Abstract
Methyl methacrylate (MMA) is classified under GHS as a weak skin sensitiser and a skin and respiratory irritant. It has recently been proposed that MMA be classified as a respiratory sensitiser (a designation that in a regulatory context embraces both true respiratory allergens, as well as chemicals that cause asthma through non-immunological mechanisms). This proposal was based primarily upon the interpretation of human data. This review, and a detailed weight of evidence analysis, has led to another interpretation of these data. The conclusion drawn is that persuasive evidence consistent with the designation of MMA as a respiratory sensitiser is lacking. It is suggested that one reason for different interpretations of these data is that occupational asthma poses several challenges with respect to establishing causation. Among these is that it is difficult to distinguish between allergic asthma, non-allergic asthma, and work-related exacerbation of pre-existing asthma. Moreover, there is a lack of methods for the identification of true chemical respiratory allergens. The characterisation and causation of occupational asthma is consequently largely dependent upon interpretation of human data of various types. Recommendations are made that are designed to improve the utility and interpretation of human data for establishing causation in occupational asthma.
1. Introduction
Asthma is best described as an inflammatory disease of the respiratory tract characterised by narrowing of the airways and wheeze. It has been estimated that asthma associated with workplace exposures (occupational asthma (OA) comprises up to 20% of adult asthma (Malo et al. Citation2015; Maestrelli et al. Citation2020).
Occupational asthma is an important problem that gives rise to health, social, and economic burdens (Kenyon et al. Citation2012; Feary et al. Citation2016; Tiotiu et al. Citation2020). It is frequently defined as a function of symptoms associated with exposures in the workplace environment, rather than in terms of the pathogenic processes through which the disease develops. In fact, occupational asthma is associated with two broad classes of mechanism. One is allergic asthma which is driven by allergic sensitisation of the respiratory tract and therefore, by definition, requires the stimulation of a specific immune response. The second is non-allergic asthma, acquired as a result of non-immunological mechanisms. The latter can take a variety of forms but is frequently associated with local irritation (Vandenplas Citation2011; Tarlo and Lemiere Citation2014; Arts and Kimber Citation2017; Maestrelli et al. Citation2020).
It is very unfortunate that there is commonly a failure to distinguish between allergic asthma and asthma associated with non-immunological mechanisms. This lack of distinction between these two broad classes of OA is reflected in regulatory definitions (Kimber et al. Citation2001). One example of this is provided by the European Chemical Agency (ECHA) guidance for the implementation of REACH (ECHA Citation2017a). That guidance defines a “respiratory sensitiser” as an agent that can cause airway hypersensitivity following inhalation exposure, where hypersensitivity is described as a term that embraces both immunological and non-immunological mechanisms that result in asthma (ECHA Citation2017a).
As has been discussed in previous reports (Arts and Kimber Citation2018; Arts Citation2020; Pemberton and Kimber Citation2021), there are a number of very important reasons why it is necessary to draw a clear distinction between allergic and non-allergic asthma associated with exposure to chemicals. Among these are considerations of risk assessment and risk management that are critical elements of ensuring safety in the workplace, and the need for a correct differential classification of asthmagens based on mechanistic characteristics (Arts Citation2020; Pemberton and Kimber Citation2021). In this context, it is also necessary to appreciate that inappropriate identification of a non-allergic chemical asthmagen as a respiratory sensitiser using the current EU classification scheme may trigger containment measures that are probably unnecessary, and possible designation as a Substance of Very High Concern (SVHC) under REACH, with all the consequences and restrictions that may subsequently follow (Arts Citation2020).
It is worth mentioning also that there is a clear precedent for considering allergic and non-allergic asthma as being different hazards. In chemical regulations, a clear distinction is drawn between allergic contact dermatitis and irritant contact dermatitis, the latter neither being associated with, nor requiring, an immune response or allergic sensitisation (Kimber et al. Citation2001; Arts and Kimber Citation2018).
It is against this background that attention is focussed here on the classification of methyl methacrylate (MMA). This chemical is an acknowledged skin sensitiser, albeit one with only a weak sensitising potency (Betts et al. Citation2006; Kimber and Pemberton Citation2014). However, MMA has recently been proposed as a respiratory sensitiser under EU CLP (Classification, Labelling, and Packaging), and it is the validity of that designation that this paper seeks to explore.
In addressing the question of whether MMA is a true chemical respiratory allergen it is necessary to review briefly the availability of relevant tools and date sources.
One long-standing hurdle in determining whether a chemical has a genuine potential to induce allergic sensitisation of the respiratory tract is the fact that there are as yet no validated, or even widely accepted, methods available for their identification. This is not the result of any lack of ambition. Adverse outcome pathways for respiratory sensitisation by low molecular weight chemicals have been proposed (Kimber et al. Citation2014b, Citation2018; Sullivan et al. Citation2017), and a wide range of in vivo, in vitro and in silico approaches have been developed and evaluated, without the emergence of a method that has gained regulatory acceptance. Strategies that have been explored are reviewed elsewhere (Holsapple et al. Citation2006; Kimber et al. Citation2007, Citation2014a; Boverhof et al. Citation2008; Isola et al. Citation2008; Cochrane et al. Citation2015; North et al. Citation2016; Arts Citation2020).
In the absence of validated predictive tests there is consequently a need to rely on the consideration of available human data, an approach that itself is not without significant interpretive challenges. The present authors have recently reviewed the value and limitations of human data for the accurate identification of true chemical respiratory allergens, and the need for careful interpretation of such data (Pemberton and Kimber Citation2021).
In addressing whether MMA is able to cause allergic sensitisation of the respiratory tract and respiratory allergy the purpose of the analyses reported here was to review rigorously and comprehensively the available human data (comprising worker surveys, case studies and clinical investigations) to reach an informed view about whether there is adequate evidence to support the classification of MMA as a respiratory sensitiser. This paper builds upon and extends a previous review of the respiratory sensitisation potential of MMA based on in vitro data, animal models and human studies (Borak et al. Citation2011).
2. Methods
In the absence of validated or widely accepted methods (in vivo, in vitro, or in silico) for the predictive identification of chemical respiratory allergens a weight of evidence (WoE), as opposed to a strength of evidence, approach has been taken for the assessment of MMA. Such WoE approaches have been described extensively in World Health Organisation/International Programme on Chemical Safety (WHO/IPCS) publications (WHO/IPCS Citation2005, Citation2010, Citation2014; Boobis et al. Citation2008), as well as by the European Food Safety Authority (EFSA 2008), the EU Scientific Committee on Emerging and Newly-Identified Health Risks (SCENHIR 2012), European Chemicals Agency (ECHA Citation2011, Citation2016, Citation2017b), the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER Citation2018), the Society of Environmental Toxicology and Chemistry (SETAC Citation2018), and others. In the context of classification of chemicals, Article 9 of the EU CLP Regulation (EC Citation2008) refers to the use of WoE and expert judgement in such cases and provides a template for constructing a WoE assessment (ECHA Citation2017c). This template is consistent with approaches taken by others and was used to organise the evidence for the assessment reported here and comprises six sections; 2.1 Problem formulation, 2.2 Collection and documentation of all information, 2.3 Assessment of quality of individual evidence, 2.4 integration and weighing of evidence (WoE analysis) and application of levels of confidence, 2.5 Uncertainty analysis, and 2.6 Conclusions.
2.1. Problem formulation
The hypothesis being tested is that:
“MMA can cause the development of occupational asthma (OA) in subjects that were not previously asthmatic.”
Based upon the framework for diagnosis of OA as proposed by the World Allergy Organisation (WAO) (), this can be broken down into three sub-questions that assist with the Weight of Evidence/uncertainty analysis.
Figure 1. Diagnosis of occupational asthma as proposed by the World Allergy Organisation (WAO) RADS: reactive airways dysfunction syndrome. https://www.worldallergy.org/education-and-programs/education/allergic-disease-resource-centre/professionals/diagnosis-of-occupational-asthma.
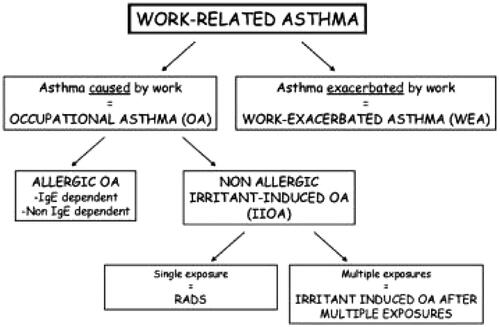
Are there instances where a diagnosis of OA can be causally related to workplace exposure to MMA, and where work exacerbated asthma (WEA), i.e. irritant-induced reactions in asthmatic subjects who had pre-existing asthma, or caused by other possible agents (e.g. exposure to other chemicals), can be excluded with moderate to high levels of confidence?
Is it possible to establish with certainty that OA where MMA has been implicated is dependent upon an immunological mechanism (allergic sensitisation resulting in allergic occupational asthma; allergic OA)? The conventional burden of proof is the requirement for two or more cases where allergic sensitisation of the respiratory tract to MMA has been confirmed with high confidence.
Is it possible to establish whether OA where MMA has been implicated is driven by an irritant mechanism (non-allergic irritant-induced OA; IIOA), the burden of proof requiring moderate confidence in the consistency of findings from different occupational settings with different levels of exposure. For classification purposes the exclusion of cases of irritant-induced WEA is appropriate since MMA is already classified in the EU as a respiratory irritant (ECHA Citation2021c).
2.2. Collection and documentation of all information
A literature search was conducted for the identification of relevant information on the issue discussed here on the 21st of June 2021 across MEDLINE, BIOSIS, and EMBASE databases using the search terms described in .
Table 1. Keywords combined to produce the search query.
Categorisation of the 368 records identified by the literature search followed the process described by Martin et al. (Citation2018). First, an initial screening of the records was conducted to remove duplicates. Second, a review of the abstracts where available, or of the full article where an abstract was not available, was undertaken to identify records that could be excluded as not being relevant on the basis that they did not refer to MMA, were unrelated to the target subject matter or reported contact allergy without any reference to respiratory effects. The remaining records represented four separate lines of evidence, namely, clinical case studies, worker health studies, quantitative structure–activity relationship (QSAR) models, and exposure studies.
A fifth line of evidence not identified from the literature search was available from Annexe I to the proposal for Harmonised Classification and Labelling (CLH) of MMA based on Regulation (EC) No 1272/2008 (CLP Regulation) submitted by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) (EC Citation2019) and from information submitted under the CLH review. This comprised clinical case studies reported to EU national surveillance networks including the French national network for the monitoring and prevention of occupational diseases (RNV3P), the Surveillance of Work-Related and Occupational Respiratory Disease (SWORD) database, the Occupational Physicians Reporting Activity (OPRA) from the UK, and the Finnish Institute of Occupational Health (FIOH). In addition, data are available for the German dental sector (BG ETEM Citation2021).
A sixth line of evidence comprised clinical evidence from Specific Inhalation Challenge (SIC) tests in patients believed to have developed OA as a result of occupational exposure to MMA from MMA-based products typically used in their workplace. Some of the records were identified from the literature search, whereas others were identified from the additional Supplementary data and information not included in the publication by Suojalehto et al. (Citation2019a), but subsequently provided by the authors to ECHA in response to an information request and included in the revised CLH opinion of 18 March 2021 (EC Citation2021). For some records information was also available on exposure levels of MMA determined in Specific Inhalation Challenge (SIC) tests performed with MMA-containing products, or other SIC tests claimed to be performed under comparable conditions to those reported as being positive with MMA.
2.3. Assessment of quality of individual evidence
The quality of clinical records was assessed using a scoring system based upon the prescriptive method described by Klimisch et al. (Citation1997), but adapted for this purpose based upon the work of Lavelle et al. (Citation2012), and following the approach taken by Money et al. (Citation2013) for epidemiological studies. The resulting scoring system considers the degree of transparency in the documentation of the methodology, analysis, and results, the degree to which potential methodological bias, such as information and selection bias, and on the extent and nature of the scientific data (e.g. whether supporting data are direct or indirect), are consistent with the approach taken by Martin et al. (Citation2018) (see ).
Table 2. Description of adapted Klimisch categories for clinical studies on OA.
2.4. Integration and weighing of evidence (WoE analysis) and application of levels of confidence
2.4.1. Weighting studies with respect to causation
The strength of the causal relationship was assessed for individual evidence (studies) using adapted (modified) Bradford Hill (BH) criteria (Hill Citation1965) within which each specific aspect of the criteria was framed in the context of the endpoint under study, according the principles illustrated by Meek et al. (Citation2014) and Becker et al. (Citation2015) (see ). The strength of the causal relationship was expressed in terms of strength i.e. high, medium, or low for each element of the criteria, and for the evidence (study) as a whole.
Table 3. Description of modified Bradford Hill criteria.
2.4.2. Application of levels of confidence
A level of confidence (Strength of Evidence) was assigned to individual evidence (study level) and expressed in terms of strength i.e. high, medium or low, based upon the score for a) quality (i.e. adequacy and reliability, see Section 2.3), and b) their score with respect to causation (i.e. modified BH criteria, see Section 2.4.1).
An assessment of the overall level of confidence in the evidence was derived by integrating the weighted level of confidence for each line of evidence (such as clinical data, QSAR analyses etc.).
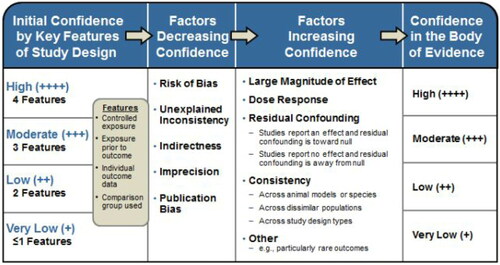
The overall level of confidence in the assessment that the body of available evidence accurately reflect the true association between exposure to MMA and causation of occupational asthma considered the strengths and weaknesses in the available studies and was rated using a four-point scoring system as described in the NTP/OHAT Handbook (NTP Citation2015, 2019) ().
Table 4. Four-point scoring system as described in the OHAT handbook.
The OHAT approach is based on guidance from the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (Balshem et al. Citation2011; Guyatt et al. Citation2011) but adapted for use across a wider range of study types and to incorporate a consideration of consistency across study designs, human populations, or animal species (Rooney et al. Citation2014).
As described in the OHAT handbook, available studies on a particular outcome are initially grouped and given a provisional confidence rating according to four key features (; column 1) which is subsequently downgraded for factors that decrease confidence in the results (risk of bias, unexplained inconsistency, indirectness or lack of applicability, imprecision, and publication bias), and upgraded for factors that increase confidence in the results (large magnitude of effect, dose response, consistency across study designs/populations/animal models or species, and consideration of residual confounding or other factors that increase our confidence in the association or effect) ().
Figure 2. Assessing confidence in the body of evidence taken from in Rooney et al. (Citation2014).
2.5. Uncertainty analysis
Any remaining uncertainties were identified and their potential impact on the assessment described in terms of Source,Nature, Magnitude, and Impact.
2.6. Conclusions regarding data adequacy
An overall conclusion was drawn as to the adequacy of the evidence for the purpose of addressing the problem formulation (see Section 2.1) based upon the completeness and level of confidence in the available evidence.
3. Results
3.1. Collection and documentation of all information
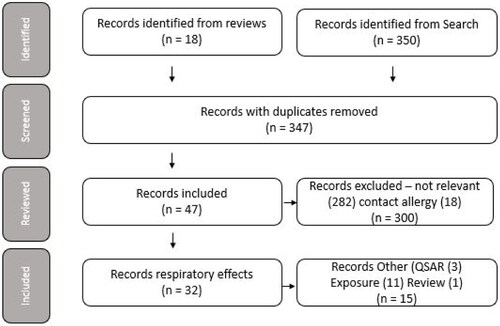
The literature search identified 350 records. In addition, a further 18 records were identified from reviews and reference lists within these articles making a total of 368 records (see Table A in the electronic appendix). Initial screening of these by article title and citation identified that 21 of these 368 records were duplicate (coloured yellow), leaving 347 unique records. Of these 347 records, review of the abstracts, where available or the full article where an abstract was not available, resulted in the identification of 282 records that were judged as being not relevant as they did not refer to MMA, or were unrelated to the subject matter, or represented reviews containing only secondary literature (coloured red). A further 18 records (coloured beige) were on contact allergy and contained no primary reference to respiratory effects. Exclusion of these 300 records resulted in 47 records (coloured white) deemed to be relevant to the aims of these analyses.
Full publications were obtained for these 47 records and reviewed. The 32 records identified on respiratory effects were categorised according to the four lines of evidence. Thirteen records described findings from worker health studies; 19 records reported case studies, of which 2 were on polymethyl methacrylate (PMMA) dust, and 17 were on MMA monomer; 3 records were on QSARs and 11 related to exposure. The remaining record was a review containing only secondary literature and was subsequently excluded.
The categorisation of the records is illustrated in .
An abstract was not available for 13 of the 47 records identified. Interpretative overviews based upon the review of the publication were written for these records, and for the other 34 records for completeness (see Table A in the electronic appendix).
The fifth line of evidence comprising clinical case studies reported to EU national surveillance networks identified 43 case records from the RNV3P database, 23 from the SWORD database, 1 from OPRA, 4 from FIOH, and 1 from BG ETEM (see Table B in the electronic appendix).
Finally, the sixth line of evidence comprising SIC tests in patients believed to have developed OA as a result of occupational exposure to MMA identified 10 case records from the literature search, 22 from the additional supplementary data and information related to the publication of Suojalehto et al. (Citation2019a), and 1 case from a personal communication from BG ETEM (BT ETEM 2021). Total case numbers related to MMA and a few case details were not reported in the Suojalehto et al. (Citation2019a) publication but subsequently provided by the authors to ECHA during the respective classification process (see Table C in the electronic appendix).
3.2. Assessment of quality of individual evidence
The 32 records identified on respiratory effects from the literature search were assessed for quality using the adapted Klimisch categories as defined in .
3.2.1. Worker health studies
While none of the 13 worker health records comprising the first line of evidence fully met all eight criteria according to ECHA R.7a guidance (ECHA Citation2017a), it was judged that eight (Monroe et al. Citation1981; Della Torre et al. Citation1982; Lindberg et al. Citation1991; Marez et al. Citation1993; Mizunuma et al. Citation1993; Pickering et al. Citation1993; Pausch Citation1994; Nishiwaki et al. Citation2001) met the majority of criteria and the information that was available was judged sufficient for assessment purposes thereby resulting in a rating of Klimisch category (K2).
Six studies (Monroe et al. Citation1981; Della Torre et al. Citation1982; Marez et al. Citation1993; Mizunuma et al. Citation1993; Pickering et al. Citation1993; Pausch Citation1994) were cross sectional worker studies on the health of workers in the cast acrylic sheet manufacturing industry by independent physicians. Additionally, the study of Della Torre et al. (Citation1982) included PMMA waste recycling before cast acrylic sheet manufacture. The studies of Pickering, Monroe, and Pausch were more comprehensive with high inclusion rates whereas those of Marez et al. (Citation1993) and Mizunuma et al. (Citation1993) were more limited. The study of Marez et al. showed evidence of mild congestion and increased incidence of chronic cough (20% compared with 1% in controls), but no asthma, in workers within the cast acrylic sheet industry where the mean atmospheric concentrations of MMA were 77.7 and 90.7 mg/m³ with ranges of 38–260 and 50–162 mg/m³ for many years. The Marez et al. (Citation1993) publication reported low exposure levels in workers whereas his PhD thesis and another paper (Marez et al. Citation1991) on the same workforce reported much higher levels, comparable to those measured by others in this industry. The study of Mizunuma et al. (Citation1993) showed that cast sheet workers exposed to 25 mg/m³ (mean) and 270 mg/m³ (short-term average) MMA complained of frequent cough and sputa and of throat irritation, but no asthma. The study of Della Torre et al. (Citation1982) showed no evidence of effects on respiratory parameters and an absence of asthma and bronchitis, but a moderate prevalence of minor nervous disorders and a slight irritant effect on mucosa, in workers involved in the recycling of PMMA and manufacture of acrylic sheets with exposures up to 736 mg/m3 (8 h-time weighted average (TWA)). The studies of Monroe et al. (Citation1981), Pickering et al. (Citation1993), and Pausch (Citation1994) showed no evidence of asthma, in workers with high MMA exposure (146 mg/m³ 8-TWA and 15 min STEL up to 749 mg/m³) over many years. In a limited study by Lindberg et al. (Citation1991), no asthma or change of lung function was observed in 10 floor layers regularly exposed to MMA at concentrations between 255 and 2476 mg/m³ for intervals of approximately 20 min followed by a period of no exposure between 30 and 60 min. Irritation of the nose or throat was observed in 3/10 workers.
Monroe (Citation1981), in an unpublished report, described normal spirometry, lower than normal prevalence of bronchitis, and no asthma in 780 cast acrylic sheet workers in the USA. Exposure levels were reported as being less than 21 mg/m³ (mean) with short-term exposures of up to 525 mg/m³, 252 mg/m³, and 563 mg/m³, respectively, prior to 1976. Nishiwaki et al. (Citation2001) reported an increased prevalence of cough and congestion (reduced forced vital capacity (FVC)) in dental technicians. The focus of the study was on MMA exposure reporting TWA levels of 0.66 to 18.0 mg/m³ with a maximum of 155.4 mg/m³ measured using passive 3 M™ badges during handling “hot-cure type resin.” The authors acknowledged that exposure to various dusts and metals could confound findings. Pausch (Citation1994) reported irritation in the eyes and the upper respiratory tract after short-term high peak exposures of MMA, but no indications for clinical symptoms of a work-related rhinopathy or any substance-related abnormalities in the nasal cavity of workers, and no asthma in 211 workers engaged in the production of acrylic sheets with exposures up to 160 mg/m³ at that time and historical peak exposures up to 2856 mg/m³. Pickering et al. (Citation1993) reported symptoms of irritation to the eyes and respiratory system, particularly following high, transient exposure to MMA, but no asthma in 384 workers (89.1% of a total workforce) involved in the manufacture of cast acrylic sheet. In a second, follow-up study of those individuals not available for the first study, a population of past leavers and those workers identified as having two or more work related respiratory symptoms in the first study, no evidence of respiratory sensitisation was found. One individual identified by the authors as presenting a medical history and peak expiratory flow measurements suggestive of occupational asthma was not subsequently confirmed. Worker turnover at the sites was reported by the company to be low and exposure to MMA as high as 420 mg/m³ (8-h TWA) in past years.
In the case of five records (Andrews et al. Citation1979; Jedrychowski and Fonte Citation1984; Piirilä et al. 1989; Jaakkola et al. Citation2007; Brisman et al. Citation2011), the available information was judged insufficient and these were rated Klimisch 3. This was mainly based upon lack of exposure information supporting the causal link of any findings to MMA.
Andrews et al. (Citation1979) reported that 6% of dental students (n = 502) answering a questionnaire and receiving spirometry had experienced respiratory symptoms and 88% of these (5.28% of dental students) had a history of either asthma or allergic rhinitis. However, there was no significant change in spirometry and symptoms in controls, asthmatics, and those with allergic rhinitis before and after a controlled exposure to MMA (concentration not stated). Brisman et al. (Citation2011) reported the incidence of asthma and lower respiratory tract symptoms were comparable between former Swedish dental technician students (n = 2139) and general population controls (n = 2288). The incidence rate ratios (IRRs) of nasal symptoms were increased during exposure to MMA, rapid glue (cyanoacrylate?) and grinding of cured acrylate material (PMMA). The response rate was relatively low i.e. 58% (1210 dental technicians and 1316 controls answered the questionnaire), leaving room for reporting bias. Jaakkola et al. (Citation2007) reported in a study in 799 female dental assistants an increased risk of adult-onset asthma (adjusted OR 2.65, 95% CI 1.14–7.24), nasal symptoms (1.37, 1.02–1.84), and work-related cough or phlegm (1.69, 1.08–2.71) with claimed exposure to methacrylates. No details of exposure levels or chemicals involved and no individual case reports of asthma were included. Jedrychowski and Fonte (Citation1984) studied chronic bronchitis, asthma, and obstructive syndrome in a group of 4717 male Polish chemical industry workers using a standardised questionnaire and spirometry and reported the incidence of asthma and obstructive syndrome, but not chronic bronchitis, to be higher in styrene, benzene, and MMA workers than in the general Polish population as was the incidence in workers of advanced age and amongst smokers. Piirilä et al. (Citation1998) reported the results of 12 SIC tests on dental workers exposed to methacrylates (monofunctional methacrylates (MMA and 2-hydroxyethyl methacrylate (2-HEMA)), multifunctional methacrylates (ethylene glycol dimethacrylate (EDGMA), triethylene glycol dimethacrylate (TREGDMA)), and acrylated and methacrylated prepolymers (bisphenol A-glycidyl methacrylate (BIS-GMA) and urethane dimethacrylate (UDMA)). The patients were exposed to dental products containing various methacrylates while their composition was not completely reported. In three of the 12 cases, there was no response to SIC testing. From the remaining nine cases, three showed an early asthmatic response (EAR) and six showed a late asthmatic response (LAR). In one of these six cases, MMA was explicitly named as ingredient in the used dental product so is included as patient 8 in Table C in the electronic appendix. This patient displayed clinical symptoms of asthma, rhinitis, and pharyngitis had elevated IgE (200 kU/L) and gave a strong LAR.
3.3. Case study records
As with the worker health studies none of the 19 records (containing 29 case studies) comprising the second line of evidence fully met all 8 criteria according to ECHA R.7a guidance (ECHA Citation2017a).
Two of the clinical records (Rumpf et al. Citation1986; Kirby et al. Citation2003) contained no indication of asthma and were consequently rated K4 and eliminated from the body of evidence. Kirby et al. (Citation2003) reported a case of a 48-year-old female radiology technologist with a history of asthma, but no prior exposure to MMA or PMMA, who developed chest tightness shortness of breath, wheeze, watery eyes, and rhinorrhea as a result of vertebroplasty using an MMA/PMMA cement. Rumpf et al. (Citation1986) reported a case study of a 33-year-old female dialysis patient that had had three attacks of anaphylaxis after treatment with dialysers that had been sterilised with ethylene oxide and oedema of the larynx, pharynx, and tongue, suggesting Quincke’s oedema, after an operation in which acrylic bone cement (Palacos®—containing gentamicin) was sterilised with ethylene oxide.
All of the remaining 27 records contained significant weaknesses to a greater or lesser extent justifying their detailed review.
Five records (Kennes et al. Citation1981; Basker et al. Citation1990; Savonius et al. Citation1993a, Citation1993b—case 2; Obando et al. Citation2013; Walters et al. Citation2017—cases 4 and 8) comprising a total of six case studies involved products that did not appear to contain liquid MMA and were consequently rated K3.
Basker et al. (Citation1990) reported a case of a 65-year-old female dental patient with a 12-year history of asthma. She developed asthma shortly after being fitted with acrylic dentures and had asthma attacks when exposed to cigarette smoke, petrol fumes and perfume, as well as wheezing and skin reactions after contact with acrylic fabrics. Kennes et al. (Citation1981) reported a case of a 33-year-old male factory worker who showed no work-related symptomology but had exposure to cutting PMMA, manipulating phosphorescent products, soldering aluminium wires, spraying epoxy resin paints, and applying Toluene diisocyanate (TDI)-based varnish and PMMA dusts out of work. He had an Early Asthmatic Reaction (EAR) (FEV1 fell 43%, FVC fell 18% at 30 min) in a provocation challenge test with PMMA dust and a greater dual asthmatic response (DAR; consisting of an EAR plus a Late Asthmatic Reaction (LAR)) in a second test two days later. A further challenge test by “painting with TDI varnish” caused an EAR. Obando et al. (Citation2013) reported a 48-year-old male plumber who developed progressive dyspnoea and dry cough after using “Tangit” adhesive. He gave a LAR with a maximal fall in FEV1 of 33% at 7 h, in a SIC with Tangit adhesive and a DAR with PVC powder (maximum drop of 17% at 30 min and 17.3% at 7 h) and MMA (maximum drop of 22% at 2 min and 20% at 9 h). “Tangit” adhesive was claimed to contain MMA in the publication but the manufacturer has confirmed in a personal communication to the author that “Tangit” was a solvent-based cement that contained dissolved PVC-U (unplasticised PVC) or PVC-C (chlorinated PVC), but not MMA monomer in the relevant time period (Henkel AG 2019; Personal communication between Henkel AG & Co. KGaA, HSA - Corporate Scientific Services – Toxicology and author; 11 February 2019). Savonius et al. (Citation1993a, erratum 1993b) reported a 32-year-old male who assembled hearing devices who gave a small maximal 15% decrease in PEF following the grinding of "a piece of methacrylate” from which it is inferred that this was PMMA polymer and not MMA. Walters et al. (Citation2017) reported a 51-year-old male that had been injection moulding acrylic polymers (case 4) and a 55-year-old female midwife that used a “methyl methacrylate tissue adhesive spray” (case 8). However, although MMA-based tissue adhesive sprays could not be identified, cyanoacrylate adhesive or surgical glue has been available since the 1950s and has become increasingly popular as a wound-closure method.
Two records (Pickering et al. Citation1986; Reynaud-Gaubert et al. Citation1991), containing one case study each, included details of the specific orthopaedic product used from which it could be determined that co-exposure occurred to an agent, namely gentamicin, an antibiotic that has been linked to OA and used in bone cements widely across Europe and USA since the 1970s (Neut et al. Citation2010). Two further records containing three case studies (Roth et al. Citation2017; Walters et al. Citation2017—cases 2 and 6a) are, by association to the orthopaedic sector, also rated K3.
Pickering et al. (Citation1986) reported a 56-year-old, smoker, orthopaedic theatre sister, who developed respiratory symptoms (persistent cough with wheezing and breathlessness) after 11 years when she handled a new cement (Palacos® with gentamicin). SIC challenge test with MMA levels up to 374 ppm during the first 2 min gave a LAR (FEV1 fell 25% at 6 h). Reynaud-Gaubert et al. (Citation1991) reported a 39-year-old female orthopaedic theatre nurse with a history of seasonal rhinitis and conjunctivitis who developed breathing difficulties during the course of mixing cement to seal prostheses with Palacos® bone cement. She gave an EAR (25% fall in FEV1 after 30 min) in SIC with Palacos® with gentamicin and “liquid MMA,” but asthma did not recur after the Palacos® with gentamicin was replaced by a different brand of bone cement (not identified) containing MMA. A pack of PALACOS® R + G (containing gentamicin) contains one or two bag(s) of gentamicin-containing cement powder (81.9% poly(methylacrylate, methyl methacrylate), 15% zirconium dioxide, 2.1% gentamicin sulphate, 1% benzoyl peroxide and colourant E141 (concentration not declared)), and one or two amber glass ampoule(s) of liquid (98% MMA and 2% N,N-dimethyl-p-toluidine). Gentamicin and Gentamicin sulphate are recognised as respiratory sensitisers by bone cement manufacturers and submitters to the classification and labelling inventory within the EU (ECHA Citation2021a, 2021b). Roth et al. (Citation2017) reported a case study of a male orthopaedic surgeon who displayed bronchial hypersensitivity, cough and dyspnoea and claimed a pattern of work-related asthma symptoms that improved when on holiday. No further details are available. Spirometry showed normal FEV1 and FVC with slightly increased airways resistance (82% of expected). He was non atopic as indicated by normal IgE levels and did not display eosinophilia. He gave a positive response to methacholine indicating bronchial hypersensitivity. Walters et al. (Citation2017) reported two cases of orthopaedic theatre nurses, one male and the other female who mixed bone cement containing MMA in an “open bowl” before developing asthma symptoms. One, a 47-year-old male worked for 20 years prior to onset of symptoms. The second was a 47-year-old female who worked for 14 years prior to symptoms which regularly occurred immediately after handling and mixing bone cement. A 2-min inhalational challenge to pre-mixed bone cement containing MMA, gave an EAR (35% fall in FEV1) that recovered over 2 h.
Seven records, comprising eleven case studies (Vallieres et al. Citation1977; Lozewicz et al. Citation1985—case 2; Savonius et al. Citation1993a, Citation1993b—cases 1 and 2; Piirilä et al. Citation1998—case 6; Thorette et al. Citation2006; Sauni et al. Citation2008; Walters et al. Citation2017—cases 3, 5, and 7; Moshe and Krakov Citation2019) contained insufficient detail on workplace exposure to both exclude co-exposure and/or in some case significant exposure to MMA i.e. the basis of the causal link of any findings to MMA was not sufficiently established.
Lozewicz et al. (Citation1985) reported a case of a 52-year-old railway cable joiner who smoked cigarettes for many years and used an acrylic curing system containing MMA. He experienced headache, sweating and lassitude during work and attacks of cough and wheeze outside work. He gave PEF measurements indicative of asthma, but a negative SIC when tested with MMA. Moshe and Krakov (Citation2019) reported a case study report of a 48-year-old female nail beautician that developed contact dermatitis to hydroxyethyl methacrylate (HEMA) and hydroxypropyl methacrylate (HPMA). Piirilä et al. (Citation1998) reported a case study of a 51-year-old female dentist, with a clinical diagnosis of occupational laryngitis that gave no SIC response but further details are available. Sauni et al. (Citation2008) reported two case studies. One was a 30-year-old female nail technician who applied sculptured nails and artificial tips to nails with cyanoacrylate and “methacrylate” based cements that was contact sensitised to 2-HEMA and EGDMA and gave a Dual Asthmatic Reaction (DAR) in a provocation test. The other was that of a 27-year-old female atopic hairdresser and nail technician who prepared artificial gel nails who also gave a DAR in a provocational test. In neither case was the composition of the products used in the workplace or provocation test described, and cyanoacrylate and gel nails do not contain MMA. Savonius et al. (Citation1993a, erratum 1993b) reported a case of a 48-year-old female using a MMA-based glue (composition unspecified) during plate engraving who developed respiratory distress at work and displayed a maximal 24% fall in PEF values (assumed to be an EAR) on challenge with the glue, although her symptoms persisted on transfer to the use of a cyanoacrylate glue, and a 46-year-old female dental technician who developed “tickling in her throat, yawning, cough, tiredness and chest tightness” and gave a DAR (peak expiratory flow rate (PEFR) fell by 26%) SIC following mixing "methacrylate powder” and “methacrylate liquid" but her symptoms persisted when using cyanoacrylate glue. Thorette et al. (Citation2006) reported a 19-year-old female prosthetic dentistry student who developed hypersensitivity pneumonitis after working with “MMA” but no details are available other than the finding of a computerised tomography (CT) scan and bronchial alveolar lavage (BAL) were described. Vallieres et al. (Citation1977) reported a 24-year-old male, smoker, paint sprayer who developed progressive rhinitis and asthma when spraying a water-based emulsion paint containing polyMMA, dimethyl ethanolamine with 1,4-dioxane and occasionally a pigment. A DAR was obtained in SIC with paint (FEV1 fell 29% immediately, and 23% at 8 h) and a 2% aqueous solution of dimethylethanolamine (DMEA) (FEV1 fell 58% immediately, and 27% at 8 h), but not with 100% MMA. Walters et al. (Citation2017) reported a 29-year-old male fabricator of aluminium tanks and radiators that used “methyl methacrylate adhesives” for seven years (case 3); a 36-year-old female nail technician (case 5) that reportedly applied “methyl methacrylate, cyanoacrylate light-curing gel nails,” however, cyanoacrylate and gel nails do not contain MMA; and a 42-year-old female dentist that reportedly used a “methyl methacrylate, cyanoacrylate dental filler” (case 7), although no such product appears to be manufactured and dentists apply a wide range of multifunctional methacrylate-based dental fillings; and a 29-year-old male fabricator of aluminium tanks and radiators that reportedly used “methyl methacrylate adhesives” but the manufacturers tend to brand a wider range of adhesives using MMA, 2-hydroxyethyl methacrylate, butyl methacrylate, epoxy systems as “MMA-based” so it is not clear to what monomers he actually was exposed.
The remaining five records (Lozewicz et al. Citation1985—case 1; Savonius et al. Citation1993a, Citation1993b—case 3; Piirilä et al. Citation1998—case 8; Scherpereel et al. Citation2004—2 cases; Wittczak et al. Citation1996), comprising six case studies came from the dental sector (three technicians, two trainees, and a dental nurse) in which it is widely recognised that MMA is used. However, the authors apparently attribute the development of OA in these cases to use of MMA without providing any evidence as to why other chemicals present in the wide range of restorative materials used in this sector were discounted resulting in their rating as K3.
Lozewicz et al. (Citation1985) reported the case of a 40-year-old male dental assistant who developed chest tightness, dyspnoea, and cough that persisted for several hours after mixing “PMMA powder with MMA liquid” and gave an EAR (24% fall in PEFR that resolved within 2 h) in SIC. Piirilä et al. (Citation1998) reported the case of a female dental nurse with a 27-year history of “mixing dental composite resin products” that contained “various acrylates.” She displayed clinical symptoms of asthma, rhinitis and pharyngitis and experienced hoarseness, sore throat, nasal stuffiness, and dyspnoea, particularly when preparing orthodontal fixatives. She displayed a “strong” LAR (mucosal changes and symptoms of the upper respiratory tract”; rhinomanometry: 70% increase in airflow resistance; FEV1 fell only 6%; PEFR fell 20% after 16 h) Paladur® self-cure resin that according to the manufacturers Safety Data Sheet contains the four contact sensitisers MMA (>90%), tetramethylene dimethacrylate (1-5%),2-(2H-benzotriazol-2-yl)-4-methylphenol (0.25-1%) and N,N-dimethyl-p-toluidine (<1%). SIC was negative after workplace simulation exposure to Scotchbond® primer containing 2-HEMA and bis-GMA (included as patient 8 in Table C in the electronic appendix 3). Savonius et al. (Citation1993a, erratum 1993b) reported a case study of a 46-year-old female dental technician who developed paraesthesia of the ulnar sides of both hands, but no dermatitis after 20 years working as a dental technician. She subsequently developed “tickling in her throat, yawning, cough, tiredness and chest tightness.” Skin prick tests (SPT); to “methacrylate and polyethyleneglycol dimethacrylate (PEGDMA)” and patch tests (acrylate series) were negative. Spirometry, PEFR, and NSIC were not reported. She elicited a DAR (PEFR fell by 26%) in SIC following mixing "methacrylate powder” and “methacrylate liquid". Scherpereel et al. (Citation2004) reported two cases of “hypersensitivity pneumonitis” involving female trainee dental technicians that developed dyspnoea and cough “within the first weeks of exposure to MMA” in a laboratory. The first was a 24-year-old female who was hospitalised for progressive severe dyspnoea and cough after six months of commencing her training. Physical examination revealed diffuse bilateral crackles, hypoxaemia and CT scan showed ground glass pattern. She responded to systemic corticosteroid treatment. One month later, after returning to work for three days, she again required hospitalisation for severe dyspnoea and hypoxaemia. Clinical investigation revealed increased BAL (cell count: 570 000 cells/ml; 10% macrophages, 60% lymphocytes, 25% neutrophils), hypoxaemia and reduced spirometry readings (FVC: 50% predicted; FEV1: 24% predicted). PEFR, NSIC were not performed. The authors refer to provocation challenge tests but provide no details. Patient 2 was a 20-year-old female who was hospitalised for acute respiratory distress a few weeks after the start of her training. She had “major dyspnoea,” cough and diffuse bilateral crackles, hypoxaemia, “pulmonary diffusion abnormality,” and chest X-ray showed bilateral ground glass pattern. Spirometry was abnormal (total lung capacity (TLC): 67% of predicted). PEFR and NSIC were not performed. Her symptoms responded to systemic corticosteroids treatment. SIC involving exposure to “aerolised particles of MMA” while in a “glass cage” was positive producing “moderate dyspnoea,” 20% fall in carbon monoxide diffusing capacity (Dlco) and 30% lymphocytes in bronchoalveolar lavage (BAL). Wittczak et al. (Citation1996) reported in a Polish publication a case study of a 40-year-old female dental technician who worked with powdered and liquid “acrylate” formations and had contact with prosthesis processing dust, who developed increasingly severe attacks of coughing and nasal secretions beginning several hours after making dentures from “Superacryl, Duracryl” moulding compound. Asthma attacks first began 6–8 months after starting work and were managed with cromolyn and corticosteroids. After almost 13 years, she received disability pension on the basis of OA. Six years later, she was hospitalised for respiratory distress after again working with MMA-containing prosthetic materials. Total IgE was 107.6 kU/L, peripheral blood eosinophil count was elevated (935 per mm3), SPT to common allergens were negative and patch tests (acrylates) were also negative. Spirometry was abnormal (FVC: 75% predicted, FEV1: 82% predicted). PEFR and NSIC were not reported. SIC involving mixing “liquid MMA” and “MMA powder” for 20 min was positive (FEV1 fell >40% at 4 h and PEFR fell > 50% at 24 h) and 24 h following later nasal lavage showed increased white blood cell (WBC) count (311.6 × 103/ml) and increased eosinophil count (54.7 × 103/ml).
Nine clinical case reports of OA due to other methacrylates have been reported in the literature. Piirilä et al. (Citation1998) described seven dentists with diagnoses of OA due to HEMA.
Case 2 was a 48-year-old male dentist with a diagnosis of occupational asthma, pharyngitis and laryngitis. He was a non-smoker with familial atopic background who worked with acrylics for 22 years and had “symptoms” (not otherwise specified) for 3 years. Total IgE was 83 kU/L. SPT was negative for common environmental allergens, latex, and methacrylates (MMA, 2-HEMA, bis-GMA, EGDMA, TREGDMA) and acrylate patch tests were negative. Spirometry, PEFR and NSIC were normal. SIC was slightly positive (no effect in FEV1, 16% in PEV) immediately after workplace simulation with 10 drops of each Rely-a-Bond paste (containing Bis-GMA, PEGDMA, Quartz, Alumina, Silica and Benzoyl peroxide) and Scotchbond™ adhesive (containing 35-45% HEMA, a copolymer of acrylic and Itaconic acid and water). Diagnosis of occupational asthma was based upon a claimed occupational effect in PEF.
The other six cases (3, 7, 9, 10, 11, and 12) reportedly used Scotchbond™ adhesive and Scotchbond™ multipurpose primer (containing 15-15% HEMA, 15-25% bisphenol A diglycidyl ether dimethacrylate (bis-GMA), 15–25% copolymer of MMA and Itaconic acid and phosphorous oxide (P2O5), 10–15% ethanol, 10–15% water, 7–13% Vitrebond™ copolymer (MMA, 3-(trimethoxysilyl)propyl ester, reaction products with vitreous silica), 1–5% acrylic and itaconic acid copolymer, <2% camphorquinone, <2% dimethylaminobenzoat(-4), and <1% (dimethylamino)ethyl methacrylate (DMAEMA)). Details of work exposures to methacrylate- containing materials were not reported in any case. SPT was negative for common environmental allergens, latex, and methacrylates (MMA, 2-HEMA, bis-GMA, EGDMA, TREGDMA), and acrylate patch tests were generally negative with one exception.
Case 3 was a 61-year-old female dentist with a clinical diagnosis of occupational asthma, pharyngitis and laryngitis. She was a non-smoker with familial atopic background who worked with acrylics for 24 years and had “symptoms” (not otherwise specified) for 22 years. Total IgE was 289 kU/L. Spirometry PEFR and NSIC were normal. She gave a LAR (20% FEV1 and 20% PEV) after workplace simulation with 20 drops of each Scotchbond™ multipurpose primer and adhesive.
Case 7 was a 53-year-old female dentist with a clinical diagnosis of occupational asthma. She was a non-smoker with no atopic background, who worked with acrylics for 22 years and had “symptoms” (not otherwise specified) for 5 years. Total IgE was 59 kU/L. Spirometry PEFR and NSIC were normal. She gave a slight EAR (5% FEV1 and 18% PEV) after workplace simulation with “4 drops of Scotchbond™ multipurpose adhesive.”
Case 9 was a 61-year-old female dentist with a clinical diagnosis of occupational asthma. She was a non-smoker with familial atopic background, who has worked with acrylics for 25 years and had “symptoms” NOS for 10 years. Total IgE was 93 kU/L. Spirometry PEFR was indicative of slight obstructive airways disease but NSIC was normal. She gave a LAR (20% FEV1 and 16% PEV) after workplace simulation with 10 drops of each Scotchbond™ multipurpose primer and adhesive.
Case 10 was a 41-year-old female dental nurse with a clinical diagnosis of rhinitis. She was a non-smoker with familial atopic background, who worked with acrylics for 22 years and had “symptoms” NOS for 6 years. Total IgE was 270 kU/L. Spirometry PEFR was indicative of slight obstructive airways disease but NSIC was normal. There was no claimed occupational effect in PEF. SIC was negative after workplace simulation with 20 drops of each Scotchbond™ multipurpose primer and adhesive.
Case 11 was a 34-year-old female dental nurse with a clinical diagnosis of asthma. She was a non-smoker with atopic background (also familiar), had been exposed to acrylics for 10 years and had “symptoms” not otherwise specified (NOS) for 9 years. Total IgE was elevated with 260 kU/L. SPT was positive for at least common environmental allergens, latex, and the above-mentioned methacrylates. Spirometry was normal whereas a non-specific SIC indicated slight hyperresponsiveness. SIC was positive (13% FEV1 and 17% PEV) with a LAR after workplace simulation with 20 drops of each Scotchbond™ multipurpose primer and adhesive. Diagnosis of occupational asthma was based upon a claimed occupational effect in PEF.
Case 12 was a 49-year-old female dental nurse with a clinical diagnosis of asthma. She was a non-smoker with a familiar atopic background, had been exposed to acrylics for 28 years and had unspecified symptoms for 1 year. Total IgE was normal with 43 kU/L. Spirometry was indicative of slight obstructive airways disease while a non-specific SIC was normal (no hyperresponsiveness). SIC was positive (24% FEV1 and 13% PEV) with a LAR after workplace simulation with 20 drops of each Scotchbond™ multipurpose primer and adhesive. Diagnosis of occupational asthma was based upon a claimed occupational effect in PEF.
Moulin et al. (Citation2009) reported 2 cases of OA claimed to be caused by HEMA in a group of 234 beauticians with allergic contact dermatitis (ACD). One patient was atopic with ACD to multiple agents and displayed reactive airways upon challenge with metacholine (0.2 mg/ml caused a 20% drop in FEV1). She gave an EAR (20% drop in PEV1 after 60 min with dyspnoea) upon challenge with HEMA. The second patient had familial and childhood asthmatiform bronchitis and seasonal spring rhinitis. She applied nail prosthesis with an activated ultraviolet gel and acrylic liquid, the Safety Data Sheet for which indicated that it contained HEMA and was diagnosed with OA based on her positive ACD to HEMA and a claimed work-related pattern of respiratory complaints. Overall, the evidence for causation of OA by HEMA in nail workers is weak.
3.4. Quantitative Structure-Activity relationship (QSAR) analyses
Three records describing QSARs for asthma and mentioning MMA have been identified. Jarvis et al. (Citation2005) reported the development of a QSAR for asthmagens (i.e. substances capable of causing occupational asthma; UK HSE Citation2001) based upon published citations and molecular fragments i.e. functional elements. While the claim by the authors that the prediction is made on the basis of functional elements might infer that the model identifies allergens, this cannot be asserted with any confidence since the precise basis for the prediction is not described. MMA is described as an “accepted respiratory sensitiser” but was predicted not to be an asthmagen with an index of 0.45 compared with the cut-off of >0.5 for positive prediction. In contrast, the same QSAR predicted an asthma index of 0.75 for the related non-asthmagen methacrylate, methacrylic acid (MAA), justifying its correct prediction on the basis of it being a “suspected” respiratory sensitiser citing Savonius et al. (Citation1993a). However, this publication makes no reference to MAA and to date no clinical case of OA has been reported for this substance. On this basis the performance of this QSAR with these two methacrylates is low. Enoch et al. (Citation2012) described the development of a QSAR for asthma based on Michael addition reactivity and identified MMA with its acryl functional group as capable of causing asthma. Although an overall predictive value of the QSAR of 79% (82/104) was reported, no positive prediction for MMA was found. Dik et al. (Citation2014) reported that the predictive performance of individual published QSAR models was lower in practice than claimed, but that the use of QSAR batteries improved the predictive power based upon a learning set comprised of low molecular weight (LMW) respiratory sensitisers identified from the literature.
In summary, QSARs have been developed based upon learning set of substances that have been cited in the literature as being the cause of OA (asthmagens) as opposed to chemicals that have been proven to be respiratory allergens. These QSARs have subsequently identified the presence of the acryl (R-C = O) functional group capable of Michael addition as a structural alert for asthma thereby forming a rationale for associating acrylics (acrylates, methacrylates and cyanoacrylates) with asthma. While the claimed predictive value against all LMW substances based upon the learning set has been optimised, their performance with substances outside the learning set is of lower confidence. Overall, these QSARs are rated as Klimisch K3 as their predictive value for MMA is low.
3.5. Exposure data
Methyl methacrylate has a vapour pressure of 37 hPa at 20 °C (ECHA Citation2021d) and vapour density quoted as between 3.45 (Air = 1) (NTP Citation1992; Bisesi Citation2001) or 4.16 (WHO/IPCS Citation1998) meaning that the vapour will settle at floor level and motions in the air created by wind and other pressure differences make the vapour rise (Stauffer et al. Citation2008).
Ten records on exposure mentioning MMA have been identified in the literature search and references therein. Three records addressed dental technicians (Nayebzadeh and Dufresne Citation1999; Golbabaei et al. Citation2005; Torbica and Krstev Citation2006), two were in dental clinics (Henriks-Eckerman et al. Citation2001; Hagberg et al. Citation2005), three were in nail salons (Spencer, Froines and Garabrant Citation1986; Hiipakka and Samimi Citation1987), and two in operating theatres (Darre et al. Citation1992; Sass-Kortsak et al. Citation1992). A further six studies on the health of workers in the cast acrylic sheet manufacturing industry reported exposure data on MMA (Della Torre et al. Citation1982; Marez et al. Citation1991; Mizunuma et al. Citation1993; Pickering et al. Citation1993; Pausch Citation1994).
In the published studies in dental technicians MMA exposures (8 h TWA) reportedly varied between 2.94 − 6.7 mg/m³ (Nayebzadeh and Dufresne Citation1999) and 327.28 ± 79.42 mg/m³ (Golbabaei et al. Citation2005). Peaks were reported up to 40.74 mg/m³ in the first study and only slightly higher than the TWA, in the second, while in the third study MMA concentrations (likely peaks) “up to 2.4 times higher” than the local MAC value of 410 mg/m3 were reported, i.e. up to 900 to 1000 mg/m3 (Torbica and Krstev Citation2006). Dust exposure of technicians was reported in the Golbabaei et al. (Citation2005) study as 2.35 ± 2.70 mg/m³ and in the Torbica and Krstev (Citation2006) study, pure silica dust was measured to be 3.6 times and 2.6 times higher than the respective local MAC of 0.1 mg/m3 during sandblasting of metals and ceramics grinding, respectively.
In a study in dental clinics, levels for MMA were not reported. Levels of another methacrylate, HEMA, were reported also as being low (0.003 mg/m³) in the breathing zone of the nurse with a maximum concentration of 0.033 mg/m³) with peaks (maximum concentration) three to five times higher (Henriks-Eckerman et al. Citation2001). In a study on HEMA and MMA exposures in five public dental clinics, 8 h TWA levels were low while short-term exposure levels were higher (0.079 mg/m³ for HEMA and 0.015 m/m³ for MMA (Hagberg et al. Citation2005). Similar findings were also reported during dental filling treatments with maximum concentrations of 0.4 mg/m3 for MMA, 45 µg/m3 for HEMA, 13 µg/m3 for EGDMA, and 45 µg/m3 for TREGDMA being reported (Marquardt et al. Citation2009).
In a study in nail worker stations, low levels of ethyl methacrylate were measured but no exposure to MMA was detected (Spencer et al. Citation1997); 8 h TWA and peak exposures to MMA during fingernail application in manicurists’ shops were 22 mg/m³ and 84 mg/m³, respectively (Froines and Garabrant Citation1986). Mean TWA concentrations of ethyl methacrylate in sculptured nail salons were 18.5 mg/m³ (mean TWA) and 64.3 mg/m³ (peak) and levels of PMMA dust in 16 personal samples were 0.9 mg/m³ and 1.4 mg/m³ for respirable dust and total dust, respectively (Hiipakka and Samimi Citation1987).
Reported workplace concentrations were between 210 and 420 mg/m³ of MMA during hip and knee replacement operations under conventional operating conditions without laminar airflow (Darre et al. Citation1992). In another study on exposure of hospital operating personnel during operations where MMA was used in surgery only in 4 of 27 cases, MMA concentrations above the detection limit (1.2 mg/m³) were found (3.7; 4.0; 4.0; 55.3 mg/m³) (Sass-Kortsak et al. Citation1992).
Exposure levels to MMA have also been reported in other studies identified that were not the primary purpose of the published study. Pickering et al. (Citation1986) measured acute exposure levels up to 1570 mg/m³ during mixing bone cement without local exhaust ventilation (Pickering et al. Citation1986). Further the review by Borak et al. (Citation2011) cites previously unpublished data from studies by Ungers and colleagues (Ungers and Vendrely Citation2006; Ungers et al. Citation2007) in which MMA levels measured by photo-acoustic spectrophotometry continue to increase for 10–20 min after mixing bone cement, reaching levels of greater than 4000 mg/m³ above the mixing bowl if emission controls were not employed (Borak et al. Citation2011, ).
Further unpublished exposure data for these identified industries are available and cited within the EU Existing Substance Risk Assessment for MMA (EU RAR) (EC Citation2002). Mean TWA exposures of 146 mg/m³, with peaks (15 min TWA) up to 749 mg/m³ were recorded during manufacture of cast sheet. The EU RAR (EC Citation2002) also reports exposures in dental laboratories and surgeries at workplaces with local exhaust ventilation being usually between 3 and 6 mg/m³ (data from the German workers compensation fund in 1990 − 1994). The Federal monitoring authorities also provided single exposure measurements in dental surgeries of <62 mg/m³, 7.5 mg/m³ and “not detected.” According to the Federal Monitoring Authority of Hamburg, Germany, the short-term values at workplaces with suitable local exhaust ventilation (LEV) are below 42 mg/m³ (original citation not provided).
As reported in the EU RAR (EC Citation2002), exposures of 197.5 mg/m³, 155 mg/m³, and 125 mg/m³ for some hours without LEV and shift averages of 110 mg/m³ and 14 mg/m³ under unfavourable conditions of small room and no ventilation have been observed. Short-term exposures (30 min, n = 4) up to 144 mg/m³ without LEV and 600 mg/m³ under unsuitable ventilation conditions have been measured. The German Federal Monitoring Authority reported significant differences depending on whether local exhaust ventilation (LEV) is used. Short-term values (5 min) of between 420 and 840 mg/m³ were measured during specific tasks (alternately humidifying orthodontic components with liquid MMA and strewing with powdery pre-polymerised PMMA) in six laboratory workplaces that did not use LEV. Sometimes even higher levels were recorded when Flame Ionisation Detectors (FIDs) capable of measuring peaks of even shorter duration were used (original citation not provided).
Workplace measurements in dental laboratories performed by the BG ETEM between 1990 and 2006 were 6.0 and 11.0 mg/m³ (mean TWA) and 35.0 and 95.3 mg/m³, with and without local exhaust ventilation. Real-time measurements with a Fourier Transform Infra-red (FTIR) detector reveal that short-term concentrations up to 370 mg/m³ occur for up to 1 min (BG ETEM 2021).
The EU RAR also included limited data on exposure to MMA during use of reactive resins as floor coatings. More recently data provided by BG-ETEM have become available revealing high exposure levels during use. Mean 50th percentiles of 241 mg/m³ without LEV (n = 78 within seven enterprises) and 141 mg/m³ with LEV (n = 34 within two enterprises). The corresponding 95th percentile values are 1045 mg/m³ (without LEV) and 625 mg/m³ (with LEV). The 95th percentiles of short-term exposures (<1 h) of 683 mg/m³ (n = 50) was measured.
The worker health studies in the cast acrylic sheet manufacturing industry also reported exposure data to MMA in the workplace. Della Torre et al. (Citation1982) reported levels of MMA in workers involved in the recycling of PMMA and manufacture of acrylic sheets with exposures of up to 736 mg/m3 (8 h-TWA). Marez et al. (Citation1991) reported mean 8 h TWA levels in four cast acrylic sheet manufacturing plants as being 2.94 mg/m³ (range “trace” to 161.7 mg/m³) with 1 h TWAs of 488.8 mg/m³ to 1680 mg/m³. The same authors reported MMA exposure in the same plants two years later, as having mean 8 h TWA levels of 77.7 and 90.7 mg/m³ with ranges of 38–260 and 50–162 mg/m³ (Marez et al. Citation1993). Mizunuma et al. (Citation1993) reported cast sheet workers exposed to 25 mg/m³ (mean) and 270 mg/m³ (short term average, duration not stated) MMA. Monroe (Citation1981) reported MMA exposure levels in acrylic sheet workers in the USA as being less than 21 mg/m³ (mean) with short term exposures of up to 525 mg/m³ with higher levels of 252 mg/m³ (mean) and 563 mg/m³ (short term) prior to 1976. Pausch (Citation1994) reported current exposures up to 160 mg/m³ and historical peak exposures up to 2856 mg/m³. Pickering et al. (Citation1993) reported exposure to MMA during cast acrylic sheet manufacture of as high as 420 mg/m³ (8-h TWA) in past years.
Extensive measured exposure data and model predictions for occupational exposures to MMA are to be found in the EU Risk assessment of MMA under Section 4.1.1 Exposure assessment (EC Citation2002).
3.6. European national surveillance data
The fifth line of evidence comprises clinical case studies reported to EU national surveillance networks. This includes 43 records (cases) from the RNV3P database, 23 from the SWORD database, 1 from Occupational Risk Appraisal (OPRA) system, 4 from the Finnish Institute for Occupational Health (FIOH), and 1 from the national Workers Compensation Funds for the vast majority of dental technicians in Germany (BG ETEM) and are given in Table B in the electronic appendix.
Of the 43 records from the RNV3P database assigned under the occupational disease table No. 82 (RG82) “Disorders caused by methyl methacrylate,” there were 27 case reports described as having high attributability. These included reports of five nail technicians (1, 19, 20, 23, and 39), four dental technicians (17, 21, 22, and 41), two reports from the automotive industry (7 and 42) and one each from the public sector (4), UV inks (8), road worker (10), silkscreen worker (14), a moulder in umbrella manufacture (15), a construction electrician (18), and a subject from the furniture industry (43). Of the remaining 16 case reports described as having moderate attributability, there were included five dental technicians (24, 25, 33, 36, and 37), three polystyrene workers (28, 29, and 30), two painters (34 and 35), and one from each of painter-decorator on glass or ceramic (32), nail technician (38), carpenter (26), furniture industry worker (27), medical instruments manufacturer involving use of PMMA (31), and one female for whom the occupation was unknown (40). There were no clinical details available apart from age, sex, job, and basis of diagnosis for any of the cases preventing comparison against ECHA R7.3 criteria, or verification of the basis for the assigned level of attributability. In the case of some entries, the level of confidence in the attribution to MMA is questionable. For example, the three polystyrene workers (28, 29, and 30) would not be exposed to MMA in their line of work. Similarly, the two painters (34 and 35) would typically be expected to use acrylic paints containing polymer and not MMA; UV inks (8) employ curing systems involving speciality acrylate resins "basic acrylate monomers (alkanes, ethers, etc.) as well as urethane acrylates, polyester acrylates, epoxy, and amine-modified acrylates; and case (41) has no details other than being a “professional.” In 2017, limitations of the RNV3P database specifically to claimed MMA exposure became public when Healthcare professionals interviewed by ANSES staff reported that they used the RG82 (MMA) code to enter cases involving exposure to (meth)acrylates other than MMA (ANSES Citation2017). It is not clear whether the coding system was subsequently changed; therefore, it is possible that the term “(meth)acrylates” continued to be used extensively by clinicians as a “catch-all” to include a wide range of chemical substances other than MMA. On this basis, without further detail at the case-study level, it is not possible to rate these higher than K3.
There was no information available on the 23 records from the SWORD database other than they were diagnosed with asthma reported by chest physicians and 61% were men working in various sectors; industry sectors: 11/23 (48%) health and social care; 8/23 (35%) manufacturing; and 1 case reported in each of the following sectors: education, construction, other service activities, and other business activities. Although it has not been possible to confirm with the authors there appears to be a likelihood that these correspond, in part of in full, to the 20 patients with occupational asthma caused by sensitisation to acrylic compounds diagnosed between 1 January 1989 and 31 December Citation2014 under the SHIELD surveillance program in the UK (Walters et al. Citation2017). However, in that paper, Walters and co-workers only reported 8 patients with OA caused by “predominantly” methyl methacrylate, the other 12 being linked to other acrylates, acrylic polymers and cyanoacrylates.
The record from OPRA was described as a man working in the manufacture of medical devices. This case bears close similarity to patient 1 described as a 58-year-old male employed manufacturing prosthetic limbs from a resin containing MMA and rigid-foam body compensations containing methylene diphenyl diisocyanate (MDI) and who gave a positive SIC to MDI (Walters et al. Citation2017).
The 4 records from FIOH included two dentists, both of whom displayed a LAR in SIC, but with no details of the product tested. One subject worked with dental primers, adhesives and fillers and what is described as prosthetic methacrylate liquid and powder. No details on the products used were available for the second. A dental technician working with prosthetic material elicited a DAR in SIC, but again no details were provided regarding exposure or products used in the workplace or in the SIC. The fourth was a production worker working with two-component lamination resin who developed asthma and gave an EAR in a SIC, but again no details are available on exposure and products used in the workplace, or used in the SIC.
The German BG ETEM investigated occupational diseases between 2015 and 2019 for dental technicians (BG ETEM 2021) and identified one case of OA out of the approximately 45 000 to 50 000 workers that have frequent contact to MMA. The technician manufactured metal-plastic dental prosthetics for 22 years involving a wide range of tasks including mixing, pouring and shaping unpolymerised material, and grinding and milling the hardened prosthetic. He displayed severe non-specific bronchial hyperreactivity and non-occupational allergic rhinoconjunctivitis and gave an EAR (significant obstructive disorder after 22 min) in a SIC with “cold polymerisation based on MMA” (stirring powder and liquid in the exposure chamber). In two other cases, causal relationship between respiratory disorders and exposure to MMA (one case) and unspecified (meth)acrylates (one case) was investigated but not confirmed. The most frequent respiratory disease for dental technicians in this investigation was silicosis caused by Quartz exposure with 21 actual or potential cases.
3.7. Specific inhalation challenge (SIC) test data
A total of 32 SIC tests in patients who claimed to have developed OA as a result of occupational exposure to MMA were identified. Ten of these records (Lozewicz et al. Citation1985 (2); Pickering et al. Citation1986; Reynaud-Gaubert et al. Citation1991; Wittczak et al. Citation1996, Citation2003; Piirilä et al. Citation1998; Uriarte et al. Citation2013; Walters et al. Citation2017(2)) were identified from a search of the literature and the CLH proposal. The remaining 22 were identified from the additional SIC test supplementary data and information related to the publication of Suojalehto et al. (Citation2019a), which were subsequently provided by the authors to ECHA. In 17 of these cases (case 7, a railway cable joiner, in Lozewicz et al. (Citation1985), and 16 of the 22 cases from the additional SIC tests supplementary data and information related to the publication of Suojalehto et al. (Citation2019a), the SIC challenge test with MMA or predominantly MMA was reported to be negative. In the case reported by Uriarte et al. (Citation2013), the product believed to have caused the OA and giving a positive response in SIC was confirmed by the manufacturer as not containing MMA. In the case of Wittczak et al. (Citation2003) the subject, a female secretary displayed a DAR (fall in FEV1 of 30% at 1 h, and 24% 4 h later) when challenged with heated (80 °C) MMA. However, the product that the patient worked with was a “black xerographic binder described as consisting of carbon black in binder resin, such as polystyrene-n-butyl methacrylate, polystyrene-n-butyl acrylate, etc.”
In a further four cases, there was indication of occupational exposure to a known respiratory sensitiser making the assertion that MMA caused the development of OA unreliable (Pickering et al. Citation1986; Reynaud-Gaubert et al. Citation1991; Walters et al. Citation2017—cases 1 and 6). The cases reported by Pickering et al. (Citation1986), Reynaud-Gaubert et al. (Citation1991), and Walters et al. (Citation2017) (case 6) were orthopaedic theatre nurses. The case reported by Pickering developed OA after 11 years when she handled a new cement (Palacos® R + G) containing gentamicin. SIC challenge test with MMA levels up to 374 ppm during the first 2 min gave a LAR (FEV1 fell 25% at 6 h). The case reported by Reynaud-Gaubert et al. (Citation1991) was a theatre nurse who developed breathing difficulties while mixing cement (Palacos® R + G—containing gentamicin) who gave an EAR (25% fall in FEV1 after 30 min) with Palacos® and “liquid MMA” but not with a different brand of bone cement (not identified) containing MMA. The report of case 6 by Walters et al. (Citation2017) contained no details of the bone cement used, but it most likely included Gentamicin as this has been established practice within Europe since the 1980s (Neut et al. Citation2010). SIC Challenge test using a 2-min inhalational challenge to “pre-mixed” bone cement containing MMA gave an EAR (fall in FEV1 of 35%, recovering over 2 h). The use of “pre-mixed” cement would have excluded the possibility of exposure to airborne polymer dust (PMMA powder component contains gentamicin). The fourth, case 1 reported by Walters et al. (Citation2017), involved manufactured prosthetic limbs from a resin containing MMA and made rigid-foam body compensations using a hardener containing MDI. The patient gave a LAR with MMA and a “positive” result to MDI. Both Gentamicin (sulphate) and MDI are known respiratory sensitisers so the assertion that MMA caused the development of OA in these cases is highly unreliable. Furthermore, the onset of asthma only after using cement with gentamicin in the cases reported by Pickering et al. (Citation1986) and Reynaud-Gaubert et al. (Citation1991), and the positive SIC to MDI in the case of Walters (Citation2017) strongly implicated gentamicin antibiotic or MDI, and not MMA, as the driver of OA in these cases. Consequently, these records were scored K3.
Following this there remained 10 cases in which the SIC was reported as being positive with MMA, or with products that were claimed to be predominantly MMA.
Isolated EAR were obtained in three cases (Lozewicz et al. Citation1985—case 6; Suojalehto et al. Citation2019a—case 6), DAR in two cases (Suojalehto et al. Citation2019a—cases 3 and 5), and isolated LAR in five cases (Wittczak et al. Citation1996; Piirilä et al. Citation1998—case 8; Suojalehto et al. Citation2019a—cases 1, 2 and 4).
Lozewicz et al. (Citation1985) reported the case of a 40-year-old male dental assistant who developed chest tightness, dyspnoea, and cough that persisted for several hours after mixing “PMMA powder with MMA liquid” and gave an EAR (24% fall in PEFR that resolved within 2 h) in SIC.
Wittczak et al. (Citation1996) reported a case study of a 40-year-old female dental technician who worked with powdered and liquid “acrylate” formations and had contact with prosthesis processing dust. SIC involving mixing “liquid MMA” and “MMA powder” for 20 min gave a LAR (FEV1 fell >40% at 4 h and PEFR fell > 50% at 24 h).
The six cases reported by Suojalehto et al. (Citation2019a) to ECHA’s Committee for Risk Assessment (RAC) include two dentists and three dental technicians, of which three gave LAR (cases 1, 2 ad 4), 2 gave DAR (cases 3 and 5) and one, a beautician applying acrylic nails (case 6), gave an EAR. Details at the individual case level as required to score under ECHA R.7a guidance are not available since they were not described in the 2019 publication or documented elsewhere. Further details were provided by the authors to the ECHA RAC in a redacted response to an information request (D(Citation2021)0116—Annexe 5 to the CLH dossier). Details were limited, but included information that in three cases (two dentists and one dental technician), the products’ main component (>90% in liquid component) was MMA; that five measurements of MMA levels (0.56, 3.6, 5.1, 5.6, and 13 mg/m3—median 5.1; method not disclosed) had been made by FIOH between 2007 and 2020 during SIC with “MMA containing products” that had negative outcomes. Two further cases were reported to have comparable SIC treatment including material, while in the sixth positive case was performed with the patient that ground a recently hardened prosthesis.
None of the 10 SIC cases studies contain sufficient details to allow a high rating against the ECHA R.7a criteria. The two published SIC cases in dental workers (Lozewicz et al. Citation1985; Wittczak et al. Citation1996) lack essential details of product identity and composition that would be required to exclude significant co-exposure justify their rating as K3. This is reported for products used by dental technicians and dental assistants using a wide range of restorative materials (composites self-cure/light cure multifunctional resins), sealants (resin or glass-ionomers), impression materials (alginate, agar, elastomers, waxes, etc.), and resin-based cements but very few, if any, of which contain MMA. The lack of documentation including any details on the patients, their clinical history, the products involved in their workplace or the SIC etc. for the 22 SIC cases on MMA reported by Suojalehto et al. (Citation2019a) to RAC fully justify their rating as K4.
The claimed 5 measurements of MMA levels in SIC tests with “similar products and SIC protocols” also require further investigation. Notwithstanding that these SIC had negative outcomes and were with different products, the authors did not state the method used for these measurements. However, the revised handbook on specific challenge testing for occupational asthma produced by Suojalehto et al. (Citation2019b) reveals that FIOH typically uses the ISO 16000-6 method for the determination of volatile organic compounds in indoor and test chamber air. This method employs active sampling on Tenax TA sorbent followed by thermal desorption and gas chromatography using mass spectrometry (MS) or mass spectrometry-flame ionisation detector MS-FID (ISO 2011). It is likely, therefore, that this method was used for the MMA determinations in these SIC tests. This method is very reliable; however, it clearly states in §8.2 of the standard that when test chamber air sampling: an appropriate sampling flow rate is in the range of 50 ml/min to 200 ml/min and under §8.3 that suitable sampling volumes when sampling VOCs from non-industrial indoor air, is 1 litre to 5 litre for sampling tubes with 200 mg of Tenax TA®. These conditions determine that the shortest recommended sample time is 5–100 min since the flow rate/sample volume specified is insufficient to allow measurement during shorter sample intervals. As the SIC tests referred by Suojalehto et al. (Citation2019a) and co-workers were claimed to follow the European Respiratory Society guideline (ERS Citation2014) on conducting SIC tests that recommend using simulated workplace exposure conditions of 20–30 min, it can be deduced that reported levels were mean (TWA) exposure concentrations and not peak, or short-term exposure levels, that have been associated with respiratory irritation by MMA.
On the basis that the cited MMA exposure levels claimed to occur in the six positive SIC reported by Suojalehto et al. (Citation2019a) are not sufficiently reliable to exclude the possibility that irritation could have occurred they are rated K3.
3.8. Integration and weighing of evidence (WoE) analysis and application of levels of confidence
The first step in integrating and weighing the evidence was to weight the individual studies with respect to causation using adapted (modified) Bradford Hill (BH) criteria (see ) and expressing this in terms of a four-point confidence (OHAT) score. Moderating factors (Rooney et al. Citation2014) were subsequently applied to the overall scoring against BH criteria to derive a final level of confidence in the individual line of evidence.
3.8.1. Line 1 worker health studies
Eight out of 13 worker health records were rated Klimisch category (K2) and 6 of these (Monroe et al. Citation1981; Della Torre et al. Citation1982; Marez et al. Citation1993; Mizunuma et al. Citation1993; Pickering et al. Citation1993; Pausch Citation1994) were cross-sectional worker health studies in the cast acrylic sheet manufacturing industry by independent physicians with Della Torre, Monroe, Pausch, and Pickering studies being more comprehensive with high inclusion rates compared with those of Marez and Mizunuma.
No increased risk of asthma was reported in the K2 rated studies. The only indication of an increased risk of asthma came from two K3 rated studies. Jaakkola et al. (Citation2007) reported an increased risk of adult-onset asthma (adjusted OR 2.65, 95% CI 1.14-7.24) in dental assistants with claimed exposure to methacrylates. Jedrychowski and Fonte (Citation1984) also reported a higher incidence of asthma and obstructive syndrome, but not chronic bronchitis, in styrene, benzene, and MMA workers. Low weight was attributed to the study of Jaakkola et al. (Citation2007), as no details of exposure levels or chemicals involved were reported, and no individual case reports of asthma were included. Moreover, the incidence of asthma and lower respiratory tract symptoms were reported to be comparable between former Swedish dental technician students (n = 2139) and general population controls (n = 2288) (Brisman et al. Citation2011). Low weight was also assigned to the study of Jedrychowski and Fonte (Citation1984) as mixed exposures were reported and any possible effect attributable to MMA alone could not be discerned. Overall, there is no convincing evidence for an increased risk of asthma in worker-health studies involving high exposures that were predominantly to MMA.
No increased risk of bronchitis (Della Torre et al. Citation1982; Jedrychowski and Fonte Citation1984; Mizunuma et al. Citation1993), or a lower-than-normal risk (Monroe et al. Citation1981) of bronchitis was reported. Additionally, mild airways obstruction (Marez et al. Citation1993), congestion (Nishiwaki et al. Citation2001), frequent cough (Marez et al. Citation1993; Mizunuma et al. Citation1993; Nishiwaki et al. Citation2001) and sputa (Mizunuma et al. Citation1993) have been reported in numerous studies.
Irritation of the eyes and the upper respiratory tract (Della Torre et al. Citation1982; Pickering et al. Citation1993; Pausch et al. Citation1994), mucosa (Della Torre et al. Citation1982) and throat (Marez et al. Citation1993) was reported consistent with the harmonised classification of MMA within the EU as irritating to the eyes (H319) and respiratory system (STOT SE 3, H335) (ECHA Citation2021c).
The report of a moderate prevalence of minor nervous disorders (Della Torre et al. Citation1982) has not been confirmed in any other worker health study on MMA and may reflect the unique nature of the PMMA depolymerisation process undertaken in that factory.
In terms of satisfaction of the BH criteria, the scoring was high confidence (++++) in the case of four criteria and moderate confidence (+++) in a further three (). Two criteria (experimental and analogy) were not considered applicable for this line of evidence. This led to an overall initial level of confidence in the line of evidence according to Rooney et al. (Citation2014) of “high (++++).” It is recognised that at least three studies (Monroe et al. Citation1981; Pickering et al. Citation1993; Pausch Citation1994) were sponsored by industry but were performed independently by respiratory physicians. The lead investigator for the study of Pickering et al. (Citation1993) was Professor C A Pickering of the UK North West Lung Centre. The study of Marez et al. (Citation1993) was fully independent of industry. Overall, this aspect of potential bias is not considered to have significantly decreased the level of confidence.
Table 5. Confidence score in adapted Bradford Hill criteria for worker health studies.
There are few unexplained inconsistencies in the line of evidence. The finding of increased risk of asthma by Jaakkola et al. (Citation2007) and Jedrychowski and Fonte (Citation1984) can possibly be explained as being due to mixed exposures in the workplace which was recognised by the authors in the case of Jedrychowski and Fonte (Citation1984). It was judged that these possible downgrading factors were sufficiently balanced by the consistency, specificity and biological gradient of the findings, thereby not warranting downgrading of the level of confidence in this line of evidence from high (++++).
3.8.2. Line 2 case study records
Nineteen records (containing 29 case studies) comprised the second line of evidence. Two K4 rated clinical records were excluded from the body of evidence. All of the remaining 27 case studies were rated K3 as they lacked essential detail on exposure within the workplace sufficient to meet the criteria according to ECHA R.7a guidance (ECHA Citation2017a), thereby limiting the maximum level of confidence that could be achieved to moderate (+++).
Five records (Kennes et al. Citation1981; Basker et al. Citation1990; Savonius et al. Citation1993a, Citation1993b—case 2; Obando et al. Citation2013; Walters et al. Citation2017—cases 4 and 8) involved products that apparently did not contain liquid MMA so did not add to the body of evidence for this chemical. Two of these case studies (Kennes et al. Citation1981; Savonius et al. Citation1993a, Citation1993b—case 2) described work conditions that involved exposure to polymer dust that may be a significant factor in the development or triggering of asthma and will be discussed later. Kennes et al. (Citation1981) reported a case of a 33-year-old male factory worker who likely developed OA due to isocyanate on the basis that he worked with TDI and gave a LAR in a SIC involving “painting with TDI varnish.” However, he also reportedly had exposure to dust when cutting PMMA and gave an EAR when challenged with PMMA dust. Savonius et al. (Citation1993a, erratum 1993b) reported a 32-year-old male who assembled hearing devices and gave a small maximal 15% decrease in PEF following the grinding of "a piece of methacrylate.”
Five clinical cases in four records (Pickering et al. Citation1986; Reynaud-Gaubert et al. Citation1991; Roth et al. Citation2017; Walters et al., Citation2017—cases 2 and 6a) involved mixing and use of orthopaedic cements that were described as containing, or likely contained (latter 2 records), gentamicin. Gentamicin is an antibiotic that has been linked to development of OA. The temporal alignment between starting work with the cement containing gentamicin and the development of OA (Pickering et al. Citation1986), and the negative SIC when challenged with cement containing MMA, but not gentamicin (Reynaud-Gaubert et al. Citation1991), points strongly towards development of OA due to gentamicin rather than MMA. However, the elicitation of LAR when mixing cement with MMA, but not when this was replaced with water (Pickering et al. Citation1986), perhaps points to MMA being able to trigger an asthmatic response in a subject that has already developed asthma due to prior exposure to another material.
In terms of consistency and the prevalence of OA in this sector, there are 22 288 orthopaedic surgeons (Madanat et al. Citation2016) with presumably a corresponding number of orthopaedic nurses within the EU conducting 750 000 hip replacements per year (OECD Citation2011). So, irrespective of the causative agent, the prevalence of OA in this sector is extremely low.
Eight records containing eleven case studies (Vallieres et al. Citation1977; Lozewicz et al. Citation1985—case 2; Savonius et al. Citation1993a, Citation1993b—cases 1 and 2; Piirilä et al. Citation1998; Thorette et al. Citation2006; Sauni et al. Citation2008; Walters et al. Citation2017—cases 3, 5, and 7; Moshe and Krakov Citation2019) contained insufficient detail on workplace exposure to indicate with any confidence a causal inference for MMA. For example, a railway cable joiner reported by Lozewicz et al. Citation1985 (case 2) was described as working with an MMA-based jointing resin, but no details of other chemicals used in his workplace are available. Furthermore, while he had PEF measurements indicative of asthma, he only experienced respiratory symptomology (cough and wheeze) outside the workplace and gave a negative SIC with MMA, making the causal link to workplace exposure to MMA very unlikely. Similarly, nail/beauty technicians are recognised as using a number of artificial nail technologies including gel-, wrap- and cyanoacrylate-nails none of which contain MMA. Exposure to hydroxyethyl methacrylate (HEMA), hydroxypropyl methacrylate (HPMA), ethylene glycol dimethacrylate (EGDMA), and cyanoacrylate used in these technologies has been reported (Sauni et al. Citation2008; Moshe and Krakov Citation2019), thereby weakening the strength of attributing the development of OA solely to MMA in these cases.
Five records containing six case studies, (Lozewicz et al. Citation1985—case 1; Savonius et al. Citation1993a, Citation1993b—case 3; Wittczak et al. Citation1996; Piirilä et al. Citation1998—case 1; Scherpereel et al. Citation2004—2 cases) were from the dental sector (three technicians, two trainees, and a dental nurse) in which use of MMA is commonly described. However, the authors apparently attribute the development of OA in these cases to use of MMA without providing any evidence as to why other chemicals present in the wide range of restorative materials used in this sector can be discounted. In terms of consistency, and the prevalence of OA cases claimed to be due to MMA, there are 40 000 dental laboratories and 210 000 dental technicians (FEPPD Citation2021) and 837 000 dental assistants (EC Citation2016) within the EU indicating that the prevalence, in any event, is extremely low.
In terms of analogous evidence, there have been seven clinical cases reports of OA due to other methacrylates in the literature. Piirilä et al. (Citation1998) reported four dentists and three nurses with clinical diagnosis of OA claimed to be due to HEMA. The complex composition of these Scotchbond™ adhesive and primer products combined with the use of other dental products renders the attribution of the development of OA to HEMA subject to some uncertainty. There is also low confidence that the two beauticians with allergic contact dermatitis who developed OA reported by Moulin et al. (Citation2009), did so as a result of working with HEMA.
In terms of satisfaction of the BH criteria, the scoring was very low confidence (+) in the case of five criteria and low confidence (++) in a further two (). Two criteria (experimental and analogy) were not considered applicable for this line of evidence. This led to an overall initial level of confidence in the line of evidence according to Rooney et al. (Citation2014) of “very low (+).” The strength of the clinical case study evidence is hampered by the understandable difficulties in recording an accurate and historical profile of workplace exposure to the suspected causative agent, as well as other agents that could be responsible. Typically, this is only achievable in the industrial setting, with the worker health studies comprising the first line of evidence.
Table 6. Confidence score in adapted Bradford Hill criteria for case studies.
The main inconsistency in the line of evidence is the reporting in some studies of exposure to other chemicals that might have equally been responsible for the development of OA. Importantly in other case studies from the same sector, no such co-exposure is reported. This is evident in case reports from both the orthopaedic and dental sectors and could reflect incomplete information being reported by the patient to the clinician, or reporting bias due to the a priori opinion held by some that “acrylates” and MMA are recognised asthmagens. This, combined with the low prevalence of effect and lack of dose response, does not warrant a change in the overall level of confidence in this line of evidence from very low (+).
3.8.3. Line 3 quantitative Structure-Activity relationship (QSAR) analyses
The two QSARs for asthma (Jarvis et al. Citation2005; Enoch et al. Citation2012) that mention MMA have been optimised in terms of predictive value based upon a learning set of substances that are described as asthmagens and include MMA. Jarvis et al. (Citation2005) predicted MMA as not being an asthmagen (false negative by their criteria) but that methacrylic acid, a related substance not linked with OA, was an asthmagen (false positive). This low predictive performance may reflect the QSAR being based upon putative asthmagens without consideration of mechanism of action (irritation or sensitisation), or of the strength of the evidence. Although Enoch et al. (Citation2012) described the acryl moiety and Michael addition reactivity as a predictor of respiratory sensitisation they only reported the overall predictive value of the QSAR as being 79% without reporting an outcome for MMA.
There is no widely accepted or validated QSAR for respiratory sensitisation hazard. The QSAR developed by Jarvis et al. (Citation2005) predicts MMA not to be an asthmagen, whereas the QSAR developed by Enoch et al. (Citation2012) predicts a potential be a respiratory sensitiser based upon to the presence of the acryl (R-C = O) functional group. However, it is not possible from the publication to derive any confidence in this prediction with respect to MMA. In this regard, the authors in recognising that known chemical respiratory allergens typically elicit strong responses in the LLNA whereas MMA is a weak allergen acknowledged that this prediction for MMA is “surprising given that acrylates and methacrylates have been reported as being relatively weak skin sensitisers” citing Gerberick et al. (Citation2005). It is recognised that QSAR models should strictly speaking only be cited for their predictive power against chemicals outside the domain of the learning set upon which they were developed. Since MMA presumably was within the learning set then the assertion of a claimed 79% overall predicted power of the model cannot be applied to MMA. Furthermore, Dik et al. (Citation2014) reporting that the predictive performance of published QSAR models was lower in practice than claimed so any assumed prediction with respect to MMA is likely to be even lower than 79%.
In terms of satisfaction of the BH criteria, the scoring was low confidence (++) in the case of four criteria and very low confidence (+) in a further two (). Three criteria (temporality, experiment and analogy) were not considered applicable for this line of evidence. This led to an overall initial level of confidence in the line of evidence according to Rooney et al. (Citation2014) of “very low (+).” The strength of the QSAR evidence is limited by the available learning set of proven low molecular weight respiratory sensitisers and the inclusion of substances that are claimed to be asthmagens without clear distinction of their likely MOA, or the extent to which irritation may have confounded their identification.
Table 7. Confidence score in adapted Bradford Hill criteria for QSARs.
Table 8. Confidence score in adapted Bradford Hill Criteria for exposure data.
The inherent low confidence in the published clinical case studies forming the learning set upon which these QSARs are developed and validated transfers a low confidence to the QSARs potentially lowering confidence even further. Overall, the level of confidence in this line of evidence is low (+).
3.8.4. Line 4 exposure data
Nine records on exposure assessments mentioning MMA were identified in the literature and references therein with a further 6 from the worker health studies.
The worker health studies in the cast acrylic sheet manufacturing industry reported relatively high shift average (8 h TWA) exposures of between less than 21 mg/m³ and up to 736 mg/m3 with extremely high shorter duration exposures of up to 2856 mg/m³. Overall, the available data are consistent, although the data reported by Marez et al. (Citation1991, Citation1993) are both internally and externally inconsistent. For example, it is not feasible that mean 8 h TWA levels can range from “trace” to 161.7 mg/m³ while 1 h TWAs range from 488.8 mg/m³ to 1680 mg/m³. Excluding the Marez data, the available exposure data for this sector is consistent with the larger body of data reported within the EU RAR for MMA (EC Citation2002).
The published exposure data for the dental industry superficially appears inconsistent. Very low mean (8 h TWA) and peak exposure levels have been reported by some authors (Nayebzadeh and Dufresne Citation1999; Hagberg et al. Citation2005; Marquardt et al. Citation2009) whereas Golbabaei et al. (Citation2005) and Torbica and Krstev (Citation2006) report much higher levels that are consistent with the ranges reported for this sector in the EU RAR (EC Citation2002), and recently by the BG ETEM (2021). In this regard, the EU RAR reported similarly divergent data but recognised that exposure levels were extremely dependent upon the nature of the task and use of local exhaust ventilation (LEV). For risk assessment purposes the RAR identified 6 mg/m³ (8-h TWA) and 42 mg/m³ (short-term) with LEV and 110 mg/m³ (8-h TWA) and 600 mg/m³ (short-term) under unfavourable conditions without LEV for this sector. Apart from this, dental technicians appear more exposed to peak exposures of MMA when mixing MMA liquid with PMMA powder, than workers in other dental occupations (like nurses & dentists) that are routinely handling dental products of complex compositions, including various other methacrylates. On this basis, this disparity likely reflects differences in work practices and not reporting if LEV is present. Additionally, observed differences in peak level measurements may be confounded by the monitoring methods used since most investigators employed thermal desorption tubes followed by GC-ID analysis or absorbent badges and charcoal tubes that would not allow determination of short duration peaks.
Published exposure data for MMA in the artificial nail applications derived from a study in the late 1980s (Froines and Garabrant Citation1986). The levels reported were lower than the upper end of the range observed in the dental sector without LEV, presumably reflecting the lower volumes of MMA used in nail applications. Ethyl methacrylate (EMA), but not MMA, was detected and this presumably reflects the widespread substitution of MMA with EMA in this sector (Hiipakka and Samimi Citation1987; Spencer et al. Citation1997). More recently the use of EMA has widely given way to use of gel-nail technology that uses neither EMA or MMA.
Disparity of exposure data was also observed for the orthopaedic sector, with higher levels of MMA detected without use of laminar airflow or LEV (Pickering et al. Citation1986; Darre et al. Citation1992; Ungers and Vendrely Citation2006; Ungers et al. Citation2007). Although the maximum levels reported vary considerably (1570 mg/m³ (Pickering et al. Citation1986); 840 mg/m³ (EC Citation2002) to greater than 4000 mg/m³ (Ungers and Vendrely Citation2006)), it is evident that extremely high peak levels are achieved over short durations of a few minutes in the absence of LEV.
Exposure data for workers in the cast acrylic sheet manufacturing industry revealed a similar picture of high, short-duration peaks of exposure, particularly during tasks where LEV was absent or ineffective. The absolute levels of MMA exposure, mean long-term (LTEL 8 h TWA), short-term average (STEL e.g. 15 min) and peaks, were higher than for the downstream professional workplaces, such as dental, medical and nail, likely reflecting the much larger volumes of MMA being handled in these industries.
Unlike in the cast acrylic sheet industry, workplace exposure in the downstream professional workplaces, such as dental and nail labs involve exposure to other volatile chemicals including solvents and other acrylic and methacrylic monomers, and dusts. Dust exposure could be a direct consequence of the use of two-part cements, containing fine PMMA particles, or the shaping (grinding and sanding) of polymerised material, e.g. dental prosthetics, nails or acrylic articles. While quantitative dust measurements are rare, Torbica and Krstev (Citation2006) reported silica dust concentrations at levels critical for respiratory health effects in dental technicians.
None of the published exposure studies on MMA was “comprehensive” with full characterisation of the range and extent of occupational control measures (i.e. use or not of LEV and its effectiveness) and exposures (i.e. all substances, their profile of exposure, levels and task duration). Rather, the studies in dental, orthopaedic, and nail sectors focussed primarily on MMA and in only in two cases included other methacrylates. This disregard for other potentially significant exposures, including dust, is a significant weakness in terms of causal attribution of clinical findings in these sectors. On balance, the exposure data for dental, orthopaedic and nail facilities can be attributed with no greater than low (++) confidence.
This concern is less significant for the data from the cast acrylic sheet industry as workplace exposure is predominantly to MMA. Furthermore, the exposure data would have been collected under the legal requirement for hygiene monitoring programs. For these reasons there is a higher level of confidence [moderate (+++)] in this data pointing to high historical exposure to MMA [mean as high as 420 mg/m³ (8-h TWA) and peaks well in excess of 1000 mg/m³)] in this industry, with lower levels in recent years as a result of introduction of improved control measures.
It is recognised that not all BH criteria are applicable to scoring the level of confidence in exposure data. For those criteria considered relevant, the scoring for one criterion was high (++++) and moderate (+++) for all others (). This led to an overall initial level of confidence in the line of evidence according to Rooney et al. (Citation2014) of “moderate (+++).” Potential bias by investigators in only monitoring and/or reporting data on MMA and no other substances present in the workplace, combined with use of methods that are inadequate for detection of peak exposures, would tend to decrease confidence in the body of data. Whereas the consistency of the data, particularly compared with the findings of the EU RAR report (EC Citation2002), increases confidence, the overall level of confidence in this line of evidence is judged to be moderate (+++).
3.8.5. Line 5 european national surveillance data
The fifth line of evidence comprises clinical case studies reported to EU national surveillance networks. With exception of the BG ETEM investigation, there were no comprehensive clinical details or exposure data available for any case study as prescribed by ECHA R.7a guidance. In some cases, details of the age, sex, sector, or job and basis of diagnosis were available, while for many details were even more limited or completely absent. The level of confidence in the diagnosis of MMA causing the development of OA in these cases is therefore low. Indeed, in a number of cases the available evidence indicated that not only was occupational exposure to other chemicals involved, but may in fact have actually caused the development of OA.
Confidence in this line of evidence may be increased if further details of workplace exposure and clinical diagnosis were to become available but these surveillance systems do not appear to be designed to collect this type of information even if it were available.
In terms of satisfaction of the BH criteria, the scoring was very low confidence (+) for seven criteria (). Two criteria (experiment and analogy) were not considered applicable for this line of evidence. This led to an overall initial level of confidence in the line of evidence according to Rooney et al. (Citation2014) of “very low (+).” The lack of details relating to the cases prevents any further modification and results in an overall level of confidence in this line of evidence of low (+).
Table 9. Confidence score in adapted Bradford Hill criteria for national surveillance data.
3.8.6. Line 6 specific inhalation challenge (SIC) test data
The fifth line of evidence initially comprised a total of 32 reported SIC tests in patients who claimed to have developed OA as a result of occupational exposure to MMA. After exclusion of those cases that did not involve MMA, or were more likely to be due to a widely recognised respiratory sensitiser, there remained 10 cases in which the SIC was reported as being positive with MMA, or with products that were claimed to be “predominantly MMA.”
This body of evidence can be broken down into three sub-lines of evidence: a) that patients were claimed to have given positive SIC in response to MMA, b) that these SIC responses comprised a combination of early and late asthmatic responses, and c) that according to a redacted response by the authors to RAC that MMA exposure during six of these positive SIC, three of which were performed at FIOH were very low, and therefore could not have been the result of irritation.
First, there is little information on the identity and levels of the substances that these patients were exposed to in both the workplace and subsequently during the SIC test. The patients are described as coming from the dental sector in which the published literature recognises that there is workplace exposure to a wide range of restorative materials including other methacrylates and acrylates as well as organic or inorganic dusts. As a consequence, establishing a causal link to MMA on the basis of work history alone can only be made with low confidence (+). This is particularly true for the case that was based on a positive SIC result observed when grinding a “recently hardened prosthesis,” i.e. without any apparent exposure to liquid MMA. Similarly, the conclusion that MMA can elicit an asthmatic response in SIC is limited to the extent that the full composition of the products used in the SIC is not disclosed beyond it being the “main component.” Considering that often these minor components are other mono and multifunctional acrylates and methacrylates and there is recognised exposure to PMMA powder, when mixing these two-part products, or other dusts including crystalline silica, some conservatism should also be displayed in this regard and therefore a low (++) to moderate (+++) confidence appears justified.
Second, in terms of the types of responses observed in the positive SIC, isolated EAR was obtained in three cases, both early and late (dual or DAR) in two cases, and isolated LAR in five cases. Although there appears to be a slightly greater tendency towards LAR, considering the small group size, this is not compelling.
Third, in terms of the strength of evidence that exposures in these positive SIC were too low to cause irritation there are two aspects that require further consideration. 1) The exposure measurements reported were not made in the positive SIC with MMA, but instead in SIC with “similar products and SIC protocols.” Since no details of these SIC are available other than they were made with different products, on other occasions with different patients, and gave negative a SIC responses. The extent of their comparability to the six positive SIC made with MMA remains uncertain. 2) The measurement of MMA levels in SIC tests made by FIOH used the ISO 16000-6 method that is stated as being unsuitable for measurement of shorter sample intervals of less than 5 min. This is significant since short peak exposures that would not be detected by this method have been shown to occur in dental workplaces and have been reported as the cause of respiratory irritation in workers.
Taken together, these aspects lessen the level of confidence from low (++) to very low (+) in the assertion that MMA exposure employed in 6 of these positive SIC performed at FIOH were so low that it could not have resulted in irritation.
In terms of satisfaction of the BH criteria, the scoring was very low confidence (+) for four criteria and low (++) for a further four (). One criterion (experiment) was not considered applicable for this line of evidence. This led to an overall initial level of confidence in the line of evidence according to Rooney et al. (Citation2014) of “low (++).” The lack of details for the case histories, workplace and SIC exposures levels and co-exposure prevents any further modification and results in an overall level of confidence in this line of evidence of low (++).
Table 10. Confidence score in adapted Bradford Hill criteria for SIC data.
4. Integrating the lines of evidence
The overall level of confidence for the 6 lines of evidence is summarised in .
Table 11. Overall level of confidence in the six lines of evidence.
Despite a certain disparity in some of the exposure evidence, there remains a high confidence in this line of evidence. The more reliable evidence from the industrial sector as reviewed in the EU RAR (EC Citation2002), combined with exposure modelling are consistent with MMA being a heavy vapour capable of producing very high peak exposures of short-duration. Several studies have reported similar very high peak exposures occurring when mixing bone cements, a task comparable to the mixing of two-part cements by dental operatives as simulated in SIC with MMA. A combination of underestimation of the impact of LEV, different work tasks with products of different composition, and use of inappropriate measurement methods that could not detect short peak exposure levels, are the likely explanation for why some studies in the dental sector and SIC reported much lower exposures.
In terms of the clinical evidence, there was very high confidence in the worker health studies. These consistently showed that MMA caused irritation of the respiratory tract, mild congestion, but no asthma in workers exposed to a combination of high shift average and very high peak levels of MMA over many years. It would be expected that this respiratory irritation observed in industrial workers would occur in asthmatics exposed to similarly high peak levels of MMA during SIC, where LEV would be absent or ineffective.
There was low confidence in the other clinical evidence (case studies, national surveillance data and SIC tests) for a combination of overlapping reasons. One of the primary weaknesses in these data is the lack of a comprehensive worker exposure history on which to base identification of the causative agent(s). In many cases there is clear evidence of selection and reporting bias reflecting a priori conclusion of causation by MMA. The characteristic smell of MMA and other meth(acrylates)may play a significant role in this respect. In some cases, this was further compounded by imprecision in the description of the chemistry involved and the clustering of acrylates, methacrylates and cyanoacrylates, without regard to their very different physical and chemical properties, and biological reactivity. In this respect, ECHA R.7a and clinicians (Shofer et al. Citation2006; Parhar et al. Citation2011; de Olim et al. Citation2015; Vandenplas et al. Citation2014, Citation2017) highlight the importance of making accurate exposure assessments both in the workplace and during SIC. Failure to follow this guidance prohibits any level of confidence in the assertion of a work-related pattern of disease causally related to a specific substance(s) and exclusion of non-specific irritation from SIC tests.
In regard to the latter, it is a commonly held view that a greater tendency for isolated LAR in SIC points to involvement of an immune mechanism. However, this is not scientifically established and in the case of MMA, the small numbers of positive (six SIC; consisting of three EAR, two Dual (DAR), and five LAR) is not sufficiently strong evidence (Pemberton and Kimber Citation2021). In any event, the relatively small numbers involved in comparison with the large number employed in this sector points to an extremely low prevalence that is inconsistent with an inherent property to cause respiratory sensitisation.
There was also low confidence in the available QSARs for respiratory sensitisation/asthma as the predictive performance is less than claimed (Dik et al. Citation2014; Arts Citation2020) and the only available prediction for MMA was that it was negative (Jarvis et al. Citation2005).
5. Uncertainty analysis
There are no generally accepted or validated animal models for sensitisation of the respiratory tract to LMW chemicals, and currently available QSARs are not sufficiently predictive to be of any value. The majority of the available clinical evidence is of such low confidence that it inevitably is associated with a high level of uncertainty. This situation is not surprising as medical authors of clinical case descriptions (may it be as worker health studies, case studies or specific diagnostic studies like SIC tests) rarely consider regulatory requirements for classification of chemicals when they plan their studies or bring observations to paper. Nevertheless, the majority of the asthma cases reviewed in this context do not even allow a clear medical assignment to the different types of OA according to WAO (see above).
A high level of uncertainty is also given for the evidence of exposure to MMA as causative agent for OA as long as co-exposure to material with the potential for relevant respiratory effects (e.g. gentamicin in the orthopaedic sector, a wide range of anorganic dusts including plaster, non-precious and precious metals (chrome, nickel, cobalt, gold, palladium etc.) ceramics (amorphous and crystalline silica, quartz, cristobalite etc.) and rare metal oxides (zirconium dioxide, hafnium and yttrium oxides etc.) in the dental sector, PMMA dust in both sectors) cannot be excluded specifically in those sectors where OA cases of interest were observed. Also, with regards to sector-specific MMA exposure, uncertainty on causality arises from the observation that OA was not consistently found in sectors of high MMA exposure (e.g. not in cast sheet production). If the underlying weaknesses in this evidence were to be addressed this could have a significant impact on the outcome of this assessment. Fundamental to this would be the conduct of a comprehensive clinical history and exposure assessment in the workplace upon which assertions of association and, ultimately, causation is drawn. The same applies to SIC testing, where current guidance on exposure measurement and its interpretation is deficient and requires revision with involvement of appropriate experts.
6. Conclusions regarding data adequacy
It is recognised that the available clinical evidence on MMA and asthma is extremely weak and of low reliability rendering it unsuitable for assessment purposes. Nevertheless, the high number of claimed cases of OA in the literature and in national surveillance databases arguably justifies its consideration under a WoE framework. Overall, the clinical evidence suggesting that MMA is the cause of OA is incongruent with the finding of an absence of asthma in the industrial (cast sheet) sector with higher exposures over many years.
Regarding the first sub-question; there are no published cases of OA for which acquisition can be causally related to workplace exposure to MMA with moderate to high confidence. In those cases identified, the causative agent is more likely to be other known chemical respiratory sensitisers, or has not been adequately established. Any work-related pattern of disease corresponding with MMA exposure is more likely the result of irritant exacerbation of pre-existing disease.
Regarding the second sub-question; the evidence in favour of the involvement of an immune-specific mechanism associated with allergic sensitisation is both weak and insufficient since no cases of “respiratory sensitisation” due to MMA have been documented. The only evidence suggestive of involvement of an immune mechanism comes in the form of a small number of OA cases with positive SIC from the dental sector. However, irritant provocation is the more likely explanation since a) exposure levels used were not sufficiently established to be below levels that cause irritation and, b) the profile of LAR in these few cases does not indicate with any confidence immune involvement. Furthermore, considering workers in the dental sector are known to have mixed exposures to other chemicals including dusts, both in their workplace and during the SIC tests, it is not possible to infer that MMA is an asthmagen, i.e. based upon causation of OA in the workplace or, indeed, that MMA is a respiratory allergen, i.e. based upon “specificity” of the asthma response in SIC.
Regarding the third sub-question; MMA is a recognised irritant chemical and has been reported to cause respiratory irritation in industrial workers exposed to high levels of MMA vapours. Strongly irritating substances are known to cause “irritant induced” asthma. However, MMA is only a weak irritant both in animals and humans and this is consistent with an absence of asthma in industries (cast sheet and floor resins) with much higher and dedicated MMA exposure levels compared with those in the dental sector.
Taken together, the available evidence does not support MMA as being the cause of OA.
7. Discussion
There is no doubt that occupational asthma provides toxicologists with a number of important challenges.
One issue that has caused considerable uncertainty is the fact that from a regulatory perspective no distinction is drawn between OA associated with allergic sensitisation of the respiratory tract induced by low molecular weight chemicals (where there is a requirement for stimulation of an adaptive immune response), and non-allergic asthma which is driven by other mechanisms, and frequently by irritant responses. An added complication is that in some instances inhalation exposure to an irritant material might provoke a reaction in a subject that had pre-existing asthma unconnected with the workplace (Vandenplas Citation2011; Vandenplas et al. Citation2014; Arts and Kimber Citation2017; Maestrelli et al. Citation2020). As cited above, one consequence of this is that the guidance from ECHA is that the term “respiratory sensitiser” embraces chemicals that are able to cause airway hypersensitivity by either an immunological or non-immunological mechanism.
It can be argued that, in practice, the failure to differentiate between true respiratory allergens and agents that cause the same or similar symptoms through non-immune mechanisms is of little significance. However, this is not the case and there are compelling reasons why a clear distinction should be drawn between different forms of OA, not least of which is in the interests of effective risk management. Moreover, the incorrect classification of a chemical as a respiratory sensitiser will trigger unnecessary and inappropriate regulatory measures, and possible designation as SVHC under REACH (Arts Citation2020).
In this context best practice would dictate that there is a real need to distinguish carefully between true chemical respiratory allergens and other substances that are able to cause OA through other, non-immunological mechanisms. Herein lies another challenge for toxicologists, insofar as there are currently available no widely accepted or validated methods (in vivo, in vitro or in silico) that can be used for the identification of true chemical respiratory allergens (Holsapple et al. Citation2006; Kimber et al. Citation2007, Citation2014a; Isola et al. Citation2008; Boverhof et al. Citation2008; Arts Citation2020). As a consequence, reliance must be placed on available human data, and this can take a number of forms, including for instance worker health studies, individual case studies, surveillance data, and the results of specific inhalation challenges (Pemberton and Kimber Citation2021).
In a recent article (Pemberton and Kimber Citation2021), the need for careful interpretation of human data for the purposes of accurate identification of chemical respiratory allergens, and for discrimination between these and other substances implicated as causes of occupational asthma, was discussed in general terms. It was one purpose of this paper to document these complex challenges with this detailed review of MMA as an actual example, by re-examination of data which were available in a recent regulatory assessment under EU CLP regulations.
Although this analysis does not, and was not intended to, fulfil the requirements of a systematic review, it certainly provides a comprehensive analysis of all the relevant data relating to any possible association between exposure to MMA and occupational asthma.
In effect, using the available data, three questions were addressed, as follows: (a) is there sufficient evidence to conclude that MMA is a respiratory allergen that is able to cause sensitisation of the respiratory tract and allergic occupational asthma? (b) is there sufficient evidence to conclude that MMA is able to cause non-allergic, irritant-induced occupational asthma?, and (c) is there sufficient evidence to conclude that in certain instances exposure to MMA is able to induce work-exacerbated reactions in subjects who have pre-existing asthma?
The conclusions drawn from these analyses indicate that there is insufficient evidence to implicate MMA as a substance that is responsible for the acquisition of asthma in the workplace. Certainly, while MMA is an allergen capable of causing contact allergy, there is no reason to conclude that MMA can cause allergic sensitisation of the respiratory tract i.e. is a respiratory sensitiser. Neither is there sufficient evidence to conclude that MMA (a weak irritant) is associated with acquisition of irritant-induced asthma. Furthermore, while MMA is irritating to the respiratory system and as such should be capable of exacerbating pre-existing asthma in workers there is insufficient evidence that in practice it is a significant cause of work-exacerbated asthma. As such, even despite the need or not of demonstrating involvement of an immune mechanism, there is no basis for classification of MMA as a respiratory sensitiser under EU CLP, which is widely consistent with UN GHS.
In reaching these conclusions, it is important to make a number of points.
First, it is unusual in a published article of this kind to conduct such an extensive review of the available data and to evaluate the strength, reliability and relevance of those data in reaching weight-of-evidence based conclusions regarding the likelihood of causation. However, this may be an approach that will be adopted more frequently when there needs to be a formal re-appraisal of decisions that are reached using data that present interpretive challenges.
Second, it might appear to some that, although the data on which the decision to classify MMA as a respiratory sensitiser are inconclusive, the decision was correct as a precautionary measure. It is the view of the present authors that such a view would be misguided. The inappropriate classification of a chemical as being a respiratory sensitiser (or any other manner of toxicant) will inevitably: (a) “devalue the currency” of chemical regulation, and engender a loss of confidence that the process is based on rigorous standards, and (b) undermine the integrity of the learning set upon which in silico, in vitro and QSAR methods are developed and validated. There are, of course, also arguments that inappropriate classification and regulation will have important commercial implications if the use of certain chemicals are subject to unnecessary restrictions.
Third, it is necessary to make it clear that although this review found the available human data insufficient to support the classification of MMA as a respiratory sensitiser, this is not intended to be a criticism of the important work undertaken by health care professionals and occupational physicians charged with investigation and diagnosis of workplace-associated asthma. Worker health monitoring, national surveillance data, individual case studies, exposure monitoring and the conduct of specific inhalation challenge tests are all very important elements of monitoring and ensuring worker safety. However, it has to be acknowledged that while such activities fulfil important clinical and occupational hygiene needs they are not designed to provide data on which clear decisions can necessarily be reached about causation, and the requirements for accurate classification of chemicals encountered in the workplace.
Finally, it is appropriate to identify recommendations that flow from these analyses, and which if adopted would have the potential to improve the utility of some aspects of human data for the purposes of establishing causation in OA. It would clearly be of great benefit if there were available a comprehensive history of previous workplace exposures and exposure levels for cases of asthma associated with the workplace. Moreover, the interpretation of the relevance of SIC tests would be improved significantly if there were detailed records of the composition and concentrations of challenge materials.
Another important recommendation is that those charged with reaching regulatory decisions in this area adhere strictly to the weight of evidence paradigm for respiratory sensitisation.
In conclusion, a detailed evaluation of the available data indicates that it is inappropriate and unnecessary to classify MMA as a respiratory sensitiser. It is hoped that the analyses reported here will provide a paradigm for the determination of causality by others.
| Abbreviations | ||
| ACD | = | allergic contact dermatitis |
| ANSES | = | Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (French Agency for Food, Environmental and Occupational Health & Safety) |
| BAL | = | BronchoAlveolar Lavage |
| BG ETEM | = | Berufsgenossenschaft Energie Textil Elektro Medienerzeugnisse |
| BH | = | Bradford Hill |
| Bis-GMA | = | bisphenol A-glycidyl methacrylate |
| CLH | = | Harmonised Classification and Labelling |
| CLP | = | Classification, Labelling, and Packaging |
| CT | = | computerised tomography |
| DAR | = | dual asthmatic response |
| DLCO | = | diffusing capacity for lung carbon monoxide |
| DMAEMA | = | dimethylaminoethyl methacrylate |
| DMEA | = | dimethylethanolamine |
| EAR | = | early asthmatic response |
| ECHA | = | European Chemical Agency |
| EU | = | European Union |
| FEV1 | = | forced expiratory volume in the first second |
| FID | = | flame ionisation detectors |
| FIOH | = | Finnish Institute of Occupational Health |
| FTIR | = | Fourier Transform infra-red |
| FVC | = | forced vital capacity |
| GHS | = | globally harmonised system |
| GRADE | = | Grading of Recommendations Assessment, Development and Evaluation |
| HEMA | = | hydroxyethyl methacrylate |
| HPMA | = | hydroxypropyl methacrylate |
| IIOA | = | non-allergic irritant-induced occupational asthma |
| LAR | = | late asthmatic reaction |
| LEV | = | local exhaust ventilation |
| MDI | = | methylene diphenyl diisocyanate |
| MMA | = | methyl methacrylate |
| MS | = | mass spectrometry |
| MS-FID | = | mass spectrometry-flame ionisation detector |
| NOS | = | not otherwise specified |
| NSIC | = | non-specific inhalation challenge |
| NTP/OHAT | = | National Toxicology Program/Office of Health Assessment and Translation |
| OA | = | occupational asthma |
| OPRA | = | occupational physicians reporting activity |
| PEFR | = | peak expiratory flow rate |
| PEGDMA | = | polyethyleneglycol dimethacrylate |
| PMMA | = | polymethyl methacrylate |
| PVC | = | polyvinyl chloride |
| QSAR | = | quantitative structure–activity relationship |
| RADS | = | reactive airways dysfunction syndrome |
| REACH | = | registration, evaluation, authorisation and restriction of chemicals |
| RNV3P | = | Le Réseau National de Vigilance et de prévention des pathologies Professionnelles |
| SCHEER | = | Scientific Committee on Health, Environmental and Emerging Risks |
| SETAC | = | Society of Environmental Toxicology and Chemicals |
| SIC | = | Specific Inhalation Challenge |
| SPT | = | skin prick test |
| STEL | = | short-term exposure limit |
| STOT SE | = | specific target organ toxicity singe exposure |
| SVHC | = | substances of very high concern |
| SWORD | = | Surveillance of Work-Related and Occupational Respiratory Disease |
| TDI | = | toluene diisocyanate |
| TLC | = | total lung capacity |
| TREGDMA | = | triethylene glycol dimethacrylate |
| TWA | = | time weighted average |
| UDMA | = | urethane dimethacrylate |
| WAO | = | World Allergy Organisation |
| WEA | = | work exacerbated asthma |
| WHO/IPCS | = | World Health Organisation/International Programme on Chemical Safety |
| WoE | = | weight of evidence |
Acknowledgements
The authors gratefully acknowledge the expert review of the manuscript by mammalian toxicologists and regulatory specialists of the Cefic Methacrylates Sector Group including Dr Stuart Hindle (Dow Europe GmbH); Drs Knut Kreuzer, Julia Scheel and Gregor Tuschl (Röhm GmbH); Ms Fiona Smith (Mitsubishi Chemical UK Ltd) and Dr Stelios Kouvroukoglou (Trinseo Deutschland Anlagengesellschaft mbH). These reviewers focused their attention primarily on improving the readability of the paper and the completeness of citations in the text and the reference list. The reviewers did not make any substantive changes to the opinions voiced by the authors. The authors would also like to acknowledge the expert views and comments of the Editor and of the anonymous peer-reviewers. The authors are cognisant that this manuscript will be shared with EU regulators in connection with the CLH review on MMA and may be referred to by Cefic Methacrylates Sector Group in a future complaint or legal action.
Declaration of interest
Dr Mark A Pemberton (MAP) is an independent consultant. MAP is a member of the Scientific Committee of ECETOC and is a freelance scientific consultant with interests in regulatory toxicology and risk assessment covering a wide range of industrial chemicals and polymers. MAP provides toxicology and regulatory consultancy services to the Cefic Methacrylates Sector Group on MMA and in relation to the ongoing classification and labelling (CLH) activity within Europe. MAP received payment from the Methacrylate Producers Association for researching and reviewing the relevant literature and for preparation of this resultant manuscript.
Professor Ian Kimber (IK) is Emeritus Professor of Toxicology at the University of Manchester, UK. IK is a freelance scientific consultant with interests focussed primarily at the interface between immunology and toxicology. IK has provided toxicology consultancy services to the Cefic Methacrylates Sector Group in relation to the ongoing classification and labelling (CLH) activity within Europe. IK received payment from the Methacrylate Producers Association for researching and reviewing the relevant literature and for preparation of this resultant manuscript.
Supplemental material
Supplemental material for this article is available online here.
the test protocol used (study design, controls);
the substance or preparation studied (should be the main, and ideally, the only substance or preparation present which may possess the hazard under investigation);
the extent of exposure (magnitude, frequency and duration);
the frequency of effects (versus number of persons exposed);
the persistence or absence of health effects (objective description and evaluation);
the presence of confounding factors (e.g. pre-existing respiratory health effects, medication; presence of other respiratory sensitisers);
the relevance with respect to the group size, statistics, documentation;
the healthy worker effect.
References
- Andrews CP, Smith JD, Johanson WG. 1979. Pulmonary effects of methyl methacrylate vapour exposure in dental students. Clin Res. 27:759A.
- [ANSES] Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail. 2017. Assessment of risks for professionals exposed to products used in nail care and decoration activities. ANSES Opinion/Collective Expertise Report on Request No. “2014-SA-0148.” [accessed 2017 October]. https://www.anses.fr/en/system/files/CONSO2014SA0148EN.pdf.
- Arts J. 2020. How to assess respiratory sensitization of low molecular weight chemicals. Int J Hyg Environ Health. 225(2020):113469.
- Arts J, Kimber I. 2017. Azodicarbonamide (ADCA): a reconsideration of classification as a respiratory sensitiser. Regul Toxicol Pharmacol. 89:268–278.
- Arts J, Kimber I. 2018. Azodicarbonamide (ADCA): a reconsideration of classification as a respiratory sensitiser. Regul Toxicol Pharmacol. 94:332–333.
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. 2011. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 64(4):401–406.
- Basker RM, Hunter AM, Highet AS. 1990. A severe asthmatic reaction to poly(methyl methacrylate) denture base resin. Br Dent J. 169(8):250–251.
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov L, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, et al. 2015. Increasing scientific confidence in adverse outcome pathways: application of tailored Bradford-Hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol. 72(3):514–537.
- Betts CJ, Dearman RJ, Heylings JR, Kimber I, Basketter DA. 2006. Skin sensitization potency of methyl methacrylate in the local lymph node assay: comparisons with guinea-pig data and human experience. Contact Dermatitis. 55(3):140–147.
- [BG ETEM] BG Energie Textil Elektro Medienerzeugnisse. 2021. Exposure data for dental technicians in Germany. Personal communication to CEFIC methacrylate sector group for submission to the CLH review on MMA. Köln, Germany: Berufsgenossenschaft Energie Textil Elektro Medienerzeugnisse (BG ETEM).
- Bisesi MS. 2001. Esters of mono- and alkenyl carboxylic acids and mono-and polyalcohols. In: Bingham E, Cohrssen B, Powell CH, editors. Patty’s toxicology. Chapter 79.
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. 2008. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38(2):87–96.
- Borak J, Fields C, Andrews LS, Pemberton MA. 2011. Methyl methacrylate and respiratory sensitization: a critical review. Crit Rev Toxicol. 41(3):230–268.
- Boverhof DR, Billington R, Gollapudi JA, Hotchkiss JA, Krieger SM, Poole A, Wiescinski CM, Woolhiser MR. 2008. Respiratory sensitization and allergy: current research approaches and needs. Toxicol Appl Pharmacol. 226(1):1–13.
- Brisman J, Andersson E, Meding B, Torén K. 2011. Increased risk of nasal symptoms in Swedish dental technicians during exposure to methyl methacrylate, rapid glue or grinding. Occup Environ Med. 2011(Suppl 1):68–A127.
- Cochrane SA, Arts JHE, Ehnes C, Hindle S, Hollnagel HM, Poole A, Suto H, Kimber I. 2015. Thresholds in chemical respiratory sensitisation. Toxicology. 333:179–194.
- Darre E, Jørgensen LG, Vedel P, Jensen JS. 1992. Breathing zone concentrations of methylmethacrylate monomer during joint replacement operations. Pharmacol Toxicol. 71(3 Pt 1):198–200.
- Della Torre L, Valsecchi F, Sala C, Cesana MA. 1982. Evaluation of occupational risk and biological effects in a factory of polymethylmethacrylate sheets. Lavoro Umano. 30:72–79.
- de Olim C, Begin D, Boulet LP, Cartier A, Gerin M, Lemiere C. 2015. Investigation of occupational asthma: do clinicians fail to identify relevant occupational exposures? Can Respir J. 22(6):341–3477.
- Dik S, Ezendam J, Cunningham AR, Carrasquer CA, van Loveren H, Rorije E. 2014. Evaluation of in silico models for the identification of respiratory sensitizers. Toxicol Sci. 142(2):385–394.
- [EC] European Chemicals Bureau. 2002. European Union - risk assessment report on methyl methacrylate. Eur Union Risk Assess Rep. 22:1–194
- [EC] European Chemicals Bureau. 2008. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. [accessed 2021 October 17]. https://echa.europa.eu/regulations/clp/legislation.
- [EC] European Chemicals Bureau. 2016. Mutual evaluation of regulated professions. Overview of the regulatory framework in the health services sector – dental hygienists and related professions. GROW/E5. [accessed 2016 April 29]. https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjfn6TW2eP0AhUk8LsIHeB9AJAQFnoECAMQAQ&url=https%3A%2F%2Fec.europa.eu%2Fdocsroom%2Fdocuments%2F17942%2Fattachments%2F1%2Ftranslations%2Fen%2Frenditions%2Fpdf&usg=AOvVaw2z-XClKQu46J5Jt0RsSsEt.
- [EC] European Chemicals Bureau. 2019. Proposal for harmonised classification and labelling based on regulation (EC) No 1272/2008 (CLP regulation), Annex VI, Part 2. International chemical identification: methyl methacrylate. Dossier submitter: ANSES (on behalf of the French MSCA), 14 rue Pierre Marie Curie, F-94701 Maisons-Alfort Cedex. [accessed 2019 February]. https://www.echa.europa.eu/documents/10162/032dc3bb-55fe-7f7f-5762-70722fa80c82.
- [EC] European Chemicals Bureau. 2021. Committee for risk assessment (RAC). Opinion proposing harmonised classification and labelling at EU level of methyl methacrylate, methyl 2-methylprop-2-enoate, methyl 2-methylpropenoate. CLH-O-0000006852-69-01/F. [accessed 2021 March 18]. https://echa.europa.eu/documents/10162/6e62f500-ac37-2875-ecf1-7776d22a30b3.
- [ECHA] European Chemicals Agency. 2011. Guidance on information requirements and chemical safety assessment Chapter R.4: evaluation of available information, ECHA-2011-G-13-EN. https://echa.europa.eu/documents/10162/13643/information_requirements_r4_en.p. df/d6395ad2-1596-4708-ba86-0136686d205e.
- [ECHA] European Chemicals Agency. 2016. Practical guide: how to use alternatives to animal testing to fulfil the information requirements for REACH registration, chapter 4.1 weight of evidence, ECHA-16-B-25-EN. https://echa.europa.eu/documents/10162/1365. 5/practical_guide_how_to_use_alternatives_en.pdf/148b30c7-c186-463c-a898-522a888a4404.
- [ECHA] European Chemicals Agency. 2017a. Guidance on information requirements and chemical safety assessment. Chapter R.7a: endpoint specific guidance version 6.0. July 2017.
- [ECHA] European Chemicals Agency. 2017b. Weight of evidence/uncertainty in hazard assessment, background document & example. https://echa.europa.eu/documents/10162/17169198/wo_eu_uncertainty_background_en.docx/4f2. b49ab-ade0-6ee3-e977-8abe00c21c23.
- [ECHA] European Chemicals Agency. 2017c. Template for weight of evidence/uncertainty in hazard assessment. https://echa.europa.eu/documents/10162/17169198/template_for_weight_of_evidence_en.docx/eb183c2e-c360-cbce-7a58-ad2d1270e5bd.
- [ECHA] European Chemicals Agency. 2021a. C&L inventory. Summary of classification and labelling: gentamicin. https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/53997.
- [ECHA] European Chemicals Agency. 2021b. C&L inventory. Summary of classification and labelling: gentamicin sulfate (salt). https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/82702.
- [ECHA] European Chemicals Agency. 2021c. C&L inventory. Summary of classification and labelling: methyl methacrylate. https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/104369.
- [ECHA] European Chemicals Agency. 2021d. Registered substances database. Methyl methacrylate. https://echa.europa.eu/registration-dossier/-/registered-dossier/15528.
- [EFSA] European Food Safety Authority. 2017. Guidance on the use of the weight of evidence approach in scientific assessments. [accessed 2017 August 3]. https://doi.org/10.2903/j.efsa.20174971/full.
- Enoch SJ, Seed MJ, Roberts DW, Cronin MT, Stocks SJ, Agius RM. 2012. Development of mechanism-based structural alerts for respiratory sensitization hazard identification. Chem Res Toxicol. 25(11):2490–2498.
- [ERS] European Respiratory Society. 2014. Task force report on occupational asthma. Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement. Eur Resp J. 43:1573–1587.
- Feary J, Pinnock H, Cullinan P. 2016. Occupational asthma. BMJ. 353:i2658.
- Froines JR, Garabrant DH. 1986. Quantitative evaluation of manicurists exposure to methyl, ethyl and isobutyl methacrylate during production of synthetic fingernails. Appl Ind Hyg. 1(2):70–74.
- [FEPPD] Fédération Européenne des Patrons Prothésistes Dentaires. 2021. FEPPD in a nutshell. https://www.feppd.eu/organisation.
- Gerberick GF, Ryan CA, Kern PS, Schlatter H, Dearman RJ, Kimber I, Patlewicz GY, Basketter DA. 2005. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis. 16(4):157–202.
- Golbabaei F, Mamdouh M, Jelyani KN, Shahtaheri SJ. 2005. Exposure to methyl methacrylate and its subjective symptoms among dental technicians, Tehran, Iran. Int J Occup Saf Ergon. 11(3):283–289.
- Guyatt GH, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, Debeer H, et al. 2011. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 64(4):383–394.
- Hagberg S, Ljungkvist G, Andreasson H, Karlsson S, Barregård L. 2005. Exposure to volatile methacrylates in dental personnel. J Occup Environ Hyg. 2(6):302–306.
- Henriks-Eckerman ML, Alanko K, Jolanki R, Kerosuo H, Kanerva L. 2001. Exposure to airborne methacrylates and natural rubber latex allergens in dental clinics. J Environ Monit. 3(3):302–305.
- Hiipakka D, Samimi B. 1987. Exposure of acrylic fingernail sculptors to organic vapors and methacrylate dusts. Am Ind Hyg Assoc J. 48(3):230–237.
- Hill AB. 1965. The environment and disease: an association of causation. Proc R Soc Med. 58:295–300.
- Holsapple MP, Jones D, Kawabata TT, Kimber I, Sarlo K, Selgrade MK, Shah J, Woolhiser MR. 2006. Assessing the potential to induce respiratory hypersensitivity. Toxicol Sci. 91(1):4–13.
- [ISO] International Organization for Standardization. 2011. ISO 16000-6: indoor air — part 6: determination of volatile organic compounds in indoor and test chamber air by active sampling on Tenax TA sorbent, thermal desorption and gas chromatography using MS or MS-FID. https://www.iso.org/standard/52213.htm.
- Isola D, Kimber I, Sarlo K, Lalko J, Sipes IG. 2008. Chemical respiratory allergy and occupational asthma: what are the key areas of uncertainty? J Appl Toxicol. 28(3):249–253.
- Jaakkola MS, Leino T, Tammilehto L, Ylostalo P, Kuosma E, Alanko K. 2007. Respiratory effects of exposure to acrylates among dental assistants. Eur Respir J. 30(Suppl. 51):999.
- Jarvis J, Seed MJ, Elton R, Sawyer L, Agius RM. 2005. Relationship between chemical structure and the occupational asthma hazard of low molecular weight organic compounds. Occup Environ Med. 62(4):243–250.
- Jedrychowski WA, Fonte R. 1984. Sintomatologia cronica respiratoria e 38 sindrome ostruttiva in lavoratori di una industria chimica [Chronic respiratory symptomatology and obstructive syndrome in workers of a chemical industry]. G Ital Med Lav. 6(5-6):225–233 (Italian).
- Kennes B, Garcia-Herreros P, Dierckx P. 1981. Asthma from plexiglas powders. Clin Allergy. 11(1):49–54.
- Kenyon NJ, Morrissey BM, Schivo M, Albertson TE. 2012. Occupational asthma. Clin Rev Allergy Immunol. 43(1-2):3–13.
- Kimber I, Agius R, Basketter DA, Corsini E, Cullinan P, Dearman RJ, Gimenez-Arnau E, Greenwell L, Hartung T, Kuper F, et al. 2007. Chemical respiratory allergy: opportunities for hazard identification and characterisation. The report and recommendations of ECVAM workshop 60. Altern Lab Anim. 35(2):243–265.
- Kimber I, Basketter DA, Roggeband R. 2001. Chemical respiratory allergy: classification and labelling. Toxicology. 167(2):159–162.
- Kimber I, Dearman RJ, Basketter DA. 2014a. Diisocyanates, occupational asthma and IgE antibody: implications for hazard characterization. J Appl Toxicol. 34(10):1073–1077.
- Kimber I, Dearman RJ, Basketter DA, Boverhof DR. 2014b. Chemical respiratory allergy: reverse engineering an adverse outcome pathway. Toxicology. 318:32–39.
- Kimber I, Pemberton MA. 2014. Assessment of the skin sensitising potency of the lower alkyl methacrylate esters. Regul Toxicol Pharmacol. 70(1):24–36.
- Kimber I, Poole A, Basketter DA. 2018. Skin and respiratory chemical allergy: confluence and divergence in a hybrid adverse outcome pathway. Toxicol Res (Camb). 7(4):586–605.
- Kirby BS, Doyle A, Gilula L. 2003. Acute bronchospasm due to exposure to polymethylmethacrylate vapors during percutaneous vertebroplasty. AJR Am J Roentgenol. 180(2):543–544.
- Klimisch HJ, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol. 25(1):1–5.
- Lavelle KS, Schnatter AS, Travis KZ, Swaen GMH, Pallapies D, Money C, Priem P, Vrijhof H. 2012. Framework for integrating human and animal data in chemical risk assessment. Regul Toxicol Pharmacol. 62(2):302–312.
- Lindberg E, Iregren A, Malmberg P, Vesterberg O, Wennberg A. 1991. Health risks of exposure to methyl methacrylate (MMA) – a pilot study. Bethesda, MD: National Institute of Occupational Health.
- Lozewicz S, Davison AG, Hopkirk A, Burge PS, Boldy DA, Riordan JF, McGivern DV, Platts BW, Davies D, Newman Taylor AJ. 1985. Occupational asthma due to methyl methacrylate and cyanoacrylates. Thorax. 40(11):836–839.
- Madanat R, Hussey D, Donahue GS, Potter HG, Wallace R, Bragdon C, Muratoglu O, Malchau H. 2016. Early lessons from a worldwide, multicenter, followup study of the recalled articular surface replacement hip system. Clin Orthop Relat Res. 474(1):166–174.
- Maestrelli P, Henneberger PK, Tarlo S, Mason P, Boschetto P. 2020. Causes and phenotypes of work-related asthma. IJERPH. 17(13):4713.
- Malo JL, Tarlo SM, Sastre J, Martin J, Jeebhay MF, Le Moual N, Heedrick D, Platts-Mills T, Planc PD, Vandenplas O, et al. 2015. An official American thoracic society workshop report; presentations and discussion of the fifth Jack Pepys workshop on asthma in the workplace. Comparisons between asthma in the workplace and non-work-related asthma. Ann Am Thorac Soc. 12:S9–S110.
- Marez T, Edme JL, Boulenguez C, Shirali P, Haguenoer JM. 1993. Bronchial symptoms and respiratory function in workers exposed to methyl methacrylate. Brit J Ind Med. 50:894–898.
- Marez T, Shirali P, Hildebrand HF, Haguenoer JM. 1991. Increased frequency of sister chromatid exchange in workers exposed to high doses of methylmethacrylate. Mutagenesis. 6(2):127–129.
- Marquardt W, Seiss M, Hickel R, Reichl FX. 2009. Volatile methacrylates in dental practices. J Adhes Dent. 11(2):101–107. PMID: 19492711.
- Martin P, Bladier C, Meek B, Bruyere O, Feinblatt E, Touvier M, Watier L, Makowski D. 2018. Weight of evidence for hazard identification: a critical review of the literature. Environ Health Perspect. 126(7):076001.
- Meek ME, Palermo CM, Bachman AN, North CM, Jeffrey Lewis R. 2014. Mode of action human relevance (species concordance) framework: evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 34(6):595–606.
- Mizunuma K, Kawai T, Yasugi T, Horiguchi S, Takeda S, Miyashita K, Taniuchi T, Moon CS, Ikeda M. 1993. Biological monitoring and possible health effects in workers occupationally exposed to methyl methacrylate. Int Arch Occup Environ Health. 65(4):227–232.
- Money CD, Tomenson JA, Penman MG, Boogaard PJ, Jeffrey Lewis RJ. 2013. A systematic approach for evaluating and scoring human data. Regul Toxicol Pharmacol. 66(2):241–247.
- Monroe C. 1981. Report of a respiratory health survey of the Knoxville facility of the Rohm and Haas Company. Philadelphia (PA): Rohm and Haas. Corporate Health and Safety, Rohm and Haas Chemicals Company in Bristol, Pennsylvania 19007, USA.
- Moshe S, Krakov A. 2019. Case report: three occupational diseases in a nail technician. Occup Environ Med. 76(Suppl 1):A92.1–A92.
- Moulin P, Magnan A, Lehucher-Michel M-P. 2009. Occupational allergic contact dermatitis and asthma due to a single low molecular weight agent. J Occup Health. 51(1):91–96.
- Nayebzadeh A, Dufresne A. 1999. Evaluation of exposure to methyl methacrylate among dental laboratory technicians. Am Ind Hyg Assoc J. 60(5):625–628.
- Neut D, Kluin OS, Thompson J, van der Mei HC, Busscher HJ. 2010. Gentamicin release from commercially-available gentamicin-loaded PMMA bone cements in a prosthesis-related interfacial gap model and their antibacterial efficacy. BMC Musculoskelet Disord. 11:258.
- Nishiwaki Y, Saitoh T, Taklbayashi T, Tanaka S, Etoh N, Eitaki Y, Omaf K. 2001. Cross-sectional study of health effects of methyl methacrylate monomer among dental laboratory technicians. J Occup Health. 43(6):375–378.
- North CM, Ezendam J, Hotchkiss JA, Maier C, Aoyama K, Enoch S, Goetz A, Graham C, Kimber I, Karjalainen A, et al. 2016. Developing a framework for assessing chemical respiratory sensitization: a workshop report. Regul Toxicol Pharmacol. 80:295–309.
- [NTP] National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health. 1992. National Toxicology Program chemical repository database. Research Triangle Park (NC): National Institutes of Health.
- [NTP] National Toxicology Program. 2015. New OHAT handbook for conducting systematic reviews. https://ntp.niehs.nih.gov/update/2015/1/ohat-handbook/index.html.
- [NTP] National Toxicology Program. 2019. Updates and clarification to the OHAT approach for systematic review and evidence integration. Research Triangle Park (NC): National Institutes of Health. https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookclarificationmarch2019_508.pdf.
- Obando SU, Fernández-Nieto M, Sastre J. 2013. Occupational asthma due to polyvinyl chloride and methy lmethacrylate, “hidden in an adhesive. Clin Transl Allergy. 3:P32.
- OECD. 2011. Health at a glance 2011: OECD indicators. Paris, France: OECD Publishing.
- Parhar A, Lemiere C, Beach JR. 2011. Barriers to the recognition and reporting of occupational asthma by Canadian pulmonologists. Can Respir J. 18(2):90–96.
- Pausch FE, Jacobi S, Clajus P, Lehr H. 1994. Medical examination of workers in acrylic sheet production exposed to methyl methacrylate. Report to Roehm GmbH. Darmstadt, Germany: Röhm Gmbh, Kirschenallee.
- Pemberton MA, Kimber I. 2021. Classification of chemicals as respiratory allergens based on human data: requirements and practical considerations. Regul Toxicol Pharmacol. 123:104925.
- Pickering CA, Bainbridge D, Birtwistle IH, Griffiths DL. 1986. Occupational asthma due to methyl methacrylate in an orthopaedic theatre sister. Br Med J (Clin Res Ed). 292(6532):1362–1363.
- Pickering CAC, Niven R, Simpson J. 1993. A study of the prevalence of occupational asthma at the ici acrylics site at Darwen, Lancashire. Manchester, UK: North West Lung Centre.
- Piirilä P, Kanerva L, Keskinen H, Estlander T, Hytonen M, Tuppurainen M, Nordman H. 1998. Occupational respiratory hypersensitivity caused by preparations containing acrylates in dental personnel. Clin Exp Allergy. 28(11):1404–1411.
- Reynaud-Gaubert M, Philip-Joet F, Arnaud A. 1991. Professional asthma due to methylmethacrylate ([French]). Presse Med. 20(8):386–386.
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 122(7):711–718.
- Roth E, Kristiansen CB, Assing KD, Weinreich UM. 2017. Work-related asthma in an orthopaedic surgeon. Ugeskr Laeger. 179(30):1682–1684.
- Rumpf KW, Rieger J, Jansen J, Scherer M, Seubert S, Seubert A, Sellin HJ. 1986. Quincke's edema in a dialysis patient after administration of acrylic bone cement: possible role of ethylene oxide allergy. Arch Orthop Trauma Surg. 105(4):250–252.
- Sass-Kortsak AM, Purdham JT, Bozek PR, Murphy JH. 1992. Exposure of hospital operating room personnel to potentially harmful environmental agents. Am Ind Hyg Assoc J. 53(3):203–209.
- Sauni R, Kauppi P, Alanko K, Henriks-Eckerman ML, Tuppurainen M, Hannu T. 2008. Occupational asthma caused by sculptured nails containing methacrylates. Am J Ind Med. 51(12):968–974.
- Savonius B, Keskinen H, Tuppurainen M, Kanerva L. 1993a. Occupational respiratory disease caused by acrylates. Clin Exp Allergy. 23(5):416–424.
- Savonius B, Keskinen H, Tuppurainen M, Kanerva L. 1993b. Erratum: occupational respiratory disease caused by acrylates. Clin Exp Allergy. 23:712.
- [SCENIHR] Scientific Committee on Emerging and Newly-Identified Health Risks. 2012. Memorandum on the use of the scientific literature for human health risk assessment purposes, weighing of evidence and expression of uncertainty. http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_s_001.pdf.
- [SCHEER] Scientific Committee on Health, Environmental and Emerging Risks. 2018. Memorandum on weight of evidence and uncertainties, revision 2018. https://ec.europa.eu/health/sites/default/files/scientific_committees/scheer/docs/scheer_o_014.pdf.
- Scherpereel A, Tillie‐Leblond I, Pommier de Santi P, Tonnel AB. 2004. Exposure to methyl methacrylate and hypersensitivity pneumonitis in dental technicians. Allergy. 59(8):890–892.
- [SETAC] Society of Environmental Toxicology and Chemistry. 2018. Weight-of-evidence in environmental risk assessment of chemicals, SETAC technical issue paper. https://cdn.ymaws.com/www.setac.org/resource/resmgr/publications_and_resources/setac_tip_weight_of_evidence.pdf.
- Shofer S, Haus BM, Kuschner WG. 2006. Quality of occupational history assessments in working age adults with newly diagnosed asthma. Chest. 130(2):455–462.
- Spencer AB, Estill CF, McCammon JB, Mickelsen RL, Johnston OE. 1997. Control of ethyl methacrylate exposures during the application of artificial fingernails. Am Ind Hyg Assoc J. 58(3):214–218.
- Stauffer E, Dolan JA, Newman R. 2008. Flammable and combustible liquids. In: Fire debris analysis. Burlington: Academic Press; p. 199–233.
- Suojalehto H, Suuronen K, Cullinan P, Lindström I, Sastre J, Walusiak-Skorupa J, Munoz X, Talini D, Klusackova P, Moore V, et al. 2019a. Phenotyping occupational asthma caused by acrylates in a multicenter cohort study. J Allergy Clin Immunol Pract. 8(3):971–979.e1. Erratum in: J Allergy Clin Immunol Pract. 2021;9(8):3234.
- Suojalehto H, Suuronen K, Cullinan P. 2019b. Specific challenge testing for occupational asthma: revised handbook. Eur Respir J. 54(2):1901026.
- Sullivan KM, Enoch SJ, Ezendam J, Sewald K, Roggen EL, Cochrane S. 2017. An adverse outcome pathway for sensitization of the respiratory tract by low-molecular weight chemicals: building evidence to support the utility of in vitro and in silico methods. Appl In Vitro Toxicol. 3(3):213–226.
- Tarlo SM, Lemiere C. 2014. Occupational asthma. N Engl J Med. 370(7):640–649.
- Thorette C, Grigoriu B, Canut E, Sobaszek A, Tonnel AB, Tillie-Leblond I. 2006. Pulmonary disease in dental laboratory technicians. Rev Mal Respir. 23(Suppl 2):4S8–4S16.
- Tiotiu AI, Novakova S, Labor M, Emelyanov A, Mihaicuta S, Novakova P, Nedeva D. 2020. Progress in occupational asthma. IJERPH. 17(12):4553.
- Torbica N, Krstev S. 2006. World at work: dental laboratory technicians. Occup Environ Med. 2006 Feb63(2):145–148.
- UK HSE. 2001. Asthmagen? Critical assessments of the evidence for agents implicated in occupational asthma. UK Health and Safety Executive, Redgrave Court, Merton Road, Bootle, Merseyside, L20 7HS, UK. https://www.hse.gov.uk/asthma/asthmagen.pdf.
- Ungers LJ, Vendrely TG. 2006. Comparison of sampling and analytical methods used during the preparation of methyl methacrylate bone cements. J Occup Environ Hyg. 3(7):351–357.
- Ungers LJ, Vendrely TG, Barnes CL. 2007. Control of methyl methacrylate during the preparation of orthopedic bone cements. J Occup Environ Hyg. 4(4):272–280.
- Uriarte SA, Fernández-Nieto M, Sastre J. 2013. Occupational asthma due to polyvinyl chloride and methyl methacrylate in a plumber. Investig Allergol Clin Immunol. 23(6):437–438.
- Vallieres M, Cockcroft DW, Taylor DM, Dolovich J, Hargreave FE. 1977. Dimethyl ethanolamine-induced asthma. Am Rev Respir Dis. 115(5):867–871. 1977
- Vandenplas O. 2011. Occupational asthma: etiologies and risk factors. Allergy Asthma Immunol Res. 3(3):157–167.
- Vandenplas O, Suojalehto H, Aasen TB, Baur X, Burge PS, de Blay F, Fishwick D, Hoyle J, Maestrelli P, Muñoz X, et al. 2014. Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement. Eur Respir J. 43(6):1573–1587.
- Vandenplas O, Suojalehto H, Cullinan P. 2017. Diagnosing occupational asthma. Clin Exp Allergy. 47(1):6–18.
- Walters GI, Robertson AS, Moore VC, Burge PS. 2017. Occupational asthma caused by acrylic compounds from SHIELD surveillance (1989-2014). Occup Med (Lond). 67(4):282–289.
- Wittczak T, Palczynski C, Szulc B, Gorski P. 1996. Bronchial asthma with inflammation of the nose mucous membrane induced by occupational exposure to methyl methacrylate in a dental technician. Med Pr. 47:259–266.
- Wittczak T, Walusiak J, Ruta U, Pałczynski C. 2003. Occupational asthma and allergic rhinitis due to xerographic toner. Allergy. 58(9):957.
- [WHO/IPCS] World Health Organisation/International Programme on Chemical Safety. 1998. World Health Organisation international programme on chemical safety. Concise international chemical assessment document no. 4. Methyl methacrylate. https://apps.who.int/iris/bitstream/handle/10665/42030/9241530049.pdf.
- [WHO/IPCS] World Health Organisation/International Programme on Chemical Safety. 2005. Chemical specific adjustment factors for interspecies differences and human variability: guidance document for use of data in dose/concentration-response assessment. https://apps.who.int/iris/handle/10665/43294.
- [WHO/IPCS] World Health Organisation/International Programme on Chemical Safety. 2010. Guidance on principles of characterising and applying PBPK models in risk assessment https://www.who.int/ipcs/methods/harmonization/areas/pbpk_models.pdf?ua=1.
- [WHO/IPCS] World Health Organisation/International Programme on Chemical Safety. 2014. Guidance on evaluating and expressing uncertainty in hazard assessment is available in harmonization project document no. 11 https://www.who.int/ipcs/methods/harmonization/uncertainty_in_hazard_characterization.pdf.