 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Formaldehyde is one of the most comprehensively studied chemicals, with over 30 years of research focused on understanding the development of cancer following inhalation. The causal conclusions regarding the potential for leukemia are largely based on the epidemiological literature, with little consideration of cancer bioassays, dosimetry studies, and mechanistic research, which challenge the biological plausibility of the disease. Recent reanalyzes of the epidemiological literature have also raised significant questions related to the purported associations between formaldehyde and leukemia. Because of this, considerable scientific debate and uncertainty remain on whether there is a causal association between formaldehyde inhalation exposure and leukemia. Further complexity in evaluating this association is related to the endogenous production of formaldehyde. Multiple modes of action (MOA) have been postulated for the development of leukemia following formaldehyde inhalation that includes unsupported hypotheses of direct or indirect toxicity to the target cell population. Herein, the available evidence relevant to evaluating the postulated MOAs for leukemia following formaldehyde inhalation exposure is organized in the IPCS MOA Framework. The integration of all the available evidence clearly highlights the limited amount of data that support any of the postulated MOAs and demonstrates a significant amount of research supporting the null hypothesis that there is no causal association between formaldehyde inhalation exposure and leukemia. These analyses result in a lack of confidence in any of the postulated MOAs, increasing confidence in the conclusion that there is a lack of biological plausibility for a causal association between formaldehyde inhalation exposure and leukemia.
Introduction
Leukemia is a cancer of blood-forming cells, particularly myeloid or lymphoid stem cells (American Cancer Society Citation2018). While it has been proposed that inhalation exposure to formaldehyde may be associated with an increased risk of leukemia, specifically acute myeloid leukemia (AML), in humans (International Agency for Research on Cancer Citation2006; Zhang et al. Citation2009; National Toxicology Program Citation2016; Zhang Citation2018), any mechanisms by which formaldehyde exposure could result in the key events leading to the development of leukemia remain elusive. For example, a study that reported hematological toxicity in workers exposed to formaldehyde (Zhang et al. Citation2010) was subsequently shown to have serious methodological flaws (Gentry et al. Citation2013). The National Academy of Sciences (National Research Council Citation2011) acknowledged that the causal conclusions regarding the potential for leukemia are largely based on a few selected studies in the epidemiological literature, with little consideration of other streams of evidence such as animal bioassays, dosimetry, or mode of action (MOA) studies. Recent reanalysis of the two key epidemiological studies that have been cited as supporting a causal association between formaldehyde and leukemia have also raised significant methodological and/or analytical concerns (Checkoway et al. Citation2015; Mundt et al. Citation2017). As such, considerable uncertainty remains as to whether there is a causal association between formaldehyde exposure and leukemia. Moreover, decades of dosimetry research indicate that inhalation of formaldehyde does not result in the elevation of the levels of formaldehyde naturally present in the blood. Therefore, in drawing any conclusions regarding the potential for leukemia or developing any postulated MOA for the development of leukemia following formaldehyde exposure, it is important to understand the relative dosimetry of exogenous and endogenous formaldehyde exposures.
Formaldehyde is considered a probable human carcinogen by some regulatory agencies, such as the European Chemicals Agency (European Chemical Agency Citation2019a) and the United States Environmental Protection Agency’s (USEPA) Integrated Risk Information System (IRIS) (United States Environmental Protection Agency Citation1989), based on the association between formaldehyde inhalation exposure and the incidence of nasal cancers observed in rats (Kerns et al. Citation1983). Some non-regulatory agencies, such as National Toxicology Program (Citation2016) and International Agency for Research on Cancer (Citation2012) have also concluded that results from occupational studies (Hauptmann et al. Citation2003, Citation2004; Beane-Freeman et al. Citation2009) support an association between formaldehyde exposure and leukemia. However, the evidence for leukemia following inhalation exposure to formaldehyde has been shown to be inconsistent or even lacking (Marsh and Youk Citation2005; Rhomberg et al. Citation2011; Checkoway et al. Citation2015, Citation2019). In addition, the proposed association between formaldehyde exposure and leukemia appears to lack the biologically plausible mechanistic evidence needed to support causation. As part of the recently published “Application of Systematic Review in the Toxic Substances Control Act (TSCA) Risk Evaluations” (United States Environmental Protection Agency Citation2018), the USEPA’s Office of Pollution and Prevention of Toxics (OPPT) recommends prioritizing the evaluation of mechanistic evidence, rather than evaluating all of the identified evidence upfront, in order to conduct a focused review of those mechanistic studies that are most relevant to the hazards under evaluation. To date, four MOAs have been postulated for the development of leukemia following formaldehyde inhalation (Zhang et al. Citation2009, Citation2010; Zhang Citation2018), each involving direct, DNA-reactive mutagenic damage to the target hematopoietic stem cells (HSCs), hematopoietic progenitor cells (HPCs) or pluripotent stem cells to initiate leukemic stem cells (LSCs). Common to each of the four postulated MOAs is the ability of formaldehyde to produce direct DNA-reactive mutagenic damage. Formaldehyde in vitro has clearly been shown to have genotoxic potential (e.g. SCEs, micronuclei) in all systems tested ranging from plasmids and bacteria to mammalian cell cultures; however, differences have been reported in the genotoxicity literature for in vivo systems (United States Environmental Protection Agency Citation2010; Albertini and Kaden Citation2017). Notably, studies that have attempted to measure genotoxicity (micronuclei) in human volunteers exposed to formaldehyde by inhalation in controlled settings reported no changes in genotoxic endpoints in buccal cells (Speit et al. Citation2007) or peripheral blood cells and nasal epithelial cells (Zeller, Neuss, et al. Citation2011).
The purpose of this evaluation was to critically evaluate the plausibility of inhaled formaldehyde causing leukemia. To this end, this analysis focuses on currently postulated MOAs for leukemia following inhalation exposure to formaldehyde and the research for formaldehyde that is relevant to the postulated key events in these hypothesized MOAs using the World Health Organization (WHO)/International Programme on Chemical Safety (IPCS) MOA framework (Meek et al. Citation2014). The framework established by the IPCS to aid in the evaluation of MOA and human relevance for both cancer and noncancer endpoints (Boobis et al. Citation2006, Bosetti et al. Citation2008; Meek et al. Citation2014), is not designed to determine “how much information is enough” to support an MOA or human relevance for an outcome but to provide guidance for considering the weight of evidence available for each hypothesized MOA. Using the IPCS framework (Boobis et al. Citation2006, Bosetti et al. Citation2008; Meek et al. Citation2014), each proposed MOA for the association of exposure to exogenous formaldehyde with increased risk of leukemia in humans was analyzed using a weight of evidence approach based on modified Bradford Hill considerations for causality (Hill Citation1965). Since comprehensive review and determination of study quality and relevance have become increasingly important in the evidence integration process (National Research Council Citation2011; National Toxicology Program Citation2015; United States Environmental Protection Agency Citation2018), the evaluation of the quality of the mechanistic studies and their relevance to the postulated MOAs was also conducted using the latest guidance developed as part of the TSCA program (United States Environmental Protection Agency Citation2018) and the European Commission’s Scientific Committee on Emerging and Newly Identified Health Risks (Scientific Committee on Emerging and newly Identified Health Risks Citation2012). United States Environmental Protection Agency (Citation2018) has developed a pre-determined set of criteria to guide the data evaluation process, which has been implemented in this evaluation to account for the study quality of the individual studies relied upon to support the postulated MOAs for leukemia. SCENIHR has developed criteria to assess the relevance of data to the postulated problem formulation which have been incorporated into a web-based reporting and evaluation resource, Science in Risk Assessment and Policy (SciRAP).
Methods
Problem formulation
The initial step in the evaluation included the development of the problem formulation or the establishment of clearly defined objectives for the assessment consistent with publicly available guidelines (National Toxicology Program Citation2015; United States Environmental Protection Agency Citation2018). This step must be completed prior to the initiation of the evaluation in order to ensure a well-planned and conducted literature search. The problem formulation step consisted of an initial broad review of the available literature, including toxicological reviews developed by authoritative or regulatory agencies, as well as recent comprehensive review articles identified in the published scientific literature or on websites for authoritative bodies.
The initial broad review resulted in the identification of four postulated MOAs for leukemia (United States Environmental Protection Agency Citation2010; Zhang Citation2018):
MOA 1: Initiation of leukemia by direct DNA damage to hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) in bone marrow
MOA 2: Direct induction of mutations or toxicity to circulating HSCs and HPCs in the blood
MOA 3: Direct DNA damage or toxicity to pluripotent nasal/oral cells
MOA 4: Direct induction of mutations or toxicity to HSCs and HPCs in the lungs
In addition, other recently published formaldehyde review articles highlighted significant issues that should be considered when assessing the postulated MOAs for leukemia associated with formaldehyde exposure (Heck and Casanova Citation2004; Swenberg et al. Citation2011, Citation2013; Albertini and Kaden Citation2017; Mundt et al. Citation2017, Citation2018). In two of the postulated MOAs for leukemia (MOA 1 and MOA 2), it is assumed that inhaled formaldehyde can be absorbed and systemically distributed where it can cause direct DNA damage or toxicity resulting in indirect damage to HSCs and/or HPCs in the blood or bone marrow that can lead to an initiated leukemia stem cell. Important considerations include the dosimetry of inhaled formaldehyde (i.e. ability of inhaled formaldehyde to pass into the systemic circulation) and the level of endogenous formaldehyde normally present in tissues.
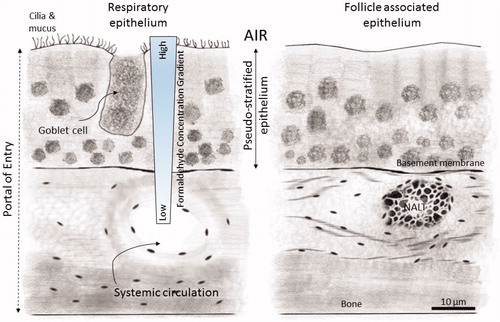
The remaining postulated MOAs (3 and 4) assume cells within the nasal tissue or lung tissue can be mutated or through toxicity be transformed into an initiated leukemia stem cell and transported to the bone marrow and replenish blood stem cells in the bone marrow. Currently, the body of evidence clearly demonstrates inhaled formaldehyde does not migrate past the portal of entry (Casanova-Schmitz, Starr, et al. Citation1984; Moeller et al. Citation2011; Swenberg et al. Citation2011, Citation2013; Edrissi et al. Citation2013; Yu et al. Citation2015; Leng et al. Citation2019). Formaldehyde is a highly reactive gas that forms reversible products with nucleophilic sites or is rapidly metabolized by enzymes at the portal of entry (i.e. upper respiratory tract) inactivating it. Inhaled formaldehyde contacts the upper respiratory tract through nose breathing and the tracheobronchial airways during mouth breathing (National Academy of Sciences Citation2014). The respiratory lining can be divided into three compartments (1) the mucous layer, (2) the epithelial layer, and (3) the submucosal layer (Franks Citation2005). The airway epithelium is metabolically active and, combined with the other compartments, serves as an effective clearance mechanism for formaldehyde, as well as blocks absorption into the blood, preventing systemic distribution (National Academy of Sciences Citation2014). , reproduced from the National Research Council (Citation2011), illustrates the multiple barriers to systemic distribution of inhaled formaldehyde in the respiratory tract tissues. A concentration gradient for inhaled formaldehyde exists with concentrations at the tissue layer closest to the portal of entry being higher and concentrations in deeper layers of the tissue being lower. The gradient is the result of direct binding of formaldehyde with tissue substrates or loss by enzymatically catalyzed metabolism. Initially, inhaled formaldehyde reversibly reacts with water in the mucus layer of the epithelium and forms formaldehyde acetal, the hydrated form of formaldehyde or formaldehyde hydrate. Rapid protein binding, such as to albumin in the mucus also acts as an additional barrier to systemic absorption. Formaldehyde that has not been bound to mucus proteins and not removed through ciliary transport and ingestion of mucus, diffuses through the mucus layer and into the epithelium where it is removed by either enzymatically catalyzed metabolism or bound through reversible nonenzymatic reactions with glutathione and covalent binding to various other nucleophiles.
Figure 1. Representation of the reaction of inhaled formaldehyde in mammalian nasal epithelium which rapidly reacts with macromolecules in the tissue and the albumin in the mucus that lines the respiratory epithelium resulting in a steep concentration gradient. After crossing the basement membrane, formaldehyde can react further with macromolecules in the submucosal layer or reach the systemic circulation. The nasal-associated lymphoid tissue (NALT) that is generally present near the ethmoid turbinates on either side of the nasal septum and near the ventral nasopharyngeal duct is one putative site of formaldehyde interactions with lymphoid tissues, but direct evidence that supports this hypothesis is lacking. (Figure reproduced from National Research Council (Citation2011)).
Formaldehyde is also produced endogenously from normal cellular metabolism and present in all human tissues (Swenberg et al. Citation2011, Citation2013; Schroeter et al. Citation2014; Albertini and Kaden Citation2017). Formaldehyde (as free formaldehyde and loosely bound formaldehyde) occurs naturally in cells throughout the body. In blood, the pre-exposure formaldehyde concentration in humans was at an average concentration of 2.61 ± 0.14 µg/g of whole blood (Heck et al. Citation1985). Similar blood concentrations (2.24 ± 0.07 µg/g) were observed in rats pre-exposure to inhaled formaldehyde. Following exposure to inhaled formaldehyde at concentrations of 14.4 ppmFootnote1 for 2 h or 1.9 ppm (2.3 mg/m3) for 40 min in rats and humans, respectively, Heck et al. (Citation1985) found no increase in blood formaldehyde concentrations. These results have been confirmed with more recent biomarker work in multiple species demonstrating that formaldehyde measured systemically is from endogenous sources (Lu et al. Citation2010; Moeller et al. Citation2011; Swenberg et al. Citation2011). Therefore, when assessing the postulated MOAs for inhaled formaldehyde to cause leukemia the data to support each key event must be based on exogenous formaldehyde exposure and steps should be taken to ensure conclusions are not confounded by endogenously produced formaldehyde (Swenberg et al. Citation2011; Farland et al. Citation2019).
Based on these considerations, the problem formulation review questions were developed keeping in mind this MOA assessment does not include an analysis of the potential for oral exposures or endogenously produced formaldehyde to cause leukemia and is limited to evaluation of the potential for inhaled formaldehyde to cause leukemia. Based on the 4 postulated MOAs, an understanding that inhaled formaldehyde does not diffuse past the portal of entry, and recognizing that formaldehyde is present in tissues from endogenous sources, the problem formulation review questions presented in were developed to be consistent with the IPCS framework.
Table 1. Problem formulation review questions.
Literature search and data screening
The initial broad literature search provided valuable information in understanding the postulated MOAs for leukemia reported in the literature and the studies cited to support the postulated key events in each hypothesized MOA. Since formaldehyde has a large database of literature and this literature has been previously critically reviewed by multiple authoritative bodies and regulatory agencies (e.g. World Health Organization Citation2010; Nielsen et al. Citation2017; European Chemical Agency Citation2019a, Citation2019b, European Chemical Agency Citation2019c), an initial assessment was performed to understand the available primary literature relied upon in comprehensive published reviews to support the postulated MOAs for leukemia. The reviews identified during the problem formulation step from authoritative bodies, regulatory agencies, and in the peer-reviewed literature were critically evaluated to determine what primary literature was cited in support of the four postulated MOAs for leukemia. Additionally, to ensure all recent relevant literature was considered in this assessment, a literature search was performed based on the problem formulation review questions presented in to identify any additional studies published since the latest comprehensive review. The literature search strategy was developed to identify peer-reviewed literature that addressed the key events in the postulated MOAs for leukemia associated with inhalation exposure to formaldehyde. According to United States Environmental Protection Agency (Citation2018), data should be collected under a defined literature search strategy developed based on the needs of the different disciplines supporting the risk evaluation and include strategies for searching and identifying relevant data published in public databases, as well as other sources containing unpublished or published data. United States Environmental Protection Agency (Citation2018) states that literature search results from recent assessments and systematic reviews, such as those conducted by USEPA Integrated Risk Information System (IRIS) can be relied upon as a starting point to identify relevant data when they are available. Therefore, the literature search focused on the postulated MOAs for leukemia and was limited to documents published after the most recent comprehensive literature review conducted as part of the draft United States Environmental Protection Agency’s (2010) IRIS Toxicological Review of Formaldehyde – Inhalation Assessment. In addition, the most recent ECHA Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) Registration Dossier for formaldehyde (European Chemical Agency Citation2019a) was reviewed for completeness of this literature search. Literature searches were conducted using the National Library of Medicine’s PubMed and TOXLINE search engines with the literature search strings provided in . Searching was limited to articles published in the English language between 1 January 2010 and 17 October 2018 and updated on 14 February 2019. However, the primary literature considered for this evaluation was not limited to only the results of the literature search, but also included primary literature cited in the comprehensive reviews from the initial broad review of the available literature, as well as, primary data cited as supporting evidence in the postulated MOAs.
Table 2. Literature search strings*.
This targeted systematic review was also informed by the development of study inclusion/exclusion criteria used to identify the highest quality data for this MOA assessment. Using the problem formulation review questions () as a guide, study eligibility criteria were determined in the form of PECO statements which stands for Population, Exposure, Comparator, and Outcome. Using the PECO framework, criteria about those characteristics in each publication that should be present in order to be eligible for inclusion in the evaluation were defined. An initial screen of the titles and abstracts identified through literature searching was conducted using inclusion and exclusion criteria based on the expected exposed population, the expected exposure route (i.e. inhalation), and the postulated outcome (i.e. leukemia). Studies were obtained for a fuller, detailed review if, based on a review of the title and/or abstract, the following inclusion criteria were met, or if there was not enough information in the title or abstract to determine the following:
Exposure was to formaldehyde only
In vivo exposure was via the inhalation route
The study was conducted in a preferred test species for the testing guideline being used; or conventional mammalian species of commonly used laboratory strains (e.g. rats, mice, dogs, rabbits, guinea pigs, nonhuman primates) or humans
In vitro studies were conducted in bacterial cells or primary cell lines.
A study was excluded from being considered for review if, based on a review of the title and/or abstract, the following could be determined:
Exposure was not to formaldehyde only (e.g. formalin containing methanol)
The route of exposure for in vivo studies was not by the inhalation route
For in vitro studies, the study was conducted in a cell line that was not bacterial or not a primary animal cell line (e.g. plant cell line) or was a cancer cell line or immortalized cell line.
The study was not in English and no English translation of a study could be obtained.
The study was not a toxicity or mechanistic study (e.g. the goal of the study was to describe testing methodology).
Several review papers were identified in the literature search and each was reviewed to ensure that the primary references cited in the reviews were also identified in our literature searches and considered for screening.
Study quality and relevance assessment
The titles and abstracts of the articles identified in the literature search were screened against the inclusion/exclusion criteria. Each article was screened by two individual reviewers and conflicts were resolved by consensus or consultation with a third reviewer. The articles determined to be relevant based on the initial screening of titles and abstracts were obtained and underwent a full-text review to determine relevance based on the inclusion/exclusion criteria and the problem formulation questions. In some cases, studies were identified based on title and abstract screening that fit the inclusion criteria; however, after reviewing the full text of the primary study it was determined that the objective and results of the study were not pertinent to the problem formulation (i.e. the data reported in the study was not related to the potential for leukemia or the key events postulated in the MOAs for leukemia following formaldehyde inhalation exposure). Because the data reported in these studies did not contribute to the MOA assessment, these studies were excluded following full-text review and were not included in the study quality evaluation. The pertinent data from each included study was extracted into data tables. This included data regarding the test species (species, strain, and sex), doses administered, and study outcome and results.
Each study considered for the MOA assessment was then evaluated for study quality using criteria recommended under the TSCA program as outlined in United States Environmental Protection Agency (Citation2018). The strategy for assessing study quality recommended by United States Environmental Protection Agency (Citation2018) uses a structured framework with predefined criteria for each type of data source. United States Environmental Protection Agency (Citation2018) developed a numerical scoring system that can be used to assess overall study quality. The structure of the scoring system is composed of evaluation domains, metrics, and criteria. The domains represent general categories of attributes evaluated in each study (e.g. test substance, test conditions, reliability). Within each domain is a unique set of metrics or sub-categories used to assess specific aspects of the study. Each metric specifies criteria for assessing confidence that, along with professional judgment, guide the identification of study strengths and limitations.
For this assessment, based on information collected in the initial broad literature review and the answers to the problem formulation questions, the study quality evaluation included consideration of methods that allow differentiation between endogenous and exogenous formaldehyde, if this differentiation was important in interpreting the results of the study. Therefore, mechanistic studies focused on key events suggested being part of the postulated MOAs for leukemia that differentiate between endogenous and exogenous formaldehyde were deemed more reliable or “relevant” than those that could not differentiate between the two. In vivo studies in uncompromised animals were also deemed more reliable than those performed with compromised systems (e.g. rats receiving radiation to destroy bone marrow; lung transplant models in mice with thrombocytopenia). In addition, studies in which exposure was to gaseous formaldehyde were deemed more reliable than studies relying upon formalin for formaldehyde generation due to the possible presence of impurities, such as methanol, that can be systemically distributed before being metabolized to formaldehyde. Based on the strengths, limitations, and deficiencies of each data source, a confidence level score of 1 (high confidence), 2 (medium confidence), 3 (low confidence), or 4 (unacceptable), as defined in detail in , was applied for each individual metric.
Table 3. Definitions of confidence levels and corresponding scores for individual study components.
Following United States Environmental Protection Agency (Citation2018) recommendations, when evaluating animal, in vitro, and human exposure studies, each metric, except for those deemed unacceptable (score of 4), is assigned a weighting factor of 1 or 2, with the higher weighting factor of 2 given to metrics deemed critical for the evaluation. United States Environmental Protection Agency (Citation2018) assigned weighting factors to each individual metric using “…expert judgement to determine the importance of a particular metric relative to others.” Metrics deemed critical for the evaluation were assigned a higher weighting factor (2). United States Environmental Protection Agency (Citation2018) judged metrics related to applicability and temporal representativeness to be the most critical and those metrics received a weighting factor of 2. Therefore, the score for each metric is the metric score multiplied by the weighting factor for the metric. The range of weighted scores for each metric was calculated by multiplying the range of metric scores (1–3) by the weighting factor for the metric. The overall quality scores for each data source of high, medium, or low, defined as shown in are calculated as follows:
Table 4. Definition of overall study quality levels and corresponding study quality scores.
Any metric scored as not applicable was not included in the overall score; therefore, the sum of weighting factors and the sum of the weighted scores may differ if some metrics are not scored (not applicable). It should also be noted that no overall score was calculated for studies that received an “unacceptable” rating (score of 4) for any metric. In these cases, the study was considered unacceptable and was not used in the assessment.
United States Environmental Protection Agency (Citation2018) states that additional criteria can be incorporated into the assessment to address chemical-specific considerations to assist in determining if the data being considered are relevant to the problem formulation. While issues regarding study relevance could have simply been noted in the TSCA study quality scoring as a comment, a more systematic approach was implemented for this evaluation to ensure each study was consistently evaluated for relevance based on the problem formulation. SciRAP, a web-based reporting and evaluation resource, was utilized along with the TSCA quality framework to evaluate the relevance of the animal in vivo and in vitro toxicity studies to the problem formulation questions. Each study considered for the MOA assessment was assessed and determined to be either directly relevant, indirectly relevant, or not relevant to the problem formulation questions using specific criteria developed by the European Commission’s Scientific Committee on Emerging and newly Identified Health Risks (Citation2012).
The SciRAP criterion used to evaluate relevance and guidance for decisions regarding relevance are presented in . In vivo animal studies were evaluated for relevancy to the problem formulation questions based on 5 criteria including (1) the identity of the tested substance, (2) the animal model used, (3) endpoint studied, (4) route of administration, and (5) administered dose levels. In vitro studies were also evaluated for relevancy to the problem formulation questions based on 4 criteria including (1) the identity of the tested substance, (2) the test system, (3) endpoint studied, and (4) concentrations tested. Studies conducted in human populations or human cell lines or tissue were all considered to be directly relevant. If the majority of the criteria for an in vivo or in vitro study (i.e. 3 or more out of 5) were determined to be directly relevant to the problem formulation for a study, the study was considered directly relevant to the problem formulation. Likewise, if the majority of the criteria were considered indirectly relevant, the study was considered indirectly relevant to the problem formulation. If one criterion for study relevancy was found to be not relevant, then the entire study was considered to be not relevant to the problem formulation. For in vitro studies, if there appeared to be a balanced conclusion for study relevancy, the specific relevancy questions were discussed by two independent reviewers and a decision for this study was made based on the relevancy to informing the problem formulation questions.
Table 5. SciRAP criterion for evaluating study relevance.
It is important to note that mechanistic evidence to support this MOA analysis came from human and animal in vivo, and in vitro toxicity studies. These data were used to provide support for biological plausibility, as well as any potential differences in tissue sensitivity, species, or gender factors. Examples of the study quality scoring sheets for each of these study types as outlined in United States Environmental Protection Agency (Citation2018) are presented in the Supplemental materials.
MOA analysis
For this evaluation, each postulated MOA for leukemia was evaluated within the World Health Organization (WHO) International Programme on Chemical Safety (IPCS) framework for the assessment of carcinogenic MOAs (Boobis et al. Citation2006; Meek et al. Citation2014). The MOA analysis takes into account study quality, using a weight of evidence approach based on the Bradford Hill considerations for causality (Hill Citation1965). The key events comprising each postulated MOA were identified in the literature and the evidence available to support each postulated key event was evaluated for concordance of dose-response relationships based on the modified Bradford Hill considerations, potential temporal associations of key events, strength, consistency, and specificity of key events, biological plausibility of the MOAs and uncertainties associated with each of the postulated MOAs. It is important to point out that the IPCS framework based on modified Bradford Hill considerations is not a checklist of criteria, but an analytical approach designed to bring transparency to the analysis of hypothesized MOAs and promote confidence through the use of defined procedures that promotes transparency and highlights potential inconsistencies and uncertainties in the available data (Boobis et al. Citation2006).
The dose-response and temporal relationship for each key event were compared with one another, as well as with the outcome to determine if the key events were observed at doses below or similar to those associated with the outcome and that they occur in the expected order. The consistency and specificity of the weight of evidence linking the key events with the outcome were also evaluated. Finally, the pattern of effects across species/strain/systems was evaluated to determine if they were consistent with the hypothesized MOA for leukemia and whether the hypothesized MOA was comparable with what is known about the biology and etiology of leukemias in order to determine biological plausibility.
Results
Problem formulation and considerations specific to formaldehyde exposure literature search and screening results
Results of the critical review of comprehensive reviews from the published literature and from websites for authoritative bodies (United States Environmental Protection Agency Citation2010; Albertini and Kaden Citation2017; Mundt et al. Citation2017, Citation2018; Zhang Citation2018) for primary literature specific to the postulated MOAs for leukemia following exposure to formaldehyde yielded a total of 78 primary studies that were obtained and critically reviewed in this assessment. Of these 78 studies, 31 provided formaldehyde-specific data to inform the MOA evaluations regarding dose-response, temporality, strength and consistency of the key events or biological plausibility specific to a key event in the postulated MOAs for leukemia.
Results of the literature searches conducted on 17 October 2018 and updated on 14 February 2019 using the PubMed and TOXLINE search engines with the literature search strings provided in identified 737 potentially relevant citations. The results of the literature searches were stored in an EndNote database. Titles and abstracts were then screened against the inclusion/exclusion criteria presented in Section 2.2 and 109 articles were determined to be potentially relevant based on title and abstract screen. Because this evaluation is intended to be a focused comprehensive review of the literature available to support the key events in the postulated MOAs for leukemia following inhalation exposure to formaldehyde, the 109 remaining articles were reviewed to determine if they were cited in the identified comprehensive reviews (United States Environmental Protection Agency Citation2010; Albertini and Kaden Citation2017; Mundt et al. Citation2017, Citation2018; Nielsen et al. Citation2017; Zhang Citation2018; European Chemical Agency Citation2019a). Following this assessment, 25 studies published since 2010 and not cited in the comprehensive reviews were identified as potentially relevant and obtained for critical review. After a full study review, only 4 of the 25 studies published since 2010 and that was not originally identified in the comprehensive reviews were relevant to the MOA analysis and were added to the list of relevant studies identified from comprehensive reviews and relied upon for this assessment.
Study quality assessment results
Each of the primary studies considered for the MOA analysis was scored for study quality based on the TSCA guidelines (United States Environmental Protection Agency Citation2018) and study relevance to the problem formulation questions () based on the SciRAP criterion. The quality scoring and relevance results for each study are presented in the Supplemental materials. A summary of the final scoring for quality and relevance is presented in . includes all of the studies considered in the MOA assessment that received a study quality score between 1 and 3 (i.e. of minimally acceptable quality). Thirteen studies reviewed for the MOA assessment received a study quality score of 4 and were considered unacceptable for inclusion in the MOA assessment. These studies are presented in along with an explanation of the unacceptable study quality results.
Table 6. Study quality and study relevance scoring results.
Table 7. List of unacceptable studies determined during study quality evaluation.
MOA analysis
Identification of the postulated MOAs for leukemia
Four postulated MOAs for the development of leukemia following formaldehyde inhalation exposure were identified in the published or publicly available literature (Zhang et al. Citation2009, Citation2010; United States Environmental Protection Agency Citation2010; Zhang Citation2018). These four postulated MOAs involve the initiation of leukemia by (1) direct damage to the stem cells in the bone marrow, (2) direct damage to HSCs or HPCs circulating in the peripheral blood, (3) direct damage to primitive pluripotent stem cells present within the nasal or oral tissue or (4) direct damage to the HSCs/HPCs present within the lung tissue. For 3 of the 4 postulated MOAs, HSCs/HPCs or other stems cells (e.g. nasal or oral) would be exposed to formaldehyde and direct DNA damage would occur outside the target tissue (bone marrow) or macromolecular binding may occur leading to toxicity or genetic damage that could result in an initiated leukemia stem cell that would then travel to the bone marrow and replenish blood stem cells in the bone marrow.
A key event common to all of the four postulated MOAs is the ability of formaldehyde to produce direct DNA damage. Formaldehyde’s intrinsic mutagenic/genotoxic profile has been extensively reviewed (Bolt et al. Citation2003; Liteplo and Meek Citation2003; International Agency for Research on Cancer Citation2006; United States Environmental Protection Agency Citation2010; Albertini and Kaden Citation2017) and formaldehyde has clearly been shown to be positive for mutations in in vitro systems tested ranging from plasmids and bacteria to mammalian cell cultures where formaldehyde has the ability to reach genetic material in these systems with direct application to the culture medium. However, a critical review of the relevant data regarding the potential mutagenicity/genotoxicity of formaldehyde following in vivo inhalation exposure to animals or humans indicates that there is no convincing evidence that exogenous exposures to formaldehyde induce mutations as a direct DNA-reactive effect at sites distant from the portal-of-entry tissue (Albertini and Kaden Citation2017). In fact, other than detection of exogenous formaldehyde–DNA adducts similar to ever-present endogenous formaldehyde–DNA adducts, studies conducted to evaluate genotoxic damage (mutation and clastogenic) in the portal of entry have failed to detect positive signs of genotoxicity (e.g. Meng et al. Citation2010; Speit et al. Citation2011) (see Thompson et al. CitationForthcoming). In addition, no genotoxicity (specifically, micronuclei) in peripheral blood cells and nasal epithelial cells (Zeller, Neuss, et al. Citation2011) or in buccal cells (Speit et al. Citation2007) was reported in human volunteers exposed to formaldehyde by inhalation in controlled settings. Other key events noted in the postulated MOAs for leukemia involve the systemic delivery and migration of formaldehyde through the blood, macromolecular binding to hematopoietic stem cells and progenitor blood cells in the bone marrow and blood, the release of damaged stem cells into the systemic circulation, and the incorporation of initiated stem cells into the bone marrow. These reflect hypotheses, most if not all of which currently lack substantiating experimental evidence.
presents an overview of the key events of each postulated MOA and demonstrates the common key events across postulated MOAs. To simplify this analysis, key events that are substantially similar are grouped, so that a single analysis of each event can be presented (). Several key factors are relevant for multiple postulated MOAs as indicated in , and these are discussed in detail in the following sections. Finally, each postulated MOA is discussed within the framework of the WHO/IPCS framework for the assessment of carcinogenic MOAs.
Table 8. Overview of postulated MOAs.
Analysis of individual events across the four postulated MOAs
The following sections will focus on the individual key events that are relevant for the multiple postulated MOAs as indicated in . Each key event is discussed in detail in the following sections.
Event 1: conversion of formaldehyde to methanediol for transport through the portal of entry
It has been suggested that absorption of formaldehyde through the tissues at the portal of entry is facilitated by conversion to a hydrated form by the addition of water (i.e. methanediol) (Zhang et al. Citation2009; United States Environmental Protection Agency Citation2010; Zhang Citation2018). This suggestion is based solely on information reported by Zhang et al. (Citation2009; Zhang Citation2018) regarding in vitro data from a tissue fixation process reported by Fox et al. (Citation1985) and Walker (Citation1964). Neither Fox et al. (Citation1985) or Walker (Citation1964) were assessed for study quality or relevance to the problem formulation questions because they did not meet our inclusion criteria for this assessment. These studies were focused on investigating formaldehyde as an agent for the fixation of tissues and cultured cells. There was no in vivo exposure to formaldehyde nor were these in vitro studies designed to investigate toxicity or mechanisms of toxicity following formaldehyde exposure. These studies were only included for the current evaluation because they were the only studies cited as part of the postulated MOAs to provide evidence for Event 1 (Zhang et al. Citation2009; United States Environmental Protection Agency Citation2010; Zhang Citation2018). However, if they had been assessed each would have received an unacceptable score based on the fact that each of the studies describes a method for tissue fixation and neither was an in vivo, in vitro, or human toxicity or mechanistic study. In addition, Fox et al. (Citation1985) did not provide necessary test substance information.
Results from a medium quality directly relevant in vitro study in human mucus (Priha et al. Citation1996), as well as results from a high quality and directly relevant in vivo study in rats (Lu et al. Citation2010) indicate neither exogenously administered formaldehyde nor exogenous formaldehyde hydrate move past the portal of entry. In addition, National Research Council (Citation2011) concluded that the available data does not support the hypothesis that the hydration of formaldehyde to methanediol enhances the delivery of exogenous formaldehyde beyond the portal of entry (National Research Council Citation2011). In conclusion, the data presented by Zhang et al. (Citation2009) and Zhang (Citation2018) to support the conversion of formaldehyde to methanediol for transport through the portal of entry, if they would have met the inclusion criteria, would have been of low study quality. The medium to high-quality relevant studies addressing this issue (Priha et al. Citation1996; Lu et al. Citation2010) indicate formaldehyde hydrate or methanediol resulting from exogenous sources does not move past the portal of entry.
Event 2: systemic delivery and transport of formaldehyde through blood
One of the key factors considered across multiple postulated MOAs involves absorption of formaldehyde through the tissues at the portal entry, followed by systemic delivery of formaldehyde through the circulatory system to the bone marrow, producing direct damage to stem and progenitor cells in the bone marrow. However, results of in vitro studies with human mucus have shown that at low concentrations comparable to ambient exposure, free formaldehyde is not expected to pass through the mucus layer to the epithelium of the respiratory tract in an unhydrated form, as it rapidly reacts with amino groups in the chemical components of mucous (Priha et al. Citation1996). What has not been considered in the proposed MOAs is that following inhalation of formaldehyde, any “free” formaldehyde in biological systems would be infinitesimal due to the chemical/physical properties of formaldehyde, and the efficient biochemical mechanisms for homeostatic control of free formaldehyde in tissues. While the dynamic equilibrium between the hydrated (i.e. methanediol) and unhydrated (i.e. formaldehyde) forms of formaldehyde in biological systems is well understood, the proposed MOAs do not consider the impact of the equilibrium constant for formaldehyde and methylene glycol (formaldehyde acetal, methanediol) on the “free” formaldehyde pool. The equilibrium constant is noted to be less than 0.001 (Priha et al. Citation1996), indicating that, in aqueous solutions, less than 0.1% of formaldehyde remains in an unhydrated form (Priha et al. Citation1996).
In addition, methanediol can further interact with glutathione (GSH) to form a thioacetal (S-hydroxymethylglutathione), removing the “pool” of methanediol that would be available to form any “free” formaldehyde. Although there might be some small concentration of “free” formaldehyde, it is also important to consider that other forms of formaldehyde, such as methanediol, predominate. However, there are biochemical mechanisms that efficiently regulate formaldehyde levels, such as its rapid metabolism by aldehyde dehydrogenases to formate (Casanova-Schmitz, David, et al. Citation1984), which is further metabolized to carbon dioxide and water, incorporated into the one-carbon pool, and/or eliminated in the urine as a sodium salt. These data are consistent with results from high quality, directly relevant in vitro and in vivo studies in rats that indicate neither formaldehyde or methanediol move past the portal of entry due to removal by both macromolecular binding and efficient metabolic processes (Priha et al. Citation1996; Lu et al. Citation2010, Citation2011). The National Research Council (National Research Council Citation2011) concluded that while equilibrium dynamics indicate that once in the tissues methanediol constitutes more than 99.9% of the totally free and hydrated formaldehyde, as noted previously, the available data do not support the hypothesis that the hydration of formaldehyde to methanediol in the respiratory tissues enhances the delivery of formaldehyde beyond the portal of entry (National Research Council Citation2011).
Following conversion to methanediol, it has been postulated that formaldehyde is delivered via systemic circulation to the bone marrow. However, in evaluating systemic levels following formaldehyde exposure, it is important to consider that formaldehyde is produced endogenously and is a common intermediate of normal metabolism and a byproduct of oxidative demethylation of histone nucleic acids in RNA and DNA which produces free formaldehyde in the immediate proximity of genetic material and therefore, potentially forming DNA–protein cross-links (Albertini and Kaden Citation2017). Formaldehyde DNA–protein cross-links, as well as mono adducts and DNA–DNA adducts, are a traditional biomarker of formaldehyde inhalation exposure, with the cross-links being proportional to the administered dose of formaldehyde, increasing at 2 ppm with a steep increase occurring at 6 ppm (Casanova-Schmitz, Starr, et al. Citation1984; Casanova and Heck Citation1987; Swenberg et al. Citation2011, Citation2013; Albertini and Kaden Citation2017). These DNA–protein cross-links were reported to occur at the portal of entry only (i.e. the nasal mucosa), but not in the nasal olfactory mucosa or bone marrow. Antibodies to formaldehyde–hemoglobin adducts and formaldehyde–albumin adducts detected in humans exposed to air concentrations of formaldehyde from residential or office buildings or occupationally have been suggested as an indication that formaldehyde can be transported through the blood to sites distal from the portal of entry (United States Environmental Protection Agency Citation2010). However, a review of the studies cited to support this suggestion (Grammer et al. Citation1990, Citation1993; Thrasher et al. Citation1990; Dykewicz et al. Citation1991; Li et al. Citation2007) does not offer conclusive evidence that these adducts were associated with exogenous formaldehyde exposure in exposed human populations.
The studies used to provide evidence to support systemic delivery and transport of formaldehyde through blood (Ye et al. Citation2013; Zhang et al. Citation2013; Wei et al. Citation2017) report increases in macromolecular binding, oxidative stress, and cytotoxicity at sites distant from the portal of entry following formaldehyde exposure. However, none of these studies provide evidence of mutagenicity and none definitively demonstrates whether the macromolecular binding and cellular effects reported at a site distal from the portal of entry were directly related to exogenous formaldehyde exposure because the methodologies used to assess the endpoints did not appropriately characterize whether these effects were associated with exogenous or endogenous formaldehyde exposure. In addition, these studies relied upon formalin to generate formaldehyde concentrations, potentially introducing confounding impurities, such as methanol which is metabolized to formaldehyde, into the studies. Therefore, Ye et al. (Citation2013), Wei et al. (Citation2017), and Zhang et al. (Citation2013) are considered not relevant to the problem formulation question (). At least 12 high quality and directly relevant studies are available in the literature that demonstrate a lack of transport of exogenous formaldehyde beyond the portal of entry ().
Table 9. Summary of available studies associated with key events 2 and 3 of the postulated MOAs.
When considering any endogenously formed chemical, such as formaldehyde, it is important to be able to differentiate the DNA adducts associated with exogenous exposure from those associated with concentrations of the chemical endogenously present in the body. Earlier DNA–protein cross-link and adduct evaluation methods used nonspecific assays, such as the dual-isotope method (Casanova-Schmitz, Starr, et al. Citation1984; Casanova and Heck Citation1987), alkaline elution or filter retention methods (Barker et al. Citation2005), potassium sodium dodecyl sulfate (K-SDS) precipitation method, or the modified comet assay. These methods measure retardation of migration in gel electrophoresis and are all nonspecific methods of DNA–protein cross-link quantification due to insufficient recovery of the product during the analysis or artifact formation (Shaham et al. Citation1997; Albertini and Kaden Citation2017). Others used formalin (a liquid of approximately 30–40% formaldehyde and 10–15% methanol) for the formation of gaseous formaldehyde (Shaham et al. Citation1996, Citation2003; Ye et al. Citation2013; Yu R et al. Citation2015; Albertini and Kaden Citation2017). The use of formalin for the development of gaseous formaldehyde potentially introduces impurities, such as methanol, which is metabolized to formaldehyde and has toxicokinetic and toxicodynamic factors that differ from formaldehyde, in addition to the formaldehyde being generated for exposure. Methanol is readily absorbed and can be rapidly metabolized to formaldehyde in vivo. Following oral exposure of rats to 500 or 2000 mg/kg for 5 days, methanol was enzymatically metabolized to formaldehyde and resulted in the detection of exogenous DNA adducts in multiple tissues, including bone marrow (Lu et al. Citation2012). Therefore, studies using formalin as the test substance rather than formaldehyde are likely to lead to erroneous conclusions regarding the potential for inhaled formaldehyde to be systemically distributed, as also noted by Whalan et al. (Citation2014).
Due to potential methanol co-exposure issues and the nonspecificity of these assays, cross-links measured using these methods could also form from endogenously produced formaldehyde, methanol exposure, or artifacts that could be misinterpreted as resulting from exogenous formaldehyde gas exposure. Some of the observations of adducts or crosslinks beyond the portal of entry in the earlier literature are likely to be erroneous since the studies did not employ differential labeling assays that would distinguish endogenously versus exogenous formaldehyde binding or address covalent versus nonspecific binding or isotope exchange. However, new methods recently available from high quality, directly relevant studies have allowed for the definitive differentiation of exogenous and endogenous specific DNA mono adducts, DNA–DNA adducts, and protein using methods of 13CD2-isotope labeling of hydroxymethyl-dG (HmdG) or hydroxymethyl-dA (HmdA) (in vitro derivative of reversible hydroxy methyl reaction products formed in vivo) (Lu et al. Citation2010, Citation2011; Moeller et al. Citation2011), or N6-formyllysine adducts (Edrissi et al. Citation2013). As mass spectrometry methods have developed, the availability of high quality, directly relevant literature considered in this assessment continue to strengthen the evidence that exogenous formaldehyde does not move past the portal of entry, as demonstrated by the absence of DNA adduct and protein cross-link formation in tissues remote from the portal of entry in multiple species (i.e. rats and non-human primates) following both acute and sub-chronic exposure across a range of air concentrations (0.001 ppm to 15 ppm; ; Speit et al. Citation2009; Lu et al. Citation2010, Citation2011; Moeller et al. Citation2011; Edrissi et al. Citation2013; Kleinnijenhuis et al. Citation2013; Yu R et al. Citation2015; Lai et al. Citation2016; Leng et al. Citation2019).
In conclusion, the only studies identified to support Event 2 (Ye et al. Citation2013; Zhang et al. Citation2013; Wei et al. Citation2017) are considered not relevant to the problem formulation question () because they cannot differentiate between whether or not the observed endpoints are due to endogenous or exogenous exposure to formaldehyde. However, as noted previously, multiple high quality and directly relevant studies are available in the literature in which formaldehyde was administered by inhalation to multiple species up to 15 ppm () that demonstrate a lack of transport of exogenous formaldehyde beyond the portal of entry, based on the absence of DNA adduct or protein cross-link formation in tissues remote from the portal of entry. Therefore, the weight-of-evidence supports the conclusion that inhaled formaldehyde is not systemically distributed into the blood.
Event 3: macromolecular binding to hematopoietic stem cells and progenitor cells in the bone marrow or blood
Two of the four postulated MOAs are built on the assumption that exogenous formaldehyde can be absorbed into the blood or transported to the bone marrow, where macromolecular binding to target cells relevant to leukemia can result in toxicity that leads to the formation of initiated cells that can then impact the bone marrow. The only study that has been cited as evidence to support the hypothesis that exogenous formaldehyde can be transported to the bone marrow is a high-quality study (Ye et al. Citation2013) that is not relevant to the problem formulation questions because the study used formalin for formaldehyde generation which introduces the possible presence of impurities, such as methanol (). In this study, significant dose-dependent increases in DNA–protein cross-links in the bone marrow (cell type not specified), peripheral blood mononuclear cells, liver, spleen, and testes were reported in the exposed mice.
The SDS/K + method used in the Ye et al. (Citation2013) study measures nonspecific protein binding to DNA. The SDS/K + method precipitates proteins and measures the fraction of the total DNA in the sample that is co-precipitated with the protein (Barker et al. Citation2005). There is nothing in this analysis that directly relates the observed effects to formaldehyde. The DNA is bathed in proteins that form covalent bonds with DNA under the influence of a multitude of exogenous and endogenous agents (Tretyakova et al. Citation2015). Included among these cross-linking agents are reactive aldehydes produced by lipid peroxidation, a process that was demonstrated in the Ye et al. (Citation2013) study by the increases in malondialdehyde (MDA) (which itself induces DNA–protein cross-links) following inhalation exposures to formaldehyde in mice.
While Ye et al. (Citation2013) reported increases in DNA–protein cross-links in the bone marrow, the authors did not report the specific cells in which the DNA–protein cross-links occurred. Cell types that would have to be affected to produce leukemia include HSCs or HPCs; therefore, this introduces uncertainty in the evidence from this study. Furthermore, this is the only study providing this type of evidence in a single species (mice), and the methods used did not appear to differentiate between endogenous and exogenous adducts, or the confounding effects of administration of formalin, a mixture of formaldehyde, water, and methanol, which can produce the formation of formaldehyde adducts that are not the result of exposure to exogenous formaldehyde. Since methanol is metabolized to formaldehyde and has much greater systemic distribution potential than inhaled formaldehyde, any systemic binding of formaldehyde observed by Ye et al. (Citation2013) is likely attributable to methanol contained in inhaled formalin being systemically distributed and then metabolized to formaldehyde, rather than inhaled formaldehyde being systemically distributed.
The results of Ye et al. (Citation2013) are also inconsistent with results from multiple studies in rats in which cytogenetic analyses were conducted on the bone marrow (Dallas et al. Citation1992) and blood (Speit et al. Citation2009) following inhalation exposure to a range of formaldehyde concentrations (0.5–15 ppm). These results demonstrate if macromolecular binding were occurring, there is no evidence of genotoxicity in the blood or bone marrow of rats following inhalation exposure to formaldehyde. This is further demonstrated in the discussion of Event 4.
In addition, results from multiple high quality, directly relevant in vivo studies conducted in rats and primates and designed to differentiate between the endogenous and exogenous formaldehyde demonstrate that exogenous adducts were not detectable in the bone marrow of rats exposed to formaldehyde (). Specifically, results have demonstrated that there are no detectable exogenous DNA–protein cross-links or DNA adducts or protein adducts in bone marrow cells following inhalation exposure to exogenous formaldehydes at concentrations ranging from 0.001 to 15 ppm for up to 4 weeks (Casanova-Schmitz, Starr, et al. Citation1984; Casanova and Heck Citation1987; Heck et al. Citation1989; Speit et al. Citation2009; Lu et al. Citation2010, Citation2011, Moeller et al. Citation2011; Edrissi et al. Citation2013; Leng et al. Citation2019).
Concentration-dependent formation of exogenous formaldehyde–DNA adducts was detected in the nasal epithelium of rats exposed to formaldehyde concentrations ranging from 0.7 to 15.2 for 6 h or 6 to 10 ppm for 1 to 5 days; however, no exogenous formaldehyde was detected in any tissues beyond the portal of entry based on the lack of detection of exogenous formaldehyde-specific DNA adducts (; Lu et al. Citation2010, Citation2011; Edrissi et al. Citation2013; Lai et al. Citation2016). Both Lu et al. (Citation2010) and Edrissi et al. (Citation2013) reported protein adducts specific to endogenous, but not exogenous, formaldehyde in tissues distant from the portal of entry including the liver, lung, thymus, bone marrow, and spleen. “In addition, no impact on endogenous product adduct levels was reported.”
In rats exposed to 2 ppm formaldehyde for 28 days, exogenous DNA–protein cross-links were reported in the nasal tissue, but not the bone marrow, liver, or peripheral blood mononuclear cells (Lai et al. Citation2016). Results from multiple high quality, directly relevant studies that measure exogenous formaldehyde and are conducted in non-human primates indicated that at formaldehyde concentrations of 2–6 ppm for 2 days, exogenous DNA–protein cross-links and adducts were found in the nasal tissue, but not the bone marrow (; Moeller et al. Citation2011; Yu et al. Citation2015; Lai et al. Citation2016).
In conclusion, there is evidence from high quality and directly relevant studies in multiple species (i.e. rats and non-human primates) that adequately characterize effects associated with exogenous formaldehyde that indicate that inhaled exogenous formaldehyde does not move past the portal of entry (Casanova-Schmitz, Starr, et al. Citation1984; Casanova and Heck Citation1987; Heck et al. Citation1989; Speit et al. Citation2009; Lu et al. Citation2010, Citation2011, Moeller et al. Citation2011; Edrissi et al. Citation2013; Kleinnijenhuis et al. Citation2013; Yu et al. Citation2015; Lai et al. Citation2016; Leng et al. Citation2019). Further, considering the dosimetry evidence and the potential for blood cells (i.e. HSCs and HPCs) to be affected, inhaled formaldehyde would have to enter the blood vessels to produce cellular damage. While there are no studies available that have sampled for the presence of formaldehyde directly at the interchange of the respiratory tissue and systemic circulation, several high quality, directly relevant studies have analyzed blood samples collected directly from blood vessels (i.e. tail vein, tarsal vein retroorbital venous plexus, and cardiac puncture) following inhalation exposure of formaldehyde in rats and mice (Speit et al. Citation2009; Lu et al. Citation2010; Kleinnijenhuis et al. Citation2013; Ye et al. Citation2013). These studies reported no macromolecular binding of exogenous formaldehyde in the blood, whereas binding of endogenous formaldehyde was detected. Therefore, while some uncertainty still exists, results do not demonstrate the transfer of inhaled formaldehyde to the blood vessels and systemic circulation.
Event 4: formaldehyde induced genotoxicity in hematopoietic stem cells and progenitor blood cells or pluripotent nasal or oral cells
In two of the postulated MOAs, it is suggested that formaldehyde could directly induce DNA damage and chromosome aberrations in HSCs and HPCs whether in the blood or bone marrow. The remaining two postulated MOAs suggest that formaldehyde can cause genotoxicity to cells in the portal of entry tissues, remote from the circulation, that then enter the circulation, reach the bone marrow and repopulate the bone marrow with altered or transformed cells.
MOA 2, which is based on the assumption of direct or indirect mutagenicity to circulating HSCs/HPCs, is suggested to be supported by the results from three in vivo animal studies (Ye et al. Citation2013; Zhang et al. Citation2013; Wei et al. Citation2017) that were considered to be not relevant to the problem formulation (). As noted previously, these studies involve the generation of formaldehyde using formalin, a mixture containing methanol, which calls into question their relevance for understanding the potential for systemic effects from inhalation of exogenous formaldehyde. Methanol is systemically distributed and then metabolized to formaldehyde, which is different from inhaled formaldehyde being systemically distributed. In the studies relying upon formalin for the generation of formaldehyde, significant increases in biomarkers of oxidative stress (i.e. reactive oxygen species (ROS) and malondialdehyde (MDA)) and endpoints, as defined by the study authors as genotoxic (i.e. DNA–protein cross-link formation and results from the CFU-GM assay) in the bone marrow, blood, and myelofibrosis were reported in mice. Animals were exposed to formaldehyde via inhalation at concentrations ranging from 0.5 to 3 mg/m3 for 8 h/day for 5 or 7 days; however, none of these studies report direct cytotoxicity or genotoxicity to HSCs or HPCs in the bone marrow. In addition, unlike other species, mice display irritant-induced reflex apnea (bradypnea), which will not only reduce the inhalation intake of formaldehyde (Chang et al. Citation1983, Citation1981; Chang and Barrow Citation1984) but may have contributed to the observed biomarkers of oxidative stress. Further, a reanalysis of one of the studies (Zhang et al. Citation2013) found substantial flaws in the design, conduct, reporting, and interpretation of one of the histopathological endpoints (Environmental Pathology Laboratories, Inc. Citation2014). The most serious of these flaws include inadequate and inaccurate description of the histopathology methods; the low number of animals sampled for histopathology (2 per group, 6 total); the lack of a defined scoring system for determining relative numbers of megakaryocytes; the apparent misdiagnosis of myelofibrosis; and the unsupported conclusion that the histopathologic results are indicative of bone marrow toxicity.
Regarding the misdiagnosis of myelofibrosis, Zhang et al. (Citation2013) indicate the presence of fibrosis in the bone marrow using a black arrow and inset image in figure 3(F) of the publication. However, the single indicated object, which is a thin-walled, tubular structure lined by endothelial cells, is clearly a normal arteriole (small artery) and not fibrosis (Environmental Pathology Laboratories, Inc. Citation2014). Furthermore, there does not appear to be evidence of myelofibrosis in other areas of Figure 3(F), or in any of the other five bone marrow images.
Further, Zhang et al. (Citation2013) suggest that changes in peripheral blood hematologic values and bone marrow histopathologic findings provide evidence of bone marrow toxicity. They state that “…changes in the numbers of circulating blood cells in several lineages may reflect toxicity to BM HSC [bone marrow hematopoietic stem cells].” They then further state that “In our study, significantly altered counts of WBC, RBC, LYM (decreased) and PLT (increased) in FA-exposed mice, the results are consistent with the clinical symptoms of some hematopoietic diseases such asaplastic [sic] anemia (AA), myelofibrosis and megakaryocytic leukemia, indirectly suggesting that FA induces BM toxicity.” However, Zhang et al. (Citation2013) neglect to acknowledge that alterations in numbers of peripheral erythrocytes and several lineages of leukocytes can also be induced by disturbances that have no obligate association with myelotoxicity, such as localized or systemic inflammatory disease (Environmental Pathology Laboratories, Inc. Citation2014).
In addition to the results from animal studies, a study conducted in workers (Zhang et al. Citation2010) compared markers of hematopoietic function and chromosomal aneuploidy in workers occupationally exposed to formaldehyde with groups of unexposed workers and reported a higher prevalence of monosomy 7 and trisomy 8 in metaphase spreads prepared from cultures of CFU-GM colony cells. The authors proposed that these changes indicated an increase in leukemia-specific chromosomal aneuploidy in vivo in the hematopoietic progenitor cells of the exposed workers. However, for this assessment, the Zhang et al. (2010) study received an unacceptable study quality score of 4 () and was not included in the MOA assessment because the study measured biomarkers of effect (i.e. aneuploidy, specifically monosomy 7 and trisomy 8) that is not specific to the health outcome of leukemia alone. In addition, a reanalysis of the Zhang et al. (2010) data by Gentry et al. (Citation2013), based on selected underlying data, suggested that factors other than formaldehyde exposure may have contributed to the effects reported. A critical review of the underlying data (Gentry et al. Citation2013) revealed that although the authors stated in their paper that “all scorable metaphase spreads on each slide were analyzed, and a minimum of 150 cells per subject was scored,” this protocol was not followed. In fact, in reviewing the raw data from the Zhang et al. (Citation2010) study, Gentry et al. (Citation2013) noted that the protocol to evaluate the presence of monosomy 7 or trisomy 8 was followed for three or fewer samples in exposed workers and six or fewer samples in non-exposed workers. In addition, the assays used (CFU-GM) do not actually measure the proposed events in primitive cells involved in the development of leukemia and the aneuploidy measured probably arose during the in vitro culture, rather than in vivo (see additional discussion in Gentry et al. Citation2013 and Albertini and Kaden Citation2017). As a follow up to this evaluation, Mundt et al. (Citation2017) obtained the average exposure (based on three measurement samples) for each of the formaldehyde-exposure workers in the Zhang et al. (2010) study, provided through a Technology Transfer Agreement with the National Cancer Institute, the sponsor of the study. This allowed exposure-response analyses to be conducted for each of the blood and aneuploidy tests. The results of the additional analyses by Mundt et al. (Citation2017) indicated that there was no association between individual average formaldehyde exposure estimates and frequency of any of the blood or aneuploidy tests as had been suggested by Zhang et al. (2010) based only on a cross-sectional comparison of groups, ignoring the actual exposures obtained.
Regarding MOAs 1, 2, and 3, a high quality directly relevant genotoxicity study performed in samples taken from 41 male volunteers exposed to formaldehyde in chambers (for 4 h per day for 5 consecutive days at concentrations of 0.3, 0.4, 0.5, or 0.7 ppm with 15-min peak exposures up to 0.8 ppm) reported no changes in genotoxic endpoints (i.e. comet assay, sister chromatid exchange (SCE), micronuclei (Mn) in peripheral blood cells or nasal epithelial cells (Zeller, Neuss, et al. Citation2011). In addition, two high-quality (Dallas et al. Citation1992; Speit et al. Citation2009) directly relevant in vivo genotoxicity studies conducted in rats exposed to concentrations of formaldehyde ranging from 0.5 to 15 ppm for up to 8 weeks report no genotoxic effects in (i.e. chromosomal aberrations, SCEs, DNA migration) in the bone marrow or in blood cells derived from the bone marrow (i.e. red blood cells). Meng et al. (Citation2010) exposed F344 rats to five concentrations of formaldehyde, including three concentrations (6, 10, and 15 ppm) known to increases cell proliferation in the nasal tissue. Despite clear signs of formaldehyde reaching the nasal tissue and increased cell proliferation, no increases in mutation frequency were observed in the nasal tissue any treatment group, supporting a lack of mutagenicity in tissues at the portal of entry.
There are some discrepancies in the reported in vivo genotoxicity results, primarily from studies not published in English (Kitaeva et al. Citation1996; Liu et al. Citation2003; Tao et al. Citation2004; Zhao et al. Citation2004; Gao et al. Citation2008) and/or studies that did not meet inclusion criteria for this assessment as noted in (Zhang et al. Citation2010; Katsnelson et al. Citation2013; Ji et al. Citation2014). However, integration of the available evidence from multiple high-quality studies that were deemed directly relevant to the problem formulation (Kligerman et al. Citation1984; Dallas et al. Citation1992; Speit et al. Citation2009; Zeller, Neuss, et al. Citation2011), along with the conclusions of other scientific groups who have assessed the available data (National Research Council Citation2011; Albertini and Kaden Citation2017), indicate that inhaled formaldehyde does not cause direct mutagenic or genotoxic effects in systemic tissues, including the blood and bone marrow).
Event 5: initiated stem cell release to systemic circulation
No studies involving exposure to formaldehyde have been reported to support this key event for either MOA 3 or 4. For MOA 3, Murrell et al. (Citation2005) is the only study reported to support this event. In one of the experiments reported in the Murrell et al. (Citation2005) study, female rat olfactory mucosa cells were injected intravenously into bone marrow irradiated male rats, with the observation of generation of leukocytes from the donor cells reported. The focus of the study was to investigate cells that might generate neurons. While the authors concluded that the results demonstrate the existence of multipotent stem-like cells in the olfactory mucosa, the animals had compromised systems (irradiated bone marrow) and the study provides no evidence of the transport of the cells from the nasal passages to the bone marrow.
Lefrancais et al. (Citation2017) is the only study that has been cited to support this key event in the postulated MOA 4 involving HSCs/HPCs in the lung tissues that are proposed to circulate from the bone marrow to the lung and then re-circulate back to the bone marrow. This study was conducted in a genetically modified PF4-mTmG reporter mouse strain that expresses codon-improved Cre recombinase (detected in megakaryocytes) under the control of the mouse platelet factor 4 promoter. These mice also underwent a single-lung transplant from mTmG reporter mice, with pro-platelet formation observed in the lung of recipient mice following transplantation. The authors indicate that the cells can migrate out of the extravascular spaces under conditions of thrombocytopenia and relative stem cell deficiency in the bone marrow. While, neither Murrell et al. (Citation2005) nor Lefrancais et al. (Citation2017) met our inclusion criteria since there are no formaldehyde specific data reported in either study, and no formaldehyde specific data in non-compromised systems are available to provide evidence for this postulated key event; both studies were included in this evaluation because they were cited by Zhang et al. (Citation2009; Zhang Citation2018) as providing supporting evidence for key events in the postulated MOAs. Because neither Murrell et al. (Citation2005) or Lefrancais et al. (Citation2017) met the inclusion criteria based on the problem formulation review questions, neither were assessed for study quality or relevance
Event 6: initiated stem cell migration and incorporation into the bone marrow
This key event is suggested based on the assumptions that (1) the genotoxicity (i.e. SCE, chromosomal aberrations, micronuclei) that has been reported to occur in peripheral lymphocytes could also potentially occur in circulating HSCs and HPCs, and (2) genotoxicity to HSCs and HPCs circulating in the blood could lead to initiated stem cells that return to the bone marrow and impact the bone marrow. As discussed in previous sections, there are currently no in vivo data identified in the published literature directly relevant to the problem formulation that demonstrates initiated stem cells resulting from exogenous formaldehyde exposure are re-incorporated back into the bone marrow. With regard to the hypotheses related to the ability of formaldehyde to damage selected circulating cells that could impact the bone marrow, no evidence is available to indicate circulating hematopoietic stem cells return to bone marrow during homeostasis (McKinney-Freeman and Goodell Citation2004). The normal human peripheral blood repopulating (stem) cells are vanishingly rare and are not measurable. In addition, circulating repopulating cells in humans are end-stage cells, making the biological plausibility of this proposed event unlikely.
There is limited general information (e.g. not formaldehyde-specific) that has been cited as part of the postulated MOAs indicating that HSCs in circulation may reenter the bone marrow (National Institutes of Health Citation2001). HSCs are primarily found in the stroma of the bone marrow; however, they are also found in the spleen, peripheral blood circulation, and other tissues. According to National Institutes of Health (Citation2001), HSCs may make brief trips out of the bone marrow into tissues and then return to the bone marrow; however, it appears that the HSCs that are mobilized into peripheral circulation are primarily non-dividing cells. Results of parabiotic studies in mice provide evidence that HSCs in circulation do not stably return to the bone marrow and those HSCs that do reenter the bone marrow do not persist in the bone marrow after returning (Abkowitz et al. Citation2003; McKinney-Freeman and Goodell Citation2004).
In addition, none of these results demonstrate damage to the bone marrow stroma, which is critical in regulating hematopoiesis (Irons and Kerzic Citation2014; Calvi and Link Citation2015). If toxicity to circulating cells were demonstrated to occur and was also demonstrated to be related to inhalation of formaldehyde, this still does not demonstrate toxicity to the bone marrow stroma, and stroma involvement would still need to be demonstrated to provide evidence of an association between formaldehyde exposure and leukemia. Therefore, the incorporation of initiated stem cells into the bone marrow and their division is not considered biologically plausible due to a lack of scientific evidence that (1) formaldehyde induces direct DNA damage to cells in nasal tissues that have been demonstrated to migrate to the bone marrow and repopulate the bone marrow (MOA 3), and (2) direct DNA damaged HSCs and HPCs in lung tissues following formaldehyde exposure have reentered the bone marrow and proliferated (MOA 4).
Adverse outcome: initiation of leukemia
Multiple authoritative bodies have evaluated the potential carcinogenicity of formaldehyde (World Health Organization Citation2010; International Agency for Research on Cancer Citation2012; Risk Assessment Committee Citation2012; Bolt et al. Citation2016; National Toxicology Program Citation2016; Nielsen et al. Citation2017). Overall, these reviews classified formaldehyde as a known or probable human carcinogen, largely based on evidence in humans related to nasopharyngeal cancer. However, there were wide discrepancies in classifying formaldehyde as a leukemogen, with International Agency for Research on Cancer (Citation2012) and National Toxicology Program (Citation2016) classifying formaldehyde as a known human leukemogen and Risk Assessment Committee (Citation2012), Bolt et al. (Citation2016), and Nielsen et al. (Citation2017) concluding that the evidence was insufficient for classifying formaldehyde as a leukemogen. In general, all reviews have noted the challenges with cancer hazard identification for formaldehyde, especially regarding the potential for leukemogenicity due to the high background incidence of various leukemia types and highly variable incidences in human populations, as well as formaldehyde’s endogenous production and data indicating it is not delivered systemically following inhalation exposure. The conclusions from several of the available assessments from authoritative bodies have suggested that the weight of evidence does not support a causal association of this endpoint with formaldehyde exposure (Health Canada Citation1999; Bundesinstitut für Risikobewertung Citation2006; European Chemical Agency Citation2016). Regardless of the conclusion related to the potential for inhaled formaldehyde to cause leukemia, World Health Organization (Citation2010) concluded that even the epidemiology studies that identified statistical associations between inhaled formaldehyde and leukemia identified those associations only at concentrations much greater than currently anticipated exposures. Thus, when deriving an acceptable indoor air concentration, World Health Organization (Citation2010) focused on more sensitive and reliable endpoints, such as irritation, that were concluded to be protective of any potential carcinogenic effects of formaldehyde exposure (Nielsen et al. Citation2017; ANSES Citation2018).
Data supporting the conclusion that formaldehyde is a leukemogen are generally based on cohort analyses of embalmers, pathologists, and anatomists (Hall et al. Citation1991; Hayes et al. Citation1990, Levine et al. Citation1984, Matanoski Citation1989, Stroup et al. Citation1986, Walrath and Fraumeni Citation1983; Beane-Freeman et al. Citation2009) despite contradictory evidence in industrial cohorts indicating no similar association between formaldehyde exposure and increased risk of leukemia (Coggon et al. Citation2003; Pinkerton et al. Citation2004, Mundt et al. Citation2018). Three meta-analyses (Bosetti et al. Citation2008, Collins and Lineker Citation2004, Zhang et al. Citation2009) have also been used to provide support for leukemogenic potential; however, these analyses primarily included evidence from the same studies, repeating the same results and not offering independent or additional evidence (Mundt et al. Citation2018). The National Research Council (Citation2011) concluded that claims that formaldehyde exposure causes leukemia, myeloid leukemia, and related hematopoietic cancer were not supported, were subjective in nature, and followed no clear scientific framework in reaching conclusion. National Research Council (Citation2011) also criticized any reliance solely on epidemiological studies to determine causality instead of integrating epidemiological evidence with toxicological and mechanistic evidence. National Research Council (Citation2011) also noted that any evaluation that relied upon grouping all lymphohematopoietic cancers (LHPs) in determining causality was inappropriate because LHPs comprises 14 biologically distinct diagnoses in humans. While there is evidence that some LHPs may originate from the same stem cell line (Mundt et al. Citation2018), there are no studies that demonstrate an effect on these stem cells following exposure to formaldehyde. An additional issue highlighted by National Research Council (Citation2011) was the uncertainty in the exposure assessments for the epidemiological literature, and the reliance on exposure metrics, such as peak exposure, in determining causality, when a different exposure metric (cumulative exposure) which demonstrated no association with the LHP cancers was used in conducting the dose-response assessment for the inhalation unit risk.
In a critical review of the epidemiological evidence, Checkoway et al. (Citation2012) reported inconsistent and sporadic associations between formaldehyde exposure and various lymphohematopoietic malignancies (LHM), and only a few of these studies considered acute myeloid leukemia (AML) specifically. The authors reported that, apart from a few exceptions, relative risks from occupational cohort and population-based case-control studies were close to the null and there was little evidence for dose-response relationships for any of the LHM and formaldehyde inhalation exposure. For the occupational cohort studies, no excesses for all leukemia (standardized mortality ratio (SMR) 1.02, 95% confidence interval (CI 0.85–1.22) or myeloid leukemia (SMR 0.90, 95% CI 0.67–1.22) were reported in the most recent follow up of the National Cancer Institute (NCI) study (Beane-Freeman et al. Citation2009), based on comparisons with national rates. There was a weak trend in relative risk (RR) with peak exposure (RR = 1.78; 95% CI 0.87–3.64 for myeloid leukemias; RR = 1.42 95% CI 0.92–2.18 other leukemias); however, the trends and individual RR estimates were concluded by the authors to be not remarkable or precise.
Checkoway et al. (Citation2012) also reported no elevation in leukemia mortality, overall or in the highest exposure groups (>2.0 ppm) of formaldehyde workers in the United Kingdom, and no excess of myeloid leukemias in a group of US embalmers. Based on the same review, SMRs for leukemia deaths (1.09, 95% CI 0.7–1.62) myeloid leukemias (1.44, 95% CI 0.9–2.37) and AMLs (1.34, 95% CI 0.66–2.54) were reported in United States garment workers with the highest SMR reported for myeloid leukemia in workers with ≥10 years exposure (SMR 2.19, 95% CI 0.95–4.32) or ≥20 years exposure (SMR 1.91, 95% CI 1.02–3.27). Among persons classified as exposed to formaldehyde in population-based case-control studies, no excesses in all leukemia or any leukemia subtypes were observed (Checkoway et al. Citation2012). Based on the review of epidemiological literature, Checkoway et al. (Citation2012) concluded that there was little or no evidence indicating excess risk overall or exposure-response associations between formaldehyde exposure and LHM including leukemia, myeloid leukemia, and acute myeloid leukemia.
Mundt et al. (Citation2018) reviewed the epidemiological evidence published after the Checkoway et al. (Citation2012) review and identified several epidemiological studies including the NIOSH garment workers study (Meyers et al. Citation2013), the UK industry-wide formaldehyde producers and users study (Coggon et al. Citation2014), a case-control study in the Nordic countries (Talibov et al. Citation2014), an occupational study in Italy (Pira et al. Citation2014) and a European occupational study (Saberi Hosnijeh et al. Citation2013). In addition, an extended analysis of the National Cancer Institute’s (NCI) cohort study (Beane-Freeman et al. Citation2009) was conducted by Checkoway et al. (Citation2015) which included data from the NCI formaldehyde industrial workers cohort to further investigate specific types of LHM, especially AML, and a more conventional definition of peak exposure. Following a review of the results of the available epidemiological data, Mundt et al. (Citation2018) concluded that there is no evidence of significant increases in LHM, specifically AML, among cohorts of workers exposed to formaldehyde.
In evaluating the results from animal studies, there is no evidence that inhalation of formaldehyde causes leukemia in exposed animals. Over the years, formaldehyde has been investigated in numerous rodent cancer bioassays (Battelle Columbus Laboratories Citation1981; Kerns et al. Citation1983; Appelman et al. Citation1988; Feron et al. Citation1988; Kamata et al. Citation1997) and as part of routine carcinogenicity protocols, multiple tissues were collected for assessment, including lymph nodes, blood and bone marrow. The lack of bone marrow toxicity and leukemia in animal studies is consistently reported. A recent study performed by the National Toxicology Program (Morgan et al. Citation2017) was conducted to investigate the role of mutations in the Trp53 gene in the pathogenesis of leukemia or lymphohematopoietic cancer. In this study, Trp53 haploinsufficient mice designed to be susceptible to developing leukemia were exposed by inhalation to 7.5 or 15 ppm formaldehyde for 6 h/day, 5 days/week, for 8 weeks. No increased prevalence of leukemia or lymphohematopoietic cancers or significant changes in hematological parameters were reported in the exposed mice. The primary formaldehyde-related findings were squamous metaplasia of the respiratory epithelium of the nose and significant injury and regenerative cell proliferation in the nasal mucosa.
Integrated assessment of postulated MOAs
In the following sections, an evaluation of each of the postulated MOAs for leukemia, based on the IPCS MOA framework (Boobis et al. Citation2006, Bosetti et al. Citation2008; Meek et al. Citation2014) and Bradford–Hill criteria (Hill Citation1965; Adami et al. Citation2011; Fedak et al. Citation2015) is conducted. The available data from all streams of evidence (i.e. epidemiological, animal, and mechanistic studies) have been integrated and evaluated using this framework. The key events for each postulated MOA were evaluated for concordance of dose-response relationships, temporal associations of key events, strength, consistency, and specificity of key events, the biological plausibility of the postulated MOA, and uncertainties associated with the MOA. Summaries of the evaluation of each MOA, the dose-response and temporal concordance, and the biological plausibility are presented in the following sections and in ). The dose-response data available for each of the key events are described in . A summary of the integration of the epidemiological, animal toxicity and in vitro mechanistic data that support the postulated MOAs, as well as evidence inconsistent with the postulated MOAs, is presented in along with the results of the study quality and study relevance assessments.
Table 10. Integration of evidence for postulated MOA 1 – initiation of leukemia by direct damage to hematopoietic stem cells in the bone marrow.
Table 11. Formaldehyde-specific dose-response and temporal concordance data for leukemia.
Table 12. Integration of Evidence for Each of the Key Events Associated with the Postulated MOAs.
Table 13. Comparative weight of evidence for postulated MOA 2 – toxicity to circulating blood stem cells and progenitors.
Table 14. Results from studies measuring endogenous and exogenous DNA-adducts and DNA–protein crosslinks in multiple species.
Postulated MOA 1 – initiation of leukemia by direct damage to hematopoietic stem cells in the bone marrow
In this postulated MOA, following inhalation exposure, formaldehyde is hypothesized to be absorbed systemically and travel in its hydrated form (methanediol) through the blood to the bone marrow where it can react with cellular proteins and DNA causing direct damage in the hematopoietic stem or early progenitor cells of the bone marrow (Zhang et al. Citation2009; United States Environmental Protection Agency Citation2010; Zhang Citation2018). The postulated MOA is comprised of Events 1 through 5 in .
The majority of the results from high-quality studies that are directly relevant to each postulated key event provide contradictory evidence, rather than supporting evidence, for this MOA. Dose-response data to support postulated key events were only available for Event 3 (macromolecular binding to hematopoietic stem cells and progenitor cells in the bone marrow) and Event 4 (formaldehyde induced genotoxicity in the hematopoietic stem cells and progenitor cells in the bone marrow) (). The supporting data are limited to short-term in vivo exposure studies conducted in one species (mice) at similar doses and durations in all studies, using methodologies to assess the endpoints that did not differentiate between endogenous and exogenous exposure and introduced potential co-exposures associated with the test substance used (i.e. formaldehyde was generated from formalin containing methanol) (Ye et al. Citation2013, Wei et al. Citation2017; Zhang et al. Citation2013). More convincingly, multiple studies in multiple species and considered to be of high quality and directly relevant to informing the problem formulation questions (Lu et al. Citation2010, Citation2011; Edrissi et al. Citation2013; Lai et al. Citation2016) provide evidence that these key events are not plausible, or the observations are not related to formaldehyde exposure (). Therefore, the majority of the available data from high quality and directly relevant studies that inform the key events for the postulated MOA provide a lack of support. This is further emphasized by the lack of dose-response concordance and temporal association for supporting evidence available for the key events in the postulated MOA ( and ).
The biological plausibility of postulated MOA 1, therefore, is improbable. Each of the key events in the postulated MOA is built on the assumption that formaldehyde can cause leukemia by directly inducing direct DNA damage in HSCs and HPCs in the bone marrow. This postulated MOA is considered improbable due to a large number of high-quality studies in multiple species demonstrating that formaldehyde is not systemically absorbed and, therefore, does not reach the bone marrow in significant quantities ( and ). Exogenous exposures to formaldehyde have not been reported to be associated with systemic absorption of formaldehyde and transport to the bone marrow where both myeloid and lymphoid stem cells originate (Albertini and Kaden Citation2017); and, as such, there is no dose-response. The only data that has been cited to support Event 1 or 2 are from studies focused on the development of a method for use of formaldehyde during the fixation process. No in vitro or in vivo studies using appropriate tissues or cell lines have been conducted that demonstrate inhaled formaldehyde distribution past the portal of entry. The actual mechanistic data from high quality, directly relevant studies for formaldehyde provide evidence that exogenous formaldehyde is not transported from the point of contact to the bone marrow or any other distant sites.
In conclusion, the available supporting evidence for the key events for this postulated MOA did not provide evidence of dose-response or temporal association between the key events in the postulated MOA (). There is evidence from multiple high-quality studies conducted in multiple species, that differentiate between endogenous production and exogenous formaldehyde inhalation exposure up to 15 ppm (Speit et al. Citation2009; Lu et al. Citation2010, Citation2011, Moeller et al. Citation2011; Edrissi et al. Citation2013; Kleinnijenhuis et al. Citation2013; Yu R et al. Citation2015; Lai et al. Citation2016; Leng et al. Citation2019), which provide directly relevant evidence to inform the problem formulation. They provide evidence that inhaled formaldehyde is not systemically distributed past tissues at the portal of entry which is necessary to result in a molecular initiating event in peripheral blood mononuclear cells or bone marrow mononuclear cells, including blood-forming stem cells.
Postulated MOA 2 – toxicity to circulating blood stem cells and progenitors
The key events associated with postulated MOA 2 were evaluated based on the IPCS MOA framework considering dose-response and temporality, consistency, and biological plausibility of both the supporting evidence and potentially inconsistent evidence related to MOA 2 (). However, data to support postulated key events were only available for Event 3 (macromolecular binding to hematopoietic stem cells and progenitor cells in the blood) and Event 6 (Incorporation of initiated stem cell into the bone marrow) (). As discussed previously for postulated MOA 1, supporting data for Event 3 are based on results from short-term in vivo high-quality studies that were determined to be not relevant to the problem formulation questions based on the use of a test substance with known confounding impurities (e.g. methanol in formalin). Studies relying on formalin may provide evidence of formaldehyde adducts systemically resulting from the metabolism of compounds to formaldehyde following uptake. In addition, these studies used methodologies that did not differentiate between exogenous and endogenous formaldehyde exposure (Ye et al. Citation2013; Zhang et al. Citation2013; Wei et al. Citation2017). There is contradictory evidence indicating the absence of macromolecular binding to HSCs/HPCs specifically in studies where exogenous formaldehyde exposure can be clearly measured or identified (). Therefore, based on the available data from high-quality studies relevant to the problem formulation, there are no dose-response or temporal relationships between formaldehyde and the key events in the postulated MOA.
The biological plausibility of the key events for postulated MOA 2 is also questionable (). Each of the key events in postulated MOA 2 is built on the assumption that formaldehyde can cause leukemia by directly inducing DNA damage and chromosome aberrations in HSCs and HPCs in the circulating blood that can then impact the bone marrow. Circulating HSCs and HPC are present at a very low frequency in the peripheral blood. In view of the concentration gradient, levels of formaldehyde are much lower in peripheral blood than in the airways. Although mutations associated with leukemia, in general, have been studied extensively, there is evidence in the literature that suggest these mutations do not occur in circulating HSCs and HPCs rather than in the bone marrow. Furthermore, there is no evidence in the available literature to indicate any exposure known to cause leukemia has occurred due to mutations in circulating HSCs and HPCs.
In the formaldehyde-specific studies which apply methodologies that differentiate between endogenous and exogenous formaldehyde, the data demonstrate that there is no detectable systemic distribution of inhaled formaldehyde to sites distal from the portal of entry (). Furthermore, there are no mechanistic data available for inhaled formaldehyde that provide evidence that exogenous formaldehyde as the acetal can be transported from the point of contact to any other distant sites in the body. There is also no available evidence of inhaled formaldehyde producing genotoxicity or cytotoxicity in HSCs or HPCs necessary to induce leukemia, and the weight of evidence of the integrated epidemiological and toxicology data indicate the inhalation of formaldehyde is not associated with an increased risk of leukemia. In conclusion, the weight of evidence for the key events for postulated MOA 2 did not satisfy the conditions of dose concordance, temporality, or biological plausibility of the IPCS MOA framework (). There does not appear to be sufficient evidence to support this postulated MOA, in contrast to considerable evidence that indicates most, or all, of the key events are not plausible.
Postulated MOAs 3 and 4 – targeting pluripotent nasal or oral cells or HSCs/HPCs in the lung tissue
The third and fourth postulated MOAs (MOA 3 and 4) are built on the hypothesis that formaldehyde directly induces mutations or pre-mutagenic lesions in primitive pluripotent cells in the nasal or oral passages or in HSCs/HSPs in the lung tissue through normal trafficking or formaldehyde-induced cytotoxicity trafficking (Zhang et al. Citation2009; Zhang Citation2018). The damaged cells in either the nasal or oral passages or the lung are then released from the tissue and circulate through the blood and are eventually incorporated into the bone marrow where they could induce leukemia.
Based on a search of the peer-reviewed literature, there is currently no available evidence in the literature of any cancer occurring due to any chemical exposure that is supported by an MOA driven by de-differentiation of pluripotent nasal/oral cells. While circulating HSCs and HPCs traffic in the blood, spleen, liver, and marrow (Jaiswal et al. Citation2009), the presence of circulating HSCs and HPCs cells in the nasal, oral, or lung tissue is extremely rare. The likelihood of exposure of these very rare cells within the nasal, oral or lungs to sufficient levels of formaldehyde to induce mutations that result in leukemia appears unlikely. Moreover, this mechanism has not been established for any inhaled carcinogen known to cause leukemia.
As discussed previously, formaldehyde can induce toxicity, DNA-adducts, and DNA–protein cross-links at the portal of entry (i.e. nasal passages) (Swenberg et al. Citation2013; Albertini and Kaden Citation2017). However, no evidence was identified that suggests formaldehyde can induce toxicity in extravascular HSCs/HSPs in the lung. During normal cell proliferation or secondary proliferation due to formaldehyde induced cytotoxicity, DNA damage and lesions in primitive pluripotent stem cells in the olfactory mucosa may be converted into mutations, but there are no reliable data to support this as a key event. This MOA is singularly supported by a recent study that did not test formaldehyde but shows that olfactory epithelial cell from irradiated rat nasal passages, an animal model with a compromised system, are capable of re-populating the hematopoietic tissues and giving rise to HSCs/HPCs including myeloid and lymphoid cells (Murrell et al. Citation2005). This event is suggested to be possible based on conclusions by Lefrancais et al. (Citation2017) that lung tissue is a site of platelet biogenesis and a reservoir for hematopoietic progenitors with the capability to replenish blood-making stem cells. However, this conclusion is based on observations in mice under conditions of thrombocytopenia and relative stem cell deficiency in the bone marrow (). The key events specific to these MOAs are Events 4, 5, and 6 (). As discussed previously, while the Lefrancais et al. (Citation2017) was included in the discussion of this MOA because it was the only study provided as support for this postulated MOA (Zhang et al. Citation2009; Zhang Citation2018), it would otherwise have been excluded from the current assessment because it did not meet the inclusion criteria (providing formaldehyde-specific information). The study also was performed in a compromised animal model (i.e. irradiated rat nasal passages).
Similar to both postulated MOA 1 and 2, postulated MOAs 3 and 4 have been evaluated based on the IPCS MOA framework considering dose-response and temporality, consistency, and biological plausibility (). However, there are no formaldehyde-specific data identified that met the inclusion criteria to support any of the key events for postulated MOA 3 or 4 ( and ). Therefore, the biological plausibility for each of the key events for postulated MOA 3 and 4 is questionable. Both Events 4 and 5 () in postulated MOA 3 and 4 rely on the hypothesis that formaldehyde can produce genotoxicity in cells in tissue at or near the portal of entry. There are no data available that indicate formaldehyde induces mutagenicity specifically in primitive pluripotent cells of the nasal tissue or HSCs/HSPs within the lung tissue. As discussed previously for postulated MOA 2, no data were identified to support Event 6 (Incorporation of initiated stem cell into the bone marrow) of postulated MOA 3 and 4 (). In conclusion, there are no formaldehyde-specific data to support the key events for postulated MOA 3 or 4 and the postulated MOAs did not satisfy the conditions of the biological plausibility of the IPCS MOA framework.
Table 15. Comparative weight of evidence for postulated MOA 3 and 4 – targeting pluripotent nasal cells or HSCs/HSPs in lung tissue.
Human relevance
According to the IPCS framework (Boobis et al. Citation2006; Meek et al. Citation2014), if the weight of evidence is sufficient and an MOA can be established for an outcome, the relevance of the outcome to human health (i.e. human relevance) should be determined. This can be accomplished by following the human relevancy framework outlined in Boobis et al. (Citation2006, Bosetti et al. Citation2008) in which human relevance is determined by answering a series of three questions: (1) Is the weight of evidence sufficient to establish an MOA in animals for the outcome in question? (2) Can human relevance of the MOA be reasonably excluded on the basis of fundamental, qualitative differences in key events between animals and humans? and (3) Can human relevance of the MOA be reasonably excluded on the basis of quantitative differences in either kinetic or dynamic factors between experimental animals and humans?
Using the IPCS MOA framework, none of the four postulated MOAs was determined to be biologically plausible, as the weight of evidence did not support the postulated MOAs (see ). Moreover, there are no animal bioassay data supporting an association between formaldehyde inhalation and leukemia. Therefore, an evaluation to determine the potential human relevance of these data is not warranted. Further, the epidemiological associations between formaldehyde inhalation and leukemia are weak and controversial. In addition, the results from the application of the IPCS framework suggest that the next step of the risk assessment process, or the quantitative dose-response assessment for formaldehyde induced leukemia, is not warranted.
Discussion
A significant amount of scientific debate surrounds the conclusions suggesting that formaldehyde exposure is associated with an increased risk of leukemia. A review of formaldehyde science conducted by the National Research Council (Citation2011) concluded that associations between formaldehyde exposure and leukemias or related hematopoietic cancers were not supported by the literature. National Research Council (Citation2011) further noted that any conclusions regarding a link between inhaled formaldehyde and leukemia were subjective in nature and no clear scientific framework has been applied when evaluating the potential for inhaled formaldehyde to cause leukemia. Furthermore, formaldehyde is a reactive compound produced endogenously by numerous biochemical pathways, with processes demonstrated to be in place to provide protection for HSCs, hepatocytes, and nephrons in mice (Pontel et al. Citation2015).
In the current assessment, the IPCS framework was applied to the identified postulated MOAs by which formaldehyde inhalation exposure may be associated with leukemia to provide a transparent framework for integration of the available evidence. A comprehensive review of the literature associated with the key events for the postulated MOAs for leukemia that address the problem formulation questions was performed, as well as a study quality assessment. The TSCA study quality framework (United States Environmental Protection Agency Citation2018) was incorporated into this assessment to ensure the highest quality studies were considered. However, during the review and application of the study quality assessment, it was noted that these guidelines did not include a study relevancy component. This type of evaluation is especially important for formaldehyde, as this compound has important chemical-specific considerations, with one main consideration being it is produced endogenously. The TSCA quality assessment allows for additional metrics to be incorporated as needed when there are chemical-specific issues that should be addressed when assessing the literature. Therefore, an approach provided by the European Commission, the SCIRAP study relevancy component, was added to determine if each study was directly, indirectly or not relevant to the problem formulation questions established for the current assessment. The addition of the SCIRAP component assisted in addressing critical formaldehyde-specific issues during the integration of the evidence to inform the postulated MOAs. Specifically, the differentiation of the total amount of formaldehyde associated with exogenous exposures compared to the total amount associated with endogenous production.
As discussed, there are unique risk assessment considerations for endogenously formed chemicals that are also present in foods, or metabolically derived from exposures to chemicals in foods or other media. In these cases, consideration of specific exogenous exposures in comparison to endogenous concentration is critical in assessing the potential for human health effects (Farland et al. Citation2019). In the case of formaldehyde, the differentiation of the total amount of formaldehyde associated with exogenous exposures compared to the total amount associated with endogenous production provides a reality check on any conclusions regarding the potential for inhalation of formaldehyde to induce systemic effects (). For example, at the current World Health Organization (Citation2010) Indoor Air Quality Standard of 0.1 mg/m3, the equivalent daily amount of formaldehyde that can be inhaled and is protective of long term health effects, including cancer, is 2 mg/day (assuming a human inhalation rate of 20 m3/day (United States Environmental Protection Agency Citation1994, Citation2009). When compared to the total endogenous turnover rate of formaldehyde in the human body of 61,460–91,700 mg/day estimated by European Food Safety Authority (Citation2014), if all of the exogenous formaldehyde associated with exposure to the WHO guideline was systemically absorbed, it would be an insignificant amount (i.e. less than 0.003% of endogenous production). Likewise, the current Occupational Safety and Health Administration’s (OSHA) Permissible Exposure Limit (PEL), an 8-h time-weighted average enforceable value derived to be protective of worker’s health of 0.75 ppm (0.92 mg/m3) (Occupational Safety and Health Administration Citation2020) is equivalent to 9 mg/day, assuming an occupational inhalation rate of 10 m3/day, which is less than 0.01 percent of estimated daily amount of endogenous formaldehyde produced in the human body. Dietary sources of formaldehyde include fruits that could result in body burdens of formaldehyde ranging from approximately 420–1,400 mg/day, which is over 100 times greater than exogenous exposure associated with the WHO guideline. Without considering metabolism, European Food Safety Authority (Citation2014) estimates that a person eating 1 kg of food per day could be directly exposed to approximately 100 mg formaldehyde per day, which would be approximately 50 times the exogenous exposure to formaldehyde associated with the WHO guideline concentration. This comparison of amounts of formaldehyde that would be associated with exogenous exposures to those amounts estimated to be produced endogenously on a daily basis provides an important reality check in consideration of the potential for formaldehyde to cause systemic health effects. As Swenberg et al. (Citation2011) stated “If one constructs a worst-case scenario by assuming that exogenous adducts were just below the LOD…. This would mean that less than one exogenous DNA adduct was present for every 13,900 endogenous formaldehyde adducts. It is difficult to conceive of a mechanism by which 1/13,900 identical DNA adducts could drive the biology that leads to carcinogenesis.” Likewise, it is hard to believe that at the Occupational Exposure Standard, inhalation of <1/10,000 of the amount of formaldehyde metabolically generated each day () would causally increase the human leukemia risk in a statistically significant manner.
Table 16. Comparison of formaldehyde regulatory standards, exogenous formaldehyde concentrations in food and drink, and endogenous production of formaldehyde in humans.
In addition, it should be noted that several compounds that are metabolized to formaldehyde have been evaluated and study results indicated no evidence of leukemia (Andersen et al. Citation2019). These include methyl chloride, methanol, caffeine, and aspartame (European Food Safety Authority Citation2014). Following oral administration of methanol (as a 15% solution in drinking water) to rats, 2- to 3-fold increases in formaldehyde DNA-reaction products were noted in bone marrow, kidney, and liver (Pontel et al. Citation2015). In another study, in which rats were dosed with 500 or 2000 mg methanol/kg/day for 5 days with mass-labeled 13CD4-methanol (Lu et al. Citation2012), increases in exogenous formaldehyde-DNA reaction products were noted in multiple tissues, including white blood cells. Thus, it is clear that following methanol administration, formaldehyde is produced in tissues throughout the body at sufficient concentrations to increase DNA-reaction products; however, evidence indicates that methanol exposure does not lead to the development of leukemia or other cancers (Cruzan Citation2009). Methyl chloride is also extensively metabolized to formaldehyde in rats (Kornbrust and Bus Citation1983). Following inhalation exposure of rats and mice to methyl chloride, significant increases in formaldehyde concentrations were reported in rat liver testes and brain, but not in nasal mucosa (Heck et al. Citation1982). Further, methyl chloride was carcinogenic only to the male mouse kidney and not in rats (Agency for Toxic Substances and Disease Registry Citation1998). Considering the available animal toxicology and human studies for formaldehyde-producing compounds provides a lack of any evidence for increased cancer in tissues in the hematopoietic system.
In evaluating the available evidence for the postulated MOAs for leukemia following inhalation exposure to formaldehyde, the key events for the first two postulated MOAs provides a lack of dose-response or temporal concordance and a lack of biological plausibility based on data reported in high-quality studies determined to be relevant to the problem formulation. Most of the supporting information for the postulated MOAs were limited to studies conducted in mice from one laboratory or studies conducted in animals with compromised systems that were not specifically exposed to formaldehyde. The data that reported inconsistent evidence with the postulated MOAs are from high-quality studies considered to be directly relevant in informing the problem formulation questions. There is also an absence of formaldehyde-specific or directly relevant supporting data for multiple key events including information to support that formaldehyde reaches the systemic circulation, formaldehyde damages circulating stem cells, or initiated circulating stem cells resulting from formaldehyde-induced toxicity migrate back into the bone marrow.
The presumption that formaldehyde can be systemically absorbed and impact tissues beyond the portal of entry have been extensively studied. Multiple studies have reported formaldehyde induced DNA–protein cross-links and adducts or other biomarkers of exposure measured in smokers and occupational workers (Shaham et al. Citation1996, Citation1997, Citation2003; Wang et al. Citation2009; Bono Citation2010; Lin et al. Citation2013; Peteffi et al. Citation2015; Albertini and Kaden Citation2017). However, there are some uncertainties in the results associated with the methodologies used for the measurement of the DNA protein crosslinks and whether these methods can differentiate between endogenous or exogenous crosslinks or adducts. None of the studies providing evidence of crosslinks, adducts, or biomarkers in occupational workers used methodologies that can distinguish between exogenously derived formaldehyde and endogenously derived formaldehyde, or adequately account for confounding co-exposure (Albertini and Kaden Citation2017). Therefore, those studies do not provide convincing in vivo evidence that inhalation of formaldehyde would trigger a molecular initiating event by reaching the systemic circulation and causing direct DNA damage to HSCs/HPCs in the blood.
Applying the IPCS framework highlights the lack of formaldehyde-specific dose-response information to support multiple key events for the four postulated MOAs. The integration of the epidemiological, animal toxicity, and in vitro mechanistic data that support the MOAs indicated that the weight of the evidence is inconsistent with, or contradicts, the postulated MOAs. Results of the study quality and study relevance (to the problem formulation questions) assessment helped ensure that those studies with the highest quality scores that are directly relevant to the problem formulation were used in drawing conclusions.
In conclusion, the IPCS framework provided a method for systematically and critically reviewing mechanistic data and integrating these data with other streams of evidence (i.e. epidemiological, animal). In addition, the framework allowed for consideration of study quality as well as considerations specific to the chemical of interest, in this case, endogenous production of formaldehyde and whether methods can distinguish exogenously inhaled from endogenously produced formaldehyde. Using the study quality criteria recommended by United States Environmental Protection Agency (Citation2018) for TSCA, as well as the SciRAP tool for determining study relevance to the problem formulation questions established by the European Commission (Scientific Committee on Emerging and newly Identified Health Risks Citation2012), the study quality and relevancy of the data indicated the highest quality and directly relevant studies provide evidence inconsistent with the postulated MOAs for leukemia. The results of this case study demonstrate that the IPCS framework will work in determining biological plausibility for endpoints for which data from multiple streams of evidence are available to provide supporting evidence for key events in a postulated MOA, as well as those for which the evidence is inconsistent. The results of this assessment are also consistent with the weight of evidence provided by recent epidemiological studies that report no significant increase in leukemia among workers exposed to formaldehyde (Meyers et al. Citation2013; Saberi Hosnijeh et al. Citation2013; Talibov et al. Citation2014) and recent re-analyses (Checkoway et al. Citation2015) and integration of the currently available epidemiological data (Mundt et al. Citation2017; Nielsen et al. Citation2017), as well as new analyses of mechanistic data (Mundt et al. Citation2018), all of which do not support a causal association between formaldehyde exposure and leukemia.
| Abbreviations | ||
| AML | = | acute myeloid leukemia |
| AOP | = | adverse outcome pathway |
| ANSES | = | Agency for Good, Environmental and Occupational Health and Safety |
| AA | = | Anemia |
| BM HSC | = | bone marrow hematopoietic stem cells |
| CFU-GM | = | colony forming unit-granulocyte, monocyte |
| DNA | = | deoxyribonucleic acid |
| ECHA | = | European Chemicals Agency |
| USFDA | = | United States Food and Drug Administration |
| FA | = | formaldehyde |
| GSH | = | glutathione |
| HPCs | = | hematopoietic progenitor cells |
| HSCs | = | hematopoietic stem cells |
| IRIS | = | integrated risk information system |
| IARC | = | International Agency for Research on Cancer |
| IPCS | = | International Programme on Chemical Safety |
| KE | = | key event |
| LSCs | = | leukemic stem cells |
| LOD | = | limit of detection |
| LYM | = | lymphocytes |
| LHPs | = | lymphohematopoietic cancers |
| LHM | = | lymphohematopoietic malignancies |
| M | = | male |
| MDA | = | malondialdehyde |
| MADLs | = | maximum allowable dose levels |
| Mn | = | micronuclei |
| MOA | = | mode of action |
| NALT | = | nasal-associated lymphoid tissue |
| NRC | = | National Research Council |
| NAS | = | National Academy of Science |
| NTP | = | National Toxicology Program |
| NSRLs | = | no significant risk levels |
| NHP | = | non-human primate |
| NS | = | not specified |
| OSHA | = | Occupational Safety and Health Administration |
| OEHHA | = | Office of Environmental Health Hazard Assessment |
| OPPT | = | Office of Pollution and Prevention of Toxics |
| PBMC | = | peripheral blood mononuclear cell |
| PEL | = | permissible exposure limit |
| PBPK | = | physiologically-based pharmacokinetic |
| PLT | = | platelets |
| K-SDS | = | potassium sodium dodecyl sulfate |
| QAPP | = | Quality Assurance Project Plan |
| ROS | = | reactive oxygen species |
| RBC | = | red blood cells |
| REACH | = | registration, evaluation, authorization, and restriction of chemicals |
| RR | = | relative risk |
| RAC | = | Risk Assessment Committee |
| SciRAP | = | Science in Risk Assessment and Policy |
| SCENIHR | = | Scientific Committee on Emerging and newly Identified Health Risks |
| SCE | = | sister chromatid exchange |
| SMR | = | standardized mortality ratio |
| RSCA | = | Toxic Substances Control Act |
| TDERs | = | toxicological data evaluation reviews |
| USEPA | = | United States Environmental Protection Agency |
| WBC | = | white blood cells |
| WHO | = | World Health Organization |
Supplementary Tables B – Study Quality Assessment Criteria for In Vivo Studies
Download MS Word (350.9 KB)Supplementary Tables C – Study Quality Assessment Criteria for in vitro studies
Download MS Word (115.6 KB)Supplementary Tables A – Study Quality Assessment Guidelines for Epidemiological Studies
Download MS Word (47.3 KB)Acknowledgments
The authors thank Drs. Melvin Andersen (Andersen ToxConsulting, LLC, Denver, NC), Harvey Clewell (Ramboll US Corporation, Raleigh, NC), Samuel Cohen (University of Nebraska Medical Center, Omaha, NE), James Sherman (Celanese Corporation, Irving, TX), and Michael Thirman (University of Chicago Medicine, Chicago, IL) for their insight and comments on this manuscript. The authors especially recognize Dr. James Swenberg (University of North Carolina, Chapel Hill, NC; retired) for his groundbreaking work on ultra-low-dose analytical techniques.
Declaration of interest
The authors’ employment affiliations are shown in the title block above. Both ToxStrategies and Ramboll are private consulting firms providing services to private and public organizations on toxicology and risk assessment issues.
This project was a concept presented jointly by Ramboll and ToxStrategies to the Formaldehyde Science Panel of the American Chemistry Council (ACC) (https://www.americanchemistry.com; https://formaldehyde.americanchemistry.com) in 2018, as it represented a data gap in the science for formaldehyde. The ACC is America’s oldest trade association of its kind, representing more than 170 companies engaged in the production, manufacture, and use of chemicals. The concept consisted of outlines for the proposed manuscripts and costs associated with development, as well as a plan to request review and input from relevant experts in the subject matter outside of Ramboll and ToxStrategies, All of the experts that provided review or input are included as coauthors or listed in the acknowledgments. This work was supported by the Foundation for Chemistry Research & Initiatives (FCRI), a 501(c)(3) tax-exempt organization established by the ACC with funding from industry (https://foundationforchemistry.org); however, no one from FCRI or ACC was involved in the preparation of the manuscript. Members of the Formaldehyde Science Panel of ACC, under the direction and coordination of Dr. Kimberly Wise White, were given the opportunity to review the draft manuscript. The purpose of this review was for the authors to receive input on the clarity of the science presented but not on the interpretation of research results. Most of the members did not provide any comments and those that did are listed in the acknowledgments. The researchers’ scientific conclusions and professional judgments were not subject to the funders’ control; the contents of this manuscript reflect solely the view of the authors.
None of the authors, with the exception of RA and KL, received direct compensation from FCRI or ACC for this project. The project was funded through contracts between FCRI and Ramboll or ToxStrategies. Ramboll is also currently contracted by FCRI or ACC on three other projects involving the evaluation of the available science and a risk assessment for formaldehyde. Both Ramboll and ToxStrategies are also currently contracted with multiple chemical panels at ACC to provide scientific consulting support for chemicals other than formaldehyde. All the scientists of Ramboll (RG, AF, JS, and TG) and ToxStrategies (CT) involved in the development of the current manuscript were provided salary compensation as part of their employment as consultants. As noted previously, relevant experts were asked for review and input and are either coauthors or listed in the acknowledgments. In addition, anyone else who provided comments for consideration are listed in the acknowledgments. RA received an honorarium from ACC for his involvement in the development of the manuscript. RA is currently under contract with other ACC Panels and is involved in genotoxicity and risk assessment issues for other chemicals. RA is an international expert in the area of mutagenicity/genotoxicity and is also a subcontractor to Ramboll on other projects related to human mutagenicity monitoring assays that best detect bone marrow effects. KL also received an honorarium for his involvement in the development of this manuscript, as well as an additional manuscript funded by FCRI/ACC on the mode of action of nasal tumors following inhalation of formaldehyde (Thompson et al. Citation2019). KL currently and has previously received funding from FCRI and ACC for formaldehyde research he is involved in that is conducted at the University of North Carolina at Chapel Hill.
There are no conflicts of interest for any of the authors to disclose related to the submission of this manuscript. None of the authors are currently engaged to testify as experts on behalf of the sponsors in litigation related to formaldehyde. Prior to the initiation of this project, RG participated in a meeting with the EPA on behalf of ACC to discuss the current state of the science for formaldehyde and the need to consider the mode of action data in a risk assessment for formaldehyde. None of the other co-authors were involved in this meeting. It is anticipated that regulatory authorities will consider the contents of this review in making regulatory decisions regarding the potential health effects of formaldehyde.
Supplemental material
Supplemental material for this article is available online here.
Notes
1 Air concentrations of formaldehyde presented in ppm can be converted to mg/m3 by multiplying the ppm concentrations by the molecular weight for formaldehyde of 30.03 grams per mole and dividing by the molar volume at 1 atm and 25 °C of 24.45 L.
References
- Abkowitz J, Robinson A, Kale S, Long M, Chen J. 2003. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 102(4):1249–1253.
- Adami HO, Berry SCL, Breckenridge CB, Smith LL, Swenberg JA, Trichopoulos D, Weiss NS, Pastoor TP. 2011. Toxicology and epidemiology: improving the science with a framework for combining toxicological and epidemiological evidence to establish causal inference. Toxicol Sci. 122(2):223–234.
- Agency for Toxic Substances and Disease Registry. 1998. Toxicological profile for chloromethane. Silver Spring (MD): USDHHS; Atlanta (GA): ATSDR.
- Albertini R, Kaden D. 2017. Do chromosome changes in blood cells implicate formaldehyde as a leukemogen? Crit Rev Toxicol. 47(2):145–184.
- American Cancer Society. 2018. Leukemia. Atlanta (GA): ACS.
- Andersen ME, Gentry PR, Swenberg JA, Mundt KA, White KW, Thompson C, Bus J, Sherman JH, Greim H, Bolt H, et al. 2019. Considerations for refining the risk assessment process for formaldehyde: results from an interdisciplinary workshop. Regul Toxicol Pharmacol. 106:210–223.
- ANSES. 2018. Opinion of the French Agency for Good, Environmental and Occupational Health and Safety on the revision of ANSES’s reference values for formaldehyde: occupational exposure limits (OELs), derived no-effect levels (DNELs) for professionals, toxicity reference values (TRVs) and indoor air quality guidelines (IAQGs). Maisons-Alfort (France): ANSES; https://www.anses.fr/en/content/indoor-air-quality
- Appelman LM, Woutersen RA, Zwart A, Falke HE, Feron VJ. 1988. One-year inhalation toxicity study of formaldehyde in male rats with a damaged or undamaged nasal mucosa. J Appl Toxicol. 8 (2):85–90.
- Barker S, Weinfeld M, Zheng J, Li L, Murray D. 2005. Identification of mammalian proteins cross-linked to DNA by ionizing radiation. J Biol Chem. 280(40):33826–33838.
- Battelle Columbus Laboratories. 1981. A chronic inhalation toxicity study in rats and mice exposed to formaldehyde. Columbus (OH): Battelle Memorial Institute.
- Beane-Freeman LE, Blair A, Lubin JH, Stewart PA, Hayes RB, Hoover RN, Hauptmann M. 2009. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: the national cancer Institute cohort. J Natl Cancer Inst. 101(10):751–761.
- Bolt HM, Johnson G, Nielsen GD, Papameletiou D, Klein CL. 2016. SCOEL/REC/125 formaldehyde recommendation from the Scientific Committee on Occupational Exposure Limits. Adopted 30 June 2016. Luxembourg (Luxembourg): Publications Office of the European Union.
- Bolt HM, Roos PH, Thier R. 2003. The cytochrome P-450 isoenzyme CYP2E1 in the biological processing of industrial chemicals: consequences for occupational and environmental medicine. Int Arch Occup Environ Health. 76(3):174–185.
- Bono F. 2010. Pleural mesothelioma in situ and its adenocarcinoma simulator. Int J Surg Pathol. 18(6):519–520.
- Boobis A, Cohen S, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, Farland W. 2006. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 36(10):781–792.
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. 2008. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38(2):87–96.
- Bosetti C, McLaughlin JK, Tarone RE, Pira E, La Vecchia C. 2008. Formaldehyde and cancer risk: a quantitative review of cohort studies through 2006. Ann Oncol. 19(1):29–43.
- Bundesinstitut für Risikobewertung. 2006. Toxicological assessment of formaldehyde. Opinion of BfR No. 023/2006 of 30 March 2006. Berlin (Germany): BfR; http://www.bfr.bund.de/cm/350/assessment_of_the_carcinogenicity_of_formaldehyde.pdf
- Calvi LM, Link DC. 2015. The hematopoietic stem cell niche in homeostasis and disease. Blood. 126(22):2443–2451.
- Casanova M, Heck H. 1987. Further studies of the metabolic incorporation and covalent binding of inhaled [3H]- and [14C]formaldehyde in Fischer-344 rats: effects of glutathione depletion. Toxicol Appl Pharmacol. 89(1):105–121.
- Casanova M, Heck HD, Everitt JI, Harrington WW, Jr, Popp JA. 1988. Formaldehyde concentrations in the blood of rhesus monkeys after inhalation exposure. Food Chem Toxicol. 26(8):715–716.
- Casanova-Schmitz M, David RM, Heck HD. 1984. Oxidation of formaldehyde and acetaldehyde by NAD+-dependent dehydrogenases in rat nasal mucosal homogenates. Biochem Pharmacol. 33(7):1137–1142.
- Casanova-Schmitz M, Starr TB, Heck H. 1984. Differentiation between metabolic incorporation and covalent binding in the labelling of macromolecules in the rat nasal mucosa and bone marrow by inhaled [14C]-and [3H]formaldehyde. Toxicol Appl Pharmcol. 76(1):26–44.
- Chang JC, Barrow CS. 1984. Sensory irritation tolerance and cross-tolerance in F-344 rats exposed to chlorine or formaldehyde gas. Toxicol Appl Pharmacol. 76(2):319–327.
- Chang JC, Gross EA, Swenberg JA, Barrow CS. 1983. Nasal cavity deposition, histopathology, and cell proliferation after single or repeated formaldehyde exposures in B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol. 68(2):161–176.
- Chang JC, Steinhagen WH, Barrow CS. 1981. Effect of single or repeated formaldehyde exposure on minute volume of B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol. 61(3):451–459.
- Checkoway H, Boffetta P, Mundt DJ, Mundt KA. 2012. Critical review and synthesis of the epidemiologic evidence on formaldehyde exposure and risk of leukemia and other lymphohematopoietic malignancies. Cancer Causes Control. 23(11):1747–1766.
- Checkoway H, Dell LD, Boffetta P, Gallagher AE, Crawford L, Lees PS, Mundt KA. 2015. Formaldehyde exposure and mortality risks from acute myeloid leukemia and other lymphohematopoietic malignancies in the US national cancer Institute cohort study of workers in formaldehyde industries. J Occup Environ Med. 57(7):785–794.
- Checkoway H, Lees PSJ, Dell LD, Gentry PR, Mundt KA. 2019. Peak exposures in epidemiologic studies and cancer risks: considerations for regulatory risk assessment. Risk Anal. 39(7):1441–1464.
- Coggon D, Harris EC, Poole J, Palmer KT. 2003. Extended follow-up of a cohort of British chemical workers exposed to formaldehyde. J Natl Cancer Inst. 95(21):1608–1615.
- Coggon D, Ntani G, Harris EC, Palmer KT. 2014. Upper airway cancer, myeloid leukemia, and other cancers in a cohort of British chemical workers exposed to formaldehyde. Am J Epidemiol. 179(11):1301–1311.
- Collins JJ, Lineker GA. 2004. A review and meta-analysis of formaldehyde exposure and leukemia. Regul Toxicol Pharmacol. 40(2):81–91.
- Cruzan G. 2009. Assessment of the cancer potential of methanol. Crit Rev Toxicol. 39(4):347–363.
- Dallas CE, Scott MJ, Ward JB, Jr, Theiss JC. 1992. Cytogenetic analysis of pulmonary lavage and bone marrow cells of rats after repeated formaldehyde inhalation. J Appl Toxicol. 12(3):199–203.
- Dykewicz M, Patterson R, Cugell DW, Harris KE, Wu AF. 1991. Serum IgE and IgG to formaldehyde-human serum albumin: lack of relation to gaseous formaldehyde exposure and symptoms. J Allergy Clin Immunol. 87(1):48–57.
- Edrissi B, Taghizadeh K, Moeller B, Kracko D, Doyle-Eisele M, Swenberg J, Dedon P. 2013. Dosimetry of N6-formyllysine adducts following [13C2H2]-formaldehyde exposures in rats. Chem Res Toxicol. 26(10):1421–1423.
- Environmental Pathology Laboratories, Inc. 2014. Review of Zhang et al. “bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde”. Draft Consultation Report. 2014. Atlanta (GA): American Chemistry Council.
- European Chemical Agency. 2016. Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products. Evaluation of active substances. Assessment report. Formaldehyde product-type 03 (Veterinary hygiene). January 2016. Helsinki (Finland): ECHA.
- European Chemical Agency. 2019a. Formaldehyde (EC number: 200-001-8; CAS number: 50-00-0). Helsinki (Finland): ECHA; [accessed 2019 October 21]. https://echa.europa.eu/registration-dossier/-/registered-dossier/15858
- European Chemical Agency. 2019b. Annex XV restriction report, proposal for a restriction (Substance Name: Formaldehyde and formaldehyde releasers; IUPAC Name: Formaldehyde and formaldehyde releasers; EC Number: 200-001-08; CAS Number: 50-00-0). Version Number: 1.1 (Dated March 20, 2019). Helsinki (Finland): ECHA
- European Chemical Agency. 2019c. Worker exposure to formaldehyde and formaldehyde releasers (Substance Name: Formaldehyde; IUPAC Name: Formaldehyde; EC Number: 200-001-08 [Formaldehyde]; CAS Number: 50-00-0 [Formaldehyde]). Version Number: 1 (Dated July 12, 2019). Helsinki (Finland): ECHA.
- European Food Safety Authority. 2014. Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources. EFSA J. 12(2):3550.
- Farland WH, Lynch A, Erraguntla NK, Pottenger LH. 2019. Improving risk assessment approaches for chemicals with both endogenous and exogenous exposures. Regul Toxicol Pharmacol. 103:210–215.
- Fedak KM, Bernal A, Capshaw ZA, Gross S. 2015. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 12:14.
- Feron VJ, Bruyntjes JP, Woutersen RA, Immel HR, Appelman LM. 1988. Nasal tumours in rats after short-term exposure to a cytotoxic concentration of formaldehyde. Cancer Lett. 39(1):101–111.
- Fleig I, Petri N, Stocker WG, Thiess AM. 1982. Cytogenetic analyses of blood lymphocytes of workers exposed to formaldehyde in formaldehyde manufacturing and processing. J Occup Med. 24(12):1009–1012.
- Fox CH, Johnson FB, Whiting J, Roller PP. 1985. Formaldehyde fixation. J Histochem Cytochem. 33(8):845–853.
- Franks SJ. 2005. A mathematical model for the absorption and metabolism of formaldehyde vapour by humans. Toxicol Appl Pharmacol. 206(3):309–320.
- Gao N, Chang Q, Chen L, Li XB, Xu Y, Ding SM. 2008. Effect of micronucleus rate in peripheral lymphocytes and liver cells of mice exposed to gaseous formaldehyde. J Pub Health Prev Med. 19:7–9.
- Gentry PR, Rodricks JV, Turnbull D, Bachand A, Van Landingham C, Shipp AM, Albertini RJ, Irons R. 2013. Formaldehyde exposure and leukemia: critical review and reevaluation of the results from a study that is the focus for evidence of biological plausibility. Crit Rev Toxicol. 43(8):661–670.
- Grammer LC, Harris KE, Cugell DW, Patterson R. 1993. Evaluation of a worker with possible formaldehyde-induced asthma. J Allergy Clin Immunol. 92(1):29–33.
- Grammer LC, Harris KE, Shaughnessy M, Sparks P, Ayars GH, Altman LC, Patterson R. 1990. Clinical and immunologic evaluation of 37 workers exposed to gaseous formaldehyde. J Allergy Clin Immunol. 86(2):177–181.
- Hall A, Harrington JM, Aw TC. 1991. Mortality study of British pathologists. Am J Ind Med. 20(1):83–89.
- Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. 2003. Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries. J Natl Cancer Inst. 95(21):1615–1623.
- Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. 2004. Mortality from solid cancers among workers in formaldehyde industries. Am J Epidemiol. 159(12):1117–1130.
- Hayes RB, Blair A, Stewart PA, Herrick RF, Mahar H. 1990. Mortality of U.S. embalmers and funeral directors. Am J Ind Med. 18(6):641–652.
- Health Canada. 1999. Priority substances list assessment report, formaldehyde. Toronto (Canada): Health Canada.
- Heck H, Casanova M. 2004. The implausibility of leukemia induction by formaldehyde: a critical review of the biological evidence on distant-site toxicity. Regul Toxicol Pharmacol. 40(2):92–106.
- Heck H, White E, Casanova-Schmitz M. 1982. Determination of formaldehyde in biological tissues by gas chromatography/mass spectrometry. Biomed Mass Spectrom. 9(8):347–353.
- Heck HD, Casanova M, Dodd PB, Schachter EN, Witek TJ, Tosun T. 1985. Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rats exposed to CH2O under controlled conditions. Am Ind Hyg Assoc J. 46(1):1–3.
- Heck HD, Casanova M, Steinhagen WH, Everitt JI, Morgan KT, Popp JA. 1989. Formaldehyde toxicity: DNA–protein cross-linking studies in rats and nonhuman primates. In: Feron VJ, Bosland MC, editors. Nasal carcinogenesis in rodents: relevance to human risk. Wageningen (The Netherlands): PUDOC; p. 159–164.
- Hill AB. 1965. The environment and disease: association or causation? Proc R Soc Med. 58(5):295–300.
- International Agency for Research on Cancer. 2006. Formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2propanol, 2006. Lyon (France): IARC.
- International Agency for Research on Cancer. 2012. IARC monographs on the evaluation of carcinogenic risks to humans. A review of human carcinogens part F: chemical agents and related occupations. vol. 100. Lyon (France): IARC.
- Irons RD, Kerzic PJ. 2014. Cytogenetics in benzene-associated myelodysplastic syndromes and acute myeloid leukemia: new insights into a disease continuum. Ann NY Acad Sci. 1310:84–88.
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. 2009. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 138(2):271–285.
- Ji Z, Li X, Fromowitz M, Mutter-Rottmayer E, Tung J, Smith MT, Zhang L. 2014. Formaldehyde induces micronuclei in mouse erythropoietic cells and suppresses the expansion of human erythroid progenitor cells. Toxicol Lett. 224(2):233–239.
- Kamata E, Nakadate M, Uchida O, Ogawa Y, Suzuki S, Kaneko T, Saito M, Kurokawa Y. 1997. Results of a 28-month chronic inhalation toxicity study of formaldehyde in male Fisher-344 rats. J Toxicol Sci. 22(3):239–254.
- Katsnelson BA, Degtyareva TD, Privalova LI, Minigaliyeva IA, Slyshkina TV, Ryzhov VV, Beresneva OY. 2013. Attenuation of subchronic formaldehyde inhalation toxicity with oral administration of glutamate, glycine and methionine. Toxicol Lett. 220(2):181–186.
- Kerns WD, Pavkov KL, Donofrio DJ, Gralla EJ, Swenberg JA. 1983. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 43(9):4382–4392.
- Kitaeva LV, Mikheeva EA, Shelomova LF, Shvartsman PI. 1996. Genotoxic effect of formaldehyde in somatic human cells in vivo. Genetika. 32(9):1287–1290.
- Kleinnijenhuis AJ, Staal YC, Duistermaat E, Engel R, Woutersen R. 2013. The determination of exogenous formaldehyde in blood of rats during and after inhalation exposure. Food Chem Toxicol. 52:105–112.
- Kligerman AD, Phelps MC, Erexson GL. 1984. Cytogenetic analysis of lymphocytes from rats following formaldehyde inhalation. Toxicol Lett. 21(3):241–246.
- Kornbrust DJ, Bus JS. 1983. The role of glutathione and cytochrome P-450 in the metabolism of methyl chloride. Toxicol Appl Pharmacol. 67(2):246–256.
- Lai Y, Yu R, Hartwell H, Moeller B, Bodnar W, Swenberg J. 2016. Measurement of endogenous versus exogenous formaldehyde-induced DNA-protein crosslinks in animal tissues by stable isotope labeling and ultrasensitive mass spectrometry. Cancer Res. 76(9):2652–2561.
- Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, et al. 2017. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 544(7648):105–109.
- Leng J, Liu C-W, Hartwell HJ, Yu R, Lai Y, Bodnar WM, Lu K, Swenberg JA. 2019. Evaluation of inhaled low-dose formaldehyde-induced DNA adducts and DNA-protein cross-links by liquid chromatography-tandem mass spectrometry. Arch Toxicol. 93(3):763–773.
- Levine RJ, Andjelkovich DA, Shaw LK. 1984. The mortality of Ontario undertakers and a review of formaldehyde-related mortality studies. J Occup Med. 26(10):740–746.
- Li GY, Lee HY, Shin HS, Kim HY, Lim CH, Lee BH. 2007. Identification of gene markers for formaldehyde exposure in humans. Environ Health Perspect. 115(10):1460–1466.
- Lin D, Guo Y, Yi J, Kuang D, Li X, Deng H, Huang K, Guan L, He Y, Zhang X, et al. 2013. Occupational exposure to formaldehyde and genetic damage in the peripheral blood lymphocytes of plywood workers. Jrnl of Occup Health. 55(4):284–291.
- Liteplo RG, Meek ME. 2003. Inhaled formaldehyde: exposure estimation, hazard characterization, and exposure-response analysis. J Toxicol Environ Health B Crit Rev. 6(1):85–114.
- Liu J, Hanchao Y, Chen M, Lui H, Wang G, Yan X. 2003. A study on the genetic toxicity of formaldehyde emitted from wood-based panels. Acta Scientiae Circumstantiae. 23:679–682.
- Lu K, Collins L, Hongyu R, Bermudez E, Swenberg J. 2010. Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol Sci. 116(2):441–451.
- Lu K, Gu GH, Upton P, Moeller B, Swenberg J. 2012. Formation of hydroxymethyl DNA adducts in rats orally exposed to stable isotope labeled methanol. Toxicol Sci. 126(1):28–38.
- Lu K, Moeller B, Doyle-Eisele M, McDonald J, Swenberg J. 2011. Molecular dosimetry of N2-hydroxymethyl-dG DNA adducts in rats exposed to formaldehyde. Chem Res Toxicol. 24(2):159–161.
- Marsh GM, Youk AO. 2005. Reevaluation of mortality risks from nasopharyngeal cancer in the formaldehyde cohort study of the National Cancer Institute. Regul Toxicol Pharmacol. 42(3):275–283.
- Matanoski G. 1989. Risk of pathologists exposed to formaldehyde. Final Report. Baltimore (MD): John Hopkins University; Department of Epidemiology, School of Hygiene and Public Health.
- McKinney-Freeman S, Goodell MA. 2004. Circulating hematopoietic stem cells do not efficiently home to bone marrow during homeostasis. Exp Hematol. 32(9):868–876.
- Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. 2014. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 34(1):1–18.
- Meng F, Bermudez E, McKinzie PB, Andersen ME, Clewell HJ, III, Parsons BL. 2010. Measurement of tumor-associated mutations in the nasal mucosa of rats exposed to varying doses of formaldehyde. Regul Toxicol Pharmacol. 57(2–3):274–283.
- Meyers AR, Pinkerton LE, Hein MJ. 2013. Cohort mortality study of garment industry workers exposed to formaldehyde: update and internal comparisons. Am J Ind Med. 56(9):1027–1039.
- Moeller BC, Lu K, Doyle-Eisele M, McDonald J, Gigliotti A, Swenberg J. 2011. Determination of N2-hydroxymethyl-dG adducts in the nasal epithelium and bone marrow of nonhuman primates following 13CD2-formaldehyde inhalation exposure. Chem Res Toxicol. 24(2):162–164.
- Morgan DL, Dixon D, King DH, Travlos GS, Herbert RA, French JE, Tokar EJ, Waalkes MP, Jokinen MP. 2017. NTP research report on absence of formaldehyde-induced neoplasia in Trp53 haploinsufficient mice exposed by inhalation. NTP RR 3. Research Triangle Park, North Carolina: National Toxicology Program; p. 1–29.
- Mundt KA, Gallagher AE, Dell LD, Natelson EA, Boffetta P, Gentry PR. 2017. Does occupational exposure to formaldehyde cause hematotoxicity and leukemia-specific chromosome changes in cultured myeloid progenitor cells? Crit Rev Toxicol. 47(7):592–602.
- Mundt KA, Gentry PR, Dell LD, Rodricks JV, Boffetta P. 2018. Six years after the NRC review of EPA’s Draft IRIS Toxicological Review of Formaldehyde: regulatory implications of new science in evaluating formaldehyde leukemogenicity. Regul Toxicol Pharmacol. 92:472–490.
- Murrell WF, Féron F, Wetzig A, Cameron N, Splatt K, Bellette B, Bianco J, Perry C, Lee G, Mackay-Sim A. 2005. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 233(2):496–515.
- National Academy of Sciences. 2014. Review of the formaldehyde assessment in the national toxicology program 12th report on carcinogens. Washington (DC): NAS.
- National Institutes of Health. 2001. Stem cells: scientific progress and future research directions. Washington (DC): Office of Science Policy.
- National Research Council. 2011. Review of Environmental Protection Agency’s draft IRIS assessment of formaldehyde, 2011. Washington (DC): Office of Science Policy.
- National Toxicology Program. 2015. Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. Washington (DC): United States Department of Health and Human Services, National Toxicology Program. https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf
- National Toxicology Program. 2016. Report on carcinogens. 14th ed. Research Triangle Park (NC): United States Department of Health and Human Services, Public Health Service.
- Neuss S, Moepps B, Speit G. 2010. Exposure of human nasal epithelial cells to formaldehyde does not lead to DNA damage in lymphocytes after co-cultivation. Mutagenesis. 25(4):359–364.
- Nielsen GD, Larsen ST, Wolkoff P. 2017. Re-evaluation of the WHO (2010) formaldehyde indoor air quality guideline for cancer risk assessment. Arch Toxicol. 91(1):35–61.
- Occupational Safety and Health Administration. 2020. 1910.1948 – Formaldehyde. Occupational Safety and Health Standards. 1910 Subpart Z (Toxic and hazardous substances). Washington (DC): United States Department of Labor, Occupational Safety and Health Administration; https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1048
- Peteffi GP, Basso da Silva L, Antunes MV, Wilhelm C, Valandro ET, Glaeser J, Kaefer D, Linden R. 2015. Evaluation of genotoxicity in workers exposed to low levels of formaldehyde in a furniture manufacturing facility. Toxicol Indust Health. 32(10):1763–1773.
- Pinkerton LE, Hein MJ, Stayner LT. 2004. Mortality among a cohort of garment workers exposed to formaldehyde: an update. Occup Environ Med. 61(3):193–200.
- Pira E, Romano C, Verga F, La VC. 2014. Mortality from lymphohematopoietic neoplasms and other causes in a cohort of laminated plastic workers exposed to formaldehyde. Cancer Causes Control. 25(10):1343–1349.
- Pontel LB, Rosado IV, Burgos-Barragan G, Garaycoechea JI, Yu R, Arends MJ, Chandrasekaran G, Broecker V, Wei W, Liu L, et al. 2015. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol Cell. 60(1):177–178.
- Priha E, Liesivuori J, Santa H, Laatikainen R. 1996. Reactions of hydrated formaldehyde in nasal mucus. Chemosphere. 32(6):1077–1082.
- Rhomberg LR, Bailey LA, Goodman JE, Hamade AK, Mayfield D. 2011. Is exposure to formaldehyde in air causally associated with leukemia?-A hypothesis-based weight-of-evidence analysis. Crit Rev Toxicol. 41(7):555–621.
- Risk Assessment Committee. 2012. Opinion proposing harmonised classification and labelling at EU level of formaldehyde. Helsinki (Finland): European Chemicals Agency; [accessed 2017 March 30]. https://echa.europa.eu/documents/10162/254a73cf-ff8d-4bf4-95d1-109f13ef0f5a
- Saberi Hosnijeh F, Christopher Y, Peeters P, Romieu I, Xun W, Riboli E, Raaschou-Nielsen O, Tjønneland A, Becker N, Nieters A, et al. 2013. Occupation and risk of lymphoid and myeloid leukaemia in the european prospective investigation into cancer and nutrition (EPIC). Occup Environ Med. 70(7):464–470.
- Schmid O, Speit G. 2007. Genotoxic effects induced by formaldehyde in human blood and implications for the interpretation of biomonitoring studies. Mutagenesis. 22(1):69–74.
- Schroeter JD, Campbell J, Kimbell JS, Conolly RB, Clewell HJ, III, Andersen ME. 2014. Effects of endogenous formaldehyde in nasal tissues on inhaled formaldehyde dosimetry predictions in the rat, monkey, and human nasal passages. Toxicolog Sci. 138(2):412–424.
- Scientific Committee on Emerging and newly Identified Health Risks. 2012. Memorandum on the use of the scientific literature for human health risk assessment purposes – weighting of evidence and expression of uncertainty. Luxembourg (Luxembourg): Scientific Committee on Emerging and newly Identified Health Risks, European Commission.
- Shaham J, Bomstein Y, Melzer A, Ribak J. 1997. DNA-protein crosslinks and sister chromatid exchanges as biomarkers of exposure to formaldehyde. Int J Occup Environ Health. 3(2):95–104.
- Shaham J, Bomstein Y, Gurvich R, Rashkovsky M, Kaufamn Z. 2003. DNA-protein crosslinks and p53 protein expression in relation to occupational exposure to formaldehyde. Occup Environ Med. 60(6):403–409.
- Shaham J, Melzer A, Kaufman Z, Ribak J. 1996. Occupation and bladder cancer. Harefuah. 131(10):382–386.
- Speit G, Kühner S, Linsenmeyer R, Schütz P. 2011. Does formaldehyde induce aneuploidy? Mutagenesis. 26(6):805–811.
- Speit G, Schmid O, Fröhler-Keller M, Lang I, Triebig G. 2007. Assessment of local genotoxic effects of formaldehyde in humans measured by the micronucleus test with exfoliated buccal mucosa cells. Mutat Res. 627(2):129–135.
- Speit G, Zeller J, Schmid O, Elhajouji A, Ma-Hock L, Neuss S. 2009. Inhalation of formaldehyde does not induce systemic genotoxic effects in rats. Mutat Res. 677(1–2):76–85.
- Stroup NE, Blair A, Erikson GE. 1986. Brain cancer and other causes of death in anatomists. J Natl Cancer Inst. 77(6):1217–1224.
- Swenberg J, Lu K, Moeller B, Gao L, Upton P, Nakamura J, Starr JB. 2011. Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology and risk assessment. Toxicol Sci. 120:130–145.
- Swenberg J, Moeller B, Lu K, Rager J, Fry R, Starr TB. 2013. Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment. Toxicol Pathol. 41(2):181–189.
- Talibov M, Lehtinen-Jacks S, Martinsen JI, Kjaerheim K, Lynge E, Sparén P, Tryggvadottir L, Weiderpass E, Kauppinen T, Kyyrönen P, et al. 2014. Occupational exposure to solvents and acute myeloid leukemia: a population-based, case-control study in four Nordic countries. Scand J Work Environ Health. 40(5):511–517.
- Tao XY, Yu SY, Kang L, Huang HX, Wei AY. 2004. Study on the genetic damage in mice induced by the volatile organic compounds of decoration materials. Zhonghua Laodong Weisheng Zhiyebing Zazhi. 22:194–196.
- Thompson CM, Gentry R, Fitch S, Lu K, Clewell HJ. III. Forthcoming. An updated mode of action and human relevance framework evaluation for formaldehyde-related nasal tumors. Crit Rev Toxicol.
- Thrasher JD, Broughton A, Madison R. 1990. Immune activation and autoantibodies in humans with long-term inhalation exposure to formaldehyde. Arch Environ Health. 45(4):217–223.
- Tretyakova NY, Groehler A, 4th, Ji S. 2015. DNA-protein cross-links: formation, structural identities, and biological outcomes. Acc Chem Res. 48(6):1631–1644.
- United States Environmental Protection Agency. 1989. Integrated Risk Information System (IRIS) formaldehyde (CASRN 50-00-0). Chemical Assessment Summary. Washington (DC): USEPA.
- United States Environmental Protection Agency. 1994. Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. EPA/600/8-90/066F. Washington (DC): USEPA.
- United States Environmental Protection Agency. 2009. Risk assessment guidance for superfund. Volume I: human health evaluation manual. (Part F, Supplemental Guidance for Inhalation Risk Assessment) EPA-540-R-070-002. OSWER 9285. Washington (DC): USEPA; p. 7–82.
- United States Environmental Protection Agency. 2010. Toxicological review of formaldehyde – inhalation assessment (CAS No. 50-00-0). External review draft. Washington (DC): USEPA.
- United States Environmental Protection Agency. 2018. Application of systematic review in TSCA risk evaluations. USEPA Document # 740-P1-8001. Washington (DC): USEPA.
- Viegas S, Ladeira C, Nunes C, Malta-Vacas J, Gomes M, Brito M, Mendonca P, Prista J. 2010. Genotoxic effects in occupational exposure to formaldehyde: a study in anatomy and pathology laboratories and formaldehyde-resins production. J Occup Med Toxicol. 5(1):25–32.
- Walker FJ. 1964. Formaldehyde. 3rd ed. New York (NY): Reinhold.
- Walrath J, Fraumeni JF. Jr. 1983. Mortality patterns among embalmers. Int J Cancer. 31(4):407–411.
- Wang M, Cheng G, Balbo S, Carmella SG, Villalta PW, Hecht SS. 2009. Clear differences in levels of a formaldehyde-DNA adduct in leukocytes of smokers and nonsmokers. Cancer Res. 69(18):7170–7174.
- Wei C, Wen H, Yuan L, Mchale CM, Li H, Wang K, Yuan J, Yang X, Zhang L. 2017. Formaldehyde induces toxicity in mouse bone marrow and hematopoietic stem/progenitor cells and enhances benzene-induced adverse effects. Arch Toxicol. 91(2):921–933.
- Whalan JE, Jarabek AM, Stanek J, Woodall G, Reinhart P, Galizia A, McLanahan E, Glenn B, David A, Kraft A, et al. 2014. Systematic evaluation of inhalation studies for exposure quality: a case study with formaldehyde. The Toxicol. 138(1):479.
- World Health Organization. 2010. WHO guidelines for indoor air quality. Selected pollutants. Copenhagen (Denmark): WHO Regional Office for Europe.
- Ye X, Ji Z, Wei C, McHale CM, Ding S, Thomas R, Yang X, Zhang L. 2013. Inhaled formaldehyde induces DNA-protein crosslinks and oxidative stress in bone marrow and other distant organs of exposed mice. Environ Mol Mutagen. 54(9):705–718.
- Yu G, Chen Q, Liu X, Guo C, Du H, Sun Z. 2014. Formaldehyde induces bone marrow toxicity in mice by inhibiting peroxiredoxin 2 expression. Mol Med Rep. 10(4):1915–1920.
- Yu R, Lai Y, Hartwell HJ, Moeller BC, Doyle-Eisele M, Kracko D, Bodnar WM, Starr TB, Swenberg JA. 2015. Formation, accumulation, and hydrolysis of endogenous and exogenous formaldehyde-induced DNA damage. Toxicol Sci. 146(1):170–182.
- Yu G, Song X, Zhao S, Liu Y, Sun Z. 2015. Formaldehyde induces the bone marrow toxicity in mice by regulating the expression of Prx3 Protein. J Huazhong Univ Sci Technolog Med Sci. 35(1):82–86.
- Zeller J, Neuss S, Mueller JU, Kuhner S, Holzmann K, Hogel J, Klingmann C, Bruckner T, Triebig G, Speit G. 2011. Assessment of genotoxic effects and changes in gene expression in humans exposed to formaldehyde by inhalation under controlled conditions. Mutagenesis. 26(4):555–561.
- Zeller J, Ulrich A, Mueller JU, Riegert C, Neuss S, Bruckner T, Triebig G, Speit G. 2011b. Is individual nasal sensitivity related to cellular metabolism of formaldehyde and susceptibility towards formaldehyde-induced genotoxicity? Mutat Res. 723(1):11–17.
- Zhang L. 2018. Formaldehyde exposure, toxicity, and health effects. Issues in Toxicology No. 37. London (UK): CPI Group (UK) Ltd.
- Zhang L, Steinmaus C, Eastmond D, Xin X, Smith M. 2009. Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat Res. 681(2–3):150–168.
- Zhang L, Tang X, Rothman N, Vermeulen R, Ji Z, Shen M, Qiu C, Guo W, Liu S, Reiss B, et al. 2010. Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol Biomarkers Prev. 19(1):80–88.
- Zhang Y, Liu X, McHale C, Li R, Zhang L, Wu Y, Ye X, Yang X, Ding S. 2013. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One. 8(9):e74974.
- Zhao Q, Duan LJ, Liu YS, Lu ZS, Qiao Y, Yan Y, Yang X. 2004. Effect of formaldehyde from artificial based boards on micronucleus of mice marrow polychromatic erythrocyte. J Public Health Prev Med. 15:18–20.
- Zhao Y, Magaña LC, Cui H, Huang J, McHale CM, Yang X, Looney MR, Li R, Zhang L. 2020. Formaldehyde-induced hematopoietic stem and progenitor cell toxicity in mouse lung and nose. Arch Toxicol. Online ahead of print. doi: 10.1007/s00204-020-02932-x.

