Abstract
Mantle cell lymphoma (MCL) is a rare non-Hodgkin lymphoma that frequently becomes chemoresistant over time. The distinct mechanisms of ibrutinib and lenalidomide provided a judicious rationale to explore the combination with anti-CD20 immunotherapy. In this phase 1b study (NCT02446236), patients (n = 25) with relapsed/refractory MCL received rituximab with escalating doses of lenalidomide (days 1–21) and ibrutinib 560 mg (days 1–28) of 28-day cycles. The MTD for lenalidomide was 20 mg; most common grade ≥3 adverse events were skin rashes (32%) and neutropenic fever (24%). The best ORR was 88%, CR rate was 83%, and median duration of response (DOR) was 36.92 months (95% CI 33.77, 51.37). Responses were seen even in refractory patients or with high-risk features (e.g. blastoid variant, TP53 mutation, Ki-67 > 30%). R2I was safe and tolerable in patients with R/R MCL.
Introduction
Though a rare subtype of non-Hodgkin lymphoma (NHL), mantle cell lymphoma (MCL) has been the focus of intense research efforts due to its poorer outcomes, typically characterized by a pattern of relapse with patients frequently becoming chemoresistant over time [Citation1, Citation2]. Such efforts over the last 2 decades have led to improvement in median progression-free survival (PFS) in excess of 7 to 8 years and overall survival (OS) of more than 10 years, especially in younger patients, after dose-intensive therapies with or without autologous stem cell transplant (ASCT) [Citation3–5]. In elderly or unfit patients, chemoimmunotherapy with BR (bendamustine and rituximab) has become the backbone of induction therapy leading to superior PFS over R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), while maintenance rituximab has shown benefit across all subgroups [Citation6, Citation7]. However, real-world data show that overall median time to second line therapy is still just over 24 months and median OS is shorter compared to trials [Citation8, Citation9]. MCL patients continue to relapse with progressively shorter clinical benefit from subsequent lines using standard therapies as the disease often evolves into blastoid variant or other high-risk molecular types [Citation10, Citation11]. Innovative treatment approaches that combine new agents with distinct mechanisms of action and consider tumor characteristics are needed for relapsed/refractory (R/R) MCL patients.
Remarkably, 7 novel agents for the treatment of MCL have been approved in the US since the mid-2000s, including Bruton’s tyrosine kinase (BTK) inhibitors, which have shown durable responses even in heavily pretreated patients. The approval of these agents has led to much interest in evaluating the safety and efficacy of potentially less toxic, ‘chemotherapy-free’ treatment regimens for R/R MCL patients [Citation12]. Lenalidomide is an immunomodulatory agent that may enhance the antitumor activity of rituximab to overcome some of the resistance seen in patients with R/R NHL [Citation13]. As monotherapy for R/R MCL, lenalidomide was shown as a promising treatment option with an overall response rate (ORR) of 28% (complete response [CR] rate 7.5%) despite patients having received a median of 4 prior lines of therapy (range 1 to 9) in the MCL-001 trial [Citation14]. Previous data suggest that synergy when lenalidomide and rituximab are used together, which may stem from lenalidomide’s ability to augment rituximab-induced antibody dependent cellular cytotoxicity by NK-cells [Citation15, Citation16]. This combination has been noted to increase trafficking of immune cells to the tumor, expand NK-cell populations, and potentiate activity in rituximab-resistant lymphomas [Citation15]. A study evaluating rituximab and lenalidomide (R2) in 44 R/R MCL patients observed an ORR of 57% (CR 36%), and median duration of response (DOR), PFS and OS of 18.9, 11.1, and 24.3 months, respectively [Citation17].
Other studies also support doublet therapy with ibrutinib and rituximab (Ib-R) in MCL patients. BTK is an enzyme downstream of the B-cell receptor (BCR) signaling pathway that is required for B-cell proliferation, BCR-induced calcium release, and activation of the nuclear factor κB pathway [Citation18]. Ibrutinib, a selective small molecule inhibitor of BTK, inhibits BCR signaling and induces cancer cell apoptosis, resulting in decreased malignant B-cell proliferation and survival [Citation18]. In 50 R/R MCL patients who received a median of 3 (range 1–9) prior lines of therapy, Ib-R led to an ORR of 88% (CR 44%) [Citation19]. Toxicities were tolerable, with the only grade 3 adverse event (AE) occurring at ≥10% incidence being atrial fibrillation (12%) [Citation19].
Doublets such as R2 or Ib-R have shown greater activity compared to single-agents, providing the rationale for the triplet combination shown here using rituximab, lenalidomide, and ibrutinib (R2I) in patients with R/R MCL. The objective of this phase 1b study was to determine the maximum tolerated dose (MTD), and to assess the safety and potential activity of R2I in R/R MCL patients who failed standard chemoimmunotherapy.
Materials and methods
Study design
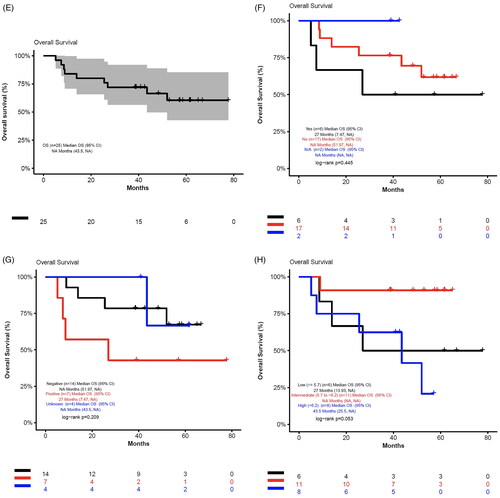
This was a phase 1b, single-center, open-label study of R2I in patients with R/R MCL. This trial was performed as a standard 3 + 3 dose-escalation design to determine the MTD of lenalidomide in combination with Ib-R. Dose escalation of lenalidomide spanned from 15 mg to 20 mg, which was selected based on the previously established MTD of lenalidomide at 20 mg when given in combination with Ib-R in patients with R/R diffuse large B-cell lymphoma [Citation20]. A dose de-escalation cohort of lenalidomide 10 mg was permitted in the event of significant toxicities [Citation20]. Cycle length was defined as 28 days. Ibrutinib was administered at 560 mg orally daily on days 1–28 of each cycle. Lenalidomide was administered orally daily on days 1–21 of each cycle. Rituximab 375 mg/m2 was administered weekly for 4 doses during cycle 1 followed by once on day 1 for cycles 2 through 6, then every other cycle for up to a total of 24 cycles (). This study was registered with ClinicalTrials.gov (NCT02446236).
Figure 1. Treatment schema. IV: intravenous; PO: orally. Ibrutinib was administered on days 1–28 of a 28-day cycle. Lenalidomide was administered on days 1–21 of a 28-day cycle. Dose-limiting toxicities were assessed during the first treatment cycle.
The primary objective of this trial was to define the MTD for the combination of R2I in patients with R/R MCL by assessing the incidence of dose-limiting toxicities (DLTs) in cycle 1. Secondary objectives included the incidence of grade ≥3 AEs, best ORR, DOR, PFS, and OS.
Key eligibility criteria
Patients were included if all of the following criteria were met: age ≥18 years old; histologically or cytologically confirmed MCL; R/R disease after ≥1 prior line of therapy (including stem cell transplant); ≥1 lymph node or mass evaluable for response; Eastern Cooperative Oncology Group (ECOG) performance status ≤2; and adequate organ and hematologic function. All study participants were required to register with the mandatory Revlimid® Risk Evaluation and Mitigation Strategies (REMS®) program and be able to comply with the statutes of the REMS® program. Patients were required to take prophylactic anticoagulation with aspirin or, for those who were intolerant to aspirin, low molecular weight heparin.
Key exclusion criteria included: prior treatment with lenalidomide or ibrutinib; use of chemotherapy, radiation therapy, or experimental drug therapy for treatment of MCL within 21 days or 5 half-lives of starting treatment; concomitant therapy with warfarin or other vitamin K antagonists; central nervous system (CNS) involvement by lymphoma; a secondary malignancy; major surgery or significant traumatic injury within 28 days of the first dose of study drug; or significant cardiac problems.
This study was approved by the institutional review board (IRB) at the John Theurer Cancer Center and Hackensack Meridian Health (Pro00005444). The trial was conducted under the International Conference on Harmonization Good Clinical Practice guidelines and according to the ethical principles from the Declaration of Helsinki. Informed consent was obtained from all patients.
Toxicity assessments
Safety assessments consisted of monitoring all AEs, including serious AEs and treatment emergent AEs (TEAEs), monitoring of hematology, blood chemistry, electrocardiogram, vital signs and physical conditions. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03. The MTD was defined as the highest dose level in which no more than 1 of 6 patients experienced unacceptable DLTs within 28 days of R2I therapy. DLTs were defined as grade ≥3 non-hematologic or grade 4 hematologic toxicities related to the study treatment (Text S1).
Response assessments
Response was assessed using Cheson Criteria 2007 [Citation21]. Radiologic tumor assessments included chest, abdomen, and pelvis computed tomography/magnetic resonance imaging, and positron emission tomography scans post cycles 2, 4, and 6, then every 3 cycles until disease progression or the patient was removed from the study. Best ORR was defined as the percentage of patients who achieved a CR or partial response (PR) as their best response to treatment. See Text S2 for definitions of response endpoints.
Statistical analysis
Sample size was based on a standard 3 + 3 design using 3 to 6 patients per each dose, and it was expected that no more than 3 dose levels would be required to determine the MTD. The maximum planned sample size was 28 participants, including up to 10 patients for phase 1b dose expansion. Among 25 enrolled patients, the intent-to-treat population to evaluate efficacy was 24 patients, as 1 patient was unevaluable due to early discontinuation of the study. All patients (n = 25) who received study treatment were included in the safety analysis. Efficacy and safety evaluations were summarized descriptively. Median PFS and OS for the whole cohort were evaluated using the Kaplan-Meier (KM) method. The median PFS and OS according to subgroups of interest (e.g. MIPI score, blastoid histology, and TP53 mutation status) were evaluated using the KM method and compared using the log-rank test. ORR was summarized with Clopper-Pearson 95% confidence intervals (CI). A two-sided significance level of 0.05 was used for statistical significance.
Results
Patient characteristics
Twenty-five subjects were enrolled in the study between November 2015 and April 2019. The median age was 61 years (range 39–79), 76% were male, received a median of 1 (range 1–4) prior lines of therapy, 12% were primary refractory, and 32% were refractory to their last line of therapy (). In this cohort, 41% had Ki-67 > 30% at baseline, 33% had overexpression of p53 detected by IHC, and 26% had blastoid histology. Most patients had either high (32%) or intermediate (44%) MIPI scores, and all (100%) patients had an ECOG of 0–1.
Table 1. Baseline characteristics.
Treatment and safety
The first escalation cohort began treatment with lenalidomide 15 mg (one dose level below the established MTD dose) as described in the Methods section [Citation20]. One of these patients discontinued the trial prior to the first assessment and was not considered evaluable. Subsequently an additional patient was enrolled into the first escalation cohort, and none of these 3 patients experienced toxicity at the first dose level within 28 days of starting treatment. The subsequent cohort of patients were enrolled into the next dose level (lenalidomide 20 mg), and 1 of 6 patients experienced a DLT of diffuse, grade 3 skin rash that was treated with intravenous and topical steroids. After this occurrence, the MTD of lenalidomide was determined to be 20 mg orally daily on days 1–21 when given in combination with Ib-R.
The most common grade ≥3 toxicities were rash (33%) and neutropenic fever (25%) (). Of the 24 patients evaluable for toxicity, 23 patients (96%) discontinued therapy because of either TEAEs (n = 9, 39%), disease progression (n = 7, 32%), patient preference (n = 5, 23%) (2 geographic relocation, 3 patient choice), or death (n = 2, 9%). Of the 9 patients who discontinued the study due to TEAEs, 1 patient experienced worsening hypertension; 1 patient developed a pulmonary infection caused by Haemophilus influenzae and Aspergillus; 1 patient developed osteomyelitis caused by methicillin-susceptible Staphylococcus aureus (MSSA) and was treated with oxacillin; 1 patient developed cellulitis and was treated with doxycycline and levofloxacin; 1 patient developed dizziness which resolved with discontinuation of ibrutinib; 1 patient experienced a syncopal event due to a drop in heart rate and dehydration but did not develop bleeding or any neurological deficits; 1 patient developed atrial fibrillation; 1 patient had an ICU admission complicated by sepsis, pneumonia, and respiratory failure; and 1 patient developed persistent headaches after 65 cycles of treatment that resolved with ibrutinib discontinuation.
Table 2. Grade ≥3 adverse events.
One patient with intermediate MIPI who was positive for TP53 and disease involvement of bone marrow, scalp, and lymph nodes, remains in CR and is still actively on the study April 2023. The patient had been previously treated with rituximab, R-HCVAD, and bortezomib, and is in the 76th cycle receiving ibrutinib 560 mg orally daily. Of the 5 patients who opted to withdraw from the study due to patient preference, Citation3 patients completed 50 cycles, 34 cycles and 31 cycles, respectively, prior to switching to standard of care treatment; and 2 patients discontinued treatment after receiving 52 and 38 cycles on the study prior to geographic relocation.
Response and survival
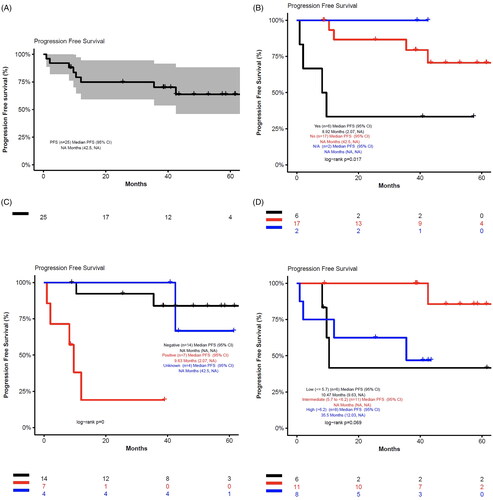
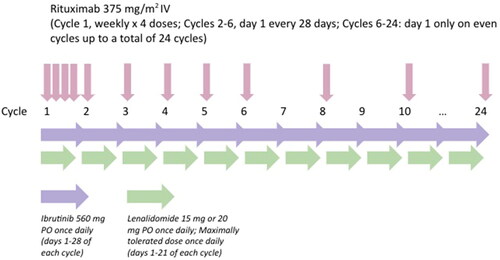
Of the 24 evaluable patients, the best ORR was 88% (95% CI 66.5, 96.7) (20 CR, 1 PR), CR rate was 83% (95% CI 61.8, 94.5), and clinical benefit rate ≥2 months was 92% (). In the overall cohort, the median PFS was not reached (95% CI 42.5, NA) and median OS was not reached (95% CI 43.5, NA) at 6 years of follow up (). The median DOR was 36.92 months (95% CI 33.77, 51.37) with all but 1 of patients achieving a CR as shown in and Figure S1A. For the 9 patients with Ki-67 ≥ 30% at baseline, the best ORR was 67% (95% CI 30.9, 90.9) (6 CR, 1 SD, 2 progressive disease [PD]).
Figure 2. Best response in evaluable patients. Response was evaluated after cycles 2, 4, 6 and then every 3 cycles thereafter until disease progression or discontinuation of study using Cheson criteria 2007. Best overall response was defined as the percentage of patients who achieved a CR or PR. Clinical benefit rate was defined as the percentage of patients who achieved CR, PR, or SD for at least 2 months. One patient who was not evaluable for response was excluded.
Figure 3. Progression-free and overall survival. Progression-free and overall survival in all patients (n = 25). Overall survival was defined as the number of months from first dose date to the date of death or censoring prior to the data cutoff. Progression-free survival was defined as the number of months from first dose date to the date of first event (disease progression or death) or censoring prior to the data cutoff. A. Progression-free survival in all patients. B. Progression-free survival in patients with blastoid/pleomorphic variant. C. Progression-free survival in patients with overexpression of p53 detected by IHC. D. Progression-free survival in patients by MIPI score. E. Overall survival in all patients. F. Overall survival in patients with blastoid/pleomorphic variant. G. Overall survival in patients with overexpression of p53 detected by IHC. H. Overall survival in patients by MIPI score.
Figure 4. Duration of response. CR: complete response; PR: partial response; SD: Stable disease; PD: progressive disease. Duration of response in 24 evaluable patients. Duration of response was defined as the number of months from first documented response to the date of first event (disease progression or death) or censoring prior to the data cutoff. One patient who was not evaluable for response was excluded.
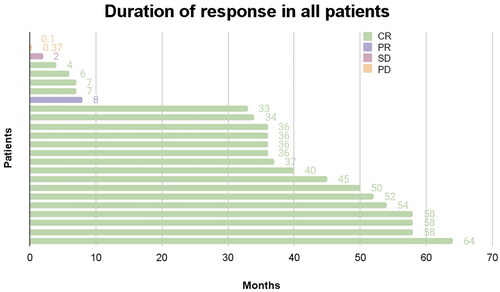
For the 6 patients with blastoid histology, of whom 5 patients received ≥2 prior lines of therapy, the best ORR was 50% (95% CI 18.8, 81.2) (33 CR, 1 SD, 2 PD) in the overall cohort of blastoid patients and 40% (95% CI 7.2, 82.9) (2 CR, 1 SD, 2 PD) in those who received ≥2 prior lines of therapy. The median DOR was 5.03 months (95% CI 0.37, NA); median PFS was 8.92 months (95% CI 2.07, NA); and median OS was 27 months (95% CI 7.47, NA) (Figure S1B, ).
There were 7 patients (28%) positive for overexpression of p53 by IHC, of whom 5 patients received ≥2 prior lines of therapy. The best ORR was 57% (95% CI 20.2, 88.2); disease control rate was 71% (95% CI 30.3, 94.9) (4 CR, 1 SD, 2 PD); and the median DOR was 4.93 months (95% CI 0.37, NA) (Figure S1C). Median PFS was 9.63 months (95% CI 2.07, NA) and median OS was 27 months (95% CI 7.47, NA) ().
In high MIPI patients (n = 8), the best ORR was 71% (95% CI 30.3, 94.9) (5/7, 5 CR, 2 PD, 1 not evaluable for response); DOR was 33.77 months (95% CI 0.37, NA); median PFS was 35.5 months (95% CI 12.03, NA); and median OS was 43.5 months (95% CI 25.5, NA) (Figure S1D, ). For intermediate MIPI patients (n = 11), the best ORR was 100% (11/11 CR); DOR was 46.4 months (95% CI 37.03, NA); and neither median PFS (95% CI NA, NA) nor the median OS were reached (95% CI NA, NA) (Figure S1D, ). For low MIPI patients (n = 6), the best ORR was 100% (6/6, 4 CR, 1 PR, 2 SD); median DOR was 8.2 months (95% CI 4.93, NA); median PFS was 10.47 months (95% CI 9.63, NA); and median OS was 27 months (95% CI 13.93, NA) (Figure S1D, ).
Discussion
A shift in the treatment paradigm for MCL is largely in part to the approval of several targeted agents that provide ‘chemotherapy-free’ options for patients unable to tolerate dose intensive conventional therapy. Previous data suggest 2-drug regimens demonstrate impressive activity in R/R MCL patients with ORR of >80% (CR 44%) for Ib-R and ORR of 57% (CR 36%) for R2, lending to the hypothesis that combining all 3 agents may provide synergy [Citation12, Citation17, Citation19, Citation22]. This phase 1b study evaluated the safety and potential activity of the R2I regimen in 25 R/R MCL patients. When given in this triplet combination, the MTD of lenalidomide was found to be 20 mg due to the occurrence of treatment-related grade 3 skin rash in 1 of 6 patients enrolled at this dose level during cycle 1.
Lenalidomide was well-tolerated in combination with Ib-R in patients with R/R MCL. The most common grade ≥3 AEs observed were skin rashes (32%) and neutropenic fever (24%). Skin rash is a known AE of lenalidomide thought to be related to amplified cytokine production, T-cell and NK-cell stimulation, and may be a positive predictive factor in some cohorts [Citation23]. In registration trials, the reported incidence of grade ≥3 lenalidomide-associated skin rash was <1% to 6% [Citation24]. In our study, there was an increased incidence of grade ≥3 skin rash in 32% of patients, but, importantly, none of these patients discontinued the trial due to this AE. This increased incidence may be attributed to an additive effect of administering lenalidomide in combination with ibrutinib. Ibrutinib use alone may cause the development of rash and is associated with an overall incidence of 15% (grade ≥3 skin rash in 2%) [Citation25]. In the PHILEMON study, which studied R2I in R/R MCL, the incidence of grade ≥3 cutaneous toxicity was 14%, supporting the rationale a slight increase in toxicity may be due to the triplet combination, but is still much lower than what was observed in our study (32%) [Citation26]. Jerkeman et al. hypothesized that cutaneous toxicity may be blunted in patients with chemotherapy exposure as their patient population was more heavily pretreated (median of 2 [range 1–7] previous therapies) compared to our patient population (median of 1 [range 1–4] previous therapies) [Citation26]. In the Alliance A051103 trial, R2I was studied in patients with untreated follicular lymphoma and resulted in an incidence of grade ≥3 cutaneous toxicity of 36%, which is comparable to our study [Citation27].
Other AEs of interest are cardiac abnormalities, including atrial fibrillation/flutter, which are common side effects associated with ibrutinib [Citation28, Citation29]. In our study, 1 patient (4%) developed paroxysmal atrial fibrillation/flutter, which is slightly lower than other studies that report an incidence of approximately 8% with ibrutinib [Citation30]. Our patient experienced episodes of paroxysmal atrial fibrillation/flutter despite titration of beta blocker therapy leading to discontinuation of ibrutinib and switching to a selective BTK inhibitor, zanubrutinib, with resolution of cardiac intolerance. Overall, the R2I regimen appeared safe, and the toxicity profile was consistent with what has been observed with these agents in prior studies [Citation26, Citation27].
In 24 evaluable patients, we observed an impressive, best ORR of 88% and median DOR of nearly 37 months. The PHILEMON study evaluated the R2I regimen at a lower dose of lenalidomide 15 mg in 50 patients with R/R MCL and observed similarly high response rates with an ORR of 76% (CR 56%) [Citation26]. At a median follow-up of 40 months, median PFS was 18 months (95% CI 6.5–28), and median OS was 47 months (95% CI 30–64) [Citation31]. Thus, the R2I combination appears to be highly active in R/R MCL. Considering our data and other available therapies for MCL, it is important to know what a patient has previously received for treatment to determine optimal sequencing. CAR T-cell therapy has shown impressive results as second line treatment in MCL patients and may be a better option for younger patients or those with a relatively aggressive presentation. Results from the recent TRIANGLE study evaluating frontline ibrutinib plus chemoimmunotherapy with or without ASCT in younger MCL patients suggest improved failure-free survival and no added toxicity with the combination [Citation32]. As this treatment approach is increasingly used, patients may require an alternate BTKi in the relapsed setting to offset possible resistance. MCL is a heterogenous disease that will potentially have different frontline treatments that can be considered SOC and more research is needed, which may include evaluating R2I in the frontline setting or with a limited treatment time and assessment of MRD.
High-risk features, specifically blastoid histology and TP53 mutations, have classically conferred a poorer prognosis in MCL patients regardless of the regimen used, even with high-dose chemotherapy [Citation11]. In the WINDOW-1 trial, 131 patients with previously untreated MCL received up to 12 cycles of Ib-R induction (part A) followed by 4 cycles of R-HCVAD (part B) [Citation33]. In the overall cohort, the ORR after Ib-R (part A) and R-HCVAD (part B) was 98% and 90%, respectively. Despite an ORR of 91%, patients with TP53 mutations seemed to experience early progression as an initial CR of 88% at the end of Ib-R lowered to a CR of 55% with R-HCVAD; subsequently, the protocol was modified to allow for ibrutinib maintenance therapy in these patients. This lends to the rationale behind our trial that a 3-drug regimen consisting of two targeted agents with known activity in MCL may be advantageous in patients with previously treated and high-risk disease to sustain response. In our study, there were 6 patients (24%) with blastoid histology and 7 patients (28%) with TP53 mutations. In this cohort of high-risk patients, there were 6 patients who experienced early progression of ≤7 months, which is consistent with the classical pattern of worse outcomes in these patients. Intriguingly, two patients with blastoid variant sustained a CR for 34 and 54 months while on study, and we observed no significant differences in OS between patients with and without blastoid variant; however, our sample size was very small. Patients with TP53 mutations had a lower ORR of 57% compared to the overall cohort with an ORR of 88%, as expected. Notably one TP53 positive patient with extranodal disease involvement remained in CR for 64 months at data cutoff and is continuing treatment on study.
Limitations of this study include the single-center design and small number of patients. In addition, we sought to identify differences in response and survival when stratified by MIPI score. However, our results were unexpected as patients with low MIPI scores had poorer OS when compared to those with intermediate or high MIPI scores. Possible explanations include the small numbers of patients in each subgroup and other disease factors impacting patient outcomes other than those comprising the MIPI score. In our cohort of patients with low MIPI scores (n = 6), 2 had blastoid histology and 3 were TP53 mutation positive, which are high-risk molecular subtypes associated with a poor prognosis not considered in the MIPI score. Another limitation of this study was it became difficult to enroll patients on this study due to the evolving treatment landscape of MCL with the availability of novel therapies or new treatment options that were approved or offered through other clinical trials.
In conclusion, this phase 1b study showed the R2I regimen was safe and tolerable in patients with R/R MCL. The regimen led to very high ORR and CR rate, even in high-risk patients, suggesting that R2I may help to overcome some of the resistance observed in these patients. The results of this study warrant further review of R2I in larger clinical trials. Furthermore, trials combining various targeted therapies are currently ongoing both in the frontline and in R/R MCL, which will help build the armamentarium of non-chemotherapy options in MCL management.
Authors’ contributions
Conception and design: AI, AHG; administrative support: JZ, LP; provision of study materials or patients: AI, TF, LAL, AHG; collection and assembly of data: AP, SG, JZ, MG, VB, AN, AD, GL; data analysis and interpretation: AP, SG, ADP, VB, AN, AHG, AI, AD, GL, JZ, JA; manuscript writing: All authors; Final approval of manuscript: All authors.
Supplemental Material
Download MS Word (237.7 KB)Data availability statement
The data that support the findings of this study are available on request from the corresponding author, AI. The data are not publicly available due to their containing information that could compromise the privacy of research participants. The study involves a review of the electronic medical records of mantle cell lymphoma patients who received R2I. The research data set has removed most, but not all protected health information (for example: actual dates of response assessments and treatment were included as needed for analysis but would be considered PHI). The dataset is also considered property of Hackensack Meridian Health and not owned by the investigators. Therefore, for both reasons, the dataset cannot be made openly available. However, upon request we are willing to share portions of the data for appropriate review. Requests can be made through the corresponding author or directly to representatives of Hackensack Meridian Health (Dr. Andrew Ip; Email: [email protected]).
Additional information
Funding
References
- Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113(4):791–798. doi:10.1002/cncr.23608
- Al-Hamadani M, Habermann TM, Cerhan JR, et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the national cancer data base from 1998 to 2011. Am J Hematol. 2015;90(9):790–795. doi:10.1002/ajh.24086
- Goy A, Kahl B. Mantle cell lymphoma: the promise of new treatment options. Crit Rev Oncol Hematol. 2011;80(1):69–86. doi:10.1016/j.critrevonc.2010.09.003
- Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the nordic lymphoma group. Blood. 2008;112(7):2687–2693. doi:10.1182/blood-2008-03-147025
- LaCasce AS, Vandergrift JL, Rodriguez MA, et al. Comparative outcome of initial therapy for younger patients with mantle cell lymphoma: an analysis from the NCCN NHL database. Blood. 2012;119(9):2093–2099. doi:10.1182/blood-2011-07-369629
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial [published correction appears in lancet. 2013 apr 6;381(9873):1184]. Lancet. 2013;381(9873):1203–1210. doi:10.1016/S0140-6736(12)61763-2
- Flinn IW, van der Jagt R, Kahl B, et al. First-Line treatment of patients with indolent Non-Hodgkin lymphoma or Mantle-Cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-Year Follow-Up study. J Clin Oncol. 2019;37(12):984–991. doi:10.1200/JCO.18.00605
- Kumar A, Martin P, Wang M, et al. Real-World treatment patterns and outcomes of 3455 previously untreated mantle cell lymphoma patients in US routine clinical practice [supplement abstract 153. ]. Hematological Oncology. 2021;39(S2):223–225. doi:10.1002/hon.65_2280
- Narkhede M, Goyal G, Shea L, et al. Evaluating real-world treatment patterns and outcomes of mantle cell lymphoma. Blood Adv. 2022;6(14):4122–4131. doi:10.1182/bloodadvances.2022007247
- Kumar A, Sha F, Toure A, et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019;9(6):50. Published 2019 May 20. doi:10.1038/s41408-019-0209-5
- Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903–1910. doi:10.1182/blood-2017-04-779736
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132(19):2016–2025. doi:10.1182/blood-2018-07-859769
- Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84(9):553–559. doi:10.1002/ajh.21468
- Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688–3695. doi:10.1200/JCO.2013.49.2835
- Ruan J, Shah B, Martin P, et al. Clinical experience with lenalidomide alone or in combination with rituximab in indolent B-cell and mantle cell lymphomas. Ann Oncol. 2016;27(7):1226–1234. doi:10.1093/annonc/mdw158
- Rule S, Dreyling MH, Goy AH, et al. Long-Term outcomes with ibrutinib versus the prior regimen: a pooled analysis in relapsed/refractory (R/R) mantle cell lymphoma (MCL) with up to 7.5 years of extended follow-up [supplement abstract 623]. Blood. 2019;134(Supplement_1):1538–1538. doi:10.1182/blood-2019-124691
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13(7):716–723. doi:10.1016/S1470-2045(12)70200-0
- Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31(1):128–130. doi:10.1200/JCO.2012.44.4281
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-Centre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):48–56. doi:10.1016/S1470-2045(15)00438-6
- Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019;134(13):1024–1036. doi:10.1182/blood.2018891598
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi:10.1200/JCO.2006.09.2403
- Giné E, de la Cruz F, Jiménez Ubieto A, et al. Ibrutinib in combination with rituximab for indolent clinical forms of mantle cell lymphoma (IMCL-2015): a multicenter, Open-Label, Single-Arm, phase II trial. J Clin Oncol. 2022;40(11):1196–1205. doi:10.1200/JCO.21.02321
- Kojima A, Tanaka Y, Kimura Y, et al. Multiple myeloma patients with Lenalidomide-Associated skin rash have a favorable prognosis [abstract 653]. Blood. 2016;128(22):4532–4532. doi:10.1182/blood.V128.22.4532.4532
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of Lenalidomide-Related rash. Clin Lymphoma Myeloma Leuk. 2015;15 Suppl: s 64–S69. doi:10.1016/j.clml.2015.02.008
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi:10.1056/NEJMoa1306220
- Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol. 2018;5(3):e109–e116. doi:10.1016/S2352-3026(18)30018-8
- Ujjani CS, Jung SH, Pitcher B, et al. Phase 1 trial of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma: alliance A051103. Blood. 2016;128(21):2510–2516. doi:10.1182/blood-2016-06-718106
- Sestier M, Hillis C, Fraser G, et al. Bruton’s tyrosine kinase inhibitors and cardiotoxicity: more than just atrial fibrillation. Curr Oncol Rep. 2021;23(10):113. Published 2021 Aug3. doi:10.1007/s11912-021-01102-1
- Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74(13):1667–1678. doi:10.1016/j.jacc.2019.07.056
- Fazal M, Kapoor R, Cheng P, et al. Arrhythmia patterns in patients on ibrutinib. Front Cardiovasc Med. 2021;8:792310. Published 2022 Jan3. doi:10.3389/fcvm.2021.792310
- Jerkeman M, Hutching M, Raty R, et al. Ibrutinib-Lenalidomide-Rituximab in Patients with Relapsed/Refractory Mantle Cell Lymphoma: final Results from the Nordic Lymphoma Group MCL6 (PHILEMON) Phase II Trial. Poster presented at the American Society of Hematology 2020 Meeting; December 5-8, 2020. https://ash.confex.com/ash/2020/webprogram/Paper133298.html.
- Dreyling M, Doordujin JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: results from the randomized TRIANGLE trial by the European MCL Network. Plenary scientific session. 64th American Society of Hematology Annual Meeting and Exposition; Dec 11, 2022; New Orleans, US.
- Wang ML, Jain P, Zhao S, et al. Ibrutinib-rituximab followed by R-HCVAD as frontline treatment for young patients (≤65 years) with mantle cell lymphoma (WINDOW-1): a single-arm, phase 2 trial. Lancet Oncol. 2022;23(3):406–415. doi:10.1016/S1470-2045(21)00638-0