Abstract
Established treatments for transplant-ineligible (TNE) patients with newly diagnosed multiple myeloma (NDMM) include melphalan and prednisone (MP) combined with either bortezomib (VMP) or thalidomide (MPT), or lenalidomide plus low-dose dexamethasone (Rd). New treatments for TNE NDMM include Rd plus bortezomib (RVd) and daratumumab plus VMP (VMP + D), daratumumab plus lenalidomide and dexamethasone (D + Rd). Relative efficacy of these treatments was compared using a network meta-analysis. Eight trials identified by a systematic literature review were included in the primary analysis; hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS) were used. Rd was superior to other MP-based regimens for OS and PFS. There was strong evidence that, compared with Rd, both D + Rd and RVd improved PFS (HR 0.57; 95% credible interval (CrI) 0.43, 0.73 and HR 0.72; 95% CrI 0.56, 0.91, respectively). However, there was strong evidence only for RVd in respect to OS (HR 0.72; 95% CrI 0.52, 0.96).
Introduction
Multiple myeloma (MM) accounts for approximately 10% of hematologic cancers and is more commonly diagnosed in older patients (median age at diagnosis 72 years in European patients) [Citation1].
Induction therapy followed by high-dose chemotherapy with autologous stem cell transplantation (ASCT) is the standard treatment for patients with newly diagnosed MM (NDMM) who are aged <65 years or fit and aged ≤70 years [Citation1]. For patients aged >70 years and those unfit for ASCT, several chemotherapy regimens are recommended [Citation1,Citation2]. The 2017 European Society for Medical Oncology (ESMO) guidelines include bortezomib, melphalan, and prednisone (VMP), lenalidomide plus dexamethasone (Rd), or lenalidomide, bortezomib, and dexamethasone (RVd) as preferred first-line options, and several alternative triplets, including melphalan, prednisone, and thalidomide (MPT), and bortezomib, cyclophosphamide, and dexamethasone (VCD) [Citation1].
The National Comprehensive Cancer Network® (NCCN®) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) include RVd, Rd, VCD, and daratumumab plus Rd (D + Rd) as preferred treatment options for NDMM patients not intended for ASCT (TNE); furthermore, NCCN Guidelines® state that older melphalan-containing regimens are no longer to be considered standard of care (SoC) in the United States because novel agents are available and accessible [Citation2]. Despite the growing treatment options for TNE patients with NDMM, very few regimens have been directly compared. Therefore, network meta-analysis (NMA), a statistical method used to simultaneously evaluate the comparative efficacy of several treatment options by direct and indirect comparisons, is a useful tool [Citation3].
A previous NMA [Citation4] evaluated the relative efficacy of Rd versus VMP, melphalan and prednisone (MP), and MPT for TNE NDMM patients. In this analysis, involving five trials published between 1988 and 2015, the authors reported statistically significant differences (p < 0.05) in overall survival (OS) and progression-free survival (PFS) in favor of Rd versus MP, MPT, and VMP.
Although newer treatment combination regimens are becoming available, their role in the management of NDMM remains undefined. We report the results of the NMA that includes the newer regimens D + Rd, RVd, and daratumumab plus VMP (VMP + D), in addition to established options Rd, MP, MPT, and VMP.
Methods
Systematic literature review
The systematic literature review (SLR) methodology was based on Weisel et al. [Citation4]. Articles from January 1, 1988 to July 2, 2019 were reviewed to identify relevant randomized controlled trials (RCT) evaluating efficacy in TNE patients with NDMM. The original searches were carried out in March 2016. Subsequent updates were carried out in November 2016, August 2017, January 2018, and July 2019.
Eligibility criteria
Articles were selected using the Population, Intervention, Comparators, Outcomes, and Study design criteria [Citation5]. The population was limited to patients with NDMM or untreated MM who were aged ≥65 years or aged <65 years and TNE. Studies with <10 patients per arm were excluded. The interventions of interest included lenalidomide, thalidomide, or bortezomib (as monotherapy or part of a combination treatment), older MP-based combination regimens, and newer regimens including D + Rd, RVd, VMP + D, and carfilzomib in combination with melphalan and prednisone (KMP). Comparators of interest were placebo, any of the previously mentioned interventions at a different dose or treatment duration, or any other active antimyeloma drug as monotherapy or as part of combination treatment. Endpoints of interest were OS, PFS, and response rate. Only published RCTs and conference abstracts were eligible for inclusion.
Search strategies
Embase, MEDLINE, and the Cochrane Central Register of Controlled Trials were searched for relevant articles published in the English language. Conference proceedings from ESMO, American Society of Clinical Oncology, American Society of Hematology, European Hematology Association, International Myeloma Workshop, and the International Society for Pharmacoeconomics and Outcomes Research were manually searched. The US National Institutes of Health Clinical Trial Registry was also searched for completed, unpublished trials with available results. Bibliographies of articles identified in the searches were manually checked.
The searches were conducted in four phases: an original search (carried out on March 14, 2016) and four updates (carried out on November 8, 2016, August 8, 2017, January 8, 2018, and July 2, 2019). The search strategy is provided in Supplementary Appendix 1.
Study selection
All abstracts identified were independently screened by two investigators. Eligible abstracts received full-text screening and review, which was carried out by the same two investigators. Discrepancies between the investigators were resolved through discussion with a third investigator. Articles meeting eligibility criteria at full-text screening were included. If there were multiple articles on a single trial population, only the most recent or most relevant data were included in the analysis.
Data collection and data items
Study characteristics, patient characteristics, treatment, and efficacy outcomes were extracted from all included articles.
Hazard ratios (HRs) were extracted for OS and PFS endpoints. If Kaplan–Meier curves were presented, they were digitized and used to estimate HRs [Citation6]. If these were unavailable, they were estimated as described by Tierney et al [Citation7]. If HRs were available but confidence intervals (CIs) were not, then these were estimated using p values and their corresponding z-scores used to calculate the standard error. If only number, probability, or percentages of patients alive were reported, then HR and CIs were estimated using formulae based on the log-rank test.
Risk of bias
The quality of individual publications was assessed by two independent investigators using the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials [Citation8]. Any differences were resolved through discussion with a third reviewer.
NMA
To determine the comparative efficacy of treatments, a Bayesian NMA was conducted on the HRs for OS and PFS using both fixed- and random-effects models. Model fit was assessed using the deviance information criterion (DIC). Parameters for the model were estimated using a Markov Chain Monte Carlo method in the OpenBUGS software package version 3.2.3 (OpenBUGS Project Management Group, Cambridge, UK; http://www.openbugs.net). The model used normal, noninformative prior distributions for the parameters to be investigated (mean = 0, variance = 10,000). Model fit was assessed by comparison of total residual deviance to the number of data points in the network; an overall assessment of complexity and fit was provided by the DIC.
Reported log HRs and their associated standard errors were synthesized. When trials have more than two arms, there is an induced correlation in the HRs because the same control arm is used more than once. For multi-arm trials, a multivariate normal likelihood with covariance equal to the log HR of the control arm was used [Citation9]. If the variance for the control arm was not reported, then it was estimated as the average of the reported variances of the log HRs [Citation10,Citation11].
Primary and extended evidence networks were plotted for OS and PFS, where nodes represent treatments and lines connecting treatment nodes represent trials comparing those treatments. Node size is proportional to the number of trials that evaluated the treatment; line thickness is proportional to the number of trials comparing the treatments [Citation12,Citation13].
Inconsistency was assessed using an independent means model that does not make assumptions about consistency of treatment effects. Inconsistency was indicated if the residual deviance and DIC of this model were substantially lower than for the base-case model [Citation14].
Analyses were carried out on an intention-to-treat (ITT) basis using Rd as the reference treatment. Results are presented as mean HRs for OS and PFS with their credible intervals (CrIs), where an HR <1 indicates a beneficial effect.
The overall ranking of each treatment in terms of OS and PFS was generated by plotting surface under the cumulative ranking (SUCRA) curves.
Sensitivity analyses
A sensitivity analysis was carried out on HRs for OS and PFS in the extended evidence network, focusing on RVd, D + Rd, Rd, MPT, MP, VMP, and VMP + D. To explore the effect of the difference in age between treatment groups in the SWOG S0777 trial (RVd vs Rd) [Citation15], a sensitivity analysis was carried out on age-adjusted HRs from this study in both the primary and extended networks. A fixed-effect model was used for sensitivity analyses of the primary network, whereas a random-effects model was used for the extended network.
Results
Study selection
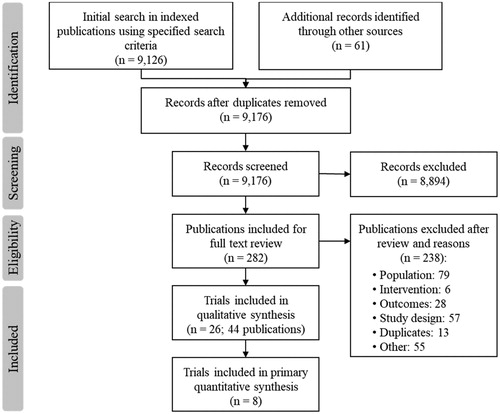
The SLR yielded 9,176 abstracts. After initial screening, 282 abstracts underwent full-text review. Of these, 238 were excluded; the most common reasons for exclusion were incorrect study population (n = 79) and inappropriate study design (n = 57). The analysis included 44 publications describing 26 RCTs ().
Risk of bias
Trials in the primary network generally had a low-risk of bias regarding blinding of outcome assessment and incomplete outcome data (Supplementary Appendix 2). In IFM 99-06, there appeared to be imbalance in the proportion of patients with chromosomal translocations in the MP versus MPT arms, and more patients in the MP arm received second-line therapies versus the MPT arm. In the SWOG S0777 trial the Rd arm contained a higher proportion of patients aged ≥65 years (48%) than the RVd arm (38%), and 10% of patients in the trial received ASCT (the proportion in each arm was not reported).
Evidence networks
A conservative approach was used to construct the primary analysis network, excluding trials based on lack of relevance to clinical practice and/or adherence to the Summary of Product Characteristics. Overall, eight trials (ALCYONE, MAIA, VISTA, IFM 01/01, IFM 99-06, MM03, FIRST, and SWOG S0777) which evaluated seven treatment regimens: D + Rd, MP, MPT, Rd, RVd, VMP, and VMP + D were included in the primary analysis [Citation11,Citation16–25] ().
Table 1. Extracted efficacy data from RCTs in the primary and extended analysis networks.
The primary analysis network had a simple geometry with a single evidence path between all treatment nodes and no clustering of nodes. As the OS data from the ALCYONE [Citation24] and MAIA [Citation25] trials were immature, only the PFS network included the D + Rd and VMP + D treatment nodes. In both the OS and PFS networks, three trials contributed to the MP versus MPT comparison; all other comparisons involved one trial each. Rd was directly compared with both D + Rd, RVd and MPT, but it was separated from other treatment options by two to four indirect steps ().
Figure 2. Evidence networks. (A) Overall survival (OS). (B) Progression-free survival (PFS). CPR + MPR: cyclophosphamide, prednisone, and lenalidomide plus melphalan, prednisone, and lenalidomide; CRD: cyclophosphamide, lenalidomide, and dexamethasone; CTD: cyclophosphamide, thalidomide, and dexamethasone; CTDa: cyclophosphamide, thalidomide, and dexamethasone (attenuated); D: dexamethasone; D + IFN: dexamethasone and interferon alpha; D + Rd: daratumumab, lenalidomide, and dexamethasone; KMP: carfilzomib, melphalan, and prednisone; MD: melphalan and dexamethasone; MP: melphalan and prednisone; MPR: melphalan, prednisone, and lenalidomide; MPR + R: melphalan, prednisone, and lenalidomide plus lenalidomide maintenance; MPT: melphalan, prednisone, and thalidomide; MPT + T: melphalan, prednisone, and thalidomide plus thalidomide maintenance; NR: not reported; PFS: progression-free survival; OS: overall survival; Rd: lenalidomide and dexamethasone; Rd18: lenalidomide in 3 of 4-week cycles; RVd: lenalidomide, bortezomib, and dexamethasone; TD: thalidomide and dexamethasone; VD: bortezomib and dexamethasone; VMP: bortezomib, melphalan, and prednisone; VMP + D: bortezomib, melphalan, prednisone, and daratumumab; VMP + S: bortezomib, melphalan, and prednisone plus siltuximab maintenance; VMPT + VT: bortezomib, melphalan, prednisone, and thalidomide plus thalidomide maintenance; VTP: bortezomib, thalidomide, and prednisone.
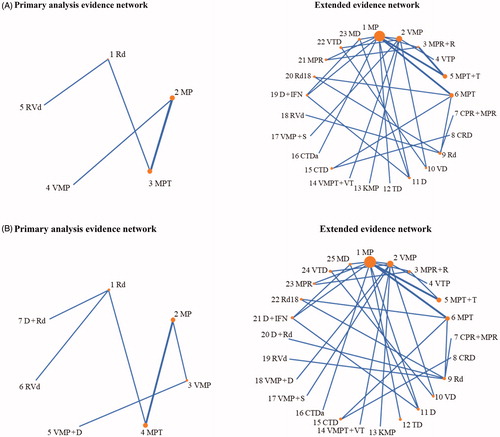
The extended networks had a more complicated geometry but only made small modifications to the evidence contributing to our key comparisons (D + Rd, RVd, Rd, MPT, MP, VMP, and VMP + D; ). The main difference was greater heterogeneity of the included studies, favoring the random-effects analyses chosen for sensitivity analyses of the overall networks.
Assessment of inconsistency
There were no closed loops of evidence in the primary analysis networks, so inconsistency could not be assessed. In the extended networks, the residual deviance and DIC for the independent-means models did not indicate that inconsistency was present in either the OS or PFS networks [Citation14], nor for either ITT or age-adjusted results from SWOG S0777 [Citation15] (Supplementary Appendix 3).
Primary analysis
Baseline age was similar across the studies (), apart from SWOG S0777 (median age 63 years vs >70 years for the other trials). The proportion of patients with International Staging Score (ISS) stage 3 disease was slightly lower (≤30%) in the three trials evaluating MPT [Citation16,Citation19–21] versus trials of bortezomib- and lenalidomide-based regimens (29%–41%) [Citation11,Citation15,Citation17,Citation18,Citation21–25].
Table 2. Baseline age and ISS stage from RCTs in the primary analysis network.
The similarity of the trial designs and small number of studies included in the primary network suggested low heterogeneity. Thus, a fixed-effects model was used. This was supported by the similarity of both the DIC and residual deviance statistics across fixed- and random-effects models (Supplementary Appendix 3).
Analysis of OS showed evidence of Rd superiority over MP, MPT, and VMP (. RVd was the only therapy with evidence of superiority over Rd (HR 0.72, 95% CrI 0.52, 0.96; . The impact on OS for VMP + D versus Rd could not be assessed due the absence of mature OS data for VMP + D.
Figure 3. Results of the primary analysis. (A) Overall survival (OS). (B) Progression-free survival (PFS). Data are HR (95% CrI). CrI: credible interval; D + Rd: daratumumab, lenalidomide, and dexamethasone; HR: hazard ratio; MP: melphalan and prednisone; MPT: melphalan, prednisone, and thalidomide; PFS: progression-free survival; OS: overall survival; Rd: lenalidomide and dexamethasone; RVd: lenalidomide, bortezomib, and dexamethasone; VMP: bortezomib, melphalan, and prednisone; VMP + D: bortezomib, melphalan, prednisone, and daratumumab.
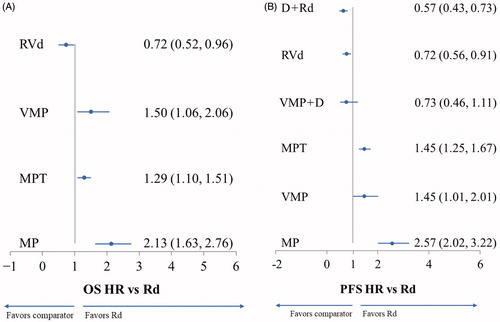
Similarly, for PFS, there was evidence that Rd was superior to MP, MPT, and VMP. Compared with Rd, there was evidence that both D + Rd and RVd improved PFS (HR 0.57; 95% CrI 0.43, 0.73 and HR 0.72; 95% CrI 0.56, 0.91, respectively; . Results for VMP + D versus Rd were inconclusive for PFS.
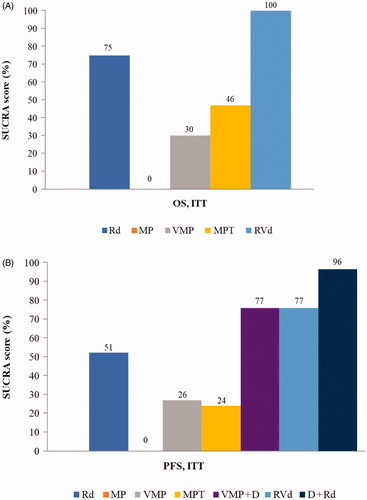
Contingency tables for the primary analyses can be found in Supplementary Appendix 4. SUCRA rankings confirmed these findings ().
Figure 4. Surface under the cumulative ranking plot for the primary analysis. (A) Overall survival (OS). (B) Progression-free survival (PFS). Higher SUCRA scores indicate a higher probability that the treatment was in the top rank or one of the top ranks. D + Rd: daratumumab, lenalidomide, and dexamethasone; ITT: intentionto-treat; MP: melphalan and prednisone; MPT: melphalan, prednisone, and thalidomide; PFS: progression-free survival; OS: overall survival; Rd: lenalidomide and dexamethasone; RVd: lenalidomide, bortezomib, and dexamethasone; SUCRA: surface under the cumulative ranking curve; VMP: bortezomib, melphalan, and prednisone; VMP + D: bortezomib, melphalan, prednisone, and daratumumab.
Sensitivity analyses
In extending the network of trials, a random-effects model for both PFS and OS was used, as suggested by greater heterogeneity across trials and supported by the lower DIC and deviance on the PFS outcome (Supplementary Appendix 3). Rd maintained the PFS improvement over MPT and VMP, as well as RVd over Rd, but the inclusion of additional indirect evidence resulted in broader CrIs. Analysis of the primary network using age-adjusted HRs from the SWOG S0777 trial also provided evidence that Rd was superior to MP, MPT, and VMP in terms of both PFS and OS (Supplementary Appendix 5). Both D + Rd and RVd improved PFS versus Rd (HR 0.57; 95% CrI 0.43, 0.73 and HR 0.73; 95% CrI 0.58, 0.92, respectively), and RVd also marginally improved OS versus Rd (HR 0.75; 95% CrI 0.55, 0.99). SUCRA rankings confirmed these findings (Supplementary Appendix 6).
Sensitivity analyses incorporating evidence from the extended network and random effects indicated none of the treatments had strong evidence of superiority to Rd in terms of PFS or OS in either the ITT analysis (Supplementary Appendix 7) or the age-adjusted analysis (Supplementary Appendix 8). Point estimates were similar to the results from the primary network, but CrIs were wider, reflecting the adoption of random effects.
Discussion
This NMA informs the relative effectiveness of established and newer therapies for the management of TNE NDMM versus current SoC. The results suggest that D + Rd, Rd, RVd, and VMP + D provide clinically relevant benefits over VMP, MPT, and MP, and that D + Rd and RVd provide benefits over Rd.
Our analysis found evidence that Rd offers a PFS advantage versus VMP. This agrees with several phase-3 studies, which show a median PFS for Rd of 26.0–31.9 months [Citation15,Citation18,Citation25] versus 18.1–20.0 months for VMP [Citation22,Citation24]. Compared with Rd alone, the Rd-based combination regimens of RVd and D + Rd provided additional benefit in terms of PFS. Moreover, RVd was the only regimen to demonstrate a significant advantage over Rd in terms of OS, which is arguably the most important efficacy endpoint when evaluating novel therapies for NDMM [Citation45]. The efficacy and safety of RVd were demonstrated in the SWOG S0777 trial, which compared RVd with Rd in patients with NDMM who were not intended for ASCT [Citation15]. A recent update showed that at a median follow-up period of 84 months (7 years), the median OS for RVd was not reached, and that there was a 12-month prolongation of median PFS with RVd over Rd [Citation46]. In addition to these findings, our NMA suggests that RVd offers OS and PFS benefits over VMP and also the older regimens, MP and MPT, which are no longer recommended by the NCCN® based on the FIRST trial data [Citation2]. Other advantages of the RVd regimen include the wide experience with the regimen in a real-world clinical practice setting (in the USA), and the relatively low cost compared with options like D + Rd [Citation45]. Taken together, these observations and the findings from our analysis provide further confidence in the outcomes that are achievable with the RVd regimen.
Clinical and safety outcomes have recently been published for D + Rd from the MAIA study, comparing D + Rd with Rd [Citation25]. As of July 2019, OS data from this study are not available, however, the risk of disease progression or death was 44% lower in the daratumumab group compared to the Rd arm. PFS data are also available for another relatively new regimen, VMP + D, from phase-3 ALCYONE study [Citation24]. Again, OS data have yet to be reported so it is unclear whether the reported 53% reduction in the risk of disease progression or death versus VMP will translate into a survival benefit [Citation47].
It is also relevant to compare the results for VMP + D with SoC, in addition to VMP. Our study aimed to address this by comparing VMP + D with Rd. However, results of this comparison were inconclusive, reflecting that Rd and VMP + D are separated by three nodes in the network, resulting in broad CrIs.
Two other NMAs have addressed the relative efficacy of treatment options for NDMM. An NMA comparing Rd with regimens investigated in RCTs that included patients with NDMM who were aged >65 years reported improved outcomes for response rate, OS, and PFS for Rd, however RVd, VMP + D, and D + Rd were not included in this analysis [Citation48]. A recently published NMA of treatments for patients with TNE NDMM [Citation49] used a random-effects model with a wide evidence network similar to our extended network. However, their conclusions differ slightly from ours; the relative ordering of VMP + D and RVd in terms of PFS impact is reversed, with VMP + D ranked ahead of RVd. The authors do not report their methodology, but the differences in findings may be due to variations in the extracted HRs for dexamethasone from Kaplan–Meier curves in IFM-95/01 [Citation37].
As in any indirect comparison, some limitations can be expected in this analysis. However, intra- and inter-trial inconsistencies and risk of bias were addressed. Risk of bias in the primary network was generally low in terms of blinding of outcome assessment (performance bias) and incomplete outcome data (detection bias). NMAs are also limited by the assumption that treatment effects are transitive [Citation50].
In the primary analysis, the main intra-trial inconsistency relates to the differences in age between treatment groups in the SWOG S0777 trial; the Rd arm contained a higher proportion of patients aged ≥65 years (48%) than the RVd arm (38%). However, sensitivity analyses showed that the results were unaffected by this imbalance, as reported by Durie et al. [Citation46] in an updated analysis. Furthermore, in the SWOG S0777 trial, a small proportion of patients received unplanned ASCT; inclusion of these patients could potentially influence the results of the analysis relating to RVd.
Inter-trial inconsistencies in the primary analysis relate to differences in the median age and proportion of patients with ISS stage 3. Differences in the proportion of patients with renal impairment or high-risk cytogenetics were also present, but these data were not included in our analysis due to inconsistent reporting between the published studies. In addition, the VMP regimen used varied between trial: in the ALCYONE trial patients received bortezomib twice weekly in cycle 1 only, whereas in the VISTA trial patients received bortezomib twice weekly during cycles 1 to 4; this may have affected the results of comparisons with VMP + D.
Similarly, differences in clinical management, monitoring, and patient follow-up likely exist between studies. OS analyses are affected by treatments given post-progression, which may differ between countries and also vary based on when the study was conducted, thereby increasing heterogeneity between studies. Many of these differences are not reported in sufficient detail by publications to allow adjustment or consideration in sensitivity analyses.
Assumptions related to the analysis are also potential limitations of this study. The assumption that HRs are constant—whereas they may be subject to change based on follow-up duration (median follow-up in the studies was 16–60 months)—can lead to poor fit, as seen in the fixed-effect models for the PFS extended network. This could be overcome by modeling Kaplan–Meier curves using fractional polynomial or piecewise-constant models [Citation51,Citation52]. Consistency of treatment effects across trials was assumed and, because there were no independent loops of evidence, could not be formally tested in the primary analysis network. However, no evidence of inconsistency was found in the extended network (Supplementary Appendix 3). Our analysis also assumed that no treatment-effect modifiers were imbalanced across the network, but a network meta-regression was not possible because only single trials were available on majority of treatment contrasts [Citation53].
This analysis supports the findings of the primary studies identified in the SLR, indicating that first-line treatment with Rd provides OS and PFS benefits over the currently approved regimens available to TNE patients with NDMM. Moreover, it establishes RVd as a promising emerging therapeutic option that extends OS and PFS compared with Rd for TNE patients with NDMM; the recent positive opinion from European Medicines Agency's Committee for Medicinal Products for Human Use on RVd supports our findings [Citation54]. Evidence also suggests a role for both D + Rd and VMP + D in the management of TNE NDMM, although additional data are required to establish whether these regimens extend OS as well as PFS versus current SoC. As data from additional phase-3 studies of Rd and Vd combinations become available, such as ixazomib plus Rd, further updates to this NMA may help define the role of new regimens in the treatment armamentarium for MM. Finally, as patients aged ≥75 years constitute up to 40% of the NDMM population [Citation55] and can be both challenging to treat and under-represented in clinical trials, studies focusing on this subgroup would help define the optimal treatment regimens for these patients.
GLAL-2019-0863-File008.docx
Download MS Word (885.7 KB)Acknowledgments
Medical writing and editorial support were provided by Mauro Locati, PhD (Excerpta Medica) and Joanna Todd, PhD (Stellar Medical Communications Limited) and were funded by Celgene Corporation. The authors are fully responsible for all content and editorial decisions for this manuscript.
Disclosure statement
K. R. has received honoraria from AbbVie, Amgen, Celgene Corporation, Janssen, Oncopeptides, Sanofi, and Takeda; and grants from Amgen, Celgene Corporation, Janssen, and Takeda. H. T. has received honoraria from Bayern AG, Celgene Corporation, Hoffman-La Roche, Janssen, Novartis, and Pfizer. V. K. D. and V. B. have received a grant and consultancy fees from Celgene Corporation. S. R. and S.D. are employees of Celgene Corporation and hold shares in Celgene Corporation. K. W. is an advisory board member for Amgen, Adaptive Biotechnologies, Bristol-Myers Squibb, Janssen, Juno, Sanofi, and Takeda; and has received honoraria from Amgen, Bristol-Myers Squibb, Celgene Corporation, Janssen, Novartis, Takeda; and grants from Amgen, Celgene Corporation, Janssen, and Sanofi.
Additional information
Funding
References
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28 (suppl_4):iv52–iv61.
- National Comprehensive Cancer Network, Inc. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.1.2020; 2019. [cited 2019 Oct 3]. Available from: NCCN.org.
- Tonin FS, Rotta I, Mendes AM, et al. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract. 2017;15(1):943.
- Weisel K, Doyen C, Dimopoulos M, et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leuk Lymphoma. 2017;58(1):153–161.
- Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York: University of York; 2009.
- Jansen JP, Cope S. Meta-regression models to address heterogeneity and inconsistency in network meta-analysis of survival outcomes. BMC Med Res Methodol. 2012;12(1):152–152.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617.
- Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759–1769.
- Facon T, Dimopoulos MA, Dispenzieri A, et al. Final analysis of overall survival from the First trial. Blood. 2016;128(22):241.
- Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682.
- Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654.
- Dias S, Sutton AJ, Welton NJ, et al. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33(5):618–640.
- Durie BGM, Hoering A, Abidi M, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–527.
- Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–1218.
- Facon T, Dimopoulos MA, Hulin C, et al. Updated overall survival analysis of the FIRST study: continuous lenalidomide plus low-dose dexamethasone vs melphalan, prednisone, and thalidomide in patients with newly diagnosed multiple myeloma. Haematologica. 2015;100(3):Abstract S105.
- Facon T, Dimopoulos MA, Dispenzieri A, et al. Final survival analysis from the FIRST trial: lenalidomide plus low-dose dexamethasone until progression (Rd cont) v melphalan, prednisone and thalidomide (MPT), and Rd for 18 cycles (Rd18) for transplant-ineligible (TNE) patients (pts) with newly diagnosed multiple myeloma. Clin Lymphoma Myeloma Leuk. 2017;17(suppl 1):e63–e64.
- Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. JCO. 2009;27(22):3664–3670.
- Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118(5):1239–1247.
- Sacchi S, Marcheselli R, Lazzaro A, et al. A randomized trial with melphalan and prednisone versus melphalan and prednisone plus thalidomide in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplant. Leuk Lymphoma. 2011;52(10):1942–1948.
- San Miguel JF, Schlag R, Khuageva NK, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. JCO. 2013;31(4):448–455.
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917.
- Mateos M-V, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–528.
- Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–2115.
- Ludwig H, Hajek R, Tothova E, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113(15):3435–3442.
- Facon T, Lee JH, Moreau P, et al. Phase 3 study (CLARION) of carfilzomib, melphalan, prednisone (KMP) v bortezomib, melphalan, prednisone (VMP) in newly diagnosed multiple myeloma (NDMM). Clin Lymphoma Myeloma Leuk. 2017;17(1):e26–e27.
- Stewart AK, Jacobus S, Fonseca R, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood. 2015;126(11):1294–1301.
- Mateos MV, Oriol A, Martínez-López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11(10):934–941.
- Mateos M-V, Oriol A, Martínez-López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014;124(12):1887–1893.
- Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107–3114.
- Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. JCO. 2014;32(7):634–640.
- Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 study. JCO. 2010;28(19):3160–3166.
- Zweegman S, van der Holt B, Mellqvist U-H, et al. Lenalidomide plus melphalan and prednisone, followed by lenalidomide maintenance versus thalidomide plus melphalan and prednisone, followed by thalidomide maintenance; results of the randomized phase 3 HOVON87/NMSG18 trial. Blood. 2016;127(9):1109–1116.
- Zweegman S, van der Holt B, Mellqvist U-H, et al. Randomized phase III trial in non-transplant eligible patients with newly diagnosed symptomatic multiple myeloma comparing melphalan-prednisone-thalidomide followed by thalidomide maintenance (MPT-T) versus melphalan-prednisone-lenalidomide followed by maintenance with lenalidomide (MPR-R); a joint study of the Dutch-Belgian Cooperative Trial Group for Hematology Oncology (HOVON) and the Nordic Myeloma Study Group (NMSG). Blood. 2014;124(21):179.
- Hungria VT, Crusoe EQ, Maiolino A, et al. Phase 3 trial of three thalidomide-containing regimens in patients with newly diagnosed multiple myeloma not transplant-eligible. Ann Hematol. 2016;95(2):271–278.
- Facon T, Mary JY, Pegourie B, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006;107(4):1292–1298.
- Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127(9):1102–1108.
- Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118(5):1231–1238.
- Pawlyn C, Davies FE, Cairns D, et al. Continuous treatment with lenalidomide improves outcomes in newly-diagnosed myeloma patients not eligible for autologous stem cell transplant: results of the Myeloma XI trial. Blood. 2017;130:1854.
- Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9):1405–1412.
- San-Miguel J, Blade J, Shpilberg O, et al. Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood. 2014;123(26):4136–4142.
- Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86(1):16–22.
- Niezivsky R, Flinn IW, Rifkin R, et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. JCO. 2015;33:3921–3929.
- Kapoor P, Rajkumar SV. MAIA under the microscope – bringing trial design into focus. Nat Rev Clin Oncol. 2019;16(6):339–340.
- Durie BGM, Hoering A, Abidi M, et al. Longer term follow up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood. 2018;132(suppl 1):Abstract 1992.
- Dimopoulos MA, Mateos MV, Cavo M, et al. One-year update of a phase 3 randomized study of daratumumab plus bortezomib, melphalan, and prednisone (D-VMP) versus bortezomib, melphalan, and prednisone (VMP) in patients (pts) with transplant-ineligible newly diagnosed multiple myeloma (NDMM): ALCYONE. Blood. 2018;132:Abstract 156.
- Liu X, Chen J, He YA, et al. Comparing efficacy and survivals of initial treatments for elderly patients with newly diagnosed multiple myeloma: a network meta-analysis of randomized controlled trials. OTT. 2016;10:121–128.
- Blommestein HM, van Beurden-Tan CHY, Franken MG, et al. Efficacy of first-line treatments for multiple myeloma patients not eligible for stem cell transplantation - a network meta-analysis. Haematologica. 2019;104(5):1026.
- Cipriani A, Higgins JO, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130–137.
- Lu G, Ades AE, Sutton AJ, et al. Meta-analysis of mixed treatment comparisons at multiple follow-up times. Statist Med. 2007;26(20):3681–3699.
- Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol. 2011;11(1):61.
- Dias S, Welton N, Sutton A, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33(5):641–656.
- CHMP post-authorisation summary of positive opinion for Revlimid (II-102-G). [cited 2019 Mar 28]. Available from: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-revlimid-ii-102-g_en.pdf.
- Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118(17):4519–4529.