Abstract
Peripheral T-cell lymphomas (PTCLs) are a rare group of lymphoid neoplasms with high relapse rates after initial therapy and poor prognosis. Most patients are aged ≥60 years and are often not candidates for aggressive salvage therapies. Romidepsin, a potent class I histone deacetylase inhibitor, has shown significant single-agent activity in relapsed/refractory PTCL. We evaluated the efficacy and tolerability of romidepsin in elderly patients in this setting. Ninety-five patients aged ≥60 years were identified from 2 prospective phase 2 registration trials of romidepsin, and comparative analyses were performed with younger patients from these trials. Response rates, progression-free survival, and overall survival were not statistically different for younger vs older patients. The toxicity profile in older and younger patients was similar in both trials. Romidepsin demonstrated similar efficacy and tolerability in younger and older patients and presents an attractive treatment option for relapsed/refractory PTCL regardless of age.
Trial registration: Clinicaltrials.gov identifiers: NCT00426764, NCT00007345.
Introduction
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of mature, post-thymic T- and natural killer-cell non-Hodgkin lymphomas (NHLs) associated with a poor prognosis in most subtypes [Citation1,Citation2]. The World Health Organization classification of hematologic malignancies recognizes >15 biologically distinct subtypes that together comprise 5–10% of the estimated 71,850 new cases of NHL diagnosed in the United States in 2015 [Citation1–3]. In an international epidemiologic PTCL study, the median age at diagnosis of PTCL was 62 years [Citation2]. A US epidemiologic study reported similar findings; the median age of all PTCL patients was 62 years and the majority of patients were 60 years or older [Citation4]. Similar to other lymphomas, advanced age has been shown to be a negative prognostic factor for survival in patients with PTCL [Citation2,Citation5].
Patients with relapsed/refractory PTCL have a poor prognosis, particularly those unable to receive salvage combination chemotherapy [Citation1,Citation2,Citation6]. Elderly patients are at increased risk of significant morbidity and mortality from aggressive salvage protocols due to cumulative comorbidities and/or poor performance status. In addition, age is a negative prognostic factor for outcomes after hematopoietic stem cell transplant [Citation7], which is the only curative strategy available after relapse.
Romidepsin is a potent class I selective histone deacetylase (HDAC) inhibitor [Citation8–10] and epigenetic modifier approved by the US Food and Drug Administration for the treatment of patients with PTCL who have received ≥1 prior therapy. Approval of romidepsin for the treatment of relapsed/refractory PTCL was based on results from 2 prospective phase 2 clinical trials [Citation11–13]. In the pivotal phase 2 study of romidepsin in patients with relapsed/refractory PTCL (NCT00426764; N = 131), the objective response rate (ORR) was 25% (15% with confirmed/unconfirmed complete response [CR/CRu]) [Citation11,Citation12]. The median duration of response (DOR) was 28 months [Citation12], with the longest response ongoing at 56 months [Citation14]. The second study, from the National Cancer Institute (NCI; NCT00007345; N = 47) [Citation13], demonstrated an ORR of 38% (18% with CR). The median DOR was 9 months, with the longest response ongoing at 76 months. The most common romidepsin-related adverse events (AEs) in both studies were gastrointestinal or asthenic conditions that were primarily grade 1/2 and did not result in drug discontinuation [Citation11,Citation13,Citation15]. Common grade ≥3 AEs included thrombocytopenia, neutropenia, anemia, leukopenia, fatigue, and pyrexia [Citation16]. The objective of this report was to analyze the efficacy and safety of romidepsin specific to older patients (≥60 years) with relapsed/refractory PTCL in the phase 2 pivotal and NCI trials.
Materials and methods
Study design
The study design and eligibility criteria for these similar multicenter, single-arm, open-label phase 2 studies have been described previously [Citation11,Citation13]. Briefly, both trials enrolled patients with PTCL who relapsed from or were refractory to ≥1 prior therapy. Patients received romidepsin 14 mg/m2 as a four-hour intravenous infusion on days 1, 8, and 15 of 28-day cycles.
Efficacy assessments, response criteria, and safety analyses
In the pivotal trial, the primary endpoint was the rate of CR/CRu as determined by an independent review committee using International Workshop Criteria (IWC) guidelines for response assessments for NHL [Citation17]. Key secondary endpoints included ORR and DOR. In the NCI trial, the primary outcomes were ORR, rate of CR, and DOR determined by investigator assessment using IWC guidelines for NHL in nodal disease and Response Evaluation Criteria in Solid Tumors for skin or visceral lesions [Citation17,Citation18]. Responses in both studies were assessed every two cycles; patients in CR were assessed every three cycles in the NCI study. Patients in the NCI study who discontinued therapy for reasons other than progressive disease (PD) or AE could restart therapy if disease recurred; response durations were censored at the time of first disease recurrence [Citation19]. PFS and OS were prospectively determined only in the pivotal trial. AE severity was graded using the NCI Common Terminology Criteria for Adverse Events, version 3.0 in the pivotal trial and 2.0 in the NCI trial. In the NCI trial, all abnormalities were reported as AEs regardless of clinical significance.
Statistical analyses
The median follow-up was 22.3 months in the pivotal trial and 67.5 months in the NCI trial. Time-to-event data in both studies were summarized by Kaplan–Meier methods. The regression-growth models were based on the assumption that change in tumor quantity during therapy results from two independent component processes: an exponential decrease/regression (d) and an exponential growth/regrowth of the tumor (g) [Citation20,Citation21]. The model for this is f(t) = exp(−d × t) + exp(g × t) – 1, where f(t) is the tumor quantity (sum of the products of perpendicular lymph node diameters) at time t in days, normalized to the starting level at day 0. For data showing continuous decrease from the start of treatment, g is eliminated and the model simplified to f(t)=exp(g×t). We modeled each data set for which >2 data points were available where the ratio of tumor quantity differed by ≥20%. Analysis and output were generated using Base SAS and SAS/STAT software, version 9.1.3 or 9.3 of the SAS System for Windows (SAS Institute Inc., Cary, NC) [Citation22].
Results
Patients
In the pivotal trial, 71 of 131 patients (54.2%) were ≥60 years old; in the NCI trial, 23 of 47 patients (48.9%) were ≥60 years old. Baseline characteristics of the older and younger patient populations were generally similar within each trial (). In the pivotal study, older patients tended to have higher Eastern Cooperative Oncology Group (ECOG) performance status and younger patients had PTCL for a longer duration and were more likely to have received prior transplant. The distribution of PTCL subtypes varied by age in both studies.
Table 1. Patient demographics and characteristics.
Efficacy
Response rates and DOR were similar between older and younger patient populations within each trial (, Supplemental Figure 1). In both trials, median times to first response were <60 days in both the older and younger populations. The median DOR in the pivotal trial was not evaluable (NE) in the older or younger patient population at this data cut (p = .99), with the longest response ongoing at 56 months in a 61-year-old woman with angioimmunoblastic T-cell lymphoma (AITL). In the NCI trial, the median DOR was 5 months and 12 months for the older and younger patient populations, respectively (p = .55), with the longest response ongoing at 76 months in a 50-year-old woman with CD30+ lymphoma.
Figure 1. Waterfall plots for best response in the (A) pivotal and (B) National Cancer Institute (NCI) trials. Blue bars represent younger patients and red bars represent older patients. For the pivotal trial, nodal responses are shown, while in the NCI trial, the composite responses including both nodal and non-nodal disease are shown. Hash marks indicate breaks in the y-axis. Patients with complete response have been normalized to −100%. It is evident that patients aged ≥60 can be found at both ends of the spectrum.
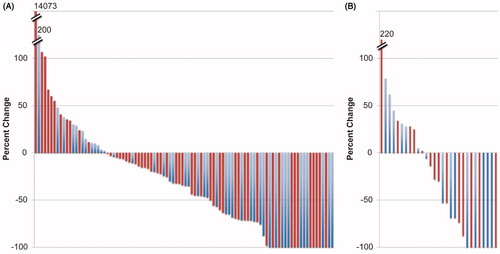
Table 2. Response rates.
Of the 10 patients ≥60 years old who achieved CR/CRu in the pivotal trial, six had a DOR of ≥12 months (Supplemental Figure 2). Of the three patients ≥60 years old who achieved CR in the NCI trial, one had a DOR of ≥12 months (55 months). Of 13 total patients ≥60 years old who achieved a CR in both trials, only one relapsed while on romidepsin treatment within <12 months.
Figure 2. Kinetic analysis for patients aged <60 and ≥60 years in the pivotal trial. Lymph node measurements obtained at baseline and restaging were summed at each time point and then equations were applied [Citation20] to determine growth and regression rate constants (g and d, respectively). (A) A patient with first regression and then progression, where both g and d could be determined. (B) A patient in whom only d, regression rate, could be determined. (C) A patient in whom only g, growth rate, could be determined. (D) Data are summarized in this panel. (E) Calculated rates of d, tumor shrinkage, and g, growth, are shown in box plots. As shown, there was no statistical difference between the two age groups. IQR: interquartile range.
![Figure 2. Kinetic analysis for patients aged <60 and ≥60 years in the pivotal trial. Lymph node measurements obtained at baseline and restaging were summed at each time point and then equations were applied [Citation20] to determine growth and regression rate constants (g and d, respectively). (A) A patient with first regression and then progression, where both g and d could be determined. (B) A patient in whom only d, regression rate, could be determined. (C) A patient in whom only g, growth rate, could be determined. (D) Data are summarized in this panel. (E) Calculated rates of d, tumor shrinkage, and g, growth, are shown in box plots. As shown, there was no statistical difference between the two age groups. IQR: interquartile range.](/cms/asset/d9d1a808-ebc4-4176-9840-a4964dd09fee/ilal_a_1295143_f0002_c.jpg)
Most evaluable patients in both trials had an overall decrease in nodal disease. Older patients were not clustered toward one end of the waterfall plot but were dispersed throughout (). A kinetic analysis was performed using the computed tomography (CT) measurements to compare response in older and younger patients (). This kinetic analysis provides rates of both tumor regression and growth (d and g, respectively) by devolving the curves obtained from CT measurements into these two components [Citation20] and offers another approach to examining the activity of a therapeutic agent [Citation21]. Three examples of tumor growth patterns were observed (). When rates of regression and growth (d and g, respectively) were calculated, no difference was found for older vs younger patients (). Together, these results suggest no inherent differences between the lymphomas and responses to romidepsin in the older and younger populations.
Survival
In the pivotal trial, the median PFS was 4.6 months in the older population and 3.7 months in the younger population (p = .92; Supplemental Figure 1E). The median OS was 11.8 months in both the older and younger populations (p = .98; Supplemental Figure 1F). Survival data were not prospectively collected in the NCI trial.
Safety
The AE profiles with romidepsin treatment were similar between the older and younger populations within each trial; the rates of any AE (p = .38 and p = 1.00, respectively) or any grade ≥3 AE (p = .46 and p = .42, respectively) were not statistically different in the pivotal and NCI trials. The most common AEs were gastrointestinal, hematologic, or infectious in origin (Supplemental Table 1). The most common grade ≥3 AEs were hematologic abnormalities (thrombocytopenia, neutropenia, leukopenia, and anemia) or infections of any kind (). No individual infection type occurred in ≥10% of patients, and most infections reported with romidepsin were not drug related [Citation11]. There were no clinically meaningful changes in median hemoglobin, neutrophils, leukocytes, or platelets across multiple treatment cycles in the younger or older populations (Supplemental Figure 3). For the majority of both older and younger patients in the pivotal trial, platelet count initially declined and then recovered between each treatment cycle. Due to differences in protocol requirements, AEs were reported more frequently in the NCI trial.
Table 3. Grade ≥3 adverse events.
Patient disposition and deaths
Older patients experienced similar dose reductions and number of cycles received compared with the younger population within each trial (Supplemental Table 2). In the pivotal trial, five of eight deaths within 30 days of the last romidepsin dose were in patients who were ≥60 years old (PD in two patients and related to infection in three patients). Death was possibly related to treatment in one patient, a 77-year-old man with stage IV PTCL-not otherwise specified (NOS). He received two doses of romidepsin prior to withdrawing consent and died of sepsis leading to multiorgan failure ≈3 weeks after his last dose. In the NCI trial, five of seven deaths within 30 days of last romidepsin dose were in patients who were ≥60 years old (PD in four patients and sudden/unexpected death in one patient). The patient who died suddenly was a 71-year-old man with a history of extensive atherosclerotic disease. He experienced CR with romidepsin but died in his sleep three days after the second dose of the fifth treatment cycle.
Discussion
Romidepsin is an effective agent in the treatment of relapsed/refractory PTCL. The data presented herein demonstrate its efficacy and safety in older patients. No safety signal was observed for cardiac effects in patients aged ≥60 years despite the known age-related increase in underlying disease. The cardiac effects of romidepsin have been carefully studied, demonstrating that clinically insignificant changes in QTc were likely exaggerated by transient increases in heart rate and concomitant administration of QT-prolonging antiemetics [Citation11,Citation12,Citation23–27]. Exclusions for significant cardiac disease were put into place in the clinical trials along with monitoring of potassium and magnesium levels; no further cardiac safety concerns were raised once these precautions were instituted.
In contrast to bone marrow toxicity, which can be dose limiting with conventional chemotherapies, thrombocytopenia associated with HDAC inhibitors is not cumulative and does not appear to result from cytotoxicity to bone marrow progenitors but has been related to decreased platelet release from megakaryocytes [Citation28]. The analysis in Supplemental Figure 3 demonstrated an impact of romidepsin on platelet count consistent with that mechanism and underscored the absence of cumulative hematologic toxicity. It should be noted that inclusion criteria for both the pivotal and NCI trials specified an ECOG performance status ≤2. Therefore, this analysis did not capture elderly (or young) patients with a relatively poorer performance status (≥3), nor did it include the potential impact of specific comorbidities.
Our analysis showing no differences in response kinetics in the older vs younger population suggests that the impact of age on treatment outcomes in PTCL was unlikely to be due to differences in disease biology but rather due to approaches to overall management and poor treatment tolerance by older patients in previous trials. Although predictive biomarkers of response to romidepsin are not yet available, these data also suggest that future identification of such biomarkers would equally benefit both older and younger patients.
The more difficult question for patients ≥60 years is what to do in those who have experienced a deep PR or a CR and are not candidates for stem cell transplant. In the pivotal trial, due to prolonged responses in some patients, the protocol was amended to allow for (but not mandate) maintenance dosing at twice per cycle for patients treated for ≥12 cycles and once per cycle for patients who had received two doses per cycle for ≥6 cycles through at least cycle 24 [Citation12]. A recent assessment of patients with AITL from the pivotal study showed that all five of the patients with responses ≥12 months received maintenance dosing, and the patient with the longest response ongoing at 56+ months had been receiving once-per-cycle dosing since cycle 23 [Citation14]. However, there have been no controlled trials to formally assess whether romidepsin should be administered on a maintenance schedule, discontinued and restarted at PD, or some hybrid of these. Understanding the molecular biology that leads to responses and developing sensitive methodology for minimal residual disease monitoring could help in answering these questions.
Together, the results presented herein support the use of romidepsin in patients ≥60 years old, suggesting that safety and efficacy are not significantly impacted by patient age. Given that the median age at diagnosis of PTCL is 62 years, and that advanced age is a negative prognostic factor for survival in patients with PTCL, further studies should focus on optimizing the use of romidepsin as a single agent or in combination regimens in older patients.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at http://dx.doi.org/10.1080/10428194.2017.1295143.
ICMJE Forms for Disclosure of Potential Conflicts of Interest
Download Zip (14 MB)Acknowledgements
The authors take full responsibility for the content of this article, but thank William Ho, PhD (MediTech Media), for providing medical editorial assistance.
References
- Horwitz SM. Management of peripheral T-cell non-Hodgkin's lymphoma. Curr Opin Oncol. 2007;19:438–443.
- Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130.
- American Cancer Society. Cancer facts & figures; 2015. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf
- Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T-cell lymphoma incidence and survival in the United States. J Clin Oncol. 2016;34:963–971.
- A predictive model for aggressive non-Hodgkin's lymphoma. The International non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994.
- Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976.
- Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32:3249–3256.
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784.
- Bradner JE, West N, Grachan ML, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243.
- Tan J, Cang S, Ma Y, et al. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol. 2010;3:5.
- Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636.
- Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11.
- Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834.
- Pro B, Horwitz SM, Prince HM, et al. Romidepsin induces durable responses in patients with relapsed or refractory angioimmunoblastic T-cell lymphoma (AITL). Hematol Oncol. [cited 2016 Jul 12]. DOI:10.1002/hon.2320
- ISTODAX (romidepsin) [package insert]; 2014. Summit, NJ: Celgene Corporation; 2014.
- Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma. [cited 2016 Nov]:[14 p.]. DOI:10.1080/10428194.2016.1247956
- Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216.
- Bates SE, Eisch R, Ling A, et al. Romidepsin in peripheral and cutaneous T-cell lymphoma: mechanistic implications from clinical and correlative data. Br J Haematol. 2015;170:96–109.
- Stein WD, Wilkerson J, Kim ST, et al. Analyzing the pivotal trial that compared sunitinib and IFN-alpha in renal cell carcinoma, using a method that assesses tumor regression and growth. Clin Cancer Res. 2012;18:2374–2381.
- Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–917.
- Burotto M, Wilkerson J, Stein W, et al. Continuing a cancer treatment despite tumor growth may be valuable: sunitinib in renal cell carcinoma as example. PLoS One. 2014;9:e96316.
- Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773.
- Sager PT, Balser B, Wolfson J, et al. Electrocardiographic effects of class 1 selective histone deacetylase inhibitor romidepsin. Cancer Med. 2015;4:1178–1185.
- Noonan AM, Eisch RA, Liewehr DJ, et al. Electrocardiographic studies of romidepsin demonstrate its safety and identify a potential role for K(ATP) channel. Clin Cancer Res. 2013;19:3095–3104.
- Shah MH, Binkley P, Chan K, et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2006;12:3997–4003.
- Cabell C, Bates S, Piekarz R, et al. Systematic assessment of potential cardiac effects of the novel histone deacetylase (HDAC) inhibitor romidepsin. Blood. 2009;114:3709.
- Bishton MJ, Harrison SJ, Martin BP, et al. Deciphering the molecular and biologic processes that mediate histone deacetylase inhibitor-induced thrombocytopenia. Blood. 2011;117:3658–3668.
