Abstract
In 2015, the International Agency for Research on Cancer (IARC) published a monograph concluding there was strong evidence for genotoxicity of glyphosate and glyphosate formulations and moderate evidence for genotoxicity of the metabolite aminomethylphosphonic acid (AMPA). These conclusions contradicted earlier extensive reviews supporting the lack of genotoxicity of glyphosate and glyphosate formulations. The IARC Monograph concluded there was strong evidence of induction of oxidative stress by glyphosate, glyphosate formulations, and AMPA. The Expert Panel reviewed the genotoxicity and oxidative stress data considered in the IARC Monograph, together with other available data not considered by IARC. The Expert Panel defined and used a weight of evidence (WoE) approach that included ranking of studies and endpoints by the strength of their linkage to events associated with carcinogenic mechanisms. Importantly, the Expert Panel concluded that there was sufficient information available from a very large number of regulatory genotoxicity studies that should have been considered by IARC. The WoE approach, the inclusion of all relevant regulatory studies, and some differences in interpretation of individual studies led to significantly different conclusions by the Expert Panel compared with the IARC Monograph. The Expert Panel concluded that glyphosate, glyphosate formulations, and AMPA do not pose a genotoxic hazard and the data do not support the IARC Monograph genotoxicity evaluation. With respect to carcinogenicity classification and mechanism, the Expert Panel concluded that evidence relating to an oxidative stress mechanism of carcinogenicity was largely unconvincing and that the data profiles were not consistent with the characteristics of genotoxic carcinogens.
Executive summary
Overall, extensive reviews of the genotoxicity of glyphosate, aminomethylphosphonic acid (AMPA) and glyphosate based formulations (GBFs) that were available prior to the development of the International Agency for Research on Cancer (IARC) Glyphosate Monograph all support a conclusion that glyphosate (and related materials) is inherently not genotoxic. Further, evidence indicative of an oxidative stress mechanism of carcinogenicity is largely unconvincing. The Expert Panel concluded that there is no new, valid evidence presented in the IARC Monograph that would provide a basis for altering these conclusions.
The differences between the conclusions of the IARC review and the Expert Panel review were in large part due to IARC exclusion of numerous available studies and in some cases differences in interpretation of study results reported in the IARC Monograph. Another significant source of difference was the Expert Panel’s weighting of different studies and endpoints by the strength of their linkage to mutagenic events associated with carcinogenic mechanisms. The Expert Panel concluded that without critically evaluating all available data, it is not possible to make an accurate weight of evidence (WoE) assessment.
The IARC review process does not allow for use of data from reports that are not published or accepted for publication in the open scientific literature or data from government reports that are not publicly available. However, detailed primary data were extracted and published in reviews such as Kier and Kirkland (Citation2013), although the study reports themselves are unpublished. The Expert Panel concluded that these data along with regulatory studies of GBFs and AMPA summarized in Williams et al. (Citation2000) should have been considered by IARC, and should be considered by all stakeholders going forward in evaluating the genetic toxicology of glyphosate and GBFs. A critical review of the complete dataset by the Expert Panel supports a conclusion that glyphosate (including GBFs and AMPA) does not pose a genotoxic hazard and therefore, should not be considered support for the classification of glyphosate as a genotoxic carcinogen.
Introduction
In 2015, IARC published the Glyphosate Monograph of Volume 112 (IARC Citation2015) which concluded that there was strong evidence supporting that “glyphosate can operate through two key characteristics of known human carcinogens” including genotoxicity and induction of oxidative stress. This was viewed as providing strong support for IARC classifying glyphosate as probably carcinogenic to humans, Group 2A. A number of published and regulatory approval reviews of the carcinogenic and genotoxic potential of glyphosate, AMPA and GBFs were available prior to the development of the IARC Monograph (Health and Welfare Canada Citation1991; US EPA Citation1993; WHO Citation1994; Williams et al. Citation2000; European Commission Citation2002; Kier & Kirkland Citation2013; US EPA Citation2013). The consensus among these reviews was that proper use of glyphosate and GBFs does not pose a genotoxic or carcinogenic hazard/risk with hazard indicating potential for adverse effects and risk indicating potential for adverse effects under actual conditions and amounts of exposure. As a result, glyphosate based herbicides have been approved for use in over 160 countries. The recent IARC conclusion was therefore inconsistent with these other reviews. Consequently, the Monsanto Company commissioned Intertek Scientific & Regulatory Consultancy to assemble a panel of experts to conduct a thorough review in the four areas considered by IARC including mechanistic data (focused on genotoxicity and oxidative stress). This review section reports the views of the Expert Panel of genetic toxicologists on the genotoxicity of glyphosate, GBFs and AMPA and discusses how they relate to the IARC opinions. The views and conclusions represent those of the Expert Panel of genetic toxicologists as independent scientific consultants and neither employees of the Monsanto Company nor attorneys reviewed this manuscript prior to submission.
Proper methods to accurately evaluate and interpret complex sets of genetic toxicology data
Characteristics of genetic toxicology tests and genetic testing data sets
Due to interest in understanding the potential to produce adverse effects, chemicals such as glyphosate, for which there is widespread human exposure, are typically subjected to extensive testing for genotoxic activity. The resultant database will contain studies that encompass diverse phylogenetic boundaries, types of genetic alterations, and exposure methods. Some of the more common test methods are often represented by multiple entries in the database. Proper evaluation of such data sets requires an approach that is both systematic and critical.
In large datasets, there are always likely to be some positive responses that are described as "false" or "misleading" positives from the standpoint of predicting carcinogenicity or relevance to carcinogenic mechanism (Waters et al. Citation1988; Mendelsohn et al. Citation1992; Jackson et al. Citation1993). False or misleading responses generally fall into one of three types:
Non-predictive – positive responses produced by non-carcinogenic agents. It is well documented that misleading positive responses are more frequent in certain genotoxicity tests (particularly in in vitro mammalian cells) due to their inherent lack of specificity (Kirkland et al. Citation2005; Pfuhler et al. Citation2011; Walmsley & Billinton Citation2011) and artifacts resulting from in vitro treatment conditions (Halliwell Citation2003).
Secondary response – the positive response is not associated with direct DNA-reactivity of the agent or metabolites of the agent but is a downstream or indirect consequence of high levels of cytotoxicity (Kirsch-Volders et al. Citation2003; Pratt & Barron Citation2003) or extreme treatment conditions such as high osmotic conditions or significant variations in pH (Scott et al. Citation1991). Such responses may not be relevant to in vivo prediction because they involve effects generated by exposures that exceed potential in vivo exposures.
Technical deficiencies – positive responses may be produced by inadequate study designs, mistakes made during the conduct of a test or inappropriate evaluation of data. This type includes cases where there is reason to question whether a positive experimental result has actually been obtained.
An understanding of possible actions leading to false or misleading responses with respect to carcinogenicity prediction or carcinogenic mechanism must be incorporated into the design, conduct, evaluation, and interpretation of genotoxicity assays. As a consequence, new standard test guidelines for in vitro mammalian assays published by the Organization for Economic Cooperation and Development (OECD) and other organizations indicate that treatment conditions must be monitored for maintenance of normal physiological parameters.
Therefore, it is expected that a chemical as heavily tested as glyphosate would exhibit some positive responses in its genotoxicity database that would be considered "misleading" and therefore not predictive of its true genotoxic or carcinogenic hazard/risk potential.
Methods applicable to evaluation and interpretation of complex data sets
The universally recommended method for evaluating the databases of the type associated with glyphosate (including GBFs and AMPA), involves the application of a WoE approach as discussed recently for genetic toxicology testing (US FDA Citation2006; Dearfield et al. Citation2011). Many of the principles of the WoE analysis indicated here are consistent with and included in the very recently issued endpoint specific guidance document of the European Chemicals Agency (ECHA Citation2015).
While numerous attempts to develop a standard WoE method to evaluate large, complex data sets have not found universal acceptance, some critical performance requirements for WoE approaches have been identified by the US EPA (Suter & Cormier Citation2011). One of the most important requirements is that individual test methods should be assigned a weight that is consistent with their contribution to the overall evidence, and different types of evidence or evidence categories must be weighted before they are combined into a WoE.
The weight of a category of evidence used in the Expert Panel evaluation is based on four considerations:
Different categories of evidence (i.e. assay types) have different weights. Genotoxicity tests measuring mutations and chromosome damage have greater weight than “indicator” assays that measure DNA damage. For example, for human pharmaceuticals, ICH S2 (R1) (ICH Citation2011) states that “fixation of damage to DNA in the form of gene mutations, larger scale chromosomal damage or recombination is generally considered to be essential for heritable effects and in the multi-step process of malignancy”. The following comments are taken from the “Overview of the Set of OECD Genetic Toxicology Test Guidelines and Updates Performed in 2014–2015” (OECD Citation2015): “There are tests that detect primary DNA damage (i.e. the first in the chain of events leading to a mutation), but not the consequences of this genetic damage. The endpoint measured in these tests does not always lead to a mutation, a change that can be passed on to subsequent generations (of cells or organisms). The DNA damage measured in the comet assay, or the unscheduled DNA synthesis (UDS) test, may lead to cell death, or it may initiate DNA repair, which can return the DNA either to its original state or result in mutation. When evaluating the mutagenic potential of a test chemical, more weight should be given to the measurement of permanent DNA changes (i.e. mutations) than to DNA damage events that are reversible.”
The aggregate strength (robustness of protocols and reproducibility) and quality of evidence in the category also influence the weight. It is generally acknowledged that studies conducted in compliance with Good Laboratory Practice (GLP) Regulations and studies conducted according to OECD guidelines have greater weight than studies lacking these attributes. These are fundamental features of the Klimisch scoring system, which is widely used to assess the reliability of study data, particularly for regulatory purposes (Klimisch et al. Citation1997).
The number of pieces of evidence within a category influences the weight. A single (or few) divergent responses (positive or negative) within a majority of studies exhibiting concordant findings would be insufficient to alter the direction and strength of the WoE. This component of the overall WoE is an aggregate of the weights of all the pieces of evidence within a single test category (e.g. tests for gene mutation).
Tests with greater ability to extrapolate results to humans carry greater weight. Test responses able to more accurately predict potential hazard in humans, such as in vivo tests, will generally be weighted more heavily than evidence developed from tests conducted in vitro or in non-mammalian models.
Human versus non-human test results
Using a variety of different methods, genotoxicity test data can be derived from human populations exposed under typical use conditions. Human population monitoring studies, if performed with sufficient sample sizes, knowledge of exposure levels and adjusted appropriately for confounding variables, can offer highly relevant information. Poorly controlled human biomonitoring studies, however, can lead to erroneous conclusions (Schmid & Speit Citation2007; Dusinska & Collins Citation2008). Adjustments that need to be considered in human biomonitoring studies for genotoxicity must extend beyond age, gender, smoking, alcohol, tobacco use, and medicines used. Diet, disease status (e.g. presence of inflammatory diseases), seasonal variation, and physical stress are all important confounding factors that influence an individual’s background level for any parameter under consideration (Moller Citation2005; Battershill et al. Citation2008; Bonassi et al. Citation2011; Fenech et al. Citation2011; Tenorio et al. Citation2013; Collins et al. Citation2014). There is evidence that different factors may have different impact depending on the specific genotoxic endpoints (e.g. Fenech et al. Citation2011 for the cytokinesis block MN endpoint; Collins et al. Citation2014 for the comet endpoint).
It is worth noting that there is currently considerable debate concerning the relevance of increased levels of micronuclei in human biomonitoring studies. Speit (Citation2013) suggested that micronuclei induced in the cytochalasin B micronucleus assay used in human biomonitoring studies, do not represent micronuclei that were induced during exposure, but rather represent DNA damage that generates micronuclei during the in vitro culturing required for the assay. As such, this bioassay could be classified as an "indicator test" of DNA damage with lower relevance for genotoxic risk. Kirsch-Volders et al. (Citation2014), however, considered gaps in the knowledge regarding the source of micronuclei observed in human biomonitoring studies, but considers the assay, especially with modifications, to have utility for human genotoxic hazard/risk measurements. For the purposes of this review, the Expert Panel adopted a conservative approach and the measurement of micronuclei detected in studies of exposed humans was assigned a high weight.
It is also possible to conduct genetic tests using human derived cell lines or in primary lymphocyte cultures. With respect to results from cell lines of different origin, the benefits of using human rather than rodent derived cell lines are not as compelling as one might presume. Cell lines (human or rodent origin) with mutations affecting how cells handle initial DNA damage (e.g. p53 mutations) are typically more susceptible to genetic damage. Consequently, human cell lines with altered responsiveness to DNA damaging mechanisms may be expected to generate results not dissimilar to those produced in rodent cell lines. At this time there are not enough data available to reliably determine if the use of p53-competent cell lines of human origin (as opposed to p53-competent rodent derived lines) or other human cells confer greater accuracy (Walmsley & Billinton Citation2011; Fowler et al. Citation2014).
The most current OECD in vitro mammalian cell chromosomal aberration and micronucleus test guidelines indicate that either human or rodent cell lines or primary cultures may be used (OECD Citation2014a, Citation2014d). These guidelines also state that: “At the present time, the available data do not allow firm recommendations to be made but suggest it is important, when evaluating chemical hazards to consider the p53 status, genetic (karyotype) stability, DNA repair capacity and origin (rodent versus human) of the cells chosen for testing.”
Thus, any in vitro mammalian cell results should be interpreted with caution, and the weight they contribute to an overall assessment of genotoxic activity should take account of the potential limitations.
A summary of assumptions, results, and conclusions regarding the IARC genotoxicity evaluation of glyphosate, GBFs, and AMPA
The Expert Panel used the considerations discussed above when assigning weights to genotoxicity endpoints and to the responses present in the glyphosate (and related materials) dataset. The results of this review indicate some areas of agreement with IARC, but also identified some major differences between the conclusions of the two assessments.
An evaluation of IARC and expert panel review processes
The Expert Panel agreed that there was sufficient evidence to conclude that glyphosate and GBFs appeared to induce DNA strand breaks and possibly micronuclei in in vitro mammalian and non-mammalian systems and sister chromatid exchanges (SCEs) in in vitro mammalian systems. These results provide some evidence of genotoxicity, but it is not possible to accurately characterize or classify genotoxic hazard/risk or carcinogenesis mechanisms based on these results alone. As noted earlier and further stated in the OECD overview comments (OECD Citation2015) regarding test weights, “When evaluating the mutagenic potential of a test chemical, more weight should be given to the measurement of permanent DNA changes (i.e. mutations) than to DNA damage events that are reversible.” Consequently, positive responses in genotoxic endpoints identified above as “indicator tests” (i.e. DNA strand breaks, SCEs) are evidence of compound exposure but not sufficient to determine compound effect. In order to determine compound effect, consideration must be given to available evidence clearly demonstrating the induction of gene mutations or stable chromosomal alterations, particularly in vivo in mammalian systems.
Evidence weighting
Weights assigned to individual assays represent the strength of evidence assigned to an endpoint or category and may be derived from validation studies supporting the endpoint’s involvement in carcinogen prediction as well as its relevance to mechanisms involved with initiation of malignancy (ICH Citation2011). In general human and in vivo mammalian systems have the highest test system weight, with a lower degree of weighting applied to in vitro mammalian cell systems and in vivo non-mammalian systems and lowest weight to in vitro non-mammalian systems (with the exception of the well validated bacterial reverse mutation “Ames” tests using mammalian metabolic activation). Other considerations, such as response reproducibility or GLP compliance, may influence the weight of a particular study result. GLP compliance indicates a high degree of, and standard for, detailed documentation of experimental conditions and data.
Section 4.2.1 of the IARC Monograph does not provide sufficient information to its readers regarding the strategy employed by IARC reviewers in assessing the WoE; therefore, it is not possible to know if, for example, studies were assigned variable weights in accordance with the criteria discussed above. While the Expert Panel agrees that data from a well conducted human population biomonitoring study might carry more weight in a WoE assessment, it appears that IARC considered in vitro studies in human cells as carrying more weight than rodent in vivo studies as evidenced by the order of discussion topics in Section 4.2.1, and the inclusion of a separate table for human in vitro studies. The overall IARC Monograph evaluation (Section 6.0) and rationale (Section 6.4) indicate that the conclusion of strong evidence of genotoxicity is based on “studies in humans in vitro and studies in experimental animals.” As discussed above, the Expert Panel evaluation considered in vitro studies using cells of human origin to be weighted as equivalent to any other in vitro mammalian cell assay using the same endpoint.
There did not, however, appear to be additional weight assigned by IARC to other criteria such as relevance of the endpoint to neoplastic initiation, quality of study performance, in vitro versus in vivo or reproducibility of responses.
summarizes the Expert Panel’s endpoint weighting assumptions. Weights represent strength, relevance and reliability of evidence and are based on a compilation of information regarding the endpoint’s reversibility and susceptibility to false or misleading positive responses with respect to carcinogenicity prediction or relevance to mechanisms involved in initiation of malignancy (Solomon et al. Citation1991; Pierotti et al. Citation2003; Petkov et al. Citation2015).
Table 1. Expert Panel’s evidence weighting assumptions for mammalian (plus selected microbial test) endpoints.
The endpoint and test system weighting categories are defined as follows:
Negligible weight – the endpoint is not linked to any adverse effect relevant to genetic or carcinogenic hazard/risk and as such is not given weight as evidence of genotoxicity.
Low weight – the end point is indicative of primary DNA damage, is not unequivocally linked to mechanisms of tumorigenicity, and the test system has low specificity.
Moderate weight – the endpoint is potentially relevant to tumorigenicity or may be subject to secondary, threshold-dependent mechanisms of induction (e.g. cytotoxic clastogens, aneugens) or the test system exhibits a high rate of misleading positives with respect to carcinogenicity predictivity or carcinogenic mechanism.
High weight – the endpoint is one that has been demonstrated with a high level of confidence to play a critical role in the process of tumorigenicity.
Chemical structure and chemistry of GBFs
Chemical structures of glyphosate and AMPA are presented in . IARC did not consider the chemical structure of glyphosate in its mechanistic section; however, IARC Monograph Section 5.3 states that glyphosate is not electrophilic. Many guidelines recommend that the presence of structural alerts be considered in evaluation of or testing for genotoxicity (Cimino Citation2006; Eastmond et al. Citation2009; EFSA Citation2011; ICH Citation2011). As reported in Kier and Kirkland (Citation2013) analysis of the glyphosate structure by DEREK software identified no structural alerts for chromosomal damage, genotoxicity, mutagenicity, or carcinogenicity. Analysis of structural alerts for genotoxicity inherently includes consideration of potential metabolites. Although formal analysis is not available, it does not appear likely that the metabolite AMPA (glyphosate without a carboxymethyl group) has structural alerts. While structural alerts are not as definitive as experimental data, they serve as part of a WoE (Dearfield et al. Citation2011). The lack of structural alerts in the glyphosate molecular structure suggests lack of genotoxicity or that genotoxic effects might well be secondary to toxicity or resulting from mechanisms other than DNA-reactivity.
Figure 1. Chemical structures of glyphosate and AMPA. Glyphosate: N-(phosphonomethyl)glycine, acid form, CAS 1071-83-6; AMPA: aminomethylphosphonic acid; CAS 1066-51-9.
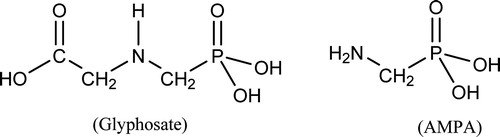
Another aspect of chemistry that should be recognized is the fact that GBFs, while containing glyphosate (often present as a sodium or potassium salt) also contain other components which frequently include surfactants. Specific formulations differ in composition and differences may exist between GBFs identified with a common brand name. Frequently, GBFs are observed to have greater toxicities than glyphosate. Evaluation of genotoxicity results for glyphosate and GBFs should always consider the possibility that effects observed with GBFs may be due to GBF components other than glyphosate and that there may be chemical differences between various GBFs.
The case for including other published results in the IARC genotoxicity evaluation
Although IARC policies and Working Group decisions excluded consideration of additional data from unpublished studies or publicly unavailable governmental reports, it was the Expert Panel’s conclusion that the genetic toxicology studies published in reviews such as Kier and Kirkland (Citation2013), in particular the supplementary primary data submitted with the paper, should have been considered by IARC in evaluating the genetic toxicology of glyphosate and GBFs. Though the primary study reports from which the data were extracted were not available to IARC, detailed data were provided in the Kier and Kirkland (Citation2013) review and exceed the weight of data in most published reports that were considered by IARC. Regulatory studies of GBFs and AMPA summarized in Williams et al. (Citation2000) should also have been considered and information on these studies is presented in Appendices A and B.
Inclusion of the studies in these publications would have filled data gaps, supplemented study categories for which there were limited numbers of test responses and would have added a very high level of confirmation to other core assay results. summarizes an additional 90 studies covering a range of test categories that were available for review if the regulatory studies in the Kier and Kirkland (Citation2013) publication and other published or publicly available studies had been included. Among the 90 studies not included in the IARC Monograph, only nine were reported as positive. Inclusion of these studies in a WoE produces a much clearer, more reliable and balanced assessment of the genotoxicity of glyphosate, GBFs and AMPA.
Table 2. Summary of test categories, number of studies, and study responses available from Kier and Kirkland (Citation2013) and other publically available studies not included in the IARC Monograph (details for all studies provided in Supplemental Information, Appendix A).
The rationale supporting the inclusion of these 90 additional studies is that the supplementary tables presented in the Kier and Kirkland (Citation2013) paper, and presented in Supplemental Information, Appendix A of this publication, do contain sufficient detail concerning the robustness of the studies. For the regulatory studies, which were the key studies not reviewed by IARC, the Kier and Kirkland (Citation2013) paper clearly states:
Each study examined was stated to have been conducted in accordance with GLP standards with almost all studies citing the OECD Principles of Good Laboratory Practice (OECD GLP 1982, 1997). Reports also cited compliance with various national and regional GLP Guidelines (e.g. European Commission GLP Directives 87/18/EEC or 88/320/EEC; U.S. Environmental Protection Agency GLP Standards, 40 CFR Part 160; Japanese Ministry of Agriculture, Forestry, and Fisheries (MAFF) GLP Standards, 11 Nousan No. 6283). Variations from GLPs were considered not to have significantly impacted the study results.
Almost all of the studies were reported to have been conducted in accordance with the relevant OECD test guidelines applicable at the time of the study. Study reports were examined to determine that the protocols and experimental methods for the report were consistent with the OECD guidelines and any deviations were noted and considered. Report data were examined to confirm the conclusion of the report regarding whether treatment-related activity had been observed.
Thus, the methods used were generally as specified in OECD guidelines, or any deviations were noted. Moreover, the studies were performed under GLP conditions, which would ensure protocol compliance and high quality data. The key aspects of each test method were detailed in the first few pages of the supplementary material in Kier and Kirkland (Citation2013) so it is easy to see how top concentrations were chosen, what measures of cytotoxicity were used, how many cells were scored etc. Links to the guidelines were provided.
The rationale given by IARC for not including the regulatory studies in Kier and Kirkland (Citation2013) was that the primary study reports were not available, and that the information provided in the supplementary tables was insufficient regarding topics such as details of statistical methods, choice of highest dose tested, and verification of the target tissue exposure.
This rationale for exclusion is unjustified for the following reasons.
For bacterial reverse mutation assays the concentrations tested were detailed in every table, as were critical aspects of the methods (e.g. plate incorporation or pre-incubation for the Ames tests, inducing agent for the S9 and its final concentration, and number of replicate cultures). Thus, it is clear what top concentrations were used, whether they complied with the maximum concentration/dose as recommended in OECD guidelines, or whether they were defined by toxicity.
Almost all of the many Ames tests on glyphosate used a top concentration of the maximum required, 5000 μg/plate unless contraindicated by toxicity. All of the required strains, including either TA102 or Escherichia coli, have been used in the regulatory studies included in Kier and Kirkland (Citation2013). The Ames tests on GBFs used quite variable top concentrations. Some went as high as the maximum required (5000 μg/plate) but others only reached <100 μg/plate, seemingly limited by toxicity. Since we know glyphosate per se is not very toxic in the bacterial tests, the toxicity is presumably caused by the other components of the formulations, which were more toxic in some GBFs than in others.
The mammalian cell assays on glyphosate generally reached top concentrations in the range 500–5000 μg/mL, even when prolonged (48 h) treatments were performed in the chromosomal aberration studies. Thus, many of these studies exceeded 10 mM (1690 μg/mL for glyphosate), the top concentration currently recommended in OECD guidelines for nontoxic substances. There were no regulatory mammalian cell tests on GBFs.
All except one of the regulatory in vivo micronucleus (MN) tests on glyphosate that used oral dosing achieved a top dose of at least 2000 mg/kg, which is the top dose for a nontoxic substance recommended in OECD guidelines. One oral study achieved a top dose of only 30 mg/kg, seemingly because severe toxicity and lethality was seen at higher doses. It is unclear why such lethal effects were seen in this study when much higher doses were tolerated in other studies using the same acute dosing regimen. Several studies using intraperitoneal (i.p.) injection had lower top doses because of greater toxicity when using the intraperitoneal route. Thus, all of the regulatory MN studies on glyphosate met or exceeded the required top dose.
The in vivo bone marrow MN and chromosomal aberration regulatory studies of Kier and Kirkland (Citation2013) generally did not report evidence of target organ toxicity (e.g. %PCE, which would be a measure of bone marrow toxicity) or include analyses to demonstrate presence of glyphosate in plasma. Therefore, the issue of whether the bone marrow was exposed needs verification by evidence other than target organ toxicity.
The IARC Monograph states that about 1/3 of glyphosate administered orally to rodents is absorbed and excreted, largely unchanged, in urine. This provides evidence that it is likely that the bone marrow, a well-perfused tissue, is exposed to glyphosate in rodents treated orally. Definitive evidence of absorption and systemic distribution of glyphosate in rodents is also contained in a summary of regulatory toxicokinetic studies (JMPR Citation2006). These studies demonstrated absorption of glyphosate and systemic distribution, including distribution in bone marrow, in rats dosed intraperitoneally or orally. Published reports have also indicated absorption and systemic distribution of glyphosate administered by the intravenous (i.v.) or oral route in rats (Brewster et al. Citation1991; Anadon et al. Citation2009) and by the oral (dietary) route in mice (Chan & Mahler Citation1992). Thus, in the regulatory rodent in vivo MN and chromosomal aberration tests, target organ exposure would have been achieved.
If statistical analysis was performed (not commonly performed or required for Ames tests) this is given as a footnote to the supplementary tables (Kier & Kirkland Citation2013, supplementary tables; Appendix B, this report), together with the statistical method used, and whether the results were significant.
Thus, in view of the Expert Panel, the exclusion of these studies was not justified. Failure to evaluate and consider the large number of results included in the publication by Kier and Kirkland (Citation2013) as well as other publicly available studies not reviewed by IARC, resulted in an inaccurate assessment of glyphosate, GBFs and AMPA’s genotoxic hazard/risk potential.
Expert panel’s critique of selected studies: impact on IARC evaluation
Genetic toxicology tests relied upon by most regulatory bodies to support decisions focus on a set of core endpoints that are known to be involved either in direct activation of genes responsible for neoplastic initiation in somatic cells or alteration of the genetic information in germ cells (EFSA Citation2011; ICH Citation2011; Kirkland et al. Citation2011). Therefore, the endpoints given the greatest weight in include gene mutation and chromosomal aberrations.
MN formation in vivo was also assigned a high weight (), as it is considered an indication of chromosome breakage but could also result from aneuploidy (Kirsch-Volders et al. Citation2003). However, aneugenic effects are usually thresholded (Parry et al. Citation1994). For instance, MN may be induced by alterations in normal mitosis produced by various kinases. It was demonstrated that GBFs activate mitotic kinase CDK-1 (Marc et al. Citation2002) which could possibly play a role in MN induction through a separate mechanism believed to be threshold based (Terasawa et al. Citation2014). Although a thresholded mechanism may be considered of less weight than a non-thresholded mechanism, most in vivo MN studies did not investigate this. In the absence of information on clastogenic or aneugenic mode of action, the panel considered that a high weight should be applied to all in vivo MN studies.
Human genotoxicity biomonitoring studies
The results provided for GBFs in (human studies) of the IARC Monograph concluded positive evidence of DNA breakage as determined by results in humans using the comet assay Paz-y-Miño et al. (Citation2007), negative induction of chromosomal aberrations (Paz-y-Miño et al. Citation2011), and positive induction of MN (Bolognesi et al. Citation2009). Due to the importance of these studies in the IARC review, these papers were critically reviewed by the Expert Panel as described in detail below.
Table 4. Comparison of test response profiles from glyphosate, GBFs, and AMPA to the profile characteristics of confirmed genotoxic carcinogens.
Paz-y-Miño et al. (Citation2007) reported increased DNA damage (comet assay) in individuals recently exposed to GBF spraying, but only “suggested” this implied a genotoxic risk. The comet assay, as discussed earlier is an “indicator” endpoint and primary DNA damage does not accumulate, so the consequences of the observed DNA breaks remain unknown (Faust et al. Citation2004).
The Expert Panel review of this study identified a number of issues that questioned the validity of the interpretation of results. For example, it is not clear which blood cells were scored for comets, or if it was all cells in the blood. Also, the observation of a median comet tail length of exactly 25.0 μm for 20/21 unexposed control individuals in this publication questions the quality of data collection. This unusual observation was not noted in the IARC Monograph. The Paz-y-Miño et al. (Citation2007) publication indicated that signs of clinical toxicity were reported in the population and that the GBF application rate was reported to be some 20 times higher than recommended. The clinical signs were consistent with acute intoxication associated with severe exposures (Menkes et al. Citation1991) and these factors suggest that comet effects might have been secondary to toxicity from very high exposure to GBF. The Paz-y-Miño et al. (Citation2007) report seems to qualify the conclusiveness of the results by indicating that the results "suggest" a genotoxic effect. Due to uncertainties regarding the negative control data, and particularly because of uncertainties regarding the mechanistic role of cytotoxicity in generating the effects the Panel regarded this study as inconclusive evidence for in vivo human genotoxic effects relevant to induction of mutations or carcinogenesis.
In a follow-up study, Paz-y-Miño et al. (Citation2011) reported negative results for induction of chromosomal changes in individuals from areas where GBF spraying had occurred two years previously. The absence of chromosomal aberrations supports the presumption that the DNA strand breaks identified in the Paz-y-Miño et al. (Citation2007) study were either repaired or lethal and did not persist as lesions which could be expressed as chromosomal aberrations in cultured lymphocytes in the follow-up study.
Bolognesi et al. (Citation2009) reported a significant but small, transient and inconsistent effect of glyphosate spraying on MN induction in individuals living in areas where aerial spray application of glyphosate occurred ( in Bolognesi et al. Citation2009), but concluded that any risk was “low”. Of greater importance however, is the observation that no statistically significant increase in the frequency of micronucleated binucleated cells (BNMN) was observed in individuals that actually reported direct exposure to the spray compared to individuals who lived in the spray area but were not present during spraying (Bolognesi et al. Citation2009, ). These results are shown graphically in (graph provided by K. Solomon). As indicated in of Bolognesi et al. (Citation2009), statistical analysis did not indicate a significant difference (p < .05, ANOVA) in post-spray BNMN frequency between different categories of self-reported spray exposure and there was no statistically significant difference (p < .05) between no exposure and any self-reported spray exposure for any of the three regions. The Valle del Cauca region, which exhibited the highest post-spraying increase, only had 1/26 persons self-reporting spray exposure and the GBF spray application rate was substantially lower than the application rates in the other two regions.
Figure 2. Mean frequency of binucleated cells with micronuclei (BNMN) in self-reported exposures to glyphosate spray in areas where aerial application occurred. From Bolognesi et al. (Citation2009); . Data from Valle del Cauca not shown in graph since only one individual reported exposure. Graph provided by K. Solomon.
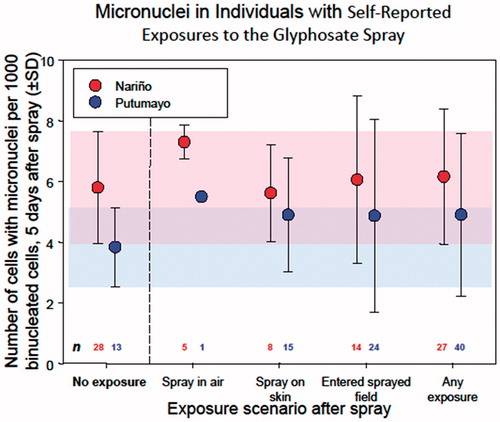
Although results were temporally consistent with GBF spraying, the lack of significant correlation between increased post-spraying BNMN frequencies and self-reported spray exposure, and inconsistency with application rates, indicate that the MN effects observed in this study cannot be associated with GBF exposure () and therefore the Expert Panel concluded the results to be negative. The panel agrees with the statement made in the discussion section of Bolognesi et al. (Citation2009) that based on the Bradford Hill criteria (Hill Citation1965) it is not possible to assign causality to the BNMN increases observed in their study and notes that elsewhere in this publication the authors seemed to qualify their conclusions with terms like “suggest” and “potentially”. Lack of clear evidence of causality indicates that it is inappropriate to conclude that GBF induces MN in humans. The Bolognesi et al. (Citation2009) results were considered negative by the Expert Panel because there were no statistically significant increases in MN frequency associated with self-reported spray exposure. This conclusion is subject to the limitation of the use of self-reporting as a measure of exposure.
The Expert Panel conclusion for the Bolognesi et al. (Citation2009) results seems to be quite different from the IARC Monograph. The qualifications about lack of consistency with exposure rates or statistically significant association with self-reported spray exposure are noted in the discussion of this study in IARC Monograph Section 4.2.1(a)(i). However, these qualifications are not evident in IARC Monograph Section 5.4 which presents these results as positive without qualification. IARC Monograph Section 6.4 not only presents the results as positive without qualification but seems to give this study a high weight in arriving at their conclusion of a genotoxic mode of action.
Due to the deficiencies cited in the biomonitoring studies above, along with the lack of scientific consensus regarding the relevance of MN found in exposed humans, the Expert Panel concluded that there was little or no reliable evidence produced in these studies that would support a conclusion that GBFs, at levels experienced across a broad range of end-user exposures, poses any human genotoxic hazard/risk.
Studies in mammalian in vitro and in vivo assays
The number of studies conducted in mammalian models both in vitro and in vivo was relatively extensive but with some notable data deficiencies and gaps. However, looking for evidence consistent with a concern for genotoxic hazard finds little or no compelling support among test methods that assess relevant endpoints.
Gene mutation
IARC noted one negative in vitro mammalian gene mutation result for glyphosate (IARC Monograph Table 4.4). Additionally there are two negative results for glyphosate in the mouse lymphoma tk locus assay (Kier & Kirkland Citation2013). These provide a clear WoE that glyphosate does not induce gene mutation in mammalian cell systems. There are no in vitro mammalian cell gene mutation results for GBFs or AMPA.
Chromosomal effects in vitro
In in vitro mammalian cell chromosomal aberration assays (IARC Monograph and 4.4) glyphosate was reported positive in one study and negative in two other studies. Regulatory studies and published studies, not considered by IARC, provide one additional positive result and five additional negative results (see Supplemental Information, Appendix A, of this paper). One of the positive studies (Lioi et al. Citation1998a) is not considered valid due to the fact that there was excessive cytotoxicity (>50% reductions in mitotic index at all concentrations tested, exceeding current regulatory guidelines for a valid assay). Several of the published studies did not include exogenous mammalian metabolic activation. Most importantly, the negative studies tested glyphosate at dose levels well in excess of those reported positive by Lioi et al. (Citation1998a, Citation1998b) and included several human and bovine lymphocyte studies. In addition to the negative chromosomal aberration assays the two negative results in the mouse lymphoma tk locus assay also add weight to a conclusion that glyphosate is not clastogenic in in vitro mammalian cell assays. Overall these results provide sufficient evidence that glyphosate is not clastogenic in mammalian cells when studied under appropriate in vitro treatment conditions.
No in vitro mammalian chromosomal aberration studies of GBFs and one positive in vitro mammalian chromosomal aberration study with AMPA were reported by IARC. The latter study by Sivikova and Dianovsky (Citation2006), reported as a GBF study in IARC, is considered to be a study of a manufacturing batch of an isopropyl salt of glyphosate from a Monsanto source (Kier & Kirkland Citation2013). An additional positive in vitro mammalian chromosomal aberration study was not considered by IARC (Amer et al. Citation2006; see Supplemental Information, Appendix A, of this paper). The positive GBF study tested an unusual GBF and employed very high dose levels. These single studies do not provide a strong WoE for induction of chromosomal aberrations for GBFs or AMPA in mammalian cells in vitro.
IARC reported two positive in vitro mammalian cell MN studies of glyphosate. However, another four positive or equivocal in vitro mammalian cell MN studies of glyphosate were identified in the literature that were not reported in IARC but were summarized in Kier and Kirkland (Citation2013). Several of the studies had weak or inconsistent responses. Piesova (Citation2004, Citation2005), not in IARC, reported statistically significant increases in MN in bovine lymphocytes only with 48-h incubation without S9 metabolic activation but the responses were not consistent between donors. Two papers by Mladinic et al. (Citation2009a, Citation2009b) reported weak responses in human lymphocytes at the highest dose tested in the presence of S9 metabolic activation. MN results for Mladinic et al. (Citation2009a) were not reported in IARC. One of these studies (Mladinic et al. Citation2009a) had a very high control MN frequency and in both publications it appears that cells were treated prior to mitogen stimulation which would mean cells would have been exposed in G0 cell stage. This treatment regimen is not considered appropriate according to current test guidelines. The MN induced at high doses were predominantly centromere positive suggesting the possibility of an aneugenic effect. These responses were considered of limited quality by IARC and the publication authors indicated that the high dose effects might have been at a dose level exceeding a threshold and possibly associated with high toxicity. Koller et al. (Citation2012), MN results not evaluated by IARC, reported positive in vitro MN results in human-derived buccal epithelial cells for glyphosate in the absence of S9 metabolic activation. An unusual feature of this paper was indication of significant cytotoxicity at very low dose levels (20 μg/mL) and with very short exposure times (20 min). Although the authors speculated their epithelial cells might be more sensitive than cells of the hematopoietic system such as lymphocytes, a large number of other studies using non-hematopoietic cells used much higher doses and longer exposure times. A study by Roustan et al. (Citation2014) reported increases in MN frequency in CHO-K1 cells only in the presence of S9 activation. There was very little dose response observed over an order of magnitude of concentrations (10–100 μg/mL). Thus, although positive (or equivocally positive) responses were observed for glyphosate in several studies these responses were not consistent in terms of dose levels or requirement for an S9 metabolic activation system. The possibility of a threshold aneugenic effect in the presence of S9 metabolic activation might be suggested by the results of Mladinic et al. (Citation2009a, Citation2009b) but other studies cannot confirm this possibility because presence or absence of centromeres was not measured. It should be noted that there is a report that glyphosate is essentially unchanged by incubation with rat liver homogenate which would indicate that S9 activation dependent responses might not be due to metabolites of glyphosate (Gohre et al. Citation1987).
Overall these studies provide only very limited evidence of the possibility of MN induction by glyphosate in in vitro mammalian cell assays and this observation, coupled with the negative profile for clastogenicity in in vitro mammalian cell assays, would suggest this low possibility is limited to aneugenic effects that are likely to be indirect and thresholded.
Although IARC reports one negative in vitro mammalian cell assay with a GBF (Sivikova & Dianovsky Citation2006), as noted above this assay is likely to have been performed with a technical glyphosate preparation rather than a formulation. Koller et al. (Citation2012) report a positive in vitro MN result for a GBF (result not included in IARC) in buccal epithelial cells derived from a human-neck metastatic tumor. The authors noted that these cells have not been used for genotoxicity assessments and the Expert Panel considered the results in this non-validated system to be of unknown relevance. IARC reported one positive result for AMPA in an in vitro mammalian cell MN assay in CHO-K1 cells (Roustan et al. Citation2014). An unusual feature of the Roustan et al. (Citation2014) study was that AMPA apparently exhibited much higher cytotoxicity than glyphosate. Although complete cytotoxicity data are not presented, the maximum AMPA concentrations evaluated for MN, appearing to produce less than 50% reduction in cytokinesis blocked proliferation index, were 1000-fold lower than glyphosate concentrations in the absence of S9 metabolic activation, 20-fold lower in the presence of S9 metabolic activation and 100,000-fold lower with light activation. These very large cytotoxicity differences are dramatically different from the relative toxicities of AMPA and glyphosate observed in other mammalian cell studies, e.g. Chaufan et al. (Citation2014); Manas et al. (Citation2009a, Citation2009b); Li et al. (Citation2013); Kwiatkowska et al. (Citation2014). These individual studies, particularly the Roustan et al. (Citation2014) study, appear to exhibit technical problems and do not present a convincing WoE for in vitro mammalian cell MN effects of GBFs or AMPA.
Chromosomal effects in vivo
As a general point, it was noted earlier that there is adequate evidence available from toxicology studies demonstrating absorption and distribution of glyphosate to bone marrow in the rat (i.p., i.v., and oral routes) and absorption and distribution of glyphosate in blood by the oral route in the mouse. This information provides evidence for target organ exposure in the rodent bone marrow studies discussed below, which is particularly important when negative results are obtained.
in the IARC Monograph reported one negative in vivo rat bone marrow chromosomal aberration result and one negative mouse dominant lethal result for glyphosate. In addition there is one negative regulatory in vivo mouse bone marrow chromosomal aberration study of glyphosate not evaluated by IARC (Suresh Citation1994; see Supplemental Information, Appendix A, ). These studies provide in vivo evidence complementing the larger number of in vitro studies (discussed above) indicating glyphosate is not clastogenic when tested in mammalian assays.
Table 3. Summary of Expert Panel’s evaluation of human, non-human mammalian, and selected microbial genotoxicity studies from IARC Section 4.2.1 and other published sources.
IARC reported two positive results and one negative result for glyphosate in in vivo MN assays. In one of the positive studies reported by IARC (Bolognesi et al. Citation1997), relatively low increases in MN frequency were observed which might well be within the historical range of many laboratories (Salamone & Mavournin Citation1994). The other positive study (Manas et al. Citation2009a) had an unusual feature in that it is reported that erythrocytes were scored for MN, but in the bone marrow and at an early sampling time. Historical control data were not reported in the publication so the relevance of this result cannot be determined. By contrast, there are an additional 13 published, publicly available or regulatory in vivo MN studies with glyphosate in the mouse (12 studies) or rat (one study), all of which gave negative results (see Supplemental Information, Appendix A, of this paper). These negative results were obtained in multiple studies at dose levels that exceeded those at which positive results had been reported in the IARC reviewed studies mentioned above using the same (i.p.) route of administration. With respect to a route of exposure, the negative MN results in a glyphosate mouse feeding study (Chan & Mahler Citation1992) that was not reported in IARC are of particular relevance to carcinogenic potential. The Expert Panel’s conclusion is that there is a strong WoE that glyphosate does not induce MN in vivo in mammals.
IARC reported one positive and one negative rodent bone marrow chromosomal aberration study for GBFs. An additional two published positive rodent chromosomal aberration studies on GBFs were identified that were not reported in IARC. One mouse study with positive results (Prasad et al. Citation2009) employed sampling times for a chromosomal aberration assay quite different from those currently recommended (OECD Citation2014c). Moreover, the GBF was administered i.p. using dimethylsulfoxide (DMSO) as a vehicle and the use of this vehicle and route has unusual toxicity properties (Heydens et al. Citation2008). This assay was also unusual in that dose-responsive increases were observed at multiple sampling times, which is difficult to explain since cells damaged at early sampling times have usually died and disappeared from the bone marrow by later sampling times. Another positive publication (Amer et al. Citation2006), not reported in IARC, found positive chromosomal aberration results in mouse bone marrow and spermatocytes with treatments that included repeated oral and i.p. dosing. The test material was reported to be a formulation containing 84% glyphosate which is very unusual and raises the possibility that observed effects were due to some unusual or unique component of this formulation. Another published positive GBF study (Helal & Moussa Citation2005) uniquely involved rabbits exposed to GBF (750 ppm) in drinking water for 60 days. Using extended repeat dosing for a bone marrow chromosomal aberration assay is questionable because cells with chromosome breaks usually do not accumulate and any cytogenetic effects would likely be due to the final one or two doses. Total aberrations reported for this study included some nonstandard and questionable categories such as gaps and centromeric attenuations. Thus, most of the positive in vivo chromosomal aberration studies with GBF’s are all subject to concerns regarding the reliability or biological relevance of the results. While they cannot be ignored, they do not warrant undue weight, and do not support a conclusion of strong evidence of genotoxicity.
IARC reported two positive and three negative in vivo rodent bone marrow MN results for GBFs. One of the two positive studies (Bolognesi et al. Citation1997) had low negative control MN frequencies and the MN frequencies in treated groups were within historical control ranges for many laboratories (Salamone & Mavournin Citation1994) although historical control ranges for the laboratory were not reported in the publication. The other positive study (Prasad et al. Citation2009) was unusual in using DMSO as a vehicle by the i.p. route which, as noted above, may have led to unusual toxicity. However, there are an additional 17 rodent bone marrow studies with GBFs that were not considered by IARC, and all were negative (see Supplemental Information, Appendix A, of this paper). The negative studies included use of both oral and i.p. routes and maximum dose levels frequently were limit doses of 2000 mg/kg (OECD Citation2014b). The overwhelming majority of in vivo MN studies on GBFs, therefore, gave negative results. In the studies reported positive, there are indications that the results may not be biologically meaningful, or that artifacts may have resulted from use of DMSO as vehicle.
For AMPA, IARC reported one positive mouse bone marrow MN study. There was one negative regulatory mouse bone marrow MN study of AMPA not reported in IARC. Both studies used the i.p. route. The positive study used a top dose of 200 mg/kg administered on two occasions, 24 h apart. The negative study used a single top dose of 1000 mg/kg which produced signs of toxicity. There is no obvious explanation for these conflicting results and the limited data do not allow reasonable WoE conclusions for AMPA in terms of the in vivo MN endpoint.
DNA damage in vitro
As noted above, the Expert Panel is in agreement with IARC reviewers that there are several in vitro mammalian cell studies of glyphosate which show DNA strand break effects (more specifically the alkaline single cell gel electrophoresis or comet endpoint). However, as also noted above, these studies should be assigned low weights compared to other more relevant endpoints in evaluating genotoxic risk, particularly when the results for relevant endpoints are more abundant. An assumption that the DNA damage observed in vitro might be secondary to toxicity rather than leading to DNA-reactive or persistent genotoxicity is underscored by cases where the same publication reports DNA damage effects but not chromosomal alterations, e.g. Sivikova and Dianovsky (Citation2006); Manas et al. (Citation2009a); Mladinic et al. (Citation2009a) without metabolic activation. Other publications reported both DNA damage and chromosomal effects, e.g. Lioi et al. (Citation1998a); Koller et al. (Citation2012).
For GBFs there are only two positive in vitro mammalian cell comet results reported by IARC. These provide limited evidence for GBF-induced DNA damage effects in vitro in mammalian cells.
There are a few positive in vitro mammalian cell SCE reports for glyphosate and GBFs reported in IARC. Since the OECD guideline for the SCE test has recently been deleted because of a lack of understanding of the mechanism(s) detected by the test, the biological relevance of SCE is unclear, and these studies have not been further considered by the Expert Panel for a WoE evaluation.
One negative primary hepatocyte UDS result is reported by IARC for glyphosate, but there are also negative primary hepatocyte UDS results for glyphosate and AMPA (one each) not reported by IARC.
DNA damage/adducts in vivo
One in vivo mammalian DNA damage and one in vivo mammalian DNA adduct study of glyphosate were reported by IARC. No additional regulatory or published studies were identified. Results for 8-hydroxydeoxyguanosine (8-OHdG) measurements are considered in the oxidative stress section (Section IIIB).
Bolognesi et al. (Citation1997) reported transient (4 h after dosing) increases in alkali-labile DNA strand breaks in liver and kidneys of mice treated i.p. with glyphosate. Interpretation of the genotoxic significance of these observations is difficult because such effects might be due to arrest of cells in S-phase or secondary to cytotoxicity (Williams et al. Citation2000). Peluso et al. (Citation1998) reported no induction of adducts in mouse liver or kidney detectable by 32P-postlabelling methodology after i.p. administration of glyphosate.
There is one positive in vivo SCE report for a GBF by Amer et al. (Citation2006) which was not evaluated by IARC. For reasons of relevancy noted above, this study has not been further considered by the Expert Panel in a WoE evaluation.
One in vivo mammalian DNA damage and one in vivo mammalian DNA adduct studies of GBFs were reported by IARC. No additional regulatory or published studies were identified.
Bolognesi et al. (Citation1997) reported transient (4 h after dosing) increases in alkali-labile DNA strand breaks in liver and kidneys of mice treated i.p. with a GBF. Similar conclusions about interpretation of these results apply as for the glyphosate results by the same authors discussed above. Peluso et al. (Citation1998) observed 32P-postlabelling adducts in liver and kidneys of mice dosed with a GBF. The source or identity of the adducts were not characterized although such adducts were not observed in studies with glyphosate in their publication.
No in vivo mammalian DNA damage studies of AMPA were reported in IARC or identified.
The paucity of data as well as the limited significance of the primary DNA damage endpoints on tumor initiation did not warrant that these observations should have a significant WoE impact.
Weight of evidence (WoE) for genotoxic effects in mammalian systems
In summary, the WoE from in vitro and in vivo mammalian tests for genotoxicity indicates that:
Glyphosate does not induce gene mutations in vitro. There are no in vitro mammalian cell gene mutation data for GBFs or AMPA, and no gene mutation data in vivo.
Glyphosate, GBFs, and AMPA are not clastogenic in vitro. Glyphosate is also not clastogenic in vivo. Some positive in vivo chromosomal aberration studies with GBFs are all subject to concerns regarding their reliability or biological relevance.
There is limited evidence that glyphosate induces MN in vitro. Although this could be a reflection of increased statistical power in the in vitro MN studies, the absence of clastogenic effects in a large majority of in vitro chromosomal studies suggests the possibility of threshold-mediated aneugenic effects. However, there is strong evidence that glyphosate does not induce MN in vivo.
Limited studies and potential technical problems do not present convincing evidence that GBFs or AMPA induce MN in vitro. The overwhelming majority of in vivo MN studies on GBFs gave negative results, but conflicting and limited data do not allow a conclusion on in vivo induction of MN by AMPA.
There is evidence that glyphosate and GBFs can induce DNA strand breaks in vitro, but these might be secondary to toxicity since they did not lead to chromosome breaks. There is limited evidence of transient DNA strand breakage for glyphosate and GBFs in vivo, but for glyphosate at least these are not associated with DNA adducts. These results are assigned a lower weight than results from other more relevant endpoints, which were in any case more abundant.
There is evidence that glyphosate and AMPA do not induce UDS in cultured hepatocytes.
Some reports of induction of SCE in vitro by glyphosate and GBFs, and one positive report of SCE induction in vivo by a GBF, do not contribute to the overall evaluation of genotoxic potential since the mechanism of induction and biological relevance of SCE are unclear.
Studies in non-mammalian test systems
With the exception of the bacterial reverse mutation test, global genotoxicity testing guidelines such as those issued by OECD (Citation2015) and other regulatory bodies do not recommend routine use of non-mammalian assays. Recently, OECD guidelines for two non-mammalian tests have been deleted because mammalian cell tests are considered more biologically relevant, and non-mammalian tests (with the exception of the bacterial reverse mutation test) are rarely used for regulatory test batteries.
Table 4.6 in the IARC Monograph summarized results from two bacterial reverse mutation test publications. One publication (Li & Long Citation1988) reviewed by IARC reported no mutagenic activity associated with glyphosate in a bacterial reverse mutation test but a publication by Rank et al. (Citation1993) indicated a positive finding with a glyphosate formulation.
Rank et al. (Citation1993) reported positive mutagenicity in TA98 only without S9 and positive mutagenicity in TA100 only with S9. At the outset this combination of responses is problematic as it is an unlikely combination and suggests that either one or both strain/S9 responses would be in error. The study data shown in of the Rank et al. (Citation1993) publication indicates that the positive responses reported for TA98 and TA100 were neither dose related nor were they reproduced in repeat data sets. The authors called the results indicative of gene mutation capabilities for a GBF; however, the data should never have been accepted for publication without additional testing over a narrower range of doses and as they currently stand, do not meet commonly used criteria for declaring Ames test results positive. The data from this one publication are not in agreement with 19 bacterial reverse mutation assays of GBFs presented in Supplemental Information, Appendix A, that were not included in the IARC Monograph. The Expert Panel considered the results of this study to be inconclusive.
A large number (20) of negative bacterial reverse mutation assays of GBFs are presented in Supplemental Information, Appendix A, . None of these were included in the IARC Monograph. There is also one negative regulatory study of AMPA.
In contrast to the two bacterial reverse studies considered in the IARC Monograph there are actually abundant data from 40 additional studies (Supplemental Information, Appendix A, ) that glyphosate and GBFs are negative in the one genetic test for gene mutation considered overall to be the best non-mammalian predictor of mammalian carcinogenesis.
Publications in which glyphosate or GBFs have been tested for genotoxicity in a variety of non-mammalian species other than bacterial reverse mutation appear to be included in the IARC Monograph, with only a few regulatory or published studies not included. With the exception of two positive and one negative chromosomal aberration assays in plants for glyphosate, chromosomal effect assay results have mainly been published for GBFs and showed predominantly positive results for MN in fish and amphibians.
A larger number of DNA damage comet assays in fish and other non-mammalian species in vitro are reported as exhibiting predominantly positive results for glyphosate. Larger numbers of positive comet results are available for GBFs in fish and amphibian/reptile studies. One positive fish comet study is reported for AMPA.
Some general features of these non-mammalian tests should be noted. First, both major endpoints measured in the majority of non-mammalian tests (i.e. MN and comet) might well be secondary to toxic effects. Second, many of these tests involve exposure by immersion in or surface contact with the test material in water. This is certainly not a standard or relevant route of exposure for in vivo mammalian systems and may introduce route-specific unique toxicity and genotoxic effects. This is particularly a concern for GBFs which commonly contain surfactants.
As a consequence, the Expert Panel did not consider data from a majority of the non-mammalian systems and nonstandard tests with glyphosate, GBF, and AMPA to have significant weight in the overall genotoxicity evaluation, especially given the large number of standard core studies in the gene mutation and chromosomal effects categories available in mammalian systems. Rationale supporting this consideration is the absence of internationally accepted guidelines for such non-mammalian test systems, lack of databases of acceptable negative control data or positive control responses, and no results from validation studies suggesting concordance with carcinogenicity. OECD guidelines specifically state that use of any nonstandard test requires justification along with stringent validation including establishing robust historical negative and positive control databases. Therefore, results in these tests, when conflicting with findings obtained in well validated test systems for which OECD guidelines exist, and where the biological relevance of the results can be evaluated, do not carry a significant WoE.
Critique of the classifications and mode of action (MoA) proposed in the IARC monograph for glyphosate and related agents
Genotoxicity classification and MoA
Based on the results of the WoE critique detailed above and the wealth of negative regulatory studies reviewed by Kier and Kirkland (Citation2013) and Williams et al. (Citation2000), the Expert Panel does not agree with IARC’s conclusion that there is strong evidence for genotoxicity across the glyphosate or GBFs database. In fact the Expert Panel WoE assessment provides strong support for a lack of genotoxicity, particularly in study categories closely associated with indications of potential genetic and carcinogenic hazard.
In order to demonstrate how the evidence from all sources was used to develop the Expert Panel’s WoE conclusions for glyphosate, GBFs, and AMPA, the results from all study types were compiled in . Wherever possible, positive or negative responses were assigned to the individual studies in according to the conclusions given in the original publication or report. In a small number of studies the Expert Panel concluded that there were significant issues regarding data analysis and interpretation of results and either changed the positive call given by IARC, e.g. Bolognesi et al. (Citation2009) or, if the impact of the issues on the overall conclusions of the study was considered inconclusive, the data from that paper were excluded from , e.g. Paz-y-Miño et al. (Citation2007) and Rank et al. (Citation1993).
It should also be noted that the weight indicated in this table primarily reflects the endpoint of the publication or report. As noted above, there are significant test system (experimental protocol and data interpretation) considerations for some specific studies that significantly lowered the weight of these studies independently of the endpoint measured.
An evaluation of the studies in according to their relative contributions to a WoE produced the following results:
Test methods identified as providing low contribution (Low Weight) to the WoE produced the highest frequency of positive responses, regardless of whether the responses were taken from the results of IARC evaluated studies alone (eight of nine) or from all studies combined (eight of 11).
The highest frequencies of positive responses were reported for test endpoints and systems considered most likely to yield false or misleading positive results with respect to carcinogenicity prediction or carcinogenic mechanism due to their susceptibility to secondary effects. This relationship was constant regardless of whether the results were taken from IARC evaluated studies alone or all studies combined.
The numbers of studies providing strong evidence of relevant genotoxicity (High Weight) were in the minority for both the IARC and Expert Panel evaluations, with six out of 15 studies identified as High Weight being positive for the IARC evaluation, and only eight out of 92 studies identified as High Weight being positive for all studies combined by the Expert Panel.
Contrary to IARC’s conclusion that there is strong evidence of genotoxicity, the Expert Panel’s WoE analysis of the complete database (or the IARC subset alone) using the weighting categories proposed in Suter and Cormier (Citation2011) indicates that glyphosate and GBFs should not be classified as genotoxic. The panel does not agree with IARC’s conclusion of moderate evidence for genotoxicity of AMPA. The data needed to make an assessment of the genetic hazard of AMPA are too limited and conflicting to reliably support such a classification.
To provide greater emphasis to the Expert Panel’s WoE conclusion, provides a comparison between a set of characteristics found in confirmed genotoxic carcinogens (Bolt et al. Citation2004; Petkov et al. Citation2015) and the genotoxic activity profiles for glyphosate, AMPA, and GBFs. There is virtually no concordance between the two sets of characteristics.
Oxidative stress classification and MoA
Oxidative stress was the second characteristic considered by IARC as operative in human carcinogens and thus supporting their classifying glyphosate as probably carcinogenic to humans. Publications investigating the relationship between oxidative DNA damage and cancer (Wu et al. Citation2004; Klaunig et al. Citation2010) have demonstrated that following exposure to oxidative stress-inducing agents, a common adaptive response induced in mammalian cells is the up-regulation of stress-response genes. The resultant toxic response is threshold dependent.
It has been shown that reactive oxygen species (ROS) are genotoxic in principle, and the question arises as to whether GBFs that increase ROS production will add to an endogenously produced background level of DNA lesions or whether compensatory mechanisms may result in non-linear dose-effects. Halliwell (Citation2003) reported that alteration to DNA molecules triggers repair, and frequent activation may increase the general repair capacity, irrespective of the cause of the damage. Thus, repeated exposure to ROS may lead to an adaptive response, mitigating the mutagenicity of oxidative DNA lesions. Moreover, as suggested by Deferme et al. (Citation2015) oxidative stress is not uniquely associated with a genotoxic carcinogens and simple measurements of ROS are insufficient evidence supporting a genotoxic causal MoA for carcinogenicity (Arai et al. Citation2006).
The evidence for oxidative stress induction summarized by IARC comes from studies employing a variety of endpoints and test systems, but in the IARC Monograph the data on oxidative stress are comingled with data from other endpoints, and data on glyphosate and GBFs are also comingled. It is therefore difficult to obtain a clear picture of the oxidative stress effects.
Indirect measures of oxidative stress vs. measures of oxidative damage
In some respects, measures (endpoints) of oxidative effects can be weighted in a manner similar to that applied to measures of genotoxicity. For example, in the majority of the studies reviewed by IARC, the endpoints assessed were only indirect measures of oxidative stress, in the form of antioxidant suppressive effects, changes in endogenous levels of protective molecules or enzymes (e.g. glutathione, superoxide dismutase) or changes in ROS (e.g. H2O2). The experiments in vitro in mammalian cells produced conflicting results and some positive results were observed only at very high dose levels which could be problematic for reliable evaluation of the potential for in vivo oxidative stress (Halliwell Citation2003). Long et al. (Citation2007) demonstrated that reactive oxygen can be produced as an artifact by chemical reactions with components of the culture media, a possibility not evaluated in the studies reviewed by IARC. Overall, IARC’s assessment did not appear to consider the relative importance of different biomarkers of oxidative stress with the exception of noting limitations of using dihydrofluorescein acetate as a marker of oxidative stress.
A more meaningful endpoint for identification of oxidative damage, particularly as it pertains to identification of a possible genotoxic mechanism of cancer, would be the identification and application of a biomarker relevant to oxidative stress-induced damage to DNA. While a number of biochemical and physiological changes in cells can be produced during oxidative stress, the most extensively studied oxidative DNA lesion produced is 8-OHdG. This adduct has been widely used as a biomarker of oxidative DNA damage, and determination of 8-OHdG levels may be useful in defining a chemical’s MoA.
Oxidative damage studies evaluated in the IARC monograph
Peluso et al. (Citation1998) reported 32P-postlabelling adducts in rats treated with GBFs (but not glyphosate). The nature or source of the adducts was not identified but Williams et al. (Citation2000) noted that the solvent system used by Peluso et al. (Citation1998) could not detect oxidative DNA damage. Evidence for increased DNA damage in Bolognesi et al. (Citation1997) as measured by 8-OHdG DNA adducts was both limited and contradictory. Glyphosate was reported to induce 8-OHdG adducts in liver but not kidney tissues whereas a GBF (with an equivalent level of glyphosate) was reported to induce 8-OHdG adducts in kidney but not in liver tissue. Results of the Bolognesi et al. (Citation1997) study are contradicted by another published study (Heydens et al. Citation2008) that was not considered by IARC. In this study no statistically significant increases in 8-OHdG were observed in liver or kidneys of mice 24 h after treatment by i.p. injection with 600 and 900 mg/kg of a GBF of the same composition as those used by Peluso et al. (Citation1998) and Bolognesi et al. (Citation1997).
The only other cited mammalian study examining oxidative DNA damage was a measurement of the effect of human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) on the comet endpoint in human lymphocytes exposed to glyphosate (Mladinic et al. Citation2009a). This study showed a small but statistically significant effect on comet tail intensity at only a low mid-dose level in the absence of an S9 metabolic activation system and at the highest dose level tested (580 μg/mL) in the presence of S9. The observation of an effect at the highest dose level only in the presence of S9 is unusual because statistically significant increases in other markers of oxidative stress were observed at the high dose levels in either the presence or absence of S9. The authors indicated that their results were not considered an unequivocal indication of the oxidative potential of glyphosate. As noted above there does not appear to be any significant in vitro metabolism of glyphosate with rat liver homogenate (Gohre et al. Citation1987).
A series of studies in eels examined oxidative DNA damage of glyphosate, GBF, and AMPA by measurement of comet endpoints with and without treatment of samples with endonucleases that cleave at sites of oxidative damage (Guilherme et al. Citation2012a, Citation2012b; Guilherme et al. Citation2014a, Citation2014b; Marques et al. Citation2014a, Citation2014b). When considering net effects of endonuclease treatment there were varied responses in different conditions, tissues, and treatments ranging from no statistically significant effect to relatively small but statistically significant effects. These studies did not provide consistent strong evidence of oxidative DNA damage in a non-mammalian system.
In addition there was a human biomonitoring study measuring blood 8-OHdG which did not indicate a statistically significant association between previous GBF exposure and high 8-OHdG levels (Koureas et al. Citation2014, not evaluated in IARC). There are concerns with this study, particularly the relationship between the timing of exposure and a presumably transient marker of exposure. While some other agents did show associations, the lack of a statistically significant association between 8-OHdG and past GBF exposure does not provide support for GBF-related oxidative DNA damage in humans.
Many more oxidative stress studies are available for GBFs than for glyphosate or AMPA. Unlike glyphosate, most of the GBF studies show evidence of oxidative stress suggesting that GBFs contain compounds that are likely to be toxic under some treatment conditions leading to ROS followed by normal cellular protective responses. Comparison of GBF oxidative stress study results with predicted human exposure levels (e.g. calculated 90th percentile for applicators of 0.064 mg/kg body weight/day and much lower for other exposures), suggests that it is not likely that GBFs would induce oxidative stress likely to exceed endogenous detoxification capacities.
IARC claims of strong evidence supporting oxidative stress from AMPA seem to result from glyphosate and particularly GBF results rather than AMPA results. In fact, oxidative stress studies of AMPA are very limited. In the section on oxidative stress, IARC only cites one negative in vitro mammalian cell study of AMPA (Chaufan et al. Citation2014) and one positive in vitro mammalian cell study (Kwiatkowska et al. Citation2014). There is one other positive human cell study (Roustan et al. Citation2014) that was not cited; however, AMPA had unusually high toxicity in this report compared to other in vitro mammalian studies (see above) and no dose response was observed over an order of magnitude concentrations. The paucity and inconsistency of cited data does not seem to justify a conclusion of strong evidence for oxidative stress induction by AMPA.
Research on oxidative stress induced genotoxicity suggests that it is often a secondary response to toxicity and characterized by a threshold (Pratt & Barron Citation2003). Therefore the most appropriate conclusion supported by the oxidative stress data presented in IARC Monograph Section 4.2 is that there is not a strong WoE that glyphosate, GBFs, or AMPA produce oxidative damage to DNA that would lead to induction of endpoints predictive of a genotoxic hazard or act as a mechanism for the induction of cancer in experimental animals or humans.
Summary and conclusions
Detection of genotoxic activity or induction of oxidative stress/damage in any test conducted with a chemical does not, a priori, mean that the agent has a carcinogenic potential, induces key events leading to tumor development or represents an in vivo genotoxic risk. A systematic and critical assessment of the WoE is required before genotoxic hazard and MoA conclusions can be reached. The IARC process leading to conclusions suggesting modes of action involving genotoxicity and oxidative stress was incomplete (excluding valuable data) and did not appear to critically evaluate some of the key studies it relied upon. A meaningful WoE evaluation depends on an assessment of all available data using an appropriate weighting process.
A number of reviews of the carcinogenicity, genotoxicity, and oxidative stress/damage for glyphosate, AMPA, and GBFs were available prior to the development of the IARC Glyphosate Monograph (see Introduction). These prior reviews included much of the data available to IARC reviewers involved in the evaluation presented in the IARC Monograph. In general, genetic toxicology data evaluated in these prior reviews all support a conclusion that glyphosate (and related materials) is inherently not genotoxic. The Expert Panel concluded that there is no new, valid evidence presented in the IARC Monograph that would provide a basis for altering these conclusions and that including the study results reviewed by Kier and Kirkland (Citation2013) would provide considerable additional support to the conclusion of absence of inherent genotoxic potential.
The Expert Panel concluded that glyphosate, GBFs, and AMPA genotoxicity response profiles are not consistent with characteristics of genotoxic carcinogens ().
There is substantial evidence, particularly in bacterial reverse mutation assays, demonstrating that glyphosate, GBFs, or AMPA do not induce gene mutation from either direct or oxidative induced mechanisms.
The evidence indicating that glyphosate can produce chromosomal aberrations in mammalian systems is very limited, conflicting, and potentially due to secondary mechanisms.
The absence of evidence indicating that glyphosate or GBFs induced lesions characteristic of genotoxic carcinogens, in well-validated test systems with robust experimental protocols, invalidates conclusions that glyphosate or GBFs might act via a genotoxic MoA.
The evidence for oxidative stress/damage as a mechanism or predictor of carcinogenesis is unconvincing. Repeated exposure to ROS most likely leads to adaptive responses, mitigating the mutagenicity of oxidative DNA lesions. Studies directed toward a better understanding of this relationship for glyphosate or GBF related exposures have not been reported.
There is little or no reliable evidence that GBFs, at levels experienced across a broad range of end-user exposures, poses any human genotoxic hazard/risk.
The Expert Panel concluded that the IARC assessment of classifications regarding strong evidence of genotoxicity and oxidative stress capabilities of glyphosate, GBFs, and AMPA is not supported by the available data. A critical review of the complete dataset by the Expert Panel supports a conclusion that glyphosate (including GBFs and AMPA) does not pose a genotoxic hazard and therefore, should not be considered support for the classification of glyphosate as a genotoxic carcinogen. These conclusions are supportive of recent reviews that have occurred during the preparation of this review. A European Food Safety Authority peer review concluded that glyphosate is unlikely to pose a carcinogenic hazard to humans (EFSA Citation2015) and a Joint FAO/WHO Meeting on Pesticide Residues concluded that glyphosate is unlikely to be genotoxic at anticipated dietary exposures and unlikely to cause a carcinogenic risk to humans from dietary exposure (JMPR Citation2016).
Supplemental material
Supplemental material for this article is available online here.
Brusick et al._Supplemental_Information_App_B.docx
Download MS Word (118.7 KB)Brusick et al._Supplemental_Information_App_A.docx
Download MS Word (63.4 KB)Acknowledgements
The authors gratefully acknowledge the extensive comments received from seven independent reviewers selected by the Editor and who were anonymous to the authors. These comments were very helpful in revising the manuscript.
Declaration of interest
The employment affiliation of the authors is as shown on the cover page. However, it should be recognized that each individual participated in the review process and preparation of this paper as an independent professional and not as a representative of their employer. Gary Williams, David Brusick, and David Kirkland have previously served as independent consultants for the Monsanto Company on the European Glyphosate Task Force. Gary Williams has consulted for Monsanto on litigation matters involving glyphosate. Larry Kier was previously an employee of the Monsanto Company. Marilyn Aardema has not previously been employed in the Monsanto Company or previously been involved in any activity involving glyphosate and as such declares no potential conflicts of interest. Furthermore, other than Gary Williams, none of the aforementioned authors have been involved in any litigation procedures involving glyphosate.
The Expert Panel Members recruitment and evaluation of the data were organized and conducted by Intertek Scientific & Regulatory Consultancy (Intertek). The Expert Panelists acted as consultants for Intertek. Intertek (previously Cantox) is a consultancy firm that provides scientific and regulatory advice, as well as safety and efficacy evaluations for the chemical, food, and pharmaceutical industries. While Intertek Scientific & Regulatory Consultancy has not previously worked on glyphosate related matters for the Monsanto Company, previous employees of Cantox had worked in this capacity.
Funding for this evaluation was provided by the Monsanto Company which is a primary producer of glyphosate and products containing this active ingredient. Neither any Monsanto company employees nor any attorney reviewed any of the Expert Panel?s manuscripts prior to submission to the journal.
This article is part of a supplement, sponsored and supported by Intertek Scientific & Regulatory Consultancy. Funding for the sponsorship of this supplement was provided to Intertek by the Monsanto Company, which is a primary producer of glyphosate and products containing this active ingredient.
References
- Amer SM, Aly FAE, Farghaly AA, Ibrahim AAE. 2006. In vitro and in vivo evaluation of the genotoxicity of the herbicide glyphosate in mice. Bull Natl Res Center Egypt (Cairo). 31:427–446.
- Anadon A, Martinez-Larranaga MR, Martinez MA, Castellano VJ, Martinez M, Martin MT, Nozal MJ, Bernal JL. 2009. Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol Lett. 190:91–95.
- Arai T, Kelly VP, Minowa O, Noda T, Nishimura S. 2006. The study using wild-type and ogg1 knockout mice exposed to potassium bromate shows no tumor induction despite an extensive accumulation of 8-hydroxyguanine in kidney DNA. Toxicology. 221:179–186.
- Battershill JM, Burnett K, Bull S. 2008. Factors affecting the incidence of genotoxicity biomarkers in peripheral blood lymphocytes: impact on design of biomonitoring studies. Mutagenesis. 23:423–437.
- Bolognesi C, Bonatti S, Degan P, Gallerani E, Peluso M, Rabboni R, Roggieri P, Abbondandolo A. 1997. Genotoxic activity of glyphosate and its technical formulation Roundup. J Agric Food Chem. 45:1957–1962.
- Bolognesi C, Carrasquilla G, Volpi S, Solomon KR, Marshall EJP. 2009. Biomonitoring of genotoxic risk in agricultural workers from five Colombian regions: association to occupational exposure to glyphosate. J Toxicol Environ Health A. 72:986–997.
- Bolt HM, Foth H, Hengstler JG, Degen GH. 2004. Carcinogenicity categorization of chemicals-new aspects to be considered in a European perspective. Toxicol Lett. 151:29–41.
- Bonassi S, Coskun E, Ceppi M, Lando C, Bolognesi C, Burgaz S, Holland N, Kirsh-Volders M, Knasmueller S, Zeiger E, et al. 2011. The human micronucleus project on exfoliated buccal cells (HUMNxL): the role of life-style, host factors, occupational exposures, health status, and assay protocol. Mutat Res. 728:88–97.
- Brewster DW, Warren J, Hopkins WE. II. 1991. Metabolism of glyphosate in Sprague-Dawley rats tissue distribution identification and quantitation of glyphosate-derived materials following a single oral dose. Fundam Appl Toxicol. 17:43–51.
- Chan P, Mahler J. 1992. Toxicity studies of glyphosate (CASRN 1071-83-6) administered in dosed feed to F344/N rats and B6C3F1 mice. Research Triangle Park (NC): National Toxicology Program (NTP) (Toxicity Report Series, No. 16; NIH Publication 92-3135). Available from: http://ntp.niehs.nih.gov/results/pubs/shortterm/reports/abstracts/tox016/index.html
- Chaufan G, Coalova I, del Carmen Rios de Molina M. 2014. Glyphosate commercial formulation causes cytotoxicity, oxidative effects, and apoptosis on human cells: differences with its active ingredient. Int J Toxicol. 33:29–38.
- Cimino MC. 2006. Comparative overview of current international strategies and guidelines for genetic toxicology testing for regulatory purposes. Environ Mol Mutagen. 47:362–390.
- Collins A, Koppen G, Valdiglesias V, Dusinska M, Kruszewski M, Moller P, Rojas E, Dhawan A, Benzie I, Coskun E, et al. 2014. The comet assay as a tool for human biomonitoring studies: the ComNet project. Mutat Res Rev Mutat Res. 759:27–39.
- Dearfield KL, Thybaud V, Cimino MC, Custer L, Czich A, Harvey JS, Hester S, Kim JH, Kirkland D, Levy DD, et al. 2011. Follow-up actions from positive results of in vitro genetic toxicity testing. Environ Mol Mutagen. 52:177–204.
- Deferme L, Wolters J, Claessen S, Briede J, Kleinjans J. 2015. Oxidative stress mechanisms do not discriminate between genotoxic and nongenotoxic liver carcinogens. Chem Res Toxicol. 28:1636–1646.
- Dusinska M, Collins AR. 2008. The comet assay in human biomonitoring: gene–environment interactions. Mutagenesis. 23:191–205.
- Eastmond DA, Hartwig A, Anderson D, Anwar WA, Cimino MC, Dobrev I, Douglas GR, Nohmi T, Phillips DH, Vickers C. 2009. Mutagenicity testing for chemical risk assessment: update of the WHO/IPCS Harmonized Scheme. Mutagenesis. 24:341–349.
- ECHA. 2015. Chapter R.7a: Endpoint Specific Guidance. In: Guidance on information requirements and chemical safety assessment. Helsinki, Finland: European Chemical Agency (ECHA) (Version 4.1 October 2015). Available from: http://echa.europa.eu/documents/10162/13632/information_requirements_r7a_en.pdf.
- EFSA. 2011. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment (EFSA Scientific Committee) (Question no. EFSA-Q-2009-00782, adopted on 13 September 2011 by European Food Safety Authority, 3 October 2012, replaces the earlier version). EFSA J. 9:2379 [69 pp.]. doi:10.2903/j.efsa.2011.2379. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/2379.htm.
- EFSA. 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate (EFSA-Q-2014-00546, EFSA-Q-2015-00279, approved on 30 October 2015 by European Food Safety Authority). EFSA J. 13:4302 [107 pp.]. doi:10.2903/j.efsa.2015.4302. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/4302.
- European Commission. 2002. Review report for the active substance glyphosate. Finalized in the Standing Committee on Plant Health at its meeting on 29 June 2001 in view of the inclusion of glyphosate in Annex I of Directive 91/414/EEC. Brussels (Belgium): European Commission (EC), Health and Consumer Protection Directorate General (6511/VI/99-Final). Available from: http://ec.europa.eu/food/fs/sfp/ph_ps/pro/eva/existing/list1_glyphosate_en.pdf
- Faust F, Kassie F, Knasmuller S, Boedecker RH, Mann M, Mersch-Sundermann V. 2004. The use of the alkaline comet assay with lymphocytes in human biomonitoring studies. Mutat Res. 566:209–229.
- Fenech M, Holland N, Zeiger E, Chang WP, Burgaz S, Thomas P, Bolognesi C, Knasmueller S, Kirsch-Volders M, Bonassi S. 2011. The HUMN and HUMNxL international collaboration projects on human micronucleus assays in lymphocytes and buccal cells-past, present and future. Mutagenesis. 26:239–245.
- Fowler P, Smith R, Smith K, Young J, Jeffrey L, Carmichael P, Kirkland D, Pfuhler S. 2014. Reduction of misleading (“false”) positive results in mammalian cell genotoxicity assays. III: Sensitivity of human cell types to known genotoxic agents. Mutat Res Genet Toxicol Environ Mutagen. 767:28–36.
- Gohre K, Casida JE, Ruzo LO. 1987. N oxidation and cleavage of the amino acid derived herbicide glyphosate and anilino acid of the insecticide fluvalinate. J Agric Food Chem. 35:388–392.
- Guilherme S, Gaivao I, Santos MA, Pacheco M. 2012a. DNA damage in fish (Anguilla anguilla) exposed to a glyphosate-based herbicide – elucidation of organ-specificity and the role of oxidative stress. Mutat Res Genet Toxicol Environ Mutagen. 743:1–9.
- Guilherme S, Santos MA, Barroso C, Gaivao I, Pacheco M. 2012b. Differential genotoxicity of Roundup® formulation and its constituents in blood cells of fish (Anguilla anguilla): considerations on chemical interactions and DNA damaging mechanisms. Ecotoxicology. 21:1381–1390.
- Guilherme S, Santos MA, Gaivao I, Pacheco M. 2014a. Are DNA-damaging effects induced by herbicide formulations (Roundup® and Garlon®) in fish transient and reversible upon cessation of exposure? Aquat Toxicol. 155:213–221.
- Guilherme S, Santos MA, Gaivao I, Pacheco M. 2014b. DNA and chromosomal damage induced in fish (Anguilla anguilla L.) by aminomethylphosphonic acid (AMPA) – the major environmental breakdown product of glyphosate. Environ Sci Pollut Res Int. 21:8730–8739.
- Halliwell B. 2003. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 10:3–6.
- Health and Welfare Canada. 1991. Preharvest application of glyphosate (Roundup) herbicide. Ottawa (ON): Health and Welfare Canada, Pest Management Regulatory Agency (PMRA), Plant Industry Directorate. Pesticide Information Division (Pesticides Directorate Discussion Document, Vol. 91, Iss. 1), 92 p.
- Helal AD, Moussa HM. 2005. Chromosomal aberrations induced by glyphosate isopropylamine herbicide and trials for diminuting its toxicity using some chemical inactivators and antioxidant. Vet Med J Giza. 53:169–187.
- Heydens WF, Healy CE, Hotz KJ, Kier LD, Martens MA, Wilson AGE, Farmer DR. 2008. Genotoxic potential of glyphosate formulations: mode-of-action investigations. J Agric Food Chem. 56:1517–1523.
- Hill AB. 1965. The environment and disease: association or causation? Proc R Soc Med. 58:295–300.
- IARC. 2015. Glyphosate. In: Some organophosphate insecticides and herbicides: diazinon, glyphosate, malathion, parathion, tetrachlorvinphos. IARC Working Group, March 3–10, 2015, Lyon (France). Lyon (France): World Health Organization (WHO), IARC (IARC Monographs on the Evaluation of Carcinogen Risks to Humans, vol. 112). p. 1–92. Available from: http://monographs.iarc.fr/ENG/Monographs/vol112/index.php.
- ICH. 2011. Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use: S2(R1). Geneva (Switzerland): International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (ICH Harmonized Tripartite Guideline – Current Step 4 version [Combines S2A & S2B]). Available from: http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html.
- Jackson MA, Stack HF, Waters MD. 1993. The genetic toxicology of putative nongenotoxic carcinogens. Mutat Res. 296:241–277.
- JMPR. 2006. Glyphosate. In: Pesticides residues in food – 2004. Evaluations 2004 Part II – Toxicological. Joint Meeting of the FAO Panel of Experts on Pesticide residues in Food and the Environment and the WHO Core Assessment Group (JMPR), Sep 20–29, 2004, Rome. Rome: Food and Agriculture Organization of the United Nations (FAO)/Geneva: World Health Organization (WHO), International Program on Chemical Safety (IPCS) (WHO/PCS/06.1). p. 95–169. Available from: http://www.inchem.org/documents/jmpr/jmpmono/v2004pr01.pdf.
- JMPR. 2016. 1.2 Glyphosate (158). In: Summary report. Joint FAO/WHO Meeting on Pesticide Residues, May 9–13, 2016, Geneva, Switz. Rome: Food and Agriculture Organization of the United Nations/Geneva, World Health Organization (WHO) (Issued May 16, 2016). Available from: http://www.who.int/foodsafety/jmprsummary2016.pdf.
- Kier LD, Kirkland DJ. 2013. Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit Rev Toxicol. 43:283–315.
- Kirkland DJ, Henderson L, Marzin D, Muller L, Parry JM, Speit G, Tweats DJ, Williams GM. 2005. Testing strategies in mutagenicity and genetic toxicology: an appraisal of the guidelines of the European scientific committee for cosmetics and non-food products for the evaluation of hair dyes. Mutat Res. 588:88–105.
- Kirkland DJ, Reeve L, Gatehouse D, Vanparys P. 2011. A core in vitro genotoxicity battery comprising the Ames test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat Res. 721:27–73.
- Kirsch-Volders M, Bonassi S, Knasmueller S, Holland N, Bolognesi C, Fenech MF. 2014. Commentary: critical questions, misconceptions and a road map for improving the use of the lymphocyte cytokinesis-block micronucleus assay for in vivo biomonitoring of human exposure to genotoxic chemicals – a HUMN project perspective. Mutat Res Rev Mutat Res. 759:49–58.
- Kirsch-Volders M, Vanhauwaert A, Eichenlaub-Ritter U, Decordier I. 2003. Indirect mechanisms of genotoxicity. Toxicol Lett. 140:63–74.
- Klaunig JE, Kamendulis LM, Hocevar BA. 2010. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 38:96–109.
- Klimisch H-J, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol. 25:1–5.
- Koller VJ, Fuerhacker M, Nersesyan A, Misik M, Eisenbauer M, Knasmueller S. 2012. Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells. Arch Toxicol. 86:805–813.
- Koureas M, Tsezou A, Tsakalof A, Orfanidou T, Hadjichristodoulou C. 2014. Increased levels of oxidative DNA damage in pesticide sprayers in Thessaly region (Greece). Implications of pesticide exposure. Sci Total Environ. 496:358–364.
- Kwiatkowska M, Huras B, Bukowska B. 2014. The effect of metabolites and impurities of glyphosate on human erythrocytes (in vitro). Pestic Biochem Physiol. 109:34–43.
- Li AP, Long TJ. 1988. An evaluation of the genotoxic potential of glyphosate. Fundam Appl Toxicol. 10:537–546.
- Li Q, Lambrechts MJ, Zhang Q, Liu S, Ge D, Yin R, Xi M, You Z. 2013. Glyphosate and AMPA inhibit cancer cell growth through inhibiting intracellular glycine synthesis. Drug Des Dev Ther. 7:635–643.
- Lioi MB, Scarfi MR, Santoro A, Barbieri R, Zeni O, Di Berardino D, Ursini MV. 1998a. Genotoxicity and oxidative stress induced by pesticide exposure in bovine lymphocyte cultures in vitro. Mutat Res. 403:13–20.
- Lioi MB, Scarfi MR, Santoro A, Barbieri R, Zeni O, Salvemini F, Di Berardino D, Ursini MV. 1998b. Cytogenetic damage and induction of pro-oxidant state in human lymphocytes exposed in vitro to glyphosate, vinclozolin, atrazine, and DPX-E9636. Environ Mol Mutagen. 32:39–46.
- Long LH, Kirkland D, Whitwell J, Halliwell B. 2007. Different cytotoxic and clastogenic effects of epigallocatechin gallate in various cell-culture media due to variable rates of its oxidation in the culture medium. Mutat Res. 634:177–183.
- Manas F, Peralta L, Raviolo J, Garcia Ovandoa H, Weyers A, Ugnia L, Gonzalez Cid M, Larripa I, Gorla N. 2009a. Genotoxicity of glyphosate assessed by the comet assay and cytogenetic tests. Environ Toxicol Pharmacol. 28:37–41.
- Manas F, Peralta L, Raviolo J, Ovando HG, Weyers A, Ugnia L, Cid MG, Larripa I, Gorla N. 2009b. Genotoxicity of AMPA, the environmental metabolite of glyphosate, assessed by the comet assay and cytogenetic tests. Ecotoxicol Environ Saf. 72:834–837.
- Marc J, Mulner-Lorillon O, Boulben S, Hureau D, Durand G, Belle R. 2002. Pesticide Roundup provokes cell division dysfunction at the level of CDK1/cyclin B activation. Chem Res Toxicol. 15: 326–331.
- Marques A, Guilherme S, Gaivao I, Santos MA, Pacheco M. 2014a. Erratum to: "Progression of DNA damage induced by a glyphosate-based herbicide in fish (Anguilla anguilla) upon exposure and post-exposure periods – insights into the mechanisms of genotoxicity and DNA repair". Comp Biochem Physiol C Toxicol Pharmacol. 168C:1. doi: 10.1016/j.cbpc.2014.10.008.
- Marques A, Guilherme S, Gaivao I, Santos MA, Pacheco M. 2014b. Progression of DNA damage induced by a glyphosate-based herbicide in fish (Anguilla anguilla) upon exposure and post-exposure periods-insights into the mechanisms of genotoxicity and DNA repair. Comp Biochem Physiol C Toxicol Pharmacol. 166:126–133.
- Mendelsohn ML, Moore DH II, Lohman PHM. 1992. A method for comparing and combining short-term genotoxicity test data results and interpretation. Mutat Res. 266:43–60.
- Menkes DB, Temple WA, Edwards IR. 1991. Intentional self-poisoning with glyphosate-containing herbicides. Hum Exp Toxicol. 10:103–107.
- Mladinic M, Berend S, Vrdoljak AL, Kopjar N, Radic B, Zeljezic D. 2009a. Evaluation of genome damage and its relation to oxidative stress induced by glyphosate in human lymphocytes in vitro. Environ Mol Mutagen. 50:800–807.
- Mladinic M, Perkovic P, Zeljezic D. 2009b. Characterization of chromatin instabilities induced by glyphosate, terbuthylazine and carbofuran using cytome fish assay. Toxicol Lett. 189:130–137.
- Moller P. 2005. Genotoxicity of environmental agents assessed by the alkaline comet assay. Basic Clin Pharmacol Toxicol. 96:1–42.
- OECD. 2014a. In vitro mammalian chromosomal aberration test. In: OECD Guidelines for the Testing of Chemicals. Paris (France): Organization for Economic Co-operation and Development (OECD) (OECD Guideline no. 473) [Adopted: 26 September 2014]. Available from: http://www.oecd-ilibrary.org/environment/test-no-473-in-vitro-mammalian-chromosomal-aberration-test_9789264224223-en.
- OECD. 2014b. Mammalian erythrocyte miconucleus test. In: OECD Guidelines for the Testing of Chemicals. Paris (France): Organization for Economic Co-operation and Development (OECD) (OECD Guideline no. 474) [Adopted: 26 September 2014]. Available from: http://www.oecd-ilibrary.org/environment/test-no-474-mammalian-erythrocyte-micronucleus-test_9789264224292-en.
- OECD. 2014c. Mammalian bone marrow chromosomal aberration test. OECD Guidelines for the Testing of Chemicals. Paris (France): Organization for Economic Co-operation and Development (OECD) (OECD Guideline no. 475) [Adopted: 26 September 2014]. Available from: http://www.oecd-ilibrary.org/environment/test-no-475-mammalian-bone-marrow-chromosomal-aberration-test_9789264224407-en.
- OECD. 2014d. In vitro mammalian cell micronucleus test. OECD Guidelines for the Testing of Chemicals. Paris (France): Organization for Economic Co-operation and Development (OECD) (OECD Guideline no. 487) [Adopted: 26 September 2014]. Available from: http://www.oecd-ilibrary.org/environment/test-no-487-in-vitro-mammalian-cell-micronucleus-test_9789264224438-en
- OECD. 2015. Overview of the Set of OECD Genetic Toxicology Test Guidelines and Updates Performed in 2014–2015. Paris (France): Organization for Economic Co-operation and Development (OECD), Environment Directorate, Health and Safety Publications Available from: http://www.oecd.org/env/ehs/testing/series-testing-assessment-publications-number.htm
- Parry JM, Fiedler RJ, McDonald A. 1994. Thresholds for aneuploidy-inducing chemicals. Advisory Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment of the UK Department of Health. Mutagenesis. 9:503–504.
- Paz-y-Miño C, Muñoz MJ, Maldonado A, Valladares C, Cumbal N, Herrera C, Robles P, Sánchez ME, López-Cortés A. 2011. Baseline determination in social, health, and genetic areas in communities affected by glyphosate aerial spraying on the northeastern Ecuadorian border. Rev Environ Health. 26:45–51.
- Paz-y-Miño C, Sánchez ME, ArévaloI M, Muñoz MJ, Wittel T, Oleas De-la-CarreraI G, Leonel PE. II. 2007. Evaluation of DNA damage in an Ecuadorian population exposed to glyphosate. Genet Mol Biol. 30:456–460.
- Peluso M, Munnia A, Bolognesi C, Parodi S. 1998. 32P-postlabeling detection of DNA adducts in mice treated with the herbicide Roundup . Environ Mol Mutagen. 31:55–59.
- Petkov PI, Patlewicz G, Schultz TW, Honma M, Todorov M, Kotov S, Dimitrov SD, Donner EM, Mekenyan OG. 2015. A feasibility study: can information collected to classify for mutagenicity be informative in predicting carcinogenicity? Regul Toxicol Pharmacol. 72:17–25.
- Pfuhler S, Fellows M, van Benthem J, Corvi R, Curren R, Dearfield K, Fowler P, Froetschl R, Elhajouji A, Le Hegarat L, et al. 2011. In vitro genotoxicity test approaches with better predictivity: summary of an IWGT workshop. Mutat Res. 723:101–107.
- Pierotti MA, Sozzi G, Croce CM. 2003. Oncongenes: mechanisms of oncogene activation. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RC Jr, Gansler TS, Holland JF, Frei E III, editors. Holland-Frei cancer medicine. 6th ed. Hamilton (ON): B.C. Decker. Available from: http://www.ncbi.nlm.nih.gov/books/NBK12538/.
- Piesova E. 2004. The influence of different treatment length on the induction of micronuclei in bovine lymphocytes after exposure to glyphosate. Folia Vet Lat. 48:130–134.
- Piesova E. 2005. The effect of glyphosate on the frequency of micronuclei in bovine lymphocytes in vitro. Acta Vet (Beogr). 55:101–109.
- Prasad S, Srivastava S, Singh M, Shukla Y. 2009. Clastogenic effects of glyphosate in bone marrow cells of Swiss albino mice. J Toxicol. 2009:308985. doi: 10.1155/2009/308985.
- Pratt IS, Barron T. 2003. Regulatory recognition of indirect genotoxicity mechanisms in the European Union. Toxicol Lett. 140: 53–62.
- Rank J, Jensen AG, Skov B, Pedersen LH, Jensen K. 1993. Genotoxicity testing of the herbicide Roundup and its active ingredient glyphosate isopropylamine using the mouse bone marrow micronucleus test, salmonella mutagenicity test, and allium anaphase-telophase test. Mutat Res. 300:29–36.
- Roustan A, Aye M, De Meo M, Di Giorgio C. 2014. Genotoxicity of mixtures of glyphosate and atrazine and their environmental transformation products before and after photoactivation. Chemosphere. 108:93–100.
- Salamone MF, Mavournin KH. 1994. Bone marrow micronucleus assay: a review of the mouse stocks used and their published mean spontaneous micronucleus frequencies. Environ Mol Mutagen. 23:239–273.
- Schmid O, Speit G. 2007. Genotoxic effects induced by formaldehyde in human blood and implications for the interpretation of biomonitoring studies. Mutagenesis. 22:69–74.
- Scott D, Galloway SM, Marshall RR, Ishidate JM, Brusick D, Ashby J, Myhr BC. 1991. Genotoxicity under extreme culture conditions: a report from ICPEMC task group 9. Mutat Res Genet Toxicol Environ Mutagen. 257:147–205.
- Sivikova K, Dianovsky J. 2006. Cytogenetic effect of technical glyphosate on cultivated bovine peripheral lymphocytes. Int J Hyg Environ Health. 209:15–20.
- Solomon E, Borrow J, Goddard AD. 1991. Chromosome aberrations and cancer. Science. 254:1153–1160.
- Speit G. 2013. Does the recommended lymphocyte cytokinesis-block micronucleus assay for human biomonitoring actually detect DNA damage induced by occupational and environmental exposure to genotoxic chemicals? Mutagenesis. 28:375–380.
- Suresh TP. 1994. Genetic toxicology – in vivo mammalian bone marrow cytogenetic test – chromosomal analysis [Unpublished Regulatory Study] (Report Identification Number: TOXI:890-MUTCH. AB).
- Suter GWII, Cormier SM. 2011. Why and how to combine evidence in environmental assessments: weighing evidence and building cases. Sci Total Environ. 409:1406–1417.
- Tenorio NM, Ribeiro DA, Alvarenga TA, Fracalossi ACC, Carlin V, Hirotsu C, Tufik S, Andersen ML. 2013. The influence of sleep deprivation and obesity on DNA damage in female Zucker rats. Clinics (Sao Paulo). 68:385–389.
- Terasawa M, Shinohara A, Shinohara M. 2014. Double-strand break repair-adox: restoration of suppressed double-strand break repair during mitosis induces genomic instability. Cancer Sci. 105:1519–1525.
- US EPA. 1993. RED facts: glyphosate. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Prevention, Pesticides, and Toxic Substances (EPA 738-F-93-011). Available from: http://archive.epa.gov/pesticides/reregistration/web/pdf/0178fact.pdf
- US EPA. 2013. Glyphosate pesticide tolerances; Final rule (40 CFR Part 180) [EPA-HQ-OPP-2012-0132; FRL-9384-3]. Fed Regist (US). 78:25396-25401. Available from: http://www.regulations.gov/#%21documentDetail;D=EPA-HQ-OPP-2012-0132-0009.
- US FDA. 2006. Guidance for industry and review staff: recommended approaches to integration of genetic toxicology study results. Rockville (MD): U.S. Food and Drug Administration (US FDA), Center for Drug Evaluation and Research (CDER). Available from: http://www.fda.gov/downloads/Drugs/…/Guidances/ucm079257.pdf.
- Walmsley RM, Billinton N. 2011. How accurate is in vitro prediction of carcinogenicity? Br J Pharmacol. 162:1250–1258.
- Waters MD, Bergman HB, Nesnow S, 1988. The genetic toxicology of Gene-Tox non-carcinogens. Mutat Res. 205:139–182.
- WHO. 1994. Glyphosate. Geneva: World Health Organization (WHO)/International Program on Chemical Safety (IPCS)/United Nations Environment Program (UNEP) (Environmental Health Criteria, no. 159). Available from: http://www.inchem.org/documents/ehc/ehc/ehc159.htm.
- Williams GM, Kroes R, Munro IC. 2000. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol. 31:117–165.
- Wu LL, Chiou C-C, Chang P-Y, Wu JT. 2004. Urinary 8-ohdg: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 339:1–9.

