Abstract
CPX-351, a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a synergistic 5:1 molar drug ratio, achieved superior efficacy compared with conventional chemotherapy in older adults with newly diagnosed, high-risk/secondary acute myeloid leukemia (AML) in phase 2 and 3 studies. Prior to CPX-351 commercialization, an expanded access program (EAP) provided CPX-351 access for this population in the United States. In this phase 4, single-arm, open-label study (NCT02533115), 52 patients were treated with CPX-351 for 1–2 induction cycles and ≤4 consolidation cycles. The primary endpoint was safety. The most common serious adverse events were febrile neutropenia (19%), pneumonia (10%), and infection (8%). The 30- and 60-d mortality rates were 0% and 6%, respectively. Remission was achieved by 44% of patients; 90% of patients were alive at study completion. Overall, these results support outcomes from prior studies and the use of CPX-351 in older adults with newly diagnosed, high-risk/secondary AML.
Introduction
Acute myeloid leukemia (AML) that arises as a late complication after prior exposure to cytotoxic chemotherapy, ionizing radiation, or immunosuppressive therapy (i.e. therapy-related AML) or that evolves from a prior hematologic disorder is generally referred to as secondary AML [Citation1,Citation2]. Approximately 25% of all individuals diagnosed with AML are found to have secondary AML, which increases with age (median age of 68 years at diagnosis) [Citation2–4]. Secondary AML is also associated with adverse/complex cytogenetics and multidrug resistance, both of which contribute to the poor outcomes observed for this disease with conventional induction chemotherapy [Citation2,Citation3,Citation5–10]. For example, induction with the 7 + 3 regimen (i.e. cytarabine continuous infusion for 7 d in combination with three daily injections of an anthracycline) has been associated with lower rates of remission, higher rates of relapse, and increased early mortality in older adults and patients with secondary AML when compared with younger adults and patients with de novo AML [Citation6,Citation11]. Therefore, improved treatment strategies are needed.
CPX-351 (Vyxeos®; daunorubicin and cytarabine liposome for injection; Jazz Pharmaceuticals, Palo Alto, CA) is a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a synergistic 5:1 molar ratio [Citation12,Citation13]. A synergistic drug ratio is maintained in human plasma for ≥24 h after CPX-351 administration, which is not possible with the administration of free cytarabine and daunorubicin (i.e. 7 + 3 regimen). A large randomized phase 3 study compared the safety and efficacy of CPX-351 vs. conventional 7 + 3 chemotherapy in older adults aged 60–75 years with newly diagnosed, high-risk/secondary AML [Citation14]. In this study, CPX-351 significantly prolonged median overall survival (9.56 vs. 5.95 months; hazard ratio = 0.69; 1-sided p = .003) and achieved a significantly higher rate of CR + CRi (47.7% vs. 33.3%; 2-sided p = .016) compared with 7 + 3. The proportion of patients who underwent hematopoietic cell transplantation (HCT) was 34.0% with CPX-351 vs. 25.0% with 7 + 3 (2-sided nominal p = .098), and an exploratory analysis of overall survival landmarked from the time of HCT favored CPX-351 (not reached vs. 10.25 months; 1-sided nominal p = .009). Furthermore, an exploratory post hoc analysis in patients who achieved CR + CRi found that those treated with CPX-351 had longer median OS (25.43 vs. 10.41 months; hazard ratio = 0.49), a higher rate of HCT (55% vs. 46%), and longer median OS landmarked from the date of HCT (not reached vs. 11.65 months; hazard ratio = 0.42) vs. 7 + 3, suggesting deeper responses with CPX-351 [Citation15]. The safety profile of CPX-351 was generally similar to that of 7 + 3. Though the duration of myelosuppression was longer with CPX-351 than with 7 + 3 (median time to neutrophil recovery ≥500/µL: 35 vs. 29 d; median time to platelet recovery ≥50,000/µL: 36.5 vs. 29 d), mortality rates were lower with CPX-351 at 30 d (5.9% vs. 10.6%; 2-sided nominal p = .149) and 60 d (13.7% vs. 21.2%; 2-sided nominal p = .097) [Citation14].
Results from this phase 3 study led to the approval of CPX-351 by the US Food and Drug Administration in 2017 and the European Medicines Agency in 2018 for the treatment of adults with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes (AML-MRC) [Citation16,Citation17]. AML-MRC is a World Health Organization (WHO)–defined category encompassing AML with prior myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN); MDS-related cytogenetic abnormalities; and/or multilineage dysplasia in >50% of ≥2 cell lineages (in the absence of NPM1 or biallelic CEBPA mutations) [Citation18].
Following completion of enrollment in the phase 3 study and prior to commercial availability, CPX-351 was made available to older adults with newly diagnosed, high-risk/secondary AML in an early access protocol (EAP) in the United States. Safety and efficacy data from the EAP are reported herein.
Methods
Study design and oversight
This phase 4, multicenter, single-arm, open-label EAP was conducted in the United States to provide access to CPX-351 treatment for older adults with newly diagnosed, high-risk/secondary AML who were candidates for treatment with intensive chemotherapy induction (ClinicalTrials.gov Identifier NCT02533115).
The study consisted of 2 phases: a treatment phase and a follow-up phase. During the treatment phase, patients were eligible to receive up to two induction cycles and up to four consolidation cycles with CPX-351. Induction consisted of CPX-351 100 units/m2 (cytarabine 100 mg/m2 and daunorubicin 44 mg/m2) via 90-min infusion on Days 1, 3, and 5. If a second induction was administered, the dose of CPX-351 was 100 units/m2 by 90-min intravenous (IV) infusion on Days 1 and 3. Patients achieving CR or CRi (confirmed by bone marrow assessment) after induction could receive up to four cycles of consolidation, consisting of CPX-351 65 units/m2 (cytarabine 65 mg/m2 and daunorubicin 29 mg/m2) via 90-min infusion on Days 1 and 3. The follow-up phase began after the completion of the treatment phase and lasted for 30 d. Depending on the dosing regimen (number of induction and consolidation cycles received), the duration of treatment for an individual patient lasted a maximum of approximately 336 d. The total duration of the study, including follow-up, was approximately 1 year.
This study was conducted in accordance with local regulations, the principles of the Declaration of Helsinki, and the International Council for Harmonisation Good Clinical Practice guidelines. The original protocol and all amendments were reviewed and approved by the Institutional Review Board at each participating center. All study participants provided written informed consent prior to enrollment.
Eligibility criteria
Enrollment criteria were generally consistent with those of the prior phase 3 study [Citation14]. In brief, patients were eligible for study inclusion if they were adults 60–75 years of age and newly diagnosed with AML according to WHO 2008 criteria (≥20% blasts in peripheral blood or bone marrow). Three patients 55–60 years of age were granted waivers for study entry. Eligible patients must have had confirmation of high-risk/secondary AML based on prior cytotoxic treatment (i.e. therapy-related AML), a history of MDS or chronic myelomonocytic leukemia (CMML), or de novo AML with myelodysplasia-related cytogenetic abnormalities. In addition, patients must have had an Eastern Cooperative Oncology Group (ECOG) performance status between 0 and 2 and the ability to tolerate intensive AML chemotherapy in the opinion of the treating physician. Patients were excluded from this study if they had acute promyelocytic leukemia t(15;17) or favorable cytogenetics at screening, or if they had received prior treatment intended as induction therapy for AML (hydroxyurea permitted). Patients with active secondary malignancies, central nervous system leukemia, or a history of MPN or combined MDS/MPN, except CMML, were also excluded from the study.
Endpoints and assessments
The primary endpoint of the study was the evaluation of the safety of CPX-351. Patients were continuously monitored for safety and the occurrence of treatment-emergent adverse events (TEAEs) from the first dose of CPX-351 until 30 d after the last dose of CPX-351. Evaluation of CPX-351 efficacy was based on the achievement of remission (CR or CRi), according to established criteria [Citation19], following induction therapy. An assessment of bone marrow was required between Days 14–28 to confirm response to initial induction and whether or not a second induction was indicated. A bone marrow biopsy was required within 14 d of peripheral blood count recovery to confirm treatment response.
Statistical analyses
There were no specific enrollment or statistical objectives for this study because this was an EAP; therefore, no power calculations were performed and enrollment continued until commercialization of CPX-351.
Both safety and efficacy analyses were based on the safety analysis set, which included all patients who received ≥1 dose of CPX-351. Counts and percentages of patients are presented for categorical variables, while mean, median, standard deviation, and minimum and maximum are presented for continuous variables. Baseline values were defined as the last recorded values prior to the first dose of study drug.
Results
Patient disposition
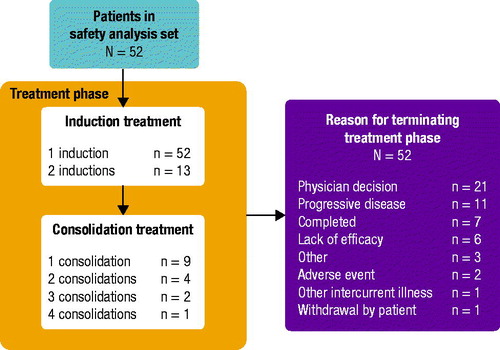
A total of 57 subjects provided informed consent and were screened for this study. Of these, four patients were screen failures and 53 subjects were enrolled; 1 enrolled patient did not receive study treatment. Therefore, a total of 52 patients from 6 sites in the United States were treated with CPX-351 and included in the safety and efficacy analyses (). All 52 patients received ≥1 induction cycle, and 13 (25%) patients received 2 induction cycles; 9 (17%), 4 (8%), 2 (4%), and 1 (2%) patients received 1, 2, 3, and 4 consolidation cycles, respectively. Patients received a mean of 4.1 infusions (standard deviation [SD]: 1.65) and had a mean duration of treatment exposure of 26.7 d (SD: 41.2). The most common reasons for discontinuation of treatment were physician decision (n = 21 [40%]) and progressive disease (n = 11 [21%]). Median follow-up time from the start of treatment was 53.5 d (range: 21–245).
Baseline demographic and clinical characteristics
Enrolled patients tended to be older within the permitted age range (), with a median age of 70 years (range: 55–75). The majority of patients had an ECOG performance status of 1 (n = 31 [60%]) or 2 (n = 12 [23%]). The median time from diagnosis to the date of informed consent was 0.3 months (range: 0.03–36.1 months), and the median myeloid blast percentage was 30% (range: 10–91%). A majority (77%) of patients had AML-MRC, including those with antecedent MDS with or without prior hypomethylating agent treatment (n = 24 [46%]), antecedent CMML (n = 4 [8%]), or de novo AML with MDS-related karyotypic abnormalities (n = 12 [23%]).
Table 1. Baseline demographic and clinical characteristics.
Safety and tolerability
A total of 50 (96%) patients experienced ≥1 TEAE during the study (). The most common TEAEs (any grade) were febrile neutropenia (n = 40 [77%]), hypoxia (n = 12 [23%]), pneumonia (n = 7 [13%]), and hypertension (n = 7 [13%]), with the majority of patients experiencing grade 3 or 4 events (). TEAEs considered to be related to CPX-351 treatment were reported for 23 (44%) patients; however, the only treatment-related TEAEs occurring in ≥5% of patients were febrile neutropenia (n = 16 [31%]) and infection (n = 3 [6%]). Serious TEAEs were experienced by 30 (58%) patients, with febrile neutropenia being the most commonly reported serious TEAE (n = 10 [19%]). Infection was reported in 25 (48%) patients. Infections that occurred in more than one patient were pneumonia (n = 7 [13%]), infection (n = 4 [8%]), lung infection (n = 4 [8%]), sepsis (n = 3 [6%]), and staphylococcal infection (n = 2 [4%]). Twenty-five (48%) patients experienced a grade ≥3 infection and 14 (27%) patients experienced an infection considered at least possibly related to treatment with CPX-351. Four bleeding events were reported (grade 3 epistaxis, grade 3 purpura, grade 4 subdural hematoma, and grade 5 intracranial hemorrhage; n = 1 each).
Figure 2. Most frequently reported TEAEs by maximum severity grade. All TEAEs reported by ≥5% of patients are included and shown by maximum Common Terminology Criteria for Adverse Events severity grade. TEAE: treatment-emergent adverse event.
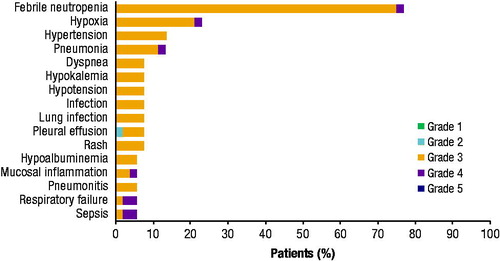
Table 2. Summary of TEAEs.
Five (10%) patients died due to a TEAE during the study. No deaths occurred within the first 30 d after starting treatment, and 3 (6%) patients died within 60 d after starting treatment; these deaths were due to aspiration, disease progression, and multiple organ dysfunction syndrome (n = 1 each). Two additional deaths were reported >60 d after starting treatment and were attributed to acute respiratory distress syndrome (n = 1; occurring on Day 70) and intracranial hemorrhage (n = 1; occurring on Day 138, after receiving platelets on Day 134 and packed red blood cells on Day 136, with a platelet count of 23 × 109/L). Only 1 of the deaths reported in the study (intracranial hemorrhage) was considered to be related to CPX-351 treatment by the treating investigator. One (2%) additional patient discontinued treatment due to a TEAE (ejection fraction decrease).
As expected for patients receiving cytotoxic chemotherapy, low neutrophil and platelet counts were observed for nearly all patients during the study. As shown in , recovery of neutrophil and platelet counts in patients who achieved a CR or CRi after receiving induction with CPX-351 was frequently prolonged.
Table 3. Median time to neutrophil and platelet recovery in patients who achieved CR or CRia.
Efficacy
Overall, 15 (29%) patients achieved CR and 8 (15%) patients achieved CRi, for a CR + CRi rate of 44% (95% confidence interval [CI]: 30.5–58.7; ). The majority (n = 22/23 [96%]) of patients achieved CR + CRi after 1 induction, and the median time to remission (CR or CRi) was 37 d (range: 15–72). Among the subgroup of patients with AML-MRC (n = 40), the CR + CRi rate was 43% (95% CI: 27.0–59.1) and included 12 (30%) patients with a CR and 5 (13%) patients with a CRi. Among the subgroup of patients with therapy-related AML (n = 12), the CR + CRi rate was 50% (95% CI: 21.1–78.9) and included 3 (25%) patients with a CR and 3 (25%) patients with a CRi. Additionally, among patients who had previously received treatment with hypomethylating agents (n = 13), the CR + CRi rate was 31% (95% CI: 9.1–61.4) and included 2 (15%) patients with a CR and 2 (15%) patients with a CRi. Eleven (21%) patients in the study underwent HCT after receiving CPX-351.
Table 4. Best induction response rates.
Discussion
Compared with patients with de novo AML, patients with secondary AML have a poorer prognosis, including lower remission rates and higher relapse rates, following conventional chemotherapy [Citation2,Citation3,Citation6,Citation9,Citation11,Citation20]. CPX-351 is a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a synergistic 5:1 molar ratio, and has a different mechanism of delivery and pharmacokinetic profile from 7 + 3 [Citation12,Citation13,Citation21]. In animal models, CPX-351 demonstrated superior anti-leukemia activity compared with free cytarabine and daunorubicin administered at the same molar ratio [Citation12,Citation13]. Drug exposure has been shown to persist for approximately 7 d in patients treated with CPX-351, with a clearance of <0.5 L/h/m2 compared with historical values of 38.6 L/h/m2 for daunorubicin and 134 L/h/m2 for cytarabine [Citation21]. Additionally, in animal models, the CPX-351 liposomes are preferentially taken up to a greater extent by leukemia cells vs. normal cells in the bone marrow [Citation12]. In phase 2 and 3 studies, CPX-351 demonstrated significantly improved survival and remission rates, and comparable safety to that of the conventional 7 + 3 regimen in patients with high-risk/secondary AML [Citation14,Citation22]. Following completion of enrollment into the phase 3 study, this EAP provided access to CPX-351 for older adults with newly diagnosed, high-risk/secondary AML prior to the commercialization of CPX-351 in the United States.
Overall, CPX-351 had an acceptable safety profile in this EAP that was generally consistent with that observed in the pivotal phase 3 trial and other clinical studies of CPX-351, as well as with the known safety profile of the 7 + 3 chemotherapy regimen, which has historically been the standard of care for most patients with AML [Citation14,Citation22]. However, while CPX-351 has been associated with prolonged neutrophil and platelet recovery compared to 7 + 3 in prior clinical studies, the recovery from myelosuppression was even further prolonged in this study. In the EAP, the median recovery times were 66 d to neutrophils ≥1000/μL, 98 d to platelets ≥50,000/μL, and not reached to platelets ≥100,000/μL. In contrast, in the phase 2 study of CPX-351, median recovery times were 36 d to neutrophils ≥1,000/μL and 37 d to platelets ≥100,000/μL, and in the phase 3 study median recovery times were 35 d to neutrophils ≥500/μL and 36.5 d to platelets ≥50,000/μL [Citation14,Citation22]. The reason for the longer recovery times in the EAP is not known but may be related to differences in scheduled assessments between the studies. The phase 2 and phase 3 study protocols required weekly hematologic assessments whereas the EAP protocol did not specify timing for hematologic assessments other than requiring them to be within 14 d of bone marrow response. Of note, the prolonged myelosuppression observed with CPX-351 vs. 7 + 3 may be related to the prolonged drug exposure observed with CPX-351 [Citation21,Citation23]. Importantly, despite prolonged myelosuppression and TEAEs of febrile neutropenia and infection, the mortality rate remained low in the EAP, consistent with prior studies of CPX-351 [Citation14,Citation22].
The CR + CRi rate of 44% reported in this EAP was similar to that reported for CPX-351 in the phase 3 study (48%) [Citation14], which was conducted in a similar population of older patients with newly diagnosed, high-risk/secondary AML. Further, the CR + CRi rates reported in this EAP were also consistent with those reported in the phase 3 study across patient subgroups, including patients with AML-MRC (CR + CRi of 43% in the EAP vs. 48% in the phase 3 study), therapy-related AML (50% vs. 47%), and/or prior treatment with a hypomethylating agent (31% vs. 36%) [Citation14,Citation24]. The rate of patients undergoing HCT in this EAP was 21%, which is lower than the rate observed in the phase 3 study (34%) [Citation14]. The slightly older median age (70 vs. 68 years) and higher proportion of patients with an ECOG performance status of 2 (23% vs. 10%) enrolled in this EAP may have contributed to the lower HCT rate [Citation14].
Some limitations of this study are inherent to the design of an EAP and include the lack of a comparator arm, which limits the nature of the conclusions that can be drawn, and the relatively small number of enrolled patients. In addition, the data collected in this EAP allow only a limited scope of analyses, since cytogenetic/molecular data and survival outcomes were not collected. The EAP was conducted in a similar patient population to the prior phase 3 study (older adults with newly diagnosed, high-risk/secondary AML) [Citation14], and the results are not generalizable to other subsets of patients with AML.
In conclusion, the results of this EAP support outcomes from prior clinical studies of CPX-351 and its use in adults with newly diagnosed, therapy-related AML or AML-MRC. Ongoing and planned clinical studies are evaluating CPX-351 in pediatric patients and younger adults, in relapsed/refractory AML, in related hematologic malignancies (e.g. MDS and AML), and as a chemotherapy backbone for combination regimens.
Geolocation information: United States
Acknowledgments
The authors would like to thank Emaryn Mancino of Jazz Pharmaceuticals for her contributions to the study. Medical writing and editorial assistance were provided by William Perlman, PhD, and Diana Avery, PhD, of SciFluent Communications, Inc., and were financially supported by Jazz Pharmaceuticals.
Disclosure statement
G.J. Roboz has received research funding from Cellectis and consulting fees from AbbVie, Actinium, Agios, Aphivena Therapeutics, Argenx, Array Biopharma, Astellas, Astex, AstraZeneca, Bayer, Celgene, Celltrion, Daiichi Sankyo, Eisai, Epizyme, Helsinn, Janssen, Jasper Therapeutics, Jazz Pharmaceuticals, MEI Pharma, Novartis, Orsenix, Otsuka, Pfizer, Roche/Genentech, Sandoz, Takeda, and Trovagene. S.E. Rubenstein reports speaker’s bureau participation for Alexion and AstraZeneca. G.J. Schiller has received research funding from Jazz Pharmaceuticals and Pharmacyclics. Q. An is an employee of and holds stock ownership/options in Jazz Pharmaceuticals. M. Chiarella and A.C. Louie are former employees of and hold stock ownership/options in Jazz Pharmaceuticals; A.C. Louie additionally holds patents and royalties with Jazz Pharmaceuticals. M.L. Larson, S.R. Solomon, and T.L. Lin have no conflicts of interest to disclose.
Data availability
All relevant data are provided within the manuscript and supporting files.
Additional information
Funding
References
- Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20(1):29–37.
- Granfeldt Ostgard LS, Medeiros BC, Sengelov H. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33:3641–3649.
- Hulegardh E, Nilsson C, Lazarevic V, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90:208–214.
- American Cancer Society. Key statistics for acute myeloid leukemia (AML). [cited 2019 Mar 28]. Available from: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html
- Miesner M, Haferlach C, Bacher U, et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC). Blood. 2010;116:2742–2751.
- Schoch C, Kern W, Schnittger S, et al. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18(1):120–125.
- Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89(9):3323–3329.
- Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365.
- Xu XQ, Wang JM, Gao L, et al. Characteristics of acute myeloid leukemia with myelodysplasia-related changes: a retrospective analysis in a cohort of Chinese patients. Am J Hematol. 2014;89(9):874–881.
- Bhatia S. Therapy-related myelodysplasia and acute myeloid leukemia. Semin Oncol. 2013;40(6):666–675.
- Kayser S, Dohner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145.
- Lim WS, Tardi PG, Dos Santos N, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res. 2010;34(9):1214–1223.
- Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129–139.
- Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692.
- Faderl S, Uy GL, Schiller GJ, et al. Outcome in older patients with newly diagnosed high-risk/secondary AML (sAML) who achieve remission with CPX-351 versus 7 + 3 induction. Presented at: International Symposium on Acute Leukemias XVII (ISAL XVII); 2019 February 24–27; Munich, Germany. Poster #39.
- VYXEOS® (daunorubicin and cytarabine injection), solution for intravenous use [package insert]. Palo Alto (CA): Jazz Pharmaceuticals, Inc.; 2019.
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Vyxeos daunorubicin/cytarabine. Opinion. [cited 2019 Sep 12]. Available from: https://www.ema.europa.eu/medicines/human/summaries-opinion/vyxeos
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474.
- Szotkowski T, Rohon P, Zapletalova L, et al. Secondary acute myeloid leukemia - a single center experience. Neoplasma. 2010;57(2):170–178.
- Feldman EJ, Lancet JE, Kolitz JE, et al. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979–985.
- Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–3246.
- Feldman EJ, Kolitz JE, Trang JM, et al. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine: daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36(10):1283–1289.
- Ryan DH, Uy GL, Cortes JE, et al. Efficacy and safety of CPX-351 versus 7 + 3 in a subgroup of older patients with newly diagnosed acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) enrolled in a phase 3 study. Blood. 2018;132(1):1425.