Abstract
Acalabrutinib studies have limited Asian participation. This phase 1/2 study (NCT03932331) assessed acalabrutinib in Chinese patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL). Primary endpoint was blinded independent central review (BICR)-assessed overall response rate (ORR). Overall, 34 patients were enrolled. Most patients were men (88%); median age was 63 years and 59% had ≥3 prior treatments. Median treatment duration was 14 months (range, 1–24). Any-grade adverse events (AEs) and grade ≥3 AEs occurred in 85.3% and 44.1% of patients, respectively. AEs causing treatment discontinuation were aplastic anemia, thrombocytopenia, and gastrointestinal infection (n = 1 each). Fatal AEs occurred in 2 patients (aplastic anemia and multiple organ dysfunction syndrome [n = 1 each]). BICR-assessed ORR was 82.4% (95% confidence interval [CI]: 65.5, 93.2); 12 (35.3%) patients achieved complete response. Estimated 12-month OS was 84.5% (95% CI: 66.6, 93.3). Acalabrutinib yielded tolerable safety and high response rates in Chinese patients with R/R MCL.
Introduction
Mantle cell lymphoma (MCL) is a rare type of B-cell non-Hodgkin lymphoma (NHL) [Citation1]. In 2016, a lymphoma burden study demonstrated that 14.9% of new cases of NHL worldwide occurred in China and that the burden of NHL in China rose more substantially from 2006 to 2016 compared with the global burden [Citation2]. Furthermore, MCL accounts for approximately 2.7% to 4.6% of NHL cases in China [Citation3–5]. Patients with MCL have a poor prognosis and a high rate of recurrence [Citation6].
Current treatments available for MCL include rituximab-based chemoimmunotherapy and newer targeted therapies such as Bruton tyrosine kinase inhibitors (BTKis) [Citation1]. BTKis demonstrated durable responses in phase 1 and 2 clinical trials for patients with relapsed/refractory (R/R) MCL [Citation7, Citation8]. Acalabrutinib is a next-generation, highly selective covalent BTKi approved by the United States Food and Drug Administration for the treatment of adult patients with R/R MCL and chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma [Citation9, Citation10]. Acalabrutinib studies conducted thus far have had limited participation of patients from Asian populations [Citation8, Citation9, Citation11].
The present study aimed to assess acalabrutinib for the treatment of R/R MCL in patients of Asian ethnicities. This phase 1/2 clinical trial (NCT03932331) is the first to assess the safety and efficacy of acalabrutinib in Chinese patients with R/R MCL and other advanced B-cell malignancies.
Methods
This phase 1/2 open-label study was conducted at 14 sites in China. In the phase 1 portion, adult patients aged 18 or older with R/R B-cell malignancies including non–germinal center B cell–like diffuse large B-cell lymphoma, follicular lymphoma, MCL, and CLL were enrolled. The phase 2 portion enrolled adult patients with R/R MCL. Patients were eligible for inclusion if they had confirmed MCL with documentation of chromosome translocation t(11;14)(q13;q32) and/or overexpression of cyclin D1 and were R/R to at least 1, but no more than 5, prior treatment regimens. Additional inclusion criteria were Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and presence of radiographically measurable lymphadenopathy or extranodal lymphoid malignancy. Patients were excluded if they had significant cardiovascular disease, history of bleeding diathesis, history of stroke or intracranial hemorrhage within 6 months before treatment, or known central nervous system involvement of lymphoma or leukemia. In addition, patients were excluded if they required or received anticoagulation with warfarin or an equivalent vitamin K antagonist within 7 days of the first dose of study treatment, and if they used a strong inhibitor or inducer of CYP3A within 7 days before a dose of study drug or were expected to require use during the first 28 days of administration of study drug.
The study protocol received Institutional Review Board and Independent Ethics Committee review approval and was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice. Informed consent was obtained from all patients.
Treatment was administered in 28-day cycles. In phase 1 and 2, patients who received acalabrutinib 100 mg twice daily continued until progressive disease or any other treatment discontinuation criteria were met. Patients who had disease progression were assessed for survival and use of alternative anticancer therapy until death or loss to follow-up. Dose modifications to 100 mg once daily were considered for toxicity. Withholding of acalabrutinib for a maximum of 28 consecutive days from expected dose was permitted. If the toxicity resolved to grade ≤1 or baseline within 28 days of onset and the patient showed clinical benefit, treatment with acalabrutinib could be restarted at the standard or reduced dose.
The primary efficacy endpoint was overall response rate (ORR) as assessed by blinded independent central review (BICR) per Lugano classification for NHL [Citation12]. Secondary endpoints included investigator-assessed ORR; BICR- and investigator-assessed duration of response (DOR); progression-free survival (PFS); time to response (TTR); overall survival (OS); and adverse events (AEs). AEs were graded according to the Common Terminology Criteria for Adverse Events version 5.0.
All patients received baseline safety assessments including hematology, clinical chemistry, and urinalysis. Throughout the study, safety evaluations including hematology and clinical chemistry were conducted at each cycle until cycle 12, then every 12 weeks thereafter, within 7 days of last dose of study drug, and 30 days after last dose. Tumor assessments, including computed tomography (CT), were performed at baseline, cycles 2, 4, 6, 9, and 12, and then every 12 weeks thereafter until disease progression (including patients who discontinued for any reason other than disease progression). Positron-emission tomography (PET)-CT was performed at baseline, at cycles 2 and 6, and to confirm complete response (CR) thereafter. Pretreatment CT scans were required for the neck, chest, abdomen, pelvis, and any other disease sites within 28 days before the first dose of acalabrutinib and a PET-CT scan was performed within 60 days before the first dose of acalabrutinib. Tumor assessments included physical examination, radiographic examination, endoscopy, and bone marrow assessment. Bone marrow assessments occurred at screening and throughout to confirm CR via aspiration and biopsy including immunohistochemistry. If gastrointestinal tract involvement was suspected, an endoscopy was obtained at baseline. Endoscopy was required to confirm CR for any patients with documented gastrointestinal tract involvement.
Sample size was calculated to achieve a 95% two-sided confidence interval (CI) centered around an expected ORR of 80% that excludes an ORR of 60% as a lower bound. The ORR and corresponding 95% two-sided CI are presented based on the Clopper-Pearson exact method. Descriptive statistics were used for best overall response. DOR, PFS, and OS were estimated using the Kaplan-Meier (KM) method. DOR was defined as the interval from the first response to progressive disease or death. PFS was defined as the interval from the start of acalabrutinib to progressive disease or death. TTR was analyzed for patients with a partial response or better and was defined as the interval between the first dose of acalabrutinib and the initial response. OS was defined as the interval from the start of acalabrutinib to death from any cause.
Results
Between April 29, 2020 and March 2, 2021, 45 patients were screened for the phase 2 portion of the study and 33 patients with MCL were enrolled. One patient with MCL from phase 1 was analyzed together with the patients from phase 2. The baseline demographic and disease characteristics are summarized in . The median age was 63 years (range, 36–75), 88% (n = 30) of patients were male, and 100% (n = 34) were of Asian race. The median number of prior anticancer therapy regimens was 3 (range, 1–5) with 59% (n = 20) of patients having 3 or more prior regimens.
Table 1. Demographics and baseline characteristics.
As of May 6, 2022, the median treatment duration was 14 months (range, 1–24). The median relative dose intensity was 100%. The most common any-grade or grade 3 or higher AEs are summarized in . Overall, 29 (85.3%) patients experienced an AE of any grade and 15 (44.1%) an AE of grade 3 or higher. The most common any-grade AEs were decreased platelet count (n = 15, 44.1%); upper respiratory tract infection (n = 9, 26.5%); anemia and increased lymphocyte count (n = 8, 23.5% each); increased alanine aminotransferase, increased aspartate aminotransferase, headache, and decreased neutrophil count (n = 7, 20.6% each); and increased blood bilirubin, diarrhea, and rash (n = 6, 17.6% each). The most common AEs of grade 3 or higher were anemia (n = 3, 8.8%), decreased neutrophil count (n = 3, 8.8%), and pneumonia (n = 2, 5.9%).
Table 2. Any-grade adverse events occurring in ≥15% of patients and grade ≥3 adverse events occurring in ≥5% of patients in the R/R MCL cohort.
Three (8.8%) patients experienced AEs leading to treatment discontinuation including aplastic anemia, thrombocytopenia, and gastrointestinal infection (n = 1 each). Seven serious AEs were reported in 6 (17.6%) patients; these included pneumonia (n = 2), and aplastic anemia, thrombocytopenia, inguinal hernia, multiple organ dysfunction syndrome, and gastrointestinal infection (n = 1 each). Fatal AEs occurred in 2 (5.9%) patients (aplastic anemia and multiple organ dysfunction syndrome in 1 patient each). The patient with multiple organ dysfunction is the same patient who discontinued treatment due to gastrointestinal infection; the patient died 22 days after the last dose of acalabrutinib, and the primary cause of death was reported as progressive disease. There were no reported cases of any-grade atrial fibrillation, major hemorrhage, hypertension, second primary malignancies (excluding non-melanoma skin cancers), or tumor lysis syndrome.
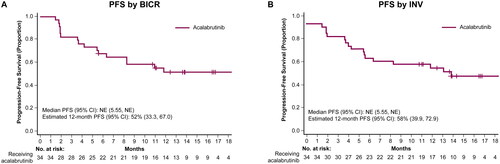
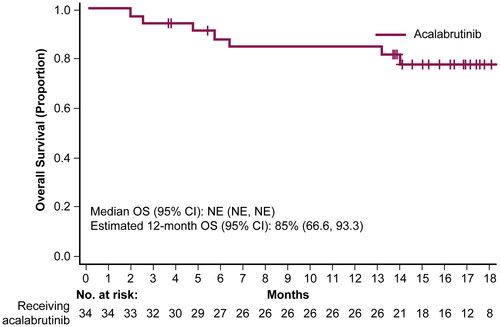
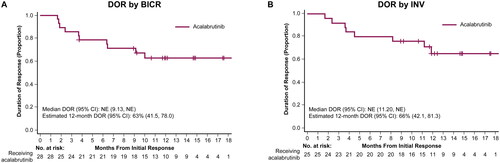
According to BICR assessment, the ORR was 82.4% (95% CI: 65.5, 93.2), 12 (35.3%) patients achieved CR, and the median TTR was 1.8 months (range, 1.6–3.7). The median DOR and median PFS were not estimable. The BICR-assessed 12-month estimated DOR and PFS rates were 62.7% (95% CI: 41.5, 78.0) and 51.5% (33.3, 67.0), respectively ( and ). Investigator-assessed ORR was 73.5% (95% CI: 55.6, 87.1), with 13 (38.2%) patients achieving a CR. Investigator-assessed TTR was 1.9 months (range, 1.6–3.7), and DOR and PFS 12-month estimates were 65.5% (95% CI: 42.1, 81.3) and 58.3% (95% CI: 39.9, 72.9), respectively ( and ). Median OS was not estimable; estimated 12-month OS was 84.5% (95% CI: 66.6, 93.3; ).
Figure 1. Duration of response. Kaplan-Meier curves for DOR with Kaplan-Meier estimates per BICR assessment (A) and INV assessment (B). BICR, blinded independent central review; CI, confidence interval; DOR, duration of response; INV, investigator; NE, not evaluable.
Discussion
This phase 1/2 study demonstrated good tolerability and efficacy of acalabrutinib in Chinese patients with R/R MCL. The results were consistent with the known safety profile of acalabrutinib, and high BICR- and investigator-assessed response rates were similar to the outcomes demonstrated in prior acalabrutinib studies that had limited representation from Asian populations. Additionally, these high response rates were observed in a heavily pretreated population with 59% of patients having 3 or more prior regimens.
Treatment with acalabrutinib yielded safety outcomes that were consistent with prior acalabrutinib trials for patients with R/R MCL. A phase 2 trial of acalabrutinib in predominantly White patients with R/R MCL reported a 1% rate of grade 3 bleeding events and no instances of atrial fibrillation [Citation8]. The present study reported no bleeding events of grade 3 or higher, no instances of major hemorrhage, and no instances of atrial fibrillation, indicating that acalabrutinib has a similar safety profile in Chinese patients with R/R MCL as the predominantly White cohorts in previous R/R MCL trials [Citation7, Citation8].
Efficacy assessments demonstrated high response rates, with more than 35% of patients achieving a CR. The BICR-assessed ORR of 82% and the investigator-assessed ORR of 74% are similar to the independent review committee-assessed ORR of 80% in the phase 2 trial of acalabrutinib [Citation8]. Furthermore, pharmacokinetic data from the previously presented phase 1 portion of this study demonstrated rapid absorption and elimination of acalabrutinib in Chinese patients with no recommendation for dose adjustment based on race [Citation13]. Taken together, these results demonstrate similar efficacy for acalabrutinib in Chinese patients and a predominantly White patient cohort with R/R MCL.
Results of several randomized studies evaluating other BTKis in patients with R/R MCL were consistent with this study [Citation7, Citation14–16]. A phase 2 trial of ibrutinib in patients with R/R MCL (N = 111), with median follow-up of 26.7 months, demonstrated an investigator-assessed ORR of 67% [Citation14]. The investigator-assessed ORR in this study was 74%. Additionally, 6% of patients treated with ibrutinib experienced grade ≥3 bleeding and 6% of patients experienced grade ≥3 atrial fibrillation. No grade ≥3 bleeding events or atrial fibrillation events of any grade were reported in patients treated with acalabrutinib in this study.
A phase 1/2 study of zanubrutinib in a predominantly White cohort of patients with R/R MCL (N = 32), with median follow-up of 19 months, reported investigator- and IRC-assessed ORRs of 91% and 84%, respectively. Rates of grade ≥3 bleeding and atrial fibrillation were 9% and 3%, respectively, in patients treated with zanubrutinib [Citation15]. A phase 2 study of zanubrutinib in Chinese patients with R/R MCL (N = 86), with median follow-up of 35 months, demonstrated an investigator-assessed ORR of 84%; rates of grade ≥3 bleeding and atrial fibrillation were 1% and 0%, respectively [Citation16].
This trial had some limitations. Patients, blinded independent central reviewers, and investigators were not blinded to treatment due to the single-arm design, which could have influenced the outcomes (BICR was undertaken with blinding to patient information). The small cohort size limits the ability to compare acalabrutinib to other BTKi regimens for MCL. Finally, longer follow-up is needed to confirm the durability of the efficacy and safety outcomes in this study.
In conclusion, the covalent BTKi acalabrutinib demonstrated high response rates and good tolerability in this first phase 1/2 study of Chinese patients with R/R MCL.
Contribution
JZ designed the study, was a study investigator, and collected and assembled data. YS, JL, KZ, XK, ZC, and HZ were study investigators, provided patients and study materials, and collected and assembled data. TY, ZX, YW, PL, and XL performed data analysis. All authors interpreted data, prepared the manuscript, and participated in the critical review and revision of this manuscript and provided approval of the manuscript for submission.
Supplemental Material
Download PDF (9.3 KB)Acknowledgments
We thank the investigators and coordinators at each of the clinical sites; the patients who participated in this trial and their families; employees of AstraZeneca who contributed to this trial, including Roser Calvo, Paulo Miranda, Simon Rule, and Jack Roos for reviewing the manuscript. The study was funded by AstraZeneca. Medical writing assistance, funded by AstraZeneca, was provided by Maria Ali, PhD, of Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors.
Disclosure statement
YS, JL, KZ, XE, ZC, HZ, JZ: No conflict of interest to declare.
TY, ZX, YW, PL, and XL: Employees of AstraZeneca.
Data sharing statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
References
- Jain P, Wang ML. Mantle cell lymphoma in 2022–a comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol. 2022;97(5):638–656. doi:10.1002/ajh.26523
- Liu W, Liu J, Song Y, et al. Burden of lymphoma in China, 2006-2016: an analysis of the global burden of disease study 2016. J Hematol Oncol. 2019;12(1):115. doi:10.1186/s13045-019-0785-7
- Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6(1):77. doi:10.1186/1746-1596-6-77
- Li X, Li G, Gao Z, et al. The relative frequencies of lymphoma subtypes in China: a nationwide study of 10002 cases by the Chinese lymphoma study group [abstract 169]. Ann Oncol. 2011;22(Suppl 4):iv141.
- Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4,638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429–434. doi:10.1309/AJCP7YLTQPUSDQ5C
- Cortelazzo S, Ponzoni M, Ferreri AJ, et al. Mantle cell lymphoma. Crit Rev Oncol Hematol. 2012;82(1):78–101. doi:10.1016/j.critrevonc.2011.05.001
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi:10.1056/NEJMoa1306220
- Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391(10121):659–667. doi:10.1016/S0140-6736(17)33108-2
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi:10.1016/S0140-6736(20)30262-2
- Calquence [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2022 August 2022.
- Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):JCO2300838–2861.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi:10.1200/JCO.2013.54.8800
- Zhu J, Song Y, Li J, et al. editors. Pharmacokinetics, safety, and efficacy of acalabrutinib in chinese patients with relapsed/refractory mantle cell lymphoma and other B-cell malignancies: an open-label, multicenter phase 1/2 trial [poster 2892]. Annual Meeting and Exposition of the American Society of Hematology; 2022 10-13 December 2022; New Orleans, LA, USA.
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739–745. doi:10.1182/blood-2015-03-635326
- Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021;5(12):2577–2585. doi:10.1182/bloodadvances.2020004074
- Song Y, Zhou K, Zou D, et al. Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood. 2022;139(21):3148–3158. doi:10.1182/blood.2021014162