Abstract
The current understanding of thyroid-related adverse outcome pathways (AOPs) with adverse neurodevelopmental outcomes in mammals has been reviewed. This served to establish if standard rodent toxicity test methods and in vitro assays allow identifying thyroid-related modes-of-action potentially leading to adverse neurodevelopmental outcomes, and the human relevance of effects – in line with the European Commission’s Endocrine Disruptor Criteria. The underlying hypothesis is that an understanding of the key events of relevant AOPs provides insight into differences in incidence, magnitude, or species sensitivity of adverse outcomes. The rodent studies include measurements of serum thyroid hormones, thyroid gland pathology and neurodevelopmental assessments, but do not directly inform on specific modes-of-action. Opportunities to address additional non-routine parameters reflecting critical events of AOPs in toxicological assessments are presented. These parameters appear relevant to support the identification of specific thyroid-related modes-of-action, provided that prevailing technical limitations are overcome. Current understanding of quantitative key event relationships is often weak, but would be needed to determine if the triggering of a molecular initiating event will ultimately result in an adverse outcome. Also, significant species differences in all processes related to thyroid hormone signalling are evident, but the biological implications thereof (including human relevance) are often unknown. In conclusion, careful consideration of the measurement (e.g. timing, method) and interpretation of additional non-routine parameters is warranted. These findings will be used in a subsequent paper to propose a testing strategy to identify if a substance may elicit maternal thyroid hormone imbalance and potentially also neurodevelopmental effects in the progeny.
Introduction
The European Commission (Citation2017, Citation2018) has adopted criteria for the determination of endocrine disrupting properties of substances regulated under the Biocidal Products Regulation (EU) No 528/2012 (EP and Council Citation2012) and the Plant Protection Products Regulation (EC) No 1107/2009 (EP and Council Citation2009). A substance shall be considered as having endocrine disrupting properties that may cause adverse effect in humans if it meets all of three criteria: (1) It shows adverse effects in an intact organism or its progeny. (2) It alters the function(s) of the endocrine system, i.e. it exhibits endocrine activity. (3) It has an endocrine mode-of-action (MoA), i.e. there is a biologically plausible link between the endocrine activity and the adverse effect (European Commission Citation2017, Citation2018; EFSA and ECHA Citation2018). However, a substance that meets all of these criteria shall not be considered an endocrine disruptor for humans if “there is evidence demonstrating that the adverse effects identified are not relevant to humans” (European Commission Citation2017, Citation2018). In the criteria, it is also clearly differentiated between specific and unspecific adverse effects. It is stated that “adverse effects that are non-specific secondary consequences of other toxic effects shall not be considered for the identification of the substance as endocrine disruptor” (European Commission, Citation2017, Citation2018). An assessment for endocrine disruption is conducted by the European Food Safety Authority (EFSA) for pesticidal active substances and the European Chemicals Agency (ECHA) for biocides. Active substances and biocides identified as endocrine disruptors cannot be approved in the European Union unless negligible exposure is demonstrated. In contrast, some jurisdictions (e.g. USA) apply risk assessment when regulating endocrine disruptors such that compounds with a sufficient margin of exposure (i.e. acceptable risk) can be used (US EPA Citation2013).
To support the implementation of the Endocrine Disruptor Criteria in the European Union, EFSA and ECHA have published Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009 (EFSA and ECHA Citation2018). While this Guidance generally covers the oestrogenic, androgenic, thyroidal and steroidogenic modalities, its Appendix A presents “Additional considerations for how to assess the potential for thyroid disruption for human health”. This Appendix A is intended to provide guidance on which additional data could be considered in a weight-of-evidence evaluation to substantiate that specific thyroid effects are not human relevant and/or secondary to a non-specific mechanism and how to address thyroid-related developmental neurotoxicity (DNT) concerns. Appendix A presupposes that, “in the absence of substance-specific data which provide proof of the contrary, humans and rodents are considered to be equally sensitive to thyroid disruption, including cases where liver enzyme induction is responsible for increased thyroid hormone clearance” (EFSA and ECHA Citation2018, EFSA Citation2020). However, Appendix A does not provide any scientific evidence to support this statement (Sauer et al. Citation2020), even though the assumption of equal species sensitivity to thyroid disruption constitutes a paradigm change as compared to what has been commonly agreed for many years, i.e. that rats are more sensitive than humans (Jahnke et al. Citation2004; Bartsch et al. Citation2018).
Appendix A generally describes a testing scheme to determine serum thyroid hormone levels and liver enzyme activities, and to exclude specific thyroid MoAs. However, neither Appendix A nor EFSA (Citation2020) indicate how the various parameters should be measured or how the data should be evaluated in a weight-of-evidence approach to reach a conclusion on whether, or not, a substance meets the Endocrine Disruptor Criteria. In this regard, Appendix A recognises that the identification of thyroid-related hazards is currently hampered by a lack of internationally validated test methods. Overall, based upon the provisions of Appendix A of EFSA and ECHA (Citation2018) and the further clarifications provided in EFSA (Citation2020), it is currently unclear how specific thyroid-related MoAs should be identified, and how the human relevance or irrelevance of thyroid effects and/or DNT observed in rats should be established.
To address these uncertainties, the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) has convened the Special T4 Task Force.
The goal of this Task Force is to review the available evidence to contribute to the development of a science-based tiered testing strategy to identify if (1) a substance has the ability to elicit maternal thyroid hormone imbalance and potentially also neurodevelopmental effects in the progeny; and (2) if effects observed in rodents are relevant for humans – in line with the European Commission (Citation2017, Citation2018) Endocrine Disruptor Criteria. In pursuing this goal, the ECETOC Special T4 Task Force is preparing a series of reviews, of which the present article constitutes the second.
The first review (Sauer et al. Citation2020) evaluated available human studies to explore how low serum thyroid hormone levels in pregnant mothers affect child neurodevelopment. This activity aimed at identifying parameters in the human studies which are most relevant for toxicological assessments, and should hence be included in the planned testing strategy. In pregnant mothers, serum levels of free thyroxine (fT4) and thyroid stimulating hormone (TSH) were the most frequently measured thyroid-related parameters. In children, a broad spectrum of different approaches was applied between the studies to establish neurodevelopmental outcomes. Depending on the study, assessments included psychomotor and mental development, cognitive function (intelligence quotient), expressive vocabulary or educational attainment, and, in single studies, brain morphology assessed by magnetic resonance imaging, or clinical diagnoses of conditions such as autism or attention deficit hyperactivity disorder. The human data are overall in support of an association between low maternal serum fT4 (and in some studies also high TSH) and increased risk for child neurodevelopmental impairment. However, the available evidence did not allow identifying the most sensitive parameter(s) to assess effects in the pregnant mothers or children, nor quantitative boundaries of effects indicative of increased risks. Clearly, none of the recorded studies included histological evaluations of the mothers’ thyroid gland. Also, none of the human studies allowed establishing a link between substance-mediated liver enzyme induction and increased thyroid hormone clearance, and the impact of these on child neurodevelopment (Sauer et al. Citation2020).
Taking into account the findings from the first review, this second review pursues three goals:
To collate information on the molecular initiating events (MIEs) and key events of thyroid-related MoAs and adverse outcome pathways (AOPs) that include adverse neurodevelopmental outcomes in mammals;
To establish how the respective MIEs, key events and adverse outcomes are being addressed in standard toxicity test methods;
To describe qualitatively and, if possible quantitatively, the biological processes underlying the MIEs and early key events of the AOPs, as they occur in rodents and/or humans
In order to identify potentially relevant additional parameters of thyroid-related effects, which are not (yet) addressed in routine toxicological assessments;
Also in view of identifying opportunities to establish species relevance of such events and of the key event relationships.
All of this information is highly relevant for the establishment of a scientific basis to determine whether a substance has an endocrine (thyroid-related) MoA and whether effects observed in rodents are, or are not, relevant for humans. There is general agreement that a weight-of-evidence approach is needed for this evaluation, e.g. following the WHO/IPCS MoA/species concordance framework (Meek, Boobis, et al. Citation2014; Meek, Palermo et al. Citation2014). However, to date, there is no specific guidance on how to evaluate thyroid-related MoAs. This is also due to a lack of agreed upon in vitro assays and of approaches to establish substance-specific species differences (rats vs. humans) in thyroid hormone metabolism and hence liver enzyme-induced thyroid hormone changes. Further, there are still uncertainties in critical key events and key event relationships of different thyroid-related AOPs, and there is no consensus on how to integrate all available data into weight-of-evidence assessments.
The focus of this review is on studies using rats (and some evidence from mouse studies), since regulatory toxicity studies by far most frequently use rodents. Also, in vitro assays that inform on MIEs and critical key events of thyroid-related AOPs are considered. By contrast, opportunities to address specific events by in silico modelling, toxicological test methods using non-rodent species (e.g. dogs and rabbits), and ecotoxicological test methods are excluded as these are out of scope. Further, only those AOPs, which address non-neoplastic thyroid effects and DNT are considered; neoplastic adverse outcomes, i.e. thyroid adenoma or thyroid carcinoma, are out of scope.
In order to investigate whether xenobiotics have the potential to cause neurodevelopmental effects through maternal thyroid hormone imbalance, it is essential to investigate potential MoAs by which such events could occur. This is also reflected in the European Commission (Citation2017, Citation2018) Endocrine Disruptor Criteria. To facilitate such investigations, a better of understanding of MIEs, key events, and key event relationships of relevant AOPs will provide further insight into whether adverse outcomes differ in incidence, magnitude, or species sensitivity – depending on the AOP in question. The underlying hypothesis is that not every thyroid-related MoA has the same propensity for an adverse outcome and that well-delineated AOPs are a useful concept to inform on such MoA differences.
Against this background, this review encompasses the following sections:
Thyroid-related AOPs including adverse neurodevelopmental outcomes: A presentation of the concepts of AOPs and MoAs, followed by a summary of current knowledge on thyroid-related AOPs and MoAs with adverse neurodevelopmental outcomes in mammals.
Thyroid and neurodevelopmental parameters included in rodent toxicity test methods: An overview on the available standard rodent toxicity test methods and the range of thyroid and neurodevelopmental parameters included therein. These parameters are compared to the MIEs, key events and adverse outcomes of the thyroid-related AOPs.
Non-routine parameters reflecting events of thyroid-related AOPs: A summary of the state-of-the art for additional parameters and assays reflecting the MIEs and early key events of thyroid-related AOPs, which are not (yet) included in routine toxicological assessments.
A discussion of the collated evidence to derive a conclusion on how thyroid-related MoAs can be established in toxicological assessments and how the human relevance of effects observed in rodents can be determined. Research needed to enable such assessments is identified.
Thyroid-related AOPs including adverse neurodevelopmental outcomes
Introduction to the MoA and AOP concepts
In the last decade, the concepts of MoAs and AOPs have emerged as powerful approaches to enhance the scientific understanding of the mechanisms by which substances may elicit toxicological effects including possible species differences in the expression of effects (Vinken et al. Citation2017). A MoA is defined as the biologically plausible sequence of substance-specific key events, starting with exposure and proceeding through the interaction of the substance or its metabolites with a cell, through functional and anatomical changes leading to an effect that is supported by robust experimental observations and mechanistic data (Meek, Boobis, et al. Citation2014; Meek, Palermo et al. Citation2014).
By comparison, an AOP is defined as a linear sequence of events beginning with a MIE that may lead to early cellular events and, ultimately, an observable adverse outcome (OECD Citation2017; Vinken et al. Citation2017). Hence, in contrast to MoAs, AOPs are not substance-specific and therefore do not include exposure or metabolism considerations. Notably, AOPs in biological reality are hardly ever truly linear. Instead, converging key events, as well as feedback loops that aim at restoring balance, play important roles in hazard outcomes (Knapen et al. Citation2018; Villeneuve et al. Citation2018).
Nonetheless, knowledge on AOPs has proven useful to help address the biological plausibility of a substance-specific MoA and to enhance an understanding of toxicological effects. An abundance of research is ongoing to develop AOPs for a broad spectrum of pathological processes. To focus this research effort, in 2012 the Organisation for Economic Co-operation and Development (OECD) established a new programme on the development of AOPs (OECD Citation2017); see also https://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm. Within the OECD AOP programme, an AOP Wiki (https://aopwiki.org [both websites accessed 2020 October]) has been established as a central AOP repository. Generally, the AOPs included therein should be considered living working documents up until endorsement by the OECD Task Force Hazard Assessment (TFHA)/Working Group of the National Coordinators of the Test Guideline Programme (WNT).
The information on specific AOPs collated in the AOP Wiki does not only refer to the given sequence of key events, but also to the corresponding key event relationships. This is “information that helps to define how much change in the upstream key event, and/or for how long, is needed to elicit a detectable and defined change in the downstream key event” (definition from the AOP Wiki). Therefore, a quantitative understanding of key event relationships will enhance the understanding of the toxicological implications of the triggering of the MIE, i.e. whether all downstream events are likely to occur thereby ultimately leading to the adverse outcome (Noyes et al. Citation2019). Further, different concentrations of the test substance might be necessary in different species to trigger not only the MIE, but also any subsequent key event up until the adverse outcome (Noyes et al. Citation2019). Therefore, evaluations of the human relevance of substance-mediated effects observed in rodents should, at best, not only consider whether the MoA in rodents is relevant for humans, but also whether there are quantitative differences in the key event relationships.
Thyroid-related AOPs including adverse neurodevelopmental outcomes
Knapen et al. (Citation2018), Villeneuve et al. (Citation2018), and Noyes et al. (Citation2019) published networks of thyroid-related AOPs, i.e. assemblies of several AOPs that share one or more MIEs, key events and/or adverse outcomes. These networks reflect the complexity of biological processes and consider that e.g. feedback loops may prevent a sequence of events from occurring up until the adverse outcome. While the AOP networks provide important overviews showing how different AOPs are interlinked, the AOP Wiki presents details on the current understanding of specific (linear) AOPs, including the evidence supporting specific events. Therefore, the information from the AOP Wiki has been selected as a starting point for the present review, while further considering additional MIEs and key events presented in the AOP networks.
Five AOPs in which thyroid hormone imbalance in mammals is linked to neurodevelopmental outcomes were included in the AOP Wiki as per 16 October 2020 and were not marked as “do not cite”. The AOP numbers and titles (as provided in the AOP Wiki), and the evidence supporting these AOPs are summarised below:
AOP 8: Upregulation of thyroid hormone catabolism via activation of hepatic nuclear receptors, and subsequent adverse neurodevelopmental outcomes in mammals
Refers to rodent studies addressing exposure to polychlorinated biphenyls (PCBs) to support the described sequence of key events (Crofton and Zoeller Citation2005). Postnatal lactation exposure is indicated as the critical period of exposure (Crofton et al. Citation2000).
AOP 42: Inhibition of thyroid peroxidase (TPO) and subsequent adverse neurodevelopmental outcomes in mammals
Supporting evidence is mostly derived from in vitro studies and rodent studies, while indicating that “it is well accepted” that specific key events also occur in humans; the main body of experimental evidence stems from studies with the very potent pharmaceutical TPO inhibitors propylthiouracil and methimazole.
AOP 54: Inhibition of Na+/I- symporter (NIS) leading to learning and memory impairment
Refers to rodent studies, human studies and in vitro studies.
AOP 134: NIS inhibition and subsequent adverse neurodevelopmental outcomes in mammals
Does not summarise underlying evidence.
AOP 152: Interference with thyroid serum binding protein transthyretin and subsequent adverse human neurodevelopmental toxicity
Refers to human studies, rodent studies, and in vitro studies.
AOP 42 and AOP 54 have been published in Crofton et al. (Citation2019) and Rolaki et al. (Citation2019). By comparison, AOP 8, AOP 134, and AOP 152 are research drafts, and AOP 8 has the author status “not under active development” indicating that it has been dormant since an early draft stage. Indeed, the five AOPs are not listed as a starting point for this review in view of establishing their scientific comprehensiveness or coherence, but with the major aim to identify potentially relevant key events that should be considered during toxicological assessments.
For each of these AOPs, presents the MIEs and the sequence of key events leading to the given adverse outcome. While four AOP titles generally refer to adverse neurodevelopment, the adverse outcomes indicated in the respective AOPs are (somewhat) more specific: For AOPs 42, 134, and 152, “cochlear function, decreased/loss” was indicated as the adverse outcome in September 2019, but had been replaced by “cognitive function, decreased” by mid-October 2019. (Since all of these AOPs were “under development” when these changes were introduced, their authors were free to make such amendments without further explanation.) For AOP 8, “cochlear function, loss” is indicated as the adverse outcome, observed in rats upon perinatal and lactation exposure to PCBs (Crofton and Zoeller Citation2005), with lactation being the critical period of exposure (Crofton et al. Citation2000). For AOP 54, “impaired learning and memory” is indicated as the adverse outcome.
Table 1. Overview of thyroid-related AOPs including neurodevelopmental outcomes in mammals in the AOP Wiki (as per 16 October 2020).
With respect to neurological events, three key events for altered hippocampal gene expression, anatomy and physiology are included in four of the five thyroid-related AOPs. The only exception is AOP 54, where key events leading to a decrease in GABAergic interneurons are described as ultimately leading to impaired learning and memory.
also presents the strength of evidence for key event relationships and their quantitative understanding, as provided by the authors of the given AOP. In the AOP Wiki, such information is provided for AOPs 42, 54, and 134. While there is a range across key events, the strength of evidence that the given key event relationship truly exists is mostly “moderate” or “high”. By comparison, the quantitative understanding on key event relationships is mostly “low” or at best “moderate”. There are only a few instances where the quantitative understanding of a key event relationship is considered “high”, and these instances mostly relate to early key event relationships.
AOPs 42, 54 and 134 describe direct MoAs leading to thyroid hormone imbalance. Their MIEs relate to the inhibition of enzymes and transporters, which are present in the thyroid gland and are necessary for thyroid hormone synthesis (i.e. (AOP 42) inhibition of TPO and (AOP 54) inhibition of NIS). For AOP 134, the MIE (inhibition of NIS) and the first key events are widely concordant with those from AOP 54, whereas the later key events and the adverse outcome are concordant with those from AOP 42.
AOP 8 and AOP 152 describe indirect MoAs leading to thyroid hormone imbalance. AOP 8 is an update of a MoA published by Crofton and Zoeller (Citation2005). Therein, the indirect effect is the increased metabolism of serum thyroid hormone. Its MIE is described as “pregnane X receptor (PXR); activation” (formerly: “PXR/constitutive androstane receptor (CAR) activation”). The MIE leads to the induction of the phase II liver enzyme uridine diphosphate glucuronyltransferase (UGT), which then leads to increased thyroid hormone clearance. In AOP 152, the indirect effect on the thyroid hormone system is caused by changes in the binding of thyroid hormone to the serum binding protein transthyretin.
Hence, the alteration of serum T4 levels is included as a key event in all five thyroid-related AOPs. Indeed, decreased serum T4 is highlighted as the “knot of a bow-tie motif” within a thyroid hormone imbalance AOP network (Villeneuve et al. Citation2018). Notably, none of the AOPs indicate whether the reduced serum T4 relates to the hormone status of the mothers/dams, foetuses or newborn offspring.
Finally, Knapen et al. (Citation2018), Villeneuve et al. (Citation2018), and Noyes et al. (Citation2019) have published thyroid-related AOP networks that include MIEs and key events that are not considered in the five linear thyroid-related AOPs, e.g. inhibition of iodotyrosine deiodinases (DIOs) leading to reduced tissue levels of thyroid hormone and binding to thyroid hormone receptors (TRs). From amongst all linear thyroid-related AOPs and the AOP networks, only the AOP network described by Noyes et al. (Citation2019) also includes key events related to upregulation of serum TSH and thyroid gland histopathology; see Figure 2 in Noyes et al. (Citation2019). While not being directly related to DNT outcomes, TSH and thyroid histopathology are particularly relevant, because these endpoints are measured in guideline toxicity studies and provide important data on the functioning of the hypothalamic–pituitary–thyroid (HPT) axis; thereby contributing to the weight-of-evidence evaluation of potential alterations of thyroid signalling.
Thyroid and neurodevelopmental parameters included in rodent toxicity test methods
On an international level, the OECD assists countries in harmonising Test Guidelines (TGs) for the testing of chemicals. The OECD TGs that address human health effects are adopted within the OECD TG 400 series; http://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm [accessed 2020 October]. Further, the US Environmental Protection Agency (US EPA) Office of Chemical Safety and Pollution Prevention (OCSPP) maintains a series of harmonised TGs. OCSPP TGs that address human health effects are described in the 870 Series, and special TGs from the US EPA Endocrine Disruption Screening Program in the 890 Series; https://www.epa.gov/sites/production/files/2019-10/documents/ocspp-testguidelines_masterlist-2019-09-24.pdf [accessed 2020 October].
In the aforementioned OECD and OCSPP TGs, mandatory parameters related to the thyroid include serum levels of triiodothyronine (T3), T4 and TSH, as well as gross inspection, organ weight and histopathological examination of the thyroid gland. Neurodevelopmental parameters include clinical observations as well as neurobehavioural, neuropathological, and neurohistopathological evaluations of the pups. None of the OECD and OCSPP TGs include either mandatory or optional parameters related to any of the MIEs of the thyroid-related AOPs presented above.
Thyroid-related parameters in rodent toxicity test methods
“T4 in serum, decrease” is included as key event in:
All five AOPs, and it is considered as the “knot of a bow-tie motif” within a thyroid hormone imbalance AOP network (Villeneuve et al. Citation2018). None of the AOPs specify if this key event relates to serum T4 levels in the dams, foetuses, or newborn pups.
“TSH, increase” and “thyroid hyperplasia/hypertrophy” are included as key events in:
The AOP network by Noyes et al. (Citation2019), but are recorded as leading to rat thyroid follicular tumours as adverse outcomes, which are out of scope of the present review.
Measurements of hormones of the HPT axis are included as mandatory parameters in five OECD TGs that address potential human health effects (OECD TGs 408, 414, 421, 422 and 443), the OCSPP 890.1450 and 890.1500 pubertal assays, as well as the US EPA (Citation2005) Guidance on the Comparative Thyroid Assay (CTA) (). Three different hormones may be measured; namely T3 and T4 produced by the thyroid gland, and TSH, produced by the pituitary gland following stimulation by the hypothalamus. The timepoint and frequency of blood sampling vary per TG, as do the specific hormones considered (). Decrements in maternal T4 are most important during critical windows of neurodevelopment and when the foetus is still fully dependent on maternal T4. By comparison, if T4 is reduced in early postnatal development, different adverse outcomes may result. Notably, the mandatory parameters included in the TGs relate to total T3 (tT3), total T4 (tT4), and TSH, whereas in pregnant women, and generally during human diagnostics, fT4 and TSH are the most frequently measured thyroid-related hormones.
Table 2. Measurements of triiodothyronine, thyroxine or thyroid stimulating hormone in standard rodent toxicity studies.
The same TGs that include measurements of hormones of the HPT axis also include morphological assessments of the thyroid gland; specifically, gross inspection (macroscopic appearance), measurement of the absolute and relative weight of the thyroid gland, and histopathological examination. These assessments are made at study termination, usually in the adult/parental animals, and optionally in the pups. An exception is US EPA (Citation2005) CTA, which requires thyroid weight and thyroid histopathology in foetuses (gestational day 20) and pups (postnatal days 4 and 21) as mandatory parameters ().
Generally, the TGs provide negligible guidance regarding the interpretation of the hormone measurements, and there are few agreed values for normal hormone levels (Beekhuijzen et al. Citation2018, Citation2019) (see Sections “Serum levels of free thyroid hormones” and “Challenges in determining when serum T3, T4 and TSH levels are altered in rodent toxicity studies – or human studies” for uncertainties related to methodology). Findings are recorded per group mean, as compared to the mean of the concurrent control group (so that a concurrent control group is a necessity). Further, findings that are statistically significant as compared to the concurrent control group should be compared to the available historical control data, which reflect the normal distribution range for the respective sub-population (covering up to several hundred individuals). Variation in the concurrent control groups, that commonly include 10 animals, is generally smaller than the normal distribution range. Therefore, findings that are statistically significant as compared to the concurrent control group may still lie within the normal distribution range, and comparison to the historical control data serves to determine if such statistically significant findings are also “abnormal”. Further, the reliability of findings can be verified by reference to data for a concurrent or historical reference substance (positive control). The abnormality of findings must be determined on a case-by-case basis with consideration of sources of variability in thyroid hormone assessments (well described in Li et al. Citation2019). In principle, animals that are hypothyroid could express decreased activity, lethargy, decreased muscle tone, hypothermia and other effects. Whether such effects are apparent will depend on the magnitude and duration of the effects on thyroid function. Notably, all of these effects are non-specific and might also evolve as a consequence of non-thyroid-related and non-neurological perturbations (Midgley et al. Citation2019).
With respect to statistical analysis, the OECD TGs advise determining the statistical significance of findings, but do not prescribe any specific procedure (e.g. OECD TG 408: “When applicable, numerical results should be evaluated by an appropriate and generally acceptable statistical method”). In the OCSPP pubertal assays, performance criteria indicate the range of mean values and acceptable coefficients of variation for control levels of TSH (male only) and T4 (male and female), requesting ANOVA with transformation if there is heterogeneity of variance. Since the sample sizes vary across TGs, the statistical power to detect thyroid hormone changes also differs (see Discussion Section).
US EPA (Citation2005) CTA has been designed to investigate whether pregnant and lactating females, foetuses, neonatal and juvenile pups are more sensitive to thyroid-active substances than animals at other life stages. For this purpose, the findings from the CTA are compared to available data from adult males and adult nulliparous, non-pregnant and non-lactating females. The CTA has not been adopted as a formal TG, and it does not include any specific neurodevelopmental parameters (apart from clinical observations). The CTA is designed to determine whether these potentially sensitive life stages are adequately protected by the points-of-departure that are used for risk assessment and not for further elucidation of (neurodevelopmental) hazard (US EPA Citation2005).
Neurodevelopmental parameters in rodent toxicity test methods
Following the scope of this review, all AOPs considered include neurodevelopmental outcomes:
AOP 8: “Cochlear function, loss”, with lactation indicated as critical period of exposure
AOPs 42, 134, and 152: “Cognitive function, decreased”
AOP 54: “Impaired learning and memory”
To different extents, the TGs that include thyroid-related parameters also include neurological, neurobehavioural, and/or neurohistopathological parameters (). OECD TG 408 (rodent 90-day oral repeated-dose toxicity study) does not include pregnant or lactating females, foetuses, newborn pups or juveniles. Hence, this TG is not designed to identify neurodevelopmental effects. In the OECD TG 421/422 developmental and reproductive toxicity screening tests, and similarly in US EPA (Citation2005) CTA, the neurodevelopmental assessment is limited to the recording of body weight and alterations in clinical behaviour of the pups. In the OCSPP pubertal assays, body and pituitary weights are measured, and alterations in clinical behaviour of the pups are recorded. In the OECD TG 414, that is terminated before delivery, assessments of the offspring are restricted to foetal body weight and the identification of external, soft tissue or skeletal alterations in the foetuses.
Table 3. Neurological and neurodevelopmental parameters included in standard rodent toxicity studies.
Hence, of all the TGs that include thyroid-related parameters, only OECD TG 443 (Extended One-Generation Reproductive Toxicity Study (EOGRTS)) includes more specific tests to investigate alterations in neurodevelopment when the DNT Cohort is included. These neurobehavioral assessments include a functional observational battery, and assessments of motor activity and auditory startle habituation, along with detailed clinical observations. Further, OECD TG 426 (DNT study) specifically addresses DNT. Since this TG does not include any thyroid-specific parameters, it has not been considered above. Mandatory parameters included in OECD TG 426 are detailed clinical observations, behavioural ontogeny, motor and sensory function, auditory startle habituation, motor activity (including habituation), as well as learning and memory.
Neuro(histo-)pathological investigations of the offspring in OECD TG 443 (when the DNT Cohort is included) and OECD TG 426 are conducted in the F1 weanlings (postnatal day 21/22) and in the F1 adults at study termination (postnatal days 77–84 (cohort 2a) and 60–70, respectively). Organs that are examined in these TGs include the brain, spinal cord and peripheral nerves (see for details). Further, the assessment of thyroid, liver and other tissues is included (TG 443) or can be added (TG 426) to the study design, based on expected biological targets associated with a given test substance or effects that are observed in the study, including evidence of delayed development.
Non-routine parameters reflecting events of thyroid-related AOPs
This section presents and discusses non-routine parameters reflecting the MIEs and early key events of thyroid-related AOPs including adverse neurodevelopmental outcomes in mammals:
Thyroid hormone synthesis: Inhibition of NIS and TPO
Serum levels of free thyroid hormones
Serum thyroid hormone transport proteins
Liver enzymes mediating enhanced thyroid hormone metabolism
Tissue levels of T3 and T4
Local regulation of thyroid hormone levels: Inhibition of DIOs, cell membrane transporters and TR transcription
Information is provided on the (patho-)physiological processes related to the given parameter, including species differences thereof, and on opportunities to assess the given parameter in rodent toxicity studies or in vitro assays, as applicable.
Generally, in vitro assays are available for parameters that reflect enzyme activities (TPO, DIOs), transporter activities (NIS), as well as serum protein and TR binding properties. Focus is on in vitro assays used within the US EPA Toxicity Forecasting Program ToxCast; https://comptox.epa.gov/index.html#/ [accessed 2020 October]. As described on this website, ToxCast uses high-throughput screening methods developed by the US EPA and computational toxicology approaches to rank and prioritise chemicals for testing. Therefore, the ToxCast assays appear especially suitable for the present review, since they are being applied for substance screening in a regulatory context. Further, relevant ongoing activities of the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) and the EU Network of Laboratories for Validation of Alternative Methods (EU-NETVAL; Zuang et al. Citation2019) are considered. These EU activities also follow up from OECD (Citation2014) New scoping document on in vitro and ex vivo assays for the identification of modulators of thyroid hormone signalling. Notably, Zuang et al. (Citation2019) provide the titles of 17 methods that have been included in a “large scale validation study of a set of mechanistically informative alternative methods to detect chemicals that disrupt normal thyroid hormone function”, but does not include any further details on e.g. the test protocols or applicability domains of these methods.
Thyroid hormone synthesis: Inhibition of Na+/I− symporter and thyroid peroxidase
Na+/I− symporter inhibition
NIS inhibition is the MIE of:
AOP 54 Inhibition of NIS leading to learning and memory impairment
AOP 134 NIS inhibition and subsequent adverse neurodevelopmental outcomes in mammals
Uptake of iodide (I−) into the thyroid gland via the NIS is the first step in the biosynthesis of thyroid hormones (Hallinger et al. Citation2017; Wang et al. Citation2018). The NIS is a glycoprotein that actively transports I− into thyroid follicular cells. This process relies on the maintenance of the Na+ electrochemical gradient by Na+/K+ ATPases (electrogenic stoichiometry 2 Na+: 1 I−). Under normal physiological conditions, NIS can mediate I− uptake into the thyroid gland at 20- to 40-fold higher concentrations than the corresponding serum levels. Malfunction of the NIS protein is known to affect thyroid hormone homeostasis. For example, patients with various gene mutations leading to aberrant NIS protein have been diagnosed with hypothyroidism (Pohlenz and Refetoff Citation1999). Generally, NIS-mediated uptake of I− as the first step in the biosynthesis of thyroid hormones is highly conserved across species, and the genomic organisation of human and rat NIS is highly homologous (Smanik et al. Citation1996). However, in vitro studies provide some indication for quantitative differences in NIS activity, since rat and mouse NIS proved to be more efficient in mediating I− uptake than human NIS (Heltemes et al. Citation2003; Dayem et al. Citation2008).
Within the US EPA ToxCast program, a previously validated Radioactive-Iodide Uptake Assay using human hNIS-HEK293T-EPA cells has been applied to screen for NIS inhibitors in the ToxCast Phase I chemical library (Hallinger et al. Citation2017; Wang et al. Citation2018). In a tiered-approach, approx. 300 substances were first tested at one single high concentration (100 μM) performing three independent measurements in distinct cell passages. If substances inhibited radioactive-iodide uptake by at least 20% in this Tier 1 (indicating potential for NIS inhibition), they were further evaluated in Tier 2 to establish concentration-response relationships (0.001–100 μM), measuring both radioactive-iodide uptake and concurrently cell viability (Wang et al. Citation2018). Since, over 1000 substances from the ToxCast Phase I and Phase II libraries have been screened for human NIS inhibition (Wang et al. Citation2019).
More recently, Buckalew et al. (Citation2020) described an in vitro Radioactive-Iodide Uptake Inhibition Screening Assay using the Fischer rat thyroid follicular cell line (FRTL-5). In this assay, 25 of 29 test substances that tested positive in the human hNIS-HEK293T-EPA assay also showed effects (IC50 values) in the rat FRTL-5 assay when tested at six concentrations from 0.001–100 μM (i.e. inhibition at the maximum concentration was at least 50%). Of these 25 substances, 18 showed less than one order of magnitude difference in the IC50 values between the human NIS cell assay and the FRTL-5 cell assays. Buckalew et al. (Citation2020) concluded that substances exhibiting NIS inhibition with minimal cytotoxicity in both assays merited further testing in short-term in vivo assays to characterise effects on thyroid hormone synthesis.
Thyroid peroxidase inhibition
TPO inhibition is the MIE of:
AOP 42 Inhibition of TPO and subsequent adverse neurodevelopmental outcomes in mammals
Once I− has entered a follicular cell via the NIS, it is transported to its apical membrane where the enzyme TPO is located. TPO is an integral membrane protein that catalyses the sequential reactions needed for the formation of the respective thyroid hormones. TPO first oxidises I- to iodine, then iodinates tyrosine residues on thyroglobulin to produce mono- and diiodotyrosine, and finally links two tyrosine molecules together to produce T3 or T4 (Rousset et al. Citation2015). Generally, TPO function is assumed to be highly conserved across species (Paul et al. Citation2013; Tietge et al. Citation2013), although species differences for some TPO-inhibiting substances have been reported (Takayama et al. Citation1986). A decrease in TPO activity leads to reduced thyroid hormone synthesis and consequently decreased serum levels of T3 and T4 (Crofton et al. Citation2019).
The US EPA has developed a high-throughput screening Amplex UltraRed-TPO Assay to measure TPO inhibition in rat microsomes via loss of the Amplex UltraRed signal (Paul Friedman et al. Citation2016). This assay uses a two-tiered approach similar to the one described above for the NIS inhibition assay. The Tier 1 screening for TPO inhibition includes testing at a single, high concentration (87.5 µM). Substances that inhibit TPO by at least 20% (and are thus identified as putative TPO inhibitors) are submitted to the Tier 2 concentration-response assessments (eight concentrations ranging from 0.00534 to 87.5 µM). To establish assay relevance and reliability, a total of 1074 substances from the ToxCast Phase I and II chemical libraries were screened in the Amplex UltraRed-TPO Assay, together with two additional assays to evaluate non-specific luciferase inhibition and cell cytotoxicity; together, these assays provide further data on specificity for the 314 chemicals positive for TPO inhibition (Paul Friedman et al. Citation2016). In the meantime, over 1800 substances have been screened at single concentrations in the Amplex UltraRed assay with 972 identified as potentially active TPO inhibitors (52%) (https://comptox.epa.gov/dashboard/assay_endpoints/?link=&search=AUR [accessed 2021 January]. The significance of these in vitro positive results is difficult to determine as TPO inhibition has been measured in vitro/ex vivo with many dietary flavonoids (e.g. Divi and Doerge Citation1996), amino acids (e.g. Carvalho et al. Citation2000), antiseptics (e.g. resorcinol; Dong et al. Citation2020), protein denaturing agents, or other bioactive compounds (e.g. glutathione, ascorbic acid; Schussler et al. Citation1961) without corresponding in vivo effects or with differences in species sensitivity (Takayama et al. Citation1986).
To account for the potentially high false-positive rate (and hence decreased assay specificity) typically observed with loss-of-signal assays such as the Amplex UltraRed-TPO Assay (Paul Friedman et al. Citation2016), the data recorded for 150 substances were compared to those from a gain-of-signal assay, the orthogonal peroxidase (guaiacol) oxidation assay. While this assay also allows identifying TPO inhibitors, it is not amenable to high-throughput screening. Paul Friedman et al. (Citation2016) reported a high overlap of positive results between the two assays, especially for TPO inhibitors with high in vitro potency. As compared to the guaiacol oxidation assay, the Amplex UltraRed-TPO Assay showed high sensitivity (88.3%), but low specificity (39.3%) since 34 of the 150 substances were false positives in the Amplex UltraRed-TPO Assay (Paul Friedman et al. Citation2016). These findings serve to illustrate the importance of using additional experimental evidence to confirm (or refute) positive results obtained in the Amplex UltraRed-TPO assay.
Serum levels of free thyroid hormones
Serum levels of free thyroid hormones relate to key event 2 of:
AOP 152 Interference with thyroid serum binding protein transthyretin and subsequent human neurodevelopmental toxicity
T3 and T4 released from the thyroid gland can be present in the blood either as free hormones or bound to binding proteins (see next section), but it is only the free thyroid hormone that can bind to the TRs in target tissues. Therefore, homeostasis of the free hormone is pertinent to biological activity, while the bound hormone is thought to serve as a reservoir to maintain the free hormone at physiological levels. As such, when free hormone is reduced, thyroid hormone is released from the binding proteins within a physiological equilibrium, and when free hormone is increased, more thyroid hormone is bound to binding proteins (Stockigt Citation2001).
The foetus is fully dependent on maternal thyroid hormone until the foetal thyroid gland starts producing thyroid hormone (approx. gestational day 17 in rats (Perez-Castillo et al. Citation1985); towards the end of the first trimester in humans (Thorpe-Beeston et al. Citation1991)), and it remains partially dependent until delivery (Morreale de Escobar et al. Citation1990; Grijota-Martinez et al. Citation2011). The stage of development when the foetus or pup is exposed to low serum levels of thyroid hormones is critical for the onset of altered neurodevelopment. Therefore, the measurement of fT4 levels in pregnant and/or lactating females, foetuses, newborns and/or juveniles may be relevant to identify substance-mediated thyroid hormone imbalance.
Analytical methods are available to measure serum concentrations of free T3 (fT3) and fT4. Indeed, in human medicine, measurement of the free hormones is considered preferable for the assessment of thyroid function, given that it represents the bioavailable, biologically active fraction (Alexander et al. Citation2017). Routinely, direct immunoassays without sample pre-treatment are also used for measurement of free thyroid hormones. Ultrafiltration or dialysis as sample pre-treatment are used only in larger hospitals with research units for separation of bound vs. free thyroid hormones. Once separated, two kinds of analytical methods are available (Jonklaas et al. Citation2014), i.e. (1) direct immunoassays, which are commonly used in clinical laboratories; and (2) analytical methods such as tandem mass spectrometry. Generally, assays must enable the quantification of very low levels of the hormones, as fT3 and fT4 (in humans) constitute only 0.2% of tT3 and 0.02% of tT4 (Stockigt Citation2001).
A potential disadvantage of using ultracentrifugation or dialysis in rodent toxicity studies is the need for greater sample volumes (approx. 200 µL, as compared to 60 µL when using direct immunoassays alone). Such sampling requirements may prevent the use of these separation techniques to assess samples from rat foetuses or very young pups, due to the limited volumes of blood that can be collected.
In human medicine, the accuracy of direct immunoassays for measuring free thyroid hormones is increasingly being questioned, since many factors might affect their protein binding characteristics (Stockigt Citation2001; Soldin and Soldin Citation2011; Jonklaas et al. Citation2014). This issue becomes even more critical when direct immunoassays that were developed for human serum are used for the assessment of rat serum. As compared to humans, adult rats have negligible levels of the high-affinity thyroid binding globulin (see below).
Since internationally agreed generic ranges for normal fT4 are unavailable, human fT4 data are usually compared to population-based reference ranges, with “population” referring to any pre-defined group of people (Sauer et al. Citation2020; ). Hence, population-based reference ranges need to be established for every single epidemiological study or clinical trial (see in Sauer et al. (Citation2020) for an overview of the population-based reference ranges that were calculated for the respective human studies considered). Therefore, comparisons of the findings from any given human study to the respective population-based fT4 reference range should consider that these reference ranges are dependent on the characteristics of the pre-defined population (including the gestational period considered since fT4 levels vary physiologically during pregnancy), and that they are further assay- and laboratory-dependent (Sauer et al. Citation2020). In toxicological assessments, the dose groups are usually compared with concurrent control groups and/or with historical control data.
Figure 4. Free T4 and tT4 reference ranges for humans (males, pregnant women, infants) as compared to tT4 normal distribution for Wistar rats (males, lactating dams, pups): Ranges from median to 2.5th percentile, relative to the median. fT4: free thyroxine; GD: gestational day; LD: lactational day; N: number of individuals; Trim: trimester; tT4: total thyroxine. Colour legend: grey columns: human fT4 data; striped columns: human tT4 data; black columns: rat tT4 data. Note that T4 variation only attains -50% as compared to TSH variation attaining up to 210% (). Age/life stages (number of individuals): human males: 20–39 years (fT4/tT4: N = 286/130); Wistar rat males: 20 weeks (N = 486); pregnant women: 1st trimester (fT4/tT4: N = 418/417) and 3rd trimester (fT4/tT4: N = 169/169); Wistar rat dams: GD20 (N = 368) LD14 (N = 56); infants: 6 days to 3 months (fT4/tT4: N = 111/99); children: 1–6 years (fT4/tT4: N = 344/341); Wistar rat pups: postnatal day 13 (males/females: N = 398/399). The human fT4/tT4 reference ranges were taken from Roche (Citation2009). The rat normal tT4 distribution ranges were measured in control rats at BASF SE, Ludwigshafen (Germany); see Supplementary Information SI-2 for methodological details and absolute data.
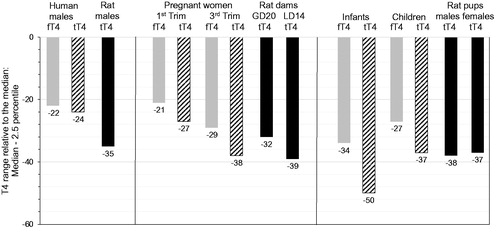
While fT3/fT4 data may be generated in rodent studies, their applicability to evaluate substance-mediated thyroid hormone imbalance is still limited. In addition to the aforementioned technical challenges (e.g. with respect to limits of quantification), it remains difficult to establish at what level lower free thyroid hormone concentrations are truly adverse. The physiological processes involved in maintaining fT3/fT4 homeostasis impair the establishment of a specific threshold at which the adaptive capacity of the thyroid hormone system has been overwhelmed and an adverse effect results (Sauer et al. Citation2020). The same stands true for TSH and tT3/tT4, since all components of the HPT axis possess adaptive capacities.
While some work has been done to correlate serum T4 with brain tT4 and tT3 in rats (O’Shaughnessy et al. Citation2018), it is still unknown how serum levels of fT3 (or fT4) relate to the tissue levels of the active T3 in either rats or humans. In particular, the discovery that active transporters play a major role in achieving specific hormone concentrations in cells challenged the previously held hypothesis that serum fT3/fT4 levels dictate the amount of hormone delivered into the cell (see comprehensive review by Holtorf (Citation2014)). Thyroid hormone activity in the tissues can be regulated locally through very complex networks, and knowledge about the extent to which the associated networks differ between humans and rodents is just beginning to evolve. Such information will be pivotal to establish the human relevance of altered serum fT3/fT4 levels recorded in rodent studies.
Thyroid hormone serum binding proteins
Thyroid hormone serum binding proteins relate to the MIE of:
AOP 152 Interference with thyroid serum binding protein transthyretin and subsequent human neurodevelopmental toxicity
In the blood, the vast majority of thyroid hormone is bound to binding proteins (or transport proteins). Primarily, these are thyroid binding globulin, transthyretin, and albumin (Schussler et al. Citation1978; Refetoff Citation2015). Hormone distribution across the three binding proteins differs between humans and rodents, as well as between different life stages of the same species. Adult human serum contains 80% T4 bound to thyroid binding globulin, 15% to transthyretin, and less than 5% to albumin (Stockigt Citation2001). In contrast, adult rats express very low levels of thyroid binding globulin, so the majority of thyroid hormone in their serum binds to albumin and transthyretin (Vranckx et al. Citation1990). Further, the binding affinity of thyroid hormone in the serum, which is highly conserved across species (Chang et al. Citation1999), follows thyroid binding globulin > transthyretin > albumin. Hence, in the rat, the distribution of thyroid hormone to the specific proteins (albumin > transthyretin > thyroid binding globulin) is inversely related to the thyroid hormone protein binding affinity. By comparison, thyroid hormone distribution and protein binding affinity are positively related in humans. In consequence, the kinetics of thyroid hormones in the blood differ considerably between humans and rats. Higher dissociation rates from the predominant, but low-affinity albumin contribute to much shorter serum half-lives of the thyroid hormones in the rats (T3: 0.25 day in rats, 1 day in humans: T4: 0.5–1 day in rats, 3–4 days in human foetuses and neonates, 5–9 days in human adults) (Vulsma et al. Citation1989; Jahnke et al. Citation2004). These rather short half-life times for thyroid hormones in rats probably also explain why the TSH levels in rats are generally much higher than in humans, i.e. 0.6–3.4 ng/mL in rats vs. 0.05–0.5 ng/mL in humans (Kaptein et al. Citation1994; Loeb and Quimby Citation1999; Lewandowski et al. Citation2004). Consequently, rats have a higher basal level of activity in the HPT axis than humans (Choksi et al. Citation2003), which would likely make them more vulnerable to perturbation of thyroid homeostasis.
Displacement of thyroid hormone from binding proteins may result in enhanced thyroid hormone clearance, since it is the free hormone that can be eliminated by the kidney or glucuronidated for kidney storage or biliary excretion (see below). Displacement of thyroid hormone from binding proteins may also affect the systemic distribution of the thyroid hormone, such that it has greater fluctuations or does not reach relevant biological targets. However, inherited abnormalities in human thyroid binding globulin indicate that while these result in a decrease of tT4, fT4 levels remain unaffected and that there are no subsequent adverse effects in humans that exhibit normal function of the HPT axis (Chakravarthy and Ejaz Citation2020). Similar results have been reported in rats lacking albumin and null mice lacking TTR (Palha et al. Citation2000, Palha Citation2002).
The substance-mediated displacement of thyroid hormones from serum binding proteins (mainly transthyretin) has been investigated in vitro and ex vivo (Brouwer and van den Berg Citation1986; Lans et al. Citation1994; Hallgren and Darnerud Citation2002; Hamers et al. Citation2008). The available in vitro screening assays use various detection methods, including displacement of either radioactive T4 or non-radioactive fluorescent T4, or surface plasmon resonance biosensing (Noyes et al. Citation2019). The EU-NETVAL is currently validating in vitro assays to address a substance’s potential to displace thyroid hormones from transthyretin and/or thyroid binding globulin (Zuang et al. Citation2019). Noyes et al. (Citation2019) highlighted that the biological relevance of any change in thyroid hormone serum binding properties observed in vitro needs to be established.
Liver enzymes mediating enhanced thyroid hormone metabolism
Receptor activation resulting in the induction of phase II liver enzymes relates to the MIE of:
AOP 8 Upregulation of thyroid hormone catabolism via activation of hepatic nuclear receptors, and subsequent adverse neurodevelopmental outcomes in mammals
The liver, which is often the target organ in toxicity studies, plays a pivotal role in thyroid hormone metabolism as well as for substance detoxification by metabolism and elimination. Due to its nearly direct connection, via the portal vein, to the intestinal tract, the liver is commonly exposed to rather high levels of the administered substance. As the major organ for biotransformation of xenobiotics, the liver frequently responds to the increased functional demand by increased tissue weight, gene expression/enzyme inductions, hepatocellular hypertrophy and, in some cases, hyperplasia.
In humans, the most prominent route for thyroid hormone metabolism is by deiodination (Cavalieri and Pitt-Rivers Citation1981), and in rats, it is by conjugation via phase II liver enzymes (Beetstra et al. Citation1991). In rats, substances have also been observed to elicit increased thyroid hormone conjugation subsequent to PXR- and CAR-mediated phase I liver enzyme induction (Visser Citation1996; Szabo et al. Citation2009; Roques et al. Citation2012). Nonetheless, phase I enzyme induction is not required to induce phase II thyroid hormone conjugation (Visser, Kaltein, Gijzel, et al. Citation1993; Visser, Kaltein, van Raaij, et al. Citation1993; Visser Citation1996), and indeed the phase II enzyme UGT is also directly induced by PXR (Gardner-Stephen et al. Citation2004), as is reflected in AOP 8. PXR has low concordance between species in the ligand binding domain (77% between human and mouse (Kliewer and Willson Citation2002)); this stands in contrast to other nuclear receptor orthologues and likely accounts for differential substrate efficacy.
The two primary types of phase II enzymes involved in thyroid hormone metabolism are UGTs and sulphotransferases (SULTs). These enzymes conjugate thyroid hormones via glucuronidation and sulphation, respectively, and typically at the phenolic hydroxyl group (Kester et al. Citation2002, Citation2003; Zhou et al. Citation2005).
UGT-mediated glucuronidation is the major metabolic pathway for thyroid hormones in the rat. It increases the solubility of these hormones thereby facilitating their renal and biliary excretion (McClain Citation1989; Beetstra et al. Citation1991; Barter and Klaassen Citation1994; Liu et al. Citation1995). While the substrate-specificity of UGTs is not strict, specific UGT isoenzymes preferentially metabolise either T3 or T4 (Beetstra et al. Citation1991). In rats, androsterone-UGT (UGT2B2) is involved in the metabolism of T3, whereas T4 is glucuronidated by phenol-UGT (UGT1A1) and bilirubin-UGT (UGT1A6) (Visser, Kaltein, Gijzel, et al. Citation1993; Visser, Kaltein, van Raaij, et al. Citation1993; Visser, Kaltein, van Toor, et al. Citation1993; Klaassen and Hood Citation2001). However, other studies have yielded divergent findings (Emi et al. Citation2007), so that further investigations are needed to fully elucidate the conditions under which specific UGT isoenzymes are involved in the metabolism of T3 and T4 in rats or humans. Once glucuronidated, thyroid hormone is excreted from the body, but it can also be hydrolysed by intestinal glucuronidases and re-enter the body through enterohepatic recirculation (Visser, Kaltein, Gijzel, et al. Citation1993; Visser, Kaltein, van Raaij, et al. Citation1993).
SULT-mediated sulphation, the second type of phase II thyroid hormone metabolism, generally occurs most readily with 3,3′-diiodothyronine, then more slowly with T3 and reverse T3 in both rat and human liver (Santini et al. Citation1992; Kester et al. Citation1999, Citation2003). In healthy human and rat adults, sulphated thyroid hormone is a more favourable substrate for DIO1 deiodination to reverse T3, a reaction that inactivates the thyroid hormone (Visser Citation1994). By contrast, human foetuses and neonates have lower levels of DIO1 activity and/or altered hepatic transporters, which allows for high serum levels of iodothyronine sulphates (i.e. T4S, T3S, reverse T3–S and 3,3′-diiodothyronine-S) (Chopra et al. Citation1992; Santini et al. Citation1993). Thus, it has been hypothesised that sulphated iodotyronines may serve as a potential reservoir for thyroid hormones in sulfatase-containing foetal tissues (Kester et al. Citation2002).
As regards species differences in phase II thyroid hormone metabolism, UGT-mediated T4 glucuronidation is a minor excretory pathway in humans, with only 10–15% elimination of glucuronidated T4 in bile (Hill et al. Citation1989), as compared to approximately 50% in rats (McClain Citation1989). In comparative in vitro assessments, basal T4-UGT levels were much higher in primary rat hepatocytes than in primary human hepatocytes (Richardson et al. Citation2014). While rodents also glucuronidate T3 (by UGT2B2), human T3 is primarily deiodinated or sulphated (Kester et al. Citation1999), yielding 3,5-diiodothyronine and inactive T3S, respectively, with recirculation of I- for de novo thyroid hormone synthesis (Findlay et al. Citation2000). Human SULT1A1 can metabolise both T3 and T4, and this reaction is not regulated by nuclear receptors (Hempel et al. Citation2004); by contrast, its rodent counterpart is regulated by CAR (Fang et al. Citation2003; Maglich et al. Citation2004; Tien and Negishi Citation2006). Hence, substance-mediated CAR activation will have different implications for SULT-mediated thyroid hormone synthesis in humans versus rodents. Also, term human placental microsomes have sulfatase activity, whereas gestational day-20 rat placenta has limited hydrolysis for iodothyronine sulphates (Kester et al. Citation2002).
As these examples show, any phase II liver enzyme induction-mediated decrease in serum thyroid hormone levels observed in rodents is less relevant for humans, and pronounced quantitative differences are to be expected (Choksi et al. Citation2003). Research work to better quantify the interspecies differences of substance-mediated T4-UGT induction would be of great importance to improve hazard assessment. Further, a better understanding of the role of sulphated thyroid hormone across different ages and species and of how enterohepatic recirculation contributes to iodothyronine recycling and the maintenance of thyroid homeostasis would be useful (Visser Citation1996; Wu et al. Citation2005).
In vitro high-throughput screening assays to assess chemical binding and activation of specific nuclear receptors, including CAR, PXR and aryl hydrocarbon receptors, are available in ToxCast (Noyes et al. Citation2019). The EU-NETVAL is currently validating a liquid chromatography/mass spectrometry assay addressing inhibition of thyroid hormone glucuronidation and a liquid chromatography assay addressing inhibition of thyroid hormone sulphation (Zuang et al. Citation2019).
Tissue levels of thyroid hormones
Thyroid hormone levels in neuronal tissue relate to:
Key event 4 in AOP 8 Upregulation of thyroid hormone catabolism via activation of hepatic nuclear receptors, and subsequent adverse neurodevelopmental outcomes in mammals
Key event 3 in AOP 42 Inhibition of TPO and subsequent adverse neurodevelopmental outcomes in mammals
Key event 4 in AOP 54 Inhibition of NIS leads to learning and memory impairment
Key event 4 in AOP 134 NIS inhibition and subsequent adverse neurodevelopmental outcomes in mammals
Key event 6 in AOP 152 Interference with thyroid serum binding protein transthyretin and subsequent human neurodevelopmental toxicity
Serum thyroid hormone levels do not necessarily reflect the hormone concentrations at their active sites in the target organs (Bianco et al. Citation2014). The thyroid hormone status in the brain is likely the most relevant endocrine parameter to inform on potential neurodevelopmental effects. Indeed, “T4 in neuronal tissue, decrease” is a key event in all five thyroid-related AOPs that include adverse neurodevelopmental outcomes.
Some attempts have been made to measure T4 in foetal rat tissues including the liver, kidney, and (specific parts of the) brain (Morreale de Escobar et al. Citation1985; Pinna et al. Citation1999; Bastian et al. Citation2010; O'Shaughnessy et al. Citation2018). When serum thyroid hormone levels are decreased, it is generally assumed that the extent of thyroid hormone alteration varies across tissues, so that the specific tissue(s) of interest would need to be assessed. Also, the relative sensitivity of various tissues to serum thyroid decrements likely varies at different life stages.
Studies in humans with severe nonthyroidal illnesses indicate that thyroid hormone tissue levels may differ by tissue and do not necessarily mirror blood levels (Arem et al. Citation1993; Peeters et al. Citation2005). Further research work is needed to determine if critical non-thyroidal illness is causative for any such altered tissue hormone levels; also, it is currently unclear how effects observed in critically ill patients translate to serum and tissue hormone levels in pregnant mothers and their children.
Despite their potential relevance for the assessment of thyroid-related neurodevelopmental effects, thyroid hormone levels in the foetal (or pup) brain are rarely measured in rodent studies (and they are generally only amenable to measurement in humans with a specific medical indication). A multi-step procedure is required to extract thyroid hormones from brain tissues, and this process has not yet been standardised (Riutta et al. Citation2019). Further, given local modulation of thyroid hormone levels, tissue (or blood) levels of the free thyroid hormones would be better indicators of thyroid status than total thyroid hormone levels (Stockigt Citation2001), but technical challenges impair the measurement of free thyroid hormone in rodents (see above). Furthermore, low levels of free thyroid hormone with limited amounts of foetal brain tissue may make this measurement extremely challenging at the current time. Finally, any measurements of thyroid hormones in foetal brain tissues needs to consider the temporal and spatial regulation of T3 bioavailability and DIO activities. Such issues may render foetal brain thyroid hormone concentrations highly variable between brain regions and gestational days (Morreale de Escobar et al. Citation2004).
Local regulation of thyroid hormone levels: inhibition of deiodinases, cell membrane transporters and thyroid hormone receptor transcription
Inhibition of deiodinases
DIO inhibition is included as MIE in:
The AOP networks (Knapen et al. Citation2018; Villeneuve et al. Citation2018; Noyes et al. Citation2019), resulting in conversion of thyroid hormone in target tissues to a more or less active metabolite
Also, DIO activity is identified as relevant parameter to identify modulators of thyroid hormone signalling in OECD (Citation2014) and US EPA (Citation2017).
There are three types of DIOs, i.e. DIO1, DIO2 and DIO3, and they are present in all vertebrates from fish to mammals. All DIOs serve to metabolise thyroid hormones, but with different specific functions, which also explains their distribution within the organism, and which may further differ between species:
DIO2 generally converts T4 by outer ring deiodination to the more bioactive T3. As such, DIO2 is expressed in tissues requiring the local production of T3. Some species differences have been recorded. For example, DIO2 expression has been recorded in skeletal muscle of humans, but not of rodents (see comprehensive reviews by Bianco et al. (Citation2002) and Arrojo E Drigo and Bianco (Citation2011)).
DIO3 converts T4 and T3 by inner ring deiodination to inactive forms (i.e. reverse T3) or diiodothyronine. In rats, DIO3 is generally found in the central nervous system, placenta and pregnant uterus (Bianco et al. Citation2002). In humans, DIO3 has been recorded in the pregnant uterus, placenta, and other maternal-foetal interfaces, e.g. umbilical arteries and vein, where it limits foetal exposures to maternal thyroid hormone (Huang et al. Citation2003). Further, during critical illness and injury, DIO3 has been documented in human tissues that are usually devoid of DIO3 (e.g. liver and skeletal muscle), presumably to inhibit T3-stimulated energy expenditure during catabolic stress (Huang and Bianco Citation2008).
DIO1 can perform both activation and inactivation reactions. In rats, DIO1 is expressed in the liver, kidney, central nervous system, pituitary, thyroid, intestine, and placenta. In humans, DIO1 activity is absent from the central nervous system, but it is present in liver, kidney, thyroid, and pituitary as well as in circulating mononuclear cells (Bianco et al. Citation2002; Darras and van Herck Citation2012).
Serum thyroid hormone homeostasis can be derived from direct release of T3 from the thyroid and/or from DIO1-/DIO2-mediated metabolism of T4 to T3 in different tissues, including the thyroid. Indeed, such DIO-mediated production of T3 supplies a significant fraction of the serum T3 in euthyroid humans (Visser Citation1996; Bianco et al. Citation2002; Köhrle Citation2002; Maia et al. Citation2005). DIO-mediated thyroid hormone metabolism plays a critical role in thyroid signalling. It allows for a dynamic, tissue-specific regulation of thyroid hormone levels, rather than the whole organism-level changes mediated by TSH release (Little (Citation2018); see Supplementary Information SI-1 for further details).
While the general characteristics of the DIOs appear well conserved across species, their relative contribution to thyroid hormone metabolism may vary, with DIO1 showing the greatest evidence for evolutionary diversity (Darras and van Herck Citation2012). Amongst the liver microsomes from eleven species, rat liver microsomes exhibited the highest DIO1 activity (approx. 9× higher than in human liver microsomes); similarly, their DIO1 content was approximately 7× higher than that of the human microsomes (Schoenmakers et al. Citation1992). Also, DIO1 structure and substrate affinity varies between humans and rats (Darras and van Herck Citation2012). Finally, there are also differences in the molecular regulation of the expression of DIO-related genes. The human gene expressing DIO1 contains two thyroid hormone response elements that are not present in the rat gene. For DIO2, humans have thyroid transcription factor 1 binding sites that have not been identified in the rat (Gereben et al. Citation2001).
DIO activities during development
Different types and/or proportions of DIOs are present in different tissues at different stages of the foetal development and control thyroid hormone activation and inactivation at the cellular level relatively independently of serum thyroid hormone levels (see reviews by Darras et al. (Citation1999); Bianco and Kim (Citation2006); Gereben et al. (Citation2008)). In tissues, the activated thyroid hormone generally drives a transition from cellular proliferation to cellular differentiation and maturation. Accordingly, DIO3 activities in the human uterus during pregnancy, at maternal-foetal interfaces, and in the foetal liver are initially high resulting in a high inactivation of thyroid hormones (Huang et al. Citation2003). This generally favours tissue growth (i.e. cell proliferation) and prevents premature cell differentiation due to over-exposure to thyroid hormone (Visser Citation2016). As the need for cell proliferation during development decreases and differentiation becomes more important, the tissue-specific need for DIO3 activity decreases. Thus, DIO3 activity in the human foetal liver decreases from high levels at gestational week 20 to pregnancy term to even lower levels in the neonatal period; whereas the adult human liver has little to no DIO3 activity (Richard et al. Citation1998; Darras et al. Citation1999).
By contrast, DIO2 levels increase during tissue differentiation since DIO2 converts T4 to active T3. In the human foetus, thyroid hormones, thyroid hormone receptors (TRs) and DIOs have been detected in the brain around gestational week 10–12, i.e. before the foetal thyroid gland begins to function, but not in other tissues (Abuid et al. Citation1974; Bernal and Pekonen Citation1984). By gestational week 18, thyroid hormone levels in the cerebral cortex of the human foetus reach peak levels (i.e. at a time when hormone synthesis in the foetal thyroid is still increasing), and DIO2 expression in the cortex mirrors cortex T3 levels (Bernal and Pekonen Citation1984; Chan et al. Citation2002).
In the rat foetus, DIO2 is first detectable on gestational day 16.5 and then increases, particularly in the brain and pituitary, to locally produce active T3, with DIO2 expression further increasing until postnatal day 15 (Calvo et al. Citation1990; Ruiz de Oña et al. Citation1991; Chan et al. Citation2002; Grijota-Martinez et al. Citation2011). Similar to humans, DIO2, T3, and T4 levels in the foetal rat brain are higher than the circulating thyroid hormone levels with circulating T3 remaining low until the end of gestation (Ruiz de Oña et al. Citation1988). By contrast, DIO activity precedes increases in T3 levels in other tissues of the rat foetus including the thyroid, lung and liver (Ruiz de Oña et al. Citation1991).
DIO1 is less relevant for neurodevelopment than DIO3 or DIO2. DIO1 is present in the liver of the human foetus at mid-gestation (Richard et al. Citation1998), and rapidly metabolises sulphated thyroid hormone; yet high levels of sulphated thyroid hormone circulate in the foetal blood. Therefore, it has been hypothesised that sulphated thyroid hormone does not readily enter the liver of the human foetus, but constitutes a reservoir for thyroid hormone (see above, Liver enzymes mediating enhanced thyroid hormone metabolism). Hepatic DIO1 levels increase postnatally and contribute to peripheral T3 formation also in human adults (see reviews by Maia et al. (Citation2011) and Visser (Citation2016) and SI-2 for further details on DIO1 activity at different life stages in humans). In the rat foetus, hepatic DIO1 activity is generally low during most of foetal life, increasing between gestational day 18–21, and further to adult levels during the postnatal period (Galton et al. Citation1991; Ruiz de Oña et al. Citation1991).
In vitro DIO inhibition assays
Although DIO inhibition is not (yet) considered in any AOP included in the OECD AOP programme, it has been identified as an important endpoint when screening for substances affecting the thyroid hormone system. An in vitro DIO inhibition assay has been developed that uses human DIO1 produced by an adenovirus expression system and non-radioactive, colorimetric determination of I− release from a hormone substrate as the detection method (Renko et al. Citation2015; Hornung et al. Citation2018). Similar to the NIS inhibition and TPO inhibition testing schemes described above, screening for DIO1 inhibition includes testing at one single high concentration (200 µM) in Tier 1. Substances that inhibit DIO1 activity by more than 50% are further assessed in Tier 2 to determine concentration-response relationships (Hornung et al. Citation2018). Subsequently, DIO2 and DIO3 inhibition assays using human DIO2 and DIO3 have been developed (Hornung et al. Citation2018).
Recently, Olker et al. (Citation2019) screened more than 1,800 substances from different ToxCast libraries for their potential to inhibit human DIO1, DIO2 or DIO3 in vitro. Tier 1 testing (at 200 µM as permissible with solubility) identified 411 putative DIO inhibitors that inhibited at least one of the three DIOs by at least 20%, including substances that had not been shown to inhibit DIOs before. Of these, the 228 substances that inhibited the respective DIO by at least 50% were further assessed in Tier 2. Comparisons across the three DIO assays identified 81 substances that produced selective inhibition of only one of the three isoenzymes by at least 50% (Olker et al. Citation2019). While false positive/false negative rates were not reported in these studies, certain substances are known to interfere with assay outcomes, including iodinated compounds, thiocyanates and metal-containing compounds (Hornung et al. Citation2018). Furthermore, maintaining consistent enzyme activity across assays may be technically challenging (Dr. M.W. Hornung; personal communication at HESI DART Committee Thyroid Hormone Assessment Workshop in Washington DC, USA; 9–10 May 2019).
Cell membrane transporters
Altered thyroid hormone transport is included as “putative MIE without high-throughput screening assay”:
• in the AOP network by Noyes et al. (Citation2019).
Also, cell membrane transporter activity is identified as relevant parameter in OECD (Citation2014) and US EPA (Citation2017). By comparison, none of the linear AOPs include events that specifically refer to cell membrane transporters. Instead, their key events proceed directly from reduced serum T4 levels to reduced tissue T4 levels.
During foetal neurodevelopment, the maternal thyroid hormones must cross numerous barriers including the placenta, the endothelial cells of the foetal blood-brain-barrier, the epithelial cells of the choroid plexus, and the cell membranes of the target cells. Increasingly, evidence is becoming available that thyroid hormones do not cross membranes passively, but that they are actively transported by specific transport proteins (Braun et al. Citation2011; Visser Citation2016). Mostly, this evidence, summarised below, has been derived from studies using rats and genetically modified mice, but sometimes also from studies using non-human primates or human foetuses (Bernal Citation2007; Visser Citation2016), which provide some indications for species differences in cell membrane transport processes (Bernal et al. Citation2015).
The organic anion transporters (OATPs) transport iodothyronines and sulphated thyroid hormone across cell membranes (Visser Citation2016). OATP1 selectively mediates T4 uptake; it is found in the placenta and, in high concentrations, in astrocytes (Bernal Citation2007; Schnell et al. Citation2015).
The L-type amino acid transporters are widely distributed, including in the placenta and brain. LAT1 and LAT2 facilitate uptake of both T4 and T3, but with relative lower affinity (Taylor and Ritchie Citation2007; Zevenbergen et al. Citation2015), whereas LAT3 and LAT4 facilitate efflux of inactive thyroid hormone (Visser Citation2016).
Thyroid hormone transport also relies on monocarboxylate transporters (MCTs). MCT8, which plays a critical role in human neurodevelopment as has been shown in human foetuses (Chan et al. Citation2014), has preference for T3, and is expressed in placenta, liver, kidney, thyroid and brain (particularly in neuronal cells) (Visser Citation2016). MCT8 also transports thyroid hormone across the blood-brain-barrier (Ceballos et al. Citation2009) as well as into developing human oligodendrocytes (in vitro) to support their differentiation and then subsequent myelination of adjacent neurons (Lee et al. Citation2017). MCT10 facilitates both uptake and efflux of T3 from numerous tissues, including placenta, liver and kidney (Visser Citation2016).
Finally, sodium/taurocholate co-transporting polypeptide transporters have also been reported to play a role in thyroid hormone transport in the liver (Friesema et al. Citation1999; Visser Citation2016).
In OECD (Citation2014) New Scoping Document, the development of screening assays for inhibition of thyroid hormone transmembrane transporters is granted secondary importance. It is explained that such screening would also be covered by TR ligand binding domain assays since the cell lines used in these assays would need to contain functional transmembrane transporters in order to inform on TR transactivation. However, OECD (Citation2014) also concludes that assessments of TR agonism/antagonism are of subordinate relevance for substance screening due to the paucity of substances interfering with TRs (Paul-Friedman et al. Citation2019). The EU-NETVAL is currently validating an in vitro assay addressing substance-mediated MCT8 inhibition (Zuang et al. Citation2019).
Thyroid hormone receptors and transcriptional regulation
TR binding is included as MIE in:
• The AOP network by Noyes et al. (Citation2019).
By contrast, the sequence of key events described in all linear AOPs proceeds directly from reduced serum T4 levels to reduced tissue T4 levels, but does not include any key events related to TRs.
Within cells, T3 can bind reversibly to specific cytosolic proteins and enter the nucleus where it can then bind to nuclear TRs thereby inducing their genomic action. Several isoforms of TRs exist with TRα1 and TRβ1-3 being transcriptionally active (Flamant et al. Citation2006; Cheng et al. Citation2010). Expression of these isoforms is tissue- and life stage-dependent with TRα1 in the rat primarily expressed from early foetal neuronal development onward, whereas TRβ expression rather occurs perinatally and in specific neuronal types (TRβ3 is rat-specific) (Bradley et al. Citation1989, Citation1992). While most neurons express both TRα and TRβ receptors, the ratios of the isoforms differ depending on the neuronal type and location (Bradley et al. Citation1992). Importantly, the HPT feedback loop involves TRβ2 (Flamant et al. Citation2006).
TRs form homodimers and heterodimers with other nuclear receptors, in particular the retinoid-X receptor (RXR), thereby forming a regulatory complex, and recruit co-activators and co-repressors in order to interact with transcriptional response elements upstream of TR-regulated genes (Paul-Friedman et al. Citation2019). Further, ligand-activated transcription factors interact with T3 thereby either activating the transcription of specific genes (e.g. to drive neuronal differentiation during development) or repressing their transcription (e.g. allowing neural progenitor cells to retain a more proliferative, undifferentiated state) (Alshehri et al. Citation2015; Mendoza and Hollenberg Citation2017). TR modulators have been developed as potential therapeutics, but are limited in number and structural diversity (Paul-Friedman et al. Citation2019).
Available in vitro assays (cell line and reporter gene assays) identified few TR agonists and antagonists (Murk et al. Citation2013; OECD Citation2014). Also, the large-scale screening of several thousand substances using a variety of assays under ToxCast revealed only 11 out of 8,305 substances as being direct TR ligands, supporting the conclusion that TR is a very restrictive receptor with limited ligand structural diversity and likely is not a relevant target for most environmental chemicals (Paul-Friedman et al. Citation2019). The EU-NETVAL is currently validating different TR transactivation assays (Zuang et al. Citation2019).
Integrated tissue responses to alterations in thyroid hormone levels
None of the AOPs or AOP networks include MIEs or key events that specifically refer to compensatory responses in tissues following decreased intracellular thyroid hormone levels.
The AOP Wiki includes a variety of linear AOPs that cover different MIEs, subsequent key events, and adverse outcomes following from altered thyroid signalling. In the organism, however, thyroid signalling involves a network of AOPs that include common key events (e.g. decreased serum T4) and further key events that are specific to individual AOPs, as well as key event relationships that impact the likelihood that altered thyroid hormone levels will progress to an adverse outcome (Knapen et al. Citation2018; Villeneuve et al. Citation2018). This section aims at presenting an integrated view on compensatory responses that may take place in thyroid signalling pathways in different tissues (e.g. the brain) in response to specific perturbations (e.g. decreases in intracellular thyroid hormone levels).
Low thyroid hormone levels in the blood can result in the removal of negative feedback signalling on the HPT axis to stimulate the release of TSH and subsequently, increased T3 and/or T4 release from the thyroid gland (Hadlow et al. Citation2013). When iodine levels are low, increased T3 (rather than T4) is synthesised and released to conserve available iodine. Potentially, substances could interfere with the HPT axis by triggering this compensatory response of low serum thyroid hormone levels, e.g. by blocking or antagonising TSH activity. However, chemical interaction with these receptors seems to be scarce (Noyes et al. Citation2019). The EU-NETVAL is currently validating a thyrotropin-releasing hormone receptor activation assay and a TSH receptor activation assay (Zuang et al. Citation2019).
Cells and tissues can up-regulate or down-regulate various components of cellular transport, DIOs and receptors/co-factors to avoid under- or over-stimulation of thyroid signalling. For example, during neurodevelopment, the placenta expresses high levels of DIO3, which converts thyroid hormone to inactive reverse T3 (see above). This inactivation process can be bypassed via T4 transport through the placenta by endocytosis via transthyretin-T4 complexes (Patel et al. Citation2011).
When brain thyroid hormone levels are low, numerous pathways serve to regain normal levels. Paracrine signalling in the central nervous system is also important for thyroid hormones as astrocytes can take up T4 via OATP1 transporters, deiodinate T4 to more active T3 via DIO2, and then release T3 for uptake by MCT8 transporters on adjacent neurons (Bernal Citation2015). Neurons have high levels of DIO3 to prevent overabundance of active T3. At the tissue level, up-regulation of membrane transport proteins and DIO2 activity has been reported in response to hypothyroidism, although this up-regulation may not be sufficient to re-establish euthyroidism (e.g. Sharlin et al. Citation2010). Finally, transthyretin has also been reported as being an exclusive thyroid hormone carrier in cerebrospinal fluid (Chanoine and Braverman Citation1992; Palha et al. Citation2000; Fleming et al. Citation2009).
Discussion
In the preceding sections, the available evidence on thyroid-related AOPs leading to adverse neurodevelopmental outcomes has been reviewed (1) to collate information on the MIEs and key events of thyroid-related MoAs and AOPs that include adverse neurodevelopmental outcomes in mammals; (2) to establish how the respective MIEs, key events and adverse outcomes are being addressed in standard toxicity test methods; (3) to describe qualitatively and, if possible quantitatively, the biological processes underlying the MIEs and early key events of the AOPs, as they occur in rodents and/or humans, in order to identify potentially relevant additional parameters of thyroid-related effects, which are not (yet) addressed in routine toxicological assessments; and opportunities to establish species relevance of such events and of the key event relationships.
Below, the collated evidence is further discussed, applying the same tripartite structure.
Thyroid-related AOPs including adverse neurodevelopmental outcomes
How useful are the thyroid-related AOPs with adverse neurodevelopmental outcomes to establish the thyroid-related MoA(s) of a substance?
The five thyroid-related AOPs with adverse neurodevelopmental outcomes in mammals presented in the AOP Wiki include four different MIEs covering a spectrum of biological processes: Both TPO inhibition (AOP 42) and NIS inhibition (AOPs 54 and 134) affect thyroid hormone synthesis in the thyroid gland and are examples of direct mechanisms causing serum thyroid hormone imbalance. PXR activation leading to UGT induction and increased thyroid hormone clearance (AOP 8) and altered binding of thyroid hormones to transthyretin (AOP 152) are indirect mechanisms leading to serum thyroid hormone imbalance. In addition, the AOP network presented by Noyes et al. (Citation2019) includes MIEs for hypothalamic-pituitary feedback, for peripheral thyroid hormone metabolism (DIO inhibition) in target tissues, for the induction of further hepatic receptors, as well as for TR binding in target organs.
Hence, the AOPs and AOP networks cover key elements of the thyroid hormone system (i.e. hormone synthesis, distribution, elimination, effects at target organs, and TSH feedback loop). Thereby, they generally provide useful concepts to support the establishment that a substance has, or does not have, a specific thyroid-related MoA causally leading to an adverse outcome in accordance with the Endocrine Disruptor Criteria (European Commission Citation2017, Citation2018).
However, to date, only AOPs 42 and 54 (presenting direct MoAs) have been endorsed by the OECD (Crofton et al. Citation2019; Rolaki et al. Citation2019), whereas AOP 8, 134, and 152 (with the latter two presenting indirect MoAs) are denoted as “under development” (). Therefore, the sequence of events described in these AOPs, up to their adverse outcomes, should not be considered conclusive yet. Indeed, the description of the AOPs has changed over the course of the few months of the writing of this review. Further, it is sometimes unclear if the knowledge underlying a specific key event or key event relationship was derived from human or rodent studies, or from in vitro or in vivo studies. For example, during the review of AOP 54, it was criticised that “for many of the key event and key event relationship descriptions, references and result description from in vitro, in vivo and epidemiology studies are mixed together, so that it is hard to discern which data is from which types of studies”, and it was suggested to reorganise key events and key event relationships to improve transparency on the type of underlying evidence (OECD Citation2018). These shortcomings currently limit the applicability of the AOPs to establish that a particular substance has a specific thyroid-related MoA, or that effects observed in rodents are, or are not, relevant for humans (European Commission Citation2017, Citation2018; EFSA and ECHA Citation2018).
Indeed, in fundamental research addressing the function of the thyroid system, knowledge is often derived from rodent studies, sometimes using genetically modified animals (Bianco and Kim Citation2006; Bernal Citation2007). While such research using rodents has its merits to enhance the understanding of patho-physiological processes occurring in humans, its informative value is limited when it comes to identifying species differences between rodents and humans. As a first step to address these shortcomings, it is recommended that whenever the MoA of a particular substance is substantiated using a specific thyroid-related AOP, it is indicated if the critical key events and key event relationships were observed in rodents, and if so, if they are likely to occur in humans – at all, or in the same manner. For example, if a substance mediates phase II liver enzyme induction in rodents, leading to increased T4-glucuronidation and subsequent increased T4 clearance, this sequence of effects is likely much less pronounced in humans where deiodination is the major metabolic pathway (Cavalieri and Pitt-Rivers Citation1981).
Ultimately, a sound quantitative understanding of the key event relationships included in the five thyroid-related AOPs (and preferably separately for rodents and humans) will enhance their applicability to substantiate the MoA of a substance. Such information considers that each key event needs to attain a certain magnitude before the subsequent key events, and ultimately, the adverse outcome, will evolve (Hassan et al. Citation2017; Noyes et al. Citation2019). A recent publication by Hassan et al. (Citation2020) examined the relationship between TPO inhibition (a MIE) in in vitro and ex vivo assays and decreases in serum T4 in male rats in vivo. While such quantitative relationships between MIEs and downstream events are valuable, the study design (e.g. only two potent TPO inhibitors examined) limits extrapolation of these data to other compounds identified for TPO inhibition in in vitro assays.
Similarly, information is required on the specific window of susceptibility (e.g. in utero versus postnatal exposures) for a given effect in rodents and humans. It is also important to keep in mind that AOPs are not substance-specific and therefore neither consider exposure, nor metabolism. As such, they are theoretical constructs and cannot be used on their own during hazard and risk assessment to establish the MoA of a particular substance.
The strength of evidence to support the later key event relationships of the five thyroid-related AOPs, and their quantitative understanding, is generally much weaker than that to support the earlier key event relationships (). While the MIEs and early key events listed in the AOPs are mostly specific to the given AOP, the later key events of the thyroid-related AOPs are widely concordant: Four of the five AOPs include the three key events for altered hippocampal gene expression, anatomy, and physiology. To the best of the ECETOC Special T4 Task Force’s knowledge, it is currently unclear if the hippocampus (as compared to other areas of the brain) truly plays a pivotal role in the evolvement of adverse effects on cognitive function caused by thyroid hormone imbalance. The strength of evidence for the respective key event relationships is generally moderate, and their quantitative understanding is generally weak ().
The only AOP that does not include the hippocampal events is AOP 54, with NIS inhibition as MIE, where events leading to reduced levels of brain-derived neurotrophic factor are followed by decreased population densities of GABAergic interneurons, reduced synaptogenesis, and decreased neuronal network functioning, which then results in impaired learning and memory. However, the same MIE of NIS inhibition is also indicated for AOP 134, where this MIE is described to lead to the hippocampal events, and ultimately decreased cognitive function. While such overlaps highlight the relevance of AOP networks (Knapen et al. Citation2018; Villeneuve et al. Citation2018), it is currently unclear under which conditions (and/or in which species) NIS inhibition might trigger the sequence of events described in AOP 54 and/or that described in AOP 134.
Moreover, for many key events, it is currently unclear how they can be assessed, or how it can be established that two events are truly correlated. Taking the example of AOP 54, standardised test methods and tools to evaluate parameters reflecting the later key events are widely unavailable. At best, a decrease in the population density of GABAergic interneurons might be identified in vitro e.g. in rodent neocortical cell cultures using immunohistochemical methods (Franchi et al. Citation2017); however, assays that are amenable to routine usage, including relevant data interpretation procedures, remain to be developed, standardised and validated. With respect to assessments of the function of the neuronal network, it is difficult to include practicable methodologies, which are truly predictive of neuronal function deficit in humans, in rat studies conducted in a regulatory setting. This issue will be further explored in the planned third manuscript. In spite of these methodological limitations, it is announced on the AOP Wiki website that AOP 54 shall be used as a basis for the “development of a mechanistically informed Integrated Approaches and Testing Assessment… to identify chemicals with potential to cause impairment of learning and memory.” While the knowledge gaps are evident, the development of such integrated approaches appears as an urgent research need.
Finally, the five AOPs cover three different adverse outcomes. Three AOPs (AOPs 42, 134, and 152) generally indicate “cognitive function, decreased” as adverse outcome. In these same AOPs, the adverse outcome had been indicated as “cochlear function, loss/decrease” up until September 2019. Possibly, this is also an indication that the knowledge on the later events is not as differentiated as the knowledge on the earlier events. Loss of cochlear function remains the adverse outcome of AOP 8 which includes liver enzyme induction as the MIE. Finally, AOP 54 includes impaired learning and memory as adverse outcome. This AOP, however, has NIS inhibition as the MIE, which is also the MIE of AOP 134, which then leads to “cognitive function, decreased” (“cochlear function, decreased” up until September 2019). Opportunities to address these adverse outcomes in toxicological assessments are presented below.
Thyroid and neurodevelopmental parameters included in rodent toxicity studies – and human studies
The thyroid-related and neurodevelopmental parameters included in the standard rodent toxicity studies are generally meaningful for the identification of thyroid-related effects which could lead to adverse neurodevelopmental outcomes. However, they do not allow the establishment of any specific thyroid-related MoA following the AOP concept. First, these parameters do not cover any MIEs or critical key events of the five thyroid-related AOPs, apart from the common key event “serum T4, reduced”. Second, a number of technical and scientific limitations prevail with respect to the measurement of specific parameters and the evaluation of findings. These issues are further discussed below.
Challenges in determining when serum T3, T4, and TSH levels are altered in rodent toxicity studies – or human studies
Reduced serum T4, reflecting the “knot of a bow-tie motif” within a thyroid-related AOP network (Villeneuve et al. Citation2018), is a key event in all five linear thyroid-related AOPs. Serum T4 is a mandatory parameter in five OECD TGs (OECD TG 408, 414, 421, 422 and 443), the OCSPP pubertal assays, and US EPA (Citation2005) CTA.
By contrast, only the AOP network described by Noyes et al. (Citation2019) includes key events related to serum TSH and T3, but none of the linear AOPs do. Clearly, TSH is a relevant parameter to identify compensatory reactions of the HPT feedback loop, and indeed, all test methods that include measurement of serum T4 also include measurement of serum TSH. Further, the OECD TGs 408 and 414 as well as the CTA include measurement of serum T3. However, none of these TGs include specific neurodevelopmental parameters.
It is currently unclear if tT4 (the standard parameter in rodent studies), fT4 (the standard parameter in humans), fT3, or tT3 is the most sensitive parameter to identify thyroid hormone imbalance leading to adverse neurodevelopmental outcomes, either in humans (Sauer et al. Citation2020) or rodents. Also, it is unclear if altered maternal, foetal, or offspring serum hormone levels are equally indicative of adverse neurodevelopmental outcomes.
In addition to these knowledge gaps, it remains challenging to determine if specific findings are indicative of pathological hormone imbalance, or if they are incidental, or if they reflect physiological adaptive reactions of the highly versatile endocrine system. To date, the interpretation of T3, T4 and TSH levels is impaired by the circumstance that internationally accepted generic ranges for “normal”, “low, but normal” or “high” TSH, T3 or T4 levels in humans do not exist (but only population-based ones). For rodents, there is also no agreed threshold, and data are insufficient to indicate at which quantitative level of thyroid hormone change DNT may be induced. In humans, overt hypothyroidism is generally diagnosed when the serum fT4 value of the individual patient undercuts the respective study-specific pre-defined population-based reference range and the serum TSH value exceeds such a range (Roche Citation2009; Alexander et al. Citation2017; Sauer et al. Citation2020). In rodent toxicity studies, hormone values are not compared between individual animals, but group differences are established between treatment and control groups. As described in further detail by Li et al. (Citation2019), assuming two groups with 10 animals each (a common group size in regulatory toxicity studies) and the maximum allowed intra-assay coefficient of variation (e.g. 25% for T3/T4 and 35% for TSH as per OECD TG 407) in one group and about half of this coefficient of variation in the other group (to reflect biological variability), T3/T4 decreases of approx. 25% and a TSH increase of approx. 40% as compared to the concurrent controls can be detected as statistically significant, and can therefore be considered “reasonably detectable” in a common regulatory setting. More generally, the reasonably detectable hormone level change depends on the group size () and the coefficient of variation (). Hence, it is critical to determine and to strive to minimise the coefficient of variation in hormone assays from rodent studies (Li et al. Citation2019). The coefficient of variation is influenced by assay technologies, in-life procedures including handling and blood sampling, as well as inherent biological variability of the investigated population. While a 25% decrease in T3/T4 and 40% increase in TSH may be detected, the biological significance for changes of this magnitude is unclear and would need to be considered on a case-by-case basis and in the context of life stage and duration of hormone change.
Figure 1. Reasonably detectable T4 and TSH level change [%] – dependent on group size. The percent T4 and TSH level change that is expected to be significant (and hence “reasonably detectable”) is shown for different group sizes. It is presumed that the concurrent control group and the treatment groups have the same maximal allowed coefficient of variation (CV) as per OECD TG 407 (25% for T3/T4 and 35% for TSH); statistical analysis: two-sided Wilcoxon test, power 75%; p < 0.05; software NQUERY.
![Figure 1. Reasonably detectable T4 and TSH level change [%] – dependent on group size. The percent T4 and TSH level change that is expected to be significant (and hence “reasonably detectable”) is shown for different group sizes. It is presumed that the concurrent control group and the treatment groups have the same maximal allowed coefficient of variation (CV) as per OECD TG 407 (25% for T3/T4 and 35% for TSH); statistical analysis: two-sided Wilcoxon test, power 75%; p < 0.05; software NQUERY.](/cms/asset/dc3ce0e3-ec49-4f29-8862-b97f308e5e6d/itxc_a_1910625_f0001_b.jpg)
Figure 2. Reasonably detectable T4 and TSH level change [%] – dependent on coefficient of variation (CV). The percent T4 and TSH level change that is expected to be significant (and hence “reasonably detectable”) assuming group sizes of N = 10 (for both the concurrent control group and the treatment group) was calculated using the formula p = effect size × CV, where p = percent decrease and effect size for N = 10 is 1.406. Statistical analysis: two-sided Wilcoxon test, power 75%; p < 0.05; software NQUERY); see Li et al. (Citation2019) for further details.
![Figure 2. Reasonably detectable T4 and TSH level change [%] – dependent on coefficient of variation (CV). The percent T4 and TSH level change that is expected to be significant (and hence “reasonably detectable”) assuming group sizes of N = 10 (for both the concurrent control group and the treatment group) was calculated using the formula p = effect size × CV, where p = percent decrease and effect size for N = 10 is 1.406. Statistical analysis: two-sided Wilcoxon test, power 75%; p < 0.05; software NQUERY); see Li et al. (Citation2019) for further details.](/cms/asset/a4dd6c5f-0d41-4fd9-a336-e6307459c6f5/itxc_a_1910625_f0002_b.jpg)
In human medicine, reference ranges for clinical pathology parameters normally encompass the 2.5th − 97.5th percentiles (i.e. the 95% confidence interval) of a pre-defined group of people (Ichihara et al. Citation2017). (for TSH) and (for fT4/tT4) compare the Roche (Citation2009) reference ranges for human males, pregnant women and infants with the normal distribution ranges (historical control data) recorded for adult male rats, pregnant and lactating dams, and pups (BASF SE, unpublished data). Generally, the TSH and tT4/fT4 variations between the respective pre-defined groups of humans and rats are roughly comparable. In consequence, TSH and T4 alterations in rats and humans that exceed normal ranges can be detected with roughly the same sensitivity. Generally, TSH variation () is higher than fT4/tT4 variation (). This is also reflected in the aforementioned “reasonably detectable” hormone level changes (25% T4 decrease versus 40% TSH increase). In addition, these reasonably detectable hormone changes demonstrate that, in rat studies with group sizes of ten, statistical analysis procedures are very sensitive compared to the variability in healthy individuals. For this reason, findings that are statistically significant as compared to the concurrent control are further compared to the historical control data to determine if they are also “abnormal”.
Figure 3. TSH reference ranges for humans (males, pregnant women, infants) compared to TSH normal distributions for Wistar rats (males, lactating dams, pups): Ranges from median to 97.5th percentile, relative to the median. GD: gestational day; LD: lactational day; N: number of individuals; Trim: trimester; TSH: thyroid stimulating hormone. Colour legend: Striped columns: human data; black columns: rat data. Note that TSH variation attains up to 210% as compared to T4 variation attaining only -50% (). Numbers of individuals; age/life stages: Human males: 20–39 years (N = 286); Wistar rat males: 20 weeks (N = 304); pregnant women: 1st trimester (N = 418) and 3rd trimester (N = 170); Wistar rat dams: GD20 (N = 364) and LD14 (N = 48); infants: 6 days to 3 months (N = 119); children: 1–6 years (N = 346); Wistar rat pups: postnatal day 13 (males/females: N = 222/222). The human TSH reference ranges were adapted from Roche (Citation2009). The rat normal TSH distribution ranges were measured in control rats at BASF SE, Ludwigshafen (Germany); see Supplementary Information SI-2 for methodological details and absolute data.
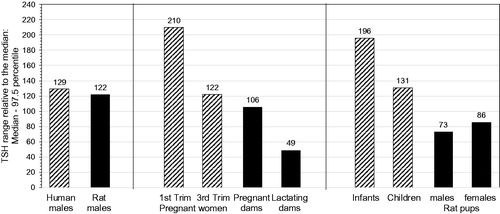
Finally, in some TGs, hormones are only measured at one time point, typically at the end of the study. Further research is needed to determine if low serum hormone levels in either the dams, the foetuses, or the pups are the most sensitive indicators of any subsequent DNT. For that a thorough characterisation of maternal (gestational and lactational), foetal and pup serum hormones assessed in the same studies will facilitate the establishment of temporal patterns in hormone changes in the dam and her progeny and the identification of essential periods for thyroid assessments (Li et al. Citation2019). However, any impact of repeated blood sampling in the same animals on the primary goal of the study and the overall wellbeing of the animal must be considered.
What is the most sensitive thyroid parameter addressed in the rodent toxicity studies?
In rodent toxicity studies, thyroid weight, gross inspection, and histopathology can be assessed in addition to the serum hormone parameters thereby enhancing the weight-of-evidence on which the evaluation is based. By comparison, human studies addressing the impact of maternal thyroid hormone imbalance on child neurodevelopment generally do not include these thyroid parameters.
Only the AOP network described by Noyes et al. (Citation2019), but none of the linear AOPs, includes a key event related to thyroid gland histopathology. Appendix A of the EFSA and ECHA (Citation2018) Endocrine Disruptor Guidance provides one unreferenced “example for a postulated MoA” for thyroid hormone imbalance, and this MoA includes thyroid histopathological changes as the ultimate of altogether five steps. These are (1) hepatic tissue doses; (2) activation of CAR/PXR; (3) hepatic phase I/II enzyme induction; (4) decrease in T4, increase in TSH; (5) thyroid histopathological changes. Hence, this MoA does not include an adverse outcome, since “thyroid histopathological changes” are not per se adverse (and clearly not neurodevelopmental). Also, this MoA in Appendix A is not referenced. Therefore, it is not possible to relate the underlying scientific evidence to that from the AOPs in the AOP Wiki or to the AOP networks.
Non-neoplastic histopathological alterations of the thyroid gland mainly include thyroid follicular cell hypertrophy, decreased colloid, and thyroid follicular cell hyperplasia. The evaluation of the toxicological implications of such alterations is impaired by the circumstance that the threshold for non-neoplastic adversity of the thyroid gland (versus physiological adaptive reaction) has not yet been clearly defined.
The European Society of Toxicologic Pathology (ESTP) Working Group has suggested the following general definition of an adverse effect, which is widely concordant with that provided by the World Health Organisation (WHO IPCS Citation2009):
“In the context of a nonclinical toxicity study, an adverse effect is a test article-related change in the morphology, physiology, growth, development, reproduction or life span of the animal model that likely results in an impairment of functional capacity to maintain homeostasis and/or impairment of the capacity to respond to an additional challenge” (Palazzi et al. Citation2016).
Following this definition, it is the “impairment of a capacity” that is the prerequisite for adversity.
These general considerations on adversity were further discussed specifically for thyroid follicular cell hypertrophy and hyperplasia at the 6th ESTP International Expert Workshop (Huisinga et al. Citation2020). Workshop participants emphasised that the thyroid gland has a physiological capacity to compensate for changes in hormonal and metabolic conditions as reflected by transient increases or decreases in activity, and proposed that:
“Diffuse follicular cell hypertrophy and hyperplasia in the absence of other morphological changes such as focal hyperplasia or neoplasia should not be considered intrinsically adverse. Additional parameters indicating impairment of cell/tissue/organ function or reserve capacity should also be considered. These may include lesion severity, associated effects, including hormonal changes, MoA, and experimental factors such as life stage” (Huisinga et al. Citation2020).
Hence, histopathological findings will generally need to be evaluated together with all available data in a weight-of-evidence approach in order to establish if the capacity of the thyroid gland is impaired. If single thyroid-related parameters are altered, this does not provide evidence for an adverse effect on the thyroid system; instead, a pattern of effects should be observed. Consideration of thyroid weight and histopathology may enhance the assessment of serum TSH, T3, and T4 levels, as these parameters are less sensitive to confounders (DeVito et al. Citation1999; Choksi et al. Citation2003; Colnot and Dekant Citation2017).
Future research work is merited to enhance the understanding for how thyroid histopathology can be used as an additional sensitive metric of the magnitude of maternal thyroid hormone alteration that will induce neurodevelopmental impairment in the offspring. Rodent studies should include all thyroid-related endpoints (serum thyroid hormone, thyroid weight and histopathology, when possible) in the dams, foetuses and pups. Further, these studies should include dose-response relationships for thyroid-related effects at different ages, which should ideally be tied to a neurodevelopmental outcome (Li et al. Citation2019). In this regard, it remains to be determined if a particular pattern of thyroid effects or a certain magnitude of hormone change in the dams, foetuses or pups can be identified that would directly link to neurodevelopmental impairment in rodents, and what their implications would be for human health (Beekhuijzen et al. Citation2019).
How are the adverse neurodevelopmental outcomes of the thyroid-related AOPs addressed in toxicological assessments?
The five AOPs cover three different adverse outcomes, i.e. decreased cognitive function (AOPs 42, 134, 152), impaired learning and memory (AOP 54), and loss of cochlear function (AOP 8). Hence, for five of the five AOPs, the adverse outcomes relate to cognitive processes for which humans and rodents, on account of their phylogenesis, exhibit very different capabilities.
In the human studies evaluated by Sauer et al. (Citation2020), significant effects recorded in children born from mothers with low serum fT4 levels included impaired psychomotor development, expressive language delay, decreased mental scores, lower intelligence quotient, autism spectrum disorders and impaired educational attainment. Generally, specific effects were only recorded in single observational studies, which also reflects the diversity of the study designs (Sauer et al. Citation2020). It remains to be established if the different neurodevelopmental findings observed in the human studies are caused by the same, or similar MoAs or if such differences are indicators of differences in timing or sequences of key events.
From amongst all TGs that include thyroid-related parameters, only OECD TG 443 (EOGRTS) also includes specific neurodevelopmental investigations (when the DNT Cohort is included), thereby addressing specific parameters of relevance for the adverse outcomes described in the five thyroid-related AOPs (notably, however, OECD TG 443 does not include assessments of learning and memory). OECD TG 426 (DNT study) also includes specific neurodevelopmental investigations (including learning and memory), but no thyroid-related parameters.
The neurobehavioural endpoints addressed in OECD TG 443 (DNT cohort) and OECD TG 426 are only crude surrogates for the complex effects on cognitive function observed in humans (Middaugh et al. Citation2003; Makris et al. Citation2009). Indeed, there is considerable concern that these endpoints are not sufficiently sensitive to detect the subtler, but important, neurodevelopmental consequences of thyroid hormone imbalance (Beekhuijzen et al. Citation2019). Recently, the EU project ATHENA (Assays for the identification of Thyroid Hormone axis-disrupting chemicals: Elaborating Novel Assessment strategies; https://cordis.europa.eu/project/rcn/219094/factsheet/en) embarked upon the identification of new endpoints, which are more sensitive to perinatal thyroid hormone imbalance. Potentially relevant further neurodevelopmental parameters (beyond those reflecting the adverse outcomes of the five thyroid-related AOPs) will be presented and discussed in the third review of the ECETOC Special T4 Task Force.
Interestingly, the human cohort studies by Korevaar et al. (Citation2016) and Jansen et al. (Citation2019) suggest that brain magnetic resonance measurements in the progeny may yield relevant and precise measurable outcomes to relate with maternal thyroid hormone imbalance. However, the findings from these studies are not yet conclusive on a causal relation between maternal thyroid function and brain morphological alterations in the child. Further research work is merited to determine how magnetic resonance imaging, as a morphometric methodology, may contribute to the assessment of the impact of maternal thyroid hormone imbalance on child cognitive function. Possibly, such a tool, which is generally applicable in both humans and rats, might be useful to establish species differences and/or concordances of effects.
Finally, loss of cochlear function is indicated as adverse outcome in AOP 8 with liver enzyme induction as the MIE, which has pronounced species-specific differences. In rats, exposure to PCBs via lactation in the early postnatal period has been recorded to elicit upregulation of hepatic UGTs, followed by increased thyroid hormone clearance and ultimately loss of cochlear function (Crofton and Zoeller Citation2005). Hence, the critical period of exposure is postnatal (Crofton et al. Citation2000). Also, in a Slovakian cohort study including 351 children, higher PCB serum concentrations at 6-, 16-, and 45-months, but not maternal or cord PCBs concentrations, were associated with reduced cochlear function in the 45-months old children, as measured by distortion product otoacoustic emissions (Jusko et al. Citation2014). From amongst 195 children with sensorineural hearing loss and 615 children selected at random (all born in 1959–1966 in the Collaborative Perinatal Project US cohort), maternal serum PCB levels were unrelated to the adjusted odds of sensorineural hearing loss or to the adjusted mean hearing threshold (Longnecker et al. Citation2004). Notably, these human studies did not include measurement of thyroid hormones, or of liver enzymes.
With respect to the measurement of cochlear function, OECD TG 443 (DNT cohort) includes performance of the auditory startle test at postnatal day 24 in the Cohort 2a pups. In principle, auditory response is also evaluated in the open field assessment, but these tests provide only very rudimentary information on cochlear/auditory function. Likewise, the auditory startle test will only detect severe impairment of auditory function, since the startle-eliciting stimulus in rats is a very loud (100–120 dB) burst of broad-spectrum sound (Pilz et al. Citation1987). By comparison, reflex modification of the startle response can be used to evaluate cochlear function, with pre-pulse stimuli that range in amplitude (e.g. 35–90 dB) and frequency (e.g. 1–40 kHz) (Crofton Citation1990). This non-invasive test could be added to the study design to supplement the assessment of the auditory startle response in rats. The cochlea could also be examined histopathologically for the loss of hair cells (Neal et al. Citation2015); however, this procedure requires highly-specialized dissection skills and the results do not necessarily inform on cochlear function.
Non-routine parameters reflecting key events of thyroid-related AOPs
None of the OECD or OCSPP TGs that include thyroid-related and/or neurodevelopmental parameters also include (mandatory or optional) parameters related to any of the MIEs or early key events of the thyroid-related AOPs, or AOP networks, with adverse neurodevelopmental outcomes in mammals. This is unsurprising since the traditional in vivo toxicity test methods were designed to investigate if a test substance has the potential to elicit an adverse outcome, but they generally do not focus on the identification of mechanisms of effects, i.e. MoAs. (Exceptions are genotoxicity studies, which are out of scope of this review.) Therefore, parameters reflecting specific MIEs or early key events of AOPs have not been standardised for application in in vivo toxicity studies.
It is the overarching goal of the ECETOC Special T4 Task Force to propose a science-based tiered testing strategy to identify if a substance elicits maternal thyroid hormone imbalance and, if so, if this may ultimately lead to neurodevelopmental impairment in the progeny. Altered serum thyroid hormone levels, on their own, do not necessarily provide proof-of-evidence for an endocrine MoA, also since serum thyroid hormone changes in rats are not necessarily specific or meaningful (see comprehensive review by Marty et al. (Citation2018)). The additional parameters reflecting MIEs and early key events of the thyroid-related AOPs may prove useful for the planned tiered testing strategy by providing supporting evidence to establish the presence of a thyroid-related MoA. As applicable, such parameters might be addressed in in vitro assays, or as supplementary parameters in rodent studies.
Similarly, since the evidence on the biological processes underlying important events of thyroid-related AOPs presented herein distinguishes between processes in rodents and humans, respectively, this information will support the inclusion of species relevance assessments in the planned testing strategy.
Inhibition of NIS and inhibition of TPO are two important MIEs that directly affect thyroid hormone synthesis. Substances that elicit pronounced and/or prolonged effects on thyroid hormone synthesis are likely to also elicit substantial thyroid hormone imbalance. Therefore, in vitro assays that address these endpoints could be valuable additions to the planned testing strategy. It is beneficial that such assays have been developed and validated in the context of the US EPA ToxCast Program thereby having demonstrated their usefulness within a regulatory setting. With respect to NIS inhibition, Radioactive-Iodide Uptake Assays are available that use cells of either human or rat origin (Hallinger et al. Citation2017; Wang et al. Citation2018; Buckalew et al. Citation2020). Comparative assessments using both human and rat cells may also serve to address possible species differences of effects. With respect to TPO inhibition, the Amplex UltraRed-TPO Assay can be used with rat microsomes (Paul Friedman et al. Citation2016), human TPO derived from a human thyroid follicular cell line (Nthy-ori 3-1; Jomaa et al. Citation2015), or recombinant human TPO expressed in cell lines (Dong et al. Citation2020). There have been reports of interspecies differences that may offer advantages to in vitro assessments using human TPO (Jomaa et al. Citation2015; Dong et al. Citation2020). For example, when testing 19 reference compounds (Dong et al. Citation2020), the Amplex-UltraRed assay yielded similar results for strong or negative compounds using rat microsomes or recombinant human TPO; however, variable responses were seen with weak-acting TPO inhibitors across studies. Generally, these in vitro TPO assays, as well as recently described three-dimensional in vitro models of the thyroid gland (Deisenroth et al. Citation2020) may provide useful testing opportunities while minimising animal use.
Further research work is also merited to determine how in vitro assays addressing NIS inhibition and TPO inhibition may be used within a testing strategy to support the determination of whether a substance has the potential to elicit thyroid hormone imbalance via these MIEs. Importantly, the observation that a substance inhibits NIS or TPO in vitro should not per se lead to the conclusion that it will also inhibit thyroid hormone synthesis in vivo. A substance’s potential to elicit an effect in the intact organism also depends on likely exposure scenarios (external exposure), internal exposure levels at the target organ, as well as toxicokinetics (absorption, distribution, metabolism and elimination (ADME)). When assessing if a test substance meets the European Commission (Citation2017, Citation2018) Endocrine Disruptor Criteria, whereby the adverse effect is observed in vivo and the endocrine activity detected in a screening assay, it needs to be shown that it is plausible that the endocrine activity and the adverse outcome are linked by a common endocrine MoA and that the endocrine activity would actually occur in vivo at doses, where the adverse effect is observed. Furthermore, the aforementioned simplicity of in vitro models favours enhanced sensitivity to a myriad of interactions and an inability to adapt or compensate for perturbations, suggesting that in vitro models will, in some cases, overpredict toxicant impact. If in vitro data (and in silico data, if available) indicate TPO inhibition as primary MoA and altered thyroid signalling is seen in in vivo studies, ex vivo thyroid TPO evaluations in treated animals might be useful to include in follow-up experiments (e.g. Hassan et al. Citation2020).
Measurements of circulating fT3/fT4 requires in vivo studies. In humans, fT4 is the serum thyroid hormone parameter which is most frequently measured (together with TSH). Nonetheless, the most frequently measured parameter is not necessarily the most relevant one. It is currently unclear if serum levels of fT4, tT4, fT3 or tT3 in pregnant mothers or their children, or in the dams, foetuses, or pups (for rodent studies), are the most sensitive parameters indicative of thyroid hormone imbalance, or what magnitude of alteration leads to neurodevelopmental impairment. Also, prevailing knowledge gaps associated with the measurement and evaluation of free hormone in the serum currently preclude their utility as additional parameters in rodent studies conducted in a regulatory setting. Therefore, resolving the prevailing scientific and technical limitations with respect to the measurement of free thyroid hormone (in serum and/or relevant tissues) is an urgent research need.
Thyroid hormone binding to serum binding proteins reflects the fraction of free, bioactive thyroid hormone in the blood. Changes in such binding properties can affect the availability of thyroid hormone at the target organs. In vitro assays to address a substance’s potential to displace thyroid hormones from transthyretin are available and have been identified as suitable “for pre-validation in the short term” (OECD Citation2014). Such assays should be useful to determine MoAs that include thyroid hormone displacement from the serum binding proteins. Nonetheless, the biological relevance of any change in thyroid hormone serum binding properties observed in vitro will need to be established (Noyes et al. Citation2019). This appears as pertinent research need. Preferably, the in vitro assays should allow addressing the biological implications of the differences in the spectrum of serum binding proteins between humans and rats.
It remains to be established if liver enzyme induction during pregnancy results in levels of (maternal and/or foetal) thyroid hormone imbalance that are sufficiently high to elicit neurodevelopmental impairment. Appendix A of the EFSA and ECHA (Citation2018) Endocrine Disruptor Guidance suggests that, to investigate whether liver enzyme induction is responsible for altered thyroid hormone levels and/or thyroid histopathology and weight, “comparative studies of enzyme activity induced by the test substance in liver in vitro systems should be measured in both the relevant test species (e.g. rat, mouse and dog) and humans”. Further, it is stated that the ADME properties in both test species and humans, and the activities of possible metabolites must be considered.
Currently, scientific and technical challenges limit the practicability of such comparative assessments in a regulatory setting. Whereas high-throughput screening in vitro assays to assess a substance’s potential to bind to relevant nuclear receptors or to inhibit SULTs or UGTs are available (Noyes et al. Citation2019; Zuang et al. Citation2019), standardised in vitro cell-based assays to assess phase II liver enzyme induction are unavailable, and it is even challenging to maintain the in vivo characteristics of hepatocytes in vitro. Also, species differences in the relative importance of metabolic pathways for thyroid hormones (e.g. metabolism by UGTs, SULTs and/or DIOs), as well as differences in serum transport proteins, need to be considered when striving to establish the human relevance of thyroid-related effects observed in rats. Further investigations are merited to enhance the understanding of such species differences, and of their biological implications. Finally, in vivo comparisons (humans versus rats) are generally limited because UGTs, SULTs and DIOs are generally measured in tissues, cells or sub-cellular fractions, but liver biopsies are rarely if ever undertaken in human studies addressing thyroid function. Accordingly, the identification and establishment of non-invasive, indirect markers of UGT activity in serum or urine is a pertinent research need (Sauer et al. Citation2020).
Generally, assessments of brain thyroid hormone levels require an intact organism since they can be affected by a substance’s systemic and toxicokinetic properties. While it is the thyroid hormone concentration present in relevant brain tissues that is decisive for physiological neurodevelopment, it is currently unclear if this parameter has an added value beyond the assessment of serum hormone levels. Important limitations of measuring tissue hormone levels in rodent studies conducted in a regulatory setting are difficulties in standardising the multi-step hormone extraction procedure and the need for inter-laboratory validation to ensure that robust and reproducible results are obtained.
DIO inhibition is an important event that may affect many aspects of maternal and/or foetal thyroid function and foetal neurodevelopment. While DIO inhibition is not included in any of the thyroid-related AOPs included in the AOP Wiki, its relevance is recognised in the AOP networks (Knapen et al. Citation2018; Villeneuve et al. Citation2018; Noyes et al. Citation2019). Also, DIO inhibition is considered in OECD (Citation2014) New Scoping Document and in the US EPA Endocrine Disruptor Screening Program (US EPA Citation2017). In vitro assays to assess DIO inhibition appear as important additions to the planned testing strategy. It is beneficial that DIO assays have been developed and validated in the context of the US EPA ToxCast Program thereby having demonstrated their usefulness within a regulatory setting. Currently, these in vitro assays include human DIO and further allow distinguishing between inhibition of DIO1, DIO2, and/or DIO3. Considering the species differences in the expression of the respective DIOs in different tissues, the development of in vitro assays using rat DIO is recommendable, since in vitro comparative assessments are likely to facilitate predictions of the human relevance of effects observed in rodent studies. Of note, DIO1 activity can also be determined in ex vivo rat liver microsomes using radioactive substrate 125I-T4 (Mol and Visser Citation1985). If a substance is shown to inhibit DIO in vitro in the early tiers of the testing strategy, such ex vivo assessment might prove useful supplements to the higher-tier rodent studies.
Finally, in vitro assays to evaluate a substance’s potential to inhibit thyroid hormone transmembrane transporters are not yet applicable in a regulatory setting. With respect to TR activation, a transactivation assay aimed at TRα and TRβ is available in ToxCast (Noyes et al. Citation2019). Due to the very limited number of substances shown to interfere with TRs, OECD (Citation2014) New Scoping Document has identified the further validation of such assays to be of subordinate relevance.
Summary and conclusions
This review has (1) collated information on the MIEs and key events of thyroid-related MoAs and AOPs that include adverse neurodevelopmental outcomes in mammals; (2) to establish how the respective MIEs, key events and adverse outcomes are being addressed in standard toxicity test methods; (3) to describe qualitatively and, if possible quantitatively, the biological processes underlying the MIEs and early key events of the AOPs, as they occur in rodents and/or humans, in order to identify potentially relevant additional parameters of thyroid-related effects, which are not (yet) addressed in routine toxicological assessments; and opportunities to establish species relevance of such events and of the key event relationships.
The available standard rodent toxicity test methods include measurements of thyroid hormones and TSH, as well as assessments of the thyroid gland (weight, gross inspection, histopathology). However, there are considerable knowledge gaps for how to establish that altered serum hormone levels and/or thyroid gland (histo-)pathology are indicative of adversity, and not of physiological adaptations of the highly versatile thyroid hormone system. As regards neurodevelopmental parameters, the OECD TG 443 EOGRTS is the only TG that includes both thyroid-related parameters and specific neurodevelopmental investigations (in the DNT cohort), and these are only crude surrogates for the subtle, but relevant, cognitive effects that might occur in humans. Generally, none of the available OECD or OCSPP TGs directly inform on a substance’s specific thyroid-related MoA.
Therefore, the ECETOC Special T4 Task Force selected further, currently non-routine parameters that reflect the MIEs and critical key events of the thyroid-related AOPs. These non-routine parameters have been evaluated herein to determine their suitability to inform on a substance’s specific thyroid-related MoA and on species differences of effects.
In vitro screening assays to assess NIS inhibition, TPO inhibition, and DIO inhibition, as important MIEs potentially leading to thyroid hormone imbalance, are available and have proven useful in regulatory assessments. Screening assays are also available to inform on a substance’s potential to displace thyroid hormones from the serum binding proteins; however, the in vivo relevance of any effects observed in vitro remains to be established. Similarly, in vitro TR activation assays are available, but generally only few substances seem to elicit effects on the level of the TRs. In vitro cell-based assays to assess substance-mediated phase II liver enzyme induction remain to be developed and/or validated for usage in a regulatory setting. Likewise, technical limitations need to be overcome to allow the measurement of free thyroid hormones in serum, or tissue hormone levels, in rodent studies.
Overall, the usefulness of the current knowledge on thyroid-related AOPs to establish a substance’s MoA is limited because the quantitative understanding of the key event relationships, and of their modulation by adaptive homeostatic responses, is often weak. Further, and importantly, the sequence of events described in some of the AOPs should not be considered conclusive. Also, species differences in all processes related to thyroid hormone signalling are evident, but the biological implications of such differences (as regards impact on maternal thyroid function and/or, subsequently, neurodevelopment of the offspring in rodents and humans) are generally unknown. Thus, evaluation of the additional non-routine parameters requires careful consideration around when and how to collect these data and how to interpret the outcomes in a regulatory setting. Research needs have been identified to enhance the applicability of the standard and non-routine parameters reflecting the MIEs and early key events of thyroid-related AOPs in toxicological assessments.
Building upon the findings from the review of the human evidence (Sauer et al. Citation2020) and the present review, the third part of the ECETOC Special T4 Task Force review series will evaluate the evidence from available rodent studies showing how specific xenobiotics affect the maternal thyroid hormone system, under what conditions such effects lead to adverse neurodevelopmental outcomes in the progeny, and how DNT can be experimentally determined - also beyond the current guideline parameters. Finally, in the planned fourth part of the ECETOC Special T4 Task Force review series, all findings will be consolidated to propose a science-based tiered testing strategy. This testing strategy shall serve to identify if a substance has the ability to elicit maternal thyroid hormone imbalance and potentially also neurodevelopmental effects in the progeny – in line with the European Commission (Citation2017, Citation2018) Endocrine Disruptor Criteria and the EFSA and ECHA (Citation2018) Endocrine Disruptor Guidance.
Supplemental Material
Download MS Word (29.2 KB)Acknowledgements
We would like to thank the members of the ECETOC Scientific Committee for their critical comments. Also, we would like to thank all members of the ECETOC Special T4 Task Force as well as all participants of the November 2019 Extended Task Force Meeting for valuable discussions that contributed to the basis for this review. We are indebted to Olivier de Matos, Secretary General of ECETOC, and his team at ECETOC (Alice Brousse, Andreea Cuciureanu, Lisa Wingate, Virginie van der Steeg) for organisational and technical assistance to the ECETOC Special T4 Task Force and for support in preparing and holding the November 2019 Extended Task Force Meeting. A special thank you for the preparation and holding of that meeting also goes to Ursel Blum and Christine Gahn (BASF SE, Germany). We thank both the reviewers selected by the Editor that were anonymous to the authors. The comprehensive and helpful comments served to improve the manuscript.
Declaration of interest
This manuscript relates to work undertaken by the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC; www.ecetoc.org) Special Thyroxine (T4) Task Force. ECETOC is a scientific organisation which provides a collaborative space for scientists from industry, academia and governments. Its mission is to develop and promote practical, trusted and sustainable solutions to scientific challenges which are valuable to industry, as well as to the regulatory community and society in general. ECETOC is financed by its membership, which are the leading companies with interests in the manufacture and use of chemicals, biomaterials and pharmaceuticals (http://www.ecetoc.org/ecetoc-membership/member-companies/). Within the ECETOC Task Forces, Task Force members work within their regular working hours, but do not receive compensation by ECETOC. In preparation of this article, the ECETOC Special T4 Task Force presented and discussed a preliminary draft with invited experts at an Extended Task Force Meeting held in Ludwigshafen, Germany. The meeting venue and catering, as well as transfers in Ludwigshafen, were sponsored by BASF SE (see below for company details). All co-authors, with the exception of UGS, a freelance scientific writer, participated at the meeting and contributed to the preparation of the manuscript without compensation.
The co-authors of this manuscript consist of Task Force members (SM, AC, NH, BRH, SJ, SM-K, LS, VS, DU), Stewards from the ECETOC Scientific Committee (PAB, BvR,), a toxicologist who was invited to the Extended Task Force Meeting (MB), and the scientific writer (UGS). The views expressed in this article are solely those of the co-authors and may not represent those of the sponsoring organisations.
SM is employed by The Dow Chemical Company. The issues of hazard identification and risk assessment of thyroid-active compounds, and how these are assessed by regulatory/other agencies, impact substances of interest to the corporation. An in-house review of this manuscript was conducted by additional scientists of The Dow Chemical Company, but only few minor, mostly editorial changes were requested. SM’s role is focussed on Dow’s science strategy and testing, which includes the assessment of endocrine-active compounds. SM received no funding in cash or kind for her contribution to this manuscript. SM's expenses for the attendance at the Extended Task Force Meeting were paid by Dow.
MB is employed by Charles River Laboratories, The Netherlands. Charles River is a Contract Research Organisation that offers a full-service portfolio for (agro-)chemical, and pharmaceutical companies. One of these services is in vivo toxicology testing according to OECD guidelines in which thyroid hormone and/or DNT measurements are included. This manuscript was subjected to the usual internal peer-review process in Charles River, in this case by the Global Head of Developmental, Reproductive and Juvenile Toxicology. This review yielded very few and minor requests for amendment. MB’s role at Charles River is focussed on scientific discussions with clients and study directors on study design and data evaluation. MB received no funding in cash or kind for her contribution to this manuscript.
AC and PAB are employed by Syngenta, an international agribusiness that markets crop protection chemicals and seeds. The Syngenta portfolio includes substances that may have to be tested for their potential to cause maternal thyroid disruption and subsequent developmental neurotoxicity. This manuscript was subjected to the usual internal peer-review process in Syngenta, in this case by the Global Head of Human Safety, but no changes were requested. AC’s responsibilities include providing scientific support to research and development activities and to regulatory toxicology projects. PAB’s responsibilities within Syngenta are to provide strategic scientific advice on product safety issues to the company’s Product Safety, Business Sustainability and Crop Protection Development organisations. AC’s and PAB’s expenses for the attendance at the Extended Task Force Meeting were paid by Syngenta; they received no funding in cash or kind for their contribution to this manuscript.
NH and LS are employed by the Crop Science Division of Bayer AG, which developed and markets products that contain some of the pesticides referenced in this paper. Further, the Bayer portfolio includes substances that may have to be tested for their potential to cause maternal thyroid disruption and subsequent developmental neurotoxicity. This manuscript was submitted to in-house review in different Bayer AG company divisions, but no changes were requested. NH’s responsibilities include being the Bayer Crop Science Division Environment Safety Ecotoxicology Terrestrial Vertebrates Team Leader. Up until January 2020, NH was Chair of the ECETOC T4 Task Force, and she chaired the Extended Task Force Meeting. NH’s expenses for the attendance at that meeting were paid by Bayer AG. LS’s responsibilities include being Senior Fellow Regulatory Toxicology at the Bayer Crop Science Division. NH and LS received no funding in cash or kind for their contributions to this manuscript.
BRH is employed by Corteva Agriscience, USA. Corteva markets products (or previously marketed products) containing some of the chemicals included in this paper. Further, the Corteva portfolio includes substances that may have to be tested for their potential to cause maternal thyroid disruption and subsequent developmental neurotoxicity. This manuscript was submitted to in-house review by the Corteva Regulatory and Stewardship Function, but no changes were requested. BRH leads the Corteva Agriscience Haskell R&D Centre Endocrine Group. BRH received no funding in cash or kind for her contribution to this manuscript. BRH's expenses for the attendance at the Extended Task Force Meeting were paid by Corteva Agriscience.
SJ is employed by Albemarle Europe SRL. The Albemarle portfolio includes substances that may have to be tested for their potential to cause maternal thyroid disruption and subsequent developmental neurotoxicity. SJ’s responsibilities include heading the corporate toxicology department of Albemarle Corporation, worldwide management of regulatory toxicology and risk assessment of the chemicals produced by Albemarle Corporation. SJ’s expenses for the attendance at the Extended Task Force Meeting were paid by Albemarle; she received no funding in cash or kind for her contribution to this manuscript.
BvR, SMK, VS and DU are employed by BASF SE, Ludwigshafen, Germany. BASF SE produces a very wide range of chemicals including some of those mentioned in this paper and/or substances that may have to be tested for their potential to cause maternal thyroid disruption and subsequent developmental neurotoxicity. This paper underwent the normal BASF SE review process of the Global Product Safety Department of Agricultural Solutions, but no changes were requested. BvR is Senior Vice President of the BASF SE Experimental Toxicology and Ecology Department, which is independent of any business unit and ISO 17020 certified. BvR is an Associate Professor of Reproduction Toxicity of the University of Wageningen, Netherlands, and the Chairman of the ECETOC Scientific Committee. SMK’s responsibilities within BASF SE include being regulatory toxicologist for agrochemicals, and she is the current Chair of the ECETOC T4 Task Force. VS is Principal Scientist and Head of the Clinical Pathology Laboratory at BASF SE. DU is regulatory toxicologist for agrochemicals. BvR, SMK, VS and DU received no funding in cash or kind for their contribution to this manuscript, any travel expenses were covered entirely by BASF.
UGS (a freelance scientific writer) was hired by ECETOC to assist in the preparation of this manuscript. This included payment of working hours and reimbursement of accommodation and travel expenses (train fare) for participation at the Extended Task Force Meeting (purpose: note taking).
Finally, this manuscript was reviewed by the ECETOC Scientific Committee consisting of representatives of academia and industry (http://www.ecetoc.org/about-ecetoc/scientific-committee/). This review yielded few comments that mainly related to the structuring of the manuscript.
Supplemental material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Abuid J, Klein AH, Foley TPJ, Larsen PR. 1974. Total and free triiodothyronine and thyroxine in early infancy. J Clin Endocrinol Metab. 39(2):263–268.
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, et al. 2017. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 27(3):315–389.
- Alshehri B, D'Souza DG, Lee JY, Petratos S, Richardson SJ. 2015. The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol. 27(5):303–323.
- Arem R, Wiener GJ, Kaplan SG, Kim HS, Reichlin S, Kaplan MM. 1993. Reduced tissue thyroid hormone levels in fatal illness. Metabolism. 42(9):1102–1108.
- Arrojo E Drigo R, Bianco AC. 2011. Type 2 deiodinase at the crossroads of thyroid hormone action. Int J Biochem Cell Biol. 43(10):1432–1441.
- Barter RA, Klaassen CD. 1994. Reduction of thyroid hormone levels and alteration of thyroid function by four representative UDP-glucuronosyltransferase inducers in rats. Toxicol Appl Pharmacol. 128(1):9–17.
- Bartsch R, Brinkmann B, Jahnke G, Laube B, Lohmann R, Michaelsen S, Neumann I, Greim H. 2018. Human relevance of follicular thyroid tumors in rodents caused by non-genotoxic substances. Regulat Toxicol Pharmacol. 98:199–208.
- Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. 2010. Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology. 151(8):4055–4065.
- Beekhuijzen M, Rijk JCW, Meijer M, de Raaf MA, Pelgrom S. 2019. A critical evaluation of thyroid hormone measurements in OECD test guideline studies: is there any added value? Reprod Toxicol. 88:56–66.
- Beekhuijzen M, Schneider S, Barraclough N, Hallmark N, Hoberman A, Lordi S, Moxon M, Perks D, Piersma AH, Makris SL. 2018. The urgency for optimization and harmonization of thyroid hormone analyses and their interpretation in developmental and reproductive toxicology studies. Reprod Toxicol. 80:126–130.
- Beetstra JB, van Engelen JG, Karels P, van der Hoek HJ, de Jong M, Docter R, Krenning EP, Hennemann G, Brouwer A, Visser TJ. 1991. Thyroxine and 3,3',5-triiodothyronine are glucuronidated in rat liver by different uridine diphosphate-glucuronyltransferases. Endocrinology. 128(2):741–746.
- Bernal J. 2007. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 3(3):249–259.
- Bernal J. 2015. Thyroid hormones in brain development and function. In: Feingold KR, Anawalt B, Boyce A, editors. Endotext [Internet]. South Dartmouth MA, USA; MDText.com, Inc.
- Bernal J, Guadaño-Ferraz A, Morte B. 2015. Thyroid hormone transporters-functions and clinical implications. Nat Rev Endocrinol. 11(12):690.
- Bernal J, Pekonen F. 1984. Ontogenesis of the nuclear 3,5,3'-triiodothyronine receptor in the human fetal brain. Endocrinology. 114(2):677–679.
- Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, et al. 2014. American Thyroid Association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 24(1):88–168.
- Bianco AC, Kim BW. 2006. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 116(10):2571–2579.
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 23(1):38–89.
- Bradley DJ, Young WII, Weinberger C. 1989. Differential expression of alpha and beta thyroid hormone receptor genes in rat brain and pituitary. Proc Natl Acad Sci U S A. 86(18):7250–7254.
- Bradley DJ, Towle HC, Young WS. 1992. Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci. 12(6):2288–2302.
- Braun D, Kinne A, Bräuer AU, Sapin R, Klein MO, Köhrle J, Wirth EK, Schweizer U. 2011. Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia. 59(3):463–471.
- Brouwer A, van den Berg KJ. 1986. Binding of a metabolite of 3,4,3',4'-tetrachlorobiphenyl to transthyretin reduces serum vitamin A transport by inhibiting the formation of the protein complex carrying both retinol and thyroxin. Toxicol Appl Pharmacol. 85(3):301–312.
- Buckalew AR, Wang J, Murr AS, Deisenroth C, Stewart WM, Stoker TE, Laws SC. 2020. Evaluation of potential sodium-iodide symporter (NIS) inhibitors using a secondary Fischer rat thyroid follicular cell (FRTL-5) radioactive iodide uptake (RAIU) assay. Arch Toxicol. 94(3):873–885.
- Calvo R, Obregón MJ, Ruiz de Oña C, Escobar del Rey F, Morreale de Escobar G. 1990. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest. 86(3):889–899.
- Carvalho DP, Ferreira ACF, Coelho SM, Moraes JM, Camacho MAS, Rosenthal D. 2000. Thyroid peroxidase activity is inhibited by amino acids. Braz J Med Biol Res. 33(3):355–361.
- Cavalieri RR, Pitt-Rivers R. 1981. The effects of drugs on the distribution and metabolism of thyroid hormones. Pharmacol Rev. 33(2):55–80.
- Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, Morte B, Bernal J. 2009. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3'-triiodo-L-thyronine. Endocrinology. 150(5):2491–2496.
- Chakravarthy V, Ejaz S. 2020. Thyroxine-binding globulin deficiency. In: StatPearls. Treasure Island FL, USA; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK544274/
- Chan S, Kachilele S, McCabe CJ, Tannahill LA, Boelaert K, Gittoes NJ, Visser TJ, Franklyn JA, Kilby MD. 2002. Early expression of thyroid hormone deiodinases and receptors in human fetal cerebral cortex. Brain Res Dev Brain Res. 138(2):109–116.
- Chang L, Munro SL, Richardson SJ, Schreiber G. 1999. Evolution of thyroid hormone binding by transthyretins in birds and mammals. Eur J Biochem. 259(1–2):534–542.
- Chan SY, Hancox LA, Martín-Santos A, Loubière LS, Walter MNM, González A-M, Cox PM, Logan A, McCabe CJ, Franklyn JA, et al. 2014. MCT8 expression in human fetal cerebral cortex is reduced in severe intrauterine growth restriction. J Endocrinol. 220(2):85–95.
- Chanoine JP, Braverman LE. 1992. The role of transthyretin in the transport of thyroid hormone to cerebrospinal fluid and brain. Acta Med Austriaca. 19(Suppl 1):25–28.
- Cheng SY, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev. 31(2):139–170.
- Choksi NY, Jahnke GD, St Hilaire C, Shelby M. 2003. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res B Dev Reprod Toxicol. 68(6):479–491.
- Chopra IJ, Wu SY, Teco GN, Santini F. 1992. A radioimmunoassay for measurement of 3,5,3'-triiodothyronine sulfate: studies in thyroidal and nonthyroidal diseases, pregnancy, and neonatal life. J Clin Endocrinol Metab. 75(1):189–194.
- Colnot T, Dekant W. 2017. Approaches for grouping of pesticides into cumulative assessment groups for risk assessment of pesticide residues in food. Regul Toxicol Pharmacol. 83:89–99.
- Crofton KM. 1990. Reflex modification and the detection of toxicant-induced auditory dysfunction. Neurotox Teratol. 12(5):461–468.
- Crofton KM, Gilbert M, Friedman KP, Demeneix B, Marty MS, Zoeller RT. 2019. Adverse outcome pathway on inhibition of thyroperoxidase and subsequent adverse neurodevelopmental outcomes in mammals. OECD Series on Adverse Outcome. Pathways, No. 13. DOI:10.1787/ea5aa069-en
- Crofton KM, Kodavanti PR, Derr-Yellin EC, Casey AC, Kehn LS. 2000. PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol Sci. 57(1):131–140.
- Crofton KM, Zoeller RT. 2005. Mode of action: Neurotoxicity induced by thyroid hormone disruption during development-hearing loss resulting from exposure to PHAHs. Crit Rev Toxicol. 35(8–9):757–769.
- Darras VM, Hume R, Visser TJ. 1999. Regulation of thyroid hormone metabolism during fetal development. Mol Cell Endocrinol. 151(1–2):37–47.
- Darras VM, van Herck SL. 2012. Iodothyronine deiodinase structure and function: from ascidians to humans. J Endocrinol. 215(2):189–206.
- Dayem M, Basquin C, Navarro V, Carrier P, Marsault R, Chang P, Huc S, Darrouzet E, Lindenthal S, Pourcher T. 2008. Comparison of expressed human and mouse sodium/iodide symporters reveals differences in transport properties and subcellular localization. J Endocrinol. 197(1):95–109.
- Deisenroth C, Soldatow VY, Ford J, Stewart W, Brinkman C, LeCluyse EL, MacMillan DK, Thomas RS. 2020. Development of an in vitro human thyroid microtissue model for chemical screening. Toxicol Sci. 174(1):63–78.
- DeVito M, Biegel L, Brouwer A, Brown S, Brucker-Davis F, Cheek AO, Christensen R, Colborn T, Cooke P, Crissman J, et al. 1999. Screening methods for thyroid hormone disruptors. Environ Health Perspect. 107(5):407–415.
- Divi RL, Doerge DR. 1996. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 9(1):16–23.
- Dong H, Godlewska M, Wade MG. 2020. A rapid assay of human thyroid peroxidase activity. Toxicol in Vitro. 62:104662.
- Emi Y, Ikushiro S, Kato Y. 2007. Thyroxine-metabolizing rat uridine diphosphate-glucuronosyltransferase 1A7 is regulated by thyroid hormone receptor. Endocrinology. 148(12):6124–6133.
- European Commission. 2017. Commission Delegated Regulation (EU) 2017/2100 of 4 September 2017 setting out scientific criteria for the determination of endocrine-disrupting properties pursuant to Regulation (EU) No 528/2012 of the European Parliament and Council. OJ EU L. 60:1–12.
- European Commission. 2018. Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out criteria for the determination of endocrine disrupting properties. OJ EU L. 101:33–36.
- European Food Safety Authority [EFSA]. 2020. Technical report on the outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology. EFSA Supporting Publication. 2020:EN-1837. DOI:https://doi.org/10.2903/sp.efsa.2020.EN-1837
- European Food Safety Authority, European Chemicals Agency [EFSA, ECHA]. 2018. European Food Safety Authority and European Chemicals Agency with the technical support of the Joint Research Centre (Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, et al.). Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. ECHA-18-G-01-EN; EFSA J. 16:1661–170.
- European Parliament and Council [EP and Council]. 2009. Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L. 309:1–50.
- European Parliament and Council [EP and Council]. 2012. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. OJ L. 167:1.
- Fang HL, Shenoy S, Duanmu Z, Kocarek TA, Runge-Morris M. 2003. Transactivation of glucocorticoid-inducible rat aryl sulfotransferase (SULT1A1) gene transcription. Drug Metab Dispos. 31(11):1378–1381.
- Findlay KA, Kaptein E, Visser TJ, Burchell B. 2000. Characterization of the uridine diphosphate-glucuronosyltransferase-catalyzing thyroid hormone glucuronidation in man. J Clin Endocrinol Metab. 85(8):2879–2883.
- Flamant F, Baxter JD, Forrest D, Refetoff S, Samuels H, Scanlan TS, Vennström B, Samarut J. 2006. International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev. 58(4):705–711.
- Fleming CE, Nunes AF, Sousa MM. 2009. Transthyretin: more than meets the eye. Prog Neurobiol. 89(3):266–276.
- Franchi SA, Macco R, Astro V, Tonoli D, Savino E, Valtorta F, Sala K, Botta M, de CI. 2017. A method to culture GABAergic interneurons derived from the medial ganglionic eminence. Front Cell Neurosci. 11:423.
- Friesema EC, Docter R, Moerings EP, Stieger B, Hagenbuch B, Meier PJ, Krenning EP, Hennemann G, Visser TJ. 1999. Identification of thyroid hormone transporters. Biochem Biophys Res Commun. 254(2):497–501.
- Galton VA, McCarthy PT, St Germain DL. 1991. The ontogeny of iodothyronine deiodinase systems in liver and intestine of the rat. Endocrinology. 128(4):1717–1722.
- Gardner-Stephen D, Heydel JM, Goyal A, Lu Y, Xie W, Lindblom T, Mackenzie P, Radominska-Pandya A. 2004. Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab Dispos. 32(3):340–347.
- Gereben B, Salvatore D, Harney JW, Tu HM, Larsen PR. 2001. The human, but not rat, dio2 gene is stimulated by thyroid transcription factor-1 (TTF-1). Mol Endocrinol. 15(1):112–124.
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 29(7):898–938.
- Grijota-Martinez C, Diez D, Morreale de Escobar G, Bernal J, Morte B. 2011. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology. 152(4):1713–1721.
- Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Lim EM, Walsh JP. 2013. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab. 98(7):2936–2943.
- Hallgren S, Darnerud PO. 2002. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats – testing interactions and mechanisms for thyroid hormone effects. Toxicol. 177(2-3):227–243.
- Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Stoker TE, Laws SC. 2017. Development of a screening approach to detect thyroid disrupting chemicals that inhibit the human sodium iodide symporter (NIS). Toxicol in Vitro. 40:66–78.
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJ, Brouwer A, Bergman A. 2008. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2',4,4'-tetrabromodiphenyl ether (BDE-47)). Mol Nutr Food Res. 52(2):284–298.
- Hassan I, El-Masri H, Ford J, Brennan A, Handa S, Paul Friedman K, Gilbert ME. 2020. Extrapolating in vitro screening assay data for thyroperoxidase inhibition to predict serum thyroid hormones in the rat. Toxicol Sci. 173(2):280–292.
- Hassan I, Hisham E-M, Kosian PA, Ford J, Degitz SJ, Gilbert ME. 2017. Neurodevelopmental and thyroid hormone synthesis inhibition in the rat: quantitative understanding within the adverse outcome pathway framework. Tox Sci. 160(1):57–73.
- Heltemes LM, Hagan CR, Mitrofanova EE, Panchal RG, Guo J, Link CJ. 2003. The rat sodium iodide symporter gene permits more effective radioisotope concentration than the human sodium iodide symporter gene in human and rodent cancer cells. Cancer Gene Ther. 10(1):14–22.
- Hempel N, Wang H, LeCluyse EL, McManus ME, Negishi M. 2004. The human sulfotransferase SULT1A1 gene is regulated in a synergistic manner by Sp1 and GA binding protein. Mol Pharmacol. 66(6):1690–1701.
- Hill RN, Erdreich LS, Paynter OE, Roberts PA, Rosenthal SL, Wilkinson CF. 1989. Thyroid follicular cell carcinogenesis. Fundam Appl Toxicol. 12(4):629–697.
- Holtorf K. 2014. Thyroid hormone transport into cellular tissue. j Restorat Med. 3(1):53–68.
- Hornung MW, Korte JJ, Olker JH, Denny JS, Knutsen C, Hartig PC, Cardon MC, Degitz SJ. 2018. Screening the ToxCast phase 1 chemical library for inhibition of deiodinase type 1 activity. Toxicol Sci. 162(2):570–581.
- Huang SA, Bianco AC. 2008. Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab. 4(3):148–155.
- Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. 2003. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 88(3):1384–1388.
- Huisinga M, Bertrand L, Chamanza R, Damiani I, Engelhardt J, Francke S, Freyberger A, Harada T, Harleman J, Kaufmann W, et al. 2020. Adversity considerations for thyroid follicular cell hypertrophy and hyperplasia in nonclinical toxicity studies: results from the 6th ESTP International Expert Workshop. Toxicol Pathol. 48(8):920–938.
- Ichihara K, Ozarda Y, Barth JH, Klee G, Qiu L, Erasmus R, Borai A, Evgina S, Ashavaid T, Khan D, et al. 2017. A global multicenter study on reference values: 1. Assessment of methods for derivation and comparison of reference intervals. Clin Chim Acta. 467:70–82.
- Jahnke GD, Choksi NY, Moore JA, Shelby MD. 2004. Thyroid toxicants: assessing reproductive health effects. Environ Health Perspect. 112(3):363–368.
- Jansen TA, Korevaar TIM, Mulder TA, White T, Muetzel RL, Peeters RP, Tiemeier H. 2019. Maternal thyroid function during pregnancy and child brain morphology: a time window-specific analysis of a prospective cohort. Lancet Diabetes Endocrinol. 7(8):629–637.
- Jomaa B, de Haan LH, Peijnenburg AA, Bovee TF, Aarts JM, Rietjens IM. 2015. Simple and rapid in vitro assay for detecting human thyroid peroxidase disruption. ALTEX. 32(3):191–200.
- Jonklaas J, Sathasivam A, Wang H, Gu J, Burman KD, Soldin SJ. 2014. Total and free thyroxine and triiodothyronine: measurement discrepancies, particularly in inpatients. Clin Biochem. 47(13-14):1272–1278.
- Jusko TA, Sisto R, Iosif AM, Moleti A, Wimmerová S, Lancz K, Tihányi J, Sovčiková E, Drobná B, Palkovičová L, et al. 2014. Prenatal and postnatal serum PCB concentrations and cochlear function in children at 45 months of age. Environ Health Perspect. 122(11):1246–1252.
- Kaptein EM, Hays MT, Ferguson DC. 1994. Thyroid hormone metabolism. Vet Clin North Am Small Anim Pract. 24(3):431–462.
- Kester MH, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W, Glatt H, Coughtrie MW, Visser TJ. 1999. Characterization of human iodothyronine sulfotransferases. J Clin Endocrinol Metab. 84(4):1357–1364.
- Kester MHA, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W, Glatt H, Coughtrie MWH, Visser TJ. 2003. Characterization of rat iodothyronine sulfotransferases. Am J Physiol Endocrinol Metab. 285(3):E592–E598.
- Kester MH, Kaptein E, van Dijk CH, Roest TJ, Tibboel D, Coughtrie MW, Visser TJ. 2002. Characterization of iodothyronine sulfatase activities in human and rat liver and placenta. Endocrinology. 143(3):814–819.
- Klaassen CD, Hood AM. 2001. Effects of microsomal enzyme inducers on thyroid follicular cell proliferation and thyroid hormone metabolism. Toxicol Pathol. 29(1):34–40.
- Kliewer SA, Willson TM. 2002. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 43(3):359–364.
- Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O'Brien JM, Pollesch N, Smith LC, et al. 2018. Adverse outcome pathway networks I: Development and applications. Environ Toxicol Chem. 37(6):1723–1733.
- Köhrle J. 2002. Iodothyronine deiodinases. Methods Enzymol. 347:125–167.
- Korevaar TIM, Muetzel R, Medici M, Chaker L, Jaddoe VWV, de Rijke YB, Steegers EAP, Visser TJ, White T, Tiemeier H, et al. 2016. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. 4(1):35–43.
- Lans MC, Spiertz C, Brouwer A, Koeman JH. 1994. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur J Pharmacol. 270(2–3):129–136.
- Lee JY, Kim MJ, Deliyanti D, Azari MF, Rossello F, Costin A, Ramm G, Stanley EG, Elefanty AG, Wilkinson-Berka JL, et al. 2017. Overcoming monocarboxylate transporter 8 (MCT8)-deficiency to promote human oligodendrocyte differentiation and myelination. EBioMedicine. 25:122–135.
- Lewandowski TA, Seeley MR, Beck BD. 2004. Interspecies differences in susceptibility to perturbation of thyroid homeostasis: a case study with perchlorate. Regul Toxicol Pharmacol. 39(3):348–362.
- Li AA, Makris SL, Marty MS, Strauss V, Gilbert ME, Blacker A, Zorrilla LM, Coder PS, Hannas B, Lordi S, et al. 2019. Practical considerations for developmental thyroid toxicity assessments: what's working, what's not, and how can we do better? Regul Toxicol Pharmacol. 106:111–136.
- Little AG. 2018. Local regulation of thyroid hormone signaling. Vitam Horm. 106:1–17.
- Liu J, Liu Y, Barter RA, Klaassen CD. 1995. Alteration of thyroid homeostasis by UDP-glucuronosyltransferase inducers in rats: a dose-response study. J Pharmacol Exp Ther. 273(2):977–985.
- Loeb WF, Quimby FW. 1999. The clinical chemistry of laboratory animals. New York: Pergamon Press.
- Longnecker MP, Hoffman HJ, Klebanoff MA, Brock JW, Zhou H, Needham L, Adera T, Guo X, Gray KA. 2004. In utero exposure to polychlorinated biphenyls and sensorineural hearing loss in 8-year-old children. Neurotoxicol Teratol. 26(5):629–637.
- Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. 2004. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem. 279(19):19832–19838.
- Maia AL, Goemann IM, Meyer EL, Wajner SM. 2011. Deiodinases: the balance of thyroid hormone: type 1 iodothyronine deiodinase in human physiology and disease. J Endocrinol. 209(3):283–297.
- Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. 2005. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. 115(9):2524–2533.
- Makris SL, Raffaele K, Allen S, Bowers WJ, Hass U, Alleva E, Calamandrei G, Sheets L, Amcoff P, Delrue N, et al. 2009. A retrospective performance assessment of the developmental neurotoxicity study in support of OECD test guideline 426. Environ Health Perspect. 117(1):17–25.
- Marty MS, Borgert C, Coady K, Green R, Levine SL, Mihaich E, Ortego L, Wheeler JR, Yi KD, Zorrilla LM. 2018. Distinguishing between endocrine disruption and non-specific effects on endocrine systems. Regul Toxicol Pharmacol. 99:142–158.
- McClain RM. 1989. The significance of hepatic microsomal enzyme induction and altered thyroid function in rats: implications for thyroid gland neoplasia. Toxicol Pathol. 17(2):294–306.
- Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. 2014. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 34(1):1–18.
- Meek MEB, Palermo CM, Bachman AN, North CM, Jeffrey Lewis R. 2014. Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 34(6):595–606.
- Mendoza A, Hollenberg AN. 2017. New insights into thyroid hormone action. Pharmacol Ther. 173:135–145.
- Middaugh LD, Dow-Edwards D, Li AA, Sandler JD, Seed J, Sheets LP, Shuey DL, Slikker W, Jr, Weisenburger WP, Wise LD, et al. 2003. Neurobehavioral assessment: a survey of use and value in safety assessment studies. Toxicol Sci. 76(2):250–261.
- Midgley JEM, Toft AD, Larisch R, Dietrich JW, Hoermann R. 2019. Time for a reassessment of the treatment of hypothyroidism. BMC Endocr Disord. 19(1):37.
- Mol JA, Visser TJ. 1985. Rapid and selective inner ring deiodination of thyroxine sulfate by rat liver deiodinase. Endocrinol. 117(1):8–12.
- Morreale de Escobar G, Calvo R, Obregon MJ, Escobar del Rey F. 1990. Contribution of maternal thyroxine to fetal thyroxine pools in normal rats near term. Endocrinology. 126(5):2765–2777.
- Morreale de Escobar G, Pastor R, Obregon MJ, Escobar del Rey F. 1985. Effects of maternal hypothyroidism on the weight and thyroid hormone content of rat embryonic tissues, before and after onset of fetal thyroid function. Endocrinol. 117(5):1890–1900.
- Morreale de Escobar GM, Obregón MJ, del Rey FE. 2004. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 18(2):225–248.
- Murk AJ, Rijntjes E, Blaauboer BJ, Clewell R, Crofton KM, Dingemans MML, Furlow JD, Kavlock R, Köhrle J, Opitz R, et al. 2013. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol in Vitro. 27(4):1320–1346.
- Neal C, Kennon-McGill S, Freemyer A, Shum A, Staecker H, Durham D. 2015. Hair cell counts in a rat model of sound damage: effects of tissue preparation & identification of regions of hair cell loss. Hear Res. 328:120–132.
- Noyes PD, Friedman KP, Browne P, Haselman JT, Gilbert ME, Hornung MW, Barone S, Jr, Crofton KM, Laws SC, Stoker TE, et al. 2019. Evaluating chemicals for thyroid disruption: opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ Health Perspect. 127(9):95001.
- Olker JH, Korte JJ, Denny JS, Hartig PC, Cardon MC, Knutsen CN, Kent PM, Christensen JP, Degitz SJ, Hornung MW. 2019. Screening the ToxCast Phase 1, Phase 2, and e1k Chemical Libraries for inhibitors of iodothyronine deiodinases. Toxicol Sci. 168(2):430–442.
- Organisation for Economic Cooperation and Development [OECD]. 2014. Series on testing and assessment No. 207. New scoping document on in vitro and ex vivo assays for the identification of modulators of thyroid hormone signaling. ENV/JM/MONO(2014)23. Paris, France: OECD.
- Organisation for Economic Cooperation and Development [OECD]. 2017. Series on testing and assessment No. 184. Revised guidance document on developing and assessing adverse outcome pathways. ENV/JM/MONO(2013)6. Paris, France: OECD.
- Organisation for Economic Cooperation and Development [OECD]. 2018. Adverse outcome pathway external review report. AOP 54: inhibition of Na+/I- symporter (NIS) decreases TH synthesis leading to learning and memory deficits in children [accessed 2020 Sep] https://aopwiki.org/system/dragonfly/production/2018/07/03/9nmpr7rl2_AOP_54_External_review_report.pdf.
- O'Shaughnessy KL, Wood C, Ford RL, Kosian PA, Hotchkiss MG, Degitz SJ, Gilbert ME. 2018. Thyroid hormone disruption in the fetal and neonatal rat: Predictive hormone measures and bioindicators of hormone action in the developing cortex. Toxicol Sci. 166(1):163–179.
- Palazzi X, Burkhardt JE, Caplain H, Dellarco V, Fant P, Foster JR, Francke S, Germann P, Gröters S, Harada T, et al. 2016. Characterizing “adversity” of pathology findings in nonclinical toxicity studies: results from the 4th ESTP International Expert Workshop. Toxicol Pathol. 44(6):810–824.
- Palha JA. 2002. Transthyretin as a thyroid hormone carrier: function revisited. Clin Chem Lab Med. 40(12):1292–1300.
- Palha JA, Fernandes R, de Escobar GM, Episkopou V, Gottesman M, Saraiva MJ. 2000. Transthyretin regulates thyroid hormone levels in the choroid plexus, but not in the brain parenchyma: study in a transthyretin-null mouse model. Endocrinology. 141(9):3267–3272.
- Patel J, Landers K, Li H, Mortimer RH, Richard K. 2011. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 22(5):164–170.
- Paul Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, Simmons SO. 2016. Tiered high-throughput screening approach to identify thyroperoxidase inhibitors within the ToxCast phase I and II chemical libraries. Toxicol Sci. 151(1):160–180.
- Paul KB, Hedge JM, Macherla C, Filer DL, Burgess E, Simmons SO, Crofton KM, Hornung MW. 2013. Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology. 312:97–107.
- Paul-Friedman K, Martin M, Crofton KM, Hsu C-W, Sakamuru S, Zhao J, Xia M, Huang R, Stavreva DA, Soni V, et al. 2019. Limited chemical structural diversity found to modulate thyroid hormone receptor in the Tox21 chemical library. Env Health Perspecti. 127(9):97009.
- Peeters RP, van der Geyten S, Wouters PJ, Darras VM, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. 2005. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab. 90(12):6498–6507.
- Perez-Castillo A, Bernal J, Ferreiro B, Pans T. 1985. The early ontogenesis of thyroid hormone receptor in the rat fetus. Endocrinology. 117(6):2457–2461.
- Pilz PK, Schnitzler HU, Menne D. 1987. Acoustic startle threshold of the albino rat (Rattus norvegicus). J Comp Psychol. 101(1):67–72.
- Pinna G, Hiedra L, Prengel H, Broedel O, Eravci M, Meinhold H, Baumgartner A. 1999. Extraction and quantification of thyroid hormones in selected regions and subcellular fractions of the rat brain. Brain Res Brain Res Protoc. 4(1):19–28.
- Pohlenz J, Refetoff S. 1999. Mutations in the sodium/iodide symporter (NIS) gene as a cause for iodide transport defects and congenital hypothyroidism. Biochimie. 81(5):469–476.
- Refetoff S. 2015. Thyroid hormone serum transport proteins. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, editors. Endotext [Internet]. South Dartmouth MA, USA: MDText.com, Inc.
- Renko K, Schäche S, Hoefig CS, Welsink T, Schwiebert C, Braun D, Becker N-P, Köhrle J, Schomburg L. 2015. An improved nonradioactive screening method identifies genistein and xanthohumol as potent inhibitors of iodothyronine deiodinases. Thyroid. 25(8):962–968.
- Richard K, Hume R, Kaptein E, Sanders JP, van Toor H, de Herder WW, den Hollander JC, Krenning E, Visser TJ. 1998. Ontogeny of iodothyronine deiodinases in human liver. J Clin Endocrinol Metab. 83(8):2868–2874.
- Richardson VM, Ferguson SS, Sey YM, DeVito MJ. 2014. In vitro metabolism of thyroxine by rat and human hepatocytes. Xenobiotica. 44(5):391–403.
- Riutta C, Hassan I, Ford J, Kosian P, O’Shaughnessy K, Degitz S, Gilbert M. 2019. Thyroid hormone measurements in rat brain by mass spectrometry. San Diego CA, USA: Development Neurotoxicol Society. p. 23–26.
- Roche. 2009. Elecsys thyroid tests. Reference intervals for children and adults. D-Mannheim: Roche Diagnostics GmbH.
- Rolaki A, Pistollato F, Munn S, Price AB. 2019. Adverse outcome pathway on inhibition of Na+/I- symporter (NIS) leads to learning and memory impairment. OECD Series on Adverse Outcome Pathways No. 14. DOI:https://doi.org/10.1787/7ca86a34-en
- Roques BB, Lacroix MZ, Puel S, Gayrard V, Picard-Hagen N, Jouanin I, Perdu E, Martin PG, Viguié C. 2012. CYP450-dependent biotransformation of the insecticide fipronil into fipronil sulfone can mediate fipronil-induced thyroid disruption in rats. Toxicol Sci. 127(1):29–41.
- Rousset B, Dupuy C, Miot F, Dumont J, 2015. Chapter 2: thyroid hormone synthesis and secretion. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, editors. Endotext [Internet]. South Dartmouth MA, USA; MDText.com, Inc.
- Ruiz de Oña C, Morreale de Escobar G, Calvo R, Escobar del Rey F, Obregon MJ. 1991. Thyroid hormones and 5'-deiodinase in the rat fetus late in gestation: effects of maternal hypothyroidism. Endocrinology. 128(1):422–432.
- Ruiz de Oña C, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. 1988. Developmental changes in rat brain 5'-deiodinase and thyroid hormones during the fetal period: the effects of fetal hypothyroidism and maternal thyroid hormones. Pediatr Res. 24(5):588–594.
- Santini F, Chopra IJ, Wu SY, Solomon DH, Chua Teco GN. 1992. Metabolism of 3,5,3'-triiodothyronine sulfate by tissues of the fetal rat: a consideration of the role of desulfation of 3,5,3'-triiodothyronine sulfate as a source of T3. Pediatr Res. 31(6):541–544.
- Santini F, Hurd RE, Lee B, Chopra IJ. 1993. Thyromimetic effects of 3,5,3'-triiodothyronine sulfate in hypothyroid rats. Endocrinology. 133(1):105–110.
- Sauer UG, Asiimwe A, Botham PA, Charlton A, Hallmark N, Jacobi S, Marty S, Melching-Kollmuss S, Palha JA, Strauss V, et al. 2020. Towards a science-based testing strategy to identify maternal thyroid hormone imbalance and neurodevelopmental effects in the progeny – part I: which parameters from human studies are most relevant for toxicological assessments? Crit Rev Toxicol. 11:1–24.
- Schnell C, Shahmoradi A, Wichert SP, Mayerl S, Hagos Y, Heuer H, Rossner MJ, Hülsmann S. 2015. The multispecific thyroid hormone transporter OATP1C1 mediates cell-specific sulforhodamine 101-labeling of hippocampal astrocytes. Brain Struct Funct. 220(1):193–203.
- Schoenmakers CHH, Pigmans IGAJ, Visser TJ. 1992. Species differences in liver type I iodothyronine deiodinase. Biochim Biophys Acta. 1121(1–2):160–166.
- Schussler GC, Ingbar SH, Eveleth P. 1961. The role of intermediary carbohydrate metabolism in regulating organic iodinations in the thyroid gland. J Clin Invest. 40:1394–1412.
- Schussler GC, Schaffner F, Korn F. 1978. Increased serum thyroid hormone binding and decreased free hormone in chronic active liver disease. N Engl J Med. 299(10):510–515.
- Sharlin DS, Gilbert ME, Taylor MA, Ferguson DC, Zoeller RT. 2010. The nature of the compensatory response to low thyroid hormone in the developing brain. J Neuroendocrinol. 22(3):153–165.
- Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, Jhiang SM. 1996. Cloning of the human sodium lodide symporter. Biochem Biophys Res Commun. 226(2):339–345.
- Soldin OP, Soldin SJ. 2011. Thyroid hormone testing by tandem mass spectrometry. Clin Biochem. 44(1):89–94.
- Stockigt JR. 2001. Free thyroid hormone measurement – a critical appraisal. Assess Thyr Funct Dis. 30(2):265–289.
- Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. 2009. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 107(1):27–39.
- Takayama S, Aihara K, Onodera T, Akimoto T. 1986. Antithyroid effects of propylthiouracil and sulfamonomethoxine in rats and monkeys. Toxicol Appl Pharmacol. 82(2):191–199.
- Taylor PM, Ritchie JW. 2007. Tissue uptake of thyroid hormone by amino acid transporters. Best Pract Res Clin Endocrinol Metab. 21(2):237–251.
- Thorpe-Beeston JG, Nicolaides KH, Felto CV, Butler J, McGregor AM. 1991. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med. 324(8):532–536.
- Tien ES, Negishi M. 2006. Nuclear receptors CAR and PXR in the regulation of hepatic metabolism. Xenobiotica. 36(10–11):1152–1163.
- Tietge JE, Degitz SJ, Haselman JT, Butterworth BC, Korte JJ, Kosian PA, Lindberg-Livingston AJ, Burgess EM, Blackshear PE, Hornung MW. 2013. Inhibition of the thyroid hormone pathway in Xenopus laevis by 2-mercaptobenzothiazole. Aquat Toxicol. 126:128–136.
- US Environmental Protection Agency [US EPA]. 2005. Guidance for thyroid assays in pregnant animals, fetuses and postnatal animals, and adult animals. Washington (DC): US EPA, Office of Pesticide Programs, Health Effects Division.
- US Environmental Protection Agency [US EPA]. 2013. Endocrine disruptor screening program tier 1 assays: considerations for use in human health and ecological risk assessments. Office of Chemical Safety and Pollution Prevention, June 2013; https://www.epa.gov/sites/production/files/2015-07/documents/use_of_tier_1_data_in_risk_assessment.pdf.
- US Environmental Protection Agency [US EPA]. 2017. Endocrine disruptor screening program. Continuing development of alternative high-throughput screens to determine endocrine disruption, focusing on androgen receptor, steroidogenesis, and thyroid pathways. FIFRA SAP November 28–30, 2017. https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=338571.
- Villeneuve DL, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O'Brien JM, Pollesch NL, Smith LC, et al. 2018. Adverse outcome pathway networks II: network analytics. Environ Toxicol Chem. 37(6):1734–1748.
- Vinken M, Knapen D, Vergauwen L, Hengstler JG, Angrish M, Whelan M. 2017. Adverse outcome pathways: a concise introduction for toxicologists. Arch Toxicol. 91(11):3697–3707.
- Visser TJ. 1994. Role of sulfation in thyroid hormone metabolism. Chem Biol Interact. 92(1–3):293–303.
- Visser TJ. 1996. Pathways of thyroid hormone metabolism. Acta Med Austriaca. 23(1–2):10–16.
- Visser TJ. 2016. Thyroid hormone transport across the placenta. Ann Endocrinol. 77(6):680–683.
- Visser TJ, Kaptein E, Gijzel AL, de Herder WW, Ebner T, Burchell B. 1993. Glucuronidation of thyroid hormone by human bilirubin and phenol UDP-glucuronyltransferase isoenzymes. FEBS Lett. 324(3):358–360.
- Visser TJ, Kaptein E, van Raaij JAGM, Joe CTT, Ebner T, Burchell B. 1993. Multiple UDP-glucuronyltransferases for the glucuronidation of thyroid hormone with preference for 3,3',5'-triiodothyronine (reverse T3). FEBS Lett. 315(1):65–68.
- Visser TJ, Kaptein E, van Toor H, van Raaij JA, van den Berg KJ, Joe CT, van Engelen JG, Brouwer A. 1993. Glucuronidation of thyroid hormone in rat liver: effects of in vivo treatment with microsomal enzyme inducers and in vitro assay conditions. Endocrinology. 133(5):2177–2186.
- Vranckx R, Rouaze M, Savu L, Nunez EA, Beaumont C, Flink IL. 1990. The hepatic biosynthesis of rat thyroxine binding globulin (TBG): demonstration, ontogenesis, and up-regulation in experimental hypothyroidism. Biochem Biophys Res Commun. 167(1):317–322.
- Vulsma T, Gons MH, De Vijlder JJM. 1989. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 321(1):13–16.
- Wang J, Hallinger DR, Murr AS, Buckalew AR, Lougee RR, Richard AM, Laws SC, Stoker TE. 2019. High-throughput screening and chemotype-enrichment analysis of ToxCast phase II chemicals evaluated for human sodium-iodide symporter (NIS) inhibition. Environ Int. 126:377–386.
- Wang J, Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Laws SC, Stoker TE. 2018. High-throughput screening and quantitative chemical ranking for sodium-iodide symporter inhibitors in ToxCast phase i chemical library. Environ Sci Technol. 52(9):5417–5426.
- World Health Organisation – International Programme on Chemical Safety. [WHO IPCS]. 2009. Principles and methods for the risk assessment of chemicals in food. World Health Organisation Environmental Health Criteria. Food and Agriculture Organisations of the United Nations. Vol. 240. p. 1–45.
- Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ. 2005. Alternate pathways of thyroid hormone metabolism. Thyroid. 15(8):943–958.
- Zevenbergen C, Meima ME, Lima de Souza EC, Peeters RP, Kinne A, Krause G, Visser WE, Visser TJ. 2015. Transport of iodothyronines by human l-type amino acid transporters. Endocrinology. 156(11):4345–4355.
- Zhou J, Zhang J, Xie W. 2005. Xenobiotic nuclear receptor-mediated regulation of UDP-glucuronosyl-transferases. Curr Drug Metab. 6(4):289–298.
- Zuang V, Dura A, Bofill DA, Barroso J, Batista Leite S, Berggren E, Bernasconi C, Bopp S, Bowe G, Campia I, et al. 2019. EURL ECVAM status report on the development, validation and regulatory acceptance of alternative methods and approaches. EUR 30100 EN, Luxembourg: Publications Office of the EU. (JRC119292).

