Abstract
Treatment-free remission (TFR) in patients with chronic myeloid leukemia in chronic phase (CML-CP) is considered a feasible option, especially with the ability of second-generation tyrosine kinase inhibitors to induce higher rates of sustained deep molecular response (DMR). DASFREE is an open-label, single-arm, multicenter phase II trial assessing TFR after dasatinib discontinuation in patients with CML-CP (N = 84). At 2 years, TFR was 46% in all patients. Multivariate analyses revealed statistically significant associations between 2-year TFR and duration of prior dasatinib (≥median; p = .0051), line of therapy (first line; p = .0138), and age (>65 years; p = .0012). No disease transformation occurred, and the most common adverse events experienced off treatment were musculoskeletal (observed in 30 patients); however, dasatinib withdrawal events were reported in nine patients (11%) by the investigator. Overall, these findings support the feasibility of discontinuing dasatinib for patients with CML-CP in sustained DMR in the first line and beyond.
Introduction
Tyrosine kinase inhibitors (TKIs) have vastly improved long-term outcomes for patients with chronic myeloid leukemia in chronic phase (CML-CP). In particular, second-generation TKIs have been shown to induce higher rates of deep molecular responses (DMR) and a more rapid decline in BCR-ABL1 transcript levels compared with imatinib in newly diagnosed patients [Citation1–3]. Consequently, DMR is now increasingly being considered as being correlative of disease burden reduction and is also the most relevant clinical endpoint for patients wishing ultimately to stop treatment [Citation4–6]. A reduction in leukemic clone burden is associated with reduced rates of transformation to accelerated phase (AP) or blast crisis (BC) CML; however, it is not clear if the absence of detectable BCR-ABL1 transcripts is synonymous with a cure for CML [Citation4–6]. Despite the persistence of the leukemic clone in such cases, a functional cure might still be possible, and the need for lifelong TKI treatment is now being challenged [Citation7–22]. Furthermore, patients might be motivated to stop therapy for reasons that include toxicity, a desire for improvement in quality of life, and the financial burden associated with indefinite TKI treatment [Citation23–25].
Accordingly, treatment-free remission (TFR) has become a treatment goal for many patients with CML-CP in DMR. Typically, TFR is attempted in selected patients in the context of DMR, as measured by a reduction in BCR-ABL1 transcript levels on the International Scale (IS), ranging from ≤0.01% (MR4) to ≤0.0032% (MR4.5) to ≤0.001% (MR5) [Citation26]. Successful TFR has been demonstrated in several clinical trials, the majority of which have included patients discontinuing long-term imatinib treatment. These studies have shown that nearly half of patients with sustained DMR successfully maintained major molecular response (MMR) after discontinuing treatment [Citation9–12,Citation14–16,Citation19,Citation21]. In nearly all cases, patients who relapsed in these trials remained sensitive to TKIs and regained MMR upon re-treatment [Citation7–22]. Based on these findings, the National Comprehensive Cancer Network [Citation27] and the European Society for Medical Oncology [Citation28] suggest the possibility of stopping TKI therapy in selected patients, in the context of frequent monitoring and availability of standardized laboratory testing.
The consensus on the use of TFR in clinical practice, however, is still evolving. Results from ongoing TFR trials will help to provide more confirmatory data on long-term outcomes. Furthermore, many clinical trials on TFR have been unable to identify strong predictors of successful TKI discontinuation consistently. The optimal duration of DMR before entering TFR also remains to be determined.
DASFREE (NCT01850004) is the largest clinical trial to date of patients with CML-CP and sustained dasatinib-induced DMR (specifically MR4.5) who discontinued dasatinib across all lines of therapy. In this ongoing, open-label, single-arm phase 2 study, the primary objective was to assess the rate of TFR (defined herein as the maintenance of MMR following treatment cessation) at 1 year. Herein we report findings for this primary objective and additional pre-specified analyses for patients with a minimum of 2 years of follow-up after stopping dasatinib.
Materials and methods
Study design and eligibility
Patients aged ≥18 years with CML-CP who received dasatinib treatment (as first- or subsequent-line therapy) for a minimum of 2 years at the time of enrollment and had confirmed dasatinib-induced MR4.5 for at least 1 year prior to study entry were eligible. Patients were required to have achieved a 1-log reduction in BCR-ABL1 transcript levels relative to baseline as determined by local standards or BCR-ABL1 ≤ 10% on the IS at 3 months with current dasatinib therapy. Additional eligibility criteria information are listed in the Online Supplementary Material.
Study endpoints and assessments
Once all eligibility criteria were met, dasatinib was discontinued. The primary objective was the rate of TFR (proportion of subjects who maintained MMR [BCR-ABL < 0.1%]) at 1 year following dasatinib discontinuation without restarting treatment. If MMR was lost, patients resumed dasatinib at the last dose received prior to stopping treatment. Key secondary endpoints include event-free survival (EFS; survival with no loss of MMR), BCR-ABL1 kinetics, rate of transformation to CML-AP/BC, and progression-free survival (PFS). Patients who discontinued from the study were censored on the date of their last molecular assessment. PFS was defined as survival without progression to CML-AP/BC or death due to any cause. Time in prior MR4.5 was assessed retrospectively; consent for 10 patients could not be collected, thus information is available for only 74 of the 84 patients enrolled in the trial. Both intolerance and resistance were determined by the investigator. Additional study endpoints and further information on assessments and molecular testing are shown in the Online Supplementary Material.
Key exploratory endpoints included the frequency of adverse events (AEs) and serious AEs (SAEs) occurring off treatment after discontinuing and after restarting on dasatinib, the rate of MMR recapture after reinitiating dasatinib, and identification of prognostic factors in relation to TFR maintenance. AEs and SAEs were assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.03 [Citation29]. Withdrawal events were defined as any AE occurring and/or worsening due to treatment cessation, as determined by the investigator.
The primary objective was presented as a percentage with an exact 95% Clopper–Pearson confidence interval (CI). Additional statistical analyses are provided in the Online Supplementary Material. Data for the primary endpoint are based on a cutoff date of October 2017, at which time all patients who discontinued dasatinib had 1 or more years of follow-up. Data presented herein are based on a cutoff date of December 2018, at which time patients had a minimum follow-up of 2 years.
Ethics
All patients provided written informed consent in accordance with the Declaration of Helsinki and local guidelines before study entry. The study protocol was approved by all Institutional Review Boards of each participating center, as well as each center’s Ethics Committee and competent national authority.
Results
Accrual and patient characteristics
A total of 84 patients enrolled between February 2014 and June 2016 at 22 study centers in six countries discontinued dasatinib treatment. Baseline patient characteristics are presented in . Thirty-seven patients (44%) who were receiving first-line dasatinib and 47 patients (56%) who were receiving dasatinib in subsequent lines prior to stopping treatment were enrolled. Of those on later lines, 53% were deemed resistant to their first-line TKI and 38% were considered intolerant of a prior TKI by the investigator, including two patients (2%) on prior imatinib and nilotinib. Four patients (9%) on later lines of dasatinib were not classified as resistant to or intolerant of prior therapy by the investigator; the status of two of these patients is unknown, while one patient switched to dasatinib following closure of a first-line ponatinib trial and the other switched from imatinib to dasatinib due to out-of-pocket costs. In 74 patients with available information on duration of prior time in MR4.5, the median duration of MR4.5 prior to discontinuation was 28 months (range, 13–116 months) and was similar regardless of line of therapy (Supplementary Figure S1). The median time from CML diagnosis to discontinuation was 69 months (range, 29–244 months), and the median dose received prior to stopping dasatinib was 100 mg (range, 20–150 mg) once daily. With a minimum follow-up of 2 years, 11 patients (13%) discontinued the study (Supplementary Table S1). Two patients discontinued the study (while maintaining MMR) due to relocation, one patient was non-compliant with study visits and discontinued the study before undergoing molecular assessments, and one patient discontinued after being diagnosed with a metastatic malignancy unrelated to CML.
Table 1. Baseline patient characteristics.
Efficacy
At 1 year, TFR was 48% (95% CI: 37, 59) in all enrolled patients (Supplementary Figure S2). By line of dasatinib, TFR at 1 year was maintained in 54% (95% CI: 37, 71) of first-line patients and 43% (95% CI: 28, 58) of subsequent line patients (broken down further, TFR was 40% [95% CI: 21, 61] in patients resistant to prior TKI therapy and 50% [95% CI: 26, 74] in patients intolerant of prior TKI therapy.
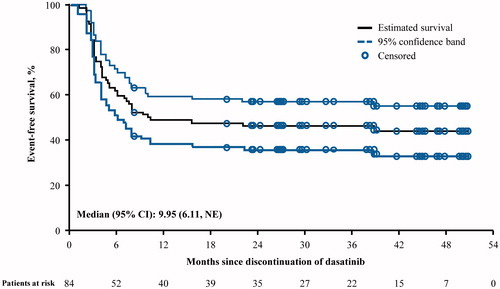
At 2 years, TFR was 46% (95% CI: 36, 57) in all patients, 51% (95% CI: 35, 67) in first-line patients, and 42% (95% CI: 28, 57) in subsequent-line patients (). TFR was maintained in 44% (95% CI: 25, 64) of patients resistant to prior TKI and 44% (95% CI: 22, 67) of patients intolerant of prior TKI.
Figure 1. TFR in all enrolled patients at 2 years (N = 84). CI: confidence interval; NE: not estimable; TFR: treatment-free remission.
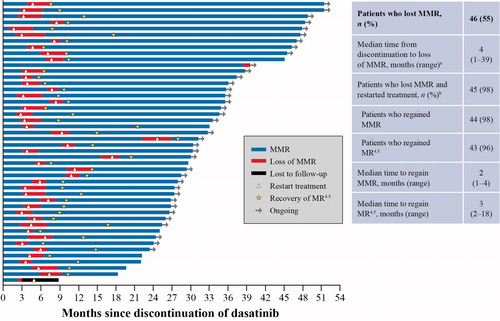
In total, 46 patients (55%) lost MMR 2 years after discontinuation (). Of the 45 patients (98%) who restarted dasatinib, 44 (98%; 95% CI: 88, 100) regained MMR after a median of 2 months (range, 1–4) and 43 (96%; 95% CI: 85, 100) regained MR4.5 after a median of 3 months (range, 2–18). One patient on first-line dasatinib for 41 months prior to discontinuation lost MMR at month 39 and had not restarted therapy at the time of this analysis. Additionally, one patient who lost MMR and discontinued the study after restarting treatment was lost to follow-up after having only one follow-up molecular assessment. Of the 40 evaluable patients in MR4.5 who remained in MMR after dasatinib discontinuation, 14 (35%) patients maintained MR4.5, while 26 (65%) patients lost MR4.5 but did not lose MMR at 12 months after dasatinib discontinuation.
Figure 2. Loss and recovery of MMR and MR4.5 at 2 years. aA 52-year-old male with low-risk CML treated with first-line dasatinib 100 mg once daily for 40 months discontinued the study after 33 months in MR4.5. This patient maintained BCR-ABL1 transcript levels between 0.0017 and 0.01 for 3 years and had an increase to 0.1% in month 39, which was confirmed on a second occasion in month 42 (0.11%). bOne patient lost MMR and restarted treatment. This patient discontinued the study after only one follow-up molecular assessment and therefore was not considered evaluable for molecular response. CML: chronic myeloid leukemia; MMR: major molecular response; MR4.5: BCR-ABL1 ≤ 0.0032% on the International Scale.
At 2 years, PFS was 99% (95% CI: 96, 100) in all patients, 100% (95% CI: 100, 100) in first-line patients, and 98% (95% CI: 93, 100) in subsequent-line patients. No patients progressed to CML-AP/BC. However, one patient was diagnosed with ovarian cancer, discontinued the study, and died 1 month later. This patient was categorized as having discontinued due to death for this database lock and is thus reflected in the survival results.
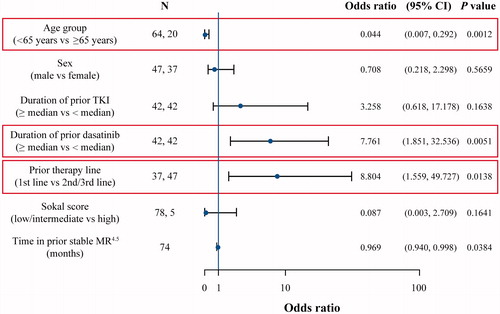
Associations between TFR and prognostic variables of clinical relevance (age [<65 vs ≥65 years], sex [male vs female], duration of prior TKI [≥median vs < median], duration of prior dasatinib [≥median vs < median], prior therapy line [first vs second/third], Sokal score [low/intermediate vs high], and time in prior stable MR4.5) were examined in a multivariate analysis to ascertain whether certain patient characteristics might be predictive of TFR maintenance. Results revealed statistically significant associations between 2-year TFR and age (>65; odds ratio [OR] 0.044; 95% CI: 0.007, 0.292; p = .0012), duration of prior dasatinib (≥median; OR 7.761; 95% CI: 1.851, 32.536; p = .0051), and line of therapy (first line; OR 8.804; 95% CI: 1.559, 49.727; p = .0138) (). Median duration of prior dasatinib was 55 months in patients aged ≥65 years compared with 45 months in patients aged <65 years. Time on prior TKIs (including prior dasatinib) was further assessed in patients who lost or maintained MMR after discontinuing first- or subsequent-line treatment, and was found to be numerically shorter in patients who lost MMR than in patients who maintained MMR (Supplementary Table S2). Seven patients had a prior history of pleural effusion while receiving dasatinib before discontinuation; all episodes were resolved at the time of study entry. None of these patients have lost MMR.
Safety
AEs of any grade and cause occurred in 67% of patients off treatment and 76% of patients after restarting treatment. All-causality AEs reported in ≥4% of patients off treatment following dasatinib discontinuation or on treatment after restarting dasatinib are described in . Dasatinib-related AEs of any grade and grade 3/4 were experienced in 22 patients (49%) and 4 patients (9%) on treatment after restarting dasatinib, respectively, with 2 of 45 patients (4%) experiencing SAEs. Pleural effusion (any grade) was reported in one patient (1%) off treatment and in three patients (7%) after restarting dasatinib; the effusion was grade 3 in one patient. After restarting treatment, three patients (7%) experienced an AE leading to treatment discontinuation; two of these patients discontinued due to pleural effusion, while a metastatic ovarian cancer unrelated to CML led to death in one patient.
Table 2. All causality adverse events in ≥4% of enrolled patients and withdrawal events in all enrolled patients.
As noted earlier, withdrawal events in this study were defined as any AE occurring and/or worsening after dasatinib discontinuation and determined by the investigator to be a result of stopping dasatinib. In total, study investigators deemed 15 events in nine patients (11%) to be related to dasatinib withdrawal (). After stopping treatment, 30 musculoskeletal events were reported; 11 of these events were attributed to dasatinib withdrawal. Two arterial hypertension episodes (observed in one patient) were also considered withdrawal events. At the time of this data cutoff, six events remain unresolved, although all are grade 1 and have not required therapy.
Discussion
While other studies have shown promising TFR data with dasatinib, DASFREE is the largest dasatinib trial to date to examine TFR in patients discontinuing any line of therapy. In DASFREE, 48% (95% CI: 37, 59) of all patients maintained TFR at 1 year following dasatinib discontinuation, and remission was found to be durable at 2 years (with one late relapse at month 39). Furthermore, patients who lost MMR and restarted treatment quickly regained their response (median time to regain MMR and MR4.5 was 2 and 3 months, respectively). Three factors identified in this study as prognostic for TFR at 2 years (duration of prior dasatinib [≥median], line of therapy [first line], and age [>65]) might help to predict which patients are more likely to remain in TFR. Overall, these findings not only provide new information on achieving improved TFR outcomes with dasatinib but also emphasize the practicality of stopping dasatinib across all lines of therapy.
The findings from the DASFREE study are comparable to other dasatinib discontinuation trials: in D-STOP, 63% maintained MR4 at 1 year, and the phase II Japanese Dasatinib Discontinuation (DADI) study reported an estimated overall rate of TFR was 44% at 3 years [Citation11,Citation14]. Findings for other second-generation TKIs have been similar: the ENESTfreedom and ENESTop trials with first- and second-line nilotinib reported TFR rates of 47% and 48%, respectively, at 144 weeks [Citation9,Citation12,Citation15,Citation19]. Finally, in STOP 2 G-TKI (dasatinib and nilotinib discontinuation), 63% of patients remained in TFR at 1 year [Citation16]. It is important to note, however, that the capability to make an in-depth, comprehensive comparison of results across all discontinuation trials is limited by differences in eligibility criteria and varying DMR and molecular relapse definitions.
Although several TKI discontinuation trials have sought to identify prognostic factors that could help predict successful TFR [Citation7,Citation30], a consensus has yet to be reached. In this study, duration of prior dasatinib (≥median; p = .0051), line of therapy (first line; p = .0138), and age (>65 years; p = .0012) represent three prognostic factors for 2-year TFR. Although prior line of therapy is considered a prognostic factor for TFR, these findings suggest that TFR is certainly achievable regardless of the line of discontinued therapy. Identification of these factors adds to the growing body of evidence in delineating key prognostic factors for successful TFR. Similar findings were also observed for age in the Imatinib Suspension and Validation (ISAV) study, where an inverse relationship between patient age and risk of relapse was reported (95% of patients aged <45 years relapsed vs 42% of patients aged ≥45 to <65 and 33% of patients aged ≥65 years), although this may be an effect of a smaller patient population [Citation30]. In addition, other discontinuation trials have established the presence of imatinib resistance as a significant risk factor for molecular relapse [Citation14,Citation16]. This association was particularly evident in the DADI trial, in which imatinib-resistant patients relapsed more quickly and with a significantly shorter doubling time of BCR-ABL1 transcript levels [Citation14]. In DASFREE, however, an important proportion of resistant patients (42%) were able to maintain TFR at 2 years. Although some prognostic factors were identified in this study, other ongoing trials are seeking to identify additional factors predictive of TFR outcomes, including the possible effect of pleural effusion [Citation22].
No significant association between TFR and duration of prior TKI therapy (other than dasatinib) or prior MR4.5 was identified in this study. However, treatment duration and duration of DMR have been identified in other studies as prognostic factors for TFR when a TKI is discontinued [Citation11,Citation30–34]. Results from the multicenter Stop Imatinib (STIM) trial, in which 40% of patients entering TFR with MR4.5 remained in remission at 18 months, indicated an imatinib treatment duration of ≥50 months as a prognostic factor of TFR [Citation11]. In addition, prognostic analysis of patients in EURO-SKI (European Stop Tyrosine Kinase Inhibitor Study) suggested that the duration of DMR had the greatest impact on the success of stopping TKI treatment [Citation21]. In EURO-SKI it was recommended that the patient should be in MR4 for 3 or more years before stopping [Citation21]. Patients in DASFREE were required to be in MR4.5 for 1 or more years (Online Supplementary Figure S1) and on dasatinib for 2 or more years prior to enrollment, which might be considered aggressive in comparison to the treatment duration and duration of response criteria in similar discontinuation trials.
Patients considering TFR are not only concerned about the risk of relapse but also fear that their disease might not remain responsive to treatment following relapse. Across TFR trial reports, all patients who have relapsed after stopping treatment have remained sensitive to re-treatment and regained molecular responses ≥ MMR [Citation22]. However, one patient (in ENESTfreedom) with loss of MMR was found to have a detectable BCR-ABL1 mutation, though the significance of this finding is unclear because the mutation might have been present prior to stopping nilotinib [Citation19]. In DASFREE, PFS was high (99%) at 2 years and no cases of disease progression to CML AP/BC were observed. All patients who relapsed and were evaluable for molecular assessment after restarting dasatinib regained MMR, and 93% regained MR4.5.
Withdrawal events after discontinuation of TKI therapy are well described in the current literature on TFR [Citation7–22]. In DASFREE, only nine patients (11%) off treatment reported symptoms that investigators considered related to withdrawal, most of which resolved on their own without the use of concomitant therapy. Furthermore, AEs observed in patients who restarted treatment were found to be consistent with the known safety profile of dasatinib, with no new safety signals being reported in this study. To our knowledge, the specific definition of withdrawal events utilized in DASFREE (investigator assessed and defined as any AE occurring and/or worsening in severity after discontinuation and related to dasatinib discontinuation) is unique to this trial and unlike other discontinuation trials that defined withdrawal events to be solely musculoskeletal [Citation16,Citation21,Citation22].
One limitation of this study is the retrospective manner in which some data were collected, which might account for some of the differences between DASFREE and other TFR studies. This might play a particularly important role with regard to the low rate of withdrawal events in this study, which might be associated with a definition of withdrawal that requires an investigator assessment of relatedness. Conversely, most patients discontinued treatment following the first published description of TKI withdrawal syndrome, so most investigators were aware of this phenomenon. A second possible limitation of this study, which is also characteristic of other discontinuation trials, is sample size. A better understanding of the long-term durability of TFR, including identification of definitive prognostic factors and longer follow-up, is needed before fully incorporating these findings into clinical decision-making, particularly given the apparently low risk of attempting TKI discontinuation. Long-term (5-year) follow-up is planned, including an assessment of the stability of MR4.5 achieved on dasatinib, recurrence rate, patients’ quality of life off treatment, and the ability to regain a molecular response in patients who relapse after discontinuation.
In summary, DASFREE results provide clinically relevant information on TFR for many patients with CML-CP who achieve stable DMR with dasatinib. Suggestions on appropriate treatment duration and duration of response criteria prior to discontinuing, as well as newly identified prognostic factors, are novel contributions to those considering TFR. Furthermore, these data support the practicality of TFR in patients with resistance to or intolerance of prior TKI treatment and, perhaps most importantly, confirm the feasibility and safety of dasatinib discontinuation in patients stopping first-line treatment.
Supplemental Material
Download JPEG Image (866.4 KB)Supplemental Material
Download JPEG Image (1.1 MB)GLAL-2019-0709-File009.docx
Download MS Word (43 KB)GLAL-2019-0709-File008.docx
Download MS Word (43.8 KB)GLAL-2019-0709-File007.docx
Download MS Word (347.3 KB)Acknowledgments
The authors thank the patients who participated in this study and the clinical study teams. The authors also thank the protocol manager, Renuka Gurnani. Medical writing and editorial support were provided by Jessica Franciosi, PhD, Andrea Lockett, and Joshua Safran of StemScientific (Lyndhurst, NJ, USA), an Ashfield Company, funded by Bristol-Myers Squibb.
Disclosure statement
N. P. S. has received research funding from ARIAD, Bristol-Myers Squibb, Daiichi-Sankyo, and Pfizer. V. G. has served as a consultant to, received research funding from, or served on the board of directors/advisors of Bristol-Myers Squibb, Incyte, Novartis, and Pfizer. A. J. and S. L. declare no conflicts to disclose. S. S. has received research funding from Bristol-Myers Squibb and Novartis, and honoraria from Bristol-Myers Squibb, Incyte, Novartis, and Pfizer. D. R. has served as a consultant to Bristol-Myers Squibb and Novartis, and received honoraria from Bristol-Myers Squibb, Incyte, Novartis, and Pfizer. F. M. has received research funding and honoraria from ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer. M. Y. L. declares no conflicts to disclose. M. T. G. has served on the speakers’ bureaus of Bristol-Myers Squibb, Incyte, Novartis, and Pfizer. FP has received honoraria from and served on the speakers’ bureau of Novartis. F. N. has served as a consultant to Bristol-Myers Squibb, received honoraria from and served on the speakers’ bureaus of Bristol-Myers Squibb and Incyte, and received research funding from Novartis. M. J. M. has served as a consultant to Bristol-Myers Squibb. O. S. and P. M. are employees of Bristol-Myers Squibb. J. H. L. has served as a consultant to, received research funding from, or served as an advisor to ARIAD, Bristol-Myers Squibb, Novartis, and Pfizer.
Data availability statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Additional information
Funding
References
- Cortes JE, Saglio G, Kantarjian HM. Final 5-year study results of DASISION: the Dasatinib versus Imatinib Study in treatment-Naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333–2340.
- Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–927.
- National Cancer Institute. 2006. Common terminology criteria for adverse events, v3.0; [cited 2018 Feb 1]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Clapp GD, Lepoutre T, El Cheikh R, et al. Implication of the autologous immune system in BCR-ABL transcript variations in chronic myelogenous leukemia patients treated with imatinib. Cancer Res. 2015;75(19):4053–4062.
- Pophali PA, Patnaik MM. The role of new tyrosine kinase inhibitors in chronic myeloid leukemia. Cancer J. 2016;22(1):40–50.
- Thielen N, Richter J, Baldauf M, et al. Leukemic stem cell quantification in newly diagnosed patients with chronic myeloid leukemia predicts response to nilotinib therapy. Clin Cancer Res. 2016;22(16):4030–4038.
- Branford S, Yeung DT, Ross DM, et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood. 2013;121(19):3818–3824.
- Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298–305.
- Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525–1531.
- Kumagai T, Nakaseko C, Nishiwaki K, et al. Discontinuation of dasatinib after deep molecular response for over 2 years in patients with chronic myelogenous leukemia and the unique profiles of lymphocyte subsets for successful discontinuation: a prospective, multicenter Japanese trial (D-STOP Trial). Blood. 2016;128:791.
- Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035.
- Mahon F-X, Boquimpani C, Takahashi N, et al. Long-term treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) after stopping second-line (2L) nilotinib: ENESTop 144-wk results. J Clin Oncol. 2018;36(15):7003.
- Nicolini FE, Noël M-P, Escoffre M, et al. Preliminary report of the STIM2 study: a multicenter Stop Imatinib trial for chronic phase chronic myeloid leukemia de novo patients on imatinib. Blood. 2013;122:654.
- Okada M, Imagawa J, Tanaka H, et al. Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin Lymphoma Myeloma Leuk. 2018;18(5):353–360. e351.
- Radich JP, Masszi T, Casares MTG, et al. Long-term treatment-free remission (TFR) following frontline (1L) nilotinib in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP): ENESTfreedom 144-wk results. J Clin Oncol. 2018;36(15 suppl):7063.
- Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–854.
- Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719–1724.
- Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522.
- Ross DM, Masszi T, Gómez Casares MT, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144(5):945–954.
- Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58–60.
- Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–757.
- Saußele S, Richter J, Hochhaus A, et al. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–1647.
- Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–311.
- Giona F, Putti MC, Micalizzi C, et al. Long-term results of high-dose imatinib in children and adolescents with chronic myeloid leukaemia in chronic phase: the Italian experience. Br J Haematol. 2015;170(3):398–407.
- Williams LA, Gonzalez AGG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122(5):641–647.
- Caldemeyer L, Akard LP. Rationale and motivating factors for treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2016;57(12):2739–2751.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): chronic myeloid leukemia. Version 1.2019; [cited 2008 Oct 8]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf
- Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(4):iv41–iv51.
- Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–430.
- Mori S, Vagge E, le Coutre P, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90(10):910–914.
- Horn M, Glauche I, Muller MC, et al. Model-based decision rules reduce the risk of molecular relapse after cessation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Blood. 2013;121(2):378–384.
- Stein AM, Bottino D, Modur V, et al. BCR-ABL transcript dynamics support the hypothesis that leukemic stem cells are reduced during imatinib treatment. Clin Cancer Res. 2011;17(21):6812–6821.
- Takahashi N, Kyo T, Maeda Y, et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica. 2012;97(6):903–906.
- Roeder I, Horn M, Glauche I, et al. Dynamic modeling of imatinib-treated chronic myeloid leukemia: functional insights and clinical implications. Nat Med. 2006;12(10):1181–1184.