Abstract
The Intergroupe Francophone du Myelome 2009 trial (NCT01191060) assessed health-related quality of life (HRQoL) in patients with newly diagnosed multiple myeloma (NDMM) receiving lenalidomide/bortezomib/dexamethasone (RVd) induction therapy followed by consolidation therapy with either autologous stem cell transplantation (ASCT) plus RVd (RVd-ASCT) or RVd-alone; both groups then received lenalidomide maintenance therapy for 1 year. Global HRQoL, physical functioning, and role functioning scores significantly improved for both cohorts from baseline to the end of consolidation and were sustained during maintenance and follow-up, with clinically meaningful changes (RVd-alone: p = .0002; RVd-ASCT: p < .001). Similarly, both groups showed clinically meaningful improvements from baseline in fatigue, pain, and disease symptom scores. Side effects of treatment scores remained stable. In the RVd-ASCT group, there was transient worsening in HRQoL immediately after ASCT. These findings suggest that the clinical improvements observed with RVd-based treatment are accompanied by overall improvements in HRQoL for patients with NDMM.
Introduction
Multiple myeloma (MM) is one of the most debilitating hematological diseases, and patients experience substantial pain and fatigue along with significant reductions in physical functioning, role functioning, and overall quality of life (QoL) [Citation1–3]. High-dose therapy (HDT) with melphalan followed by autologous stem cell transplantation (ASCT), and subsequent lenalidomide maintenance therapy is one of the standard treatment approaches for newly diagnosed MM (NDMM) in adults who are < 65 years of age and clinically fit [Citation4]. This is based on a large body of evidence [Citation5–11]. A meta-analysis reported a median overall survival (OS) beyond 7 years after a follow-up of 69.5 months, and a median progression-free survival (PFS) of > 4 years versus patients who received placebo or observation-only following ASCT [Citation10]. An updated analysis showed that this approach could extend OS to > 9 years [Citation11]. Combination triplet therapies, such as lenalidomide, bortezomib, and dexamethasone (RVd) are used as induction therapy prior to ASCT [Citation4,Citation5,Citation12,Citation13] and have shown promising results as consolidation therapy after ASCT [Citation12]. In patients not intended for immediate ASCT, RVd or lenalidomide and dexamethasone may be used as induction therapy [Citation4,Citation13,Citation14]. Research into additional options for induction therapy, including the combination of lenalidomide, dexamethasone, and daratumumab (Dara-Rd), is ongoing.
While current standards of care induce remission in a high proportion of patients and prolong survival, the persistent disease sequelae and adverse effects of therapy can affect health-related quality of life (HRQoL). Undergoing ASCT, for example, is associated with a short-term worsening of HRQoL although symptom scores may recover within 1–2 months and typically continue to improve over time [Citation15]. Investigation of new therapies should focus not only on clinical efficacy, safety, and tolerability but also on the effects of treatment on HRQoL and other patient-reported outcomes.
The Intergroupe Francophone du Myelome (IFM) 2009 trial demonstrated the clinical benefits of RVd as induction and consolidation therapy, with or without ASCT, in patients with NDMM (ClinicalTrials.gov number, NCT01191060) [Citation5]. Overall survival at 4 years was similar in both treatment groups (adjusted hazard ratio [HR] for death [95% confidence interval, CI]: 1.16 [0.80, 1.68]; p = .87); however, RVd therapy plus ASCT was associated with significantly longer median PFS compared with RVd therapy alone (50 months versus 36 months; adjusted HR for disease progression or death [95% CI]: 0.65 [0.53, 0.80]; p < .001) [Citation5]. To better understand the impact of RVd with or without ASCT on HRQoL, we assessed changes in HRQoL among patients in the IFM 2009 trial.
Materials and methods
Data source
The IFM 2009 trial was a phase 3, multicenter, randomized, open-label study in patients with NDMM aged > 65 years (N = 700) [Citation5]. The study was conducted in France, Belgium, and Switzerland with the formal approval of the institutional ethics committee (Purpan Hospital, Toulouse, France) and was registered according to legislature requirements. All patients provided written informed consent. Details of trial design, enrollment criteria, and treatment have been published elsewhere [Citation5]. Briefly, patients were randomized (1:1) and stratified by International Staging System (ISS) stage and cytogenetic risk to either RVd (lenalidomide 25 mg orally on days 1–14; bortezomib 1.3 mg per square meter of body surface area intravenously on days 1, 4, 8, and 11; dexamethasone 20 mg orally on days 1, 2, 4, 5, 8, 9, 11, and 12) for three 3-week cycles as induction therapy followed by 5 cycles as consolidation therapy (RVd-alone [dexamethasone 10 mg]); or RVd for three 3-week cycles as induction therapy followed by ASCT and then RVd for consolidation (RVd-ASCT) [dexamethasone 10 mg]) for 2 cycles. Both treatment groups initiated lenalidomide maintenance therapy (10 mg/day for the first 3 months with a possible dose increase to 15 mg, based on safety profile) within the first 3 weeks after completion of consolidation therapy, and continued maintenance therapy for a maximum of 1 year or until disease progression, unacceptable adverse events (AEs), or withdrawal of patient consent.
HRQoL measures
Patient-reported outcomes, including HRQoL were assessed using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life-Core 30 Questionnaire (QLQ-C30) and the EORTC Quality of Life Questionnaire for Patients with Multiple Myeloma (QLQ-MY20) [Citation16–19]. In both treatment groups, HRQoL assessments were performed at baseline, during induction, consolidation, and maintenance, at the end of treatment, and during follow-up visits (Supplementary Table S1). This analysis focused on the following domains considered to be most relevant for this treatment setting, namely QLQ-C30 (i.e. global QoL, physical functioning, role functioning, fatigue, and pain) and side effects of treatment and disease symptoms domains from the QLQ-MY20 [Citation3]. Results from other QLQ-C30 and QLQ-MY20 domains are presented in Supplementary Tables S2 and S3.
All the scales in the QLQ-C30 and QLQ-MY20 range in score from 0 to 100. A higher score on a functional scale or the global QoL scale of the QLQ-C30 represents a better level of functioning or health status. A higher score on a symptom scale represents worsening symptoms. For QLQ-MY20, a higher score for the disease symptoms and side effects of treatment domains represents worsening symptoms or side effects.
Mean QLQ-C30 scores at baseline and over the course of treatment were benchmarked against those reported for the general population from an international study involving 15,386 individuals, of whom approximately 80% were < 69 years of age [Citation20].
Changes from baseline were estimated for all time points and were considered clinically meaningful if the magnitude of changes exceeded the minimally important difference (MID) threshold of 10 points or greater for both QLQ-C30 and QLQ-MY20, selected based on the findings from previous studies [Citation21,Citation22].
Statistical analyses
The data cutoff date for this analysis was 1 September 2015. Analysis of HRQoL scores was performed using SAS software version 9.4 or higher (SAS® Institute Inc., Cary, NC).
Compliance rates were determined for each time point based on the patients for whom clinical data were available. For the QLQ-C30 [Citation16,Citation23] and QLQ-MY20 questionnaires [Citation17], a patient was considered compliant if ≥ 50% of the questionnaire items were completed for a given assessment visit. Comparisons between groups were conducted only if ≥ 50% of patients completed the questionnaires [Citation24].
Analyses of key demographic and disease characteristics and HRQoL outcomes were based on the HRQoL-evaluable population, defined as randomized patients who completed the QLQ-C30 assessment at baseline and for ≥ 1 post-baseline assessment visit. Descriptive statistics were used to summarize patients’ clinical characteristics at baseline and HRQoL results at baseline and follow-up. Within-group differences in mean changes from baseline were compared using the one-sample and two-sample t-test, respectively. Evaluation of statistical significance was not adjusted for multiplicity. A supplementary analysis of within-group differences in least squares (LS) mean change (and 95% CI) from baseline at each post-baseline visit for all domains of the QLQ-C30 and QLQ-MY20 was carried out using analysis of covariance (ANCOVA) models, adjusting for baseline domain scores and stratification factors (i.e. ISS stage and cytogenetic risk) (Supplementary Table S4). To assess the treatment effects on HRQoL, a mixed model repeated measure (MMRM) analysis of LS mean changes from baseline in HRQoL was performed for all visits during which patients remained progression free.
Results
Patients
Of the 700 randomized patients (intent-to-treat [ITT] population), 604 (86%) had both a baseline and ≥ 1 post baseline assessment and were included in the HRQoL-evaluable population (Supplementary Figure S1). Baseline demographics and clinical characteristics were similar between the ITT and HRQoL-evaluable populations, and between treatment groups (). The mean age was 56.7 years, most patients (59.9%) were male, and 90.5% had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1. For patients who remained on the study, compliance rates with the QLQ-C30 were high at baseline for both treatment groups (RVd-alone, 90.3%; RVd-ASCT, 88.6%), remained high at the end of the induction period (RVd-alone, 77.1%; RVd-ASCT, 73.5%), and were ≥ 59% through the last treatment visit in both groups with the exception of visit 2 (day 1 of cycle 2; C2D1), which was added after study initiation (Supplementary Table S5). Compliance rates were similar between both arms; however, a lower compliance rate was observed for the RVd-ASCT group at the first post-ASCT visit (46.1% on day 20 post-autograft versus 79.8% for the RVd-alone group on visit 4, day 1 of cycle 5 (C5D1); p < .05). Compliance rates for the QLQ-MY20 were similar to those for the QLQ-C30 for both treatment groups.
Table 1. Baseline demographics and clinical characteristics of HRQoL-evaluable patients.
HRQoL scores
Baseline HRQoL scores were similar between treatment groups ( and Supplementary Table S2). Scores in both groups were generally worse than scores in the general population [Citation20] for global QoL, most functional domains, and all symptoms – scores were similar in the treatment groups and the general population for diarrhea, nausea and vomiting, cognitive functioning, and financial difficulties.
Table 2. Mean (SD) HRQoL scores at baseline for the HRQoL-evaluable population.
Global health status and functional domains
Over the course of the study, both treatment groups had improvements in HRQoL scores related to global health status and functional domains. Mean scores from the QLQ-C30 for global QoL and functional domains (physical functioning and role functioning) indicated poor HRQoL at baseline that improved steadily, reaching levels comparable to the reference population [Citation20] by the final follow-up visit ().
Figure 1. Mean observed scores for the QLQ-C30 domains in the IFM 2009 trial compared with the general population of: global QoL (a); physical functioning (b); and role functioning (c).a ASCT: autologous stem cell transplantation; C: cycle; D: day; EOT: end of treatment; FU: follow-up; IFM: Intergroupe Francophone du Myelome; M: maintenance; PA: post-autograft; PreMob: premobilization; QLQ-C30: Quality of Life Questionnaire Core-30; QoL: quality of life; RVd: lenalidomide, bortezomib, and dexamethasone; SD: standard deviation. aEnd of induction is end of cycle 3/prior to PreMob; follow-up visit 1 is 2 years after initial dosing; follow-up visit 2 is 3 years after initial dosing. bGeneral population (N = 11,343). Mean global QoL value for general population is 65.5 (weighted score). Reference values for general population were used as a benchmark to help interpret findings [Citation19]. cGeneral population (N = 11,343). Mean value for general population is 84.9 (weighted score). dGeneral population (N = 11,343). Mean value for general population is 84.2 (weighted score).
![Figure 1. Mean observed scores for the QLQ-C30 domains in the IFM 2009 trial compared with the general population of: global QoL (a); physical functioning (b); and role functioning (c).a ASCT: autologous stem cell transplantation; C: cycle; D: day; EOT: end of treatment; FU: follow-up; IFM: Intergroupe Francophone du Myelome; M: maintenance; PA: post-autograft; PreMob: premobilization; QLQ-C30: Quality of Life Questionnaire Core-30; QoL: quality of life; RVd: lenalidomide, bortezomib, and dexamethasone; SD: standard deviation. aEnd of induction is end of cycle 3/prior to PreMob; follow-up visit 1 is 2 years after initial dosing; follow-up visit 2 is 3 years after initial dosing. bGeneral population (N = 11,343). Mean global QoL value for general population is 65.5 (weighted score). Reference values for general population were used as a benchmark to help interpret findings [Citation19]. cGeneral population (N = 11,343). Mean value for general population is 84.9 (weighted score). dGeneral population (N = 11,343). Mean value for general population is 84.2 (weighted score).](/cms/asset/32ff2a2d-23b8-4bd1-a927-2665c867c25a/ilal_a_1719091_f0001_c.jpg)
In the RVd-alone group, QLQ-C30 global QoL scores increased from baseline to the end of the consolidation phase (6.4 points; p = .0002) and were sustained during maintenance therapy and further follow-up (Supplementary Figure S2a). A similar pattern was observed for functional domains, where statistically significant increases were observed from baseline to the end of the consolidation period for physical functioning (11.4, p < .0001; Supplementary Figure S2b) and role functioning (20.8, p < .0001; Supplementary Figure S2c).
In the RVd-ASCT group, an overall increase in QLQ-C30 global QoL (13.8, p < .0001) was observed from baseline to the end of the consolidation period, which was sustained during maintenance therapy and further follow-up (Supplementary Figure S2a). Scores were similar to those reported for the RVd-alone group, except for the first post-ASCT visit. As expected, a temporary decrease in QLQ-C30 global QoL was observed immediately following ASCT; however, mean scores improved by the next visit at the end of the consolidation period. Similarly, statistically significant increases from baseline to the end of the consolidation period were noted for physical functioning (11.1, p < .0001; Supplementary Figure S2b) and role functioning (18.5, p < .0001; Supplementary Figure S2c).
Longitudinal analyses of mean changes from baseline in global QoL and functional domains (e.g. role and physical functioning) showed significant improvements in the key domains of interest by the end of RVd induction therapy (week 6; premobilization [PreMob]), as well as during consolidation (week 21; cycle 8 day 1 [C8D1]) and follow-up (week 72; end of treatment [EOT]) in both treatment groups ( and Supplementary Table S3).
Table 3. MMRM analyses for LS mean changes from baseline in HRQoL.
Symptom domains
Overall, both treatment groups reported improvements in the QLQ-C30 symptom domains of fatigue and pain (,b)). Both groups had clinically meaningful decreases in fatigue score of > 10 points from baseline over the course of treatment (). In the RVd-alone group, fatigue decreased by 4.7 points (p = .0088) by the end of induction therapy, was 1.9 points (p = .3368) lower than baseline at the end of consolidation therapy, and > 8 points lower than baseline from visit 6 onward (indicating improvement). From this time point onward, mean fatigue scores approached that of the general population (Supplementary Figure S3a). In the RVd-ASCT group, an increase in fatigue was observed immediately following ASCT but improved by visit 5 at the end of consolidation (9 points lower than at baseline and continued to improve). Both groups also showed clinically significant decreases in pain scores of approximately 15 points from visit 5 onward, which was sustained (despite a transient increase in pain scores immediately after ASCT) and was approximately 5 points greater than the score for the general population ( and Supplementary Figure S3b).
Figure 2. Mean changes from baseline in the IFM 2009 trial for: the fatigue domain of QLQ-C30 (a); and the pain domain of QLQ-C30 in the IFM 2009 trial (b).a ASCT: autologous stem cell transplantation; C: cycle; CI: confidence interval; D: day; FU: follow-up; IFM: Intergroupe Francophone du Myelome; LS: least squares; M: maintenance; MID: minimally important difference; PA: post-autograft; PreMob: premobilization; QLQ-C30: Quality of Life Questionnaire Core-30; RVd: lenalidomide, bortezomib, and dexamethasone. aEnd of induction is end of cycle 3/prior to PreMob; follow-up visit 1 is 2 years after initial dosing; follow-up visit 2 is 3 years after initial dosing. *Significance between the groups at p < .05 based on a two-sample t-test. A positive value indicates improvement from baseline and vice versa.
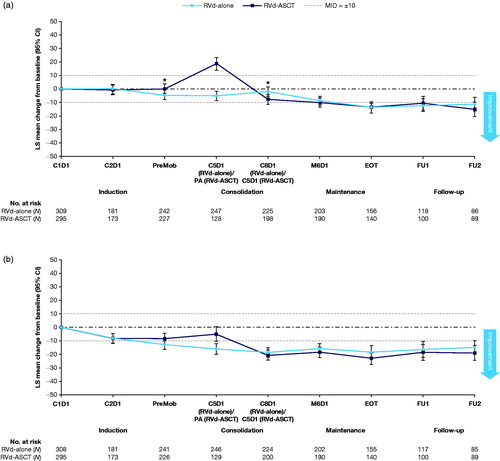
Results for the QLQ-MY20 domains of disease symptoms and side effects of treatment showed favorable changes over the course of the study. Disease symptoms scores showed similar decreases by the end of the consolidation period in both groups (RVd-alone 10.2, p < .0001; RVd-ASCT 8.9, p < .0001). The side effects of treatment scores from the QLQ-MY20 were relatively stable over time, despite a transient worsening following ASCT in the RVd-ASCT group.
Longitudinal analyses showed significant improvements in pain, side effects, and disease symptoms starting at the end of RVd induction therapy (week 6; PreMob), as well as during consolidation (week 21; C8D1) and follow-up (week 72; EOT) in both treatment groups; improvements in fatigue were significant only at follow-up (week 72, EOT) in the RVd-ASCT group ( and Supplementary Table S3).
Discussion
Current guidelines recommend RVd as an induction therapy in patients with NDMM undergoing ASCT and as a first-line treatment in patients ineligible for ASCT [Citation4,Citation13]. These recommendations are mainly based on the SWOG S0777 study that demonstrated a clinically significant improvement in median OS (75 vs. 64 months; p = .250) and PFS (43 versus 30 months; p = .0037) for RVd over lenalidomide plus dexamethasone (Rd), one of the current standards of care in patients not intended for ASCT [Citation14]. The clinical data from the IFM 2009 trial suggest that use of RVd in conjunction with ASCT is a valuable option in patients able to tolerate the more intensive therapy as the RVd-ASCT strategy was associated with a statistically significant prolongation in PFS (50 versus 36 months; p < .001) [Citation5]. The results reported here further substantiate this by showing that the more intensive regimen does not adversely affect HRQoL, except in the immediate post-ASCT period.
This analysis shows that both RVd treatment strategies followed by 1 year of lenalidomide maintenance therapy provided clinically meaningful improvements in the most distressing disease-related symptoms from a patient perspective, namely fatigue and pain, and that these translated into clinically meaningful improvements in physical functioning, role functioning, and global HRQoL. The data are consistent with reports from other clinical trials showing that regimens that induce clinical responses and prolong PFS also provide sustained improvements in HRQoL, suggesting that a clinically meaningful improvement in relevant symptoms outweighs the adverse effects of treatment [Citation22,Citation25–28]. In addition, these findings are also supported by data from a cross-sectional observational study on clinical treatment of MM in France [Citation29], and a systematic literature review into the impact of lenalidomide, thalidomide, and bortezomib therapy on HRQoL in patients with MM [Citation30].
In this study, 1 year of lenalidomide maintenance therapy was not associated with clinically significant toxicities and did not adversely affect HRQoL. Scores for fatigue, pain, physical functioning, role functioning, and global HRQoL did not change substantially during the follow-up period when patients were off treatment, compared with the preceding phase of maintenance therapy. This is consistent with results from the Connect® MM Disease Registry, a large observational, prospective registry of more than 3000 patients with NDMM, with a median follow-up of 39 months and median duration of maintenance therapy of 24 months [Citation30]. In this registry study, no deterioration in HRQoL was observed in patients who received post-ASCT maintenance therapy, specifically lenalidomide maintenance therapy, compared with no maintenance therapy. Similarly, a multicenter cross-sectional, non-interventional study involving sites in the USA and Canada [Citation1] found that maintenance therapy (lenalidomide in 75% of patients) after ASCT did not negatively affect HRQoL in patients as measured by the QLQ-C30 and QLQ-MY20.
Lenalidomide maintenance therapy until progression has been shown to improve outcomes and is currently the main therapy approved for use as continuous maintenance treatment; however, optimum duration of therapy data are lacking. In the UK-based phase 3 Myeloma XI trial (median follow-up: 31 months; median duration of lenalidomide maintenance: eighteen 28-day cycles [interquartile range: 6–30 cycles]), PFS in the overall cohort was significantly prolonged for lenalidomide versus observation in transplantation-eligible patients (39 months versus 20 months; HR 0.46 [95% CI 0.41–0.53], p < .0001) [Citation31]. Similar findings were reported in an updated survival analysis of the USA-based CALGB 100104 randomized phase 3 trial (median follow-up: 91 months; median duration of lenalidomide maintenance: 30 months [range: 0–108]) – median time to progression was 57.3 months for patients receiving lenalidomide maintenance compared with 28.9 months for placebo [Citation11]. In other trials, lenalidomide maintenance was associated with clinically relevant HRQoL improvements versus no maintenance, with a longer time on maintenance highlighted as an important factor for improvement [Citation25,Citation32]. Real-world evidence from the Connect® MM Disease Registry (median duration of lenalidomide maintenance: 35.2 months) and other North American observational studies confirm the survival benefits of lenalidomide maintenance seen in clinical trials [Citation33–35]. Such real-world data support the use of lenalidomide maintenance in patients with NDMM. Ultimately, physicians must determine the optimal duration of maintenance therapy, with dose modifications an option for those patients who are unable to tolerate continuous lenalidomide at 10 or 15 mg until progression [Citation36].
A number of potential limitations may affect interpretation of the findings of this study. Missing data are a limitation in most HRQoL studies. Analyses were based on the HRQoL-evaluable population, which was 86% of the ITT population. However, baseline characteristics of the HRQoL-evaluable population were largely consistent with the ITT population and generally balanced between the 2 treatment groups. Thus, the HRQoL-evaluable population is likely representative of the total trial population. Compliance with HRQoL assessments was high (approximately 90%) in both groups at baseline, and remained over 70% until the end of induction, before decreasing to approximately 60% by the end of treatment. This reduction over time may result in an overestimation of HRQoL effects if patients with better HRQoL are more likely to be compliant. Equally, as patients who did not benefit from the treatments were more likely to withdraw from the trial, the HRQoL benefit reported reflects the experience of patients who remained on treatment. No imputation of missing data was performed for the HRQoL analyses; this may have overestimated HRQoL benefits and underestimated the impact of symptoms, given that patients with more severe symptoms that could have a greater impact on HRQoL may be more likely to discontinue treatment or show lower compliance, as indicated by the sharp reduction in compliance following ASCT. However, this limitation is less likely to have affected the results for the phase of the study during which patients received RVd (with or without ASCT), which was the focus of the study. In addition, HRQoL assessments were performed at specific time points during the trial and may not provide a comprehensive evaluation of the symptoms and HRQoL burden throughout all stages of treatment, including the follow-up period. In addition, maintenance therapy was only given for 1 year in this trial, rather than continued until progression, as recommended, which may underestimate the benefits. However, the primary reason for this approach was to reduce unacceptable AEs as the data available on the optimal duration of lenalidomide maintenance therapy are limited [Citation5]. In the ongoing randomized trial (the DETERMINATION study; ClinicalTrials.gov number, NCT01208662), with a similar study design to the IFM 2009 trial, lenalidomide maintenance therapy is administered via a continuous dosing regimen until progression. Findings from this trial, when available, may provide insights into the optimal duration of maintenance therapy and greater understanding of patient outcomes associated with lenalidomide maintenance [Citation5]. Together with the IFM 2009 study, this study will provide a valuable framework for physicians looking to apply these results to clinical practice.
Nevertheless, the data from the current study provide a valuable insight into the impact of triplet therapy with or without ASCT on HRQoL in patients receiving treatment for NDMM.
Conclusions
In an era where patient QoL is becoming increasingly important in healthcare decision-making, this analysis of data from the IFM 2009 trial demonstrated that RVd-based strategies followed by 1 year of lenalidomide maintenance therapy are a valuable option for the management of patients with NDMM. Furthermore, the inclusion of ASCT in the strategy can improve clinical outcomes without compromising HRQoL. Since the introduction of lenalidomide and bortezomib, clinicians have gained considerable experience in using these treatments, and the RVd regimen is now considered standard of care in both Europe and the USA [Citation4,Citation13]. Taken together with previous studies, the results from this clinical trial suggest that these HRQoL benefits can also be achieved in a real-world setting.
GLAL-2019-0996-File002.docx
Download MS Word (809.6 KB)Acknowledgments
The authors would like to thank the study investigators, participating sites, patients, nurses, and personnel who were involved in the study. The IFM 2009 study was supported by the French Programme Hospitalier de Recherche Clinique, by the French National Research Agency (ANR-11-PHUC-001-CAPTOR project), and by a grant from Celgene Corporation, and Janssen (ClinicalTrials.gov number, NCT01191060). The authors received writing and editorial assistance in the preparation of this manuscript funded by Bristol-Myers Squibb. Writing assistance was provided by Evidera (Kim Poinsett-Holmes, PharmD, and Saurabh Aggarwal, PhD), and editorial assistance by Excerpta Medica (Rosie Morland, PhD) and AccuScript Consultancy (Rowena Hughes, PhD).
Disclosure statement
The authors are fully responsible for all content and editorial decisions for this manuscript. Dr. Dhanasiri reports other from Celgene, A Bristol-Myers Squibb Company, during the conduct of the study.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Tay J, Vij R, Norkin M, et al. Health related quality of life for multiple myeloma patients according to treatment strategy after autologous stem cell transplant: a cross-sectional study using EORTC, EQ-5D and MY-20 scales. Leuk Lymph. 2019;60(5):1275–1282.
- Despiégel N, Touboul C, Flinois A, et al. Health-related quality of life of patients with multiple myeloma treated in routine clinical practice in France. Clin Lymphoma Myeloma Leuk. 2019;19(1):e13–e28.
- Hatswell AJ, Burns D, Baio G, et al. Frequentist and Bayesian meta-regression of health state utilities for multiple myeloma incorporating systematic review and analysis of individual patient data. Health Econ. 2019;28(5):653–665.
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv52–iv61.
- Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311–1320.
- Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16(16):1617–1629.
- Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905.
- Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–1791.
- McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781.
- McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279–3289.
- Holstein SA, Jung SH, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol. 2017;4(9):e431–e442.
- Roussel M, Lauwers-Cances V, Robillard N, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myélome. J Clin Oncol. 2014;32(25):2712–2717.
- Kumar SK, Callander NS, Baljevic M, et al. Multiple myeloma, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. 2020. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.2.2020. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. [cited 2019 Dec 17]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–527.
- Chakraborty R, Hamilton BK, Hashmi SK, et al. Health-related quality of life after autologous stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2018;24(8):1546–1553.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Cocks K, Cohen D, Wisløff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43(11):1670–1678.
- Stead ML, Brown JM, Velikova G, et al. Development of an EORTC questionnaire module to be used in health-related quality-of-life assessment for patients with multiple myeloma. European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Br J Haematol. 1999;104(3):605–611.
- Nolte S, Liegl G, Petersen MA, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153–163.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144.
- Musoro JZ, Bottomley A, Coens C, et al. Interpreting European Organisation for Research and Treatment for Cancer Quality of life Questionnaire core 30 scores as minimally importantly different for patients with malignant melanoma. Eur J Cancer. 2018;104:169–181.
- Royle KL, Gregory WM, Cairns DA, et al. Quality of life during and following sequential treatment of previously untreated patients with multiple myeloma: findings of the Medical Research Council Myeloma IX randomised study. Br J Haematol. 2018;182(6):816–829.
- EORTC.org [Internet]. Brussels: European Organisation for Research and Treatment of Cancer; [cited 2019 Nov 06]. Available from: https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf.
- Delforge M, Minuk L, Eisenmann J, et al. Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica. 2015;100(6):826–833.
- Dimopoulos MA, Delforge M, Hájek R, et al. Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health-related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: results of a randomized phase III trial. Haematologica. 2013;98(5):784–788.
- Dimopoulos MA, Palumbo A, Hajek R, et al. Factors that influence health-related quality of life in newly diagnosed patients with multiple myeloma aged ≥ 65 years treated with melphalan, prednisone and lenalidomide followed by lenalidomide maintenance: results of a randomized trial. Leuk Lymphoma. 2014;55(7):1489–1497.
- Stewart AK, Dimopoulos MA, Masszi T, et al. Health-related quality-of-life results from the open-label, randomized, phase III ASPIRE trial evaluating carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma. J Clin Oncol. 2016;34(32):3921–3930.
- Ludwig H, Moreau P, Dimopoulos MA, et al. Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2019;9(3):23.
- Sonneveld P, Verelst SG, Lewis P, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27(10):1959–1969.
- Abonour R, Wagner L, Durie BGM, et al. Impact of post-transplantation maintenance therapy on health-related quality of life in patients with multiple myeloma: data from the Connect® MM Registry. Ann Hematol. 2018;97(12):2425–2436.
- Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57–73.
- Nielsen LK, Stege C, Lissenberg-Witte B, et al. Health-related quality of life in transplant ineligible newly diagnosed multiple myeloma patients treated with either thalidomide or lenalidomide-based regimen until progression: a prospective, open-label, multicenter, randomized, phase 3 study. Haematologica. 2019 Sep 12. DOI:10.3324/haematol.2019.222299 [Epub ahead of print].
- Jagannath S, Abonour R, Durie BGM, et al. Impact of post-ASCT maintenance therapy on outcomes in patients with newly diagnosed multiple myeloma in Connect MM. Blood Adv. 2018;2(13):1608–1615.
- Cherniawsky H, Kukreti V, Reece D, et al. The impact of lenalidomide maintenance on second line chemotherapy in transplant eligible patients with multiple myeloma in the Canadian setting. Clin Lymphoma Myeloma Leuk. 2019;19(10):e250.
- Hari P, Ung B, Abouzaid S, et al. Lenalidomide maintenance post-transplantation in newly diagnosed multiple myeloma: real-world outcomes and costs. Future Oncol. 2019;15(35):4045–4056.
- Reece D, Kouroukis CT, Leblanc R, et al. Practical approaches to the use of lenalidomide in multiple myeloma: a Canadian consensus. Adv Hematol. 2012;2012:621958.
