Abstract
Despite advances in treatment, a significant proportion of patients with chronic lymphocytic leukemia (CLL) will relapse with drug-resistant disease. The imipridones, ONC-201 and ONC-212, are effective against a range of different cancers, including acute myeloid leukemia (AML) and tumors of the brain, breast, and prostate. These drugs induce cell death through activation of the mitochondrial protease, caseinolytic protease (CIpP), and the unfolded protein response (UPR). Here we demonstrate that the novel imipridone analog, TR-57, has efficacy as a single agent and synergises with venetoclax against CLL cells under in vitro conditions that mimic the tumor microenvironment. Changes in protein expression suggest TR-57 activates the UPR, inhibits the AKT and ERK1/2 pathways and induces pro-apoptotic changes in the expression of proteins of the BCL-2 family. The study suggests that TR-57, as a single agent and in combination with venetoclax, may represent an effective treatment option for CLL.
Introduction
Chronic lymphocytic leukemia is the most common form of adult leukemia, accounting for approximately one third of all diagnoses world-wide [Citation1]. CLL is characterized by the clonal expansion of CD5-positive B-cells in the peripheral blood, bone marrow, lymph nodes, and spleen [Citation2]. Regions within lymph nodes and bone marrow populated by CLL cells are collectively known as the tumor microenvironment (TME). CLL proliferation in the TME is driven by the interaction of leukemic cells with ‘accessory’ cells, other immune cells and by a range of cytokines and growth factors [Citation3].
Ibrutinib [Citation4] and venetoclax [Citation5,Citation6], which target the BTK and BCL-2 proteins, respectively, have proven effective in both front-line and relapsed/refractory settings. However, relapse and drug resistance remains common, highlighting the need for new treatment strategies.
To survive and proliferate within the TME, cancer cells rely on pathways that are collectively known as the unfolded protein response (UPR) [Citation7]. Recent studies suggest that targeting the UPR may represent an effective treatment approach, resulting in apoptosis in a variety of cancers [Citation8]. In healthy cells, the UPR functions to limit protein translation and cell proliferation in response to a variety of cellular stresses and can trigger apoptosis. However, the proliferation and survival of cancer cells is dependent on their ability to adapt to the stress associated with rapid cellular growth and limited availability of nutrients and oxygen. Several key proteins and pathways have been identified that regulate the UPR. Endoplasmic reticulum (ER) stress activates the Grp78 protein, which in turn activates protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) [Citation9]. PERK phosphorylates eIF2α, which generally attenuates protein synthesis. However, the UPR also increases translation of certain proteins through activity of transcription factors, including ATF4 [Citation7].
Several recent studies have demonstrated that a class of drugs that target the UPR, known as imipridones, have efficacy against a range of cancers [Citation10,Citation11], including acute myeloid leukemia (AML) [Citation12]. The mechanisms of action of the imipridones include inhibition of mitochondrial function [Citation13] and activation of the mitochondrial protease, caseinolytic protease (CIpP) proteolytic subunit [Citation14–16]. Paradoxically, ClpP overexpression has been observed in several cancers that are sensitive to imipridones, including AML [Citation12], prostate and breast [Citation17].
We recently demonstrated that the imipridone ONC-212 (TR-31) induces expression of ClpP, inhibits signaling downstream of the B-cell receptor (BCR) and induces a pro-apoptotic shift in expression of BCL-2 family proteins in CLL cells, under in vitro conditions that mimic the TME [Citation18]. In this study, we investigated the efficacy of the CIpP activator, TR-57, against CLL cells under conditions that mimic pro-survival effects of the TME. Our observations that MCL-1 expression is increased in cells treated with venetoclax [Citation19] and that ONC-212 decreases MCL-1 expression [Citation18], provided the rationale for investigating synergy between TR-57 and venetoclax.
Materials and methods
Patient samples
Samples were collected from CLL patients managed at Royal North Shore Hospital, Sydney following written consent. CLL was diagnosed according to the international workshop on CLL (iwCLL) guidelines [Citation20]. Peripheral blood mononuclear cells (PBMCs) were isolated by ficoll-density gradient centrifugation. All patient samples contained >85% CD5+/CD19+ (CLL) cells, as determined by flow cytometry (data not shown). PBMCs were cryopreserved in fetal calf serum (FCS), containing 10% dimethyl sulphoxide (DMSO). ATM/TP53 function and ZAP-70 and CD38 expression were assessed using previously published methods [Citation21,Citation22]. Category 1 and 3 ATM/TP53 dysfunction are consistent with mutations in TP53 [Citation21] and the presence of a small TP53 dysfunctional clone [Citation23], respectively. Deletions of the 17p13 (TP53) and 11q23 (ATM) chromosomal regions were detected by fluorescence in-situ hybridization (FISH).
Cell culture
All cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% FCS, 2 mM L-glutamine and 1% penicillin/streptomycin (complete medium). The OSU-CLL cell line was obtained under a materials transfer agreement with Ohio State University. Details of the derivation of the OSU-CLL line are described in [Citation24].
Primary CLL cells were seeded at 1 × 105 or 2 × 106 cells per well of a 96- or 24-well plate, respectively. Mouse L-fibroblasts, human CD40-ligand (CD40L-fibroblasts) were seeded at a density of 250 cells/µL one day prior to co-culture with primary CLL cells.
Generation of a TP53 knock-out OSU-CLL cell line
Knock-out of the TP53 gene in the OSU-CLL cell line was achieved using a lentiviral CRISPR-Cas9 technology developed at the Walter and Eliza Hall Institute (WEHI), Melbourne, Australia, as described by Aubrey et al. [Citation25]. The guide RNA sequence used was as follows: 5′-AAACGAAAACAACGTTCTGGTAAG-3′. Sanger sequencing and immunoblotting were performed to confirm knockout of the TP53 gene and protein (Supplementary Figure 1(A)). Flow cytometry was used to confirm that the processes involved in the generation of the OSU-CLLTP53ko line had no effect on CD5/CD19 expression (data not shown).
Assessment of cell viability
Cell viability was assessed by flow cytometry on an LSR Fortessa instrument (Becton Dickinson, Franklin Lakes, NJ) by staining cells for 20 min at 37 °C with 0.05 µM of the mitochondrial membrane potential dye, 1,1′,3,3,3′,3′-hexamethylindodicarbocyanine iodide (DiIC1(5); Thermo Fisher Scientific) and 10 µM propidium iodide (PI; Sigma-Aldrich, St Louis, MI). Loss of mitochondrial membrane potential is an early event in apoptosis [Citation26], which can be detected with dyes such as DiIC1(5).
Drug concentrations that induced a 50% decrease in cell viability compared to untreated controls (IC50 values) were calculated using GraphPad Prism software (GraphPad Software, San Diego, CA).
The cytotoxic effects of TR-57 and venetoclax toward CLL and autologous T-cells were assessed by flow cytometry using antibodies against CD5 and CD19 and the viability dyes, DiIC1(5) and PI.
Assessment of drug synergy
TR-57 and venetoclax were combined at ratios based on their IC50 values as single agents (Supplementary Table 1). CompuSyn software (www.combosyn.com) was used to calculate combination indices (CI) for the drugs against the cell lines and primary CLL cells, according to the methods of Chou and Talalay [Citation27]. CI values of <1, 1, and >1 are indicative of synergy, additivity, and antagonism, respectively.
Cell cycle and proliferation
Proliferation of the OSU-CLL and OSU-CLL-TP53ko cells was assessed using carboxyfluorescein succinimidyl ester (CFSE; Sigma-Aldrich). Cells were stained with 2 µM CFSE prior to treatment with IC25 doses of TR-57 and venetoclax. The rate of decay of the mean fluorescence intensity (MFI) of CFSE was analyzed by flow cytometry and used to calculate the rate of proliferation.
The CpG oligonucleotide, Dsp30, and interleukin-2 (IL-2) were used to stimulate primary CLL cells to cycle. Peripheral blood-derived CLL cells were cultured with 2 µM Dsp30 (Integrated DNA Technologies, Coralville, IO), and 200 U/ml IL-2 (Peprotech, Rocky Hill, NJ) for 4 d, followed by treatment with TR-57 and venetoclax for a further 48 h. Samples were then harvested and stained with 40 µg/ml PI, 100 µg/ml RNase, and 0.1% Triton in PBS at 37 °C for 30 min. Data were acquired by flow cytometry and analyzed using ModFit software (Verity Software House, Topsham, ME). Data are expressed as the proportion of CLL cells in the S and G2/M phases.
Integrin and cytokine-receptor expression and cell migration
Primary CLL cells in co-culture with CD40L-fibroblasts were treated with 100 nM TR-57 or 10 nM venetoclax for 24 h. The cells were then harvested and stained with phycoerythrin (PE) conjugated antibodies to CD49d or CXCR4 (Biolegend, San Diego, CA). Expression of both proteins was assessed by flow cytometry. Results are expressed as fold-changes in expression relative to untreated cells.
The effects of the drugs on the migratory capacity of CLL cells were assessed using the CXCR4 ligand, SDF-1α, as a chemoattractant. Primary CLL cells were treated for 24 h with TR-57 and venetoclax. Cell viability was then assessed by trypan blue exclusion and 3 × 106 viable cells from each condition were loaded into the upper chambers of Transwell culture inserts (Merck, Burlington, MA). Cells were allowed to migrate toward the lower well containing medium, with or without 200 ng/ml SDF1α, for 3 h. Viable cells harvested from the lower well were enumerated using flow cytometry by staining with DiIC1(5) and PI with a fixed acquisition time of 120s. Data are expressed as a fold-change in the number of viable CLL cells that migrated through the insert, relative to untreated controls.
Immunoblotting
Primary CLL cells cultured in medium alone or in co-culture with CD40L-fibroblasts were treated with TR-57 (50 nM) and venetoclax (50 nM) for 24 h. The percentage of intact, viable cells prior to lysis was assessed using PI and flow cytometry and exceeded 70% for all samples. An equal number of viable cells from each treatment were lysed in radioimmunoprecipitation assay buffer (RIPA; 50 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris–HCl, pH 8.0) containing phosphatase and protease inhibitors (MSSafe; Sigma Aldrich, St Louis, MI). Methods used for immunoblotting and data analysis were as described previously [Citation28].
Analysis of RNA expression by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
Gene expression changes following treatment with TR-57 (50 nM), venetoclax (50 nM) or the drugs in combination were assessed by RT-qPCR. Primary CLL cells (3 × 106 per condition) were cultured in medium alone or co-cultured with CD40L fibroblasts (as described earlier) and treated 50 nM TR-57, 50 nM venetoclax or the drugs in combination for 4 h. Following treatment, CLL cells were harvested into Tri-Reagent (Sigma Aldrich) according to the manufacturers recommendations and stored at −80 °C until required. The viability of all samples prior to storage was >80%, as assessed using PI and flow cytometry. RNA was extracted from the cell lysates and cDNA generated using Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) or M-MuLV Reverse Transcriptase (New England Biolabs, Ipswich, MA). Primer sequences are shown in Supplementary Figure 4(B). Primer efficiency was confirmed via RT-qPCR using a four point, 10-fold serial dilution of universal cDNA. Gene expression was then determined via RT-qPCR using SYBR Green Master Mix (Applied Biosystems, Waltham, MA). Delta-delta Ct calculations were performed and normalized to expression of the house-keeping gene, HPRT1. Data from the cells co-cultured with CD40L-fibroblasts are expressed as fold-changes in expression relative to cells cultured in media. Drug induced changes are expressed as fold changes relative to control CLL cells co-cultured with CD40L-fibroblasts.
Data presentation and statistics
Graph Pad Prism software was used to generate all graphs and perform statistical analyses using the T-test function (paired or unpaired as appropriate), following confirmation of normal distribution (data not shown). p Values of <0.05 were considered significant.
Results
TR-57 is cytotoxic toward CLL cells cultured under in vitro conditions that mimic the TME
TR-57 was effective at inducing apoptosis of primary CLL cells (n = 15 patients; CLL# 1,2,4,5,7,12,13,15–19, and 20–22) cultured in medium alone or with CD40L-expressing fibroblasts (). CLL cells cultured in medium alone were significantly more sensitive to TR-57 than cells co-cultured with CD40L-fibroblasts; the IC50 values were 23.5 ± 3.35 and 147.8 ± 22.3 nM, respectively (Supplementary Table 1). No significant difference (p = 0.70) in sensitivity to TR-57 was observed between samples with (n = 5; CLL# 2,7,15,19,21) or without (n = 8; CLL# 1,4,5,16,17,18,20, and 22) TP53 dysfunction ().
Figure 1. TR-57 induces apoptosis in primary CLL cells and in the OSU-CLL and OSU-CLL-TP53ko cell lines. CLL cell viability was assessed by flow cytometry using DiIC1(5) and PI. Viability is expressed relative to vehicle-treated controls and error bars represent the standard deviation. (A) Primary CLL cells (n = 15 patients), cultured in medium alone or in co-culture with CD40L-fibroblasts, were treated with TR-57 for 48 h. (B) Dose response analysis of TR-57 against TP53 wild-type (n = 8) and TP53 dysfunctional (n = 5) CLL patient samples following 48 h treatment in co-culture with CD40L-fibroblasts. (C) OSU-CLL and OSU-CLL-TP53ko cell lines were treated with TR-57 for 48 h. Data are the mean of 4 biological replicates.
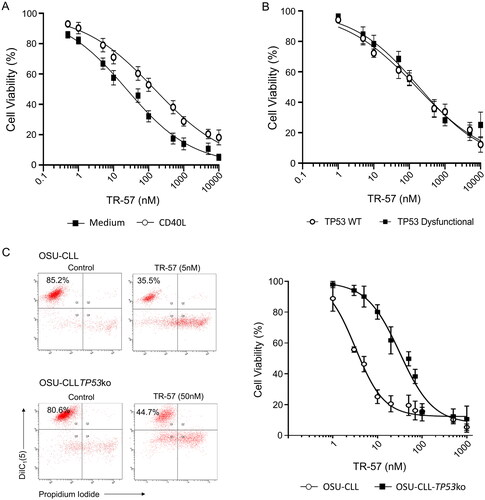
TR-57 is cytotoxic toward TP53-deficient CLL cells
To further examine the effects of TR-57 on TP53-deficient CLL cells, we generated an OSU-CLL cell line in which TP53 was knocked-out using CRISPR-Cas9 (OSU-CLL-TP53ko). OSU-CLL cells transfected with CRISPR-Cas9 and guide RNA, but not activated with doxycycline, were used as a control for effects of the transfection processes. No significant difference (p = 0.25) in the sensitivity of these control cells and OSU-CLL cells to TR-57 was observed (Supplementary Figure 1(A)).
TR-57 was cytotoxic toward OSU-CLL and OSU-CLL-TP53ko cells in a dose-dependent manner, however, the IC50 for TR-57 was significantly (p = 0.004) higher against the TP53ko (90.71 ± 3.51 nM) than wild-type line (3.98 ± 1.03 nM; ).
TR-57 and venetoclax are synergistic against primary CLL cells and OSU-CLL and OSU-CLL-TP53ko cells
Next, we explored the effects of combining TR-57 with venetoclax against primary CLL cells from 8 patients co-cultured with CD40L-expressing fibroblasts, including 5 samples from patients with ATM/TP53 deletions or dysfunction (CLL# 2,7,15,19,21; ), and the OSU-CLL and OSU-CLL-TP53ko lines. Dose-response analyses and CI suggest a high degree of synergy between TR-57 and venetoclax in all primary CLL samples () and both cell lines (); CI values at a fractional effect of 0.5 (50% cell killing) for primary CLL cells, OSU-CLL and OSU-CLL-TP53ko cells, were 0.13, 0.10, and 0.10, respectively (Supplementary Table 1).
Figure 2. TR-57 and venetoclax are synergistic in their cytotoxic effects toward CLL cells.
Cell viability was assessed by flow cytometry using DiIC1(5) and PI following treatment with TR-57 and venetoclax as single agents and in combination. Drugs were combined at fixed ratios based on IC50 values as single agents. Ratios of TR57: venetoclax were 1:1 for primary CLL cells (A) and the OSU-CLL cell line (B) and 1:10 for the OSU-CLLTP53ko line (C). Synergy was assessed by calculating CI at a range of fractional effects using the CompuSyn software. CI values of >1, 1, and <1 are indicative of antagonism, additivity and synergy, respectively. Dose-response analyses and assessment of synergy in (A) CLL patient samples (n = 15). Representative data from one CLL patient sample is shown. (B) OSU-CLL and (C) OSU-CLL-TP53ko cell lines. Error bars represent the standard deviation. Data in (B) and (C) are the mean of 3 biological replicates.
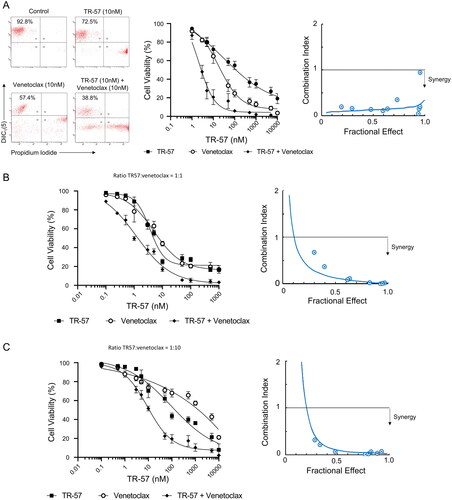
Table 1. Details of CLL patient samples studied.
Healthy PBMCs were significantly (p < 0.005) less sensitive to both drugs as single agents or in combination (Supplementary Figure 2(A) and Supplementary Table 1). TR-57 and venetoclax, alone and in combination, also significantly (p < 0.05) reduced the proportion of viable CLL cells, with a concomitant increase in the proportion of viable T-cells, in CLL patient samples (n = 5, CLL# 2,8,9,14,21; Supplementary Figure 2(B)).
TR-57 and venetoclax in combination arrest the proliferation and cell cycle progression of CLL cells
TR-57 and venetoclax, alone or in combination, significantly decreased the rate of proliferation of both the OSU-CLL and OSU-CLL-TP53ko cells (). After 72 h, a significant (p = 0.01 and 0.03 in the OSU-CLL and OSU-CLLTP53ko lines, respectively) reduction in cell proliferation was observed in cells treated with TR-57 and venetoclax in combination, compared to cells treated with either drug as a single agent.
Figure 3. TR-57 and venetoclax reduce the proliferative and migratory capacity of CLL cells.
(A) Proliferation of the OSU-CLL and OSU-CLL-TP53ko cells was assessed using flow cytometry in cells stained with CFSE. Cells were treated with TR-57 and venetoclax, alone or in combination for 0–72 h (2 and 2 nM and 25 and 250 nM for TR-57 and venetoclax against the OSU-CLL and OSU-CLLTP53ko lines, respectively). (B) Cell cycle analysis by flow cytometry of primary CLL cells (n = 7 patients) following culture in medium alone or stimulation with Dsp30/IL2. The proportion of CLL cells stimulated into S or G2/M phases and the effects of TR-57 and venetoclax, alone or in combination, on the stimulation were assessed. Representative histograms from one patient sample are shown. Data were analyzed using ModFit software. (C) The effects of TR-57 and venetoclax, alone or in combination, on the expression of CD49d (left) and CXCR4 (center) and on the migratory capacity (right) of primary CLL cells from 7 patients were assessed by flow cytometry. Data are expressed as fold-changes relative to cells cultured in medium alone (control). Asterix indicate the following: *p < 0.05, **p < 0.01, and ***p < 0.001.
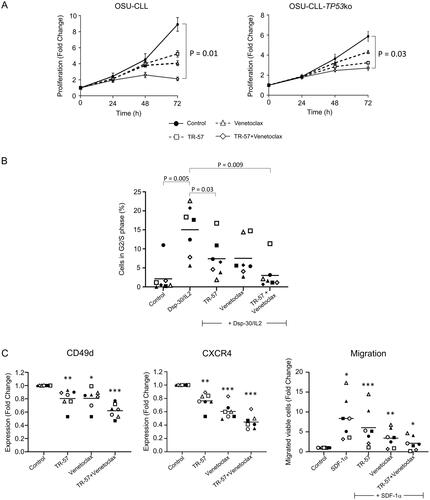
Stimulation of primary CLL cells from seven patients (CLL# 3,6,7,10,11,19,23) with Dsp30/IL2 induced a significant (p = 0.005) increase in the percentage of cells in G2/M/S-phase (). TR-57, alone (p = 0.03) and in combination with venetoclax (p = 0.009), significantly reduced the proportion of cells in the G2/M/S-phases.
TR-57 and venetoclax in combination decrease expression of CD49d and CXCR4 and attenuate the migratory capacity of CLL cells
The G-coupled protein, chemokine receptor CXCR4 and the integrin CD49d play important roles in the homing and retention of CLL cells within the TME [Citation29–31]. TR-57 and venetoclax significantly decreased expression of CD49d and CXCR4, compared to vehicle-treated cells (n = 7, CLL# 1,10,14,15,18,19, and 23; ). Expression of both proteins was significantly lower (p < 0.01) on cells treated with both drugs than on cells treated with either drug alone.
The functional consequence of CXCR4 downregulation was assessed in CLL samples from 7 patients (CLL# 1,7,10,11,14,19, and 23) using the CXCR4 ligand, SDF-1α, as a chemoattractant. The number of viable CLL cells that migrated was significantly (p = 0.04) higher in the presence of SDF-1α than in medium alone (, right). Consistent with the effects on expression of CXCR4 (, center), TR-57 and venetoclax significantly decreased the number of viable CLL cells that migrated under the influence of SDF-1α (p = 0.01). TR-57 and venetoclax in combination had a significantly (p = 0.02) greater effect on migration than either drug as a single agent, and completely attenuated SDF-1α-induced migration (p = 0.14). The downregulation of CXCR4 expression and activity observed is consistent with the study by Kline et al. which demonstrated antagonism of the G-coupled protein dopamine receptor DRD2 by the imipridone ONC-201 [Citation32].
TR-57 and venetoclax in combination induce expression of CIpP and ATF4 and decrease HSP60 in CLL cells
Next, changes in protein expression following treatment with TR-57 and venetoclax were examined by immunoblotting. Fold changes in expression are the mean of all samples assessed. Co-culture of CLL patient samples (n = 4; CLL# 1,7,14, and 19) with CD40L-expressing fibroblasts decreased expression of CIpP and increased expression of ATF4 and Grp78 in all samples (). CD40L-fibroblast co-culture also increased expression of HSP60 in 3 of the 4 samples.
Figure 4. TR-57, alone and in combination with venetoclax, induces changes in proteins involved in the UPR, inhibits AKT and ERK1/2-MAPK signaling, and induces a pro-apoptotic shift in BCL-2 family proteins. Immunoblotting of CLL patient samples, examining changes in (A) UPR-related proteins, CIpP and HSP60, (B) AKT and ERK1/2-MAPK signaling, and (C) BCL-2 family proteins. Primary CLL cells were cultured in medium alone or with CD40L-fibroblasts and were treated either with vehicle, 50 nM TR-57 or venetoclax, as single agents or in combination, for 24 h. The ratios of proteins to β-actin (A and B) or anti-apoptotic BCL-2 proteins (C) are shown under the immunoblots and in the histograms. *indicates p < 0.05 for changes in expression of the UPR or AKT and ERK1/2 proteins relative to CLL cells co-cultured with CD40L-fibroblasts.
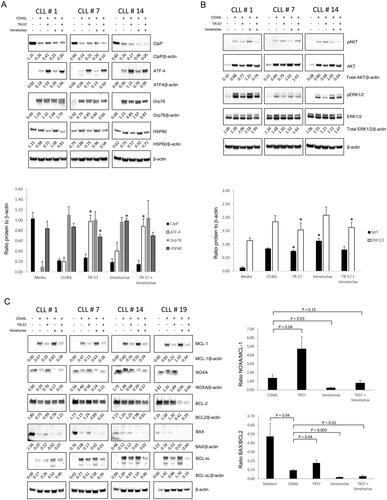
Treatment of CLL cells with TR-57 increased expression of the CIpP (3 samples: 1.60-fold) and ATF4 (4 samples: 7.02-fold) proteins and decreased expression of HSP60 protein (4 samples: 0.75-fold).
Venetoclax had variable effects on the expression of CIpP, but increased expression of ATF4 protein in all 4 samples (1.74-fold). Decreased levels of Grp78 protein were also observed in 2/4 samples following treatment with venetoclax. In cells treated with TR-57 and venetoclax in combination, decreased expression of CIpP (3/4 samples; 0.51-fold) and increased ATF4 expression were observed (5.17-fold; ). Expression of Grp78 and HSP60 was decreased in 3/4 (0.88-fold) and 4/4 (0.79-fold) samples, respectively, following treatment with the drug combination.
Changes in RNA levels of CIpP and ATF4 were inconsistent between the 4 patient samples assessed (Supplementary Figure 4(B)) and did not correlate with the protein changes observed. Relative to cells cultured with CD40L-fibroblasts, no change in expression of CIpP was observed, while ATF4 levels decreased in 3/4 samples with all treatments. Expression of the other UPR-related genes, CHOP, PERK, and EIF-2A was also inconsistent; expression of CHOP and PERK increased in 2/4 and 3/4 samples, respectively with all treatments. EIF-2A expression did not appear to be significantly affected by TR-57 or venetoclax but was increased in 3/4 samples following treatment with TR-57 in combination with venetoclax.
TR-57 and venetoclax in combination reduce phosphorylation of AKT and ERK1/2-MAPK in CLL cells
TR-57 reduced expression of total (phosphorylated and pan-protein) AKT and ERK1/2-MAPK by 0.89- and 0.82-fold, respectively, in all 4 CLL patient samples (). In contrast, venetoclax increased expression of total AKT (1.34-fold) and ERK-1/2 (1.14-fold) protein. TR-57 and venetoclax in combination reduced total expression of AKT (3/4 samples) and ERK1/2-MAPK (4/4 samples).
TR-57 and venetoclax in combination induce a pro-apoptotic shift in the expression of the BCL2-family
TR-57 treatment decreased expression of MCL-1 (0.37-fold) and BCL-xL (0.92-fold) protein in all 4 samples assessed but only decreased expression of BCL-2 protein in 1 sample, with increased expression observed in the remaining 3 samples (). In 3/4 samples, increased expression of the pro-apoptotic NOXA (1.32-fold) and BAX (2.02-fold) proteins was also observed. A significant increase in both the NOXA/MCL-1 and BAX/BCL2 ratios was observed in cells treated with TR-57 (, histograms). Venetoclax decreased expression of BCL-2 in 3/4 samples, but interestingly increased expression of MCL-1 and BCL-xL in 4 and 2 samples, respectively. Venetoclax reduced levels of both NOXA and BAX, which contributed to a decrease in the NOXA/MCL-1 and BAX/BCL-2 ratios. Treatment with TR-57 and venetoclax in combination resulted in decreased expression of MCL-1 (0.43-fold), BCL-2 (0.83-fold), and BCL-xL (0.89-fold) in at least 3 of the 4 samples.
Changes in the expression of MCL1 and BCL2 at the mRNA level were inconsistent between the 4 samples assessed. MCL1 levels decreased in 2/4 samples following treatment with TR-57 but were increased in 3/4 samples following treatment with venetoclax or TR-57 plus venetoclax. BCLXL expression was decreased in all 4 samples following treatment with TR-57, venetoclax or the drugs in combination. An increase in BCL2 expression was observed in 2/4 samples treated with TR-57, while venetoclax, alone and in combination with TR-57, reduced expression in 3/4 samples.
Discussion
Despite high response rates and improved outcomes associated with BTK and BCL-2-targeted therapies, relapse rates remain high among CLL patients.
Studies suggest drugs that target the UPR and upregulate ClpP activity may be an effective treatment option for several cancers [Citation14]. Our previous study [Citation18] and development of more potent CIpP-activating, TR-compounds prompted the current study, in which we examined the effects of TR-57 against CLL cells, as a single agent and in combination with venetoclax.
TR-57 had both cytotoxic () and cytostatic () effects against primary CLL cells and CLL cell lines, including a line in which TP53 was knocked-out using CrispR-Cas9 (OSU-CLL-TP53ko). TR-57 was significantly (p = 0.0001) more cytotoxic toward CLL cells than ONC-212 [Citation18], with IC50 values of 147.8 ± 22.3 and 404 ± 70.6 nM, respectively. Furthermore, TR-57 and venetoclax appeared to more effective against CLL cells than healthy PBMCs or T-cells (Supplementary Figure 2).
The cytotoxic effects of TR-57 against CLL cells were consistent with a pro-apoptotic shift in expression of MCL-1, BCL-xL, NOXA, and BAX and decreased expression of AKT and ERK1/2-MAPK (). Importantly, TR-57 was effective against primary CLL cells from patients with ATM or TP53 dysfunction () and OSU-CLL-TP53ko cells (). Co-culture of CLL cells with stromal cells decreased expression of CIpP, NOXA, and BAX and increased expression of AKT, ERK1/2-MAPK, MCL-1, and BCL-xL, which may explain the reduced sensitivity to TR-57 (). Similarly, reduced sensitivity of the OSU-CLLTP53ko line may be associated with the role of TP53 in the induction of the pro-apoptotic BAX protein [Citation33]; the BAX/BCL-2 ratio in the OSU-CLLTP53ko cells was approximately 1/3 of that observed in the wild-type line following treatment (data not shown). Unlike TP53 ablation in the OSU-CLLTP53ko cells, TP53 mutations often result in a protein with some residual activity or a ‘gain of function’, which may only be present in a small CLL subclone. This may explain why TP53ko () and not TP53 dysfunction () significantly decreases sensitivity to TR-57. Nevertheless, results from the OSU-CLLTP53ko line suggest TR-57 is effective and synergises with venetoclax even in the complete absence of TP53.
The marked increase in ATF4 protein, but not transcript, expression in CLL cells treated with TR-57 is consistent with the study by Ishizawa et al. who demonstrated that ONC-201 induces activation of the UPR in mantle cell lymphoma (MCL) and AML cells [Citation34]. Down-regulation of heat shock protein 60 (HSP60) by TR-57 () may also be important, given that heat shock proteins play key roles in tumorigenesis [Citation35] and have been proposed as therapeutic targets for a range of cancers [Citation36–38] including CLL [Citation39,Citation40]. HSP60 has important roles in regulating cellular stress [Citation41] and mitochondrial dysfunction [Citation42] and can act as both a tumor suppressor and promoter [Citation38]. Reduced HSP60 expression in cells treated with TR-57 () supports the notion that regulation of protein trafficking at the ER plays a crucial role in CLL-cell survival.
Emerging evidence suggests that a distinct UPR within the mitochondrial proteome (UPRmit), regulates cellular energy metabolism [Citation43]. TR-57 may target the UPRmit by regulating activity of CIpP [Citation15], resulting in the proteolysis of respiratory complexes and elevated levels of reactive oxygen species (ROS) [Citation44]. ATF4 is known to regulate transcription of BCL-2 family proteins [Citation45] and may, therefore, play a role in mediating the pro-apoptotic effects of TR-57 on the ratios of NOXA/MCL-1 and BAX/BCL-2 (). However, the discrepancy between the effects of TR-57 on protein and transcript expression of MCL-1 and BCL-2 suggests the effects may be mediated by degradation of the protein rather than a decrease in the rate of transcription. In contrast, BCL-xL appears to be downregulated at both the RNA and protein level. It is conceivable that the effects of TR-57 on expression of the BCL-2 family proteins may be mediated by an increase in the protease activity of CIpP, which may degrade MCL-1, BCL-2, and BCL-xL protein.
The Increase in MCL-1 expression we observed in CLL cells treated with venetoclax () appears to be consistent with findings of a recent study in AML, showing that MCL-1 expression is associated with decreased sensitivity to the BH3-mimetic [Citation46]. TR-57 decreased MCL-1 and BCL-xL expression induced by co-culture with stromal cells and countered the effects of venetoclax on MCL-1 expression, suggesting that synergy with venetoclax may be mediated by the effects of TR-57 on BCL-2 family proteins, other than BCL-2.
Inhibition of signaling via AKT and ERK1/2-MAPK () may also be significant in the cytotoxic effects of TR-57 against CLL cells. AKT plays a crucial role in CLL-cell survival and inhibition of ERK1/2-MAPK signaling downstream of the BCR is a key determinant of ibrutinib efficacy [Citation47]. Although we did not have access to samples from patients who were refractory to ibrutinib, down-regulation of signaling in the AKT and ERK-MAPK pathways suggest TR-57 may be effective against CLL cells with mutations of BTK or PLC-γ2, which confer resistance to ibrutinib [Citation48].
Collectively, the effects of TR-57 on signaling downstream of the BCR, protein trafficking and homeostasis and on the balance of expression of the BCL-2 family proteins demonstrate a multi-faceted mechanism of action of this drug against CLL cells. The synergy of TR-57 with concentrations of venetoclax below the reported steady state plasma concentrations [Citation49], suggest that the benefits of this drug combination may be achieved in vivo with reduced doses of the BH3-mimetic. The effects of the drugs in combination on the proliferative and migratory capacities of CLL cells also raises the possibility that this drug combination may be effective against CLL cells in the TME and may represent a treatment option for patients with poor risk features, including those with TP53 aberrations.
Author contributions
O.G.B., S.Pm, R.I.C., E.J.I., H.L., and D.S.K. designed the study and wrote the manuscript. N.F., Y.S., K.C., O.B., L.T., and O.G.B. generated and analyzed the data.
Supplemental Material
Download Zip (7.1 MB)Disclosure statement
E.J.I. and H.L. have financial interests in Madera Therapeutics, LLC. D.S.K. is a consultant for Madera Therapeutics, LLC. All other authors have no competing interests.
Additional information
Funding
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48.
- Gatt ME, Izraeli S. Lymphoid leukemias. Clinical immunology. Amsterdam, Netherlands: Elsevier; 2019. p. 1049–1063. e1.
- Caligaris-Cappio F, Bertilaccio MT, Scielzo C, editors. How the microenvironment wires the natural history of chronic lymphocytic leukemia. Seminars in cancer biology. Amsterdam, Netherlands: Elsevier; 2014. doi:10.1016/j.semcancer.2013.06.010
- Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi:10.1182/blood-2011-01-328484
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–322. doi:10.1056/NEJMoa1513257
- Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi:10.1038/nm.3048
- Wang M, Wey S, Zhang Y, et al. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11(9):2307–2316. doi:10.1089/ars.2009.2485
- McConkey DJ. The integrated stress response and proteotoxicity in cancer therapy. Biochem Biophys Res Commun. 2017;482(3):450–453. doi:10.1016/j.bbrc.2016.11.047
- Lee ASJM. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35(4):373–381. doi:10.1016/j.ymeth.2004.10.010
- Prabhu VV, Talekar MK, Lulla AR, et al. Single agent and synergistic combinatorial efficacy of first-in-class small molecule imipridone ONC201 in hematological malignancies. Cell Cycle. 2018;17(4):468–478. doi:10.1080/15384101.2017.1403689
- Allen JE, Krigsfeld G, Patel L, et al. Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol Cancer. 2015;14(1):1–10.
- Cole A, Wang Z, Coyaud E, et al. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2015;27(6):864–876. doi:10.1016/j.ccell.2015.05.004
- Greer YE, Porat-Shliom N, Nagashima K, et al. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget. 2018;9(26):18454–18479. doi:10.18632/oncotarget.24862
- Graves PR, Aponte-Collazo LJ, Fennell EMJ, et al. Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem Biol. 2019;14(5):1020–1029. doi:10.1021/acschembio.9b00222
- Ishizawa J, Zarabi SF, Davis RE, et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35(5):721–737. e9. doi:10.1016/j.ccell.2019.03.014
- Wang S, Dougan DA. The direct molecular target for imipridone ONC201 is finally established. Cancer Cell. 2019;35(5):707–708. doi:10.1016/j.ccell.2019.04.010
- Seo JH, Rivadeneira DB, Caino MC, et al. The mitochondrial Unfoldase-Peptidase complex ClpXP controls bioenergetics stress and metastasis. PLoS Biol. 2016;14(7):e1002507. doi:10.1371/journal.pbio.1002507
- Fatima N, Shen Y, Crassini K, et al. The ClpP activator ONC-212 (TR-31) inhibits BCL2 and B-cell receptor signaling in CLL. eJHaem. 2021;2(1):81–93. doi:10.1002/jha2.160
- Shen Y, Crassini K, Fatima N, et al. IBL-202 is synergistic with venetoclax in CLL under in vitro conditions that mimic the tumor microenvironment. Blood Adv. 2020;4(20):5093–5106. doi:10.1182/bloodadvances.2019001369
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the national cancer institute–working group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi:10.1182/blood-2007-06-093906
- Best OG, Gardiner AC, Majid A, et al. A novel functional assay using etoposide plus nutlin-3a detects and distinguishes between ATM and TP53 mutations in CLL. Leukemia. 2008;22(7):1456–1459. Jul doi:10.1038/sj.leu.2405092
- Orchard JA, Ibbotson RE, Davis Z, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004;363(9403):105–111. doi:10.1016/S0140-6736(03)15260-9
- Tracy I, Tapper W, Parker A, et al. Type C TP53-CDKN1A pathway dysfunction occurs independently of CDKN1A gene polymorphisms in chronic lymphocytic leukaemia and is associated with TP53 abnormalities. Br J Haematol. 2017;178(5):824–826. doi:10.1111/bjh.14172
- Hertlein E, Beckwith KA, Lozanski G, et al. Characterization of a new chronic lymphocytic leukemia cell line for mechanistic in vitro and in vivo studies relevant to disease. PLoS One. 2013;8(10):e76607. doi:10.1371/journal.pone.0076607
- Aubrey BJ, Kelly GL, Kueh AJ, et al. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep. 2015;10(8):1422–1432. doi:10.1016/j.celrep.2015.02.002
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513–519. doi:10.1038/74994
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi:10.1016/0065-2571(84)90007-4
- Shen Y, Crassini K, Sandhu S, et al. Dual inhibition of MEK1/2 and AKT by binimetinib and MK2206 induces apoptosis of chronic lymphocytic leukemia cells under conditions that mimic the tumor microenvironment. Leuk Lymphoma. 2019;60(7):1632–1643. Jul doi:10.1080/10428194.2018.1542148
- Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma. 2002;43(3):461–466. doi:10.1080/10428190290011921
- Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood J Am Soc Hematol. 1999;94(11):3658–3667.
- Kriston C, Plander M, Márk Á, et al. In contrast to high CD49d, low CXCR4 expression indicates the dependency of chronic lymphocytic leukemia (CLL) cells on the microenvironment. Ann Hematol. 2018;97(11):2145–2152. doi:10.1007/s00277-018-3410-x
- Kline CLB, Ralff MD, Lulla AR, et al. Role of dopamine receptors in the anticancer activity of ONC201. Neoplasia. 2018;20(1):80–91. doi:10.1016/j.neo.2017.10.002
- Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–1014. doi:10.1126/science.1092734
- Ishizawa J, Kojima K, Chachad D, et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal. 2016;9(415):ra17–ra17.
- Merendino AM, Bucchieri F, Campanella C, et al. Hsp60 is actively secreted by human tumor cells. PLoS One. 2010;5(2):e9247. doi:10.1371/journal.pone.0009247
- Hsu HS, Lin JH, Huang WC, et al. Chemoresistance of lung cancer stemlike cells depends on activation of Hsp27. Cancer. 2011;117(7):1516–1528. doi:10.1002/cncr.25599
- Lawson DA, Bhakta NR, Kessenbrock K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526(7571):131–135. doi:10.1038/nature15260
- Yun CW, Kim HJ, Lim JH, et al. Heat shock proteins: agents of cancer development and therapeutic targets in anti-cancer therapy. Cells. 2019;9(1):60. doi:10.3390/cells9010060
- Frezzato F, Raggi F, Martini V, et al. HSP70/HSF1 axis, regulated via a PI3K/AKT pathway, is a druggable target in chronic lymphocytic leukemia. Int J Cancer. 2019;145(11):3089–3100. doi:10.1002/ijc.32383
- Guo A, Lu P, Lee J, et al. HSP90 stabilizes B-cell receptor kinases in a multi-client interactome: PU-H71 induces CLL apoptosis in a cytoprotective microenvironment. Oncogene. 2017;36(24):3441–3449. doi:10.1038/onc.2016.494
- Hall L, Martinus RDJS. Hyperglycaemia and oxidative stress upregulate HSP60 & HSP70 expression in HeLa cells. SpringerPlus. 2013;2(1):1–10.
- Wu J, Liu T, Rios Z, et al. Heat shock proteins and cancer. Trends Pharmacol Sci. 2017;38(3):226–256. doi:10.1016/j.tips.2016.11.009
- Deng P, Haynes CM, editors. Mitochondrial dysfunction in cancer: potential roles of ATF5 and the mitochondrial UPR. Seminars in cancer biology. Amsterdam, Netherlands: Elsevier; 2017. doi:10.1016/j.semcancer.2017.05.002
- Zhang Y, Huang Y, Yin Y, et al. ONC206, an imipridone derivative, induces cell death through activation of the integrated stress response in serous endometrial cancer in vitro. Front Oncol. 2020;10:2299.
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi:10.1038/nrm3270
- Carter BZ, Mak PY, Tao W, et al. Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica 2022;107:58–76.
- Cheng S, Ma J, Guo A, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28(3):649–657. doi:10.1038/leu.2013.358
- Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–2294. doi:10.1056/NEJMoa1400029
- Salem AH, Dunbar M, Agarwal SK. Pharmacokinetics of venetoclax in patients with 17p deletion chronic lymphocytic leukemia. Anticancer Drugs. 2017;28(8):911–914. doi:10.1097/CAD.0000000000000522

