ABSTRACTS
We explored the incidence of Epstein-Barr virus (EBV) and cytomegalovirus (CMV) infections in 131 patients with multiple myeloma (MM), 53 of whom received daratumumab (Dara) treatments. The Dara group had more RRMM patients than the group without Dara. CMV infection was significantly more common in patients treated with Dara (16.98%) than in patients treated with regimens without Dara (2.56%). During Dara treatments, 24.53% of patients developed CMV and/or EBV infections. Patients who developed infections had significantly lower levels of albumin and lymphocytes in their peripheral blood. The median time from the first Dara infusion to infection was 27 days. We observed NK cell depletion and T cell expansion during Dara-treatment. Patients with CMV and/or EBV infections had significantly lower numbers of NK cells, total T cells, and CD8 + T cells at 1 month, and lower numbers of CD8 + T cells at 2 months after the first Dara infusion than those without infections.
Introduction
Multiple myeloma (MM) is a malignant hematological disease that is characterized by the clonal proliferation of malignant plasma cells in the bone marrow (BM) microenvironment [Citation1]. The approval of proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs) has improved disease control and extended overall survival, while MM remains an incurable disease. Many novel targeted treatments have been developed in recent years, including anti-CD38 monoclonal antibodies (e.g. daratumumab), selective inhibitors of nuclear export (SINE), histone deacetylase (HDAC) inhibitors, BCL-2 inhibitors, and so on [Citation2].
Daratumumab (Dara) is a human immunoglobulin G1 k monoclonal antibody targeting CD38 [Citation3]. CD38 is relatively highly expressed in hematopoietic tissues, with plasma cells, NK cells, and B cells being the most abundant in humans [Citation4]. Dara has been widely used in MM treatment, either as monotherapy or in combination with other drugs, with satisfactory effects [Citation5–7]. Dara has several antitumor mechanisms, including Fc-dependent immune effector pathways (CDC, ADCC, and ADCP), direct effects, and immunomodulatory effects [Citation8].
It was observed that patients treated with Dara-containing regimens had a relatively high rate of infections [Citation9,Citation10]. Infection is a common complication and the leading cause of death in MM patients [Citation11]. The risk of infection in MM patients was appropriately 7-fold higher than in controls [Citation12,Citation13]. Several cases have been reported of Epstein-Barr virus (EBV), cytomegalovirus (CMV), other herpes viruses, and hepatitis B infection and reactivation during Dara-containing regimens [Citation14–17]. This research included 131 patients with MM, 53 of whom were treated with Dara-containing regimens) and 78 of whom were treated with classical regimens for MM and reviewed 13 cases of MM patients who developed EBV and/or CMV infections during Dara-containing treatments.
Methods
Patients
This retrospective study involved 131 patients with MM, including 78 patients who were treated with classical regimens and 53 patients who were treated with Dara-containing regimens), at the First Affiliated Hospital of Zhejiang University from December 2018 to May 2022. The diagnosis of MM was following the International Myeloma Working Group criteria. Patients were assessed in staging using International Staging System (ISS). This study protocol was approved by the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Treatments
78 MM patients received classical combination therapy, including bortezomib plus lenalidomide plus dexamethasone (VRD), bortezomib plus cyclophosphamide plus dexamethasone (VCD), bortezomib plus doxorubicin plus dexamethasone (VAD), and bortezomib plus pomalidomide plus dexamethasone (VPD). 53 patients received Dara-based combination therapy, including Dara plus dexamethasone (DD) and triplet combinations of DD with bortezomib (DVD), pomalidomide (DPD), or Selinexor, and quadruple combinations of Dara plus VRD (Dara + VRD), Dara plus VCD (Dara + VCD), Dara plus VPD (Dara + VPD), or Dara plus bortezomib plus melphalan plus steroids (Dara + VMP). One cycle lasted 28 days. Patients received Dara 16 mg/kg intravenously weekly in cycles 1 and 2 and then every 2 weeks in cycles 3 to 6 and every 4 weeks thereafter.
CMV-DNA and EBV-DNA monitoring
The CMV-DNA and EBV-DNA loads in the blood were measured regularly using real-time quantitative polymerase chain reaction. The threshold for EBV-DNA and CMV-DNA copies in the blood provided by the manufacturer (ZJ Bio-Tech, Shanghai, China) was <500 copies/ml. The CMV-DNA and EBV-DNA loads in the blood were measured before the initiation of treatments and at each administration. All patients had undetectable CMV-DNA and EBV-DNA in their blood before the initiation of treatments.
Flow cytometry analysis
The phenotypic characteristics of the lymphocytes (CD4+ and CD8+ T-cells, as well as B-cells and NK cells) were detected in samples of EDTA anticoagulated peripheral blood (5 mL) from patients with MM prior to treatment initiation and at each administration. The cells were analyzed using multiple-color flow cytometry (BD FACS Canto II) with CD3/CD16 + 56/CD45/CD4/CD19/CD8 TruC. The gating strategy of CD4+ T cells, CD8+ T cells, B cells, and NK cells were respectively executed as CD3+CD4+, CD3+CD8+, CD3-CD19+, and CD3-CD56+.
Statistical analysis
The software SPSS and Graphpad Prism were used for data analysis and data visualization. Continuous variables were expressed as median and range, and qualitative variables were summarized in terms of frequencies. The Fisher’s exact test and the chi-squared test for categorical variables, and the Mann-Whitney U test for continuous variables were used to compare patients and disease-related factors between groups. All probability values were two-sided, and statistical significance was indicated by a p value of 0.05.
Results
Clinical features of MM patients and the prevalence of infections in them
This study included 131 MM patients, 53 of whom were treated with Dara-containing regimens and 78 of whom were treated with classical regimens (VCD, VRD, VPD, or VAD) (). There were more RRMM patients in the Dara group than in the classical group. The median duration of follow-up was 13 months (range: 1–48 months). 13 (24.53%) patients developed CMV and/or EBV infections during Dara-containing treatments, whereas 7 (8.97%) patients developed CMV and/or EBV infections during classical treatments (p = 0.015). CMV infection was significantly more common in MM patients treated with Dara-containing regimens than in MM patients treated with classical regimens without Dara (9 (16.98%) vs. 2 (2.56%), p = 0.007). The incidence of EBV infection was comparable in MM patients treated with Dara-containing regimens and MM patients treated with classical regimens (6 (11.32%) vs. 6 (7.69%), p = 0.480). According to these findings, Dara might increase the incidence of CMV infection but not the incidence of EBV infection in MM patients.
Table 1. Baseline characteristics of patients with multiple myeloma.
When comparing the clinical characteristics of MM patients treated with Dara who developed viraemia or not, we observed that the levels of albumin (29.40 (23.00–37.50) vs. 39.05 (24.00–48.10), p < 0.001) and absolute number of lymphocytes in peripheral blood (PB) (0.68 (0.10–1.15) vs. 1.01 (0.42–3.53), p = 0.002) were significantly lower in patients with CMV and/or EBV infections (). No significant differences were observed in age, sex, disease type, disease relapse, ISS stage, beta 2 microglobulins (β2-MG), the ratio of free light chain (FLC), serum M protein, hemoglobin, absolute neutrophil counts (ANC), and platelets. Moreover, no differences were observed in high-risk cytogenetic changes, lines of therapy, prior treatments (drug treatments and ASCT), and combined drugs with Dara.
Table 2. Baseline characteristics of patients treated with daratumumab-containing regimens.
Features of viral infections, treatments, and outcomes
Prophylaxis for Herpes Zoster Virus (VZV) (famciclovir, po, 0.25 g tid) was regularly used before and during the treatment of Dara as directed by the prescribing information, while prophylaxis for CMV was not used. After starting Dara-containing regimens, single infections of EBV occurred in 4/13 patients, single infections of CMV occurred in 7/13 patients, and dual infections of EBV and CMV occurred in 2/13 patients (). The median time between the diagnosis of EBV and/or CMV infections and the start of Dara treatment was 27 days (ranging from 8 to 115). The median time between infections diagnosis and initial Dara treatment was 27 days (range: 14–82) for CMV infection, and 29.5 days (ranging from 8–115) for EBV infection.
Table 3. Clinical characteristics of patients with EBV and/or CMV infections during daratumumab-containing regimens.
Table 4. Characteristics, treatments, and outcomes of the EBV/CMV infections during daratumumab treatment.
The main symptom of EBV/CMV infection was persistent and repeated fever that was difficult to control with empiric antibiotics. We excluded bacterial infection, fungal infection, and other virus infections before the diagnosis. The median level of EBV-DNA at the time of the first detection was 3.48 × 103 copies/ml. The median level of CMV-DNA at the time of the first detection was 4.36 × 103 copies/ml. Other symptoms such as neutropenia and/or thrombocytopenia were hard to evaluate because of the primary disease. There was no EBV-related posttransplant lymphoproliferative disorder (PTLD) in patients who had EBV infection by the last day of follow-up.
7 cases with CMV infections were treated with antiviral drugs (ganciclovir, 300 mg q12h intravenous for 2 weeks; 500 mg tid po until CMV-DNA turned negative on 2 consecutive measurements) in combination with IVIG (400 mg/kg qd 5 days; 10 g biw maintenance until 1 week after DNA undetectable). One patient with dual infection of EBV and CMV received only IVIG treatment. Another patient with dual infection of EBV and CMV neither received IVIG nor Ganciclovir treatment, instead receiving only empirical antibiotics and expectant treatment (such as antipyretic). There is currently no effective antiviral drug approved for the treatment of EBV infections. 4 patients with single EBV infections were treated with IVIG (400 mg/kg qd 5 days; 10 g biw maintenance until 1 week after DNA undetectable). The median duration of viremia was 34 days (range: 12–180) for CMV infection and 103.50 days (range: 16–180) for EBV infection, respectively.
Correlation between viral activation and subpopulations of lymphocytes
As mentioned above, the absolute number of lymphocytes in PB at baseline was significantly lower in patients with CMV and/or EBV infections. Therefore, we further analyzed the subpopulation of lymphocytes in PBMCs from patients treated with Dara-containing regimens (n = 32, 2 with single EBV infection, 6 with single CMV infection, 1 with dual infection, and 23 without infection) and those with classical regimens (n = 50, 4 with single EBV infection, 1 with single CMV infection, 1 with dual infection, and 44 without infection) (. CD56+NK cell depletion and T cell (especially CD8+T cells) expansion were observed in cases after the treatment of Dara, which were not observed in MM patients treated with classical regimens, while B cell depletion was observed in both groups. Furthermore, the level of CD8+T cell progressively increased through the Dara-containing treatment, and surpassed the level of CD8+T cell in the classical-treatment group since about 1 months after the first dose of Dara.
Figure 1. Longitudinal monitoring of lymphocytes and neutrophils in MM patients treated with classical regimens or Dara-containing regimens. A) B cell depletion, NK cell depletion and T cell expansion (particularly CD8 + T cell) were observed in MM patients treated with Dara-containing regimens, which were not observed in MM patients treated with classical regimens. B) Comparison of lymphocyte subpopulations at different time points in the course of daratumumab treatment between MM patients with infections and MM patients without infections. C) The levels of ANC in MM patients in the two groups were comparable in the process of Dara treatments.
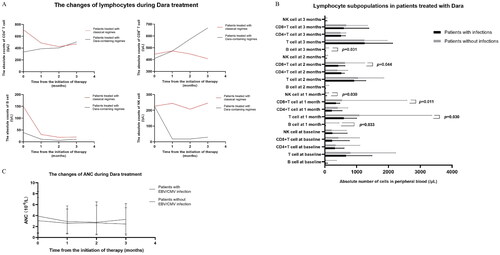
Moreover, in longitudinal analysis of lymphocytes subpopulations (, the absolute cell number in PB (/μL) in patients who developed EBV/CMV infection before Dara administration was 317.00 (72.00–604.00) for CD4 + T cell, 229.00 (92.00–771.00) for CD8 + T cell, 28.00 (4.00–82.00) for B cell and 237.00 (11.00–700.00) for NK cell, respectively, while the absolute cell number when the infection was detected was 453.00 (195.00–633.00) for CD4 + T cell, 429.50 (349.00–495.00) for CD8 + T cell, 6.00 (2.00–23.00) for B cell and 16.50 (6.00–66.00) for NK cell, respectively. Furthermore, we observed that the absolute numbers of CD56+NK cells (12.00 (5.00–55.00) vs. 45.00 (9.00–121.00), p = 0.030), total T cells (597.00 (332.00–997.00) vs. 1083.50 (197.00–3357.00), p = 0.030), CD8+T cells (349.00 (102.00–472.00) vs. 539.50 (114.00–2576.00), p = 0.011) and B cells (3.00 (1.00–23.00) vs. 16.50 (1.00–448.00), p = 0.033) at 1 month after the first Dara infusion were significantly lower in patients with infections, compared with patients without infections. At 2 months after the first Dara infusion, only the absolute numbers of CD8+T cells (426.00 (298.00–632.00) vs. 630.00 (194.00–1250.00), p = 0.044) were significantly lower in patients with infections, compared to patients without infections. At 3 months after the first Dara infusion, no significant difference was observed in T cell populations and NK cells between patients with or without infections, indicating a relatively delayed CD8+T cell expansion in patients with infections. Besides the change in lymphocytes, neutropenia was another common side effect in patients treated with Dara-containing regimens [Citation18–20]. We observed that the levels of ANC in MM patients in two groups were comparable in the process of Dara treatments (). Our findings indicated the potential involvement of CD56+NK cell depletion and delayed CD8+T cell expansion in the viral infections during Dara treatment, particularly in the early stage of treatment.
Discussion
Here, we investigated the prevalence of EBV and CMV infections in 131 patients with MM, and found that the incidence of CMV infection in MM patients treated with Dara was significantly higher than the incidence of CMV infection in MM patients treated with classical regimens without Dara. 24.53% of MM patients had CMV and/or EBV infections during Dara treatments. We reviewed the features of infection, treatments, and outcomes, and reported the first case encountered EBV infection treated with Dara in combination with Selinexor. Albumin and the absolute number of lymphocytes in PB were significantly lower in patients with infections. The median time between infections diagnosis and initial Dara treatment was 27 days for CMV infection and 29.5 days for EBV infection, respectively, which was consistent with the median time (30 days) of CMV reactivation from the initiation of Dara treatment in a prior study [Citation21]. In longitudinal monitored the subpopulations of lymphocytes in PB, we observed NK cell depletion and progressive CD8+T cell expansion after the treatment of Dara. Significant lower absolute numbers of NK cells and CD8+T cells at 1 month and absolute numbers of CD8+T cells at 2 months after the first Dara infusion was observed in patients with CMV and/or EBV infections.
The research result indicated that patients treated with Dara encountered higher infection risk than patients in the control group in clinical trials [Citation10]. A retrospective study including MM patients treated with multiple Dara-containing regimens (monotherapy, or combination therapies) indicated that infections occurred in 36.5% of included patients, and viruses accounted for the majority of infections in nearly all groups [Citation22]. Mechanisms underlying increased viral infections during Dara's treatment remain unknown, though there are some possible explanations.
A prior small-sample study indicated that the absolute lymphocyte counts after Dara treatment might influence the tendency of CMV infection [Citation17]. Dara-induced NK cell depletion could be one explanation. Total NK cells in PBMCs were rapidly reduced in a dose-dependent manner after the first Dara infusion, remained depleted, and showed partial recovery until month 3–6 [Citation23,Citation24]. The relationship between Dara-induced NK cell reduction and increasing infection risk remained controversial. A prior study observed that patients with infection had significantly lower absolute lymphocyte counts and CD56+ NK cells than patients who did not have infection [Citation22]. However, another in vitro study found that although ADCC induced by PBMCs in vitro was reduced after Dara treatment, there was no correlation between the NK-cell depletion and increased incidence of overall infections or VZV infection [Citation23]. These findings indicate that there are additional underlying mechanisms, such as compensation from other immune cells. Indeed, Dara promoted the expansion of helper and cytotoxic T cells, particularly cytotoxic T cells. It was observed that the absolute number of CD8 T cells and the CD8+/CD4+ T cell ratio significantly increased at week 8 after the initial infusion and progressively increased every 100 days. These cytotoxic T cells responded strongly to viral antigens which included CMV, EBV, and influenza peptides, and the virus-reactive subpopulation showed increasing proliferative capacity during Dara treatment [Citation25]. These hypotheses partially explained the result in this study that EBV/CMV activation mostly occurred in the early phase of Dara when NK depletion occurred while CD8+ T cell expansion was inapparent. Further surveillance of T cell subpopulation in PBMCs might have clinical benefits during the treatment of Dara.
Although no difference was observed in the agents that patients were treated prior to Dara treatments or in the combined drugs between patients with or without CMV or EBV infections after Dara treatments in our analysis, combined drug was still an important factor that influenced the incidence of EBV and CMV infections. Bortezomib was found to significantly increase the risk of CMV reactivation in CMV-seropositive patients with MM or AL amyloidosis, particularly during the first two cycles of treatment [Citation26]. Lenalidomide alone would not increase the risk of CMV reactivation in MM patients undergoing HSCT [Citation27], while the role of IMiDs in EBV reactivation was controversial. One research in vitro observed that IMiDs reactivated the EBV lytic cycle (pomalidomide > lenalidomide > thalidomide), and this function was enhanced when lenalidomide was combined with dexamethasone, rituximab, or melphalan [Citation28]. Selinexor is a novel selective inhibitor of nuclear export with no data yet showing its effects on viral infection/reactivation.
Herpesvirus accounted for the majority of viral infections during Dara treatment [Citation29]. Prescribing information noted that VZV infection was observed in 3%–5% of RRMM patients treated with Dara, and recommended prophylaxis for VZV during Dara treatment [Citation5–7]. However, no large-scale studies on the clinical benefits of CMV surveillance and prophylaxis during Dara treatment have been conducted. Further research comparing the incidence of CMV infection in patients treated with Dara with and without CMV-prophylaxis could find out an effective and specific regimen to reduce the incidence of CMV activation. There is no effective anti-viral drug for EBV. In recent years, several approaches including rituximab, EBV-specific T cells, and bortezomib have shown effects on EBV infection and related diseases. Rituximab is a monoclonal anti-CD20 antibody that has been shown to eliminate B cells, including those infected with EBV. Pre-transplant rituximab therapy significantly reduced the incidence of EBV primary infection and reactivation following stem cell and solid organ transplantation [Citation30–32]. Preemptive rituximab therapy induced significant decreases in EBV-DNA loads and reduced the incidence and mortality of EBV-related posttransplant lymphoproliferative disorders (PTLD) in patients who developed EBV viraemia after transplantation [Citation33–44]. Furthermore, rituximab treatment improved symptoms and decreased viral loads in patients with EBV-associated hemophagocytic lymphohistiocytosis [Citation45,Citation46]. However, it was observed that the levels of EBV-DNA load and EBV-positive B cells increased after several days or months from the rituximab therapy [Citation47,Citation48]. Rituximab could not eliminate EBV-infected CD20-CD19+ B cells and T/NK cells [Citation46,Citation47]. CD20- lymphoproliferative lesions developed in some patients with EBV infection even after rituximab therapy [Citation49]. Rituximab achieved short-term remissions but did not cure the disease, indicating that further research on novel drugs was needed [Citation50–52]. Although rituximab has been recommended for the preemptive therapy in patients who developed EBV infection or EBV-related PTLD after allogeneic hematopoietic stem cell transplantation by several guidelines [Citation50,Citation53], little previous research has focused on the rituximab treatment for EBV infection in MM patients who received active drug treatment. Because there was no patient acquired EBV-related PTLD during the follow-up, we did not use off-label rituximab, which could increase the economic burden and infection risk, to clear the virus in the MM patients in this study.
Our research has some limitations. Regimens containing PIs and/or IMiDs and steroids were the first-line therapy for MM in our institution, while Dara was always used as ≥2 lines of therapy for MM. The proportion of RRMM patients in the Dara group was significantly higher than those in the classical group, which might influence the difference in viral infection rates between two groups. Further research comparing the incidence of viral infection in NDMM patients treated with Dara versus classical regimens would provide strong evidence of the relationship between Dara-containing treatments and CMV infection. Due to the small sample size, we did not divide the patient into EBV group and CMV group, and we did not compare the characterization of each group. More research is needed to examine the incidence of EBV and CMV infections during Dara treatment in a larger cohort, as well as to identify the associated clinical factors in each group. Although the incidence of CMV and EBV infection after ASCT is lower than after allogeneic stem cell transplantation [Citation54,Citation55], CMV and EBV infection in the early stage after stem cell reinfusion was reported by several studies [Citation56,Citation57]. The time from ASCT to the start of Dara administration might be a potential factor that influences the viral infection during Dara treatment. Since there were only 2 patients who underwent ASCT before the start of Dara administration in the infection group in this study, the sample size was too small to compare the time from ASCT to infection between the infection group and the non-infection group. Further research is needed to explore this problem. Further research is also required to investigate the underlying mechanisms of EBV/CMV activation after Dara treatment, risk stratification of viral reactivation in MM patients treated with Dara, and the clinical benefits of CMV-prophylaxis. Further research would be valuable to explore the utility of rituximab in the treatment of EBV infection in MM patients, the influence of rituximab on the anti-tumor response, and the EBV-DNA levels threshold for preemptive therapy of rituximab in MM patients who received active drug treatment.
In conclusion, this study shows a considerable risk of CMV infection after Dara treatment in MM patients, particularly in the early phase after the initial infusion, indicating the importance of viral surveillance and prophylactic/preemptive therapy for CMV during the Dara treatment, and speculates potential involvements of NK cells depletion and delayed CD8+T cells expansion in these infections in early stage of treatment. Furthermore, our observations indicate that EBV and CMV-DNA monitoring and antiviral prophylaxis in the early stage of Dara treatment might benefit early detection of viral infections, reduce the incidence of viral infections, and lessen the adverse effects of viral infections. More research was needed to determine the clinical benefits of anti-CMV prophylaxis during Dara treatment.
Ethical approval
This study was conducted in compliance with the Declaration of Helsinki and was approved by the Institutional Review Board of Zhejiang University School of Medicine First Affiliated Hospital.
Patient consent statement
Written informed consent was given by all the included participants.
Author contributions
Shuchan Li: collected and analyzed the clinical data, wrote the manuscript; reviewed literature.
Xiaoyan Han: gave critical suggestion for the article, managed the patients, and critically revised the work.
Zhen Cai: supervised and formulated the article.
Gaofeng Zheng, Jingsong He, Wenjun Wu, Qingxiao Chen, Yang Yang, Donghua He, Yi Zhao: managed the patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Palumbo A, Anderson K. Multiple Myeloma. N Engl J Med. 2011;364(11):1046–1060.
- Bazarbachi AH, Al Hamed R, Malard F, et al. Relapsed refractory multiple myeloma: a comprehensive overview. Leukemia. 2019;33(10):2343–2357.
- Sanchez L, Wang Y, Siegel DS, et al. Daratumumab: a first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. 2016;9(1):51.
- Piedra-Quintero ZL, Wilson Z, Nava P, et al. CD38: an immunomodulatory molecule in inflammation and autoimmunity. Front Immunol. 2020;11(597959)
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766.
- Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560..
- van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29.
- Johnsrud A, Susanibar S, Jo Kamimoto J, et al. Infectious complications of daratumumab-containing therapy for multiple myeloma. Blood. 2017;130(Supplement 1):3148.
- Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–528.
- Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom medical research council trials between 1980 and 2002—medical research council adult leukaemia working party. J Clin Oncol. 2005;23(36):9219–9226.
- Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–113.
- Li L, Wang L. Multiple myeloma: what do We do about immunodeficiency? J Cancer. 2019;10(7):1675–1684.
- Ferreira LM, Cerezer JL, Gehrcke M. Do cytomegalovirus infections affect the daratumumab treatment course in multiple myeloma patients? – Literature review. Hematol Transfus Cell Ther. 2021;43(2):185–190.
- Frerichs KA, Bosman PWC, Nijhof IS, et al. Cytomegalovirus reactivation in a patient with extensively pretreated multiple myeloma during daratumumab treatment. Clin Lymphoma Myeloma Leuk. 2019;19(1):e9–e11.
- Lee SK, Sung PS, Park SS, et al. Reactivation of resolved hepatitis B after daratumumab for multiple myeloma. Clin Infect Dis. 2021;73(6):e1372–e5.
- Nakagawa R, Onishi Y, Kawajiri A, et al. Preemptive therapy for cytomegalovirus reactivation after daratumumab-containing treatment in patients with relapsed and refractory multiple myeloma. Ann Hematol. 2019;98(8):1999–2001.
- Facon T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066–1077.
- Sonneveld P, Chanan-Khan A, Weisel K, et al. Overall survival with daratumumab, bortezomib, and dexamethasone in previously treated multiple myeloma (CASTOR): a randomized, Open-Label, phase III trial. J Clin Oncol. 2022; JCO2102734.
- Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582–1596.
- Tabata R, Sato N, Yamauchi N, et al. Cytomegalovirus reactivation in patients with multiple myeloma administered daratumumab-combination regimens. 2022;101(2):467.
- Johnsrud AJ, Johnsrud JJ, Susanibar SA, et al. Infectious and immunological sequelae of daratumumab in multiple myeloma. Br J Haematol. 2019;185(1):187–189.
- Casneuf T, Xu XS, Adams HC, 3rd, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017;1(23):2105–2114.
- Pierceall WE, Amatangelo MD, Bahlis NJ, et al. Immunomodulation in pomalidomide, dexamethasone, and daratumumab-treated patients with relapsed/refractory multiple myeloma. Clin Cancer Res. 2020;26(22):5895–5902.
- Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394.
- Sharpley FA, De‐Silva D, Mahmood S, et al. Cytomegalovirus reactivation after bortezomib treatment for multiple myeloma and light chain amyloidosis. Eur J Haematol. 2020;104(3):230–235.
- Marchesi F, Mengarelli A, Giannotti F, et al. High incidence of post-transplant cytomegalovirus reactivations in myeloma patients undergoing autologous stem cell transplantation after treatment with bortezomib-based regimens: a survey from the rome transplant network. Transpl Infect Dis. 2014;16(1):158–164.
- Jones RJ, Iempridee T, Wang X, et al. Lenalidomide, thalidomide, and pomalidomide reactivate the Epstein-Barr virus lytic cycle through phosphoinositide 3-Kinase signaling and ikaros expression. Clin Cancer Res. 2016;22(19):4901–4912.
- Drgona L, Gudiol C, Lanini S, et al. ESCMID Study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (agents targeting lymphoid or myeloid cells surface antigens [II]: CD22, CD30, CD33, CD38, CD40, SLAMF-7 and CCR4). Clin Microbiol Infect. 2018;24(Suppl 2): S83–S94.
- Burns DM, Rana S, Martin E, et al. Greatly reduced risk of EBV reactivation in rituximab-experienced recipients of alemtuzumab-conditioned allogeneic HSCT. Bone Marrow Transplant. 2016;51(6):825–832.
- Wei X, Xie Y, Jiang R, et al. The impact of rituximab administered before transplantation in patients undergoing allogeneic hematopoietic stem cell transplantation: a real-world study. Front Immunol. 2022;13:967026.
- Schachtner T, Reinke P. Pretransplant prophylactic rituximab to prevent Epstein-Barr virus (EBV) viremia in EBV-seronegative kidney transplant recipients from EBV-seropositive donors: results of a pilot study. Transpl Infect Dis. 2016;18(6):881–888.
- Worth A, Conyers R, Cohen J, et al. Pre-emptive rituximab based on viraemia and T cell reconstitution: a highly effective strategy for the prevention of Epstein-Barr virus-associated lymphoproliferative disease following stem cell transplantation. Br J Haematol. 2011;155(3):377–385.
- Ahmad I, Cau NV, Kwan J, et al. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation. 2009;87(8):1240–1245.
- Coppoletta S, Tedone E, Galano B, et al. Rituximab treatment for Epstein-Barr virus DNAemia after alternative-donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):901–907.
- Liu Q, Xuan L, Liu H, et al. Molecular monitoring and stepwise preemptive therapy for Epstein-Barr virus viremia after allogeneic stem cell transplantation. Am J Hematol. 2013;88(7):550–555.
- Carpenter B, Haque T, Dimopoulou M, et al. Incidence and dynamics of Epstein-Barr virus reactivation after alemtuzumab-based conditioning for allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(5):564–570.
- van Esser JW, Niesters HG, van der Holt B, et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99(12):4364–4369.
- van der Velden WJ, Mori T, Stevens WB, et al. Reduced PTLD-related mortality in patients experiencing EBV infection following allo-SCT after the introduction of a protocol incorporating pre-emptive rituximab. Bone Marrow Transplant. 2013;48(11):1465–1471.
- Stocker N, Labopin M, Boussen I, et al. Pre-emptive rituximab treatment for Epstein-Barr virus reactivation after allogeneic hematopoietic stem cell transplantation is a worthwhile strategy in high-risk recipients: a comparative study for immune recovery and clinical outcomes. Bone Marrow Transplant. 2020;55(3):586–594.
- Savoldo B, Rooney CM, Quiros-Tejeira RE, et al. Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am J Transplant. 2005;5(3):566–572.
- Choquet S, Varnous S, Deback C, et al. Adapted treatment of Epstein-Barr virus infection to prevent posttransplant lymphoproliferative disorder after heart transplantation. Am J Transplant. 2014;14(4):857–866.
- Walti LN, Mugglin C, Sidler D, et al. Association of antiviral prophylaxis and rituximab use with posttransplant lymphoproliferative disorders (PTLDs): a nationwide cohort study. Am J Transplant. 2021;21(7):2532–2542.
- Martinez-Calle N, Alfonso A, Rifon J, et al. First-line use of rituximab correlates with increased overall survival in late post-transplant lymphoproliferative disorders: retrospective, single-Centre study. Eur J Haematol. 2017;98(1):38–43.
- Chellapandian D, Das R, Zelley K, et al. Treatment of Epstein Barr virus-induced haemophagocytic lymphohistiocytosis with rituximab-containing chemo-immunotherapeutic regimens. Br J Haematol. 2013;162(3):376–382.
- Meng G-Q, Wang J-S, Wang Y-N, et al. Rituximab-containing immuno-chemotherapy regimens are effective for the elimination of EBV for EBV-HLH with only and mainly B lymphocytes of EBV infection. Int Immunopharmacol. 2021;96:107606.
- Comoli P, Basso S, Zecca M, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007;7(6):1648–1655.
- Ohta K, Shimizu M, Nakai A, et al. Rituximab therapy for Epstein-Barr virus-related chronic hepatitis following living donor kidney transplantation. Am J Kidney Dis. 2006;48(6):986–989.
- Cohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117(22):5835–5849.
- Styczynski J, van der Velden W, Fox CP, et al. Management of Epstein-Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth european conference on infections in leukemia (ECIL-6) guidelines. Haematologica. 2016;101(7):803–811.
- Roddie C, Peggs KS. Immunotherapy for transplantation-associated viral infections. J Clin Invest. 2017;127(7):2513–2522.
- Yoshimori M, Shibayama H, Imadome KI, et al. Antineoplastic and anti-inflammatory effects of bortezomib on systemic chronic active EBV infection. Blood Adv. 2021;5(7):1805–1815.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238.
- Ferrari V, Cacere CR, Machado CM, et al. Distinct patterns of regeneration of central memory, effector memory and effector TCD8+ cell subsets after different hematopoietic cell transplant types: possible influence in the recovery of anti-cytomegalovirus immune response and risk for its reactivation. Clin Immunol. 2006;119(3):261–271.
- Czyzewski K, Dziedzic M, Salamonowicz M, et al. Epidemiology, outcome and risk factors analysis of viral infections in children and adolescents undergoing hematopoietic cell transplantation: antiviral drugs do not prevent Epstein-Barr virus reactivation. Infect Drug Resist. 2019;12:3893–3902.
- Rossini F, Terruzzi E, Cammarota S, et al. Cytomegalovirus infection after autologous stem cell transplantation: incidence and outcome in a group of patients undergoing a surveillance program. Transpl Infect Dis. 2005;7(3–4):122–125.
- Hussein AA, Al-Antary ET, Najjar R, et al. Incidence and risk factors for cytomegalovirus (CMV) reactivation following autologous hematopoietic stem cell transplantation in children. Pediatr Blood Cancer. 2015;62(6):1099–1101.
