ABSTRACT
Background
Preeclampsia (PE) is a pregnancy disorder that represents a major cause of maternal and perinatal morbidity and mortality.
Methods
This network meta-analysis was registered with PROSPERO. We searched the PubMed, ClinicalTrials.gov. and Embase databases for studies published from inception to the 31st of March 2023. RevMan5.3 software provided by the Cochrane Collaboration was used for direct meta-analysis (DMA) statistical analysis. Funnel maps, network meta-analysis (NMA), the surface under the cumulative ranking curve (SUCRA) to rank the different interventions and publication bias were generated by STATA 17.0 software.
Results
We included eight randomized controlled trials (RCTs) involving a total of 1192 women with PE; two studies were of high quality and six were of moderate quality. Eight interventions were addressed in the NMA. In the DMA, we found that blood pressure in the Ketanserin group were significantly higher than those in the Nicardipine group. NMA showed that blood pressure in the Dihydralazine group was significantly higher than that in the Methyldopa, Labetalol, Nicardipine and Diltiazem groups. And the blood pressure in the Labetalol group was significantly lower than that in the Nicardipine group. SUCRA values showed that Diltiazem was more effective in lowering blood pressure than other drugs looked at in this study.
Conclusion
According to the eight RCTs included in this study, Diltiazem was the most effective in reducing blood pressure in PE patients; Labetalol and Nicardipine also had good effects. Diltiazem is preferred for the treatment of patients with severe PE and high blood pressure.
Introduction
Preeclampsia (PE) is a pregnancy disorder associated with new-onset hypertension, a pregnancy-induced hypertension disorder that usually occurs after 20 weeks of gestation, with new-onset albuminuria and potential dysfunction in other organs (Citation1). PE affects 3 to 5% of all pregnant women and is a major cause of maternal and perinatal morbidity and mortality, killing 76 000 women and 500 000 infants worldwide each year (Citation2,Citation3). There is a critical need to develop new tools for the prediction, early recognition and effective intervention of PE.
PE is characterized by multi-factor heterogeneity, multi-mechanism pathogenesis heterogeneity, and multi-pathway non-parallelism of pathological changes and clinical manifestations (Citation4). PE has more than one subtype and has multiple pathophysiological pathways that can lead to maternal and fetal mortality and morbidity (Citation3). Early-onset PE is commonly associated with placental dysfunction, reduced placental volume, intrauterine growth restriction, abnormal uterine and umbilical artery Doppler assessment, low birth weight, multiple organ dysfunction, perinatal death, and poor maternal and neonatal outcomes (Citation5). PE is more commonly associated with a normal placenta, greater placental volume, normal fetal growth, normal uterine and umbilical artery Doppler evaluation, normal birth weight, and more favorable maternal and neonatal outcomes due to underlying maternal physical disorders. Compared with term PE, premature PE is associated with higher levels of PE-related liver and kidney dysfunction and a higher incidence of neonatal diseases (Citation6). Severe preeclampsia may be complicated by kidney, heart, lung, liver, and nerve dysfunction, hematological disorders, fetal growth restriction, stillbirth, and maternal death (Citation7). Furthermore, PE is associated with higher rates of hypertension, ischemic heart disease, recurrent acute coronary syndrome, heart failure, stroke, and death (Citation8).
Cyclooxygenase (COX)-1 and COX-2-dependent effects play an important role in the early stages of abnormal placental development and the next stage leading to the clinical syndrome of preeclampsia, while aspirin, as a COX inhibitor, can play a role in PE prevention (Citation9). The current guidelines for PE recommend that pregnant women with high risk factors for PE should use aspirin to prevent the development of PE (Citation1,Citation3,Citation10–14). However, recent research results led to new concepts with regards to the application of aspirin in PE prevention. A randomized controlled study from Shanghai, China found that low-dose aspirin (25/50/75 mg per day) can prevent PE (especially early-onset preeclampsia), and that its efficacy is dose-dependent (Citation15). A study by Mirabito Colafella et al. proposed that higher doses of aspirin inhibit COX-1 and COX-2 simultaneously to prevent PE and that low doses of aspirin inhibit COX-1 in a selective manner (Citation16). A multicenter, randomized, double-blinded study conducted by Daniel Rolnik found that prophylactic aspirin therapy in women at high risk for PE was protective against preterm PE, but not against full-term PE (Citation17). A study by Lan et al. found that aspirin had no significant protective effect against late-onset PE in high-risk groups (Citation18). Another study by Rolnik et al. found that aspirin doses exceeding 100 mg, starting before 16 weeks of gestation, were highly effective in preventing preterm eclampsia, further highlighting the timing and dosage of aspirin in the prevention of preterm PE in high-risk groups (Citation19). Van Doorn et al. recently performed a meta-analysis of the literature relating to aspirin doses <150 mg/d and found that aspirin had no significant protective effect against premature PE (Citation20). At present, preventative measures for PE are limited and the relevant effects are controversial; this may be related to insufficient screening of the relevant population (Citation21).
Timely identification, effective treatment, termination of pregnancy when necessary, and perinatal management, are key to reducing adverse maternal and fetal outcomes associated with PE. The diagnosis of PE is based on criteria recommended by the International Society for the Study of Hypertension in Pregnancy (ISSHP) (Citation3): a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg after20 weeks of gestation, accompanied by any of the following: urinary protein ≥0.3 g/24 h or urinary protein/creatinine ratio ≥+; no albuminuria but accompanied by the involvement of any one organ or system, heart, lung, liver, kidney and other important organs; abnormal changes in the blood system, digestive system, nervous system, or involvement of the placental fetus. PE can also occur postpartum.
Various blood pressure medications have been used to control blood pressure in pre – and post-natal women with PE and chronic hypertension. Methyldopa, Labetalol, Hydralazine, and Nifedipine are first-line drugs for severe PE, while Nicardipine and Nodium nitroprusside are second-line drugs (Citation22). However, there is no consensus on the treatment of non-severe PE. Salt restriction, bed rest, and physical activity restriction are not recommended for the prevention or treatment of PE without severe features. Furthermore, there is no meta-analysis of the efficacy of various drugs against PE. In this study, we summarized the randomized controlled trials (RCTs) carried out in the past and conducted network meta-analysis (NMA) on blood pressure control in PE patients taking antihypertensive drugs. Our aim was to provide new evidence to support the development of medication strategies for patients with PE.
Methods
This study followed the Network Meta-Analysis extension of the Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISMA-NMA) reporting guideline (Citation23). This network meta-analysis was registered with PROSPERO (Reference: CRD42023406777).
Search strategy
We searched the PubMed, ClinicalTrials.gov., and Embase databases for relevant studies published from inception to the 31st of March 2023. Study retrieval involved a combination of subject-heading and keyword searches. Search terms included “preeclampsia,” “anti-hypertension” and “drug.” The publication type of the retrieved studies was limited to randomized controlled trials (RCTs), with no language or site restrictions.
The diagnostic criteria for PE was in accordance with ISSHP guidelines (Citation3): pregnant women at 20 gestational weeks with a systolic blood pressure of140 mmHg or over, and/or diastolic blood pressure of 90 mmHg or over, or associated with any one of the following: a quantitative acuity of 0.3 g/24 h urinary protein, or a urinary albumin/creatinine ratio of0.3 or higher, or random urine protein (+) or the unconditional protein quantitative inspection method; no proteinuria but associated with any one of the following organs or systems: abnormalities of the heart, lungs, liver, kidney, or of the blood, digestive, and nervous systems, and involvement of the placental fetus. The diagnostic criteria were adjusted according to the year in which the study was conducted.
Inclusion and exclusion criteria
Studies included in the NMA met the following criteria (Citation1): patients were diagnosed with PE (Citation2); the study was an RCT, and (Citation3) all control trials involving anti-hypertensive drug treatment for preeclampsia. Any comparison of one or more anti-hypertensive drug with either placebo or a non-anti-hypertensive drug was included, as were comparisons of one anti-hypertensive drug with another, including those among the same drug class; and (Citation4) studies that reported maternal and (or) fetal outcomes.
The exclusion criteria were as follows (Citation1): the design of the study did not qualify it as a randomized controlled study (thus excluding reviews, letters, and others) (Citation2); the study did not meet the diagnostic criteria for PE (such as gestational hypertension) (Citation3); patients had comorbidities such as severe kidney disease, or liver disease (Citation4); anti-hypertensive therapy was defined as any pharmacological intervention intended to reduce BP. As such, we also excluded trials of drugs that were prescribed to reduce the risk of PE.
Data extraction and quality evaluation
Data extraction: Data was abstracted with a review-specific form to collect the following information: (i) study characteristics (including country, year of publication, eligibility criteria, and definition of non-severe hypertension in pregnancy); (ii) characteristics of the women (including age, body mass index (BMI), co-morbidities, parity, gestational age at enrollment, smoking, past obstetric history); (iii) details of the intervention (including anti-hypertensive drug, dosage, and route of administration; target BP) and co-interventions (including place of care); and (iv) definitions of trial outcomes. Abstraction was undertaken by two reviewers and disagreement was addressed by consulting a third author and through consensus; if consensus could not be reached, the principal investigator adjudicated.
We will include all participants randomized to each group in the analyses who had known outcomes. For multi-arm studies, double-counting was avoided by selecting appropriate pair-wise comparisons. For any information that is unclear, we contacted the authors of the original reports for further details. The methodological quality of the study was evaluated in accordance with the Cochrane Collaboration’s tool for assessing risk of bias (Citation24). The evaluation included random sequence generation, hidden distribution hiding, subject and intervention provider blinding, outcome evaluation blinding, outcome data integrity, selective outcome reporting, and other sources of bias. Disagreement was judged by public discussion. In accordance with the Cochrane Collaboration Group criteria, we divided the studies into three categories (Citation1): low bias risk (low bias risk in all key areas) (Citation2), unclear bias risk (unclear bias risk in one or more key areas), and (Citation3) high bias risk (high bias risk in one or more key areas).
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework was used to assess the quality of evidence for the primary outcomes contributing to each network estimate (Citation25). All disagreements of quality evaluation were resolved through discussion between all authors.
Statistical analysis
Direct meta-analysis (DMA)
RevMan5.3 software (https://revman.cochrane.org/), provided by the Cochrane Collaboration, was used for DMA statistical analysis, and the relative risk (RR) and 95% confidence interval (CI), and the mean difference and 95% CI, were used as evaluation indices. First, the Chi-squared test was used to assess heterogeneity, and the existence of heterogeneity (I2) was quantitatively analyzed (I2 ≥50%). Meta-analysis was performed without heterogeneity; when statistical heterogeneity existed among study results, the source of heterogeneity was further analyzed, and the influence of obvious clinical heterogeneity was excluded and a random effects model was adopted. When there was no statistical heterogeneity, a fixed effects model was applied. Funnel maps were created by STATA 17.0 (StataCorp, College Station, TX, USA) software to detect publication bias.
Network meta-analysis (NMA)
We performed a frequentist NMA using STATA 17.0 software. NMA can combine direct and indirect comparisons to further analyze the effects of different treatment options on maternal-fetal outcomes for PE women. The results of the comparisons were expressed as RRs and 95% CIs. Moreover, we built a network diagram using STATA 17.0 software and calculated the surface under the cumulative ranking curve (SUCRA) to rank the different interventions (Citation26). If one intervention had a higher SUCRA value than the others, this indicated that the greater the treatment effect, the lower the incidence of adverse reactions. The assumption of consistency between direct and circumstantial evidence was evaluated using the node splitting method (Citation27). When the direct evidence and indirect evidence were consistent (p > 0.05), a consistency model was used, otherwise we used an inconsistency model.
Results
Retrieved results
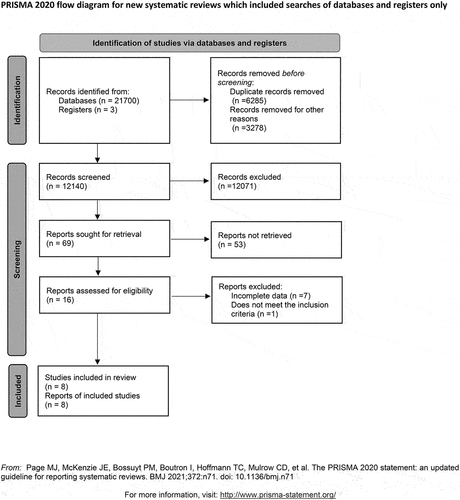
Following a predesigned literature retrieval strategy, 217 003 articles were retrieved, but this included 6285 duplicate articles. After reviewing the titles, abstracts, and full texts, we included eight RCTs involving a total of 1192 women with PE. The literature screening process and the results are shown in .
Features incorporated into the study
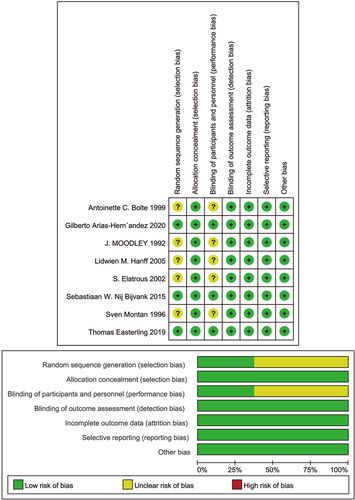
The basic features of the included studies are shown in . The included studies were published between 1992 and 2020; two studies (Citation28,Citation29) were of high quality and six studies (Citation30–35) were of moderate quality.
Table 1. Characteristics of included RCT studies.
Eight interventions were reported in the eight studies: Dihydralazine, Epoprostenol, Methyldopa, Isradipine, Ketanserin, Nicardipine, Labetalol and Diltiazem.
Quality evaluation
The quality of the included studies was evaluated, and the results showed that the blinding of subjects and intervention providers was the main source of potential bias (). This was because blinding was not possible in trials, as intravenous drip and oral drugs were administered by completely different routes.
Network evidence diagram
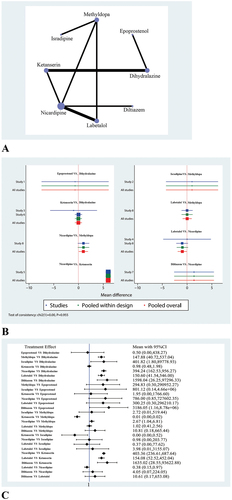
shows a network evidence diagram that includes the eight interventions addressed in the NMA: Dihydralazine, Epoprostenol, Methyldopa, Isradipine, Ketanserin, Nicardipine, Labetalol and Diltiazem. In , the size of the circles is proportional to sample size, the lines between circles represent direct comparative evidence, and the width of the lines is proportional to the number of trials.
Direct meta-analysis and network meta-analysis results
show the DMA and NMA results relating to blood pressure level. shows the contribution of direct and indirect comparisons to final estimates. All eight of the included studies reported the effects of different interventions on blood pressure in pregnant women with PE.
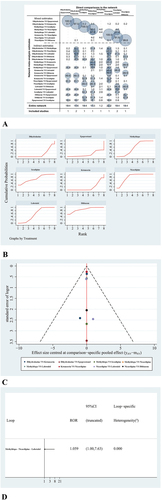
Figure 4. (a) Contribution of DMA and NMA. Interventions.(b) The surface under the cumulative ranking curve (SUCRA). (c) Comparison-adjusted funnel plot (points with different colors represent different interventions). (d) Loop-consistency plot.
In the DMA, we found that blood pressure in the Ketanserin group was significantly higher than those in the Nicardipine group (P < 0.05). No significant differences were observed between the Dihydralazine, Epoprostenol, Methyldopa, Isradipine, Labetalol and Diltiazem groups.
In the NMA, we found that blood pressure in the Dihydralazine group was significantly higher than that in the Methyldopa group (147.88, 95% CI: 40.72–537.04), Labetalol (394.24, 95% CI: 162.53–956.27), Nicardipine (150.60, 95% CI: 41.54–546.00) and Diltiazem (1598.04, 95% CI: 26.25–97296.33) groups. Blood pressure in the Ketanserin group was significantly higher than that in the Labetalol group (403.36, 95% CI: 236.61–687.64), Nicardipine group (154.08, 95% CI: 52.52–452.04) and Diltiazem group (1635.02, 95% CI: 28.55–93622.88). Blood pressure in the Ketanserin group was significantly lower than that in the Methyldopa group (0.01, 95% CI: 0.00–0.02) and Isradipine group (0.00, 95% CI: 0.00–0.52). Blood pressure in the Labetalol group was significantly lower than that in the Nicardipine group (0.38, 95% CI: 0.15–0.97). No significant differences were observed between the other groups.
SUCRA
NMA can evaluate the best effect of each intervention for different results and sort the interventions by SUCRA value; a higher SUCRA value indicates a better intervention or a lower incidence of adverse reactions. shows the detailed ranking results. According to , blood pressure in the Epoprostenol group was the lowest, and diltiazem was more effective, compared to other drugs that were looked at in this study
Publication bias
shows a comparison-adjusted funnel diagram. All studies on the funnel map are symmetrically distributed with respect to the vertical lineX = 0, indicating that there were no significant small-sample effects or publication bias. Finally, shows that no loop inconsistency existed in this NMA.
Discussion
In this study, NMA revealed that Diltiazem had the best effect on reducing blood pressure in patients with; Labetalol and Nicardipine were also effective. At present, Diltiazem is preferentially used to treat patients with severe PE and hypertension.
Diltiazem is an alternative calcium antagonist that is 1000-fold less potent than Nifedipine and has selective vasodilator arterial bed properties (Citation36–40). Therefore, Diltiazem has little effect on venous return and cardiac function, thus allowing for better cell perfusion than Nifedipine (Citation36,Citation37). Many studies have reported that Diltiazem has a large safety margin and fewer side effects due to its pharmacological properties (Citation36,Citation38). Arias-Hernández’s et al. (Citation35) reported that Diltiazem met international standards for blood pressure in women with PE, and that both systolic and diastolic blood pressure were reduced in a uniform manner. The incidence of hypotension in the Diltiazem group was 0.036–0.296-fold times lower than in the Nifedipine group. With regards to the effects of Diltiazem used during pregnancy on maternal and fetal outcomes, calcium channel blocking antihypertensive drugs, including Nifedipine and Diltiazem, are currently considered safe (Citation41).
Labetalol lowers blood pressure by blocking beta- and alpha-adrenergic receptors. In addition, labetalol protects uterine placental blood flow better than other beta-blockers. Compared to methyldopa, Labetalol works faster (2 h). Randomized clinical trials comparing Labetalol with Methyldopa or Nifedipine have shown that Labetalol is safe for use during pregnancy (Citation42,Citation43). Labetalol has been shown to cause maternal hepatotoxicity. It is important to recognize this side effect because it can be confused with HELLP (elevated hemolysis, liver enzymes, and low platelet count) syndrome. Most of the liver toxicity caused by Labetalol is reversible, although deaths have been reported (Citation44).
Nicardipine is considered as a second-generation dihydropyridine calcium antagonist that dilates peripheral blood vessels, cerebral vessels, coronary arteries, and renal arterioles (Citation45). Nicardipine can also increase blood flow to different organs, thus reducing lower blood pressure. In clinical application, Nicardipine can significantly reduce arterial pressure, while maintaining no significant change in uterine and placental perfusion. In addition, nicardipine was well tolerated by mothers and their fetuses. Nicardipine, however, is highly irritating to blood vessels (Citation46). The symptoms caused by phlebitis mainly include pain, redness, sclerosis and a cord-like appearance, which may occur along the vein, thus increasing difficulty and risk; this is of great significance for clinical follow-up management (Citation47–49).
In the process of inclusion in the screening study, we observed the auxiliary effects of supplements (such as fatty preparations) and non-antihypertensive drugs (including pravastatin and proton pump inhibitors) on PE. Prenatal lipid supplementation has been shown to improve maternal-fetal outcomes in underweight patients with PE (Citation50,Citation51). Pravastatin is a lipid-lowering drug that is widely used to reduce the risk of cardiovascular events and has received the most attention of all drug candidates (other than aspirin and calcium) over the past decade because of its potential to treat or prevent PE (Citation52,Citation53). Proton pump inhibitors are widely used to relieve symptomatic acid reflux by reducing acid production in the stomach, including during pregnancy. We published preclinical evidence that in pregnancy and similar to pravastatin, proton pump inhibitors (lansoprazole, rabeprazole, and esomeprazole) can reduce placental release of sFlt-1 and sEng (but at lower concentrations than pravastatin) in vitro (Citation54–56). However, studies on the effects of these other drugs and supplements on PE are very limited.
This study has the certain limitations that need to be considered. We only selected RCTs in this study and the inclusion requirements were relatively strict. The total number of included studies and the number of sample cases were limited, and the representativeness of the results was limited. In this study, NMA was only conducted on the blood pressure control of PE patients with various antihypertensive drugs. All antihypertensive drugs can cross the placenta. Currently, there are no randomized controlled trials to recommend the use of an antihypertensive drug. However, certain medications are effective in lowering blood pressure and have an acceptable safety profile during pregnancy. Due to the limitation of inclusion in this study, NMA was not conducted on the maternal-fetal outcome and possible side effects under the action of different drugs. In addition, parenteral or oral therapy must be considered as the preferred option when selecting a specific drug. Due the limited body of literature, subgroup analysis of drug routes was not conducted in this study. Finally, due to the large time span of the included literature, differences in PE diagnostic criteria inevitably occurred, as well as bias caused by the iteration of detection technology accompanied by scientific and technological progress. Therefore, the results of this study should be interpreted with caution.
Conclusion
According to the eight RCTs included in this study, Diltiazem was the most effective in reducing PE blood pressure, although Labetalol and Nicardipine also had good effects. Diltiazem is preferred for treating patients with severe PE and high blood pressure levels. However, the side effects of drugs were not considered in this study, and the selection of antihypertensive drugs for PE in the clinic should also consider the specific situation of patients to develop personalized programs. The selection of highly effective drugs for PE with low side effects still needs to be investigated in large multicenter RCTs.
Authors’ contributions
Conception and design: Ting Wang, Ruoan Jiang, Yingsha Yao
Acquisition and data: Ruoan Jiang, Yingsha Yao, Ting Xu
Analysis and interpretation of data: Ruoan Jiang, Yingsha Yao, Ting Xu
Drafting of the manuscript: Ruoan Jiang, Yingsha Yao, Na Li
Critical revision of the manuscript for important intellectual content: Ting Wang
Statistical analysis: Ting Wang, Yingsha Yao
Supervision: Ting Wang
Consent for publication
The authors declare that they agree with the publication of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Further inquiries can be directed to the corresponding author/s.
Additional information
Funding
References
- Gestational hypertension and preeclampsia: ACOG practice bulletin summary, number 222. Obstet Gynecol. 2020;135(6):149–11. doi: 10.1097/AOG.0000000000003892
- Chappell LC, Cluver CA, Kingdom J, et al. Pre-eclampsia. Lancet. 2021;398(10297):341–54. doi: 10.1016/S0140-6736(20)32335-7
- Poon LC, Shennan A, Hyett JA, et al. The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019; 145 (Suppl 1):1–33.10.1002/ijgo.12802
- Roberts JM, Rich-Edwards JW, McElrath TF, et al. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. 2021;77(5):1430–41. doi: 10.1161/HYPERTENSIONAHA.120.14781
- Obed S, Patience A. Birth weight and ponderal index in pre-eclampsia: a comparative study. Ghana Med J. 2006;40(1):8–13.
- Chandra I, Sun L. Preterm and term preeclampsia: differences in biochemical parameter and pregnancy outcomes. Postgrad Med. 2018;130(8):703–7. doi: 10.1080/00325481.2018.1527169
- Ives CW, Sinkey R, Rajapreyar I, et al. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 76(14):1690–1702. 10.1016/j.jacc.2020.08.014
- Ananth CV, Duzyj CM, Yadava S, et al. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension. 2019;74(5):1089–95. doi: 10.1161/HYPERTENSIONAHA.119.12968
- Loussert L, Vidal F, Parant O, et al. Aspirin for prevention of preeclampsia and fetal growth restriction. Prenat Diagn. 2020;40(5):519–27. doi: 10.1002/pd.5645
- Webster K, Fishburn S, Maresh M, et al. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366:15119. doi: 10.1136/bmj.l5119
- Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014; 36(5):416–441. 10.1016/S1701-2163(15)30588-0
- Lowe SA, Bowyer L, Lust K, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015;55(5):e1–29. doi: 10.1111/ajo.12399
- Butalia S, Audibert F, Côté AM, et al. Hypertension Canada’s 2018 guidelines for the management of hypertension in pregnancy. Can J Cardiol. 2018;34(5):526–31. doi: 10.1016/j.cjca.2018.02.021
- WHO Guidelines Approved by the Guidelines Review Committee. WHO recommendations: policy of interventionist versus expectant management of severe pre-eclampsia before term. Geneva: World Health Organization © World Health Organization 2018; 2018.
- Gu W, Lin J, Hou YY, et al. Effects of low-dose aspirin on the prevention of preeclampsia and pregnancy outcomes: a randomized controlled trial from Shanghai, China. Eur J Obstet Gynecol Reprod Biol. 2020;248:156–63. doi: 10.1016/j.ejogrb.2020.03.038
- Mirabito Colafella KM, Neuman RI, Visser W, et al. Aspirin for the prevention and treatment of pre-eclampsia: a matter of COX-1 and/or COX-2 inhibition? Basic Clin Pharmacol Toxicol. 2020;127(2):132–41. doi: 10.1111/bcpt.13308
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613–22. doi: 10.1056/NEJMoa1704559
- Lan PG, Gillin AG, Pelosi M, et al. Effect of early use of low-dose aspirin therapy on late-onset preeclampsia. J Matern Fetal Neonatal Med. 2019;32(13):2137–42. doi: 10.1080/14767058.2018.1427718
- Rolnik DL, Nicolaides KH, Poon LC Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. 2022; 226(2s):S1108–s19. 10.1016/j.ajog.2020.08.045
- Van Doorn R, Mukhtarova N, Flyke IP, et al. Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: a systematic review and meta-analysis. PLoS One. 2021;16(3):e0247782. doi: 10.1371/journal.pone.0247782
- Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50(4):492–5. doi: 10.1002/uog.18816
- Odigboegwu O, Pan LJ, Chatterjee P. Use of antihypertensive drugs during preeclampsia. Front Cardiovasc Med. 2018;5:50. doi: 10.3389/fcvm.2018.00050
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. doi: 10.7326/M14-2385
- Savović J, Weeks L, Sterne JA, et al. Evaluation of the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3(1):37. doi: 10.1186/2046-4053-3-37
- Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349(sep24 5):g5630. doi: 10.1136/bmj.g5630
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–17. doi: 10.1177/0272989X12458724
- Yu-Kang T. Node-splitting generalized linear mixed models for evaluation of inconsistency in network meta-analysis. Value Health. 2016;19(8):957–63. doi: 10.1016/j.jval.2016.07.005
- Bijvank SW, Visser W, Duvekot JJ, et al. Ketanserin versus dihydralazine for the treatment of severe hypertension in early-onset preeclampsia: a double blind randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2015;189:106–111. doi: 10.1016/j.ejogrb.2015.02.002
- Easterling T, Mundle S, Bracken H, et al. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet. 2019;394(10203):1011–21. doi: 10.1016/S0140-6736(19)31282-6
- Moodley J, Gouws E. A comparative study of the use of epoprostenol and dihydralazine in severe hypertension in pregnancy. Br J Obstet Gynaecol. 1992;99(9):727–30. doi: 10.1111/j.1471-0528.1992.tb13872.x
- Montan S, Anandakumar C, Arulkumaran S, et al. Randomised controlled trial of methyldopa and isradipine in preeclampsia–effects on uteroplacental and fetal hemodynamics. J Perinat Med. 1996;24(2):177–84. doi: 10.1515/jpme.1996.24.2.177
- Bolte AC, van Eyck J, Kanhai HH, et al. Ketanserin versus dihydralazine in the management of severe early-onset preeclampsia: maternal outcome. Am J Obstet Gynecol. 1999; 180(2 Pt 1):371–377. 10.1016/S0002-9378(99)70216-4
- Elatrous S, Nouira S, Ouanes Besbes L, et al. Short-term treatment of severe hypertension of pregnancy: prospective comparison of nicardipine and labetalol. Intensive care Med. 2002;28(9):1281–6. doi: 10.1007/s00134-002-1406-3
- Hanff LM, Vulto AG, Bartels PA, et al. Intravenous use of the calcium-channel blocker nicardipine as second-line treatment in severe, early-onset pre-eclamptic patients. J Hypertens. 2005;23(12):2319–26. doi: 10.1097/01.hjh.0000188729.73807.16
- Arias-Hernández G, Vargas-De-León C, Calzada-Mendoza CC, et al. Efficacy of diltiazem for the control of blood pressure in puerperal patients with severe preeclampsia: a randomized, single-blind, controlled trial. Int J Hypertens. 2020;2020:5347918. doi: 10.1155/2020/5347918
- Ding Y, Vaziri ND. Nifedipine and diltiazem but not verapamil up-regulate endothelial nitric-oxide synthase expression. J Pharmacol Exp Ther. 2000;292(2):606–9.
- Pepine CJ, Feldman RL, Whittle J, et al. Effect of diltiazem in patients with variant angina: a randomized double-blind trial. Am Heart J. 1981;101(6):719–25. doi: 10.1016/0002-8703(81)90606-2
- Balasubramaniam R, Chawla S, Mackenzie L, et al. Nifedipine and diltiazem suppress ventricular arrhythmogenesis and calcium release in mouse hearts. Pflugers Arch - Eur J Physiol. 2004;449(2):150–8. doi: 10.1007/s00424-004-1321-2
- Klinke WP, Kvill L, Dempsey EE, et al. A randomized double-blind comparison of diltiazem and nifedipine in stable angina. J Am Coll Cardiol. 1988;12(6):1562–7. doi: 10.1016/S0735-1097(88)80026-3
- Glasser SP, Gana TJ, Pascual LG, et al. Efficacy and safety of a once-daily graded-release diltiazem formulation dosed at bedtime compared to placebo and to morning dosing in chronic stable angina pectoris. Am Heart J. 2005;149(2):e1–9. doi: 10.1016/j.ahj.2004.08.002
- Ringholm L, Damm JA, Vestgaard M, et al. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep. 2016;16(2):12. doi: 10.1007/s11892-015-0705-3
- Peacock WF, Hilleman DE, Levy PD, et al. A systematic review of nicardipine vs labetalol for the management of hypertensive crises. Am J Emerg Med. 2012; 30(6):981–993. 10.1016/j.ajem.2011.06.040
- Molvi SN, Mir S, Rana VS, et al. Role of antihypertensive therapy in mild to moderate pregnancy-induced hypertension: a prospective randomized study comparing labetalol with alpha methyldopa. Arch Gynecol Obstet. 2012;285(6):1553–62. doi: 10.1007/s00404-011-2205-2
- Clark JA, Zimmerman HJ, Tanner LA Labetalol hepatotoxicity. Ann Intern Med. 1990; 113(3):210–213. 10.7326/0003-4819-113-3-210
- Whiting RL, Dow RJ, Graham DJ, et al. An overview of the pharmacology and pharmacokinetics of nicardipine. Angiology. 1990;41(11 Pt 2):987–91.
- Ye F, Lu Q, Kong B, et al. Clinical efficacy and safety of two concentrations of intravenous nicardipine hydrochloride for nicardipine-related phlebitis in patients with preeclampsia. Can J Physiol Pharmacol. 2022;100(4):291–4. doi: 10.1139/cjpp-2021-0387
- Park I, Jeong MH, Park CJ, et al. Clinical features and management of “phlebitis-like abnormal reaction” after cyanoacrylate closure for the treatment of incompetent saphenous veins. Ann Vasc Surg. 2019;55:239–245. doi: 10.1016/j.avsg.2018.07.040
- Igarashi A, Okuno T, Shimizu T, et al. Mechanical stimulation is a risk factor for phlebitis associated with peripherally inserted central venous catheter in neonates. Pediatr Int. 2021;63(5):561–4. doi: 10.1111/ped.14476
- Murphy K, Murphy J, Fischer-Cartlidge E Reducing the Incidence of amiodarone-related phlebitis through utilization of evidence-based practice. Worldviews Evid Based Nurs. 2020; 17(5):385–392. 10.1111/wvn.12470
- Mohammad NS, Nazli R, Zafar H, et al. Effects of lipid based multiple micronutrients supplement on the birth outcome of underweight pre-eclamptic women: a randomized clinical trial. Pak J Med Sci. 2022; 38(1):219–226. 10.12669/pjms.38.1.4396
- Sher N, Mubaraki MA, Zafar H, et al. Effect of lipid-based multiple micronutrients supplementation in underweight primigravida pre-eclamptic women on maternal and pregnancy outcomes: randomized clinical trial. Medicina (Kaunas). 2022;58(12):1772. doi: 10.3390/medicina58121772
- Yebyo HG, Aschmann HE, Kaufmann M, et al. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: a systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am Heart J. 2019;210:18–28. doi: 10.1016/j.ahj.2018.12.007
- Tong S, Kaitu’u-Lino TJ, Hastie R, et al. Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: new horizons for the prevention or treatment of preeclampsia. Am J Obstet Gynecol. 2022; 226(2s):S1157–S1170. 10.1016/j.ajog.2020.09.014
- Brownfoot FC, Tong S, Hannan NJ, et al. Effects of pravastatin on human placenta, endothelium, and women with severe preeclampsia. Hypertension. 2015;66(3):687–97. discussion 445. doi: 10.1161/HYPERTENSIONAHA.115.05445
- Onda K, Tong S, Beard S, et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension. 2017;69(3):457–68. doi: 10.1161/HYPERTENSIONAHA.116.08408
- Saleh L, Samantar R, Garrelds IM, et al. Low soluble fms-like tyrosine kinase-1, endoglin, and endothelin-1 levels in women with confirmed or suspected preeclampsia using proton pump inhibitors. Hypertension. 2017;70(3):594–600. doi: 10.1161/HYPERTENSIONAHA.117.09741