Abstract
Nearly all patients with multiple myeloma eventually relapse or become refractory to treatment. Lenalidomide is increasingly administered in the frontline until disease progression or intolerance to therapy, resulting in the need for highly effective, lenalidomide-sparing options. In this study, carfilzomib plus daratumumab and dexamethasone were evaluated against lenalidomide-sparing, pomalidomide-containing triplets using matching-adjusted indirect comparison in the absence of head-to-head data. The analyses utilized long-term follow-up data from the CANDOR study (NCT03158688). Treatment with carfilzomib, daratumumab, and dexamethasone resulted in significantly longer progression-free survival (hazard ratio 0.60 [95% confidence interval: 0.37, 0.88])vs. pomalidomide plus bortezomib and dexamethasone, and numerically longer progression-free survival (hazard ratio 0.77 [95% confidence interval: 0.50, 1.08]) vs. daratumumab plus pomalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma and previous lenalidomide exposure, the majority of whom were lenalidomide refractory. Carfilzomib plus daratumumab and dexamethasone offers a highly effective, lenalidomide-sparing treatment option for this population.
Introduction
Multiple myeloma (MM) is an incurable blood cancer that represents 1% of all new cancer cases and 10% of all hematologic malignancies globally [Citation1]. In 2020, approximately 176,404 people were diagnosed with MM worldwide and 117,077 died from the disease [Citation2,Citation3]. MM, which is most frequently diagnosed in older populations (median age of 69 years [Citation4]), is characterized by uncontrolled proliferation of malignant plasma cells in the bone marrow [Citation5–7]. Patients with the disease typically experience a pattern of relapse and remission [Citation5,Citation8,Citation9].
There have been significant therapeutic advancements in the treatment of MM in the past two decades, including the development of novel agents, comprising immunomodulatory drugs (IMiD) and proteasome inhibitors (PI). The use of these therapies surged from 8.7% in 2000 to 61.3% in 2014 [Citation10], and significant improvement in survival outcomes has been documented since the beginning of that span [Citation11]. More recently, anti-CD38 monoclonal antibodies have been investigated and approved in combination with these agents, demonstrating improved efficacy when added to previously established standards of care [Citation12–26]. Despite this progress, nearly all patients with MM eventually relapse or become refractory to treatment and experience disease-related complications [Citation27].
Lenalidomide (a type of IMiD) is a cornerstone treatment that is increasingly administered in the frontline setting as part of initial induction, post-transplant consolidation, and/or very often as a maintenance regimen [Citation11,Citation28]. Since lenalidomide treatment is administered until disease progression or until the patient becomes intolerant to therapy [Citation29–31], a high proportion of patients will become lenalidomide refractory following first-line therapy. These patients are poorly suited to receive lenalidomide-based triplet combinations in second-line therapy [Citation32,Citation33]. The proportion of lenalidomide-refractory patients is expected to grow [Citation34], therefore, highly effective, lenalidomide-sparing therapies for patients with relapsed and/or refractory MM (RRMM) are needed.
Carfilzomib is a second-generation PI approved for the treatment of RRMM in many countries worldwide. The therapy inhibits the proteasome irreversibly, leading to sustained response as well as showing a well-tolerated safety profile [Citation35]. Carfilzomib is authorized in Europe and the US for use in combination with dexamethasone (Kd), with lenalidomide and dexamethasone, with daratumumab and dexamethasone (KdD), as well as with isatuximab and dexamethasone [Citation36–39]. Carfilzomib as a single agent is also indicated to treat patients with RRMM in the US [Citation37].
The approval of the lenalidomide-sparing KdD therapy with carfilzomib 56 mg/m2 twice-weekly was based on the CANDOR study (NCT03158688; first subject enrolled: 13 June 2017) [Citation12,Citation13,Citation18], an open-label, phase 3, randomized controlled trial that evaluated KdD vs. Kd. The KdD arm of CANDOR included 312 patients, 123 (32.0%) of whom had received prior lenalidomide. In lenalidomide-exposed patients, 99 (80.5%) were lenalidomide refractory and 39 (31.7%) were bortezomib refractory; 52.0% of the lenalidomide-exposed population was older than 65 years and 6.5% was older than 75 years. A blinded independent review committee assessed progression-free survival (PFS) as the primary endpoint [Citation12,Citation13,Citation18]; PFS was assessed centrally by a validated computerized algorithm (Onyx Response Computational Assessment) subsequent to the primary analysis.
In the CANDOR study, KdD significantly prolonged PFS vs. Kd (median PFS not reached for KdD vs. 15.8 months for Kd, hazard ratio [HR] 0.63; 95% confidence interval [CI]: 0.46, 0.85, p = 0.0027) [Citation12] at the primary analysis (data cutoff date: 14 July 2019) and showed a consistent benefit for this outcome at the second interim analysis (data cutoff date: 15 June 2020; HR 0.59; 95% CI: 0.45, 0.78; median PFS of 28.6 months for KdD vs. 15.2 months for Kd) [Citation13]. The PFS benefit of KdD over Kd was consistent across clinically relevant patient subgroups; the estimated HR for KdD vs. Kd was 0.49 (95% CI: 0.33, 0.74) in patients exposed to lenalidomide (KdD: N = 123; Kd: N = 74) and 0.46 (95% CI: 0.28, 0.73) in the majority subset of patients also refractory to lenalidomide (KdD: N = 99; Kd: N = 55) [Citation13]. After a median follow-up of 39.7 months (data cutoff date: 14 June 2021), similar results were observed for KdD within the intent-to-treat population (median PFS 28.4 months) and within lenalidomide-exposed and -refractory groups (median PFS 27.0 months in both subgroups [data on file]).
Several other anti-myeloma drugs have been clinically developed and have been utilized in combination therapy regimens since the introduction of lenalidomide. Pomalidomide was developed as a more potent and potentially less toxic IMiD than lenalidomide [Citation40]. Combination regimens (particularly triplet regimens) containing pomalidomide have demonstrated efficacy benefit vs. comparators in clinical trials [Citation41,Citation42] and real-world studies [Citation43] in patients with RRMM, and are becoming commonly used treatment options in clinical practice [Citation44]. Published data on pomalidomide-containing regimens in a lenalidomide-exposed population may contextualize the outcomes observed for KdD in lenalidomide-exposed patients in the CANDOR study and inform optimal treatment selection for patients with RRMM; however, trials directly comparing these combination treatments with IMiD-sparing options have not been conducted. Data from a substantial number of patients in the representative phase 3 registrational OPTIMISMM (NCT01734928) [Citation45] and APOLLO (NCT03180736)[Citation14] trials can be aligned with CANDOR based on study and patient characteristics (e.g. study design, inclusion/exclusion criteria, availability and comparability of efficacy outcomes of interest).
OPTIMISMM [Citation45] was an open-label study that evaluated pomalidomide, bortezomib, and dexamethasone (PVd) vs. bortezomib and dexamethasone (Vd). The trial enrolled (start date: 7 January 2013) lenalidomide-exposed patients with RRMM who were ≥18 years old, had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 to 2, and received one to three previous regimens. APOLLO [Citation14] investigated daratumumab in combination with pomalidomide and dexamethasone (DPd) vs. pomalidomide and dexamethasone (Pd). This open-label, multi-center study (start date: 12 June 2017) included patients with RRMM who had received ≥1 prior line of therapy including lenalidomide and a PI, had responded to prior treatment, and progressed on or after their last regimen. Patients in the APOLLO study with only one prior line were required to be refractory to lenalidomide.
The analysis presented here directly compared KdD vs. PVd and KdD vs. DPd in patients with RRMM using the most mature available data from the CANDOR study (data cutoff date: 14 June 2021).
Materials and methods
Network meta-analysis (NMA) has become the gold standard to indirectly evaluate evidence in the absence of head-to-head comparisons [Citation46]. An NMA feasibility assessment was performed to evaluate the heterogeneity with respect to trial design and patient population across the CANDOR (lenalidomide-exposed population), OPTIMISMM, and APOLLO trials. There was substantial heterogeneity in terms of important patient characteristics that are considered prognostic and/or treatment effect modifiers, including the proportion of patients >75 years old (6.5% in CANDOR, 16.4% in OPTIMISMM, and 16.6% in APOLLO), refractory to bortezomib therapy (31.7% in CANDOR, 8.5% in OPTIMISMM, and not reported in APOLLO), ECOG PS score of 0 (44.7% in CANDOR, 53.0% in OPTIMISMM, and 60.3% in APOLLO), and who received two or more prior lines of therapy (78.0% in CANDOR, 60.5% in OPTIMISMM, and 89.4% in APOLLO). In terms of study design, all three were phase 3, open-label, multicenter, randomized trials.
Given the presence of these differences in important prognostic and/or predictive factors, an NMA approach was deemed inappropriate as it was likely to yield unreliable and biased efficacy estimates. This finding is aligned with those of a recent critical evaluation of NMAs in RRMM [Citation47] as well as with precedents established for using matching-adjusted indirect comparison (MAIC) methodology in RRMM [Citation48–51]. Therefore, MAIC was considered a more appropriate method to apply (as has been previously detailed in the literature [Citation52,Citation53]) to estimate the relative efficacy between treatments for the current analyses. In the MAIC analyses, cross-trial differences (i.e. reported baseline population characteristics) were accounted for according to the approach outlined in National Institute for Health and Care Excellence (NICE) Decision Support Unit Technical Support Document (TSD) 18 [Citation54]. However, since there are inherent limitations to using MAIC (e.g. unobserved differences cannot be adjusted for) that make it challenging to assess the totality of potential bias [Citation54], results were interpreted in this context.
Baseline characteristics and PFS data were used to conduct naïve pairwise comparisons and MAICs for the lenalidomide-exposed patients who received KdD (CANDOR) vs. PVd (OPTIMISMM) and KdD (CANDOR) vs. DPd (APOLLO). A stepwise approach following MAIC methodology was used to analyze outcomes in each pairwise comparison according to NICE guidance for population-adjusted indirect treatment comparisons [Citation54].
In the first step, patient selection criteria were aligned to minimize any confounding bias in each comparison (i.e. KdD [CANDOR] vs. PVd [OPTIMISMM] and KdD [CANDOR] vs. DPd [APOLLO]). In the base-case comparison, matching (using propensity score weighting of individual patient data from CANDOR and aggregate data from OPTIMISMM and APOLLO) was performed on the following variables: time from diagnosis, International Staging System disease stage (at diagnosis), number of prior lines of treatment, age, creatinine clearance (APOLLO only), ECOG PS, prior stem cell transplantation (SCT), prior bortezomib (APOLLO only), refractory to last prior treatment, refractory to lenalidomide, and refractory to PI (OPTIMISMM only). Baseline characteristics were considered by clinical experts to be prognostic for clinical outcomes; details of the variable selection process have been described previously [Citation51,Citation55].
Due to the high proportion of patients with unknown/missing data in CANDOR (>50%), cytogenetic risk status was not included in the matching analysis; 29.5% (n = 83) of patients in OPTIMISMM and 31.8% (n = 48) in APOLLO were also missing cytogenetic risk status.
In the second step, published DPd and PVd Kaplan-Meier (KM) curves for PFS were digitized using WebPlotDigitizer [Citation56] to generate virtual patient-level data by applying the Guyot algorithm that replicated the PFS curves [Citation57]. Finally, Cox regression models were fitted to the matched CANDOR data and the reconstructed virtual patient-level data to estimate HRs for PFS between KdD vs. PVd and KdD vs. DPd. A similar process was followed for the comparison of response outcomes (i.e. overall response rate [ORR], very good partial response [VGPR] or better, complete response [CR] or better) where the matched CANDOR data and the extracted response data for the comparator therapies were used in a logistic regression model to estimate odds ratios (OR) between the treatments.
Matching characteristics (e.g. including cytogenetic risk status or excluding prior bortezomib exposure) were varied in additional scenario analyses to determine the robustness of the results. Lenalidomide-refractory patients were considered as an exploratory subgroup. However, only naïve, unmatched comparisons were possible for these analyses, mainly due to the lack of available reported baseline characteristics in OPTIMISMM and APOLLO.
Safety outcomes were compared descriptively in an unmatched manner because it is less understood if prognostic factors relevant for clinical outcomes are equally relevant for adverse events (AE). Adverse events for the comparisons were selected based on available data and included all-grade AEs as well as grade ≥3 AEs.
Results
KdD (CANDOR) vs. PVd (OPTIMISMM)
The OPTIMISMM trial included 281 patients. With a median follow-up of 15.9 months, median PFS for PVd was 11.2 months (HR for PFS vs. Vd was 0.61; 95% CI: 0.49, 0.77). Baseline characteristics for lenalidomide-exposed KdD and lenalidomide-exposed PVd patients are displayed in .
Table 1. Baseline characteristics.
Prior to matching, lenalidomide-exposed patients receiving KdD (N = 123) and PVd (N = 281) treatments differed considerably in terms of the proportion of patients who were >75 years old (KdD 6.5% vs. PVd 16.4%), previously exposed to bortezomib (KdD 94.3% vs. PVd 71.5%), refractory to bortezomib (KdD 31.7% vs. PVd 8.5%), ECOG PS score of 0 (KdD 44.7% vs. PVd 53.0%), refractory to lenalidomide (KdD 80.5% vs. PVd 71.2%), and with two or more lines of prior therapy (KdD 78.0% vs. PVd 60.5%).
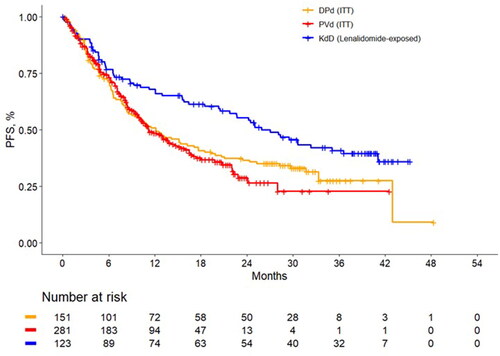
In the naïve comparison of PFS among lenalidomide-exposed patients, the median PFS was 27.0 months for KdD vs. 11.2 months for PVd with an HR of 0.58 (95% CI: 0.41, 0.76). Unadjusted KM curves are presented in .
Figure 1. Unadjusted progression-free survival Kaplan-Meier curves among lenalidomide-exposed patients. DPd: daratumumab plus pomalidomide and dexamethasone; ITT: intention to treat; KdD: carfilzomib, dexamethasone, and daratumumab; PFS, progression-free survival; PVd, pomalidomide, bortezomib, and dexamethasone.
In a MAIC analysis of KdD vs. PVd, differences were minimized, and the average baseline characteristics were balanced after matching. Of the 123 lenalidomide-exposed KdD patients from CANDOR, 10 patients were excluded because of missing data on variables used for matching. The effective sample size (ESS, calculated as the squared sum of weights divided by the sum of squared weights) was 32.3. The distribution of matching weights in the base-case analysis is presented in Supplemental Figure 1.
In matched populations, the PFS rate at 24 months was 48.5% for KdD and 28.8% for PVd. Landmark PFS estimates at 6-, 12-, 24- and 36-month timepoints are presented in . The MAIC estimated an overall PFS HR of 0.60 (95% CI: 0.37, 0.88) favoring KdD. Summary results for the base-case MAIC analysis are presented in .
Figure 2. Landmark analyses at 6-, 12-, 24- and 36-month timepoints and progression-free survival Kaplan-Meier curves for the matched population for KdD (lenalidomide-exposed subgroup) and PVd (full trial population). HR: hazard ratio; ITT: intention to treat; KdD: carfilzomib, dexamethasone, and daratumumab; PFS: progression-free survival; PVd: pomalidomide, bortezomib, and dexamethasone.
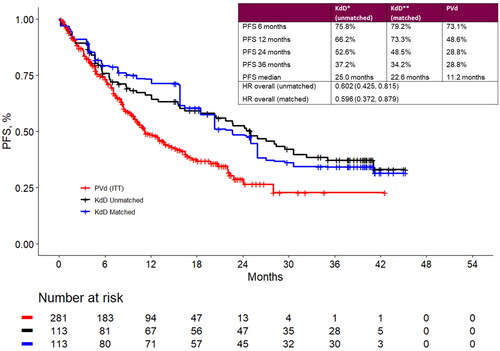
Table 2. Summary results for base-case and scenario matching-adjusted indirect treatment comparison analyses.
Several scenarios were conducted to test the robustness of the results. In the base-case analysis, the matching algorithm did not include cytogenetic risk status which was missing for a large number of patients in the trials. A scenario analysis was performed where cytogenetic risk (high vs. standard vs. unknown) was included in the matching procedure. The analysis yielded an overall HR of 0.59 (95% CI: 0.38, 0.93) indicating that potential differences in cytogenetic risk distribution across KdD and PVd patients minimally impacted the comparative efficacy results. Other analyses that included refractoriness to bortezomib (HR 0.66 [95% CI: 0.44, 1.01]) or excluded prior bortezomib therapy from the matching procedure (HR 0.61 [95% CI: 0.40, 0.88]) suggested that the impact of these potential differences on comparative efficacy results were minor. Additional scenarios as listed in also resulted in minimal differences from the HR for the overall matched and naïve comparisons.
The analyses indicated a numerically higher ORR with KdD vs. PVd. The ORR in matched patient populations was 85.0% for KdD and 82.2% for PVd, resulting in an OR of 1.22 (95% CI: 0.51, 2.91). Larger increases in response rates were estimated for patients treated with KdD vs. PVd with respect to VGPR or better and CR or better; the estimated ORs were 1.73 (95% CI: 0.82, 3.66) and 2.39 (95% CI: 0.98, 5.84), respectively (Supplemental Table 1).
The incidence of grade ≥3 neutropenia was lower for KdD than for PVd (16.4% vs. 41.7%) although grade ≥3 anemia and thrombocytopenia occurred more frequently in KdD-treated patients than in PVd-treated patients (22.1% vs. 13.7% and 32.0% vs. 27.3%, respectively). The occurrence of grade ≥3 non-hematologic AEs was similar overall across the treatment arms (Supplemental Table 2).
KdD (CANDOR) vs. DPd (APOLLO)
In the APOLLO trial, all patients (n = 151 for DPd) were lenalidomide exposed and 79.5% were refractory to lenalidomide; all patients were exposed to a PI and 47.0% were PI refractory (the study did not report bortezomib-refractory data). The median PFS was 12.4 months for DPd (HR for PFS vs. Pd was 0.63; 95% CI: 0.47, 0.85) with a median follow-up of 16.9 months.
Baseline characteristics for KdD and DPd patients can be found in . Prior to the matching procedure for the MAIC, there were considerable differences in proportion of patients who were >75 years old (KdD 6.5% vs. DPd 16.6%), refractory to PI therapy (KdD 39.0% vs. DPd 47.0%), had ECOG PS score of 0 (KdD 44.7% vs. DPd 60.3%), and had ≥2 number of prior lines of therapy (KdD 78.0% vs. DPd 89.4%).
In the naïve comparison of lenalidomide-exposed patients without matching, the median PFS was 27.0 months for KdD vs. 12.1 months for DPd with an HR of 0.66 (95% CI: 0.48, 0.90). Unadjusted analysis results are presented in .
Differences were minimized, and the average baseline characteristics were balanced after matching. Of the 123 lenalidomide-exposed KdD patients in CANDOR, five patients were excluded due to a lack of prior PI exposure (APOLLO eligibility required previous PI treatment). Nine additional patients were excluded due to missing data on variables used for matching. The ESS was 68.1. The distribution of matching weights in the base-case analysis is presented in Supplemental Figure 1.
After matching, the PFS rates at 24 months in the MAIC were 47.4% for KdD and 36.6% for DPd. Landmark PFS estimates at additional timepoints are presented in .
Figure 3. Landmark analyses at 6-, 12-, 24- and 36-month timepoints and progression-free survival Kaplan-Meier curves for the matched population for KdD (lenalidomide-exposed subgroup) and PVd (full trial population). DPd: daratumumab plus pomalidomide and dexamethasone; HR: hazard ratio; ITT: intention to treat; KdD: carfilzomib, dexamethasone, and daratumumab; PFS: progression-free survival.
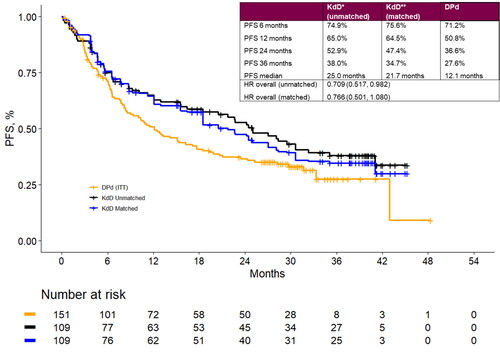
The MAIC estimated an overall PFS HR of 0.77 (95% CI: 0.50, 1.08) favoring KdD. Summary results for the base-case MAIC analysis are presented in . Robustness of the results was assessed via several scenario analyses.
Similar to the indirect comparison of KdD vs. PVd, a scenario analysis was performed which included cytogenetic risk (high vs. standard vs. unknown) in the matching procedure. The analysis yielded an overall HR of 0.71 (95% CI: 0.44, 1.03), indicating that comparative efficacy results were minimally affected by potential differences in cytogenetic risk across KdD and DPd patients. A scenario analysis was conducted to address the large, pre-matching difference across the treatment arms in the proportion of patients with an ECOG PS score of 0 (KdD 44.7% vs. DPd 60.3%). When ECOG PS was not adjusted for, the overall HR was 0.77 (95% CI: 0.50, 1.08), indicating that potential differences in ECOG PS had little impact on the comparative efficacy results. There was minimal change in the overall HR as demonstrated by scenarios which explored refractoriness to last prior regimen, prior SCT, and all three combined (i.e. ECOG PS, refractoriness to last prior regimen, and prior SCT [see ]). Similar to the other analyses, minor deviations from the overall HR were observed with and without adjustment for these differences, suggesting that the impact of the potential differences on the comparative efficacy results was minimal.
Overall response rates were numerically higher for KdD vs. DPd; the ORR in matched populations was 79.2% for KdD and 68.9% for DPd, resulting in an OR of 1.72 (95% CI: 0.83, 3.56). Consistently higher, although smaller increases in response rates were estimated for patients treated with KdD vs. DPd concerning VGPR or better and CR or better; the estimated ORs were 1.30 (95% CI: 0.72, 2.37) and 1.15 (95% CI: 0.61, 2.16), respectively (Supplementary Table 1).
Similar to the KdD vs. PVd comparison, the incidence of grade ≥3 neutropenia was considerably lower for KdD than for DPd (16.4% vs. 69.1%); however, grade ≥3 anemia and thrombocytopenia occurred somewhat more frequently in KdD-treated patients than in DPd-treated patients (22.1% vs. 18.1% and 32.0% vs. 18.1%, respectively). The incidence of grade ≥3 non-hematologic AEs was similar across the two treatment arms (Supplemental Table 2).
Discussion
Lenalidomide is increasingly used in the frontline setting for patients with RRMM up to and including the time of eventual relapse, creating a need for lenalidomide-sparing treatment options as more patients become refractory to the IMiD. KdD and other triplet regimens—PVd and DPd as evaluated in this study, as well as DVd as reported in Weisel et al. 2022 [Citation58]—have shown varying degrees of efficacy in patients with previous exposure to lenalidomide.
In the current analysis, the indirect efficacy comparisons of KdD vs. PVd and KdD vs. DPd were each evaluated using naïve comparisons and MAICs that adjusted for potential imbalances in the observed baseline characteristics among lenalidomide-exposed patients. Consistent with the naïve comparisons, the MAIC results showed that KdD was associated with a 40.4% reduction in the risk of progression or death vs. PVd that was statistically significant, with PFS rates at 24 months about 70% greater with KdD vs. PVd (). The estimated 33.4% reduction in the risk of progression or death with KdD compared with DPd may also be considered clinically meaningful, consistent with an approximately 30% higher PFS rate at 24 months with KdD than DPd within these study populations.
In scenario analyses of the KdD vs. PVd comparison (), the effect size was estimated to be robust with overall HRs varying between 0.57 and 0.66 and upper bounds of 95% CIs generally remaining less than one. In scenario analyses of the KdD and DPd comparison, the effect size was estimated to vary between 0.71 and 0.78, although sample sizes were small. There was greater uncertainty around estimated HRs, with 95% CIs narrowly inclusive of one. A similar previous analysis based on shorter follow-up from each study showed HRs with 95% CI upper bounds below one [Citation59].
Response rates (i.e. ORR, VGPR or better, CR or better) were numerically higher for KdD than for comparator therapies in the matched populations with estimated ORs of ≥1.22 for KdD vs. PVd and ≥1.15 for KdD vs. DPd. With respect to safety outcomes, grade ≥3 AEs were similar overall across the treatments.
Sample sizes were insufficient to perform robust analyses on some subgroups, for example, in one prior line of therapy. However, a comparison of results for median PFS across CANDOR, OPTIMISMM, and APOLLO still favored KdD. For example, in patients with two or more lines of therapy, median PFS was slightly better for KdD (24.2 months) vs. PVd (22.4 months) and more than double that for DPd (10.7 months).
Although only unmatched analysis was feasible in lenalidomide-refractory subgroups due to the lack of published baseline characteristics, the analyses suggested that the treatment benefit of KdD over PVd may be largest in the lenalidomide-refractory populations (overall HR = 0.48; Supplemental Table 3), that is, KdD may be particularly effective in patients with the greatest need for lenalidomide-sparing therapy. Median PFS for patients refractory to lenalidomide was estimated to be 27.0 months (95% CI: 20.3, 41.1) with KdD in CANDOR and reported as 9.9 months with DPd in APOLLO (Supplemental Table 3). Statistical comparison of overall PFS in the lenalidomide-refractory subgroups between CANDOR and APOLLO was not possible in the absence of published KM curves for DPd. However, the other comparisons presented herein may be generally representative because there was little difference between the treatment arms in the proportion of lenalidomide-refractory patients, and most lenalidomide-exposed patients were also refractory, consistent with real-world application of the IMiD for the treatment of newly diagnosed MM (NDMM).
Each of the phase 3 studies included in this analysis reported a significant improvement in PFS alongside deeper responses with experimental triplet regimens vs. corresponding doublet comparators. High rates of patients with VGPR or better were reported in CANDOR (KdD 69.0% vs. Kd 49.0%), OPTIMISMM (PVd 61.0% vs. Vd 23.0%), and APOLLO (DPd 51.0% vs. Pd 20.0%). Further, the rate of achievement of minimal residual disease (MRD) negativity was 18.0% for KdD vs. 4.0% for Kd in CANDOR (at 12 months, p < 0.0001) and 9.0% for DPd vs. 2.0% for Pd in APOLLO (p = 0.01) (MRD was not reported in OPTIMISMM). Depth of response is increasingly recognized as an important clinical outcome in MM [Citation60]. In NDMM, statistically significant correlations have been demonstrated between median PFS and response outcomes, including ORR, stringent CR, and MRD negativity, with stronger correlations observed with deeper responses [Citation61,Citation62].
Overall survival (OS) data were not analyzed in the current analysis since mature OS data were not available from the analysis sets corresponding to each study. The proportions of patients with prior exposure to bortezomib and those who were refractory to bortezomib differed in the OPTIMISMM and APOLLO trials, suggesting that these patient populations may not have received the same subsequent treatments, making comparisons of OS data challenging. Though OS data were not available, PFS and response endpoints are increasingly recognized as important early indicators of efficacy and survival benefits in MM [Citation63,Citation64].
Indirect treatment comparisons evaluating the efficacy and safety of isatuximab, one of the two anti-CD38 monoclonal antibodies approved for the treatment of RRMM, have been conducted using MAIC methodology similar to the current analysis. Specifically, isatuximab plus Kd from the IKEMA trial was compared with daratumumab plus lenalidomide/dexamethasone (DRd) from the POLLUX trial and it was found that isatuximab plus Kd was associated with longer PFS than DRd [Citation65]. While the results for isatuximab plus Kd may be generalizable to KdD, the comparison was considered less relevant in the context of the current study because the POLLUX population was mostly lenalidomide naïve (82.0%). In another MAIC analysis, isatuximab plus Pd from the ICARIA trial was compared with DPd and it was found that isatuximab plus Pd was associated with a numerically longer PFS than DPd [Citation66]. The comparison was, however, affected by a number of challenges due to differences in trial populations that could not be adjusted for (e.g. in the APOLLO study, the range of prior lines of treatments was one to five, whereas in the ICARIA study, no patients received one or five prior lines of therapy). While in the present study, no comparisons were performed for KdD vs. isatuximab plus Pd, given isatuximab plus Pd and DPd may be associated with similar outcomes, the comparisons presented here for KdD vs. DPd may be generalizable to KdD vs. isatuximab plus Pd.
Health-related quality of life, convenience, avoidance of specific toxicities, method of administration, and affordability play an important role in treatment decision-making. However, the focus of this study was that of the comparative effectiveness using a well-established ITC methodology. Future research on a wider range of treatment decision-making criteria is warranted to provide a well-rounded picture of patient care.
Limitations
The MAIC analysis was undertaken in alignment with guidance from NICE, but results should be interpreted with caution due to the inherent limitations of this approach (as acknowledged in NICE TSD 18) associated with the implicit assumption that all observed and unobserved prognostic and/or predictive factors for a given outcome are accounted for. While these may be strong assumptions in certain circumstances, MAIC is increasingly being used for health technology assessments and economic evaluations as it provides an approach that adjusts for imbalances in patient populations under study; nonetheless, the validity of the indirect comparisons must be carefully considered and extensive scenario analyses should be conducted to assess any potential bias.
The OPTIMISMM and APOLLO trials used simulated patient-level data, although any potential bias was likely to be small since KM estimates were closely reproduced.
There were differences in the proportion of patients receiving different numbers of prior lines of therapies in each clinical trial. In CANDOR, the median PFS was 25.9 months for lenalidomide-exposed patients with only one prior line of therapy (22.0%). In OPTIMISMM and APOLLO, the median PFS was 20.7 months and 14.1 months for patients with only one prior line of therapy (39.5% and 10.6%), respectively. Although the MAIC corrected for differences in the proportion of patients with one prior line of therapy, some residual confounding may have remained within patients receiving ≥2 prior therapies that could have introduced some bias in the comparisons. Given that in each pairwise comparison, there was no common arm (e.g. placebo), an unanchored approach was used. While unanchored analyses require all prognostic variables and effect modifiers to be included in the matching procedure to ensure unbiased indirect comparisons, for the current study, any potential bias was deemed to be small given the results of the extensive scenario analyses.
Finally, a recent literature review summarized the profile of grade ≥3 cardiotoxicity of certain IMiDs and PIs. The IMiDs thalidomide and lenalidomide had an OR of 2.05 (95% CI: 1.30, 3.26) vs. no IMiDs; in the latter category, bortezomib and carfilzomib demonstrated more frequent high-grade cardiotoxicity compared with controls (OR 1.67; 95% CI: 1.17, 2.40 and OR 2.68; 95% CI: 1.63, 4.40, respectively) [Citation67]. Multiple myeloma typically affects older populations whose age make them at an increased risk for cardiovascular events. CANDOR enrolled a lower proportion of patients ≥75 years old (6.5%) compared with the OPTIMISMM (16.4%; >75) and APOLLO (16.6%) trials. While all studies (CANDOR, OPTIMISMM, and APOLLO) excluded patients based on certain cardiac-related criteria (e.g. congestive heart failure, clinically significant electrocardiogram abnormalities, myocardial infarction within four, 12, and six months prior to therapy, respectively), additional differences in patient selection criteria (e.g. exclusion of patients with uncontrolled hypertension in CANDOR) may have introduced some bias into the MAIC results. However, since the analyses adjusted for age, which is strongly correlated with cardiovascular comorbidity, any potential bias is expected to be limited.
Conclusions
In this MAIC study using long-term follow-up data from the CANDOR study, treatment with KdD resulted in significantly longer PFS than PVd and numerically longer PFS than DPd in patients with RRMM and previous lenalidomide exposure, the majority of whom were also refractory to lenalidomide. KdD offers a highly effective, lenalidomide-sparing treatment option for patients with RRMM. Although the optimal treatment of RRMM should be tailored to individual patient characteristics and preferences, KdD can be considered as a standard therapeutic option at first relapse, especially among the growing population of patients with refractoriness and/or prior exposure to lenalidomide.
Supplemental Material
Download Zip (160 KB)Acknowledgements
The study was sponsored and funded by Amgen. The authors thank Hoora Moradian, PhD, and Colleen Dumont, BS, of Cytel, Inc. for methodological support and medical writing assistance in the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Qualified researchers may request de-identified data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Additional information
Funding
References
- Rajkumar SJ. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018; 93(8):981–1114.
- Cancer.net. Multiple Myeloma: statistics. 2023 [May 2023].
- International Agency for Research on Cancer. Multiple myeloma (Source: globocan 2020) 2020 May 2023]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/35-Multiple-myeloma-fact-sheet.pdf.
- National Cancer Institute. Cancer Stat Facts: myeloma November 2022]. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html.
- Durie B. Concise review of the disease and treatment options: multiple myeloma. North Hollywood: International Myeloma Foundation (IMF) 2018 October 2022]. Available from: https://imf-d8-prod.s3.us-west-1.wasabisys.com/resource/ConciseReview.pdf.
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. doi:10.1056/NEJMra1011442
- Multiple Myeloma Research Foundation. Multiple myeloma disease overview. 2017. Available from: https://themmrf.org/multiple-myeloma/.
- Jakubowiak AJ. Novel therapies for relapsed/refractory multiple myeloma: how can we improve on "salvage" therapy?–introduction. Semin Hematol. 2012;49 Suppl 1(Suppl 1): S1–S2. doi:10.1053/j.seminhematol.2012.05.002
- Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol. 2016; 175(2):252–264. doi:10.1111/bjh.14213
- Fonseca R, Abouzaid S, Bonafede M, et al. Trends in overall survival and costs of multiple myeloma, 2000-2014. Leukemia. 2017; 31(9):1915–1921. doi:10.1038/leu.2016.380
- Holstein SA, Suman VJ, McCarthy PL. Update on the role of lenalidomide in patients with multiple myeloma. Ther Adv Hematol. 2018; 9(7):175–190. doi:10.1177/2040620718775629
- Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020; 396(10245):186–197. doi:10.1016/S0140-6736(20)30734-0
- Usmani SZ, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol. 2022; Jan23(1):65–76. doi:10.1016/S1470-2045(21)00579-9
- Dimopoulos MA, Terpos E, Boccadoro M, et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021; 22(6):801–812. doi:10.1016/S1470-2045(21)00128-5
- Dimopoulos MA, Oriol A, Nahi H, et al. Overall survival with daratumumab, lenalidomide, and dexamethasone in previously treated multiple myeloma (POLLUX): a randomized, open-label, phase III trial. J Clin Oncol. 2023; 41(8):1590–1599. doi:10.1200/JCO.22.00940
- Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397(10292):2361–2371. doi:10.1016/S0140-6736(21)00592-4
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016; 375(8):754–766. doi:10.1056/NEJMoa1606038
- Usmani SZ, Quach H, Mateos MV, et al. Final analysis of carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone in the CANDOR study. Blood Adv. 2023,Jul 25;7(14):3739–3748. doi: 10.1182/bloodadvances.2023010026
- Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica. 2018;103(12):2079–2087. doi:10.3324/haematol.2018.194118
- Mateos MV, Sonneveld P, Hungria V, et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with previously treated multiple myeloma: three-year follow-up of CASTOR. Clin Lymphoma Myeloma Leuk. 2020; 20(8):509–518. doi:10.1016/j.clml.2019.09.623
- Sonneveld P, Chanan-Khan A, Weisel K, et al. Overall survival with daratumumab, bortezomib, and dexamethasone in previously treated multiple myeloma (CASTOR): a randomized, open-label, phase III trial. J Clin Oncol. 202341(8):1600–1609. doi:10.1200/JCO.21.02734
- Bahlis NJ, Dimopoulos MA, White DJ, et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020;34(7):1875–1884. doi:10.1038/s41375-020-0711-6
- Martin T, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in patients with relapsed multiple myeloma: updated results from IKEMA, a randomized phase 3 study. Blood Cancer J. 2023; 13(1):72. doi:10.1038/s41408-023-00797-8
- Martin T, Mikhael J, Hajek R, et al. Depth of response and response kinetics of isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma. Blood Adv. 2022;6(15):4506–4515. doi:10.1182/bloodadvances.2021006713
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016; 375(14):1319–1331. doi:10.1056/NEJMoa1607751
- Dimopoulos MA, San-Miguel J, Belch A, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018; 103(12):2088–2096. doi:10.3324/haematol.2018.194282
- Leukemia & Lymphoma Society. Multiple myeloma: refractory and Relapsed June 2023]. Available from: https://www.lls.org/myeloma/treatment/refractory-and-relapsed.
- Nijhof IS, van de Donk N, Zweegman S, et al. Current and new therapeutic strategies for relapsed and refractory multiple myeloma: an update. Drugs. 2018;78(1):19–37. doi:10.1007/s40265-017-0841-y
- Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up(dagger). Ann Oncol. 2021; 32(3):309–322. doi:10.1016/j.annonc.2020.11.014
- Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the international myeloma working group. Lancet Oncol. 2021; 22(3):e105–e118. doi:10.1016/S1470-2045(20)30756-7
- Moreau P, Zamagni E, Mateos MV. Treatment of patients with multiple myeloma progressing on frontline-therapy with lenalidomide. Blood Cancer J. 2019;9(4):38. doi:10.1038/s41408-019-0200-1
- Lecat CSY, Taube JB, Wilson W, et al. Defining unmet need following lenalidomide refractoriness: real-world evidence of outcomes in patients with multiple myeloma. Front Oncol. 2021;11:703233. doi:10.3389/fonc.2021.703233
- Touzeau C, Quignot N, Meng J, et al. Survival and treatment patterns of patients with relapsed or refractory multiple myeloma in France - a cohort study using the French National healthcare database (SNDS). Ann Hematol. 2021;100(7):1825–1836. doi:10.1007/s00277-021-04522-y
- Botta C, Martino EA, Conticello C, et al. Treatment of lenalidomide exposed or refractory multiple myeloma: network meta-analysis of lenalidomide-sparing regimens [opinion]. Front Oncol. 2021;11:643490. doi:10.3389/fonc.2021.643490
- Khan ML, Stewart AK. Carfilzomib: a novel second-generation proteasome inhibitor. Future Oncol. 2011; 7(5):607–612. doi:10.2217/fon.11.42
- European Medicines Agency. Kyprolis (carfilzomib) summary of product characteristics; 2020 [updated 2020 Jun 25] 2020 [October 2022]. Available from: https://www.ema.europa.eu/en/documents/product-information/kyprolis-epar-product-information_en.pdfhttps://www.ema.
- US Food and Drug Administration. KYPROLIS (carfilzomib) for injection, for intravenous use; 2020 [updated 2020 Aug] 2020 [October 2022]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202714s030lbl.pdf.
- US Food and Drug Administration. FDA approval of Sarclisa (isatuximab-irfc) in combination with carfilzomib and dexamethasone for adult patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy 2021 December 2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-sarclisa-isatuximab-irfc-combination-carfilzomib-and.
- European Medicines Agency. SARCLISA (isatuximab) Summary of Product Characteristics December 2022]. Available from: https://www.ema.europa.eu/en/documents/product-information/sarclisa-epar-product-information_en.pdf.
- Lacy MQ, McCurdy AR. Pomalidomide. Blood. 2013; 2013/10/03/122(14):2305–2309. doi:10.1182/blood-2013-05-484782
- Fotiou D, Gavriatopoulou M, Terpos E, et al. Pomalidomide- and dexamethasone-based regimens in the treatment of refractory/relapsed multiple myeloma. Ther Adv Hematol. 2022;13:20406207221090089. doi:10.1177/20406207221090089
- Paludo J, Mikhael JR, LaPlant BR, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed lenalidomide-refractory multiple myeloma. Blood. 2017;130(10):1198–1204. doi:10.1182/blood-2017-05-782961
- Mark T, Falkenstein A, Kish J. Real-world outcomes of pomalidomide therapy after lenalidomide induction in relapsed/refractory multiple myeloma. Future Oncol. 2021; 18(5):553–564. doi:10.2217/fon-2021-1176
- Jagannath S, Abonour R, Durie BGM, et al. Heterogeneity of second-line treatment for patients with multiple myeloma in the connect MM registry (2010-2016). Clin Lymphoma Myeloma Leuk. 2018;18(7):480–485 e3. doi:10.1016/j.clml.2018.04.007
- Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019; 20(6):781–794. doi:10.1016/S1470-2045(19)30152-4
- Laws A, Kendall R, Hawkins N. A comparison of national guidelines for network meta-analysis. Value Health. 2014;17(5):642–654. doi:10.1016/j.jval.2014.06.001
- Cope S, Toor K, Popoff E, et al. Critical appraisal of published indirect comparisons and network meta-analyses of competing interventions for multiple myeloma. Value Health. 2020; 23(4):441–450. doi:10.1016/j.jval.2019.11.003
- Majer I, van de Wetering G, Polanyi Z, et al. Panobinostat plus bortezomib versus lenalidomide in patients with relapsed and/or refractory multiple myeloma: a matching-adjusted indirect treatment comparison of survival outcomes using patient-level data. Appl Health Econ Health Policy. 2017; 15(1):45–55. doi:10.1007/s40258-016-0271-0
- Rael M, Benedict A, Ishak J, et al. Indirect comparison to assess the relative efficacy of carfilzomibþlenalidomideþdexamethasone versus bortezomibþthalidomideþdexamethasone: a matching adjusted indirect comparison. Blood. 2015;126(23):5622–5622. doi:10.1182/blood.V126.23.5622.5622
- Van Sanden S, Ito T, Diels J, et al. Comparative efficacy of daratumumab monotherapy and pomalidomide plus low-dose dexamethasone in the treatment of multiple myeloma: a matching adjusted indirect comparison. Oncologist. 2018; Mar23(3):279–287. doi:10.1634/theoncologist.2017-0103
- Weisel K, Nooka AK, Terpos E, et al. Carfilzomib and dexamethasone versus eight cycles of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma: an indirect comparison using data from the phase 3 ENDEAVOR and CASTOR trials. Leuk Lymphoma. 2020; 63(8):1887–1896. doi:10.1080/10428194.2019.1648806
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi:10.1016/j.jval.2012.05.004
- Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–945. doi:10.2165/11538370-000000000-00000
- Phillippo DA, Dias S, et al. NICE DSU Technical Support Document 18: methods for population-adjusted indirect comparisons in submissions to NICE. (Technical Support Documents). NICE Decision Support Unit 2016 October 2022]. Available from: https://research-information.bris.ac.uk/ws/portalfiles/portal/94868463/Population_adjustment_TSD_FINAL.pdf.
- Majer IM, Castaigne JG, Palmer S, et al. Modeling covariate-adjusted survival for economic evaluations in oncology. Pharmacoeconomics. 2019;37(5):727–737. doi:10.1007/s40273-018-0759-6
- WebPlotDigitizer. Home page June 2023]. Available from: https://automeris.io/WebPlotDigitizer/.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. doi:10.1186/1471-2288-12-9
- Weisel K, Nooka AK, Terpos E, et al. Carfilzomib 56 mg/m(2) twice-weekly in combination with dexamethasone and daratumumab (KdD) versus daratumumab in combination with bortezomib and dexamethasone (DVd): a matching-adjusted indirect treatment comparison. Leuk Lymphoma. 2022;63(8):1887–1896. doi:10.1080/10428194.2022.2047962
- Chari A, Dimopoulos M-A, Beksac M, et al. P-191: comparison of efficacy outcomes for carfilzomib plus dexamethasone and daratumumab (KdD) versus pomalidomide plus bortezomib and dexamethasone (PVd) and D-Pd in relapsed or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2021 ;21:S142. doi:10.1016/S2152-2650(21)02318-1
- Lahuerta JJ, Paiva B, Vidriales MB, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35(25):2900–2910. doi:10.1200/JCO.2016.69.2517
- Avet-Loiseau H, Ludwig H, Landgren O, et al. Minimal residual disease status as a surrogate endpoint for progression-free survival in newly diagnosed multiple myeloma studies: a meta-analysis. Clin Lymphoma Myeloma Leuk. 2020; 20(1):e30–e37. doi:10.1016/j.clml.2019.09.622
- Daniele P, Mamolo C, Cappelleri JC, et al. Response rates and minimal residual disease outcomes as potential surrogates for progression-free survival in newly diagnosed multiple myeloma. PLOS One. 2022;17(5):e0267979. doi:10.1371/journal.pone.0267979
- Cartier S, Zhang B, Rosen VM, et al. Relationship between treatment effects on progression-free survival and overall survival in multiple myeloma: a systematic review and meta-analysis of published clinical trial data. Oncol Res Treat. 2015;38(3):88–94. doi:10.1159/000375392
- Dimopoulos M, Sonneveld P, Nahi H, et al. Progression-free survival as a surrogate endpoint for overall survival in patients with relapsed or refractory multiple myeloma. Value in Health. 2017;20(9):A408. doi:10.1016/j.jval.2017.08.064
- Richter J, Lin PL, Garcia-Horton V, et al. Matching-adjusted indirect comparison of isatuximab plus carfilzomib and dexamethasone with daratumumab plus lenalidomide and dexamethasone in relapsed multiple myeloma. Cancer Med. 2023; 12(7):8005–8017. doi:10.1002/cam4.5584
- Leleu X, Delea T, Guyot P, et al. POSC34 comparative efficacy and safety of isatuximab plus pomalidomide and dexamethasone versus daratumumab plus pomalidomide and dexamethasone in patients with multiple myeloma using a matching-adjusted indirect comparison. Value in Health. 2022;25(1):S39. doi:10.1016/j.jval.2021.11.180
- El-Cheikh J, Moukalled N, Malard F, et al. Cardiac toxicities in multiple myeloma: an updated and a deeper look into the effect of different medications and novel therapies. Blood Cancer J. 2023; 13(1):83. doi:10.1038/s41408-023-00849-z

