Abstract
Relapsed/refractory multiple myeloma (RRMM) patients have poor overall survival (OS). Pomalidomide plus low-dose dexamethasone (POM + LoDEX) significantly extends OS in RRMM vs. high-dose dexamethasone. Survival of patients with stable disease (SD) was compared to patients with progressive disease (PD) or ≥ partial response (≥PR) at cycles (C) 3, 5, and 7. Among 302 patients randomized to POM + LoDEX, at C3 19.2% achieved ≥ PR, 38.4% SD, and 14.6% PD. Patients with SD at C3 (17.4%) and C5 (13.6%) showed improved responses at C7. Median OS from randomization by response at C3 was 22.4 months for ≥ PR (n = 58, HR 0.66; 95% CI 0.40–1.08, p = 0.0976 vs. SD), 16.2 months for SD (n = 116), and 6.3 months for PD (n = 44, HR 3.43; 95% CI 2.23–5.27, p < 0.0001 vs. SD). Similar patterns were observed for C5 and C7. Results show that POM + LoDEX should be a standard treatment after lenalidomide and bortezomib, including in SD patients.
Keywords:
Introduction
Multiple myeloma (MM) is an incurable malignancy that forms in plasma cells of bone marrow. Despite a better understanding of disease biology and the introduction of autologous hematopoietic stem cell transplantation in the early 1990s, and improved outcomes due to availability of new effective therapies such as thalidomide (THAL), bortezomib (BORT) and lenalidomide (LEN), the vast majority of responding patients eventually relapse or become refractory to available treatments.[Citation1,Citation2] Introduction of these agents has improved the five-year relative survival in MM patients from 47.3% (2002–2004) to 53.8% (2008–2010) in Germany and from 39.8% to 53.2% in the United States (US) in the same time intervals.[Citation3]
Patients with refractory or relapsed and refractory multiple myeloma (RRMM) have a poor prognosis and short overall survival (OS) after failure of BORT and immunomodulatory agents (THAL or LEN). Indeed, an expected median event-free survival of five months and a median OS of nine months has been reported in RRMM patients; survival is even shorter in the absence of further treatment after failure of BORT and immunomodulatory agents (THAL or LEN).[Citation4] Further to a decrease in survival outcomes, RRMM is characterized by an increasing symptom burden due to disease and cumulative effects of treatments, highlighting the need for therapies that provide disease control and preserve quality of life.[Citation5–8]
Pomalidomide (POM) is a novel second-generation immunomodulatory drug that is efficacious in patients after BORT and LEN therapy.[Citation9–13] It was approved in 2013 in the US for use in combination with dexamethasone in RRMM patients who have received at least two prior therapies, including LEN and a proteasome inhibitor and have demonstrated disease progression on or within 60 days of completing the last therapy.[Citation14] Similarly, in Europe, POM is indicated in combination with dexamethasone in RRMM patients who have received at least two prior treatment regimens, including both LEN and BORT, and have demonstrated disease progression on their last therapy.[Citation15]
The main evidence for POM efficacy in RRMM patients is based on the phase III multicenter, randomized, open-label MM-003 (NIMBUS) study that compared POM plus low-dose dexamethasone therapy (POM + LoDEX) to high-dose dexamethasone alone (HiDEX). Some patients were double refractory, and this population has a high unmet need. The NIMBUS trial showed that POM is effective even in this hard to treat population. With a median follow-up of 15.4 months, POM + LoDEX showed significant and clinically meaningful improvements in OS (13.1 vs. 8.1 months; hazard ratio [HR], 0.72; p = 0.009) and progression-free survival (PFS; HR 0.49; 95% CI, 0.40–0.61], p < 0.001) compared with HiDEX.[Citation16] Moreover, POM + LoDEX treatment provided consistent efficacy regardless of the number and type of prior treatments, including in patients who were refractory to LEN.[Citation16]
We present a post hoc analysis of the NIMBUS study, whereby OS and safety were investigated based on response status at early assessments (using the International Myeloma Working Group [IMWG] criteria [Citation17]) at different landmark treatment cycles, with a focus on patients in the POM + LoDEX arm achieving stable disease (SD).
Methods
The methodology was based on earlier studies that also evaluated survival according to response.[Citation18–20] The primary objective of this post hoc analysis was to investigate the relationship between response, as assessed using IMWG criteria,[Citation17] and OS. The survival data cutoff date was 1 September 2013 (median follow-up 15.4 months). The analysis focused on whether the response status of patients at early assessments (first day of cycles [C] 3, 5 and 7) was predictive of OS, and how the survival of patients classified as having SD compared to those with overall response (defined as at least partial response, ≥PR) and progressive disease (PD). These time points were chosen because the median duration of therapy from the NIMBUS trial is approximately five 28-day cycles, so if assessments were done too early, little difference may have been observed, whereas if they were done too late, the sample size would have been too small to allow for robust comparison. Landmark analyses were used as the primary analysis methods.[Citation18]
The relationship between IMWG response and the pattern of IMWG response over time were investigated as secondary objectives. Specifically, response over time analyses focused on whether patients with SD at early assessments sustained SD or improved to achieve ≥ PR. Furthermore, safety data on day 1 of C3 were also collected and analyzed.
Patient profiles and NIMBUS inclusion criteria have been described previously.[Citation13] Briefly, patients were refractory to their last prior treatment (PD during or within 60 days) and failed BORT and LEN after at least two consecutive cycles (alone or in combination). Patients were randomized 2:1 to receive 28-day cycles of POM 4 mg on days 1–21 + LoDEX 40 mg (20 mg for patients aged >75 years) weekly or HiDEX 40 mg (20 mg for patients aged >75 years) on days 1–4, 9–12, and 17–20. Treatment continued until confirmed PD or unacceptable toxicity, death, loss to follow-up, or other protocol-specified reasons. Subjects stayed in the study treatment phase after treatment discontinuation for reasons other than PD.
Landmark analyses at the start of C3, C5, and C7 were performed using Kaplan–Meier graphs, adjusted and unadjusted Cox regression models using non crossover adjusted datasets. Survival of patients with SD was compared to that of patients with PD or ≥ PR at the same point in their treatment. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC). These were not pre-specified in the protocol, and no adjustment for multiplicity was made. Efficacy assessments were done in the intent-to-treat population and the safety assessment was done in all patients who received at least one dose of POM + LoDEX.
A landmark analysis identifies patients who survive to a certain time point and only includes those patients in the survival comparison. Assessments were performed every 28 days following randomization with a ±3 day window. End periods were calculated as halfway between the scheduled response assessment and the next response assessment. In the case of C3, C5 and C7, patients must be alive at the start of specific time intervals centered around these cycles (days 56, 112, and 186, respectively), and must provide an assessment within these intervals (otherwise patients were classified as missing). If several assessments fell within these time windows, the assessment used for the classification of response was the first evaluable response assessment following the start of the window (unless the response was PD, which overrides all other responses). Patients who had PD prior to the landmark timeframe would be included in the missing group, rather than in the PD group. Using these sets of patients, Kaplan-Meier graphs of OS and PFS were created. Furthermore, HRs, 95% CIs and p values were calculated for comparing SD patients to patients with ≥ PR or PD. A HR <1.0 indicates that survival is improved vs. SD. Hazard ratios and p values were based on Cox proportional hazards modeling.
As previously described, severity of adverse events (AEs) was graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).[Citation13]
Results
Patient demographics
Between March 2011 and August 2012, the NIMBUS trial randomized 455 patients using a 2:1 allocation ratio. In total, 302 patients received POM + LoDEX and 153 received HiDEX. As expected in this population, patient numbers decreased as the study progressed, thus limiting statistical interpretability at later cycles. In particular, patients in the HiDEX group discontinued from the study earlier than those in the POM + LoDEX group, with reasons for discontinuation being comparable between the treatment arms.[Citation13,Citation21] There were 82, 36, and 23 evaluable patients at the start of C3, C5 and C7, respectively in the HiDEX arm (due to the lower response rate, the 2:1 allocation ratio, and shorter OS vs. POM + LoDEX). Given the limited data for the HiDEX arm, the analysis focused on data from the POM + LoDEX arm.
Based on the NIMBUS trial, baseline patient characteristics showed that treatment groups were balanced, with similar median time from diagnosis, and median number of prior treatments. The majority of the patients were refractory to LEN (95% in the POM + LoDEX group and 92% in the HiDEX group). Two thirds of patients were also refractory to both LEN and BORT.[Citation13] Among the patients who survived to the start of C3 and have a response assessment, in the POM + LoDEX arm, patient characteristics were well balanced across response groups. However, there were trends towards a difference in refractoriness to LEN and LEN/BORT (with PD and SD groups having higher proportions of patients refractory to LEN and both LEN/BORT), and ECOG performance status (PS) (with ≥ PR and PD groups having higher proportion of patients with ECOG PS ≥2 than the SD group) []. Some of these differences may have an impact on the efficacy outcome.
Table 1. Patient demographics in the POM + LoDEX arm at the start of C3Table Footnote*.
Landmark analysis
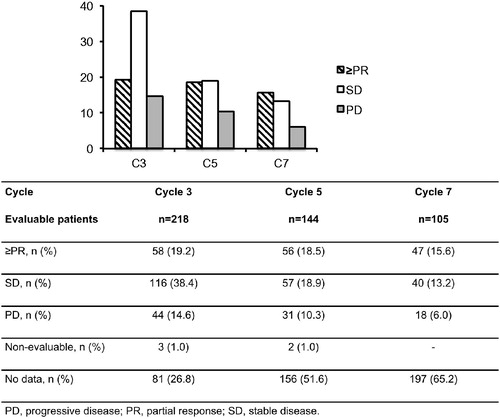
At the start of C3, 19.2% (58/302) of patients randomized to the POM + LoDEX treatment arm had achieved ≥ PR, 38.4% (116/302) had SD, and 14.6% (44/302) had PD. Eighty-four patients (27.8%) had no response data, as defined in the “Methods” section, most due to early discontinuation (including patients who had progressed or died prior to the cycle, or otherwise stopped being assessed for response). At the start of C5, 18.5% (56/302) had ≥ PR, 18.9% (57/302) of POM + LoDEX patients had SD, and 10.3% (31/302) had PD. Respective response rates for the start of C7 were 15.6% (47/302) for ≥ PR, 13.2% (40/302) for SD, and 6.0% (18/302) for PD [].
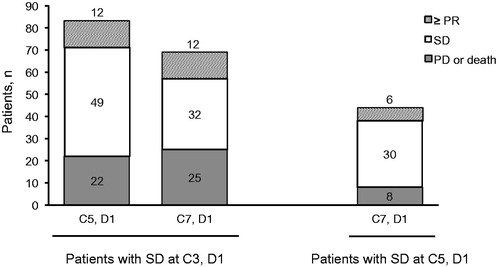
A proportion of patients with SD showed improved responses at later treatment cycles. When evaluating response status, 17.4% (12/69) of patients randomized to POM + LoDEX who had SD after two treatment cycles went on to demonstrate a response (≥PR) by the end of C6, and 13.6% (6/44) of patients who maintained SD for at least four cycles went on to demonstrate a response by the end of C6 (compared with none in the HiDEX treatment group) [].
Overall survival
The median OS from randomization by response status at C3 was 22.4 months for patients who achieved ≥ PR (n = 58, HR 0.66; 95% CI 0.40–1.08, p = 0.0976 vs. SD), 16.2 months for patients who achieved SD (n = 116), and 6.3 months for patients with PD (n = 44, HR 3.43; 95% CI 2.23–5.27, p < 0.0001 vs. SD). There was a significant difference in OS at C3 between the SD and PD group, which was not seen when SD was compared to the ≥ PR group [], even when adjusting for ECOG PS and refractoriness to both LEN and BORT.
Figure 3. Landmark analysis. (A) POM + LoDEX arm; (B) Pooled POM + LoDEX and HiDEX arms.
Start of C7 analysis excludes patients with no data, or who were recorded as progressed/died prior to end of Cycle 2, 4, or 6, respectively. Performed with Kaplan-Meier method and unadjusted Cox regression model. HR: hazard ratio; OS: overall survival; PD: progressive disease; PR: partial response; SD: stable disease
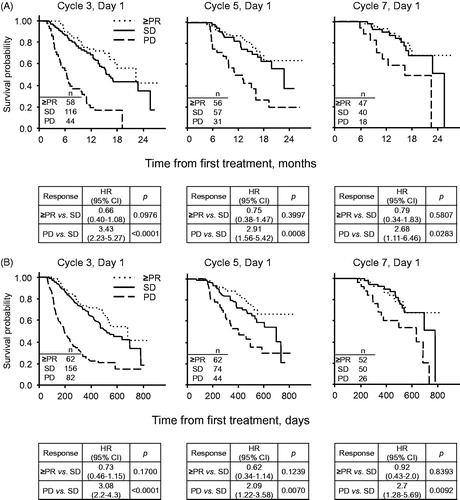
A similar pattern was observed at the start of C5 and C7 []. Median OS by response status at C5 was not reached for patients who had ≥ PR (n = 56, HR 0.75; 95% CI 0.38–1.47, p = 0.3997 vs. SD), 22.9 months for patients with SD (n = 57), and 13.1 months for those with PD (n = 31, HR 2.91; 95% CI 1.56–5.42, p = 0.0008 vs. SD). At the start of C7, median OS by response status was not reached for ≥ PR (n = 47, HR 0.79; 95% CI 0.34–1.83 p = 0.5807 vs. SD), 25.7 months for SD (n = 40), 16.4 months for PD (n = 18, HR 2.68; 95% CI 1.11–6.46, p = 0.0283 vs. SD) []. Patients in the POM + LoDEX arm who had SD after 2, 4 or 6 treatment cycles had similar OS values to responding patients at these time points. After 2, 4, and 6 treatment cycles, patients who had SD had significantly different OS to patients with PD at those same time points.
When analyzing the survival by response status from the pooled data of patients across both treatment arms, the pattern was the same: there were no significant differences in median OS values between patients who achieved SD and those with ≥ PR at all tested time points. For ≥ PR vs. SD comparisons, these were 22.4 months vs. 16.6 months at C3 (HR 0.73; 95% CI 0.46–1.15, p = 0.1700), not reached vs. 22.9 months at C5 (HR 0.62; 95% CI 0.34–1.14, p = 0.1239), and not reached vs. 25.7 months at C7 (HR 0.92; 95% CI 0.43–2.00, p = 0.8393). Moreover, significantly longer survival was observed in patients with SD vs. PD at C3 (16.6 months vs. 6.3 months, HR 3.08; 95% CI 2.2–4.3, p < 0.0001), C5 (22.9 months vs. 13.1 months, HR 2.09; 95% CI 1.22–3.58, p = 0.0070), and C7 (25.7 months vs. 20.9 months, HR 2.7; 95% CI 1.28–5.69, p = 0.0092) [].
Progression-free survival
This analysis also included a PFS evaluation, but due to patient selection into response groups, and to the fairly quick disease progression in PD patients (within two weeks), PFS comparisons across response groups were not useful (data not shown).
Safety
At the start of C3, of the 300 safety population patients randomized to the POM + LoDEX arm, 175 (58.3%) experienced at least one ≥ grade 3 specified treatment-emergent AE (TEAE), including 45/58 (77.6%) of subjects with ≥ PR, 93/116 (80.2%) with SD, and 35/44 (79.5%) with PD []. With the exception of ≥ grade 3 febrile neutropenia, which was 13.8% in patients with ≥ PR, 12.1% in SD patients, and 0% in PD patients, ≥ grade 3 hematological TEAEs were higher in PD patients compared with ≥ PR and SD subgroups. Grade 3/4 hematological TEAEs were as follows in ≥ PR vs. SD patients: neutropenia (46.6% vs. 48.3%), anemia (20.7% vs. 31.0%), thrombocytopenia (13.8% vs. 15.5%), and leukopenia (6.9% vs. 5.2%). No notable differences between subgroups were observed in the remaining non-hematological ≥ grade 3 TEAEs that occurred in ≥5% of patients (pneumonia, fatigue, bone pain, dyspnea, and hypercalcemia) [].
Table 2. Grade ≥3 treatment-emergent adverse events (≥5%) on day 1 of C3.
Discussion
POM has demonstrated good clinical responses with manageable toxicity in patients who are refractory to LEN, BORT, or both when combined with low-dose dexamethasone.[Citation13,Citation16] The current post hoc analysis from the NIMBUS study indicated that treatment with POM + LoDEX provided clinical benefit in SD patients, with similar survival results to responding patients and much better survival than progressing patients. In the POM + LoDEX treatment arm, patients with SD at the start of C3, C5, and C7 had no significant difference in OS compared with patients who achieved at least a PR at those time points. Compared with PD patients, those with SD had a significantly lower risk of death. It is recognized that this analysis is limited by its retrospective design, in which the sample size of pre-defined subgroups reduces as the study progresses, especially in later landmark time points. In addition, the imbalance in certain baseline characteristics (e.g. refractoriness, ECOG PS) among these non-randomized subgroups may have some impact on the results, although none were observed when adjusting for these differences.
In recent years, the proportion of MM patients achieving improved responses in the salvage setting has steadily increased. Other treatment combinations incorporating novel agents are emerging as valuable additions to the limited treatment options available to RRMM patients. Defining optimal treatment for these patients is a dynamic field of research. Aside from POM + LoDEX,[Citation12,Citation13,Citation22] addition of the second-generation proteasome inhibitor carfilzomib to LEN and dexamethasone has shown promise in early relapse based on the results of the ASPIRE trial in patients with relapsed MM who have received 1–3 prior treatments,[Citation23] and the combination of histone deacetylase inhibitor panobinostat with bortezomib and dexamethasone was recently granted approval by the U. S. Food and Drug Administration for the treatment of MM patients who have received at least two prior regimens, including BORT and an immunomodulatory agent.[Citation24,Citation25]
In a study that assessed survival according to the level of reduction in M-protein levels, it was shown that depth of response correlates with improved OS in RRMM patients treated with POM + LoDEX.[Citation16] In the relapsed or refractory setting, several studies have also shown associations between response and clinical outcomes,[Citation20,Citation26–32] including studies that have demonstrated a survival benefit in achieving a minor response.[Citation29,Citation33] In this analysis, we demonstrate that in heavily pretreated RRMM patients, avoiding disease progression has a positive impact on survival outcomes. An individual risk-to-benefit approach that controls disease and ensures quality of life with sequential regimens is worth considering.[Citation34] In selected patients, including heavily pretreated RRMM patients, achievement of any response may be adequate to prolong survival. In addition to the survival and response outcomes reported by San Miguel and colleagues,[Citation13,Citation16] the POM + LoDEX regimen has further been shown to confer sustained quality of life in RRMM patients compared with HiDEX.[Citation21,Citation35] In the present analysis, we further demonstrate that there is a survival benefit of remaining on POM + LoDEX treatment in patients who achieve at least SD.
The main reported grade 3/4 adverse events associated with POM + LoDEX in the NIMBUS trial were neutropenia, anemia and thrombocytopenia.[Citation16] This was confirmed in the current analysis of safety data on the first day of C3, and most grade 3/4 infections were of a respiratory nature (pneumonia, upper respiratory tract infections and bronchitis). Discontinuation of POM + LoDEX because of adverse events was uncommon (9%), suggesting that the combination was generally well tolerated. With appropriate dose modifications, the safety profile of POM + LoDEX was manageable in this heavily pretreated population.[Citation13] No unexpected AEs were observed in the current analysis, with no notable differences reported between the SD and ≥ PR subgroups in grade ≥3 TEAEs occurring at a rate of ≥5%.
The introduction of novel agents in the treatment of MM has improved responses and survival. Although clinical trials are often not designed to assess the correlation between response and survival, response quality is generally accepted to correlate with improved disease control and longer survival. As suggested in quality of life evaluations,[Citation35] some RRMM patients may derive benefit from any response and achieve prolonged survival without facing unnecessary morbidity.[Citation36] The current analysis confirms that heavily pretreated RRMM patients that achieved stabilization of the disease with POM + LoDEX derived a survival benefit similar to responding patients at early assessments.
Summary
In summary, this post hoc analysis showed that continuing POM + LoDEX treatment benefits patients who maintain SD for an extended period of time. Patients who achieved SD at the start of C3, C5 and C7 showed no significant difference in OS benefit compared to responding patients at the same time points. Moreover, some patients with SD at C3 and C5 showed improved responses at later cycles.
Together with the primary efficacy results of the NIMBUS trial, we further demonstrate that POM + LoDEX has manageable toxicity with no unexpected AEs. Myeloma patients are increasingly achieving improved responses to therapy, which translate to better PFS and OS clinical outcomes. In patients with poor responses, typically poor outcomes are observed. In this landmark analysis, we show that POM + LoDEX is an effective treatment option for RRMM patients, including those with SD as best response, highlighting the key importance of treatment response assessment. POM + LoDEX should be a standard of treatment for patients that have received therapy with both LEN and BORT.
ICMJE Forms for Disclosure of Potential Conflicts of Interest
Download Zip (9.3 MB)Acknowledgements
We thank the patients who participated in this study, the staff members at the study sites who cared for them, and the representatives of the sponsors who were involved in data gathering and analyses. We also thank BresMed for providing the statistical analysis, and Dr Vanessa Gray-Schopfer, OmniScience SA, for providing medical writing services funded by Celgene.
Potential conflict of interest
The authors are fully responsible for content and editorial decisions for this manuscript. The authors made substantial contributions to the concept and design of the manuscript; they participated in collection, data analysis, interpretation, and assembly of the data. All the authors revised the article critically for important intellectual content and have given final approval of the version of the manuscript for submission. Disclosure forms provided by the authors are available with the full text of this article at http://dx.doi.org/10.1080/10428194.2016.1180685.
Funding information
Celgene Corporation provided funding for the analysis and for medical writing. Celgene did not collect nor analyze the data.
References
- Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128.
- Dimopoulos MA, Richardson PG, Moreau P, et al. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat Rev Clin Oncol. 2015;12:42–54.
- Pulte D, Jansen L, Castro FA, et al. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol. 2015;171:189–196.
- Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157.
- Boland E, Eiser C, Ezaydi Y, et al. Living with advanced but stable multiple myeloma: a study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J Pain Symptom Manage. 2013;46:671–680.
- Molassiotis A, Wilson B, Blair S, et al. Unmet supportive care needs, psychological well-being and quality of life in patients living with multiple myeloma and their partners. Psychooncology. 2011;20:88–97.
- Mols F, Oerlemans S, Vos AH, et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: results from a population-based study using the PROFILES registry. Eur J Haematol. 2012;89:311–319.
- Poulos AR, Gertz MA, Pankratz VS, et al. Pain, mood disturbance, and quality of life in patients with multiple myeloma. Oncol Nurs Forum. 2001;28:1163–1171.
- Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27:5008–5014.
- Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia. 2010;24:1934–1939.
- Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–1832.
- Leleu X, Attal M, Arnulf B, et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myélome 2009-02. Blood. 2013;121:1968–1975.
- Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066.
- Pomalyst® (pomalidomide) [prescribing information]. Summit, NJ: Celgene Corporation. April 2015.
- European Medicines Agency. Imnovid (pomalidomide). Summary of product characteristics; [cited 2015 May]. Available from: http://www.ema.europa.eu.
- San Miguel JF, Weisel KC, Song KW, et al. Impact of prior treatment and depth of response on survival in MM-003, a randomized phase 3 study comparing pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed/refractory multiple myeloma. Haematologica. 2015;100:1334–1339.
- Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473.
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719.
- Gore SD, Fenaux P, Santini V, et al. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica. 2013;98:1067–1072.
- Harousseau JL, Dimopoulos MA, Wang M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95:1738–1744.
- Song KW, Dimopoulos MA, Weisel KC, et al. Health-related quality of life from the MM-003 trial of pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed and/or refractory multiple myeloma. Haematologica. 2015;100:e63–e67.
- Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961–1967.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152.
- San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–1206.
- Panobinostat. U.S. Food and drug administration; [cited 2015 May 6]. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm435339.htm.
- Niesvizky R, Richardson PG, Rajkumar SV, et al. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol. 2008;143:46–53.
- Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008;19:1160–1165.
- Pineda-Roman M, Zangari M, van Rhee F, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22:1419–1427.
- Quach H, Mileshkin L, Seymour JF, et al. Predicting durable remissions following thalidomide therapy for relapsed myeloma. Leuk Lymphoma. 2009;50:223–229.
- Alegre A, Aguado B, Giraldo P, et al. Lenalidomide is effective as salvage therapy in refractory or relapsed multiple myeloma: analysis of the Spanish compassionate use registry in advanced patients. Int J Hematol. 2011;93:351–360.
- Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560.
- Hussein MA, Baz R, Srkalovic G, et al. Phase 2 study of pegylated liposomal doxorubicin, vincristine, decreased-frequency dexamethasone, and thalidomide in newly diagnosed and relapsed-refractory multiple myeloma. Mayo Clin Proc. 2006;81:889–895.
- Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825.
- Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: a clash of philosophies. Blood. 2011;118:3205–3211.
- Weisel K, Dimopoulos M, Song KW, et al. Pomalidomide and low-dose dexamethasone improves health-related quality of life and prolongs time to worsening in relapsed/refractory patients with multiple myeloma enrolled in the MM-003 randomized phase III trial. Clin Lymphoma Myeloma Leuk. 2015;15:519–530.
- Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28:258–268.