Abstract
Cytokine release syndrome (CRS) occurs frequently after haplo-identical allogeneic stem cell transplantation (alloSCT) with post-transplant cyclophosphamide (PTCy), increasing nonrelapse mortality (NRM) and decreasing survival. Data on CRS in HLA-matched alloSCT are limited and effects of specific HLA-mismatches on CRS development unknown. We hypothesized that in HLA-matched alloSCT increasing degrees of HLA-mismatching influence CRS incidence, NRM and survival. Retrospective analysis of 126 HLA-matched PTCy-alloSCT patients showed that higher degrees of HLA-mismatching significantly increased CRS incidence (26%, 75% and 90% CRS with 12/12, 10/10 and 9/10 matched donors, respectively). Maximum temperature during CRS increased with higher HLA-mismatch. Specific associations between HLA-mismatches and CRS could be determined. Grade 2 CRS and CRS-induced grade 3 fever were associated with significantly increased NRM (p < 0.001 and p = 0.003, respectively) and inferior survival (p < 0.001 and p = 0.005, respectively). NRM was mainly caused by disease conditions that may be considered CRS-induced inflammatory responses (encephalopathy, cryptogenic organizing pneumonia and multi-organ failure).
Introduction
Post-transplant cyclophosphamide (PTCy) administered at days 3 and 4 after allogeneic stem cell transplantation (alloSCT) effectively reduces the incidence of severe acute and chronic graft-versus-host disease (GVHD) [Citation1,Citation2]. PTCy most likely acts through different working mechanisms, including reduction of alloreactive T-cell proliferation, functional impairment of surviving alloreactive T cells and preferential recovery of regulatory T cells [Citation3,Citation4]. Also, when using PTCy early immune reconstitution takes place, most likely by naïve donor T cells [Citation4].
In haplo-identical PTCy-alloSCT, fever of unknown origin can develop early after the infusion of the unmanipulated graft, which typically starts in the first 5 days after transplantation and quickly resolves upon administration of PTCy [Citation5–22]. It is believed to be caused by an alloreactive immune response inducing a severe systemic inflammatory reaction with the development of a cytokine release syndrome (CRS). Significant increases in multiple cytokines, such as interferon gamma, interleukin (IL) 6, IL2, IL2 receptor, IL8, IL10, IL17, and tumor necrosis factor (TNF) have been observed during this CRS [Citation21].
In haploidentical PTCy-alloSCT, CRS develops in 73–94% of patients [Citation5–22]. Literature on CRS in PTCy-alloSCT with matched related donors (MRD) and matched unrelated donors (MUD) is limited [Citation9,Citation23], hinting toward a much lower CRS incidence in patients transplanted with 10/10 HLA-matched donors (14–23%). One study showed that in a mixed group of 29 patients transplanted with a 7/10, 8/10, or 9/10 MUD, incidences of CRS approached those seen after haplo-SCT (62% grade 1 CRS and 3% grade 2 CRS) [Citation23].
When experiencing CRS, most patients develop mild grade 1–2 CRS (fever with or without hypoxia or hypotension, responding to therapy). In haploidentical PTCy-alloSCT 12–17% of patients develop severe grade 3–4 CRS, occurring more in patients transplanted with peripheral blood versus bone marrow grafts [Citation8,Citation11,Citation13,Citation19,Citation22]. Severe CRS is a life-threatening complication necessitating Intensive Care Unit support. In haploidentical PTCy-alloSCT, severe CRS is associated with increased NRM and reduced overall survival (OS) [Citation6,Citation12,Citation13,Citation19]. Interestingly, in PTCy-alloSCT with a 10/10 HLA match, the development of CRS appears to have no negative effects on NRM and OS [Citation9,Citation23].
Although these studies together give the impression that incidence and severity of CRS increases in transplantations with higher degree of HLA mismatch between patient and donor, specific data on the effects of the number and different types of HLA mismatches on CRS are not available. Furthermore, it is unknown whether HLA mismatches that are generally accepted and are considered to be less important in alloSCT (HLA-DPB1 and HLA-DRB3, 4 and 5) influence CRS development.
Our center performs PTCy-alloSCT using either 12/12 MRD or MUD with a 9/10 or 10/10 match. We hypothesized that in these patients increasing degrees of HLA-mismatching influence CRS incidence and severity, NRM and survival. Therefore, we retrospectively analyzed a cohort of 126 transplanted patients to determine effects of different HLA mismatches on the development of CRS after PTCy-alloSCT.
Subjects and methods
Study population and treatment protocols
All consecutive patients who underwent myeloablative or nonmyeloablative alloSCT with PTCy GVHD prophylaxis using a MRD or a 9/10 or 10/10 MUD at the Leiden University Medical Center between March 2020 and July 2022 were included. Donors and patients were molecularly HLA typed for HLA-A, B, C, DR, DQ and DP loci. Donor–patient matching was performed on HLA-A, B, C, DR, DQ according to common practice, disregarding mismatches in HLA-DPB1 and HLA-DRB3/4/5. For this analysis, we categorized HLA-DPB1 mismatches into permissive and non-permissive according to the IMGT/HLA-matching algorithm version 2.0 (TCE-4) (https://www.ebi.ac.uk/ipd/imgt/hla/matching/) [Citation24,Citation25].
All patients gave written informed consent for the treatment and scientific evaluation before transplantation and data collection. Data analysis was approved by the LUMC Biobank Review Committee (RP22.002) and performed as of August 2023.
Transplantation protocol
Patients over 50 years were transplanted with a nonmyeloablative conditioning regimen consisting of cyclophosphamide, fludarabine and low dose TBI [Citation1], patients up to 50 years with a myeloablative conditioning regimen consisting of thiotepa, busulfan and fludarabine [Citation26]. All patients received peripheral blood grafts. GVHD prophylaxis consisted of PTCy (40 mg/kg) twice administered 72 and 96 h after SCT, mycophenolate 15 mg/kg three times per day between day +5 and +28, and tacrolimus twice daily 0.04 mg/kg starting on day +5. Filgrastim 5 ug/kg/day was started on day +5 until neutrophil recovery. Rapid tapering of tacrolimus started on day +70 in patients transplanted with a 10/10 or 12/12 matched donor and on day +90 in patients transplanted with a 9/10 matched donor. All patients received standard supportive care including antimicrobial prophylaxis. Patients with fever > 38.5°C received antipyretic therapy with acetaminophen. All patients were scheduled to receive prophylactic donor lymphocyte infusion at 6 months after transplantation in the absence of active graft-versus-host-disease (GVHD), poor risk patients were scheduled for additional donor lymphocyte infusion one month after tacrolimus discontinuation.
Study endpoints and definitions
The primary objective of the study was to determine the effect of different degrees of HLA mismatching on the incidence and severity of CRS. The secondary objective was to determine the effect of CRS severity on NRM and OS.
Fever was defined as a single temperature measurement of 38.3°C or higher or sustained temperature of greater than 38°C lasting for at least 1 h. CRS was graded according to published criteria using the ASTCT consensus for fever and CRS manifestations [Citation27]. Fever was graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 5 [Citation28]. A diagnosis of CRS was made when patients developed fever in the first 5 days after transplantation and infectious etiology of this fever was ruled out by comprehensive clinical, radiographic, microbiological, and laboratory analysis. Graft versus host disease (GVHD) was diagnosed and graded according to standard criteria [Citation29,Citation30]. Refined disease risk index (DRI), comorbidity index (HCT-CI) and EBMT risk score were determined as published [Citation31–33].
Statistical analysis
Categorical variables were compared by Fisher’s exact test. Continuous variables were compared between groups by two-tailed Mann–Whitney test or Kruskal–Wallis test. Cumulative incidence was used to estimate endpoints of neutrophil and platelet engraftment, acute and chronic GVHD, NRM, relapse, cryptogenic organizing pneumonia (COP) and encephalopathy. For each endpoint, death without the endpoint of interest was the competing event. Associations with fever and CRS were tested using Gray’s test. The Kaplan–Meier method was used to determine probabilities of overall survival (OS), relapse-free survival (RFS) and GVHD and relapse free survival (GRFS) [Citation34]. The log-rank test was used to compare OS by CRS grade and maximum grade fever during CRS. Since all cases of CRS and fever took place before the first event in the dataset occurred, these variables could be treated as time-fixed (i.e. known at the moment of transplantation) without causing immortal time bias.
Multivariable analysis was performed to adjust for potential confounders to obtain a net effect of CRS and fever during CRS on OS. This analysis could not be performed on NRM because of the limited number of NRM cases (n = 15) [Citation35]. Because it is conceivable that many different patient (age, CMV status, type of disease, remission status, co-morbidities), donor (age, HLA mismatch, patient-donor sex mismatch) and transplant variables (type of conditioning, cell number, freezing of graft) may affect the remaining T cell repertoire and/or vigorousness of T-cell responses early after transplantation and thereby influence CRS occurrence, the impact of these variables on OS and NRM was first assessed. Variables showing difference in OS with a significance level of p < 0.10 were entered into a multivariable Cox regression model. Results were expressed as hazard ratios (HR) with 95% confidence intervals (95% CI).
For statistical analysis R 4.1.2 (packages ‘survival’, ‘prodlim’ and ‘cmprsk’) and IBM SPSS version 25 were used.
Results
Patient and transplantation characteristics
Between April 2020 and July 2022, 133 consecutive patients received PTCy-alloSCT. Seven patients showed evidence of infection within the first 5 days after SCT (three cases of pneumonia, one phlebitis, one cellulitis of the eye, one cellulitis of the skin, one rhinoviral infection). Since clinically manifest infections are a confounding cause for the development of fever, these seven patients were excluded from further analysis.
Characteristics of the remaining 126 patients are shown in . Median age was 61 years (range 19–77), 71% of patients were male, 76% of patients were transplanted for acute leukemia or myelodysplastic syndrome. All patients received peripheral blood grafts (median 6.9 × 10^6/kg, range 1.9–6.5). Twenty patients (16%) were transplanted with a sibling related donor and 106 (84%) with an unrelated donor. Median follow-up in alive patients was 720 days (range 382–1196). Transplantation outcomes are shown in Supplemental Table 1.
Table 1. Patient, donor, disease and transplant characteristics.
The effect of HLA matching on CRS incidence and severity
For the primary and secondary endpoint analysis, patients were categorized in three different HLA-matching groups, a 9/10 group (38 patients transplanted with 9/10 MUD and 1 with a related donor with a single mismatch), a 10/10 group (60 patients transplanted with 10/10 MUD with one or two HLA-DP mismatches) and a 12/12 group (19 patients transplanted with MRD and 8 with 10/10 MUD without HLA-DP mismatches).
Eighty-six of the 126 transplanted patients (68%) developed fever in the first 5 days after transplantation without evidence of active infection. Seventy-five patients (87%) experienced only fever (grade 1 CRS) and 11 patients (13%) developed grade 2 CRS (fever combined with hypotension requiring intravenous fluids and/or hypoxia requiring oxygen therapy). No grade 3-4 CRS was observed.
The incidence of CRS after transplantation increased with higher degrees of HLA mismatching between patient and donor (12/12, 10/10 or 9/10) (). Significantly more patients with a 10/10 or 9/10 match (75% and 87%, respectively) developed CRS compared to patients with a 12/12 match (26%) (). Also, the incidence of CRS grade 2 was significantly higher in patients with a 9/10 matched (26%) compared to a 10/10 (2%) or 12/12 matched donor (0%) (Fisher’s exact p < 0.001 and p = 0.004, respectively).
Figure 1. Significant effects of HLA matching differences on CRS and maximum temperature developing after PTCy-alloSCT in patients transplanted with 12/12, 10/10 and 9/10 matched donors.
Horizontal bars show median values.
A) Differences in CRS. P-values indicate differences between groups with combined CRS grade 1 and 2 by Fisher’s exact test.
B) Differences in maximum temperature. Temperatures of 37, 38, 39, 40 and 41°C correspond with 98.6, 100.4, 102.2, 104 and 105.8°F, respectively. P-values indicate differences between groups by Kruskal-Wallis test with pairwise comparisons after Bonferroni correction (global p-value p < 0.001).
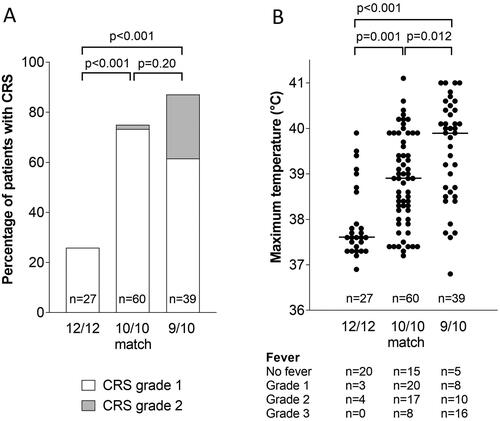
Because 87% of patients developing CRS experienced the same severity of CRS (grade 1), we next used the maximum temperature developing during CRS to bring more differentiation within this group with regard to CRS severity. Median maximum temperature was lowest in 12/12 matched patients (37.6°C) and significantly increased in 10/10 (38.9°C) and 9/10 (39.9°C) matched patients (). In particular, grade 3 fever (>40°C) occurred predominantly in patients with a 9/10 match (16 of 39 patients, 41%), significantly more than in the 12/12 (0 of 27 patients) and 10/10 matched groups (8 of 60 patients, 13%) (Fisher’s Exact p < 0.001 and p = 0.003, respectively).
The effects of HLA matching on fever were further analyzed (). In 12/12 matched patients, no clear difference between patients transplanted with a related or unrelated donor was observed (median maximum temperatures 37.6 and 37.7°C). In this 12/12 matched group we observed 4 outliers with maximum temperatures above 39°C. The two 12/12 matched patients reaching the highest temperatures (39.5 and 39.9°C) were found to have an HLA-DRB3/4/5 mismatch, mismatches that are generally disregarded in patient-donor matching [Citation36]. However, in this 12/12 matched group also, a patient with an HLA-DRB3/4/5 mismatch was identified that did not develop fever.
Figure 2. Effects of differences in HLA matching on maximum temperature developing after alloSCT with PTCy.
Temperatures of 37, 38, 39, 40 and 41°C correspond with 98.6, 100.4, 102.2, 104 and 105.8°F, respectively. Horizontal bars show median values. Patients transplanted with 12/12 matched donors are subdivided in RD (related donor) and UD (unrelated donor). Patients transplanted with 10/10 matched donors are subdivided in DP P (HLA-DPB1 permissive mismatch) and DP NP (HLA-DPB1 non-permissive mismatch), two-sided Mann-Whitney shows significant difference between these groups. Patients transplanted with 9/10 matched donors are subdivided according to MM (mismatch) in HLA-A, B, C, DR (DRB1) or DQ (DQB1), Kruskal–Wallis test shows statistical difference within these groups (global p-value = 0.003), post hoc testing with Bonferroni adjustment shows significant difference between HLA-DRB1 and HLA-C subgroups.
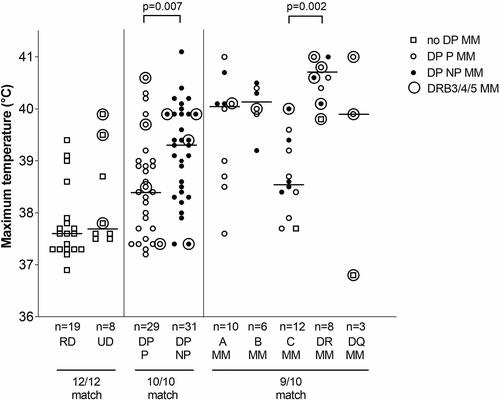
In the 10/10 matched group, patients with a nonpermissive DPB1-mismatch experienced significantly higher maximum temperatures than patients with a permissive DPB1-mismatch (median 39.3°C versus 38.4°C, p = 0.007). In the permissive group, 21% of patients developed grade 2-3 fever (>39°C), in the non-permissive group 61% (p = 0.002 Fischer’s Exact). HLA-DRB3/4/5 mismatches were observed in patients that developed high temperatures as well as in patients that did not.
In the 9/10 matched group, maximum temperatures were >39°C in all patients with HLA-DRB1 and HLA-B mismatches (median 40.6 and 40.2°C, respectively). Maximum temperatures were more variable in patients with HLA-A, HLA-DQB1 and HLA-C mismatches (median 40.1, 39.9 and 38.6°C, respectively) (). Statistical analysis showed significantly higher temperatures in the HLA-DRB1 group compared to the HLA-C group. Concomitant HLA-DRB3/4/5 mismatches were predominantly observed in the high maximum temperature ranges.
Because of the COVID pandemic approximately half of the grafts had been cryopreserved before infusion. Cryopreservation did not influence the incidence or severity of CRS (Supplemental Table 2). Also, the composition of the graft (CD34 and total nucleated cell number) did not influence CRS (Supplemental Figure 1). Rapid immune reconstitution occurred after transplantation, in a pattern similar as published [Citation37]. CRS only marginally slowed the immune reconstitution of CD4, CD8 and B-cells in the first weeks after transplantation (Supplemental Table 3).
Effect of CRS on clinical outcome after alloSCT with PTCy
Fifteen patients died from NRM, as specified in . Six of these patients (40%) had experienced grade 2 CRS, 8 of these patients (53%) CRS with grade 3 fever. In the majority of patients, this NRM occurred early after transplant with the underlying disorder being diagnosed already before day 100 (patients 1–11). As most frequent causes of death in these 11 patients, four cases of encephalopathy without identifiable cause and 2 cases of cryptogenic organizing pneumonia (COP) were observed, all in patients developing symptoms before day 100 after transplantation. In the 111 patients without NRM we could not identify any further encephalopathy cases, however two other cases of COP early after transplantation were identified; one patient at day +52 (after experiencing grade 3 fever with grade 2 CRS), another patient at day +75 (after experiencing grade 3 fever with grade 1 CRS). Both patients were successfully treated with prednisolone. In addition, two patients developed a chronic GVHD-related COP at days +207 and +438 after transplant (both had not experienced either grade 3 fever or grade 2 CRS).
Table 2. Description of patients with non-relapse mortality after PTCy-alloSCT.
The 1-year cumulative incidence of COP was 16% and 18.1% in patients experiencing grade 3 fever and grade 2 CRS, compared to 0% and 1.7% in patients experiencing grade 0–2 fever and grade 0–1 CRS, respectively. The 1-year cumulative incidence of encephalopathy was 12% and 18.1% in patients experiencing grade 3 fever and grade 2 CRS, compared to 1% and 1.7% in patients experiencing grade 0–2 fever and grade 0–1 CRS, respectively. Statistical analysis of all these differences was significant (p-values < 0.01), however considering the low number of COP and encephalopathy cases these results must be interpreted with caution.
Two of the NRM patients died from severe GVHD, both had not experienced CRS after transplant. None of the other NRM patients had developed acute or chronic GVHD (data not shown). Patients who developed CRS did not experience a higher incidence of acute or chronic GVHD in the first 180 days after transplantation (Supplemental Table 4).
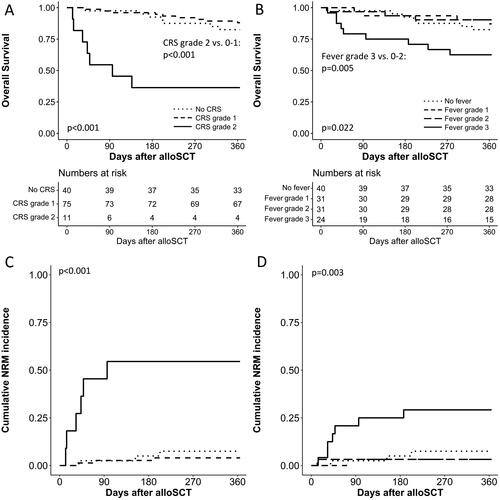
Grade 2 CRS was associated with a significantly inferior OS and increased NRM compared to grade 0-1 CRS (). Grade 3 fever occurring during CRS was also associated with a significantly inferior OS and increased NRM compared to grade 0–2 fever (). Relapse rates in patients with grade 3 fever (12% at day 180 (95% C.I. 9–24%), 16% at day 360 (95% C.I. 13–29%)) were not statistically different from patients with grade 0–2 fever (16% at day 180 (95% C.I. 3–28%), 21% at day 360 (95% C.I. 5–33%) (p = 0.562). Relapse rates in patients with grade 2 CRS (9% at day 180 (95% C.I. 0–37%), 9% at day 360 (95% C.I. 0–37%)) were not statistically different from patients with grade 0–1 CRS (16% at day 180 (95% C.I. 10–23%), 21% at day 360 (95% C.I. 14–28%) (p = 0.365). Relapse rates were also not statistically different between patients transplanted with HLA-matched versus HLA-mismatched grafts (p = 0.929).
Figure 3. Effect of the grade of CRS and maximum grade of fever during CRS on survival and non-relapse mortality (NRM) after PTCy-alloSCT.
Figures A and C show effects of grade of CRS on survival and NRM, figures B and D effects of maximum grade of fever during CRS on survival and NRM. Both for the log-rank test (survival) and for Grays’s test (NRM), global p-values are shown. Also p-values for the comparison of grade 3 fever versus grade 0-2 fever and grade 2 CRS versus grade 0-1 CRS are shown.
Univariable analysis of the impact of different variables known to influence OS, NRM or relapse was performed (Supplemental Figure 2). Patient age, donor age, disease activity at SCT, severity of CRS and grade of fever during CRS were significantly associated with 1-year OS. Severity of CRS, grade of fever during CRS and disease activity at SCT were the only variables to be significantly associated with NRM.
We fitted a multivariable Cox model for OS with the significant variables in the univariable analysis (). CRS and height of fever during CRS were analyzed separately because of the high correlation between these variables. CRS grade 2 (versus CRS grade 0–1) and fever grade 3 (versus fever grade 0–2) were associated with poorer OS with HR of 8.3 (95% CI: 3.2 to 21.8; p < 0.001) and 4.5 (95% CI: 1.9 to 11.2; p = 0.001), respectively.
Table 3. Multivariable analysis of overall survival.
Discussion
The hypothesis that in MRD/MUD PTCy-alloSCT, the incidence and severity of CRS are dependent on the degree of HLA-mismatching between patient and donor proved correct. Also, significantly increased NRM and inferior survival was observed in patients developing grade 2 CRS or CRS-induced grade 3 fever.
A low incidence of mild CRS with limited clinical relevance has been reported in MRD/MUD PTCy-alloSCT [Citation9,Citation23]. Our findings show that in patients transplanted with 9/10 and 10/10 MUDs, CRS incidence can be much higher, depending on the type of HLA-mismatch, and can influence NRM and survival. This is especially important considering the increasing popularity of PTCy-alloSCT in the matched related and unrelated setting. Furthermore, recognition of CRS as a possible cause for early NRM offers an opportunity for interventions to diminish NRM, either by choosing patient-donor HLA combinations with a low chance of inducing CRS or by using anti-inflammatory drugs, such as tocilizumab or dexamethasone. Successful administration of tocilizumab in patients with severe CRS after alloSCT has already been published [Citation6,Citation16,Citation18], but warrants confirmation in prospective trials to demonstrate subsequent reduction in NRM risk.
The use of the height of fever for CRS grading may have further clinical implications. An HLA-DPB1 mismatch permissiveness model based on in vivo and in vitro data has been built to improve donor selection [Citation25,Citation38,Citation39]. The observed heterogeneity in fever responses in patients transplanted with 10/10 matched donors with permissive and non-permissive HLA-DPB1 mismatches () may be used for further improvement of this model. Also, it may offer more information on the clinical relevance of specific HLA-DRB3/4/5 mismatches, mismatches generally considered to be unimportant for HLA matching.
The CRS incidence we observed in patients transplanted with 10/10 matched donors (75%) was much higher than the reported 14-23% from the literature. Our standardized temperature measurements multiple times a day may have resulted in a higher incidence. Alternatively, differences in HLA typing strategy may have played a role. We do not search for specific patient-donor HLA-DPB1 combinations; centers that specifically target for 10/10 matched donors with a HLA-DPB1 permissive match will experience lower CRS incidence.
Many patients died from conditions that may considered to be excessive inflammatory responses secondary to CRS (encephalopathy, COP, multi-organ failure). We observed 4 cases of COP, all associated with grade 3 fever, of which 50% died. COP normally has a median time of onset of 8.2 months after alloSCT (predominantly after 3 months) and is highly correlated with graft-versus-host disease [Citation40]. The COP patients described here already developed symptoms after a median 64 days in the absence of graft-versus-host-disease. We therefore think that this early CRS related COP is different from the already known post-transplantation COP.
Encephalopathy was observed in 4 of the NRM patients. Encephalopathy is the most common noninfectious neurological complication after transplant, occurring a median 28 days after alloSCT [Citation41]. Because we could not find an underlying cause and tacrolimus discontinuation had no effect, we postulate that the encephalopathy in our patients may have been caused by CRS. Clinically these patients resemble the immune effector cell-associated neurotoxicity syndrome (ICANS), which is frequently observed after CAR-T cell therapy and believed to be cytokine related [Citation42].
This study has several limitations. We showed with multivariable analysis that the observed effects of CRS and CRS-associated fever on OS were not influenced by confounding variables. Because of the limited number of patients and death events, this analysis was performed only with a limited set of variables obtained from univariable analysis. It would have been preferable to perform this analysis with an extended set of possible confounders. Also, our hypothesis that CRS-induced NRM is caused by CRS-induced inflammatory responses is based on a limited number of NRM cases.
Bias may have been introduced by the exclusion of 7 patients who were found to have an infection in the first days after alloSCT. Analysis with these patients included, as if they had also experienced CRS, resulted in essentially identical results and significance levels (data not shown).
Because of reported improved recovery and lower cyclophosphamide-associated complications, we used a lower dose of cyclophosphamide (80 mg/kg instead of 100 mg/kg) [Citation43,Citation44]. Because CRS develops before cyclophosphamide administration [Citation21], we do not think that CRS data were influenced by this lower dose. Furthermore, in the study of Duléry et al. the incidence of NRM was not significantly influenced by the use of a lower dose of cyclophosphamide [Citation44].
Author contributions
PvdB designed the study. BK and AB provided HLA data and interpretation. PvdB, JV and HV wrote the manuscript draft and final version. LW provided statistical advice and analysis. BK, LW, AB, TS, DL, JT, AS, MO, CH, PvB, WM and JT contributed to the manuscript and critically reviewed the final version.
Supplemental Material
Download Zip (1.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi:10.1016/j.bbmt.2008.03.005
- Williams L, Cirrone F, Cole K, et al. Post-transplantation cyclophosphamide: from HLA-haploidentical to matched-related and matched-unrelated donor blood and marrow transplantation. Front Immunol. 2020;11:636. doi:10.3389/fimmu.2020.00636
- Wachsmuth LP, Patterson MT, Eckhaus MA, et al. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;129(6):2357–2373. doi:10.1172/JCI124218
- Nunes NS, Kanakry CG. Mechanisms of graft-versus-host disease prevention by post-transplantation cyclophosphamide: an evolving understanding. Front Immunol. 2019;10:2668. doi:10.3389/fimmu.2019.02668 eCollection 2019.
- O'Donnell P, Raj K, Pagliuca A. High fever occurring 4 to 5 days post-transplant of haploidentical bone marrow or peripheral blood stem cells after reduced-intensity conditioning associated with the use of post-transplant cyclophosphamide as prophylaxis for graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(1):197–198. doi:10.1016/j.bbmt.2014.10.008
- Abboud R, Keller J, Slade M, et al. Severe cytokine-release syndrome after T cell-replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti-IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant. 2016;22(10):1851–1860. doi:10.1016/j.bbmt.2016.06.010
- Arango M, Combariza JF. Fever after peripheral blood stem cell infusion in haploidentical transplantation with post-transplant cyclophosphamide. Hematol Oncol Stem Cell Ther. 2017;10(2):79–84. doi:10.1016/j.hemonc.2017.03.001
- Raj RV, Hamadani M, Szabo A, et al. Peripheral blood grafts for T cell-replete haploidentical transplantation increase the incidence and severity of cytokine release syndrome. Biol Blood Marrow Transplant. 2018;24(8):1664–1670. doi:10.1016/j.bbmt.2018.04.010
- McCurdy SR, Muth ST, Tsai HL, et al. Early fever after haploidentical bone marrow transplantation correlates with class II HLA-mismatching and myeloablation but not outcomes. Biol Blood Marrow Transplant. 2018;24(10):2056–2064. doi:10.1016/j.bbmt.2018.06.004
- Sugita J, Kagaya Y, Miyamoto T, et al. Myeloablative and reduced-intensity conditioning in HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant. 2019;54(3):432–441. doi:10.1038/s41409-018-0279-1
- Solh MM, Dickhaus E, Solomon SR, et al. Fevers post infusion of T cell replete HLA mismatched haploidentical hematopoietic stem cells with posttransplant cyclophosphamide: risk factors and impact on transplant outcomes. Bone Marrow Transplant. 2019;54(11):1756–1763. doi:10.1038/s41409-019-0522-4
- Imus PH, Blackford AL, Bettinotti M, et al. Severe cytokine release syndrome after haploidentical peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(12):2431–2437. doi:10.1016/j.bbmt.2019.07.027
- Mariotti J, Taurino D, Marino F, et al. Pretransplant active disease status and HLA class II mismatching are associated with increased incidence and severity of cytokine release syndrome after haploidentical transplantation with posttransplant cyclophosphamide. Cancer Med. 2020;9(1):52–61. doi:10.1002/cam4.2607
- Abid MB, Hamadani M, Szabo A, et al. Severity of cytokine release syndrome and its association with infections after T cell-replete haploidentical related donor transplantation. Biol Blood Marrow Transplant. 2020;26(9):1670–1678. doi:10.1016/j.bbmt.2020.06.006
- Modi D, Albanyan O, Kim S, et al. Grade 3-4 cytokine release syndrome is associated with poor survival in haploidentical peripheral blood stem cell transplantation. Leuk Lymphoma. 2021;62(8):1982–1989. doi:10.1080/10428194.2021.1891231
- Jayakumar I, Uppuluri R, Lakshmanan C, et al. Risk-adapted therapy for the management of cytokine release syndrome in children undergoing unmanipulated haploidentical stem cell transplantation. Pediatr Transplant. 2021;25(5):e13964.
- Kurita N, Sakamoto T, Kato T, et al. Early administration of cyclosporine may reduce the incidence of cytokine release syndrome after HLA-haploidentical hematopoietic stem-cell transplantation with post-transplant cyclophosphamide. Ann Hematol. 2021;100(5):1295–1301. doi:10.1007/s00277-021-04439-6
- Cho C, Perales MA. Rapid identification of cytokine release syndrome after haploidentical PBSC transplantation and successful therapy with tocilizumab. Bone Marrow Transplant. 2016;51(12):1620–1621. doi:10.1038/bmt.2016.229
- Abboud R, Wan F, Mariotti J, et al. Cytokine release syndrome after haploidentical hematopoietic cell transplantation: an international multicenter analysis. Bone Marrow Transplant. 2021;56(11):2763–2770. doi:10.1038/s41409-021-01403-w
- Otoukesh S, Elmariah H, Yang D, et al. Cytokine release syndrome following peripheral blood stem cell haploidentical hematopoietic cell transplantation with post-transplantation cyclophosphamide. Transplant Cell Ther. 2022;28(2):111.e1-111–e8. doi:10.1016/j.jtct.2021.11.012
- Wang L, Dai B, Gao W, et al. Clinical significance of haplo-fever and cytokine profiling after graft infusion in allogeneic stem cell transplantation from haplo-identical donors. Front Med (Lausanne). 2022;9:820591. doi:10.3389/fmed.2022.820591
- Solán L, Landete E, Bailén R, et al. Cytokine release syndrome after allogeneic stem cell transplantation with posttransplant cyclophosphamide. Hematol Oncol. 2020;38(4):597–603. doi:10.1002/hon.2772
- Rappazzo KC, Zahurak M, Bettinotti M, et al. Nonmyeloablative, HLA-mismatched unrelated peripheral blood transplantation with high-dose post-transplantation cyclophosphamide. Transplant Cell Ther. 2021;27(11):909.e1-909–e6. doi:10.1016/j.jtct.2021.08.013
- Fernández-Viña MA, Klein JP, Haagenson M, et al. Multiple mismatches at the low expression HLA loci DP, DQ, and DRB3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood. 2013;121(22):4603–4610. doi:10.1182/blood-2013-02-481945
- Fleischhauer K, Shaw BE, Gooley T, et al. Effect of T-cell-epitope matching at HLA-DPB1 in recipients of unrelated-donor haemopoietic-cell transplantation: a retrospective study. Lancet Oncol. 2012;13(4):366–374. doi:10.1016/S1470-2045(12)70004-9
- Soltermann Y, Heim D, Medinger M, et al. Reduced dose of post-transplantation cyclophosphamide compared to ATG for graft-versus-host disease prophylaxis in recipients of mismatched unrelated donor hematopoietic cell transplantation: a single-center study. Ann Hematol. 2019;98(6):1485–1493. doi:10.1007/s00277-019-03673-3
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi:10.1016/j.bbmt.2018.12.758
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5. Published: november 27. US department of health and human services, national institutes of health. National Cancer Institute.
- Przepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1994;15(6):825–828. 1995
- Jagasia MH, Greinix HT, Arora M, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: i. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e1. doi:10.1016/j.bbmt.2014.12.001
- Armand P, Kim HT, Logan BR, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi:10.1182/blood-2014-01-552984
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi:10.1182/blood-2005-05-2004
- Gratwohl A. The EBMT risk score. Bone Marrow Transplant. 2012;47(6):749–756. doi:10.1038/bmt.2011.110
- Ruggeri A, Labopin M, Ciceri F, et al. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWPEBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51(4):610–611. doi:10.1038/bmt.2015.305
- Iacobelli S, On behalf of the EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the european group for blood and marrow transplantation. Bone Marrow Transplant. 2013; Mar48 Suppl 1: s 1–37. doi:10.1038/bmt.2012.282
- Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134(12):924–934. doi:10.1182/blood.2019001212
- Khimani F, Ranspach P, Elmariah H, et al. Increased infections and delayed CD4+ T cell but faster B cell immune reconstitution after post-transplantation cyclophosphamide compared to conventional GVHD prophylaxis in allogeneic transplantation. Transplant Cell Ther. 2021;27(11):940–948. doi:10.1016/j.jtct.2021.07.023
- Crivello P, Heinold A, Rebmann V, et al. Functional distance between recipient and donor HLA-DPB1 determines nonpermissive mismatches in unrelated HCT. Blood. 2016;128(1):120–129. doi:10.1182/blood-2015-12-686238
- van Balen P, Kester MGD, de Klerk W, et al. Immunopeptidome analysis of HLA-DPB1 allelic variants reveals new functional hierarchies. J Immunol. 2020;204(12):3273–3282. doi:10.4049/jimmunol.2000192
- Patel SS, Ahn KW, Khanal M, et al. Noninfectious pulmonary toxicity after allogeneic hematopoietic cell transplantation. Transplant Cell Ther. 2022;28(6):310–320. doi:10.1016/j.jtct.2022.03.015
- Balaguer-Rosello A, Bataller L, Piñana JL, et al. Noninfectious neurologic complications after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(9):1818–1824. doi:10.1016/j.bbmt.2019.05.024
- Gust J, Ponce R, Liles WC, et al. Cytokines in CAR T cell-associated neurotoxicity. Front Immunol. 2020; Dec 1611:577027. doi:10.3389/fimmu.2020.577027
- Sugita J, Kamimura T, Ishikawa T, et al. Reduced dose of posttransplant cyclophosphamide in HLA-haploidentical peripheral blood stem cell transplantation. Bone Marrow Transplant. 2021;56(3):596–604. doi:10.1038/s41409-020-01065-0
- Duléry R, Goudet C, Mannina D, et al. Reduced post-transplant cyclophosphamide doses in haploidentical hematopoietic cell transplantation for elderly patients with hematological malignancies. Bone Marrow Transplant. 2023;58(4):386–392. doi:10.1038/s41409-022-01908-y

