Abstract
This phase II clinical trial investigates a one-time oromucosal dose of tetrahydrocannabinol/cannabidiol (THC/CBD) in 23 patients with indolent leukemic B cell lymphomas. Primary endpoint was a significant reduction in leukemic B cells. Grade 1 − 2 adverse events were seen in 91% of the patients; most common were dry mouth (78%), vertigo (70%), and somnolence (43%). After THC/CBD a significant reduction in leukemic B cells (median, 11%) occurred within two hours (p = .014), and remained for 6 h without induction of apoptosis or proliferation. Normal B cells and T cells were also reduced. CXCR4 expression increased on leukemic cells and T cells. All effects were gone by 24 h. Our results show that a single dose of THC/CBD affects a wide variety of leukocytes and only transiently reduce malignant cells in blood. Based on this study, THC/CBD shows no therapeutic potential for indolent B cell lymphomas (EudraCT trial no. 2014-005553-39).
Introduction
Indolent B cell leukemias are malignant diseases with several available treatment options [Citation1,Citation2]. However, some patients do not respond to or do not tolerate these interventions, particularly the elderly. For these patients, the treatment contributes to a reduced quality of life and survival. Therefore, novel treatments and identification of factors influencing outcome are needed. Previous experimental studies have indicated that cannabinoids may induce cell death in lymphoma [Citation3–5].
Two G-protein coupled receptors (CB1 and CB2) mediate most of the effects seen by cannabinoids [Citation6–9]. CB1 is highly expressed in synapses within the central nervous system and to a lesser degree in the peripheral nerves and the enteric nervous system of the gastro-intestinal tract. CB1 regulates synaptic signaling and it is through this receptor that delta-9-tetrahydrocannabinol (THC) produces the psychoactive effects associated with Cannabis sativa [Citation8,Citation10]. While THC is an agonist to CB1, the other major component of Cannabis sativa, cannabidiol (CBD), acts as a CB1 antagonist [Citation11], counteracting the psychotropic effects of THC [Citation12]. CB2 is expressed in the immune system including B cells, T cells, monocytes/macrophages, and dendritic cells [Citation7,Citation13]. In mice, CB2 signaling is important for retention of immature B cells in bone marrow sinusoids [Citation14] and for the positioning and retention of marginal zone B cells in splenic marginal zones [Citation15,Citation16].
A key mechanism for lymphocyte migration and tissue localization is the chemokine receptor CXCR4 and its ligand CXCL12 [Citation14]. Specifically, CXCR4 expression is high on lymphocytes in blood and these lymphocytes migrate toward CXCL12 which is produced by stroma cells in lymph nodes and bone marrow. CXCR4 is a G-protein coupled receptor, known to interact with other G-protein coupled receptors, such as CB2, to modulate the CXCL12-induced effects on cell migration and homing [Citation17,Citation18].
THC and CBD have shown various effects on the immune system, such as inhibition of mitogen-stimulated lymphocyte cell replication [Citation19] and T-cell proliferation and cytokine production by signaling via CB2 [Citation20,Citation21].
Many B-cell lymphomas express CB1 and CB2. Extensive screening for CB1 mRNA expression across different B-cell leukemias/lymphomas demonstrated increased CB1 expression in most cases of mantle cell lymphoma, follicular lymphoma, and in approximately half of CLL cases compared to normal B cells [Citation22,Citation23]. Low CB1 expression levels have been associated with lymphocytosis in mantle cell lymphoma [Citation24] and longer survival in CLL [Citation25]. Micromolar concentrations of synthetic agonists to both CB1 and CB2 have been demonstrated to induce cell death of CB1- or CB2-expressing lymphoid cell-lines in vitro and in xenografts [Citation3,Citation5,Citation26].
In view of these findings, we conducted a clinical trial to investigate the therapeutic potential of THC/CBD in indolent B cell leukemia/lymphoma. In this phase II clinical trial, patients received a single administration of an oromucosal spray approved for treating pain and spasticity in multiple sclerosis, Sativex® (THC/CBD, in a molecular ratio 1/1) [Citation27], to investigate 1) whether THC/CBD would reduce leukemia/lymphoma cells in blood and 2) how an elderly population would tolerate THC/CBD.
Methods
Study design
Asymptomatic patients with CLL or leukemic (lymphocytes > 5 × 109/L) mantle, follicular or marginal zone lymphomas received a single dose of THC/CBD. The primary endpoint was a reduction of malignant (clonal) B cells in blood. Blood samples were collected at four timepoints (9 am, 11 am, 1 pm, and 3 pm) on a day prior to THC/CBD and at the same timepoints on a day with THC/CBD (administered at 9 am) and 24 and 168 h after THC/CBD. The maximum tolerated dose was established by stepwise increasing the dose of THC/CBD in every third patient until two adverse events grade 2 were seen in two or more consecutive patients (using common terminology criteria for adverse events, version 4.0), starting from 2.7 mg THC and 2.5 mg CBD (one actuation of Sativex) to 18.9 mg THC and 17.5 mg CBD (seven actuations). At the scheduled interim analysis when the seven actuation-dose was identified, 10 patients had been treated and we saw an early decrease of leukemic and normal lymphocytes already 2 h (11 am) after THC/CBD, which continued to 1 pm (4 h) and started to resolve by 3 pm. To ascertain that the changes were not due to a hitherto unknown diurnal rhythm of normal lymphocytes or leukemic cells, we sampled all subsequent 13 patients (12 with CLL) also on a date prior to THC/CBD (median 6 d before [range, 1–26]), and we included an additional measurement at 10 am on the THC/CBD day (Supplementary Figure S1).
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee in Stockholm (2015/281-31/2 with amendments 2016/210-32 and 2017/556-32) and by the Swedish Medical Products agency and registered as EudraCT trial no. 2014-005553-39. Patients were included, after providing informed consent, between April 2016 and February 2019. The date of last follow-up was 25 June 2020.
Adverse events and peak plasma concentrations of THC and CBD will also be reported in a parallel pharmacologic manuscript describing the evaluation of measuring THC and CBD in other fluids than plasma (Melén et al., manuscript submitted).
Chemistry, pharmacology assays, and flow cytometry
Complete blood counts and other routine chemistry analyses were conducted using the facilities of the Karolinska University Laboratory. Assays of THC and CBD concentration measurement were conducted at the Department of Pharmacology using liquid chromatography–mass spectrometry method as described previously [Citation28]. Multiparameter flow cytometry on peripheral blood samples for characterizing lymphoid and myeloid cell populations, as well as for assessing caspase-3 activation (by cleavage of active caspase substrate – PhiPhiLux) for apoptosis in CD19 + CD5+ cells, was done in the routine flow cytometry unit at the Dept. of Clinical Pathology and Cytology (Supplementary Tables S1 and S2; Supplementary Methods). Surface expression of the homing chemokine receptor CXCR4 was analyzed using flow cytometry (Supplementary Figure S2) and CXCR4high/CXCR4low ratios were calculated.
Enrichment of malignant B cells and analysis of cell proliferation
Blood samples were enriched for B cells by negative selection using RosetteSep™, collected using Ficoll-Paque PLUS (GE Healthcare Life Science, Marlborough, MA). The flow cytometer BD FACSCanto II (BD Biosciences, San Diego, CA) was used to determine the purity of B cells (Supplementary Methods) and data analysis was performed using the FlowJo software version 10 (Ashland, USA). Cell proliferation of enriched malignant B cells was assessed by incorporation of 3H-Thymidine (Supplementary Methods).
RNA isolation and real-time PCR
mRNA expression levels of genes encoding for CB1 (CNR1) and CB2 (CNR2) were assessed by real-time PCR [Citation24] after RNA isolation and complementary DNA synthesis from enriched B cells (Supplementary Methods). Custom-made primers were purchased from Invitrogen, sequences for respective genes are provided in Supplementary Methods.
Statistics
Comparisons between repeated measurements in the same individuals were done, using the Wilcoxon matched-pairs signed-ranks test. The 9 am results from the day prior to, and the day of, THC/CBD were used as separate baselines. Survival was analyzed with Kaplan–Meier curves. Assessments of other associations were conducted using Spearman, Fisher’s exact, or Mann–Whitney–Wilcoxon tests, according to the nature of the variables. p values are two-tailed and calculated using Stata version 14.2 (StataCorp LLC, College Station, TX). Apoptosis and proliferation data were analyzed using GraphPad Prism version 8 (GraphPad software Inc., La Jolla, CA). p < .05 was considered significant.
Results
Twenty-three (20 with CLL) elderly patients with indolent B cell malignancy received a one-time administration of THC/CBD. The patients are described in .
Table 1. Clinical characteristics at inclusion.
Adverse events
The maximum tolerated dose was seven actuations (18.9 mg THC/17.5 mg CBD); this dose was given to 15 patients. Transient adverse events were seen in 21/23 patients, all grade 1 or 2; most common were dry mouth (78%), vertigo (70%), and somnolence (43%; ). The adverse events occurred within the first 6 h, did not require hospitalization and all patients returned home at 4 pm.
Table 2. Adverse events.
THC/CBD concentrations in plasma
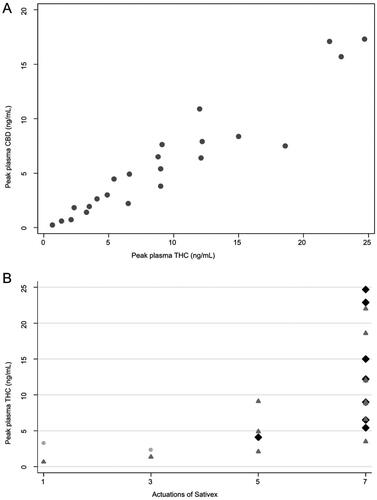
The peak plasma concentrations of THC and CBD were reached at different time points for different patients. For THC, the peak concentration in plasma (median 8.8 ng/mL) was seen at 1 h (n = 5), 2 h (n = 12), and 4 h (n = 6) and ranged from 0.7 to 24.7 ng/mL. The peaks of CBD (median 4.9 ng/mL) were at 1 h (n = 7), 2 h (n = 8), 4 h (n = 7), and 6 h (n = 1), with a range of 0.2–17.3 ng/mL. The relation between the plasma concentrations of THC and CBD was linear (R2 = 0.90; p < .00005; ). THC and CBD levels correlated with the number of actuations (p < .00005) and with the severity of adverse events (p = .043; ).
Figure 1. Peak plasma levels of THC and CBD, number of actuations and adverse events. (A) Peak plasma levels of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), each dot representing a patient. (B) Peak plasma levels of THC and the number of actuations of THC/CBD and the worst adverse event per patient (gray dot, no adverse event; dark gray triangle, adverse event grade 1; black diamond, adverse event grade 2).
Effects of THC/CBD on blood cells
Baseline cell counts and relevant fold changes are presented in , and representative graphs of the median values are shown in . As expected, there was an increase of neutrophils, 4 h after THC/CBD, climaxing at 126% by 6 h (; ). Serum cortisol levels also increased 4 h after THC/CBD (, ). After THC/CBD, leukemic B cells decreased already by 10 am, reaching equal nadir at 11 am and 1 pm (11% decrease), and remained reduced at 3PM (8%; ; ). There was a similar but larger reduction in normal B cells after THC/CBD, from 10 am with a nadir at 3 pm (37%; ; ). There was also a decrease in CD3+ cells (nadir 35% at 3 pm; ; ) but there was no change in CD4/CD8 ratio (). Decreases in CD56+ cells and in platelets were also observed (). Twenty-four hours after THC/CBD, no effects remained on any leukocyte subset ().
Figure 2. Changes in median levels of blood leukocyte subsets and serum cortisol levels during the days without and with THC/CBD. For simplicity, only median values are shown here, detailed data are represented in . (A) Leukemic B cells. (B) Normal B cells. (C) CD3+ T cells. (D) CD4/CD8 ratio. (E) CD56+ NK cells. (F) Platelets. (G) Neutrophils. (H) Serum cortisol. Dashed black lines are from the day without treatment (n = 13) and solid gray lines are from the day with treatment (n = 23). All results are presented in relation to sampling at baseline (9 am) for each day. All cell-subset analyses were calculated from absolute blood counts, except CD4/CD8 (ratio). One circumflex (^) indicates significant changes at the day without treatment, with respect to baseline with p < .05. One asterisk (*) and two asterisks (**) indicate significant changes at the day with treatment, with respect to baseline with p < .05 and p < .005, respectively.
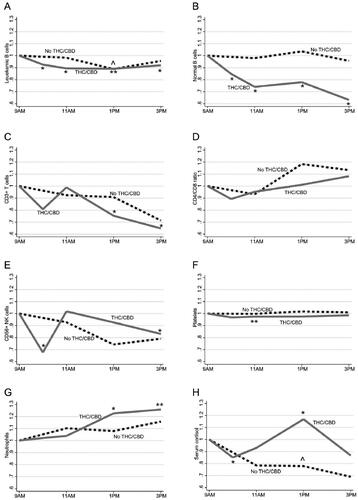
Table 3. Changes with and without THC/CBD.
Influence of cannabinoid receptor expression
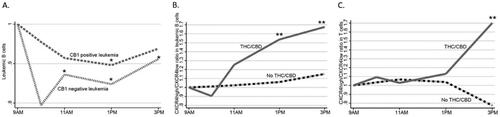
The leukemic B cells in all 23 patients expressed CB2 while 17/23 expressed CB1 (). Neither CB1 nor CB2 mRNA levels changed after the administration of THC/CBD at any timepoint (p > .05 for every comparison). The six CB1-negative cases, all CLL, showed a deeper reduction after THC/CBD (15% decrease at 1 pm; p = .028) than in the CB1-positive cases (10% decrease; p = .013; ).
Figure 3. Reduction of CB1-expressing and non-expressing malignant B cells and CXCR4 surface expression. For simplicity, only median values are shown here, detailed data are represented in . (A) Reduction of leukemic B cells after THC/CBD in CB1 positive (dashed gray line; n = 17) and CB1 negative (dotted gray line; n = 6) cases. Changes in CXCR4+/CXCR4- ratio in (B) leukemic B cells and (C) T cells.
Effects on CXCR4 expression, apoptosis, and proliferation
Ratios of CXCR4high/CXCR4low were similar in CB1-negative and CB1-positive cases (median 9.4 and 9.5, respectively; p = .54) and were stable in leukemic cells and T cells when no drug had been given. However, after THC/CBD administration CXCR4high/CXCR4low ratios increased in leukemic B cells () and T cells () at 1 and 3 pm (). Apoptosis and cell proliferation did not change at any timepoint (Supplementary Figure S3).
Existence of a diurnal rhythm in patients with CLL
Thirteen patients (12 with CLL) were sampled on a date prior to THC/CBD (median 6 d before [range, 1–26]), to ascertain any hitherto unknown diurnal rhythm of normal lymphocytes or leukemic cells. Without THC/CBD, there was a late significant reduction in leukemic cells at 1 pm only (11%; ); other leukocyte subsets including T cells did not change (; ).
Long-term follow-up
Leukemic cell counts moderately increased between the non-THC/CBD day (approximately one week before therapy), the THC/CBD day, and one week after THC/CBD. The 9 am leukocyte counts went from 23.2 (day without THC/CBD) to 24.7 (immediately before THC/CBD) to 26.0 (a week after THC/CBD) x 109/L (Supplementary Figure S4(A)). Thus, the increase in leukemic cells that was observed one week after THC/CBD was considered a natural, gradual increase caused by the disease.
Median follow-up was 2.8 years (range, 1.4–4.2). Seven patients required treatment after the trial, six with rituximab-bendamustine and one with ibrutinib. One patient died from a cerebral stroke and one from kidney cancer, both >2 years after the trial and without any evidence of leukemia progression. Overall survival and time to next treatment are shown in Supplementary Figure S4(B).
Discussion
Cannabinoids are increasingly prescribed for treatment of spasticity, pain, and epilepsy [Citation29]. Some patients use cannabinoids during chemotherapy, to alleviate adverse events, such as nausea and fatigue [Citation30,Citation31]. A recent study showed that almost one-fifth of cancer patients use cannabinoids as self-treatment [Citation32]. The effects these cannabinoids have on benign and malignant cells in vivo are not fully known, since most knowledge comes from descriptive studies and animal models.
In this trial, repeated sampling after a single dose of THC/CBD showed a quick decrease in circulating leukemic and normal B and T cells in patients with indolent B cell leukemia. To investigate whether this was due to diurnal rhythms, the same repeated samplings were conducted on a day prior to THC/CBD administration on subsequent patients. We then found that CLL cells in untreated patients display a diurnal fluctuation, with similarities to circadian rhythms. Circadian rhythms are 24-h variations in physiological processes and are generated by the expression of circadian clock genes in humans. In the CLL cells, the significant diurnal change in untreated patients might be due to an aberrant expression of clock genes. The transcription factor BMAL1 and the gene CRY1, both part of the circadian system, are described as being epigenetically inactivated in CLL [Citation33]. We did not detect any diurnal changes in normal B or T cells within the time frame of 9–3 pm.
It was safe to administrate a single dose of THC/CBD to these elderly patients. Still, psychotropic adverse events were frequent, being the main dose-limiting toxicity. For most patients, the side effects were unpleasant, but they all could return home at the end of the day. After the first 24 h, there were neither beneficial nor adverse effects of this single THC/CBD dose, and the natural course of the disease remained unperturbed.
Sativex, containing a mixture of THC/CBD, was the only approved cannabinoid compound available to us, therefore we could not separate the effects of THC and CBD. This is a limitation of the study. Our trial did not reproduce in vitro findings of induced cell death caused by high concentrations of synthetic [Citation3,Citation5,Citation26] and natural [Citation4,Citation34] cannabinoids. Indeed, the psychotropic side effects prohibited the attainment of tumoricidal plasma concentrations of cannabinoids. We cannot, however, exclude that THC/CBD acts differently than other cannabinoids previously used for in vitro studies.
Patients with CB1-negative leukemia showed a faster and deeper reduction of leukemic cell counts compared to CB1-positive cases. Normal B cells, which also have very low CB1 expression [Citation7], behaved similarly, with a more profound decrease than leukemic B cells. We cannot in this study mechanistically explain the slower reduction of the leukemic cells in blood when both cannabinoid receptors are expressed, but it has been shown that CB1 and CB2 can form heterodimers in neurons and when such heterodimers were formed, stimulation of one receptor led to negative modulation of its partner [Citation35]. If this also would occur in leukemic cells, CB1 stimulation would impair the stimulation of CB2. Since CB2 is involved in homing of leukocytes to secondary lymphoid tissues, this could partly explain why CB1-negative cases and normal B cells, which have very low CB1 expression, had a more profound decrease after exposure to the study drug [Citation14–16].
We detected an increased expression of CXCR4 on the cell surface of leukemic cells and T cells from blood, at 6 h after THC/CBD. CXCR4, together with other chemokine receptors and integrins, plays an important role in the interaction between lymphoma cells and the microenvironment, increasing cell survival and drug resistance [Citation36,Citation37]. We also found that the administration of THC/CBD increased cortisol levels. Cortisol is known to affect circadian rhythms in T cells [Citation38], and to increase CXCR4 expression in T lymphocytes [Citation39]. It is likely that the increase of cortisol was secondary to stress from adverse events rather than directly induced by the THC/CBD [Citation40]. Based on our findings we believe that the increase of CXCR4high expressing cells (both leukemic B cells and T cells) seen 6 h after THC/CBD is a secondary effect due to elevated cortisol levels and that this increased expression of CXCR4 is associated to a redistribution of lymphocytes from blood at 6 h. The increase of neutrophils after THC/CBD is probably also a secondary effect of cortisol and has also been observed in a study investigating the chronic use of synthetic cannabinoids [Citation41].
However, our main finding, the fast decrease of circulating leukemic cells 1–2 h after a single dose of THC/CBD, cannot be explained by the later changes in CXCR4 expression or cortisol levels. Furthermore, THC/CBD did not affect cell proliferation or apoptosis on leukemic cells in blood. Therefore, we propose that cannabinoids induce a redistribution of malignant B cells away from blood. However, we do not know where the cells relocate to. It is possible that the cells adhere to the endothelium of blood vessels, or migrate from blood to spleen, lymph nodes, and/or bone marrow. Nevertheless, our data point toward the involvement of the cannabinoid receptors in regulation of the tissue localization of lymphocytes, as previously suggested in experimental studies in mice [Citation14–16]. Whether chronic use of cannabinoids might result in a sustained redistribution of lymphocyte subsets is not known. In a small study of 20 multiple sclerosis patients, Sativex intake for up to six weeks did not significantly affect the levels of different blood cells populations [Citation42].
Cannabinoids may also impact immune checkpoint control: a recent study of cancer patients with solid tumors given immune checkpoint inhibitors showed that concomitant cannabinoid use correlated with inferior time to progression and overall survival [Citation43]. Our study demonstrates that THC/CBD affects a wide variety of immune cells in vivo, and this should be taken into consideration when cannabinoids are used to treat, for example, nausea from chemotherapy, because cannabinoid-induced immune modulation could have unforeseen effects.
We conclude that a single dose of THC/CBD causes considerable adverse events in elderly, cannabis-naïve patients but did not affect apoptosis or proliferation of the malignant cells. Instead, THC/CBD probably induced an early redistribution of malignant and benign blood cells away from blood. If the malignant cells home to lymphoid tissues and bone marrow, such a redistribution would be opposite of that induced by successful anti-tumoral therapies such as inhibitors of Bruton’s tyrosine kinase and phosphoinositide 3-kinase, where malignant cells egress from the bone marrow to the peripheral blood [Citation44,Citation45]. It is important to consider that cannabinoids might negatively interfere with anti-leukemia/lymphoma treatment. Based on our findings, this mixture of cannabinoids should not be considered as treatment for indolent lymphomas.
Author contributions
C.M.M., B.E.W., K.S., and H.R.J. recruited patients; C.M.M. collected clinical data; M.M. and A.M.W. performed and analyzed ex vivo experiments; B.E.W. did the statistical analysis; M.M. and B.E.W. prepared figures; O.B. and G.P. performed the pharmacology assays; C.M.M., B.S., and B.E.W. designed the study; M.M., C.M.M., A.M.W., B.C., B.S., and B.E.W. wrote the article; all authors have read and agreed to the published version of the manuscript.
GLAL-2021-0657-File002.docx
Download MS Word (664.7 KB)Acknowledgments
The authors thank the Department of Clinical Pathology personnel for their assistance with flow cytometry analyses and Professor Eva Kimby for comments on the manuscript.
Disclosure statement
The authors have no conflict of interest.
Additional information
Funding
References
- Apostolidis J, Mokhtar N, Al Omari R, et al. Follicular lymphoma: update on management and emerging therapies at the dawn of the new decade. Hematol Oncol. 2020;38(3):213–222.
- Sharma S, Rai KR. Chronic lymphocytic leukemia (CLL) treatment: so many choices, such great options. Cancer. 2019;25(9):1432–1440.
- Gustafsson K, Christensson B, Sander B, et al. Cannabinoid receptor-mediated apoptosis induced by R(+)-methanandamide and Win55,212-2 is associated with ceramide accumulation and p38 activation in mantle cell lymphoma. Mol Pharmacol. 2006;70(5):1612–1620.
- McKallip RJ, Lombard C, Fisher M, et al. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002;100(2):627–634.
- Wasik AM, Almestrand S, Wang X, et al. WIN55,212-2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells [research support, Non-U.S. Cell Death Dis. 2011;2:e225.
- Devane WA, Dysarz FA, 3rd, Johnson MR, et al. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–613.
- Galiegue S, Mary S, Marchand J, et al. Expression of Central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232(1):54–61.
- Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564.
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215.
- Thomas A, Baillie GL, Phillips AM, et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–623.
- Klein C, Karanges E, Spiro A, et al. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl). 2011;218(2):443–457.
- Castaneda JT, Harui A, Kiertscher SM, et al. Differential expression of intracellular and extracellular CB(2) cannabinoid receptor protein by human peripheral blood leukocytes. J Neuroimmune Pharmacol. 2013;8(1):323–332.
- Pereira JP, An J, Xu Y, et al. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10(4):403–411.
- Basu S, Ray A, Dittel BN. Cannabinoid receptor 2 is critical for the homing and retention of marginal zone B lineage cells and for efficient T-independent immune responses. J Immunol. 2011;187(11):5720–5732.
- Muppidi JR, Arnon TI, Bronevetsky Y, et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208(10):1941–1948.
- Coke CJ, Scarlett KA, Chetram MA, et al. Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J Biol Chem. 2016;291(19):9991–10005.
- Ghosh S, Preet A, Groopman JE, et al. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43(14):2169–2179.
- Klein TW, Newton CA, Widen R, et al. The effect of Delta-9-tetrahydrocannabinol and 11-hydroxy-Delta-9-tetrahydrocannabinol on T-lymphocyte and B-lymphocyte mitogen responses. J Immunopharmacol. 1985;7(4):451–466.
- Börner C, Smida M, Höllt V, et al. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. 2009;284(51):35450–35460.
- Klein TW, Lane B, Newton CA, et al. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225(1):1–8.
- Gustafsson K, Wang X, Severa D, et al. Expression of cannabinoid receptors type 1 and type 2 in non-Hodgkin lymphoma: growth inhibition by receptor activation. Int J Cancer. 2008;123(5):1025–1033.
- Islam TC, Asplund AC, Lindvall JM, et al. High level of cannabinoid receptor 1, absence of regulator of G protein signalling 13 and differential expression of cyclin D1 in mantle cell lymphoma [comparative study research support, Non-U.S. Gov’t]. Leukemia. 2003;17(9):1880–1890.
- Wasik AM, Nygren L, Almestrand S, et al. Perturbations of the endocannabinoid system in mantle cell lymphoma: correlations to clinical and pathological features. Oncoscience. 2014;1(8):550–557.
- Freund P, Porpaczy EA, Le T, et al. Cannabinoid receptors are overexpressed in CLL but of limited potential for therapeutic exploitation. PLoS One. 2016;11(6):e0156693.
- Flygare J, Gustafsson K, Kimby E, et al. Cannabinoid receptor ligands mediate growth inhibition and cell death in mantle cell lymphoma. FEBS Lett. 2005;579(30):6885–6889.
- Collin C, Davies P, Mutiboko IK, et al. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14(3):290–296.
- Beck O, Stephanson N, Sandqvist S, et al. Detection of drugs of abuse in exhaled breath using a device for rapid collection: comparison with plasma, urine and self-reporting in 47 drug users. J Breath Res. 2013;7(2):026006.
- Keating GM. Delta-9-tetrahydrocannabinol/cannabidiol oromucosal spray (Sativex®): a review in multiple sclerosis-related spasticity. Drugs. 2017;77(5):563–574.
- Davis MP. Cannabinoids for symptom management and cancer therapy: the evidence. J Natl Compr Canc Netw. 2016;14(7):915–922.
- Javid FA, Phillips RM, Afshinjavid S, et al. Cannabinoid pharmacology in cancer research: a new hope for cancer patients? Eur J Pharmacol. 2016;775:1–14.
- Martell K, Fairchild A, LeGerrier B, et al. Rates of cannabis use in patients with cancer. Curr Oncol. 2018;25(3):219–225.
- Taniguchi H, Fernandez AF, Setien F, et al. Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 2009;69(21):8447–8454.
- Kampa-Schittenhelm KM, Salitzky O, Akmut F, et al. Dronabinol has preferential antileukemic activity in acute lymphoblastic and myeloid leukemia with lymphoid differentiation patterns. BMC Cancer. 2016;16:25.
- Callén L, Moreno E, Barroso-Chinea P, et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J Biol Chem. 2012;287(25):20851–20865.
- Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94(11):3658–3667.
- Kurtova AV, Tamayo AT, Ford RJ, et al. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specific targeting. Blood. 2009;113(19):4604–4613.
- Dimitrov S, Benedict C, Heutling D, et al. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113(21):5134–5143.
- Besedovsky L, Linz B, Dimitrov S, et al. Cortisol increases CXCR4 expression but does not affect CD62L and CCR7 levels on specific T cell subsets in humans. Am J Physiol Endocrinol Metab. 2014;306(11):E1322–9.
- Zuardi AW, Guimarães FS, Moreira AC. Effect of cannabidiol on plasma prolactin, growth hormone and cortisol in human volunteers. Braz J Med Biol Res. 1993;26(2):213–217.
- Guzel D, Yazici AB, Yazici E, et al. Alterations of the hematologic cells in synthetic cannabinoid users. J Clin Lab Anal. 2017;31(6):e22131.
- Centonze D, Mori F, Koch G, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci. 2009;30(6):531–534.
- Bar-Sela G, Cohen I, Campisi-Pinto S, et al. Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers. 2020;12(9):2447.
- Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81.
- Chen SS, Chang BY, Chang S, et al. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2016;30(4):833–843.
