Abstract
The development of clinically functional chimeric antigen receptor (CAR) T cell therapy is the culmination of multiple advances over the last three decades. Axicabtagene ciloleucel (formerly KTE-C19) is an anti-CD19 CAR T cell therapy in development for patients with refractory diffuse large B cell lymphoma (DLBCL), including transformed follicular lymphoma (TFL) and primary mediastinal B cell lymphoma (PMBCL). Axicabtagene ciloleucel is manufactured from patients’ own peripheral blood mononuclear cells (PBMC) during which T cells are engineered to express a CAR that redirects them to recognize CD19-expressing cells. Clinical trials have demonstrated the feasibility of manufacturing axicabtagene ciloleucel in a centralized facility for use in multicenter clinical trials and have demonstrated potent antitumor activity in patients with refractory DLBCL. Main acute toxicities are cytokine release syndrome and neurologic events. Axicabtagene ciloleucel holds promise for the treatment of patients with CD19-positive malignancies, including refractory DLBCL.
Introduction
Rationale for and description of axicabtagene ciloleucel
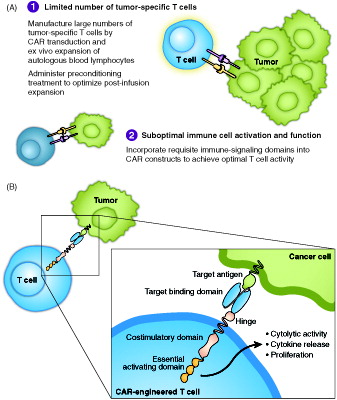
The immune system is the body’s main defense against infection and has been shown to harbor the potential to recognize and kill cancer cells [Citation1]. T cells have emerged as central players in the immune response to cancer [Citation2]. Upon engagement of tumor cells by T cells expressing tumor antigen-specific T cell receptors (TCRs), the T cells are activated, undergo clonal proliferation to broaden the attack, and release cytokines that can enhance antitumor activity, ultimately leading to the lysis of tumor cells. However, the immune system’s ability to eradicate cancer cells is limited by several obstacles, including insufficient numbers of T cells specific to tumor-specific antigens, a series of immune checkpoints that limit full T cell activation against cancer, and the immune suppressive tumor microenvironment (). Chimeric antigen receptor (CAR) T cell therapy may overcome some of the main limitations of the endogenous immune system and of other cancer immunotherapies, providing a new avenue to treat cancer. CARs are synthetic immunoreceptors whose extracellular domain is typically an antibody-derived single chain variable fragment (scFv) that recognizes a tumor cell surface protein. The scFv is linked to intracellular signaling components derived from T lymphocytes, including the key intracellular signaling domain of the TCR CD3ζ subunit [Citation3]. Although essential for T cell activation, signaling via CD3ζ is not sufficient for optimal T cell function. Therefore, current CARs also include the intracellular signaling domain of a costimulatory receptor, such as CD28 or 4-1BB, which provides additional signals to optimize performance () [Citation4,Citation5]. CARs redirect T cells to target tumor cells in a major histocompatibility complex-independent fashion, ideally sparing normal cells, but at a minimum limiting on-target/off-tumor activity to nonessential tissues, depending on the antigen target. CARs are typically introduced to patients’ blood-derived T cells ex vivo via a viral vector system. CAR T cells are then expanded and administered back to patients who have been conditioned with a lymphodepleting chemotherapy regimen that primes the patient for optimal CAR T cell in vivo expansion and antitumor activity [Citation6–8].
Figure 1. Key hurdles of antitumor immunity in B cell malignancies and how CAR technology may solve them (A), and schematic representation of a CAR (B).
The development of axicabtagene ciloleucel originated from earlier preclinical work conducted by James Kochenderfer and Steven A. Rosenberg at the Surgery Branch of the National Cancer Institute (NCI, Bethesda, MD). The CD19-specific CAR of axicabtagene ciloleucel comprises an extracellular scFv specific for CD19 and the signaling domains of CD3ζ and CD28, and was first described by Kochenderfer and colleagues in 2009 [9]. That study demonstrated that primary human T cells expressing this anti-CD19 CAR could produce cytokines specifically in response to CD19 + target cells and efficiently kill primary chronic lymphocytic leukemia (CLL) cells in vitro. In a later study, a CAR of similar design, but specific for murine CD19 and bearing intracellular signaling domains of murine origin, was evaluated in an immune competent syngeneic mouse model of lymphoma [Citation10]. Potent antilymphoma activity of anti-CD19 CAR T cells was observed together with the expected on-target/off-tumor effect of normal B-cell aplasia. These preclinical studies laid the groundwork for the first clinical report to describe successful anti-CD19 CAR T cell therapy [Citation11].
Based on the early CD19 CAR experience from the NCI, axicabtagene ciloleucel has been generated using a modified manufacturing process optimized for multicenter clinical trials in aggressive non-Hodgkin lymphoma (NHL) and other indications and for potential future commercial applications.
Epidemiology and treatment of aggressive B cell lymphomas
NHL is the seventh most common cancer in the United States, with an estimated 73,000 new cases in 2016 [Citation12] and several subtypes. The most prevalent subtype of NHL is diffuse large B cell lymphoma (DLBCL), an aggressive disease that accounts for 30–40% of NHL cases [Citation13]. Transformed follicular lymphoma (TFL) and primary mediastinal B cell lymphoma (PMBCL) are rarer types of NHL [Citation14,Citation15]. TFL is an aggressive form of the generally indolent follicular lymphoma (FL) that develops in 2–3% of FL cases per year [Citation16]. Although the median overall survival (OS) of FL is 15 to >20 years [Citation17,Citation18], the median OS of TFL is poor, ranging from 2.5 months to 2.5 years [Citation14]. PMBCL constitutes 2–4% of NHL cases [Citation15] and is also associated with poor outcomes [Citation15,Citation19].
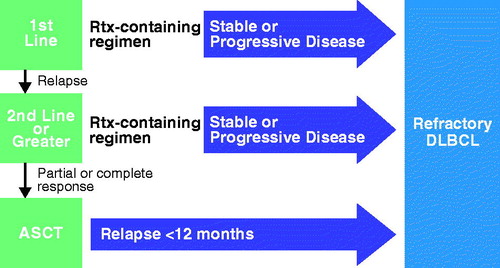
First-line treatment for patients with DLBCL, PMBCL, or TFL generally consists of combination therapy with rituximab and chemotherapy [Citation13,Citation19,Citation20]. Most new cases of DLBCL are cured with standard of care front-line therapy, but 10–15% of patients experience primary refractory disease and 20–30% of patients who do respond to front-line treatment will relapse [Citation13]. Patients who fail treatment with standard rituximab and chemotherapy regimens can receive a second course of remission-induction (salvage) chemoimmunotherapy followed by autologous stem cell transplant (ASCT; ) [Citation13], but outcomes are variable. More than two-thirds of patients (64–80%) do not achieve a satisfactory response to salvage therapy [Citation21] and are thus ineligible for consolidation with ASCT. Even with ASCT, 3-year PFS is approximately 50% [Citation22], and overall, only about 10% of patients who relapse after first-line R-CHOP are cured of DLBCL with ASCT [Citation23]. Patients with relapsed DLBCL who are ineligible for ASCT because of comorbidities have no remaining potentially curative options. In contrast to outcomes in patients who experience a first relapse, outcomes for patients who are refractory to first-line treatment, who become refractory to second- or later-line salvage therapy, or who relapse within 12 months after ASCT are homogeneous and poor. A recent, large pooled analysis of patient-level data inclusive of 635 patients with refractory DLBCL called SCHOLAR-1 reported an overall response rate (ORR) to the next line of treatment of 26% and a median OS of 6.3 months in patients with refractory DLBCL [Citation21]. This uniformly poor outcome represents a significant unmet medical need, and patients in this clinical predicament are in need of novel and efficacious treatment approaches. It is in patients with refractory aggressive NHL that axicabtagene ciloleucel is being investigated in the ZUMA-1 clinical trial.
Manufacturing axicabtagene ciloleucel
CAR T cell production processes at academic centers conducting single-center trials have common features, including collection of T cell-containing starting material, selection and activation of T cells, introduction of a new genetic element containing the CAR gene, expansion of cells, and final formulation of the CAR T cell product for patient administration. The manufacturing process for axicabtagene ciloleucel is based on an improvement of this general approach that is aligned with current Good Manufacturing Practices (GMP) and has potential for use on a commercial scale.
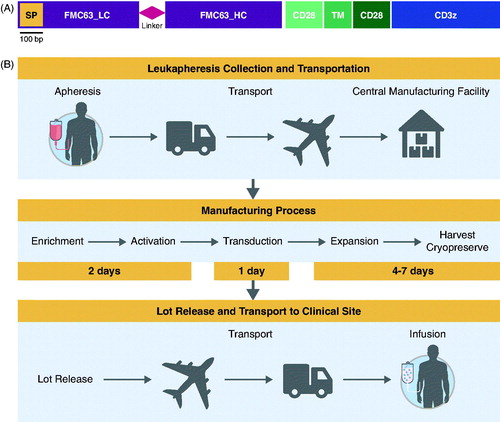
In general, CAR T cell manufacturing begins with outpatient T cell harvesting through leukapheresis, during which peripheral blood mononuclear cells (PBMC) are collected from blood, usually via peripheral venous access. T lymphocytes can be selected and activated using magnetic beads or, alternatively, separated from other blood cells on a density gradient followed by stimulation through their TCR. Delivery of the CAR gene () into activated T cells has most frequently involved a viral transfer vector, usually retroviral or lentiviral vectors. Alternative strategies including electroporation of vector-plasmid DNA and transposon-based systems [Citation24,Citation25] have had limited success due to inefficiency in stably expressing CAR genes. Viral vectors are incapable of self-replication but can infect and stably integrate the CAR gene into the T cell genome via a process known as transduction, yielding T cells that are permanently genetically modified to express the CAR molecule. To achieve the final dose, cells are expanded in culture bags, flasks, or bioreactor systems. Many of the frequently used production systems have been recently reviewed [Citation26]. Furthermore, manufacturing processes have been improved for current GMP compliance and optimized to support multicenter clinical trials.
Figure 3. CAR gene (A) and manufacturing process (B) for axicabtagene ciloleucel. (A) Graphical representation of the CAR gene introduced into human T cells using a retroviral vector to make axicabtagene ciloleucel. The gene encodes a protein of ∼54 kDa. SP indicates the position of the signal peptide. CD28-TM-CD28 indicates the position of the extracellular, transmembrane (TM), and intracellular regions of CD28, respectively. bp: base pairs; LC: light chain; HC: heavy chain. (b) Schematic overview of the vein to vein axicabtagene ciloleucel production process. The process begins with collection of blood cells at the clinical center. Patient material is then transported to the central manufacturing site where axicabtagene ciloleucel is produced. T cells in the incoming leukapheresis material are enriched on a closed-system density gradient. T cells are then activated with anti-CD3 antibody in the presence of IL-2 for 48 h, after which time they become receptive to transduction with a gamma-retroviral vector that encodes the anti-CD19 CAR gene. After transduction, cells are expanded until a target dose is achieved (2 × 106 CAR-positive cells per kg body weight). After product release, the final product is returned to the clinical center. Door-to-door turn around time is approximately 2 weeks.
The anti-CD19 CAR T cell production method [Citation9] used to support initial trials at the NCI, as well as at other academic institutions, has largely relied on numerous manual, open-process steps, and cell culture media supplemented with human serum to achieve the clinical dose. This approach limits the ability to support large, multicenter clinical trials, and to scale for commercial cell production where many autologous products will need to be produced concurrently. Therefore, studies were completed to streamline and optimize the original NCI production process. Human serum was removed to minimize risk of viral contamination, process steps were moved from open- to closed-system operations to minimize the risk of microbial contamination, and additional steps were standardized to maximize process consistency. Furthermore, an extended ex vivo expansion of T cells was felt to be potentially detrimental by driving them into exhaustion [Citation27] and thus, a shorter engineered manufacturing process was desirable [Citation28]. The optimized axicabtagene ciloleucel manufacturing method is now able to produce CAR T cells on a large scale in a rapid 6–8 d functionally closed process. [Citation29].
In the ongoing ZUMA-1 clinical trial, apheresis material is collected at the investigational site using standard apheresis equipment, such as Cobe® Spectra, Spectra Optia®, Fenwal™, Amicus®, or equivalent. The apheresis process generally takes 3–4 h, corresponding to approximately 10–20 L of recirculated blood and generates approximately 100–500 mL of apheresis material collection. The apheresis material collection bag is then shipped at 1–10 °C to the central manufacturing facility using validated shipping containers, where it is processed to enrich for the T cell-containing PBMC fraction on a closed-system Ficoll™ gradient (). T cells in the PBMC fraction are then activated using an anti-CD3 monoclonal antibody and cultured in serum-free medium containing 300 IU/mL of interleukin (IL)-2. Magnetic beads are not used for either cell selection or activation. Activated T cells are transduced with a retroviral vector bearing the anti-CD19 CAR gene and are then further expanded [Citation30]. As the CAR T cells are cultured and expanded, the total number of CAR T cells is monitored for attainment of the target dose, which, for the ZUMA-1 clinical trial, is 2 × 106 CAR T cells/kg of body weight. Once the target dose of axicabtagene ciloleucel has been achieved, the product is then washed and cryopreserved. Each CAR T cell lot undergoes a series of batch-release tests to ensure the product is functional, sterile, and free of other contaminants, including replication-competent retroviral particles. After acceptance and product-release criteria are met, the CAR T cell product is prepared for shipment back to the investigational clinical sites using a validated cryoshipper. This process is GMP compliant and is being scaled to meet potential commercial demand.
The axicabtagene ciloleucel manufacturing process achieves a very high success rate and allows for the generation of CAR T cells despite interpatient variability in apheresis material composition and baseline lymphocyte counts (baseline ALC ≥100/μL), even in cases of severe lymphopenia [Citation29,Citation30]. In Phase 2 of ZUMA-1, product was delivered to the site for the 111 patients in the full analysis set, including product manufactured from a second apheresis procedure for one patient. The cell product for one patient who was enrolled, but not treated, was lost during manufacture due to equipment failure. In an analysis of apheresis and the axicabtagene ciloleucel final product, the final product contains a population of CAR T cells that express markers associated with central memory or effector memory T cells [Citation29].
Clinical experience with axicabtagene ciloleucel
Conditioning for receipt of axicabtagene ciloleucel: optimizing lymphodepletion
While axicabtagene ciloleucel is being produced in the central manufacturing facility, the patient is given a 3-d course of conditioning chemotherapy in preparation for the CAR T cell infusion. The choice of conditioning chemotherapy regimen before CAR T cell reinfusion has emerged as a critical determinant of CAR T cell efficacy. The main mechanisms by which conditioning increases antitumor responses are believed to be the removal of cellular sinks of prosurvival and T cell-activating cytokines, such as IL-15, driving their serum concentration up, and possibly creating a favorable cytokine environment for CAR T cell expansion and survival [Citation31,Citation32]. Other beneficial effects of conditioning chemotherapy include decreasing immunosuppressive regulatory T cells, activating antigen-presenting cells, and inducing proinflammatory tumor cell damage [Citation31].
Many different conditioning chemotherapy regimens have been utilized in patients treated in CAR T studies. However, it appears that not all such regimens are equally effective at creating the ideal environment for CAR T cell engraftment and target cell killing. Early work performed at the NCI led to the hypothesis that cyclophosphamide and fludarabine were critical in creating the optimal environment for robust and rapid engraftment of adoptively transferred T cells [Citation8,Citation33,Citation34]. To reduce toxicity and enable easier evaluation of CAR T cell therapy compared with the conditioning regimen, Kite Pharma, Inc., Santa Monica, CA, in collaboration with the NCI systematically evaluated lower doses of conditioning therapy [Citation32,Citation35]. It was discovered that anti-CD19 CAR T cell therapy after low-dose cyclophosphamide (300–500 mg/m2) and fludarabine (30 mg/m2) daily for 3 d resulted in significant activity against advanced lymphomas, with comparable response rates to high-dose conditioning [Citation32,Citation35].
Additionally, low-dose conditioning with cyclophosphamide and fludarabine results in greater in vivo expansion and higher response rates compared with cyclophosphamide alone [Citation36]. Although the exact mechanism of optimal expansion is still under investigation, cyclophosphamide and fludarabine conditioning leads to increased levels of systemic IL-15 as well as induction of certain proinflammatory cytokines and chemokines that could facilitate CAR T cell activity [Citation37]. Although elevated IL-15 levels are associated with high-grade cytokine release syndrome (CRS) and neurologic events (NEs), elevated IL-15 levels have also been correlated with increased CAR T cell expansion and disease remissions [Citation32]. Improved understanding of the underlying mechanism of in vivo CAR T cell expansion is needed to further maximize responses and reduce adverse events.
Once the outpatient conditioning chemotherapy regimen is complete, the dose of CAR T cells is then thawed at the bedside and infused into the patient. This is often performed in an inpatient setting so that patients can be monitored for toxicity [Citation37].
Initial clinical experience with CAR T cells in B cell malignancies
As manufacturing of axicabtagene ciloleucel evolved to a process ultimately amenable to multicenter clinical use, parallel clinical testing at the NCI was aimed at exploring the activity of the modified anti-CD19 CAR T cell product in small, single-center clinical trials. The first patient to be treated with an anti-CD19 CAR T cell product to achieve a clinical response was reported in 2010 by James Kochenderfer and colleagues at the NCI [Citation11]. Further follow-up on this patient was also subsequently reported as part of a group of eight patients treated at the NCI with the same CAR construct that was eventually used to make axicabtagene ciloleucel [Citation38]. These patients were treated for multiple-relapsed indolent B-cell NHL and CLL and were given high doses of lymphodepleting chemotherapy prior to CAR T cell infusion consisting of 60 mg/kg of cyclophosphamide on days −7 and −6 and 25 mg/m2 of fludarabine on days −5 through −1. A single CAR T cell dose of 3 to 30 × 106 CAR T cells/kg of body weight was infused on day 0 and was followed 3 h later by an intravenous infusion of IL-2 at a dose of 720,000 international units/kg that was repeated every 8 h until toxicity precluded additional doses [Citation38]. The NCI team reported objective responses in six out of eight patients (75%) that lasted a minimum of 7 months with 2 ongoing beyond a year (1 PR ongoing ≥18 months and 1 CR ongoing ≥15 months). Toxicity was significant and consisted of prolonged B-cell aplasia lasting 6 months or more in four out of eight patients as well as several acute toxicities including hypotension, fever, fatigue, renal failure, and obtundation that generally peaked by 8 d after cell infusion and resolved over time. One patient of this first group of eight died 18 d after cell infusion due to culture-proven influenza-A pneumonia, nonbacterial thrombotic endocarditis, and cerebral infarction.
In an effort to reduce the acute toxicity associated with this regimen, the next group of patients treated at the NCI with this CAR construct was given lower cell doses (1–5 × 106 CAR T cells/kg of body weight) without postinfusion IL-2 [Citation35]. Additionally, the manufacturing process used to generate these CAR T cell products was modified in several ways to make the process faster and more reliable. These patients had either multiple-relapsed low-grade NHL or CLL as in the first group or, notably, aggressive NHL relapsed from or refractory to prior therapies. The rate and durability of objective responses in this group was similar to that of the first group with 12 of 13 evaluable treated patients achieving a complete response (CR) or partial response (PR) with duration of response ranging from 1 to 23+ months. Of the evaluable patients with aggressive NHL (4 with DLBCL and 3 with PMBCL), 5 achieved a CR (). Significant toxicity, including B-cell aplasia and acute toxicities, was again observed. Four of the 15 patients evaluable for safety experienced grade 3 or 4 hypotension, and 3 out of the 15 had significant NEs including confusion and obtundation. One patient died 16 d after cell infusion from a suspected cardiac arrhythmia and was not experiencing signs of CRS at the time of death. As in the first group, acute toxicities generally resolved within 3 weeks after cell infusion. In a long-term follow up study of these patients, duration of 4 of the 5 CRs ranged from 38+ to 56+ months [Citation39].
Table 1. Axicabtagene ciloleucel in single-center and national clinical trials.
More recently, the NCI, with research support from Kite Pharma, reported similar results with anti-CD19 CAR T cell therapy and low-dose conditioning like that used in the ZUMA-1 trial (300 or 500 mg/m2 of cyclophosphamide and 30 mg/m2 of fludarabine, each given daily on days −5, −4, and −3) in 22 patients with aggressive B cell lymphomas (19 with DBLCL, 2 with TFL, 1 with mantle cell lymphoma) [Citation32]. The ORR for DLBCL was 73%, including 55% of patients achieving a CR. CRs appeared to be durable and, at the time of the report, all were ongoing with a range of ≥7 to ≥24 months () [Citation32]. Although 4 out of 22 patients (18%) had grade 3 or 4 hypotension and 12 of 22 patients (55%) experienced grade 3 or 4 NEs, including confusion, dysphasia, encephalopathy, and gait disturbances, these acute toxicities generally resolved [Citation32].
Using the same anti-CD19 CAR construct as the NCI used but incorporating the optimized manufacturing process for axicabtagene ciloleucel discussed above in a multicenter phase 1–2 clinical trial, Locke and colleagues reported rapid and durable responses with axicabtagene ciloleucel in refractory aggressive B cell NHL in the phase 1 portion of ZUMA-1 [Citation40]. The ORR was 71% (5/7) with 57% (4/7) of patients achieving a CR, consistent with results from the earlier NCI studies [Citation32,Citation35,Citation38]. All responding patients achieved a response by 1 month after infusion, and three of four patients with CRs had ongoing CRs of 12+ months at publication (). All patients experienced NEs, and 86% of patients experienced CRS, with 57 and 14% experiencing grade 3 or 4 NEs and CRS, respectively [Citation40]. One patient experienced a dose-limiting toxicity consisting of life-threatening NE and CRS that was complicated by pseudomonal sepsis and profound thrombocytopenia and neutropenia. This patient subsequently experienced a fatal intracranial hemorrhage 0.5 months after cell infusion. In all other patients, all axicabtagene ciloleucel-related CRS and NEs resolved.
Consistent results were reported at the 101-patient primary analysis of phase 2 of ZUMA-1, with an ORR of 82%, including a CR rate of 54% in patients with DLBCL (n = 77) and TFL or PMBCL (n = 24) and ≥6 months of follow-up [Citation41]. Across all patients, 13 and 28% of patients experienced grade ≥3 CRS or NE, respectively. The same anti-CD19 CAR construct is being evaluated in three other studies of standalone CAR T cell therapy following conditioning chemotherapy for the treatment of mantle cell lymphoma and adult and pediatric acute lymphoblastic leukemia (NCT02601313, NCT02614066, and NCT02625480, respectively) [Citation42–44]. Additionally, axicabtagene ciloleucel is currently being investigated in a study of patients with refractory DLBCL evaluating conditioning chemotherapy and CAR T cells followed by a limited course of PD-L1 blockade with atezolizumab (NCT02926833).
Pharmacokinetic and pharmacodynamic effects of axicabtagene ciloleucel
Across CAR constructs, specific biomarkers provide insights into the mechanism of action of anti-CD19 CAR T cell therapy and may emerge as predictors of outcome. Anti-CD19 CAR T expansion in the blood occurs during the first 1 to 2 weeks postinfusion, followed by a contraction phase. Of note, the magnitude of peak anti-CD19 CAR T cell expansion following infusion is emerging as a key predictor of clinical response across CAR T cell products and in different CD19-expressing malignancies, underscoring the importance of CAR T cell expansion postinfusion [Citation32,Citation36,Citation45–47].
A related concept is the length of time CAR T cells remain in circulation following infusion, frequently referred to as persistence. Data regarding the role of long-term persistence in durable remissions are inconclusive. Researchers at the NCI showed complete eradication of CD19-positive CLL cells along with elimination of normal B cells only to observe a spontaneous repopulation of normal B cells at 13 months after infusion and continued absence of the CLL cells beyond 23 months in one patient [Citation35]. In contrast, in patients treated with CAR T cells for acute lymphoblastic leukemia (ALL), the recovery of normal B cells may portend an impending disease relapse [Citation45,Citation46]. Ultimately, the requirement for long-term CAR T persistence and continued B cell aplasia for durable disease control may prove to be disease- and/or CAR-specific.
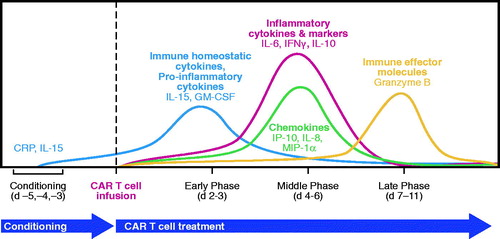
Emerging clinical data also support a key role for certain cytokines in modulating CAR T cell activity and expansion and may provide important insights into optimal CAR design and product composition and the need for careful selection of conditioning regimens (). In studies of anti-CD19 CAR T cells at the NCI and with axicabtagene ciloleucel, four major cytokine programs were modulated throughout treatment: homeostatic, effector, chemotactic, and inflammatory. The homeostatic cytokines IL-15 and granulocyte-macrophage colony-stimulating factor (GM-CSF) were upregulated early following conditioning therapy and peaked 5–6 d after completion of the conditioning regimen, which was usually 2–3 d after CAR T infusion [Citation48–50]. Greater induction of IL-15 was associated with response to treatment [Citation32,Citation36,Citation48]. C-reactive protein (CRP), an inflammatory marker produced by hepatocytes in response to IL-6 [51], has been shown to peak 3–5 d after CAR T cell infusion [Citation37,Citation40]. Granzyme B, an effector serine protease and key mediator of T cell cytotoxic effects, and IL-10, an inflammatory/immune-modulating cytokine, have been shown to peak at day 6–7 post-CAR T cell infusion [Citation50], and higher peak levels of IL-10 have been associated with CR and PR vs. stable and progressive disease [Citation32].
Safety and toxicity of axicabtagene ciloleucel
As a form of adoptive cell transfer, anti-CD19 CAR T cell therapy has the capacity to elicit a class-specific set of adverse events (AEs) [Citation51]. The most concerning acute toxicities, occurring within hours to a few days after infusion of CAR T cells, namely CRS and NEs, are generally reversible and usually resolve within 2 weeks without lasting sequelae in the majority of patients [Citation35,Citation40,Citation51,Citation52]. Clinical manifestations of CRS can be evident within 12 h after infusion, and initial symptoms include fever, and constitutional symptoms. The syndrome may progress to involve hypoxemia, hemodynamic instability, and end-organ damage, including cardiac and renal toxicity [Citation52]. Lee and colleagues devised a CRS classification and management system graded 1–5 based on symptom severity [Citation52]. NEs including encephalopathy, delirium, aphasia, and seizures have been reported in patients who received anti-CD19 CAR T cell therapy [Citation32,Citation40,Citation46]. Importantly, both CRS and NEs have also been reported with other T cell-directed treatments, such as blinatumomab, a bispecific T cell engager that simultaneously binds CD3+ T cells and CD19+ target cells [Citation53].
CRS is thought to result from widespread and simultaneous activation of T cells and the subsequent release of soluble inflammatory mediators, such as cytokines, chemokines, and immune effector molecules [Citation51]. Similarly, although the underlying mechanism of NEs in CAR T therapy is not fully understood, emerging data indicate a correlation with elevated inflammatory cytokines [Citation51,Citation54]. Specific cytokine profiles have been associated with CRS and NEs. CRS has been associated with elevated inflammatory cytokines, including interferon (IFN)-γ, GM-CSF, ferritin, IL-6, IL-10, and CRP [Citation51,Citation55] and, specifically, levels of IFN-γ, IL-6, CRP, ferritin, and soluble IL-2 receptor were higher in patients with severe CRS compared with those who had less severe CRS [Citation46]. In addition to being associated with better clinical responses, higher peak levels of IL-15, granzyme B, and IL-10 were associated with higher grade NEs (grade 3 or 4 vs. <3) [Citation32,Citation48], and elevated IFN-γ and/or IL-6 levels have been shown to coincide with peak incidence of toxicities, including NEs [Citation35]. In such cases of CRS, administration of the IL-6–receptor antagonist tocilizumab and, if necessary, corticosteroids is recommended [Citation51,Citation52]. Treatment with tocilizumab results in a rapid reversal of symptoms [Citation51,Citation52], and it has been hypothesized that prophylactic treatment, including early tocilizumab administration, may play a role in reducing CAR T therapy-related CRS [Citation56]. In the ZUMA-1 trial, 43% of patients received tocilizumab and 27% received corticosteroids for management of cytokine release syndrome and/or neurologic events [Citation41]. Additionally, preliminary clinical evidence suggests that early intervention with tocilizumab and/or steroids may decrease the rates of severe CRS in ALL, possibly without negatively impacting efficacy outcomes [Citation57], although these results require confirmation.
Because CD19 is the target for axicabtagene ciloleucel and several other leading CAR T cell therapies under investigation for the treatment of B cell malignancies, and CD19 is expressed on malignant and healthy B cells [Citation58], the risk of so-called on-target, off-tumor toxicity is restricted to normal B cell aplasia [Citation51]. Patients lacking B cells after CAR T therapy may require immunoglobulin supplementation [Citation51,Citation59]. The length of time anti-CD19 CAR T cells persist in the circulation can influence duration of normal B cell aplasia, because active anti-CAR T cells can target B cells [Citation40,Citation60], thereby hindering B cell recovery and extending the need for immunoglobulin treatment. However, it does not appear that indefinite normal B-cell aplasia is a prerequisite for ongoing disease remission. Kochenderfer et al. reported a patient who remained in an ongoing remission from CLL for 23 months post-CAR T cell treatment [Citation35]. This patient had no evidence of disease recurrence 10 months after recovery of polyclonal CD19+ B cells that occurred 13 months after CAR T cell infusion [Citation35].
Conclusions and future directions
Refractory DLBCL is a difficult clinical problem with a high unmet medical need. Existing high-intensity salvage therapies, such as ASCT cure a minority of patients and, thus, there is a dire need for novel treatment modalities. Tumor immunotherapy has emerged as an exciting new frontier in the treatment of malignancy, and CAR T cells, as a unique type of immunotherapy with particular potency in hematologic cancers, harness the cytolytic capabilities of a patient’s own T cells to seek out and eradicate tumor cells that have proved resistant to more conventional approaches.
Nearly 25 years after Eshhar and colleagues first reported that the antigen specificity of T cells could be redirected by a cancer-specific CAR [Citation61,Citation62], it is likely that in the near future there will be commercially available CD19-targeted CAR T cells as a treatment option for patients with refractory B cell malignancies. Multiple groups have made impressive strides by not only investigating and optimizing CAR construct design, but also in generating critical tools and techniques, such as the development of efficient gene transfer technology and improved manufacturing processes, to create an accessible therapy [Citation24–26].
Future efforts will be directed toward further improving benefit-risk by mitigating toxicity while preserving, or even enhancing, efficacy. Several approaches are being investigated, including CAR T cells with additional functionality, novel CAR T dosing strategies, and mechanistic studies of observed clinical correlates of safety and efficacy (). For example, results of preclinical studies have demonstrated the viability of various switch mechanisms for CAR T cells [Citation51,Citation63–65]. Switch technology may give clinicians real-time, tunable control of CAR T cell activity and the ability to widen the CAR T therapeutic window. Additionally, engineering CAR T cells to express more than one CAR may lessen the ability of tumor cells to downregulate surface proteins targeted by monospecific CAR therapies. The development of bispecific CAR T cells, such as those that recognize both CD19 and CD20, another generally B-cell–specific surface antigen, may increase response rates and improve durability of responses by preventing antigen-loss tumor escape mechanisms [Citation66,Citation67].
Figure 5. Major future directions in the development of next-generation CAR products. CAR: chimeric antigen receptor; PD-1: programed death receptor; PD-L1: programed death receptor ligand; scFv: single chain variable fragment.
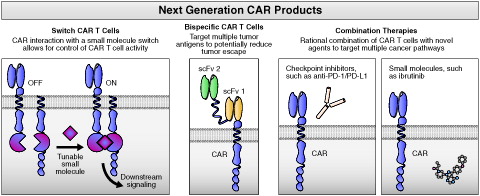
Modified dosing, specifically, repeat dosing with human (or humanized) CARs may be effective in driving up response rates. Additionally, a more granular understanding of efficacy and toxicity mechanisms through further biomarker analysis may enable rational, targeted, and proactive safety management and may provide new avenues to reprogram CAR T cells away from toxicity-related immune programs while preserving efficacy.
Moreover, combination approaches with CAR T cells and other agents hold great potential to further improve patient outcomes. Like their endogenous counterparts, CAR T cells express and upregulate programed cell death protein 1 (PD-1) [Citation68], a pathway that can be co-opted by tumor cells to impair T cell function. Results of preclinical experiments in mouse models have demonstrated that combining CAR T cell therapy with PD-1 pathway blockade maximizes CAR T cell activity and results in increased tumor reduction [Citation69,Citation70]. The first interventional clinical studies of CAR T cells in combination with checkpoint blockade agents are accruing. Combining CAR T cells with targeted agents that have immune modulating activity may also prove beneficial. For example, recent work has demonstrated that CAR T cells made from patients with CLL have greater proliferative potential when harvested after prolonged treatment with ibrutinib and that ibrutinib enhances CAR T cell activity in mouse models [Citation71].
The last several decades have seen dramatic innovation in CAR design, manufacturing, and clinical applications. Even as the field continues to evolve at remarkable speed, patients, and oncologists are likely to see one or more CAR T products in the clinic within the next few years. Continuing the pioneering efforts to bring the promise of this truly novel therapy will place CARs on the road to routine implementation in the management of cancer.
Practice points
Anti-CD19 CAR T cell therapy is a promising therapeutic modality for patients with refractory aggressive B cell NHL.
Although efficacy in refractory aggressive NHL is impressive, anti-CD19 CAR T cell therapy is associated with unique and reversible toxicity syndromes, such as CRS and neurologic events.
Engineered T cells may serve as a platform to treat additional hematologic and solid malignancies in the coming years.
Practice points
Anti-CD19 CAR T cell therapy is a promising therapeutic modality for patients with refractory aggressive B cell NHL.
Although efficacy in refractory aggressive NHL is impressive, anti-CD19 CAR T cell therapy is associated with unique and reversible toxicity syndromes, such as CRS and neurologic events.
Engineered T cells may serve as a platform to treat additional hematologic and solid malignancies in thecoming years.
Research agenda
Evaluate the role of anti-CD19 CAR T cells earlier in the treatment course of aggressive B cell NHL.
Explore rational combinations of CAR T cells with other immunotherapies and novel cancer treatments.
Delineate differences in the cellular and molecular mechanisms of efficacy and toxicity to bring about product optimizations to improve the therapeutic index of CAR T cells.
Expand the proof of concept established with anti-CD19 CAR T cells to other hematologic malignancies and solid tumors.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1387905.
ICMJE Forms for Disclosure of Potential Conflicts of Interest
Download Zip (5.6 MB)Acknowledgments
Medical writing assistance was provided by Skye Geherin of Nexus Global Group Science, LLC funded by Kite Pharma.
References
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–3337.
- Khalil DN, Smith EL, Brentjens RJ, et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–290.
- Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13:370–383.
- Jensen MC, Riddell SR. Designing chimeric antigen receptors to effectively and safely target tumors. Curr Opin Immunol. 2015;33:9–15.
- Priceman SJ, Forman SJ, Brown CE. Smart CARs engineered for cancer immunotherapy. Curr Opin Oncol. 2015;27:466–474.
- Brentjens RJ, Curran KJ. Novel cellular therapies for leukemia: CAR-modified T cells targeted to the CD19 antigen. Hematol Am Soc Hematol Educ Program. 2012;2012:143–151.
- Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828.
- Muranski P, Boni A, Wrzesinski C, et al. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go?. Nat Clin Pract Oncol. 2006;3:668–681.
- Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702.
- Kochenderfer JN, Yu Z, Frasheri D, et al. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886.
- Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102.
- Howlader NNA, Krapcho M, Miller D, et al., editors. SEER cancer statistics review, 1975–2013. Bethesda (MD): National Cancer Institute; 2017.
- Chaganti S, Illidge T, Barrington S, et al. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174:43–56.
- Lossos IS, Gascoyne RD. Transformation of follicular lymphoma. Best Pract Res Clin Haematol. 2011;24:147–163.
- Petkovic I. Current trends in the treatment of primary mediastinal large B-cell lymphoma – an overview. Contemp Oncol (Pozn). 2015;19:428–435.
- Kridel R, Mottok A, Farinha P, et al. Cell of origin of transformed follicular lymphoma. Blood. 2015;126:2118–2127.
- Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1–2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122:981–987.
- Provencio M, Sabin P, Gomez-Codina J, et al. Impact of treatment in long-term survival patients with follicular lymphoma: a Spanish Lymphoma Oncology Group registry. PLoS One. 2017;12:e0177204.
- Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood. 2015;125:33–39.
- Wagner-Johnston ND, Link BK, Byrtek M, et al. Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS). Blood. 2015;126:851–857.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017. [in press]. doi: 10.1182/blood-2017-03-769620
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. JCO. 2010;28:4184–4190.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Program. 2011;2011:498–505.
- Jin Z, Maiti S, Huls H, et al. The hyperactive Sleeping Beauty transposase SB100X improves the genetic modification of T cells to express a chimeric antigen receptor. Gene Ther. 2011;18:849–856.
- Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271.
- Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015.
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–684.
- Chodon T, Comin-Anduix B, Chmielowski B, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20:2457–2465.
- Better M, Chiruvolu V, Oliver J, et al. Manufacturing and characterization of KTE-C19 in a multicenter trial of subjects with refractory aggressive non-Hodgkin lymphoma (NHL) (ZUMA-1). Proceedings of the 105th Annual Meeting American Association Cancer Research; New Orleans (LA): AACR; 2015. p. 2308.
- Better M, Chiruvolu V, Oliver J, et al. Production of KTE-C19 (anti-CD19 CAR T cells) for ZUMA-1: a phase 1/2 multi-center study evaluating safety and efficacy in subjects with refractory aggressive non-Hodgkin lymphoma (NHL). Mol Ther. 2016;24:287.
- Klebanoff CA, Khong HT, Antony PA, et al. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005; 26:111–117.
- Kochenderfer JN, Somerville RP, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum Interleukin-15 levels. JCO. 2017;35:1803–1813.
- Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. JCO. 2008;26:5233–5239.
- Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854.
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. JCO. 2015;33:540–549.
- Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8:355ra116.
- Siddiqi T, Neelapu S, Locke F, et al. Updated phase 1 results from ZUMA-1: a phase 1–2 multicenter study evaluating the safety and efficacy of KTE-C19 (anti-CD19 CAR T cells) in refractory aggressive B-cell non-Hodgkin lymphoma (NHL). Mol Ther. 2016;21:S791.
- Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720.
- Kochenderfer JN, Somerville RPT, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther. 2017;25:2245–2253.
- Locke F, Neelapu S, Bartlett N, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2016;25:285–295.
- Locke FL, Neelapu SS, Bartlett NL, et al. Primary results from ZUMA-1: a pivotal trial of axicabtagene ciloleucel (Axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL). Proceedings of the 107th Annual Meeting American Association Cancer Research; Washington (DC): AACR; 2017. p. CT019.
- Wang M, Locke F, Siddiqi T, et al. ZUMA-2: a phase 2 multicenter study evaluating the efficacy of KTE-C19 (anti-CD19 CAR T cells) in patients with relapsed/refractory mantle cell lymphoma (R/R MCL). Ann Oncol. 2016;27:943TiP.
- Wayne A, Sender L, Lee D, et al. ZUMA-4: a phase 1/2 multicenter study evaluating the safety and efficacy of KTE-C19 (anti-CD19 CAR T cells) in pediatric and adolescent subjects with relapsed/refractory B-precursor acute lymphoblastic leukemia (R/R ALL). J Clin Oncol. 2016;34:TPS7075.
- Shah B, Castro J, Wierda W, et al. ZUMA-3: a phase 1/2 multicenter study evaluating the safety and efficacy of KTE-C19 (anti-CD19 CAR T cells) in adult patients with relapsed/refractory B precursor acute lymphoblastic leukemia (R/R ALL). Ann Oncol. 2016;27:415TiP.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528.
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517.
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139.
- Bot A, Rossi J, Yizhou J, et al. Cyclophosphamide and fludarabine conditioning chemotherapy induces a key homeostatic cytokine profile in patients prior to CAR T cell therapy. ASH Ann Meeting Abstracts. 2015;126:4426.
- Neelapu S, Locke F, Bartlett N, et al. Ongoing complete remissions (CR) in the phase 1 of ZUMA-1: a phase 1-2 multicenter study evaluating the safety and efficacy of KTE-C19 (anti-CD19 CAR T cells) in subjects with refractory aggressive B-cell non-Hodgkin lymphoma (NHL). Vol. 34. ASCO Annual Meeting; 2016; Chicago, IL.
- Rossi J, Sherman M, Xue A, et al. Low dose conditioning chemotherapy and CD19-directed CAR T cells May elicit distinct immune programs associated with clinical responses. SITC Meeting Abstracts; 2016; Los Angeles, CA.
- Bonifant C, Jackson H, Brentjens RJ, et al. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3:16011.
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195.
- Benjamin JE, Stein AS. The role of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Ther Adv Hematol. 2016;7:142–156.
- Dai H, Wang Y, Lu X, et al. Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst. 2016;108:pii:djv439.
- Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122.
- Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023.
- Gardner R, Leger K, Annesley C, et al. Decreased rates of severe CRS seen with early intervention strategies for CD19 CAR-T cell toxicity management. Blood. 2016:128:586.
- Levine BL. Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Ther. 2015;22:79–84.
- Dunbar CE. Blood’s 70th anniversary: CARs on the blood highway. Blood. 2016;128:1–3.
- Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73.
- Stancovski I, Schindler DG, Waks T, et al. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993;151:6577–6582.
- Dotti G, Gottschalk S, Savoldo B, et al. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–126.
- Cao Y, Rodgers DT, Du J, et al. Design of switchable chimeric antigen receptor T cells targeting breast cancer. Angew Chem Int Ed. 2016;55:7520–7524.
- Cartellieri M, Feldmann A, Koristka S, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6:e458.
- Rodgers DT, Mazagova M, Hampton EN, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci USA. 2016;113:E459–E468.
- Zah E, Lin MY, Silva-Benedict A, et al. ADDENDUM: T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:639–641.
- Zah E, Lin MY, Silva-Benedict A, et al. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:498–508.
- Abate-Daga D, Hanada K, Davis JL, et al. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood. 2013;122:1399–1410.
- Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144.
- John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2:e26286.
- Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–1127.