WoE approach (US EPA Citation1986c; ECHA Citation2010). Methods for evaluating the results of an extensive database of toxicology and carcinogenicity bioassays, as exist for glyphosate, have evolved from the application of WoE approaches (US EPA, Citation2005; Suter and Cormier, Citation2011) to approaches built on the systematic and rigorous methods of systematic evidence-based reviews (James et al. Citation2015). These approaches recommend that all reliable information be evaluated. Transparent descriptions of studies to be included and excluded are a key component of this approach. In any review, if certain studies are judged to be unreliable and thus not included, the reasons for this should be provided. The carcinogenicity Expert Panel reviewed the incidences of the tumors in the various studies with respect to dose-response, rate of occurrence relative to known spontaneous rates in control animals, and on the basis of biological plausibility. Additional details of the Expert Panel’s considerations and conclusions are presented in Williams et al. (Citation2016).
In contrast to the results of past reviews (see ), IARC (Citation2015) concluded that there is sufficient evidence in experimental animals for the carcinogenicity of glyphosate, based on the following:
A significant positive trend in the incidence (p = .037) of renal tubule carcinomas and of adenomas and carcinomas (p = .034) in male CD-1 mice of one study only. This is a rare tumor type.
In a second feeding study in the same strain of mice, a significant positive trend in the incidence (p < .001) of hemangiosarcomas occurred in males.
In two dietary studies in SD rats, a significant positive trend (p < .05) in the incidence of pancreatic islet cell adenomas occurred in males.
In a dietary study in SD rats, a significant positive trend (p = .016) in the incidence of hepatocellular adenomas occurred in males.
In a dietary study in SD rats, a significant positive trend (p = .031) in the incidence of thyroid C-cell adenomas occurred in females.
Table 4. Regulatory agency reviews of three studies evaluated by IARC.
Kidney tubular – cell neoplasia in mice
In regard to the rare renal tubular tumors in male CD-1 mice, the Expert Panel noted that the conclusions of the IARC were based on only one 2-year oral mouse carcinogenicity study, (Monsanto Citation1983) excluding two additional 18-month oral studies in CD-1 mice (Arysta Life Sciences Citation1997; Nufarm Citation2009) and one 18-month oral study in Swiss Albino mice (Feinchemie Schwebda Citation2001). All of the studies were considered by authoritative bodies to have met the guidelines for a carcinogenicity bioassay in mice (US EPA Citation1990; ICH Citation1997).
In the study conducted by Monsanto (Citation1983) considered by IARC (Citation2015) to show evidence of renal tubular neoplasia associated with glyphosate dosing, male (M) and female (F) CD-1 mice received 0 (M0/F0 mg/kg/day, control), 1000 (157/190, LD), 5000 (814/955, MD), or 30,000 (4841/5874, HD) ppm in the diet. The incidence by dose of renal neoplasms in male mice was as follows: 1/49, 0/49, 1/50, and 3/50. The important non-neoplastic renal findings of hyperplasia were as follows: 3/49, 0/49, 4/50, and 2/50, indicating lack of a dose-response, with the highest incidence in the mid-dose (MD) group, followed by the control group, and the high-dose (HD) group. The low-dose (LD) group had no renal findings. Females had neither neoplasia nor hyperplasia. Absence of hyperplasia indicates that all renal proliferative and neoplastic lesions, which occurred in all experimental groups (including controls) occurred de novo, i.e. were spontaneous or background lesions and were not compound related.
Factors to assess whether an association between exposure and an effect (two variables) is causal include strength, consistency, and specificity of the association, the temporal (latency) and dose-response relationships present, plausibility of effect, and coherence of the available data. When applied to the study by Monsanto (Citation1983), several conclusions were drawn, as follows:
The association was not strong because the incidence of rare renal neoplasms was not statistically significant in any exposed group when compared to the control group.
The association is not consistent, since four out of five mouse studies did not find similar renal neoplasms at similar doses.
The association is not specific, since females of this pivotal study, which were exposed to higher levels of glyphosate, did not develop renal neoplasms. Also, there were no renal findings (hyperplasia, neoplasia) in the LD group, whereas the control group had four.
The time required between exposure and effect, i.e. the latency time, was not reduced; all tumors were observed only at termination. Also, no mouse with neoplasia had also hyperplasia.
The biological gradient of association or the dose-response curve was absent, since the females and the males in the LD group had no neoplasms, whereas there was one in the control group.
A plausible explanation for the association was absent, since the mode of action for induction of these renal neoplasms was not established.
Coherence of the association was also absent, as female mice and male and female rats did not display kidney effects. Also in the other four mouse carcinogenicity studies (three of which were not considered in the IARC monograph), the mice did not develop similar neoplastic renal lesions.
The association does not demonstrate a dose-response pattern (see #5, 6), and furthermore the “in-study” females had neither neoplasms nor any of the other renal lesions, although they were exposed to higher levels of glyphosate.
Consequently, under the conditions of this assessment, the renal neoplastic effects are not plausibly associated with glyphosate exposure. This conclusion is in agreement with that of JMPR (Citation1987, 2006), US EPA (Citation1993), and EFSA (2015).
Hemangiosarcomas in mice
With respect to the common liver hemangiosarcoma in male mice, in the CD-1 mouse study reported by Cheminova (Citation1993) there were no statistically significant increases in the incidence of any tumors when compared with the in-study and historical (for both sexes 2–12%) control groups and no dose response was apparent (Williams et al. Citation2016). IARC, based on their own statistical analysis, indicated/reported that there was an increase in the incidence of hemangiosarcoma in males [p < .001, Cochran–Armitage trend test] based on the incidence of the HD group (). In addition, IARC (Citation2015) did not comment on the lack of hemangiosarcomas in females which have received higher doses of glyphosate, and also of renal tumors in this mouse study.
Table 5. Tumor incidence/number of animals examined (mg/kg bw/day)Table Footnote*.
It is clear that the association between glyphosate treatment and hemangiosarcoma in mice is weak since pairwise comparisons are not significant, there is no consistency (some mouse studies show no tumors of this type at all at comparable doses), and a dose response effect is not seen (some HD groups have a lower incidence than lower doses). In addition, the recorded incidences are within the historical control range.
Given the foregoing analysis, the Expert Panel concludes that overall the evidence does not support the conclusion that glyphosate exposure results in increased incidence of hemangiosarcoma in mice.
Pancreatic tumors in rats
In two of the seven carcinogenicity studies in rats that were evaluated by IARC, tumors of islet cells of the pancreas were diagnosed in both males and females. Both studies were made available to IARC by the US EPA (Citation1991a,b,c).
In the first study Sprague-Dawley rats received doses of 0, 30 (3), 100 (10), and 300 (31 mg/kg bw/day) ppm in the diet for 26 months. No pancreatic islet carcinomas were observed. Adenomas were found having a positive trend (p < .05) in the study. The level of significance for an increase in common tumors in the trend test should be p < .005. The tumor incidences for controls, low, mid, and high doses respectively were: males – 0/50, 5/49 (10%), 2/50 (4%), 2/50 (4%), and females – 2/50 (4%), 1/50 (2%), 1/50 (2%) 0/50. This incidence demonstrates no dose-response pattern, and an absence of pre-neoplastic effects. In addition, in the first study in males, the adenomas did not progress to carcinomas.
In the second study Sprague-Dawley rats received 0, 2000, 8000, and 20,000 ppm glyphosate (96.5% purity) in the diet, fed ad libitum for 24 months. In males, the following pancreatic islet cell tumor incidences were observed in the controls and three dose groups (low to high): adenoma: 1/58 (2%), 8/57 (14%), 5/60 (8%), 7/59 (12%); carcinoma: 1/58 (2), 0/57, 0/60, 0/59. Corresponding incidence values in females were: 5/60 (8%), 1/60 (2%), 4/60 (7%), 0/59, and 0/60, 0/60, 0/60, 0/59. The historical control rates for pancreatic islet cell tumors at the testing laboratory were in the range 1.8–8.5%. The Panel disagrees with the conclusion of IARC that there is a significant positive trend (p < .05) in the incidence of pancreatic adenomas in males, since here again the level of significance should be p < .005 (US FDA, Citation2001; Williams et al. Citation2014). Moreover, there was no progression of adenomas to carcinomas.
Four additional studies in rats, described by Greim et al. (Citation2015) not evaluated by IARC, similarly did not show pancreatic islet cell tumors. Based on this information the Expert Panel concludes that there is no evidence that glyphosate induces islet cell tumors in the pancreas.
Liver tumors in rats
Hepatocellular neoplasms are common for the SD rat (about 5% in males and 3% in female controls) (Williams et al. Citation2014).
The IARC evaluation indicated that there was “…a significant (p = .016) positive trend in the incidences of hepatocellular adenoma in males…” (IARC Citation2015). This opinion was based on its interpretation of the Stout and Ruecker (Citation1990) study as presented by the US EPA’s Peer Review of Glyphosate (US EPA Citation1991a,Citationb) (see ). The Stout and Ruecker (Citation1990) study has been reviewed twice by the US EPA (Citation1991a,Citationb). The final interpretation of the US EPA Review committee was: “Despite the slight dose-related increase in hepatocellular adenomas in males, this increase was not significant in the pair-wise comparison with controls and was within the historical control range. Furthermore, there was no progression from adenoma to carcinoma and incidences of hyperplasia were not compound-related. Therefore, the slight increased occurrence of hepatocellular adenomas in males is not considered compound-related” (US EPA Citation1991b). The US EPA ultimately concluded that glyphosate should be classified as a Group E (evidence of non-carcinogenicity for humans) chemical (US EPA Citation1991a,b).
Table 6. Sprague-Dawley male rats, hepatocellular tumor rates+, and Cochran–Armitage trend and Fisher’s exact tests results (p values).
There are other aspects of the Stout and Ruecker (Citation1990) data that support the conclusion that glyphosate did not exert an oncogenic effect on the liver of SD rats. For example, chemically induced rat hepatocellular carcinogenesis is a multiple stage process characterized by progressive functional, morphological, and molecular changes that indicate or precede the full establishment of neoplasia, such as enzyme induction, hepatocyte hypertrophy, degeneration and necrosis, hepatocyte proliferation, altered hepatocellular foci, etc. (Williams Citation1980; Bannasch et al. Citation2003; Maronpot et al. Citation2010). Identification and analyses of these liver changes – that span from adaptive to irreversible toxic effects – can help support characterization of key events along the carcinogenesis process and inform the mode of action of the tested chemical (Williams & Iatropoulos Citation2002; Holsapple et al. Citation2006; Carmichael et al. Citation2011). These changes were not apparent in this study.
In the last 30 years, the systemic carcinogenic potential of glyphosate has been assessed in at least eight studies in Sprague-Dawley or Wistar rats, which were not all included within the IARC monograph (Greim et al. Citation2015); a ninth could not be evaluated because of a high mortality and the low doses used (Chruscielska et al. Citation2000). Considered jointly, the animals were exposed through the diet to 24 different doses distributed across a wide range (3.0–1290 mg/kg bw/day). In exposed males, the incidences of hepatocellular adenomas across the doses showed no dose-response relationship and varied within the same range as the controls. Similar rates were also seen for hepatocellular carcinomas. These observations confirm that glyphosate is not carcinogenic to the rat liver.
Thyroid tumors in rats
C-cell tumors of the thyroid are a common tumor in the SD rat (Williams et al. Citation2014).
The incidence of thyroid C-cell adenoma was reported in the Monograph (IARC Citation2015), to have a significant positive trend (p = .031) in females. IARC based their opinion, again, on their interpretation of the Stout and Ruecker’s (Citation1990) study and the US EPA’s Second Peer Review of Glyphosate (US EPA Citation1991a). In the Stout and Ruecker’s study (1990), no statistically significant difference (group comparison) was reported in the incidence of thyroid C-cell neoplasms, as shown in . Additionally, the US EPA (Citation1991a) concluded that “the C-cell adenomas in males and females are not considered compound-related.” Although the C-cell adenomas were slightly numerically greater in male and female MD and HD groups, there was no dose-related progression to carcinoma and no significant dose-related increase in severity of grade or incidence of hyperplasia in either sex. However, IARC concluded that “there was a statistically significant positive trend in the incidence of thyroid, C-cell adenomas in females” (p = .031 but, because this is a common tumor type, the trend significance value should be p < .005 (US FDA Citation2001; Williams et al. Citation2014)). Thus, this tumor is not significant.
Table 7. Tumor incidence/number of animals examined (mg/kg bw/day)*.
Therefore, in one of the two evaluated studies, the significant trend in the incidence of thyroid C-cell adenomas in female rats did not materialize, and there was no progression to carcinomas. The adenomas were within the historical ranges.
Genetic toxicity and oxidative stress data
The genetic toxicology Expert Panel (Brusick et al. Citation2016) considered published studies reviewed in the IARC monograph and additional published studies identified by literature searches or from review articles, not considered by IARC. These included both genetic toxicology studies and studies of oxidative stress. A large number of core genetic toxicology regulatory studies were also considered by the Expert Panel for which information was available from review publication supplements. These regulatory studies were not considered in the IARC monograph, but the Expert Panel concluded that sufficient test-related information was available to justify including these studies. In addition, some unpublished regulatory studies not reviewed previously were included in the Expert Panel evaluation.
The universally recommended method for evaluating the databases of the type associated with glyphosate (including GBFs and AMPA), involves the application of a WoE approach as discussed recently for genetic toxicology testing (US FDA Citation2006; Dearfield et al. Citation2011). One of the most important requirements of a WoE approach is that individual test methods should be assigned a weight that is consistent with their contribution to the overall evidence, and different types of evidence or evidence categories must be weighted before they are combined into a WoE.
The weight of a category of evidence used in the Expert Panel evaluation is based on four considerations: (i) different categories of evidence (i.e. assay types) have different weights, (ii) the aggregate strength (robustness of protocols and reproducibility) and quality of evidence in the category also influence the weight (Klimisch et al. Citation1997), (iii) the number of items of evidence within a category influences the weight, and (iv) tests with greater potential to extrapolate results to humans carry greater weight. In general, human and in vivo mammalian systems have the highest test system weight, with a lower weight applied to in vitro mammalian cell systems and in vivo non-mammalian systems and lowest weight to in vitro non-mammalian systems (with the exception of the well-validated bacterial reverse mutation-[Ames] test using mammalian metabolic activation). Typically, the results of in vivo assays supersede the results of in vitro assays (EFSA Citation2011).
In contrast to the standard WoE approach used by the Expert Panel, IARC’s process for evaluating/weighting the genotoxicity data for glyphosate, GBF and AMPA was not defined. IARC’s process may be inferred by how the data were summarized and described, and indicate a number of differences from current standard procedures for WoE. For instance, it appears that IARC considered in vitro studies in human cells as carrying more weight than rodent in vivo studies as evidenced by the order of discussion topics in Section 4.2.1, and the inclusion of a separate table for human in vitro studies. Further, the IARC conclusion of strong evidence of genotoxicity was stated as based on “studies in humans in vitro and studies in experimental animals.” In contrast, the Expert Panel evaluation considered in vitro studies using cells of human origin to be weighted as equivalent to any other in vitro mammalian cell assay using the same endpoint. IARC also gave weight to publications in which glyphosate or GBFs have been tested for genotoxicity in a variety of nonstandard non-mammalian species (fish, insects). The Expert Panel did not consider data from these non-mammalian systems and nonstandard tests with glyphosate, GBF and AMPA to have weight in the overall genotoxicity evaluation, especially given the large number of standard core studies assessing the more relevant gene mutation and chromosomal effects categories available in mammalian systems. In addition, nonstandard tests lack internationally accepted guidelines for design and conduct, databases that document acceptable negative control data or positive control responses are absent, and validation with respect to concordance with rodent or human carcinogenicity has yet to be completed. OECD guidelines specifically state that use of any nonstandard tests require justification along with stringent validation including establishing adequate historical negative and positive control databases (OECD Citation2014).
In addition, the IARC review seemed to apply significant weight to “indicator” tests such as DNA damage (comet assay) or sister chromatid exchange (SCE) studies. These tests are identified as indicators because the measured endpoint is reversible and does not always lead to mutation, a key event in cancer development. As stated by OECD (Citation2015), when evaluating potential genotoxicants, more weight should be given to the measurement of permanent DNA changes than to DNA damage events that are reversible. Therefore, the Expert Panel also considered that the data from these “indicator” tests with glyphosate, GBFs and AMPA should not have significant weight in the overall genotoxicity evaluation, especially given the large number of standard core studies in the more relevant gene mutation and chromosomal effects categories available in mammalian systems.
IARC did not consider the chemical structure of glyphosate in its mechanistic section. Many guidelines recommend that the presence of structural alerts be considered in evaluation of or testing for genotoxicity (Cimino Citation2006; Eastmond et al. Citation2009; EFSA Citation2011; ICH Citation2011). As reported in Kier and Kirkland (Citation2013), analysis of the glyphosate structure by DEREK software identified no structural alerts for chromosomal damage, genotoxicity, mutagenicity, or carcinogenicity. The lack of structural alerts in the glyphosate molecular structure suggests lack of genotoxicity and that genotoxic effects observed might be secondary to toxicity or resulting from mechanisms other than DNA reactivity.
Genetic toxicology tests relied upon by most regulatory bodies to support decisions regarding safety focus on a set of core endpoints that are known to be involved either in direct activation of genes responsible for neoplastic initiation in somatic cells or alteration of the genetic information in germ cells (EFSA Citation2011; ICH Citation2011; Kirkland et al. Citation2011). Therefore, the endpoints given the greatest weight in consist of gene mutation and chromosomal aberrations.
Table 8. Summary of the Panel’s evaluation of human, non-human mammalian and selected microbial genotoxicity studies from IARC section 4.2.1 and other published sources.
An evaluation of the studies in according to their relative contributions to a WoE produced the following results:
Test methods identified as providing low contribution to the WoE (low weight) produced the highest frequency of positive responses, regardless of whether the responses were taken from the results of IARC-evaluated studies alone (8 of 9) or from all studies combined (8 of 11).
The highest frequencies of positive responses were reported for test endpoints and systems considered most likely to yield false or misleading positive results due to their susceptibility to secondary effects. This relationship was constant regardless of whether the results were taken from IARC-evaluated studies alone or all studies combined.
The numbers of studies providing strong evidence of relevant genotoxicity (high weight) were in the minority for both the IARC and the Expert Panel’s evaluations, with 6 out of 15 studies identified as high weight being positive for the IARC evaluation, and only 8 out of 92 studies identified as high weight being positive for all studies combined.
In summary, the WoE from in vitro and in vivo mammalian tests for genotoxicity indicates that:
Glyphosate does not induce gene mutations in vitro. There are no in vitro mammalian cell gene mutation data for GBFs or AMPA, and no gene mutation data in vivo.
Glyphosate, GBFs, and AMPA are not clastogenic in vitro. Glyphosate is also not clastogenic in vivo. Some positive in vivo chromosomal aberration studies with GBFs are all subject to concerns regarding their reliability or biological relevance.
There is limited evidence that glyphosate induces micronuclei (MN) in vitro. Although this could be a reflection of increased statistical power in the in vitro MN studies, the absence of clastogenic effects suggests the possibility of threshold-mediated aneugenic effects. However, there is strong evidence that glyphosate does not induce MN in vivo.
Limited studies and potential technical problems do not present convincing evidence that GBFs or AMPA induce MN in vitro. The overwhelming majority of in vivo MN studies on GBFs gave negative results, but conflicting and limited data do not allow a conclusion on in vivo induction of MN by AMPA.
There is evidence that glyphosate and GBFs can induce DNA strand breaks in vitro, but these are likely to be secondary to toxicity since they did not lead to chromosome breaks. There is limited evidence of transient DNA strand breakage for glyphosate and GBFs in vivo, but for glyphosate at least these are not associated with DNA adducts. These results are assigned a lower weight than results from other more relevant endpoints, which were more abundant.
There is evidence that glyphosate and AMPA do not induce unscheduled DNA synthesis (UDS) in cultured hepatocytes.
Reports of the induction of SCE in vitro by glyphosate and GBFs, and one positive report of SCE induction in vivo by a GBF, do not contribute to the overall evaluation of genotoxic potential since the mechanism of induction and biological relevance of SCE are unclear.
Although IARC policies prohibited the inclusion of additional data from unpublished studies or governmental reports, it was the Expert Panel’s conclusion that the regulatory genetic toxicology studies published in reviews such as Kier and Kirkland (Citation2013) () should be included in a WoE assessment. The rationale supporting the inclusion of these additional studies is that the supplementary tables presented in the Kier and Kirkland (Citation2013) paper, contain sufficient detail supporting the reliability of the studies. Failure to evaluate and consider the large number of results included in the publication by Kier and Kirkland (Citation2013), as well as other publicly available studies not reviewed by IARC, results in an inaccurate assessment of glyphosate, GBFs and AMPA’s genotoxic hazard/risk potential.
Table 9. Summary of studies presented in Kier and Kirkland (Citation2013) and of other publicly available studies not included in the IARC review.
Based on the results of the WoE critique detailed above and the wealth of regulatory studies reviewed by Kier and Kirkland (Citation2013) and Williams et al. (Citation2000), the Panel concluded that the available data do not support IARC’s conclusion that there is strong evidence for genotoxicity across the glyphosate or GBFs database. In fact, the Panel’s WoE assessment provides strong support for a lack of genotoxicity, particularly in the relevant mechanism categories (mutation, chromosomal effects) associated with carcinogen prediction. As additional support for the Panel’s WoE conclusion, provides a comparison between a set of characteristics associated with confirmed genotoxic carcinogens (Bolt et al. Citation2004; Petkov et al. Citation2015) and the genotoxic activity profiles for glyphosate, AMPA, and GBFs. There is virtually no concordance between the two sets of characteristics.
Table 10. Comparison of test response profiles from glyphosate, GBFs, and AMPA to the profile characteristics of confirmed genotoxic carcinogens.
Beyond the standard genetic toxicity assays, IARC concluded for humans exposed to GBFs that there was positive evidence of DNA breakage as determined using the comet assay (Paz-y-Miño et al. Citation2007), negative induction of chromosomal aberrations (Paz-y-Miño et al. Citation2011), and positive induction of MN (Bolognesi et al. Citation2009). These papers were critically reviewed by the Expert Panel and were found to be deficient as evidence for GBF genetic effects for many reasons (e.g. identification of cells scored for comets, inconsistent observations, uncertainties with respect to “negative controls,” lack of statistical significance, and lack of effect relative to self-reported exposure). In addition to questions about the significance of the comet endpoint there is also a lack of scientific consensus regarding the relevance of MN found in exposed humans (Speit Citation2013; Kirsch-Volders et al. Citation2014). Importantly, for the Bolognesi study, increases in MN were not significantly correlated with self-reported GBF spray exposure and were not consistent with application rates. The Expert Panel concluded that there was little or no reliable evidence produced in these studies that would support a conclusion that GBFs, at levels experienced across a broad range of end-user exposures, poses any human genotoxic hazard/risk.
With respect to oxidative stress and genotoxic potential of glyphosate and its formulations, it is noted that many more oxidative stress studies are available for GBFs than for glyphosate or AMPA. A higher proportion of the GBF studies show evidence of oxidative stress. This might be consistent with induction of oxidative stress by GBF components such as surfactants. IARC’s statement that there is strong evidence supporting oxidative stress from AMPA seems to result from glyphosate and particularly GBF results rather than AMPA results. In fact, oxidative stress studies of AMPA are very limited. The paucity of cited data does not seem to justify a conclusion of strong evidence for oxidative stress induction by AMPA.
One mechanism connecting oxidative stress to induction of carcinogenicity is oxidative damage to DNA and the generation of mutagenic lesions. Most of the endpoints used in oxidative stress studies cited by IARC are indirect response endpoints and the number of studies examining direct oxidative DNA damage are very few and presented mixed results. Further, research on oxidative stress-induced genotoxicity suggests that it is often a secondary response to toxicity and characterized by a threshold (Pratt & Barron Citation2003). Comparison of GBF oxidative stress study results with predicted human exposure levels of less than 0.064 mg/kg bw/day, suggests that it is improbable that GBFs would induce levels of oxidative stress likely to exceed endogenous detoxication capacities.
The most appropriate conclusion supported by the oxidative stress data is, based on a WoE approach, that there is no strong evidence that glyphosate, GBFs, or AMPA produce oxidative damage to DNA that would lead to induction of endpoints predictive of a genotoxic hazard or act as a mechanism for the induction of cancer in experimental animals or humans.
A thorough WoE review of genotoxicity data does not indicate that glyphosate, GBFs, or AMPA possess the properties of genotoxic hazards or genotoxic mechanisms of carcinogenesis.
Discussion and conclusions
Four Expert Panels conducted detailed reviews of glyphosate exposure, animal carcinogenicity, genotoxicity, and epidemiologic studies. With respect to exposure, even when using a number of worst-case assumptions, systemic doses of glyphosate in human applicators, bystanders, and the general public are very small. Exposures of the general public are three or more orders of magnitude less than the US EPA’s RfD (1.75 mg/kg/day) as well the ADIs established by JMPR (1 mg/kg/day) and EFSA (0.5 mg/kg/day). The RfD is the allowable limit of daily exposure derived from toxicity studies, and even in the most exposed applicators (90th centile) the systemic dose was estimated at 20-fold less that the RfD. Exposures to the public are in the range of 0.00001–0.001 mg/kg bw/day while occupational exposures can range up to 0.01 mg/kg bw/day. Systemic exposures are even lower than the reported ranges since oral and dermal absorption of glyphosate is low.
With respect to the animal cancer bioassay data, the Expert Panel conducted a thorough overall WoE evaluation that considered a much wider range of studies than IARC, all of which met Good Laboratory Practice (GLP) guidelines and were submitted to support glyphosate Annex I renewal in the European Union. These studies provided evidence that neoplasms naturally occurring in rodents are widely represented in non-exposed animals, as well as those exposed to doses well below those that might be expected in regulatory studies. The pattern of occurrence of these tumors was found to be inconsistent across and within species and no “novel” neoplasms appeared; progression of non-neoplastic to neoplastic lesions also was not seen. Further, the comparatively large number of studies performed would be expected to generate several numerical imbalances by chance. In fact, Haseman (Citation1983) has estimated that the overall false positive rate for animal bioassays that tested both sexes in two species, because of multiple comparisons, corresponds to 7–8% significance level for the study as a whole; the US Food and Drug Administration has estimated that the overall rate can approach 10%.
After review of all available glyphosate rodent carcinogenicity data, the Panel concludes:
The mouse renal neoplastic effects are not associated with glyphosate exposure, because they lack statistical significance, consistency, specificity, a dose-response pattern, plausibility, and coherence;
The association of hemangiosarcomas in the livers of mice is weak, lacks consistency, and there was no dose-response effect;
The association of pancreatic islet-cell adenomas in male SD rats is weak, not seen in the majority of rat studies, lacks a dose-response pattern (the highest incidence is in the low dose followed by the high dose), plausibility and pre-neoplastic/malignant effects;
In one study, the significant positive trend in the incidence of hepatocellular adenomas in male rats did not materialize, no progression to malignancy was evident and no glyphosate-associated pre-neoplastic lesions were present;
In one study, the significant positive trend in the incidence of thyroid C-cell adenomas in female rats did not materialize, the adenomas were only slightly increased in mid- and high doses, and there was no progression to malignancy.
Overall, extensive reviews of the genotoxicity of glyphosate, AMPA, and GBFs that were available prior to the development of the IARC Glyphosate Monograph all support a conclusion that glyphosate (and related materials) is inherently not genotoxic. Further, evidence indicative of an oxidative stress mechanism of carcinogenicity is largely unconvincing. The Expert Panel concluded that there is no new, valid evidence presented in the IARC Monograph that would provide a basis for altering these conclusions.
Lastly, the Expert Panel’s review of the glyphosate epidemiologic literature and the application of commonly applied causal criteria did not indicate a relationship with glyphosate exposure and NHL. In addition, the Panel considered the evidence for MM to be inadequate to judge a relationship with glyphosate. The extremely large margin of safety found in exposure monitoring studies is considered to be supportive of these conclusions.
In summary, the totality of the evidence, especially in light of the extensive testing that glyphosate has received, as judged by the Expert Panels, does not support the conclusion that glyphosate is a “probable human carcinogen” and, consistent with previous regulatory assessments, the Expert Panels conclude that glyphosate is unlikely to pose a carcinogenic risk to humans.
Acknowledgements
The authors gratefully acknowledge the extensive comments received from nine independent reviewers selected by the Editor and who were anonymous to the authors. These comments were very helpful in revising the manuscript.
Declaration of interest
The employment affiliation of the authors is as shown on the cover page. However, it should be recognized that each individual participated in the review process and preparation of this paper as an independent professional and not as a representative of their employer.
The Expert Panel Members recruitment and evaluation of the data was organized and conducted by Intertek Scientific & Regulatory Consultancy (Intertek). The Expert Panelists were engaged by, and acted as consultants to, Intertek, and were not directly contacted by the Monsanto Company. Funding for this evaluation was provided to Intertek by the Monsanto Company which is a primary producer of glyphosate and products containing this active ingredient. Neither any Monsanto company employees nor any attorneys reviewed any of the Expert Panel?s manuscripts prior to submission to the journal.
Intertek (previously Cantox) is a consultancy firm that provides scientific and regulatory advice, as well as safety and efficacy evaluations for the chemical, food, and pharmaceutical industries. While Intertek has not previously worked on glyphosate-related matters for the Monsanto Company, previous employees (Ian Munro, Barry Lynch) of Cantox, have worked in this capacity. These employees of Cantox, and Gary M. Williams, prepared a safety and risk assessment, including the carcinogenicity, of Roundup herbicide (glyphosate), which was published in 2000 (Williams et al. Citation2000).
Gary M. Williams, Sir Colin Berry, David Brusick, João Lauro Viana de Camargo, Helmut A. Greim, David J. Kirkland, Keith R. Solomon, and Tom Sorahan have previously served as independent consultants for the Monsanto Company on the European Glyphosate Task Force. John Acquavella and Larry D. Kier have also served as independent consultants and were previously employees of the Monsanto Company. John Acquavella was employed by Monsanto between the years 1989 and 2004 while Larry D. Kier was employed between 1979 and 2000. David Garabrant serves on a scientific advisory board to Dow Agro Sciences, which markets pesticides including glyphosate, and has consulted on behalf of Bayer Corp. on litigation matters concerning glyphosate and leukemia. Gary Williams and Tom Sorahan have consulted for Monsanto on litigation matters involving glyphosate. Tom Sorahan has received consultancy fees and travel grants from Monsanto Europe SA/NV as a member of the European Glyphosate Toxicology Advisory Panel and participated in the IARC Monograph Meeting for volume 112, as an Observer for the Monsanto Company. Douglas L. Weed has consulted on litigation matters concerning Monsanto that did not involve glyphosate. Marilyn Aardema, Michele M. Burns, Gary Marsh, and Ashley Roberts have not previously been employed by the Monsanto Company or previously been involved in any activity involving glyphosate and as such declare no potential conflicts of interest. Furthermore, other than David Garabrandt, Gary Williams and Tom Sorahan, none of the aforementioned authors have been involved in any litigation procedures involving glyphosate.
This article is part of a supplement, sponsored and supported by Intertek Scientific & Regulatory Consultancy. Funding for the sponsorship of this supplement was provided to Intertek by the Monsanto Company, which is a primary producer of glyphosate and products containing this active ingredient.
References
- Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M. 2004. Glyphosate for farmers and their families: results from the Farm Family Exposure Study. Environ Health Perspect. 112:321–326.
- Acquavella J, Garabrant D, Marsh G, Sorahan T, Weed DL. 2016. Glyphosate epidemiology expert panel review: a weight of evidence systematic review of the relationship between glyphosate exposure and non-Hodgkin?s lymphoma or multiple myeloma. Crit Rev Toxicol, in this issue. doi:10.1080/10408444.2016.1214681.
- APVMA. 2013. A review of the Earth Open Source (EOS) report “Roundup and birth defects: is the public being kept in the dark?” Prepared by Canberra (Australia): Scitox Assessment Services for Canberra (Australia): Australian Pesticides and Veterinary Medicine Authority (APVMA). Available from: http://archive.apvma.gov.au/news_media/docs/glyphosate_scitox_review_july_2013.pdf.
- Arysta Life Sciences. 1997. HR-001: 18-month oral oncogenicity study in mice. Tokyo (Japan): The Institute of Environmental Toxicology. Cited In: Greim et al. 2015 [As: Arysta Life Sciences 1997a].
- Aschengrau A, Seage GR. III. 2003a. Bias. In: Essentials of epidemiology in public health. Sudbury (MA): Jones and Bartlett Publishers; p. 251–279.
- Aschengrau A, Seage GR. III. 2003b. Guide to the critical review of epidemiologic studies. In: Essentials of epidemiology in public health. Sudbury (MA): Jones and Bartlett Publishers; p. 348–374.
- Bannasch P, Haertel T, Su Q. 2003. Significance of hepatic preneoplasia in risk identification and early detection of neoplasia. Toxicol Pathol. 31:134–139.
- Bolognesi C, Carrasquilla G, Volpi S, Solomon KR, Marshall EJP. 2009. Biomonitoring of genotoxic risk in agricultural workers from five Colombian regions: association to occupational exposure to glyphosate. J Toxicol Environ Health. A. 72:986–997. Cited In: IARC 2015.
- Bolt HM, Foth H, Hengstler JG, Degen GH. 2004. Carcinogenicity categorization of chemicals-new aspects to be considered in a European perspective. Toxicol Lett. 151:29–41.
- Brown LM, Burmeister GD, Everett GD, Blair A. 1993. Pesticide exposures and multiple myeloma in Iowa men. Cancer Causes Control. 4:153–156. Cited In: IARC 2015.
- Brusick D, Aardema M, Kier L, Kirkland D, Williams G. 2016. Genotoxicity Expert Panel review. Weight-of-evidence evaluation of the genotoxicity of glyphosate, glyphosate-based formulations and aminomethylphosphonic acid. Crit Rev Toxicol, in this issue. doi:10.1080/10408444.2016.1214680.
- Cantor KP, Blair A, Everett G, Gibson R, Burmeister LF, Brown LM, Schuman L, Dick FR. 1992. Pesticides and other agricultural risk factors for Non-Hodgkin’s Lymphoma among men in Iowa and Minnesota. Cancer Res. 52:2447–2455.
- Carmichael N, Bausen M, Boobis AR, Cohen SM, Embry M, Fruijtier-Pölloth C, Greim H, Lewis R, Meek ME, Mellor H, et al. 2011. Using mode of action information to improve regulatory decision-making: an ECETOC/ILSI RF/HESI workshop overview. Crit Rev Toxicol. 41:175–186.
- Chang FC, Simcik MF, Capel PD. 2011. Occurrence and fate of the herbicide glyphosate and itsdegradateaminomethylphosphonic acid in the atmosphere. Environ Toxicol Chem. 30:548–555.
- Cheminova. 1993. 104 week combined chronic feeding/oncogenicity study in rats with 52 week interim kill (results after 104 weeks) [Unpublished Report] Tranent (Scotland): Inveresk Research International, Ltd. Submitted to WHO by Lemvig (Denmark): Cheminova A/S (Report No. 7867, IRI project No. 438623). Cited In: Greim et al. 2015 [As: Cheminova 1993a]. Cited In: JMPR 2006 [As: Atkinson et al. 1993b].
- Chruscielska K, Brzezinski J, Kita K, Kalhorn D, Kita I, Graffstein B, Korzeniowski P. 2000. Glyphosate – evaluation of chronic activity and possible far-reaching effects. Part 1. Studies on chronic toxicity. Pestycydy (Warsaw) (3/4):11–20. Cited In: Greim et al. 2015 [As: Chruscielska et al. 2000a].
- Cimino MC. 2006. Comparative overview of current international strategies and guidelines for genetic toxicology testing for regulatory purposes. Environ Mol Mutagen. 47:362–390.
- Cocco P, Satta G, Dubois S, Pili C, Pilleri M, Zucca M, ‘t Mannetje AM, Becker N, Benavente Y, de Sanjosé S, et al. 2013. Lymphoma risk and occupational exposure to pesticides: results of the Epilymph study. Occup Environ Med. 70:91–98.
- Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja MC. 2007. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. Ann Occup Hyg. 51:53–65.
- de Roos AJ, Zahm SH, Cantor KP, Weisenburger DD, Holmes FF, Burmeister LF, Blair A. 2003. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup Environ Med. 60:E11. doi:10.1136/oem.60.9.e11.
- de Roos AJ, Blair A, Rusiecki JA, Hoppin JA, Svec M, Dosemeci M, Sandler DP, Alavanja MC. 2005. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environ Health Perspect. 113:49–54. Cited In: IARC 2015 [As de Roos et al. 2005a].
- Dearfield KL, Thybaud V, Cimino MC, Custer L, Czich A, Harvey JS, Hester S, Kim JH, Kirkland D, Levy DD, et al. 2011. Follow-up actions from positive results of in vitro genetic toxicity testing. Environ Mol Mutagen. 52:177–204.
- Eastmond DA, Hartwig A, Anderson D, Anwar WA, Cimino MC, Dobrev I, Douglas GR, Nohmi T, Phillips DH, Vickers C. 2009. Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS Harmonized Scheme. Mutagenesis. 24:341–349.
- ECHA. 2010. European Chemicals Agency. Practical Guide 2: How to Report Weight of Evidence. 24 March. ECHA-10-B-05-EN. Available from: http://echa.europa.eu/documents/10162/13655/pg_report_weight_of_evidence_en.pdf.
- EFSA. 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA Scientific Committee) (Question no EFSA-Q-2009-00782, adopted on 13 September 2011 by European Food Safety Authority, 3 October 2012, replaces the earlier version). EFSA J. 9:2379 [69 p.]. doi:10.2903/j.efsa.2011.2379. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/2379.htm.
- EFSA. 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate (EFSA-Q-2014-00546, EFSA-Q-2015-00279, approved on 30 October 2015 by European Food Safety Authority). EFSA J. 13:4302. [107 p.]. doi:10.2903/j.efsa.2015.4302. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/4302.
- Eriksson M, Hardell L, Carlberg M, Akerman M. 2008. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer. 123:1657–1663. Cited In: IARC 2015.
- European Commission. 2002. Review report for the active substance glyphosate. Finalised in the Standing Committee on Plant Health at its meeting on 29 June 2001 in view of the inclusion of glyphosate in Annex I of Directive 91/414/EEC. Brussels (Belgium): European Commission (EC), Health and Consumer Protection Directorate General (6511/VI/99-Final). Available from: http://ec.europa.eu/food/fs/sfp/ph_ps/pro/eva/existing/list1_glyphosate_en.pdf.
- Feinchemie Schwebda. 2001. Carcinogenicity study with glyphosate technical in Swiss Albino mice [Unpublished Report]. Bangalore (India): Rallis India, Ltd. Cited In: Greim et al. 2015.
- Greim H, Saltmiras D, Mostert V, Strupp C. 2015. Evaluation of carcinogenic potential of the herbicide glyphosate, drawing on tumor incidence data from fourteen chronic/carcinogenicity rodent studies. Crit Rev Toxicol. 45:185–208.
- Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K. 2015. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate International Agency for Research on Cancer Monograph Working Group, IARC, Lyon, France. Lancet Oncol. 16:490–491.
- Hardell L, Eriksson M. 1999. A case-control study of non-Hodgkin lymphoma and exposure to pesticides. Cancer. 85:1353–1360.
- Hardell L, Eriksson M, Nordström M. 2002. Exposure to pesticides as risk factor for non-Hodgkin’s lymphoma and hairy cell leukemia: pooled analysis of two Swedish case-control studies. Leuk Lymphoma. 43:1043–1049. Cited In: IARC 2015.
- Haseman JK. 1983. A reexamination of false-positive rates for carcinogenesis studies. Fundam Appl Toxicol. 3:334–339.
- Health and Welfare Canada. 1991. Preharvest application of glyphosate (Roundup) herbicide. Ottawa (ON): Health and Welfare Canada, Pest Management Regulatory Agency (PMRA), Plant Industry Directorate. Pesticide Information Division (Pesticides Directorate Discussion Document, Vol. 91, Iss. 1, 92 p.
- Health Canada. 2015. Proposed re-evaluation decision PRVD2015-01, glyphosate. Ottawa (ON): Health Canada, Pest Management Regulatory Agency (PMRA). Available from: http://www.hc-sc.gc.ca/cps-spc/pest/part/consultations/_prvd2015-01/prvd2015-01-eng.php [Archived June 17, 2015].
- Hill AB. 1965. The environment and disease: association or causation. J R Soc Med. 58:295–300.
- Hill AB. 1971. Statistical evidence and inference. In: A short textbook of medical statistics. London: Hodder and Stoughton; p. 283–296.
- Hohenadel K, Harris SA, McLaughlin JR, Spinelli JJ, Pahwa P, Dosman JA, Demers PA, Blair A. 2011. Exposure to multiple pesticides and risk of non-Hodgkin Lymphoma in men from six Canadian provinces. Int J Environ Res Public Health. 8:2320–2330.
- Holsapple MP, Pitot HC, Cohen SM, Boobis AR, Klaunig JE, Pastoor T, Dellarco VL, Dragan YP. 2006. Mode of action in relevance of rodent liver tumors to human cancer risk. Toxicol Sci. 89:51–56.
- Honeycutt Z, Rowlands H. 2014. Glyphosate testing report: findings in American mothers’ breast milk, urine and water. Moms Across America & Sustainable Pulse, 19 p. Available from: https://d3n8a8pro7vhmx.cloudfront.net/yesmaam/pages/774/attachments/original/1396803706/Glyphosate__Final__in_the_breast_milk_of_American_women_Draft6_.pdf?1396803706.
- Hoppe H-W. 2013. Determination of glyphosate residue in human urine samples from 18 European countries. Bremen (Germany): Medical Laboratory Bremen (Report Glyphosate MLHB-2013-06-06), 18 p. Available from: https://www.bund.net/fileadmin/bundnet/pdfs/gentechnik/130612_gentechnik_bund_glyphosat_urin_analyse.pdf.
- IARC. 2006. Data for the Monographs. Preamble to the IARC Monographs (amended January 2006). Lyon (France): World Health Organization (WHO), International Agency for Research on Cancer (IARC). Available from: http://monographs.iarc.fr/ENG/Preamble/index.php.
- IARC. 2015. Glyphosate. In: Some organophosphate insecticides and herbicides: diazinon, glyphosate, malathion, parathion, tetrachlorvinphos. IARC Working Group, March 3–10, 2015, Lyon (France). Lyon (France): World Health Organization (WHO), International Agency for Research on Cancer (IARC) (IARC Monographs on the Evaluation of Carcinogen Risks to Humans, Vol. 112), p. 1–92. Available from: http://monographs.iarc.fr/ENG/Monographs/vol112/index.php.
- ICH. 1997. Testing for carcinogenicity of pharmaceuticals: S1B. Geneva: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (ICH Harmonised Tripartite Guideline – Current Step 4 version dated 16 July 1997). Available from: http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html [Open S1B].
- ICH. 2011. Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use: S2(R1). Geneva (Switzerland): International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (ICH Harmonised Tripartite Guideline – Current Step 4 version [Combines S2A & S2B]). Available from: http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html.
- James RC, Britt JK, Halmes NC, Guzelian PS. 2015. Evidence-based causation in toxicology: a 10-year retrospective. Hum Exp Tox. 34:1245–1252.
- JMPR. 1987. Glyphosate. In: Pesticide residues in food – 1986: part II – toxicology. Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment (JMPR) and the WHO Expert Group on Pesticide Residues, September 29–October 8, 1986, Rome. Rome: Food and Agriculture Organization of the United Nations (FAO)/FAO Panel of Experts on Pesticide Residues in Food and the Environment/WHO Expert Group on Pesticide Residues Joint FAO/WHO Meeting on Pesticide Residues (JMPR) (FAO Plant Production and Protection Paper, Vol. 78), p. 63–76. Available from: http://www.inchem.org/documents/jmpr/jmpmono/v86pr08.htm.
- JMPR. 2006. Glyphosate. In: Pesticides residues in food – 2004. Evaluations 2004 Part II–Toxicological. Joint Meeting of the FAO Panel of Experts on Pesticide residues in Food and the Environment and the WHO Core Assessment Group (JMPR), Sep 20–29, 2004, Rome. Rome: Food and Agriculture Organization of the United Nations (FAO)/Geneva: World Health Organization (WHO), International Programme on Chemical Safety (IPCS) (WHO/PCS/06.1), p. 95–169. Available from: http://www.inchem.org/documents/jmpr/jmpmono/v2004pr01.pdf.
- JMPR. 2014. 5.21. Glyphosate (158) and metabolites. In: Pesticide residues in food 2013. Joint FAO/WHO Meeting on Pesticide Residues and the WHO Core Assessment Group on Pesticide Residues, Geneva,17 to 26 September 2013. Rome: Food and Agriculture Organization of the United Nations/Geneva, World Health Organization (WHO) (FAO Plant Production and Protection Paper No. 219), p. 225–228, 484–486. Available from: http://www.fao.org/publications/card/en/c/299ca869-ae51-5093-8407-9cb30782b9f5/.
- JMPR. 2016. Glyphosate (158). In: Pesticide residues in food 2016. Special Session of the Joint FAO/WHO Meeting on Pesticide Residues, Geneva, 9 to 13 May 2016. Rome: Food and Agriculture Organization of the United Nations/Geneva, World Health Organization (WHO) (FAO Plant Production and Protection Paper No. 227), p. 19–28, 45, 72–82. Available from: http://www.fao.org/3/a-i5693e.pdf.
- Jönsson J, Camm R, Hall T. 2013. Removal and degradation of glyphosate in water treatment: a review. Aqua. 62:395–408.
- Kachuri L, Demers PA, Blair A, Spinelli JJ, Pahwa M, McLaughlin JR, Pahwa P, Dosman JA, Harris SA. 2013. Multiple pesticide exposures and the risk of multiple myeloma in Canadian men. Int J Cancer. 133:1846–1858. Cited In: IARC 2015.
- Kier LD. 2015. Review of genotoxicity biomonitoring studies of glyphosate-based formulations. Crit Rev Toxicol. 45:209–918.
- Kier LD, Kirkland DJ. 2013. Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit Rev Toxicol. 43:283–315.
- Kirkland D, Reeve L, Gatehouse D, Vanparys P. 2011. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat Res. 721:27–73.
- Kirsch-Volders M, Bonassi S, Knasmueller S, Holland N, Bolognesi C, Fenech MF. 2014. Commentary: critical questions, misconceptions and a road map for improving the use of the lymphocyte cytokinesis-block micronucleus assay for in vivo biomonitoring of human exposure to genotoxic chemicals-a HUMN project perspective. Mutat Res Rev Mutat Res. 759:49–58.
- Klimisch H-J, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol. 25:1–5. Cited In: Greim et al. 2015.
- Krüger M, Schledorn P, Shrödl W, Wolfgang Hoppe H, Lutz W, Shehata AA. 2014. Detection of glyphosate residues in animals and humans. J Environ Anal Toxicol. 4:210. doi: 10.4172/2161-0525.1000210.
- Landgren O, Kyle RA, Hoppin JA, Freeman LEB, Cerhan JR, Katzmann JA, Rajkumar SV, Alavanja MC. 2009. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood. 113:6386–6391.
- Markard C. 2014. Ergebnisse der Vorstudie HBM von Glyphosat. Dessau-Roßlau (Germany): Federal Environmental Agency (UBA), Umweltprobenbank des Bundes [Unpublished Report provided to] Berlin (Germany): German Federal Institute for Risk Assessment (BfR).
- Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, Singh B, Ward JM. 2010. Hepatic enzyme induction: histopathology. Toxicol Pathol. 38:776–795.
- McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW. 2001. Non-Hodgkin’s lymphoma and specific pesticide exposures in men: Cross-Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev. 10:1155–1163. Cited In: IARC 2015.
- Mesnage R, Moesch C, Grand R, Lauthier G, Vendômois J, Gress S, Séralini G. 2012. Glyphosate exposure in a farmer’s family. J Environ Protect. 3:1001–1003.
- Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Ann Intern Med. 151:264–269. W264.
- Monsanto. 1983. A chronic feeding study of glyphosate (Roundup® technical) in mice [Unpublished Report]. East Millstone (NJ): Bio/dynamics Inc. (Project #77-2062, 1981). Cited In: Greim et al. 2015.
- Niemann L, Sieke C, Pfeil R, Solecki R. 2015. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J Verbr Lebensm. 10:3–12.
- Nordstrom M, Hardell L, Magnuson A, Hagberg H, Rask-Andersen A. 1998. Occupational exposures, animal exposure and smoking as risk factors for hairy cell leukaemia evaluated in a case-control study. Br J Cancer. 77:2048–2052.
- Nufarm. 2009. Glyphosate technical: dietary carcinogenicity study in the mouse [Unpublished Report] Derbyshire (UK): Harlan Laboratories Ltd. Cited In: Greim et al. 2015 [As: Nufarm 2009a].
- OECD. 2009. Combined chronic toxicity\carcinogenicity studies. In: OECD guidelines for the testing of chemicals. Paris (France): Organisation for Economic Co-operation and Development (OECD) (OECD Guideline No. 453) [Adopted: 7 September 2009]. Available from: http://www.oecd-ilibrary.org/environment/test-no-453-combined-chronic-toxicity-carcinogenicity-studies_9789264071223-en
- OECD. 2014. Guidance document for describing non-guideline in vitro test methods. Paris, France: Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides, and Biotechnology. Paris (France): Organisation for Economic Co-operation and Development (OECD), Environment Directorate, Health and Safety Publications (Series on Testing and Assessment no 211; ENV/JM/MONO(2014)35). Available from: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2014)35&doclanguage = en.
- OECD. 2015. Overview of the set of OECD genetic toxicology test guidelines and updates performed in 2014-2015. Paris (France): Organisation for Economic Co-operation and Development (OECD), Environment Directorate, Health and Safety Publications. Available from: http://www.oecd.org/chemicalsafety/testing/Genetic%20Toxicology%20Guidance%20Document%20Aug%2031%202015.pdf.
- Orsi L, Delabre L, Monnereau A, Delval P, Berthou C, Fenaux P, Marit G, Soubeyran P, Huguet F, Milpied N, et al. 2009. Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case-control study. Occup Environ Med. 66:291–298. Cited In: IARC 2015.
- Pahwa P, Karunanayake CP, Dosman JA, Spinelli JJ, McDuffie HH, McLaughlin JR. 2012. Multiple myeloma and exposure to pesticides: a Canadian case-control study. J Agromedicine. 17:40–50.
- Paz-y-Miño C, Muñoz MJ, Maldonado A, Valladares C, Cumbal N, Herrera C, Robles P, Sánchez ME, López-Cortés A. 2011. Baseline determination in social, health, and genetic areas in communities affected by glyphosate aerial spraying on the northeastern Ecuadorian border. Rev Environ Health. 26:45–51. Cited In: IARC 2015.
- Paz-y-Miño C, Sánchez ME, ArévaloI M, Muñoz MJ, Wittel T, Oleas De-la-CarreraI G, Leonel PEII. 2007. Evaluation of DNA damage in an Ecuadorian population exposed to glyphosate. Genet Mol Biol. 30:456–460. Cited In: IARC 2015.
- Petkov PI, Patlewicz G, Schultz TW, Honma M, Todorov M, Kotov S, Dimitrov SD, Donner EM, Mekenyan OG. 2015. A feasibility study: can information collected to classify for mutagenicity be informative in predicting carcinogenicity? Regul Toxicol Pharmacol. 72:17–25.
- Pratt IS, Barron T. 2003. Regulatory recognition of indirect genotoxicity mechanisms in the European Union. Toxicol Lett. 140/141:53–62.
- Sanderson S, Tatt LD, Higgins JPT. 2007. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 36:666–676.
- Solomon K. 2016. Glyphosate in the general population and in applicators: a critical review of studies on exposures. Crit Rev Toxicol, in this issue. doi:10.1080/10408444.2016.1214678.
- Sorahan T. 2015. Multiple myeloma and glyphosate use: a re-analysis of US agricultural health study (AHS) data. Int J Environ Res Public Health. 12:1548–1559.
- Speit G. 2013. Does the recommended lymphocyte cytokinesis-block micronucleus assay for human biomonitoring actually detect DNA damage induced by occupational and environmental exposure to genotoxic chemicals? Mutagenesis. 28:375–380.
- Stout LD, Ruecker FA. 1990. Chronic study of glyphosate administered in feed to Albino rats [Unpublished Report] St Louis (MO): Monsanto Agricultural Company (No. MSL-10495, job/project No. ML-87-148/EHL 87122). Cited In: JMPR 2006.
- Suter GWII, Cormier SM. 2011. Why and how to combine evidence in environmental assessments: weighing evidence and building cases. Sci Total Environ. 409:1406–1417.
- US EPA. 1985. Glyphosate; EPA Reg. #: 524-308; mouse oncogenicity study [memo]. Washington (DC): U.S. Environmental Protection Agency (US EPA) (Document No. 004370). Available from: http://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/html/103601.html.
- US EPA. 1986a. Glyphosate; EPA Reg. #: 524-308; Roundup; additional histopathological evaluations of kidneys in the chronic feeding study of glyphosate in mice [memo]. Washington (DC): U.S. Environmental Protection Agency (US EPA) (Document No. 005590). Available from: http://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/html/103601.html.
- US EPA. 1986b. Transmittal of the final FIFRA Scientific Advisory Panel reports on the February 11–12, 1986 Meeting. Washington (DC): Environmental Protection Agency (US EPA), Office of Pesticides and Toxic Substances. Available from: http://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/html/103601.html.
- US EPA. 1986c. Guidelines for carcinogen risk assessment. Washington (DC): U.S. Environmental Protection Agency (US EPA) (EPA/630/P-03/001FMarch 2005). Available from: http://www.epa.gov/sites/production/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf.
- US EPA. 1990. Determination of glyphosate in drinking water by direct-aqueous-injection HPLC, post column derivatization, and fluorescence detection. In: Methods for the determination of organic compound in drinking water – supplement I. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Research and Development (EPA/600/4-90/020). Available from: http://nepis.epa.gov/Exe/ZyPDF.cgi/30000UX8.PDF?Dockey =30000UX8.PDF.
- US EPA. 1991a. Second peer review of glyphosate [memo]. Washington (DC): U.S. Environmental Protection Agency (US EPA). Available from: http://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/html/103601.html.
- US EPA. 1991b. Glyphosate; 2-year combined chronic toxicity/carcinogenicity study in Sprague-Dawley rats – list a pesticide for reregistration [memo]. Washington (DC): U.S. Environmental Protection Agency (US EPA) (Document No. 008390). Available from: http://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/html/103601.html.
- US EPA. 1991c. Peer review on glyphosate [Memo]. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Pesticides, and Toxic Substances. Cited In: IARC 2015 [As: EPA 1991c].
- US EPA. 1993. Reregistration Eligibility Decision (RED): glyphosate. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Prevention, Pesticides, and Toxic Substances (EPA 738-R-93-014). Available from: http://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-417300_1-Sep-93.pdf.
- US EPA. 2005. Guidelines for Carcinogen Risk Assessment [EPA/630/P-03/001F]. Washington (DC): U.S. Environmental Protection Agency (U.S. EPA), Risk Assessment Forum, National Center for Environmental Assessment (NCEA). Available from: http://www.epa.gov/risk/guidelines-carcinogen-risk-assessment.
- US EPA. 2009. Exposure factors handbook: review draft. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Research and Development, National Center for Environmental Assessment (No. EPA/600/R-09/052A), 1265 p. Available from: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid =209866 [Archived].
- US EPA. 2012. Glyphosate. section 3 registration concerning the application of glyphosate to carrots, sweet potato, teff, oilseeds (crop group (CG) 20) and to update the CG definitions for bulb vegetable (CG 3-07), fruiting vegetable (CG 8-10), citrus fruit (CG 10-10), porne fruit (CG 11-10), berry (CG 13-07), human health risk assessment. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Chemical Safety and Pollution Prevention (Decision No.: 459870), 28 p.
- US EPA. 2013. Glyphosate pesticide tolerances; Final rule (40 CFR Part 180) [EPA–HQ–OPP–2012–0132; FRL–9384–3]. Fed Regist (US). 78:25396–25401. Available from: http://www.regulations.gov/#%21documentDetail;D=EPA-HQ-OPP-2012-0132-0009.
- US FDA. 2001. Draft guidance for industry on the statistical aspects of the design, analysis, and interpretation of chronic rodent carcinogenicity studies of pharmaceuticals; Availability [Docket No. 01N–0006]. Fed Regist (US). 66:23266–23267.
- US FDA. 2006. Guidance for industry and review staff: recommended approaches to integration of genetic toxicology study results. Rockville (MD): US Food and Drug Administration (US FDA), Center for Drug Evaluation and Research (CDER). Available from: http://www.fda.gov/downloads/Drugs/…/Guidances/ucm079257.pdf.
- USGS. 2015. NAWQA Database. Reston (VA): United States Geological Survey (USGS). Available from: http://cida.usgs.gov/nawqa_public/apex/f?p=136:1:0, Accessed September 2 2015.
- Weed DL. 2005. Weight of evidence: a review of concept and methods. Risk Anal. 25:1545–1557.
- WHO. 1994. Glyphosate. Geneva: World Health Organization (WHO)/International Programme on Chemical Safety (IPCS)/United Nations Environment Program (UNEP) (Environmental Health Criteria, no 159). Available from: http://www.inchem.org/documents/ehc/ehc/ehc159.htm.
- Williams GM. 1980. Classification of genotoxic and epigenetic hepatocarcinogens using liver culture assays. Ann NY Acad Sci. 349:273–282.
- Williams GM, Iatropoulos MJ. 2002. Alteration of liver cell function and proliferation: differentiation between adaptation and toxicity. Toxicol Pathol. 30:41–53.
- Williams GM, Iatropoulos MJ, Enzmann HG, Deschl UF. 2014. Carcinogenicity of chemicals. In: Hayes AW, Kruger CL, editors. Hayes’ principles and methods of toxicology. Boca Raton (FL): CRC Press Taylor & Francis Group; p. 1251–1303.
- Williams GM, Berry C, Burns M, de Camargo JLV, Greim H. 2016. Glyphosate rodent carcinogenicity bioassay expert panel review. Crit Rev Toxicol, in this issue. doi: 10.1080/10408444.2016.1214679.
- Williams GM, Kroes R, Munro IC. 2000. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol. 31:117–165.