Abstract
Information on Hodgkin lymphoma (HL) is mostly limited to Europe and North America. This real-world, retrospective study assessed treatment pathways and clinical outcomes in adults with stage IIB–IV classical HL receiving frontline treatment (n = 1598) or relapsed/refractory HL (RRHL, n = 426) in regions outside Europe and North America between January 2010 and December 2013. The primary endpoint was progression-free survival (PFS) in the RRHL group. Among patients with RRHL, 89.0% received salvage chemotherapy; most common regimen was etoposide, methylprednisolone, cytarabine, cisplatin (ESHAP; 26.3%). Median PFS in the RRHL group was 13.2 months (95% confidence interval [CI]: 9.9–20.2) and was longer in patients with vs. without stem cell transplantation (SCT; 20.6 vs. 7.5 months; p = 0.0071). This large-scale study identified a lower PFS for RRHL in the rest of the world compared with Europe and North America, highlighting the need for novel targeted therapies and SCT earlier in the treatment continuum.
Clinical trial registration: NCT03327571.
Introduction
The incidence and clinical outcome of Hodgkin lymphoma (HL) vary according to age, sex, race, genetics, and geographic location [Citation1–3]. More than 80% of patients with classical HL (cHL) achieve long-term remission with contemporary frontline chemotherapy that is now response-adapted using positron emission tomography/computed tomography (PET-CT) scans [Citation4]. However, 30% of patients with advanced-stage disease (IIB–IV) experience relapse or have primary refractory disease [Citation5]. For patients with relapsed/refractory HL (RRHL), salvage chemotherapy followed by stem cell transplantation (SCT) is the current standard of care [Citation6,Citation7]. Despite this, ∼50% of patients experience progressive disease, with poor prognosis and survival [Citation8,Citation9].
Disease characteristics, treatment pathways, and clinical outcomes in HL vary significantly between developed and developing regions, which may be related to differences in the availability and access to resources, country-level healthcare policies, and quality of cancer treatment [Citation10]. In developing regions, the burden of RRHL is further complicated by late diagnosis, lack of awareness, gaps in using diagnostic techniques and novel therapeutic agents, and financial issues [Citation11–13]. Moreover, in developing countries there is a distinct lack of reliable statistics and proper documentation of geographic differences in treatment and outcomes [Citation10,Citation14]. Health data on epidemiology, treatment, and outcome in HL from diverse geographic regions is important to understand the disease, determine if healthcare programs and treatment protocols from developed countries can be extrapolated to resource-limited settings, and to guide country-specific healthcare infrastructure.
Real-world information regarding treatment pathways and clinical outcomes in RRHL is mostly confined to studies in Europe and North America [Citation9,Citation15–19]. Extrapolating these results to patient populations outside Europe and North America is confounded by the limited provision of basic healthcare and the availability of lifesaving chemotherapy regimens [Citation10]. The few studies that have been undertaken in developing countries are limited by sample size, single-center experiences, data quality, or lack of comprehensive information on patients, treatment, or clinical outcomes [Citation11,Citation20–22].
In the absence of robust real-world data outside Europe and North America, the B-CD30+ HOdgkin Lymphoma International Multi-center Retrospective Study of Treatment PractIces and OutComes (B-HOLISTIC) study assessed treatment pathways and clinical outcomes in RRHL and cHL in a large cohort of patients from East Asia, Latin America, the Middle East, and South Africa, Australia, and Russia. The primary results of the study are presented here with an emphasis on RRHL.
Materials and methods
Study design and patients
B-HOLISTIC (ClinicalTrials.gov, NCT03327571) is an international, multicenter, observational study involving the retrospective review of medical records of patients with advanced-stage IIB–IV cHL who received frontline chemotherapy (frontline cHL), with or without radiotherapy, and/or patients diagnosed with RRHL. Participating centers (Supplementary Table 1) were selected based on the physician’s interest, startup information, resource availability, and accessibility to a defined minimum data set (Supplementary Table 2) for all patients with RRHL and frontline cHL. Highly specialized treatment centers and physicians able to provide extensive retrospective data from hospitals, cancer institutes, and academic medical centers were included. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. The relevant independent ethics committees or institutional review boards at each center approved the study. Patients provided written informed consent according to local regulations.
Table 1. Baseline patient characteristics in the RRHL group.
Table 2. Treatment pathways in the RRHL group.
Data were collected retrospectively for all patients (≥18 years) with RRHL or frontline cHL between 1 January 2010 and 31 December 2013, until death or last follow-up (whichever occurred first). Patients initially diagnosed as cHL, with subsequent progression to RRHL during the study, were included in both groups. Patients without a minimum study dataset or with participation in an interventional clinical trial at any stage of their HL management were ineligible. Patients were identified at each center using central management information systems or paper-based medical charts. Pseudonymized data were collected using a web-based electronic case report form and assessed for random selection, pooled analysis, and reporting. To adequately compare progression-free survival (PFS) across regions, the initial target sample size in each country was 50 patients in each group. However, because target patient numbers were not achievable in every center and country, oversampling was allowed in countries with larger populations of eligible patients.
Study endpoints
The primary endpoint was PFS in patients with RRHL, defined as the time from initiation of first salvage treatment for RRHL to first documentation of relapse or disease progression or death. Patients without a relapse or disease progression, and who did not die, were censored on the date when they were last known to be alive.
Secondary endpoints in the RRHL group included overall survival (OS), best clinical response (e.g. complete remission [CR], partial remission [PR], stable disease [SD], or progressive disease [PD]) to first salvage treatment, time to achieve objective response (CR or PR), number of lines of chemotherapy to achieve first response, duration of response, adverse events (AEs, assessed by severity and line of treatment), PFS in patients with and without SCT, and treatment pathways. OS in the RRHL group was defined as either (a) the time from the first relapse after frontline treatment to death in the relapsed group or (b) the time from initial diagnosis of cHL to death in the entire RRHL group to include patients with refractory disease and those not receiving any salvage treatment. Additionally, an exploratory analysis was performed for the RRHL group only (i.e. excluding patients initially diagnosed as cHL who subsequently progressed to RRHL) to understand the impact of inclusion criteria on PFS.
All the above parameters were also assessed in the frontline cHL group. In this group, OS was defined as the time from diagnosis of cHL to death, and PFS was defined as the time from initiation of frontline treatment to the first documentation of relapse, disease progression, or death.
Statistical analysis
All analyses for the overall population and individual regions were performed separately. Patient and disease characteristics, treatment pathways, and AEs were presented as descriptive statistics, including measures of central tendency (median) and spread (range or interquartile range) for continuous variables and frequency distributions (number, %) for categorical variables using the number of subjects with available data. PFS and OS were analyzed using the Kaplan–Meier method. PFS and OS between patient groups were compared by calculating p-values using the log-rank test from the Kaplan–Meier analysis, considering a 5% significance level. Both univariate and multivariate analyses were performed in each group to identify various patient- and disease-related risk factors for PFS and OS. For the multivariate analysis, only significant factors in the univariate analysis with a p-value of <0.2 were retained in the final model, and hazard ratios (HRs) were estimated from Cox regression models. All analyses were conducted using Statistical Analysis System (SAS®) Software, Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 1703 patients (RRHL: n = 426; frontline cHL: n = 1598) from 74 centers in 13 countries across five regions participated in the study: East Asia (n = 426), Latin America (n = 366), the Middle East and South Africa (n = 694), Australia (n = 56), and Russia (n = 161). The data cutoff date was 4 March 2020, with a median follow-up of 4.4 years (range: 0.03–134.7 months) for the RRHL group and 5.4 years (range: 0.2–118.6 months) for the frontline cHL group.
Patient demographics and characteristics in the RRHL group
The median age of patients with RRHL was 31.0 years (range: 15.0–92.0). At the regional level, Russia had the lowest median age (28.5 years [range: 18.0–54.0]) and Latin America had the highest (35.0 years [range: 18.0–85.0]) (). Overall, there were more males (56.8%), and the most prominent ethnicity was White/Caucasian (38.3%). The prevalence of stage III and stage IV disease at presentation was not very different (30.5 and 37.5%, respectively). PET or PET-CT scans at baseline were performed in 32.8% of patients in the RRHL group. The proportion of baseline PET or PET-CT scans performed was lowest in Russia (1/11; 9.1%) and highest in East Asia (33/81; 40.7%). In total, 177 (41.5%) patients had primary refractory disease only, 182 (42.7%) had relapsed disease only, and 67 (15.7%) had both refractory and relapsed disease.
Progression-free survival in the RRHL group
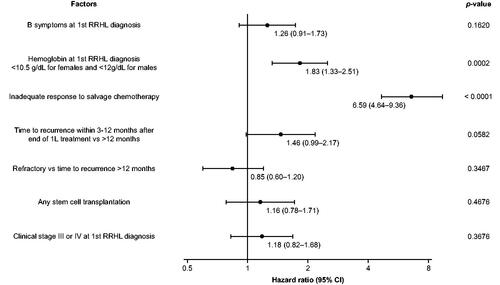
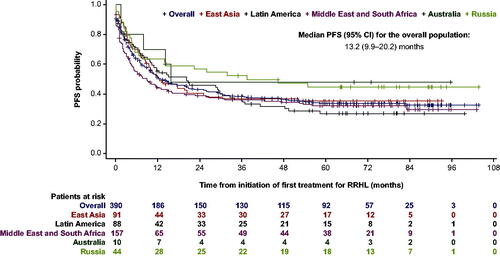
Median PFS in the RRHL group was 13.2 months (95% confidence interval [CI]: 9.9–20.2), ranging from 8.8 months (95% CI: 5.0–15.0) in the Middle East and South Africa to 37.4 months (95% CI: 6.5–not reached [NR]) in Russia (). A univariate analysis revealed that the following were associated with a higher risk of disease progression, relapse, or death: time to recurrence within 3–12 months after the end of first-line treatment, clinical stage III/IV at first RRHL diagnosis, hemoglobin level at first RRHL diagnosis (<10.5 g/dL for females and <12 g/dL for males), B symptoms at first RRHL diagnosis, and inadequate response to first salvage chemotherapy (defined as response < PR or PET positivity). However, in the adjusted multivariate model the risk of disease progression, relapse, or death was only statistically significant for the ‘hemoglobin level at the time of RRHL diagnosis’ (p = 0.0002) and ‘inadequate response to salvage chemotherapy’ (p < 0.0001) ().
Figure 1. PFS from initiation of first salvage treatment in the RRHL group. CI: confidence interval; PFS: progression-free survival; RRHL: relapsed/refractory Hodgkin lymphoma. Thirty-six patients with missing dates were excluded from the Kaplan–Meier analysis. East Asia comprises: Republic of Korea, Singapore, Taiwan, China, and Hong Kong; Latin America comprises: Argentina, Colombia, and Mexico; the Middle East and South Africa comprise: Saudi Arabia, Turkey, and South Africa.
Treatment pathways in the RRHL group
Intensive first salvage chemotherapy was given to 89.0% of patients with RRHL. The most common salvage regimens were etoposide, methylprednisolone, cytarabine, cisplatin (ESHAP; 26.3%), followed by dexamethasone, cytarabine, cisplatin (DHAP; 17.5%) (). The choice of salvage regimens was generally similar across the five regions. Of the 302 patients eligible for SCT (292 at RRHL diagnosis and 10 initially ineligible who subsequently became eligible), 222 (73.5%) underwent SCT (52.1% of the overall RRHL cohort). Autologous SCT was the most preferred modality (84.7% of cases). The most common reasons for SCT ineligibility were chemoresistance (15.7%), comorbid conditions (10.7%), and advanced age (9.9%). The proportion of patients undergoing SCT was lower in Russia (61%) and Latin America (68%) than that in other regions (range: 75.0–87.5%). Only half the patients with RRHL (49%) achieved a CR before SCT. Among the 201 patients not undergoing SCT, 157 (78.1%) received second-line salvage therapy, which was similar across all regions.
Clinical outcomes in the RRHL group
Following first salvage chemotherapy, 28.6% of patients with RRHL achieved a CR, and the median time to response (CR or PR) following first treatment for RRHL was 2.5 months (interquartile range [IQR]: 1.3–4.9) (). Median PFS was significantly longer in patients with SCT than in patients without SCT: 20.6 months (95% CI: 13.2–31.1) vs. 7.5 months (95% CI: 4.6–11.6; p = 0.0071). Median PFS following SCT ranged from 11.8 months (95% CI: 6.5–31.1) in the Middle East and South Africa region (n = 99) to 31.9 months (95% CI: 4.4–NR) in Russia (n = 17) and was not significantly different across regions (p = 0.8117). Furthermore, compared with patients without SCT, patients with SCT were younger (mean age: 31.1 years vs. 35.7 years), with a larger proportion having more favorable Josting scores (SCT: 0 [14.4%], 1 [44.4%], 2 [33.3%], and 3 [7.8%] vs. non-SCT: 0 [13.7%], 1 [39.0%], 2 [37.9%], and 3 [9.3%]). The median OS, measured from the time of the first relapse, was not reached in patients undergoing SCT compared with 54.8 months (95% CI: 27.3–NR) patients without SCT (p < 0.0001).
Table 3. Clinical outcomes in the RRHL group.
Exploratory analysis in patients with initial RRHL-only diagnosis showed that the median PFS (data available for 103/105 patients) was 22.7 months (95% CI: 11.5–52.4). PFS rates at 1, 3, and 5 years were 59.7, 43.7, and 38.0%, respectively.
Safety outcomes in the RRHL group
In total, 113/426 (26.5%) and 114/374 (30.5%) patients in the RRHL group experienced at least one treatment-related AE during frontline and first salvage treatments, respectively. Serious AEs (SAEs) were reported in 103/426 (24.2%) patients. The most common SAEs were febrile neutropenia (5.9%) and pyrexia (3.8%).
Baseline characteristics, treatment pathways, and clinical outcomes in the frontline cHL group
In the frontline cHL group, the median age was 33.0 years (range: 18.0–90.0; ranging from 28.0 years [range: 18.0–66.0] in Russia [n = 147] to 42.5 years [range: 19.0–83.0] in Australia [n = 52]), 55.0% of patients were male, and the most common ethnicity was Asian (35.0%) followed by White/Caucasian (32.9%) (). The prevalence of stages III and IV disease at presentation were similar (35.3 and 37.2%, respectively). Overall, 27.0% of patients with frontline cHL had a poor baseline International Prognostic Score (IPS). All patients in the frontline cHL group received chemotherapy. The most common frontline regimen was doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD; 85.3%) in all regions except Russia, where bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone (BEACOPP) was the most common regimen (38.1%). In all other regions, BEACOPP was the second most common frontline regimen (overall: 6.5%). Radiotherapy was administered as part of frontline treatment in 22.3% of patients.
Table 4. Baseline characteristics, treatment pathways, and clinical outcomes in the frontline cHL group.
Median PFS was not reached and CR was achieved in 60.6% of patients following frontline treatment, and the median time to response (CR or PR) following frontline treatment was 3.9 months (IQR: 2.6–5.6). The 5-year OS rate was 84.7% (95% CI: 82.8–86.5), ranging from 79.4% (95% CI: 74.2–83.7) in Latin America to 93.4% (95% CI: 87.6–96.5) in Russia. In an adjusted multivariate analysis model, only the following were identified as statistically significant factors associated with an increased risk of disease progression, relapse, or death: White/Caucasian ethnicity (HR: 1.5; 95% CI: 1.1–2.0; p = 0.0044); Other ethnicity, defined as Black or African American, American Indian or Alaska Native, Native Hawaiian or another Pacific Islander, or Other (HR: 1.5; 95% CI: 1.1–2.0; p = 0.0070); WBC count ≥15 × 109/L at diagnosis (HR: 1.4; 95% CI: 1.1–1.8; p = 0.0222); and age at cHL diagnosis (HR: 1.0; 95% CI: 1.0–1.0; p = 0.0009). In an adjusted model of HRs for PFS from initiation of frontline treatment, where the IPS score was included as a covariate (instead of all 7 components of the IPS), factors significantly associated with a lower risk of disease progression, relapse, or death included IPS-fair risk (HR: 0.7; 95% CI: 0.5–0.8) and IPS-good risk (HR: 0.6; 95% CI: 0.4–0.8) (Supplementary Table 3).
Discussion
The B-HOLISTIC study is the largest observational study assessing treatment pathways and long-term clinical outcomes in patients with HL in East Asia, Latin America, the Middle East and South Africa, Australia, and Russia. Considering the paucity of long-term clinical data in HL, particularly in regions outside Europe and North America, the present study contributes additional and relevant real-world data to the literature.
The salvage chemotherapy regimens used in the current study (ESHAP, DHAP, and ICE) were generally consistent with those reported in other real-world studies in RRHL from developed [Citation9,Citation18] and developing regions [Citation20,Citation23] around the time of this study. To improve outcomes in RRHL, novel agents and their combinations as salvage regimens have been increasingly used, especially in developed regions [Citation15,Citation16,Citation18,Citation19]. Real-world evidence suggests that, despite the advancement in treatment options, some developing regions still rely heavily on conventional salvage regimens for RRHL due to limitations associated with the availability, costs, and access to novel targeted therapies [Citation10,Citation20,Citation23]. Given that the European Society for Medical Oncology and National Comprehensive Cancer Network guidelines do not recommend any specific salvage regimen for RRHL [Citation6,Citation7], and given the existing limitations with modern diagnostic techniques and novel agents in resource-limited settings [Citation10,Citation11,Citation20,Citation23], our study findings can help guide salvage treatment choices in RRHL in these developing regions.
In our RRHL cohort, the overall proportion of PET or PET-CT scans performed at baseline was low (32.8%) because the now-standard practice of PET-guided treatment was not common during 2010–2013. The low proportion of PET or PET-CT-guided treatment for RRHL still holds true for some developing regions with limited availability of PET-CTs [Citation10,Citation11,Citation24].
The clinical outcomes of patients with RRHL in the current study were suboptimal compared with those reported in European and North American real-world studies conducted during a similar period [Citation9,Citation18,Citation19,Citation25]. This difference may be partly attributed to the lower use of novel agents in salvage settings in the current study. Additionally, the proportion of patients with refractory disease (41.5%) was higher than that reported in other studies (15%) [Citation26–28]. This may have contributed to higher treatment resistance and inferior clinical outcomes. However, the 5-year PFS rate (33.9%) was in line with that reported in real-world studies in developing regions [Citation11,Citation21]. The risk of disease progression in RRHL was significantly associated with the hemoglobin level at the time of RRHL diagnosis and inadequate response to salvage chemotherapy, both of which have been previously reported as prognostic factors in RRHL patients [Citation29,Citation30]. Real-world clinical outcomes from regions outside Europe and North America are expected to be less favorable than outcomes in randomized controlled trials in Western countries where conventional and novel therapies and more advanced diagnostic tools are easily accessible. Nevertheless, they provide a benchmark for comparing outcomes in clinical practice.
We observed a significant improvement in PFS for patients with RRHL who underwent SCT compared with those who did not undergo SCT, which concurs with other real-world studies [Citation21,Citation25,Citation31]. SCT following salvage chemotherapy is the current standard of care for patients with RRHL [Citation6,Citation7] and was undertaken in 52.1% of patients in the RRHL cohort of this study. Although the proportion of SCT was consistent with that in developed countries (44%) [Citation16], the clinical outcomes with SCT were less optimal compared with those from developed regions [Citation16,Citation26,Citation31]. The most important parameter predicting SCT outcomes is the pre-SCT complete metabolic response rate based on PET/PET-CT imaging [Citation32,Citation33], which was achieved in only 49% of patients with RRHL in this study. Prior evidence suggests that the 4-year event-free survival rates were better in transplanted patients with negative (77%) vs. positive (33%) functional imaging [Citation34]. Accordingly, the low pre-SCT response rates are likely to have contributed to the lower PFS rates post-SCT in this study. Additionally, the delay in undergoing SCT due to limitations with its access in some of the developing countries could have influenced the suboptimal post-SCT outcomes.
The most common treatment regimens in the frontline cHL group were ABVD and BEACOPP, which is in line with treatment recommendations for cHL [Citation6,Citation7]. The 5-year PFS and OS rates (65.0 and 84.7%, respectively) were largely comparable to those reported in real-world studies of patients with advanced-stage cHL in Europe and North America [Citation35,Citation36] and in developing regions [Citation10,Citation22]. With Asian ethnicity as a reference, White/Caucasian and Other ethnicities were statistically associated with a longer time-to-first event from initiation of frontline treatment. However, this likely reflects socioeconomic differences and access to healthcare rather than observed ethnic differences.
This study has several limitations due to its retrospective nature. The data set spans from 2010 to 2013 and may not be applicable to the current HL treatment landscape, especially in developed regions. However, this study evaluated a large body of patients from several regions hereto underrepresented in the literature. The patients enrolled were predominantly from specialized treatment centers with the necessary resources to undertake clinical trials. Therefore, the results may not reflect the entire patient population in a certain region. Selection bias due to the retention of patients with minimal data was unavoidable. Clinical outcomes were based on the study investigator’s assessment, without specific criteria provided. Country- and institution-specific differences in the choice of initial and subsequent therapies may have influenced clinical outcomes, making it difficult to generalize results. Additionally, information on the relative dose intensities (RDIs) for different chemotherapy regimens was not collected, which could have influenced overall clinical outcomes. However, current evidence in HL suggests that reduced RDI does not significantly impact outcomes [Citation37], and generally RDI <85% is considered an issue in patients aged >60 years [Citation38]. In our study, most patients were <60 years old. Additionally, this study does not address the delays from diagnosis to treatment initiation resulting from different referral patterns that may have influenced the poor clinical outcomes. However, limited evidence suggests that the diagnosis to treatment interval is not a significant prognostic factor for HL [Citation39]. The AE data presented here may only include actively managed SAEs and AEs recorded in the medical records, and this may not include common or expected AEs not formally recorded, such as alopecia or nausea. Lastly, although this study focused on developing regions, some developed countries like Australia and South Korea are included, which may have influenced the overall results.
In conclusion, PFS rates for RRHL patients from the developing regions in this study are lower than those reported in real-world studies from Europe and North America. Recent evidence has shown that using novel agents in earlier treatment lines has clinical benefits in HL patients [Citation15,Citation18,Citation19]. The results among high-risk RRHL patients emphasize the importance of considering the use of novel targeted therapies in salvage settings as well as timely SCT. However, given that the management of patients with HL has changed dramatically since 2013, further investigation into diagnostic modalities and treatment pathways in developing regions is needed.
Author contributions
All authors had access to and verified all the data reported in this manuscript and accept responsibility for this publication. All authors were involved in study design, methodology, data collection, and contributed equally toward analysis, interpretation of results, and manuscript development. All authors have reviewed and approved this manuscript for publication.
Acknowledgments
The authors would like to thank all patients, participating principal and co-investigators, and study-site staff for their contributions to the B-HOLISTIC study, with special mention to the principal investigators from the participating centers: Saad Akhtar, King Faisal Specialist Hospital and Research Center, Saudi Arabia; Sevgi Besisik, Istanbul University Medical Faculty, Turkey; Alvaro Hernandez, Unidad Médica de Alta Especialidad, Dr. Antonio Fraga Mouret, Centro Médico La Raza, IMSS, Mexico; Muhit Ozcan, Ankara University Medical Faculty, Turkey; Irina Kryuchkova, Federal State Budgetary Scientific Institution Research Institute of Fundamental and Clinical Immunology, Russia; Can Boga, Baskent University Adana Application and Research Center, Turkey; Mehmet Turgut, Ondokuz Mayis University Medical Faculty, Turkey; Mohsen Al Zahrani, National Guard Hospital–King Abdulaziz Medical City, Saudi Arabia; Dorotea Fantl, Hospital Italiano de Buenos Aires, Argentina; Ruben Salazar, Clinica De Oncologia Astorga, Colombia; Chien-Yuan Chen, National Taiwan University Hospital, Taiwan; Kenny Galvez, Hospital Pablo Tobón Uribe, Colombia; Moosa Patel, Chris Hani Baragwanath Hospital, South Africa; Seok-Goo Cho, The Catholic University of Korea, Seoul St. Mary’s Hospital, South Korea; and Juan Antonio Flores Jimenez, Centro de Investigación Farmacéutica Especializada de Occidente, S.C, Mexico. We would also like to acknowledge the continuous support of Frances Quek, who was the Clinical Study Manager at Takeda Pharmaceuticals during the time of this study. Data analysis was provided by IQVIA, and editorial assistance was provided by Brad Dalton BAppSc, MSc, and Sandra Kurian, MPharm, of Synergy Vision Ltd (London, UK), with funding from Takeda Pharmaceuticals International AG–Singapore Branch.
Disclosure statement
Burhan Ferhanoglu serves as a member of a board of directors or advisory committee of Takeda, Roche, AbbVie, and Janssen. Tae Min Kim has consulting and advisory roles for AstraZeneca, Boryung, Hanmi, Novartis, Takeda, Sanofi, and Roche/Genentech; receives a grant from AstraZeneca-KHIDI outside this work; and has received consulting fees from AstraZeneca, Hanmi, Novartis, and Takeda. Amado Karduss has received honoraria from Takeda, Amgen, and Janssen, and serves as a member of a board of directors or advisory committee of Novartis. David Brittain has received honoraria from Takeda, Roche, AbbVie, and Janssen, and serves as a member of a board of directors or advisory committee for Takeda. Gayane Tumyan and Mubarak Al-mansour serve as members of a board of directors or advisory committee for Takeda. Marta Zerga has received conference fees from Bristol Myers Squibb, Janssen, Roche, and Takeda; has received support for attending meetings from Sandoz, Amgen, AbbVie, Teva, and Pint Pharma; has received honoraria from Takeda, AbbVie, AstraZeneca, Teva, and Sandoz; and has participated on the advisory board for Takeda, Janssen, and Merck. Yuqin Song has received conference fees from Takeda and has participated on the advisory boards for Roche, Takeda, and Janssen. Silvia Rivas-Vera has received honoraria from Roche and serves on the advisory board for Takeda. Yok Lam Kwong serves as a consultant and has received honoraria or research funding from Amgen, Astellas, Bayer, BeiGene, Bristol Myers Squibb, Celgene, Gilead, Janssen, Merck, Novartis, Roche, and Takeda. Soon Thye Lim's institution has received honoraria from Takeda. Su-Peng Yeh has received honoraria from AbbVie, Amgen, Janssen, Astellas, AstraZeneca, Novartis, Sanofi, Roche, Bristol Myers Squibb, and Takeda, and has served in an advisory role for AbbVie, Amgen, Janssen, Astellas, Astex, Novartis, Sanofi, and Takeda. Arif Abdillah was an employee of Takeda Pharmaceuticals during the time of this study and is currently affiliated with GlaxoSmithKline Pte Limited. Zhongwen Huang is an employee of Takeda Pharmaceuticals and holds shares in the company. Hui Wan was an employee of Takeda Pharmaceuticals at the time of the study and is currently employed with Cellular Biomedicine Group. Mehul Dalal is an employee of Takeda Pharmaceuticals and owns equity in the company. Mark Hertzberg has received honoraria, consulting fees, and serves as a member of a board of directors or advisory committee of Takeda, Roche, BMS, Gilead, and MSD.
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article will be made available within 3 months from the initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Additional information
Funding
References
- Salati M, Cesaretti M, Macchia M, et al. Epidemiological overview of Hodgkin lymphoma across the Mediterranean basin. Mediterr J Hematol Infect Dis. 2014;6(1):e2014048.
- Evens AM, Antillón M, Aschebrook-Kilfoy B, et al. Racial disparities in Hodgkin’s lymphoma: a comprehensive population-based analysis. Ann Oncol. 2012;23(8):2128–2137.
- Shenoy P, Maggioncalda A, Malik N, et al. Incidence patterns and outcomes for Hodgkin lymphoma patients in the United States. Adv Hematol. 2011;2011:1–11.
- Hoppe RT, Advani RH, Ai WZ, et al. NCCN guidelines insights: Hodgkin lymphoma, version 1.2018. J Natl Compr Canc Netw. 2018;16(3):245–254.
- Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–4554.
- Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv19–iv29.
- Hoppe R, Advani R, Ai W, et al. Hodgkin lymphoma, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(6):755–781.
- Moskowitz AJ, Perales M-A, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146(2):158–163.
- Bair SM, Strelec L, Nagle SJ, et al. Outcomes of patients with relapsed/refractory Hodgkin lymphoma progressing after autologous stem cell transplant in the current era of novel therapeutics: a retrospective analysis. Am J Hematol. 2017;92(9):879–884.
- Hewamana S, Kandabadage L, Skandarajah T, et al. Applicability of protocols from high-income countries in a resource limited setting; real world data of histopathology, clinical features and long-term outcome of Hodgkin lymphoma in Sri Lanka. eClinicalMedicine. 2021;38:100998.
- Jaime-Pérez JC, Gamboa-Alonso CM, Padilla-Medina JR, et al. High frequency of primary refractory disease and low progression-free survival rate of Hodgkin’s lymphoma: a decade of experience in a Latin American center. Rev Bras Hematol Hemoter. 2017;39(4):325–330.
- Maddi RN, Linga VG, Iyer KK, et al. Clinical profile and outcome of adult Hodgkin lymphoma: experience from a tertiary care institution. Indian J Med Paediatr Oncol. 2015;36(4):255–260.
- Price AJ, Ndom P, Atenguena E, et al. Cancer care challenges in developing countries. Cancer. 2012;118(14):3627–3635.
- Arakelyan J, Movsisyan A, Sargsyan L, et al. Incidence patterns and review of Hodgkin lymphoma in the Republic of Armenia. Ecancermedicalscience. 2021;15:1319.
- Bair SM, Strelec LE, Feldman TA, et al. Outcomes and toxicities of programmed death-1 (PD-1) inhibitors in Hodgkin lymphoma patients in the United States: a real-world, multicenter retrospective analysis. Oncologist. 2019;24(7):955–962.
- Collins GP, Rueda A, Salles G, et al. Management of Hodgkin lymphoma in the era of brentuximab vedotin: real-world data from five European countries. Leuk Lymphoma. 2018;59(9):2113–2120.
- Driessen J, Visser O, Zijlstra JM, et al. Primary therapy and relative survival in classical Hodgkin lymphoma: a nationwide population-based study in The Netherlands, 1989–2017. Leukemia. 2021;35(2):494–505.
- Král Z, Michalka J, Móciková H, et al. Treatment of relapsed/refractory Hodgkin lymphoma: real-world data from the Czech Republic and Slovakia. J Cancer. 2019;10(21):5041–5048.
- Zagadailov EA, Corman S, Chirikov V, et al. Real-world effectiveness of brentuximab vedotin versus physicians’ choice chemotherapy in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplantation in the United Kingdom and Germany. Leuk Lymphoma. 2018;59(6):1413–1419.
- Bhurani D, Nair R, Rajappa S, et al. Real-world outcomes of Hodgkin lymphoma: a multi-centric registry from India. Front Oncol. Front Oncol. 2022 Feb 11;11:799948.
- Ramirez P, Ocqueteau M, Rodriguez A, et al. Outcomes in relapsed Hodgkin’s lymphoma treated with autologous and allogeneic hematopoietic cell transplantation at the Pontificia Universidad Católica de Chile. Rev Bras Hematol Hemoter. 2015;37(3):184–189.
- Shamoon RP, Ali MD, Shabila NP. Overview and outcome of Hodgkin’s lymphoma: experience of a single developing country’s oncology centre. PLOS One. 2018;13(4):e0195629.
- Sánchez-Valledor LF, Habermann TM, Murrieta-Alvarez I, et al. Long-term results of the treatment of Hodgkin’s lymphoma in a resource-constrained setting: real-world data from a single center. World J Clin Oncol. 2021;12(9):800–807.
- Global atlas of medical devices. Geneva: World Health Organization; 2017 [cited 2022 Mar 15]. Licence: CC BY-NC-SA 3.0 IGO. Available from: https://apps.who.int/iris/handle/10665/255181.
- Fernández de Larrea C, Martínez C, Gaya A, et al. Salvage chemotherapy with alternating MINE & ESHAP regimen in relapsed or refractory Hodgkin’s lymphoma followed by autologous stem-cell transplantation. Ann Oncol. 2010;21(6):1211–1216.
- Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12(10):1065–1072.
- Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14(10):943–952.
- Thomas RK, Re D, Zander T, et al. Epidemiology and etiology of Hodgkin’s lymphoma. Ann Oncol. 2002;13:147–152.
- Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Société Française de Greffe de Moëlle. Bone Marrow Transplant. 1997;20(1):21–26.
- Cuccaro A, Bartolomei F, Cupelli E, et al. Prognostic factors in Hodgkin lymphoma. Mediterr J Hematol Infect Dis. 2014;6(1):e2014053.
- Veilleux O, Claveau J-S, Alaoui H, et al. Real-World outcomes of autologous and allogeneic hematopoietic stem cell transplantation for relapsed/refractory Hodgkin lymphoma in the era of novel therapies: a Canadian perspective. Transplantation and cellular therapy. Transplant Cell Ther. 2022;28(3):145–151.
- Devillier R, Coso D, Castagna L, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin’s lymphoma responding to prior salvage therapy. Haematologica. 2012;97(7):1073–1079.
- Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–1670.
- Moskowitz CH, Yahalom J, Zelenetz AD, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148(6):890–897.
- Andre MPE, Carde P, Viviani S, et al. Long-term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: a pooled analysis of four randomized trials. Cancer Med. 2020;9(18):6565–6575.
- Guru Murthy GS, Szabo A, Hamadani M, et al. Contemporary outcomes for advanced-stage classical Hodgkin lymphoma in the U.S.: analysis of surveillance, epidemiology, and end results database. Oncologist. 2019;24(11):1488–1495.
- Bowhay-Carnes E, Fountzilas C, Janania Martinez M, et al. Chemotherapy relative dose intensity and therapy efficacy in Hodgkin and non-Hodgkin lymphoma: the effect of dose reductions and delays on cure. Blood. 2015;126(23):5092–5092.
- Böll B, Görgen H, Fuchs M, et al. ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin study group HD10 and HD11 trials. J Clin Oncol. 2013;31(12):1522–1529.
- Brooks EG, Connors JM, Sehn LH, et al. Impact of time from diagnosis to initiation of curative-intent chemotherapy on clinical outcomes in patients with classical Hodgkin lymphoma. Leuk Lymphoma. 2016;57(4):872–879.