Abstract
The Ramazzini Institute (RI) has been conducting animal carcinogenicity studies for decades, many of which have been considered by authoritative bodies to determine potential carcinogenicity in humans. Unlike other laboratories, such as the U.S. National Toxicology Program (NTP), the RI does not provide a report or record of historical control data. Transparently documenting historical control data is critical in the interpretation of individual study results within the same laboratory. Historical control data allow an assessment of significant trends, either increasing or decreasing, resulting from changes in laboratory methods or genetic drift. In this investigation: (1) we compiled a dataset of the tumors reported in control groups of Sprague-Dawley rats and Swiss mice based on data included in published RI studies on specific substances, and (2) conducted case studies to compare data from this RI control dataset to the findings from multiple RI studies on sweeteners and corresponding breakdown products. We found considerable variability in the tumor incidence across multiple tumor types when comparing across control groups from RI studies. When compared to the tumor incidence in treated groups from multiple studies, the incidence of some tumors considered to be treatment-related fell within the variability of background incidence from the RI control dataset.
Introduction
Since before its formal founding in 1987, the Cancer Research Center of the Ramazzini Institute (RI) has been performing and publishing the results of their carcinogenicity studies in rats or mice (Soffritti, Belpoggi, Minardi, et al. Citation2002). The RI has conducted studies investigating the carcinogenicity of vinyl chloride, benzene, formaldehyde, oxygenated gasoline additives, World Trade Center dust, extremely low frequency electromagnetic fields (ELFEMF), along with many other potentially carcinogenic agents (https://www.istitutoramazzini.it/about/). In the distant past, some of these studies had been considered by authoritative bodies to determine, both qualitatively and quantitatively, the potential for carcinogenicity in humans of the substances tested. In recent decades, however, studies conducted by the RI have been called into question by numerous regulatory and authoritative bodies, including the United States Food and Drug Administration (USFDA), the United States Environmental Protection Agency (USEPA), the European Food Safety Authority (EFSA), and the United States National Institutes of Health’s (NIH) National Toxicology Program (NTP), among others (Gift et al. Citation2013).
According to the RI, their carcinogenicity bioassay project is second only to that of the NTP and includes studies on a greater number of agents than in any other single laboratory (Soffritti, Belpoggi, Minardi, et al. Citation2002). Most of the agents studied by the RI were selected based on their presence in the environment and the number of people potentially exposed. The carcinogenicity bioassays performed by the RI are conducted following a basic design protocol with exposure beginning either during embryonal life (12th day of pregnancy), perinatal, or at 6–9 weeks of age and continuing for either 104 weeks, as in the case of the studies conducted for formaldehyde (Soffritti, Belpoggi, Lambertin, et al. Citation2002) and methyl alcohol (Soffritti, Belpoggi, Cevolani, et al. Citation2002), or to end-of-life as in the case of studies conducted with aspartame (Belpoggi et al. Citation2006; Soffritti, Belpoggi, Esposti, Lambertini, Tibaldi, et al. Citation2006; Soffritti et al. Citation2007), with animals allowed to live until spontaneous death to account for tumors that may occur late in life. The routes of exposure for these studies are reportedly chosen to mimic the routes of human exposure including inhalation, intraperitoneal or intrapleural injection, ingestion (dietary or gavage), intratracheal instillation, and external exposure (radiation).
For nearly 40 years, the animals typically used in the RI studies were Sprague-Dawley (SD) rats from the Cesare Maltoni Cancer Research Center (CMCRC) of the European Ramazzini Foundation (ERF) colony (Soffritti, Belpoggi, Minardi, et al. Citation2002). RI explains that the basic tumor incidences of this SD strain are well known, and the cancer susceptibility is not different from humans. According to the RI, their experimental research has been performed in highly standardized control conditions by largely the same team over decades.
In spite of claims by RI of highly standardized control conditions, several of the RI lifetime bioassays have been heavily criticized by authoritative bodies due to study design (and protocol differences) and study conduct and analyses from other laboratories (EFSA Citation2006; Gift et al. Citation2013). A key criticism is that no information is provided for either the existence of a health monitoring surveillance program or the health status of RI’s source animals used over several decades (i.e. internal CMCRC/ERF rat colony) (Gift et al. Citation2013; Elmore et al. Citation2023a, Citation2023b). Perceived health problems in the RI rat colony could have caused inflammatory changes in the lung, prompting discussions regarding the role of respiratory infections in the formation of selected tumors reported by RI (e.g. lymphoma/leukemia) (Gift et al. Citation2013). It has been suggested that the observed lesions are confounded by possible pervasive infection and may be either neoplastic or inflammatory in origin; if neoplastic, the lesions may be the consequence of infection and not necessarily be treatment related.
In a review by USEPA scientists (Gift et al. Citation2013), it was noted that the fraction of RI control groups with a lymphoma/leukemia rate of more than 10% has increased from 7% (three out of 43 studies) of RI studies conducted between 1988 and 1989 to upwards of 82% (18 out of 22 studies) of studies conducted from 2002 to 2006. The USEPA scientists indicated this noted increase may have been due to a “genetic drift associated with inbreeding of the colony and a more active immune system in the non-pathogen-free RI rats” (Gift et al. Citation2013). This explanation underscores a fundamental issue with the RI laboratories, which is further compounded by rampant infections confirmed by the detection of antibodies and noted by Dr. Belpoggi in deposition (Rodricks and Turnbull Citation2010). Mycoplasma pulmonis infection persists despite immune and antibody responses (Schoeb et al. Citation1996, Citation2009).
In 2010, NTP pathologists and technicians conducted their preliminary review of selected RI pathology tissue sections (Malarkey et al. Citation2010), showing differences between NTP and Ramazzini scientists’ diagnoses of cancer types (e.g. concurrent lung infection vs. lymphomas/leukemias in methanol (Soffritti, Belpoggi, Cevolani, et al. Citation2002) and methyl-tertiary-butyl ether (MTBE) (Belpoggi et al. Citation1995) studies, inflammatory infiltrates vs. ear/cranium neoplasms in methanol study (Soffritti, Belpoggi, Cevolani, et al. Citation2002)). Separately in 2011, USEPA and the United States National Institute of Environmental Health Sciences (NIEHS) jointly sponsored an independent group of scientists, the Pathology Working Group (PWG), to review RI-supplied tissue sections (a partial sampling was supplied) from a larger group of animal studies. The PWG concluded that the presence of respiratory infections rendered some RI diagnoses invalid (e.g. leukemias and lymphomas) (NTP Citation2011; Gift et al. Citation2013). Additionally, in response to a much earlier request in 2004, a pathology review demonstrated that RI’s diagnosis of mammary gland adenocarcinomas in one of the aspartame studies (Soffritti et al. Citation2007; Chiozzotto et al. Citation2011) were considered by select NTP reviewers to instead be fibroadenomas (NIEHS Citation2004).
In 2009, the RI was to open a European Experimental Laboratory (EEL) following the Organization for Economic Co-operation and Development (OECD) guidelines to maintain Good Laboratory Practice (GLP) certification from the Italian Minister of Health, the Italian GLP compliance monitoring authority (Gift et al. Citation2013). The outcome from the RI’s establishment of the EEL is not clear, as publications that could showcase the results from the RI animal studies conducted in the EEL after 2009 have not been identified.
In view of outstanding questions surrounding RI studies, historical control data become even more critical in providing context for observed lesions. NTP and other laboratories provide historical control data to understand background incidence of both cancerous and noncancerous lesions, “drift” or an increase in certain lesions over time, and how these changes over time might impact the interpretation of findings from these studies conducted by said laboratory. NTP compares study findings to their historical control data to assess whether noted observations fall within the range of normal background variability. Except for a select group of RI historical controls from 1984 to 2001 reported in Bua et al. (Citation2018) and historical control data for a select group of tumor types reported in Maltoni et al. (Citation1983), the majority of historical control incidence from RI is not publicly available, contrary to what others have suggested (Soffritti, Belpoggi, Esposti, Lambertini, Tibaldi, et al. Citation2006; Bua et al. Citation2018; Falcioni et al. Citation2018).
The purpose of this evaluation was to attempt to create an RI historical control dataset to provide the means to compare observations in any RI study against RI historical control data. The goal of this evaluation was to better understand the normal background variability in tumor incidence within SD rats and Swiss mice across control groups across RI studies and assess how findings in the RI treated groups for multiple compounds (aspartame, methyl alcohol, formaldehyde, and sucralose) compare. This evaluation is based solely on the experimental methods and tumor incidence data reported in published articles from the RI and does not take into account any deficiencies in experimental methodology or diagnostic processes or criteria of the RI that have been identified in other publications (Gift et al. Citation2013; Elmore et al. Citation2023a, Citation2023b), such as infection, lack of pathology expert peer review, analysis of total tumors in some instances, and overt autolysis. Some of the latter considerations are noted in the discussion of the current evaluation. The compounds that are the focus of these comparative analyses were selected as they not only represent the potential for dietary exposure but also have been conducted by the oral route in multiple species. In addition, some recent RI mouse studies were conducted with specific pathogen-free (SPF) Swiss mice acquired from other laboratories. There was no indication that microbiological monitoring was performed during the course of these experiments conducted at the RI using acquired SPF mice. As part of the current evaluation, additional comparisons were conducted to determine if background incidence between the early RI mouse colony and background incidence in acquired SPF mice is different. Implications of any identified differences are discussed.
Materials and methods
Identification of relevant studies for historical control dataset
As there are no comprehensive lists of the studies conducted by the RI, literature searches were conducted using PubMed and Google Scholar to identify all 2-year RI bioassays or RI lifespan carcinogenicity studies from which control data could be extracted, including the studies conducted for the case study chemicals. Search terms used for the literature searches included the last names of Ramazzini authors, taken from the RI webpage (https://www.istitutoramazzini.it/about/) or from other identified studies published by RI authors, combined with terms including “Ramazzini”, “carcinogenicity”, “lifetime” or “lifespan”, and “rat” or “mouse”.
Data extraction and organization of the RI control dataset
NTP has developed a dataset reporting historical pathology results in control groups across cancer bioassays in different test species and strains (NTP Citation2023). The current evaluation to develop a dataset of control tumor incidence data for RI followed NTP practices for data extraction and organization, reporting data by study type and other relevant factors that include species, sex, route of administration, vehicle, study type, and laboratory. Tumor incidence is typically defined as the number of animals exhibiting a tumor type divided by the number of animals examined and is expressed as both raw counts and percentage. NTP historical control pathology tables also present the mean and standard deviation of tumor incidence and preneoplastic lesions, as well as the range of tumor incidence and preneoplastic lesions across studies, along with the number of studies summarized.
Study characteristics for RI studies in which control data were reported were tabulated according to study citation, substance tested, year of publication, experiment number, species/strain and gender, study duration, route of exposure and vehicle, number of animals per control group, tumor or preneoplastic lesion type, number of tumors or preneoplastic lesions in the control group,Footnote1 and percent tumor or preneoplastic lesion incidence within a study-specific control group. The compilation of these study characteristics from the control animals of the available RI studies is referred to hereafter as the RI control dataset. Control data from studies conducted in rats and mice from 1987 to the present were chosen for comparisons as it brackets the time frame of when the selected case studies (case studies for comparison include aspartame, sucralose, formaldehyde, and methyl alcohol) were conducted and therefore should be representative of the potential background tumor incidence in control animals during the time these studies were conducted. Keeping the timeframe similar to the studies selected for a case study comparison decreases the chance of variability observed among control groups due to genetic drift within the SD rat or Swiss mouse colony of the RI. Also, it is likely that the methods used for pathological evaluation and the nomenclature used to identify tumors would not have changed significantly within this limited timeframe as compared to studies conducted decades before.
For the RI studies selected for the case study comparison, additional data collected included the number of animals, tumor incidence, and percent tumor incidence (including preneoplastic lesions) for the control groups, as well as the treated groups. Some discussion of these results is provided in the results section, with additional detailed information provided in supplemental files.
Control comparison
To compare individual study results to the assembled RI controls in the RI control dataset, summary statistics were first derived for the combined control group data across the published RI studies (with the same route of administration) separately for each tumor type reported for male and female rats or mice. The summary statistics determined for each tumor type were the mean incidence rate (reported as a percentage and equal to the incidence of the tumor type divided by the number of control animals), the standard deviation of the incidence rate, and the minimum and maximum incidence rates seen across all the control groups being combined.
RI control data for all administration routes were compiled; however, the comparative analyses were limited to the oral route (dietary, drinking water, or gavage exposures), as the case studies were all conducted by the oral route. A pairwise comparison of the incidence of each tumor type reported in male or female control rats was conducted across the RI studies for all oral routes of administration. This pairwise comparison allowed for investigating oral route, vehicle, or diet, as a contributing factor to variability in the control incidence. Following NTP’s approach to reporting historical control information, we then combined the control data from the RI control dataset (developed as part of this evaluation) for all oral routes and compared the range of control incidence to the incidence for tumor types reported by the RI authors to be treatment-related in the treatment groups from the designated case studies (aspartame, formaldehyde, and methyl alcohol). In these individual studies, treatment-related effects were considered those reported as significantly different from concurrent controls or showed a statistically significant trend. By following the NTP approach, we were able to rely on tumor incidence data from a larger pool of RI control animals to represent the range of background incidence.
The RI historical control data reported in Bua et al. (Citation2018) – covering studies conducted between 1984 and 2001 – and Maltoni et al. (Citation1983) were considered for limited tumor types in RI SD rats, and it is likely these control data were already considered as part of the studies included in our RI control dataset. The mean and standard deviation of the reported controls in Bua et al. (Citation2018) fell within the range of values for each tumor type included in the RI control dataset. Further, Maltoni et al. (Citation1983) did not disclose the date range for the historical control incidence data they reported, and, therefore, these data were not included in the RI control dataset.
For each case study (aspartame, formaldehyde, or methyl alcohol), several different comparisons were conducted. Initially, the percent incidence for each tumor type reported by the study authors to be statistically significantly increased compared to concurrent controls or reported to have a statistically significant trend, was compared to the range of percent incidence from the RI control dataset for the years 1987 to the present. Next, the relevant RI control data were subdivided into the following date ranges − 1992–1997, 1997–2006, or 1992–2006 – to better represent the time period during which the studies for aspartame, formaldehyde, or methyl alcohol were conducted. The percent incidence reported for each treated group was then compared to the RI control dataset percent incidence using both trends analysis and pairwise comparisons to confirm the conclusions reached regarding purported treatment-related effects. For these analyses, statistical significance was determined by performing (i) the Cochran–Armitage trend test on the incidence data (Cochran Citation1954; Armitage Citation1955) and (ii) Fisher’s exact test (Fisher Citation1935; Irwin Citation1935) between the concurrent control and each of the treated groups. Tumor incidence trends were considered significant if the Cochran–Armitage trend test had a p value of less than .05. If Fisher’s exact test p value was less than .05 for one or more of the treated groups (compared to the concurrent control), the incidence was considered significantly increased. Comparative analyses for Swiss mice data were also conducted based on available studies, and designated case studies were for aspartame and sucralose.
Results
RI control incidence data
The literature search identified 119 possibly relevant RI carcinogenicity studies. A summary of the studies conducted in SD rats and Swiss mice are presented in and . Because of the large number of studies identified, further details from these studies including the author and year of publication, year the study was conducted, test substance, and the lab from which the test animals were obtained, are presented in Supplemental Tables. The supplemental tables are categorized by rodent species: SD rats (Supplemental Table 1), Swiss mice (Supplemental Table 2), and other rodent species including Wistar rats, Golden hamsters, and B6C3F1 and RF/J mice (Supplemental Table 3). Of the 119 studies identified as possibly relevant, there were multiple publications that either did not report any original data (e.g. reviews, commentaries), were not available for purchase, or were published in Italian only (Supplemental Table 4). Most accessible studies were performed years prior to publication, drawing from statements in the publications (e.g. referring to study start dates or references to current Italian laws regulating study conduct). The year of study conduct (if available) is included in Supplemental Tables 1–4. In the absence of a clear indication of when the study was performed, a study was assumed to have been conducted approximately 8 years prior to publication, based on the pattern noted for other RI studies.
Table 1a. Published Ramazzini Institute (RI) studies in SD rats.
Table 1b. Published Ramazzini Institute (RI) studies in Swiss mice.
Table 2. Variability of tumor incidence from SD rats dietary and drinking water studies.
Table 3. Summary statistics for tumors of interest across RI control groups in SD rats for studies conducted between 1987 and 2006 (oral route of administration).
RI SD rats control dataset
Of the 119 studies identified, 60 studies were conducted in SD rats and published between 1957 and 2005, the same species and strain RI used to evaluate the case study compounds (aspartame, formaldehyde, or methyl alcohol). RI studies considered relevant for the RI control dataset include carcinogenicity studies with multiple routes of administration (i.e. drinking water, diet, oral gavage, intraperitoneal injection, intrapleural injection, and intratracheal instillation). Exposure in some RI rat studies, including those for formaldehyde and methyl alcohol, began at around 8 weeks of age and continued for approximately 104 weeks, after which the exposure to treatment stopped and the rats were allowed to live until spontaneous death. In other RI studies, exposure began in utero and continued until spontaneous death, such as for aspartame (Soffritti et al. Citation2007; Chiozzotto et al. Citation2011), electromagnetic fields (Bua et al. Citation2018), and mobile phone radiofrequency (Falcioni et al. Citation2018). All RI SD rat control tumor incidence data are presented in Supplemental Table 5, otherwise known as the RI SD rat control group dataset.
The methods and results of each RI case study (i.e. aspartame, formaldehyde, methyl alcohol in SD rats, and aspartame and sucralose in mice) are discussed further below.
Outcomes from relevant RI studies conducted in SD rats
Aspartame
There are two RI aspartame carcinogenicity studies conducted in SD rats, with the results reported in several publications (Belpoggi et al. Citation2006; Soffritti, Belpoggi, Esposti, Lambertini, Tibaldi, et al. Citation2006; Soffritti et al. Citation2007; Chiozzotto et al. Citation2011). In the first study (Belpoggi et al. Citation2006; Soffritti, Belpoggi, Esposti, Lambertini, Tibaldi, et al. Citation2006), male and female SD rats (100–150/sex/group) were administered 0, 80, 400, 2,000, 10,000, 50,000, or 100,000Footnote2 ppm aspartame in the diet equivalent to a daily intake of 0, 4, 20, 100, 500, 2500, or 5000 mg/kg-bw/day, respectively, beginning at 8 weeks of age until spontaneous death (week 159) at which time cancer incidence was assessed. The authors reported a significantly increased dose-related trend in the incidence of total malignant tumors in both males and females, with a significant increase in incidence following administration of 50,000 ppm in females. A significant trend in the incidence of hyperplasia of the olfactory epithelium was reported by the authors in males and females, with significant increases in incidence reported following administration of 10,000 ppm and higher in both males and females. A significant trend in the incidence of preneoplastic and neoplastic lesions of the renal pelvis and ureter in female rats was reported with significant increases in the incidence of preneoplastic and neoplastic lesions of the renal pelvis and ureter reported following administration of 2000 ppm and higher. The incidence of total lymphomas and leukemias was significantly increased following administration of 400 ppm and higher in female rats compared to concurrent controls, and a significant trend in the incidence of lymphomas and leukemias was reported in both males and females. Statistically significantly increased tumor incidence or trends reported by the study authors are presented in Supplemental Table 6.
In the second study in which exposure was initiated in utero, male and female SD rats (70–95/sex/group) were administered 0, 400, or 2000 ppm dietary aspartame equivalent to daily intake of 0, 20, or 100 mg/kg-bw/day aspartame, respectively (Soffritti et al. Citation2007; Chiozzotto et al. Citation2011). The study was initiated with adult females (designated as breeders by the study authors) administered the test diet from the 12th day of pregnancy through weaning, and then sacrificed. The offspring continued treatment until spontaneous death (around week 144). The study authors reported a significant increase in the incidence of uterine polyps following administration of 400 ppm in females; however, the incidence of uterine polyps was not significantly increased following administration of 2000 ppm. In male rats, the authors reported a significant trend in the incidence of total malignant tumors along with a significant increase following administration of 2000 ppm. In females, there was a significant trend in the incidence of mammary adenocarcinomas and a significant increase in incidence of mammary adenocarcinomas following administration of 2000 ppm. The incidence of lymphomas and leukemias was significantly increased following administration of 2000 ppm in both males and females, and there was a significant trend reported in female rats. Statistically significantly increased tumor incidence or trends reported by the study authors are presented in Supplemental Table 7.
In response to criticisms of the RI carcinogenicity studies, including the inability to histologically distinguish inflammatory lesions from neoplasia (Schoeb et al. Citation2009), Gnudi et al. (Citation2023) recently reevaluated lesions from these aspartame studies. Unfortunately, this re-analysis relied upon past flawed methods employed in the study which could not differentiate neoplastic lesions from non-neoplastic lymphoid lesions in rodents (Elmore et al. Citation2023a, Citation2023b).
Formaldehyde
Formaldehyde was administered to groups of 50 male and 50 female SD rats in drinking water at concentrations of 0, 10, 50, 100, 500, 1000, or 1500 mg/L (0, 1.4, 7, 14, 70, 140, or 210 mg/kg-bw/da)Footnote3 (Soffritti, Belpoggi, Lambertin, et al. Citation2002). The control group included 100 males and 100 females that received tap water only. Exposure began at seven weeks of age and continued for 104 weeks, with study termination and cancer incidence assessment following spontaneous death. Tumor incidences noted by the study authors included a significant increase in the incidence of total malignant tumors in males following administration of 1500 mg/L, and a significant increase in the incidence of testicular interstitial cell adenomas following administration of 1000 mg/L in males, but not following administration of 1500 mg/L. There was a significant increase in the incidence of lymphomas/leukemias following administration of 100 mg/L and higher in males and following administration of 1000 and 1500 mg/L in females. Other tumor incidences noted by the study authors included significant increases in the incidence of malignant mammary tumors in females following administration of 100 mg/L and higher, as well as the presence of rare stomach leiomyosarcomas, intestine leiomyomas, and leiomyosarcomas. Statistically significantly increased tumor incidence or trends reported by the study authors are presented in Supplemental Table 8.
Methyl alcohol
Soffritti, Belpoggi, Cevolani, et al. (Citation2002) administered methyl alcohol in drinking water at concentrations of 0, 500, 5000, or 20,000 ppm (equivalent to 0, 70, 700, or 2800 mg/kg-bw/day)Footnote4 to groups of 100 male and 100 female SD rats. Test article administration began when the animals were 8 weeks of age and continued for 104 weeks, with study termination and cancer incidence assessment following spontaneous death. Significantly increased tumor incidences noted included total malignant tumor-bearing animals and ear duct carcinomas at all doses in both males and females; and lymphomas and leukemias at all doses in females. Statistically significantly increased tumor incidence or trends reported by the study authors are presented in Supplemental Table 9.
RI control dataset for RI Swiss mice
Of the 119 studies identified, 11 were conducted in Swiss mice, the same species and strain used in RI aspartame and sucralose mice studies. Routes of administration for the 11 studies included drinking water, diet, oral gavage, and inhalation (see Supplemental Table 2). While most studies had exposures beginning around 8 weeks of age, continuing for approximately 78 weeks and ended weeks later at spontaneous death in the case of vinyl acetate monomer (Maltoni, Ciliberti, Lefemine, et al. Citation1997), both sucralose (Soffritti, Padovani, et al. Citation2016) and aspartame (Soffritti et al. Citation2010) exposure continued until spontaneous death. The aspartame and sucralose studies were conducted in 1997 (Soffritti et al. Citation2010; Soffritti, Padovani, et al. Citation2016). In contrast, the Swiss mice control dataset was comprised primarily of studies conducted from 1989 or earlier. All compiled historical tumor incidence control data in Swiss mice are presented in Supplemental Table 10.
Both the sucralose and aspartame studies were conducted with SPF Swiss mice obtained from the Charles River Laboratory (CRL) in Milan, Italy. There was no indication that microbiological monitoring was performed during the experiments conducted at the RI. Although no historical control oncology or carcinogenicity data are provided on the CRL website for the Milan, Italy, facility, historical control data (assumed to be from the CRL Swiss mice colony) are provided in both the sucralose and aspartame publications (Soffritti et al. Citation2010; Soffritti, Padovani, et al. Citation2016). Comparative analyses with the Swiss mice control incidence in the RI control dataset and with the historical control data included in the sucralose and aspartame studies were conducted.
Review of the sucralose and aspartame studies indicated that the same control group was used for both of these studies, so a comparison of the most recent controls from the RI mouse colony (Maltoni, Ciliberti, Lefemine, et al. Citation1997) to the concurrent CRL control group from the sucralose and aspartame studies was also conducted to determine if background incidence between the early RI mouse colony and background incidence from recent studies conducted with acquired mice from the CRL were different. This comparison indicated the ranges of tumor incidence across controls in the RI mouse colony (Maltoni, Ciliberti, Lefemine, et al. Citation1997) and the control groups from the sucralose and aspartame studies (Soffritti et al. Citation2010; Soffritti, Padovani, et al. Citation2016) obtained from CRL were comparable.
Outcomes from relevant RI studies conducted in Swiss mice
Aspartame
Soffritti et al. (Citation2010) administered aspartame to Swiss mice at dietary concentrations of 0, 200, 800, 16,000, or 32,000 ppm (0, 250, 1000, 2000, or 4000 mg/kg-bw/day) from day 12 of gestation until death. The authors reported increased tumor incidence only in males: a significant dose-related trend in the incidence of hepatocellular carcinomas, with a significant increase in the incidence of hepatocellular carcinomas following administration of 16,000 or 32,000 ppm, compared to concurrent controls; a significant increase in the incidence of hepatocellular adenomas and carcinomas combined was reported following administration of 16,000 ppm; a significant dose-related trend in the incidence of alveolar/bronchiolar carcinomas, with a significant increase in the incidence of alveolar/bronchiolar carcinomas and alveolar/bronchiolar adenomas and carcinomas combined reported following administration of 32,000 ppm. Statistically significantly increased tumor incidence or trends reported by the study authors are presented in Supplemental Table 11.
Sucralose
Five groups of male and female Swiss mice were fed diets containing 0, 500, 2000, 8000, or 16,000 ppm (equivalent to 0, 400, 1600, 6400, or 12,800 mg/kg-bw/day)Footnote5 sucralose from day 12 of gestation until death (Soffritti, Padovani, et al. Citation2016). The tumor incidence from the control and treated groups is presented in Supplemental Table 12. The authors reported a significant dose-related trend in total malignant tumor bearing male animals. There was also a significant increase in the incidence of total hematopoietic tumors following administration of 2000 or 16,000 ppm and leukemias following administration of 2000, 8000, or 16,000 ppm in males. A significant dose-related trend in the incidence of histiocytic sarcomas was also reported in males. Statistically significantly increased tumor incidence or trends reported by the study authors are presented in Supplemental Table 12.
Statistical comparisons
Comparative analyses across RI control groups
Data were collected for approximately 600 different tumor types (including preneoplastic lesions) across RI studies identified in SD rats and Swiss mice, including all routes of exposure. The summary statistics for each study’s control data including sex, tumor type, and mean incidence rate reported as a percentage (i.e. the incidence of the tumor type divided by the number of animals in the control group) are presented in Supplemental Table 5 (SD rats) and Supplemental Table 10 (Swiss mice).
Pairwise comparisons were also conducted between control groups from studies within the RI control dataset in rats from 1987 to the present by different methods of oral administration (diet, drinking water, oral gavage). Statistically significant differences in tumor incidences were observed across control groups between diet and drinking water studies () suggesting high variability in control tumor incidences across these routes of exposure. These control animals would be receiving the same diet and drinking water; therefore, differences related to route of exposure among these control animals would not be expected. This finding suggests that any differences in incidence between control animals in drinking water or dietary study, as well as any differences observed for other routes of oral exposure (e.g. gavage) may be due to background variability in tumor development and not related to vehicle. Because these are control animals exposed to the same diet and drinking water, controls from all oral groups (i.e. diet, drinking water, and gavage) were combined (similar to how NTP approaches historical controls). Swiss mice controls were limited to only one study (Maltoni, Ciliberti, Lefemine, et al. Citation1997).
Of the approximately 600 different tumor types identified in the published studies, only a subset of tumors were used in the comparative analyses based on relevant ones from the case studies being evaluated here (see ). provides the mean percent incidence, standard deviation, minimum and maximum percent incidence, and the total number of animals considered for each tumor type being considered. A high degree of variability across controls was observed for most tumor types. For example, in female rats, the range of percent incidence for lymphomas and leukemias, reported in 21 studies, was 0–21.6%. For total malignant tumor bearing animals, the percent incidence in females ranged from 15.0% to 59.5% (22 studies), and in males the range was 18.3–50.0% (20 studies).
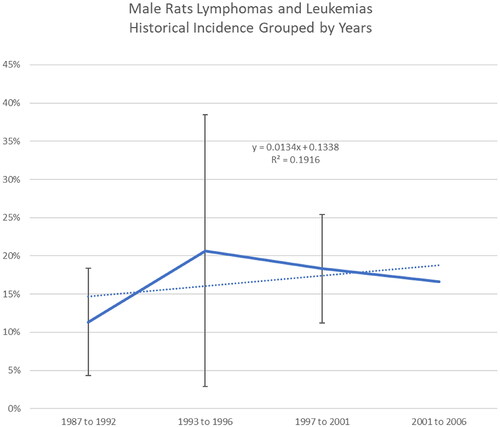
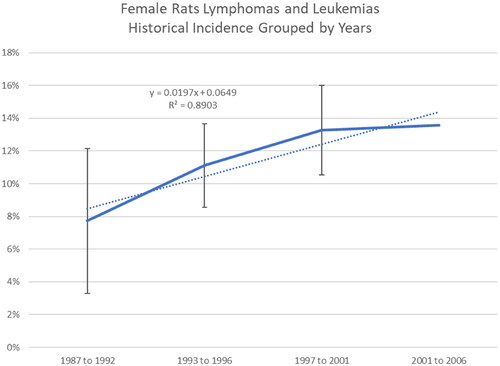
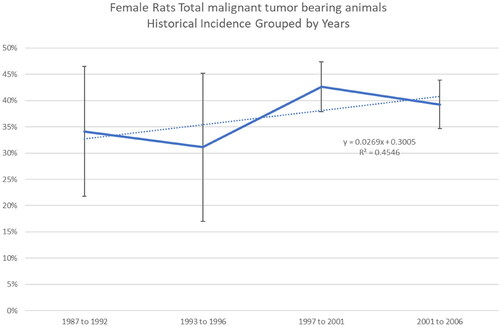
Additionally, comparative analyses across date ranges (i.e. 1987–1992, 1993–1996, 1997–2001, 2002–2006) showed a high degree of variability over time in tumor incidence across control groups relative to the different tumor types (). Examples are provided for lymphomas and leukemias in male and female rats ( and , respectively) and total malignant tumors in female rats (). For example, the range of percent tumor incidence of total malignant tumors in female rats was 20–59.5% from 1987 to 1992, 15–41.3% from 1993 to 1996, 36.7–48% from 1997 to 2001, and 36–42.5% from 2001 to 2006. These comparisons show wide variability in background tumor incidence in male and female control rats across RI experiments (32 studies).
Table 4. Comparative analyses of tumor incidence reported in RI control groups of SD rats.
Aspartame in SD rats
Tumor types reported to be treatment-related in the RI aspartame studies are listed in (Belpoggi et al. Citation2006; Soffritti, Belpoggi, Esposti, Lambertini, Tibaldi, et al. Citation2006; Soffritti et al. Citation2007; Chiozzotto et al. Citation2011). Statistically significant increased incidences of renal pelvis and ureter transitional cell carcinoma and dysplastic carcinoma were reported following administration of aspartame at 100,000 ppm (5000 mg/kg-bw/day). As noted by Elmore et al. (Citation2023a), these incidences were obtained by inappropriately combining lesions in the renal pelvis and ureter. According to Elmore et al. (Citation2023a), epithelial tumors present in distinct organs, renal pelvis (kidney) and ureter, should be reported separately unless the same tumor appears to occur in both sites, then an attempt should be made to determine the organ of the tumor origin. Also, significantly increased incidences of renal pelvis and ureter dysplastic hyperplasia were reported following administration of 2000, 10,000, 50,000, or 100,000 ppm (equivalent to 100, 500, 2500, or 5000 mg/kg-bw/day). Additionally, the urothelial lesions may possibly represent chronic progressive nephropathy (i.e. not tumors of the kidney pelvis and ureter), which is a manifestation of renal tubular disease and, very common in SD rats. These tumor types were not reported in other RI studies.
Table 5a. Comparative analyses of percent tumor incidence in SD rats between RI aspartame-treated groups and RI control dataset.
Most purported treatment-related tumor incidences fall within the variability of RI control incidence (e.g. lymphomas and leukemias in male rats, total malignant tumor bearing animals in males and females) (). The incidence of lymphomas and leukemia in female rats were reported to be significantly increased at doses of 400 ppm and higher when compared to concurrent controls (Soffritti et al. Citation2005; Belpoggi et al. Citation2006); however, when comparing to the range of control incidence in the RI control dataset, only the two highest dose groups (50,000 and 100,000 ppm) fell outside of the historical control range ().
Similar findings to those noted across all time periods in the RI control dataset were seen when comparing the incidence of lymphomas and leukemias to control incidences assembled for the following time periods: 1992–1997 (), 1997–2006 (), and 1992–2006 (), indicating the incidence of lymphomas and leukemias in the control dataset is variable across time periods. Other tumor incidences (e.g. adrenal gland pheochromoblastoma in males, lymphomas and leukemias in males) were no longer statistically significant when compared to historical controls during the time periods 1992–1997, 1997–2006, or 1992–2006.
Table 5b. Comparative analyses of percentage of SD rats between RI aspartame treated groups and RI control dataset for studies conducted from 1992 to 1997.
Table 5c. Comparative analyses of percentage of SD rats between RI aspartame treated groups and RI control dataset for studies conducted from 1997 to 2006.
Table 5d. Comparative analyses of percentage of SD rats between RI aspartame treated groups and RI control dataset for studies conducted from 1992 to 2006.
Formaldehyde in SD rats
When compared to the control incidence in the RI control dataset, only the incidence of intestine leiomyomas (1500 mg/L) and lymphomas and leukemias (1000 mg/L) in female rats, and lymphomas and leukemias and total malignant tumor bearing animals in male rats (1500 mg/L) was noted to fall above the range of RI control tumor incidence (see ). When the incidence of lymphomas and leukemias in treated males and females was compared to the control incidences across the various time periods, only the incidence in the females (1500 mg/L) was significantly greater than the controls over all time ranges (). The incidence of testicular interstitial cell adenoma was significantly increased at concentrations of 50 mg/L and higher when compared to the control incidences from 1992 to 1997 () and 1992 to 2006 (). However, when compared to the range from 1997 to 2006, incidence among treated groups was significantly increased only following exposures of 500, 1000, or 1500 mg/L.
Table 6a. Comparative analyses of percent tumor incidence in SD rats between RI formaldehyde-treated groups and RI control dataset.
Table 6b. Comparative analyses of percent tumor incidence in SD rats between RI formaldehyde-treated groups and RI control dataset from 1992 to 1997.
Table 6c. Comparative analyses of percent tumor incidence in SD rats between RI formaldehyde-treated groups and RI control dataset from 1997 to 2006.
Table 6d. Comparative analyses of percent tumor incidence in SD rats between RI formaldehyde-treated groups and RI control dataset from 1992 to 2006.
Methyl alcohol in SD rats
Except for lymphomas and leukemias in the female rats and total malignant tumor bearing male animals, all other purported treatment-related tumors were outside of the range of incidence of the RI control dataset at the highest concentration tested (20,000 ppm) (). When compared to historical controls from specific time periods – i.e. 1992–1997 (), 1997–2006 (), or 1992–2006 () – the increased incidence of lymphomas and leukemias in females and total malignant tumors in males observed across all doses were significant. The increased incidence of ear duct carcinomas in males and females was significant only at lower concentrations.
Table 7a. Comparative analyses of percent tumor incidence in SD rats between RI methyl alcohol-treated groups and RI control dataset.
Table 7b. Comparative analyses of percent tumor incidence in SD rats between RI methyl alcohol-treated groups and RI control dataset from 1992 to 1997.
Table 7c. Comparative analyses of percent tumor incidence in SD rats between RI methyl alcohol-treated groups and RI control dataset from 1997 to 2006.
Table 7d. Comparative analyses of percent tumor incidence in SD rats between RI methyl alcohol-treated groups and RI control dataset from 1992 to 2006.
Aspartame and sucralose in Swiss mice
Historical controls from Maltoni, Ciliberti, Lefemine, et al. (Citation1997) are relevant for comparisons with controls from the aspartame and sucralose RI studies in Swiss mice. The tumor types noted in the historical controls that were also noted in either the aspartame (Soffritti et al. Citation2010) or sucralose (Soffritti, Padovani, et al. Citation2016) RI study are listed in . Swiss mice used in the aspartame and sucralose RI studies were purchased from CRL, and only one set of controls was used for both studies. Comparative analyses of control groups are provided in (aspartame) and (sucralose).
Table 8. Summary statistics for relevant tumor incidence across RI control groups in Swiss mice.
Table 9. Comparative analyses of percent tumor incidence in Swiss mice between RI aspartame-treated groups and RI control dataset.
Table 10. Comparative analyses of percent tumor incidence in Swiss mice between RI sucralose-treated groups and RI control dataset.
Statistically significant increased tumor incidences when compared to concurrent controls were only reported in male mice in either the aspartame or sucralose studies. Available RI control data for comparison were limited, except for total malignant tumor bearing animals. There was a high degree of incidence variability across controls (53.9–96.3%). Incidence of hepatocellular carcinoma in male in the RI control dataset was 23.9% (21.4–26.3%). All tumor incidences reported for the aspartame or sucralose treated groups fell below or within the range of historical controls.
Discussion
Historical control data are typically documented to assess background variability over time. High variability could signify that the animals may be prone to greater than usual spontaneous tumors or could suggest other underlying issues with laboratory conditions. In these situations, it is necessary to evaluate and understand any high spontaneous rate of tumors, as it could impact any conclusions regarding potential effects from treatment.
Numerous RI cancer studies conducted over several decades have been considered by a multitude of authoritative bodies and subsequently criticized for their methods (e.g. a lack of a rodent health surveillance monitoring program). Elmore et al. (Citation2023a, Citation2023b) have described the significant flaws with the RI study design, conduct, analyses, and interpretation. Further, reporting of findings was found to be inadequate and historical control data were generally lacking.
As the RI has conducted numerous studies over the years and these studies have been and are considered by multiple authoritative bodies, the absence of historical control tumor incidence data represents a significant data gap. To better understand the potential background variability in tumor incidence across RI studies and to fill this data gap, this assessment focused on compiling available published incidence data on control groups across RI studies to build RI historical control datasets for the SD rats and Swiss mice used in their studies. We then set out to assess whether the tumor incidence reported to be statistically significantly increased following treatment in selected case studies when compared against concurrent controls would retain their significance when compared to the incidence in the RI control dataset.
Twenty-one RI studies were conducted likely between 1990 and 2009, which includes the timeframe when the aspartame (1997), sucralose (1997), methanol (1990), and formaldehyde (1990) RI studies were conducted. In Soffritti et al. (Citation2007) and Chiozzotto et al. (Citation2011), the authors discussed 20 years’ worth of historical control data for 2415 male SD rats and 2424 female SD rats, the same as those up to 2001 reported in Bua et al. (Citation2018). Our historical control dataset aggregates incidence rates reported across 3445 male and 3707 female SD rats, and 52 male and 85 female Swiss mice.
We found inconsistent approaches across RI studies. It is important to note that the historical control datasets compiled in this evaluation are based solely on purported tumor incidence data reported in published articles from the RI. The historical control datasets do not account for the many deficiencies in experimental methodology or diagnostic processes or criteria employed by the RI, such as infection, lack of pathology expert peer review, analysis of total tumors in some instances, or overt autolysis. In addition, the nomenclature used to identify tumor types was not standardized (e.g. mammary gland benign vs. mammary gland adenomas, malignant tumors vs. mammary gland carcinoma). Some studies provided a complete list of all tumor types identified and others, only purported treatment-related tumors. In contrast, NTP documents all tumor incidence including in controls and across controls (i.e. historical controls).
We also found considerable variability in tumor incidence across RI study control groups. Significant differences were noted in tumor incidences across control groups from diet versus drinking water studies and for different time periods, indicating high variability in control tumor incidences among studies in which the animals would be receiving the same diet and drinking water. This type of variability related to route of exposure would not be expected among control animals, which suggests background variability in tumor development not related to the route of exposure or vehicle. A high degree of variability was indicated by the ranges of percent tumor incidence in both SD rats and Swiss mice. In some cases, the percent incidence spanned 19% or greater. In SD rats, the range of percent incidence of lymphomas and leukemias was greater than 20% in females (0–21.6%) and 40% (0–40%) in males. The range of percent incidence of total malignant tumors in males and female SD rats and male Swiss mice was greater than 30% (18.3–50%), 40% (15–59.5%), and 42% (53.9–96.3%), respectively. Yet, the increased incidence of these same tumor types in both SD rats and Swiss mice was the same as those tumor types the RI relies on to suggest treatment-related tumor incidence in the designated case studies for aspartame, formaldehyde, and methyl alcohol-treated rats and sucralose-treated mice.
Most purported treatment-related tumor incidences for aspartame and sucralose were found to be within the background variability of the RI control dataset. The purported treatment-related tumor incidences for formaldehyde and methanol that were found to be statistically significantly increased when compared to concurrent controls were shown to be outside of the range of incidence when assessed against the RI control dataset. It is worth restating that the historical control datasets culled together from the various RI articles do not account for the deficiencies in experimental methodology or diagnostic processes or criteria identified by others (e.g. infection, lack of pathology expert peer review, analysis of total tumors in some instances, overt autolysis and more).
It should also be noted that the tumor incidence reported by Soffritti, Belpoggi, Esposti, Lambertini, Tibaldi, et al. (Citation2006) and Soffritti, Belpoggi, Esposti, Lambertini (Citation2006) for rats exposed to concentrations of aspartame in drinking water from 8 weeks of age until natural death were evaluated statistically by the study authors using a poly-3 survival adjustment technique. While useful for 2-year studies, the poly-3 technique has never been validated for lifetime studies such as those conducted by the RI (Kissling et al. Citation2008; Gift et al. Citation2013). Applying the poly-3 technique to lifetime or longer term studies without regard for limitations could have resulted in incorrect estimates by Soffritti, Belpoggi, Esposti, Lambertini, Tibaldi, et al. (Citation2006) and Soffritti, Belpoggi, Esposti, Lambertini (Citation2006) of statistical significance (Kissling et al. Citation2008). For this evaluation, we were not able to apply a survival adjusted test such as the poly-3 test because individual animal data required for the test (i.e. tumor response and time of death) were not available. However, no significant survival issues were reported in the RI studies evaluated in this assessment; therefore, the results of the Cochran–Armitage trend test (Cochran Citation1954; Armitage Citation1955) on the incidence data used in this assessment should provide results similar to those of the poly-3 test.
Here, we have compiled the incidence from available RI control groups that may assist others in the future. Clearly and transparently documenting historical control incidence data is critical to the interpretation of study results from a particular laboratory. Reputable bodies like the NTP continue this best practice. By doing so, this will allow for a proper assessment of treatment-related tumor incidence within the context of the variability of background incidence over time.
The strength of our paper is a complete account of reported tumor incidence in control groups across accessible published RI studies. Our analyses were limited by the inadequate reporting of when the RI studies were conducted and of what RI chose to include in their publications (as the historical control data are not on their website).
While the results of the current evaluation fill a significant data gap in the evaluation of results from RI studies, other critical considerations to draw conclusions on relevance to human health remain. As noted by Elmore et al. (Citation2023a, Citation2023b), the methods used in the RI studies, specifically selected immunohistochemical (IHC) markers, are not able to differentiate between neoplastic and non-neoplastic lymphoid lesions in rodents, calling into question selected results from RI studies. Further, questions surrounding a mycoplasma infection in the RI rodent colony persist. All major rodent producers have Mycoplasma pulmonis on their pathogen exclusion lists due to effects, both clinical and subclinical, that may invalidate the study results, including the inability to histologically distinguish inflammatory lesions from neoplasia (Schoeb et al. Citation2009). The accuracy of the diagnoses of the pathological lesions reported in some RI studies may also have been affected by autolysis. Pathological examinations performed by the RI were performed on tissues collected after natural death due to the methodology used by RI that allowed treated and control animals to live until spontaneous death. In studies with scheduled necropsy time, the time between death and placement of tissue specimens in preservative is minimized; however, when the animals are allowed to die naturally (possibly hours prior to clinical observation times), tissue preparation may not occur for hours after death increasing tissue autolysis and compromising the quality of the pathological examination (Gift et al. Citation2013; Elmore et al. Citation2023a). In addition to these issues, many other significant shortcomings have been noted including, but not limited to, ineffective tissue preservation, inappropriate combination of tumors (e.g. malignant tumors, lymphomas/leukemias), misdiagnoses of lesions, double counting of lesions, etc. (Brix et al. Citation2010; Elmore et al. Citation2023a, Citation2023b).
| Abbreviations | ||
| CMCRC | = | Cesare Maltoni Cancer Research Center |
| CRL | = | Charles River Laboratory |
| DIPE | = | di-isopropyl-ether |
| EEL | = | European Experimental Laboratory |
| EFSA | = | European Food Safety Authority |
| ELFEMF | = | extremely low frequency electromagnetic fields |
| EPA | = | United States Environmental Protection Agency |
| ERF | = | European Ramazzini Foundation |
| ETBE | = | ethyl tert-butyl ether |
| FDA | = | United States Food and Drug Administration |
| GLP | = | Good Laboratory Practice |
| mg/L | = | milligram per liter |
| mg/kg-bw/day | = | mg per kg body weight per day |
| MTBE | = | methyl-tertiary-butyl ether |
| NIEHS | = | United States National Institute of Environmental Health Sciences |
| NIH | = | United States National Institutes of Health |
| NTP | = | National Toxicology Program |
| OECD | = | Organization for Economic Co-operation and Development |
| ppm | = | part per million |
| PWG | = | Pathology Working Group |
| RI | = | Ramazzini Institute |
| SD | = | Sprague-Dawley |
| SPF | = | specific pathogen-free |
| TAME | = | tert-amyl-methyl-ether |
Supplemental Material
Download MS Excel (337.9 KB)Acknowledgements
The authors acknowledge and appreciate the comments of the Editor and the external reviewers (selected by the Editor) who were anonymous to the authors. Reviewers’ constructive comments helped strengthen the manuscript.
Declaration of interest
Ramboll is a private consulting firm providing services to private and public organizations on toxicology and risk assessment issues.
This project was a concept presented by Ramboll to the American Beverage Association (ABA). This work was supported by ABA, a 501(c)(6) tax-exempt organization; however, no one from ABA was involved in the preparation of the manuscript. ABA’s Dr. Maia Jack, Chief Science and Regulatory Officer, was given the opportunity to review the draft manuscript. The purpose of this review was for the authors to receive input on the clarity of the science presented but not on the interpretation of research results. The researchers’ scientific conclusions and professional judgments were not subject to the funders’ control; the contents of this manuscript reflect solely the view of the authors.
None of the authors received direct compensation from ABA for this project. The project was funded through contracts between ABA and Ramboll. All the scientists of Ramboll (RG, TG, HB, CVL, JR, and HC) involved in the development of the current manuscript were provided salary compensation as part of their employment as consultants.
There are no conflicts of interest for any of the authors to disclose related to the submission of this manuscript. None of the authors are currently engaged to testify as experts on behalf of the sponsors in litigation related to non-sugar sweeteners. It is anticipated that regulatory authorities may consider the contents of this review in making regulatory decisions regarding potential health effects of non-sugar sweeteners.
Additional information
Funding
Notes
1 Number of animals (when reported) with each tumor type within the control group. If the number of animals with a tumor type was not reported, then it was calculated based on the total number of animals and the percent tumor incidence reported in the study.
2 The aspartame dietary concentration of 100,000 ppm exceeds the limit dose for dietary studies recommended by the FDA as reported in “Toxicological Principles for the Safety Assessment of Food Ingredients Redbook 2000 Chapter IV.C.6”.
3 Doses in ppm drinking water were converted using a default daily drinking water intake and body weight for rats of 0.049 L/day and 0.35 kg, respectively.
4 Doses in ppm drinking water were converted assuming 1 ppm = 1 mg/L and using a default daily drinking water intake and body weight for rats of 0.049 L/day and 0.35 kg, respectively.
5 Equivalent doses in units of mg/kg bw/day were not provided by the authors. The equivalent doses provided for the sucralose study were estimated based on the ratio of doses in ppm to doses in mg/kg bw/day provided in the aspartame mouse study (Soffritti et al. Citation2010).
References
- Armitage P. 1955. Tests for linear trends in proportions and frequencies. Biometrics. 11(3):375. doi: 10.2307/3001775.
- Belpoggi F, Soffritti M, Guarino M, Lambertini L, Cevolani D, Maltoni C. 2002. Results of long-term experimental studies on the carcinogenicity of ethylene-bis-dithiocarbamate (mancozeb) in rats. Ann N Y Acad Sci. 982(1):123–136. doi: 10.1111/j.1749-6632.2002.tb04928.x.
- Belpoggi F, Soffritti M, Maltoni C. 1995. Methyl-tertiary-butyl ether (MTBE) – a gasoline additive – causes testicular and lymphohaematopoietic cancers in rats. Toxicol Ind Health. 11(2):119–149. doi: 10.1177/074823379501100202.
- Belpoggi F, Soffritti M, Minardi F, Bua L, Cattin E, Maltoni C. 2002. Results of long-term carcinogenicity bioassays on tert-amyl-methyl-ether (TAME) and di-isopropyl-ether (DIPE) in rats. Ann N Y Acad Sci. 982(1):70–86. doi: 10.1111/j.1749-6632.2002.tb04925.x.
- Belpoggi F, Soffritti M, Padovani M, Degli Esposti D, Lauriola M, Minardi F. 2006. Results of long-term carcinogenicity bioassay on Sprague-Dawley rats exposed to aspartame administered in feed. Ann N Y Acad Sci. 1076(1):559–577. doi: 10.1196/annals.1371.080.
- Belpoggi F, Tibaldi E, Lauriola M, Bua L, Falcioni L, Chiozzotto D, Manservisi F, Manservigi M, Soffritti M. 2011. The efficacy of long-term bioassays in predicting human risks: mesotheliomas induced by fluoro-edenitic fibres present in lava stone from the Etna Volcano in Biancavilla, Italy. Eur J Oncol. 16:185–195.
- Brix AE, Hardisty JF, McConnell EE. 2010. Combining neoplasms for evaluation of rodent carcinogenesis studies. In: Hsu CH, Stedeford T, editors. Cancer risk assessment chemical carcinogenesis, hazard evaluation, and risk quantification. Hoboken (NJ): John Wiley & Sons, Inc.; p. 699–715.
- Bua L, Tibaldi E, Falcioni L, Lauriola M, De Angelis L, Gnudi F, Manservigi M, Manservisi F, Manzoli I, Menghetti I, et al. 2018. Results of lifespan exposure to continuous and intermittent extremely low frequency electromagnetic fields (ELFEMF) administered alone to Sprague Dawley rats. Environ Res. 164:271–279. doi: 10.1016/j.envres.2018.02.036.
- Chiozzotto D, Soffritti M, Falcioni L, Tibaldi E, Manservisi F, Manservigi M, Bua L, Belpoggi F. 2011. Results of life span carcinogenicity bioassay on Sprague-Dawley rats exposed to aspartame since foetal life. Eur J Oncol. 16(2):81–97.
- Ciliberti A, Maltoni C, Perino G. 1988. Long-term carcinogenicity bioassays on propylene administered by inhalation to Sprague-Dawley rats and Swiss mice. Ann N Y Acad Sci. 534(1):235–245. doi: 10.1111/j.1749-6632.1988.tb30113.x.
- Cochran WG. 1954. Some methods for strengthening the common Chi-squared tests. Biometrics. 10(4):417–451. doi: 10.2307/3001616.
- Conti B, Maltoni C, Perino G, Ciliberti A. 1988. Long-term carcinogenicity bioassays on styrene administered by inhalation, ingestion and injection and styrene oxide administered by ingestion in Sprague-Dawley rats, and para-methylstyrene administered by ingestion in Sprague-Dawley rats and Swiss mice. Ann N Y Acad Sci. 534(1):203–234. doi: 10.1111/j.1749-6632.1988.tb30112.x.
- Cotti G, Maltoni C, Lefemine G. 1988. Long-term carcinogenicity bioassay on vinylidene chloride administered by inhalation to Sprague-Dawley rats. New results. Ann N Y Acad Sci. 534(1):160–168. doi: 10.1111/j.1749-6632.1988.tb30109.x.
- EFSA. 2006. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to a new long-term carcinogenicity study on aspartame. EFSA J. 4(5):44.
- Einbond LS, Soffritti M, Degli Esposti D, Tibaldi E, Lauriola M, Bua L, He K, Genovese G, Su T, Huggins L, et al. 2012. Chemopreventive potential of black cohosh on breast cancer in Sprague-Dawley rats. Anticancer Res. 32(1):21–30.
- Elmore S, Rehg JE, Schoeb JR, Everitt JL, Bolon B. 2023b. Is statistical reevaluation of hemolymphoreticular neoplasms from aspartame studies valid? Toxicol Sci. 195(2):143–144. doi: 10.1093/toxsci/kfad070.
- Elmore SA, Rehg JE, Schoeb TR, Everitt JI, Bolon B. 2023a. Pathologists’ perspective on the study design, analysis, and interpretation of proliferative lesions in lifetime and prenatal rodent carcinogenicity bioassays of aspartame. Food Chem Toxicol. 171:113504. doi: 10.1016/j.fct.2022.113504.
- Falcioni L, Bua L, Tibaldi E, Lauriola M, De Angelis L, Gnudi F, Mandrioli D, Manservigi M, Manservisi F, Manzoli I, et al. 2018. Report of final results regarding brain and heart tumors in Sprague-Dawley rats exposed from prenatal life until natural death to mobile phone radiofrequency field representative of a 1.8 GHz GSM base station environmental emission. Environ Res. 165:496–503. doi: 10.1016/j.envres.2018.01.037.
- Fisher R. 1935. The logic of inductive inference. J R Stat Soc A. 98(1):39–54. doi: 10.2307/2342435.
- Gift JS, Caldwell JC, Jinot J, Evans MV, Cote I, Vandenberg JJ. 2013. Scientific considerations for evaluating cancer bioassays conducted by the Ramazzini Institute. Environ Health Perspect. 121(11–12):1253–1263. doi: 10.1289/ehp.1306661.
- Gnudi F, Panzacchi S, Tibaldi E, Iuliani M, Sgargi D, Bua L, Mandrioli D. 2023. Hemolymphoreticular neoplasias from the Ramazzini Institute long-term mice and rat studies on aspartame. Ann Glob Health. 89(1):43. doi: 10.5334/aogh.4163.
- Irwin J. 1935. Tests of significance for differences between percentages based on small numbers. Metron. 12:83–94.
- Kissling G, Portier C, Huff J. 2008. MrBE and cancer in animals: statistical issues with poly-3 survival adjustments for lifetime studies. Regul Toxicol Pharmacol. 50(3):428–429. doi: 10.1016/j.yrtph.2007.07.008.
- Malarkey D, Herbert R, Nyska A, Sutphin ME, Pernika K. 2010. Memorandum from D Malarkey, R Herbert, A Nyska, ME Sutphin, and K Pernika, National Toxicology Program, to J Bucher, Associate Director, National Toxicology Program. Report on visit (4/25/2010–4/30/2010) and assessment of the pathology procedures performed at the Ramazzini Institute (RI), Bentivoglio, Italy, 11 June 2010. In: Summary report of the National Toxicology Program and Environmental Protection Agency sponsored review of pathology materials from Selected Ramazzini Institute rodent cancer bioassays. Research Triangle Park (NC); p. 21 [accessed 2013 Sep 25]. http://ntp.niehs.nih.gov/NTP/About_NTP/Partnerships/International/SummaryPWG_Report_RI_Bioassays.pdf.
- Maltoni C, Belpoggi F, Soffritti M, Minardi F. 1999. Comprehensive long-term experimental project of carcinogenicity bioassays on gasoline oxygenated additives: plan and first report of results from the study on ethyl-tertiary-butyl ether (ETBE). Eur J Oncol. 4(5):493–508.
- Maltoni C, Ciliberti A, Cotti G, Conti B, Belpoggi F. 1989. Benzene, an experimental multipotential carcinogen: results of the long-term bioassays performed at the Bologna Institute of Oncology. Environ Health Perspect. 82:109–124. doi: 10.1289/ehp.8982109.
- Maltoni C, Ciliberti A, Cotti G, Perino G. 1988. Long-term carcinogenicity bioassays on acrylonitrile administered by inhalation and by ingestion to Sprague-Dawley rats. Ann N Y Acad Sci. 534(1):179–202. doi: 10.1111/j.1749-6632.1988.tb30111.x.
- Maltoni C, Ciliberti A, Lefemine G, Soffritti M. 1997. Results of a long-term experimental study on the carcinogenicity of vinyl acetate monomer in mice. Ann N Y Acad Sci. 837(1):209–238. doi: 10.1111/j.1749-6632.1997.tb56876.x.
- Maltoni C, Ciliberti A, Pinto C, Soffritti M, Belpoggi F, Menarini L. 1997. Results of long-term experimental carcinogenicity studies of the effects of gasoline, correlated fuels, and major gasoline aromatics on rats. Ann N Y Acad Sci. 837(1):15–52. doi: 10.1111/j.1749-6632.1997.tb56863.x.
- Maltoni C, Conti B, Cotti G, Belpoggi F. 1985. Experimental studies on benzene carcinogenicity at the Bologna Institute of Oncology: current results and ongoing research. Am J Ind Med. 7(5–6):415–446. doi: 10.1002/ajim.4700070508.
- Maltoni C, Conti B, Cotti G. 1983. Benzene: a multipotential carcinogen. Results of long-term bioassays performed at the Bologna Institute of Oncology. Am J Ind Med. 4(5):589–630. doi: 10.1002/ajim.4700040503.
- Maltoni C, Conti B, Perino G, Di Maio V. 1988. Further evidence of benzene carcinogenicity. Results on Wistar rats and Swiss mice treated by ingestion. Ann N Y Acad Sci. 534(1):412–426. doi: 10.1111/j.1749-6632.1988.tb30131.x.
- Maltoni C, Cotti G, Morisi L, Chieco P. 1977. Carcinogenicity bioassays of vinylidene chloride. Research plan and early results. Med Lav. 68(4):241–262.
- Maltoni C, Cotti G, Perino G. 1988. Long-term carcinogenicity bioassays on methylene chloride administered by ingestion to Sprague-Dawley rats and Swiss mice and by inhalation to Sprague-Dawley rats. Ann N Y Acad Sci. 534(1):352–366. doi: 10.1111/j.1749-6632.1988.tb30122.x.
- Maltoni C, Cotti G, Valgimigli L, Mandrioli A. 1982. Zymbal gland carcinomas in rats following exposure to benzene by inhalation. Am J Ind Med. 3(1):11–16. doi: 10.1002/ajim.4700030104.
- Maltoni C, Cotti G. 1986. Results of long-term carcinogenicity bioassays of tetrachloroethylene on Sprague-Dawley rats administered by Ingestion. Acta Oncol. 7(1):11–26.
- Maltoni C, Cotti G. 1988. Carcinogenicity of vinyl chloride in Sprague-Dawley rats after prenatal and postnatal exposure. Ann N Y Acad Sci. 534(1):145–159. doi: 10.1111/j.1749-6632.1988.tb30108.x.
- Maltoni C, Lefemine G, Ciliberti A, Cotti G, Carretti D. 1981. Carcinogenicity bioassays of vinyl chloride monomer: a model of risk assessment on an experimental basis. Environ Health Perspect. 41:3–29. doi: 10.1289/ehp.81413.
- Maltoni C, Lefemine G, Cotti G, Perino G. 1988. Long-term carcinogenicity bioassays on trichloroethylene administered by inhalation to Sprague-Dawley rats and Swiss and B6C3F1 mice. Ann N Y Acad Sci. 534(1):316–342. doi: 10.1111/j.1749-6632.1988.tb30120.x.
- Maltoni C, Lefemine G, Cotti G. 1986. Experimental research on trichloroethylene carcinogenesis. In: Archives of research on industrial carcinogenesis. Princeton (NJ): Scientific Publishing Company, Inc.; p. 45–153.
- Maltoni C, Lefemine G, Tovoli D, Perino G. 1988. Long-term carcinogenicity bioassays on three chlorofluorocarbons (trichlorofluoromethane, FC11; dichlorodifluoromethane, FC12; chlorodifluoromethane, FC22) administered by inhalation to Sprague-Dawley rats and Swiss mice. Ann N Y Acad Sci. 534(1):261–282. doi: 10.1111/j.1749-6632.1988.tb30116.x.
- Maltoni C, Lefemine G. 1974. Carcinogenicity bioassays of vinyl chloride: I. Research plan and early results. Environ Res. 7(3):387–405. doi: 10.1016/0013-9351(74)90040-1.
- Maltoni C, Lefemine G. 1975. Carcinogenicity bioassays of vinyl chloride: current results. Ann N Y Acad Sci. 246(1):195–218. doi: 10.1111/j.1749-6632.1975.tb51094.x.
- Maltoni C, Minardi F, Pinto C, Belpoggi F, Bua L. 1997. Results of three life-span experimental carcinogenicity and anticarcinogenicity studies on tamoxifen in rats. Ann N Y Acad Sci. 837(1):469–512. doi: 10.1111/j.1749-6632.1997.tb56895.x.
- Maltoni C, Minardi F. 1988. First available results of long-term carcinogenicity bioassay on detergency zeolites (MS 4A and MS 5A). Ann N Y Acad Sci. 534:978–985. doi: 10.1111/j.1749-6632.1988.tb30189.x.
- Minardi F, Belpoggi F, Soffritti M, Ciliberti A, Lauriola M, Cattin E, Maltoni C. 2002. Results of long-term carcinogenicity bioassay on vinyl acetate monomer in Sprague-Dawley rats. Ann N Y Acad Sci. 982(1):106–122. doi: 10.1111/j.1749-6632.2002.tb04927.x.
- Minardi F, Maltoni C. 1988. Results of recent experimental research on the carcinogenicity of natural and modified asbestos. Ann N Y Acad Sci. 534(1):754–761. doi: 10.1111/j.1749-6632.1988.tb30164.x.
- Minardi F, Maltoni C. 1998. Results of long-term carcinogenicity bioassays of rockwool on Sprague-Dawley rats. Eur J Oncol. 3(3):251–260.
- NIEHS. 2004. Pathology Working Group Chairperson’s Report. Lifetime study in rats conducted by the Ramazzini Foundation. Research Triangle Park (NC): National Institute of Environmental Health Sciences.
- NTP. 2011. Summary report of the National Toxicology Program and Environmental Protection Agency‐sponsored review of pathology materials from Selected Ramazzini Institute Rodent Cancer Bioassays. National Toxicology Program.
- NTP. 2023. Historical controls. National Toxicology Program. https://ntp.niehs.nih.gov/data/controls.
- Perino G, Conti B, Ciliberti A, Maltoni C. 1988. Incidence of pancreatic tumors and tumor precursors in Sprague-Dawley rats after administration of olive oil. Ann N Y Acad Sci. 534(1):604–617. doi: 10.1111/j.1749-6632.1988.tb30151.x.
- Rodricks JV, Turnbull D. 2010. Comments on the draft toxicological review of methanol (CAS No. 67-56-1). In: Support of summary information on the Integrated Risk Information System (IRIS). Prepared for the National Petrochemical and Refiners Association. Washington (DC). Posted by the United States Environmental Protection Agency. https://www.regulations.gov/comment/EPA-HQ-ORD-2009-0398-0021.
- Schoeb TR, Davis JK, Lindsey JR. 1996. Murine respiratory mycoplasmosis, rat and mouse. In: Jones TC, Dungworth DL, Mohr U, editors. Monographs on the pathology of laboratory animals. Respiratory system. Vol. 199. 2nd ed. New York (NY): Springer-Verlag; p. 117–131.
- Schoeb TR, McConnell EE, Juliana MM, Davis JK, Davidson MK, Lindsey JR. 2009. Mycoplasma pulmonis and lymphoma in bioassays in rats. Vet Pathol. 46(5):952–959. doi: 10.1354/vp.08-VP-0240-S-COM.
- Soffritti M, Belpoggi F, Cevolani D, Guarino M, Padovani M, Maltoni C. 2002. Results of long-term experimental studies on the carcinogenicity of methyl alcohol and ethyl alcohol in rats. Ann N Y Acad Sci. 982(1):46–69. doi: 10.1111/j.1749-6632.2002.tb04924.x.
- Soffritti M, Belpoggi F, Esposti DD, Lambertini L, Tibaldi E, Rigano A. 2006. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats. Environ Health Perspect. 114(3):379–385. doi: 10.1289/ehp.8711.
- Soffritti M, Belpoggi F, Esposti DD, Lambertini L. 2005. Aspartame induces lymphomas and leukaemias in rats L’aspartame Induce Linfomi e Leucemie Nei Ratti. Eur J Oncol. 10(2):107–116.
- Soffritti M, Belpoggi F, Esposti DD, Lambertini L. 2006. Results of a long-term carcinogenicity bioassay on Sprague-Dawley rats exposed to sodium arsenite administered in drinking water. Ann N Y Acad Sci. 1076(1):578–591. doi: 10.1196/annals.1371.075.
- Soffritti M, Belpoggi F, Lambertin L, Lauriola M, Padovani M, Maltoni C. 2002. Results of long-term experimental studies on the carcinogenicity of formaldehyde and acetaldehyde in rats. Ann N Y Acad Sci. 982(1):87–105. doi: 10.1111/j.1749-6632.2002.tb04926.x.
- Soffritti M, Belpoggi F, Lenzi A, Maltoni C. 1997. Results of long-term carcinogenicity studies of chlorine in rats. Ann N Y Acad Sci. 837(1):189–208. doi: 10.1111/j.1749-6632.1997.tb56875.x.
- Soffritti M, Belpoggi F, Manservigi M, Tibaldi E, Lauriola M, Falcioni L, Bua L. 2010. Aspartame administered in feed, beginning prenatally through life span, induces cancers of the liver and lung in male Swiss mice. Am J Ind Med. 53(12):1197–1206. doi: 10.1002/ajim.20896.
- Soffritti M, Belpoggi F, Minardi F, Maltoni C. 2002. Ramazzini Foundation Cancer Program: history and major projects, life-span carcinogenicity bioassay design, chemicals studied, and results. Ann N Y Acad Sci. 982(1):26–45. doi: 10.1111/j.1749-6632.2002.tb04923.x.
- Soffritti M, Belpoggi F, Padovani M, Lauriola M, Esposti DD, Minardi F. 2004. Life-time carcinogenicity bioassays of toluene given by stomach tube to Sprague-Dawley rats. Eur J Oncol. 9(2):91–102.
- Soffritti M, Belpoggi F, Tibaldi E, Esposti DD, Lauriola M. 2007. Life-span exposure to low doses of aspartame beginning during prenatal life increases cancer effects in rats. Environ Health Perspect. 115(9):1293–1297. doi: 10.1289/ehp.10271.
- Soffritti M, Falcioni L, Bua L, Tibaldi E, Manservigi M, Belpoggi F. 2013. Potential carcinogenic effects of World Trade Center dust after intratracheal instillation to Sprague-Dawley rats: first observation. Am J Ind Med. 56(2):155–162. doi: 10.1002/ajim.22109.
- Soffritti M, Giuliani L. 2019. The carcinogenic potential of non-ionizing radiations: the cases of S-50 Hz MF and 1.8 GHz GSM radiofrequency radiation. Basic Clin Pharmacol Toxicol. 125(Suppl. 3):58–69. doi: 10.1111/bcpt.13215.
- Soffritti M, Maltoni C, Maffei F, Biagi R. 1989. Formaldehyde: an experimental multipotential carcinogen. Toxicol Ind Health. 5(5):699–730. doi: 10.1177/074823378900500510.
- Soffritti M, Minardi F, Bua L, Esposti DD, Belpoggi F. 2004. First experimental evidence of peritoneal and pleural mesotheliomas induced by fluoro-edenite fibres present in Etnean volcanic material from Biancavilla (Sicily, Italy). Eur J Oncol. 9(3):169–175.
- Soffritti M, Padovani M, Tibaldi E, Falcioni L, Manservisi F, Lauriola M, Bua L, Manservigi M, Belpoggi F. 2016. Sucralose administered in feed, beginning prenatally through lifespan, induces hematopoietic neoplasias in male Swiss mice. Int J Occup Environ Health. 22(1):7–17. doi: 10.1080/10773525.2015.1106075.
- Soffritti M, Tibaldi E, Bua L, Padovani M, Falcioni L, Lauriola M, Manservigi M, Manservisi F, Belpoggi F. 2015. Life-span carcinogenicity studies on Sprague-Dawley rats exposed to γ-radiation: design of the project and report on the tumor occurrence after post-natal radiation exposure (6 weeks of age) delivered in a single acute exposure. Am J Ind Med. 58(1):46–60. doi: 10.1002/ajim.22391.
- Soffritti M, Tibaldi E, Padovani M, Hoel DG, Giuliani L, Bua L, Lauriola M, Falcioni L, Manservigi M, Manservisi F, et al. 2016a. Synergism between sinusoidal-50 Hz magnetic field and formaldehyde in triggering carcinogenic effects in male Sprague-Dawley rats. Am J Ind Med. 59(7):509–521. doi: 10.1002/ajim.22598.
- Soffritti M, Tibaldi E, Padovani M, Hoel DG, Giuliani L, Bua L, Lauriola M, Falcioni L, Manservigi M, Manservisi F, et al. 2016b. Life-span exposure to sinusoidal-50 Hz magnetic field and acute low-dose γ radiation induce carcinogenic effects in Sprague-Dawley rats. Int J Radiat Biol. 92(4):202–214. doi: 10.3109/09553002.2016.1144942.
- Tibaldi E, Gnudi F, Panzacchi S, Mandrioli D, Vornoli A, Manservigi M, Sgargi D, Falcioni A, Bua L, Belpoggi F. 2020. Identification of aspartame-induced haematopoietic and lymphoid tumours in rats after lifetime treatment. Acta Histochem. 122(5):151548. doi: 10.1016/j.acthis.2020.151548.
- Vornoli A, Tibaldi E, Gnudi F, Sgargi D, Manservisi F, Belpoggi F, Tovoli F, Mandrioli D. 2022. Evaluation of toxicant-associated fatty liver disease and liver neoplastic progress in Sprague-Dawley rats treated with low doses of aflatoxin B1 alone or in combination with extremely low frequency electromagnetic fields. Toxins. 14(5):325. doi: 10.3390/toxins14050325.