Abstract
Genomic abnormalities, including del(17p)/TP53 mutation, del(11q), unmutated IGHV, and mutations in BIRC3, NOTCH1, SF3B1, and XPO1 predict poor outcomes with chemoimmunotherapy in chronic lymphocytic leukemia. To better understand the impact of these high-risk genomic features on outcomes with first-line ibrutinib-based therapy, we performed pooled analysis of two phase 3 studies with 498 patients randomized to receive ibrutinib- or chlorambucil-based therapy with median follow-up of 49.1 months. Ibrutinib-based therapy improved overall response rates (ORRs), complete response rates, and progression-free survival (PFS) versus chlorambucil-based therapy across all subgroups. In ibrutinib-randomized patients with versus without specified genomic features, ORR and PFS were comparable across subgroups. PFS hazard ratio (95% CI) for del(17p)/TP53 mutated/BIRC3 mutated: 1.05 (0.54–2.04); del(17p)/TP53 mutation, del(11q), and/or unmutated IGHV: 1.11 (0.69–1.77); unmutated IGHV: 1.79 (0.99–3.24); and NOTCH1 mutated 1.05 (0.65–1.69). This integrated analysis demonstrated efficacy of first-line ibrutinib-based treatment irrespective of cytogenetic and mutational risk features.
Registered at ClinicalTrials.gov (NCT01722487 and NCT02264574).
Introduction
Chronic lymphocytic leukemia (CLL) is a B-cell malignancy that is characterized by a variable clinical course and heterogeneous biology [Citation1]. A variety of genomic features have been identified that are associated with inferior prognosis in patients with CLL [Citation1]. Dohner et al identified a hierarchical model of chromosomal abnormalities with prognostic value in CLL, with the shortest survival estimates observed in patients with del(17p), followed by those with del(11q) in the absence of del(17p) [Citation2]. Subsequently, Rossi et al developed a revised hierarchical classification integrating chromosomal abnormalities with recurrent gene mutations, refining the prognostic order of relevance to implicate the high-risk subgroup of TP53 mutation and/or BIRC3 mutation and the intermediate-risk subgroup of NOTCH1 and/or SF3B1 mutation and/or del (11q) as independent risk factors for inferior overall survival (OS) [Citation3]. Mutational status of the variable region of the immunoglobulin heavy chain (IGHV) gene has also been identified as a prognostic factor in CLL, with better prognosis in patients with mutated versus unmutated IGHV [Citation4,Citation5]. In addition to genomic abnormalities, such as del(17p)/TP53 mutation, del(11q), and unmutated IGHV, mutations in BIRC3, NOTCH1, SF3B1, and XPO1 have been recently associated with poor outcomes in patients treated with chemoimmunotherapy [Citation6,Citation7]. Despite the known association between inferior outcomes and chemoimmunotherapy in the presence of these high-risk genomic features [Citation8–12], patients with CLL are frequently treated with such therapies regardless of genomic status [Citation13].
Ibrutinib, a once-daily Bruton’s tyrosine kinase inhibitor, is the only targeted therapy to demonstrate significant progression-free survival (PFS) benefit and OS benefit in multiple randomized phase 3 studies in both previously untreated and relapsed/refractory CLL/small lymphocytic lymphoma (SLL) [Citation14–19]. Of note, patients with and without high-risk genomic features known to confer inferior outcomes with chemoimmunotherapy have shown consistently enhanced PFS with single-agent ibrutinib or ibrutinib-based combination therapy, including combinations with anti-CD20 antibodies, compared to those treated with established therapies [Citation14–16,Citation19,Citation20]. In the RESONATE-2 study, first-line ibrutinib was associated with superior PFS and OS compared with chlorambucil, with PFS benefit for ibrutinib observed across all patient subgroups, including those with TP53 mutation, del(11q), and/or unmutated IGHV [Citation14]. In the iLLUMINATE study, first-line ibrutinib plus obinutuzumab was associated with superior PFS compared with chlorambucil plus obinutuzumab [Citation16]. Significant PFS benefit with ibrutinib-obinutuzumab over chlorambucil-obinutuzumab was observed in patients with del(17p), TP53 mutations, del(11q), and/or unmutated IGHV [Citation16].
Previously, in the phase 3 RESONATE study, mutations in BIRC3, NOTCH1, SF3B1, or XPO1 had no significant impact on the PFS benefit conferred by ibrutinib in patients with relapsed/refractory CLL/SLL treated with ibrutinib or ofatumumab [Citation21,Citation22]. However, limited evidence is available regarding the efficacy of ibrutinib in patients with these single-gene mutations in the first-line setting. To better understand outcomes in patients with previously untreated CLL with various high-risk genomic features, including integrated gene mutations and chromosomal abnormalities, we performed a pooled analysis of two large registrational phase 3 studies (RESONATE-2 and iLLUMINATE) with extended follow-up of ibrutinib-based therapy for first-line treatment of CLL/SLL. Safety analyses were performed to help inform the benefit–risk profile in patients with high-risk genomic features.
Materials and methods
Pooled analysis
Detailed methods for RESONATE-2 [Citation23] and iLLUMINATE [Citation16] were previously reported. Briefly, in RESONATE-2 (PCYC-1115/1116), patients aged ≥65 years with previously untreated CLL/SLL without del(17p) were randomized 1:1 to receive single-agent oral ibrutinib (420 mg once daily) until progressive disease or unacceptable toxicity, or up to 12 cycles of chlorambucil (0.5 mg/kg, increased up to 0.8 mg/kg as tolerated, on days 1 and 15 of each 28-day cycle). In iLLUMINATE (PCYC-1130), patients with previously untreated CLL/SLL aged ≥65 years or <65 years with either coexisting conditions or del(17p)/TP53 mutation were randomized 1:1 to receive ibrutinib (420 mg once daily) until progressive disease or unacceptable toxicity plus six cycles of obinutuzumab (100 mg on day 1, 900 mg on day 2, and 1,000 mg on days 8 and 15 in cycle 1, then 1,000 mg on day 1 of each 28-day cycle) or six cycles of chlorambucil (0.5 mg/kg on days 1 and 15 of each 28-day cycle) plus obinutuzumab (as described for the ibrutinib plus obinutuzumab arm). Both studies were approved by institutional review boards at each participating institution and were conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. RESONATE-2 and iLLUMINATE were registered at ClinicalTrials.gov (numbers NCT01722487 and NCT02264574, respectively).
Data were pooled for patients randomized to receive ibrutinib-based therapy (single-agent ibrutinib or ibrutinib-obinutuzumab) and for those randomized to receive chlorambucil-based therapy (single-agent chlorambucil or chlorambucil-obinutuzumab). High-risk genomic features were evaluated by central laboratory testing. Cytogenetics (del(17p) and del(11q)) were assessed using fluorescence in situ hybridization (FISH). IGHV mutational status was assessed by somatic hypermutation assay (LymphoTrack Dx IGHV; Invivoscribe, Inc., San Diego, CA, USA). TP53, BIRC3, SF3B1, NOTCH1, and XPO1 mutations were assessed by targeted next-generation sequencing (ACE Extended Cancer Panel; Personalis, Menlo Park, CA, USA).
Clinical outcomes of interest were PFS, OS, overall response rates (ORRs), complete response (CR) rate including CR with incomplete bone marrow recovery (CRi), and safety. Response was assessed by investigators per 2008 International Workshop on Chronic Lymphocytic Leukemia criteria [Citation24]. Outcomes were analyzed for various subgroups as defined by FISH cytogenetics or single-gene mutations alone and in combination, including subgroups defined by hierarchical classification after Dohner et al. [Citation2]; revised hierarchical classification after Rossi et al. [Citation3]; high-risk population per ibrutinib US prescribing information [Citation25] with del(17p)/TP53 mutation, del(11q), and/or unmutated IGHV; IGHV mutational status; and single-gene mutations in TP53, BIRC3, SF3B1, NOTCH1, and XPO1. As patients with B-cell receptor (BCR) stereotype subset 2 (IGHV3-21/IGLV3-21) predominantly carry mutated IGHV but have similar prognosis to those with unmutated IGHV [Citation26], we performed a sensitivity analysis that included seven additional patients with BCR stereotype subset 2 in the unmutated IGHV subgroup.
Outcomes were compared between (1) ibrutinib- versus chlorambucil-based therapies and (2) ibrutinib-randomized patients with versus without specified high-risk genomic features.
Statistical analysis
Efficacy analyses included all intention-to-treat patients from both studies; safety analyses included patients who received ≥1 dose of study treatment. PFS and OS were estimated using Kaplan-Meier methodology; subgroups were compared using hazard ratios (HRs) with P values based on unstratified log-rank test. OS was estimated without censoring or adjustment for crossover. ORRs were compared between subgroups using rate ratios with P values based on the chi-square test. No multiplicity adjustments were performed.
Results
Patients
Pooled analyses included 498 patients randomized to receive ibrutinib-based therapy (n = 249) or chlorambucil-based therapy (n = 249). One patient randomized to ibrutinib-based therapy and two patients randomized to chlorambucil-based therapy did not receive the assigned study treatment; thus, pooled safety populations included 248 ibrutinib-treated patients and 247 chlorambucil-treated patients. Within each study, baseline characteristics were balanced across treatment arms [Citation16,Citation23]. Genomic risk subgroups are described in Supplementary Table S1. In patients randomized to receive ibrutinib-based therapy, baseline characteristics were generally similar across genomic risk subgroups (Supplementary Table S2). At the time of analysis, median follow-up for all patients in the pooled analysis was 49.1 months (range, 0.1–78.7).
Table 1. Progression-free survival in patients randomized to ibrutinib- versus chlorambucil-based therapy by specified genomic risk features.
Outcomes with ibrutinib- versus chlorambucil-based therapy by specified genomic risk features
Ibrutinib-based therapy improved ORR and CR rates compared with chlorambucil-based therapy across patients with different genomic risk features. ORRs were 83%–97% across genomic risk subgroups in patients randomized to ibrutinib-based therapy, compared with 50%–71% across subgroups of patients randomized to chlorambucil-based therapy, with rate ratios of 1.28–1.84 (). CR rates were 22%–47% versus 6%–20% with ibrutinib- versus chlorambucil-based therapy across genomic risk subgroups (). With a median follow-up of 49 months (up to 79 months), ibrutinib-randomized patients had longer PFS compared with chlorambucil-randomized patients, regardless of genomic risk features. Median PFS was not reached in any subgroup in ibrutinib-randomized patients and ranged from 11.2 to 20.2 months across high-risk genomic subgroups in chlorambucil-randomized patients (). At 42 months, PFS rates were significantly higher across high-risk genomic subgroups in ibrutinib-randomized patients (63%–87%) compared with chlorambucil-randomized patients (6%–34%) (). Consistent PFS benefit with ibrutinib- versus chlorambucil-based therapy was observed across all high-risk genomic subgroups, with HRs ranging from 0.06 to 0.30 (). Kaplan-Meier curves for PFS in patients randomized to ibrutinib- versus chlorambucil-based therapy by NOTCH1 and SF3B1 mutational status are shown in Supplementary Figure S1. OS results had not reached maturity at the time of analysis, with median OS not reached in any subgroup in either ibrutinib- or chlorambucil-randomized patients. Although no differences were observed in OS between ibrutinib- vs chlorambucil-based therapy (), OS analyses were confounded by the high rate of crossover after progression; in total, 123 of 249 patients (49%) initially assigned to chlorambucil-based therapy crossed over to receive ibrutinib-based therapy.
Figure 1. Response with ibrutinib- versus chlorambucil-based therapy by specified genomic risk features. CI: confidence interval; CR/CRi: complete response/complete response with incomplete bone marrow recovery; ORR: overall response rate. aNeither del(17p)/TP53 mutation nor del(11q). bNeither del(17p)/TP53 mutation/BIRC3 mutation nor del(11q)/SF3B1 mutation/NOTCH1 mutation. cRate ratio for ORR with ibrutinib-based therapy versus chlorambucil-based therapy.
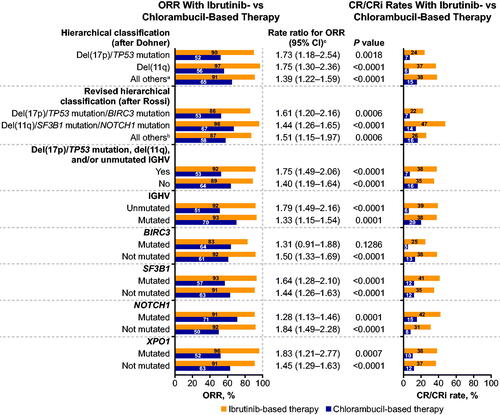
Figure 2. Forest plots of (A) progression-free survival and (B) overall survival with ibrutinib- versus chlorambucil-based therapy by specified genomic risk features. OS: overall survival; PFS: progression-free survival. aNeither del(17p)/TP53 mutation nor del(11q). bNeither del(17p)/TP53 mutation/BIRC3 mutation nor del(11q)/SF3B1 mutation/NOTCH1 mutation. cHazard ratio for PFS or OS with ibrutinib-based therapy versus chlorambucil-based therapy.
Outcomes with ibrutinib-based therapy in patients with versus without specified genomic risk features
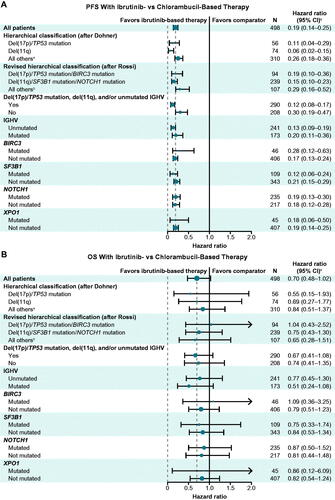
In patients randomized to ibrutinib-based therapy, ORR and CR rates were generally comparable between patients with versus without specified high-risk genomic features (), with rate ratios for ORR of 0.90–1.10. Similarly, PFS was generally comparable between patients with versus without specified high-risk genomic features (), including those with the highest risk classification of del(17p)/TP53 mutated/BIRC3 mutated per Rossi et al. (HR, 1.05; 95% CI, 0.54–2.04; p = 0.8962); the high-risk population with del(17p)/TP53 mutation, del(11q), and/or unmutated IGHV per ibrutinib US prescribing information (HR, 1.11; 95% CI, 0.69–1.77; p = 0.6729); those with unmutated IGHV (HR, 1.79; 95% CI, 0.99–3.24; p = 0.0512); and those with NOTCH1 mutations (HR, 1.05; 95% CI, 0.65–1.69; p = 0.8555). A sensitivity analysis including patients with BCR stereotype subset 2 provided similar results to the overall comparison of unmutated versus mutated IGHV, with a slight shift in the HR for PFS in favor of the mutated IGHV subgroup in patients randomized to ibrutinib-based therapy (HR, 1.85; 95% CI, 0.99–3.45; p = 0.0489). Kaplan-Meier curves for PFS in patients randomized to ibrutinib-based therapy according to subgroups defined by hierarchical classification after Dohner et al (del(17p) versus del(11q) versus all others), by revised hierarchical classification after Rossi et al (del(17p)/TP53 mutation/BIRC3 mutation versus del(11q)/SF3B1 mutation/NOTCH1 mutation versus all others) are shown in Supplementary Figure S2. Of note, a sustained PFS rate of 79% was observed at 42 months in patients with del(17p)/TP53 mutation randomized to ibrutinib-based therapy (). Median OS was not reached in any subgroup and OS was generally similar between ibrutinib-randomized patients with versus without specified genomic high-risk features ().
Figure 3. Response rates with ibrutinib-based therapy in patients with versus without specified high-risk genomic features. CI: confidence interval; CR/CRi: complete response/complete response with incomplete bone marrow recovery; NA: not applicable; ORR: overall response rate. aWithout high-risk feature = all others (neither del(17p)/TP53 mutation nor del(11q)). bWithout high-risk feature = all others (neither del(17p)/TP53 mutation/BIRC3 mutation nor del(11q)/SF3B1 mutation/NOTCH1 mutation). cRate ratio for ORR with versus without high-risk features.
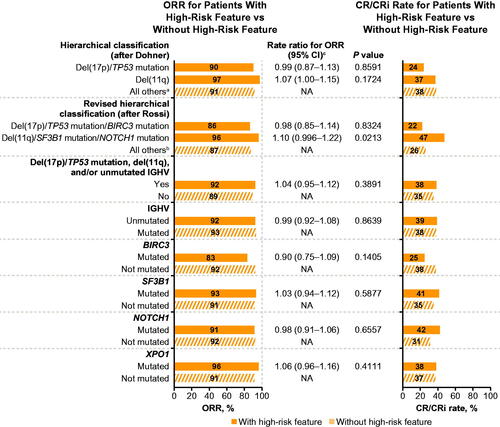
Figure 4. Forest plots of (A) progression-free survival and (B) overall survival with ibrutinib-based therapy in patients with versus without specified high-risk genomic features. CI: confidence interval; OS: overall survival; PFS: progression-free survival. aWithout high-risk feature = all others (neither del(17p)/TP53 mutation nor del(11q)). bWithout high-risk feature = all others (neither del(17p)/TP53 mutation/BIRC3 mutation nor del(11q)/SF3B1 mutation/NOTCH1 mutation). cHazard ratio for PFS or OS with versus without high-risk features.
Safety of ibrutinib-based therapy
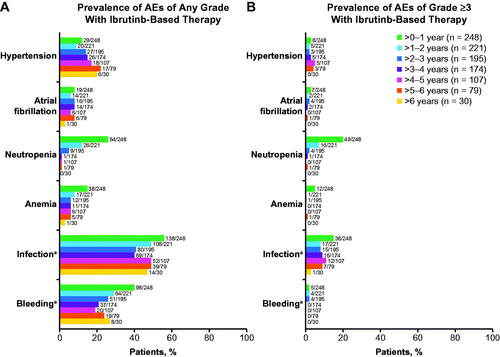
The median duration of ibrutinib-based treatment was 40.8 months (range, 0.1–74.0) and ranged from 35.7 to 43.8 months across genomic risk subgroups (Supplementary Table S3). In the overall population of patients treated with ibrutinib-based therapy, prevalence of adverse events (AEs) of clinical interest (hypertension, atrial fibrillation, neutropenia, anemia, infection, and bleeding) of any grade () or grade ≥3 () demonstrated some variability but generally decreased over time, with the exception of hypertension. Bleeding events of any grade declined from 40% during the first year to 27% after year 6; no grade ≥3 bleeding events occurred after year 3. No clinically meaningful differences in the rates of these grade ≥3 AEs were observed across high-risk genomic subgroups compared to the overall population (Supplementary Table S3). Similarly, no clinically meaningful differences were seen in any grade AEs compared to the overall population (Supplementary Table S3).
Figure 5. Prevalence of (A) any-grade AEs of clinical interest and (B) grade ≥3 AEs of clinical interest by yearly intervals in ibrutinib-treated patients. AEs: adverse events. aCombined terms. Infection was identified using the MedDRA System Organ Class term for Infections and infestations. Bleeding was identified using the Standardized MedDRA Query for Hemorrhage, excluding laboratory terms.
Discussion
Overall, this integrated analysis of patients undergoing first-line ibrutinib-based treatment, with median follow-up of 49 months (up to 79 months), confirmed significant PFS and ORR benefits with ibrutinib (with or without obinutuzumab) versus chlorambucil (with or without obinutuzumab) irrespective of high-risk genomic features. Additionally, the analyses generally demonstrated relatively similar PFS and ORR for ibrutinib-randomized patients with or without high-risk genomic features associated with inferior outcomes with chemoimmunotherapy, including del(17p), del(11q), TP53 mutation, or unmutated IGHV [Citation8–12] and single-gene mutations in BIRC3, NOTCH1, SF3B1, and XPO1 [6,7]. While PFS data have been previously published separately for the RESONATE-2 and iLLUMINATE studies according to FISH cytogenetics, IGHV status, and TP53 mutation status [Citation14,Citation16], we report here for the first time a pooled cross-trial analysis on the single-gene mutations of BIRC3, NOTCH1, SF3B1, and XPO1 and introduce novel data according to the revised Rossi hierarchical classification. The presence of high-risk genomic features did not appear to have a discernible impact on rates of treatment-emergent AEs.
Patients with CLL bearing TP53 aberrations (del (17p) and/or TP53 mutations) have poor outcomes on chemoimmunotherapy, with 3-year PFS and OS rates of only 18% and 38%, respectively, in those treated with first-line fludarabine, cyclophosphamide, and rituximab [Citation11]. In contrast, the 42-month PFS rate for patients with TP53 aberrations randomized to first-line ibrutinib-based therapy was 79% in the current pooled analysis. These findings are consistent with those from a pooled analysis of patients with TP53 aberrations with median follow-up of 4 years (up to 8 years) across four clinical trials of first-line ibrutinib-based therapy, including the two trials in this current analysis (RESONATE-2 and iLLUMINATE) as well as two trials sponsored by the National Institutes of Health (PCYC-1122e and ECOG-1912), that demonstrated 4-year PFS and OS rates of 79% and 88%, respectively [Citation27]. With a median follow-up of 6.5 years, the PCYC-1122e study demonstrated 6-year PFS and OS rates of 61% and 79%, respectively, in patients with TP53 aberrations treated with first-line ibrutinib [Citation28]. Additionally, the 2-year PFS rate in the CLL14 study was 74% for patients with TP53 aberrations receiving first-line treatment with venetoclax plus obinutuzumab [Citation29]. While previous findings in patients receiving single-agent ibrutinib in the relapsed/refractory setting suggested that patients with del(17p) and/or TP53 mutations tended to have shorter PFS than patients without TP53 aberrations [Citation15,Citation22], the current pooled analyses demonstrated comparable PFS between patients with and without TP53 aberrations receiving ibrutinib-based therapy in the first-line setting. It should be noted that the proportion of patients with TP53 aberrations is small in these first-line studies relative to the relapsed/refractory setting in which the prevalence of TP53 aberrations is increased as a result of expansion of refractory TP53-aberrant subclones under selective pressure of chemoimmunotherapy [Citation30,Citation31].
Unmutated IGHV predicts inferior outcomes with first-line chemoimmunotherapy in patients with CLL, whereas patients with mutated IGHV can achieve long-term PFS with chemoimmunotherapy [Citation9–12]. Consistent with previous findings [Citation14–16,Citation20,Citation32], we found that PFS benefit with ibrutinib-based therapy versus comparators was similar between patients with and without unmutated IGHV and ibrutinib-based therapy substantially abrogated the negative prognostic impact of unmutated IGHV status. Because the BCR stereotype subset 2 is enriched in the mutated IGHV population but carries a prognosis similar to that conferred by unmutated IGHV [Citation26], we performed a sensitivity analysis that included patients with BCR stereotype subset 2 in the unmutated IGHV subgroup. Although patient numbers were small, inclusion of these seven additional subset 2 patients in the unmutated IGHV subgroup resulted in a slight shift in the HR for PFS in favor of the mutated IGHV subgroup.
More novel mutations in BIRC3, NOTCH1, SF3B1, and XPO1 have also been associated with poor outcomes with chemoimmunotherapy in patients with CLL [Citation6,Citation7]. BIRC3 and SF3B1 appear to be associated with refractoriness to chemotherapy [Citation7], whereas NOTCH1 mutations appear to be associated with refractoriness to anti-CD20 antibodies [Citation9,Citation33,Citation34]. The role of XPO1 mutations is less clear as these frequently co-occur with NOTCH1 and/or TP53 mutations [Citation7]. Consistent with previous analyses in patients treated with ibrutinib in the relapsed/refractory setting [Citation21,Citation22], we found no significant differences in PFS or OS in patients randomized to first-line ibrutinib-based therapy according to the presence or absence of mutations in NOTCH1, SF3B1, BIRC3, or XPO1. However, it should be noted that these analyses are limited by the small numbers of patients in some of these subgroups, with only 24 patients each in the BIRC3-mutated and XPO1-mutated subgroups. Conversely, the prevalence of NOTCH1 mutations was relatively high in our study population, likely due to enrollment for other high-risk features, including TP53 mutation and unmutated IGHV, both of which are known to correlate frequently with NOTCH1 mutation [Citation35].
Integration of cytogenetic and mutational features using the revised hierarchical classification after Rossi et al. affords a more nuanced approach to CLL prognostication compared to analyses based on FISH cytogenetics alone [Citation3]. In this integrated model, patients with del(17p)/TP53 mutation and/or BIRC3 mutations comprise the highest risk group with the least favorable survival outcomes on chemotherapy or chemoimmunotherapy [Citation3]. Patients with del(11q) and/or SF3B1 and/or NOTCH1 mutations have intermediate risk, whereas patients without any of these cytogenetic or mutational lesions have lower risk [Citation3]. In the current analysis, efficacy outcomes in patients randomized to first-line ibrutinib-based therapy were generally comparable across subgroups defined by the revised hierarchical classification, including those in the highest risk subgroup.
Despite the robust efficacy of novel targeted therapy, an interim analysis from the prospective, observational informCLL registry (N = 840) showed that chemotherapy and chemoimmunotherapy were commonly used as first-line treatment in patients with high-risk prognostic factors, including those with del (17p) (34%), TP53 mutation (36%), or unmutated IGHV (57%) [Citation13]. Data from the current analysis confirm that these high-risk genomic features have less prognostic significance with first-line ibrutinib-based therapy. Additionally, numerous studies of patients with CLL/SLL receiving single-agent ibrutinib or ibrutinib-based combination therapies in the real-world setting have shown that clinical outcomes are generally consistent with efficacy observed in clinical studies, including in patients with high-risk disease features, such as del(17p), del (11q), TP53 mutations, and/or unmutated IGHV [Citation32,Citation36–40].
This analysis pooling two registrational phase 3 studies demonstrated the efficacy of first-line ibrutinib-based treatment irrespective of cytogenetic and mutational risk features, including those with unmutated IGHV, with NOTCH1 mutation, and with the highest risk classification of del(17p)/TP53 mutation/BIRC3 mutation. Evidence to date indicates that ibrutinib-based therapy achieves consistent efficacy across multiple patient subgroups defined by clinical characteristics [Citation14–17] and high-risk genomic features, which may help inform treatment decisions for patients with previously untreated CLL/SLL.
Author contributions
JAB, TJK, and AT designed the analyses in collaboration with representatives of the sponsor; JAB, TR, FD, OB, CM, DSi, TM, DAS, TJK, and AT contributed to data collection; SD, LWKC, and KK performed the data analyses; SD, LWKC, KK, IL, and EH confirmed the accuracy of the data and compiled it for analysis; all authors had access to the data and were involved in the interpretation of data, contributed to the manuscript review and revisions, and approved the final version for submission.
GLAL-2021-1034-File007.docx
Download MS Word (1.4 MB)Acknowledgments
The authors thank all the patients who participated in the RESONATE-2 and iLLUMINATE studies and their families. We also thank Jennifer Lin, MA, MS, for biometrics support and Melanie Sweetlove, MSc, for medical writing support, funded by Pharmacyclics LLC, an AbbVie Company.
Disclosure statement
JAB: honoraria from and consulting/advisory role for Janssen; research funding from AstraZeneca, BeiGene, and Pharmacyclics LLC, an AbbVie Company; and speakers bureau for and travel/accommodations/expenses from Gilead, Janssen, Novartis, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company. TR: honoraria from AstraZeneca and Janssen; and consulting/advisory role for and research funding from Acerta, AstraZeneca, and Janssen. FD: consulting/advisory role for AbbVie, Amgen, AstraZeneca, and Roche; research funding from AbbVie, AstraZeneca, Janssen, and Pharmacyclics LLC, an AbbVie Company; speakers bureau for AbbVie, Amgen, and Janssen; and travel/accommodations/expenses from AbbVie, Amgen, Janssen, and Pfizer. OB: consulting/advisory role for AbbVie, AstraZeneca, and Janssen; and research funding from Janssen. CM: consulting/advisory role for AbbVie, AstraZeneca, BeiGene, and Janssen; research funding from AbbVie and Janssen; and speakers bureau for Janssen. DSi: employment and stock or other ownership with BeiGene; honoraria and travel/accommodations/expenses from AbbVie and Janssen; research funding from AbbVie, Acerta, Amgen, BeiGene, Celgene, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Roche, Sanofi, and Pharmacyclics LLC, an AbbVie Company. TM: honoraria from AbbVie, AstraZeneca, Gilead, Janssen, and Novartis; consulting/advisory role for MorphoSys and Sunesis; and travel/accommodations/expenses from AbbVie, Gilead, and Janssen. DAS: consulting/advisory role for Amgen and MorphoSys. SD: current employment with Horizon Therapeutics and previously employed with Pharmacyclics LLC, an AbbVie Company; and stock or other ownership with AbbVie, Bristol Myers Squibb, Exelixis, Gilead, GlaxoSmithKline, Horizon Therapeutics, Myovant Sciences, and Revance Therapeutics. LWKC: employment with Pharmacyclics LLC, an AbbVie Company; stock or other ownership with AbbVie; and other relationships with Pfizer and Tizona Therapeutics. KK: current employment with BioSplice Therapeutics and previously employed with Pharmacyclics LLC, an AbbVie Company; and stock or other ownership with AbbVie, BioSplice Therapeutics, and Gilead. IL: current employment with Gilead Sciences and previously employed with Pharmacyclics LLC, an AbbVie Company; spouse employment with The Permanente Medical Group; and stock or other ownership with AbbVie, Clovis, Gilead Sciences, Infinity, Reviva Pharmaceuticals, and The Permanente Medical Group. EH: employment with Pharmacyclics LLC, an AbbVie Company; and stock or other ownership with AbbVie. TJK: employment with Moores Cancer Center; stock or other ownership with Oncternal; honoraria from AbbVie, Celgene, DAVA Oncology, Genentech, Gilead, Janssen, Roche, and Pharmacyclics LLC, an AbbVie Company; consulting/advisory role with AbbVie, Celgene, DAVA Oncology, Genentech-Roche, Gilead, Janssen, and Pharmacyclics LLC, an AbbVie Company; research funding from Celgene, CIRM, MD Anderson Cancer Center, Oncternal, Velos, and Pharmacyclics LLC, an AbbVie Company; speakers bureau for AbbVie, DAVA Pharmaceuticals, Genentech, Gilead, Janssen, Verastem, and Pharmacyclics LLC, an AbbVie Company; patents/royalties/other intellectual property for development of cirmtuzumab, which is licensed by Oncternal from the University of California; and travel/accommodations/expenses from AbbVie, Bionest Partners, Celgene, DAVA Oncology, G-Therapeutics Genentech, Gilead, Indy Hematology Review, Janssen, OncLive, Roche, Verastem, and Pharmacyclics LLC, an AbbVie Company. AT: consulting/advisory role and speakers bureau for AbbVie, AstraZeneca, BeiGene, and Janssen.
Data availability statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu
Additional information
Funding
References
- Burger JA. Treatment of chronic lymphocytic leukemia. N Engl J Med. 2020;383(5):460–473.
- Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916.
- Rossi D, Rasi S, Spina V, et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013;121(8):1403–1412.
- Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847.
- Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–1854.
- Foa R, Del Giudice I, Guarini A, et al. Clinical implications of the molecular genetics of chronic lymphocytic leukemia. Haematologica. 2013;98(5):675–685.
- Jain P, Kanagal-Shamanna R, Wierda W, et al. Clinical and molecular characteristics of XPO1 mutations in patients with chronic lymphocytic leukemia. Am J Hematol. 2016;91(11):E478–E479.
- Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24(3):437–443.
- Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123(21):3247–3254.
- Thompson PA, Tam CS, O’Brien SM, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3):303–309.
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174.
- Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942.
- Mato AR, Barrientos JC, Ghosh N, et al. Prognostic testing and treatment patterns in chronic lymphocytic leukemia in the era of novel targeted therapies: results from the inform CLL registry. Clin Lymphoma Myeloma Leuk. 2020;20(3):174–183.e173.
- Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–798.
- Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–1363.
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56.
- Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443.
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528.
- Fraser G, Cramer P, Demirkan F, et al. Updated results from the phase 3 HELIOS study of ibrutinib, bendamustine, and rituximab in relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma. Leukemia. 2019;33(4):969–980.
- Kipps TJ, Fraser G, Coutre SE, et al. Long-term studies assessing outcomes of ibrutinib therapy in patients with del(11q) chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2019;19(11):715–722.e716.
- Byrd JC, Hillmen P, O’Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133(19):2031–2042.
- Brown JR, Hillmen P, O'Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83–91.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the national cancer institute-working group 1996 guidelines. Blood. 2008;111(12):5446–5456.
- IMBRUVICA (ibrutinib) [prescribing information]. Sunnyvale, CA: Pharmacyclics LLC; 2020.
- Baliakas P, Agathangelidis A, Hadzidimitriou A, et al. Not all IGHV3-21 chronic lymphocytic leukemias are equal: prognostic considerations. Blood. 2015;125(5):856–859.
- Allan JN, Shanafelt T, Wiestner A, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukemia (CLL) with 4 years of follow-up in patients with TP53 aberrations (del(17p) or TP53 mutation): a pooled analysis from 4 clinical trials. Poster presented at: 62nd ASH Annual Meeting and Exposition; December 5-8, 2020; Virtual Meeting.
- Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med. 2020;383(5):498–500.
- Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236.
- Landau DA, Tausch E, Taylor-Weiner AN, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526(7574):525–530.
- Rossi D, Khiabanian H, Spina V, et al. Clinical impact of small TP53 mutated subclones in chronic lymphocytic leukemia. Blood. 2014;123(14):2139–2147.
- Mato AR, Roeker LE, Allan JN, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am J Hematol. 2018;93(11):1394–1401.
- Dal Bo M, Del Principe MI, Pozzo F, et al. NOTCH1 mutations identify a chronic lymphocytic leukemia patient subset with worse prognosis in the setting of a rituximab-based induction and consolidation treatment. Ann Hematol. 2014;93(10):1765–1774.
- Tausch E, Beck P, Schlenk RF, et al. NOTCH1 mutation and treatment outcome in CLL patients treated with chlorambucil (chl) or ofatumumab-Chl (O-Chl): results from the phase III study complement 1 (OMB110911). Poster presented at: American Society of Hematology 55th Annual Meeting; December 7-10, 2013; New Orleans, LA.
- Rosati E, Baldoni S, De Falco F, et al. NOTCH1 aberrations in chronic lymphocytic leukemia. Front Oncol. 2018;8:229.
- Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879.
- Aarup K, Enggaard L, Pedersen RS, et al. Real-world outcomes for 205 Danish patients with chronic lymphocytic leukemia treated with ibrutinib. Poster presented at: 61st ASH Annual Meeting & Exposition; December 7-10, 2019; Orlando, FL.
- UK CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101:1563–1572.
- Olszewski AJ, Davids MS, Yakirevich I, et al. Early adoption and outcomes of ibrutinib as treatment for older patients with chronic lymphocytic leukemia (CLL): a population-based study. Poster presented at: 61st ASH Annual Meeting & Exposition; December 7-10, 2019; Orlando, FL.
- Costa A, Loscertales J, Terol MJ, et al. Retrospective observational study of the treatment of chronic lymphocytic leukemia (CLL) with ibrutinib in routine clinical practice in Spain. Poster presented at: 25th Congress of the European Hematology Association; June 11-21, 2020; Virtual Congress.
