Abstract
Venetoclax is a first-in-class B-cell lymphoma-2 (BCL-2) inhibitor approved as continuous monotherapy and in combination with rituximab as fixed-treatment duration for relapsed and refractory chronic lymphocytic leukemia (R/R CLL). DEVOTE was a 24-week, multicenter observational study (NCT03310190) evaluating the safety, healthcare resource utilization (HCRU) and health-related quality of life (HRQoL) of patients initiating venetoclax for R/R CLL in Canada. Overall, 89 patients received 1 dose of venetoclax; 80% had prior exposure (42% resistant) to ibrutinib. Biochemical tumor lysis syndrome (TLS) occurred in five patients. We observed differences in hospitalization across Canadian provinces including in patients at low risk for TLS with no clear impact on TLS incidence. Additionally, a rapid and sustained improvement in several domains of HRQoL was observed during venetoclax initiation. Early adoption of venetoclax was mainly for R/R CLL patients with few treatment options; nonetheless, acceptable toxicity and a positive impact on HRQoL were observed.
Introduction
Venetoclax is a selective and orally bioavailable small-molecule inhibitor of BCL-2 used for the treatment of chronic lymphocytic leukemia (CLL). In Canada, successive approvals for venetoclax monotherapy, and in combination with rituximab, for the treatment of relapsed and refractory (R/R) CLL were received in 2016 and 2018, respectively [Citation1]. These approvals were based on demonstrated efficacy in registrational trials, where treatment with venetoclax monotherapy resulted in an objective response rate (ORR) of 79.4% in patients with R/R CLL and deletion of chromosome 17p (del17p)[Citation2] and the combination of venetoclax and rituximab reduced the risk for disease progression or death by 81% in patients with R/R CLL compared with bendamustine and rituximab [Citation1].
Notwithstanding, the main safety concern with the use of venetoclax has been the occurrence of tumor lysis syndrome (TLS). Therefore, a combined strategy of gradual, 5-week ramp-up dosing schedule and other TLS mitigation measures was conceived and incorporated in the aforementioned trials. Briefly, recommendations in the product monograph include frequent laboratory monitoring, the use of antihyperuricemic agents, and prophylactic hospitalization of patients at high (and potentially intermediate) risk for TLS [Citation1]. However, there has been uncertainty around the adoption of these recommendations in routine clinical practice.
The DEVOTE study (NCT03310190) was conducted to determine early real-world safety and healthcare resource utilization (HCRU) patterns of venetoclax for R/R CLL in Canada. It set out to describe the characteristics and health-related quality of life (HRQoL) of patients started on venetoclax monotherapy and later in combination with rituximab.
Materials and methods
The DEVOTE study was a Canadian prospective multicenter, open label, single arm, 24-week observational study that assessed the real-life management and HCRU following the initiation of venetoclax monotherapy or in combination with rituximab. Venetoclax was prescribed per clinical considerations and as part of routine care. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice guidelines. The study protocol was approved by the ethics committee at each participating institution.
Patients
Eligible patients were adults who had provided written informed consent, had CLL and had received at least one prior line of therapy, and whose physician had decided to prescribe venetoclax as per the Canadian product monograph [Citation1]. Those participating in an interventional study were excluded. The first patient was enrolled in January 2018 and the last patient visit took place in April 2022.
Study design
The DEVOTE study comprised of three phases: pretreatment, ramp-up, and maintenance, with treatment and follow-up performed as per routine clinical care. Venetoclax was initiated at a starting dose of 20 mg QD for 7 days, increasing to 50 mg, 100 mg, and 200 mg QD, each for 7 days, until the maximum dose (400 mg QD) is initiated and maintained. This ramp-up dosing schedule was accompanied by TLS mitigation measures (based on tumor burden: low, medium, or high), potentially including prophylactic hospitalization of patients with intermediate or high TLS risk (emergent hospitalizations were not captured). The study protocol did not mandate any interventions or assessments, and clinical management was at the discretion of the investigators.
Endpoints
Patient management following initiation of venetoclax was assessed using variables related to TLS risk assessment, TLS risk mitigation (e.g. prophylaxis treatments, prophylactic hospitalization, fluid replacement therapy), TLS monitoring (e.g. changes in serum potassium, creatinine, uric acid, phosphorus [phosphate], and calcium, and presence of clinical or laboratory TLS as assessed using Howard’s criterion) [Citation3]. Of note, we included the total (and not corrected) concentration of calcium, potentially leading to an overestimation of hypocalcemia. Lastly, changes to the standard ramp-up algorithm (e.g. dose interruptions or delays in scheduled dose increases), and permanent discontinuation of venetoclax (with the reason for discontinuation), were also captured.
HCRU was assessed at baseline, at the end of the ramp-up period and at the end of study visit using a descriptive questionnaire that captured clinic visits related to CLL, visits to hospital ER, hired assistance to help at home with regular activities, medical/surgical procedures or laboratory tests, and healthcare-related transportation costs. Demographics and baseline characteristics, including disease history, treatment history, tumor burden (and method of assessment), cytogenetic test results, major comorbidities, and Rai and Binet staging were recorded. Lastly, center-related parameters such as rural versus urban hospital, and the distance between the patient’s residence and the center, were also captured.
HRQoL was assessed using the EORTC-QLQ-C30 and the EORTC-QLQ-CLL17 tools at baseline, at the end of the ramp-up phase, and at the end of the maintenance phase, either at the end-of-study visit (24 weeks) or at an early discontinuation visit. The EORTC-QLQ-C30 is a 30-item cancer questionnaire with 5 functional scales (cognitive, social, physical, emotional, and role functioning), a global health status/QoL scale, 3 symptom scales (fatigue, nausea and vomiting, and pain) and 6 single items (insomnia, appetite loss, constipation, diarrhea, dyspnea, and financial difficulties). The EORTC QLQ-CLL17 is a 17-item module specific to CLL consisting of three multi-item scales (symptom burden, physical condition/fatigue, and worries/fears health and functioning). Scores for both questionnaires, and their functional/symptoms scales, range from 0–100. For functional scales, a higher score represents an improvement in HRQoL while for symptom scales, a higher score denotes more intense symptoms. The minimum important difference (MID) from B/L was defined for both questionnaires as a 5-point change, the lower bound of the 5 to 10-point range considered a ‘small’ change for EORTC-QLQ-C30 [Citation4].
Safety variables collected included adverse events (AEs) including those related to TLS, AEs leading to treatment discontinuation, serious AEs (SAEs), unusual failure in efficacy, and pregnancies.
Statistical analysis
All patients administered at least one dose of the drug were included in the analysis. All patients with available data were included in the respective analyses and there was no imputation for missing values. Given the observational nature of this study, statistical analysis was limited to descriptive statistics for the main analysis data set, and also for patient subgroups determined by tumor burden, TLS risk, and geographic region.
Results
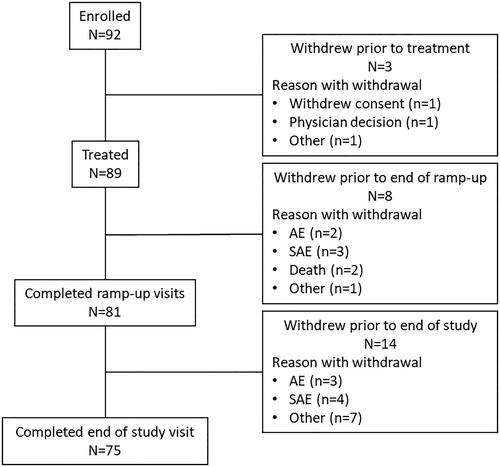
A total of 92 patients from 11 study sites were enrolled, of whom 89 got at least one dose of venetoclax and were included in the analysis data set (). Out of 11 study sites, seven and four were academic and community centers, respectively. Of the three patients enrolled but not treated, one withdrew consent due to patient decision, another withdrew due to physician advice, and the third due to an undetermined reason. Overall, 81 (91.0%) patients completed the ramp-up phase and 75 (84.3%) completed the study. Of the 14 patients who discontinued prematurely, seven experienced an AE that resulted in discontinuation.
The median time from diagnosis to receipt of first dose of venetoclax was 8.4 years (). Overall, the majority of patients with available test results had high risk features, including del17p in 31.4% and unmutated IgHV in 72.7%.
Table 1. Baseline characteristics.
Patients had received a median (range) of 2 (0–7) prior lines of therapy. Prior exposure to ibrutinib was recorded in 71 (79.8%) patients, of whom in 68 patients ibrutinib was the immediate prior line. The most common reason for discontinuing ibrutinib was disease progression, which occurred in 38 (53.5%) patients, while intolerance was the reported reason in 23 (32.4%) patients. Interestingly, 15 patients continued to receive ibrutinib after initiation of venetoclax (for a median of 21 days).
Median (range) age was 73 (39–91) years, 67 (75.3%) were male and 78 (87.6%) were white (). In general, demographics and baseline characteristics were consistent across regions (Table S1). One parameter that showed considerable discordance between regions was the distance patients lived from their treatment center: the proportion of patients living >50 km from their treatment center ranged from zero (out of six patients) in Quebec to 66.7% (6/9 patients) in Atlantic Canada. Overall, 23 (26.1%) of patients lived >50 km from their treatment center (Table S1).
Overall TLS risk was assessed as high in 15 (16.9%) patients at baseline; the prevalence of individual risk factors is presented in Table S2.
Venetoclax exposure
During the ramp-up phase, the median number of days of each dose level was 7 days. Between 98.8% (20 mg dose) and 96.1% (100 mg dose) of patients were fully compliant with each ramp-up dose strength, and the maximum venetoclax dose of 400 mg daily was achieved by >80% of patients in all regions except Atlantic Canada (n/N = 6/9, 66.7%) (Table S3). The most common reason given for dose discontinuation was AEs (15 patients, 75.0%).
Occurrence of tumor lysis syndrome
There were no reported cases of clinical TLS during the study. There were six instances of laboratory TLS (using Howard’s criteria conservatively modified to include total calcium in place of ionized or corrected calcium) in five patients: two patients met Howard’s criteria at the 20 mg ramp-up visit, one each at 50 and 200 mg, and one patient met Howard’s criteria for laboratory TLS at the 20 and 400 mg ramp-up visits. The TLS laboratory abnormalities were hyperphosphatemia in all six events, with co-existent hypocalcemia, hyperkalemia and hyperuricemia in two instances each. There was no observable trend in terms of baseline overall TLS risk or baseline tumor burden, although all five patients had an absolute lymphocyte count of at least 25 × 109/L and four of the patients had evidence of renal dysfunction (Table S4).
Prophylaxis for tumor lysis syndrome
Most (96%) patients received allopurinol for a median of 78 days after the first dose of venetoclax (Data not shown). Three patients were treated prophylactically with rasburicase for elevated urate levels, all administered to hospitalized patients. One patient received rasburicase at the 20 and 50 mg venetoclax dose levels, another patient at the 20 mg venetoclax dose only, and the third patient at the 20 mg and at the 200 mg venetoclax dose levels.
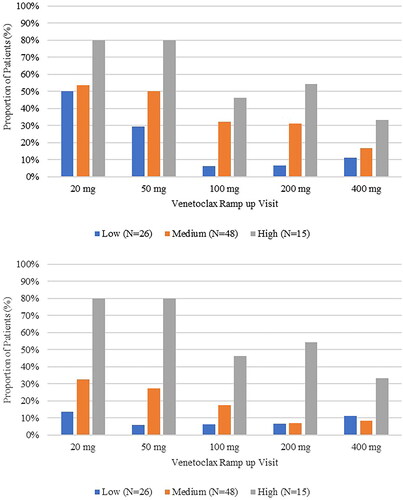
Overall, 82 instances of hospitalization for TLS prophylaxis were reported in 30 patients. There was no notable difference in admission rates over the duration of the study. At the initial 20 mg venetoclax ramp-up visit 29 (36.3%) patients were hospitalized for TLS prophylaxis, decreasing to 7 (15.6%) patients at the 400 mg ramp-up visit duration of hospitalization range: 1–37 days, data not shown). Prophylactic IV hydration and prophylactic hospitalization both decreased as venetoclax ramp-up visits progressed (). Ontario was responsible for the majority of hospitalizations and IV hydration for TLS prophylaxis (Data not shown).
Healthcare resource utilization
The median (range) number of times a patient traveled for regular appointments in the previous month increased from 2 (0, 32) at baseline (N = 89) to 5 (0, 26) at the 400 mg ramp-up visit (N = 68) while the number of patients who received extra healthcare services fell from 64 (71.9%) patients at baseline to 42 (59.2%) (Data not shown). Of note, costs associated with venetoclax, hospitalizations, and any medications provided while hospitalized, were covered by provincial health care departments. There were no notable differences in any of the other HCRU parameters assessed.
Health-related quality of life
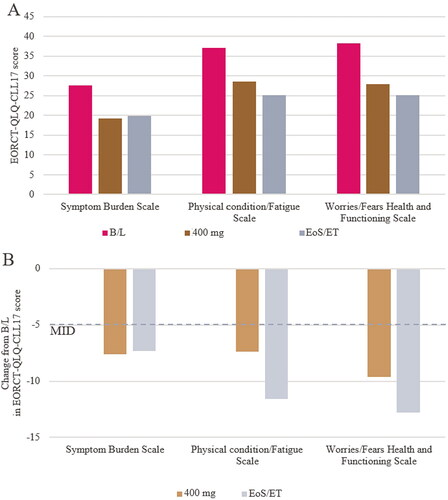
EORTC-QLQ-C30 and (86/89 at baseline, 66/81 at ramp-up, and 60/79 at end-of-study visits) and EORTC-QLQ-CLL17 (86/89 at baseline, 67/81 at ramp-up, and 62/79 at end-of-study visits) completion rates were all in excess of 75%. Baseline scores for EORTC-QLQ-30 and EORTC-CLL-17 domains were aligned with previous reports in the literature for pretreated patients with CLL, and were lower than domain scores reported for the general population (Table S5) [Citation5]. Treatment with venetoclax was associated with improvements in HRQoL overall, across all five EORTC-QLQ-C30 functioning scales, and across most of the symptom scales. The improvements were sustained from the 400 mg ramp-up visit until end of study participation (). The mean improvement in scores surpassed the MID of 5 for all functioning scales other than cognitive functioning, and the fatigue, pain, dyspnea and insomnia symptom scales. The nausea and vomiting and the constipation symptom scores both worsened marginally, and the diarrhea score was variable (). Similar results were observed for all 3 EORCT-QLQ-CLL17 domains ( and ).
Figure 3. Mean EORTC-QLQ-C30 functioning and symptoms scales (A) and corresponding change from baseline (B).
EORTC QLQ-C30 domain scores are derived from raw answers of QLQ-C30 questionnaire. The MID from B/L was defined as a 5-point change, the lower bound of the 5–10 point range considered a ‘small’ change. B/L, baseline; EoS, end of study; ET, early termination; MID minimal important difference; QoL, quality of life.
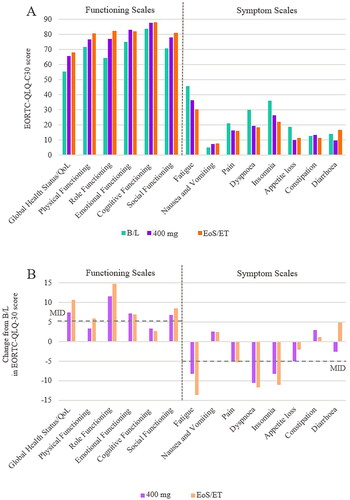
Figure 4. Mean EORTC-QLQ-CLL17 functioning and symptoms scales (a), and corresponding change from baseline (B).
EORTC QLQ-CLL17 domain scores are derived from raw answers of QLQ-CLL17 questionnaire. The MID from B/L was defined as a 5-point change, the lower bound of the 5–10 point range considered a ‘small’ change for the EORTC-QLQ-C30. B/L, baseline; EoS, end of study; ET, early termination; MID minimal important difference.
Adverse events
During the course of the study, 61 (68.5%) patients experienced at least one Grade ≥3 AE and 26 (29.2%) experienced at least one SAE. Ten (11.2%) patients discontinued treatment permanently and 7 (7.9%) withdrew from the study due to an AE. Grade ≥3 AEs reported in >2 patients were neutropenia (N = 24), thrombocytopenia (N = 7), febrile neutropenia (N = 6) and pneumonia (N = 4). Twenty subjects received at least one dose of colony stimulating factor over the course of the study. Eight (9.0%) patients died during the course of the study; three from disease progression and one each from asthenia, bacterial sepsis, pneumonia, ischemic stroke, and respiratory failure. Serious AEs were generally aligned with the Grade ≥3 AEs described above (Table S6).
Discussion
The DEVOTE study provides insights into early management of R/R CLL with venetoclax in routine Canadian practice, with particular focus on prophylactic measures taken to prevent TLS. In this analysis of real-world use, venetoclax was well tolerated and its overall safety profile reflected clinical trial experience [Citation2, Citation6, Citation7].
The rate of TLS in this study (5/89 patients, 5.6%) is more reflective of early experience with venetoclax monotherapy in the dose escalation phase [Citation6], than in registrational trial experience [Citation2, Citation7]. While all TLS events were strictly laboratory, some explanations may be offered for this observation. First, the high median age of the study population (73 years), high prevalence of comorbidities, and abundance of risk factors for TLS; specifically, only 11% of study participants had no TLS risk factor. Second, most patients had prior exposure to ibrutinib, and more than half of those experienced disease progression on this medication, which is indicative of a poor prognosis and associated with a risk for rapid disease progression, especially with ibrutinib discontinuation [Citation8, Citation9]. In this context it is noteworthy that only 25% of patients with ibrutinib as their last line of therapy were kept on this drug during venetoclax ramp-up, and even then for part of the ramp-up period only. In contrast, there have been recent recommendations to continue ibrutinib during the entire ramp-up period to mitigate TLS [Citation10, Citation11]. Third, this study represents early experience with this drug for Canadian healthcare providers. Additionally, two of the reported biochemical TLS events relied on hypocalcemia, which was likely overestimated due to the use of total (and not corrected) calcium.
Most patients achieved the 400 mg target maintenance dose in approximately 4 weeks as per standard dose-escalation recommendations [Citation1]. Geographic location, TLS risk and tumor burden at baseline had no discernable effect on maximum venetoclax dose achieved, time to reach maximum dose, or time on maximum dose.
Almost all patients in this study received prophylaxis throughout the ramp-up phase, with most patients receiving either allopurinol or rasburicase. Other than in Ontario, preemptive hospitalization was limited to the 20 mg and 50 mg venetoclax ramp-up visits, and was generally used for patients with medium or high baseline TLS risk. Use of IV hydration was also more common for the 20 mg and 50 mg ramp-up visits; as would be expected.
Baseline EORTC-QLQ-C30 scores were aligned with previous reports for a pretreated CLL population and showed a rapid improvement following initiation of venetoclax both in terms of functional and symptom scales. Most improvements were sustained from the end of the ramp-up phase until the end of study participation. The venetoclax ramp-up period was not associated with a negative impact on HRQoL. This early improvement in patient reported outcomes provides a backdrop for the frequent use of healthcare resources and reported biochemical abnormalities that accompanied the initiation of venetoclax in routine care of R/R CLL in Canada.
Limitations
The DEVOTE study was a non-randomized, observational study and as such the potential for selection bias, in terms of the representativeness of both study sites and patients enrolled, is an inherent factor. The 11 study sites were from seven of the ten Canadian provinces (British Columbia, Alberta, Manitoba, Ontario, Quebec, New Brunswick, and Nova Scotia) with none of the three Canadian Territories represented. The study was started in January 2018, shortly after venetoclax was approved as continuous monotherapy in Canada and was active when its use in combination with rituximab as fixed-treatment duration was approved in the R/R CLL setting. This may have influenced the demographics and baseline characteristics of the patients enrolled in the study as patients who require more urgent treatment with novel therapies are likely to be prioritized in terms of physician decision-making and therefore more likely to have been enrolled in this study. Also, as experience with venetoclax increased following approval, it is likely that TLS mitigation strategies may have evolved. Although the investigational sites for the DEVOTE study included both academic and community treatment centers, it is possible that treatment protocols across Canada evolved at different rates, thereby excluding some sites (and therefore patients) from participation. No data were collected related to treatment efficacy. The rapid and sustained improvements in HRQoL, however, provide a surrogate measure for improved outcomes, albeit with the caveat that self-administered questionnaires provide subjective data as opposed to objective data. Finally, the reason for hospitalization may have reflected concerns other than TLS, for example the distance from the patient’s home to the treatment center in the event of an adverse event, or the presence of other coexisting conditions that may have increased the patient’s risk for an adverse event.
In conclusion, this real-world study demonstrated the feasibility and tolerability of venetoclax as a treatment for R/R CLL, utilizing the standard ramp-up process, in a broad spectrum of community and academic cancer centers. While TLS was encountered in an early timepoint of venetoclax adoption, patient reported outcomes showed rapid improvement indicative of disease control.
Supplemental Material
Download MS Word (30.9 KB)Acknowledgments
AbbVie and the authors thank the patients who participated in the study and all study investigators for their contributions. AbbVie Corp. participated in the study design, data review, and the development and approval of this manuscript for publication. All authors had access to the data; participated in the development, review, and approval of the manuscript. AbbVie funded the research for this study. The authors wish to thank, from Alimentiv Inc., Hong Chen and Jim Wang for statistical analysis and support, and John Howell, of John Howell Consulting Inc. for medical writing and editing services in the development of this manuscript, which was funded by AbbVie. The authors also wish to thank Kelly Boyd, Rima El-Hadi, Luiza Manasyan, Karla Manzano Vargas and Vivianne Mourani from AbbVie for clinical operational support. No author has received funding for developing the manuscript. AbbVie provided financial support for the study.
Data sharing
The data that support the findings of this study are available from the corresponding author, VB, upon reasonable request.
Disclosure statement
Nizar Abdel-Samad has served as a consultant to AbbVie, Amgen, Celgene, Janssen Biotech and Roche. Andrew Aw has received honoraria from Abbvie and AstraZeneca accepted into a separate account within the Ottawa Hospital Research Institute, for research/academic use only. Versha Banerji has received research funding from CIHR, LLSC, CancerCare Manitoba Foundation, Research Manitoba, Janssen and AbbVie which are independent of this study. VB has served as a consultant to AbbVie, Janssen AstraZeneca, Gilead, Roche. VB is the PI on Clinical trials from AstraZeneca, Lily Oncology and a SWOG/CCTG cooperative group study. Marie-Pierre Bernard has participated in advisory board for GSK Canada and received honorarium from Janssen. Sathish Gopalakrishnan has received research funding from AbbVie. Annette Hay has received institutional research funding from AbbVie, Roche, Karyopharm, Janssen, Merck and Seattle Genetics. Nathalie Johnson has provided consultancy for Abbvie, Merck, Roche, BMS, Janssen, Seattle Genetics, Lundbeck and has received honoraria from Roche and Lundbeck. Nicole Laferriere has received honoraria from AbbVie, Alexion, Amgen, Astellas, BMS, Gilead, Leo Pharma, Janssen, Novartis, Roche, Sanofi, Takeda, and Teva. Carolyn Owen has received honoraria from AbbVie, AstraZeneca, Janssen, Roche, Merck, Servier, and Incyte. Anthea Peters has participated in advisory boards with AbbVie, AstraZeneca, Roche, Janssen, Incyte; has received honoraria from AbbVie, AstraZeneca and Janssen. Sue Robinson has received honoraria from AbbVie, AstraZeneca, Biogene, Janssen, Roche, Seattle Genetics. Sarah-Jane Bull, Pierre-André Fournier and Adi Klil-Drori are AbbVie employees and may own AbbVie Stocks.
Additional information
Funding
References
- Health Canada. 2016 21 January. VENCLEXTA® (venetoclax tablets) Product Monograph. AbbVie Corporation https://pdf.hres.ca/dpd_pm/00059710.PDF. Accessed 2021 21 January.
- Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–778. doi:10.1016/S1470-2045(16)30019-5
- Howard SC, Jones DP, Pui C-H. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844–1854. doi:10.1056/NEJMra0904569
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi:10.1200/JCO.1998.16.1.139
- Holzner B, Kemmler G, Kopp M, et al. Quality of life of patients with chronic lymphocytic leukemia: results of a longitudinal investigation over 1 yr. Eur J Haematol. 2004;72(6):381–389. doi:10.1111/j.1600-0609.2004.00233.x
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–322. doi:10.1056/NEJMoa1513257
- Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-Rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi:10.1056/NEJMoa1713976
- Hampel PJ, Rabe KG, Call TG, et al. Clinical outcomes in patients with chronic lymphocytic leukemia with disease progression on ibrutinib. Blood Cancer J. 2022;12(9):124. doi:10.1038/s41408-022-00721-6
- Hampel PJ, Ding W, Call TG, et al. Rapid disease progression following discontinuation of ibrutinib in patients with chronic lymphocytic leukemia treated in routine clinical practice. Leuk Lymphoma. 2019;60(11):2712–2719. doi:10.1080/10428194.2019.1602268
- Ferrarini I, Gandini F, Zapparoli E, et al. Two distinct clinical patterns of ibrutinib-to-venetoclax transition in relapsed chronic lymphocytic leukemia patients. Curr Oncol. 2022;29(4):2792–2797. doi:10.3390/curroncol29040227
- Gribben JG. Practical management of tumour lysis syndrome in venetoclax-treated patients with chronic lymphocytic leukaemia. Br J Haematol. 2020;188(6):844–851. doi:10.1111/bjh.16345