ABSTRACT
Background
Several studies indicate that the cystathionine β-synthase (CBS) gene T833C, G919A and 844ins68 polymorphisms in the 8th exon region may be correlated with coronary artery disease (CAD) susceptibility, but the results have been inconsistent and inconclusive. Thus, a meta-analysis was conducted to provide a comprehensive estimate of these associations.
Methods
On the basis of searches in the PubMed, EMBASE, Cochrane Library, Wanfang, VIP, and CNKI databases, we selected 14 case – control studies including 2123 cases and 2368 controls for this meta-analysis. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated accordingly using a fixed-effect or random-effect model.
Results
The results indicated an increased risk between the CBS T833C gene polymorphisms and susceptibility to CAD under the dominant model (CC+CT vs. TT: OR = 1.92, 95% CI: 1.11 ~ 3.32), recessive model (CC vs. CT+TT: OR = 1.88, 95% CI: 1.17 ~ 3.03), and homozygous model (CC vs. TT: OR = 2.46, 95% CI: 1.04 ~ 5.83). In these three genetic models, no significant association was identified for CBS G919A (AA+AG vs. GG: OR = 1.48, 95% CI: 0.45 ~ 4.82),(AA vs. AG+GG: OR = 1.58, 95% CI: 0.93 ~ 2.70),(AA vs. GG: OR = 1.66, 95% CI: 0.40 ~ 6.92) or CBS 844ins68 (II+ID vs. DD: OR = 1.04, 95% CI: 0.80 ~ 1.35),(II vs. ID+DD: OR = 1.09, 95% CI: 0.51 ~ 2.36),(II vs. DD: OR = 1.10, 95% CI: 0.51 ~ 2.39).
Conclusions
This meta-analysis suggests that the CBS T833C gene polymorphism is significantly associated with the risk of CAD and it shows a stronger association in Asian populations. Individuals with the C allele of the CBS gene T833C polymorphism might be particularly susceptible to CAD.
Background
Coronary artery disease (CAD) is a disease involving the formation of atherosclerotic plaques in coronary vessels caused by a variety of factors and may be characterized by stable angina, unstable angina, myocardial infarction, and sudden cardiac deathCitation1,Citation2. CAD has become a public health challenge and is a leading cause of morbidity and mortality in adults in both industrialized and developing countries around the worldCitation3. According to the World Health Organization (WHO), approximately 7.3 million people die of CAD each yearCitation4. Both genetic and environmental factors are involved in the pathogenesis of CAD, and genetic factors can explain 30% to 60% of the CAD riskCitation5,Citation6. Genome-wide association studies (GWAS) have identified some genetic variants that contribute to the risk of CAD among the general populationCitation7.
Hyperhomocysteinemia has been associated with a high risk of CADCitation8. Homocysteine (Hcy) damages vascular endothelial cells, stimulates vascular smooth muscle cell proliferation, increases platelet adhesion and aggregation, promotes thrombosis and is an important risk factor for atherosclerosis and cardiovascular diseaseCitation9–11. Cystathionine-β-synthase (CBS) is one of the key enzymes in the Hcy metabolic pathway. CBS catalyzes the transformation of serine and homocysteine to cystathionine and water. This enzyme exists in the form of a homotetramer, and each submit is combined with one heme and one pyridoxal 5’-phosphate (LPL) in the active form of CBSCitation12. KrugerCitation13 showed that specific CBS alleles are risk factors for vascular disease, and CBS gene mutations may affect the folding and stability of the encoded protein, reducing the enzyme activity of CBS, which affects the metabolism of Hcy and further leads to CADCitation14.
The CBS gene consists of 23 exons, is located on chromosome 21q22.3 and is 25–30 kbp long, encoding a protein of 551 amino acidsCitation15. Approximately 80% of CBS alleles are missense mutations, while the remainder are deletion alleles. To date, 164 pathogenic genetic mutations in CBS have been confirmed, most of which are located in the 3rd and 8th exons. Among the above mutations, the frequency of the T833C, G919A and 844ins68 mutations in the 8th exon is very high, and the 844ins68 mutation occurs in approximately 5% of CaucasiansCitation16. The role of CBS gene polymorphisms in the development of CAD has been widely studied in patients, and studies on the correlation between the T833C, G919A and 844ins68 gene mutations and CAD have been reported. DilleyCitation17 showed that the T833C gene polymorphism exhibited no significant correlation with CAD. Xu and ChenCitation18 showed that the T833C gene polymorphism was an important genetic factor in CAD. XuCitation19 showed that the G919A gene polymorphism presented no significant correlation with CAD. ChenCitation20 considered the G919A mutation to be a risk factor for CAD, and both IqbalCitation21 and ZhangCitation22 found that the 844ins68 gene polymorphism was not associated with CAD.
These gene polymorphisms (T833C, G919A and 844ins68) associated with CAD all occur in the 8th exon region of the CBS gene. Their mutation rates are higher and better studied than those of other polymorphisms, but the results are still controversial and conflicting. It is very possible that a single study may be underpowered for the detection of polymorphisms related to disease risk, especially when the sample size is relatively small. Different types of study populations and study designs may also contribute to the disparate findings. To confirm the correlation between the CBS gene T833C, G919A and 844ins68 polymorphisms and CAD, we performed this meta-analysis by pooling all eligible studies to calculate an estimate of the overall CAD risk.
Methods
Search strategy
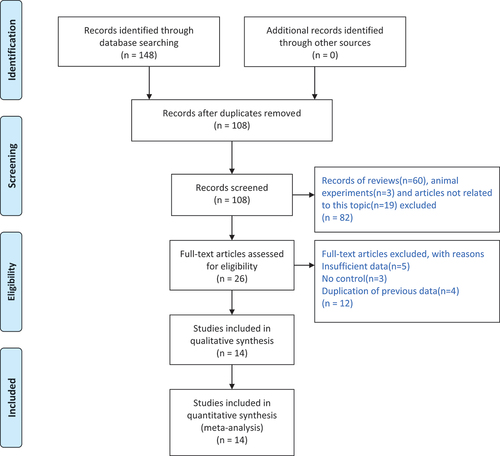
All studies on the association between the above three CBS gene polymorphisms (G919A, T833C, 844ins68) and CAD were identified via computer-based searches of the PubMed, Cochrane Library, Web of Science, CNKI, Wanfang and VIP databases, and the time period for the reference searches was from the first available article to January 27, 2024. The following key words were used for the searches: “cystathionine β synthase,” “cystathionine-β-synthase,” “cystathionine-β synthase,” “cystathionine β-synthase,” “cystathionine beta Synthase,” “cystathionine-beta-Synthase,” “cystathionine-beta Synthase,” “cystathionine beta-Synthase,” “CBS,” “myocardial infarction,” “coronary heart disease,” and “coronary artery disease.” The selection process is illustrated in .
Inclusion and exclusion criteria
Inclusion criteria: (1) study on the association between CBS gene polymorphisms and CAD; (2) case-control design; (3) CAD patients were the case group, and healthy or non-CAD patients were the control group;(4) evaluation standard: the risk of CAD; (5) for repeatedly published studies, we chose the most realistic and accurate study.
Exclusion criteria: (1) multiple publications reporting the same finding; (2) abstracts, reviews, comments and animal studies; (3) a lack of sufficient data.
Data extraction
In this study, two evaluators independently screened the literature and extracted relevant information according to the inclusion and exclusion criteria by using EndNote software. In the case of disagreement, a decision was reached between these two evaluators or made by another evaluator. The information extracted from the studies included the first author’s name, the year of publication, the country of the study, the ethnicity of the subjects, the diagnostic method, the number of cases in the coronary artery disease group and the control group, and the numbers of each genotype identified.
Study quality assessment
The quality of the included studies was independently assessed by the Newcastle-Ottawa Quality Assessment Scale (NOS)Citation23. The evaluation project consisted of 8 parts, and except for the fifth evaluation criterion of 2 points, the scores of the other items were all 1 point. The total scores of the NOS range from 0 points to 9 points. If the total score of the 8 items is greater than or equal to 7 points, the quality of studies is considered reliable.
Statistical analysis
The statistical analysis was performed by using Review Manager 5.3. Pooled odds ratios and 95% confidence intervalsCitation24 were applied to estimate the strength of associations between the CBS gene polymorphisms and CAD risk. The heterogeneity between the included studies was judged on the basis of P and I2 values. Values of p > .10 and I2 <50% indicated that the fixed effect model could be used in this meta-analysis, however, values of p < .10 and I2 >50% indicated the existence of significant heterogeneityCitation25. We identified the source of heterogeneity and performed further analysis, and the random effects model was used after excluding the effects of significant clinical heterogeneity. To further study the effect of sample size, diagnosis methods and ethnicity on heterogeneity, subgroup analysis was performed. The potential publication bias of this literature was investigated by Begg‘s test and Egger’s liner regression test, a p value of less than 0.05 was considered to showed statistically significant publication bias
Results
Study characteristics
A total of 148 studies were identified through database searching. After duplicates removed, leaving 108 studies for screening. Then, 82 studies were excluded since they were reviews, animal experiments and studies not related to this topic. After detailed evaluation, 12 studies were excluded because of insufficient data, no control and duplication of previous data. Ultimately, a total of 14 publications involving 2123 CAD cases and 2368 controls finally met the inclusion criteria and were analyzed. The subjects of 13 studies were Asian, and only 1 studyCitation17, on the CBS T833C gene polymorphism, examined North American subjects. Among the 14 studies, 4 studies involving 612 cases and 500 controls addressed the CBS T833C gene polymorphism, 3 studies including 427 cases and 331 controls addressed the CBS G919A gene polymorphism, and 4 studies comprising 1084 cases and 1537 controls addressed the CBS 844ins68 gene polymorphism. Study characteristics is shown in .
Table 1. Characteristics of the included studies in the meta-analysis.
Quality evaluation results
According to the NOS quality evaluation system, the quality of the 14 case-control studies was further evaluated in detail. The results showed that the total scores of the 14 studies included were all ≥ 7, which indicated that the 14 included studies were reliable. Detailed information on the studies is listed in .
Table 2. NOS quality evaluation form.
CBS T833C polymorphism
Four studiesCitation17,Citation18,Citation20,Citation26 addressing the correlation between 844ins68 and CAD were included in the analyses, among which 3 papers were from China, and 1 paper was from America, involving 612 CAD cases and 500 controls. In these 4 studies, there were 64 CC genotypes and 340 TT genotypes in the CAD group, and there were 28 CC genotypes and 348 TT genotypes in the control group (). Taking CC as the exposure factor and TT as the nonexposure factor, a random effects model was used to combine the data. The results showed that the T833C CC genotype was associated with susceptibility to CAD (CC vs. TT: OR = 2.46, 95% CI: 1.04 ~ 5.83). There were 272 CC+CT genotypes and 340 TT genotypes in the CAD group, and there were 152 CC+CT genotypes and 348 TT genotypes in the control group. Taking CC+CT as the exposure factor and TT as the nonexposure factor, a random effects model was used to combine the data. The results showed that the T833C CC+CT genotype was associated with susceptibility to CAD (CC+CT vs. TT: OR = 1.92, 95% CI: 1.11 ~ 3.32). There were 64 CC genotypes and 348 CT+TT genotypes in the CAD group, and there were 28 CC genotypes and 472 CT+TT genotypes in the control group. Taking CC as the exposure factor and CT+TT as the nonexposure factor, a fixed effects model was used to combine the data. The results showed that the T833C CC genotype was associated with susceptibility to CAD (CC vs. CT+TT: OR = 1.88, 95% CI: 1.17 ~ 3.03).
Table 3. Distributions of the genotypes of the CBS T833C、G919A、844ins68.
Overall, the results showed a significant association between the CBS T833C gene polymorphism and the risk of CAD under the homozygous model (CC vs. TT: OR = 2.46, 95% CI: 1.04 ~ 5.83), the dominant model (CC+CT vs. TT: OR = 1.92, 95% CI: 1.11 ~ 3.32) and the recessive model (CC vs. CT+TT: OR = 1.88, 95% CI: 1.17 ~ 3.03). (,Figure S2)
Table 4. Summary of odds ratios (95% CI) in the analysis of the association between the CBS polymorphism and CAD susceptibility in different models.
CBS G919A polymorphism
Three studiesCitation19,Citation20,Citation27 addressing the correlation between G919A and CAD were included in the analyses, all of which were from China, involving 427 CAD cases and 331 controls. Among these 3 studies, there were 46 AA genotypes and 138 GG genotypes in the CAD group, and there were 22 AA genotypes and 143 GG genotypes in the control group (). Taking AA as the exposure factor and GG as the nonexposure factor, a random effects model was used to combine the data. The results showed that the G919A AA genotype was not associated with the risk of CAD (AA vs. GG: OR = 1.66, 95% CI: 0.40 ~ 6.92). There were 289 AA+AG genotypes and 143 GG genotypes in the CAD group, and there were 188 AA+AG genotypes and 143 GG genotypes in the control group. Taking AA+AG as the exposure factor and GG as the nonexposure factor, a random effects model was used to combine the data. The results showed that the G919A AA+AG genotype was not associated with susceptibility to CAD (AA+AG vs. GG: OR = 1.48, 95% CI: 0.45 ~ 4.82). There were 46 AA genotypes and 381 AG+GG genotypes in the CAD group, and there were 22 AA genotypes and 309 AG+GG genotypes in the control group. Taking AA as the exposure factor and AG+GG as the nonexposure factor, a fixed effects model was used to combine the data. The results showed that the G919A AG+GG genotype was not associated with susceptibility to CAD (AA vs. AG+GG: OR = 1.58, 95% CI: 0.93 ~ 2.70).
Overall, the results showed that there was no association between the CBS G919A gene polymorphism and the risk of CAD under the homozygous model (AA vs. GG: OR = 1.66, 95% CI: 0.40 ~ 6.92), the dominant model (AA+AG vs. GG: OR = 1.48, 95% CI: 0.45 ~ 4.82) or the recessive model (AA vs. AG+GG: OR = 1.58, 95% CI: 0.93 ~ 2.70). (,Figure S2)
CBS 844ins68 polymorphism
Seven studiesCitation21,Citation22,Citation28–32 addressing the correlation between T833C and CAD were included in the analyses, among which 6 papers were from China, and 1 paper was from Pakistan, involving 1084 CAD cases and 1537 controls. Among these seven studies, there were 12 II genotypes and 972 DD genotypes in the CAD group, and there were 15 II genotypes and 1382 DD genotypes in the control group (). Taking II as the exposure factor and DD as the nonexposure factor, a fixed effects model was used to combine the data. The results showed that the 844ins68 II genotype was not associated with the risk of CAD (II vs. DD: OR = 1.10, 95% CI: 0.51 ~ 2.39). There were 112 II+ID genotypes and 972 DD genotypes in the CAD group, and there were 155 II+ID genotypes and 1382 DD genotypes in the control group. Taking II+ID as the exposure factor and DD as the nonexposure factor, a fixed effects model was used to combine the data. The results showed that the 844ins68 II+ID genotype was not associated with susceptibility to CAD (II+ID vs. DD: OR = 1.04, 95% CI: 0.80 ~ 1.35). There were 12 II genotypes and 1072 ID+DD genotypes in the CAD group, and there were 15 II genotypes and 1522 ID+DD genotypes in the control group. Taking II as the exposure factor and ID+DD as the nonexposure factor, a fixed effects model was used to combine the data. The results showed that the 844ins68 II genotype was not associated with susceptibility to CAD (II vs. ID+DD: OR = 1.09, 95% CI: 0.51 ~ 2.36).
Overall, the results showed that there was no association between the CBS G919A gene polymorphism and the risk of CAD under the homozygous model (II vs. DD: OR = 1.10, 95% CI: 0.51 ~ 2.39), the dominant model (II+ID vs. DD: OR = 1.04, 95% CI: 0.80 ~ 1.35) or the recessive model (II vs. ID+DD: OR = 1.09, 95% CI: 0.51 ~ 2.36). (,Figure S2)
Subgroup analysis results
We performed a subgroup analysis of the sample size, diagnosis methods and ethnicities included in the studies, and we found a significant association between T833C gene polymorphism and CAD in Chinese subjects but not in Americans under the homozygous, dominant and recessive genetic models (CC vs. TT: OR = 2.38, 95% CI: 1.38 ~ 4.10) (CC+CT vs. TT: OR = 2.12, 95% CI: 1.56 ~ 2.87) (CC vs. CT+TT: OR = 2, 95% CI: 1.17 ~ 3.43). In addition, a significant association between the CBS T833C gene polymorphism and the risk of CAD was observed in a large sample (CC vs. TT: OR = 1.93, 95% CI: 1.16 ~ 3.21) (CC+CT vs. TT: OR = 1.6, 95% CI: 1.21 ~ 2.10) (CC vs. CT+TT: OR = 1.69, 95% CI: 1.02 ~ 2.79) (). We also performed subgroup analysis on G919A and 844ins68, but there was no association between the G919A or 844ins68 gene polymorphism and CAD.
Table 5. Meta-analysis of the association between CBS T833C polymorphism and risk of CAD.
Publication bias
The Begg’s funnel plot and Egger’s linear regression test were performed to assess publication biases. As shown in Figure S3 and , this bias was observed under two genetic models (G919A’s Homozygote model and 844ins68’s Dominant model), the other showed no publication bias. Therefore, there was no strong statistical evidence of publication bias.
Table 6. The effect of publication bias was assessed by Begg’s and Egger’s linear regression tests.
Discussion
We performed a meta-analysis involving 14 case-control studies comprising 2123 cases and 2368 controls to comprehensively evaluate the effect of CBS gene polymorphism on CAD susceptibility. The results of our studies suggested that the CBS T833C gene polymorphism might be associated with the risk of CAD. There was no significant relationship between the CBS G919A and 844ins68 gene polymorphisms and the risk of CAD in the three genetic models examined in our study.
In the subgroup analysis of ethnicity, we found a significant association with Chinese ethnicity under the homozygous, dominant and recessive genetic models (OR = 2.38, 95% CI: 1.38 ~ 4.10) (OR = 2.12, 95% CI: 1.56 ~ 2.87) (OR = 2, 95% CI: 1.17 ~ 3.43). The subgroup analysis results suggested that CBS T833C may be an ethnicity-dependent factor in CAD progression. In addition, a significant association between the CBS T833C gene polymorphism and the risk of CAD was observed in a large sample under a homozygous genetic model (OR = 1.93, 95% CI: 1.16 ~ 3.21). Four studies on the CBS T833C gene polymorphism were included in the analyses, but the subjects of all but one of these studies were Chinese, while the fourth was conducted in American subjects. Based on the large sample, the results are likely to be more accurate and more credible.
According to some related studies, several possible molecular mechanisms can explain the above conclusion. T833C refers to a T to C transition at nucleotide position 833 in exon 8, causing a substitution of threonine for isoleucine at codon 278 (I278T). This mutation may change the conformation of the pyridoxal 5’-phosphate (coenzyme) binding site in CBS to affect the binding of the coenzyme to the enzyme, resulting in low CBS enzyme activity and high plasma homocysteine levels, leading to CADCitation14,Citation33. Many previous studies have resulted in similar conclusions, Nienaber’s research demonstrated that CBS T833C had no significant impact on homocysteine levels among black South AfricansCitation34. Nan’s studyCitation35, on the other hand, revealed that individuals with homozygous and heterozygous mutant genotypes of CBS T833C had higher homocysteine levels compared to healthy individuals with wild genotypes, specifically in patients with cerebral arterial thrombosis. Additionally, for children with idiopathic mental retardation, those with the wild homozygous CBS T833C genotype had higher homocysteine levels compared to those with the heterozygous mutant genotypeCitation36. Furthermore, Chang’s study illustrated that individuals carrying the CBS T833C allele, specifically those with the CT and CC genotypes, had a higher prevalence compared to individuals with the TT genotypeCitation37. Furthermore, the identification of the T833C mutation in the CBS gene revealed a notable disparity in both genotype and allele frequency distribution when compared to the control group. These findings provide evidence to suggest that the T833C mutation in the CBS gene, which is associated with elevated homocysteine levels, may serve as a significant genetic risk factor for cerebral infarctionCitation38. In a case-control study conducted by Song, it was observed that plasma homocysteine levels varied across different genotypes of the CBS gene T833C in a cohort of 570 patients with ischemic cerebrovascular disease and healthy individualsCitation39, their results are consistent with our results. G919A is a G to A transition at nucleotide position 919 in exon 8 of CBS, resulting in the substitution of serine for glycine at codon 307 (G307S). This mutation may be located at the homocysteine binding site, which makes it difficult for the CBS enzyme to bind homocysteine, causing a high level of plasma homocysteineCitation14. It is generally believed that the G919A mutation leads to low activity of CBS and hypercysteinemia, but the results of our meta-analysis do not support this conclusion, and studies with a large sample size still need to be carried out. The 844ins68 variant is a 68-bp insertion consisting of an exact duplication at the intron-exon boundary of exon 8, and it is a common mutation among normal individuals. Since this mutation produces a new splice site, it may lead to the introduction of another splicing method that still forms the wild-type sequence of CBS. The mRNA size is still correct and does not affect transcription and translation, so CBS is not affected by this mutation. The activity and amount of the CBS enzyme remain normal, which will not lead to a high plasma homocysteine level or CADCitation40. The results of studies on 844ins68 are different, with many studies showing that 844ins68 is a protective factor for CAD, which is not consistent with the results of our study; therefore, additional studies will be needed to reach more convincing conclusions.
In recent years, there have been many studies on homocysteine metabolic enzyme gene polymorphisms and CAD susceptibility. A study by Singh and LeleCitation41 indicated MTRR and BHMT gene polymorphisms may not be associated with CAD, while ChenCitation42 showed that the MTR A2756G gene polymorphism is associated with the risk of CAD in a European population. Our study shows that the CBS T833C gene polymorphism is associated with CAD. The family aggregation phenomenon of CAD and the observed racial differences suggest that genes play an important role in the risk of CAD, and the prevalence of CAD differs widely among different populations. These studies provide objective medical evidence for the initial identification of individuals susceptible to CAD and suggest comprehensive diagnostic and prevention measures. In the future, we can use technology such as gene chip or gene sequencing approaches to help identify groups showing a high risk for CAD and perform early interventions to reduce the burden of CAD patients on society.
This study has some limitations. It involved a meta-analysis of only 14 case-control studies, so the sample size was relatively small, and false positive phenomena may have occurred. Most of the included studies did not provide detailed basic information that could be used for subgroup analysis, so additional factors affecting the prevalence of CAD were not accurately identifiedCitation43. In the 14 included studies, 13 consisted of Asian subjects, and only one consisted of an American population; therefore, no more accurate ethnic correlation could be performed. In addition, there was a difference in the severity of coronary artery disease among the patients in the control group. Therefore, additional well-designed studies are needed to further verify the association of CBS polymorphism with the risk of CAD, which may be helpful for formulating individualized prevention and treatment strategies for CAD in the futureCitation44.
Conclusion
In conclusion, the CBS T833C gene polymorphism is significantly associated with the risk of CAD and shows a stronger association in Asian populations. Individuals with the C allele of the CBS gene T833C polymorphism might be particularly susceptible to CAD. Neither CBS G919A nor 844ins68 present an association with CAD in the three applied models. Considering the limitations indicated above, more studies with larger sample sizes are needed to definitively determine the association between the CBS T833C, G919A and 844ins68 gene polymorphisms and the risk of CAD.
Author’ contributions
Conceptualization: ZJ Z, YH G, LM.
Data curation: ZJ Z, YH G, LM, YM M, KY Y.
Formal analysis: RT C, JL L, KY Y.
Fundingacquisition: ZJZ.
Investigation: ZJ Z, YH G, LM, RT C, JL L.
Methodology: ZJ Z, YH G.
Supervision: ZJ Z.
Writing – original draft: ZJ Z, YHG.
Writing – review& editing: ZJ Z, YH G, LM.
Acknowledgments: ZJ Z, YH G, LM, KY Y, YM M, RT C, JL L.
Consent for publication
All data relevant to the study are included in the article or uploaded as online supplementary information.
Ethics approval and consent to participate
Ethical approval was not necessary for this work.
Supplemental Material
Download Zip (526.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10641963.2024.2328147
Additional information
Funding
References
- Dai X, Wiernek S, Evans JP, Runge MS. Genetics of coronary artery disease and myocardial infarction. World J Cardiol. 2016;8(1):1–9. doi:10.4330/wjc.v8.i1.1.
- Elnaggar IZ, Hussein S, Amin MI, Abdelaziz EA. Association of 584C/T polymorphism in endothelial lipase gene with risk of coronary artery disease. J Cell Biochem. 2019;120(9):14414–14420. doi:10.1002/jcb.28697.
- Ross R, Epstein FH. Atherosclerosis — an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi:10.1056/NEJM199901143400207.
- Hamidizadeh L, Haji Hosseini Baghdad Abadi R, Babaee Baigi MA, Dastsooz H, Khazaei Nejhad A, Fardaei M. Impact of kif6 polymorphism rs20455 on coronary heart disease risk and effectiveness of statin therapy in 100 patients from southern iran. Arch Iran Med. 2015;18(10):683–687. PMID: 26443250.
- Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330(15):1041–1046. doi:10.1056/NEJM199404143301503.
- Girelli D, Martinelli N, Peyvandi F, Olivieri O. Genetic architecture of coronary artery disease in the genome-wide era: implications for the emerging “golden dozen” loci. Semin Thromb Hemost. 2009;35(7):671–682. doi:10.1055/s-0029-1242721.
- Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AFR, Barbalic M, Gieger C, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–338. doi:10.1038/ng.784.
- Belkahla R, Omezzine A, Kchok K, Rebhi L, Ben Hadj Mbarek I, Rejeb J, Ben Rejeb N, Slimane N, Nabli N, Ben Abdelaziz A, et al. Effet des polymorphismes des enzymes clés du métabolisme de l’homocystéine sur l’homocystéinémie et le risque coronarien chez une population tunisienne. Annales de Cardiologie et d’Angéiologie. 2008;57(4):219–24. doi:10.1016/j.ancard.2008.05.018.
- Zhongjun W, Defeng X, Zhengqin Y. Relationship between changes of the levels of plasma oxidized low density lipoprotein, homocysteine and circulating endothelial cells, endothelin in patients with coronary heart disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2003;31(1):46–48.
- Li F, Chen Q, Song X, Zhou L, Zhang J. MiR-30b is involved in the homocysteine-induced apoptosis in human coronary artery endothelial cells by regulating the expression of caspase 3. Int J Mol Sci. 2015;16(8):17682–17695. doi:10.3390/ijms160817682.
- Ungvari Z, Sarkadi-Nagy E, Bagi Z, Szollár L, Koller A. Simultaneously increased TxA(2) activity in isolated arterioles and platelets of rats with hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 2000;20(5):1203–08. doi:10.1161/01.ATV.20.5.1203.
- Jhee KH, Kruger WD. The role of cystathionine β-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005;7(5–6):813–822. doi:10.1089/ars.2005.7.813.
- D KW, A EA, Wang L, Malinow MR, Duell PB, Anderson PH, Block PC, Hess DL, Graf EE, Upson B, et al. Polymorphisms in the CBS gene associated with decreased risk of coronary artery disease and increased responsiveness to total homocysteine lowering by folic acid. Mol Genet Metab. 2000;70(1):53–60. doi:10.1006/mgme.2000.2993.
- Miles EW, Kraus JP. Cystathionine β-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004;279(29):29871–29874. doi:10.1074/jbc.R400005200.
- Tang FM, Li F. Correlation between polymorphism of cystathionine β-synthase C770T gene and coronary heart disease and its syndromes. Guangdong Med J. 2012;33(17):2673–75.
- Zhu H, Blake S, Chan KT, Pearson RB, Kang J. Cystathionine β-synthase in physiology and cancer. Biomed Res Int. 2018;2018:1–11. doi:10.1155/2018/3205125.
- Dilley A, Hooper W, C E-J, Mary Renshaw M, Wenger NK, Evatt BL. Mutations in the genes regulating methylene tetrahydrofolate reductase (MTHFR C→ T677) and cystathione β-synthase (CBS G→ A919, CBS T→ c833) are not associated with myocardial infarction in African Americans. Thromb Res. 2001;103(2):109–15. doi:10.1016/S0049-3848(01)00278-X.
- Xu HY, Chen ZJ. Study on polymorphism of homocysteine metabolism related enzyme gene in patients with coronary heart disease. Chin J Med. 1999;(6):13–15.
- Haiyan X, Yunxiang J, Zaijia C, Haibo L, Xiaoning L, Rutai H. Polymorphisms of methylenetetrahydrofolate reductase A1298C and cystathionine condensation enzyme G919A gene in patients with coronary heart disease. Liaoning J Med. 2007;21(2):57–58.
- Chen X, Liu KQ, Mu H. Study on the pathogenesis of cystathionine-β-synthase (CBS) gene and coronary heart disease. Tianjin Med J. 2003;31(3):158–160.
- Iqbal MP, Iqbal K, Tareen AK, Parveen S, Mehboobali N, Haider G, Iqbal SP. Polymorphisms in MTHFR, MS and CBS genes and premature acute myocardial infarction in a Pakistani population. Pak J Pharm Sci. 2016;29(6):1901.
- Zhang G, Dai C. Gene polymorphisms of homocysteine metabolism-related enzymes in Chinese patients with occlusive coronary artery or cerebral vascular diseases. Thromb Res. 2001;104(3):187–195. doi:10.1016/S0049-3848(01)00352-8.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis[J]. Stat Med. 2002;21(11):1539–58. doi:10.1002/sim.1186.
- Chen YL. Relationship between polymorphism of homocysteine and its key enzymes in coronary heart disease and coronary heart disease[D]. Urumqi: Xinjiang Medical University; 2006.
- Li LF, Li JP, Guo LG. Mutation frequency of cystathionine β-synthase gene G919A and C572T in patients with coronary heart disease. Med J. 2008;29:317–19.
- Yang LY, He Y, Yang DH, et al. Detection of homocysteine metabolic enzyme gene polymorphism in patients with coronary heart disease in Henan han population. J Zhengzhou Univ: Med Ed. 2011;46(1):67–70.
- Luo D, Yan SK, Cheng XQ, Song, YH. Relationship between homocysteine and cystathionine β-synthase gene polymorphisms and type 2 diabetes mellitus complicated with coronary heart disease. Lab Med. 2009;24(3):182–85.
- Li YH, Jiang BZ, Li HM, Li CS, Liu XH. Relationship between homocysteine level and cystathionine synthase (CBS 844ins 68) gene polymorphism and coronary heart disease. Anatomy. 2005;27(4):257–59.
- Jiao KL. Study on polymorphism of homocysteine-related metabolic enzymes in patients with coronary heart disease in Henan han population [D]. Zhengzhou: Zhengzhou University; 2006.
- He GJ. Correlation between atherosclerosis and homocysteine in populations in northeastern Sichuan [D]. Nanchong: North Sichuan Medical College; 2015.
- E SV, M FJ, Mandell R, Kraus JP, Berry GT, Heidenreich RA, Korson MS, Levy HL, Ramesh V. A missense mutation (I278T) in the cystathionine beta-synthase gene prevalent in pyridoxine-responsive homocystinuria and associated with mild clinical phenotype. Am J Hum Genet. 1995;57(1):34.
- Nienaber-Rousseau C, Ellis SM, Moss SJ, Melse-Boonstra A, Towers GW. Gene-environment and gene-gene interactions of specific MTHFR, MTR and CBS gene variants in relation to homocysteine in black south Africans. Gene. 2013;530(1):113–18. doi:10.1016/j.gene.2013.07.065.
- Nan GX, Wang LP. The correlation between MTHFR C677T and CBS T833C G919A gene mutations and ischemic stroke in young adults. Chin J Lab Diagn. 2007;(11):1538–40.
- Dutta S, Chatterjee A, Sinha S, Chattopadhyay A, Mukhopadhyay K. Correlation between cystathionine beta synthase gene polymorphisms, plasma homocysteine and idiopathic mental retardation in Indian individuals from Kolkata. Neurosci Lett. 2009;453(3):214–218. doi:10.1016/j.neulet.2009.02.040.
- Chang CD. The meta-analysis of the association between the key synthase in its metabolism and MTHFR, CBS gene variation with ischemic cerebral vascular diseases (ICVD) [D]. Changchun: Jilin University; 2014.
- Liu JZ, Cao CZ, Bao CM, Guo CH, Ji GD. The correlation between T833C mutation of the cystathionine ß-synthase and cerebral infarction. Chin J Cerebrovascular Dis. 2006;(1):31–33.
- Song YQ, Liu FQ, Zhang C. Correlation between cystathionineβ-synthase T833C gene polymorphism and ischemic cerebral vascular disease. Chin J Neurol. 2008;41(11):731–33.
- Franco RF, Elion J, Lavinha J, Krishnamoorthy R, Tavella MH, Zago MA. Heterogeneous ethnic distribution of the 844ins68 in the cystathionine β-synthase gene. Hum Hered. 1998;48(6):338–342. doi:10.1159/000022826.
- Singh PR, Lele SS. Folate gene polymorphisms MTR A2756G, MTRR A66G, and BHMT G742A and risk for coronary artery disease: a meta-analysis. Genet Test Mol Biomarkers. 2012;16(6):471–475. doi:10.1089/gtmb.2011.0237.
- Chen L, Liu L, Hong K, Hu J, Cheng X. Three genetic polymorphisms of homocysteine-metabolizing enzymes and risk of coronary heart disease: a meta-analysis based on 23 case–control studies. DNA Cell Biol. 2012;31(2):238–249. doi:10.1089/dna.2011.1281.
- Gao Y, Ge L, Ma X, Shen X, Liu M, Tian J. Methodology and reporting quality of Cochrane network meta-analyses provides the room to improve the network geometry and inconsistency. J Clin Epidemiol. 2019;113:214–27. doi:10.1016/j.jclinepi.2019.05.022.
- Chen Y, Yang K, Jing T, Tian J, Shen X, Xie C, Ma B, Liu Y, Yao L, Cao X, et al. Use of text messages to communicate clinical recommendations to health workers in rural China: a cluster-randomized trial. Bull World Health Organ. 2014;92(7):474–481. doi:10.2471/BLT.13.127076.