Abstract
A comparison of clinical outcomes in the third or subsequent line (3 L+) of systemic therapy between a real-world data (RWD) external control cohort and a mosunetuzumab single-arm clinical trial cohort is presented. Data for 3 L + patients with relapsed/refractory follicular lymphoma (FL) were obtained from the mosunetuzumab single-arm trial (n = 90) and a US electronic health records database (n = 158), with patients meeting key eligibility criteria from the trial, balanced on pre-specified prognostic factors. Overall response and complete response rates were 80% and 60% in the mosunetuzumab cohort and 75% and 33% in the RWD cohort, odds ratios of 1.23 (95% CI, 0.52–2.93) and 3.18 (95% CI, 1.41–7.17), respectively. Hazard ratios for progression-free survival and overall survival were 0.82 (95% CI, 0.53–1.27) and 0.43 (95% CI, 0.19–0.94). These findings support a clinically meaningful benefit of mosunetuzumab monotherapy as a chemotherapy-free option for the 3 L + FL population.
Introduction
Follicular lymphoma (FL) is one of the most common forms of non-Hodgkin’s lymphoma globally, accounting for between one-tenth and one-quarter of all cases by region [Citation1, Citation2]. FL is an incurable indolent disease, with significant improvements in life expectancy seen over the past few decades following the introduction of rituximab in combination with chemotherapy [Citation3]. However, the clinical and biological course of disease varies widely following frontline therapy, with relapses experienced in roughly one in five patients within 2 years after the start of initial treatment [Citation4]. Many patients with FL will require multiple lines of therapies, with generally increasing refractoriness and decreasing duration of response to each [Citation5]. Despite recent approvals of newer therapeutic agents, such as phosphoinositide 3-kinase (PI3K) inhibitors and anti-CD19 chimeric antigen receptor (CAR) T-cell therapies, major barriers to treatment remain. The accelerated US Food and Drug Administration approvals for three PI3K inhibitors (idelalisib, duvelisib, and umbralisib) for relapsed/refractory (r/r) FL were withdrawn in 2022 due to safety concerns, with access to only one PI3K inhibitor, copanlisib, remaining in the United States (US) [Citation6]. The high cost, manufacturing complexity, and toxicity concerns of CAR T-cell therapies have left significant access barriers both in the US and globally [Citation7–9]. Consequently, the treatment landscape for r/r FL remains highly heterogeneous, with no clear standard of care, especially in the third or subsequent line (3 L + FL) of systemic therapy.
Mosunetuzumab is a CD20xCD3 bispecific antibody that engages and redirects T cells to eliminate malignant B cells [Citation10, Citation11]. Mosunetuzumab showed manageable safety and high rates of complete response (CR) in patients with 3 L + FL in a single-arm phase 2 trial [Citation12], leading to its recent approval in Europe and the US for the treatment of patients with r/r FL who have received at least 2 prior systemic therapies [Citation13, Citation14]. The single-arm trial relied on a historical control (a similar patient population receiving the PI3K inhibitor copanlisib, with a 14% CR rate) to provide a benchmark for outcomes [Citation15]. However, additional evidence from contemporary patient cohorts with similar baseline characteristics as the trial can improve interpretations of mosunetuzumab’s efficacy.
To strengthen the interpretation of efficacy data in single-arm trials, large volumes of real-world data (RWD), collected from patients receiving treatment in routine clinical practice, are increasingly used as external control arms [Citation16]. Proof-of-concept studies have demonstrated the ability of these data to replicate clinical trial control arms in various oncology settings, through proper bias adjustment, to ensure comparability between real-world and clinical trial cohorts [Citation17, Citation18].
Here, we compare clinical outcomes of fixed-duration mosunetuzumab monotherapy in the single-arm trial [Citation12] to an external control arm comprising patients treated with commonly available treatments, primarily from community cancer centers in the US.
Methods
Study design and data sources
Mosunetuzumab efficacy data in 3 L + FL were taken from the single-arm trial (NCT02500407; data cutoff: August 27, 2021) [Citation12, Citation19, Citation20]. In brief, this single-arm trial was an open-label, phase 1/2, multicenter, dose-escalation, and expansion study evaluating the safety, efficacy, and pharmacokinetics of mosunetuzumab in patients with r/r B-cell lymphoma. We used data from an expansion cohort of this study in patients with r/r FL who were treated with mosunetuzumab monotherapy at the dose intended for registration after at least 2 prior lines of systemic therapy. The primary endpoint of the mosunetuzumab trial was the proportion of participants with a CR, based on independent review facility (IRF) assessment. Secondary endpoints included overall response rate (ORR), duration of response, progression-free survival (PFS), and overall survival (OS). The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. The protocol was approved by the ethics committees of participating centers and registered at ClinicalTrials.gov. All patients provided written informed consent.
The RWD cohort was created using the US nationwide Flatiron Health (FH) electronic health record (EHR)-derived de-identified database (data cutoff: January 31, 2022). FH is a longitudinal database, comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction [Citation21, Citation22]. This database includes de-identified data from over 280 cancer clinics (800 sites of care). Most patients in the database originate from community oncology settings; relative community/academic proportions may vary depending on the study cohort. Briefly, the patient-level data included structured data (e.g. laboratory values and prescribed drugs) in addition to unstructured data collected via technology-enabled chart abstraction such as physician’s notes and other unstructured documents (e.g. radiology or pathology reports). The generation and validation of the database, as well as methods for clinical data extraction, de-identification of patient data, and linkage of clinical and genomic data have been described elsewhere [Citation21, Citation22].
To derive the RWD cohort, patients from the FH database were extracted by applying key eligibility criteria of the mosunetuzumab expansion cohort from the single-arm trial. In alignment with the trial, patients in the RWD cohort had to be aged ≥18 years diagnosed with FL grade 1, 2, or 3a and initiating 3 L + systemic therapy. At least 1 prior regimen containing an alkylating agent and at least 1 prior regimen containing an anti-CD20-directed therapy was also required for inclusion. Additional therapy-specific eligibility criteria were applied, including Eastern Cooperative Oncology Group performance status ≤1, no prior treatment with CAR T-cell therapy within 30 days prior to index line (defined below) initiation, including at least 1 agent recommended by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) v4.2022 [Citation23, Citation24], no autologous stem cell transplantation (SCT) within 100 days prior to initiation of index line, or any prior allogeneic SCT recorded anytime in patients’ records, no receipt of clinical study drugs any time prior to and through the end of the index line, no evidence of transformation to a high-grade or diffuse large B-cell lymphoma on or prior to initiation of index therapy, and initiation of index line must have occurred prior to August 27, 2021. These criteria were applied whenever data were reported; patients were not excluded because of missing data. Data elements abstracted from unstructured parts of the patients’ EHR (e.g. response and progression by line of therapy) were also provided by FH.
Defining the index line
The primary analysis considered a RWD treatment arm representing commonly used treatments in 3 L + FL based on NCCN Guidelines® v4.2022 [Citation23] (Table S1). The index therapy was defined as the latest eligible line of systemic therapy, after the patient had received ≥2 previous systemic therapies, including an alkylating agent and an anti-CD20 therapy. The latest line of therapy, with complete data on prognostic factors of interest, was chosen for patients with >1 eligible line in an attempt to align with the heavily pretreated mosunetuzumab single-arm trial cohort.
Endpoints
All endpoints in the RWD cohort were assessed up to the maximum observed follow-up of the single-arm trial, which at the clinical data cutoff date (August 27, 2021) was 27.5 months. All endpoints for the mosunetuzumab single-arm trial and RWD cohorts were assessed with data collected as of their respective data cutoff dates, as described in the previous section.
The primary outcome measure for the RWD cohort was ORR, defined as the proportion of patients who had a partial response or CR as best response to a given line of therapy. While in the mosunetuzumab cohort, ORR was assessed among all enrolled patients, in the FH RWD cohort, ORR was estimated only among patients who had non-missing information regarding response to therapy allowing for a conservative comparison of the ORR estimates between the two cohorts. In the mosunetuzumab trial, ORR was assessed by IRF. For the RWD cohort, the real-world response endpoint was curated via clinical and radiographic evidence, which has shown strong correlations with trial response variables in other oncology settings [Citation25].
CR rate was considered a secondary endpoint for the RWD cohort, defined as the proportion of patients with best documented response of CR among patients evaluable for response during the available follow-up for a given line of therapy. CR determined by IRF was estimated among all enrolled patients in the mosunetuzumab single-arm trial cohort, while real-world response was estimated for patients with non-missing information in the RWD cohort.
Additional secondary endpoints included PFS and OS. PFS was defined as time from initiation of the given line of therapy until first documented progression or death. The RWD cohort considered evidence of progressive disease as well as evidence of stable disease accompanied by clinical intervention as progression. OS was defined as time from initiation of the given line of therapy to date of death from any cause. If an event of interest was not documented in a patient’s chart (progression or death), the patient was censored at the earlier of last contact in the RWD cohort or the end of follow-up for the mosunetuzumab single-arm trial cohort. IRF-determined PFS was used for the mosunetuzumab single-arm trial cohort.
Time-to-next-treatment (TTNT) was evaluated as an exploratory endpoint in both the RWD cohort and mosunetuzumab single-arm trial cohort. TTNT was defined as time from initiation of the index line of therapy until initiation of the next subsequent line of anti-lymphoma therapy or death. If no subsequent line of therapy was documented, the patient was censored at the earliest of last contact or end of follow-up.
Statistical analysis
Descriptive statistics were used to summarize demographic and clinical characteristics of the patients in the mosunetuzumab single-arm trial cohort and the RWD cohort. Descriptive statistics included frequency distributions for categorical variables, means/medians, and standard deviation/range for continuous variables.
To ensure the external RWD cohort was comparable to the mosunetuzumab single-arm trial cohort in the absence of randomization, propensity score methods were used to adjust for potential imbalance between the cohorts. A propensity score model was fitted to the data to estimate the probability for each patient to be enrolled into the mosunetuzumab trial cohort, compared with the RWD cohort, conditional upon a set of pre-specified prognostic factors. This was done by fitting a logistic regression model to trial enrollment status as a function of the following pre-specified prognostic covariates: age in years at initiation of treatment, an indicator for progression of disease within 24 months of frontline systemic therapy, double refractory status (i.e. refractory to prior anti-CD20 and prior alkylator), refractory status to prior therapy, and number of prior lines of systemic therapy. Time in months from initial FL diagnosis to initiation of the index line was removed from the pre-specified model due to extreme imbalance between cohorts, and to achieve better balance on all other variables. Time-to-index line initiation was adjusted for in the outcome models. The RWD cohort was then re-weighted using inverse probability of treatment weights (IPW) to estimate average treatment effect in the treated population [Citation26]. The standardized mean difference was calculated for each variable in the set of prognostic factors, prior to and after applying IPW, to assess the extent of imbalance between the cohorts. Literature suggests a cutoff of up to 0.25 for the standardized mean difference between the two comparator cohorts to indicate adequate balance [Citation27, Citation28].
Two stopping rules were implemented for the propensity score model analysis: 1) the propensity score adjustment does not result in achieving adequate balance between the cohorts, defined as the majority of variables having a standardized mean difference > 0.25; and 2) the effective sample size (sum of weights squared divided by the sum of squared weights) for the RWD cohort is ≤50% of the mosunetuzumab single-arm trial cohort (n = 45). If either of the stopping rules were met, the inference would be deemed not meaningful and no comparative analysis on the outcomes of interest would be performed.
A weighted logistic regression model was fit to ORR as a function of study cohort (mosunetuzumab vs RWD) and time-to-index line initiation. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported using a robust (sandwich) estimator for the standard error. A similar approach was used to estimate OR for CR rate.
Weighted Kaplan-Meier survival curves were used to illustrate comparisons between mosunetuzumab and RWD cohorts for PFS, OS, and TTNT. A weighted Cox proportional hazards model was fit to each time-to-event outcome as a function of study cohort (mosunetuzumab vs RWD) and time-to-index line initiation. Hazard ratios (HRs) and 95% CIs were reported using a robust (sandwich) estimator for the standard errors.
The E-value, or evidence for causality, was computed for each endpoint and CI to assess the extent of potential unmeasured confounding required to nullify the observed ORs or HRs, as well as to move the CI to include the null [Citation29].
Several sensitivity analyses were performed to assess the robustness of the primary findings. Unless otherwise specified, the selected cohort and eligibility criteria were the same as those defined in the primary analysis. Each sensitivity analysis re-applied the propensity score method prior to conducting the specific analysis. TTNT was excluded from all sensitivity analyses because it was an exploratory endpoint. The first sensitivity analysis stratified the RWD cohort into five regimen-specific cohorts: anti-CD20-monotherapy, anti-CD20-chemotherapy, anti-CD20-lenalidomide, PI3K inhibitors, and Other (which included tazemetostat, CAR T-cell therapy, any regimen followed by stem cell transplant, and any other regimens recommended for the treatment of FL (regimens defined in Table S1). Patients were re-sampled to choose the earliest eligible line within each regimen, and the same stopping rules were applied to the regimen-specific cohorts. Endpoints were then assessed separately in each regimen-specific cohort. The second sensitivity analysis selected the earliest, instead of latest, eligible index line for the primary RWD cohort. The third sensitivity analysis applied a stricter set of eligibility criteria to the RWD cohort in addition to those applied in the primary analysis (Table S2). The fourth sensitivity analysis conducted the primary analysis but allowed for the full available follow-up time in the RWD cohort instead of truncating follow-up at the maximum observed follow-up time in the trial. The fifth sensitivity analysis updated the eligibility criteria with FH’s abstracted tumor grade information beyond the initial FL diagnosis. The final sensitivity analysis updated the line of therapy table with FH’s additional abstracted line confirmation information. Forest plots were generated to provide a visual comparison of all sensitivity analyses against the primary analysis for ORR, CR rate, PFS, and OS.
All analyses were conducted using R v4.1.2 software [Citation30]. As no formal hypothesis testing was conducted, statistical significance was not explicitly assessed.
Results
Cohort characteristics
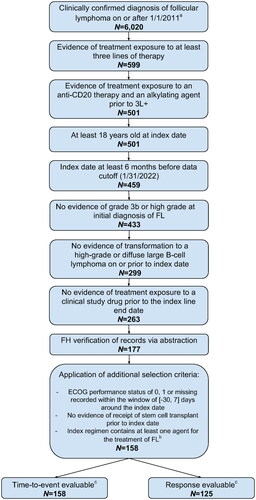
Of 6,020 patients with a confirmed diagnosis of FL in the FH database, 599 patients had received at least three lines of therapy (3 L+). In total, 158/599 patients met the eligibility criteria for inclusion in the RWD cohort (). All 158 patients had progression and/or survival data available. However, 33 patients did not have response data available for their index line of systemic therapy, resulting in 125 patients being evaluable for response and 158 evaluable for time-to-event.
Figure 1. Patient attrition from FH database. 3L+: third or subsequent line of systemic therapy; ECOG: Eastern Cooperative Oncology Group; FH: Flatiron Health; FL: follicular lymphoma. aPatients were probabilistically sampled from an overall cohort of N = 207,263 patients with an ICD code for FL in the Flatiron Health network. bDefined as a regimen including at least one agent recommended by the NCCN Guidelines v4.2022 for the treatment of FL. cOf 158 patients, all had progression and/or survival data available but 33 did not have response data available in their index line of systemic therapy, resulting in N = 125 response-evaluable patients and N = 158 time-to-event evaluable patients.
Median follow-up time after initiation of index therapy was 18 months for the mosunetuzumab cohort and 15 months for the truncated RWD cohort. The distribution of regimens by prior line of therapy in both cohorts are provided in Table S3. While higher use of anti-CD20 monotherapy was observed in the Flatiron cohort during frontline therapy, overall higher use of CAR T-cell therapy as well as regimens captured in the ‘Other’ therapy category were observed in the mosunetuzumab cohort during prior lines of therapy (Table S3). After weighting, balance (standardized mean difference < 0.10) was achieved between the mosunetuzumab and RWD cohorts for all variables included in the propensity score model (, Table S4). Notably, patients in the mosunetuzumab cohort were more heavily pretreated, with a longer duration from initial FL diagnosis to start of index therapy compared with those in the RWD cohort. The most common index treatment regimen in the RWD cohort was anti-CD20 in combination with chemotherapy (37%).
Table 1. Baseline demographic and clinical characteristics of patients from the mosunetuzumab and RWD cohorts, before (pre) and after (post) PSM weighting.
Response outcomes
The sample size for the 125 response-evaluable patients in the RWD cohort was reduced to an effective sample size of 47 and weighted sample size of 87, after imbalance adjustment. Mosunetuzumab was associated with a significant treatment benefit with a CR rate of 60%, compared with 33% in the RWD cohort (OR, 3.18; 95% CI, 1.41–7.17; E-value, 2.97 for the OR and 1.66 for the CI) (, ). ORR was comparable between the mosunetuzumab (80%) and RWD (75%) cohorts with an OR of 1.23 (95% CI, 0.52–2.93) and E-value of 1.46 for the OR and 1 for the CI. The estimate for CR rate among the sensitivity analyses was consistent with that of the primary analysis in directionality and magnitude, while the estimate for ORR fluctuated but remained comparable between the two arms ().
Figure 2. Weighted response rates in mosunetuzumab single-arm trial and real-world data cohorts. Bar plots show weighted complete and overall response rates by study cohort. Numbers above each bar correspond to the percentage of participants responding with 95% confidence intervals (also represented by the error bars).
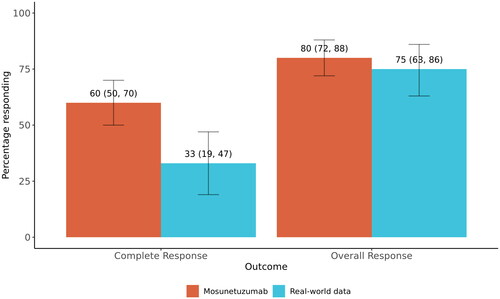
Figure 3. Forest plots of sensitivity analyses for (A) overall response rate, (B) complete response rate, (C) progression-free survival, and (D) overall survival. Each Forest plot shows the weighted association between study cohort and each outcome of interest, adjusting for months from diagnosis to index line initiation. Each row in the Forest plot corresponds to a separate sensitivity analysis conducted. The blue square represents the estimate (HR or or) while the horizontal line represents the 95% confidence interval. Upper or lower confidence limits that are outside the plot range are depicted with an arrow.
CI: confidence interval; HR: hazard ratio; I/E: inclusion/exclusion criteria; LoT: line of therapy; M: mosunetuzumab; OR: odds ratio; SS: sample size.
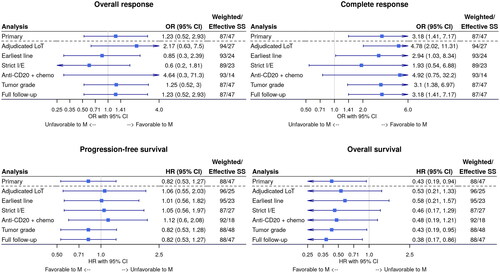
Table 2. Comparison of clinical outcomes between mosunetuzumab and RWD cohorts.
Outcomes
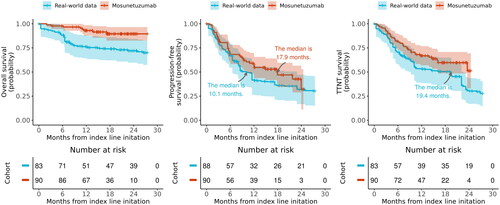
The sample size for the 158 time-to-event evaluable patients from the RWD cohort was reduced to an effective sample size of 47 and a weighted sample size of 88, after imbalance adjustment. OS was substantially longer in the mosunetuzumab cohort compared with the RWD cohort, with a HR of 0.43 (95% CI, 0.19–0.94) and E-value of 4.14 for the HR (, ). At 12 months after index therapy initiation, 93% (95% CI, 88–98%) of patients in the mosunetuzumab cohort and 76% (95% CI, 64–88%) in the RWD cohort had survived. PFS and TTNT were comparable between the mosunetuzumab and RWD cohorts, with HRs of 0.82 (95% CI, 0.53–1.27) and 0.77 (95% CI, 0.47–1.26), respectively. The E-values were 1.56 for the PFS HR and 1.68 for the TTNT HR. Both median PFS and TTNT were longer in the mosunetuzumab single-arm trial cohort compared with the RWD cohort (PFS: 17.9 months vs 10.1 months, respectively; TTNT: not reached vs 19.4 months, respectively). The estimate for OS amongst the sensitivity analyses was consistent with that of the primary analysis in directionality and magnitude, while the estimate for PFS fluctuated but remained comparable between the two arms ().
Figure 4. Weighted Kaplan-Meier plots comparing mosunetuzumab single-arm trial to RWD for (A) overall survival, (B) progression-free survival, and (C) time-to-next treatment. Solid lines represent Kaplan-Meier survival probabilities; shading represents 95% confidence intervals. PFS: progression-free survival; RWD: real-world data; TTNT: time-to-next treatment.
Discussion
This study, comparing mosunetuzumab monotherapy to commonly available treatments in the US in the 3 L + FL real-world setting, suggests a treatment benefit associated with mosunetuzumab for CR and OS. Patients treated with mosunetuzumab in the single-arm trial had more than a 3-fold increase in the OR for CR and a longer OS compared with patients treated in routine clinical practice in the US. Sensitivity analyses confirmed the consistency of the results across multiple iterations of the analytic design, including in a subgroup of patients treated only with anti-CD20 plus chemotherapy, and in a subgroup of patients who met the strictest set of eligibility criteria applicable in the real-world setting. Overall response, PFS, and TTNT were comparable between cohorts.
There is currently no single standard of care therapy for patients with 3 L + FL. Treatment options for r/r FL include rituximab as monotherapy or in combination with chemotherapy, obinutuzumab in combination with bendamustine, and rituximab in combination with lenalidomide, which can be used in the 3 L + setting, although published data on the efficacy of these therapies specifically in the 3 L + setting are limited. Therapies recently approved in the US for 3 L + r/r FL include the PI3K inhibitor copanlisib (ORR 59%, CR rate 14%) [Citation31], the EZH2 inhibitor tazemetostat (EZH2-wild-type FL: ORR 34%, CR rate 4%; EZH2-mutant FL: ORR 69%, CR rate 12%) [Citation32], and the CD19-directed CAR T-cell therapies axicabtagene ciloleucel (ORR 91%, CR rate 60%) [Citation33], and tisagenlecleucel (ORR 86%, CR rate 68%) [Citation34]. Given the heterogeneity of treatments administered in the RWD 3 L + FL cohort, including PI3K inhibitors and CAR T-cell therapies, the observed ORs for mosunetuzumab for ORR and CR are broadly consistent with the published data on 3 L + FL therapies. It is difficult to perform cross-trial comparisons, especially for single-arm trials, for response and time-to-event endpoints due to differences in enrollment criteria and era of treatment. The RWD cohort attempts to adjust for potential confounders with individual patient matching and a clinical cutoff-date similar to the mosunetuzumab single-arm trial.
In order to make unbiased comparisons with the clinical trial, the external control arm was selected using key eligibility criteria from the phase 2 trial and balanced with the trial cohort on known prognostic factors of r/r FL. The volume, richness, and longitudinal nature of the EHRs enabled the control for these sources of selection bias and confounding bias in the study; nonetheless, sources of unmeasured confounding may limit the generalizability and interpretation of findings. For example, implicit differences in patient care and attributes of patients who choose to participate in clinical trials are not captured in these data and remain possible sources of unmeasured confounding.
To assess the extent to which unmeasured confounding could explain away the observed outcome associations, we computed the E-value, or ‘evidence for causality’, for each estimate [Citation29]. The E-value requires no prior knowledge of or assumptions about the unmeasured confounder(s), serving as a useful bias assessment metric. The E-values for endpoints assessed in this comparative study suggest that a considerably strong degree of unmeasured confounding would be required, beyond the set of prognostic factors already considered, to explain away the significant treatment associations for CR and OS. For CR, unmeasured confounder(s) would need to be associated with both mosunetuzumab treatment and CR by a 3-fold risk ratio to explain away the observed OR of 3.18. To move the CI to include the null, the association of the unmeasured confounder with treatment and CR would need to be 1.7-fold. For OS, an even stronger degree of unmeasured confounding is required, beyond the set of prognostic factors already considered, to be associated with both survival and mosunetuzumab treatment (approximately 4-fold for the HR, 1.3-fold for the CI) in order to explain the observed effect of mosunetuzumab treatment on survival. Any weaker unmeasured confounder would not negate the observed CR or OS benefit. The existence of such a confounder above and beyond what was measured in the study is unlikely, suggesting these findings are relatively robust to unmeasured confounding bias, although this remains a possible limitation in this and most observational studies.
Other limitations of this study include relatively small effective sample sizes for the FH response-evaluable and time-to-event cohorts of 47 compared with the mosunetuzumab cohort of 90. Unlike in a clinical trial, where the protocol and schedule of assessments govern the standardized capture of data for all patients, data collected in real-world settings are prone to high variability in frequency, timing, and quality, affecting the measurement of endpoints. There were also differences in response assessment between the cohorts. The mosunetuzumab trial utilized International Harmonization Project 2007 revised response criteria [Citation35], as assessed by an IRF, while the FH cohort likely used a mix of response criteria based on physician and imaging assessment, reflecting variability in assessing response in real-world settings. There were no comparisons of safety endpoints; mosunetuzumab and the treatment regimens administered in the FH cohort have their own unique safety and tolerability profiles that are important for benefit-risk assessment.
The generalizability of these findings to other geographies should also be considered, as the treatment landscape for r/r FL may vary in different regions. Nonetheless, the analysis pooled many common regimens in the 3 L + setting into the external control cohort that reflect global treatment recommendations.
Considering their strength and robustness, these results support a clinically meaningful benefit for mosunetuzumab monotherapy compared to other commonly available regimens used in the 3 L + setting. These findings, as well as other attributes, such as a manageable safety profile; novel, chemotherapy-free, CD20-targeted T-cell engaging mechanism of action; and fixed duration, outpatient, and off-the-shelf administration suggest that mosunetuzumab could be a promising treatment option for the 3 L + FL population.
Authors’ contributions
Conception and design: SFM, NS, MJ, BS, MHBZ, BL, JP, AS. Collection and assembly of data: SFM, NS, JW, AS. Data analysis and interpretation: SFM, NS, JW, MCW, AS. Manuscript writing, final approval of manuscript, and accountable for all aspects of the work: all authors.
Supplemental Material
Download MS Word (30.5 KB)Acknowledgments
NCT02500407 is sponsored by F. Hoffmann-La Roche Ltd. Third party medical writing assistance was provided by Louise Profit, PhD, of Ashfield MedComms, an Inizio company, and was funded by Genentech, Inc. The authors would like to thank the patients and their families, as well as the study investigators, study coordinators, nurses, and representatives of the sponsor who were involved in data collection and analyses.
Disclosure statement
SFM, NS, JW, AS: employment (Genentech), stock or stock options (Roche), BL: employment (Roche), MHBZ, MJ, BS, JP: employment and stock or stock options (Roche), MCW: employment (Genentech), stock or stock options, and patents and royalties (Roche).
Data availability statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Additional information
Funding
References
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by world health organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi:10.3322/caac.21357
- Perry AM, Diebold J, Nathwani BN, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the international Non-Hodgkin lymphoma classification project. Haematologica. 2016;101(10):1244–1250. doi:10.3324/haematol.2016.148809
- Freedman A. Follicular lymphoma: 2015 update on diagnosis and management. Am J Hematol. 2015;90(12):1171–1178. doi:10.1002/ajh.24200
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national LymphoCare study. J Clin Oncol. 2015;33(23):2516–2522. doi:10.1200/JCO.2014.59.7534
- Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10(7):74. doi:10.1038/s41408-020-00340-z
- Richardson NC, Kasamon Y, Pazdur R, et al. The Saga of PI3K inhibitors in haematological malignancies: survival is the ultimate safety endpoint. Lancet Oncol. 2022;23(5):563–566. doi:10.1016/S1470-2045(22)00200-5
- Mikhael J, Fowler J, Shah N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. J Clin Oncol Oncol Pract. 2022;18(12):800–807. doi:10.1200/OP.22.00315
- Gajra A, Zalenski A, Sannareddy A, et al. Barriers to chimeric antigen receptor T-cell (CAR-T) therapies in clinical practice. Pharmaceut Med. 2022;36(3):163–171. doi:10.1007/s40290-022-00428-w
- Jommi C, Bramanti S, Pani M, et al. CAR T-cell therapies in Italy: patient access barriers and recommendations for health system solutions. Front Pharmacol. 2022;13:915342. doi:10.3389/fphar.2022.915342
- Assouline SE, Kim WS, Sehn LH, et al. Mosunetuzumab shows promising efficacy in patients with multiply relapsed follicular lymphoma: updated clinical experience from a phase I dose-escalation trial. Blood. 2020;136(Supplement 1):42–44. doi:10.1182/blood-2020-135839
- Sun LL, Ellerman D, Mathieu M, et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7(287):287ra70. doi:10.1126/scitranslmed.aaa4802
- Budde LE, Sehn LH, Matasar M, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055–1065. doi:10.1016/S1470-2045(22)00335-7
- US Food and Drug Administration. FDA grants accelerated approval to mosunetuzumab-axgb for relapsed or refractory follicular lymphoma. 2022 [cited 2023 Jan 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mosunetuzumab-axgb-relapsed-or-refractory-follicular-lymphoma.
- Kang C. Mosunetuzumab: first approval. Drugs. 2022;82(11):1229–1234. doi:10.1007/s40265-022-01749-5
- Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905. doi:10.1200/JCO.2017.75.4648
- Ghione P, Palomba ML, Patel AR, et al. Comparative effectiveness of ZUMA-5 (axi-cel) vs SCHOLAR-5 external control in relapsed/refractory follicular lymphoma. Blood. 2022;140(8):851–860. doi:10.1182/blood.2021014375
- Carrigan G, Whipple S, Capra WB, et al. Using electronic health records to derive control arms for early phase single-arm lung cancer trials: proof-of-concept in randomized controlled trials. Clin Pharmacol Ther. 2020;107(2):369–377. doi:10.1002/cpt.1586
- Schröder C, Lawrance M, Li C, et al. Building external control arms from patient-level electronic health record data to replicate the randomized IMblaze370 control arm in metastatic colorectal cancer. J Clin Oncol Clin Cancer Inform. 2021;5:450–458. doi:10.1200/CCI.20.00149
- Budde LE, Assouline S, Sehn LH, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J Clin Oncol. 2022;40(5):481–491. doi:10.1200/JCO.21.00931
- Budde LE, Sehn LH, Matasar MJ, et al. Mosunetuzumab monotherapy is an effective and well-tolerated treatment option for patients with relapsed/refractory (R/R) follicular lymphoma (FL) who have received ≥2 prior lines of therapy: pivotal results from a phase I/II study. Blood. 2021;138(Supplement 1):127–127. doi:10.1182/blood-2021-145872
- Ma X, Long L, Moon S, et al. Comparison of population characteristics in real-world clinical oncology databases in the US: flatiron health. SEER, and NPCR. medRxiv. 2020; doi:10.1101/2020.03.16.20037143
- Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv. 2020; doi:10.48550/arXiv.2001.09765
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-cell Lymphomas V.4.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed January 18, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-cell Lymphomas V.5.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed August 15, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Ma X, Bellomo L, Magee K, et al. Characterization of a real-world response variable and comparison with RECIST-based response rates from clinical trials in advanced NSCLC. Adv Ther. 2021;38(4):1843–1859. doi:10.1007/s12325-021-01659-0
- Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303. doi:10.1111/1475-6773.12090
- Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15(3):234–249. doi:10.1037/a0019623
- Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84–S90.e1. doi:10.1016/j.jclinepi.2013.01.013
- VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi:10.7326/M16-2607
- R-Project. The R Project for Statistical Computing. 2023 [cited 2023 Jan 6]. Available from: https://www.R-project.org/.
- Prescribing information for ALIQOPA (copanlisib). 2017 cited 2023 Jan 6]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209936s000lbl.pdf.
- Prescribing information for TAZVERIK (tazemetostat). 2020 [cited 2023 Jan 6]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213400s000lbl.pdf.
- Prescribing information for YESCARTA (axicabtagene ciloleucel). 2022 [cited 2023 Jan 6]. Available from: https://ce.mayo.edu/sites/default/files/yescarta-pi.pdf.
- Prescribing information for KYMRIAH (tisagenlecleucel). 2022 cited 2023 Jan 6]. Available from: https://www.novartis.com/us-en/sites/novartis_us/files/kymriah.pdf.
- Cheson BD. The international harmonization project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21(5):841–854. doi:10.1016/j.hoc.2007.06.011
