Abstract
The Myelofibrosis and Essential Thrombocythemia Observational STudy (MOST; NCT02953704) is an ongoing, noninterventional study assessing clinical characteristics and patient-reported outcomes (PROs) of patients with myelofibrosis (MF) or essential thrombocythemia (ET). This analysis assessed PROs at enrollment; symptom burden and quality of life (QoL), work productivity, and activity were assessed using validated questionnaires in patients with low- or intermediate-1-risk (age-alone) MF, or high- or low-risk ET (receiving ET-directed therapy) at enrollment. In MF and ET cohorts, fatigue had highest mean symptom score. Women had higher mean total symptom scores (TSS), mean symptom scores, and reduced QoL versus men. In patients with MF, mean TSS and symptom scores were similar between risk groups. Patients with low-risk ET had higher mean TSS and symptom scores than patients with high-risk ET. In conclusion, patients with lower risk MF and low- or high-risk ET experience significant symptom burden affecting QoL and ability to work.
Introduction
Myelofibrosis (MF) and essential thrombocythemia (ET) are acquired Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs), a rare group of malignant blood disorders characterized by abnormal clonal proliferation of at least one myeloid cell line [Citation1,Citation2]. Hallmark characteristics of MF include splenomegaly, burdensome constitutional symptoms, cytopenia, and progressive bone marrow fibrosis [Citation3,Citation4]. ET is associated with increased platelet and megakaryocyte production, and increased risk of vascular events such as thrombosis and bleeding [Citation1,Citation3].
Patients with MF or ET report a range of symptoms such as abdominal discomfort, bone pain, fatigue, itching, night sweats, unexplained weight loss, and/or fever [Citation5–9]. Additional symptoms common to MF are often related to spleen enlargement and include abdominal pain, left subcostal pain, and early satiety; additional ET symptoms are commonly vasomotor in nature and include headaches, dizziness, erythromelalgia, and concentration problems [Citation1]. The MPN Landmark study demonstrated that these disease-related symptoms result in a reduced quality of life (QoL) in a majority of patients with MF and ET (81% and 57%, respectively) [Citation7]. Notably, symptoms adversely affect patients’ QoL in not only those with the most severe disease, but also in those with low prognostic scores and in those in the lowest symptom severity quartile [Citation7].
Lower-risk patients with MF or ET have previously been reported to have a lower incidence of symptoms [Citation10]; however, the severity and impact of MPN-associated symptoms is not well-recognized in lower-risk patients. Although treatments for both MF and ET are risk-adapted, and are directed at managing the disease and minimizing or improving symptoms [Citation11,Citation12], symptom burden is not included as a risk stratification factor for either MF or ET [Citation12–15]. A practice of observation and monitoring of signs/symptoms for disease progression is recommended for lower-risk asymptomatic MF or low-risk ET [Citation16,Citation17]; however, patients with lower-risk symptomatic MF may receive cytoreductive therapy, ruxolitinib, or interferon at the physician’s discretion [Citation17]. Therefore, characterizing symptom burden in these patients may help guide more effective disease management and treatment strategies.
Patient-reported outcome (PRO) instruments are validated questionnaires often used in observational studies and clinical trials to assess the effect of a treatment or condition from the patient’s perspective [Citation18]. PRO instruments provide important and otherwise clinically difficult to obtain measures of the patient’s perception of their physical, social, and psychological wellbeing [Citation19]. Although most PRO-based assessments of patients with MF have focused on those with intermediate (INT) or higher risk disease, limited PRO data from patients with lower risk MF have been published. For example, the MPN Landmark study recruited patients with MPNs irrespective of risk category or treatment; of note, most recruited patients with MF had INT-2- or high-risk disease [Citation7]. Therefore, although evidence exists for the effects of symptom burden on QoL in patients with INT-2 and high-risk MF, there are limited data describing the effects of symptom burden on QoL in patients with lower-risk MF. Similarly, little is known about the comparison of PROs in patients with high- versus low-risk ET, and the extent to which ET-directed treatment received relieves symptom burden in these patients. Despite the importance of assessing PROs, a review of a registry of clinical trials (initiated between 2006 and 2016) in patients with MPNs reported that only 19/35 reported on at least one PRO assessment as a study endpoint; of the 19 trials, only nine published a detailed analysis of PRO data [Citation20]. Thus, there is an unmet need for further investigation into the extent to which symptom burden affects the daily lives and QoL of patients with lower risk MF and ET.
The Myelofibrosis and Essential Thrombocythemia Observational STudy (MOST; NCT02953704) is an ongoing noninterventional study designed to collect clinical characteristics, PROs, and treatment patterns of patients with specific risk categories of clinically diagnosed MF and ET in community and academic centers throughout the United States. This analysis assessed patient-reported symptom burden and its effect on QoL, work productivity, and activity in patients with MF and ET at the time of enrollment in MOST.
Methods
Study design
Details of the MOST study design have been published previously [Citation21,Citation22]. Patients were enrolled at 124 academic and community sites across the United States over a 24-month enrollment period. PRO data were collected at the time of enrollment and during usual-care visits over a planned 36-month observation period. All patient-care decisions were made at the discretion of the treating physician.
The study protocol and amendments were approved by review boards or independent ethics committees of the relevant institutions. The study is being conducted in accordance with the Declaration of Helsinki and Good Clinical Practice as designed by the International Conference on Harmonization. All patients provided written informed consent before participating in the study.
Patient eligibility
Eligible patients had a clinical diagnosis of either MF or ET as per their treating hematology/oncology physician. As such, not all patients met the strict World Health Organization (WHO) diagnostic criteria [Citation21,Citation22]. Patients were also required to be under the current supervision of a treating physician for MF or ET, and willing and capable of providing written informed consent and filling out patient assessment questionnaires with minimal assistance.
Patients in the MF cohort were eligible if they were ≥18 years of age with a clinical diagnosis of MF (including primary MF, post-polycythemia vera (PV) MF, and post-ET MF). Eligible patients had low risk or INT-1 risk by reason of age alone according to the Dynamic International Prognostic Scoring System (DIPSS) criteria [Citation15]. Patients in the ET cohort were eligible if they had high-risk ET (≥60 years of age and/or had a history of thrombotic events) or low-risk ET if they were currently receiving ET-directed therapy (not including aspirin-only) [Citation12].
Key exclusion criteria for both the MF and ET cohorts included participation in an active, blinded study or an investigational/interventional drug trial funded by Incyte Corporation; a life expectancy of ≤6 months; or a diagnosis of secondary acute myeloid leukemia, myelodysplastic syndrome, chronic myelogenous leukemia, or secondary thrombocytosis.
Patient-reported outcomes assessments
PROs were assessed via validated questionnaires completed by patients at the time of enrollment, and every 6 months at follow-up appointments. Patient-reported symptom burden was assessed using the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) [Citation6], consisting of 10 items (fatigue, early satiety, abdominal discomfort, inactivity, concentration problems, night sweats, itching, bone pain, fever [>100 °F], and weight loss). Symptom severity was graded from 0 (absent) to 10 (worst imaginable); scores ≥7 were considered ‘severe,’ scores of 4–6 were considered ‘moderate,’ scores of 1–3 were considered ‘mild,’ and a score of 0 was considered ‘none’. TSS was calculated as the sum of the 10 items (range, 0–100). Numbness/tingling was included on the questionnaire but was not included in the TSS calculation [Citation6].
Health-related QoL was assessed using the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) (v3) [Citation23], consisting of 30 items arranged into five multi-item functional scales (physical, cognitive, role, emotional, and social), three multi-item symptom scales (pain, fatigue, and nausea and vomiting), one multi-item global health/QoL scale, and six additional single-symptom items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Each item has four response options: (i) ‘not at all,’ (ii) ‘a little,’ (iii) ‘quite a bit,’ and (iv) ‘very much,’ except for the global health-status/quality of life scale, which has response options ranging from (i) ‘very poor’ to (vii) ‘excellent’. Higher scores on the symptoms scale indicate higher symptom burden. Higher scores on the functional and global health/QoL scale indicate higher functioning and better health/QoL, respectively.
The effect of patient health and specific symptoms on work productivity was assessed using the Work Productivity and Activity Impairment Questionnaire-Specific Health Problem (WPAI-SHP) Questionnaire [Citation24], consisting of six items based on work productivity, absenteeism, presenteeism, and activity impairment. Data are presented as a proportion with 0% representing no impairment and 100% representing maximal impairment/productivity loss.
Statistical analysis
All enrolled patients were included in the data analysis; MF and ET cohorts were analyzed separately. Data were summarized using descriptive statistics. Student’s t-tests were used to determine the statistical significance of differences between sex and risk groups with the nominal significance level of p≤0.05. PRO data analysis was performed using SAS® statistical software, version 9.4 (SAS Institute, Cary, NC). Although all efforts were made to collect data, missing data were not imputed. Before the analysis was conducted, all medical conditions, events, and medications entered in the database were coded using the Medical Dictionary for Regulatory Activities and WHO-drug reference list.
Results
Patient demographics and clinical characteristics
The MOST study enrolled 233 patients with MF (between November 2016 and March 2019) and 1235 patients with ET (between November 2016 and December 2018). Of these patients, 125 (53.6%) patients with MF and 801 (64.9%) patients with ET completed at least one PRO assessment at enrollment, and therefore qualified for inclusion in the current analysis.
MF cohort
At the time of enrollment in the MF cohort (n = 125), the median age was 68 years (range, 36–88), about half of the patients were men (52.8%), and the majority of patients were white (91.2%). The median time from MF diagnosis to enrollment was 2.1 years (range, 0–38), and 53 (42.4%) and 72 (57.6%) patients were categorized as low risk and INT-1 risk (by age alone), respectively, at the time of enrollment. Six patients (4.8%) had a documented family history of MF, PV, or ET (). One-third of patients (33.0%) were employed full- or part-time, over half (56.5%) were retired, and one patient (0.8%) reported an inability to work because of MF. At the time of enrollment, 12.1% and 46.0% of patients with MF were current or former smokers, respectively.
Table 1. Patient demographics at the time of enrollment.
ET cohort
Among the ET cohort (n = 801), the median age was 70 years (range, 19–93), about one-third were men (32.2%), and the majority of patients were white (89.6%). The median time from ET diagnosis to enrollment was 4.2 years (range, 0–37); 105 (13.1%) and 696 (86.9%) patients were categorized as low risk and high risk, respectively. Thirty patients (3.8%) had a documented family history of MF, PV, or ET (). Almost one-third of patients (29.7%) were employed full- or part-time, over half (56.7%) were retired, and 11 (1.4%) patients reported an inability to work because of ET. Sixty-seven (8.4%) and 301 (37.6%) patients were current or former smokers, respectively.
Patient-reported outcomes
MPN-SAF
MF cohort
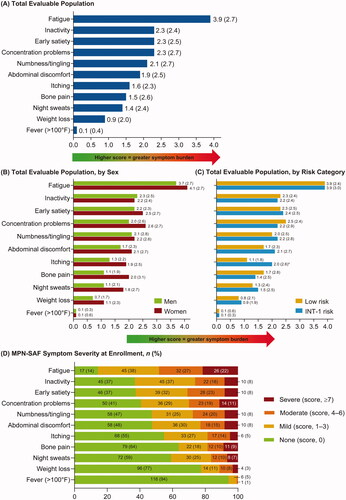
At enrollment, 124 (99.2% of patients who completed at least one PRO assessment) completed the MPN-SAF. The mean TSS (standard deviation [SD]) was 18.1 (15.1) and 36% of patients had a TSS ≥20. The highest mean (SD) individual symptom score was recorded for fatigue (3.9 [2.7]), followed by inactivity (2.3 [2.4]), early satiety (2.3 [2.5]), and concentration problems (2.3 [2.7]) (). Except for inactivity and fever, women had higher mean scores than men for all individual symptoms (p > 0.05) (). In addition, women had a higher mean (SD) TSS than men (20.4 [15.8] vs. 16.0 [14.2]; p > 0.05). Few differences were observed in the mean individual scores and TSS between low-risk and INT-1-risk groups (p > 0.05), except for itching, which was significantly higher in the INT-1-risk compared with low-risk MF cohort (p < 0.05) (). The most common severe symptoms (i.e. score ≥7) were fatigue (21.7%) and concentration problems (11.4%) ().
Figure 1. Patients with MF: mean (SD) MPN-SAF TSS patient-reported outcome scores for (A) total evaluable population, (B) total evaluable population, by sex, and (C) total evaluable population, by risk category; (D) MPN-SAF symptom severity at enrollment. *p < 0.05. INT: intermediate; MF: myelofibrosis; MPN-SAF TSS: Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score; SD: standard deviation.
ET cohort
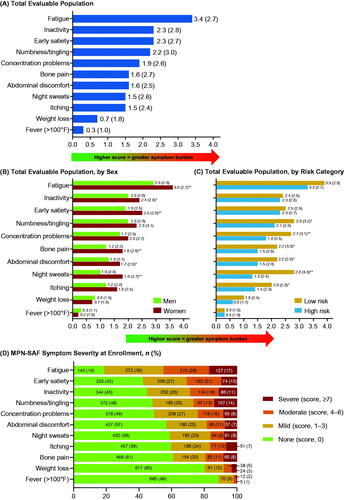
Among the ET cohort, 772 (96.4% of patients who completed at least one PRO assessment) completed the MPN-SAF at enrollment. The mean (SD) TSS was 17.1 (15.6) and 33% of patients had TSS ≥20. The highest mean (SD) individual symptom score was recorded for fatigue (3.4 [2.7]), followed by early satiety (2.3 [2.7]), inactivity (2.3 [2.8]), and numbness/tingling (2.2 [3.0]) (). Except for unintentional weight loss and fever, women had higher mean scores than men for all individual symptoms; fatigue, satiety, abdominal discomfort, night sweats, and bone pain symptoms were significantly higher in women compared with men (p < 0.05) (). In addition, women had a significantly higher mean (SD) TSS than men (18.4 [15.8] vs. 14.2 [14.9]; p < 0.01). Mean scores were higher for patients with low-risk ET than for patients with high-risk ET for the majority of individual symptoms measured (abdominal discomfort, problems with concentration, numbness/tingling, itching, bone pain, and night sweats; p < 0.05) (). Severe symptoms (i.e. score ≥7) were most commonly fatigue (17.0%) and numbness/tingling (13.9%) ().
Figure 2. Patients with ET: mean (SD) MPN-SAF TSS patient-reported outcome scores for (A) total evaluable population, (B) total evaluable population, by sex, and (C) total evaluable population, by risk category; (D) MPN-SAF symptom severity, at enrollment. *p < 0.05, **p < 0.01. ET: essential thrombocythemia; MPN-SAF TSS: Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score; SD: standard deviation.
Current therapies and TSS at enrollment
MF cohort
Of 205 patients included in this analysis, 119 were receiving MF-directed therapy at enrollment, of whom 114 were receiving MF-directed monotherapy (low risk: 100%, 48/48; INT-1 risk: 93.0%, 66/71) and five were receiving at least one MF-directed therapy (low risk: 0%, 0/48; INT-1 risk: 7.0%, 5/71). The most common MF-directed monotherapy received by patients with low-risk MF was hydroxyurea (56.3%, 27/48), followed by ruxolitinib (33.3%, 16/48), interferon (8.3%, 4/48), and anagrelide (2.1%, 1/48). For patients with INT-1-risk MF, the most common MF-directed monotherapy received was ruxolitinib (45.5%, 30/66), followed by hydroxyurea (39.4%, 26/66), interferon (12.1%, 8/66), and anagrelide (3.0%, 2/66).
An analysis of TSS at enrollment by risk status and treatment (in 123/124 patients who completed the MPN-SAF) showed that the mean (SD) TSS was lower in patients with low-risk MF (treated: 19.7 [13.3], n = 34; untreated: 13.7 [13.8], n = 18) compared with patients with INT-1-risk MF (treated: 20.6 [16.6], n = 43; untreated 15.2 [15.2], n = 28). In both risk groups, the proportion of patients with a TSS ≥20 was higher in patients who were treated (low risk: 35.3%, n = 12; INT-1 risk: 46.5%, n = 20) compared with untreated patients (low risk: 22.2%, n = 4; INT-1 risk: 28.6%, n = 8) (low risk vs. INT-1 risk, p > 0.05).
ET cohort
Among 1210 patients included in this analysis, 1046 were receiving ET-directed therapy at enrollment, of whom 996 patients were receiving ET-directed monotherapy (low risk: 94.3%, 150/159; high risk: 95.4%, 846/887) and 50 patients were receiving at least one ET-directed therapy (low risk: 5.7%, 9/159; high risk: 4.6%, 41/887). The most common ET-directed monotherapy received by patients with low-risk ET was hydroxyurea (76.7%, 115/150), followed by interferon (10.0%, 15/150), anagrelide (9.3%, 14/150), and ruxolitinib (4.0%, 6/150). The most common ET-directed monotherapy received by patients with high-risk ET was hydroxyurea (82.4%, 697/846), followed by anagrelide (10.5%, 89/846), ruxolitinib (4.1%, 35/846), interferon (2.4%, 20/846), and busulfan (0.6%, 5/846).
An analysis of TSS at enrollment by risk status and treatment (of 772 patients who completed the MPN-SAF) showed that the mean (SD) TSS was higher in patients with low-risk ET (21.9 [18.4]) compared with patients with high-risk ET (treated: 16.2 [14.7]; untreated: 16.9 [16.7]) (low risk vs. high risk, p < 0.01). The proportion of patients with a TSS ≥20 was highest in patients with low-risk (43.3%, n = 45) compared with high-risk ET (treated: 30.8%, n = 173; untreated: 35.5%, n = 38) (low risk vs. high risk, p < 0.05).
EORTC QLQ-C30 symptom scales and subscales
MF cohort
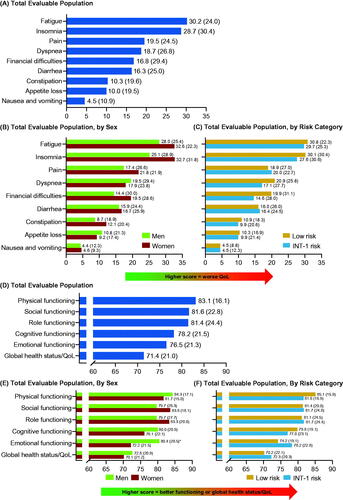
At enrollment, 123 (98.4% of patients who completed at least one PRO assessment) completed the EORTC QLQ-C30. The highest mean (SD) individual symptom score was for fatigue (30.2 [24.0]) followed by insomnia (28.7 [30.4]), both of which were higher in women than in men (p > 0.05) (). The mean (SD) global health status QoL score was 71.4 (21.0); mean functional scale scores ranged from 76.5 for emotional functioning to 83.1 for physical functioning (). The mean scores for global health status/QoL and physical, cognitive, and emotional functioning were better for men than women (emotional functioning; p < 0.05), whereas social and role functioning scores were better for women than men (p > 0.05) ().
Figure 3. Patients with MF: mean (SD) EORTC QLQ-C30 scores at enrollment for symptom scale scores for (A) total evaluable population, (B) total evaluable population, by sex, and (C) total evaluable population, by risk category; subscale scores for (D) total evaluable population, (E) total evaluable population, by sex and, (F) total evaluable population, by risk category. *p < 0.05. EORTC QLQ-C30: European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire; INT: intermediate; MF: myelofibrosis; QoL: quality of life; SD: standard deviation.
ET cohort
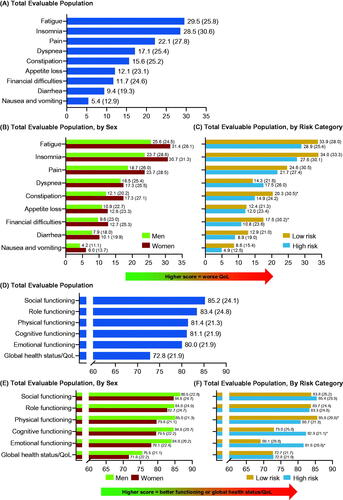
Among the ET cohort at enrollment, 798 (99.6% of patients who completed at least one PRO assessment) completed the EORTC QLQ-C30 questionnaire. The highest mean (SD) individual symptom score was for fatigue (29.5 [25.8]), followed by insomnia (28.5 [30.6]), both of which were higher in women than in men (p < 0.05) (). The mean (SD) global health status QoL score was 72.8 (21.9); mean functional scores ranged from 80.0 for emotional functioning to 85.2 for social functioning (). The mean scores for all five functioning scales were better in men than women (physical, emotional, and cognitive functioning; p < 0.05) ().
Figure 4. Patients with ET: mean (SD) EORTC QLQ-C30 scores at enrollment for symptom scale scores for (A) total evaluable population, (B) total evaluable population, by sex and, (C) total evaluable population, by risk category; subscale scores for (D) total evaluable population, (E) total evaluable population, by sex and, (F) total evaluable population, by risk category. *p < 0.05. EORTC QLQ-C30: European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire; ET: essential thrombocythemia; QoL: quality of life; SD: standard deviation.
WPAI-SHP
MF cohort
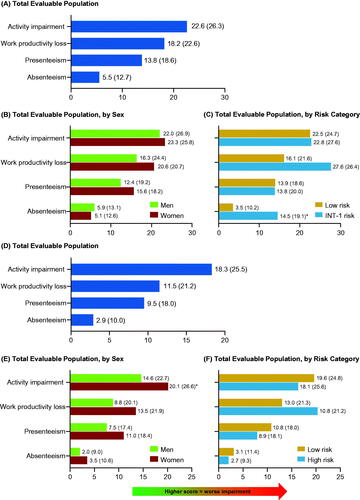
At enrollment, 121 (96.8% of patients who completed at least one PRO assessment) completed the WPAI-SHP questionnaire, out of whom 42 (34.7%) patients reported that they were currently employed. Overall, the mean (SD) WPAI-SHP scores for absenteeism, presenteeism, and overall work productivity loss were 5.5 (12.7), 13.8 (18.6), and 18.2 (22.6), respectively (). The mean (SD) activity impairment score at enrollment, regardless of employment status, was 22.6 (26.3). Average work productivity loss, presenteeism, and activity impairment were higher in women than in men (p > 0.05) (), whereas average absenteeism was higher in men than in women (p > 0.05), and higher in INT-1-risk (by age alone) than in low-risk patients with MF (p < 0.05) ().
Figure 5. Patients with MF (A–C) or ET (D–F): mean (SD) WPAI-SHP scores for (A, D) total evaluable population, (B, E) total evaluable population, by sex and, (C, F) total evaluable population, by risk category. *p < 0.05. ET: essential thrombocythemia; INT: intermediate; MF: myelofibrosis; SD: standard deviation; WPAI-SHP: Work Productivity and Activity Impairment-Specific Health Problem.
ET cohort
Among the ET cohort at enrollment, 791 (98.8% of patients who completed at least one PRO assessment) completed the WPAI questionnaire, out of whom 231 (29.2%) patients reported that they were currently employed. Overall, the mean (SD) WPAI-SHP scores for absenteeism, presenteeism, and overall work productivity loss were 2.9 (10.0), 9.5 (18.0), and 11.5 (21.2), respectively (). Average work productivity loss, absenteeism (p > 0.05), and activity impairment (p < 0.01) were higher in women than in men (), and higher in low-risk than in high-risk patients with ET (p > 0.05) ().
Discussion
MOST is the first prospective, longitudinal, observational study assessing patient-reported disease burden in patients with low- and INT-1-risk (by age alone) MF, and patients with high-risk ET or low-risk ET who are receiving ET-directed therapy, from community and academic centers across the United States. The demographic characteristics of patients enrolled in MOST were similar to those of prior real-world analyses of patients with MF and ET in the United States, suggesting a potentially representative sample [Citation5–7,Citation25]. To better understand the unmet needs of patients with respect to disease burden and its effect on QoL, the current analysis focused on PROs at the time of enrollment in MOST. The overall findings of this analysis highlight that MPN-related symptoms adversely affect patient QoL, even in MPN subtypes that are typically viewed as being less severe.
Data obtained from the MPN-SAF and EORTC QLQ-C30 symptom scale scores for both the MF and ET cohorts identified fatigue as the most common and severe symptom. This finding is consistent with those of previous real-world studies in patients with MF and ET [Citation5–7,Citation9,Citation26]. Reflecting this, fatigue is often the symptom that patients most commonly want to resolve, and treatment strategies that address disease-related fatigue may contribute to improved QoL [Citation7,Citation27]. Inactivity, early satiety, and problems with concentrating were also commonly reported symptoms on the MPN-SAF; insomnia and pain were commonly reported on the EORTC QLQ-C30. Notably, numbness/tingling, which is not included in the validated MPN-SAF TSS score calculation in this study, is a common symptom experienced by 59% and 58% of patients with ET and MF, respectively [Citation9]. In the current study, numbness/tingling was the fourth most frequently experienced symptom out of 11 symptoms assessed in patients with ET and the fifth most commonly experienced symptom out of 11 symptoms assessed in patients with MF; the mean (SD) symptom score for patients with ET and MF were 2.2 (3.0) and 2.1 (2.7), respectively. These scores are similar to those reported in the international MPN-SAF survey of 161 patients with ET and 96 patients with MF [Citation9]. This suggests that by not capturing numbness/tingling in the MPN-SAF TSS calculation, symptom burden of MF or ET may be underestimated in patients enrolled in this study.
In our study, MPN-specific symptoms such as bone pain and itching appeared to be less bothersome (i.e. lower scores on the MPN-SAF) compared with more generalized symptoms such as fatigue and concentration problems. These findings are similar to those from a large international survey of patients with MPN where the most commonly reported symptom was fatigue in both patients with low- and INT-1-risk MF and in patients with low- or high-risk ET [Citation28]. As the MF cohort in our study was comprised of lower-risk patients with less advanced disease, it is not surprising that patients had lower bone-pain scores than patients with higher-risk disease [Citation9]. Moreover, itching has been shown to increase in severity over time; because lower-risk patients may be at the earlier stages of disease, itching may have a lower symptom burden in these patients compared with those with higher-risk disease [Citation29]. Generalized symptoms including fatigue and sleep disturbances are commonly encountered with other chronic diseases, such as diabetes [Citation30] and cardiovascular diseases [Citation31,Citation32]. Therefore, it is possible that comorbidities of diabetes and hypertension experienced by patients enrolled in the MOST study (13–17.1% and 49.8–56%, respectively) may have contributed to the observed symptom burden of fatigue, etc. [Citation21,Citation22]. Nevertheless, the MOSAICC UK case-control survey reported that MPN-SAF symptom burden in patients with MPN was significantly higher compared with that of the age- and gender-matched controls (adjusted for potential confounders (age, gender, educational status, presence of chronic comorbidities, smoking status, alcohol consumption, and body mass index)); fatigue was reported as the most common symptom (92.4% in MPN vs. 78.1% in controls) [Citation26].
The observation that women reported lower global health status and QoL scores in both the MF and ET cohorts, is consistent with a growing body of evidence indicating that women with MPNs experience worse overall health and QoL than men [Citation10,Citation26,Citation33]. Thus, here, women had a higher mean MPN-SAF TSS and higher scores in the majority of EORTC QLQ-C30 symptom scales. Few studies have investigated the causes of the apparent differences between men and women in their perceived effect of MPN symptoms [Citation26,Citation33]. Women may be more likely than men to recognize and report symptoms such as pain because of inherent gender-specific differences in behavior and social acceptance [Citation34]. In general, it has been reported that women also have a lower pain threshold and increased perception of pain compared with men, which has been attributed in part to differences between men and women in the distribution of opioid receptors within the nervous system, and differences in concentrations of sex steroid hormones (including estrogen, progesterone, and testosterone) and their receptors [Citation35,Citation36]. In addition to modulating pain, decreases in estrogen have also been associated with an increased prevalence and severity of vasomotor symptoms including headache and night sweats [Citation37,Citation38]. Consistent with Geyer et al. [Citation33], women with MF or ET in our study reported higher scores for vasomotor symptoms, microvasculature-related symptoms, and bone pain. Notably, the majority of the enrolled patients were older than 65 or 60 years (for MF or ET, respectively); given that the mean age of menopause onset in the United States is 51.5 years [Citation39], it might be speculated that, for some women in this study, vasomotor symptoms associated with MF or ET might have been exacerbated by those associated with estrogen depletion. Women also experience higher levels of abdominal pain/discomfort and early satiety compared with men [Citation33]. This observation might, in part, be explained by the finding that among patients with MPNs, thrombi are predominantly abdominally localized in women, whereas in men these tend to be cardiac- and deep venous-specific [Citation40]. The observation that fatigue scores were significantly higher in women compared with men is consistent with a cluster analysis study of patients with ET by Geyer et al. [Citation28]. This study identified a female-predominant cluster that was associated with the highest reported score for fatigue (7.0), highest occurrence of anemia (33.3%), and largest average spleen size. Taken together, the apparent differences between men and women in the perceptions of their MF and ET symptoms suggest a potential role for sex-specific management of symptoms, particularly for women who experience poorer QoL.
The finding that patients enrolled in this study were predominantly white is a limitation that is seen widely in both observational and clinical trials, and clearly demonstrates a need to improve trial recruitment of, and opportunity to enroll, underrepresented US populations to avoid reporting bias in clinical outcomes in general and PROs in particular [Citation41–43]. Thus, because of this underrepresentation of minority races and ethnicities, few studies have investigated race-related differences in PROs in patients with MPNs. McFarland et al. [Citation44] compared symptom burden in white (76.1%) versus non-white (23.9%) patients diagnosed with an MPN (including MF, ET, and PV) based on patient responses on the 22-item Distress Thermometer and Problem List [Citation45]. Non-white patients with MPN were 1.8, 2.3, and 2.6 times more likely to report problems associated with sleep (p = 0.05), pain (p = 0.016), and tingling (p = 0.026), respectively, compared with white patients. Another study showed that, given equal access to healthcare, non-white patients with an MPN had a significantly higher platelet count (p = 0.026) at diagnosis compared with white patients [Citation46]. This suggests that there may be an underappreciation of the severity of vascular-related symptoms in non-white populations. Elsewhere, significant differences in awareness and recognition of cancer symptoms have been reported among ethnic minorities [Citation47]. Therefore, patients of ethnic minorities may present with more advanced stage disease at diagnosis, which may contribute to worse symptoms compared with white patients. Taken together, these results illustrate the importance for healthcare providers to appreciate the role that race, ethnicity, and socioeconomic status may play in affecting patients’ perceptions of symptom burden and overall QoL. Moreover, further development of PRO instruments that are validated across different ethnic and socioeconomic groups is warranted to better address the symptom-management needs of different patient groups.
The breadth and severity of individual symptoms experienced by patients with low- and INT-1-risk (by age alone) MF, and low-risk (receiving ET-directed therapy) and high-risk ET in MOST are consistent with published data for each cohort [Citation6,Citation7,Citation9]. A cluster analysis by Geyer et al. [Citation28] showed that symptom heterogeneity exists within and across MPN risk categories. Consistent with results from our study, DIPSS risk (as well as by some clinical abnormalities) differed significantly across clusters of patients with MF, but high symptom burden was observed in clusters of both low- and INT-1-risk patients. For patients with ET, no significant differences in clusters were observed based on the international prognostic score for thrombosis in ET risk; however, TSS significantly increased across the five clusters identified (p < 0.001) [Citation28]. In the MF cohort of the present study, the observed total mean (SD) MPN-TSS score for the MF cohort was lower compared with that reported by a previous study of patients with MF (18.1 [15.1] vs. 25.3 [17.2]) [Citation6], which may reflect that the previous study did not recruit patients based on risk, whereas MOST only recruited lower risk patients. Consistent with this, results from the MPN Landmark survey showed that patients with higher risk MF had a higher mean MPN-SAF TSS compared with lower risk MF patients (30.8 vs. 8.1) [Citation7]. Contrasting with the MF cohort, the mean MPN-SAF TSS in the ET cohort in the present study was higher for low-risk versus high-risk ET patients. This apparently counterintuitive observation is also apparent in results from the MPN Landmark study [Citation7], and might reflect that patients with low-risk ET were required to be receiving ET-directed therapy at enrollment in MOST. Taken together, these data provide evidence that risk status alone might not capture the scope of disease burden and may result in an underestimation of disease effect on a patient’s QoL [Citation48].
The observation that patients who were treated with MF-directed therapy had a higher mean TSS compared with untreated patients, suggests that high symptom burden may have provided a rationale for physicians to initiate treatment in patients with lower risk MF when they would otherwise be under a ‘watch-and-wait’ approach for disease progression. Findings from the Landmark MPN survey showed that 53% and 14% of physicians recognize symptom management to be the main goal for managing MF and ET, respectively, whereas only 7% and 9% of patients with MF and ET, respectively, agree that this is the most important treatment goal [Citation48]. Both MF and ET patients in the Landmark MPN survey placed greater importance on slowing/delaying progression of their condition, on healthy blood counts, and on better QoL than on symptom improvement [Citation7]. Moreover, the results suggest that patients with MF and ET may not recognize their symptoms as being MPN-related [Citation48]. Increased awareness and better recognition of MPN-related symptoms from patients and physicians alike may improve patient QoL, illustrating an important role for PRO instruments such as the MPN-SAF and EORTC QLQ-C30 in assessing disease burden throughout the course of disease management.
Overall, the burden of symptoms associated with MF and ET had a negative effect on daily living and work productivity. As most of the MF cohort was >65 years of age (56.8%) and the ET cohort was ≥60 years (79.5%) at the time of enrollment, it is not unexpected that a higher percentage of patients were not employed because of retirement (MF, 56.5%; ET, 56.7%). However, among the patients who were employed at the time of enrollment (MF, 34.7%; ET, 29.3%), more than one-quarter experienced activity impairment, and ∼20% of patients in each cohort experienced work productivity loss. A previous study showed a similar deleterious effect of MPN disease burden on the ability to work effectively, with ∼30% of patients with MF and ET experiencing activity impairment, work productivity loss, and work presenteeism [Citation49], likely largely attributed to MPN-related pain and discomfort [Citation7]. These findings again underscore the importance of recognizing the effect of symptom burden on patients’ lives using validated PRO tools to improve patient care and QoL, and in turn, reduce potential work-related losses.
In conclusion, patients with lower risk MF and low- or high-risk ET experience significant symptom burden that affects QoL and ability to work. Whereas current therapies effectively reduce the risk of thrombosis in patients with ET (the major cause of mortality and morbidity) [Citation50], and splenomegaly in patients with MF [Citation51], the results presented here provide further evidence that they do not fully address symptom burden. Thus, despite lower prognostic scores or perception of lower severity disease, symptom burden reduction should be included in MF and/or ET treatment strategies. The observed higher symptom burden perceived by women compared with men suggests that gender could play an important role in the presentation of MF and ET and should be incorporated into disease management decisions [Citation33]. Future analyses from MOST will continue to increase understanding of the disease-related symptom burden and its effect on QoL in patients with lower risk MF and ET.
Author contributions
All authors were involved in conceptualization, methodology, data curation, writing review, and editing drafts. RS, PK, and HR were involved in compiling and analyzing the study data and interpreted data in collaboration with all authors. All authors had full access to all the data in the study and provided input on additional analyses before the writing of the report.
Acknowledgements
The authors wish to thank the patients and their families, the investigators, and the site personnel who participated in this study. Medical writing and editorial assistance were provided by Rachel Shparberg, PhD, of Envision Pharma Group (Philadelphia, PA, USA), and was funded by Incyte Corporation.
Disclosure statement
Ellen Ritchie has a consulting role at Celgene, Incyte Corporation, Novartis, and Pfizer; participated in the speakers’ bureau for Ariad, Celgene, Incyte Corporation, and Novartis; received research funding from Astellas, Bristol Myers Squibb, Novartis, NS Pharma, and Pfizer; received travel support from Celgene and Novartis. Anas Al-Janadi holds honoraria from GSK and Karyopharm Therapeutics. Craig Kessler sits on the advisory board for Incyte Corporation and received research support from Incyte Corporation. Patricia Kalafut and Haobo Ren hold employment and stock ownership from Incyte Corporation. Robyn Scherber was a previous employee and stock owner of Incyte Corporation. Ruben Mesa has a consulting role at Incyte Corporation, La Jolla Pharmaceutical Company, Novartis, and Sierra Oncology; received research funding from AbbVie, Celgene, CTI Biopharma, Genotech Pharma, Incyte Corporation, Promedior, and Samus.
Data availability statement
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g. United States, European Union, Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960
Additional information
Funding
References
- Briere JB. Essential thrombocythemia. Orphanet J Rare Dis. 2007;2:3.
- Scherber RM, Mesa RA. Managing myelofibrosis (MF) that "blasts" through: advancements in the treatment of relapsed/refractory and blast-phase MF. Hematology Am Soc Hematol Educ Program. 2018;2018(1):118–126.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Zahr AA, Salama ME, Carreau N, et al. Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica. 2016;101(6):660–671.
- Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76.
- Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103.
- Mesa R, Miller CB, Thyne M, et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients' overall health and productivity: the MPN Landmark survey. BMC Cancer. 2016;16:167.
- Mitra D, Kaye JA, Piecoro LT, et al. Symptom burden and splenomegaly in patients with myelofibrosis in the United States: a retrospective medical record review. Cancer Med. 2013;2(6):889–898.
- Scherber R, Dueck AC, Johansson P, et al. The myeloproliferative neoplasm symptom assessment form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–408.
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653–1665.
- Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–770.
- Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015;5:e369.
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelo Fibrosis Research and Treatment. Blood. 2009;113(13):2895–2901.
- Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392–397.
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708.
- Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(12):1599–1613.
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology, Myeloproliferative Neoplasms, version 2, Plymouth Meeting, PA:NCCN; 2022.
- U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Health Qual Life Outcomes. 2009;4:79.
- U.S. Department of Health Human Services FDA Center for Drug Evaluation Research, U.S. Department of Health Human Services FDA Center for Biologics Evaluation Research, U.S Department of health human services FDA center for devices radiological health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79.
- Messina A, O'Brien Q, Andrianov V, et al. Under reporting of patient reported outcomes (PROs) in myeloproliferative neoplasm (MPN) clinical trials. Blood. 2019;134(Suppl. 1):4754.
- Gerds AT, Lyons RM, Colucci P, et al. Disease and clinical characteristics of patients with a clinical diagnosis of myelofibrosis enrolled in the MOST study. Clin Lymphoma Myeloma Leuk. 2022;22(7):e532–e540.
- Yacoub A, Lyons R, Verstovsek S, et al. Disease and clinical characteristics of patients with a clinical diagnosis of essential thrombocythemia enrolled in the MOST study. Clin Lymphoma Myeloma Leuk. 2021;21(7):461–469.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365.
- Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001–12. Br J Haematol. 2016;174(3):382–396.
- Anderson LA, James G, Duncombe AS, et al. Myeloproliferative neoplasm patient symptom burden and quality of life: evidence of significant impairment compared to controls. Am J Hematol. 2015;90(10):864–870.
- Scherber RM, Kosiorek HE, Senyak Z, et al. Comprehensively understanding fatigue in patients with myeloproliferative neoplasms. Cancer. 2016;122(3):477–485.
- Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014;123(24):3803–3810.
- Scherber RM, Geyer H, Harrison CN, et al. Impact of disease duration upon symptom burden amongst patients with myeloproliferative neoplasms (MPNs). Blood. 2015;126(23):4073–4073.
- Sudore RL, Karter AJ, Huang ES, et al. Symptom burden of adults with type 2 diabetes across the disease course: diabetes & aging study. J Gen Intern Med. 2012;27(12):1674–1681.
- Djarv T, Wikman A, Lagergren P. Number and burden of cardiovascular diseases in relation to health-related quality of life in a cross-sectional population-based cohort study. BMJ Open. 2012;2(5):e001554.
- Zambroski CH, Moser DK, Bhat G, et al. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4(3):198–206.
- Geyer HL, Kosiorek H, Dueck AC, et al. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: an analysis by the MPN QOL International Working Group. Haematologica. 2017;102(1):85–93.
- Samulowitz A, Gremyr I, Eriksson E, et al. "Brave men" and "emotional women": a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag. 2018;2018:6358624.
- Zubieta JK, Smith YR, Bueller JA, et al. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22(12):5100–5107.
- Paller CJ, Campbell CM, Edwards RR, et al. Sex-based differences in pain perception and treatment. Pain Med. 2009;10(2):289–299.
- Delaruelle Z, Ivanova TA, Khan S, et al. Male and female sex hormones in primary headaches. J Headache Pain. 2018;19(1):117.
- Glinskii OV, Huxley VH, Glinsky VV. Estrogen-dependent changes in dura mater microvasculature add new insights to the pathogenesis of headache. Front Neurol. 2017;8:549.
- Minkin MJ. Menopause: hormones, lifestyle, and optimizing aging. Obstet Gynecol Clin North Am. 2019;46(3):501–514.
- Stein BL, Rademaker A, Spivak JL, et al. Gender and vascular complications in the JAK2 V617F-positive myeloproliferative neoplasms. Thrombosis. 2011;2011:874146.
- Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870.
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726.
- Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242.
- McFarland DC, Shaffer KM, Polizzi H, et al. Prevalence of physical problems detected by the distress thermometer and problem list in patients with myeloproliferative disorders. J Natl Compr Canc Netw. 2017;15(12):1503–1508.
- Riba MB, Donovan KA, Andersen B, et al. Distress management, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(10):1229–1249.
- Peseski AM, Saliba AN, Althouse SK, et al. Does race play a role in complications and outcomes of Philadelphia chromosome-negative myeloproliferative neoplasms? Hematol Oncol Stem Cell Ther. 2021. Epub ahead of print.
- Niksic M, Rachet B, Warburton FG, et al. Ethnic differences in cancer symptom awareness and barriers to seeking medical help in England. Br J Cancer. 2016;115(1):136–144.
- Mesa RA, Miller CB, Thyne M, et al. Differences in treatment goals and perception of symptom burden between patients with myeloproliferative neoplasms (MPNs) and hematologists/oncologists in the United States: findings from the MPN Landmark survey. Cancer. 2017;123(3):449–458.
- Yu J, Parasuraman S, Paranagama D, et al. Impact of myeloproliferative neoplasms on patients' employment status and work productivity in the United States: results from the living with MPNs survey. BMC Cancer. 2018;18(1):420.
- Robinson AJ, Godfrey AL. Low-risk essential thrombocythemia: a comprehensive review. Hemasphere. 2021;5(2):e521.
- Iurlo A, Cattaneo D. Treatment of myelofibrosis: old and new strategies. Clin Med Insights Blood Disord. 2017;10:1179545X17695233.
