Abstract
This open-label, multicenter, single-arm, phase 2 study assessed the safety and efficacy of blinatumomab consolidation therapy in adult patients with newly diagnosed, high-risk diffuse large B-cell lymphoma (DLBCL; International Prognostic Index 3–5 and/or double-/triple-hit or double MYC/BCL-2 expressors) who achieved complete response (CR), partial response (PR), or stable disease (SD) following run-in with 6 cycles of R-chemotherapy (NCT03023878). Of the 47 patients enrolled, 28 received blinatumomab. Five patients (17.9%) experienced grade 4 treatment-emergent adverse events of interest (neutropenia, n = 4; infection, n = 1). Two deaths reported at the end of the study were unrelated to treatment with blinatumomab (disease progression, n = 1; infection, n = 1). 3/4 patients with PR and 4/4 patients with SD after R-chemotherapy achieved CR following blinatumomab. Consolidation with blinatumomab in patients with newly diagnosed, high-risk DLBCL who did not progress under R-chemotherapy was better tolerated than in previous studies where blinatumomab was used for treatment of patients with lymphoma.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL). DLBCL accounts for approximately 31% of all NHLs in Western countries [Citation1]. The addition of rituximab to chemotherapy (R-chemotherapy; rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) around 20 years ago significantly improved outcomes in patients with DLBCL and has since been the standard of care (SOC) [Citation2–6]. Despite success with R-chemotherapy, 30‒40% of patients with DLBCL relapse, and 10% of patients are refractory to R-chemotherapy [Citation7]. Furthermore, outcomes are poor among patients who relapse within 1 year of treatment; overall survival (OS) is estimated at less than 17% at the end of 3 years [Citation8].
A subset of patients with DLBCL harbor genetic abnormalities such as translocations/rearrangement involving myc and bcl-2 or bcl-6 (double-hit) or myc and bcl-2 and bcl-6 (triple-hit), which are associated with dismal clinical outcomes following R-chemotherapy [Citation9]. Patients with these disease subtypes progress rapidly and are generally refractory to salvage therapy. Additionally, double protein expression of MYC and BCL-2 (activated by mechanisms such as nuclear factor κB activation) in the absence of rearrangement has also been associated with poor outcomes following R-chemotherapy [Citation10]. Given the challenges associated with poor outcomes following salvage therapy, novel frontline regimens are needed to extend survival in patients with these difficult-to-treat subtypes of DLBCL.
Blinatumomab, an immunotherapy based on the BiTE® (bispecific T-cell engager) immuno-oncology platform, redirects CD3-positive T-cells to engage and lyse CD19-expressing target cells. Blinatumomab has demonstrated initial efficacy as salvage therapy in patients with relapsed/refractory (R/R) DLBCL, which suggests that addition of blinatumomab to the frontline regimen could improve outcomes in patients with newly diagnosed DLBCL [Citation8,Citation11–13]. This open-label, multicenter, phase 2 study assessed the safety and efficacy of blinatumomab consolidation therapy in patients with newly diagnosed, high-risk DLBCL who did not progress under R-chemotherapy as part of a frontline regimen.
Methods
Patients
Patients aged ≥18 years who had untreated, histologically proven high-risk DLBCL defined by an International Prognostic Index (IPI) score of 3–5 [Citation14] and/or double-hit/triple-hit (rearrangement involving myc and bcl-2 and/or bcl-6) disease and/or double protein expression of MYC and BCL-2 per local laboratory analysis, and an Eastern Cooperative Oncology Group performance status of ≤2 were enrolled (N = 47). The study was conducted in accordance with the International Council for Harmonization Good Clinical Practice Guideline and conformed to the provisions of the Declaration of Helsinki.
Study design and treatment
This open-label, multicenter, single-arm, phase 2 study was conducted at 24 centers across North America and Europe (ClinicalTrials.gov, NCT03023878) and was designed to investigate the safety and efficacy of treatment with blinatumomab following induction with R-chemotherapy in patients with newly diagnosed, high-risk DLBCL. The study protocol was approved by the ethics committee or the institutional review board at each clinical site. Enrolled patients were required to complete a run-in period, wherein patients were administered six cycles of R-chemotherapy (). Patients who demonstrated complete response (CR), partial response (PR), or stable disease by positron emission tomography/computed tomography (PET/CT) done 3 weeks (±3 days) after six cycles of R-chemotherapy and assessed by a central radiology reviewer per Lugano classification and size of the target lesions went on to receive treatment with blinatumomab [Citation15]. Patients with adequate organ and bone marrow function were included per criteria described in Supplemental Methods. Fluorescence in situ hybridization, histological, and immunochemical analyses were performed by local laboratories. Patients were excluded per criteria described in Supplemental Methods. Blinatumomab was administered by continuous intravenous (cIV) infusion in a single 84-day (12-week) cycle 1 (9 μg/day for 7 days, 28 μg/day for 7 days, and 112 μg/day for 42 days, followed by a 28-day treatment-free interval). For patients without disease progression assessed by PET/CT after blinatumomab cycle 1, an optional 28-day cycle 2 (9 μg/day for 7 days, 28 μg/day for 7 days, and 112 μg/day for 14 days) was administered at the discretion of the investigator.
Figure 1. Study design and patient disposition. The figure shows (A) Study design. (B) Patient disposition. *All patients (whether enrolled prior to cycle 1 or prior to cycle 2 of R-chemotherapy) who completed 6 cycles of SOC R-chemotherapy. †In patients who were receiving radiation therapy to bulky disease, this occurred after cycle 6 SOC R-chemotherapy and PET/CT was completed. ‡PET/CT was performed 3 weeks (±3 days) after cycle 6 SOC R-chemotherapy. §PET/CT must be performed 3 weeks (+3 days) after the last blinatumomab dose of cycle 1 (day 78 of cycle 1). ǁAt the discretion of the investigator, a second cycle of blinatumomab was administered to patients who did not have progressive disease. ¶PET/CT was performed 3 weeks (±3 days) after the last blinatumomab dose of cycle 2 (day 50 of cycle 2). #Safety follow-up visit was completed 30 days (±3 days) after the last dose of blinatumomab. ∗∗Patients should be followed for 1 year since the first dose of blinatumomab. ††If only cycle 1 of blinatumomab was given, long-term follow-up began 3 months (±3 weeks) after the last scan (day 78 of cycle 1). ‡‡If cycle 2 was given, long-term follow-up began 3 months (±3 weeks) after cycle 2 day 50. DLBCL: diffuse large B-cell lymphoma; IV: intravenous; PET/CT: positron emission tomography/computed tomography; R-chemotherapy: rituximab with chemotherapy; SOC: standard of care.
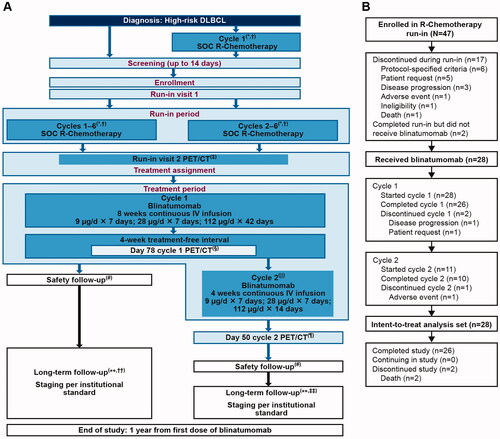
Blinatumomab treatment period was followed by a 30-day safety follow-up visit. A long-term follow-up period began after the safety follow-up visit and ended 1 year from the first dose of blinatumomab or patient death, whichever came first. During this period patients were assessed every 3 months (±3 weeks) for detection of relapse per institutional SOC disease evaluation, concomitant anti-lymphoma medication, serious adverse events (SAEs) related to blinatumomab, and levels of lactate dehydrogenase.
The primary endpoint was the incidence and severity of adverse events (AEs) during the blinatumomab treatment period per Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Secondary endpoints included objective response rate (ORR; CR + PR) based on PET/CT scans for 28 patients treated with blinatumomab using pre-blinatumomab disease status as the baseline, OS, progression-free survival (PFS), duration of response (DoR), and blinatumomab pharmacokinetic (PK) parameters.
Study assessments
Safety
AEs, graded per CTCAE and coded per Medical Dictionary for Regulatory Activities (MedDRA) version 22.0, were recorded for all patients. Treatment-emergent AEs (TEAEs) experienced between the first administration of blinatumomab and 30 days after the last administration of blinatumomab were also recorded.
Efficacy
Clinical response was assessed by PET/CT at screening and 3 weeks after the R-chemotherapy run-in. Clinical response to blinatumomab treatment was assessed by PET/CT 3 weeks (±3 days) after completion of blinatumomab on day 78 of cycle 1 and on day 50 of cycle 2 using the Lugano classification and size of the target lesion [Citation15]. Bone marrow samples were collected at screening; an additional bone marrow sample was required to confirm a CR at the end of treatment with blinatumomab if occult bone marrow involvement was suspected with an ambiguous or negative PET/CT.
T-cell kinetics
Lymphocytes from the peripheral blood of patients treated with blinatumomab were analyzed by fluorescence-activated cell sorting (FACS). Briefly, blood samples were drawn at the end of run-in with R-chemotherapy (baseline), and at 6, 24, and 48 h after the start of treatment with blinatumomab on day 1, and subsequent dose-steps on days 8, 15, and 57. Samples were stained with fluorescence-labeled antibodies against CD19 as a marker for B-cells; CD3, CD4, and CD8 as markers for subpopulations of T-cells; and CD69 as a marker for T-cell activation. Samples were analyzed on a BD FACSCanto™ cytometer (BD Biosciences). The percentage of a specific lymphocyte subpopulation was correlated with the absolute lymphocyte number from a differential blood count to calculate the absolute subpopulation numbers for that type of lymphocyte.
Pharmacokinetics
Blood samples for blinatumomab PK measurements were taken on day 1 at pre-dose, on day 2 at least 24 h after blinatumomab was started, and on days 9 and 16 at least 24 h after blinatumomab dose was increased in cycles 1 and 2. A validated enzyme-linked immunosorbent assay was used to quantify serum blinatumomab concentrations.
Statistical analysis
The primary analysis of safety was based on data from all patients who received at least one dose of blinatumomab. Objective response in patients who received at least one dose of blinatumomab was summarized with exact binomial 95% confidence intervals (CIs). The Kaplan-Meier (KM) method was used to estimate OS, PFS, and DoR. All other data were summarized descriptively. To normalize the error of flow cytometry endpoints for regression analyses, the results were presented as a fold-change from baseline for cell counts or as an absolute change from baseline for percentage of a parent population.
Results
Patients
Between 28 March 2017, and 17 May 2018, 47 patients with newly diagnosed DLBCL were enrolled (data cutoff 4 April 2019) to receive R-chemotherapy run-in. Patient demographics and baseline disease characteristics are summarized in .
Table 1. Patient demographics and baseline disease characteristics.
Disposition and exposure
Overall, 47 patients were enrolled in the R-chemotherapy run-in period, of which 17 (36%) patients discontinued treatment. Patient disposition is shown in and Supplemental Figure 1. Twenty-eight patients proceeded to receive blinatumomab, of which 26 completed cycle 1. Eleven of the 26 patients who completed cycle 1 of blinatumomab proceeded to receive cycle 2, of which 10 patients completed cycle 2 and one patient discontinued blinatumomab due to an AE. Overall, 26 patients completed the study and two deaths were reported at the end of the study. The fatalities (disease progression, n = 1; infection, n = 1) were not related to blinatumomab. Twenty-six (93%) patients received ≥80% of the intended dose and the mean dose received was 92.9% of the intended dose (Supplemental Table 1).
Safety and tolerability
Of the 28 patients treated with blinatumomab, 5 (17.9%) patients experienced TEAEs of interest at grade 4 (infection, n = 1; neutropenia and febrile neutropenia, n = 4); none of the patients experienced neurologic events or cytokine release syndrome (CRS) at grade 4 (). Grade ≥3 TEAEs of interest occurred in 9 (32.1%) patients (infections, n = 3; neurologic events, n = 3; neutropenia and febrile neutropenia, n = 4; 1 patient had more than 1 TEAE). Infections and neurologic events at grade ≥3 were experienced by 3 patients each; however, these events were resolved in all patients (infections, median duration [Q1, Q3] of 11 days [7, 16; range, 7–16]; neurologic events, median duration [Q1, Q3] of 2 days [2, 3; range, 2‒3]). Treatment-emergent SAEs were experienced by 7 (25.0%) patients; however, no fatal TEAEs were reported (Supplemental Tables 2 and 3).
Table 2. Incidence of TEAEs of interest.
Response and survival
Twenty-eight patients had CR, PR, or stable disease upon treatment with frontline R-chemotherapy and prior to initiation of treatment with blinatumomab (). At the end of 12 weeks of treatment with blinatumomab, the ORR in patients who had a CR in response to prior treatment with frontline R-chemotherapy was 90% (18/20; 95% CI, 68.3, 98.8). Seven of 8 patients who demonstrated persistence of disease (3/4 patients with PR and 4/4 patients with stable disease) at the end of treatment with R-chemotherapy achieved CR following treatment with one cycle of blinatumomab. Median OS, PFS, and DoR were not reached. The median follow-up time for OS and PFS was 12 months and the KM estimate for OS and PFS at 12 months was 92.9% (95% CI, 74.3, 98.2) and 82.0% (95% CI, 62.0, 92.1), respectively (Supplemental Figures 2(A,B); Supplemental Table 4). The median follow-up time for DoR was 11.5 months and the KM estimate for DoR at 12 months was 90.9% (95% CI, 68.1, 97.7) (Supplemental Figure 2(C) and Supplemental Table 5). Of the 28 patients who received at least one dose of blinatumomab, 26 (92.9%) patients were alive at a median follow-up of 12 months.
Table 3. Summary of best response before and at the end of cycle 1 of blinatumomab.
T-cell kinetics
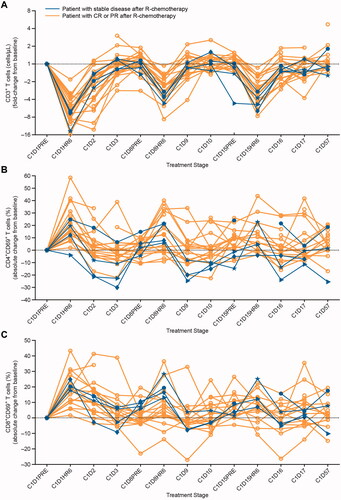
Of the 27 patients treated with blinatumomab for whom data for T-cell counts were available, the median (Q1, Q3) T-cell count for 23 responders (patients with CR or PR following treatment with R-chemotherapy) was 628 (442, 816; range, 44‒1298) cells/µL (Supplemental Table 6). The T-cell count for the four patients who had stable disease following treatment with R-chemotherapy was 448 (329, 511; range, 260‒523) cells/µL (normal range, 100‒600 cells/µL [Citation16]). Similar to the effect seen in the responders, peripheral T-cells in patients who had stable disease redistributed rapidly after the start of blinatumomab infusion; a swift drop of T-cell counts within the first few hours of exposure to blinatumomab was followed by a recovery to pretreatment levels within subsequent days (). Interestingly, the T-cell counts in most patients in both groups (responders and patients with stable disease after R-chemotherapy) not only returned to baseline in the days following the drop in the count, but also modestly exceeded baseline levels. Importantly, blinatumomab also induced a transient increase in the percentage of activated circulating T-cells as was assessed by FACS analysis of the surface expression of immediate early activation marker CD69. Similar to the effect seen in the responders, the percentage of peripheral CD4+CD69+ and CD8+CD69+ T-cells in the four patients with stable disease following treatment with R-chemotherapy increased transiently, with peak activation occurring within 6–24 h after the start of infusion with blinatumomab ()). A comparison of T-cell redistribution and expansion profiles between patients who achieved PR/stable disease and those who progressed during or at the end of one cycle of blinatumomab did not show any notable difference in T-cell redistribution and expansion profiles. Two patients who progressed on blinatumomab had slightly lower percentage of activated T-cells that expressed CD69 during cycle 1 (Supplemental Figures 3(A–C)).
Figure 2. Effect of cIV infused blinatumomab on peripheral T-cells during the first treatment cycle. (A) T-cell counts in four patients with stable disease (blue lines) and in other patients for whom data were available (gold lines) are presented as a fold-change from baseline during the first treatment cycle with blinatumomab. The expression of the immediate early activation marker CD69 on the surface of gated (B) CD4+ and (C) CD8+ T-cell subpopulations was determined by flow cytometry at baseline and throughout treatment for four patients with stable disease (blue lines) and other patients for whom data were available (gold lines). The percentages of activated, CD69+ T-cell subpopulations were calculated and presented as an absolute fold-change from baseline during the first treatment cycle with blinatumomab. Fold-change was calculated as c × 2 raised to the power of the c × log2 ratio of the post-treatment result over the pretreatment result, where c = +1 for log2 ratios ≥0 or c = −1 for log2 ratios <0. Absolute change for percentage endpoints was calculated as a difference between the post-treatment and pretreatment result. C: cycle; CD: cluster of differentiation; cIV: continuous intravenous; CR: complete response; D: day; HR: hour; PR: partial response; R-chemotherapy: rituximab with chemotherapy.
Pharmacokinetics
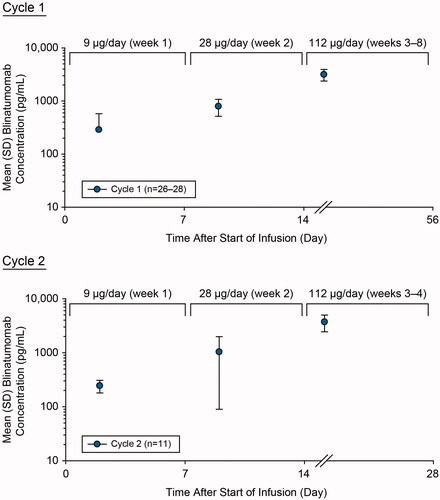
Over the 8-week cIV infusion in cycle 1 at dose-steps of 9 µg/day (week 1), 28 µg/day (week 2), and 112 µg/day (weeks 3–8), mean (standard deviation [SD]) steady-state concentration (Css) values of serum blinatumomab were 288 (289), 795 (280), and 3160 (782) pg/mL, respectively (). Mean Css in cycle 1 increased approximately dose proportionally over the dose range of 9–112 µg/day, with an 11.0-fold increase for a 12.4-fold increase in dose. Over the 4-week cIV infusion in cycle 2 at dose-steps of 9 µg/day (week 1), 28 µg/day (week 2), and 112 µg/day (weeks 3–4), mean (SD) Css values of serum blinatumomab were 244 (64.5), 1040 (945), and 3710 (1270) pg/mL, respectively. Similar to cycle 1, mean Css in cycle 2 also increased approximately dose proportionally over the dose range of 9–112 µg/day, with a 15.2-fold increase for a 12.4-fold increase in dose.
Figure 3. Assessment of pharmacokinetics of blinatumomab during cycle 1 and cycle 2. Mean (SD) blinatumomab serum concentration-time profiles after cIV blinatumomab at 9 µg/day (week 1), 28 µg/day (week 2), and 112 µg/day (week 3 onward) for cycles 1 and 2 in adult patients with newly diagnosed, high-risk DLBCL. cIV: continuous intravenous; DLBCL: diffuse large B-cell lymphoma; SD: standard deviation.
Discussion
This phase 2 pilot study, which assessed the safety of single-agent blinatumomab consolidation therapy following induction with R-chemotherapy as part of the frontline regimen in patients with newly diagnosed, high-risk DLBCL, is the first study of its kind. Patients who completed a ‘run-in’ with SOC R-chemotherapy received one cycle of blinatumomab and an optional second cycle at the discretion of the on-site investigator, which was intended as a bridge for autologous hematopoietic stem cell transplant (HSCT). Most patients did not proceed to cycle 2 of blinatumomab since they were in remission upon completion of 8 weeks of cycle 1.
Five of 28 (17.9%) patients treated with blinatumomab experienced TEAEs at grade 4. Notably, none of the patients experienced neurologic TEAEs at grade 4 or CRS at grade ≥3. Furthermore, infections and neurologic events at grade ≥3 resolved within a median duration of 11 and 2 days, respectively, following discontinuation of treatment. The percentage of patients who had grade ≥3 TEAEs in the current study (39.3%) was lower compared with the percentage reported in a phase 2 study (95.7%), which evaluated the safety and efficacy of blinatumomab in patients with R/R DLBCL [Citation13]. The percentage of patients with grade ≥3 neurologic TEAEs observed in the current study (10.7%) was lower than the percentage reported in patients with R/R DLBCL (21.7%) [Citation13], and in previous studies in patients with R/R B-cell NHL (22%–24%) [Citation12,Citation17]. Furthermore, the incidence of grade ≥3 neurologic events in the current study was consistent with the incidence reported in patients with R/R Philadelphia chromosome–negative acute lymphoblastic leukemia (ALL; 9.4%) from the phase 3 TOWER study despite the difference in the dose of blinatumomab administered (112 µg/day in the current study versus 28 µg/day for patients in the TOWER study) [Citation18]. Thus, consolidation therapy with blinatumomab was better tolerated in patients with newly diagnosed high-risk DLBCL treated with six cycles of induction SOC R-chemotherapy compared with patients with R/R DLBCL or other lymphomas. However, a direct comparison of the incidence of TEAEs observed in patients with R/R DLBCL or other aggressive lymphomas with the rate of incidence in the current study should be treated with caution since these patients have a worse prognosis. In a separate study based on a randomized phase 3 trial which compared lenalidomide as maintenance therapy with placebo in elderly patients with untreated DLBCL who achieved a CR or PR in response to R-chemotherapy, the most common grade ≥3 TEAEs were neutropenia (lenalidomide, 56% vs placebo, 22%), infection (lenalidomide, 8% vs placebo, 6%), and cardiac disorders (lenalidomide, 6% vs placebo, 3%) [Citation19]. Incidence of most common grade ≥3 TEAEs—neutropenia, infections, and cardiac disorders in this study by Thieblemont et al. was greater than or comparable to the incidence reported in the current study (neutropenia, 56.0% vs 14.3%; infections, 8.0% vs 10.7%; cardiac disorders, 6.0% vs 0%). Since many patients with DLBCL are cured with frontline R-chemotherapy alone, careful evaluation of the risks and benefits rationale should be undertaken at the time of introduction of a new agent into frontline R-chemotherapy. Predictive parameters are currently being studied to identify a subgroup of patients with high-risk DLBCL and a poor prognosis where even though addition of a novel agent such as blinatumomab to the frontline regimen may lead to additional toxicity compared with R-chemotherapy alone, but it could also provide a beneficial outcome [Citation20].
PK parameters of blinatumomab were approximately linear over the dose range of 9–112 µg/day cIV and were similar between the two cycles. These results were consistent with those in the previous studies [Citation21]. At the end of 12 weeks of treatment with blinatumomab (cycle 1), 17 of 20 (85.0%) patients who had a CR in response to induction therapy with R-chemotherapy maintained their status of CR. Seven of 8 patients who demonstrated persistence of disease after induction with R-chemotherapy (4 patients with PR and 4 patients with stable disease) achieved CR upon consolidation therapy with blinatumomab. Treatment responses for all patients from the current study who were treated with R-chemotherapy and subsequently with blinatumomab are shown in Supplemental Table 7. Although the number of patients treated with blinatumomab in the current study were limited, and since this was the first study where blinatumomab was used as consolidation therapy, the results from this study could form a basis for a future randomized trial with a large patient pool.
The pharmacodynamic profile for the peripheral CD3+ T-cells and the CD4+CD69+ and CD8+CD69+ T-cell subtypes for patients treated with one cycle of blinatumomab for whom data were available, including the four patients with stable disease after treatment with R-chemotherapy who achieved CR following treatment with one cycle of blinatumomab, was consistent with the pharmacodynamic profile of T-cells in patients treated with blinatumomab described in previous studies [Citation22–24]. The CD3+ T-cell counts in a few patients not only returned to baseline in the days following the drop, but also modestly exceeded baseline levels, which could be indicative of a beneficial effect considering that these patients were heavily immunocompromised after treatment with R-chemotherapy. Additionally, treatment with blinatumomab induced an increase in the percentage of activated circulating T-cells, which was consistent with its previously reported BiTE® mechanism of action [Citation22,Citation25].
This trial has several limitations such as small sample size, median PFS, OS, and DoR not reached, and lack of a control arm. Furthermore, 17 of 47 patients did not proceed to blinatumomab after run-in with R-chemotherapy. Of these 17 patients, a subset of patients discontinued the run-in with R-chemotherapy due to disease progression (7 patients) or AEs (3 patients) and thus could not be treated with blinatumomab, which may have introduced a pre-selection bias against these patients. In the current study, blinatumomab demonstrated efficacy in patients with persistent disease; 7 of 8 patients who had stable disease or PR after six cycles of R-chemotherapy achieved CR after treatment with blinatumomab. Thus, it could be speculated that the introduction of blinatumomab in the frontline regimen at an earlier cycle (such as after cycle 2 or cycle 4) of R-chemotherapy may result in more patients achieving a CR.
Consolidation with blinatumomab therapy following induction with R-chemotherapy in patients with newly diagnosed, high-risk DLBCL was better tolerated with no new safety signals compared with previous studies in patients with R/R lymphomas. In patients with newly diagnosed high-risk DLBCL and suboptimal response to 6 cycles of R-chemotherapy, treatment with blinatumomab converted PR/stable disease responses to CR in 7 of 8 patients. This study demonstrates that blinatumomab may be active in patients with DLBCL and future randomized trials to demonstrate the activity of blinatumomab in combination with standard chemotherapy are warranted.
Authorship contributions
All authors contributed in writing/review of the manuscript and provided approval for publication. D.A.K. contributed to the study design, data collection, data analysis, and data interpretation. J.D.M., A.A. Anderson, A.A. Avilion, H.L.W., Y.C., T.D., W.K., and Y.K., contributed to the study design, data analysis, and data interpretation. M.P.C., K.A.D., C.T., N.J.M., S.S.K., A.V., A.M.G.-S., G.R.-G., M.B.-O., S.T.L., and E.G.-B. contributed to the data collection, analysis, and interpretation.
GLAL-2021-1075-File008.tif
Download TIFF Image (599.3 KB)GLAL-2021-1075-File007.jpg
Download JPEG Image (600 KB)GLAL-2021-1075-File006.jpg
Download JPEG Image (1.4 MB)Acknowledgments
The authors thank all the site investigators and patients who participated in this study. Swapnil Kher, PhD, of Cactus Life Sciences (part of Cactus Communications) and Ben Scott, PhD (Scott Medical Communications, LLC) provided medical writing assistance funded by Amgen Inc. Graphics support was provided by Robert Dawson of Cactus Communications and funded by Amgen Inc. This study was funded by Amgen Inc and Astellas Pharma Inc. The funders contributed to study design, data collection, data analysis, and data interpretation, and funded a professional medical writer to assist with writing the report.
Disclosure of interest
D.A.K. received honoraria from and fees for consulting or advisory role and speakers’ bureau from AbbVie and Stemline. M.P.C. received fees for consulting from Teva Pharmaceuticals. C.T. received fees for consulting or advisory role from Amgen, Celgene, Jazz Pharmaceuticals, Kite/Gilead, Novartis, Servier, and Roche and received research funding from Roche, Celgene, and Hospira. N.J.M. received fees and honoraria from Kite/Gilead, Janssen, and Amgen Inc. A.V. received honoraria from and fees for consulting or advisory role and speakers’ bureau from Amgen, Roche, Kite/Gilead, and Novartis. A.M.G.-S. received honoraria from and fees for consulting or advisory role and speakers’ bureau from Celgene, Servier, Gilead, EUSA Pharma, MorphoSys, Kyowa Kirin, iQone, and Roche; research grant from Celgene and Janssen; and non-financial support/travel fees from Celgene, Janssen, Servier, Roche, and Celltrion. G.R.-G. received consultancy fees and/or honoraria from Janssen, Takeda, Celgene, and Roche. M.B.-O. received research funding from Roche; received honoraria from and fees for consulting or advisory role and speakers’ bureau from Roche, Gilead/Kite, Takeda, and Celgene. E.G.-B. received honoraria from and fees for consulting or advisory role and speakers’ bureau from Janssen, Gilead, Celgene, Kyowa Kirin, Celltrion, AbbVie, Takeda, and Roche. A.A. Avilion is a stockholder of Amgen. J.D.M., W.K., Y.C., H.L.W., A.A. Anderson, Y.K., and T.D. are Amgen employees and report stock ownership. K.A.D., S.S.K., and S.T.L. have nothing to disclose.
Data availability statement
Qualified researchers may request data from Amgen clinical studies. Deidentified individual participant data are available indefinitely at http://www.amgen.com/datasharing
References
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues, fourth edition. Lyon (France): IACR; 2008.
- Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242.
- Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127.
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–116.
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5(Suppl 5):v116–125.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Diffuse Large B-Cell Lymphomas Version 4.2020. [cited 2020 Sept 30]. https://www.nccn.org/patients/guidelines/content/PDF/nhl-diffuse-patient.pdf.
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045.
- Broséus J, Chen G, Hergalant S, et al. Relapsed diffuse large B-cell lymphoma present different genomic profiles between early and late relapses. Oncotarget. 2016;7(51):83987–84002.
- Dunleavy K. Aggressive B cell lymphoma: optimal therapy for MYC-positive, double-hit, and triple-hit DLBCL. Curr Treat Options Oncol. 2015;16(12):58.
- Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from the International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021–4031.
- Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–1861.
- Goebeler ME, Knop S, Viardot A, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34(10):1104–1111.
- Viardot A, Goebeler ME, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127(11):1410–1416.
- Thieblemont C, Gisselbrecht C. Second-line treatment paradigms for diffuse large B-cell lymphomas. Curr Oncol Rep. 2009;11(5):386–393.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068.
- Hoffman R, Furie B, McGlave P, et al. Hematology: basic principles and practice. 5th ed. Philadelphia (PA): Churchill Livingstone; 2009.
- Coyle L, Morley NJ, Rambaldi A, et al. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2020;61(9):2103–2112.
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847.
- Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-cell lymphoma treated with first-line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2017;35(22):2473–2481.
- Thieblemont C, Bernard S, Meignan M, et al. Optimizing initial therapy in DLBCL. Best Pract Res Clin Haematol. 2018;31(3):199–208.
- Zhu M, Wu B, Brandl C, et al. Blinatumomab, a bispecific T-cell engager (BiTE®) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–1288.
- Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226–6233.
- Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498.
- Zugmaier G, Gökbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood. 2015;126(24):2578–2584.
- Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977.
