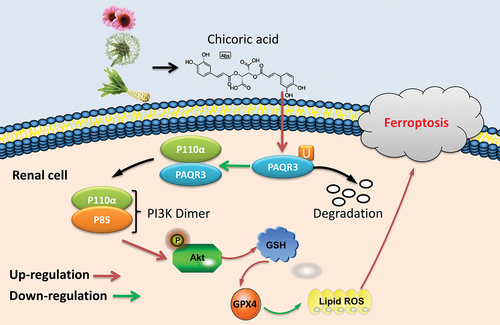
ABSTRACT
Purpose
This study aimed to examine the impact of CA on DN and elucidate its underlying molecular mechanisms of inflammation.
Methods
We fed C57BL/6 mice injected with streptozotocin to induce diabetes. In addition, we stimulated NRK-52E cells with 20 mmol/L d-glucose to mimic the diabetic condition.
Results
Our findings demonstrated that CA effectively reduced blood glucose levels, and improved DN in mice models. Additionally, CA reduced kidney injury and inflammation in both mice models and in vitro models. CA decreased high glucose-induced ferroptosis of NRK-52E cells by inducing GSH/GPX4 axis. Conversely, the ferroptosis activator or the PI3K inhibitor reversed positive effects of CA on DN in both mice and in vitro models. CA suppressed PAQR3 expression in DN models to promote PI3K/AKT activity. The PAQR3 activator reduced the positive effects of CA on DN in vitro models. Moreover, CA directly targeted the PAQR3 protein to enhance the ubiquitination of the PAQR3 protein.
Conclusion
Overall, our study has uncovered that CA promotes the ubiquitination of PAQR3, leading to the attenuation of ferroptosis in DN. This effect is achieved through the activation of the PI3K/AKT signaling pathways by disrupting the interaction between PAQR3 and the P110α pathway. These findings highlight the potential of CA as a viable therapeutic option for the prevention of DN and other forms of diabetes.
Graphical abstract
Introduction
Diabetes is a common occurring disease that seriously endangers human health. The prevalence rate of diabetes among adults aged ≥18 years in China is as high as 11.6%, with 114 million patients; In addition, according to the prediction of the World Health Organization, there will be more than 130 million type 2 diabetes patients in China by 2025, which will impose a huge burden on China’s society and economy. With the aging of the population and the aggravation of urbanization, the incidence rate of diabetes has been steadily increasing in China.Citation1 Although the prevalence of diabetes is increasing year by year, it is still a preventable and curable disease, which has been listed as the key prevention and treatment content in the medical and health system reform of China and Shanghai. The development mode of diabetes is from the normal population to the high-risk population, and then hyperglycemia occurs. Complications may occur in any of these links, which will directly lead to death.Citation2 This represents a sixteen-fold increase in prevalence over a span of nearly four decades.Citation3 Hierarchical management, screening and different intervention measures at all stages of diabetes development, especially at high-risk stage and early stage of disease, can not only delay or even reverse the progress of diabetes. Effective health intervention for high-risk groups of diabetes can prevent or delay their progression to diabetes and improve their quality of life.Citation3 With the rapid societal development and changes in dietary habits, has been steadily increasing.Citation4,Citation5 Previous studies have shown that the body’s micro-inflammatory response plays a significant role in the onset of diabetic nephropathy.Citation4,Citation6
Diabetic nephropathy (DN) is a common microvascular complication of diabetes, with an incidence rate as high as 20% to 40%. It is also a significant cause of end-stage renal disease.Citation7 Under the condition of high blood sugar level for a long time, diabetes patients have proteinuria and the complications of diabetes nephropathy with reduced glomerular filtration rate In China, the occurrence of this disease is increasing year by year, becoming the second factor leading to end-stage renal disease. Patients with diabetes nephropathy are often accompanied by renal function damage, but the disease starts insidiously, progresses slowly, and does not have typical clinical characteristics when it comes to early onset. Therefore, when patients have persistent proteinuria, the disease progress is relatively serious, and patients’ renal function is often irreversibly damaged. Currently, the clinical prevention and treatment methods for DN mainly focus on controlling blood sugar, while also considering the supplementation of vitamins and minerals to alleviate clinical symptoms. However, there is currently no direct and effective treatment method for DN.Citation8
Iron death has received widespread attention in recent years. Many studies have found that iron death exists in diabetes and its complications.Citation9,Citation10 Inhibiting iron death can greatly slow down the occurrence and progress of diabetes and its complications. Traditional Chinese medicine is a unique medical treasure in China.Citation11 It has the advantages of multi-channel, multi-directional, inexpensive, and low adverse reactions. It may be a new direction to treat diabetes and its complications in the future from the perspective of regulating iron death by traditional Chinese medicineCitation11 Fundamentally, Ferroptosis is due to the disorder of the metabolism of lipid oxide in the cell, that is, because the activity of glutathione peroxidase 4 (GPX4) decreases, the lipid oxide cannot be metabolized into non-oxidative toxic substances, and then catalyzed by the way of Fenton reaction under the action of divalent iron ions, resulting in a large amount of iron deposition in the cell, seriously damaging the balance of oxidation and reduction in the cell, attacking biological macromolecules, and triggering cell death.Citation12,Citation13 Ferroptosis plays a crucial role in kidney disease as well as other multi-system diseases.Citation14
PI3K/AKT signaling pathway can regulate cell survival, autophagy, apoptosis, oxidative stress, and inflammatory response. Inhibiting its expression can reduce cellular inflammatory response and alleviate renal tissue damageCitation15. PI3K/Akt pathway can promote Nuclear factor E2-related factor 2 (Nrf2) activation.Citation16–18 Therefore, activation of Akt/Nrf2/GPX4 pathway holds great significance in inhibiting the occurrence of Ferroptosis.
Progestin and adipo Q acceptor 3 (PAQR3) is a transmembrane protein reported to be located on the Golgi apparatus, and is a member of the PAQR family.Citation19.Citation20 The deletion of PAQR3 promotes the degradation of Nrf2 protein through the ubiquitination process mediated by Keap1, thus enhancing the sensitivity of cancer cellsCitation21. In mechanism, the down-regulation of PAQR4 blocked the signal transmission of PI3K/AKT pathway.Citation22
In clinical practice, dandelion alone or in combination with other herbs is effective in treating diabetes nephropathy.Citation23 The application of dandelion compatibility in traditional Chinese medicine indicates that it is considered a valuable herb with potential therapeutic effects on this disease.Citation23–25 Cichoric acid (CA) is a kind of hydroxycinnamic acid, which is an important active ingredient in many natural plants such as dandelion, echinacea, chicory, etc.Citation26 In plants, CA plays a crucial role in protecting against insects, viruses, bacteria, fungi, and nematodes.Citation27 Modern pharmacological studies show that CA has significant biological activities in anti-inflammatory, antioxidant, immune regulation, antibacterial, antiviral, anti-tumor, and other aspects.Citation28 The biological activity of CA is its inhibitory effect on human immunodeficiency virus. With the development and application of molecular biological technology, the inhibitory activity, and mechanism of CA against other viruses have been continuously reportedCitation29; Furthermore, CA has also exhibited significant inhibitory effects against various pathogenic bacteria.Citation27 CA is a kind of phenylpropionic acid compound, which mainly exists in Echinacea purpurea and Cichorium chicory. It has antibacterial, antiviral, anti-inflammatory, and immunomodulatory effects. Notably, CA has displayed inhibitory effects against numerous viruses.Citation30 Here, this experiment investigated the effects of CA in DN and its molecular mechanisms of inflammation.
Materials and methods
Animal model, histological examination, and kidneys function
All C57BL/6 mice (5–6 weeks, 18–20 g) were randomly assigned to five groups: sham group (n = 8), model group (n = 8), Low/Med/High CA group (n = 8), ME group (n = 8). DN mice were fed with a high-fat diet (HFD) for 12 weeks, and then injected with STZ (30 mg/kg of streptozotocin; Sigma-Aldrich, St Louis, MO, USA) i.p. for 7 consecutive days. Blood glucose levels were measured using 16.7 mmol/l after one week of the final injection. Low/Med/High CA group were administration with 7.5, 15 or 30 mg/kg/d (dissolved in water) for 6 weeks as literature.Citation30 ME group were administration with 195 mg/kg metformin for 6 weeks. Mice were killed under anesthesia, and kidneys and serum taken for analysis. This study was approved by the Animal Care and Use Committee of Yijishan Hospital of Wannan Medical College (WNWC-AWE-2023008).
Kidney tissue samples were fixed in 4% paraformaldehyde, paraffn-embedded and then sectioned into 5 μM slices for HE, Masson staining, PAS staining. Kidney tissue samples were observed using fluorescence microscope (Zeiss Axio Observer A1, Germany).
Vitro model and treatment with CA
Rat renal tubular epithelial (NRK-52E, Cell Bank of Chinese Academy of Sciences) cells were seeded in culture dish with RPMI 1640 (Gibco) supplemented with 10% FBS (Gibco) under a humidified 5% (v/v) CO2 atmosphere at 37°C. NRK-52E stimulated with 20 mmol/L d-glucose for DN model. Next, the transfections were performed using Lipofectamine 2000 (Thermo Fisher Scientific). After 48 h of transfection, NRK-52E stimulated with 20 mmol/L d-glucose and CA (5, 10 and 20 μM) as literatureCitation31 or ME (250 μM of metformin).
Cell counting kit‑8 (CCK8), ELISA assay and quantitative polymerase chain reaction (qPCR)
CCK8, ELISA assay and qPCR were executed as described previously.Citation32 NRK-52E with different Polysaccharide were cultured in a 96-well plate for corresponding time points and then incubated with CCK-8 reagent. LDH activity, GSH, INF-γ, TNF-α, IL-6, and IL-1β levels using Corresponding ELIAS reagent kitS (Nanjing Jiancheng Biological Engineering Institute, Nanjing, China) following the manufacture’s instructions.
qPCR were performed with the ABI Prism 7500 sequence detection system according to the Prime-ScriptTM RT detection kit. Relative levels of the sample mRNA expression were calculated and expressed as 2-△△Ct.
Flow cytometry for apoptosis
Cells were resuspended with 100 µL binding buffer, through centrifugation at 1000 rpm for 5 min followed by 3 washes with PBS. Cells were stained with propidium iodide (556547, BD Pharmingen, San Diego, USA) for 15 min at room temperature. 200 µl PBS was added to each tube of samples and then these samples were analyzed by FACS flow cytometer (BD Biosciences, San Jose, USA).
Western blotting analysis
Membranes were incubated with PAQR3 (sc -515 831, 1:500, Santa Cruz Biotechnology, Inc), PI3K (4257, 1:2000, Cell Signaling Technology, Inc.), p-PI3K (17366, 1:1000, Cell Signaling Technology, Inc.), Akt (4691, 1:2000, Cell Signaling Technology, Inc.), p-Akt (4060, 1:1000, Cell Signaling Technology, Inc.), GPX4 (ab41787, 1:2000, abcam), GSDMD (ab209845, 1:2000, abcam), and β-Actin (BS6007MH, 1:5000, Bioworld Technology, Inc.) at 4°C overnight. The membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (sc-2004 or sc-2005, 1:5000, Santa Cruz, USA) for 1 h at 37°C after washing with TBST for 15 min. Protein was measured using an enhanced chemiluminescence system (ECL, Beyotime) and analyzed using an Image Lab 3.0 (Bio-Rad Laboratories, Inc.).
PI staining, Calcein/PI stainin, gImmunofluorescence, and electron microscope
PI staining, Calcein/PI staining, Immunofluorescence and Electron microscope were executed as described previously.Citation32 Cells were incubated with PI staining (ST512, Beyotime Biotechnology) or Calcein/PI staining (C2015S, Beyotime Biotechnology). Cells were incubated with PI3K (ab32089, 1:100, Abcam) and PAQR3 (sc -515 831, 1:500, Santa Cruz Biotechnology, Inc) at 4°C overnight after blocking with PBS supplemented with 5% BSA for 2 h at room temperature. Cell samples were observed using fluorescence microscope (Zeiss Axio Observer A1, Germany). Ultra-thin sections were observed using a Hitachi H7650 transmission electron microscope (Tokyo, Japan).
Microscale thermophoresis (MST), thermal shift assay (TSA), and cellular thermal shift assay (CETSA)
MST, TSA and CETSA were executed as described previously.Citation33 0.10 mg/mL WT PAQR3 protein was used with or without 0.30 mmol/L CA in PBS. 20 μL supernatant was used to Western blotting. CETSA, and the thermal stability were performed using GraphPad Prism software (GraphPad Diego, CA, USA).
Statistical analysis
Data were expressed as mean ± standard deviation (SD) used GraphPad Prism 8. Multiple comparisons performed by one-way ANOVA followed by Tukey’s posttest. p values < 0.05 were considered statistically significant.
Results
CA improved DN in mice model
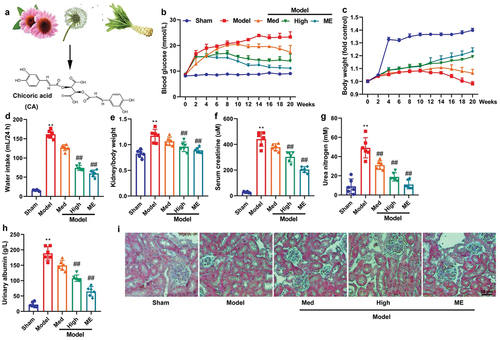
The current study aimed to examine the impact of CA on STZ-induced DN in mice. The chemical structure of CA is illustrated in . Treatment with CA or ME resulted in reduced blood glucose levels, increased body weight, suppressed water intake, and kidney/body weight ratio, decreased serum creatinine levels, lowered urea nitrogen and urinary albumin levels, and alleviated glomerulus injury in the STZ-induced mouse model of DN (as depicted in ). Notably, these findings suggest that CA may have a beneficial effect in ameliorating DN in mice models.
Figure 1. CA improved DN in mice model.
Then, CA had a negligible impact on cell viability of CT26, HUVEC and RAW 264.7 cells (Figure S1A-S1C). We did not find any obvious systemic toxicity and pathological signs in the major organs (Figure S1D).
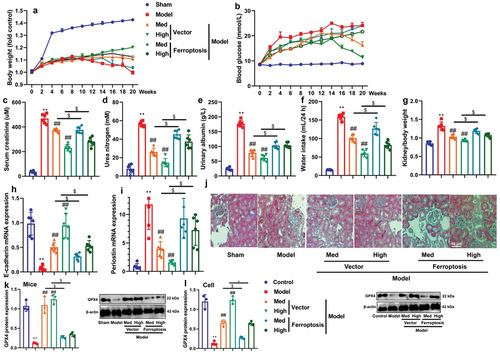
CA reduced kidney injury in amodel of DN
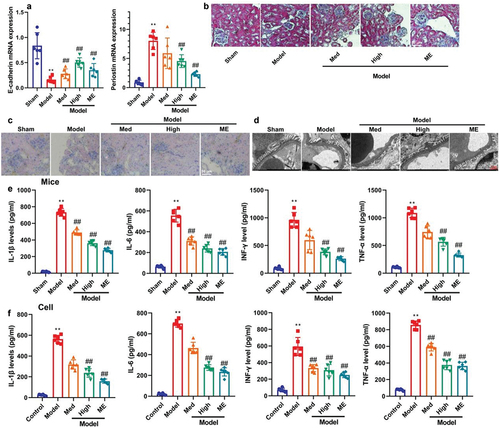
Next, this study further investigated the regulatory effects of CA on kidney injury in a model of DN. Treatment with CA or ME increased E-cadherin mRNA expression and reduced periostin mRNA expression in mice models of DN (). Additionally, MASS and PAS staining demonstrated that CA or ME ameliorated glomerular injury in STZ-induced DN (). Furthermore, electron microscopy revealed that CA or ME inhibited the masson-positive area and effacement of the podocyte foot processes in STZ-induced mice models of DN (). Importantly, CA or ME also effectively reduced inflammation levels in STZ-induced mice models of DN and in vitro models (). Collectively, CA may have the potential to mitigate kidney injury and inflammation in models of DN.
Figure 2. CA reduced acute kidney injury (AKI) and inflammation in mice model or in vitro model.
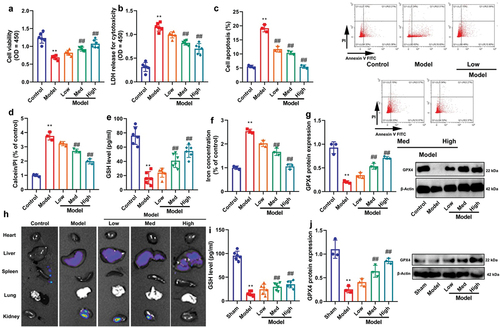
CA reduced high glucose-induced ferroptosis of NRK-52E through the inhibition of mitochondrial damage
This experiment investigated the biological function of CA in DN models. Treatment with CA was found to have several beneficial effects. Firstly, it promoted cell growth and reduced LDH activity levels and apoptosis rates in high glucose-treated NRK-52E cells (). Additionally, CA also increased GSH activity levels and GPX4 protein expression, and inhibited iron concentration in high glucose-treated NRK-52E cells (). Moreover, live imaging demonstrated that CA reduced ROS levels in the kidneys of STZ-induced DN mice models (). Furthermore, CA upregulated GSH activity levels and GPX4 protein expression in the kidney tissue of STZ-induced DN mice models (). CA increased calcein levels and JC-1 disaggregation, and reduced mitochondrial damage in high glucose-treated NRK-52E cells (). Overall, these findings suggest that CA may attenuate high glucose-induced ferroptosis of NRK-52E cells through the inhibition of mitochondrial damage by inducing the GSH/GPX4 axis, thereby protecting against kidney injury in models of DN.
Figure 3. CA reduced high glucose-induced ferroptosis of NRK-52E through the induction ferroptosis of GSH/GPX4 axle.
Ferroptosis activator reduced the effects of CA on DN
However, it remained uncertain whether ferroptosis played a crucial role in the mechanisms by which CA exerted its effects on DN. To address this, a ferroptosis activator (50 mg/kg of Sorafenib Tosylate) was administered to DN mice models in conjunction with CA treatment. The results showed that the ferroptosis activator reversed the effects of CA on body weight, blood glucose levels, and AKI (). Additionally, treatment with a ferroptosis activator (5 μM of CIL56 or 50 mg/kg of Sorafenib Tosylate) suppressed GPX4 protein expression in mice models or in vitro models treated with CA (). Similarly, in an in vitro model, treatment with a ferroptosis activator (5 μM of CIL56) hindered the effects of CA on high glucose-induced ferroptosis of NRK-52E cells (Figure S1). These findings suggest that CA may mitigate high glucose-induced ferroptosis of NRK-52E cells in DN models.
Figure 4. Ferroptosis activator reduced the effects of CA on DN in mice model or in vitro model.
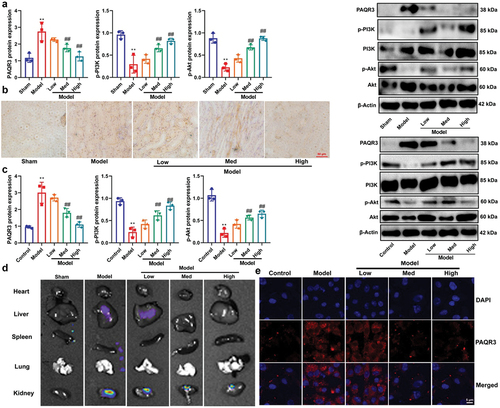
CA suppressed PAQR3 expression in model of DN to promote PI3K/AKT axle
Next, we investigated the mechanism by which CA reduces ferroptosis in DN. CA was found to suppress the expression of PAQR3 mRNA/protein and induce the expression of p-PI3K/p-AKT proteins in the kidney tissue of STZ-induced DN mice models or in an in vitro model of DN (). Meanwhile, immunohistochemistry and immunofluorescence showed that CA reduced PAQR3 expression level in kidney tissue of STZ-induced mice model of DN or in NRK-52E by high glucose (). Bioluminescent imaging showed the CA suppressed PAQR3 expression level in kidney tissue of STZ-induced mice model of DN (). Notably, these findings suggest that CA suppresses PAQR3 expression in DN models, thereby promoting the PI3K/AKT pathway.
Figure 5. CA suppressed PAQR3 expression in model of DN to promote PI3K/AKT axle.
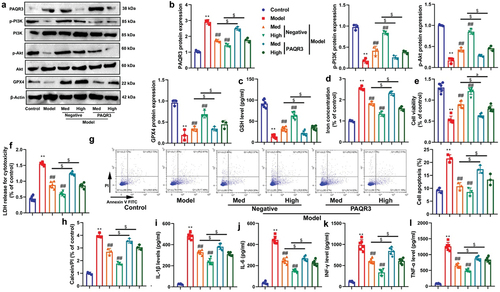
PAQR3 activator reduced the effects of CA on DN in vitro model
To investigate the functional involvement of PAQR3 in the effects of CA in DN models, we conducted experiments in which we upregulated PAQR3 expression levels through PAQR3 plasmid transfection and treated NRK-52E cells induced with glucose with CA. Our results showed that PAQR3 upregulation inhibited the activation of the PI3K/AKT/GPX4 axis induced by CA treatment, as evidenced by the suppression of protein expression levels (as shown in ). Additionally, PAQR3 upregulation reduced GSH levels and cell growth, while increasing iron concentration and LDH activity in NRK-52E cells induced with glucose by CA treatment (). Moreover, PAQR3 upregulation increased apoptosis and inflammation levels, and promoted the proportion of PI positive cells in NRK-52E cells induced with glucose by CA treatment (). Collectively, our findings suggest that PAQR3 plays an activator role in the effects of CA on DN.
Figure 6. PAQR3 activator reduced the effects of CA on DN in vitro model.
CA directly targeted the PAQR3 protein to advance ubiquitination of PAQR3 protein
This experiment aimed to elucidate the mechanism of CA targeting the PAQR3 protein. As shown in , analysis of the drug-protein linkage revealed that CA binds to PAQR3 protein. We mutated the PAQR3 protein at positions 13-GLU, 14-GLU, 29-ARG, 30-LEU, 35-GLN, 211-SER, 244-TYR, and 254-TYR (). Upon binding with CA, the thermophoretic motion of PAQR3 was affected, and the melting temperature of PDPK1 was elevated from ~ 55 to ~ 60°C (). This enhancement in thermal stability was observed in the wild-type PAQR3, while CA did not affect the thermal stability of the mutant PAQR3 at positions 13-GLU, 14-GLU, 29-ARG, 30-LEU, 35-GLN, 211-SER, 244-TYR, and 254-TYR (). In in vitro models of ALI, CA was found to accelerate PDPK1 ubiquitination (). These results suggest that CA reduces PAQR3 ubiquitination to induce PAQR3 activity, which may contribute to the effects of CA in the DN model.
Figure 7. CA directly targeted the PAQR3 protein to advance ubiquitination of PAQR3 protein.
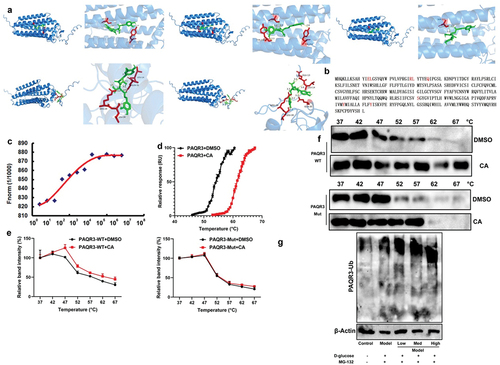
PI3K inhibitor the effects of CA on DN in a model
Based on the above results, it is likely that the effects of CA on DN in a DN model are mediated through the PI3K signaling pathway. Treatment with PI3K inhibitor LY294002 (at 25 mg/kg or 50 μM) suppressed the expression levels of p-PI3K/p-AKT/GPX4 proteins in the kidney tissue of STZ-induced DN mice models that were treated with CA or in vitro models treated with CA ( and S2A). Furthermore, treatment with PI3K inhibitor reversed the detrimental effects of CA on AKI and inflammation in STZ-induced DN mice models (). Similarly, treatment with PI3K inhibitor (at 50 μM of LY294002) impeded the positive effects of CA on GSH, iron concentration, cell growth, and increased LDH activity levels in NRK-52E cells induced by glucose (as illustrated in Figure S2B-S2G). Collectively, these findings suggest that CA activates the PI3K/AKT/GPX4 axis in both mice models and in vitro models of DN.
Figure 8. PI3K inhibitor the effects of CA on DN in mice model.
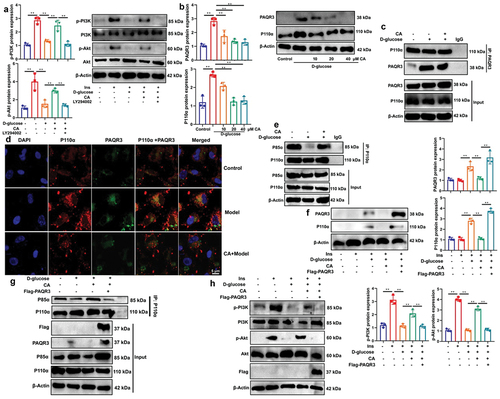
CA reduced the interaction between PAQR3 and P110α pathway to induce PI3K/AKT signaling pathway
we conducted experiments using a PI3K inhibitor (LY294002) in d-glucose-induced NRK-52E cells to elucidate the role of PAQR3 in the effects of CA on PI3K/AKT signaling pathway. As shown in , LY294002 reversed the effects of CA on p-PI3K/p-AKT proteins, indicating that CA functionally interacted with PI3K to induce the insulin pathway. Additionally, CA treatment reduced the distribution of PAQR3 and p110α in d-glucose-induced NRK-52E cells (). Moreover, co-immunoprecipitation (co-IP) verified that CA stimulation decreased the interaction between P110α protein and PAQR3 protein in NRK-52E cells, while treatment with CA diminished the interaction between P110α protein and PAQR3 protein (). Furthermore, immunofluorescence also showed that CA treatment reduced the co-localization of PAQR3 protein and p110αprotein in NRK-52E cells (). Meanwhile, co-IP results indicated that CA also increased the interaction between p110α protein and p85α protein in d-glucose-induced NRK-52E cells, implying that CA treatment promoted the formation of a PI3K dimer (). Furthermore, PAQR3 up-regulation reversed the effects of CA on the inhibition of PAQR3/P110α distribution in d-glucose-induced NRK-52E cells (). Additionally, PAQR3 up-regulation abolished the formation of the PI3K dimer (P110α/P85α) induced by CA treatment (). As expected, PAQR3 up-regulation also reversed the CA-induced activation of the PI3K/AKT axis (). Therefore, CA promotes the PI3K/AKT signaling pathway by inhibiting the interaction between PAQR3 protein and the P110α protein in DN.
Figure 9. CA reduced the interaction between PAQR3 and P110α pathway to induce PI3K/AKT signaling pathway.
Discussion
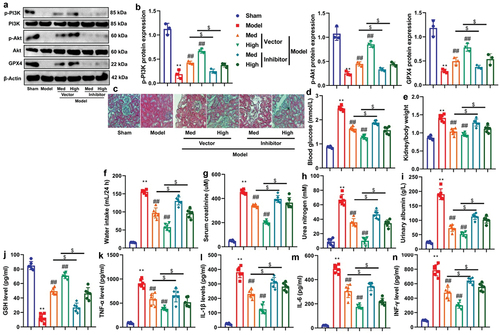
Diabetes can cause acute life-threatening complications such as ketoacidosis, non-ketotic hyperosmolar coma, and chronic complications such as coronary heart disease, retinopathy, nephropathy, neuropathy, dermatopathy, infection, and osteoporosis, seriously damaging the health of patients. At the same time, the incidence rate of diabetes is high and the number of patients is large, so the prevention and treatment of diabetes is very important.Citation34 On the one hand, people who have suffered from diabetes need to do a good job in chronic disease management, control blood sugar and reduce complications; On the other hand, timely screening should be carried out to find high-risk groups of diabetes. The risk of high-risk groups suffering from diabetes is higher than that of healthy people. Early guidance and intervention and good health management are beneficial to the prevention of diabetes. Currently, there are 24 million patients in China suffering from chronic kidney disease caused by diabetes, with diabetes nephropathy being the primary cause of progression to end-stage renal disease.Citation34 From 1990 to 2016, the mortality rate of all age diabetes nephropathy increased by 33%, which seriously affected the quality of life of patients.Citation35 The pathogenesis of diabetes nephropathy is not clear yet.Citation4 How to diagnose diabetes nephropathy early is the key link to prevent diabetes nephropathy from further aggravating.Citation36 Interestingly, our experiment revealed that CA or ME administration led to reductions in blood glucose levels, and prevented DN in a streptozotocin-induced mouse model of DN. Schlernitzauer et al. indicated that CA is one having potential anti-diabetic properties.Citation37 Therefore, these findings demonstrate for the first time that CA can ameliorate DN in a mouse model, suggesting a protective role for CA in DN.
In clinical research, diabetes nephropathy is the main factor leading to death and renal failure of diabetes patients; In a broad sense, diabetes nephropathy covers a variety of diseases, including vascular diseases and infectious diseases, among which vascular diseases include large vessels and microvascular diseasesCitation7. Among patients with microvascular diseases, glomerulosclerosis is the main representative, which can be divided into diffuse, nodular, and exudative diseases according to the specific pathological conditions of patients. Research has confirmed that patients with diabetes nephropathy are prone to renal failure in the late stage, even serious cardiovascular adverse events.Citation7 Therefore, in clinical treatment, emphasis is placed on the early diagnosis results of patients with diabetes nephropathy, which has a good guiding role in patient condition control and treatment. With the improvement of living standards, the prevalence of diabetes nephropathy has also increased.Citation7.Citation8 In the present study, CA demonstrated a reduction in kidney injury and inflammation in both mouse and in vitro models. Lee et al. suggest that CA has an anti-inflammatory effect in model of allergy-related inflammatory disorders.Citation38 Ding et al. showed that CA reduced renal tubular injury in model of chronic kidney disease.Citation39 Hence, it can be inferred that CA improves kidney injury by suppressing inflammation in the model of diabetic nephropathy, although the specific mechanism remains unclear. CA might been a potential drug for DN, however, the efficacy of CA is not as good as that of ME. In the next experiment, we will improve its activity and enhance its targeting effect.
Iron death, as a new type of programmed cell death, involves complex mechanisms. It has been proven that iron metabolism disorders, abnormalities in the SystemXc -/GSH/GPX4 pathway, oxidative stress caused by Nrf2 pathway inhibition, membrane lipid peroxidation, targeted induction of tumor suppressor p53, and abnormalities in the NADPH/FSP1/CoQ10 pathway are closely related to complications such as DM and DCM, DN, NAFLD, DPN, DR, etc, The above diseases can be treated from the perspective of intervention in iron death.Citation40 Compared to Western medicine, traditional Chinese medicine has the advantage of multiple pathways and targets, which can greatly improve the therapeutic effect, reduce the harm of toxic side effects to the body, and bring good news to the vast number of patients.Citation41 Ferroptosis is directly characterized by mitochondrial shrinkage and increased mitochondrial membrane density.Citation40.Citation41 This experiment demonstrated that CA reduced high glucose-induced ferroptosis in NRK-52E cells. Mechanistically, the ferroptosis activator attenuated the effects of CA on DN. Wang et al. suggested that CA inhibited ferroptosis in mice of LPS-induced endometritis.Citation42 These results demonstrate that CA may alleviate ferroptosis in DN models by inducing the GSH/GPX4 axis.
Mitochondria, as an important energy supplying organelle, perform important metabolic functions in cells and provide driving force for them. The types of cell death also include necrotic apoptosis, pyroptosis, and ferroptosis As for the mechanism of Ferroptosis, it is generally believed that it is related to the iron overload in the body and the disorder of oxidation/antioxidant system.Citation43 The biochemical manifestations are mainly antioxidant depletion, ROS accumulation, and the production of toxic lipid peroxidation product MDA.Citation43 Studies have shown that GPX4 is the key antioxidant enzyme used for lipid peroxide reduction in cells, and GSH is its substrate.Citation44.Citation44.Citation45 Additionally, we showed that CA suppressed PAQR3 expression in model of DN to promote PI3K/AKT/Nrf2/GPX4 axle. Importantly, PI3K inhibitor attenuated the effects of CA on DN. The present study is the first to report such a finding, CA directly targeted the PAQR3 protein to advance PAQR3 protein ubiquitination. Importantly, CA decreased the binding between PAQR3 and P110α protein, leading to an increase in P110α protein level or activity, which in turn enhanced the interaction between PAQR3 and P85 protein, resulting in the formation of PI3K dimer.
PI3K/AKT can regulate oxidative stress, cell apoptosis, inflammatory factors in the body, and regulate the development of diabetes nephropathy.Citation46 PI3K/AKT is the target for clinical treatment of diabetes nephropathy, which can inhibit the inflammatory reaction of renal tissue, alleviate proteinuria symptoms, and improve kidney damage.Citation47 Phosphorylated Akt can promote Keap1/Nrf2 nuclear transfer and antioxidant response element binding, promote downstream antioxidant system expression regulation of GPX4, thereby clearing oxygen-free radicals and inhibiting intestinal mucosal cell ferroptosis; At the same time, the clearance of oxygen-free radicals helps to restore the normal energy metabolism function of mitochondria, promote ATP production and increase ATPase activity.Citation48 Consequently, this facilitated the rapid formation of p-PI3K to induce PI3K/AKT signaling pathway. Ding et al. that CA alleviated acute lung injury through the promotion of GSH activity.Citation49 In this study, CA promoted PI3K/AKT/GPX4 axle through the inhibition of PAQR3 in model of DN. In this study, we have discovered that PAQR3 plays a crucial role as a target for CA. The next experiment uses mass spectrometry CETSA to achieve high-throughput detection of more targets. Meanwhile, the half-life of PAQR3 with or without CA is unclear. Our next experiment will mainly investigate the impact of CA on the half-life of PAQR3. Indeed, we did not use the PAQR3 CKO mice in this study to investigate the antidiabetic nephrotic activity of CA on PAQR3, which is a deficiency of this study. Our next step will be to supplement relevant research and improve the relevant conclusions.
Conclusions
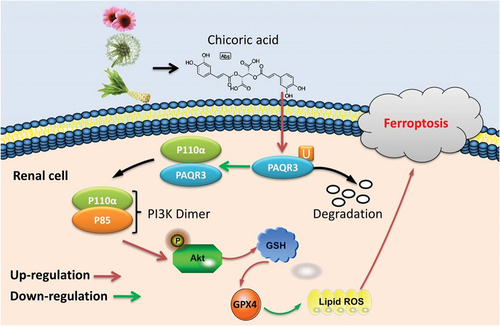
Our study revealed a new target of CA in inhibiting high glucose-induced ferroptosis in renal tubular epithelial cells. CA advanced PAQR3 ubiquitination to ameliorate mitochondrial damage-dependent Ferroptosis in DN through the activation of PI3K/AKT signaling pathways by the relieving of the interaction between PAQR3 protein and P110α protein (). Importantly, CA is a possible therapeutic option to relieve glucose-induced ferroptosis in diabetes for DN or other diabetes.
Authors’ contribution
Zhichen Pu and Yong Liu conceived the study, designed the study and prepared the manuscript. Yong Liu and Jiajun Zhou conducted the experiments and data analysis, involved in preparation of the figures and manuscript. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Abbreviations
| DN | = | Diabetic nephropathy |
| CA | = | Cichoric acid |
| P AQR3 | = | Progestin and adipo Q acceptor 3, |
| GPX4 | = | glutathione peroxidase 4 |
| PI3K | = | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| Akt | = | protein kinase B |
| Nrf2 | = | Nuclear factor E2-related factor 2 |
| HFD | = | high-fat diet |
| STZ | = | streptozotocin |
| Qpcr | = | Quantitative polymerase chain reaction |
| CCK8 | = | Cell counting kit‑8 |
| MST | = | Microscale thermophoresis |
| TSA | = | Thermal shift assay |
| CETSA | = | cellular thermal shift assay |
| co-IP | = | co-immunoprecipitation |
Acknowledgments
This work was supported by National Natural Science Foundation of China (811731333); Key Natural Science Projects of the Department of Education of Anhui province (2022AH051239, 2023AH051767); Talent Introduction Program of Yijishan Hospital of Wannan Medical College (YR202005); Science and Technology Innovation Team of Yijishan Hospital of Wannan Medical College (YPF2019016, YR20230105), and Key projects of Wannan Medical (WK2021ZF11, KY23740650). Thanks to the central laboratory for its support to this research.
Disclosure statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Funding
References
- Guo F, Song Y, Wu L, Zhao Y, Ma X, Wang J, Shao M, Ji H, Huang F, Fan X, et al. SUMO specific peptidase 6 regulates the crosstalk between podocytes and glomerular endothelial cells in diabetic kidney disease. Biochim Biophys Acta Mol Bas Dis. 2023;1869(5):166685. doi:10.1016/j.bbadis.2023.166685.
- Cook S, Schmedt N, Broughton J, Kalra PA, Tomlinson LA, Quint JK. Characterising the burden of chronic kidney disease among people with type 2 diabetes in England: a cohort study using the clinical practice research datalink. BMJ Open. 2023;13(3):e065927. doi:10.1136/bmjopen-2022-065927.
- Buckley LF, Schmidt IM, Verma A, Palsson R, Adam D, Shah AM, Srivastava A, Waikar SS. Associations between kidney histopathologic lesions and incident cardiovascular disease in adults with chronic kidney disease. JAMA Cardiol. 2023;8(4):357. doi:10.1001/jamacardio.2023.0056.
- Schmidt C. Confronting racial and ethnic disparities in diabetic kidney disease. Nature. 2023;615(7951):S8–14. doi:10.1038/d41586-023-00651-8.
- Wang Y, Liu L, Ge M, Cui J, Dong X, Shao Y. Acacetin attenuates the pancreatic and hepatorenal dysfunction in type 2 diabetic rats induced by high-fat diet combined with streptozotocin. J Nat Med. 2023;77(3):446–454. doi:10.1007/s11418-022-01675-6.
- Yin D, Guo Z, Zhang X. Identification of biomarkers and prediction of upstream miRnas in diabetic nephropathy. Front Endocrinol. 2023;14:1144331. doi:10.3389/fendo.2023.1144331.
- Aleksandrov N, Audibert F, Bedard MJ, Mahone M, Goffinet F, Kadoch IJ. Gestational diabetes insipidus: a review of an underdiagnosed condition. J Obstetrics And Gynaecology Canada: JOGC = Journal d’obstetrique Et Gynecologie du Canada: JOGC. 2010;32(3):225–31. doi:10.1016/S1701-2163(16)34448-6.
- Almalki MH, Ahmad MM, Brema I, Almehthel M, AlDahmani KM, Mahzari M, Beshyah SA. Management of diabetes insipidus following surgery for pituitary and suprasellar tumours. Sultan Qaboos Univ Med J. 2021;21(3):354–64. doi:10.18295/squmj.4.2021.010.
- Lv S, Li H, Zhang T, Su X, Sun W, Wang Q, Wang L, Feng N, Zhang S, Wang Y, et al. San-Huang-Yi-Shen capsule ameliorates diabetic nephropathy in mice through inhibiting ferroptosis. Biomed Pharmacother Biomed Pharmacotherapie. 2023;165:115086.
- Wei M, Liu X, Tan Z, Tian X, Li M, Wei J. Ferroptosis: a new strategy for Chinese herbal medicine treatment of diabetic nephropathy. Front Endocrinol. 2023;14:1188003. doi:10.3389/fendo.2023.1188003.
- Zhang S, Li Y, Liu X, Guo S, Jiang L, Huang Y, Wu Y. Carnosine alleviates kidney tubular epithelial injury by targeting NRF2 mediated ferroptosis in diabetic nephropathy. Amino Acids. 2023;55(9):1141–55. doi:10.1007/s00726-023-03301-5.
- Ma J, Li C, Liu T, Zhang L, Wen X, Liu X, Fan W. Identification of markers for diagnosis and treatment of diabetic kidney disease based on the ferroptosis and immune. Oxid Med Cell Longev. 2022;2022:1–21. doi:10.1155/2022/9957172.
- Wang YH, Chang DY, Zhao MH, Chen M, Zoratti M. Glutathione peroxidase 4 is a predictor of diabetic kidney disease progression in type 2 diabetes mellitus. Oxid Med Cell Longev. 2022;2022:1–10. doi:10.1155/2022/2948248.
- Lu Q, Yang L, Xiao JJ, Liu Q, Ni L, Hu JW, Yu H, Wu X, Zhang B-F. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radical Biol Med. 2023;195:89–102. doi:10.1016/j.freeradbiomed.2022.12.088.
- Pu Z, Shen C, Zhang W, Xie H, Wang W. Avenanthramide C from oats protects pyroptosis through dependent ROS-Induced mitochondrial damage by PI3K ubiquitination and phosphorylation in pediatric pneumonia. J Agric Food Chem. 2022;70(7):2339–53. doi:10.1021/acs.jafc.1c06223.
- Zhang W, Wang W, Xu M, Xie H, Pu Z. GPR43 regulation of mitochondrial damage to alleviate inflammatory reaction in sepsis. Aging. 2021;13(18):22588–610. doi:10.18632/aging.203572.
- Liu Y, Chou FJ, Lang F, Zhang M, Song H, Zhang W, Davis DL, Briceno NJ, Zhang Y, Cimino PJ, et al. Protein kinase B (PKB/AKT) protects IDH-mutated glioma from ferroptosis via Nrf2. Clin Cancer Res. 2023;29(7):1305–1316.
- Cheng Y, Gao Y, Li J, Rui T, Li Q, Chen H, Jia B, Song Y, Gu Z, Wang T, et al. TrkB agonist N-acetyl serotonin promotes functional recovery after traumatic brain injury by suppressing ferroptosis via the PI3K/Akt/Nrf2/Ferritin H pathway. Free Radical Biol Med. 2023;194:184–98.
- Yang H, Fang X, Shen Y, Yao W, Chen D, Shen L. MiR-153-3p reduces extracellular matrix accumulation in high glucose-stimulated human glomerular mesangial cells via targeting PAQR3 in diabetic nephropathy. Endocrinologia, diabetes y nutricion. 2022;69(1):34–42. doi:10.1016/j.endinu.2021.03.005.
- Qiao S, Yang D, Li X, Li W, Zhang Y, Liu W. Silencing PAQR3 protects against oxygen-glucose deprivation/reperfusion-induced neuronal apoptosis via activation of PI3K/AKT signaling in PC12 cells. Life Sci. 2021;265:118806. doi:10.1016/j.lfs.2020.118806.
- Xiao H, Sun X, Lin Z, Yang Y, Zhang M, Xu Z, Liu P, Liu Z, Huang H. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm Sin B. 2022;12(6):2887–904. doi:10.1016/j.apsb.2021.12.023.
- Li X, Li M, Chen D, Shi G, Zhao H. PAQR3 inhibits proliferation via suppressing PI3K/AKT signaling pathway in non-small cell lung cancer. Arch Med Sci. 2018;14(6):1289–97. doi:10.5114/aoms.2017.72220.
- Tian L, Fu P, Zhou M, Qi J. Dandelion sterol improves diabetes mellitus-induced renal injury in in vitro and in vivo study. Food Sci Nutr. 2021;9(9):5183–97. doi:10.1002/fsn3.2491.
- Liu XJ, Hu XK, Yang H, Gui LM, Cai ZX, Qi MS, Dai C-M. A review of traditional Chinese medicine on treatment of diabetic nephropathy and the involved mechanisms. Am J Chin Med. 2022;50(7):1739–79. doi:10.1142/S0192415X22500744.
- Zhang L, Miao R, Yu T, Wei R, Tian F, Huang Y, Tong X, Zhao L. Comparative effectiveness of traditional Chinese medicine and angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and sodium glucose cotransporter inhibitors in patients with diabetic kidney disease: a systematic review and network meta-analysis. Pharmacol Res. 2022;177:106111. doi:10.1016/j.phrs.2022.106111.
- Jabłońska-Trypuć A, Krętowski R, Świderski G, Cechowska-Pasko M, Lewandowski W. Cichoric acid attenuates the toxicity of mesotrione. Effect on in vitro skin cell model. Environ Toxicol Pharmacol. 2020;77:103375. doi:10.1016/j.etap.2020.103375.
- Lai TW, Cheng HL, Su TR, Yang JJ, Su CC. Cichoric acid May play a role in protecting hair cells from ototoxic drugs. Int J Mol Sci. 2022;23(12):6701. doi:10.3390/ijms23126701.
- Wang Q, Lin B, Li Z, Su J, Feng Y, Gu J. Cichoric acid ameliorates monosodium urate-induced inflammatory response by reducing NLRP3 inflammasome activation via inhibition of NF-kB signaling pathway. Evidence-based complementary and alternative medicine: eCAM 2021. Evid Based Complementary Altern Med. 2021;2021:1–12. doi:10.1155/2021/8868527.
- Luo Z, Gao G, Ma Z, Liu Q, Gao X, Tang X, Gao Z, Li C, Sun T. Cichoric acid from witloof inhibit misfolding aggregation and fibrillation of hIAPP. Int J Biol Macromol. 2020;148:1272–79. doi:10.1016/j.ijbiomac.2019.10.100.
- Ding X, Jian T, Li J, Lv H, Tong B, Li J, Meng X, Ren B, Chen J. Chicoric acid ameliorates nonalcoholic fatty liver disease via the AMPK/Nrf2/NFκB signaling pathway and restores gut microbiota in high-fat-diet-fed mice. Oxid Med Cell Longev. 2020;2020:1–20.
- Kim M, Yoo G, Randy A, Kim HS, Nho CW. Chicoric acid attenuate a nonalcoholic steatohepatitis by inhibiting key regulators of lipid metabolism, fibrosis, oxidation, and inflammation in mice with methionine and choline deficiency. Mol Nutr Food Res. 2017;61(5). doi:10.1002/mnfr.201600632.
- Pu Z, Sui B, Wang X, Wang W, Li L, Xie H. The effects and mechanisms of the anti-COVID-19 traditional Chinese medicine, dehydroandrographolide from andrographis paniculata (Burm.F.) wall, on acute lung injury by the inhibition of NLRP3-mediated pyroptosis. Phytomed: Inter J Phytothera And Phytopharmacol. 2023;114:154753. doi:10.1016/j.phymed.2023.154753.
- Xiao H, Sun X, Lin Z, Yang Y, Zhang M, Xu Z, Liu P, Liu Z, Huang H. Gentiopicroside targets PAQR3 to activate PI3K/AKT signaling pathway and ameliorate glucose and lipid metabolism. Acta Pharm Sin B. 2022;12(6):2887–904. doi:10.1016/j.apsb.2021.12.023.
- Kim DL, Lee SE, Kim NH. Renal protection of mineralocorticoid receptor antagonist, finerenone, in diabetic kidney disease. Endocrinology And Metabolism (Seoul, Korea). 2023;38(1):43–55. doi:10.3803/EnM.2022.1629.
- Liu MC, Li JL, Wang YF, Meng Y, Cai Z, Shen C, Wang M-D, Zhao W-J, Niu W-Q. Association between thyroid hormones and diabetic kidney disease in Chinese adults. BMC Endocr Disord. 2023;23(1):56. doi:10.1186/s12902-023-01299-1.
- Wang X, Li Q, Sui B, Xu M, Pu Z, Qiu T, Zhang Z. Schisandrin a from Schisandra chinensis attenuates ferroptosis and NLRP3 inflammasome-mediated pyroptosis in diabetic nephropathy through mitochondrial damage by AdipoR1 ubiquitination. Oxid Med Cell Longev. 2022;2022:1–23. doi:10.1155/2022/5411462.
- Schlernitzauer A, Oiry C, Hamad R, Galas S, Cortade F, Chabi B, Casas F, Pessemesse L, Fouret G, Feillet-Coudray C, et al. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in caenorhabditis elegans. PLoS One. 2013;8(11):e78788. doi:10.1371/journal.pone.0078788.
- Lee NY, Chung KS, Jin JS, Bang KS, Eom YJ, Hong CH, Nugroho A, Park H-J, An H-J. Effect of chicoric acid on mast cell-mediated allergic inflammation in vitro and in vivo. J Nat Prod. 2015;78(12):2956–62. doi:10.1021/acs.jnatprod.5b00668.
- Ding XQ, Jian TY, Gai YN, Niu GT, Liu Y, Meng XH, Li J, Lyu H, Ren B-R, Chen J, et al. Chicoric acid attenuated renal tubular injury in HFD-Induced chronic kidney disease mice through the promotion of mitophagy via the Nrf2/PINK/Parkin pathway. J Agric Food Chem. 2022;70(9):2923–35. doi:10.1021/acs.jafc.1c07795.
- Wu Y, Chen Y. Research progress on ferroptosis in diabetic kidney disease. Front Endocrinol. 2022;13:945976. doi:10.3389/fendo.2022.945976.
- Wu Z, Li D, Tian D, Liu X, Wu Z, Gomes AV. Aspirin mediates protection from diabetic kidney disease by inducing ferroptosis inhibition. PLoS One. 2022;17(12):e0279010. doi:10.1371/journal.pone.0279010.
- Wang K, Gao S, Wang J, Yu F, Ye C. Protective effects of chicoric acid on LPS-induced endometritis in mice via inhibiting ferroptosis by Nrf2/HO-1 signal axis. Int Immunopharmacol. 2022;113(Pt B):109435. doi:10.1016/j.intimp.2022.109435.
- Wang Y, Bi R, Quan F, Cao Q, Lin Y, Yue C, Cui X, Yang H, Gao X, Zhang D, et al. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur J Pharmacol. 2020;888:173574.
- Li S, Zheng L, Zhang J, Liu X, Wu Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radical Biol Med. 2021;162:435–49. doi:10.1016/j.freeradbiomed.2020.10.323.
- Tan H, Chen J, Li Y, Li Y, Zhong Y, Li G, Liu L, Li Y. Glabridin, a bioactive component of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol Med. 2022;28(1):58. doi:10.1186/s10020-022-00481-w.
- Ghaffari-Nasab A, Ghiasi F, Keyhanmanesh R, Roshangar L, Salmani Korjan E, Nazarpoor N, Mirzaei Bavil F. Bone marrow-derived c-kit positive stem cell administration protects against diabetes-induced nephropathy in a rat model by reversing PI3K/AKT/GSK-3β pathway and inhibiting cell apoptosis. Mol Cell Biochem. 2023. doi:10.1007/s11010-023-04750-y.
- Kong Z, Xiao M, Wang B, Zhang W, Che K, Lv W, Wang Y, Huang Y, Zhao H, Zhao Y, et al. Renoprotective effect of isoorientin in diabetic nephropathy via activating autophagy and inhibiting the PI3K-AKT-TSC2-mTOR Pathway. Am J Chin Med. 2023;51(5):1269–91. doi:10.1142/S0192415X23500581.
- Zhao T, Xiang Q, Lie B, Chen D, Li M, Zhang X, Yang J, He B, Zhang W, Dong R, et al. Yishen Huashi granule modulated lipid metabolism in diabetic nephropathy via PI3K/AKT/mTOR signaling pathways. Heliyon. 2023;9(3):e14171. doi:10.1016/j.heliyon.2023.e14171.
- Ding H, Ci X, Cheng H, Yu Q, Li D. Chicoric acid alleviates lipopolysaccharide-induced acute lung injury in mice through anti-inflammatory and anti-oxidant activities. Int Immunopharmacol. 2019;66:169–76. doi:10.1016/j.intimp.2018.10.042.