Abstract
In newly diagnosed multiple myeloma (MM), patients ineligible for front-line autologous stem cell transplantation (ASCT), melphalan and prednisone (MP) with thalidomide (MPT) or bortezomib (VMP) are standard first-line therapeutic options. Despite new treatment regimens incorporating bortezomib or lenalidomide, MM remains incurable. The FIRST study demonstrated significant improvement in progression-free survival (PFS) and overall survival (OS) for the combination of lenalidomide and low-dose dexamethasone (Rd) until progression vs. MPT in transplant-ineligible ndMM patients. However, to date no head-to-head randomized controlled trials (RCTs) have compared Rd or MPT versus VMP. We conducted a network meta-analysis using RCTs identified through a systematic literature review to evaluate the relative efficacy of Rd versus other regimens on survival endpoints in previously untreated MM patients ineligible for ASCT. In this analysis, Rd was associated with a significant PFS and survival advantage versus other first-line treatments (VMP, MPT, MP), challenging the role of alkylators in this setting.
Introduction
Multiple myeloma (MM) is currently one of the most common hematological malignancies, mainly affecting individuals over 65 years of age.[Citation1] In recent years, the introduction of new therapeutic options coupled with advances in the molecular understanding of the disease have improved patient survival; however, MM remains incurable.[Citation2]
High-dose chemotherapy paired with autologous stem cell transplantation (ASCT) is the standard front-line treatment for younger and fit patients with newly diagnosed MM (ndMM). However, in patients who are ineligible for ASCT due to age/frailty, comorbidities, impaired fitness or disability, or who are otherwise unwilling/unable to receive transplant, melphalan and prednisone (MP) combined with either thalidomide (MPT) or bortezomib (VMP) are current standard first-line therapeutic options,[Citation3] whose prescribing frequencies vary by country. This includes, in some cases, patients who are eligible for ASCT, but the treating physician or patient instead choose a therapeutic option, potentially postponing ASCT.[Citation4]
New treatment combinations incorporating proteasome inhibitors and immunomodulatory drugs are challenging the role of alkylators in ndMM treatment. For instance, lenalidomide (R) combined with low-dose dexamethasone (Rd) is an effective therapeutic option for these patients and is an approved therapeutic option by the US Food and Drug Administration and European Medicines Agency. The phase III MM-020 (FIRST) trial, a randomized, open-label, three-arm study determined the efficacy and safety of Rd versus MPT in transplant-ineligible ndMM patients and demonstrated that Rd treatment until progression significantly improved progression-free survival (PFS) and overall survival (OS) compared with the MPT regimen.[Citation5] However, there are currently no direct head-to-head clinical trials of Rd or MPT versus VMP, another commonly used regimen for the treatment of ndMM.
Head-to-head randomized controlled trials (RCTs) are considered the gold standard approach for comparing the efficacy of different interventions, but are not always available for all treatment options of interest, especially as new treatments enter the market. Network meta-analysis (NMA) is a statistical method to simultaneously evaluate the comparative efficacy of multiple treatment options through the use of direct and indirect comparisons. This method allows for a robust comparison of treatments that have not been compared head-to-head.[Citation6–8] In this study, we conducted a NMA of RCTs identified through a systematic literature review (SLR) to evaluate the relative efficacy of Rd versus VMP and other agents for the treatment of newly diagnosed or previously untreated MM patients ineligible for ASCT (referred to as ndMM patients hereafter) used according to their recommended dosing schedules as per European Summary of Product Characteristics (SmPC).
Materials and methods
Systematic literature review
Articles published in English from 1 January 1988 to 28 May 2015 were reviewed to identify relevant RCTs evaluating safety and efficacy endpoints for ndMM patients. The SLR adhered to established guidelines [Citation9] and was based on a study protocol developed specifically for this review.
Eligibility criteria
Eligibility criteria were defined in terms of the population, interventions, comparisons, outcomes, and study design (PICOS) criteria. The study population of interest was untreated adult MM patients aged 65 years or older or not eligible for stem cell transplantation. Studies with fewer than 10 patients per treatment arm were excluded. Treatment regimens searched included: lenalidomide, thalidomide, bortezomib, bendamustine, or interferon, as monotherapy or part of a combination therapy, or MP combination treatment. Comparators of interest included placebo, any of the above-listed interventions at a different dose or duration, or any other active drug provided as monotherapy or as part of a combination therapy. Outcomes of interest included: PFS, OS, and safety (grade 3/4 adverse events [AEs], serious adverse events [SAEs], and discontinuations due to AEs). Fully published RCTs as well as conference abstracts presenting the results of RCTs were considered eligible for inclusion.
Literature search strategies
Literature searches were conducted in Medline, Embase and the Cochrane Central Register of Controlled Trials. Additionally, we manually searched conference proceedings from the following meetings occurring between January 2013 and June 2015 to identify additional relevant conference abstracts: ASCO, ASH, EHA, ESMO and IMW. A manual check of the bibliographies of recent relevant reviews and meta-analyses ensured optimal and complete literature retrieval. The specific terms used in the database searches are provided in the Supplementary Information.
Study selection
All abstracts were independently screened by two investigators. Those eligible for inclusion were selected for full text screening and independent review. Discrepancies were resolved by involving a third investigator and reaching consensus. Articles meeting criteria at the full-text stage were included in the analysis. When multiple publications and/or conference abstracts reported on a single trial population, only the most recent or relevant data for the analyses were selected for inclusion in the SLR.
Data collection process and data items
Study-level data, patient characteristics, treatment details, and efficacy and safety endpoints were extracted from the included trials. Data were also extracted on length of follow-up and study inclusion criteria. Hazard ratios (HRs) were extracted for OS and PFS endpoints. When HRs were not available, these values were estimated as described in Tierney et al. [Citation10] When HRs were given but confidence intervals (CIs) were not reported, these were estimated by using p values and their corresponding Z-score to calculate the standard error. When only number, probability, or proportions of patients alive were reported, HR and 95% CI were estimated using formulas based on the log-rank test.
Risk of bias in individual studies
The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials was used.[Citation11] This assessment was conducted by two investigators working independently. Any differences were resolved through discussion with a third reviewer.
Analysis
Overview
A NMA was conducted on HRs for OS and PFS to determine the comparative efficacy of treatments in ndMM patients. Trials identified by the SLR were eligible for inclusion in the statistical analysis if they reported HRs for these survival endpoints. In order to compare VMP with Rd using MPT and MP regimens as common comparators, study comparability and treatment relevance were assessed. The primary analysis was limited to trials with a dosing schedule in line with the respective regimens’ Summary of Product Characteristics (SmPC) in order to minimize heterogeneity and provide evidence approximating actual use of the interventions of interest (fixed duration of treatment for MP, MPT, and VMP; treatment until progression for Rd).[Citation12–14] A sensitivity analysis included all trials that were connected by one- or two-degree linkages and formed a closed loop with melphalan, prednisone and continuous thalidomide (MPT-T).
Network meta-analysis
Network meta-analyses were performed in the Bayesian framework using the standard procedures described by the National Institute for Health and Care Excellence (NICE) decision support unit.[Citation15] Relative treatment effects and modeled outcomes were summarized by the median and 95% credible intervals (CrIs), constructed from the 2.5th and 97.5th percentiles. In this SLR, the evidence networks consisted of a limited number of studies relative to the number of treatments under consideration and nearly all connections in the networks consisted of single trials. Additionally, included studies comparing MPT versus MP, [Citation16–18] showed no heterogeneity (I2 = 0%). Thus, a fixed effect model was considered the most appropriate analysis.
The parameters of the different models were estimated using a Markov Chain Monte Carlo method as implemented in the OpenBUGS software package.[Citation19,Citation20] All analyses were performed using OpenBUGS version 3.2.3 (OpenBUGS Project Management Group Cambridge, UK, http://www.openbugs.net/w/FrontPage). Celgene Corporation provided funding for the analysis (Supplementary Information gives further statistical details).
Results
Study selection
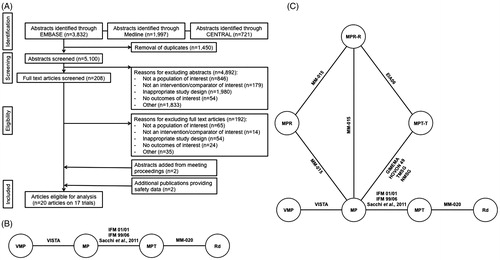
A total of 6550 abstracts were identified from the literature searches. After exclusion of 1450 duplicates, 5100 unique abstracts were eligible for review. Of the unique abstracts, a further 4892 abstracts were excluded from further review: 846 did not include a population of interest; 179 did not investigate interventions of interest; 54 did not report on outcomes of interest; 1980 had an inappropriate study design; and 1833 abstracts were excluded due to other reasons (such as being comments or review articles). Of the 208 full text articles screened and selected for further review, 192 were excluded for the following reasons: 65 did not include a population of interest; 14 did not evaluate an intervention or comparator of interest; 24 did not report on outcomes of interest; 54 had an inappropriate study design; and 35 due to other reasons. The resulting number of RCTs included was 16 (15 presented in full publications,[Citation5,Citation16–18,Citation21–33] and one presented in a conference abstract [Citation34]). Two additional publications were identified for inclusion through manual searches of conference abstracts, [Citation21,Citation29] for a total of 18 publications reporting on 17 trials.[Citation5,Citation16–18,Citation21–36] A further two publications were identified providing safety data not available in the principal publications.[Citation35,Citation36] Of note, Fayers et al. was excluded due to study design (meta-analysis); however, this study provided HR data for Beksac et al. and Waage et al. that was not otherwise available.[Citation22,Citation32,Citation37] A diagram of the study flow is presented in .
Figure 1. Methodology figures. (A) Study selection for meta-analysis. CENTRAL, Cochrane Central Register of Controlled Trials. (B) Network diagram including studies with similar MPT doses and schedules in the primary analysis network. (C) Network diagram for sensitivity analysis of MPT and MPT-T studies and studies with 1- to 2-degree linkages. MP: melphalan and prednisone; MPR: melphalan and prednisone with lenalidomide; MPR-R: melphalan and prednisone with lenalidomide followed by lenalidomide maintenance; MPT: melphalan and prednisone with thalidomide; MPT-T: melphalan and prednisone with thalidomide followed by thalidomide maintenance; Rd: lenalidomide and low-dose dexamethasone; VMP: melphalan and prednisone with bortezomib.
Risk of bias
Overall, included trials presented minimal risk of bias. The greatest risk of bias was posed by randomization and allocation concealment. Details regarding method of sequence generation for randomization were not reported in two studies.[Citation27,Citation34] Further, allocation concealment was only reported in 5 of 17 studies.[Citation22,Citation24–26,Citation33] Inclusion/exclusion criteria were not fully reported in one case due to the publication type (abstract).[Citation29] Although details on blinding were inadequately reported in a majority of studies, this is likely due to ethical considerations surrounding the treatment of MM. Baseline characteristics were presented in all studies. Finally, all but three studies [Citation22,Citation23,Citation29] reported using the intention-to-treat population in analyses (the risk of bias assessment Table of each included trial is presented in the Supplementary Information).
Primary analysis network
Following review of the network and treatment characteristics, several treatments were eliminated from inclusion in the analysis based on clinical relevance and treatment schedule. For example, only studies evaluating MPT received as fixed treatment duration were included in the primary analysis (), in line with treatment recommendations outlined in the current SmPC for thalidomide.[Citation14]
Table 1. Extracted data from RCTs in the primary analysis and sensitivity analysis networks.
The primary analysis network was thus composed of five trials evaluating Rd, VMP, MP and MPT (). [Citation5,Citation16–18,Citation21,Citation30,Citation36] Comparisons were evaluated using Rd as the reference treatment. Baseline characteristics were well distributed across trials, in terms of patient age and disease severity (). Finally, endpoint analyses were performed based on the intention-to-treat population within trials.
Table 2. Summary of baseline patient characteristics from the primary analysis network.
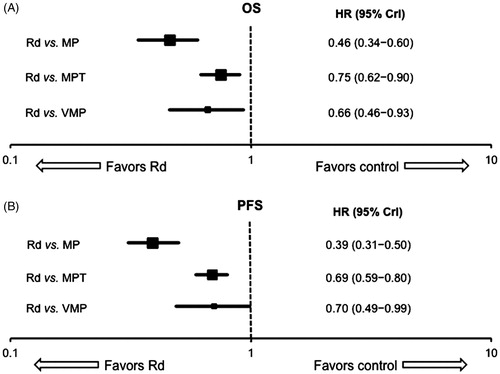
Analyses of OS using fixed effects NMA models documented a significantly lower risk of death with Rd treatment until progression compared to all tested treatment regimens (HRs [95% credible interval (CrI)]: VMP, 0.66 [0.46–0.93]; MPT, 0.75 [0.62–0.90]; MP, 0.46 [0.34–0.60]) (). Similarly, a fixed effects analysis of PFS results showed a significantly lower risk of progression or death with Rd treatment until progression compared to all tested treatment regimens (HR [95% CrI]: VMP, 0.70 [0.49–0.99], MPT, 0.69 [0.59–0.80]; MP, 0.39 [0.31–0.50]) (). Of note, the HRs and CrIs for MPT and VMP substantially overlapped for both OS and PFS evaluations, suggesting little difference between these regimens, although direct comparisons were not made.
Figure 2. Mixed treatment comparison survival data: fixed effects analyses with Rd as reference. (A) overall survival (OS); (B) progression free survival (PFS). CrI: credible interval; HR: hazard ratio; MP: melphalan and prednisone; MPT: melphalan and prednisone with thalidomide; Rd: lenalidomide and low-dose dexamethasone; VMP: melphalan and prednisone with bortezomib.
Sensitivity analyses
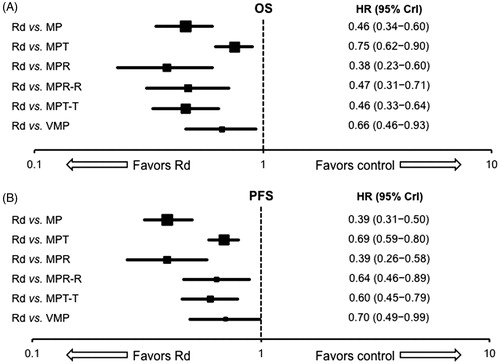
A sensitivity analysis was conducted on a broader network of 11 trials to evaluate the effect of combining all trials that evaluated MPT and MPT-T treatments as well as any study comparator with a one- to two-degree linkage to either of these treatments in the network. This analysis added six trials to the primary analysis, including five arms assessing MPT-T, one assessing MPR, and two assessing MPR-R (, ).
The sensitivity analysis for OS indicated a higher level of heterogeneity, as the CrIs were somewhat wider compared with the primary network analysis, although all HR values significantly favored Rd (). This analysis showed a significantly lower risk of death with Rd dosed until progression compared with all other investigated interventions, including MP, MPT, MPR, MPR-R, MPT-T, and VMP (HR [95% CrI]): MP 0.46 [0.34–0.60]; MPT 0.75 [0.62–0.90]; MPR 0.38 [0.23–0.60]; MPR-R 0.47 [0.31–0.71]; MPT-T: 0.46 [0.33–0.64]; VMP 0.66 [0.46–0.93]. In terms of PFS, the sensitivity analysis also showed that all HR values significantly favored Rd, including MP, MPT, MPR, MPR-R, MPT-T, and VMP (HR [95% CrI]): MP 0.39 [0.31–0.50]; MPT 0.69 [0.59–0.80]; MPR 0.39 [0.26–0.58]; MPR-R 0.64 [0.46–0.89]; MPT-T: 0.60 [0.45–0.79]; VMP 0.70 [0.49–0.99] ().
Figure 3. Mixed treatment comparison survival data: fixed-effects analyses with Rd as reference. (A) overall survival (OS); (B) progression free survival (PFS). CrI: credible interval; HR: hazard ratio; MP: melphalan and prednisone; MPR: melphalan and prednisone with lenalidomide; MPR-R: melphalan and prednisone with lenalidomide followed by lenalidomide maintenance; MPT: melphalan and prednisone with thalidomide; MPT-T: melphalan and prednisone with thalidomide followed by thalidomide maintenance; Rd: lenalidomide and low-dose dexamethasone; VMP: melphalan and prednisone with bortezomib.
An additional sensitivity analysis was conducted evaluating all 17 trials identified from the systematic literature search (). This analysis demonstrated a statistically significant lower risk of death with Rd treatment until progression compared to all thalidomide- or bortezomib-based regimens, regardless of treatment duration (data not shown).
Discussion
The availability of new combination regimens including the novel agents’ thalidomide, bortezomib, and lenalidomide, has improved treatment options in ndMM patients. In the absence of available RCTs directly comparing Rd versus VMP, the clinical decision-making process can be complex. In such a situation, NMAs can be useful. The present NMA results indicate that the Rd regimen is a more effective treatment option for ndMM patients ineligible for transplantation compared with melphalan-containing regimens VMP, MPT and MP. These results reinforce the improved OS and PFS benefit reported for Rd directly compared with MPT.[Citation5] In terms of clinical decision-making, these results are meaningful, since the Rd doublet therapy shows superior efficacy to VMP and MPT triplet therapies, and to MP.
Moreover, Rd is a fully oral, alkylator-free regimen that avoids/reduces risk of some grade 3/4 AEs associated with oral melphalan and triplet therapies, such as peripheral neuropathy, fatigue, and gastrointestinal AEs (diarrhea) with VMP [Citation30,Citation36] or infection, fatigue, peripheral neuropathy, neurotoxicity, gastrointestinal AEs (diarrhea, constipation), and venous thromboembolism with MPT.[Citation5,Citation16–18,Citation33,Citation38,Citation35] Although no NMA was conducted on safety outcomes, the proportion of patients discontinuing treatment due to AEs and the reported ≥ grade 3/4 AEs from the 11 studies included in the sensitivity analysis was overall higher in triplet combinations compared with doublets.[Citation16–18] Similar results were observed in the FIRST trial, where 44% of patients randomized to MPT reported grade 3/4 neutropenia compared with 28% in the Rd until progression group [Citation5]. Where reported, grade 3/4 neuropathic events were consistently higher in patients receiving triplet versus doublet regimens.[Citation5,Citation16–18,Citation22,Citation32,Citation33,Citation36,Citation35] In terms of other non-hematological AEs, the most commonly reported grade 3/4 AE in patients randomized to Rd was infection, seen in 29% of patients vs. 17% in MPT-treated patients. Most cases of infection in the Rd group (80%) occurred in the absence of neutropenia.[Citation5]
Previously published indirect comparisons have reported that both lenalidomide and bortezomib seem to be more efficacious than thalidomide, and that addition of these agents to MP is superior to MP alone in ndMM patients. Indirect comparisons of lenalidomide versus thalidomide maintenance after ASCT and MPR-R versus MPT-T demonstrated a statistically significant PFS benefit (p < 0.001 in both comparisons), but no survival difference when using observation/placebo as the common comparator. Data also indicated that the discontinuation rate from thalidomide trials appeared higher than in lenalidomide trials, suggesting that lenalidomide is less toxic than thalidomide.[Citation39] Compared with MPT, one indirect comparison analysis showed that VMP statistically significantly prolonged both PFS and OS (p < 0.001 in each case). However, the OS benefit in the VMP group did not apply to elderly patients (≥75 years).[Citation40] Further, this previous analysis excluded the VISTA trial of VMP versus MP,[Citation36] used for regulatory approval of VMP, as well as excluding IFM 01/01 [Citation17] and IFM 99/06,[Citation16] all of which were included in the present analysis. Two other studies evaluating VMP versus MPT showed no difference in survival outcomes.[Citation41,Citation42]
Meta-analyses are limited by the assumption that trials and outcomes are similar enough for data pooling. This assumption is given credibility by using specific pre-defined inclusion criteria during the systematic review trial selection process, ensuring that the retrieved trials show comparable patient populations and clinically relevant endpoints. Although between-study differences are inevitable, and heterogeneity in the analysis may provide a measure of uncertainty in effects due to differences in clinical practice, the current NMA showed very little heterogeneity by including only RCTs evaluating clinically relevant treatments that reflect clinical practice. For example, we included all studies identified from our literature search that included MPT dosed according to SmPC dosing description (i.e. for a fixed treatment duration). Further, Cochrane’s risk of bias tool was also applied to ensure study quality. Strict PICOS criteria also limited the risk of bias due to inconsistency. Finally, the distributions of baseline characteristics across trials were considered sufficiently homogenous for inclusion in a single NMA. Although missing survival data were imputed, most imputations were done through calculations based on Kaplan–Meier curves. The largest source of inconsistency was the maturity of the studies at the time points at which outcomes were reported, which varied between 12 and 96 months of median follow up. Based on the network size on which the analyses were performed, the fixed effect analysis is considered the most robust estimate of treatment effects.
In the current analyses, Rd treatment until progression was associated with a significant advantage in OS and PFS compared with the first-line treatments VMP, MPT and MP. The sensitivity analysis also showed a significantly lower risk of death and progression with Rd compared with all investigated interventions, including MPR, MPR-R, and MPT-T. The results suggest that Rd treatment until progression is likely the best treatment option for untreated patients with ndMM ineligible for stem cell transplantation and/or over 65 years of age. The analysis did not allow for examination of patient subgroups, such as ISS stage, age, renal impairment or cytogenetically high-risk patients. We expect treatment decisions continue to be made on an individual basis, taking disease features and co-morbidities into consideration; certain patient subgroups may better benefit from one regimen over another. However, these data are clinically important in a setting in which alkylating agents have long been considered treatment standards. Of note, in addition to favorable efficacy and safety parameters,[Citation5] the Rd regimen has shown significant improvements in clinically relevant quality of life measurements,[Citation43] which is of considerable value in the context of elderly patients with an incurable disease such as MM.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at http://dx.doi.org/10.1080/10428194.2016.1177772.
ICMJE_Forms_for_Disclosure_of_Potential_Conflicts_of_Interest.zip
Download Zip (18.9 MB)Supplementary information
Download MS Word (192 KB)Acknowledgements
We thank Evidera and Redwood Outcomes for providing the statistical analysis, and Dr Vanessa Gray-Schopfer, OmniScience SA, for providing medical writing services funded by Celgene. The authors were fully responsible for content and editorial decisions for this manuscript.
References
- McCarthy PL, Palumbo A. Maintenance therapy for multiple myeloma. Hematol Oncol Clin North Am. 2014;28:839–859.
- Faiman B, Richards T. Innovative agents in multiple myeloma. J Adv Pract Oncol. 2014;5:193–202.
- Engelhardt M, Terpos E, Kleber M, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99:232–242.
- Moreau P, Rajkumar SV. Should all eligible patients with multiple myeloma receive autologous stem-cell transplant as part of initial treatment? Leuk Res. 2012;36:677–681.
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906–917.
- Ades AE. A chain of evidence with mixed comparisons: models for multi-parameter synthesis and consistency of evidence. Stat Med. 2003;22:2995–3016.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Bmj. 2005;331:897–900.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Summary of Product Characteristics, REVLIMID, February 2015.
- Summary of Product Characteristics, VELCADE, April 2014.
- Summary of Product Characteristics, THALIDOMIDE, April 2013.
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617.
- Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218.
- Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:3664–3670.
- Sacchi S, Marcheselli R, Lazzaro A, et al. A randomized trial with melphalan and prednisone versus melphalan and prednisone plus thalidomide in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplant. Leuk Lymphoma. 2011;52:1942–1948.
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455.
- Gelman A. Inference and monitoring convergence. In: Gilks WR, Richardson S, Spiegelhalter DJ, editors. Markov chain Monte Carlo in practice. London: Chapman & Hall; 1996. p. 1311–1143.
- Facon T, Dimopoulos MA, Hulin C, et al. Updated overall survival analysis of the FIRST study: continuous lenalidomide plus low-dose dexamethasone vs melphalan, prednisone, and thalidomide in patients with newly diagnosed multiple myeloma. Haematologica. 2015;100:3 (Abstract S105).
- Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16–22.
- Facon T, Mary JY, Pegourie B, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006;107:1292–1298.
- Mateos MV, Oriol A, Martinez-Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11:934–941.
- Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118:1231–1238.
- Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–3114.
- Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101–5109.
- Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–1769.
- Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127:1102–1108.
- San Miguel JF, Schlag R, Khuageva NK, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol. 2013;31:448–455.
- San-Miguel J, Blade J, Shpilberg O, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. 2014;123:4136–4142.
- Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405–1412.
- Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28:3160–3166.
- Stewart AK, Jacobus S, Fonseca R, et al. E1A06: A phase III trial comparing melphalan, prednisone, and thalidomide (MPT) versus melphalan, prednisone, and lenalidomide (MPR) in newly diagnosed multiple myeloma (MM). Haematologica. 2014;99:220 (Abstract S642).
- Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831.
- San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917.
- Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118:1239–1247.
- Palumbo A, Waage A, Hulin C, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: a meta-analysis of data from individual patients in six randomized trials. Haematologica. 2013;98:87–94.
- Zou Y, Sheng Z, Niu S, et al. Lenalidomide versus thalidomide based regimens as first-line therapy for patients with multiple myeloma. Leuk Lymphoma. 2013;54:2219–2225.
- Morabito F, Bringhen S, Larocca A, et al. Bortezomib, melphalan, prednisone (VMP) versus melphalan, prednisone, thalidomide (MPT) in elderly newly diagnosed multiple myeloma patients: A retrospective case-matched study. Am J Hematol. 2014;89:355–362.
- Kumar A, Hozo I, Wheatley K, et al. Thalidomide versus bortezomib based regimens as first-line therapy for patients with multiple myeloma: a systematic review. Am J Hematol. 2011;86:18–24.
- NICE technology appraisal guidance 228. Bortezomib and thalidomide for the first-line treatment of multiple myeloma; [cited 2011 Jul]. Available from: http://www.nice.org.uk/guidance/ta228.
- Delforge M, Minuk L, Eisenmann JC, et al. Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica. 2015;100:826–833.
