Abstract
The symptoms of acute myeloid leukemia (AML) and its treatment can negatively impact patient functioning and quality of life. Through concept elicitation interviews, we sought to evaluate the experience of patients with AML in remission following hematopoietic stem cell transplant (HSCT). Thirty patients with AML in remission post-HSCT, and eight clinicians with experience treating such patients, were asked to identify symptoms and impacts associated with AML and/or its treatment. The findings were used to develop an AML conceptual disease model to reflect the experience of these patients. We identified five symptoms and six impacts that were salient to patients with AML in remission post-HSCT. Although clinician and patient perspectives largely aligned, emotional and cognitive impacts were most important to patients, whereas clinicians focused on physical impacts. This model could be used to ensure patient-reported outcome measures included in clinical trials are reflective of the post-HSCT AML patient experience.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy resulting from the clonal expansion of undifferentiated myeloid blasts, impacting hematopoiesis and bone marrow function [Citation1,Citation2]. This disease has a considerable impact on patients and is associated with a poor prognosis, having the lowest survival rate of all leukemias [Citation1,Citation3]. Chemotherapy has been the standard AML treatment for over four decades, but 10–40% of younger patients with AML and 40–60% of patients >60 years of age are refractory to induction therapy, and relapse is common [Citation1,Citation4,Citation5]. To reduce the incidence of relapse, many patients receive post-induction consolidation therapy, such as hematopoietic stem cell transplant (HSCT) [Citation1,Citation6]. Although HSCT is curative for many patients with AML, approximately 40% will relapse after transplant, resulting in a poor prognosis and <20% probability of long-term survival [Citation1,Citation4,Citation6]. As such, there is a need for post-transplant therapies to diminish this risk of relapse, eradicate minimal residual disease, and improve overall survival [Citation7].
The symptoms of AML can be debilitating for patients; impacting daily functioning, causing considerable distress, and adversely impacting quality of life (QoL) [Citation8–10]. Understanding the experience of these patients is important for assessing unmet care needs, helping clinicians identify and monitor symptoms that are important to them, and assessing the true value of medical interventions [Citation11,Citation12].
Several patient-reported outcome (PRO) measures have been used to evaluate the experience of patients with AML, including instruments focusing on health-related QoL, cancer-specific measures, and AML-specific measures [Citation8,Citation9,Citation13–15]. The AML patient experience has also been captured directly through qualitative interviews where participants have described their experience of diagnosis, important factors for making treatment decisions, expectations for treatment outcomes, symptoms experienced, and the impact of disease and/or treatment on daily functioning, mental health, and QoL [Citation11,Citation16–20]. However, to our knowledge, no studies have solely focused on the experience of patients in remission post-HSCT [Citation11,Citation18–20].
We used findings from concept elicitation interviews conducted previously with patients with R/R AML [Citation20], in combination with a literature search mentioned in a previous publication [Citation19], to develop a preliminary conceptual model to capture the experience of patients with AML. To specifically evaluate the experience of patients with AML in remission post-HSCT, we conducted further concept elicitation interviews with clinicians and patients to identify symptoms and impacts relating to AML and/or its treatment. From these interviews, the preliminary conceptual model was updated to capture the experience of patients with AML in remission post-HSCT more accurately.
Methods
Study design
In this prospective, noninterventional, qualitative study, patients with AML in remission post-HSCT were recruited from the US and Germany by patient advisory organizations and a third-party recruitment vendor from March to October 2021.
Initial assessment of eligibility was carried out using information provided by the patient. Eligible patients were ≥18 years old, English language speakers in the US, or German language speakers in Germany, and with access to a phone or high-speed internet connection. Patients were excluded from the study if they had received a transplant <3 months or >1 year from the date of the initial assessment of eligibility or were not in remission at the time of the assessment.
Eligible patients provided information using a form completed by their treating clinician or by sharing their medical records. Information included AML diagnosis confirmation (in the past 3 months), confirmation of date of transplant, remission status, and treatment history. The maintenance therapy subpopulation was defined as all patients receiving maintenance therapy following their transplant.
Patients were also asked to provide information on FMS-like tyrosine kinase 3 (FLT3) mutation status, as these are the most common driver mutations associated with AML, and the most frequently identified FLT3 mutation, internal tandem duplication (ITD), is associated with a higher risk of relapse and lower survival rates [Citation1,Citation2,Citation21,Citation22].
Preliminary conceptual model
Prior to the present study, concept elicitation interviews were carried out with patients with R/R AML to elucidate their experience and evaluate the symptoms and impacts associated with AML and/or its treatment [Citation20]. A targeted literature search was also conducted previously using PubMed, Cochrane, PsychInfo, Google, PROQOLID, and ClinicalTrials.gov to identify literature on AML signs, symptoms, and impacts. From this search, 20 publications were used to compile a literature review, and the findings have been summarized in a previous publication [Citation19].
In the present study, transcripts from the previous interviews with patients with R/R AML [Citation20] were reviewed, and concepts that were referenced post-transplant were used in combination with the findings of the previous AML literature review [Citation19] to create an AML preliminary conceptual model (Supplementary Figure 1).
Clinician interviews
Concept elicitation interviews were conducted via telephone with clinicians who had been practicing in the US, Germany, or France for 5–25 years. Clinicians were recruited by a third-party recruitment vendor during September–October 2020. Clinicians were considered if they specialized in oncology, hematology, or hematology–oncology; devoted at least one-third of their time to clinical patient care; spent ≥75% of their clinical time treating patients with hematologic malignancies; treated ≥5 patients with AML per month; and if ≥10% of their patients with AML had FLT3 mutations (FLT3m+). For inclusion in the study, clinicians should have at least considered HSCT as an option for their patients previously, seen patients with AML in remission post-HSCT, and considered post-HSCT maintenance therapy for their patients with AML.
During the semi-structured interviews, the clinicians were asked, without probing, to describe the symptoms and impacts (concepts) experienced by their patients with AML in remission post-HSCT; define if these concepts were treatment-related, disease-related, or both; and comment on the impact of FLT3 mutation status on these concepts. The clinicians were then asked to comment on how well the concepts included in the preliminary conceptual model represent the post-HSCT AML patient experience. Based on the findings of these interviews, the preliminary conceptual model was revised. Symptoms were assigned to ‘disease-related’, ‘treatment-related’, or ‘disease or treatment-related’ based on their assignment in the preliminary conceptual disease model or answers given by clinicians during interviews.
Patient interviews
Concept elicitation interviews consisted of 60–90-minute telephone calls conducted by trained moderators. The interview discussion guide was approved by the WIRB-Copernicus Group® Institutional Review Board (Review #: 20204118), and interviews were conducted in accordance with International Society for Pharmacoeconomics and Outcomes Research guidelines [Citation23].
Patients were asked to describe the symptoms and impacts (concepts) of AML and/or its treatment. Initially, concepts were obtained spontaneously using open-ended questions, but if patients did not mention a concept from the preliminary conceptual model, they were asked specifically (probed) about these. For the concepts mentioned, patients were asked to describe their duration, frequency, and severity. For each concept mentioned, patients were asked to provide a peak bother rating (for symptoms) or a peak disturbance rating (level of impact on daily life; for impacts) for how they experienced the concept when it was at its worst during their disease journey. These were rated on a scale of 0–10, where 10 was the most severe. The clinician-revised conceptual model was amended to incorporate the findings of the patient interviews. Symptoms were assigned to ‘disease-related’, ‘treatment-related’ and ‘disease or treatment-related’ based on their assignment in the preliminary conceptual model or based on answers given by clinicians or patients during interviews.
Patient interviews were scheduled ≥3 months post-transplant to minimize the possibility of interference from acute graft-versus-host-disease (GVHD), defined according to National Institutes of Health (NIH) criteria as GVDH occurring <100 days post-transplant [Citation24].
Qualitative analysis
Patient demographics and data from the transcripts were summarized using descriptive statistics. A codebook captured concepts identified in the preliminary conceptual model and information relating to patient diagnosis and treatment history. Transcripts were independently coded by two researchers according to this codebook, using ATLAS.ti v8. Data were exported to Microsoft Excel for quality assurance, further analysis, and data processing. Patient transcripts were arranged chronologically and analyzed in six waves, with data from five patients assessed per wave. Concepts mentioned by each wave of patients were compared with the concepts mentioned in the previous waves. If new concepts appeared in the final wave, saturation was deemed unmet. A concept was deemed salient if ≥50% of patients mentioned the concept, either upon probing or spontaneously, and the average bother/disturbance rating was ≥5. Sensitivity analyses were conducted to evaluate the impact of changing the threshold of concept saliency from ≥50% to ≥40%, ≥30% and ≥20% of patients mentioning the concept.
Results
Patient characteristics
Overall, 133 patients with AML in remission post-HSCT were assessed for eligibility; 37 met the eligibility criteria and consented to participate; and 30 were interviewed. The median (interquartile range) age was 58 years (53–63), and 17 (56.7%) patients identified as female (). Twenty patients were from the US and ten were from Germany (). The mean (range) time from transplant was 7 (3–12) months, and 27 (90%) patients were FLT3m+ (). Ten patients were included in the maintenance therapy subpopulation, and maintenance therapy included midostaurin (n = 6), gilteritinib (n = 2), crenolanib (n = 1), and cytarabine (n = 1) (). No patients had experienced a previous transplant-related relapse.
Table 1. Baseline demographics of patients with AML in remission post-HSCT.
Clinician interviews
Eight clinicians were interviewed who mentioned 25 symptoms and 16 impacts experienced by their patients with AML in remission post-HSCT (Supplementary Tables 1 and 2). The symptoms ‘fatigue’, ‘fever’ and ‘weakness’ (n = 7) and the impact ‘decreased ability to function’ (n = 6) were mentioned most frequently (Supplementary Table 2). ‘Fatigue’ was rated as the most bothersome symptom by the most clinicians (n = 4), and ‘decreased ability to function’ the most bothersome impact (n = 2) (Supplementary Table 2). One symptom and three impacts received votes for ‘disease-related’, and 24 symptoms and 11 impacts received votes for ‘treatment-related’ (Supplementary Table 2).
The preliminary conceptual model was revised following clinician interviews: 11 symptoms were added to reflect the clinicians’ experiences of their patients’ symptoms, two were revised to include specific examples, and five were removed since they were not mentioned by any clinicians (). No impacts were removed, but six were added, and seven were revised to be more specific, more broadly capture the patient experience, or include more patient-friendly language ().
Figure 1. Conceptual model revised according to clinician interviews. Concepts included in preliminary conceptual model are in black text. Concepts added from clinician interviews are in red text. Concepts included in the preliminary conceptual model but revised following clinician interviews are in blue text. Symptoms were assigned to ‘disease-related’, ‘treatment-related’, or ‘disease or treatment-related’ based on their assignment in the preliminary conceptual disease model or based on answers given by clinicians during concept elicitation interviews. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant.
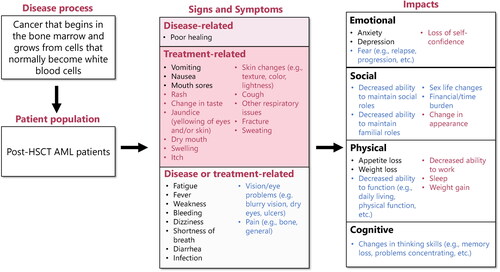
The clinicians noted that FLT3 mutation status affects the severity of symptoms and rate of disease progression in patients with AML in remission post-HSCT, but patients have similar symptoms regardless of mutation status. It was also mentioned that FLT3 mutation status influences the treatment regimen prescribed, and the additional drug prescribed for patients with FLT3m + AML incurs toxicity which would not be experienced by patients with a different mutation status.
Patient interviews
Symptoms
Patients mentioned 41 symptoms associated with AML and/or its treatment ( and Supplementary Table 3). Concept saturation was initially considered achieved in Wave 6, so interviews were halted after 30 of the eligible patients were interviewed (Supplementary Table 4). However, the new concept reported in Wave 6 (Jaundice) was already reflected in the clinician-revised conceptual model, meaning concept saturation is considered achieved in Wave 5 (Supplementary Table 4).
Figure 2. Frequency maps of symptoms mentioned by patients with AML in remission post-HSCT versus average peak bother rating: a) Total post-HSCT AML population; b) Maintenance therapy subpopulation. Bother was rated on a scale of 0–10, where 10 is the most severe. A symptom was deemed ‘salient’ if ≥50% of patients mentioned the symptom, either probed or spontaneous, and the average peak bother rating was ≥5. Some patients provided qualitative descriptions and would not provide a quantitative number, even after probing. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant
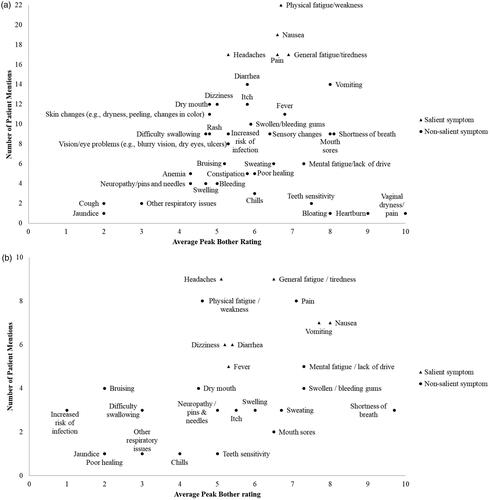
Five symptoms were found to be salient, and descriptions of these symptoms varied, including differences in when the symptom was experienced, duration of the symptom, whether the symptom improved with time from transplant, subsequent impact of the symptom, conditions that exacerbated the symptom, and its manageability (, Supplementary Tables 3 and 5).
Thirty-three symptoms were mentioned by the maintenance therapy subpopulation, with seven of these deemed to be salient ( and Supplementary Table 6). ‘General fatigue/tiredness’, ‘nausea’, and ‘headaches’ were salient for both the maintenance therapy subpopulation and total post-HSCT population, and ‘pain’ and ‘physical fatigue/weakness’ were deemed salient in the total population but not in the maintenance therapy subpopulation, where they were rated less bothersome ( and Supplementary Table 6).
Impacts
Patients mentioned 30 impacts relating to AML and/or its treatment ( and Supplementary Table 7). Six impacts were found to be salient, and descriptions of how these impacts manifested in daily life varied between patients, including differences in the symptoms or aspects of disease that patients attributed as the cause of the impacts (, Supplementary Table 7 and 8).
Figure 3. Frequency maps of impacts mentioned by patients with AML in remission post-HSCT versus average peak disturbance rating: a) Total post-HSCT AML population; b) Maintenance therapy subpopulation. Disturbance was rated on a scale of 0–10, where 10 is the most severe. An impact was deemed ‘salient’ if ≥50% of patients mentioned the impact, either probed or spontaneous, and the average peak disturbance rating was ≥5. Some patients provided qualitative descriptions and would not provide a quantitative number, even after probing. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant
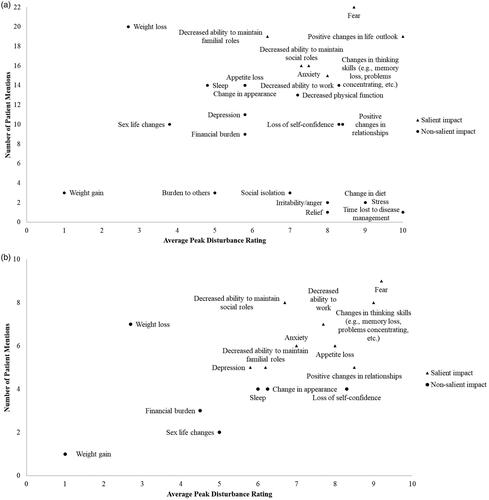
Twenty-four impacts were mentioned by the maintenance therapy subpopulation, with nine deemed to be salient ( and Supplementary Table 9). ‘Fear’, ‘anxiety’, ‘changes in thinking skills’, ‘decreased ability to maintain social roles’, and ‘decreased ability to maintain family roles’ were salient for both groups, and ‘appetite loss’ was salient in the maintenance therapy subpopulation but not the total population, where it was rated as less disturbing ( and Supplementary Table 9).
Saliency sensitivity analyses
When the definition of salient concepts was changed from ≥50% to ≥40% of patients mentioning the concept, 9 symptoms and 10 impacts were deemed to be salient (Supplementary Tables 3 and 7). When the definition was changed to ≥30% of patients mentioning the concept, 15 symptoms and 14 impacts were deemed to be salient, and, when changed to ≥20%, 19 symptoms and 14 impacts were deemed to be salient (Supplementary Tables 3 and 7).
Indicators of relapse
Some patients (n = 9) discussed concepts that may indicate relapse, with eight mentioning that they monitored themselves for the symptoms and impacts they had experienced pre-diagnosis (Supplementary Table 10). Symptoms that were indicative of relapse included ‘general fatigue/tiredness’ (n = 6), ‘pain and bruising’ (n = 3), ‘shortness of breath’ (n = 2), ‘poor healing’ (n = 1), ‘headaches’ (n = 1), and ‘decreased ability to do daily activities’ (n = 1) (Supplementary Table 10).
Final conceptual model
The clinician-revised conceptual model was amended based on the concepts mentioned by patients with AML in remission post-HSCT (). Six symptoms were added since they were mentioned by ≥5 patients, and six symptoms were removed because they were spontaneously mentioned by <5 patients (). Six symptoms were amended by including specific examples, combining symptoms with similar patient descriptions, using more generic language to capture the breadth of the patient experience, or splitting into several symptoms to reflect different ways patients experience them ().
Figure 4. Conceptual model amended according to concept elicitation interviews with patients with AML in remission post-HSCT. Concepts included in the clinician-revised conceptual model are in black text. Concepts added from patient concept elicitation interviews are in red text. Concepts included in the clinician-revised conceptual model but amended following patient interviews are in blue text. Symptoms were assigned to ‘Disease-related’, ‘Treatment-related’, and ‘Disease or treatment-related’ based on their assignment in the preliminary conceptual model or based on answers given by clinicians or patients during concept elicitation interviews. AML: acute myeloid leukemia; HSCT: hematopoietic stem cell transplant.
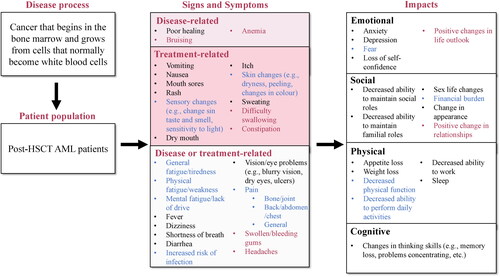
Two impacts were added to the final conceptual model since they were spontaneously mentioned by >5 patients, and one impact was removed because it was mentioned by <5 patients (). Three impacts were amended to remove wording not reflected in the patient interviews or split into several impacts to more accurately reflect the way that patients experienced them ().
Discussion
In this study, qualitative interviews were conducted to evaluate the experience of patients with AML in remission post-HSCT. Only patients in remission were selected with a view to enriching the identification of transplant survival experiences. The patient interviews captured 41 symptoms and 30 impacts experienced by 30 patients with AML in remission post-HSCT, with five symptoms and six impacts found to be salient (mentioned by ≥50% of patients with an average bother/disturbance rating ≥5/10). The findings of these interviews were used to update a conceptual model constructed on the basis of previous interviews with patients with R/R AML [Citation20] which had been further refined by clinician interviews, ensuring that the final conceptual model reflected the patient voice. Not all patients included in the study were FLT3m+, and the clinicians interviewed reported that patients have similar symptoms regardless of FLT3 mutation status, even though mutation status affects severity of symptoms and rate of disease progression. The impact of FLT3 mutation status on rate of disease progression is supported by several studies which have demonstrated that the FLT3-ITD mutation is associated with a higher risk of relapse and lower survival rates [Citation21,Citation22]. Regardless, given the reported minimal impact of FLT3 mutation status on the symptoms experienced by patients with AML, the final conceptual model generated applies to patients with and without FLT3 mutations.
Most concepts remained unchanged following patient interviews, indicating that clinicians were largely aware of the symptoms and impacts experienced by patients with AML in remission post-HSCT. However, clinicians’ perspectives did not fully align with the patient experience, as clinicians considered physical impacts of AML and its treatments to be the most bothersome (e.g. diminished functional status, weight loss, appetite loss), rather than the emotional and cognitive impacts that were important for patients (e.g. anxiety, fear, diminished cognitive function).
The concept prevalence and average peak bother and disturbance ratings the maintenance therapy subpopulation differed minimally to those of the total population of patients with AML in remission post-HSCT. This suggests that symptoms and impacts can persist even with maintenance therapy, highlighting an area of focus for therapeutic strategies.
All salient concepts identified in this study have been reported in previous studies that included post-transplant patients in their AML patient cohort [Citation18,Citation19]; however, there are notable differences between these studies and ours, precluding a direct comparison. In one study, patients with AML were asked about the symptoms and impacts associated with AML, and they rated how disturbing they found these concepts [Citation19]. Tomaszewski et al. provided separate data for patients with AML in remission post-HSCT, but the subpopulation also included patients with R/R AML who were excluded from our study [Citation19]. Consequently, it is difficult to distinguish symptoms and impacts resulting from relapse in the previous study from those resulting from initial disease and/or its treatment [Citation19]. In another study including patients with AML post-transplant, interviews were conducted to evaluate positive and negative concepts relating to their experience with AML, and the impact of these on QoL [Citation18]. However, Buckley et al. did not provide separate data for the post-transplant subgroup, meaning a direct comparison with the current study is not possible [Citation18].
In the current study, concept saturation was achieved for both symptoms and impacts, suggesting that an adequate sample population was used, and the findings can be generalized as representative of the post-HSCT AML population experience. Another strength of this study is that the final conceptual model has a robust basis, as it was built not only on the expertise of clinicians with extensive experience treating patients with AML in remission post-HSCT, but also incorporated information from patient interviews.
This study had several limitations. Results were contingent on the assumption that it is reasonable to consider concepts mentioned by a majority of patients (≥50%) as salient, with sensitivity analyses demonstrating that the number of salient concepts varied by percentage threshold. Moreover, the assignment of symptoms to disease, treatment, or a combination of both may have been limited by difficulties distinguishing between symptoms caused by AML and those caused by its treatment.
There was a lack of diversity in the study population: the majority of the US population was white, and for both populations the majority of patients were >50 years of age. This means that the final conceptual model may not be reflective of the experiences of patients from other races or ethnicities, or younger patients. Only a small number of German patients were recruited for the study, minimizing the impact of this subgroup on the overall concept prevalence, average peak bother rating, and average peak disturbance ratings. Furthermore, as some patients were excluded from this study due to disease progression or poor health status, their experiences were not captured and may not be fully reflected in the final conceptual model. Additionally, only a subset of patients was receiving maintenance therapy, introducing a degree of heterogeneity to the study population.
Moreover, as patients were asked to discuss experiences that occurred during months of the year prior to the interview, this may have resulted in recall bias. This could have been exacerbated by the fact that some patients reported changes in thinking skills, such as memory loss, as symptoms of their disease and/or its treatment.
It is possible that patients may not be able to distinguish symptoms of GVHD from concepts relating to their disease and/or its treatment. In this study, patient interviews were scheduled to minimize the possibility of interference from acute GVHD. The NIH criteria define acute GVHD as occurring <100 days post-transplant, which is considerably less than the 7 months average time (range 3–12 months) since transplant reported in the current study, suggesting that the symptoms and impacts reported herein are unlikely to be due to acute GVHD [Citation24]. However, chronic GVHD, and acute GVHD in some cases, can occur >100 days post-transplant, meaning that GVHD could still have impacted patients’ described experiences of AML [Citation24].
In conclusion, this study identified salient impacts and symptoms relevant to patients with AML in remission post-HSCT, including 10 patients undergoing maintenance therapy. The conceptual model created following patient and clinician interviews is representative of the experience of these patients. Clinicians were largely aware of the symptoms and impacts experienced by patients with AML in remission post-HSCT, although they tended to focus on physical impacts rather than the psychosocial and cognitive impacts that were important to patients. Further work is required to validate the final conceptual model in a more diverse post-HSCT patient population. However, this model could aid selection of PRO measures that are reflective of the post-HSCT AML patient experience for use in future clinical trials evaluating the impact of novel treatments in this patient population.
Ethical approval
Interviews were approved by the WIRB-Copernicus Group® Institutional Review Board (Review #: 20204118). Each patient provided informed consent online for screening and study participation prior to the interviews.
Author contributions
All authors met the International Committee of Medical Journal Editors criteria for authorship. All authors substantially contributed to at least one of the following: study design (DC, KK, AdlM, FT, and MVS), acquisition (DC, KK, AdlM, FT, and MVS), analysis (BJP, DC, KK, AdlM, MVS, and TWL), or interpretation of study data (BJP, DC, KK, AdlM, MVS, and TWL). All authors have seen and contributed to the revision of the manuscript. All authors read and approved the final manuscript for publication and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (207.9 KB)Disclosure statement
All authors report non-financial support from Astellas during the conduct of the study. KK and FT are employees of IQVIA, a CRO contracted to generate data on behalf of Astellas. AdlM was an employee of IQVIA during the conduct of the study. BJP is an employee of Astellas. MVS was an employee of Astellas during the conduct of the study. TWL received grants from AstraZeneca, CareVive, GSK, Janssen, Bristol Myers Squibb, and Jazz Pharmaceuticals; royalties from UpToDate; consulting fees from AbbVie, Astellas, Agios/Servier, Bristol Myers Squibb/Celgene, Flatiron, GSK, Genentech, Pfizer, BlueNote Therapeutics, Novartis, and AstraZeneca; and honoraria and support for attending meetings from AbbVie, Agios/Servier, and Bristol Myers Squibb/Celgene.
Data availability statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Additional information
Funding
References
- Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026.
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–2221.
- Leukemia & Lymphoma Society. Facts and statistics overview. 2022. https://www.lls.org/facts-and-statistics/facts-and-statistics-overview
- Thol F, Ganser A. Treatment of relapsed acute myeloid leukemia. Curr Treat Options Oncol. 2020;21(8):66.
- Yilmaz M, Wang F, Loghavi S, et al. Late relapse in acute myeloid leukemia (AML): clonal evolution or therapy-related leukemia? Blood Cancer J. 2019;9(2):7.
- Kassim AA, Savani BN. Hematopoietic stem cell transplantation for acute myeloid leukemia: a review. Hematol Oncol Stem Cell Ther. 2017;10(4):245–251.
- Reville PK, Kadia TM. Maintenance therapy in AML. Front. Oncol. 2021;10:1–10.
- Salas M, Henderson M, Wientzek-Fleischmann A, et al. Validated instruments of quality of life (QOL) in patients with acute myeloid leukemia (AML) and other cancers. Front. Pharmacol. 2020;11:1109.
- Stauder R, Lambert J, Desruol-Allardin S, et al. Patient-reported outcome measures in studies of myelodysplastic syndromes and acute myeloid leukemia: literature review and landscape analysis. Eur J Haematol. 2020;104(5):476–487.
- Kayastha N, Wolf SP, Locke SC, et al. The impact of remission status on patients’ experiences with acute myeloid leukemia (AML): an exploratory analysis of longitudinal patient-reported outcomes data. Support Care Cancer. 2018;26(5):1437–1445.
- Boucher NA, Johnson KS, LeBlanc TW. Acute leukemia patients’ needs: qualitative findings and opportunities for early palliative care. J Pain Symptom Manage. 2018;55(2):433–439.
- Brédart A, Marrel A, Abetz-Webb L, et al. Interviewing to develop Patient-Reported outcome (PRO) measures for clinical research: eliciting patients’ experience. Health Qual Life Outcomes. 2014;12:15.
- Cella D, Jensen SE, Webster K, et al. Measuring health-related quality of life in leukemia: the functional assessment of cancer therapy - Leukemia (FACT-Leu) questionnaire. Value Health. 2012;15(8):1051–1058.
- He J, Pierson R, Loefgren C, et al. Patient-Reported outcomes validation of the FACT-Leu in acute myeloid leukemia: a review of baseline characteristics in AML2002. Blood. 2018;132(Supplement 1):3590.
- Peipert JD, Efficace F, Pierson R, et al. Patient-reported outcomes predict overall survival in older patients with acute myeloid leukemia. J Geriatr Oncol. 2022;13(7):935–939.
- LeBlanc TW, Russell NH, Hernandez-Aldama L, et al. Patient, family member and physician perspectives and experiences with AML treatment Decision-Making. Oncol Ther. 2022;10(2):421–440.
- LeBlanc TW, Fish LJ, Bloom CT, et al. Patient experiences of acute myeloid leukemia: a qualitative study about diagnosis, illness understanding, and treatment decision-making. Psychooncology. 2017;26(12):2063–2068.
- Buckley SA, Jimenez-Sahagun D, Othus M, et al. Quality of life from the perspective of the patient with acute myeloid leukemia. Cancer. 2018;124(1):145–152.
- Tomaszewski EL, Fickley CE, Maddux L, et al. The patient perspective on living with acute myeloid leukemia. Oncol Ther. 2016;4(2):225–238.
- Shah M, Crooks P, New M. Psy218 - symptom and impact disturbance: findings from a qualitative assessment of patients with acute myeloid leukemia (aml). Value Health. 2018;21(Supplement 3):S473–S474.
- Kennedy VE, Smith CC. FLT3 mutations in acute myeloid leukemia: key concepts and emerging controversies. Front Oncol. 2020;10:612880.
- Testa U, Pelosi E. The impact of FLT3 mutations on the development of acute myeloid leukemias. Leuk Res Treatment. 2013;2013:275760.
- Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity - Establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1 - Eliciting concepts for a new PRO instru. Value Health. 2011;14(8):967–977.
- Filipovich AH, Weisdorf D, Pavletic S, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956.
