ABSTRACT
Objective
Reducing maternal morbidity and mortality has been a challenge for low and middle-income countries, especially in the setting of hypertensive disorders of pregnancy. Improved strategies for treating obstetric patients with resistant hypertension are needed. We sought to explore whether hemodynamic parameters may be used to identify patients that develop resistant hypertension in pregnancy.
Methods
Retrospective cohort study among pregnant patients with gestational hypertension or preeclampsia that experienced severe blood pressure elevations. Hemodynamic variables were evaluated, including cardiac output (CO), and total peripheral resistance (TPR). The primary endpoint was resistant hypertension. An exploratory logistic regression was performed to evaluate the association between the hemodynamic profile and the development of resistant hypertension. Adverse maternal and fetal outcomes were additionally described according to the presence of resistant hypertension.
Results
Fifty-seven patients with severe pregnancy hypertension were included, of whom 34 developed resistant hypertension (59.7%). The resistant hypertension group, in comparison to those without resistant hypertension, presented with a hypodynamic profile characterized by reduced CO < 5 L/min (41.2% vs. 8.7%, p: 0.007), and increased TPR > 1400 dyn-s/cm5 (64.7% vs. 39.1%, p: 0.057). Logistic regression analysis revealed an association between a hypodynamic profile and resistant hypertension (OR 3.252, 95% CI 1.079–9.804; p = 0.035). Newborns of the resistant hypertension group had more frequent low birth weight (<2500 g), low Apgar scores, ICU admissions, and acute respiratory distress syndrome.
Conclusion
Patients experiencing hypertensive crisis during pregnancy and exhibiting a hypodynamic profile (TPR ≥1400 dyn·s/cm5 and CO ≤ 5 L/min) developed higher rates of resistant hypertension.
Introduction
Maternal mortality rate is considered an indicator of a country’s level of development, and it is a global health priority to reduce this rate to less than 70 per 100,000 live births in alignment with the United Nations’ Sustainable Development Goals (Citation1). Despite current efforts, many low- and middle-income countries have not yet achieved the expected target for maternal mortality. The accomplishment of this objective is one of the most complex public health problems worldwide, primarily related to complications from hypertensive disorders of pregnancy, postpartum hemorrhage, and maternal sepsis (Citation2). Critical areas to address include the overall quality of obstetric care, standardization of clinical protocols, and management networks between hospitals (Citation3,Citation4).
Hypertensive disorders are the second leading cause of preventable maternal mortality worldwide (Citation5). Preeclampsia complications occur in 2 to 8% of all pregnancies, and at least 42,000 maternal deaths each year are related to complications of this disease (Citation6). In Colombia, preeclampsia is the leading cause of maternal death, accounting for 26.7% of deaths in 2022 (Citation7). Multiple interventions have been developed to either prevent or make an early diagnosis of hypertensive disorders in pregnancy. However, in cases where the disease is already established, strategies to avoid the development of severe disease and maternal complications are not fully effective (Citation8–10). Since patients in low and middle-income countries frequently present to care with established disease, prediction of disease severity and progression is critical to reducing maternal complications (Citation3,Citation4).
Hemodynamic variables can be assessed through noninvasive monitoring techniques, and such measures have proven useful to guide treatment in the setting of severe preeclampsia and hypertensive crisis (Citation11,Citation12). Hemodynamic assessment may reduce adverse maternal and perinatal outcomes by guiding use of a specific antihypertensive agent, with a mechanism of action that best addresses the identified hemodynamic profile (Citation13,Citation14).
One of the predictors of maternal morbidity is the development of “difficult-to-manage” or resistant hypertension. In the non-pregnant population, it is associated with the development of cardiovascular disease, chronic kidney disease and an increase in overall mortality (Citation15). However, resistant hypertension is an underused and poorly studied concept in obstetric population, mainly due to the lack of consensus on the diagnostic criteria. This study aims to explore whether hemodynamic parameters may be used to identify patients with severe preeclampsia that develop resistant hypertension, and to describe the proportion of adverse pregnancy outcomes related to resistant blood pressure status.
Methods
Design and population
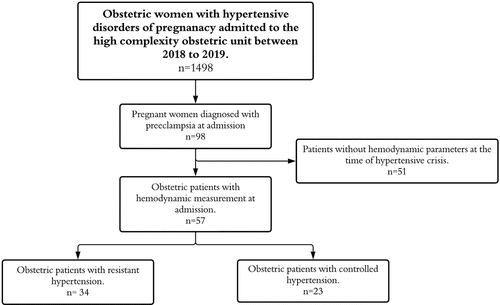
A retrospective cohort study was conducted among pregnant women who were diagnosed with preeclampsia or gestational hypertension according to the criteria established by the American College of Obstetrics and Gynecology (ACOG) and the International Society for the Study of Hypertension in Pregnancy (ISSHP) and who developed hypertensive crisis, which was defined as systolic blood pressure (SBP) equal to or greater than 160 mmHg and/or diastolic blood pressure (DBP) equal to or greater than 110 mmHg [9,10]. The patients included in the study were treated at the high complexity obstetric unit (HCOU) in Fundación Valle del Lili (Cali, Colombia) or at the ESE Clinica Maternidad Rafael Calvo (Cartagena, Colombia) between the years 2018 and 2019. Patients were excluded if they were in the early stages of pregnancy (<25 weeks gestation), had multifetal gestation, chronic hypertension, cardiovascular, metabolic, or hematologic comorbidities, or other conditions that could potentially influence the measured hemodynamic variables assessed by the bioreactance device (). The main exposure variable was the hemodynamic profile, and the main outcome variable was resistant hypertension status.
Ethical approval for the study protocol was obtained from the Institutional Ethics Committee (IRB No. 700–2022; Act No. 25 of 7 December 2022). Informed consent was not required, as this study was retrospective, and the data were already collected in the clinical records. The preparation of this article followed the STROBE guidelines of Enhancing the Quality and Transparency of Health Research (EQUATOR). (Additional file 1).
Hemodynamic evaluation and blood pressure measurement at hospital admission
A blood pressure recording was performed following a 10-minute rest period, considering the appropriate arterial cuff assignment based on each patient’s size and following the recommendations from the American College of Obstetrics and Gynecology (Citation16).
Subsequently, each patient underwent an initial hemodynamic evaluation using noninvasive transthoracic Bioreactance™ technology (Starling v5.5 device, Baxter International Inc., Deerfield, Illinois, USA). This device allows for the estimation of hemodynamic indices using thoracic bioreactance technology. The stroke volume is calculated by recording the relative phase changes that occur when an alternating electric current crosses the thorax; this is measured by placing electrodes on the anterior and posterior parts of the patient’s thorax, following the manufacturer’s instructions and considering the weight and height of the patients, which were measured at the time of admission. The participants remained in a semi-recumbent position immobile for two to three minutes while calibration and recording were performed. The hemodynamic variables of primary interest were stroke volume (SV), cardiac output (CO), and total peripheral resistance (TPR).
Blood pressure control and management during follow-up
Uncontrolled hypertensive crisis during follow-up was determined as persistent SBP ≥160 mmHg and/or DBP ≥110 mmHg after the management with two first-line medications (intravenous labetalol, fast acting oral nifedipine or hydralazine) following the treatment algorithm described in the ACOG Severe Hypertension intervention package, or the occurrence of a new episode of hypertensive crisis within 24 hours (Citation15,Citation17). Urapidil and ketanserin are not currently available in Colombia for the treatment of hypertensive crises.
After the acute management of the hypertensive crisis, treatment with long-acting antihypertensives such as extended-release nifedipine, alpha-methyldopa, or metoprolol were used to maintain a long-lasting effect. In addition, ACE inhibitors (captopril), calcium channel blockers (long acting nifedipine), and beta-blockers (propranolol) were used during the postpartum period. In patients with difficult blood pressure control, prazosin, furosemide, or clonidine were added to the treatment regimen. Patients requiring more than two long-acting antihypertensive medications to achieve blood pressure goals of SBP <150 mm Hg and a DBP <100 mm Hg throughout hospitalization were considered to have resistant hypertension.
Variables and data collection
Sociodemographic information, personal history, clinical characteristics of the current pregnancy, hemodynamic variables, and perinatal and maternal outcomes were collected, including information on organ dysfunction (Supplementary table S1). Multiorgan dysfunction was defined as more than 2 organs dysfunction. Data were obtained from the medical records and recorded in a digital database (BDClinic software) by trained personnel. At the same time, another investigator performed quality audits by cross-checking random information with the corresponding medical records.
Statistical analysis
Initially, a descriptive analysis was conducted in the entire study population. Qualitative variables were presented in both absolute and relative frequencies. The parametric distribution of quantitative variables was ascertained using the Shapiro-Wilk test and reported as means and medians with standard deviation (SD) or interquartile ranges (IQR) as appropriate. A bivariate analysis was subsequently executed based on the presence of resistant hypertension. Medians and IQR were used and compared using the Wilcoxon Mann-Whitney test. Qualitative variables were contrasted utilizing the Chi-square or Fisher’s exact test, contingent upon pending on the expected count of subjects in each category.
Considering the anticipated small sample size, an exploratory univariate logistic regression analysis was proposed. Dichotomous cutoff points were set for CO and TPR variables to delineate the hypodynamic profile, drawing upon prior literature (a CO <5 L/min and TPR > 1400 dyn·s/cm^5 (Citation13). In estimating the odds ratios (OR) with a 95% confidence interval (CI), the hypodynamic profile was designated as the independent variable. At the same time, the occurrence of resistant hypertension was treated as the dependent variable. A p-value less than 0.05 was deemed statistically significant at a 95% confidence level. Data analysis was executed using the Stata 18.0 software (Stata Corp, Texas, USA).
Results
Of the 1498 patients admitted with a diagnosis of hypertensive disorders related to pregnancy during the study period, 98 had hypertensive crisis (). Within this group, 41 were excluded as they did not have hemodynamic parameters. A total of 57 patients were included in the final analysis, of whom 34 cases developed resistant hypertension (59.7%). summarizes the sociodemographic characteristics of the population evaluated; maternal age older than 35 years was found to be more frequent in the resistant hypertension group (0, 0% vs. 7, 20.6%, p = 0.034) as well as gestational age less than 34 weeks (6, 26.1% vs. 26, 76.5%, p < 0.001).
Table 1. Sociodemographic characteristics according to the presence of resistant hypertension in women with severe preeclampsia hospitalized between 2018 and 2019.
In terms of hemodynamic parameters at admission (), the resistant hypertension group exhibited lower CO values than the nonresistant hypertension group (5.70 L/min [IQR 4.20–7.10] vs. 6.50 L/min [IQR 5.70–8.20]; p = 0.028) and elevated TPR values (1489.00 [IQR 1295.00–2026.00] vs. 1364.00 dyn-s/cm-5 [IQR 1150.00–1500.00]; p = 0.032). When setting dichotomous cutoff points for CO and TPR, 15 patients were identified with a hypodynamic profile (CO <5 L/min and TPR > 1400 dyn-s/cm-5). Of these, 13 (86.6%) belonged to the resistant group (). An exploratory logistic regression analysis revealed an association between the presence of a hypodynamic profile and resistant hypertension (OR 3.252, 95% CI 1.079–9.804; p = 0.035).
Figure 2. Scatter plot distribution among patients with resistant and nonresistant postpartum hypertension.
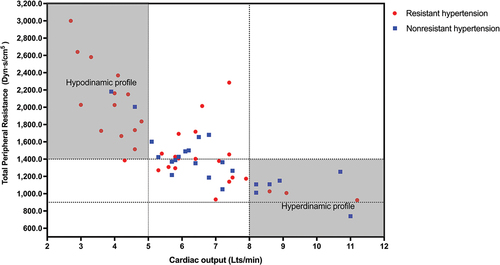
Table 2. Hemodynamic parameters according to the presence of resistant hypertension in women with severe preeclampsia at hospital admission from 2018 to 2019.
The proportion of patients with uncontrolled hypertensive crisis did not show statistically significant differences between the groups. However, it was nearly 10% more frequent in the nonresistant hypertension group, possibly associated with the need for earlier gestational termination with repercussions on the initial hemodynamic profile. On the other hand, in the resistant hypertension group, there was a higher proportion of patients who required intravenous vasodilator management with nitroprusside to achieve control of hypertensive crisis. Severe maternal outcomes including eclampsia, severe obstetric hemorrhage, acute renal failure, and organ dysfunction were also more frequent in the resistant hypertension group. No maternal deaths were recorded in either group ().
Table 3. Maternal complications according to the presence of resistant hypertension in women with severe preeclampsia hospitalized between 2018 and 2019.
Finally, there was evidence of greater neonatal morbidity in the resistant hypertension group, with a greater number of neonates with low birth weight (<2500 g), a lower Apgar score at one minute and 5 minutes after birth, ICU requirement, and Acute Respiratory Distress Syndrome (ARDS). Additionally, this group had more neonatal deaths ().
Table 4. Neonatal complications according to the presence of resistant hypertension in women with severe preeclampsia and hospitalized between 2018 and 2019.
Discussion
This study identified that patients with resistant hypertension during the hypertensive crisis, presented a hypodynamic profile characterized by low CO and high TPR more frequently than patients who achieved blood pressure goals with two or less antihypertensive medications, throughout hospitalization. The resistant hypertensive group showed higher rates of maternal and neonatal morbidity compared to the nonresistant counterparts.
It is widely recognized that the primary causes of severe hypertension in pregnancy are preeclampsia and chronic hypertension. The main reason for maintaining a target blood pressure is to prevent maternal cerebrovascular and cardiovascular complications and to enable delay of delivery (Citation18,Citation19). Despite advances in understanding the pathophysiology of these conditions, integrating this knowledge into current protocols and management guidelines remains deficient, potentially contributing to the persistently high rates of morbidity and mortality associated with hypertensive disorders of pregnancy.
Blood pressure reduction in hypertensive crisis is essential in pregnancy due to the potentially negative impact of sustained elevated blood pressure on cerebral vascular homeostasis. Autoregulation of cerebral circulation is a mechanism designed to maintain constant perfusion pressure in response to changes in systemic pressure mediated by myogenic, neurogenic, metabolic, or endothelial factors (Citation20). This mechanism involves protective vasoconstriction of cerebral vessels in the presence of elevated blood pressure. During pregnancy, the increase in plasma volume and CO promotes the adaptation of cerebral circulation distinct from other organs to maintain stable cerebral pressure. In preeclampsia, there is a lack of endothelial remodeling as an adaptative response to hypertension, making cerebral blood vessels more susceptible to blood-brain barrier (BBB) disruption. The failure of autoregulation during severe hypertension leads to increased hydrostatic pressure, reduced cerebral vascular resistance, and damage to the microvasculature, resulting in increased BBB permeability, microbleeds, focal cerebral edema, neuroinflammation, and neuronal impairment (Citation21).
To identify cerebrovascular dysregulation at an early stage and prevent its effects, new monitoring methods have been developed, focusing on hemodynamic assessment as a variable that reflects the dynamism of myogenic and neurogenic factors. Valensise H et al. initially proposed the hypothesis of different hemodynamic origins and behaviors in preeclampsia (Citation22). More recently, Gyselaers explains the deterioration process of overall circulatory function through noninvasive assessment of maternal cardiovascular function, categorizing this condition in two profiles, one characterized by the predominance of elevated CO and the other by the predominance of elevated TPR (Citation23). These profiles correlate with the development of venous hemodynamic dysfunction in scenarios of gestational hypertension, early and late-onset preeclampsia (Citation23).
We found that patients with hypodynamic profile showed an increased odd of resistant hypertension, which in turn presented a lower gestational age at the end of pregnancy. As expected, newborns of the resistant hypertension group had worse perinatal outcomes, lower birth weights, lower APGAR scores, and higher rates of admission to the neonatal ICU and ARDS. Complications and hemodynamic profiles in the resistant hypertension group were similar to that observed in early-onset preeclampsia (Citation22,Citation23).
It has been previously shown that patients with early-onset preeclampsia often have a hypodynamic profile characterized by low CO and high TPR; these findings can be observed as early as the first trimester (Citation23). Elvira Di Pasquo et al. found that women with preeclampsia who did not initially meet severity criteria but progressed to severe preeclampsia were characterized by lower CO and higher TPR (Citation24). This result is consistent with our findings and suggests a possible correlation between a hypodynamic profile and a higher disease burden which necessitates earlier delivery.
Considering the dynamism of variables such as CO and TPR in specific populations, and their role as critical factors in preserving autoregulation of cerebral perfusion, there is a strong rationale for their use in the management of hypertensive crisis and resistant hypertension (Citation14). However, only a few studies have specifically incorporated CO and TPR measures in the management of pregnant women receiving antihypertensive therapy. Stott et al. and Vasapollo et al. describe the implications of guided and stratified antihypertensive management based on this approach, proposing a therapeutic strategy focused on modulating TPR rather than solely reducing arterial pressures (Citation11,Citation25,Citation26). These studies demonstrate that patients with early-onset hypertensive disorders, who exhibit an hypodynamic profile, experience a reduction in maternal and fetal complications when TPR is reduced through combined use of nitric oxide donors and nifedipine.
To date, there is limited evidence regarding hemodynamic profiles in the Latin pregnant population, including the context of hypertensive disorders of pregnancy (Citation11,Citation25). Bioreactance represents a noninvasive and low-cost tool that could change the outcome of maternal morbidity and mortality in low- and middle-income countries by allowing a more rigorous study of hemodynamic profiles and their impact on the intervention of high blood pressure levels that are difficult to manage, as is the case of resistant hypertension (Citation13,Citation23).
Regarding resistant hypertension, this term does not have a strict definition in the obstetric patient or a consensus for its applicability within current management guidelines (Citation8,Citation27). However, we believe it is relevant to consider this term, not only due to its possible association with a worse prognostic profile, but also in considering the limitations and additional costs involved in the delayed discharge and rigorous postpartum follow-up of patients with difficult-to-manage blood pressure levels. This group may also be at greater risk of long-term morbidity from cardiovascular complications.
Definition of resistant hypertension differs in obstetric and non-obstetric populations, and its diagnosis may have different repercussions on morbidity and mortality. However, it is important to highlight that the obstetric population does not have the same variety of antihypertensive drugs evaluated and approved for managing high blood pressure compared to the general population. In addition, it should be considered that access to antihypertensive drugs with higher potency, longer half-life, and better safety profiles is sometimes limited, mainly in low- and middle-income countries.
Our findings contribute to a better understanding of the disease’s natural history and associated complications. However, we recognize some limitations of our study. In particular, the small sample size does not allow us to make generalizable associations concerning resistant hypertension in obstetric patients. Nevertheless, this evaluation will enable us to delve deeper into the behavior of hemodynamic profiles in our population and to set way for future studies and analyses in this area.
Although standardized management approaches for patients with severe hypertension or hypertensive crisis may have great utility for acute intervention and stabilization of the emergency, it is also important to consider new therapeutic proposals developed for higher-risk populations that ultimately require management in high complexity units. The integration of both clinical (maternal age, ethnicity, and resistant hypertension) and paraclinical (angiogenic biomarkers, hemodynamic variables, fetoplacental Doppler) measures directed by a logical application of what we understand of the pathophysiology and natural history of the disease, taking into account the time of onset (early or late onset), will be the key to develop specific algorithms for treatment and long-term follow-up (Citation28).
Conclusion
Patients experiencing hypertensive crisis during pregnancy and presenting with a hypodynamic profile (TPR ≥1400 dyn·s/cm5 and CO ≤5 L/min) developed higher rates of resistant hypertension. Additionally, the resistant hypertensive group showed higher rates of maternal complications, small for gestational age and low-birth weight newborns (<2500 gms), who required admission to the neonatal ICU compared to those without resistant hypertension. Further studies are warranted to validate the clinical utility of bioreactance in the management of hypertensive crisis in pregnancy.
Authors’ contributions
María F. Escobar, Javier A. Carvajal, y María P. Echavarría conceived the idea and collaborated in drafting the protocol. María A. Zambrano, Laura Sofia Gutierrez-Puerto, and Felipe Aguilar-Cano drafted the protocol and collected the data. José A. Rojas, Jose A. Santacruz, and Merida Rodriguez analyzed and interpreted the data. Evelyn E. Peña y María A. Zambrano wrote the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author (MFE), upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641955.2023.2272176
Additional information
Funding
References
- World Health Organization [Internet]. [citado 6 de sep 2023]. SDG Target 3.1 Maternal Mortality. https://www.who.int/data/gho/data/themes/topics/sdg-target-3-1-maternal-mortality
- UNICEF. Maternal mortality rates and statistics - [internet]. 2023 [citado 17 de jul 2023]. https://data.unicef.org/topic/maternal-health/maternal-mortality/
- Chakhtoura N, Chinn JJ, Grantz KL, et al. Importance of research in reducing maternal morbidity and mortality rates. Am J Obstet Gynecol. 2019 sep;221(3):179–9. doi: 10.1016/j.ajog.2019.05.050
- Belizán JM, Gibbons L, Cormick G. Maternal mortality reduction: a need to focus actions on the prevention of hypertensive disorders of pregnancy. Int J Equity Health. 2021 ago 28 de;20(1):194.
- Wang W, Xie X, Yuan T, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population‐based study. Bmc Pregnancy Childbirth. 2021 may 8 de;21(1):364. doi: 10.1186/s12884-021-03809-2
- Chappell LC, Cluver CA, Kingdom J, et al. Lancet. jul 24 de, 2021;398(10297):341–354. doi:10.1016/S0140-6736(20)32335-7
- Instituto Nacional de Salud. Boletín Epidemiológico Semanal, semana epidemiológica 52 2022. 2023 [citado 17 de jul 2023]; https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2022_Bolet%C3%ADn_epidemiologico_semana_52.pdf
- ACOG Committee Opinion No. 767: emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. feb 2019;133(2):e174–80. doi: 10.1097/AOG.0000000000003075
- Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020 jun;135(6):e237–60. doi: 10.1097/AOG.0000000000003891
- Clark SL, Hankins GDV. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. feb 2012;119(2 Pt 1):360–364. doi: 10.1097/AOG.0b013e3182411907
- Stott D, Bolten M, Salman M, et al. A prediction model for the response to oral labetalol for the treatment of antenatal hypertension. J Hum Hypertens. feb 2017;31(2):126–131. doi: 10.1038/jhh.2016.50
- Stott D, Bolten M, Paraschiv D, et al. Longitudinal hemodynamics in acute phase of treatment with labetalol in hypertensive pregnant women to predict need for vasodilatory therapy. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. ene 2017;49(1):85–94. doi: 10.1002/uog.17335
- Masini G, Foo LF, Tay J, et al. Preeclampsia has two phenotypes which require different treatment strategies. Am J Obstet Gynecol. feb 2022;226(2S):S1006–18. doi: 10.1016/j.ajog.2020.10.052
- McLaughlin K, Scholten RR, Kingdom JC, et al. Should maternal hemodynamics guide antihypertensive therapy in preeclampsia? Hypertension. abr 2018;71(4):550–556. doi: 10.1161/HYPERTENSIONAHA.117.10606
- Dudenbostel T, Siddiqui M, Oparil S, et al. Refractory hypertension: a novel phenotype of antihypertensive treatment failure. Hypertens Dallas Tex 1979. jun 2016;67(6):1085–1092. doi: 10.1161/HYPERTENSIONAHA.116.06587
- Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for International practice. Hypertens Dallas Tex 1979. 2018 jul;72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803
- Townsend R, O’Brien P, Khalil A. Current best practice in the management of hypertensive disorders in pregnancy. Integr Blood Press Control. 2016;9:79–94. doi: 10.2147/IBPC.S77344
- Wiles K, Damodaram M, Frise C. Severe hypertension in pregnancy. Clin Med. sep 2021;21(5):e451–6. doi: 10.7861/clinmed.2021-0508
- Podymow T, August P. Hypertension in pregnancy. Adv Chronic Kidney Dis. abr 2007;14(2):178–190. doi: 10.1053/j.ackd.2007.01.008
- Jones-Muhammad M, Warrington JP. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci. 2019 sep 4 de;9(9):224.
- Fishel Bartal M, Sibai BM. Eclampsia in the 21st century. Am J Obstet Gynecol. feb 2022;226(2S):S1237–53. doi: 10.1016/j.ajog.2020.09.037
- Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertens Dallas Tex 1979. nov 2008;52(5):873–880. doi: 10.1161/HYPERTENSIONAHA.108.117358
- Gyselaers W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am J Obstet Gynecol. feb 2022;226(2S):S988–1005. doi: 10.1016/j.ajog.2021.11.022
- Di Pasquo E, Ghi T, Dall’asta A, et al. Maternal cardiac parameters can help in differentiating the clinical profile of preeclampsia and in predicting progression from mild to severe forms. Am J Obstet Gynecol. 2019 dic;221(6):.e633.1–.e633.9. doi: 10.1016/j.ajog.2019.06.029
- Stott D, Papastefanou I, Paraschiv D, et al. Serial hemodynamic monitoring to guide treatment of maternal hypertension leads to reduction in severe hypertension. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. ene 2017;49(1):95–103. doi: 10.1002/uog.17341
- Vasapollo B, Novelli GP, Valensise H. Hemodynamic guided treatment of hypertensive disorders in pregnancy: is it time to change our mind? J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. nov 2021;34(22):3830–3831. doi: 10.1080/14767058.2019.1695771
- Bernstein PS, Martin JN, Barton JR, et al. National partnership for maternal safety: consensus bundle on severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2017 ago;130(2):347–357. doi: 10.1097/AOG.0000000000002115
- Phenotypes of pregnant women who subsequently develop hypertension in pregnancy | journal of the American heart association [internet]. [citado 17 de jul 2023]. https://www.ahajournals.org/doi/10.1161/JAHA.118.009595