Despite new treatments leading to improved survival, patients with multiple myeloma often relapse. Many are refractory to and/or cycle through the available treatments, indicating considerable unmet need in patients with relapsed/refractory multiple myeloma (RRMM) [Citation1]. Novel treatments are needed for patients who are triple-class exposed (TCE) to an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 antibody [Citation2].
Idecabtagene vicleucel (ide-cel) is the first B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cell therapy approved for patients after ≥3 (in Europe) or ≥4 (in the United States) prior lines of therapy (LOT) [Citation3,Citation4]. Belantamab mafodotin (bela-maf), an antibody–drug conjugate targeting BCMA, is approved by the European Medicines Agency (EMA) for TCE RRMM after ≥4 prior LOT [Citation5]. Bela-maf is no longer available for TCE RRMM in the United States [Citation6].
In the absence of head-to-head evidence, population-adjusted indirect comparisons accommodate between-study differences in patient characteristics when comparing trials. A previous matching-adjusted indirect comparison (MAIC) of the KarMMa (NCT03361748) and DREAMM-2 (NCT03525678) trials demonstrated that ide-cel improved overall response rate (ORR), extended progression-free survival (PFS), and overall survival (OS), and improved health-related quality of life (HRQoL) in comparison to bela-maf [Citation7,Citation8]. We performed a MAIC to compare the longer-term outcomes of KarMMa [Citation9] and DREAMM-2 [Citation10] in TCE RRMM patients.
The analysis used individual patient-level data (IPD) from the single-arm, phase 2 KarMMa trial evaluating the efficacy and safety of ide-cel in patients with RRMM who had received ≥3 prior regimens, were TCE, and refractory to their last regimen. HRQoL was evaluated as a secondary objective [Citation11]. Median follow-up was 24.8 months for surviving patients (an additional 11.8 months compared to the previous MAIC [Citation7]); 90 (70.3%) patients were followed for ≥12 months from infusion [Citation9]. Patients completed European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 – general cancer (QLQ-C30) and Quality of Life Questionnaire Multiple Myeloma 20 (QLQ-MY20) assessments at screening, baseline (within 72 h before or on day of lymphodepletion), day 1 (ide-cel infusion), monthly during months 1–6, and then every 3 months up to 24 months or end of study. Scores for each scale were calculated if responses were provided for ≥50% of the questions; the scale was otherwise deemed missing.
Study-level aggregate data were extracted by two independent reviewers from the two-arm, phase 2 DREAMM-2 trial evaluating efficacy, safety, and HRQoL of bela-maf in RRMM patients who had received ≥3 prior regimens, were refractory to an IMiD or PI, and refractory/intolerant to an anti-CD38 monoclonal antibody [Citation10,Citation12]. The current analysis was restricted to the 2.5 mg/kg bela-maf approved dose [Citation5]. Median follow-up was 13 months for the bela-maf population (an additional 6.7 months in comparison to the previous MAIC [Citation7]). At the time of analysis, scores from the EORTC QLQ-C30 global health status/quality of life (QoL), pain, and fatigue domains, and change from baseline scores for the EORTC QLQ-MY20 disease symptoms domain, were the only published HRQoL data for DREAMM-2. Change from baseline scores was published at baseline (cycle 1, day 1) and every 6 weeks. The Kaplan–Meier (KM) curves for DREAMM-2 were digitized for PFS and OS, including the number of patients at risk over time, and IPD were then reconstructed based on an established algorithm [Citation13]. Change from baseline scores for HRQoL outcomes was digitized using Digitizelt® software version 2.5 (Braunschweig, Germany) based on published plots.
Between-study differences (Supplementary Materials) in patient characteristics that may influence the outcomes or treatment allocation were adjusted for using MAIC. Covariates included were: receipt of ≥7 prior LOT; refractoriness to bortezomib; refractoriness to carfilzomib; refractoriness to lenalidomide; refractoriness to pomalidomide; International Staging System (ISS) stage ≤ II; high-risk cytogenetics; extramedullary disease; ≥5.49 years since diagnosis; and effective sample size (ESS) [Citation14]. For each outcome of interest, a logistic propensity score model was used to estimate weights for the IPD from KarMMa to have weighted mean baseline characteristics that matched those observed for the DREAMM-2 bela-maf population. These weights were then applied to the observed outcomes for ide-cel to estimate the effect assuming ide-cel had been used in the DREAMM-2 bela-maf population.
A weighted Cox regression was used for PFS and OS. Weighted linear regressions were used for the EORTC QLQ-C30 and QLQ-MY20 outcomes. Sandwich estimators were used to calculate the variance of the comparative treatment effects. Calculated treatment effects were hazard ratios (HRs) for PFS and OS, and difference in mean change from baseline scores for HRQoL, given a population similar to the DREAMM-2 bela-maf population. Naïve comparisons (models without individual weights) and MAIC-adjusted comparisons (models with individual weights) were performed for each outcome of interest for ide-cel versus bela-maf. Statistical significance was defined as p < 0.05. Additional details are given in the Supplementary Materials.
Baseline patient characteristics were well balanced between the ide-cel and the bela-maf populations after matching (Supplementary Materials). For efficacy outcomes, the ESS were as follows: 45 for the KarMMa treated population (N = 128 patients); 59 for the KarMMa overall population (N = 140 patients); and 12 for the KarMMa target dose population (N = 54 patients). For analyses involving the KarMMa treated population, the MAIC-adjusted comparison was associated with a 4.88-fold increase (95% confidence interval (CI): 2.25, 10.59) in ORR. MAIC-adjusted estimates for the KarMMa overall and target dose populations were 2.98 (95% CI: 1.51, 5.86) and 12.03 (95% CI: 3.53, 41.03), respectively (Supplementary Materials). For HRQoL outcomes, the ESS was 80 for the KarMMa treated population, 91 for the KarMMa overall population, and 32 for the KarMMa target dose population (Supplementary Materials).
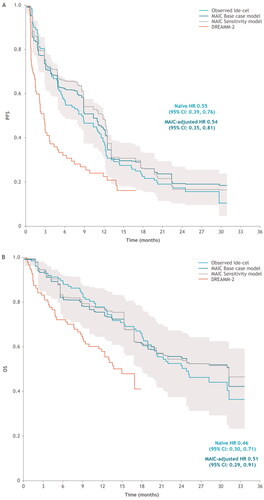
Ide-cel was associated with improved PFS and OS relative to bela-maf in both the naïve and MAIC-adjusted comparisons (for analyses involving the KarMMa treated population) (). Specifically, ide-cel extended PFS relative to bela-maf in the naïve comparison (HR 0.55 [95% CI: 0.39, 0.76]) (), with a similar result for the MAIC-adjusted comparison (HR 0.54 [95% CI: 0.35, 0.81]). PFS was also improved for ide-cel versus bela-maf for both naïve and MAIC-adjusted estimates for the KarMMa overall and target dose populations (Supplementary Materials). For the KarMMa treated population, ide-cel extended OS relative to bela-maf in the naïve comparison (HR 0.46 [95% CI: 0.30, 0.71]), which slightly shifted upwards in the MAIC-adjusted comparison (HR 0.51 [95% CI: 0.29, 0.91]) (). While the OS HR for the KarMMa overall population favored ide-cel, the estimate was not statistically significant. Inclusion of additional covariates in a sensitivity analysis led to consistent results for both PFS and OS. Exploration of the structural uncertainty in results due to inclusion of different combinations of covariates showed that MAIC-adjusted findings consistently demonstrated improved PFS and OS for ide-cel in comparison with bela-maf (). ORR data are presented in the Supplementary Materials.
Figure 1. Observed and MAIC adjusted outcomes: (A) PFS KM and (B) OS KM overlay curves for KarMMa ide-cel-treated population versus DREAMM-2 2.5 mg/kg bela-maf population, including structural uncertainty data points. Sensitivity analysis curve includes the addition of the following covariates: race, sex, and age. Gray curves indicate different combinations of selected covariates for the base case and sensitivity analyses, as well as the replacement of refractoriness to specific agents with the proportion of triple-class refractory patients. bela-maf: belantamab mafodotin; CI: confidence interval; HR: hazard ratio; ide-cel: idecabtagene vicleucel; KM: Kaplan–Meier; MAIC: matching-adjusted indirect comparison; OS: overall survival; PFS: progression-free survival.
For the KarMMa treated population, ide-cel was associated with better HRQoL than bela-maf at all time points of evaluation for the naïve and MAIC-adjusted analyses involving the QLQ-C30 global health status/QoL domain (). Additionally, ide-cel was associated with better HRQoL than bela-maf at 4, 6, and 9 months for both the naïve and MAIC-adjusted analyses of the QLQ-C30 pain domain (). For the QLQ-C30 fatigue domain, ide-cel was associated with better HRQoL than bela-maf at 6 and 9 months in the MAIC analysis (). Lastly, for the QLQ-MY20 disease symptoms domain, ide-cel was associated with better HRQoL than bela-maf at 3, 4, and 6 months in the MAIC analysis ().
Figure 2. Observed and MAIC-adjusted change from baseline for (A) QLQ-C30 global health status/QoL score, (B) QLQ-C30 pain score, (C) QLQ-C30 fatigue score, and (D) QLQ-MY20 disease symptoms score for KarMMa ide-cel-treated population versus DREAMM-2 2.5 mg/kg bela-maf population over time. Restricted to time points with ≥10 patients. Change from baseline and 95% CIs are plotted. The x-axis includes information for both months and weeks. For KarMMa, bar charts are presented to note the percent of responders (patients who achieved stringent complete response (sCR), complete response (CR), partial response (PR), or very good partial response (VGPR)) at each time point of evaluation. Horizontal dotted lines indicate MID for improvement/deterioration. Bolded mean difference scores (naïve and MAIC-adjusted) are indicative of statistical significance. bela-maf: belantamab mafodotin; CI: confidence interval; EORTC: European Organisation for Research and Treatment of Cancer; ide-cel: idecabtagene vicleucel; HRQoL: health-related quality of life; MAIC: matching-adjusted indirect comparison; MID: minimally important difference; N: number of patients; QLQ-C30: EORTC Quality of Life Questionnaire C30 – general cancer; QLQ-MY20: EORTC Quality of Life Questionnaire Multiple Myeloma 20 – multiple myeloma specific; QoL: quality of life.
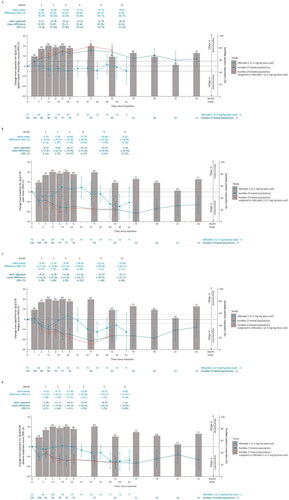
Similar findings favoring ide-cel in comparison with bela-maf were observed for analyses involving the KarMMa overall and target dose populations across the evaluated QLQ-C30 and QLQ-MY20 domains, with MAIC-adjusted point estimates favoring ide-cel more in the target dose population analysis versus analyses involving the treated or overall populations (Supplementary Materials).
Our goal was to have an inclusive model to minimize potential biases. The patient characteristics included in the propensity model were based on a literature review and clinical expert opinion, considering variables associated with the questionnaires used for HRQoL analyses. The ESS in our study aligned broadly with previous MAICs regarding absolute ESS and relative reductions in ESS (range of 12 [58% reduction] to 45 [65% reduction] for efficacy outcomes and range of 32 [41% reduction] to 91 [35% reduction] for HRQoL outcomes) [Citation15]. Results for PFS and OS were consistent across different combinations of included covariates.
This study has several limitations. Other covariates could have been considered; we were unable to adjust for differences not reported in DREAMM-2 and could not examine potential residual bias; unanchored MAICs rely on all effect modifiers and prognostic factors being measured; smaller ESS group may have less power and potential type I errors (Supplementary Materials).
In summary, findings from this unanchored MAIC using IPD from KarMMa and aggregate-level data from DREAMM-2 suggest that after adjusting for important prognostic factors and treatment effect modifiers, a single infusion of ide-cel offers clinically relevant improvements over bela-maf in both clinical efficacy and HRQoL outcomes for TCE RRMM patients. Hence, ide-cel may address an unmet need for improved response and HRQoL in TCE RRMM patients.
Author contributions
P.R.O., F.E.D., M.D., K.W., and K.K. conceived and designed the study, and contributed to the interpretation and analysis of data. K.T., S.C., C.C., D.A., and A.M. contributed to the acquisition, interpretation, and analysis of data. T.M. and J.B. contributed to the interpretation and analysis of data. J.F. and D.D. conceived and designed the study, and contributed to the acquisition, interpretation, and analysis of data. All authors have read and agreed to the final published version of the manuscript.
Supplemental Material
Download PDF (361.6 KB)Acknowledgments
The authors received editorial assistance in the preparation of this manuscript from Eilish McBurnie, PhD, of Excerpta Medica, funded by Bristol Myers Squibb. The authors are fully responsible for all content and editorial decisions for this manuscript.
Disclosure statement
P.R.O. has served on advisory boards for Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Kite Pharma, Oncopeptides, Pfizer, Sanofi, and Takeda, has provided consultancy to AbbVie, Bristol Myers Squibb, and Roche, and has received honoraria from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Regeneron, Sanofi, and Takeda. K.T., S.C., C.C., and A.M. are employees of PRECISIONheor, which received funding from Bristol Myers Squibb to conduct this study. F.E.D. has served on advisory boards for and provided consultancy to Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Oncopeptides, Sanofi, and Takeda. M.D. has served on advisory boards for Bristol Myers Squibb and Takeda, has provided consultancy to and received honoraria from Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Sanofi, and Stemline, and has received grants from Janssen. K.W. has received honoraria from AbbVie, Adaptive Biotechnologies, Amgen, AstraZeneca, BeiGene, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Novartis, Oncopeptides, Pfizer, Roche Pharma, Sanofi, Stemline, and Takeda, has provided consultancy to AbbVie, Adaptive Biotechnologies, Amgen, BeiGene, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Oncopeptides, Pfizer, Roche Pharma, Sanofi, and Takeda, and has received research funding (institution) from AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen, and Sanofi. T.M., K.K., J.B., and D.D. are employees of and have equity in Bristol Myers Squibb. D.A. was formerly an employee of PRECISIONheor, which received funding from Bristol Myers Squibb to conduct this study. J.F. was formerly an employee of and has equity in Bristol Myers Squibb and is currently an employee of Pfizer.
Data availability statement
Bristol Myers Squibb details the data sharing process via the following website: https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html
Additional information
Funding
References
- Dimopoulos MA, Richardson P, Lonial S. Treatment options for patients with heavily pretreated relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2022;22(7):460–473. doi:10.1016/j.clml.2022.01.011
- Kumar S, Baizer L, Callander NS, et al. Gaps and opportunities in the treatment of relapsed–refractory multiple myeloma: consensus recommendations of the NCI Multiple Myeloma Steering Committee. Blood Cancer J. 2022;12(6):98. doi:10.1038/s41408-022-00695-5
- European Medicines Agency (EMA). ABECMA (idecabtagene vicleucel); 2021 [updated 2022 Dec 21]. Available from: https://www.ema.europa.eu/en/documents/product-information/abecma-epar-product-information_en.pdf
- Food and Drug Administration. ABECMA (idecabtagene vicleucel); 2021 [updated 2021 Apr 21]. Available from: https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel
- European Medicines Agency (EMA). BLENREP (belantamab mafodotin): EU summary of product characteristics; 2020. Available from: https://ec.europa.eu/health/documents/community-register/2020/20200825148987/anx_148987_en.pdf
- GlaxoSmithKline. GSK provides an update on Blenrep (belantamab mafodotin-blmf) US marketing authorization [press release]; 2023 [cited 2023 Jun 19]. Available from: https://www.gsk.com/en-gb/media/press-releases/gsk-provides-update-on-blenrep-us-marketing-authorisation/
- Rodriguez-Otero P, Ayers D, Cope S, et al. Matching adjusted indirect comparisons of efficacy outcomes for idecabtagene vicleucel (ide-cel, bb2121) versus selinexor + dexamethasone and belantamab mafodotin in relapsed and refractory multiple myeloma. Leuk Lymphoma. 2021;62(10):2482–2491. doi:10.1080/10428194.2021.1913143
- Shah N, Ayers D, Cope S, et al. Health-related quality of life in patients with relapsed/refractory multiple myeloma (RRMM) treated with idecabtagene vicleucel vs belantamab mafodotin: a matching-adjusted indirect comparison study. HemaSphere. 2022;6(Suppl. 3):1621–1622. doi:10.1097/01.HS9.0000849816.07364.24
- Anderson LD Jr., Shah N, Jagannath S, et al. OAB-027: idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T-cell therapy, for the treatment of patients with relapsed and refractory multiple myeloma (RRMM): updated results from KarMMa [abstract]. Clin Lymphoma Myeloma Leuk. 2021;21(Suppl. 2):S17–S18. doi:10.1016/S2152-2650(21)02101-7
- Lonial S, Lee HC, Badros A, et al. Longer term outcomes with single‐agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13‐month follow‐up from the pivotal DREAMM‐2 study. Cancer. 2021;127(22):4198–4212. doi:10.1002/cncr.33809
- Delforge M, Shah N, Miguel JSF, et al. Health-related quality of life with idecabtagene vicleucel in relapsed and refractory multiple myeloma. Blood Adv. 2022;6(4):1309–1318. doi:10.1182/bloodadvances.2021005913
- Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–221. doi:10.1016/S1470-2045(19)30788-0
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi:10.1186/1471-2288-12-9
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211. doi:10.1177/0272989X17725740
- Li X, Liu J, Chen M, et al. Health‐related quality of life of patients with multiple myeloma: a real‐world study in China. Cancer Med. 2020;9(21):7896–7913. doi:10.1002/cam4.3391
