Abstract
Primary central nervous system lymphoma (PCNSL) is a rare and highly aggressive lymphoma entirely localized in the central nervous system or vitreoretinal space. PCNSL generally initially responds to methotrexate-containing chemotherapy regimens, but progressive or relapsing disease is common, and the prognosis is poor for relapsed or refractory (R/R) patients. PCNSL is often characterized by activation of nuclear factor kappa B (NF-κB) due to mutations in the B-cell receptor (BCR) or toll-like receptor (TLR) pathways, as well as immune evasion. Targeted treatments that inhibit key PCNSL mechanisms and pathways are being evaluated; inhibition of Bruton’s tyrosine kinase (BTK) downstream of BCR activation has demonstrated promising results in treating R/R disease. This review will summarize the evidence and potential for targeted therapeutic agents to improve treatment outcomes in PCNSL. This includes immunotherapeutic and immunomodulatory approaches and inhibitors of the key pathways driving PCNSL, such as aberrant BCR and TLR signaling.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare and highly aggressive extranodal form of diffuse large B-cell lymphoma (DLBCL) confined to the central nervous system (CNS), including the brain, spinal cord, and eyes without evidence of systemic spread [Citation1–4]. PCNSL can occur in both immunocompetent and immunosuppressed individuals, with substantial differences in etiology, biology, presentation, and behavior [Citation5]. Overall, the incidence of PCNSL has remained generally at 0.4/100,000 population and increases with age, ranging from 0.08/100,000 in individuals 20–29 years of age to a high of 4.32/100,000 in those 70–79 years of age [Citation2]. A higher incidence of PCNSL is observed in patients who have received solid organ transplants and those living with HIV/AIDS. However, incidence in the latter group has been drastically reduced with the widespread use of antiretroviral therapy [Citation4, Citation5]. This review is focused on PCNSL in the immunocompetent population.
The clinical presentation of PCNSL can exhibit a high degree of variation. While a narrow majority of patients present with focal neurologic deficits, it is nearly as common for patients with PCNSL to develop nonspecific cognitive or behavioral changes over a matter of weeks to months [Citation6, Citation7]. Generalized signs of intracranial pressure, such as headache, vomiting, or nausea, are present in about one-third of patients but may not always be readily linked to neurologic pathology [Citation6, Citation7]. The symptoms associated with primary intraocular lymphoma can be indolent and occur years before the involvement of additional CNS compartments [Citation7].
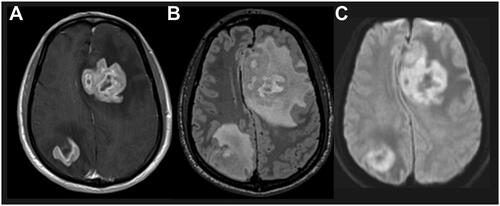
The preferred imaging modality for diagnosis, staging, and response assessment in immunocompetent patients is magnetic resonance imaging (MRI) with and without gadolinium contrast [Citation7] (). MRI provides the best resolution for visualization of characteristic PCNSL radiographic features, including blood–brain barrier (BBB) disruption, tumor burden, and vasogenic edema [Citation7, Citation8]. The International PCNSL Collaborative Group recommends whole-body fluorodeoxyglucose-positron emission tomography (PET) imaging as an approach to rule out systemic involvement, which excludes the diagnosis of PCNSL [Citation7, Citation8].
Figure 1. Characteristic PCNSL imaging pattern on magnetic resonance imaging. A) T1 sequence with gadolinium contrast demonstrates enhancing brain lesion. B) Fluid-attenuated inversion recovery sequence visualizes a mass edema surrounding the mass lesion. C) Diffusion-weighted imaging demonstrates restricted diffusion within the tumor lesions. Abbreviation: PCNSL, primary central nervous system lymphoma.
Two models are commonly used to determine PCNSL prognosis. In 2003, the International Extranodal Lymphoma Study Group (IELSG) devised a system of prognostic scoring based upon five independent predictors of survival: age, Eastern Cooperative Oncology Group (ECOG) performance score, serum lactate dehydrogenase levels, cerebrospinal fluid (CSF) protein concentration, and the involvement of deep brain structures [Citation9]. In 2006, the Memorial Sloan Kettering Cancer Center model streamlined this into three prognostic groups, based on age and the Karnofsky Performance Scale, to predict progression-free survival (PFS) and overall survival (OS) regardless of the treatment received [Citation10].
Current treatment options and unmet needs
PCNSL is highly aggressive but generally responds favorably to high-dose methotrexate (HD-MTX)-based chemotherapy; remission can often be achieved [Citation2, Citation7, Citation8]. However, patients with PCNSL have shorter survival compared with those with non-CNS lymphomas, with up to half relapsing after initial therapy. Additionally, PCNSLs do not generally respond to regimens for systemic DLBCL [Citation2, Citation11].
Newly diagnosed patients
Management of the newly diagnosed patient is best served by a multidisciplinary approach that includes radiology assessments, ophthalmologic evaluations, and the determination of general patient fitness [Citation12]. Current guidelines recommend that induction therapy in patients with PCNSL includes an HD-MTX-based regimen if the patient is suitable and tolerant but unsuitable for participation in a clinical trial [Citation13, Citation14], (). Treatment choice is determined by a combination of age, comorbidities, organ function, frailty, risk of having neurotoxic effects from treatment, performance status, and patient priorities [Citation4]. Patients deemed fit typically receive induction therapy with HD-MTX with an alkylating agent with or without rituximab and with or without high-dose cytarabine [Citation1, Citation4]. Though historically a predominant first-line approach, whole-brain radiotherapy (WBRT) is less commonly used due to neurotoxicity concerns and the emergence of HD-MTX-containing regimens [Citation1, Citation7, Citation14]. Currently, the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) suggest WBRT only if the patient is not a candidate for systemic therapy [Citation13]. The European Association of Neuro-Oncology (EANO) guidelines do not recommend first-line radiotherapy and note that WBRT should be avoided in elderly patients due to delayed neurotoxicity [Citation14]. HD-MTX can cause dramatic responses but prospective data assessing different chemotherapy regimens are lacking, with no current consensus on the optimal chemotherapy regimen [Citation7].
Figure 2. Treatment flow for patients with newly diagnosed PCNSL. A. Frontline therapy for PCNSL for fit patients includes HD-MTX regimens as induction therapy, with ASCT consolidation therapy. Fitness is determined by a combination of age, comorbidities, organ function, frailty, risk of having neurotoxic effects from treatment, performance status, and patient priorities. For patients who are not deemed fit for this therapy, alternative induction and consolidation strategies include non-myeloablative therapies, WBRT, or ifosamide- or cytarabine-containing regimens. B. For patients with R/R PCNSL, treatment options are guided by the previous therapy received and the length of time to progression from prior therapy. aWBRT should be used with caution due to neurotoxicity concerns, especially in elderly patients or in combination with chemotherapy. bHD-MTX regimens typically include maximum tolerated dose with an alkylating agent with or without rituximab and with or without high-dose cytarabine. cFor patients for whom consolidation therapy with ASCT is not recommended, potential consolidation strategies include nonmyeloablative high-dose chemotherapy alone or with etoposide, or maintenance therapy with temozolomide, rituximab, or MTX. dSalvage therapy may include retreatment with HD-MTX with or without rituximab or ibrutinib; ibrutinib, temozolomide, rituximab with or without temozolomide; lenalidomide with or without rituximab; high-dose cytarabine; high-dose ifosfamide; or pemetrexed; patients with R/R PCNSL are recommended to be enrolled into clinical trials if eligible. Adapted from information in [Citation4, Citation7, Citation13, Citation14]. Abbreviations: ASCT, autologous stem cell therapy; BSC, best supportive care; CR, complete response; CT, chemotherapy; HD, high-dose; HD-MTX, high-dose methotrexate; mo, months; PCNSL, primary central nervous system lymphoma; PR, partial response; R/R, relapsed/refractory; RT, radiotherapy; SD, stable disease; TTP, time to progression; WBRT, whole-brain radiotherapy.
![Figure 2. Treatment flow for patients with newly diagnosed PCNSL. A. Frontline therapy for PCNSL for fit patients includes HD-MTX regimens as induction therapy, with ASCT consolidation therapy. Fitness is determined by a combination of age, comorbidities, organ function, frailty, risk of having neurotoxic effects from treatment, performance status, and patient priorities. For patients who are not deemed fit for this therapy, alternative induction and consolidation strategies include non-myeloablative therapies, WBRT, or ifosamide- or cytarabine-containing regimens. B. For patients with R/R PCNSL, treatment options are guided by the previous therapy received and the length of time to progression from prior therapy. aWBRT should be used with caution due to neurotoxicity concerns, especially in elderly patients or in combination with chemotherapy. bHD-MTX regimens typically include maximum tolerated dose with an alkylating agent with or without rituximab and with or without high-dose cytarabine. cFor patients for whom consolidation therapy with ASCT is not recommended, potential consolidation strategies include nonmyeloablative high-dose chemotherapy alone or with etoposide, or maintenance therapy with temozolomide, rituximab, or MTX. dSalvage therapy may include retreatment with HD-MTX with or without rituximab or ibrutinib; ibrutinib, temozolomide, rituximab with or without temozolomide; lenalidomide with or without rituximab; high-dose cytarabine; high-dose ifosfamide; or pemetrexed; patients with R/R PCNSL are recommended to be enrolled into clinical trials if eligible. Adapted from information in [Citation4, Citation7, Citation13, Citation14]. Abbreviations: ASCT, autologous stem cell therapy; BSC, best supportive care; CR, complete response; CT, chemotherapy; HD, high-dose; HD-MTX, high-dose methotrexate; mo, months; PCNSL, primary central nervous system lymphoma; PR, partial response; R/R, relapsed/refractory; RT, radiotherapy; SD, stable disease; TTP, time to progression; WBRT, whole-brain radiotherapy.](/cms/asset/6306ae4c-9178-4431-9779-963c5387a15c/ilal_a_2342560_f0002_c.jpg)
While biopsies that may involve deep brain structures are an essential diagnostic procedure, current evidence does not support surgical resection as a therapeutic approach in PCNSL [Citation7, Citation15, Citation16]. Gross or subtotal resection increase the risk of severe and permanent neurological sequelae, in addition to tumor spill through meningeal spaces [Citation6, Citation15].
If patients achieve complete response (CR), partial response (PR), or stable disease (SD), induction therapy is most often followed by consolidation therapy that aims to eliminate any residual disease. Consolidation comprises a patient-specific combination of systemic therapy, radiation, or intensive chemotherapy with hematopoietic stem cell rescue (SCT) [Citation7, Citation13, Citation14]. High-dose chemotherapy and autologous SCT (ASCT) is the typical approach for younger patients with few comorbidities who respond to induction therapy [Citation7, Citation13, Citation14]. Alternative consolidation strategies include nonmyeloablative high-dose chemotherapy, with or without etoposide, and low-dose WBRT. Maintenance therapy with temozolomide, rituximab, or MTX is currently being assessed [Citation7, Citation13, Citation14]. Due to neurotoxicity concerns, combinations of radiation and intensive chemotherapy are typically not recommended [Citation7, Citation14].
Fifteen to 25% of patients with PCNSL are refractory to HD-MTX-based chemotherapy induction. Another 25–50% of patients experience relapses, typically within 2 years from CR [Citation7]. Relapses of ≥5 years are uncommon but have been reported after ≥10 years [Citation7, Citation17, Citation18]. Early recurrence tends to occur in patients who are elderly or frail, which may be the result of less-intensive choices for induction therapy and limitations in consolidation strategies due to associated risks, including myelotoxicity with high-dose chemotherapy and neurotoxicity with WBRT [Citation7].
Relapsed/refractory (R/R) patients
The prognosis of patients with R/R PCNSL is extremely poor, with a median OS from relapse of 6.8 months and 1- and 3-year OS rates of 38% and 25%, respectively [Citation19]. A subset of patients with SD or progressive disease during first-line therapy (n = 163) had a median OS of 2.1 months from the date of first progression [Citation20].
The best therapeutic option for patients with R/R PCNSL is enrollment in an appropriate prospective clinical trial (), [Citation4, Citation13, Citation14]. When a clinical trial is not available, key parameters used to choose salvage therapy are the type of first-line treatment received, time to progression after the first line (TTP1), and patient characteristics such as age, performance score, comorbidity, frailty, and life expectancies [Citation4, Citation13, Citation14]. Late relapses can be managed using HD-MTX retreatment (with the same or similar combination) followed by consolidation with ASCT or WBRT if not previously given [Citation13, Citation14]. After a short TTP1, young and fit patients are suitable candidates for high-dose ifosfamide/cytarabine-containing chemotherapy followed by consolidation with ASCT or WBRT [Citation4]. Elderly or unfit patients are often treated with palliative goals. This is done via WBRT or monotherapy with drugs that have been assessed in large prospective trials (see below), and best supportive care should be considered in select patients [Citation4, Citation7]. Overall, only a small proportion of patients with R/R PCNSL benefit from salvage therapy, usually with short-lasting responses [Citation7].
Unmet needs
While incidence of PCNSL has especially increased among elderly individuals, treatment strategies with durable response rates are lacking in this group [Citation4]. In an analysis of treatment regimens by age in French patients, younger and older patients received similar induction therapy but differed in consolidation therapies received, with patients <60 years of age more likely to receive consolidation with WBRT (54%) or high-dose-chemotherapy/ASCT (23%) than those >60 years (9% WBRT, 2% HD-chemotherapy/ASCT) [Citation19].
Due to the risk of delayed neurotoxicity, EANO treatment guidelines consider the risk of treatment with WBRT, especially if following HD-MTX, to be unacceptably high in elderly patients [Citation14]. Effective treatments without neurotoxic effects are needed, especially in the elderly population who are often ineligible for effective consolidation therapy [Citation4].
To effectively treat PCNSL, therapies must be able to penetrate the BBB. The BBB comprises endothelial cells connected by tight junctions that line cerebral capillaries and are surrounded by pericytes and astrocyte endfeet [Citation21]. Some small molecules (<500 Da) can pass through the BBB by passive diffusion and others by active transport. However, circulating antibodies and many small molecules are unable to reach the CNS. This inhibits the effectiveness of regimens typically used for non-CNS lymphomas, such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) [Citation22, Citation23]. The CSF concentration of rituximab has been found to reach only 0.1% of serum concentration, potentially due to its large size (143 kDa) [Citation24]. When tested, vincristine was undetected in the CSF [Citation23]. The activity of bispecific antibodies in PCNSL is unknown [Citation26]. Antibody-drug conjugates such as trastuzumab emtansine have demonstrated limited effectiveness in treating brain metastases, potentially due to their large size. However, approaches to permeabilize the BBB have had promising results in preclinical and small clinical studies [Citation27].
MTX and cyclophosphamide have demonstrated relatively high penetration of the BBB, with CSF/blood glucose ratios of 2–20% and 50%, respectively [Citation25]. CSF concentration may not be fully correlative with parenchymal drug levels, which may differ based on the vascular permeability of the tumor [Citation28]. Drugs that may have limited or moderate BBB cross capability, such as MTX, ifosfamide, and cytarabine, can be delivered at high doses in patients with PCNSL without dose-limiting toxicity [Citation4, Citation29].
New therapies should be explored urgently to improve survival in patients with PCNSL, potentially including the investigation of new therapeutic targets and novel pharmacodynamic mechanisms with positive safety and efficacy profiles.
Efforts to cross the BBB
Various strategies have been employed to allow systemically administered treatments to penetrate the BBB, including disruptors of BBB integrity [Citation21]. Permeabilization of the BBB using administration of tumor necrosis factor (TNF)-alpha coupled with a tumor vasculature-homing peptide, NGR (NGR-hTNF), was assessed in combination with administration of R-CHOP administered every 21 days in a phase 2 trial of 28 patients with R/R PCNSL (NCT03536039) [Citation23]. An objective response rate (ORR) was achieved in 21/28 patients (75%), including 11/28 patients (39%) achieving a CR. Effects of NGR-hTNF were specific for the tumor area, as imaging showed increased vascular permeability in the perilesional areas, but concentrations of doxorubicin, cyclophosphamide, and rituximab in the CSF (<2.5 ng/mL, 15.5 ± 4.8 mg/mL, and <1.0 ng/mL, respectively) were not significantly different than samples collected after R-CHOP without NGR-hTNF treatment [Citation30].
Osmotic disruption of the BBB by mannitol intra-arterial infusion combined with HD-MTX-based cerebral intra-arterial chemotherapy was assessed in a retrospective study of patients with newly diagnosed PCNSL (n = 44) [Citation31]. This regimen demonstrated a CR in 34/44 patients (77%), median OS of 45 months, and median PFS of 24 months; the regimen was well-tolerated. However, this methodology is not suited for patients with a high tumor burden, requires a multidisciplinary team, and is a procedurally invasive treatment [Citation31, Citation32]. These considerations, along with the lack of head-to-head studies demonstrating the superiority of this approach to intravenous HD-MTX, have prevented its wide adoption to date.
Disruption of the BBB via ultrasound is a promising approach that can allow ordinarily-impermeable drugs targeted access in preclinical and clinical studies [Citation33]. Multiple phase 1 and 2 clinical trials across multiple disease states such as glioblastoma, Alzheimer’s, and Parkinson’s disease are underway to assess the feasibility of this methodology for drug delivery [Citation33, Citation34].
Beyond PCNSL, in a preclinical model of intracranial human epidermal growth factor receptor 2-positive breast cancer, a study determined that intra-arterial NEO100 (perillyl alcohol) could be used to reversibly open the BBB and increase the effectiveness of trastuzumab emtansine [Citation27]. A phase 1 study of NEO100 in recurrent glioblastoma demonstrated promising initial results, but this therapeutic approach has not yet been tested in patients with PCNSL [Citation35].
Additionally, patients with PCNSL may have disruptions in the BBB that result in a more permeable brain-tumor barrier characterized by a reduction in tight junctions induced by vascular endothelial growth factor (VEGF) secretion from tumor cells [Citation21]. Analysis of VEGF-expressing cells in samples from patients with PCNSL (n = 19) showed increased microvessel density and a loss of BBB markers in VEGF-positive samples [Citation36]. A study of radiolabeled intravenously administered anti-CD20 antibodies suggested that disruption of the BBB due to PCNSL may be sufficient to allow delivery of anti-CD20 [Citation37]. Two phase 1 trials assessed the administration of intraventricular rituximab, both as monotherapy and in combination with intraventricular MTX, via an Ommaya reservoir [Citation38]. Treatment with 10- and 25-mg doses of rituximab were well-tolerated and elicited responses in the CSF, intraocular compartments, and small lesions within the brain, and appeared effective in patients with a high burden of leptomeningeal disease. In patients with R/R CNS lymphoma (either PCNSL or secondary CNS lymphoma), intraventricular rituximab combined with MTX demonstrated a CR rate in the leptomeningeal compartment of 75% (9/12) of patients [Citation39]. Stratification of patients based on BBB integrity could be a potential method to predict the effectiveness of less BBB-permeable therapies such as rituximab, and approaches to disrupt the BBB (such as ultrasound or osmotic agents) could potentially further increase access.
Mechanistic and genetic clues
Recent advances in understanding the pathogenesis of PCNSL are currently leading to the identification of new potential therapeutic targets and the development of novel agents to target them (). PCNSL histologically and immunohistologically resembles the activated B-cell-like and non-germinal center DLBCL subtypes; however, recent studies suggest a divergent pathogenesis and distinct expression profile between DLBCL with secondary involvement of the CNS and PCNSL [Citation1, Citation7, Citation40–42].
Table 1. Selected agents in recent trials for PCNSL by target or MOA.
Most PCNSLs express multiple B-cell markers, including CD20, in ≥90% of cases; however, unlike systemic DLBCL, the benefit of adding rituximab to HD-MTX-based chemotherapy remains debatable in PCNSL [Citation43, Citation44]. Adding rituximab and thiotepa to an HD-MTX and high-dose cytarabine regimen was associated with a significant improvement in 7-year OS and PFS in the IELSG32 study [Citation45]. In contrast, the phase 3 HOVON 105/ALLG NHL 24 study assessing MTX, carmustine, teniposide, and prednisone with or without rituximab in patients with PCNSL found no clear benefit in event-free survival with the addition of rituximab [Citation43]. However, a meta-analysis of the two trials determined that while there was no evidence for improvement of OS, adding rituximab to MTX-based chemotherapy may improve PFS [Citation46].
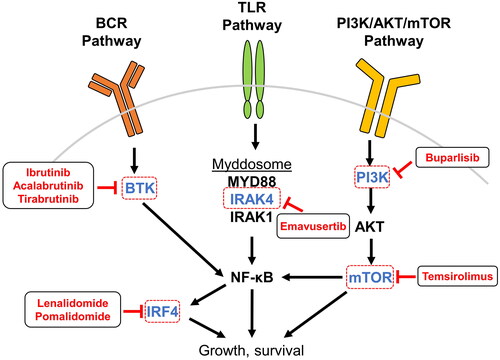
Advances in understanding key mutations and pathways in PCNSL inform the assessment of new therapeutic possibilities. Genetic alterations in PCNSL often lead to activation of the B-cell receptor (BCR) and toll-like receptor (TLR) signaling pathways and cause activation of nuclear factor kappa B (NF-κB), a transcription factor that promotes proliferation while hindering apoptosis [Citation44] (). Multiple targeted therapies that aim to interrupt aberrant signaling pathways or increase the involvement of the immune system in PCNSL are being assessed in both preclinical models and clinical trials [Citation4].
Figure 3. Inhibition of PCNSL-associated signaling pathways. PCNSL is frequently driven by overactivation of pathways leading to NF-κB, and many therapeutic agents in use and development for patients with PCNSL target components upstream and downstream of NF-κB. BCR signaling to NF-κB is transduced by BTK and is targeted by the small-molecule inhibitors ibrutinib, acalabrutinib, and tirabrutinib. TLR activation by ligand binding leads to assembly and activation of the myddosome protein complex that incorporates IRAK4, IRAK1 phosphorylation, and downstream activation of NF-κB; the small molecule emavusertib inhibits IRAK4. Within the PI3K pathway, mTOR is inhibited by temsirolimus and PI3K by the pan-PI3K-inhibitor buparlisib. NF-κB itself regulates the expression of IRF4, which is a target of the IMiDs lenalidomide and pomalidomide. Abbreviations: AKT, protein kinase B; BCR, B-cell receptor; BTK, Bruton’s tyrosine kinase; IMiD, immunomodulatory imide drugs; IRAK, interleukin-1 receptor-associated kinase; IRF4, interferon regulatory factor 4; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphatidylinositol-3-kinase; TLR, toll-like receptor.
Potential targeted treatment approaches in PCNSL
Targeting the BCR and/or TLR pathways is a promising approach
Bruton’s tyrosine kinase (BTK) inhibition
Genes encoding NF-κB pathway regulators along the BCR and TLR pathways, including MYD88 and CD79B, represent the most frequently altered genes in PCNSL [Citation47–49]. CD79B encodes a BCR subunit essential for signaling that leads to NF-κB activation, and MYD88 encodes a signaling adaptor protein that induces NF-κB after the stimulation of TLR [Citation48]. Mutations of CARD11 and TNFAIP3 are also relatively common and drive activation of NF-κB [Citation47, Citation48, Citation50]. Cumulatively, mutations affecting the BCR/TLR pathways, common in systemic DLBCL, are present in approximately 90% of PCNSL [Citation49–51]. A high frequency of concurrent CD79B and MYD88 mutations (83% and 76%, respectively) distinguishes PCNSL from systemic DLBCL; CD79B mutations have been observed in 8–18% of systemic DLBCL and MYD88 mutations in 29% of systemic activated B-cell like-DLBCL [Citation47, Citation50]. The prominent role of BCR and TLR signaling to NF-κB in PCNSL suggests BTK inhibition as a rational targeted therapeutic strategy; this approach has since demonstrated efficacy in clinical trials and is reflected in current treatment guidelines [Citation13, Citation52, Citation53].
The selective and irreversible BTK inhibitor ibrutinib has exhibited high response rates in patients with R/R PCNSL, demonstrating clinical, radiological, and biological activity in the brain, intraocular compartment, and CSF [Citation52–54]. In a study of 44 evaluable patients with R/R PCNSL or primary vitreoretinal lymphoma (PVRL), patients achieved an ORR of 61% (27/44), including 10 CRs (19%), after 2 months of ibrutinib monotherapy [Citation54]. After a median follow-up of 25.7 months, the median PFS was 4.8 months, and the median OS was 19.2 months [Citation54].
In a study of 44 Japanese patients with R/R PCNSL treated with tirabrutinib monotherapy, patients achieved an ORR of 64% (28/44 patients) including CRs in 16/44 patients (36%) with a median follow-up of 37.1 months [Citation55]. Median PFS was 2.9 months and median OS was not reached. Another study assessing 18 patients with PCNSL treated with ibrutinib monotherapy during a 14-day window reported 17/18 (94%) patients with disease reductions and PRs in 15/18 (83%) patients [Citation53]. During this time, however, two patients died from Aspergillus infection [Citation53].
While ibrutinib has been added to the NCCN Guidelines® for R/R PCNSL, with or without HD-MTX, it is not approved for treatment of PCNSL, and the risk of Aspergillus infection remains a lingering concern [Citation13]. Reports of primary and secondary resistance have emerged for ibrutinib, but the mechanisms leading to this resistance are poorly understood; in PCNSL this may be due in part to de novo resistance mechanisms [Citation56]. An additional consideration is the potential for development of de novo mechanisms of ibrutinib resistance in PCNSL. In a phase 1 trial of ibrutinib in 13 cases of R/R PCNSL, an unanticipated result was a lack of CR in patients with concurrent mutations in MYD88 and CD798B [Citation52]. These mutations frequently occur together in PCNSL and may serve to attenuate BTK “addiction” by providing redundant survival signals [Citation52]. Other tumors in this study exhibiting incomplete responsiveness to ibrutinib had mutations in CARD11 or inactivating lesions in TNFAIP3, a negative regulator of NF-κB [Citation52]. Considering resistance concerns, a relatively short PFS when used as monotherapy, and a response that appears more durable when used in combination, ibrutinib is under investigation as a component of several combination regimens [Citation44].
Second-generation BTK inhibitors are now being investigated in the recurrent PCNSL setting, including tirabrutinib (NCT04947319) as monotherapy or in combination with HD-MTX and rituximab-containing regimens and acalabrutinib as monotherapy (NCT04548648) or in combination with rituximab and durvalumab (NCT04688151). Tirabrutinib has demonstrated favorable efficacy in a phase 1/2 study including 4 confirmed and 11 unconfirmed CRs across 44 patients, with no marked differences in ORR among patients harboring MYD88, CD79B, or CARD11 mutations and the corresponding wild type [Citation57]. Acalabrutinib has demonstrated reduced off-target kinase inhibition in cell lines and increased potency in canine models of DLBCL [Citation56].
Interleukin-1 receptor-associated kinase 4 (IRAK4) inhibition
The promising outcomes of targeting BCR signaling pathways have prompted the investigation of approaches to inhibit TLR signaling pathways, the other major pathway commonly activated with driver mutations in PCNSL [Citation44]. The activation of most TLRs drives the assembly and activation of the myddosome protein complex that incorporates the IRAK4 kinase [Citation58]. Activation of the myddosome, including IRAK4, initiates a chain of downstream phosphorylation events that lead to the activation of NF-κB. Oncogenic mutations such as those in MYD88, observed in 70–80% of PCNSLs, result in constitutive activation of this pathway [Citation59–63]. Emavusertib (CA-4948) is an investigational small-molecule inhibitor of IRAK4 as well as fms-like tyrosine kinase 3 (FLT3) kinase activity that blocks downstream phosphorylation events associated with both the myddosome and FLT3 [Citation60] (). Emavusertib was shown to achieve therapeutically relevant concentrations in the brain parenchyma with a ratio of plasma to naïve CSF of 1.5%, to naïve brain parenchyma of 4.3%, and to tumor-bearing brain parenchyma of 5.0% in an aggressive PCNSL preclinical model [Citation61]. Emavusertib also showed preclinical single-agent activity, including increased median survival while decreasing mitogen-activated protein kinase and NF-κB activation [Citation61, Citation64]. Recent preclinical studies of emavusertib have shown synergy with BTK inhibitors and re-sensitization after BTK resistance [Citation65, Citation66]. A phase 1/2 clinical trial of emavusertib and ibrutinib in patients with R/R hematologic malignancies (TakeAim Lymphoma, NCT03328078) included five response-evaluable patients with PCNSL as of October 12, 2023 [Citation67]. Of these patients, all of whom previously received a BTK inhibitor, 3 achieved a CR, including one patient with a durable response for approximately 7 months at the time of data cutoff [Citation67]. Part B of TakeAim Lymphoma is ongoing and will assess the combination of emavusertib and ibrutinib in patients with PCNSL.
Immunotherapy approaches
Genetic evidence suggests that immune evasion plays a role in the pathogenesis of PCNSL. Still, to date, treatment with programmed cell death ligand 1 (PD-L1) inhibitors has shown only limited efficacy [Citation68, Citation69]. Genetic studies reveal that PD-L1/PD-L2 copy gains and translocations that lead to increased expression of these ligands and the resulting immune evasion are frequent in PCNSL [Citation47]. Focal deletions of 6p21, which contains the human leukocyte antigen (HLA)-D locus, are more frequent in PCNSL than in systemic DLBCL. Loss of the HLA-D locus may reduce major histocompatibility complex class II gene expression and suggest an additional potential mechanism of reduced immune surveillance [Citation42]. In systemic DLBCL, loss of the HLA-D locus is an independent prognostic variable of poor survival [Citation42, Citation70]. Furthermore, the tumor microenvironment of PCNSL contains tumor-infiltrating lymphocytes and tumor-associated macrophages (TAMs), which can serve as localized sources of both programmed cell death protein 1 (PD-1) and PD-L1, respectively [Citation26]. The potential benefits of PD-1 blockade were demonstrated in a small (n = 4) population of patients with R/R PCNSL, in which treatment with nivolumab yielded three CRs and one partial radiographic response following a median of three rounds of treatment, and all patients were alive at a median follow-up of 17 months [Citation71]. However, a phase 2 trial of nivolumab in patients with R/R PCNSL (n = 47) demonstrated an ORR of only 6.4% with a PFS of 1.4 months and OS of 8.6 months [Citation68]. Patients with R/R PCNSL receiving pembrolizumab in the AcSé study (n = 50) demonstrated an ORR of 26% (13/50 patients) and SD in 10% (5/50 patients) [Citation69].
Reports of successful treatment outcomes are emerging with the use of CD19-directed chimeric antigen receptor T-cell (CAR-T) products. In 2017, both tisagenlecleucel and axicabtagene ciloleucel received United States (US) Food and Drug Administration (FDA) approval for targeting cells expressing CD19; these drugs account for the majority of CAR-T use in the US [Citation72]. Both agents demonstrated favorable activity in licensing trials conducted in patients with R/R large B-cell lymphomas; however, due to concerns that CNS disease may increase the incidence and severity of immune effector cell-associated neurotoxicity syndrome (ICANS), patients with a history of prior CNS lymphoma or active secondary CNS lymphoma at the time of CAR-T infusion were excluded from the pivotal trials [Citation72, Citation73]. However, emerging data on outcomes of lymphoma patients with CNS involvement support the potential of a CAR-T approach in PCNSL [Citation73, Citation74]. In a cohort of eight patients with B-cell lymphoma and secondary CNS involvement, no grade >1 neurotoxicities were observed, and early response assessments demonstrated the favorable activity of tisagenlecleucel [Citation74]. A phase 1/2 single-arm clinical trial of tisagenlecleucel therapy following these findings (NCT02445248) included 12 relapsed patients with PCNSL, all of whom received prior treatment with a BTK inhibitor, who were treated and followed for a median of 12 months [Citation73]. Grade 1 cytokine release syndrome (CRS) was observed in 7/12 patients (58%), low-grade ICANS in 5/12 patients (42%), and grade 3 ICANS in only a single patient. Responses were seen in 7/12 patients (58%), including a CR in 6/12 patients (50%). There were no treatment-related deaths, and three patients had ongoing complete remission at the data cutoff with a median follow-up of 12.2 months [Citation73]. In a series of nine patients with R/R PCNSL treated with tisagenlecleucel (n = 7) or axicabtagene ciloleucel (n = 2), the best response was a PR in one patient (tisagenlecleucel) and CR in five patients (three tisagenlecleucel; two axicabtagene ciloleucel); seven patients experienced CRS (including one grade 3 after tisagenlecleucel) and five patients experienced ICANS (including one grade 3 after tisagenlecleucel and one grade 4 after axicabtagene ciloleucel) [Citation75]. In a study of patients receiving CD19-directed CAR-T cell therapy, the subset of patients with PCNSL (n = 5) all developed CRS and neurotoxicity that was tolerable and reversible and demonstrated an initial disease response of CR in three patients (one with progression at day 273, one who went on maintenance therapy at day 43, and one in follow-up without maintenance therapy at day 520) and SD in two patients [Citation76]. A phase 1 study of axicabtagene ciloleucel in combination with fludarabine and cyclophosphamide in R/R PCNSL or secondary CNS lymphoma (SCNSL) is ongoing (NCT04608487). Additionally, dual antigen targeting is being evaluated in PCNSL and other B-cell lymphomas to prolong responses and reduce relapse due to antigen escape [Citation77–79].
Immunomodulatory imide drugs (IMiDs)
IMiDs, such as lenalidomide and pomalidomide, have the potential to exert both direct and indirect antineoplastic effects in PCNSL. The IMiDs suppress interferon regulatory factor 4 (IRF4), which interfaces with NF-κB and Myc and is frequently upregulated in PCNSL [Citation44]. The IMiDs also block the phosphatidylinositol-3-kinase (PI3K)/protein kinase B pathway, resulting in anti-angiogenic effects, and appear to exert significant effects on the tumor microenvironment through increased populations of TAMs and natural killer cells [Citation44, Citation80].
Lenalidomide, a second-generation IMiD, has been studied as monotherapy for the treatment of relapsed PCNSL (n = 6) and SCNSL (n = 8) [Citation81]. Regression was seen in 9/14 patients (64%), including those with leptomeningeal disease (2/6, 33%) and intraocular lymphoma (2/4, 50%). Dose-dependent increases in CSF penetration were observed, with CSF/plasma partition coefficients for lenalidomide >20% at the 15 and 20 mg dose levels [Citation81]. Lenalidomide is also being considered in combination with rituximab for R/R PCNSL or PVRL, with a prospective phase 2 study (n = 45) yielding an ORR of 36% (16/45; 13 were confirmed or unconfirmed CRs), and a median PFS and OS of 7.8 and 17.7 months, respectively, after a median follow-up of 19.2 months [Citation82]. Multiple combination regimens incorporating lenalidomide with agents such as rituximab or MTX are currently under study for both newly diagnosed and relapsed disease [Citation44]. Though data are not yet clear, an additional potential role for lenalidomide exists as a maintenance agent. Maintenance with lenalidomide increased the duration of response to salvage therapy in a small (n = 10) retrospective analysis. However, another study of 18 patients following induction therapy with lenalidomide and rituximab reported only limited benefits of this strategy, with two patients with ongoing CR at the final follow-up [Citation81, Citation82].
The third-generation IMiD pomalidomide was studied in combination with dexamethasone in a phase 1 study that included 25 patients with R/R PCNSL and PVRL [Citation83]. After a median follow-up of 16.5 months, the ORR was 48% (12/25), with CR reported for 6/25 patients and a median PFS of 5.3 months. The CNS pharmacokinetics of the 3 mg/day dose of pomalidomide was assessed in one patient, and the CSF/plasma concentration ratio was determined to be 17–19%; combined with an acceptable toxicity profile, the feasibility of this regimen was confirmed [Citation83].
Agents targeting the PI3K/mammalian target of rapamycin (mTOR) signaling pathways
The PI3K-related family of kinases, of which mTOR is a ubiquitously expressed member, participate in multiple signal transduction pathways that can result in the activation of NF-κB as well as other targets. This can lead to adjustments in the machinery of protein expression to regulate cell growth and proliferation [Citation44, Citation84, Citation85]. mTOR inhibition has shown promising clinical activity in mantle cell lymphoma and systemic DLBCL [Citation86, Citation87].
In a study of 37 patients with R/R, CRs were seen with the mTOR inhibitor temsirolimus in five (14%) patients, unconfirmed CRs in three (8%) patients, and PRs in 12 (32%) patients, yielding an ORR of 54% (20/37) [Citation84]. However, the high response rate was accompanied by a short median PFS of only 2.1 months and failed to translate into durable benefits. These findings were accompanied by a lack of meaningful concentration of the drug or its metabolites in the CSF [Citation84]. The results of mTOR inhibition in PCNSL are broadly similar to the findings of a phase 2 study of the pan-PI3K inhibitor buparlisib in four patients with recurrent/refractory PCNSL/SCNSL [Citation84, Citation88]. The ORR to buparlisib was 25% (1/4), the median PFS was only 39 days, and CSF concentrations were below the levels required to induce cell death in lymphoma cells in vitro [Citation88]. Additional studies are underway exploring agents that target this pathway, with preclinical data suggesting the induction of synergistic cell death when dual PI3K inhibition is combined with the BTK inhibitor ibrutinib [Citation52].
Conclusions
Despite significant advances and improved survival in the management of PCNSL, relapse is a frequent occurrence that poses a significant clinical challenge. New insights have led to the introduction of novel targeted agents, expanding upward from the salvage setting into newly diagnosed patients in clinical trials. With an improved understanding of the molecular drivers of PCNSL, the microenvironment of the brain, and the underlying mechanisms of resistance, the number of therapeutic options should only increase. These advances in knowledge have yielded a wealth of targeted strategies that warrant further clinical investigation to help improve the frequency and quality of response in PCNSL patients with agents that achieve therapeutic dose concentrations in the CNS and generate durable responses. Routes of delivery to bypass the BBB or enhance its permeability may further broaden future therapeutic options. The use of combination therapies further points toward strategies that may avoid early resistance. At the same time, advances in the genetic characterizations of PCNSL pathology may ultimately lead to tailored treatments for appropriate patients with the best likelihood of response.
Author contributions
All authors contributed equally to writing, editing, and approving the final version of this article.
Acknowledgments
Medical writing and editorial assistance were funded by Curis, Inc. and provided by Karl Zawadzki and Alexandra Mascaro of BOLDSCIENCE, Inc.
Disclosure statement
CVR: Grant support: Curis, Inc. AJMF: Speaker fees: Adienne. Grant support: ADC Therapeutics, Amgen, Beigene, BMS, Genmab, Gilead, Hutchison Medipharma, Novartis, Pfizer, and Pharmacyclics. Advisory boards: Gilead, Juno, Novartis, and PletixaPharm. Patents: inventor of patents on NGR-hTNF/R-CHOP in relapsed or refractory PCNSL and SNGR-hTNF in brain tumors. CS: Consultant: Gossamer Bio and Curis Inc. Research Support: AstraZeneca. HWT: Grant support: Acrotech Biopharma, Curis, Inc., and Gossamer Bio. CG: Consultant: BTG, Kite, ONO, and Roche. Research support: Bayer, BMS, Pharmacyclics.
Additional information
Funding
References
- Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–2418. doi:10.1200/JCO.2017.72.7602
- Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary Central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018;20(5):687–694. doi:10.1093/neuonc/nox187
- Raval V, Binkley E, Aronow ME, et al. Primary central nervous system lymphoma: inter‐compartmental progression. EJHaem. 2022;3(2):362–370. doi:10.1002/jha2.303
- Ferreri AJM, Calimeri T, Cwynarski K, et al. Primary central nervous system lymphoma. Nat Rev Dis Primers. 2023;9(1):29. doi:10.1038/s41572-023-00439-0
- Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417–424. doi:10.1111/bjh.14073
- Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261–266. doi:10.3171/jns.2000.92.2.0261
- Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood. 2022;140(9):971–979. doi:10.1182/blood.2020008377
- Barajas RF, Politi LS, Anzalone N, et al. Consensus recommendations for MRI and PET imaging of primary central nervous system lymphoma: guideline statement from the International Primary CNS Lymphoma Collaborative Group (IPCG). Neuro Oncol. 2021;23(7):1056–1071. doi:10.1093/neuonc/noab020
- Ferreri AJM, Blay J-Y, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–272. doi:10.1200/JCO.2003.09.139
- Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. doi:10.1200/JCO.2006.08.2941
- Grommes C, Rubenstein JL, DeAngelis LM, et al. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21(3):296–305. doi:10.1093/neuonc/noy192
- Calimeri T, Steffanoni S, Gagliardi F, et al. How we treat primary central nervous system lymphoma. ESMO Open. 2021;6(4):100213. doi:10.1016/j.esmoop.2021.100213
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Central Nervous System Cancers V1; 2023. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- Hoang-Xuan K, Deckert M, Ferreri AJM, et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro Oncol. 2023;25(1):37–53. doi:10.1093/neuonc/noac196
- Reni M, Ferreri AJ, Garancini MP, et al. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol. 1997;8(3):227–234. doi:10.1023/a:1008201717089
- Bellinzona M, Roser F, Ostertag H, et al. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31(1):100–105. doi:10.1016/j.ejso.2004.10.002
- Tao K, Wang X, Tian X. Relapsed primary Central nervous system lymphoma: current advances. Front Oncol. 2021;11:649789. doi:10.3389/fonc.2021.649789
- Nayak L, Hedvat C, Rosenblum MK, et al. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13(5):525–529. doi:10.1093/neuonc/nor014
- Houillier C, Soussain C, Ghesquières H, et al. Management and outcome of primary CNS lymphoma in the modern era: an LOC network study. Neurology. 2020;94(10):e1027–e1039. doi:10.1212/WNL.0000000000008900
- Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–1303. doi:10.1093/neuonc/now033
- Mo F, Pellerino A, Soffietti R, et al. Blood-brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci. 2021;22(23):12654. doi:10.3390/ijms222312654
- Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–2305. doi:10.1200/JCO.2006.09.9861
- Ferreri AJM, Calimeri T, Ponzoni M, et al. Improving the antitumor activity of R-CHOP with NGR-hTNF in primary CNS lymphoma: final results of a phase 2 trial. Blood Adv. 2020;4(15):3648–3658. doi:10.1182/bloodadvances.2020002270
- Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466–468. doi:10.1182/blood-2002-06-1636
- Yuan Y, Ding T, Wang S, et al. Current and emerging therapies for primary central nervous system lymphoma. Biomark Res. 2021;9(1):32. doi:10.1186/s40364-021-00282-z
- Alcantara M, Fuentealba J, Soussain C. Emerging landscape of immunotherapy for primary central nervous system lymphoma. Cancers (Basel). 2021;13(20):5061. doi:10.3390/cancers13205061
- Wang W, He H, Marín-Ramos NI, et al. Enhanced brain delivery and therapeutic activity of trastuzumab after blood-brain barrier opening by NEO100 in mouse models of brain-metastatic breast cancer. Neuro Oncol. 2021;23(10):1656–1667. doi:10.1093/neuonc/noab041
- de Lange ECM. Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn. 2013;40(3):315–326. doi:10.1007/s10928-013-9301-9
- Ferreri AJM. How I treat primary CNS lymphoma. Blood. 2011;118(3):510–522. doi:10.1182/blood-2011-03-321349
- Ferreri AJM, Calimeri T, Conte GM, et al. R-CHOP preceded by blood-brain barrier permeabilization with engineered tumor necrosis factor-α in primary CNS lymphoma. Blood. 2019;134(3):252–262. doi:10.1182/blood.2019000633
- Iorio-Morin C, Gahide G, Morin C, et al. Management of primary central nervous system lymphoma using intra-arterial chemotherapy with osmotic blood-brain barrier disruption: retrospective analysis of the sherbrooke cohort. Front Oncol. 2020;10:543648. doi:10.3389/fonc.2020.543648
- Doolittle ND, Muldoon LL, Culp AY, et al. Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol. 2014;71:203–243. doi:10.1016/bs.apha.2014.06.002
- Gandhi K, Barzegar-Fallah A, Banstola A, et al. Ultrasound-mediated blood–brain barrier disruption for drug delivery: a systematic review of protocols, efficacy, and safety outcomes from preclinical and clinical studies. Pharmaceutics. 2022;14(4):833. doi:10.3390/pharmaceutics14040833
- Rezai AR, D'Haese P-F, Finomore V, et al. Ultrasound blood-brain barrier opening and aducanumab in Alzheimer’s disease. N Engl J Med. 2024;390(1):55–62. doi:10.1056/NEJMoa2308719
- Schönthal AH, Peereboom DM, Wagle N, et al. Phase I trial of intranasal NEO100, highly purified perillyl alcohol, in adult patients with recurrent glioblastoma. Neurooncol Adv. 2021;3(1):vdab005.
- Takeuchi H, Matsuda K, Kitai R, et al. Angiogenesis in primary central nervous system lymphoma (PCNSL). J Neurooncol. 2007;84(2):141–145. doi:10.1007/s11060-007-9363-x
- Iwamoto FM, Schwartz J, Pandit-Taskar N, et al. Study of radiolabeled indium-111 and yttrium-90 ibritumomab tiuxetan in primary central nervous system lymphoma. Cancer. 2007;110(11):2528–2534. doi:10.1002/cncr.23077
- Rubenstein JL, Gupta NK, Mannis GN, et al. How I treat CNS lymphomas. Blood. 2013;122(14):2318–2330. doi:10.1182/blood-2013-06-453084
- Rubenstein JL, Li J, Chen L, et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121(5):745–751. doi:10.1182/blood-2012-07-440974
- Hernández-Verdin I, Kirasic E, Wienand K, et al. Molecular and clinical diversity in primary central nervous system lymphoma. Ann Oncol. 2023;34(2):186–199. doi:10.1016/j.annonc.2022.11.002
- Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190–196. doi:10.1182/blood-2005-03-1024
- Radke J, Ishaque N, Koll R, et al. The genomic and transcriptional landscape of primary central nervous system lymphoma. Nat Commun. 2022;13(1):2558. doi:10.1038/s41467-022-30050-y
- Bromberg JEC, Issa S, Bakunina K, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019;20(2):216–228. doi:10.1016/S1470-2045(18)30747-2
- Schaff LR, Grommes C. Update on novel therapeutics for primary CNS lymphoma. Cancers (Basel). 2021;13(21):5372. doi:10.3390/cancers13215372
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia. 2022;36(7):1870–1878. doi:10.1038/s41375-022-01582-5
- Schmitt AM, Herbrand AK, Fox CP, et al. Rituximab in primary central nervous system lymphoma-a systematic review and meta-analysis. Hematol Oncol. 2019;37(5):548–557. doi:10.1002/hon.2666
- Chapuy B, Roemer MGM, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–881. doi:10.1182/blood-2015-10-673236
- Bruno A, Boisselier B, Labreche K, et al. Mutational analysis of primary central nervous system lymphoma. Oncotarget. 2014;5(13):5065–5075. doi:10.18632/oncotarget.2080
- Vater I, Montesinos-Rongen M, Schlesner M, et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia. 2015;29(3):677–685. doi:10.1038/leu.2014.264
- Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol. 2016;42(3):279–290. doi:10.1111/nan.12259
- Braggio E, Van Wier S, Ojha J, et al. Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21(17):3986–3994. doi:10.1158/1078-0432.CCR-14-2116
- Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. doi:10.1158/2159-8290.CD-17-0613
- Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843.e5. doi:10.1016/j.ccell.2017.04.012
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II “proof-of-concept” iLOC study by the lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. doi:10.1016/j.ejca.2019.05.024
- Asai K, Narita Y, Nagane M, et al. Final three-year follow-up analysis of phase I/II study on tirabrutinib in patients with relapsed or refractory primary central nervous system lymphoma. J Clin Oncol. 2023;41(16 Suppl.):7548–7548. doi:10.1200/JCO.2023.41.16_suppl.7548
- Profitós-Pelejà N, Santos JC, Marín-Niebla A, et al. Regulation of B-cell receptor signaling and its therapeutic relevance in aggressive B-cell lymphomas. Cancers (Basel). 2022;14(4):860. doi:10.3390/cancers14040860
- Narita Y, Nagane M, Mishima K, et al. Phase I/II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2021;23(1):122–133. doi:10.1093/neuonc/noaa145
- Motshwene PG, Moncrieffe MC, Grossmann JG, et al. An oligomeric signaling platform formed by the toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284(37):25404–25411. doi:10.1074/jbc.M109.022392
- Smith MA, Choudhary GS, Pellagatti A, et al. U2AF1 mutations induce oncogenic IRAK4 isoforms and activate innate immune pathways in myeloid malignancies. Nat Cell Biol. 2019;21(5):640–650. doi:10.1038/s41556-019-0314-5
- Gummadi VR, Boruah A, Ainan BR, et al. Discovery of CA-4948, an orally bioavailable IRAK4 inhibitor for treatment of hematologic malignancies. ACS Med Chem Lett. 2020;11(12):2374–2381. doi:10.1021/acsmedchemlett.0c00255
- Von Roemeling CA, Doonan BP, Klippel K, et al. Oral IRAK-4 inhibitor CA-4948 is blood-brain barrier penetrant and has single-agent activity against CNS lymphoma and melanoma brain metastases. Clin Cancer Res. 2023;29(9):1751–1762. doi:10.1158/1078-0432.CCR-22-1682
- Ferreri AJM, Calimeri T, Lopedote P, et al. Clinical relevance of MYD88 L265P mutation and interleukin-10 level in cerebrospinal fluid of patients with both newly diagnosed and relapsed primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2019;134(Suppl. 1):4114–4114. doi:10.1182/blood-2019-128298
- Watanabe J, Natsumeda M, Okada M, et al. High detection rate of MYD88 mutations in cerebrospinal fluid from patients with CNS lymphomas. J Clin Oncol Precis Oncol. 2019;3:1–13. doi:10.1200/PO.18.00308
- Von Roemeling C, Iqbal M, Doonan B, et al. P1298: the IRAK-4 inhibitor emavusertib (CA-4948) for the treatment of primary CNS lymphoma. Hemasphere. 2022;6(Suppl).:1183–1184. doi:10.1097/01.HS9.0000848056.06353.f1
- Guidetti F, Arribas AJ, Sartori G, et al. Targeting IRAK4 with emavusertib in lymphoma models with secondary resistance to PI3K and BTK inhibitors. J Clin Med. 2023;12(2):399. doi:10.3390/jcm12020399
- Guidetti F, Arribas AJ, Cannas E, et al. The IRAK4 inhibitor emavusertib (CA-4948) synergizes with second generation BTK inhibitors acalabrutinib and zanubrutinib in MYD88-L265P mutated lymphoma cell lines. Mol Cancer Ther. 2023;22(12 Suppl.):C139. doi:10.1158/1535-7163.TARG-23-C139
- Grommes C, Tun H, Rosenthal AC, et al. Takeaim lymphoma: an open-label, dose escalation and expansion trial of emavusertib (CA-4948) in combination with ibrutinib in patients with relapsed or refractory hematologic malignancies. Blood. 2023;142(Suppl. 1):4497–4497. doi:10.1182/blood-2023-189746
- Zhai Y, Zhou X, Wang X. Novel insights into the biomarkers and therapies for primary central nervous system lymphoma. Ther Adv Med Oncol. 2022;14:17588359221093745. doi:10.1177/17588359221093745
- Hoang-Xuan K, Houot R, Soussain C, et al. First results of the AcSé pembrolizumab phase II in the primary CNS lymphoma (PCNSL) cohort. Blood. 2020;136(Suppl. 1):15–16. doi:10.1182/blood-2020-141773
- Rimsza LM, Roberts RA, Miller TP, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the leukemia and lymphoma molecular profiling project. Blood. 2004;103(11):4251–4258. doi:10.1182/blood-2003-07-2365
- Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. doi:10.1182/blood-2017-01-764209
- Wu X, Zhang X, Xun R, et al. Efficacy and safety of axicabtagene ciloleucel and tisagenlecleucel administration in lymphoma patients with secondary CNS involvement: a systematic review. Front Immunol. 2021;12:693200. doi:10.3389/fimmu.2021.693200
- Frigault MJ, Dietrich J, Gallagher K, et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022;139(15):2306–2315. doi:10.1182/blood.2021014738
- Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–866. doi:10.1182/blood.2019001694
- Alcantara M, Houillier C, Blonski M, et al. CAR T-cell therapy in primary central nervous system lymphoma: the clinical experience of the French LOC network. Blood. 2022;139(5):792–796. doi:10.1182/blood.2021012932
- Siddiqi T, Wang X, Blanchard MS, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 2021;5(20):4059–4063. doi:10.1182/bloodadvances.2020004106
- Plaks V, Rossi JM, Chou J, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021;138(12):1081–1085. doi:10.1182/blood.2021010930
- Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20(6):359–371. doi:10.1038/s41571-023-00754-1
- Tu S, Zhou X, Guo Z, et al. CD19 and CD70 dual-target chimeric antigen receptor T-cell therapy for the treatment of relapsed and refractory primary central nervous system diffuse large B-cell lymphoma. Front Oncol. 2019;9:1350. doi:10.3389/fonc.2019.01350
- Li Z, Qiu Y, Personett D, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One. 2013;8(8):e71754. doi:10.1371/journal.pone.0071754
- Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595–1607. doi:10.1182/bloodadvances.2017014845
- Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective “proof of concept” phase II study of the French Oculo-Cerebral lymphoma (LOC) network and the lymphoma study association (LYSA). Ann Oncol. 2019;30(4):621–628. doi:10.1093/annonc/mdz032
- Tun HW, Johnston PB, DeAngelis LM, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. 2018;132(21):2240–2248. doi:10.1182/blood-2018-02-835496
- Korfel A, Schlegel U, Herrlinger U, et al. Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34(15):1757–1763. doi:10.1200/JCO.2015.64.9897
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23(18):3151–3171. doi:10.1038/sj.onc.1207542
- Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: the University of Chicago phase II consortium. J Clin Oncol. 2010;28(31):4740–4746. doi:10.1200/JCO.2010.29.2813
- Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822–3829. doi:10.1200/JCO.2008.20.7977
- Grommes C, Pentsova E, Nolan C, et al. Phase II study of single agent buparlisib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL). Ann Oncol. 2016;27:vi106. doi:10.1093/annonc/mdw367.13

