Abstract
Prior studies evaluating ibrutinib discontinuation are limited to clinical trials and selected medical centers and hence may not reflect real-world practice. This study used Medicare claims (2013–2019) to examine ibrutinib discontinuation and associated factors among elderly patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Over a median follow-up of 2.1 years, two-thirds (65.2%) of the 11,870 new ibrutinib initiators were discontinued, with half (45.1%) of patients discontinuing within 12 months of initiation. Factors such as advanced age, lack of Part D low-income subsidy, evidence of prior CLL/SLL treatment, and cardiovascular comorbidities (e.g. atrial fibrillation) were associated with higher risk of discontinuation. Over a median of 1.2 years from discontinuation, 40% of discontinuers initiated another CLL/SLL treatment after ibrutinib discontinuation; 25% of patients restarted ibrutinib treatment at some point over follow-up. Our findings point to a large unmet need with the widely used BTKi ibrutinib and underscore the importance of ongoing development of efficacious and well-tolerated CLL/SLL therapies.
Introduction
Chronic lymphocytic leukemia (CLL), a malignancy of auto-reactive mature B cells, is the most common type of leukemia among adults in the United States [Citation1]. Small lymphocytic lymphoma (SLL) is the same disease as CLL, but with cancer cells mostly found in the lymph nodes rather than in the blood or bone marrow [Citation1]. Treatment for CLL/SLL historically took the form of cytotoxic chemotherapy as monotherapy or in combination with other agents; later, monoclonal antibodies such as rituximab were added to treatment regimens [Citation2]. The past decade has witnessed extraordinary breakthroughs in the treatment of CLL/SLL [Citation3]. Oral targeted therapies, such as the first-generation Bruton’s tyrosine kinase inhibitor (BTKi) ibrutinib approved for CLL/SLL in February 2014, have demonstrated significantly improved progression-free and overall survival (OS) relative to traditional chemotherapies [Citation4–7]. BTKis are typically used as monotherapy and taken continuously until disease progression or intolerable toxicity, with ibrutinib-related adverse events (such as atrial fibrillation and bleeding) a key concern for patients and may result in premature treatment discontinuation [Citation8–10].
Most prior studies of ibrutinib discontinuation have relied on data from clinical trials and/or selected medical centers, which are based on small, non-representative study samples and poorly reflect real-world clinical practice at large [Citation6,Citation8,Citation11–13]. Furthermore, there have been limited real-world studies examining ibrutinib discontinuation rates and the factors associated with discontinuation in the elderly U.S. Medicare population. This is a significant gap in the knowledge base for two reasons: (1) CLL/SLL is primarily a disease of the elderly (median age at diagnosis between 67 and 72 years) and increases in prevalence with age, and (2) Medicare patients often face unique circumstances (multiple comorbidities, polypharmacy, limited financial means, etc.) that do not permit generalizability of results obtained from younger and/or commercially insured patients.
A better understanding of ibrutinib discontinuation rates and characteristics of elderly patients more likely to discontinue can help clinicians identify patients who may require targeted clinical management upon ibrutinib initiation. Additionally, these patients may also be appropriate candidates for direct initiation of alternative novel therapies such as B-cell lymphoma 2 (BCL-2) inhibitors or newer generation BTKis. Furthermore, an assessment of the treatments received after ibrutinib discontinuation, which has not been well studied to date, can add to our understanding of the unmet need among elderly patients with CLL/SLL. In this study, we used a nationally representative sample of elderly Medicare beneficiaries with CLL/SLL to examine ibrutinib discontinuation rates and identify patient factors associated with discontinuation.
Methods
Study design and data source
This retrospective cohort study used Chronic Conditions Warehouse (CCW) Medicare Parts A, B, and D claims (100%) from 2013 to 2019, the latest year of data available at the time of the analysis. The CCW Medicare (100%) files contain data on all beneficiaries with fee-for-service Medicare coverage in the United States, including medical (inpatient, outpatient, skilled nursing facility, hospice, home health, durable medical equipment) and prescription (pharmacy) claims. The Medicare Parts A, B, and D claims files are linked to Medicare personal summary files that contain patient demographics, eligibility information, and date of death information.
Study sample
All older adult fee-for-service Medicare beneficiaries who newly initiated ibrutinib between 1 January 2014 and 31 December 2018 (index date = first ibrutinib prescription claim date) were identified based on the following criteria: (1) continuous Medicare Parts A, B, and D coverage in 12 months before and at least 4 months after the index date; (2) the presence of a diagnosis of CLL/SLL (ICD-9: 200.8x, 204.1x; ICD-10: C83.0x, C91.1x) in the 12-month pre-index period and at any time over follow-up; (3) ≥66 years on index date; (4) the absence of ≥2 diagnoses for another FDA-approved indication of ibrutinib (); and (5) the absence of ibrutinib prescription in the 12-month pre-index period.
Outcomes
Discontinuation was measured from ibrutinib initiation until the end of the study period (i.e. 31 December 2019), death, or censoring (i.e. entry into Medicare Advantage). Discontinuation was captured as a dichotomous measure indicating the presence (or absence) of a continuous period of ≥60 days without any supply of ibrutinib after the days’ supply of the most recent ibrutinib prescription was exhausted in the post-index period; this 60-day consecutive gap was based on prior literature [Citation14,Citation15] and approved by the clinical advisor on the study. Time from ibrutinib initiation to discontinuation was defined as the time from the index date to the date of discontinuation (i.e. beginning of the ≥60-day consecutive gap without ibrutinib treatment). In addition to identifying any discontinuers of ibrutinib, we also identified two subgroups based on timing of discontinuation, namely discontinuers ≤12 months and discontinuers >12 months. Discontinuers ≤12 months included all patients who had evidence of ibrutinib discontinuation within 12 months of initiating treatment. Conversely, discontinuers >12 months included all patients who discontinued treatment after 12 months from treatment initiation. The 12-month cutoff was chosen as prior research has shown that many patients discontinue for toxicity-related reasons within 12 months of ibrutinib initiation [Citation6,Citation8].
Evidence of initiation of another CLL/SLL treatment after discontinuation of ibrutinib was measured as the time from ibrutinib discontinuation to the next treatment initiation, defined as the number of days from the date of discontinuation of ibrutinib to the date of initiation of a different CLL/SLL treatment. In addition, we evaluated whether the next CLL/SLL treatment was initiated within 60 days of ibrutinib discontinuation or not. We also explored the type of CLL/SLL treatment initiated after ibrutinib discontinuation, based on the first medication received after ibrutinib discontinuation. We also measured whether patients who discontinued ibrutinib restarted ibrutinib at any point over follow-up, regardless of whether or not they initiated another CLL/SLL treatment in the interim.
Finally, we measured median OS as well as 1-, 2-, and 3-year OS rates from the date of initiation of ibrutinib treatment.
Analysis
We used summary statistics including mean (standard deviation [SD]) and/or median (interquartile range [IQR]) for continuous data and relative frequencies for categorical data to describe the study sample. All descriptive results were stratified by discontinuation status and timing of discontinuation (any discontinuers, discontinuers ≤12 months, discontinuers >12 months).
Multivariable regressions were used to identify factors associated with discontinuation status and timing of discontinuation. Cox regressions were used to examine factors associated with ibrutinib discontinuation in the overall sample. Logistic regression was used to examine factors associated with discontinuation ≤12 months versus discontinuation >12 months among patients who were discontinuers. Model covariates included sociodemographic characteristics (age, sex, race, census region, metropolitan status, Part D low-income subsidy [LIS] status, Part D drug benefit type), ibrutinib treatment characteristics on initiation date (monotherapy vs. combination therapy, dose [<420 mg vs. ≥420 mg], year of index ibrutinib prescription), and clinical characteristics in the 12-month pre-index period (NCI-adjusted Charlson comorbidity score, all-cause hospitalization, evidence of any prior CLL treatment, and cardiovascular comorbidities).
All analyses were performed using SAS Enterprise Guide Version 9.4 (SAS Institute, Cary, NC, USA). This study was deemed exempt from review by Pearl IRB.
Results
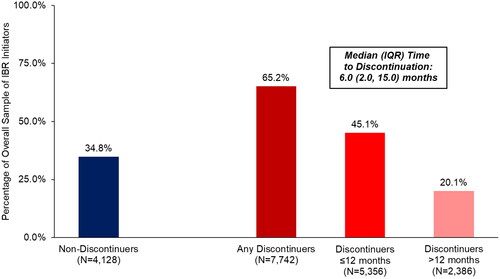
Our final sample included 11,870 Medicare beneficiaries with CLL who newly initiated ibrutinib treatment between 1 January 2014 and 31 December 2018 (). Over a median (IQR) follow-up of 2.2 (1.2, 3.4) years, 65.2% of beneficiaries had evidence of ibrutinib discontinuation (). Median (IQR) time to discontinuation was 6.0 (2.0, 15.0) months; 45.1% of patients discontinued within 12 months of ibrutinib initiation, and 20.1% of patients discontinued ibrutinib >12 months after ibrutinib initiation ().
Figure 1. Discontinuation status and timing of discontinuation among all elderly Medicare beneficiaries with CLL/SLL initiating ibrutinib.
Median (IQR) follow-up from ibrutinib initiation date was 2.1 (1.2, 3.3) years in the overall sample of 11,870 patients.
Sample characteristics by discontinuation status are presented in . The mean age of the entire cohort was 77.1 years (SD: 6.9) and 58.5% were male. Relative to non-discontinuers, patients who were in any discontinuers group were slightly older (mean age 77.6 vs. 76.4) and more likely to have evidence of prior CLL treatment (35.9% vs. 27.5%) within the 12 months prior to ibrutinib initiation. No major differences in sex, race, and urban residence were observed across the groups. Between 11.4% and 14.6% of the patients across all groups received an LIS under Medicare Part D. The vast majority (86.1–90.3%) of patients initiated ibrutinib as monotherapy; few patients (6.5–10.1%) initiated a dose <420 mg. Over one in three patients (32.8–40.5%) had evidence of all-cause hospitalization within 12 months prior to ibrutinib initiation across all groups.
Table 1. Sample characteristics by discontinuation status among all elderly Medicare beneficiaries with CLL/SLL initiating ibrutinib.
The Cox regression results for factors associated with discontinuation are presented in . Increasing age was associated with a higher hazard of discontinuation with patients aged 80+ years compared to those aged 65–69 years (hazard ratio [HR]: 1.51; 95% confidence interval [CI]: 1.41–1.63). Not receiving a LIS to cover all or part of their prescription out-of-pocket costs was also associated with higher hazard of discontinuation relative to those who received LIS (HR: 1.08; 95% CI: 1.01–1.16). Other baseline clinical characteristics that were associated with a higher hazard of discontinuation included initiation of ibrutinib as part of combination therapy (HR: 1.16, 95% CI: 1.08–1.25), NCI-adjusted Charlson comorbidity score (HR: 1.06, 95% CI 1.05–1.08), all-cause hospitalization (HR:1.10, 95% CI:1.04–1.16) in the 12-month pre-index period, evidence of prior history of CLL treatment (HR: 1.14, 95% CI 1.08–1.20), atrial fibrillation (HR: 1.27, 95% CI 1.19–1.35), conduction disorders (HR: 1.11, 95% CI 1.03–1.20), and heart failure (HR:1.09, 95% CI: 1.01–1.17). Logistic regressions showed several of the same factors such as advanced age, initiation of ibrutinib as combination therapy, NCI-adjusted Charlson comorbidity score, atrial fibrillation, heart failure, and evidence of hospitalization were associated with higher odds of ibrutinib discontinuation ≤12 months (vs. >12 months) of treatment initiation among the subset of patients who discontinued ibrutinib ().
Table 2. Cox regression results for factors associated with discontinuation among all elderly Medicare beneficiaries with CLL/SLL initiating ibrutinib.
presents the initiation of another CLL/SLL treatment after discontinuation of ibrutinib by discontinuation status. Among the discontinuers group, 39.3% initiated another CLL/SLL treatment over a median follow-up of 1.2 years after ibrutinib discontinuation. The median time to initiation of another CLL/SLL treatment was 54.0 days. Of the discontinuers, 20.2% initiated another treatment within 60 days and the median time to the next treatment in this group was less than a week (4.0 days). Conversely, the median time to the next treatment was over 9 months (287.0 days) among those who initiated another CLL/SLL treatment >60 days after ibrutinib discontinuation. Among any discontinuers, the top three drug classes initiated after ibrutinib discontinuation were chemotherapy (12.7%), anti-CD20s (11.7%), and BCL-2s (8.4%); less than 2% of the discontinuers initiated a second-generation BTKi (i.e. acalabrutinib and zanubrutinib). Differences were observed in the rate of initiation of another CLL/SLL treatment and the class of treatment initiated based on the timing of discontinuation. For instance, patients who discontinued >12 months (12.2%) had a higher rate of BCL-2 use relative to discontinuers ≤12 months (6.7%). Across all groups, about one-fourth (22.6–26.6%) of patients who discontinued ibrutinib restarted treatment with ibrutinib at some point during follow-up.
Table 3. Initiation of another CLL/SLL treatment after discontinuation of ibrutinib among elderly Medicare beneficiaries with CLL/SLL by discontinuation status.
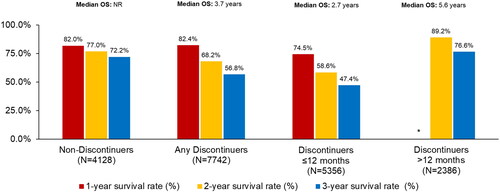
OS from ibrutinib initiation varied by discontinuation status (). While median OS was not reached among non-discontinuers, it was 3.7 years in any discontinuers group, 2.7 years in the discontinuers ≤12 months group, and 5.6 years in the discontinuers >12 months group. The 1-, 2-, and 3-year OS rates were the lowest among patients in the discontinuers ≤12 months group (74.5%, 58.6%, 47.4%).
Figure 2. Overall survival from ibrutinib initiation among all elderly Medicare beneficiaries with CLL/SLL by discontinuation status.
*One-year OS rate not shown for discontinuers >12 months since by definition 100% of these patients would need to be alive for at least 12 months to qualify for inclusion in this group.
Discussion
To the best of our knowledge, this real-world study of a nationally representative cohort of elderly Medicare beneficiaries examined the largest sample to date of patients initiating ibrutinib for CLL/SLL. As such, it provides important insights into ibrutinib discontinuation, post-ibrutinib treatment use, and OS.
One of the key findings of our study was that nearly two-thirds of the 11,870 elderly patients who initiated ibrutinib had discontinued treatment, as defined by a continuous 60-day gap in ibrutinib prescription supply, over a median follow-up of 2 years. Among patients who discontinued ibrutinib, half had discontinued within 6 months of treatment initiation and over 70% did so within 12 months of initiating treatment. While direct comparisons are difficult due to differences in study samples, measurement of discontinuation, and duration of follow-up, in general, the discontinuation rate observed in our real-world sample of elderly Medicare beneficiaries is much higher than what was observed in clinical trials (e.g. RESONATE, RESONATE-2) and prior real-world studies [Citation5,Citation6,Citation16]. Taken together, these findings suggest that there is still a significant unmet need among elderly patients with CLL/SLL receiving this first-generation BTKi.
While our claims-based study is unable to examine the reasons for discontinuation, prior research has shown adverse events to be a frequent cause in the real-world setting [Citation8]. Hence, our study findings that advanced age, history of atrial fibrillation and heart failure, higher comorbidity burden, and any hospitalization in the prior year were more likely to be associated with ibrutinib discontinuation are consistent with findings from prior studies [Citation8,Citation16,Citation17]. Patients who are older and more frail are likely to have a higher susceptibility to and a lower tolerability for adverse events [Citation16,Citation18]. We also found that patients who had evidence of prior CLL/SLL treatment in the 12-month pre-index period had increased odds of discontinuation. It is possible that this measure served as a proxy for refractory CLL/SLL, suggesting that refractory patients have a greater risk of discontinuation than individuals having 12+ months without treatment prior to ibrutinib initiation. Similarly, we found several key factors associated with early discontinuation (≤12 months) among those who discontinue ibrutinib. Taken together, our findings suggest that there are several key characteristics – age, cardiovascular comorbidities, frailty, and refractory status – that may increase the likelihood of premature ibrutinib discontinuation.
Another key finding of our study was that patients who lack an LIS to cover their Medicare Part D cost-sharing had a higher risk of discontinuation relative to patients receiving the subsidy. Patients who do not receive a LIS face substantial out-of-pocket costs for ibrutinib under Medicare Part D – as high as $8,000 for each year of ibrutinib treatment [Citation19], unless they receive help from charitable assistance organizations [Citation20]. Prior literature has established the association between high out-of-pocket costs and discontinuation of oral anticancer agents [Citation21–23].
Our study reflects the largest analysis to date of treatments initiated after ibrutinib discontinuation in the real-world setting. We found that less than 40% of patients received other CLL/SLL therapies over a median follow-up of 1.2 years after discontinuing ibrutinib despite the availability of other treatment options. It is possible that some of the patients who discontinued ibrutinib due to toxicity did not have progression of their CLL for an extended period and hence did not need to initiate another treatment. Among those who did initiate another treatment, the median time to the next treatment was less than 2 months, with many of these individuals starting a new line within a few days of discontinuation. While we cannot know the reason for discontinuation from the claims data, these findings suggest that some patients initiate a new therapy after stopping ibrutinib treatment likely due to disease progression. Our examination of the type of new treatment initiated after ibrutinib discontinuation revealed that the top three drug classes were traditional chemotherapies, anti-CD20s, and BCL-2s during our study period. While some patients did receive a second-generation BTKis (i.e. acalabrutinib) after discontinuing ibrutinib, use was relatively low during our study. Given acalabrutinib was only approved for CLL in November 2019 and our study period ended in December 2019, low rates of acalabrutinib use are unsurprising [Citation24]. Furthermore, around one in four discontinuers had evidence of restarting ibrutinib at some point after initial ibrutinib discontinuation, possible evidence of a drug holiday or ibrutinib rechallenge. Overall, our findings on the initiation of new treatments and restart of ibrutinib add to the evidence suggesting a high unmet need for elderly patients with CLL/SLL.
Our descriptive findings suggest that early discontinuation may be associated with shorter OS. Specifically, median OS from ibrutinib initiation was almost half for patients who discontinued within 12 months of starting ibrutinib treatment (2.7 years) compared to non-discontinuers (5.6 years). Prior work has shown that patients discontinuing ibrutinib earlier due to CLL/SLL progression or Richter’s transformation have poor survival, especially in heavily pretreated patient populations with few alternative options [Citation13]. More recent data suggest that patients stopping ibrutinib due to adverse events do not have as poor survival when compared to earlier more heavily pretreated cohorts receiving ibrutinib after many lines of CLL-directed therapy [Citation8]. Although we were unable to examine the reasons for worse survival among patients who discontinue ibrutinib within 12 months of starting treatment, these descriptive findings further underscore the potential need for more vigilant patient monitoring to prevent early discontinuation and/or initiation of alternative novel agents, especially in the first year after treatment initiation.
Our study has several limitations. First, Medicare claims data do not include reasons for discontinuation and lack detailed clinical information (lab values, relapsed/refractory status, etc.) that would allow for greater nuance in the analysis. However, an advantage of the Medicare claims data is that it permits efficient and timely population-level analyses of a nationally representative sample of elderly patients with CLL/SLL compared to studies using data sources with smaller sample sizes and/or non-representative samples. Second, our measure of discontinuation was based on a 60-day continuous gap in the availability of ibrutinib observed using prescription claims data as has been used in prior studies [Citation14,Citation15]; we do not have patient or provider confirmation that ibrutinib was discontinued. Third, given that the latest available data at the time of our analysis was 31 December 2019, we were unable to assess discontinuation among elderly patients initiating newer second-generation BTKis that were more recently approved. Fourth, our analysis of the initiation of CLL/SLL treatment after ibrutinib discontinuation was limited in two respects. Our study period spanned from 1 January 2014 to 31 December 2019, whereas many of the newer agents (BCL-2s, second-generation BTKis) were only available for CLL after 2018, meaning the proportion of patients using these newer agents may be higher in more recent years than what was reported in our study. We also only assessed the first treatment received after ibrutinib discontinuation and did not attempt to assess entire treatment regimens. Fifth, our identification of patients with evidence of prior CLL treatment was limited by the use of a 12-month pre-index window; given that CLL patients can go many years between treatments, patients in our sample with evidence of prior CLL treatment within the last 12 months likely have refractory disease rather than relapsed CLL. Sixth, our study sample was limited to the fee-for-service Medicare population and hence our results cannot be generalized to elderly patients with CLL/SLL under Medicare Advantage. However, between 60% and 70% of the U.S. Medicare population is covered under the fee-for-service Medicare program. Finally, our real-world study was descriptive in nature and attempts were not made to examine the causal relationship between ibrutinib discontinuation status/timing and OS.
In conclusion, this largest real-world study to date of elderly patients with CLL/SLL initiating ibrutinib shows high rates of discontinuation and low rates of initiation of another CLL/SLL treatment. Discontinuation was more likely in those with advanced age, frailty, refractory CLL/SLL, lack of a Part D LIS, and cardiovascular comorbidities such as atrial fibrillation. Taken together, our findings point to a large unmet need with the widely used BTKi ibrutinib. Clinicians should be made aware of the patient characteristics associated with ibrutinib discontinuation and closely monitor patients accordingly. Alternatively, these patients who have higher risk of discontinuation on ibrutinib may be ideal candidates for other novel agents that offer a better safety and/or efficacy profile. Future research should examine how newer novel agents (e.g. BCL-2s, next-generation BTKis) address this unmet need in elderly patients with CLL/SLL.
Disclosure statement
SFH: consultancy for Janssen, Pharmacyclics, AbbVie, AstraZeneca, Flatiron Health Inc., Novartis, SeaGen, Genentech, Merck, TG Therapeutics, ADC Therapeutics, Epizyme, Servier, Arvinas, and Thyme Inc.; research funding from Celgene, DTRM Biopharm, and TG Therapeutics; honoraria form Pharmacyclics and AstraZeneca, Bayer. EDN, MF, KR, ES, SL, and XY are employees of Merck & Co., Inc. JP and SKB are employees of COVIA Health Solutions, a consulting firm with clients in the biotech/pharmaceutical industry. JAD: consultancy for AbbVie, Acadia, Allergan, Boehringer Ingelheim, Catabasis, Ironwood Pharmaceuticals, Janssen, Kite Pharma, MeiraGTx, Merck, Otsuka, Regeneron, Sarepta, Sage Therapeutics, Sanofi, Takeda, The Medicines Company, and Vertex; research funding from AbbVie, Biogen, Humana, Janssen, Merck, Novartis, Pfizer, PhRMA, Regeneron, Sanofi, and Valeant.
Additional information
Funding
References
- American Cancer Society. What is chronic lymphocytic leukemia? [cited 2023 Jan 9]. Available from: https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/about/what-is-cll.html
- Gomes LC, Ferrão ALM, Evangelista FCG, et al. Advances in chronic lymphocytic leukemia pharmacotherapy. Biomed Pharmacother. 2018;97:349–358. doi:10.1016/j.biopha.2017.10.105
- Iovino L, Shadman M. Novel therapies in chronic lymphocytic leukemia: a rapidly changing landscape. Curr Treat Options Oncol. 2020;21(4):24. doi:10.1007/s11864-020-0715-5
- Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–1363. doi:10.1002/ajh.25638
- Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–798. doi:10.1038/s41375-019-0602-x
- O'Brien SM, Byrd JC, Hillmen P, et al. Outcomes with ibrutinib by line of therapy and post-ibrutinib discontinuation in patients with chronic lymphocytic leukemia: phase 3 analysis. Am J Hematol. 2019;94(5):554–562. doi:10.1002/ajh.25436
- Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–2506. doi:10.1182/blood-2014-10-606038
- Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879. doi:10.3324/haematol.2017.182907
- Lipsky AH, Farooqui MZH, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;100(12):1571–1578. doi:10.3324/haematol.2015.126672
- Brown JR, Moslehi J, O'Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102(10):1796–1805. doi:10.3324/haematol.2017.171041
- Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067. doi:10.1182/blood-2014-09-603670
- Jain P, Thompson PA, Keating M, et al. Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer. 2017;123(12):2268–2273. doi:10.1002/cncr.30596
- Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80–87. doi:10.1001/jamaoncol.2014.218
- Mato AR, Samp JC, Gauthier G, et al. Drivers of treatment patterns in patients with chronic lymphocytic leukemia stopping ibrutinib or idelalisib therapies. Cancer Biol Ther. 2018;19(7):636–643. doi:10.1080/15384047.2018.1449616
- Hampel PJ, Ding W, Call TG, et al. Rapid disease progression following discontinuation of ibrutinib in patients with chronic lymphocytic leukemia treated in routine clinical practice. Leuk Lymphoma. 2019;60(11):2712–2719. doi:10.1080/10428194.2019.1602268
- Hardy-Abeloos C, Pinotti R, Gabrilove J. Ibrutinib dose modifications in the management of CLL. J Hematol Oncol. 2020;13(1):66. doi:10.1186/s13045-020-00870-w
- Sharman J, Kabadi SM, Clark J, et al. Treatment patterns and outcomes among mantle cell lymphoma patients treated with ibrutinib in the United States: a retrospective electronic medical record database and chart review study. Br J Haematol. 2021;192(4):737–746. doi:10.1111/bjh.16922
- Goyal RK, Nagar SP, Kabadi SM, et al. Overall survival, adverse events, and economic burden in patients with chronic lymphocytic leukemia receiving systemic therapy: real-world evidence from the Medicare population. Cancer Med. 2021;10(8):2690–2702. doi:10.1002/cam4.3855
- Walker J. Patients struggle with high drug prices – WSJ [cited 2023 Jan 9]. Available from: https://www.wsj.com/articles/patients-struggle-with-high-drug-prices-1451557981
- Olszewski AJ, Zullo AR, Nering CR, et al. Use of charity financial assistance for novel oral anticancer agents. J Oncol Pract. 2018;14(4):e221–e228. doi:10.1200/JOP.2017.027896
- Kaisaeng N, Harpe SE, Carroll NV. Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J Manag Care Spec Pharm. 2014;20(7):669–675. doi:10.18553/jmcp.2014.20.7.669
- Doshi JA, Li P, Pettit AR, et al. Reducing out-of-pocket cost barriers to specialty drug use under Medicare part D: addressing the problem of “too much too soon”. Am J Manag Care. 2017;23(3 Suppl):S39–S45.
- Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017;35(2):166–174. doi:10.1200/JCO.2016.68.2856
- Lymphoma Research Foundation. U.S. Food and Drug Administration approves acalabrutinib (CALQUENCE) for chronic lymphocytic leukemia/small lymphocytic lymphoma. Lymphoma Research Foundation [cited 2022 Dec 8]. Available from: https://lymphoma.org/news/u-s-food-and-drug-administration-approves-acalabrutinib-calquence-for-chronic-lymphocytic-leukemia-small-lymphocytic-lymphoma/
Appendix
Appendix Table A1. ICD-9 and ICD-10 codes for other FDA-approved indications of ibrutinib.
Appendix Table A2. Sample attrition.
Appendix Table A3. Logistic regression results for discontinuation within 12 months (vs. disk >12 months) in the subset of patients who are discontinuers among Medicare beneficiaries with CLL/SLL initiating ibrutinib.
